
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 21 October 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1002642
 Guanwen Lin1,2
Guanwen Lin1,2 Fei Lan1
Fei Lan1 Duozhi Wu2
Duozhi Wu2 Guanglei Cao3
Guanglei Cao3 Zheng Li3
Zheng Li3 Zhigang Qi4
Zhigang Qi4 Yang Liu1
Yang Liu1 Shuyi Yang1
Shuyi Yang1 Jie Lu4*
Jie Lu4* Tianlong Wang1*
Tianlong Wang1*Objective: This study is designed to investigate the brain function changed regions in elderly patients with knee osteoarthritis (KOA) and to explore the relationship between neuropsychological tests and resting-state functional magnetic resonance imaging (rs-fMRI) network to clarify the possible mechanism underlying cognitive changes in KOA patients.
Materials and methods: Fifty-two patients aged ≥ 65 with KOA and twenty-two healthy-matched controls were recruited in this study. All participants were given rs-fMRI check. We used graph theory analysis to characterize functional connectivity (FC) and topological organization of the brain structural network. The relationship between FC values, topological properties, and the neuropsychological test scores was analyzed.
Results: Compared with the controls, fourteen edges with lower functional connectivity were noted in the KOA group. Local efficiency and small-worldness of KOA patients decreased compared to the healthy controls. No significant alterations of nodal topological properties were found between the two groups. There was a significant positive correlation between the AVLT-H (L) and the internetwork of default mode network (DMN) (left/right orbitofrontal Superior cortex) and limbic/cortical areas (left/right caudate, right amygdala). AVLT-H(L) was positively correlated with small-worldness and local efficiency.
Conclusion: The results indicated that for elderly KOA patients with declined cognition, topological properties, FC between DMN and subcortical limbic network related regions are significantly decreased compared to healthy controls. These alterations demonstrated a significant correlation with the neuropsychological test scores.
Knee osteoarthritis (KOA) is a highly prevalent and disabling joint disease that is more common in elderly patients. Long-term consequences of KOA may result in declined physical activity, deconditioning, impaired sleep, fatigue, depression, and disability (Sharma, 2021). The percentage of elderly patients with KOA receiving surgical treatment is increasing rapidly. The postoperative cognitive disorder is one of the most common postoperative complications in aged patients, including postoperative delirium, delayed neurocognitive recovery, and postoperative neurocognitive dysfunctions. These complications can result in decreased quality of life. Preventing perioperative neurocognitive disorders is a priority for patients and families. The cognitive reserve of elderly KOA patients before surgery was associated with postoperative functional network and neurocognitive function changes (Jones et al., 2016; Huang et al., 2018). Thus, a preoperative objective evaluation of cognitive function remains very important.
Research showed that as a clinical neuroimaging technique, resting-state functional connectivity (FC) might be a biomarker substitution to detect neurodegenerative diseases (Hohenfeld et al., 2018). The brain was shown to function as several neural networks by analyzing the blood-oxygen-level-dependent signal fluctuations (Rubinov and Sporns, 2010; Bassett and Sporns, 2017). FC is the temporal correlation of a neurophysiological index measured in different brain areas (Biswal et al., 1995) and has been widely used in resting-state functional MRI (rs-fMRI) studies. Altered FC was visible during cognitive impairment (Chabran et al., 2020), anesthesia (Liu et al., 2020), and aging (Pistono et al., 2021) by setting one/multi-seed regions and making the FC a significant indicator for brain functional changes.
Recently, graph theory-based network analysis has been applied to explore brain connectivity within whole-brain networks. It was used to analyze the brain function in larger scale network, considering both the contributions of FC and topology structures (e.g., global efficiency, local efficiency, and degree centrality) (Wang et al., 2010), which may offer important new insights into the structure and function of networked brain systems (Sporns, 2018). A more concise and completed brain network can be presented for studying brain function changes in physiological conditions and disease development. In some cognitively impaired diseases, findings from rs-fMRI have shown impaired connectivity of the default mode network (Koch et al., 2015). Some studies that employed theoretical graph measures to assess alteration of brain networks have demonstrated a reduced degree of centrality parameter in patients with neurodegenerative diseases (Guo et al., 2016). The disassociation of cognitive change from functional brain connectivity change postoperatively was observed (Browndyke et al., 2021). However, few studies have demonstrated the relationship between cognitive function disorder and brain functional network alterations in patients with KOA.
Therefore, this study aimed to investigate the regions of brain function changes to reveal the relationship between neuropsychological tests and rs-fMRI network analysis and clarify the possible mechanism underlying cognitive changes in patients with KOA. We hypothesize that brain FC and topology structure changes in elderly patients with KOA can potentially predict cognitive function degeneration.
This is a cross-sectional study conducted at Xuanwu Hospital, Capital Medical University, from 1 September 2020 to 31 August 2021 and registered at the Chinese Clinical Trial Registry (Identifier: ChiCTR2000036310). All the procedures have been approved by the Institutional Review Board of Xuanwu Hospital, Capital Medical University (approved No. [2019]-112). All study procedures were carried out in accordance with the Declaration of Helsinki. All patients received written informed consent.
In this study, fifty-two patients with knee osteoarthritis with indication for joint replacement were recruited between January 2019 and August 2021 through Xuanwu Hospital, Capital Medical University. Meanwhile, we also recruited twenty-two healthy volunteers for healthy control. The inclusion criteria are as follows: (1) age ≥ 65 years old, (2) more than 6 years of school education, and (3) have knee osteoarthritis with indication for joint replacement. Exclusion criteria are as follows: (1) Montreal Cognitive Assessment-Basic (MoCA-B) score < 19, (2) any history of drug abuse, severe cardiac, cerebrovascular disease, and diseases associated with cognitive impairment, (3) history of neurosurgery or head trauma, (4) severe mental disorders, (5) left-handedness, and (6) contraindications for MRI or unwillingness to complete the MRI.
