
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci., 04 January 2022
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.786897
This article is part of the Research TopicGenomic Instability and NeurodegenerationView all 7 articles
 Eugenie Peze-Heidsieck
Eugenie Peze-Heidsieck Tom Bonnifet
Tom Bonnifet Rania Znaidi
Rania Znaidi Camille Ravel-Godreuil
Camille Ravel-Godreuil Olivia Massiani-Beaudoin
Olivia Massiani-Beaudoin Rajiv L. Joshi
Rajiv L. Joshi Julia Fuchs*
Julia Fuchs*
The etiology of aging-associated neurodegenerative diseases (NDs), such as Parkinson’s disease (PD) and Alzheimer’s disease (AD), still remains elusive and no curative treatment is available. Age is the major risk factor for PD and AD, but the molecular link between aging and neurodegeneration is not fully understood. Aging is defined by several hallmarks, some of which partially overlap with pathways implicated in NDs. Recent evidence suggests that aging-associated epigenetic alterations can lead to the derepression of the LINE-1 (Long Interspersed Element-1) family of transposable elements (TEs) and that this derepression might have important implications in the pathogenesis of NDs. Almost half of the human DNA is composed of repetitive sequences derived from TEs and TE mobility participated in shaping the mammalian genomes during evolution. Although most TEs are mutated and no longer mobile, more than 100 LINE-1 elements have retained their full coding potential in humans and are thus retrotransposition competent. Uncontrolled activation of TEs has now been reported in various models of neurodegeneration and in diseased human brain tissues. We will discuss in this review the potential contribution of LINE-1 elements in inducing DNA damage and genomic instability, which are emerging pathological features in NDs. TEs might represent an important molecular link between aging and neurodegeneration, and a potential target for urgently needed novel therapeutic disease-modifying interventions.
Age-associated neurodegenerative diseases (NDs) such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) have become a global burden due to the continued increase in life expectancy with obvious socio-economic implications (Yang et al., 2020). The support and care of people with NDs, for which age is the main known risk factor, poses a major challenge. Unfortunately, currently available treatments only alleviate some of the symptoms and there is still no disease-modifying treatment (Van Bulck et al., 2019).
Neurodegenerative diseases are clinically separated into specific syndromes based on typical clinical manifestations. For instance, the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) leads to the cardinal motor symptoms in PD (Kalia and Lang, 2015), whereas the degeneration of hippocampal and cortical neurons results in memory impairment, cognitive dysfunction, and dementia in AD (Oboudiyat et al., 2013). In spite of these clinical differences, NDs present substantial neuropathological and genetic overlap (Naz et al., 2017; Gan et al., 2018; Karch et al., 2018). Pathways altered in various NDs include protein quality control, the autosomal-lysosome pathway, mitochondrial homeostasis, protein seeding, propagation of stress granules, synaptic toxicity, and network dysfunction (Gan et al., 2018). Among genetic and environmental factors, age remains the major risk factor for the development of the most prevalent NDs like PD and AD, as well as for other NDs like amyotrophic lateral sclerosis (ALS), multisystem atrophy (MSA), Lewy body disease (LBD), frontotemporal dementia (FTD), and Huntington’s disease (HD) (Kritsilis et al., 2018; Hou et al., 2019).
This review will highlight some striking similarities between the aging process and known pathways involved in the pathogenesis of NDs, discuss how the failure of multiple layers of LINE-1 repression, related to the pathogenesis of NDs, could explain an age-related derepression of TEs and particularly focus on LINE-1 as a source of genomic instability, an emerging pathway triggering neurodegeneration. We will also discuss a hypothetical role of LTR (long terminal repeat) retrotransposons as a possible additional source of DNA damage in the brain. Many other pathogenic mechanisms through which TEs might act as pathogenic drivers in human diseases including neurodegenerative diseases have been extensively reviewed elsewhere (Gorbunova et al., 2021; Ravel-Godreuil et al., 2021b).
To date, despite enormous efforts and a tremendous increase in knowledge about the fundamental nature of NDs, it remains widely unknown how aging and NDs might be linked at the molecular level. Aging is defined as a progressive loss of physiological integrity, leading to impaired functions and increased vulnerability to death (Gilbert, 2000; López-Otín et al., 2013). Aging, as defined by the “disposable theory of aging” put forward by Thomas Kirkwood in 1977, is due to a gradual, life-long accumulation of faults (e.g., DNA damage) in human cells and tissues, leading to organ dysfunction, disease, and ultimately death. So far, nine hallmarks of aging have been defined which comprise genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intracellular communication (López-Otín et al., 2013). The first four hallmarks are considered causative primary hallmarks, which initiate cellular damage. Since age is the major risk factor for NDs, it is important to understand whether and how aging-related processes participate in the pathogenesis of NDs. Table 1 presents a compilation of existing evidence that links NDs with what has been defined as hallmarks of organismal aging. Evidence for the presence of all the nine aging hallmarks has been documented either in brain aging or in the context of NDs (Mattson and Arumugam, 2018; Hou et al., 2019), but we will highlight here the two primary aging hallmarks, namely, genomic instability and epigenetic alterations, as these are most relevant to TE biology.
It is well documented that aging cells accumulate persistent DNA damage throughout life (Sedelnikova et al., 2004) and DNA repair activity declines in neurons with aging (Lu et al., 2004; Vyjayanti and Rao, 2006). Post-mitotic neurons in the central nervous system are particularly susceptible to this type of damage. This is due mainly to two factors related to the specificities of neuronal cell functioning. First, post-mitotic neurons do not dispose of the full repertoire of DNA repair pathways to repair DNA double-strand breaks (DSBs) (Chow and Herrup, 2015). Indeed, in mammals, there are four main DNA repair pathways, namely, nucleotide excision repair (NER) and base excision repair (BER) to repair single-strand lesions and base alterations, respectively, and homologous recombination (HR) and non-homologous end-joining (NHEJ) to repair DSBs (Chatterjee and Walker, 2017). HR requires DNA replication during cell division. However, neurons are post-mitotic, hence non-dividing and therefore cannot rely on the HR repair pathway but instead exclusively depend on NHEJ known to be more error prone. The second main specificity of neurons is the high metabolic rate which renders them prone to metabolic stress, resulting in elevated oxidative stress through the production of free radicals leading to DNA damage (Ismail and Hendzel, 2008). DNA damage can also be exacerbated by the defective functioning of topoisomerases in the context of NDs. In a physiological context, topoisomerases release torsional stress during DNA transcription, particularly on extremely long genes enriched in neuronal functions (King et al., 2013) and coordinate transcription from the promoter of immediate early genes associated with learning and memory (Madabhushi et al., 2015). These studies suggest that DNA strand breaks, if rapidly repaired at steady-state levels, can participate in physiological neuronal functions. However, when DNA repair becomes altered during aging, the transcription-associated and topoisomerase-linked DNA damage can accumulate and have indeed been reported as elevated in the context of AD (Suberbielle et al., 2013). This illustrates the importance of endogenous sources of genomic instability. The particular vulnerability of neurons to DNA damage is underscored by the fact that numerous diseases, linked to mutations in DNA repair factors, manifest with neurological symptoms (Katyal et al., 2014; Madabhushi et al., 2014). Furthermore, each neuronal population, or even subpopulation, carries cell-type-specific vulnerabilities. For instance, dopaminergic neurons are particularly vulnerable to oxidative stress due to their specific physiology and morphology (Surmeier et al., 2017), especially in humans where dopaminergic neurons increased in number and arborization throughout evolution (Vernier, 2004; Bolam and Pissadaki, 2012).
DNA damage has been documented in experimental models of NDs, and in PD and AD patients (Mullaart et al., 1990; Adamec et al., 1999; Sepe et al., 2016; Mitra et al., 2019; Shanbhag et al., 2019). Interestingly, mice deficient in the serine/threonine kinase ataxia telangiectasia (ATM) show a selective degeneration of dopaminergic neurons in the SNpc (Eilam et al., 1998). Following rapid recruitment to DSBs, ATM not only coordinates several aspects of the cellular DNA damage response (Shiloh and Ziv, 2013) but also averts DNA damage by preventing the accumulation of topoisomerase-dependent DNA lesions (Katyal et al., 2014). This indicates that dopaminergic neurons are particularly sensitive to defects in the DSB repair pathway. The NER pathway is equally important in dopaminergic neurons as NER-deficiency induces a PD-related pathology (Sepe et al., 2016). More recently, DSBs were shown to precede all pathological hallmarks in a mouse model of AD (CK-p25). DNA damage in these mice coincided with a reduction in HDAC1 (histone deacetylase 1), and neuronal loss could be rescued by HDAC1 (Kim et al., 2008) and the NAD+-dependent deacetylase SIRT1 (Sirtuin 1) (Kim et al., 2007). A complex interplay between HDAC1, SIRT1, and ATM was identified, suggesting that unrepaired DSBs, due to a dysfunctional DNA repair pathway, could underline neurodegeneration in AD (Dobbin et al., 2013). DNA damage has also been shown to accumulate in other NDs such as ALS (Kwiatkowski et al., 2009; Vance et al., 2009; Wang et al., 2013).
Taken together, neurons seem particularly susceptible to DNA damage due to neuron-specific features. Increasing experimental evidence suggests that genomic instability is related to the aging process in neurons and sufficient to trigger neurodegeneration. The life-long accumulation of DNA damage together with a decline in DNA repair mechanisms might be initiating or at least contributing to neurodegeneration (Madabhushi et al., 2014; Martínez-Cué and Rueda, 2020).
We discuss below the emerging concept that LINE-1 elements might be an additional source of DNA damage and genomic instability and that LINE-1 activation in the brain could be part of the aging process as shown in somatic cells (Gasior et al., 2006; Belancio et al., 2010; De Cecco et al., 2019; Simon et al., 2019) and lead to DNA damage and neurodegeneration (Blaudin de Thé et al., 2018). Through induction of genomic instability and other recently discovered consequences of LINE-1 activation, e.g., neuroinflammation (reviewed in Saleh et al., 2019; Gorbunova et al., 2021), LINE-1 represents a so far unsuspected new pathogenic driver in NDs.
The complete human genome sequencing revealed that about 50% of human DNA consists of repetitive sequences (Lander et al., 2001), most of which are remnants of an ancient activity of TEs (Figures 1A,B and Table 2). These mobile elements comprise DNA transposons, LTR-retrotransposons (mammalian apparent LTR retrotransposon, MaLRs; endogenous retroviruses, ERVs), and non-LTR retrotransposons (LINE-1; Short INterspersed Elements, SINEs, and the composite element SINE/VNTR/Alu, SVA). LINE-1 elements have massively expanded in mammalian genomes and are the only autonomous retrotransposons in humans that encode their own mobilization machinery to move from one genomic location to another. As shown in Figure 1C, a full-length LINE-1 contains two open reading frames: ORF1 coding for ORF1p (an RNA binding protein) and ORF2 encoding ORF2p (with endonuclease, EN, and reverse transcriptase, RT, activity). The number of LINE-1 elements that have retained their full coding potential in the human genome is currently estimated to be 146 in the human reference genome GRCh38/hg38 and 2811 in the mouse reference genome GRCm38/mm10 (euL1db: L1Basev2 Mir et al., 2015; Penzkofer et al., 2017). The mechanism of LINE-1 retrotransposition, the “LINE-1 life cycle,” is depicted in Figure 2 and described in more detail in the figure legend. TEs have self-amplified and shaped mammalian genomes during evolution and possibly conferred evolutionary benefit (Goodier and Kazazian, 2008). A physiological role of LINE-1 retrotransposition in neuronal mosaicism during adult neurogenesis has also been proposed; this aspect is not discussed here and has been reviewed elsewhere (Erwin et al., 2014; Richardson et al., 2014; Faulkner and Garcia-Perez, 2017). Neverthless, LINE-1 mobilization at the level of an individual represents a threat to genome integrity. As an example, LINE-1 insertions account for 1 in every 250 pathogenic mutations in human diseases (Kazazian, 1998; Kazazian and Moran, 2017). In addition, LINE-1 unsilencing in culture can lead to numerous DSBs in the genome of human cells (Belgnaoui et al., 2006; Gasior et al., 2006), thereby decreasing cellular viability by inducing a senescence-like state (Wallace et al., 2008) or inducing apoptosis (Belgnaoui et al., 2006). ORF2p seems to nick chromosomal DNA at hundreds of different loci before each successful integration event (Gasior et al., 2006). Indeed, mutations in the EN domain of the LINE-1 ORF2 resulted in complete loss of γ-H2AX (phosphorylated histone H2A) foci, a marker of DNA damage, in HeLa cells (Gasior et al., 2006), indicating that DNA damage is mediated by the ORF2 encoded EN activity. This was also experimentally demonstrated in mouse fibroblasts (Belancio et al., 2010). Thus, all LINE-1 elements that have retained coding potential for at least the ORF2p EN are potential endogenous sources of genomic instability when unsilenced. Based on the L1Basev2 annotation (Penzkofer et al., 2017), there are about 253 individual LINE-1 elements with a complete open reading frame for ORF2p in addition to the full-length LINE-1 elements which, when activated, could constitute an additional source of genome instability in somatic cells.
