- 1Department of Neurology, Minhang Hospital, Fudan University, Shanghai, China
- 2State Key Laboratory of Medical Neurobiology, MOE Frontier Center for Brain Science, and Institutes of Brain Science, Fudan University, Shanghai, China
Background: To determine whether dizziness can contribute to stroke as a main cause still remains challenging. This study aims to explore clinical biomarkers in the identification of ischemic stroke patients from people with dizziness and the prediction of their long-term recovery.
Methods: From January 2018 to June 2019, 21 ischemic stroke patients with a main complaint of dizziness, 84 non-stroke dizziness patients and 87 healthy volunteers were recruited in this study. Then, their peripheral blood samples were collected, and the percentages of circulating lymphocytes T cells, CD4+ T cells, CD8+ T cells, T−/− cells (DNTs), CD4+ regulatory T cells (Tregs), CD8+ Tregs, B cells and regulatory B cells (Bregs) were examined to identify biomarkers with clinical value.
Results: According to our data, a significant difference in the DNTs proportion was detected between non-stroke dizziness and ischemic stroke patients with dizziness (p = 0.0009). The Bregs proportion in ischemic stroke patients with dizziness was lower than that in non-stroke dizziness patients (p = 0.035). In addition, the percentage of Bregs and DNTs within lymphocytes in patients’ peripheral blood exhibited a significant negative correlation with stroke occurrence (Bregs, p = 0.039; DNTs, p = 0.046). Moreover, the Bregs and DNTs within lymphocytes were negatively related to participants’ age, while presented a weak relationship with clinical risks like smoking, hypertension, and diabetes. Then, area under the receiver operating characteristic curve (AUC) of Bregs and DNTs together was 0.768, the risk factors and Bregs or DNTs ranged from 0.795 and 0.792, respectively, and the AUC value of risk factors, Bregs and DNTs combination was further increased to 0.815. Furthermore, the Bregs percentage within lymphocytes at admission was also a potential predictor of repair at discharge and the following 3 months.
Conclusion: Bregs and DNTs could be the clinical biomarkers together in the identification of ischemic stroke patients from people with dizziness.
Introduction
As a major cause of mortality and morbidity worldwide, stroke has been prioritized by World Health Organization in their efforts to reduce the burden of non-communicable diseases (Feigin et al., 2015, 2017). Stroke chameleons is a situation in which it is manifested by atypical stroke symptoms as opposed to hemiparesis, facial droop, or dysarthria (Arch et al., 2016; Venkat et al., 2018; Moulin and Leys, 2019). As one of the most common symptoms among stroke chameleons, dizziness is responsible for an estimated 4.4 million visits of emergency department in American annually, while about only 3%–5% of such visits are attributed to stroke (Saber Tehrani et al., 2013; Newman-Toker and Edlow, 2015). This situation is usually associated with a high cost and medical resource expenditure, because of neuroimaging examination in roughly half the patients (Newman-Toker et al., 2013b; Newman-Toker, 2016), high misdiagnosis rate of posterior circulation stroke with dizziness and the great impact on patients’ prognosis (Liberman and Prabhakaran, 2017). Currently, intravenous alteplase and mechanical thrombectomy are two recommended effective treatments for ischemic stroke patients (Yaghi et al., 2017; Munich et al., 2019). However, these two treatments are highly time-sensitive (Saver et al., 2016). Failure of correct stroke diagnosis in time may place barriers in the initiation of secondary stroke prevention, thus missing the opportunity to avoid a poor prognosis (Richoz et al., 2015). Therefore, an evidence-based and cost-effective approach to distinguish stroke categories is needed.
The immune response plays a key role in the pathophysiology of ischemic stroke (Macrez et al., 2011; Li et al., 2018). Previous studies have proved that the pathophysiological process of ischemic stroke is not only involved inflammatory in local brain, but also related to that in the peripheral inflammatory system (Buck et al., 2008; McColl et al., 2009). Additionally, markers of immune activation were observed in the patients’ peripheral blood after stroke, and activated lymphocytes were found in peripheral blood before presenting in the brain (Urra et al., 2009; Yan et al., 2009). Furthermore, the local inflammatory changes in the central nervous system of stroke patients can be reflected by the dynamic change of peripheral blood lymphocyte subpopulations (Guoping et al., 2015). At present, peripheral blood lymphocyte subpopulations have been reported to be predictors of short-term and long-term stroke outcomes (Li et al., 2021). However, there is no study on the value of lymphocyte subpopulations in the identification of cerebral infarction in patients with dizziness as the main complaint.
Herein, the aim of the present study is to analyze the characteristics of non-stroke dizziness patients and stroke patients with dizziness, and investigate the difference of the peripheral blood lymphocyte subpopulations in the two groups for reliable biomarkers in the identification of ischemic stroke patients from all dizziness patients.
Materials and methods
Ethics and participants
The clinical study was approved by the Ethical Review Board of Central Hospital of Minhang District (Shanghai, China). From January 2018 to June 2019, 21 stroke patients with a chief complaint of dizziness, and 84 patients with non-stroke dizziness were included according to following criteria: (1) more than 18 years old; (2) stroke patients with a final diagnosis of “cerebral infarction” confirmed by head CT or MRI; (3) non-stroke dizziness patients with a final diagnosis of “dizziness and vertigo”; (4) first onset; (5) onset time no more than 5 days; (6) informed consent was obtained from the patients or their legal representatives. Patients with brain hemorrhage, neoplasms, or acute infections after stroke were excluded. Clinical information of stroke patients was collected at admission, including age, gender, risk factors (hypertension, diabetes, disorder of lipid metabolism, atrial fibrillation, smoking, alcohol consumption, and cerebral infarction history). Control subjects were 87 age- and gender-matched healthy volunteers without a history of stroke or other vascular diseases.
Isolation of peripheral blood mononuclear cells
After the informed consent was obtained from participants or their legal representatives, peripheral blood samples were collected through forearm veins from the participants, and Ethylene Diamine Tetraacetic Acid (EDTA) vacutainer blood collection tube was used to keep those samples. For non-stroke dizziness and stroke patients, blood samples were collected within 24 h from admission. For healthy volunteers, blood samples were collected when they attended the physical examination. PBMCs were isolated by density gradient centrifugation within 3 h after receipt of peripheral blood samples. Technically, the blood samples were firstly diluted in a 1:1 ratio with PBS buffer, and then layered over Ficoll-Paque (GE Healthcare Bio Sciences) in a 15 ml centrifuge tube. After 30 min of centrifugation at 400g and 20°C, PBMCs were finally collected from the plasma-Ficoll interphase.