All participants were given a battery of neuropsychological assessments by a trained neuropsychologist blinded to the study design. Montreal Cognitive Assessment-Basic (MoCA-B) (Chen et al., 2016) assesses nine cognitive domains (executive function, language, orientation, calculation, conceptual thinking, memory, visuoperception, attention, and concentration). Auditory verbal learning test-Hua Shan (AVLT-H) (Guo et al., 2007) was adapted from the California Verbal Learning Test, presenting 12 words over five trials, and the scores on immediate recall, short-delay free recall (5 min), and long-delay free recall (20 min). The Hamilton Rating Scale for Depression (HAM-D17) assesses depression severity (Hamilton, 1967). The Hamilton Anxiety Scale (HAM-A) was adapted to present the severity of anxiety neurosis (Maier et al., 1988).
For all the seventy-four subjects (fifty-two KOA patients and twenty-two healthy controls), rs-fMRI was finished before the neuropsychological assessment. All the data were collected by the clinical 3.0 Tesla MRI machine (Verio; Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil. The head coil was fitted with foam padding and headphones to minimize the influence of scanning noise and head motion before starting. All the participants were told to stay relaxed with eyes closed and not to think of anything. Three-dimensional T1-weighted images were acquired using the following parameters: repetition time (TR) = 1,900 ms, echo time (TE) = 2.2 ms, inversion time (TI) = 900 ms, flip angle = 90°, resolution = 256 × 256 matrix, 176 slices with a thickness of 1.0 mm, slice gap = 0, and voxel size = 1 × 1 × 1 mm. For the BOLD sequences, a rapid-gradient echo sequence was generated for each subject with the following parameters: TR = 2,000 ms, TE = 40 ms, field of view = 240 × 240 mm2, flip angle = 90°, section thickness = 4 mm, acquisition matrix = 64 × 64, and 28 slices with the slice gap = 0.
Data preprocessing was conducted using a Python-based pipeline tool involving AFNI, ANTs, FSL, and custom python code, known as the Configurable Pipeline for Analysis of Connectomes (C-PAC1). The whole analysis was simplified and accelerated by NeuroScholarTM platform (2 Beijing Intelligent Brain Cloud, Inc.).
Structural data preprocessing was conducted by the following steps, such as image de-obliquing, re-orientation (right-to-left posterior-to-anterior inferior-to-superior, RPI), skull stripping, normalizing the individual stripped brain to a Montreal Neurological Institute 152 stereotactic space (1 mm 3 isotropic) using linear/non-linear registrations; segmenting the brain into gray matter, white matter, and cerebral fluid (CSF); and constraining the tissue segmentation of individual subjects by tissue priors from standard space obtained from the FMRIB Software Library (FSL3).
Functional data preprocessing was conducted by the following steps, including removing the first 10 images and doing the slice-time correction. After re-oblique the images, we re-oriented all the images to RPI orientation. When the skull stripping was finished, the global intensity was normalized to 10,000. After all the above, functional images were registered to anatomical space with a linear transformation and then a white-matter boundary-based transformation and the prior white-matter tissue segmentation from FSL. To remove the head motion artifact, ICA-AROMA was used with partial component regression. To obtain a better data-preprocessing quality, nuisance signal regression was applied, including (1) mean values from the signal in the white matter and CSF derived from the prior tissue segmentations transformed from anatomical to functional space, (2) motion parameters (6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items), (3) linear trends, and (4) global signal only for one set of strategies.
The whole-brain functional connectivity (FC) network was constructed as matrix consisting of nodes and edges based on the anatomical automatic labeling (AAL) template, which includes 90 brain regions in the cortex and 26 brain regions in the subcortex. The overall 116 brain regions are represented as “node,” while the correlation between each node is represented as “edge.” The correlation is defined as Pearson’s correlation between the regional mean time series of all possible pairs of brain regions (R-value). Prior to statistical analysis, all the correlations were transformed to z-score (Fisher’s r-to-z transformation) to improve normality. In this way, a 116 × 116 correlation matrix based on AAL template was constructed for each subject.
The graph theory was used to analyze the topological and regional properties of brain networks with MATLAB-based software Gretna Toolbox (Wang et al., 2015)4 and visualized by BrainNet Viewer toolbox (Xia et al., 2013)5.
We evaluated the global properties of brain network (Achard and Bullmore, 2007) by following measures as clustering coefficient (Cp), shortest path length (Lp), small-worldness, global efficiency, and local efficiency for all participants. The global clustering coefficient is defined as the average of the likelihood of a neighbor-to-neighbor connection. Greater value of Cp represented a stronger local interconnection within a network. The global shortest path length is defined as the average of all of the shortest lengths between each pair of nodes in the network. Smaller value of Lp means a faster brain’s ability to transfer information. The global efficiency is represented as the information transfer efficiency of the network. The local efficiency represents a fault tolerance of the network.
A small-worldness network can be defined by high local clustering, characterized by a high clustering coefficient and low minimum path length between any pair of nodes. Gamma, lambda, and sigma were indices of small-worldness. Gamma (γ) = Creal/Crandom > 1 (C represented cluster coefficient), lambda (λ) = Lreal/Lrandom ∼ 1 (L represented shortest path length), and sigma (σ) = γ/λ > 1 (Watts and Strogatz, 1998). A high value of sigma indicates a high efficiency of information delivery.
In addition, we used nodal clustering coefficient, nodal shortest path length, nodal efficiency, and nodal local efficiency to describe the regional properties of the functional network (Achard and Bullmore, 2007; Hagmann et al., 2008). The clustering coefficient of a node measured interconnectability of its neighbors. The nodal local efficiency indicated the communication efficiency among its first neighbors when the node is removed. The shortest path length and efficiency of a node quantified the efficiency of parallel information transfer of that node in the network.
Clinical characteristics were compared between the patient and healthy groups by two-sample t-tests and chi-squared tests according to data type using GraphPad 8.0 (GraphPad Software; San Diego, CA, USA). Group differences in functional connectivity (FC) were calculated using two-sample t-tests in GRETNA. To explore group differences in topological properties, we applied a series of sparsity thresholds (from 0.05 to 0.5, interval 0.05) consistent with a previously published study (Watts and Strogatz, 1998). Two sample t-tests were performed on the global and nodal characteristics. False discovery rate (FDR, q < 0.05) was used to correct the differences in FC and topological properties. Partial correlation analysis was used to examine associations between FC values, topological properties, and the neuropsychological test scores, conducting with age, gender, and education as covariates (Bozzali et al., 2015; Goldstone et al., 2016).