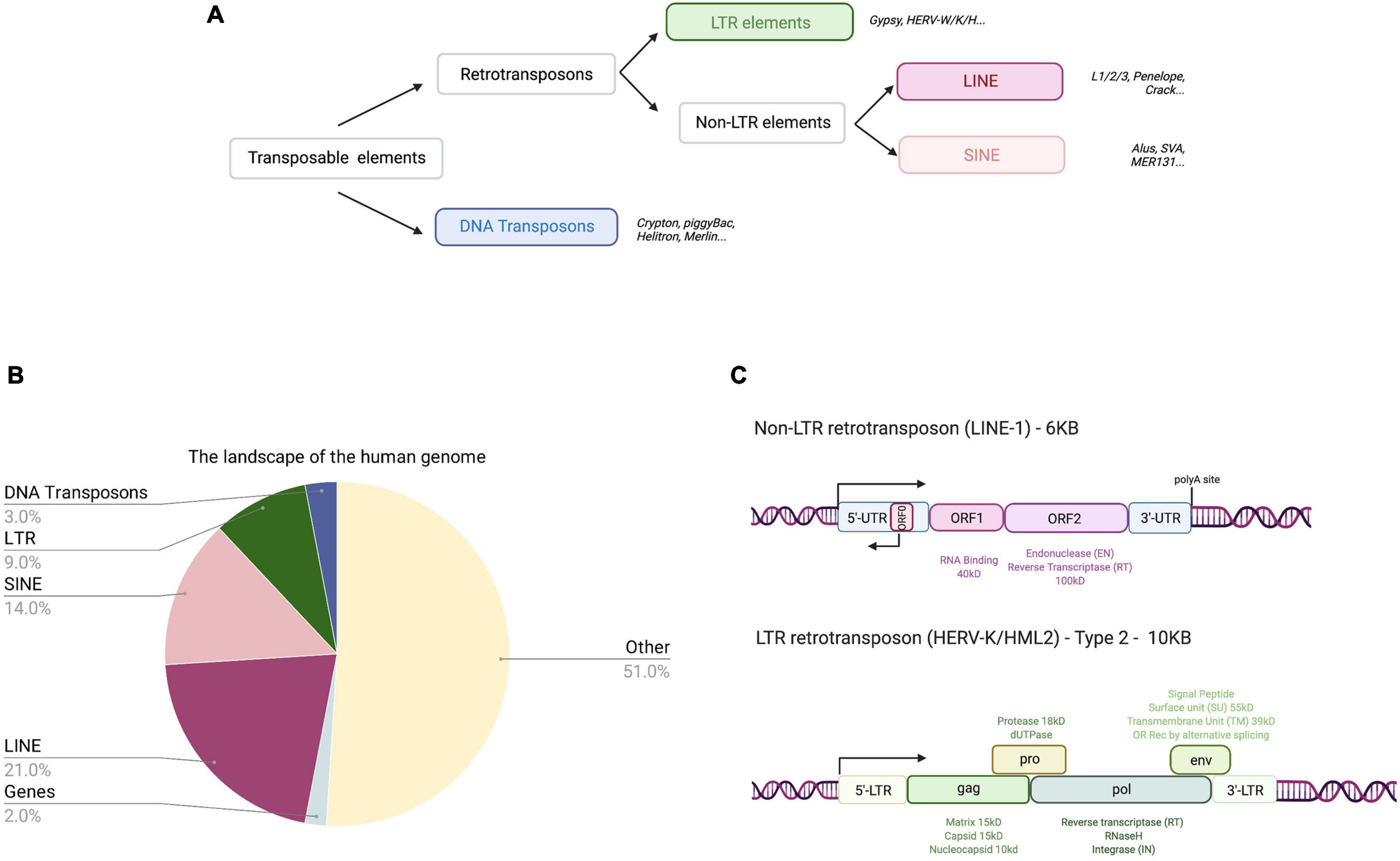
Figure 1. Categories of TEs in the human genome and their coding potential. (A) TE classification. TEs in humans can be classified into four categories belonging to two classes, retrotransposons (class 1) and DNA transposons (class 2). Retrotransposons include non-LTR retrotransposons (autonomous LINEs and non-autonomous SINEs) and LTR retrotransposons. Examples of elements are written in italics and boxed families are represented in the pie chart (B) indicating relative% abundance in the human genome. (B) The landscape of the human genome. The human genome consists of sequences derived from TEs (47%), coding sequences (or exons; 2%), and “other” sequences (promoters, enhancers, introns, non-coding RNA, telomeres, centromeres, and pseudogenes; 51%). (C) Human retrotransposons with coding potential. Non-LTR retrotransposons (approximately 6kB long) are composed of a 5′UTR (containing sense and antisense promoters), two open reading frames (ORF1 and ORF2), and a 3′UTR containing a poly(A) site. ORF1 encodes for an RNA binding protein and ORF2p has EN and RT activities. Human and primate LINE-1 also encode an antisense ORF, termed ORF0 present in the 5′UTR. LTR (HERV-K/HML-2) retrotransposons (approximately 10 kB long) are flanked by two LTRs. They contain a 5′UTR promoter within the LTR, a primer binding site and four main ORFs (gag, pro, pol, and env) giving rise to Gag, Gag-Pro-Pol, and Gag-Pro polypeptides (via ribosomal frameshift) and Env. Gag is cleaved by the encoded viral protease into the matrix, capsid and nucleocapsid proteins, which have structural functions. The protease is autocleaved into the viral protease and a dUTPase. Gag-Pol polyprotein cleavage by the viral protease gives rise to RT with polymerase activities, RNaseH, and IN. The Env protein is generated from a spliced mRNA and cleaved in the endoplasmatic reticulum by a cellular protease into signal peptide, surface unit, and transmembrane unit. Alternative splicing of Env generates two other proteins depending on the HERV-K/HML-2 type: rec or Env (type 2) or np9, but no Env (type 1, not shown).
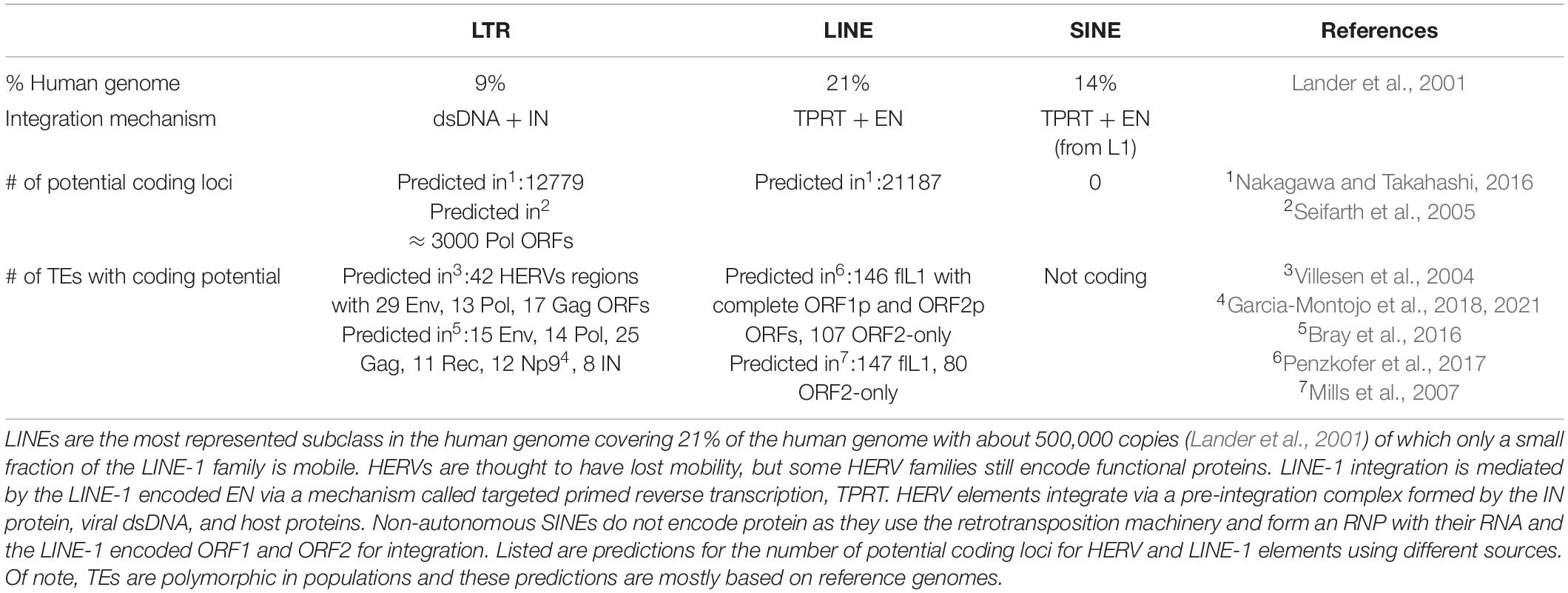
Table 2. Summary of the frequency of retrotransposons and estimates about the number of open reading frames for their encoded proteins.
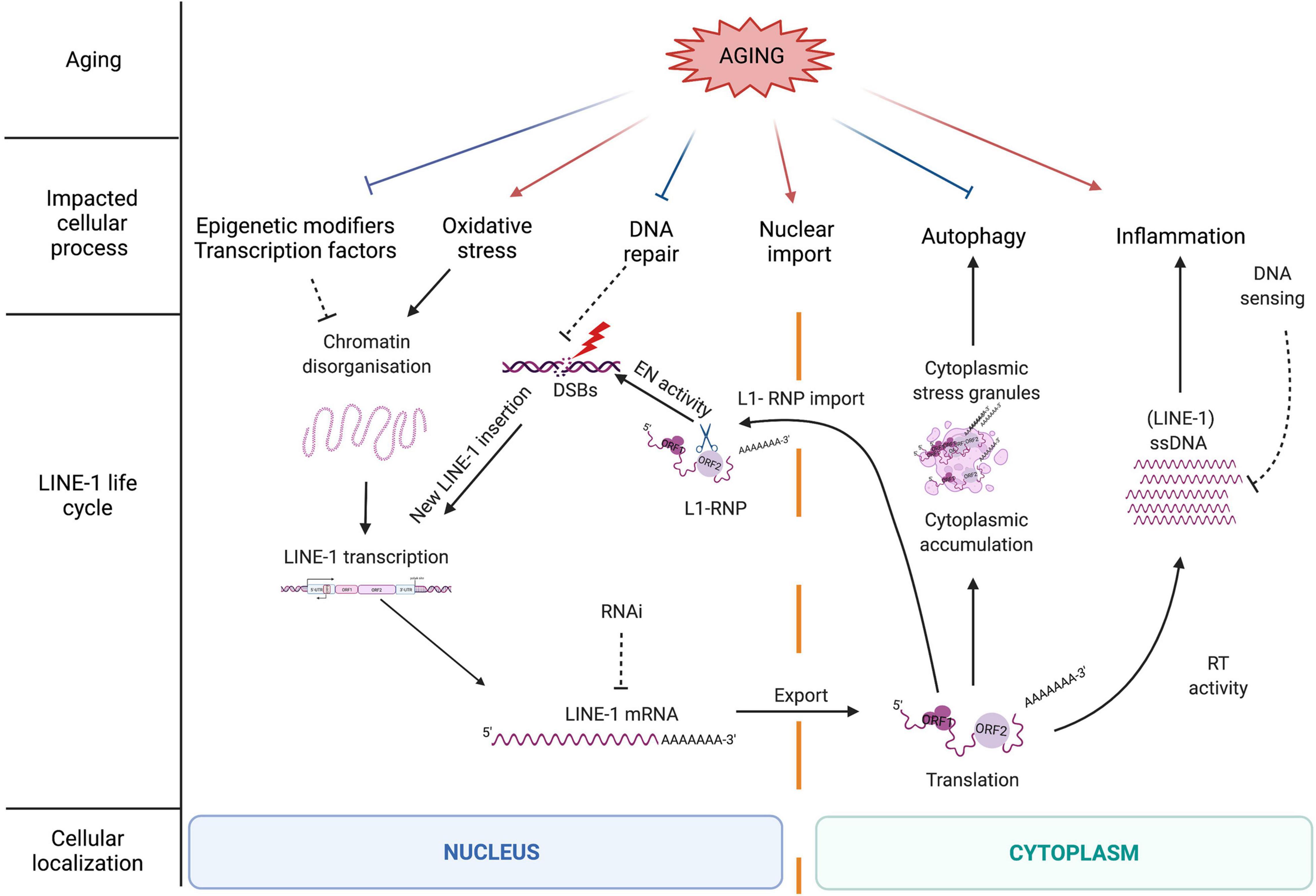
Figure 2. The LINE-1 retrotransposition life cycle as a source of DNA damage and the influence of the aging process on LINE-1 repressive mechanisms. The LINE-1 life cycle is controlled by different cellular processes many of which are negatively impacted by aging (described in more detail in the main text). Aging might thus contribute to a release of repression of LINE-1 by cellular control mechanisms including epigenetic repression, DNA repair, and autophagy. On the other hand, other cellular processes impacted by aging favor either LINE-1 expression (oxidative stress) or possibly LINE-1 nuclear import (increase in nuclear pore permeability). Aging-related inflammation might be amplified by LINE-1 expression through the generation of cytoplasmic nucleic acids via the LINE-1 RT and the generation of ssDNA which is repressed by DNA sensing (i.e., via the exonuclease Trex1 or other proteins involved in the modulation of cytosolic nucleic acid species). When cellular repressive mechanisms are alleviated, full-length LINE-1 elements become expressed, which initiates the LINE-1 life cycle starting with the transcription of a full-length LINE-1 element from the endogenous promoter contained in the 5′UTR (Figure 1C) and the export of the polyA + LINE-1 mRNA into the cytoplasm. Once translated, the LINE-1 encoded proteins ORF1p and ORF2p reassemble in “cis” with the LINE-1 mRNA to form a ribonucleoparticle (RNP). The LINE-1 RNP can accumulate in the cytoplasm in stress granules. Through a widely unknown mechanism, the RNP enters the nucleus where the ORF2 EN creates DNA strand breaks and a new LINE-1 copy (often 5′truncated) is reverse transcribed into the genome via target-primed reverse transcription (TPRT). ORF2p can also create DNA strand breaks independent of retrotransposition. As a bystander of cytoplasmic RT activity and via an unknown primer, ORF2 RT can reverse transcribe RNA into ssDNA which triggers the innate immune system and inflammation.