Flow cytometry analysis
Trypan blue exclusion method was used to quantify PBMCs, then 106–107 cells were labeled with fluorochrome-conjugated antibodies (BD Biosciences), including FITC Mouse Anti-Human CD3, APC-H7 Mouse Anti-Human CD4, APC Mouse Anti-Human CD25, Percp-cy5.5 Mouse Anti-Human CD19, PE Mouse Anti-Human CD24, PE Mouse Anti-Human CD127, APC Mouse Anti-Human CD27, Percp-cy5.5 Mouse Anti-Human CD8, PE Mouse Anti-Human CD45RA, APC Mouse Anti-Human CD62L, and PE-cy7 Mouse Anti-Human CD183. All samples were run on LSRFortessa (BD Biosciences) using FACSDiva software (version 8.0), and FlowJo (version 9.9.6) was used to analyze the data.
Statistical analysis
Patient characteristics and experimental data were presented as means ± standard deviations (SDs) or numbers (%). Correlations between cell proportion and multiple characteristics were analyzed using Mann–Whitney U test and Spearman’s correlation analysis as appropriate. The statistical significance of differences between experimental groups was assessed with the Brown-Forsythe and Welch ANOVA tests. All above statistical analyses were performed with GraphPad prism software (version 9.0). A forward stepwise binary logistic regression was performed with SPSS 25.0 software package (SPSS Inc., Chicago, Illinois). Variables showing significant association with stroke occurrence in univariate analysis (p < 0.1) and important confounders were included in the multivariate logistic regression analysis. Area under the receiver operating characteristic curve (AUC) was calculated and visualized with R-3.5.2 software1 using R package pROC (receiver operating characteristic curve). If not specified above, a two-tailed p < 0.05 was considered statistically significant.
Results
Clinical characteristics of participants
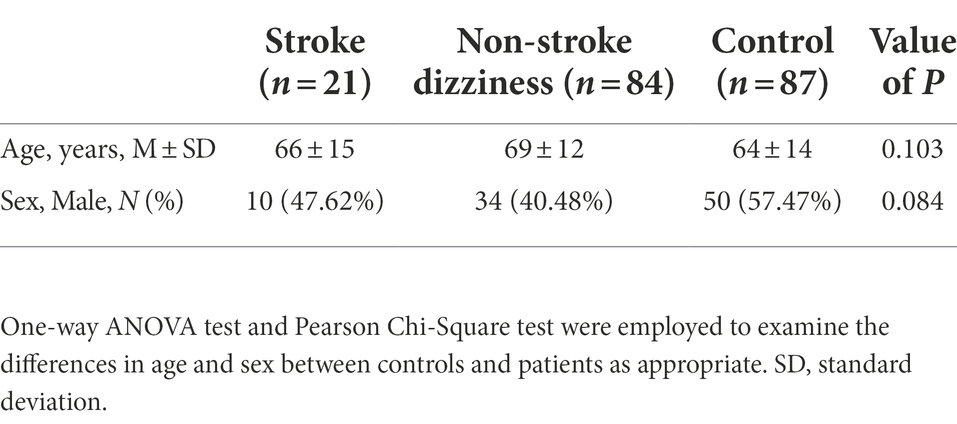
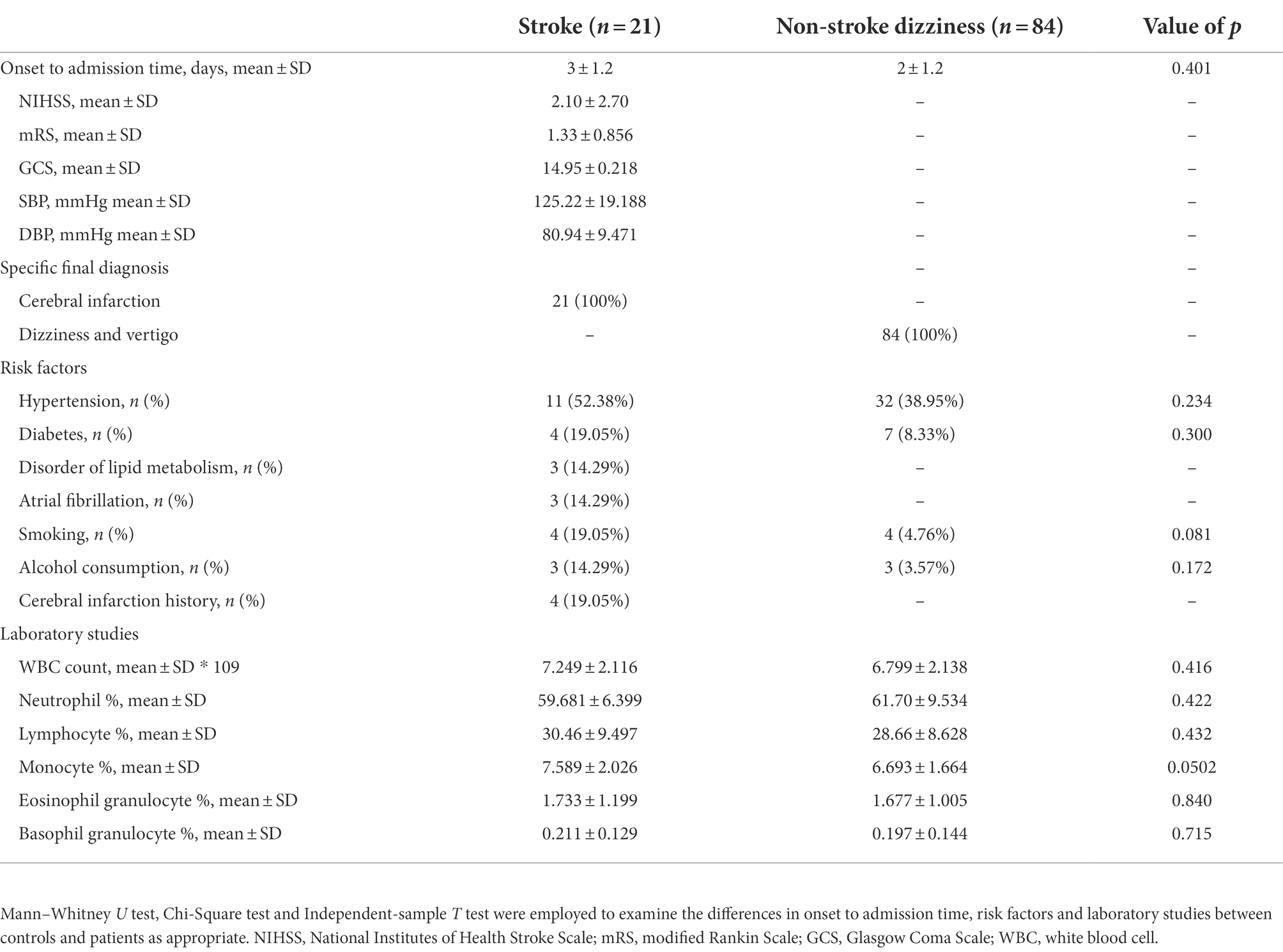
A total of 105 patients and 87 healthy controls were enrolled in our study, whose demographic characteristics were described in Table 1. No statistically significant difference was observed in either age or sex between the controls and patients. Among the 105 dizziness patients, 21 patients were diagnosed with ischemic stroke and 84 with dizziness and vertigo. The clinical