The final sample included fifty-two elderly patients with KOA (forty women and twelve men) and twenty-two healthy controls (thirteen women and nine men). Demographic characteristics are shown in Table 1. No significant differences between both groups in demographic variables (age and gender) were observed. Education level was significantly higher in the HC group’s patients than that in the KOA group (P = 0.023). MoCA-B, AVLT-H (S), and AVLT-H (L) scores in the KOA group’s patients showed significantly lower than that in the HC group (P < 0.05).
Compared with those in healthy controls, fourteen edges were identified with significantly lower FC in the KOA group’s patients (p = 1.12 × 10–4, FDR-corrected, Figures 1, 2). Twelve of these edges originating from the default mode network (DMN)-related brain regions (left/right orbitofrontal cortex, left middle temporal cortex) were connected to the limbic/subcortical regions (left/right caudate, left/right amygdala) and olfactory cortex. Two edges were connected within DMN network. Nodes showing significant difference in functional connectivity analysis between the two groups are shown in Table 2.
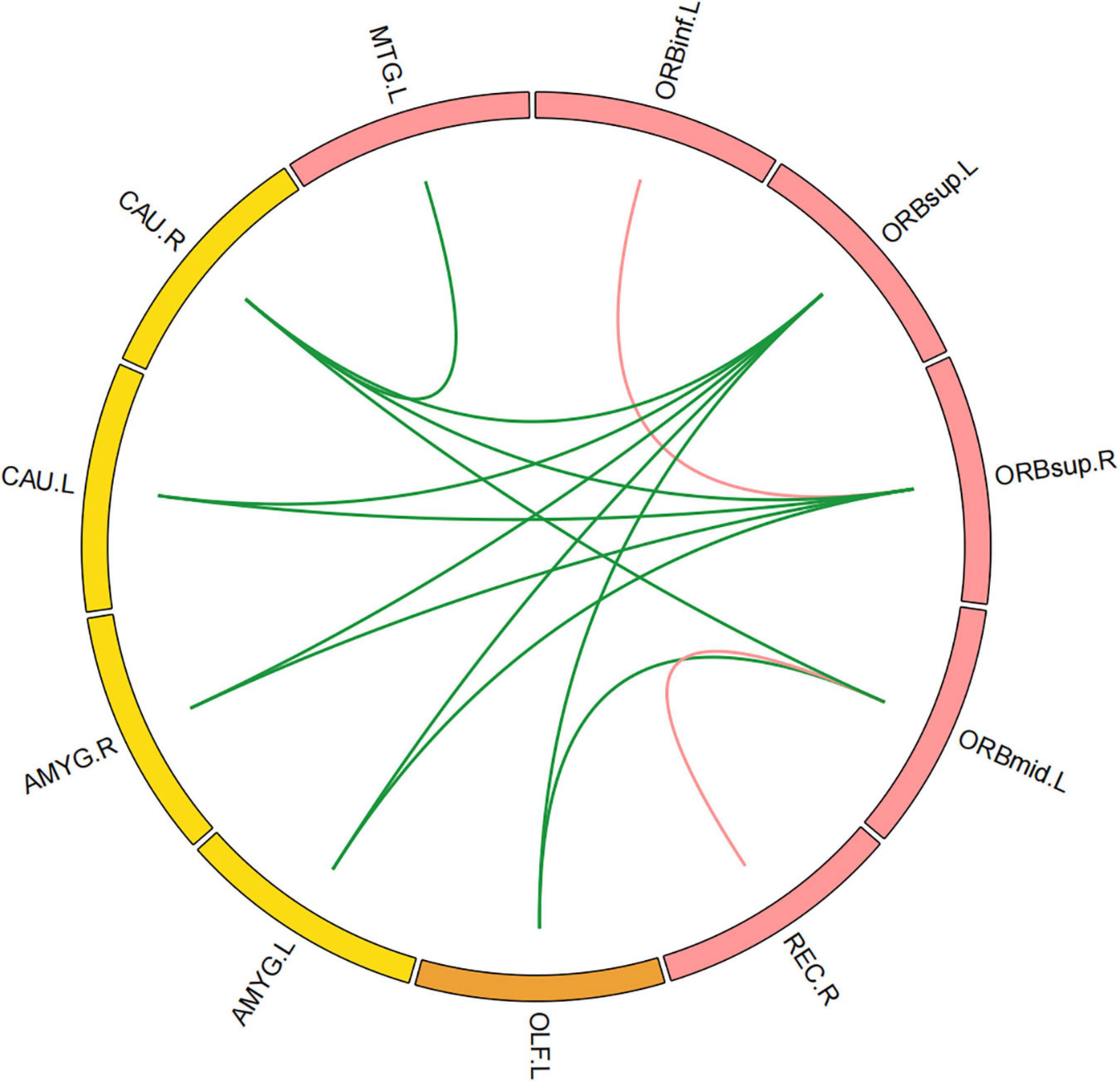
Figure 1. Significant decrease in functional connectivity (FC). The red block represents the default mode network (DMN) regions; the yellow block represents the limbic/subcortical cortex regions; the orange block represents the olfactory cortex. The green line represents the functional connectivity originated from DMN regions; the red line represents the functional connectivity within DMN regions; ORBinf.L, left inferior orbitofrontal cortex; ORBsup.L, left superior orbitofrontal cortex; ORBsup.R, right superior orbitofrontal cortex; ORBmid.L, left middle orbitofrontal cortex; OLF.L, left olfactory cortex; REC.R, right gyrus rectus; MTG.L, left middle temporal cortex; AMYG.L, left amygdala; AMYG.R, right amygdala; CAU.L, left caudate; CAU.R, right caudate.