Since LINE-1 activity represents a potential threat for genome integrity, several cellular factors and pathways keep these elements in check at almost every level of the LINE-1 life cycle (Figure 2). These repressive mechanisms include regulation at the epigenetic level by the binding of epigenetic modifiers to the LINE-1 sequence, at the transcriptional level by sequence-specific repressive transcription factors binding to the LINE-1 promoter in the 5′UTR (5′ untranslated region), at the post-transcriptional level by degradation mechanisms (splicing, RNA interference or RNAi, autophagy, and stress granules), at the translational level via RNA binding proteins, at the level of the nuclear import of the RNA, and at the integration level by several factors belonging to the DNA repair machinery (Pizarro and Cristofari, 2016). Repression at the epigenetic level is mostly accomplished by the addition of repressive histone marks, mainly trimethylation of lysine 9 of histone H3 (H3K9me3) (Bulut-Karslioglu et al., 2014; Liu et al., 2014; He et al., 2019), trimethylation of lysine 20 of histone H4 (H4K20me3) (Ren et al., 2021), and histone H1 on LINE-1 loci (Healton et al., 2020), and by DNA methylation (Hata and Sakaki, 1997; Muotri et al., 2010). This is mediated by several sequence-specific repressors that directly bind to TEs and recruit epigenetic modulators. One example of a rather general repressor of TEs is the Kruppel-associated box Zinc finger protein family (KRAB-ZFPs) which repress LTR retrotransposons (Ecco et al., 2017; Yang et al., 2017), but also LINE-1 and SVA elements (Castro-Diaz et al., 2014; Jacobs et al., 2014). KRAB-ZFPs provide a scaffold for the formation of heterochromatin on TEs. Another example for a repressive transcription factor is the homeobox protein Engrailed, which binds to the 5′UTR of LINE-1 elements and represses their expression in midbrain dopaminergic neurons (Blaudin de Thé et al., 2018). As Engrailed expression is regionally restricted (Di Nardo et al., 2018), this provides an example of region-specific TE control.
When TEs become derepressed and transcribed, the next level of repression consists of small RNAs targeted against TE transcripts. In the germline, where specific developmental states require the relaxation of epigenetic repression, the PIWI (P-element Induced WImpy testis in Drosophila)-piRNA (Piwi-interacting RNA) pathway plays an important role in TE control (Zamudio and Bourc’his, 2010). Outside of the germline, piRNA expression has been documented in somatic tissues, including the mouse brain (Lee et al., 2011), but so far the role of piRNA expression in the brain has not yet been identified. However, as a proof-of-principle, PIWIL1 (PIWI-like protein 1) overexpression from a viral vector was sufficient to repress LINE-1 induced neurodegeneration of midbrain dopaminergic neurons during oxidative stress and in the En1± heterozygous mouse model of PD (Blaudin de Thé et al., 2018). In addition to the specific piRNA pathway, endogenous siRNAs (small interfering RNAs) suppressing TE expression have been identified in both gonadal and non-gonadal tissues. They derive from bidirectional transcription of TE-containing loci (Saito and Siomi, 2010). Other RNAi pathways, capable of degrading LINE-1 mRNA directly via the microprocessor complex (Drosha/DGCR8), have also been described (Heras et al., 2013, 2014). Furthermore, several cellular proteins have been identified that positively or negatively regulate LINE-1 activity through proteomic screens for cellular interactors of ORF1p or ORF2p (Pizarro and Cristofari, 2016).
Several other cellular pathways alter LINE-1 activity. Among these, autophagy is an important cellular mechanism used to degrade LTR and non-LTR retrotransposon RNA and thereby prevent retrotransposition events. Depending on the level of expression, LINE-1 mRNA localizes either to RNA granules (endogenously expressed LINE-1) or cytoplasmic stress granules (exogenous overexpressed LINE-1 RNA), but in either case, LINE-1 RNA can be degraded via autophagy (Guo et al., 2014). ORF1p is located in distinct foci in the cytoplasm which have been (in conditions of exogenous expression) identified as stress granules (Goodier et al., 2007, 2013). It is noteworthy that stress granules formed in the absence of an exogenous stress, indicating that ORF1p overexpression itself is recognized as a stress by the host cell. Based on their data, the authors suggest that stress granules could sequester and possibly degrade LINE-1 RNPs (ribonucleoproteins consisting of ORF1p/ORF2p and the LINE-1 mRNA) via P (processing)-bodies (Goodier et al., 2007), consistent with what was found for LINE-1 RNA (Guo et al., 2014). Within the stress granules, ORF1p interacts with a large number of RNA-binding proteins but interestingly, the authors also identified RNAi factors colocalizing with ORF1p, suggesting an additional layer of repression within this membraneless cytoplasmic compartment.
The proteomic screens for LINE-1 interactors also identified proteins activated through the interferon response pathway by the innate immune system. These findings are consistent with the role of the innate immune response as one of the first cellular responses activated upon viral infections (MacMicking, 2012) and the parasitic nature of LINE-1 elements. Some of these proteins colocalize with stress granules and degrade LINE-1 RNA (e.g., MOV10, Moloney leukemia virus 10 homolog). However, the exact mechanism for LINE-1 repression remains unknown for other proteins (e.g., cytidine deaminase APOBEC3) (Pizarro and Cristofari, 2016). This inflammatory response, although essential, can have pathological consequences if not regulated (Kassiotis and Stoye, 2016). Mutations in TREX1 (three prime repair exonuclease 1) or SAMHD1 (SAM domain and HD domain-containing protein 1) (Hu et al., 2015), for instance, lead to the same autoimmune disease, Aicardi-Goutières syndrome (AGS). TREX1 detects and degrades cytoplasmic ssDNA (single-stranded DNA) fragments arising from aberrant RT activity of LINE-1 or ERV (Stetson et al., 2008; Thomas et al., 2017) and possibly also released from mitochondrial damage (Sliter et al., 2018). The loss of TREX1 activity results in the accumulation of LINE-1-derived ssDNA in the cytoplasm of astrocytes, initiating (through the cGAS-STING pathway) a neurotoxic release of interferon in the extracellular medium, and leads to neurodegeneration (Thomas et al., 2017).
Finally, at the level of LINE-1 insertions or EN-mediated DSBs, the DNA repair pathway comes into play. However, while some DNA repair pathway proteins restrict LINE-1 activity (e.g., DNA excision repair protein 1, ERCC1), others such as poly(ADP-ribose) polymerase 2 (PARP2) bind to LINE-1 integration sites leading to subsequent recruitment of the replication protein A (RPA) complex to facilitate retrotransposition (for review, Pizarro and Cristofari, 2016; Miyoshi et al., 2019).
Taken together, this complex interplay of repressive mechanisms controlling TE expression ensures genomic integrity and has been shown to be of great importance in the context of neuronal cell survival (Thomas et al., 2017; Blaudin de Thé et al., 2018). However, these mechanisms seem to fail over time. We will discuss below how the aging process weakens this multi-layer cellular response (Figure 2), increasing genomic instability in the host cell and igniting (neuro)inflammation.
Somatic cells express low, basal levels of LINE-1 (Faulkner et al., 2009; Belancio et al., 2010). The brain seems to provide a particularly permissive environment for LINE-1 activity (Muotri et al., 2005, 2010; Coufal et al., 2009; Macia et al., 2017) and supports higher retrotransposition rates compared to other tissues (Muotri et al., 2005; Coufal et al., 2009; Baillie et al., 2011; Evrony et al., 2012; Goodier, 2014; Suarez et al., 2018). While LINE-1 RNA expression is well documented in the human brain (Guo et al., 2018; Pereira et al., 2018; Sun et al., 2018; Savage et al., 2019), knowledge about the expression of LINE-1 encoded proteins in the brain remains scarce. Although limited by sample size, the only study, to our knowledge, using immunohistochemistry to characterize ORF1p expression in human post-mortem brain provides evidence of higher ORF1p expression levels in the brain compared to peripheral tissues and suggests that patterns of ORF1p expression might vary depending on the age of the individual, notably with regard to the subcellular localization of ORF1p (Sur et al., 2017). The mobility of TEs increases with aging in several species [Saccharomyces cerevisiae (Maxwell et al., 2011); Caenorhabditis elegans (Dennis et al., 2012); human cells in culture (De Cecco et al., 2013); mice (De Cecco et al., 2019) and mouse tissues including brain (Van Meter et al., 2014)]. As discussed above, aging is characterized by several hallmarks impacting normal cellular functions and inducing a secretory phenotype affecting surrounding cells (Di Micco et al., 2021). Aging alters repressive epigenetic modifiers, leading to heterochromatin disorganization (Misteli, 2010; Lee et al., 2020), induces autophagy inhibition (Barbosa et al., 2019) and cytoplasmic stress granule dysfunction (Cao et al., 2020), increases permeability of the nuclear membrane (Li and Lagier-Tourenne, 2018), triggers a dysfunctional innate immune response (Shaw et al., 2010) and a decline of DNA repair (Maynard et al., 2015). As shown in Figure 2, aging generally leads to a decrease in TE repressive factors (Van Meter et al., 2014), which allows TE expression. Finally, aging also favors certain environments which increase LINE-1 expression, notably oxidative stress (Rockwood et al., 2004; Blaudin de Thé et al., 2018).
The regulation of LINE-1 by SIRT6 (sirtuin 6) particularly illustrates the failure of a major LINE-1 repressive mechanism following cellular stress or aging and thus impacting cellular fitness. Indeed, during aging or in response to DNA damage, the heterochromatin-inducing protein SIRT6, a histone deacetylase, becomes depleted from LINE-1 loci, leading to derepression of LINE-1 elements (Van Meter et al., 2014). SIRT6 is a key regulator of mammalian lifespan as illustrated by the severe premature aging phenotype of Sirt6 knockout mice (Mostoslavsky et al., 2006) and the increase in lifespan of mice overexpressing Sirt6 (Kanfi et al., 2012). Interestingly, of all organs tested, the brain showed the highest upregulation of LINE-1 transcripts with aging, together with a partial loss of Sirt6 binding to the LINE-1 5′UTR. This, combined with reports of higher retrotransposition rates in the brain compared to other tissues (Coufal et al., 2009; Baillie et al., 2011) and several neurological diseases associated with a dysregulation of LINE-1 activity (Baillie et al., 2011; Jakovcevski and Akbarian, 2012), suggests that the brain might be a particularly susceptible organ to LINE-1 related aging.
Another sirtuin, Sirt7 (sirtuin 7), safeguards genome stability and cell viability (Tang et al., 2021). Among its cellular functions, Sirt7 also acts as a tethering factor, partly via acetylation of H3K18 (histone H3 lysine 18), between the nuclear lamina protein laminA/C and young LINE-1 sequences in mice and human cells. LINE-1 elements are thus enriched at lamin-associated domains (LADs), which are heterochromatic regions at the nuclear periphery (Zuo and Rocha, 2020), and this tethering by Sirt7 ensures LINE-1 transcriptional silencing (Vazquez et al., 2019). However, Sirt7 is downregulated during aging in hematopoietic stem cells (Mohrin et al., 2015), suggesting that this repressive mechanism of LINE-1 could be altered. Furthermore, it has been reported that aging is correlated with the decrease of LINE-1 retrotransposon promoter methylation in purified cell-free DNA from human blood (Mahmood et al., 2020).
The visionary “LINEage” theory has been postulated more than a decade ago (St. Laurent et al., 2010). It hypothesized that L1 acts as an “endogenous clock” which slowly erodes genomic integrity by competing with DNA break repair mechanisms and thereby negatively impacting longevity. Accumulating experimental evidence since then only confirms that LINE-1 activation might not only be an important universal hallmark of aging in various tissues, including the brain, but also a mechanistic driver of aging.