characteristics of all patients were demonstrated in Table 2. There was no significant group difference between the 21 stroke patients (3 ± 1.2 days) and 84 non-stroke dizziness patients (2 ± 1.2 days) from the onset to admission time. All the neurological scores of stroke patients were recorded at admission by National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS), Glasgow Coma Scale (GCS). Additionally, all patients’ risk factors including hypertension, diabetes, smoking and alcohol consumption were also recorded. The data showed that no statistically difference was observed between the stroke and non-stroke dizziness patients in the history of various risk factors, including hypertension (p = 0.23), diabetes (p = 0.30), smoking (p = 0.08), and alcohol consumption (p = 0.17). Furthermore, no significant group difference existed between the stroke patients and non-stroke dizziness patients in the laboratory tests, namely white blood cell count (p = 0.42), neutrophil proportion (p = 0.42), lymphocyte proportion (p = 0.43), monocyte proportion (p = 0.05), eosinophil granulocyte proportion (p = 0.84) and basophil granulocyte proportion (p = 0.72). The specific final diagnosis and detailed radiological characteristics of stroke patients were described in Supplementary Table 1.
Changes of lymphocytic subpopulations in different groups
With the use of flow cytometry, different lymphocytic subpopulations among all controls, non-stroke dizziness and stroke patients were analyzed, including CD19+ cells (B cells), CD3+ cells (T cells), CD3+CD4+ cells (CD4+ T cells), CD3+CD8+ cells (CD8+ T cells), CD3+CD4−CD8− cells (Double Negative T cells, DNTs), and immunoregulatory lymphocytes, namely CD19+CD24+CD27+ cells (Bregs), CD3+CD4+CD25+CD127− cells (CD4+ Tregs) and CD3+CD45RA−CD8+CD183+CD62L+ cells (CD8+ Tregs, Figure 1A). It was found that no significant group difference was observed between the non-stroke dizziness and stroke patients in the aspects of B cells (12.1 ± 5.54 vs.12.4 ± 5.38, p = 0.995), T cells (64.4 ± 9.25 vs. 65.3 ± 12.72, p = 0.98), CD4+ T cells (37.3 ± 9.77 vs. 39.8 ± 13.55, p = 0.80) and CD8+ T cells (20.6 ± 8.02 vs. 19.3 ± 7.44, p = 0.86, Supplementary Figure 1). The cell proportion analysis of