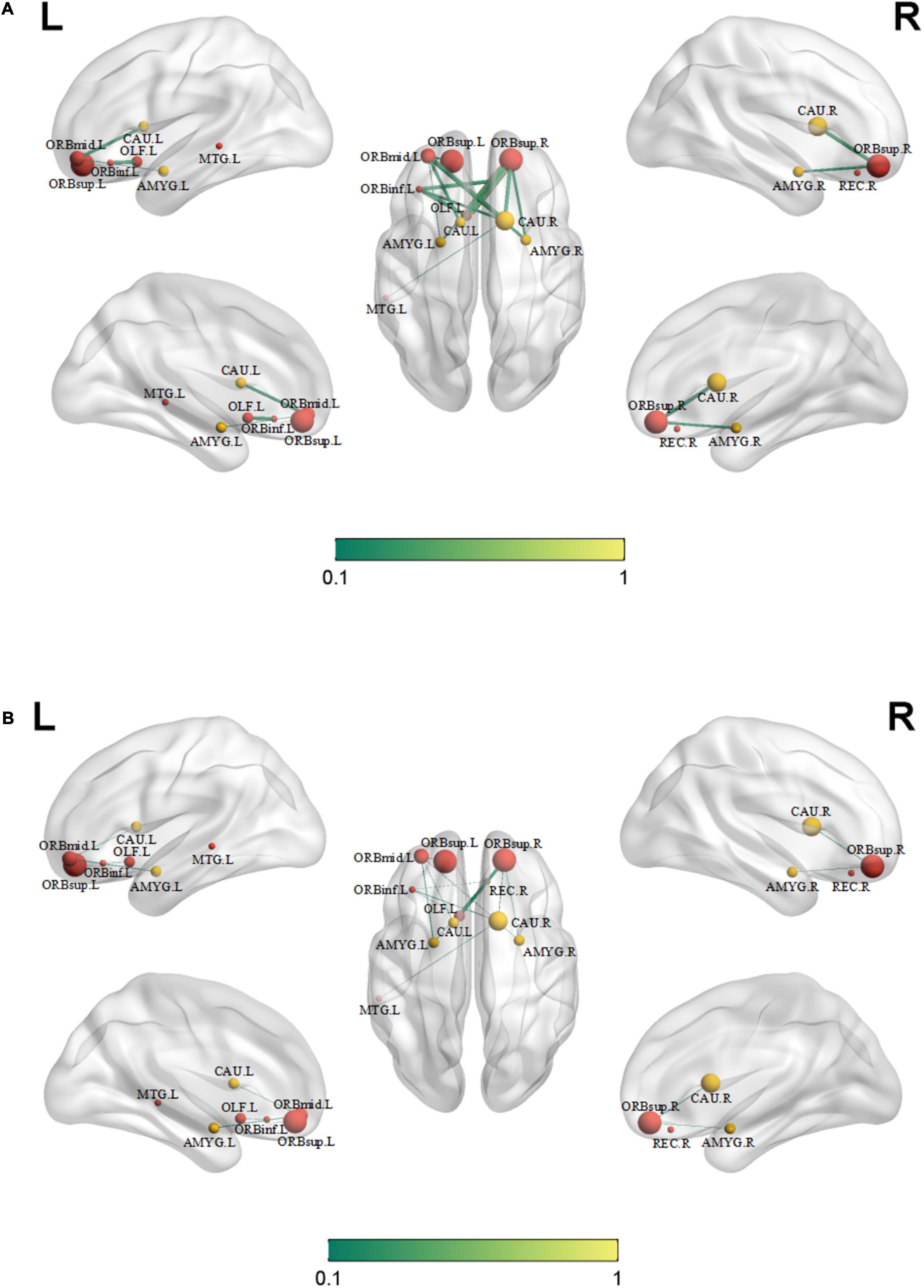
Figure 2. Significant decreased functional connectivity. (A) Healthy controls and (B) knee osteoarthritis patients. ORBinf.L, left inferior orbitofrontal cortex; ORBsup.L, left superior orbitofrontal cortex; ORBsup.R, right superior orbitofrontal cortex; ORBmid.L, left middle orbitofrontal cortex; OLF.L, left olfactory cortex; REC.R, right gyrus rectus; AMYG.L, left amygdala; AMYG.R, right amygdala; CAU.L, left caudate; CAU.R, right caudate; MTG.L, left middle temporal cortex.
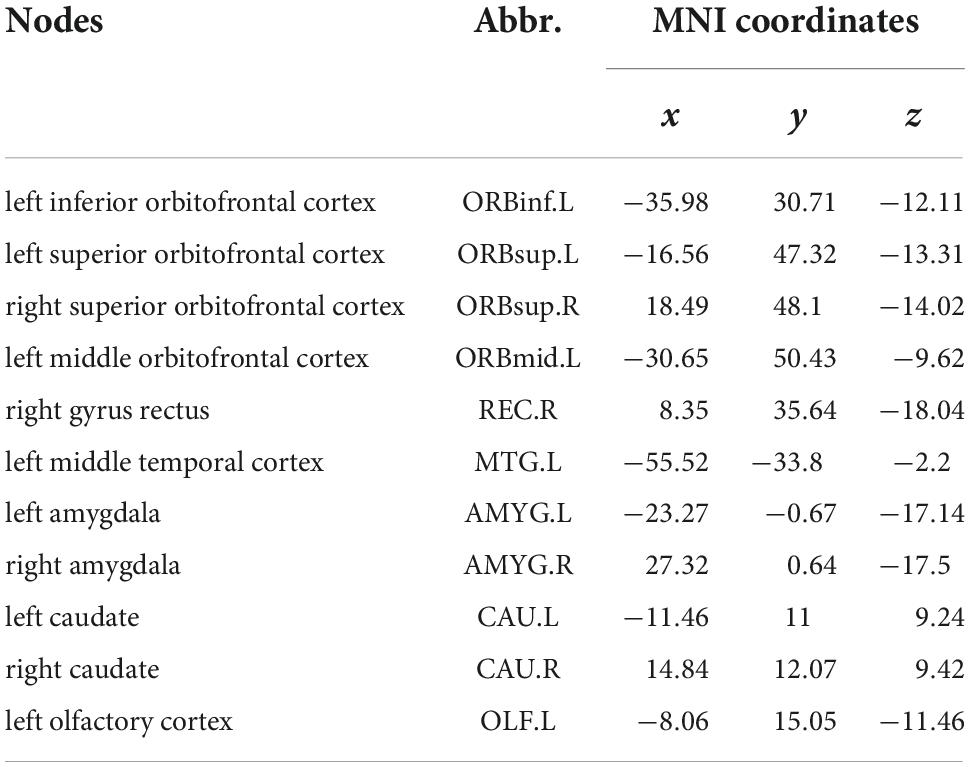
Table 2. Nodes showing significant difference in functional connectivity (FC) analysis between HC and KOA groups’ patients (P < 0.05, FDR correction).