Overexpression of LINE-1 in human embryonic stem cells differentiated into hippocampal neurons led to an increase in DNA DSBs (marked by γ-H2AX) which was abolished when the LINE-1 EN and RT domains were mutated (Erwin et al., 2016). The fact that LINE-1 activation can lead to DSBs in adult neurons in vivo and induce neurodegeneration was recently established in a mouse model of PD carrying only one allele of the homeodomain transcription factor Engrailed-1 (En1) (Blaudin de Thé et al., 2018). The homeoprotein Engrailed plays an important role in the development and survival of dopaminergic neurons during development (Simon et al., 2001; Di Nardo et al., 2007; Rekaik et al., 2015). En1 expression persists in adult midbrain dopaminergic neurons and continues to be required for the survival of these neurons. Indeed, En1± mice lose dopaminergic neurons in the SNpc starting from 6 weeks of age (Sonnier et al., 2007). This loss is progressive and mutant mice develop PD-like motor symptoms. These mice also show early dysfunctions in nerve termini and the autophagy-lysosome pathway, reminiscent of early perturbances and retrograde degeneration patterns observed in PD. En1± mice thus represent a valuable model for PD (Sgadò et al., 2008), used for preclinical drug testing (Ghosh et al., 2016). Further characterization of this model revealed that En1± mice exhibit loss of repressive chromatin marks, increased LINE-1 expression, and DSBs accumulation in dopaminergic neurons in the SNpc. Importantly, it was shown that part of these DSBs result from LINE-1 activation (Blaudin de Thé et al., 2018). In line with this, DNA damage and neuronal cell death in either En1± mice or a toxicological model of PD (direct injection of 6-hydroxydopamine in the SNpc) could be rescued by anti-LINE-1 strategies such as overexpression of PIWIL1, siRNAs targeting ORF2, or a reverse transcriptase (RT) inhibitor developed in the context of HIV/AIDS treatment, but also active against the RT enzyme encoded by ORF2p (Dai et al., 2011; Banuelos-Sanchez et al., 2019). Altogether, these studies indicate that LINE-1 activity contributes to neurodegeneration in En1± mice and in an acute oxidative stress model of dopaminergic neurodegeneration via the induction of DNA damage. These studies also showed that LINE-1 retrotransposons are inducible in postmitotic dopaminergic neurons under stress conditions and stress-induced LINE-1 increase is associated with DNA damage and linked to neurodegeneration. More recently, it was shown that heterochromatin destructuration following changes in DNA methylation in dopaminergic neurons in the SNpc also resulted in LINE-1 derepression, DNA damage and neurodegeneration (Ravel-Godreuil et al., 2021a).
The active expression of TEs, especially LINE-1 retrotransposons, has now been documented in other NDs such as AD (Guo et al., 2018; Sun et al., 2018) or ALS (Pereira et al., 2018; Savage et al., 2019). Although LINE-1 retrotransposition has been reported in healthy tissues (Muotri et al., 2005, 2010; Coufal et al., 2009; Macia et al., 2017) and in the context of a large spectrum of neurological diseases (Reilly et al., 2013; Suarez et al., 2018), the question as to whether retrotransposition events take place in postmitotic neurons is still a matter of debate. In particular, the relative contribution of LINE-1 retrotransposition events in inducing genomic instability and neurodegeneration remains to be established. However, as discussed above, even in the absence of LINE-1 mobilization, the DNA strand breaks induced by LINE-1 activity can be sufficient to jeopardize genome integrity in the brain and contribute to neurodegeneration (Blaudin de Thé et al., 2018). In addition to being a source of DNA damage and genomic instability, LINE-1 could be pathogenic drivers for other pathological features of NDs. Indeed, the activity of LINE-1 loci can modulate host gene expression in various ways (Elbarbary et al., 2016; Liu et al., 2018). In addition, TE-derived proteins can be neurotoxic (Antony et al., 2004; Douville and Nath, 2017) and an increase in TE encoded proteins can be an important source of neuroinflammation. Both, gene dysregulation and neuroinflammation are common hallmarks of NDs. These aspects have recently been reviewed in depth elsewhere (Saleh et al., 2019; Tam et al., 2019a; Gorbunova et al., 2021). Further, exciting evidence suggests that some proteins linked to AD and two other neurodegenerative diseases, namely, ALS and FTD, control or interact with TEs at different levels. The protein Tau, mutated in specific forms of FTD and aggregating in several neurodegenerative diseases including AD (Orr et al., 2017) is involved in epigenetic repression of TEs (Sun et al., 2018; Ramirez et al., 2021) and TDP-43 binds TE sequences and TE transcripts (Li et al., 2012; Tam et al., 2019b).
Genomic DNA damage can also be associated with other types of cellular response, such as cellular senescence. In recent years, a role of senescent cells has been recognized in aging and aging-related pathologies (Childs et al., 2017; Kritsilis et al., 2018). It is well documented that persistent accumulation of unrepairable DNA damage in dividing cells leads to either apoptosis or senescence, characterized by permanent cell cycle arrest (d’Adda di Fagagna, 2008; Herranz and Gil, 2018) and a senescence-associated secretory phenotype (SASP). The secretion of various factors such as pro-inflammatory cytokines or matrix remodeling factors by senescent cells can promote the induction of the same phenotype in surrounding cells and drive aging and aging-related diseases. LINE-1 expression induces cellular senescence in MCF7 cells (Wallace et al., 2008), and in turn, senescence induces LINE-1 expression which activates a type-I interferon (IFN-I) response (De Cecco et al., 2019). Furthermore, several factors, such as FOXA1 (Forkhead box protein A1), TREX1, and RB1 (retinoblastoma 1), have altered expression during senescence and are also LINE-1 regulators (De Cecco et al., 2019).
While neurons cannot divide, recent evidence suggests that post-mitotic neurons could re-enter the cell cycle in the context of neurodegeneration (Nandakumar et al., 2021). Since mature neurons cannot fully terminate the cell cycle by cell division, they might rapidly exit the cell cycle and acquire a senescent-like phenotype (Jurk et al., 2012). The DNA damage resulting from the activation of LINE-1 elements could contribute to the accumulation of senescent-like neurons in various brain areas during aging and neurodegeneration. Such cells might spread this senescence-like state through SASP to neighboring neurons and thereby contribute to the chronic neuroinflammation observed in NDs. Selective ablation of senescent cells is thought to have therapeutic potential in neuroprotection (Childs et al., 2017).
As documented above, accumulating evidence suggests that LINE-1 retrotransposons can be a source of genomic instability through the activity of the encoded EN. As DNA cleavage is an integral part of transposition, we will examine in the following section whether other TEs in the human genome could be accountable for genomic instability in specific cellular contexts. While LINE-1 are the only currently mobile TEs in the human genome, some copies of LTR retrotransposons, which comprise 8 to 9% of the human genome (Figure 1B), have retained transcriptional activity and coding potential and have been associated with human diseases, including neurodegenerative diseases. A comprehensive review on HERV pathogenicity in neurodegenerative diseases was published recently (Tam et al., 2019a).
Human LTR retrotransposons can be broadly divided into the non-autonomous MaLR and human endogenous retroviruses (HERVs), which originate from exogenous retroviruses that have infected hominoid germline cells millions of years ago (Boeke and Stoye, 1997). In contrast to endogenous retroviruses in mice, HERVs are considered non-replicating, but HERV-RNA and encoded proteins have been identified in various tissues (Garcia-Montojo et al., 2018). Although differences in structure and processing exist, ERVs follow globally the classical life cycle of retroviruses, namely, transcription from the so-called nuclear DNA provirus, a complex translation pattern of the compact RNA which includes two frameshifts, assembly of encoded proteins and RNA to envelope-mediated budding from the plasma membrane, processing of polyproteins into functional protein units by the encoded protease within the capsid, entry into a new cell, and post capsid disassembly and reverse transcription of the viral RNA into cDNA (dsDNA via the activity of the polymerase, Pol). Finally, a pre-integration complex containing an integrase (IN) processed from the Pol polyprotein within the capsid, “viral” dsDNA, and host proteins, enter the nucleus and, following DNA strand breaks, the viral dsDNA integrates into the host genome. Different HERV families arose through independent infections of the human germ line 10 millions of years ago (Tristem, 2000).
Very few complete HERV sequences with open reading frames for all encoded proteins have been identified in current human genomes so far (Turner et al., 2001; Belshaw et al., 2005a) as most HERV sequences have been mutated or recombined to solo-LTRs during hominoid evolution. It is clear, however, that TEs are highly polymorphic in populations (including humans) and polymorphic HERVs are linked to diseases (Wallace et al., 2018). It cannot be excluded that complete HERV sequences that are replication-competent might exist at low frequency (Belshaw et al., 2005a). The release of virus-like particles in the context of cancer has been documented (Brodsky et al., 1993; Contreras-Galindo et al., 2015), but no human HERV elements and very few mouse ERVs so far have been shown to complete the integration process. However, several loci show potential for coding for one or the other HERV protein (Table 2; Seifarth et al., 2005; Nakagawa and Takahashi, 2016), which theoretically could recombine to form a functional element or through a process combining recombination and trans-complementation (Dewannieux et al., 2006). Indeed, incomplete HERVs might retrotranspose through a process called “complementation in trans” (Mager and Freeman, 1995; Belshaw et al., 2005b), which requires the simultaneous expression of HERV elements encoding a polyprotein with ORFs for both Gag (group antigen) and Pol, complemented by another element (or the same), encoding a functional Env (envelop) protein. Trans complementation of HERVs has been experimentally demonstrated by reconstituting a HRV-K(HML-2) element, which was infectious and depended on a capsid-involving infection of another cell and could led to the reintegration of a new synthetic HERV copy in vitro (Dewannieux et al., 2006). Capsid-independent mobilization and amplification through retrotransposition has been suggested (Belshaw et al., 2005b), but the mechanisms by which this could be possible remain unknown. Retrotransposition in “cis” uses the proteins encoded by a given HERV element (or several HERVs via trans complementation) to “copy-and-paste” elsewhere in the genome, which requires a functional Gag and Pol, but not Env protein (as this process does not require infection). This is known for the LTR retrotransposons Ty1 and Drosophila copia (Dewannieux and Heidmann, 2005) as well as for mouse IAP elements (Ribet et al., 2008), but has not been documented for HERVs and was absent in the synthetic HERV-K Phoenix (Dewannieux et al., 2006). Another described mechanism for HERV amplification is the trans-mobilization of HERV-W elements by the LINE-1 machinery (Costas, 2002; Pavlícek et al., 2002; Grandi and Tramontano, 2017). Thus, although experimental evidence in vivo is lacking, HERV retrotransposition (within the same cell or via a capsid-involving infection of another cell) cannot be completely excluded (Romanish et al., 2010; Xue et al., 2020). Together, this theoretical framework of possible HERV retrotransposition activity implies that IN-induced genomic instability might be possible. The presence of a functional IN protein, which has been demonstrated for HERV-K (Kitamura et al., 1996) for instance, is dependent on the processing of the Pol polyprotein. However, not much is known concerning this process and whether it is possible in the absence of an infectious capsid. Some recent evidence in the context of another Pol encoded RT protein suggests that protease cleavage of the Pol polyprotein is possible in neurons and astrocytes, generating full-length RT proteins (Manghera et al., 2015) and possibly a functional IN protein. Supposing that IN is processed, it can form a so-called pre-integration complex together with the reverse transcribed dsDNA and host proteins. IN prepares the linear dsDNA ends for integration and joins these ends to the host DNA through a strand transfer reaction leaving behind DNA lesions needing to be repaired by the host DNA repair machinery (reviewed in Lesbats et al., 2016). During this process, aberrant IN activity or a failure in DNA repair (as in aging) renders the cell prone to the formation of DSB (Bray et al., 2016), which can lead to the loss of cellular functionality and, ultimately, when the cell is overwhelmed, to cell death (Hanahan and Weinberg, 2011). It remains however unclear whether this genomic instability stems from TE mobilization or the expression of the IN protein alone.
Human ERVs are expressed at low levels in the brain (Kurth and Bannert, 2010) and can be activated in several neurological diseases (Küry et al., 2018; Gröger et al., 2021). One example is ALS. HERV-K loci containing open reading frames for IN are increasingly transcribed in ALS cortical tissues (Douville et al., 2011) and RT (and Env) proteins are specifically expressed in ALS cortical neurons (Douville et al., 2011; Li et al., 2015), suggesting that IN might also be expressed. Genomic instability has been observed in ALS (Deng et al., 2014; Maizels, 2015) as well as in other neurological diseases (like schizophrenia) where HERV-K expression is upregulated (Smith et al., 2010; Kushima et al., 2017). While formal evidence of HERV-K IN-mediated DNA damage is lacking, this evidence provides a fertile ground for further studies in this direction.
As we have developed above, the activation of coding LINE-1 and potentially HERV retrotransposons can permit the production of functional DNA cleaving proteins like the LINE-1 encoded EN or the HERV encoded IN, the presence and activity of which endanger the host genome and might contribute to aging and neurodegeneration. In the following section, we will discuss the potential of targeting retrotransposons to prevent DNA damage in the context of aging and age-related NDs.
Inhibition of retrotransposon activity can be achieved at multiple levels (Figure 2), namely, transcription, mRNA stability, translation, degradation, and enzymatic activities of the encoded proteins. Modifying environmental regulators of TEs or specific drugs intervening at various steps of the TE lifecycle are already partly available and constitute attractive tools to explore as a possible treatment of NDs.