DNTs indicated a significant difference between non-stroke dizziness and stroke patients (2.6 ± 1.32 vs.1.8 ± 0.59, p = 0.0009), while there was no statistically difference between non-stroke dizziness patients and health controls (2.6 ± 1.32 vs. 2.4 ± 0.88, p = 0.59; Figures 1B,C). Besides, the immunoregulatory lymphocytes analysis indicated that the cell proportion of Bregs in stroke patients was lower than that in non-stroke dizziness patients (2.9 ± 1.41 vs. 2.0 ± 0.66, p = 0.035). However, there was no group difference found in CD4+ Tregs (2.2 ± 1.18 vs. 2.4 ± 0.87, p = 0.53) and CD8+ Tregs (1.7 ± 1.14 vs. 1.5 ± 0.65, p = 0.56; Figures 1B,C).
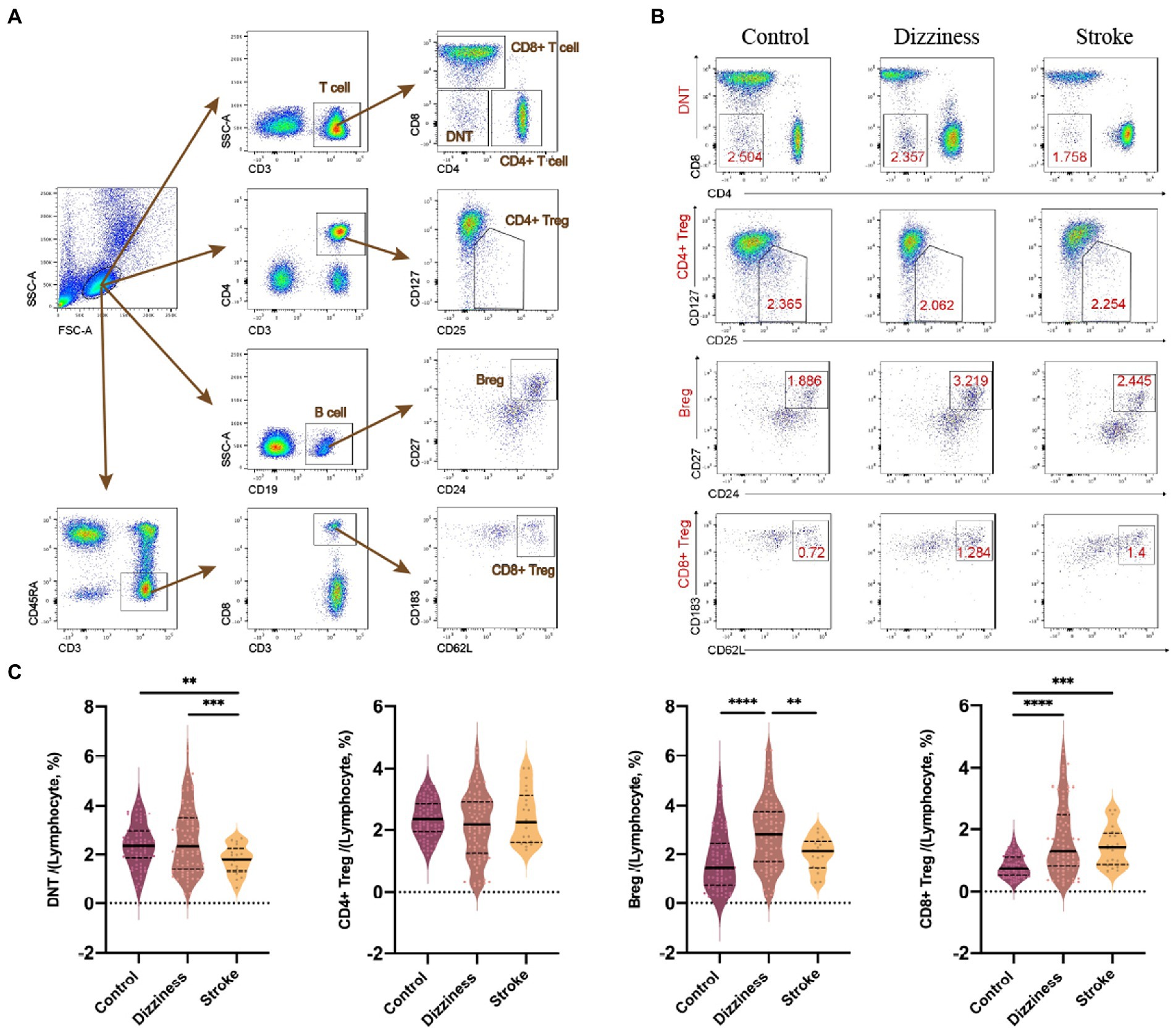
Figure 1. Changes of lymphocytic subpopulations in the control, dizziness and stroke groups. (A) Representative flow cytometry analyses on the total lymphocytes, B cells, T cells, CD4+ T cells, CD8+ T cells, Bregs, CD4+ Tregs, CD8+ Tregs, and DNTs. (B) Representative flow cytometry plot of DNTs, CD4+ Tregs, Bregs, and CD8+ Tregs in peripheral blood of control, dizziness, and stroke groups. (C) Violin plots of DNTs, CD4+ Tregs, Bregs, and CD8+ Tregs within lymphocytes of control (n = 87), dizziness (n = 84), and stroke (n = 21). **p < 0.01, ***p < 0.001, ****p < 0.0001.
Relationships between the lymphocytic subpopulations and stroke
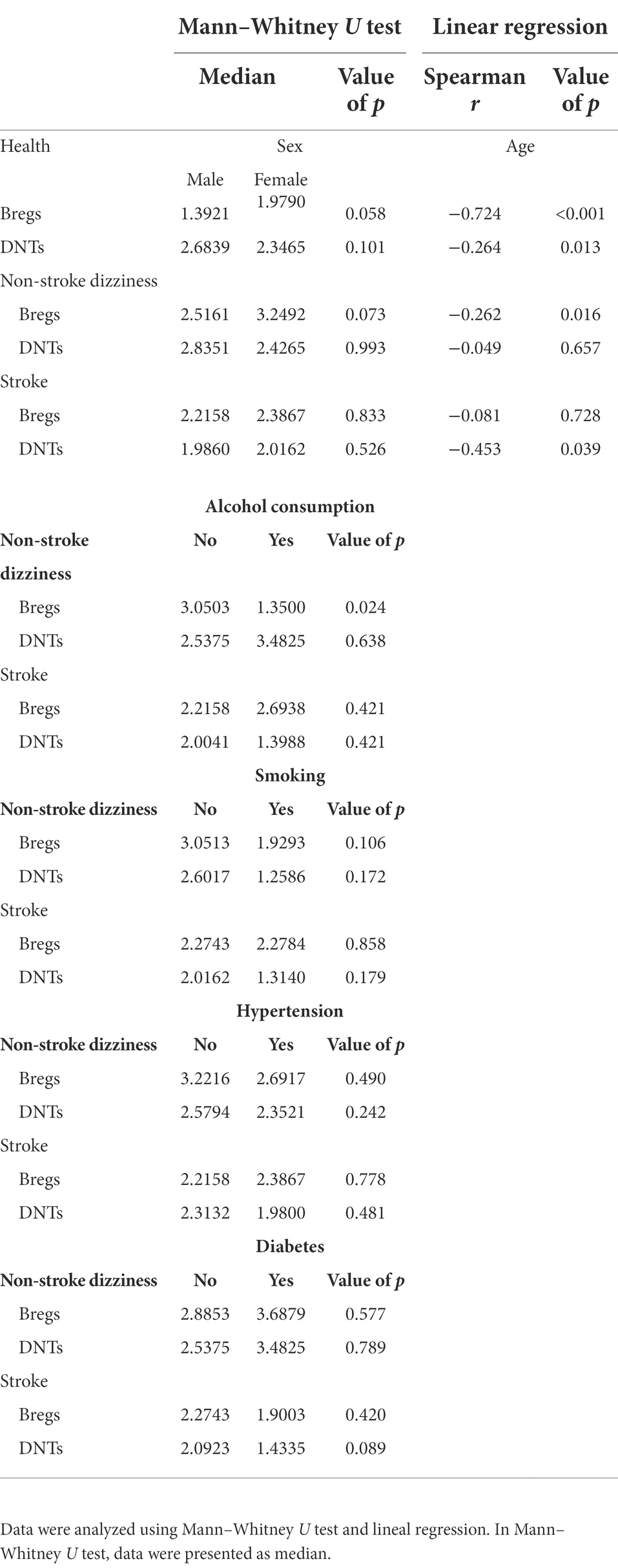
To further explore the potential value of regulatory lymphocytes in distinguishing stroke patients from dizziness patients, 5 independent variables (Bregs, DNTs, age, gender and smoking) were included in the forward stepwise binary logistic regression analyses. As shown in the Table 3, among the 5 independent variables, Bregs and DNTs had statistical significance with the stroke occurrence, while age, gender and smoking did not. The percentage of Bregs within lymphocytes in patients’ peripheral blood samples exhibited a significant negative relationship with stroke occurrence (p = 0.04). Similarly, the percentage of DNTs within lymphocytes also showed a significant negative relationship with stroke occurrence (p = 0.05). These results suggested that the lower level of Bregs or DNTs in dizziness patients at admission, the higher likelihood of stroke occurrence.