Patients in both KOA and HC groups were presented small-world organization (σ > 1). Compared with HC group’s patients, small-worldness decreased in the KOA group’s patients (p = 0.017, FDR-corrected, Figure 3). Local efficiency in the KOA group’s patients decreased compared with the HC group’s patients (p = 0.008, FDR-corrected, Figure 3). No statistical significance was observed in the global efficiency, cluster coefficient, and shortest path length between the two groups.
The nodal properties including the nodal clustering coefficient, nodal shortest path length, nodal efficiency, and nodal local efficiency were compared. No significant alterations of nodal topological properties were found between the two groups (Supplementary Figure 1).
There was a significant positive correlation between the AVLT-H (L) and the internetwork of DMN (left/right orbitofrontal superior cortex) and limbic/cortical areas (left/right caudate, right amygdala) (Figure 4). AVLT-H(L) was positively correlated with small-worldness and local efficiency (Figure 5).
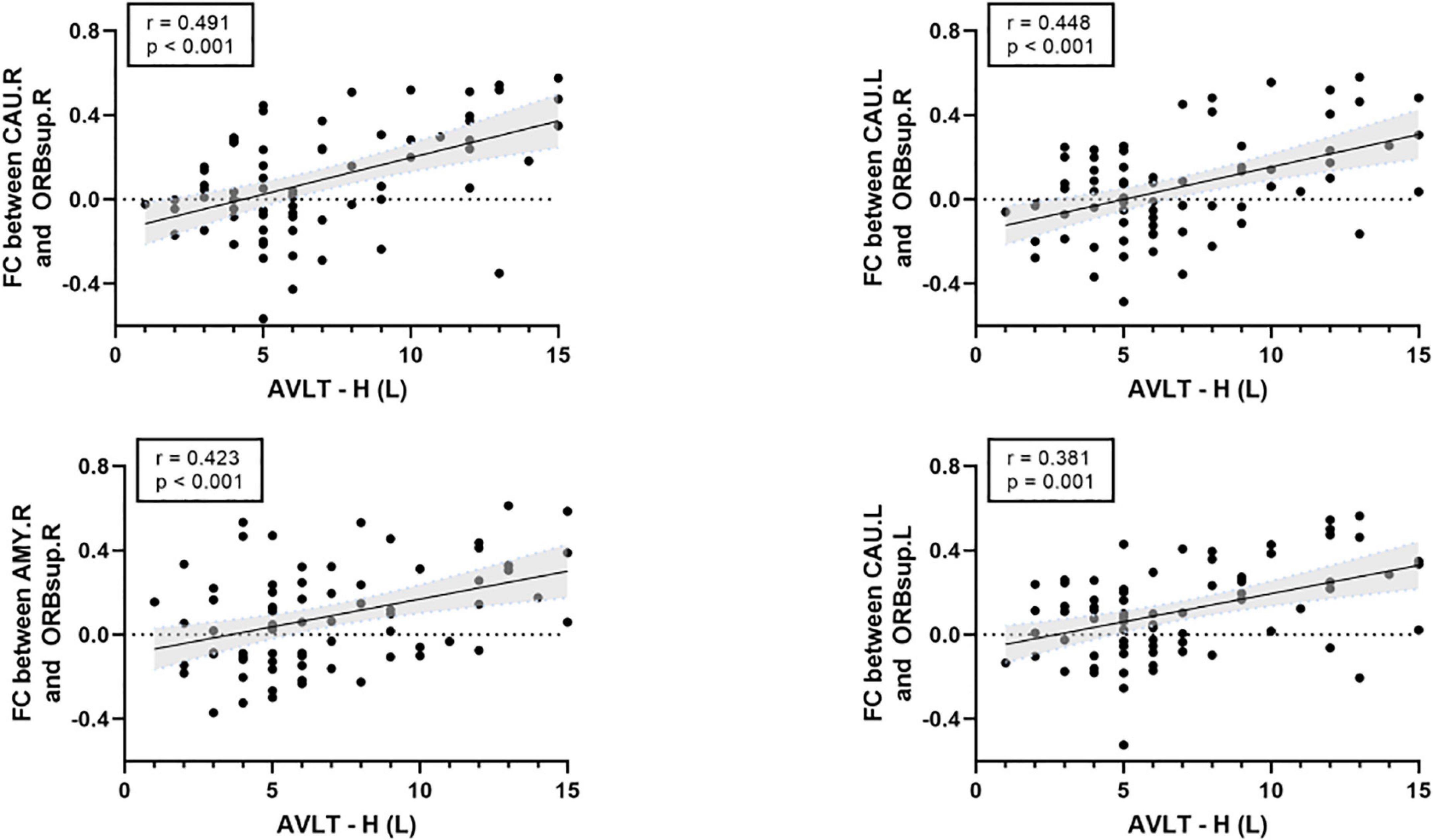
Figure 4. Correlations between cognitive test score and the functional connectivity between default mode network (DMN) and limbic/subcortical cortex. FC, functional connectivity; AVLT-H (L), Auditory verbal learning test-Huashan version for long term; ORBsup.R, right superior orbitofrontal cortex; ORBsup.L, left superior orbitofrontal cortex; CAU.L, left caudate; CAU.R, right caudate; AMYG.R, right amygdala.