A potentially interesting environmental regulator of RTs is caloric restriction. The impact of dietary restriction on lifespan and/or healthspan (length of life deprived of any age-related disease) in various organisms, ranging from invertebrates to non-human primates, is well documented (Fontana and Partridge, 2015). This is thought to be mediated by a downturn in the metabolic rate which, among many other effects (reviewed in Martin et al., 2007), reduces the generation of reactive oxidative species (Bianchi et al., 2016) and inflammation (Ma et al., 2020) and increases DNA repair (Rao, 2003); pathways with relevance for RT activation. Indeed, caloric restriction in mice results in a drastic decrease in the expression of TEs in liver and muscle, accompanied by an aging-related restructuration of chromatin, suggesting that dietary restriction might also modulate aging-induced chromatin remodeling and thereby reduce the transcription of TEs (De Cecco et al., 2013). Many sirtuins positively affect lifespan (Kanfi et al., 2012; Satoh et al., 2013), and are positively regulated by caloric restriction (Wątroba and Szukiewicz, 2016). The sirtuins might thus be one of the hubs for the effects of caloric restrictions linking TE regulation and aging-induced chromatin alterations.
Two sirtuins, Sirt1 and Sirt6, are of particular interest since they act as NAD+-dependent deacetylases of H3K9, thereby favoring the formation of H3K9me3 repressive chromatin (Zhong et al., 2010; Poulose and Raju, 2015) on LINE-1 and HERVs (Kato et al., 2018). In addition, Sirt1 activity regulates the histone methyltransferase SUV39H1 (suppressor of variegation 3-9 homolog 1) (Vaquero et al., 2004, 2007), which is also known to repress LINE-1 (Bulut-Karslioglu et al., 2014). A correlation between Sirt1 expression and LINE-1 promoter methylation levels has been identified in the human retina in the context of age-related macular degeneration (AMD) (Maugeri et al., 2019). As described above, Sirt6, in addition to being associated with aging and neurodegeneration (Kaluski et al., 2017; Portillo et al., 2021), negatively regulates LINE-1 in an age-dependent manner (Van Meter et al., 2014; Simon et al., 2019) and promotes genomic stability (Mostoslavsky et al., 2006).
Mice deficient for the DNA repair enzyme Ercc1 display an accelerated aging phenotype. Caloric restriction in these mice significantly decreases DNA damage, leads to increased lifespan and shows a protective effect on neuronal function (Vermeij et al., 2016). This is consistent with other studies showing that caloric restriction and intermittent fasting protect from cognitive decline in mouse models of AD (Halagappa et al., 2007; Stekovic et al., 2019). It is thus tempting to speculate that some of the life span-regulating effects of caloric restriction, namely, a reduction in DNA damage and inflammation, could be partly due to a decrease in RT activation upon caloric restriction via epigenetic containment of TEs. First evidence for a beneficial effect of caloric restriction in humans (Il’yasova et al., 2018; Redman et al., 2018) suggests that dietary interventions, including caloric restriction, could be a non-specific strategy to reduce RT expression, decrease DNA damage and inflammation, and, in turn, in the context of age-related diseases, prevent neurodegeneration.
On a transcriptional level, the homeodomain protein Engrailed could be an interesting target for LINE-1 repression in dopaminergic neurons. Engrailed is a conserved homeoprotein transcription factor involved in ventral midbrain dopaminergic neuron and cerebellum development and, in the adult ventral midbrain, specifically expressed in dopaminergic neurons and important for their survival (Alvarez-Fischer et al., 2011). Upon acute oxidative stress inflicted on midbrain dopaminergic neurons in vivo, Engrailed blocks cell death and restores epigenetic marks disrupted by oxidative stress (Rekaik et al., 2015). Chromatin immunoprecipitation experiments showed that Engrailed specifically binds to LINE-1 promoters and is a transcriptional repressor of LINE-1 in mice in vivo. By blocking LINE-1 expression, Engrailed prevented DNA damage and neuronal death induced by oxidative stress in mouse dopaminergic neurons (Blaudin de Thé et al., 2018). Importantly, the Engrailed protein can be internalized into live cells, enabling its use as a therapeutic protein (Di Nardo et al., 2018).
A more specific way to inhibit TE activity is using inhibitors of one of their encoded functional protein subunits. The one protein shared by LINE-1 and HERVs, and to which they owe their name, is the RT. Several FDA-approved drugs used in AIDS (acquired immunodeficiency syndrome) therapies that target HIV (human immunodeficiency virus) RT also efficiently inhibit the RT enzymes of LINE-1 (Dai et al., 2014; Krug et al., 2017; Liu et al., 2019) and the RT of HERVs (Garcia-Montojo et al., 2021). Blocking LINE-1 with the nucleoside reverse transcriptase inhibitor (NRTI) stavudine in the context of an acute oxidative stress, reduced DNA damage and mitigated the neurodegeneration of dopaminergic neurons (Blaudin de Thé et al., 2018). Lamivudine was shown to almost completely block the synthesis of LINE-1 cDNA, leading to a reduction in the expression of IFN-1 response genes and LINE-1 associated neuroinflammation (De Cecco et al., 2019). Similarly, inhibition of LINE-1 activity with NRTIs in Sirt-6-deficient mice (exhibiting upregulation of LINE-1, see above) rescued DNA damage (Simon et al., 2019). These results are surprising, as NRTIs are reverse-transcriptase inhibitors and do not inhibit LINE-1 EN activity. It is not clear how NRTIs can reduce EN-mediated DNA damage. It was proposed that NRTIs could terminate an integration attempt, thereby facilitating the access of the DNA damage response (DDR) machinery to damaged sites, as NRTI treatments result in less LINE-1 RNA bound to chromatin (Blaudin de Thé et al., 2018). NRTIs have been shown to be efficient in in vitro cell cultures or mouse models, but whether they can confer protection against neurodegeneration in diseases linked to RT activation is currently under investigation. Clinical safety and tolerability in humans have been investigated in the context of ALS in the “Lighthouse trial” (Gold et al., 2019). In this trial, a combination of NRTIs, already used in the context of AIDS, called Triumeq (abacavir, lamivudine, and dolutegravir) was tested. Triumeq was safe and well tolerated by patients and a phase 3 clinical trial has been scheduled (Gold et al., 2019). The efficacy of NRTIs to treat other NDs is currently ongoing in AD (Pizarro and Cristofari, 2016; Salloway, 2021) and continuing in ALS (National Institute of Neurological Disorders and Stroke [NINDS], 2021). Based on the current data on LINE-1 as a source of genomic instability in neurons, LINE-1 EN inhibitors would be of interest but are unfortunately not available.
In addition to RT inhibitors, efficient inhibitors of HIV IN were developed to treat AIDS. Although with lower efficacy, these inhibitors are also effective against the HERV-K/HML-2 IN in vitro (Contreras-Galindo et al., 2017; Tyagi et al., 2017). As discussed above, based on the scarcity of evidence for HERV-mediated genomic instability, the use of IN inhibitors is of less interest in the context of NDs.
In view of the evidence discussed in this review, a novel pathogenic axis for NDs emerges, linking age and neurodegeneration based on the aging-induced alteration of heterochromatin organization, subsequent LINE-1 derepression, and LINE-1-related genomic instability leading to neurodegeneration. This hypothesis reconciles the transposon theory (Driver and McKechnie, 1992), extended by the LINEage theory stipulating the importance of non-retrotransposition dependent DNA damage in the aging process (St. Laurent et al., 2010), with the heterochromatin theory of aging (Villeponteau, 1997) and provides a molecular basis to explain why age is the major risk factor for NDs. Derepression of transposable elements including LINE-1 can have multiple consequences on cellular functions of which genomic instability appears to be of major importance. Examples of neurodegenerative processes favoring TE derepression are emerging (Guo et al., 2018; Sun et al., 2018) and suggest that genetic risk together with environmental factors and age in combination might determine the individual risk to develop a neurodegenerative disease (Figure 3). As such, this pathogenic axis could be shared by many age-related NDs and regional susceptibility could be determined by the combination of TE regulatory factors specific for certain neuronal populations or brain regions. Of specific interest is the potential of this emerging axis not only to foster the understanding of the pathogenesis of NDs but also to provide a point of attack for the development of urgently needed disease-modifying treatments. Much is still to learn and the coming years will likely increase our understanding of the role that TEs play in the physiology and pathophysiology of the brain.
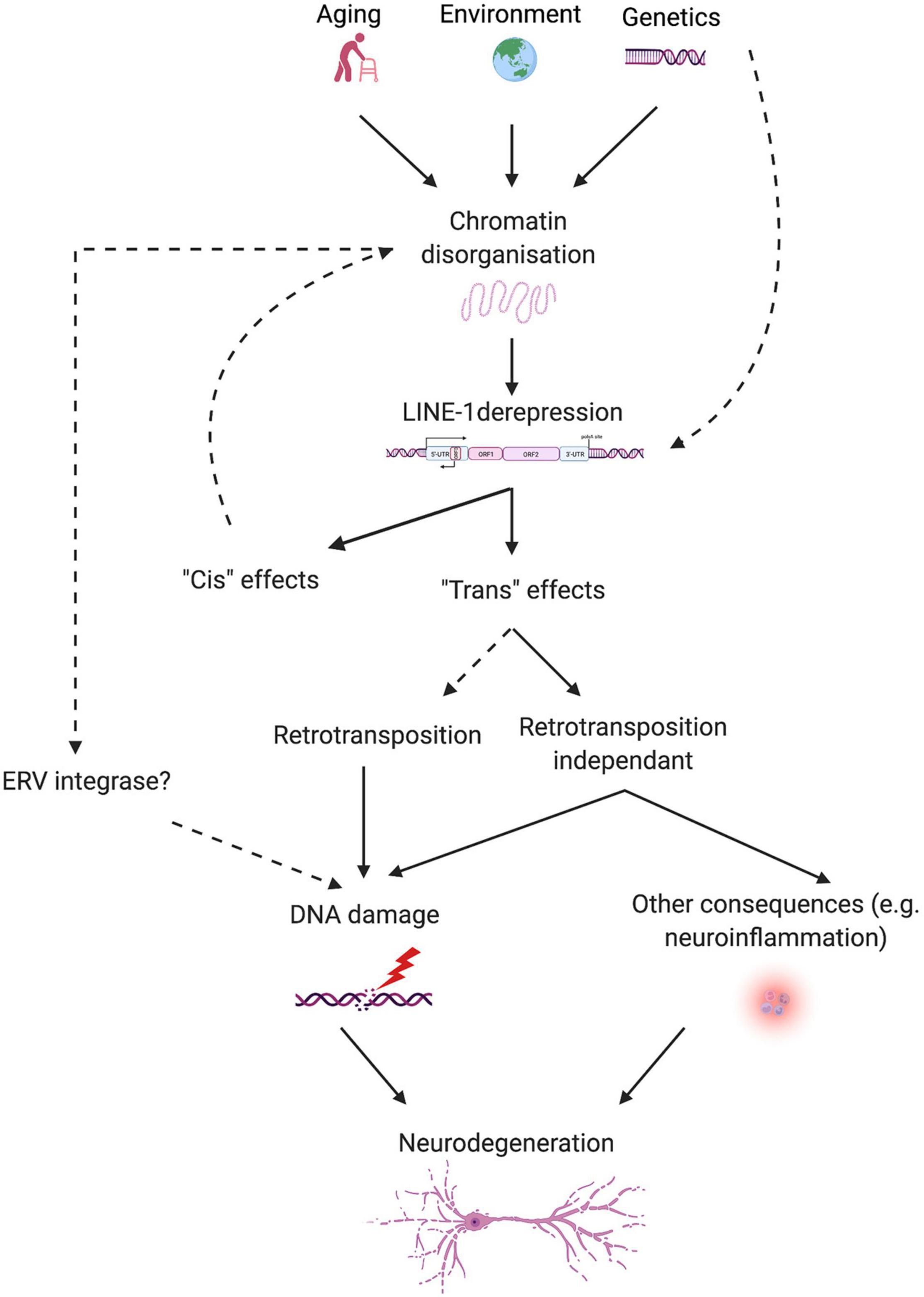
Figure 3. Schematic overview of the proposed link between aging, LINE-1 elements, and neurodegeneration. Aging, environment, and possibly genetic predisposition lead to chromatin disorganization, which releases epigenetic repression on LINE-1 elements. Some proteins relevant for neurodegenerative diseases (i.e., Tau and TDP-43) control TEs, including LINE-1, at several levels. LINE-1 transcriptional activation can lead to “cis” and “trans” effects. “Cis” effects concern local effects on chromatin organization or gene expression. Intronic LINE-1 might lead to local gene dysregulation by protein truncations via premature polyA signaling, alterations in splicing of the hosting gene, generation of antisense transcripts, etc., or, in the case of intergenic LINE-1, to the demasking of enhancers, transcription factor binding sites, changes in the 3D organization of the chromatin, etc. “Trans” effects can be mediated by actual retrotransposition events or retrotransposition-independent consequences of LINE-1 activation like DNA damage and neuroinflammation. Other sources of DNA damage can add onto this, stemming from environmental influences, the aging process, neurodegenerative processes, and possibly other TEs, like ERVs, resulting in neurodegeneration.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by grants from the Fondation de France (00086320 to JF), Fondation du Collège de France (to JF), and National French Agency for Research (Agence Nationale de la Recherche (ANR-20-CE16-0022 NEURAGE to JF). RZ and TB are enrolled in the Ecole Doctorale 3C at the Sorbonne Université, Paris, France. RZ acknowledges the “Fondation pour la Recherche sur Alzheimer’s” (FRA) for a fellowship. TB acknowledges the “Fondation du Collège de France” for a fellowship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the Fondation Bettencourt Schueller for their support. Images were created with BioRender.com (publishing license to EP-H).