Relationships between lymphocytic subpopulations and clinical risks
The occurrence and progression of stroke are associated with multiple factors, including age, gender, and basic diseases. Blood leukocytes are also known to be influenced by a series of diseases or lifestyle habits. Thus, the relationships between regulatory lymphocytes and clinical risks were further assessed in order to clarify the dependability of Bregs and/or DNTs as biomarkers in predicting stroke occurrence. As shown in the Table 4, the percentage of Bregs and DNTs within lymphocytes in the healthy controls were negatively related to age, not gender difference. Similarly, the percentages of Bregs in the non-stroke dizziness patients and DNTs in the stroke patients were negatively related to age, not gender difference. Additionally, Bregs and DNTs presented a weak relationship with clinical risks like smoking, hypertension, and diabetes. It was found that only the percentage of Bregs in the non-stroke dizziness patients showed a significant difference in alcohol consumption. Non-stroke dizziness patients with alcohol consumption habit had significant lower Bregs than those without alcohol consumption habit. These results indicate that Bregs and DNTs are independent with most clinical risks, which proves their dependability as clinical biomarkers in stroke.
The dependability of Bregs and DNTs in stroke diagnosis
In order to evaluate the dependability of Bregs and DNTs in distinguishing stroke patients from non-stroke dizziness patients, the percentages of these two cells along with the clinical features were used to calculate ROC curves and AUC. The AUC of the Bregs and DNTs combination was 0.77, which was larger than that of clinical risk factors that related to stroke including age, gender, past history of hypertension, diabetes, habits of alcohol consumption and smoking (AUC = 0.72). Additionally, the AUC values of risk factors combined with Bregs or DNTs were 0.795 and 0.792, respectively. Moreover, the combination of Bregs, DNTs and clinical risk factors further elevated the AUC to 0.82 (Figure 2). These suggested that Bregs and DNTs could be used to facilitate the diagnosis of ischemic stroke in dizziness patients.
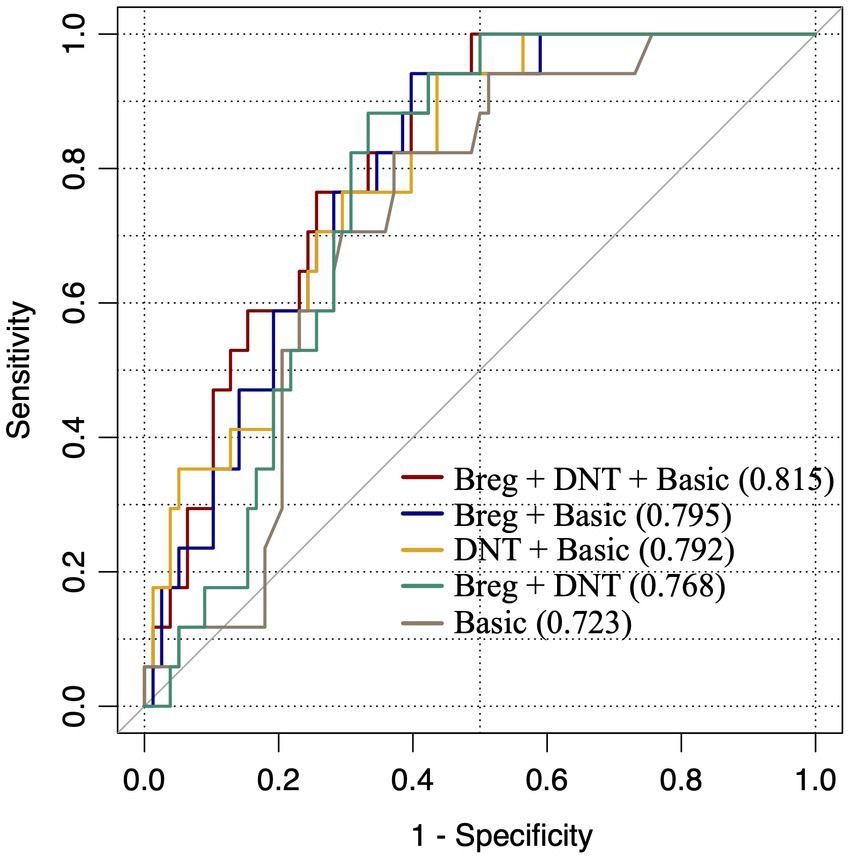
Figure 2. ROC curves to assess dependability of lymphocytic subpopulations in distinguishing stroke patients from dizziness patients. “Basic”: factors including gender, age, past history of hypertension, diabetes and habits of alcohol consumption and smoking; “Breg+DNT + Basic”: “Basic” adding DNTs/lymphocytes and Bregs/lymphocytes; “Breg+Basic”: “Basic” adding Bregs/lymphocytes; “DNT + Basic,” “Basic” adding DNTs/lymphocytes; “Breg+DNT”: Bregs/lymphocytes adding DNTs/lymphocytes.
Relationships between lymphocytic subpopulations and neural repair
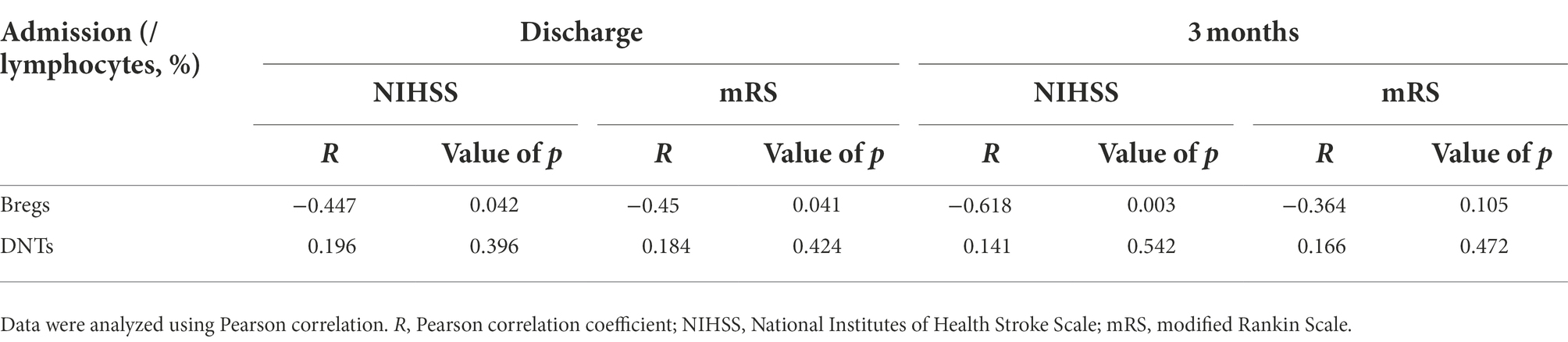
The values of Bregs and DNTs in predicting neural repair of stroke patients were further evaluated by Pearson correlation analysis (Table 5). The results suggested that stroke patients with higher percentage of Bregs within lymphocytes at admission had better neural repair at discharge [NIHSS: R = −0.45, p = 0.04; mRS: R = −0.5, p = 0.04] and the 3rd month [NIHSS: R = −0.62, p = 0.003; mRS: R = −0.36, p = 0.105], while DNTs were not related the repair process.