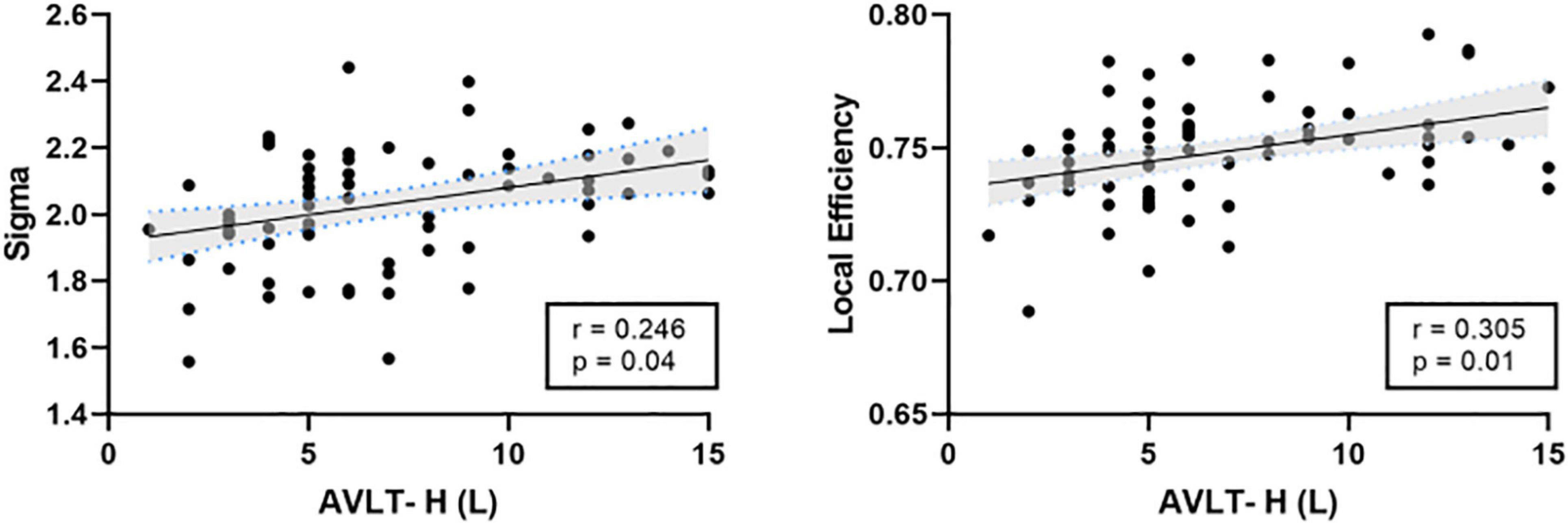
Figure 5. Correlations between cognitive test score and the topological properties. AVLT-H (L), Auditory verbal learning test-Huashan version for long term.
The current study explored the alterations in resting-state FC in patients with KOA by analyzing the rs-fMRI data through graph theory analysis. Our findings indicated that both groups displayed a small-world structure, and global properties of KOA patients, including small-worldness and local efficiency, decreased. Furthermore, these brain network alterations were related to the neuropsychological assessments.
Small-worldness is an important characteristic of a network because it represents an optimal balance between segregation and integration, which is essential for high synchronizability and fast information transmission in a complex network (Watts and Strogatz, 1998). The networks of both HC and KOA groups exhibited characteristic small-world organization, which is consistent with previous studies (Barroso et al., 2021). However, the small-worldness was significantly lower in the KOA group compared to the controls, and this finding indicated the functional brain network of KOA patients is less efficiently organized.
Global efficiency and local efficiency measure a network’s ability to transmit information (Latora and Marchiori, 2001). The local efficiency of the KOA group was significantly lower than that of healthy controls at the whole-brain level, which proved that patients with KOA had abnormal networks with lower local information processing speed, leading to less local efficiency. Interestingly, the two groups found no significant difference in global efficiency. This observation might be due to an over-recruitment of brain regions of mobility declined patients to process complex tasks based on the Related Compensation Utilization of Neural Circuits Hypothesis theory (Reuter-Lorenz and Cappell, 2008). Brain network architecture reorganizes in patients with OA that results from disrupting the whole-brain and local FC by chronic pain (Barroso et al., 2021). Our previous study revealed that end-stage older patients with KOA had decreased amplitude of low-frequency fluctuations (ALFF) in DMN (Lan et al., 2020). The decreased ALFF also proved a local efficiency damage in patients with KOA.
We found the FC network between the DMN [inferior, superior, or middle orbitofrontal cortex (OFC)] and the subcortical limbic network (amygdala, caudate) decreased in older patients with KOA compared to the healthy controls. OFC has a central role in mediating the impact of emotional context on inhibitory control (Buckner and DiNicola, 2019). It is involved in learning stimulus-reward associations (Burke et al., 2008; Zhuang et al., 2021), which receive highly processed sensory information about the current bodily state and emotional and social information (Rudebeck and Rich, 2018). In addition, evidence suggested that the OFC served as a mediator between the prefrontal cortex involved in higher order processing of emotional information and limbic regions involved in emotion perception and generation (Phillips et al., 2003a,b; Beauregard, 2007). Emotional dysregulation and concomitant neurocognitive impairment may be caused by decreased FC between OFC and amygdala of patients with KOA, which was found in our study. Caudate also played a critical role in supporting the planning and execution of strategies and behavior required for achieving complex goals (Grahn et al., 2008). Decreased FC between OFC and caudate may be related to impaired executive function. Patients with KOA suffer from long-term pain, mood disorders, and neurocognitive impairment (Lee et al., 2013). The corticolimbic system, which is involved in emotion and mood processing (Sah, 2017), is also a mediator of chronic pain. KOA pain, as a nociceptive signal, persistently activated corticolimbic circuitry and altered cortex FC due to the structure and function plasticity of the corticolimbic system (McCarberg and Peppin, 2019), resulting in pain chronification.
Functional connectivity also decreased between left olfactory cortex and left superior/middle orbitofrontal cortex. Research showed that olfactory cortex degeneration was correlated with behavioral tests in Alzheimer’s disease and mild cognitive impairment (Vasavada et al., 2015). The entorhinal cortex, which is part of olfactory cortex, has been associated frequently with the DMN (Raichle, 2015). Providing the major input to the hippocampus, entorhinal cortex plays a vital role in memory (Zola-Morgan et al., 1989). These may be a potential reason for memory decline in KOA patients.
The present study used MoCA-B (Luis et al., 2009) to detect cognitive changes and AVLT to detect memory loss (Zhao et al., 2015). Significantly worse scores on neuropsychological tests of MoCA-B and AVLT in the KOA group were observed. These findings suggested that the cognitive function of older patients with advanced KOA decreased compared to healthy controls, consistent with a previous study (Innes and Sambamoorthi, 2018). This study presented that the educational level of the KOA group was significantly lower than the HC group. Evidence that education contributed to cognition changes was controversial. Higher educational level was associated with delayed onset of accelerated cognitive decline (Hall et al., 2007). However, education contributed little to cognitive reserve in old age (Wilson et al., 2019). The exploratory analysis indicated impaired connectivity networks and topological properties significantly correlated with the results of cognitive tests after controlling education as a covariate. FC between the OFC and the limbic or subcortical network positively correlated with AVLT-H (L). Local efficiency and small-worldness were both positively correlated with AVLT-H (L). These results further support the idea that FC and topological structure damages were closely related to memory loss. Altogether, these findings suggested that decreased FC in these important brain area of elderly patients with KOA could contribute to cognitive declination.