AD, Alzheimer’s disease; DSBs, DNA double-strand breaks; EN, endonuclease; En1, Engrailed-1; ERV, endogenous retrovirus; HERV, human ERV; IN, integrase; LINE-1, long interspersed element-1; NDs, neurodegenerative diseases; NRTI, nucleoside reverse transcriptase inhibitor; PD, Parkinson’s disease; RT, reverse transcriptase; SNpc, substantia nigra pars compacta; TEs, transposable elements.
Adamec, E., Vonsattel, J. P., and Nixon, R. A. (1999). DNA strand breaks in Alzheimer’s disease. Brain Res. 849, 67–77. doi: 10.1016/s0006-8993(99)02004-1
Alvarez-Fischer, D., Fuchs, J., Castagner, F., Stettler, O., Massiani-Beaudoin, O., Moya, K. L., et al. (2011). Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nat. Neurosci. 14, 1260–1266. doi: 10.1038/nn.2916
Antony, J. M., van Marle, G., Opii, W., Butterfield, D. A., Mallet, F., Yong, V. W., et al. (2004). Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 7, 1088–1095. doi: 10.1038/nn1319
Baillie, J. K., Barnett, M. W., Upton, K. R., Gerhardt, D. J., Richmond, T. A., De Sapio, F., et al. (2011). Somatic retrotransposition alters the genetic landscape of the human brain. Nature 479, 534–537. doi: 10.1038/nature10531
Baker, D. J., and Petersen, R. C. (2018). Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J. Clin. Invest. 128, 1208–1216. doi: 10.1172/JCI95145
Banuelos-Sanchez, G., Sanchez, L., Benitez-Guijarro, M., Sanchez-Carnerero, V., Salvador-Palomeque, C., Tristan-Ramos, P., et al. (2019). Synthesis and characterization of specific reverse transcriptase inhibitors for mammalian LINE-1 retrotransposons. Cell Chem. Biol. 26, 1095–1109.e14. doi: 10.1016/j.chembiol.2019.04.010
Barbosa, M. C., Grosso, R. A., and Fader, C. M. (2019). Hallmarks of aging: an autophagic perspective. Front. Endocrinol. 9:790. doi: 10.3389/fendo.2018.00790
Belancio, V. P., Roy-Engel, A. M., Pochampally, R. R., and Deininger, P. (2010). Somatic expression of LINE-1 elements in human tissues. Nucleic Acids Res. 38, 3909–3922. doi: 10.1093/nar/gkq132
Belgnaoui, S. M., Gosden, R. G., Semmes, O. J., and Haoudi, A. (2006). Human LINE-1 retrotransposon induces DNA damage and apoptosis in cancer cells. Cancer Cell Int. 6:13. doi: 10.1186/1475-2867-6-13
Belshaw, R., Dawson, A. L. A., Woolven-Allen, J., Redding, J., Burt, A., and Tristem, M. (2005a). Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): implications for present-day activity. J. Virol. 79, 12507–12514. doi: 10.1128/JVI.79.19.12507-12514.2005
Belshaw, R., Katzourakis, A., Paces, J., Burt, A., and Tristem, M. (2005b). High copy number in human endogenous retrovirus families is associated with copying mechanisms in addition to reinfection. Mol. Biol. Evol. 22, 814–817. doi: 10.1093/molbev/msi088
Berson, A., Nativio, R., Berger, S. L., and Bonini, N. M. (2018). Epigenetic regulation in neurodegenerative diseases. Trends Neurosci. 41, 587–598. doi: 10.1016/j.tins.2018.05.005
Bianchi, V. E., Falcioni, G., Bianchi, V. E., and Falcioni, G. (2016). Reactive oxygen species, health and longevity. AIMS Mol. Sci. 3, 479–504. doi: 10.3934/molsci.2016.4.479
Blaudin de Thé, F., Rekaik, H., Peze-Heidsieck, E., Massiani-Beaudoin, O., Joshi, R. L., Fuchs, J., et al. (2018). Engrailed homeoprotein blocks degeneration in adult dopaminergic neurons through LINE-1 repression. EMBO J. 37:e97374. doi: 10.15252/embj.201797374
Boeke, J. D., and Stoye, J. P. (1997). “Retrotransposons, Endogenous Retroviruses, and the Evolution of Retroelements,” in Retroviruses, eds J. M. Coffin, S. H. Hughes, and H. E. Varmus (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
Bolam, J. P., and Pissadaki, E. K. (2012). Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov. Disord. 27, 1478–1483. doi: 10.1002/mds.25135
Bray, S., Turnbull, M., Hebert, S., and Douville, R. N. (2016). Insight into the ERVK Integrase - Propensity for DNA Damage. Front. Microbiol. 7:1941. doi: 10.3389/fmicb.2016.01941
Brodsky, I., Foley, B., Haines, D., Johnston, J., Cuddy, K., and Gillespie, D. (1993). Expression of HERV-K proviruses in human leukocytes. Blood 81, 2369–2374.
Bulut-Karslioglu, A., De La Rosa-Velázquez, I. A., Ramirez, F., Barenboim, M., Onishi-Seebacher, M., Arand, J., et al. (2014). Suv39h-Dependent H3K9me3 marks intact retrotransposons and silences LINE elements in mouse embryonic stem cells. Mol. Cell 55, 277–290. doi: 10.1016/j.molcel.2014.05.029
Cao, X., Jin, X., and Liu, B. (2020). The involvement of stress granules in aging and aging-associated diseases. Aging Cell 19:e13136. doi: 10.1111/acel.13136
Castro-Diaz, N., Ecco, G., Coluccio, A., Kapopoulou, A., Yazdanpanah, B., Friedli, M., et al. (2014). Evolutionally dynamic L1 regulation in embryonic stem cells. Genes Dev. 28, 1397–1409. doi: 10.1101/gad.241661.114
Chatterjee, N., and Walker, G. C. (2017). Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 58, 235–263. doi: 10.1002/em.22087
Childs, B. G., Gluscevic, M., Baker, D. J., Laberge, R.-M., Marquess, D., Dananberg, J., et al. (2017). Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735. doi: 10.1038/nrd.2017.116
Chow, H., and Herrup, K. (2015). Genomic integrity and the ageing brain. Nat. Rev. Neurosci. 16, 672–684. doi: 10.1038/nrn4020
Contreras-Galindo, R., Dube, D., Fujinaga, K., Kaplan, M. H., and Markovitz, D. M. (2017). Susceptibility of human endogenous retrovirus type K to reverse transcriptase inhibitors. J. Virol. 91:e01309-17. doi: 10.1128/JVI.01309-17
Contreras-Galindo, R., Kaplan, M. H., Dube, D., Gonzalez-Hernandez, M. J., Chan, S., Meng, F., et al. (2015). Human endogenous retrovirus Type K (HERV-K) particles package and transmit HERV-K-Related Sequences. J. Virol. 89, 7187–7201. doi: 10.1128/JVI.00544-15
Costas, J. (2002). Characterization of the intragenomic spread of the human endogenous retrovirus family HERV-W. Mol. Biol. Evol. 19, 526–533.
Coufal, N. G., Garcia-Perez, J. L., Peng, G. E., Yeo, G. W., Mu, Y., Lovci, M. T., et al. (2009). L1 retrotransposition in human neural progenitor cells. Nature 460, 1127–1131. doi: 10.1038/nature08248
d’Adda di Fagagna, F. (2008). Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522. doi: 10.1038/nrc2440
Dai, L., Huang, Q., and Boeke, J. D. (2011). Effect of reverse transcriptase inhibitors on LINE-1 and Ty1 reverse transcriptase activities and on LINE-1 retrotransposition. BMC Biochem. 12:18. doi: 10.1186/1471-2091-12-18
Dai, L., Mahajan, S. D., Guo, C., Zhang, T., Wang, W., Li, T., et al. (2014). Spectrum of central nervous system disorders in hospitalized HIV/AIDS patients (2009-2011) at a major HIV/AIDS referral center in Beijing, China. J. Neurol. Sci. 342, 88–92. doi: 10.1016/j.jns.2014.04.031
De Cecco, M., Criscione, S. W., Peckham, E. J., Hillenmeyer, S., Hamm, E. A., Manivannan, J., et al. (2013). Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell 12, 247–256. doi: 10.1111/acel.12047
De Cecco, M., Ito, T., Petrashen, A. P., Elias, A. E., Skvir, N. J., Criscione, S. W., et al. (2019). L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566, 73–78. doi: 10.1038/s41586-018-0784-9
Deng, Q., Holler, C. J., Taylor, G., Hudson, K. F., Watkins, W., Gearing, M., et al. (2014). FUS is phosphorylated by DNA-PK and accumulates in the cytoplasm after DNA damage. J. Neurosci. 34, 7802–7813. doi: 10.1523/JNEUROSCI.0172-14.2014
Dennis, S., Sheth, U., Feldman, J. L., English, K. A., and Priess, J. R. (2012). C. elegans germ cells show temperature and age-dependent expression of Cer1, a Gypsy/Ty3-related retrotransposon. PLoS Pathog. 8:e1002591. doi: 10.1371/journal.ppat.1002591
Dewannieux, M., Harper, F., Richaud, A., Letzelter, C., Ribet, D., Pierron, G., et al. (2006). Identification of an infectious progenitor for the multiple-copy HERV-K human endogenous retroelements. Genome Res. 16, 1548–1556. doi: 10.1101/gr.5565706
Dewannieux, M., and Heidmann, T. (2005). LINEs, SINEs and processed pseudogenes: parasitic strategies for genome modeling. Cytogenet. Genome Res. 110, 35–48. doi: 10.1159/000084936
Di Micco, R., Krizhanovsky, V., Baker, D., and d’Adda di Fagagna, F. (2021). Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95. doi: 10.1038/s41580-020-00314-w
Di Nardo, A. A., Fuchs, J., Joshi, R. L., Moya, K. L., and Prochiantz, A. (2018). The Physiology of Homeoprotein Transduction. Physiol. Rev. 98, 1943–1982. doi: 10.1152/physrev.00018.2017
Di Nardo, A. A., Nedelec, S., Trembleau, A., Volovitch, M., Prochiantz, A., and Luz Montesinos, M. (2007). Dendritic localization and activity-dependent translation of Engrailed1 transcription factor. Mol. Cell. Neurosci. 35, 230–236. doi: 10.1016/j.mcn.2007.02.015
Dobbin, M. M., Madabhushi, R., Pan, L., Chen, Y., Kim, D., Gao, J., et al. (2013). SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 16, 1008–1015. doi: 10.1038/nn.3460
Douville, R., Liu, J., Rothstein, J., and Nath, A. (2011). Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann. Neurol. 69, 141–151. doi: 10.1002/ana.22149
Douville, R. N., and Nath, A. (2017). Human endogenous retrovirus-K and TDP-43 expression bridges ALS and HIV neuropathology. Front. Microbiol. 8:1986. doi: 10.3389/fmicb.2017.01986
Driver, C. J., and McKechnie, S. W. (1992). Transposable elements as a factor in the aging of Drosophila melanogaster. Ann. N. Y. Acad. Sci. 673, 83–91. doi: 10.1111/j.1749-6632.1992.tb27439.x
Ecco, G., Imbeault, M., and Trono, D. (2017). KRAB zinc finger proteins. Development 144, 2719–2729. doi: 10.1242/dev.132605
Eilam, R., Peter, Y., Elson, A., Rotman, G., Shiloh, Y., Groner, Y., et al. (1998). Selective loss of dopaminergic nigro-striatal neurons in brains of Atm-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 95, 12653–12656. doi: 10.1073/pnas.95.21.12653
Eitan, E., Hutchison, E. R., and Mattson, M. P. (2014). Telomere shortening in neurological disorders: an abundance of unanswered questions. Trends Neurosci. 37, 256–263. doi: 10.1016/j.tins.2014.02.010
Elbarbary, R. A., Lucas, B. A., and Maquat, L. E. (2016). Retrotransposons as regulators of gene expression. Science 351:aac7247. doi: 10.1126/science.aac7247
Erwin, J. A., Marchetto, M. C., and Gage, F. H. (2014). Mobile DNA elements in the generation of diversity and complexity in the brain. Nat. Rev. Neurosci. 15, 497–506. doi: 10.1038/nrn3730
Erwin, J. A., Paquola, A. C. M., Singer, T., Gallina, I., Novotny, M., Quayle, C., et al. (2016). L1-associated genomic regions are deleted in somatic cells of the healthy human brain. Nat. Neurosci. 19, 1583–1591. doi: 10.1038/nn.4388
Evrony, G. D., Cai, X., Lee, E., Hills, L. B., Elhosary, P. C., Lehmann, H. S., et al. (2012). Single-Neuron Sequencing Analysis of L1 Retrotransposition and Somatic Mutation in the Human Brain. Cell 151, 483–496. doi: 10.1016/j.cell.2012.09.035
Faulkner, G. J., and Garcia-Perez, J. L. (2017). L1 Mosaicism in mammals: extent, effects, and evolution. Trends Genet. 33, 802–816. doi: 10.1016/j.tig.2017.07.004
Faulkner, G. J., Kimura, Y., Daub, C. O., Wani, S., Plessy, C., Irvine, K. M., et al. (2009). The regulated retrotransposon transcriptome of mammalian cells. Nat. Genet. 41, 563–571. doi: 10.1038/ng.368
Fidaleo, M., Cavallucci, V., and Pani, G. (2017). Nutrients, neurogenesis and brain ageing: from disease mechanisms to therapeutic opportunities. Biochem. Pharmacol. 141, 63–76. doi: 10.1016/j.bcp.2017.05.016
Fontana, L., and Partridge, L. (2015). Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. doi: 10.1016/j.cell.2015.02.020
Fragola, G., Mabb, A. M., Taylor-Blake, B., Niehaus, J. K., Chronister, W. D., Mao, H., et al. (2020). Deletion of Topoisomerase 1 in excitatory neurons causes genomic instability and early onset neurodegeneration. Nat. Commun. 11:1962. doi: 10.1038/s41467-020-15794-9
Gan, L., Cookson, M. R., Petrucelli, L., and La Spada, A. R. (2018). Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 21, 1300–1309. doi: 10.1038/s41593-018-0237-7
Gao, J., Wang, L., Liu, J., Xie, F., Su, B., and Wang, X. (2017). Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants 6:E25. doi: 10.3390/antiox6020025
Garcia-Montojo, M., Doucet-O’Hare, T., Henderson, L., and Nath, A. (2018). Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit. Rev. Microbiol. 44, 715–738. doi: 10.1080/1040841X.2018.1501345
Garcia-Montojo, M., Fathi, S., Norato, G., Smith, B. R., Rowe, D. B., Kiernan, M. C., et al. (2021). Inhibition of HERV-K (HML-2) in amyotrophic lateral sclerosis patients on antiretroviral therapy. J. Neurol. Sci. 423:117358. doi: 10.1016/j.jns.2021.117358
Gasior, S. L., Wakeman, T. P., Xu, B., and Deininger, P. L. (2006). The Human LINE-1 retrotransposon creates DNA double-strand breaks. J. Mol. Biol. 357, 1383–1393. doi: 10.1016/j.jmb.2006.01.089
Ghosh, A., Tyson, T., George, S., Hildebrandt, E. N., Steiner, J. A., Madaj, Z., et al. (2016). Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinsons disease. Sci. Transl. Med. 8:368ra174. doi: 10.1126/scitranslmed.aag2210
Gilbert, S. F. (2000). Aging: The Biology of Senescence. Developmental Biology. 6th edition. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK10041/ (accessed July 30, 2021).