Discussion
According to previous studies, dizziness is a common reason for the emergency department (ED) visit, and stroke patients only account for about 3%–5% of all ED patients with dizziness (Newman-Toker and Edlow, 2015). Accurate screening of potential stroke patients from all dizziness patients is of paramount importance to ensure timely and appropriate treatment (Newman-Toker et al., 2013a,c). However, the identification of this small subset of stroke patients from dizziness patients represents a great challenge, and cost a large amount of medical resource (Lapchak and Zhang, 2017). The HINTS exam (Head Impulse, Nystagmus, Test of Skew) is a useful clinical tool with a series of ocular motor tests to discriminate the central and peripheral causes of dizziness (Newman-Toker and Edlow, 2015; Zwergal and Dieterich, 2020). Nevertheless, HINTS test is not appropriate for patients with acute transient vestibular syndrome, as the typical vestibular symptoms had already disappeared when they arrived at the hospital (Choi et al., 2017). Additionally, the HINTS test is of limited utility in ED without a neuro-ophthalmologist, as most ED physicians may be out of the ability to identify appropriate candidates and interpret the results of the exam (Choi et al., 2018; Dmitriew et al., 2021). Besides, the diffusion-weighted imaging sequence in MRI plays an important role in brain examination, and it has been proven to be sensitive in detecting cerebral infarction (Chalela et al., 2007). However, it is not cost-effective to take MRI scanning in all dizziness patients in order to screen out the suspected cerebral infarction patients (Wardlaw et al., 2004). Furthermore, it may not always be available for all patients to receive an MRI scan (Wardlaw et al., 2004). Therefore, it is necessary to find a reliable biomarker to assist with existing tests like HINTS, or to screen out most probable ischemic stroke patients who require further head MRI examination from all dizziness patients.
Routine blood test is an easy and convenient test in clinical practice. In the present study, 84 non-stroke dizziness patients and 21 stroke patients with dizziness were recruited, and the clinical data including white blood cells count, percentage of neutrophils, lymphocytes, monocytes, eosinophils and basophils were collected for further analysis. Although a significant difference of these inflammatory cells was found between non-stroke dizziness patients and controls, and stroke patients and controls, no significance difference existed between non-stroke dizziness patients and stroke patients. These results revealed that these inflammatory cells could reflect the unhealthy state of patients, but were unable to distinguish non-stroke dizziness and stroke dizziness. Then, we further collected the peripheral blood samples of patients within 24 h at admission after onset, and used flow cytometry to detect the change of different lymphocytic subpopulations. Notably, the changes in lymphocytic subpopulations were significant. Not only the differences of B cells, Bregs, CD8+ Tregs and DNTs between patients and controls, but also the differences of Bregs and DNTs between non-stroke dizziness and stroke groups were statistically significant.
Bregs are a subset of B cells that secrete interleukin-10 (IL-10; Seifert et al., 2018). Recent study has proven that Bregs can limit central nervous system inflammation in experimental stroke model, which benefits stroke patients (Bodhankar et al., 2013). In the present study, the level of Bregs was decreased in stroke patients, which was consistent with the previous study (Li et al., 2021). The Bregs level of non-stroke dizziness patients was higher than that of stroke patients, which indicated that Bregs could be a potential biomarker for distinguishing dizziness and stroke. DNTs are a small subset of T cells with diverse roles in peripheral immune-related diseases, as they can regulate immunological and inflammatory homeostasis (Ford et al., 2007; D'Acquisto and Crompton, 2011). The sources of DNTs in the diseased brain of ischemic stroke patients are still unknown, and few studies focus on the roles of DNTs in the brain. Our study found that the percentage of DNTs within lymphocytes was significant decreased in stroke patients, consistent with the previous study (Li et al., 2021). These results indicated that DNTs could be a suitable biomarker for clinical physicians to differentiate non-stroke dizziness and stroke, because it was normal in non-stroke dizziness patients but decreased in stroke patients with dizziness. The change of the DNTs level is differ from that in the previous study (Meng et al., 2019), but it could be clearly explained. Firstly, the previous study with all 47 stroke patients ignores their chief complaint, while only stroke patients with dizziness as the main complaint are enrolled in our study. Therefore, the clinical characteristics were different in these two populations. Secondly, the elevated level of DNTs is observed only in 68.09% (32/47) of stroke patients, instead of all 47 patients in the previous study, so we cannot exclude the possibility that some stroke patients show decreased level of DNTs.
Further, we explored the relationship of regulatory lymphocytes, stroke occurrence and clinical risks. The results indicated that both Bregs and DNTs exhibited a significant negative relationship with stroke occurrence. It means that the lower the level of Bregs or DNTs, the higher the risk of stroke in those patients with a chief complaint of dizziness. Additionally, Bregs or DNTs had a weak correlation with smoking, hypertension and diabetes, while they were closely related to age and alcohol consumption. As alcohol consumption status was unrecorded in controls and the percentage of alcohol consumption person was very low in both non-stroke dizziness and stoke patients, their relationship was not further explored. As for age, Bregs and DNTs showed a negative relationship with it, which indicates that young dizziness patients with low level of Bregs and/or DNTs are high-risk group. In addition, we assessed the abilities of Bregs and DNTs in predicting neural repair of stroke patients. The results indicated that the percentage of Bregs within lymphocytes at admission was a potential predictor of neural repair at discharge and the following 3 months. Further studies are needed to investigate the underlying mechanisms of Bregs in affecting the long-term prognosis of ischemic stroke patients.