The identified positive result in this situation should be reliable with a multiple comparison correction, although the number of patients was limited. The present cross-sectional study cannot address causality. A further longitudinal study is needed to confirm the underlying mechanisms of brain changes in patients and the relationship between cognition and brain network changes in elderly patients with KOA. Future studies combining neuroimaging and specific cognitive domains of older patients with KOA, such as visuospatial abilities, executive functions, and working memory, will be more helpful in exploring their intrinsic association.
The results indicated that for elderly KOA patients with declined cognition, topological properties, FC between DMN and subcortical limbic network related regions are significantly decreased compared to healthy controls. These alterations correlated with the neuropsychological test scores. Our findings help to improve the understanding of functional network alterations in elderly patients with KOA. Rs-fMRI FC might be employed as a potential biomarker for detecting early cognitive impairment, depression, and anxiety stages. However, this possibility requires further investigation in future studies.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Institutional Review Board of Xuanwu Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
TW, JL, and FL: study design. GL, YL, GC, and ZL: study performance. GL, YL, and ZQ: data analysis. GL and YL: manuscript writing. TW, FL, ZQ, DW, and SY: manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by Beijing Municipal Health Commission (Jing2019-2) and Hainan Province Clinical Medical Center.
We acknowledge and thank all participating doctors and research assistants (from the Department of Orthopedics Xuanwu Hospital) and the nurses (from the Department of Operating Room of Xuanwu Hospital) for their invaluable help and cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1002642/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Nodal topological properties. No significant alterations of nodal topological properties were found between two groups.
Achard, S., and Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Barroso, J., Wakaizumi, K., Reis, A. M., Baliki, M., Schnitzer, T. J., Galhardo, V., et al. (2021). Reorganization of functional brain network architecture in chronic osteoarthritis pain. Hum. Brain Mapp. 42, 1206–1222. doi: 10.1002/hbm.25287
Bassett, D. S., and Sporns, O. (2017). Network neuroscience. Nat. Neurosci. 20, 353–364. doi: 10.1038/nn.4502
Beauregard, M. (2007). Mind does really matter: Evidence from neuroimaging studies of emotional self-regulation, psychotherapy, and placebo effect. Prog. Neurobiol. 81, 218–236. doi: 10.1016/j.pneurobio.2007.01.005
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Bozzali, M., Dowling, C., Serra, L., Spano, B., Torso, M., Marra, C., et al. (2015). The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. J. Alzheimers Dis. 44, 243–250. doi: 10.3233/JAD-141824
Browndyke, J. N., Wright, M. C., Yang, R., Syed, A., Park, J., Hall, A., et al. (2021). Perioperative neurocognitive and functional neuroimaging trajectories in older APOE4 carriers compared with non-carriers: Secondary analysis of a prospective cohort study. Br. J. Anaesth. 127, 917–928. doi: 10.1016/j.bja.2021.08.012
Buckner, R. L., and DiNicola, L. M. (2019). The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 20, 593–608. doi: 10.1038/s41583-019-0212-7
Burke, K. A., Franz, T. M., Miller, D. N., and Schoenbaum, G. (2008). The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature 454, 340–344. doi: 10.1038/nature06993
Chabran, E., Noblet, V., Loureiro, D. E. S. O. U. S. A., Demuynck, C., Philippi, N., Mutter, C., et al. (2020). Changes in gray matter volume and functional connectivity in dementia with Lewy bodies compared to Alzheimer’s disease and normal aging: Implications for fluctuations. Alzheimers Res. Ther. 12:9. doi: 10.1186/s13195-019-0575-z
Chen, K. L., Xu, Y., Chu, A. Q., Ding, D., Liang, X. N., Nasreddine, Z. S., et al. (2016). Validation of the Chinese version of montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 64, e285–e290. doi: 10.1111/jgs.14530
Goldstone, A., Mayhew, S. D., Przezdzik, I., Wilson, R. S., Hale, J. R., and Bagshaw, A. P. (2016). Gender specific re-organization of resting-state networks in older age. Front. Aging Neurosci. 8:285. doi: 10.3389/fnagi.2016.00285
Grahn, J. A., Parkinson, J. A., and Owen, A. M. (2008). The cognitive functions of the caudate nucleus. Prog. Neurobiol. 86, 141–155. doi: 10.1016/j.pneurobio.2008.09.004
Guo, Q. H., Sun, Y. M., Pei-Min, Y. U., and Hong, Z. (2007). Norm of auditory verbal learning test in the normal aged in China community. Chin. J. Clin. Psychol. 15, 132–134, 141.