Gold, J., Rowe, D. B., Kiernan, M. C., Vucic, S., Mathers, S., van Eijk, R. P. A., et al. (2019). Safety and tolerability of Triumeq in amyotrophic lateral sclerosis: the Lighthouse trial. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 595–604. doi: 10.1080/21678421.2019.1632899
Goodier, J. L. (2014). Retrotransposition in tumors and brains. Mob. DNA 5:11. doi: 10.1186/1759-8753-5-11
Goodier, J. L., Cheung, L. E., and Kazazian, H. H. (2013). Mapping the LINE1 ORF1 protein interactome reveals associated inhibitors of human retrotransposition. Nucleic Acids Res. 41, 7401–7419. doi: 10.1093/nar/gkt512
Goodier, J. L., and Kazazian, H. H. (2008). Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell 135, 23–35. doi: 10.1016/j.cell.2008.09.022
Goodier, J. L., Zhang, L., Vetter, M. R., and Kazazian, H. H. (2007). LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex. Mol. Cell. Biol. 27, 6469–6483. doi: 10.1128/MCB.00332-07
Gorbunova, V., Seluanov, A., Mita, P., McKerrow, W., Fenyö, D., Boeke, J. D., et al. (2021). The role of retrotransposable elements in ageing and age-associated diseases. Nature 596, 43–53. doi: 10.1038/s41586-021-03542-y
Grandi, N., and Tramontano, E. (2017). Type W human endogenous retrovirus (HERV-W) integrations and their mobilization by L1 machinery: contribution to the human transcriptome and impact on the host physiopathology. Viruses 9:E162. doi: 10.3390/v9070162
Gröger, V., Emmer, A., Staege, M. S., and Cynis, H. (2021). Endogenous retroviruses in nervous system disorders. Pharmaceuticals 14:70. doi: 10.3390/ph14010070
Guo, C., Jeong, H.-H., Hsieh, Y.-C., Klein, H.-U., Bennett, D. A., De Jager, P. L., et al. (2018). Tau Activates Transposable Elements in Alzheimer’s Disease. Cell Rep. 23, 2874–2880. doi: 10.1016/j.celrep.2018.05.004
Guo, H., Chitiprolu, M., Gagnon, D., Meng, L., Perez-Iratxeta, C., Lagace, D., et al. (2014). Autophagy supports genomic stability by degrading retrotransposon RNA. Nat. Commun. 5:5276. doi: 10.1038/ncomms6276
Halagappa, V. K. M., Guo, Z., Pearson, M., Matsuoka, Y., Cutler, R. G., Laferla, F. M., et al. (2007). Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 26, 212–220. doi: 10.1016/j.nbd.2006.12.019
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Hata, K., and Sakaki, Y. (1997). Identification of critical CpG sites for repression of L1 transcription by DNA methylation. Gene 189, 227–234. doi: 10.1016/s0378-1119(96)00856-6
He, J., Fu, X., Zhang, M., He, F., Li, W., Abdul, M. M., et al. (2019). Transposable elements are regulated by context-specific patterns of chromatin marks in mouse embryonic stem cells. Nat. Commun. 10:34. doi: 10.1038/s41467-018-08006-y
Healton, S. E., Pinto, H. D., Mishra, L. N., Hamilton, G. A., Wheat, J. C., Swist-Rosowska, K., et al. (2020). H1 linker histones silence repetitive elements by promoting both histone H3K9 methylation and chromatin compaction. Proc. Natl. Acad. Sci. U.S.A. 117, 14251–14258. doi: 10.1073/pnas.1920725117
Heras, S. R., Macias, S., Cáceres, J. F., and Garcia-Perez, J. L. (2014). Control of mammalian retrotransposons by cellular RNA processing activities. Mob. Genet. Elem. 4:e28439. doi: 10.4161/mge.28439
Heras, S. R., Macias, S., Plass, M., Fernandez, N., Cano, D., Eyras, E., et al. (2013). The Microprocessor controls the activity of mammalian retrotransposons. Nat. Struct. Mol. Biol. 20, 1173–1181. doi: 10.1038/nsmb.2658
Herranz, N., and Gil, J. (2018). Mechanisms and functions of cellular senescence. J. Clin. Invest. 128, 1238–1246. doi: 10.1172/JCI95148
Hill, A. F. (2019). Extracellular vesicles and neurodegenerative diseases. J. Neurosci. 39, 9269–9273. doi: 10.1523/JNEUROSCI.0147-18.2019
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi: 10.1038/s41582-019-0244-7
Hu, S., Li, J., Xu, F., Mei, S., Le Duff, Y., Yin, L., et al. (2015). SAMHD1 Inhibits LINE-1 retrotransposition by promoting stress granule formation. PLoS Genet. 11:e1005367. doi: 10.1371/journal.pgen.1005367
Il’yasova, D., Fontana, L., Bhapkar, M., Pieper, C. F., Spasojevic, I., Redman, L. M., et al. (2018). Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: the CALERIE 2 randomized clinical trial. Aging Cell 17:e12719. doi: 10.1111/acel.12719
Ismail, I. H., and Hendzel, M. J. (2008). The γ-H2A.X: Is it just a surrogate marker of double-strand breaks or much more? Environ. Mol. Mutagen. 49, 73–82. doi: 10.1002/em.20358
Iturria-Medina, Y., and Evans, A. C. (2015). On the central role of brain connectivity in neurodegenerative disease progression. Front. Aging Neurosci. 7:90. doi: 10.3389/fnagi.2015.00090
Jacobs, F. M. J., Greenberg, D., Nguyen, N., Haeussler, M., Ewing, A. D., Katzman, S., et al. (2014). An evolutionary arms race between KRAB zinc-finger genes ZNF91/93 and SVA/L1 retrotransposons. Nature 516, 242–245. doi: 10.1038/nature13760
Jakovcevski, M., and Akbarian, S. (2012). Epigenetic mechanisms in neurological disease. Nat. Med. 18, 1194–1204. doi: 10.1038/nm.2828
Johnson, J., Mercado-Ayon, E., Mercado-Ayon, Y., Dong, Y. N., Halawani, S., Ngaba, L., et al. (2021). Mitochondrial dysfunction in the development and progression of neurodegenerative diseases. Arch. Biochem. Biophys. 702:108698. doi: 10.1016/j.abb.2020.108698
Jurk, D., Wang, C., Miwa, S., Maddick, M., Korolchuk, V., Tsolou, A., et al. (2012). Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response: a senescence-like phenotype in postmitotic neurons. Aging Cell 11, 996–1004. doi: 10.1111/j.1474-9726.2012.00870.x
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Kaluski, S., Portillo, M., Besnard, A., Stein, D., Einav, M., Zhong, L., et al. (2017). Neuroprotective functions for the histone deacetylase SIRT6. Cell Rep. 18, 3052–3062. doi: 10.1016/j.celrep.2017.03.008
Kanfi, Y., Naiman, S., Amir, G., Peshti, V., Zinman, G., Nahum, L., et al. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature 483, 218–221. doi: 10.1038/nature10815
Karch, C. M., Wen, N., Fan, C. C., Yokoyama, J. S., Kouri, N., Ross, O. A., et al. (2018). Selective genetic overlap between amyotrophic lateral sclerosis and diseases of the frontotemporal dementia spectrum. JAMA Neurol. 75, 860–875. doi: 10.1001/jamaneurol.2018.0372
Kassiotis, G., and Stoye, J. P. (2016). Immune responses to endogenous retroelements: taking the bad with the good. Nat. Rev. Immunol. 16, 207–219. doi: 10.1038/nri.2016.27
Kato, M., Takemoto, K., and Shinkai, Y. (2018). A somatic role for the histone methyltransferase Setdb1 in endogenous retrovirus silencing. Nat. Commun. 9:1683. doi: 10.1038/s41467-018-04132-9
Katyal, S., Lee, Y., Nitiss, K. C., Downing, S. M., Li, Y., Shimada, M., et al. (2014). Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nat. Neurosci. 17, 813–821. doi: 10.1038/nn.3715
Kazazian, H. H. (1998). Mobile elements and disease. Curr. Opin. Genet. Dev. 8, 343–350. doi: 10.1016/s0959-437x(98)80092-0
Kazazian, H. H., and Moran, J. V. (2017). Mobile DNA in health and disease. N. Engl. J. Med. 377, 361–370. doi: 10.1056/NEJMra1510092
Kim, D., Frank, C. L., Dobbin, M. M., Tsunemoto, R. K., Tu, W., Peng, P. L., et al. (2008). Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 60, 803–817. doi: 10.1016/j.neuron.2008.10.015
Kim, D., Nguyen, M. D., Dobbin, M. M., Fischer, A., Sananbenesi, F., Rodgers, J. T., et al. (2007). SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179. doi: 10.1038/sj.emboj.7601758
King, I. F., Yandava, C. N., Mabb, A. M., Hsiao, J. S., Huang, H.-S., Pearson, B. L., et al. (2013). Topoisomerases facilitate transcription of long genes linked to autism. Nature 501, 58–62. doi: 10.1038/nature12504
Kitamura, Y., Ayukawa, T., Ishikawa, T., Kanda, T., and Yoshiike, K. (1996). Human endogenous retrovirus K10 encodes a functional integrase. J. Virol. 70, 3302–3306. doi: 10.1128/JVI.70.5.3302-3306.1996
Kritsilis, M., Rizou, S. V., Koutsoudaki, P., Evangelou, K., Gorgoulis, V., and Papadopoulos, D. (2018). Ageing, cellular senescence and neurodegenerative disease. IJMS 19:2937. doi: 10.3390/ijms19102937
Krug, L., Chatterjee, N., Borges-Monroy, R., Hearn, S., Liao, W.-W., Morrill, K., et al. (2017). Retrotransposon activation contributes to neurodegeneration in a Drosophila TDP-43 model of ALS. PLoS Genet. 13:e1006635. doi: 10.1371/journal.pgen.1006635
Kurth, R., and Bannert, N. (2010). Beneficial and detrimental effects of human endogenous retroviruses. Int. J. Cancer 126, 306–314. doi: 10.1002/ijc.24902
Küry, P., Nath, A., Créange, A., Dolei, A., Marche, P., Gold, J., et al. (2018). Human endogenous retroviruses in neurological diseases. Trends Mol. Med. 24, 379–394. doi: 10.1016/j.molmed.2018.02.007
Kushima, I., Aleksic, B., Nakatochi, M., Shimamura, T., Shiino, T., Yoshimi, A., et al. (2017). High-resolution copy number variation analysis of schizophrenia in Japan. Mol. Psychiatry 22, 430–440. doi: 10.1038/mp.2016.88
Kwiatkowski, T. J., Bosco, D. A., Leclerc, A. L., Tamrazian, E., Vanderburg, C. R., Russ, C., et al. (2009). Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208. doi: 10.1126/science.1166066
Lander, E. S., Linton, L. M., Birren, B., Nusbaum, C., Zody, M. C., Baldwin, J., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. doi: 10.1038/35057062
Lee, E. J., Banerjee, S., Zhou, H., Jammalamadaka, A., Arcila, M., Manjunath, B. S., et al. (2011). Identification of piRNAs in the central nervous system. RNA 17, 1090–1099. doi: 10.1261/rna.2565011
Lee, J.-H., Kim, E. W., Croteau, D. L., and Bohr, V. A. (2020). Heterochromatin: an epigenetic point of view in aging. Exp. Mol. Med. 52, 1466–1474. doi: 10.1038/s12276-020-00497-4
Lesbats, P., Engelman, A. N., and Cherepanov, P. (2016). Retroviral DNA Integration. Chem. Rev. 116, 12730–12757. doi: 10.1021/acs.chemrev.6b00125
Li, N., and Lagier-Tourenne, C. (2018). Nuclear pores: the gate to neurodegeneration. Nat. Neurosci. 21, 156–158. doi: 10.1038/s41593-017-0066-0
Li, W., Jin, Y., Prazak, L., Hammell, M., and Dubnau, J. (2012). Transposable Elements in TDP-43-mediated neurodegenerative disorders. PLoS One 7:e44099. doi: 10.1371/journal.pone.0044099