Next, ROC curves reveled that both Bregs and DNTs combined with the clinical risk factors can be used to distinguish non-stroke dizziness and stroke patients with dizziness to some extent. Previous studies indicated that clinical risk factors related to stroke included age, gender, past history of hypertension, diabetes and habits of alcohol consumption and smoking (Aigner et al., 2017; Qi et al., 2020). In present study, the AUC of these risk factors was 0.723, showing a moderate quality for discriminating stroke from non-stroke dizziness, consistent to the views of previous study (Aigner et al., 2017). The AUC of Bregs and DNTs combination was 0.768 with a moderate specificity and a good sensitivity, which was larger than that of risk factors. It indicates that the dependability of Bregs and DNTs in distinguishing stroke from non-stroke dizziness is better than risk factor to a certain extent, and the high sensitivity of this model is more conducive to screening potential stroke patients. We further evaluated the AUC values when Bregs or DNTs combined with risk factors. The results showed that both Bregs and DNTs could improve the AUC of basic model significantly. However, the combination of Bregs and DNTs can improve the AUC of basic model to 0.815, which makes it a high-quality model and indicates the great utility value of Bregs and DNTs. Therefore, the present study suggests that Bregs and DNTs possess a good ability to assess the occurrence of ischemic stroke among patients with a chief complaint of dizziness. The application of these peripheral blood markers would be beneficial for screening potential posterior circulation infarction patients effectively, which could not be limited by under-developed equipments of grassroots hospitals. At present, it is still difficult to apply these biomarkers in clinic, but they have great application value as the technology improves. Additionally, further studies are needed to investigate the roles, cooperation and antagonism of these regulatory lymphocytes in ischemic stroke patients to clarify the underlying mechanisms and facilitate the novel targets search for treatment.
This study has some limitations. First, only lymphocyte levels in the peripheral blood were analyzed in the present study, not including lymphocyte levels in the brain. The evaluation of peripheral markers can mask events coming from the central nervous system due to the contamination from systemic sources. Second, the stroke risk factors of healthy persons in control group are not recorded as they are volunteers without intact medical records. Third, the sample size of our study is small. Prospective studies with larger samples are needed to further validate our findings.
Conclusion
Bregs and DNTs can be the clinical biomarkers for the diagnosis of ischemic stroke among dizziness patients, and Bregs could also be a potential predictor of neural repair. These findings will lay scientific foundation for Bregs and DNTs application in clinic, which facilitates the diagnosis and subsequent recovery of ischemic stroke patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethical Review Board of Central Hospital of Minhang District (Shanghai, China). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YW, JZ, and YG conceived and designed the experiments. YH and SL performed the experiments. YW and JL analyzed the data. YW and YL prepared the figures. YW wrote the paper. YW and YH revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Nature Science Foundation of China (grant nos. 81973157, 82173646, and 81870971), the Chinese State Ministry of Science and Technology grants (2021ZD0201704), and Supported by Shanghai Municipal Science and Technology Major Projects (22ZR1413700 and 2018SHZDZX01), and Shanghai Center for Brain Science and Brain-Inspired Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1042123/full#supplementary-material
Footnotes
References
Aigner, A., Grittner, U., Rolfs, A., Norrving, B., Siegerink, B., and Busch, M. A. (2017). Contribution of established stroke risk factors to the burden of stroke in young adults. Stroke 48, 1744–1751. doi: 10.1161/strokeaha.117.016599
Arch, A. E., Weisman, D. C., Coca, S., Nystrom, K. V., Wira, C. R. 3rd, and Schindler, J. L. (2016). Missed ischemic stroke diagnosis in the emergency department by emergency medicine and neurology services. Stroke 47, 668–673. doi: 10.1161/strokeaha.115.010613
Bodhankar, S., Chen, Y., Vandenbark, A. A., Murphy, S. J., and Offner, H. (2013). IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab. Brain Dis. 28, 375–386. doi: 10.1007/s11011-013-9413-3
Buck, B. H., Liebeskind, D. S., Saver, J. L., Bang, O. Y., Yun, S. W., Starkman, S., et al. (2008). Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 39, 355–360. doi: 10.1161/strokeaha.107.490128
Chalela, J. A., Kidwell, C. S., Nentwich, L. M., Luby, M., Butman, J. A., Demchuk, A. M., et al. (2007). Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 369, 293–298. doi: 10.1016/s0140-6736(07)60151-2
Choi, J. Y., Lee, S. H., and Kim, J. S. (2018). Central vertigo. Curr. Opin. Neurol. 31, 81–89. doi: 10.1097/wco.0000000000000511
Choi, J. H., Park, M. G., Choi, S. Y., Park, K. P., Baik, S. K., Kim, J. S., et al. (2017). Acute transient vestibular syndrome: prevalence of stroke and efficacy of bedside evaluation. Stroke 48, 556–562. doi: 10.1161/strokeaha.116.015507
D'Acquisto, F., and Crompton, T. (2011). CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem. Pharmacol. 82, 333–340. doi: 10.1016/j.bcp.2011.05.019
Dmitriew, C., Regis, A., Bodunde, O., Lepage, R., Turgeon, Z., McIsaac, S., et al. (2021). Diagnostic accuracy of the HINTS exam in an emergency department: a retrospective chart review. Acad. Emerg. Med. 28, 387–393. doi: 10.1111/acem.14171
Feigin, V. L., Krishnamurthi, R. V., Parmar, P., Norrving, B., Mensah, G. A., Bennett, D. A., et al. (2015). Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology 45, 161–176. doi: 10.1159/000441085
Feigin, V. L., Norrving, B., and Mensah, G. A. (2017). Global burden of stroke. Circ. Res. 120, 439–448. doi: 10.1161/circresaha.116.308413