Guo, Z., Liu, X., Hou, H., Wei, F., Liu, J., and Chen, X. (2016). Abnormal degree centrality in Alzheimer’s disease patients with depression: A resting-state functional magnetic resonance imaging study. Exp. Gerontol. 79, 61–66. doi: 10.1016/j.exger.2016.03.017
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. doi: 10.1371/journal.pbio.0060159
Hall, C. B., Derby, C., Levalley, A., Katz, M. J., Verghese, J., and Lipton, R. B. (2007). Education delays accelerated decline on a memory test in persons who develop dementia. Neurology 69, 1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 6, 278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x
Hohenfeld, C., Werner, C. J., and Reetz, K. (2018). Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? Neuroimage Clin. 18, 849–870. doi: 10.1016/j.nicl.2018.03.013
Huang, H., Tanner, J., Parvataneni, H., Rice, M., Horgas, A., Ding, M., et al. (2018). Impact of total knee arthroplasty with general anesthesia on brain networks: Cognitive efficiency and ventricular volume predict functional connectivity decline in older adults. J. Alzheimers Dis. 62, 319–333. doi: 10.3233/JAD-170496
Innes, K. E., and Sambamoorthi, U. (2018). The association of perceived memory loss with osteoarthritis and related joint pain in a large Appalachian population. Pain Med. 19, 1340–1356. doi: 10.1093/pm/pnx107
Jones, R. N., Marcantonio, E. R., Saczynski, J. S., Tommet, D., Gross, A. L., Travison, T. G., et al. (2016). Preoperative cognitive performance dominates risk for delirium among older adults. J. Geriatr. Psychiatry Neurol. 29, 320–327. doi: 10.1177/0891988716666380
Koch, K., Myers, N. E., GöTTLER, J., Pasquini, L., Grimmer, T., FöRSTER, S., et al. (2015). Disrupted intrinsic networks link amyloid-β pathology and impaired cognition in prodromal Alzheimer’s disease. Cereb. Cortex 25, 4678–4688. doi: 10.1093/cercor/bhu151
Lan, F., Lin, G., Cao, G., Li, Z., Ma, D., Liu, F., et al. (2020). Altered intrinsic brain activity and functional connectivity before and after knee arthroplasty in the elderly: A resting-state fMRI study. Front. Neurol. 11:556028. doi: 10.3389/fneur.2020.556028
Latora, V., and Marchiori, M. (2001). Efficient behavior of small-world networks. Phys. Rev. Lett. 87:198701. doi: 10.1103/PhysRevLett.87.198701
Lee, A. S., Ellman, M. B., Yan, D., Kroin, J. S., Cole, B. J., Van Wijnen, A. J., et al. (2013). A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527, 440–447. doi: 10.1016/j.gene.2013.05.069
Liu, Y., Fu, H. Q., Wu, Y., Du, Z. S., Li, B. R., Gao, X., et al. (2020). Influence of three different anesthesia protocols on aged rat brain: A resting-state functional magnetic resonance imaging study. Chin. Med. J. 134, 344–352. doi: 10.1097/CM9.0000000000001126
Luis, C. A., Keegan, A. P., and Mullan, M. (2009). Cross validation of the Montreal cognitive assessment in community dwelling older adults residing in the Southeastern US. Int. J. Geriatr. Psychiatry 24, 197–201.
Maier, W., Buller, R., Philipp, M., and Heuser, I. (1988). The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 14, 61–68. doi: 10.1016/0165-0327(88)90072-9
McCarberg, B., and Peppin, J. (2019). Pain pathways and nervous system plasticity: Learning and memory in pain. Pain Med. 20, 2421–2437. doi: 10.1093/pm/pnz017
Phillips, M. L., Drevets, W. C., Rauch, S. L., and Lane, R. (2003a). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol. Psychiatry 54, 504–514. doi: 10.1016/S0006-3223(03)00168-9
Phillips, M. L., Drevets, W. C., Rauch, S. L., and Lane, R. (2003b). Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry 54, 515–528. doi: 10.1016/s0006-3223(03)00171-9
Pistono, A., Guerrier, L., Peran, P., Rafiq, M., Gimeno, M., Bezy, C., et al. (2021). Increased functional connectivity supports language performance in healthy aging despite gray matter loss. Neurobiol. Aging 98, 52–62. doi: 10.1016/j.neurobiolaging.2020.09.015
Reuter-Lorenz, P. A., and Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 17, 177–182. doi: 10.1111/j.1467-8721.2008.00570.x
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Rudebeck, P. H., and Rich, E. L. (2018). Orbitofrontal cortex. Curr. Biol. 28, R1083–R1088. doi: 10.1016/j.cub.2018.07.018
Sharma, L. (2021). Osteoarthritis of the Knee. N. Engl. J. Med. 384, 51–59. doi: 10.1056/NEJMcp1903768
Sporns, O. (2018). Graph theory methods: Applications in brain networks. Dialogues Clin. Neurosci. 20, 111–121. doi: 10.31887/DCNS.2018.20.2/osporns
Vasavada, M. M., Wang, J., Eslinger, P. J., Gill, D. J., Sun, X., Karunanayaka, P., et al. (2015). Olfactory cortex degeneration in Alzheimer’s disease and mild cognitive impairment. J. Alzheimers Dis. 45, 947–958.
Wang, J., Wang, X., Xia, M., Liao, X., Evans, A., and He, Y. (2015). GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 9:386. doi: 10.3389/fnhum.2015.00386
Wang, J., Zuo, X., and He, Y. (2010). Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 4:16. doi: 10.3389/fnsys.2010.00016
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of ‘small-world’ networks. Nature 393, 440–442. doi: 10.1038/30918
Wilson, R. S., Yu, L., Lamar, M., Schneider, J. A., Boyle, P. A., and Bennett, D. A. (2019). Education and cognitive reserve in old age. Neurology 92, e1041–e1050.
Xia, M., Wang, J., and He, Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. doi: 10.1371/journal.pone.0068910
Zhao, Q., Guo, Q., Liang, X., Chen, M., Zhou, Y., Ding, D., et al. (2015). Auditory verbal learning test is superior to Rey-Osterrieth complex figure memory for predicting mild cognitive impairment to Alzheimer’s disease. Curr. Alzheimer Res. 12, 520–526. doi: 10.2174/1567205012666150530202729
Zhuang, Q., Xu, L., Zhou, F., Yao, S., Zheng, X., Zhou, X., et al. (2021). Segregating domain-general from emotional context-specific inhibitory control systems - ventral striatum and orbitofrontal cortex serve as emotion-cognition integration hubs. Neuroimage 238:118269. doi: 10.1016/j.neuroimage.2021.118269
Keywords: elderly patients, knee osteoarthritis, resting-state functional connectivity, cognitive function, graph theory
Citation: Lin G, Lan F, Wu D, Cao G, Li Z, Qi Z, Liu Y, Yang S, Lu J and Wang T (2022) Resting-state functional connectivity alteration in elderly patients with knee osteoarthritis and declined cognition: An observational study. Front. Aging Neurosci. 14:1002642. doi: 10.3389/fnagi.2022.1002642
Received: 25 July 2022; Accepted: 26 September 2022;
Published: 21 October 2022.
Edited by:
Xiaobo Chen, Shandong Institute of Business and Technology, ChinaReviewed by:
Zhen Yuan, University of Macau, Macao SAR, ChinaCopyright © 2022 Lin, Lan, Wu, Cao, Li, Qi, Liu, Yang, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianlong Wang, d190bDU1OTVAaG90bWFpbC5jb20=; Jie Lu, aW1hZ2luZ2x1QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.