Ford, M. S., Chen, W., Wong, S., Li, C., Vanama, R., Elford, A. R., et al. (2007). Peptide-activated double-negative T cells can prevent autoimmune type-1 diabetes development. Eur. J. Immunol. 37, 2234–2241. doi: 10.1002/eji.200636991
Guoping, P., Wei, W., Xiaoyan, L., Fangping, H., Zhongqin, C., and Benyan, L. (2015). Characteristics of the peripheral T cell immune response of patients at different stages of vascular cognitive impairment. Immunol. Lett. 168, 120–125. doi: 10.1016/j.imlet.2015.09.015
Lapchak, P. A., and Zhang, J. H. (2017). The high cost of stroke and stroke Cytoprotection research. Transl. Stroke Res. 8, 307–317. doi: 10.1007/s12975-016-0518-y
Li, S., Huang, Y., Liu, Y., Rocha, M., Li, X., Wei, P., et al. (2021). Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J. Cereb. Blood Flow Metab. 41, 2280–2294. doi: 10.1177/0271678x21995694
Li, Y., Zhu, Z. Y., Huang, T. T., Zhou, Y. X., Wang, X., Yang, L. Q., et al. (2018). The peripheral immune response after stroke-a double edge sword for blood-brain barrier integrity. CNS Neurosci. Ther. 24, 1115–1128. doi: 10.1111/cns.13081
Liberman, A. L., and Prabhakaran, S. (2017). Stroke chameleons and stroke mimics in the emergency department. Curr. Neurol. Neurosci. Rep. 17:15. doi: 10.1007/s11910-017-0727-0
Macrez, R., Ali, C., Toutirais, O., Le Mauff, B., Defer, G., Dirnagl, U., et al. (2011). Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 10, 471–480. doi: 10.1016/s1474-4422(11)70066-7
McColl, B. W., Allan, S. M., and Rothwell, N. J. (2009). Systemic infection, inflammation and acute ischemic stroke. Neuroscience 158, 1049–1061. doi: 10.1016/j.neuroscience.2008.08.019
Meng, H., Zhao, H., Cao, X., Hao, J., Zhang, H., Liu, Y., et al. (2019). Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc. Natl. Acad. Sci. U. S. A. 116, 5558–5563. doi: 10.1073/pnas.1814394116
Moulin, S., and Leys, D. (2019). Stroke mimics and chameleons. Curr. Opin. Neurol. 32, 54–59. doi: 10.1097/wco.0000000000000620
Munich, S. A., Vakharia, K., and Levy, E. I. (2019). Overview of mechanical Thrombectomy techniques. Neurosurgery 85, S60–s67. doi: 10.1093/neuros/nyz071
Newman-Toker, D. E. (2016). Missed stroke in acute vertigo and dizziness: it is time for action, not debate. Ann. Neurol. 79, 27–31. doi: 10.1002/ana.24532
Newman-Toker, D. E., and Edlow, J. A. (2015). TiTrATE: a novel, evidence-based approach to diagnosing acute dizziness and vertigo. Neurol. Clin. 33, 577–599, viii. doi: 10.1016/j.ncl.2015.04.011
Newman-Toker, D. E., Kerber, K. A., Hsieh, Y. H., Pula, J. H., Omron, R., Saber Tehrani, A. S., et al. (2013a). HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad. Emerg. Med. 20, 986–996. doi: 10.1111/acem.12223
Newman-Toker, D. E., McDonald, K. M., and Meltzer, D. O. (2013b). How much diagnostic safety can we afford, and how should we decide? A health economics perspective. BMJ Qual. Saf. 22, ii11–ii20. doi: 10.1136/bmjqs-2012-001616
Newman-Toker, D. E., Saber Tehrani, A. S., Mantokoudis, G., Pula, J. H., Guede, C. I., Kerber, K. A., et al. (2013c). Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke 44, 1158–1161. doi: 10.1161/strokeaha.111.000033
Qi, W., Ma, J., Guan, T., Zhao, D., Abu-Hanna, A., Schut, M., et al. (2020). Risk factors for incident stroke and its subtypes in China: a prospective study. J. Am. Heart Assoc. 9:e016352. doi: 10.1161/jaha.120.016352
Richoz, B., Hugli, O., Dami, F., Carron, P. N., Faouzi, M., and Michel, P. (2015). Acute stroke chameleons in a university hospital: risk factors, circumstances, and outcomes. Neurology 85, 505–511. doi: 10.1212/wnl.0000000000001830
Saber Tehrani, A. S., Coughlan, D., Hsieh, Y. H., Mantokoudis, G., Korley, F. K., Kerber, K. A., et al. (2013). Rising annual costs of dizziness presentations to U.S. emergency departments. Acad. Emerg. Med. 20, 689–696. doi: 10.1111/acem.12168
Saver, J. L., Goyal, M., van der Lugt, A., Menon, B. K., Majoie, C. B., Dippel, D. W., et al. (2016). Time to treatment with endovascular Thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 316, 1279–1288. doi: 10.1001/jama.2016.13647
Seifert, H. A., Vandenbark, A. A., and Offner, H. (2018). Regulatory B cells in experimental stroke. Immunology 154, 169–177. doi: 10.1111/imm.12887
Urra, X., Cervera, A., Villamor, N., Planas, A. M., and Chamorro, A. (2009). Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience 158, 1174–1183. doi: 10.1016/j.neuroscience.2008.06.014
Venkat, A., Cappelen-Smith, C., Askar, S., Thomas, P. R., Bhaskar, S., Tam, A., et al. (2018). Factors associated with stroke misdiagnosis in the emergency department: a retrospective case-control study. Neuroepidemiology 51, 123–127. doi: 10.1159/000491635
Wardlaw, J. M., Keir, S. L., Seymour, J., Lewis, S., Sandercock, P. A., Dennis, M. S., et al. (2004). What is the best imaging strategy for acute stroke? Health Technol. Assess. 8, 1–180. iii, ix-x. doi: 10.3310/hta8010
Yaghi, S., Willey, J. Z., Cucchiara, B., Goldstein, J. N., Gonzales, N. R., Khatri, P., et al. (2017). Treatment and outcome of hemorrhagic transformation after intravenous Alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48, e343–e361. doi: 10.1161/str.0000000000000152
Yan, J., Greer, J. M., Etherington, K., Cadigan, G. P., Cavanagh, H., Henderson, R. D., et al. (2009). Immune activation in the peripheral blood of patients with acute ischemic stroke. J. Neuroimmunol. 206, 1-2, 112–1-117. doi: 10.1016/j.jneuroim.2008.11.001
Keywords: ischemic stroke, lymphocytes, neural repair, dizziness, biomarkers
Citation: Wang Y, Huang Y, Li S, Lin J, Liu Y, Gao Y and Zhao J (2022) The value of circulating lymphocytic subpopulations in the diagnosis and repair of ischemic stroke patients with dizziness. Front. Aging Neurosci. 14:1042123. doi: 10.3389/fnagi.2022.1042123
Edited by:
Guo-Yuan Yang, Shanghai Jiao Tong University, ChinaReviewed by:
Ying Xu, University at Buffalo, United StatesXijin Wang, Tongji Hospital Affiliated to Tongji University, China
Copyright © 2022 Wang, Huang, Li, Lin, Liu, Gao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqin Gao, eXFnYW9Ac2htdS5lZHUuY24=; Jing Zhao, emhhb19qaW5nQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work
 Yong Wang
Yong Wang Yichen Huang
Yichen Huang Sicheng Li
Sicheng Li Jixian Lin
Jixian Lin Yang Liu
Yang Liu Yanqin Gao
Yanqin Gao Jing Zhao
Jing Zhao