Li, W., Lee, M.-H., Henderson, L., Tyagi, R., Bachani, M., Steiner, J., et al. (2015). Human endogenous retrovirus-K contributes to motor neuron disease. Sci. Transl. Med. 7:307ra153. doi: 10.1126/scitranslmed.aac8201
Li, Z., Zhou, D., Zhang, D., Zhao, J., Li, W., Sun, Y., et al. (2021). Folic acid inhibits aging-induced telomere attrition and apoptosis in astrocytes in vivo and in vitro. Cereb. Cortex 2021:bhab208. doi: 10.1093/cercor/bhab208
Liu, E. Y., Russ, J., Cali, C. P., Phan, J. M., Amlie-Wolf, A., and Lee, E. B. (2019). Loss of Nuclear TDP-43 Is Associated with Decondensation of LINE Retrotransposons. Cell Rep. 27, 1409–1421.e6. doi: 10.1016/j.celrep.2019.04.003
Liu, N., Lee, C. H., Swigut, T., Grow, E., Gu, B., Bassik, M. C., et al. (2018). Selective silencing of euchromatic L1s revealed by genome-wide screens for L1 regulators. Nature 553, 228–232. doi: 10.1038/nature25179
Liu, S., Brind’Amour, J., Karimi, M. M., Shirane, K., Bogutz, A., Lefebvre, L., et al. (2014). Setdb1 is required for germline development and silencing of H3K9me3-marked endogenous retroviruses in primordial germ cells. Genes Dev. 28, 2041–2055. doi: 10.1101/gad.244848.114
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., and Kroemer, G. (2013). The Hallmarks of Aging. Cell 153, 1194–1217. doi: 10.1016/j.cell.2013.05.039
Lu, T., Pan, Y., Kao, S.-Y., Li, C., Kohane, I., Chan, J., et al. (2004). Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891. doi: 10.1038/nature02661
Ma, S., Zhang, B., LaFave, L. M., Earl, A. S., Chiang, Z., Hu, Y., et al. (2020). Chromatin Potential Identified by Shared Single-Cell Profiling of RNA and Chromatin. Cell 183, 1103–1116.e20. doi: 10.1016/j.cell.2020.09.056
Macia, A., Widmann, T. J., Heras, S. R., Ayllon, V., Sanchez, L., Benkaddour-Boumzaouad, M., et al. (2017). Engineered LINE-1 retrotransposition in nondividing human neurons. Genome Res. 27, 335–348. doi: 10.1101/gr.206805.116
MacMicking, J. D. (2012). Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 12, 367–382. doi: 10.1038/nri3210
Madabhushi, R., Gao, F., Pfenning, A. R., Pan, L., Yamakawa, S., Seo, J., et al. (2015). Activity-Induced DNA breaks govern the expression of neuronal early-response genes. Cell 161, 1592–1605. doi: 10.1016/j.cell.2015.05.032
Madabhushi, R., Pan, L., and Tsai, L.-H. (2014). DNA Damage and Its Links to Neurodegeneration. Neuron 83, 266–282. doi: 10.1016/j.neuron.2014.06.034
Mager, D. L., and Freeman, J. D. (1995). HERV-H endogenous retroviruses: presence in the New World branch but amplification in the Old World primate lineage. Virology 213, 395–404. doi: 10.1006/viro.1995.0012
Mahmood, W., Erichsen, L., Ott, P., Schulz, W. A., Fischer, J. C., Arauzo-Bravo, M. J., et al. (2020). Aging-associated distinctive DNA methylation changes of LINE-1 retrotransposons in pure cell-free DNA from human blood. Sci. Rep. 10:22127. doi: 10.1038/s41598-020-79126-z
Maizels, N. (2015). G4-associated human diseases. EMBO Rep. 16, 910–922. doi: 10.15252/embr.201540607
Manghera, M., Ferguson, J., and Douville, R. (2015). ERVK polyprotein processing and reverse transcriptase expression in human cell line models of neurological disease. Viruses 7, 320–332. doi: 10.3390/v7010320
Martin, C. K., Heilbronn, L. K., de Jonge, L., DeLany, J. P., Volaufova, J., Anton, S. D., et al. (2007). Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 15, 2964–2973. doi: 10.1038/oby.2007.354
Martínez-Cué, C., and Rueda, N. (2020). Cellular senescence in neurodegenerative diseases. Front. Cell. Neurosci. 14:16. doi: 10.3389/fncel.2020.00016
Mattson, M. P., and Arumugam, T. V. (2018). Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 27, 1176–1199. doi: 10.1016/j.cmet.2018.05.011
Maugeri, A., Barchitta, M., Fallico, M., Castellino, N., Reibaldi, M., and Agodi, A. (2019). Characterization of SIRT1/DNMTs Functions and LINE-1 methylation in patients with age-related macular degeneration. J. Clin. Med. 8:159. doi: 10.3390/jcm8020159
Maxwell, P. H., Burhans, W. C., and Curcio, M. J. (2011). Retrotransposition is associated with genome instability during chronological aging. Proc. Natl. Acad. Sci. U.S.A. 108, 20376–20381.
Maynard, S., Fang, E. F., Scheibye-Knudsen, M., Croteau, D. L., and Bohr, V. A. (2015). DNA Damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 5:a025130. doi: 10.1101/cshperspect.a025130
Mills, R. E., Bennett, E. A., Iskow, R. C., and Devine, S. E. (2007). Which transposable elements are active in the human genome? Trends Genet. 23, 183–191. doi: 10.1016/j.tig.2007.02.006
Mir, A. A., Philippe, C., and Cristofari, G. (2015). euL1db: the european database of L1HS retrotransposon insertions in humans. Nucleic Acids Res. 43, D43–D47. doi: 10.1093/nar/gku1043
Misteli, T. (2010). Higher-order genome organization in human disease. Cold Spring Harb. Perspect. Biol. 2:a000794. doi: 10.1101/cshperspect.a000794
Mitra, J., Guerrero, E. N., Hegde, P. M., Liachko, N. F., Wang, H., Vasquez, V., et al. (2019). Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc. Natl. Acad. Sci. U.S.A. 116, 4696–4705. doi: 10.1073/pnas.1818415116
Miyoshi, T., Makino, T., and Moran, J. V. (2019). Poly(ADP-Ribose) Polymerase 2 Recruits Replication Protein A to Sites of LINE-1 Integration to Facilitate Retrotransposition. Mol. Cell 75, 1286–1298.e12. doi: 10.1016/j.molcel.2019.07.018
Mohd Murshid, N., Aminullah Lubis, F., and Makpol, S. (2020). Epigenetic changes and its intervention in age-related neurodegenerative diseases. Cell. Mol. Neurobiol. doi: 10.1007/s10571-020-00979-z [Epub ahead of print].
Mohrin, M., Shin, J., Liu, Y., Brown, K., Luo, H., Xi, Y., et al. (2015). Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 347, 1374–1377. doi: 10.1126/science.aaa2361
Mostoslavsky, R., Chua, K. F., Lombard, D. B., Pang, W. W., Fischer, M. R., Gellon, L., et al. (2006). Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329. doi: 10.1016/j.cell.2005.11.044
Mullaart, E., Boerrigter, M. E., Ravid, R., Swaab, D. F., and Vijg, J. (1990). Increased levels of DNA breaks in cerebral cortex of Alzheimer’s disease patients. Neurobiol. Aging 11, 169–173. doi: 10.1016/0197-4580(90)90542-8
Muotri, A. R., Chu, V. T., Marchetto, M. C. N., Deng, W., Moran, J. V., and Gage, F. H. (2005). Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 435, 903–910. doi: 10.1038/nature03663
Muotri, A. R., Marchetto, M. C. N., Coufal, N. G., Oefner, R., Yeo, G., Nakashima, K., et al. (2010). L1 retrotransposition in neurons is modulated by MeCP2. Nature 468, 443–446. doi: 10.1038/nature09544
Nakagawa, S., and Takahashi, M. U. (2016). gEVE: a genome-based endogenous viral element database provides comprehensive viral protein-coding sequences in mammalian genomes. Database 2016:baw087. doi: 10.1093/database/baw087
Nandakumar, S., Rozich, E., and Buttitta, L. (2021). Cell cycle re-entry in the nervous system: from polyploidy to neurodegeneration. Front. Cell Dev. Biol. 9:698661. doi: 10.3389/fcell.2021.698661
National Institute of Neurological Disorders and Stroke [NINDS] (2021). HERV-K Suppression Using Antiretroviral Therapy in Volunteers With Amyotrophic Lateral Sclerosis (ALS). Available online at: https://clinicaltrials.gov/ct2/show/NCT02437110 (accessed July 29, 2021).
Naz, M., Younesi, E., and Hofmann-Apitius, M. (2017). Systematic analysis of GWAS data reveals genomic hotspots for shared mechanisms between neurodegenerative diseases. J. Alzheimers Dis. Park. 7, 460–2161. doi: 10.4172/2161-0460.1000368
Oboudiyat, C., Glazer, H., Seifan, A., Greer, C., and Isaacson, R. (2013). Alzheimer’s disease. Semin. Neurol. 33, 313–329. doi: 10.1055/s-0033-1359319
Orr, M. E., Sullivan, A. C., and Frost, B. (2017). A brief overview of tauopathy: causes, consequences, and therapeutic strategies. Trends Pharmacol. Sci. 38, 637–648. doi: 10.1016/j.tips.2017.03.011
Papanikolopoulou, K., and Skoulakis, E. M. C. (2020). Altered proteostasis in neurodegenerative tauopathies. Adv. Exp. Med. Biol. 1233, 177–194. doi: 10.1007/978-3-030-38266-7_7
Pavlícek, A., Paces, J., Elleder, D., and Hejnar, J. (2002). Processed pseudogenes of human endogenous retroviruses generated by LINEs: their integration, stability, and distribution. Genome Res. 12, 391–399. doi: 10.1101/gr.216902
Penzkofer, T., Jäger, M., Figlerowicz, M., Badge, R., Mundlos, S., Robinson, P. N., et al. (2017). L1Base 2: more retrotransposition-active LINE-1s, more mammalian genomes. Nucleic Acids Res. 45, D68–D73. doi: 10.1093/nar/gkw925
Pereira, G. C., Sanchez, L., Schaughency, P. M., Rubio-Roldán, A., Choi, J. A., Planet, E., et al. (2018). Properties of LINE-1 proteins and repeat element expression in the context of amyotrophic lateral sclerosis. Mob. DNA 9:35. doi: 10.1186/s13100-018-0138-z
Pizarro, J. G., and Cristofari, G. (2016). Post-Transcriptional Control of LINE-1 retrotransposition by cellular host factors in somatic cells. Front. Cell Dev. Biol. 4:14. doi: 10.3389/fcell.2016.00014
Portillo, M., Eremenko, E., Kaluski, S., Garcia-Venzor, A., Onn, L., Stein, D., et al. (2021). SIRT6-CBP-dependent nuclear Tau accumulation and its role in protein synthesis. Cell Rep. 35:109035. doi: 10.1016/j.celrep.2021.109035
Poulose, N., and Raju, R. (2015). Sirtuin regulation in aging and injury. Biochim. Biophys. Acta 1852, 2442–2455. doi: 10.1016/j.bbadis.2015.08.017
Ramirez, P., Sun, W., Zuniga, G., Thomas, E. O., DeVos, S. L., Hyman, B., et al. (2021). Pathogenic tau accelerates aging-associated activation of transposable elements in the mouse central nervous system. bioRxiv [Preprint]. doi: 10.1101/2021.02.25.432716
Rao, K. S. (2003). Dietary calorie restriction. DNA-repair and brain aging. Mol. Cell. Biochem. 253, 313–318. doi: 10.1023/a:1026084420943
Ravel-Godreuil, C., Znaidi, R., Bonnifet, T., Joshi, R. L., and Fuchs, J. (2021b). Transposable elements as new players in neurodegenerative diseases. FEBS Lett. 595, 2733–2755. doi: 10.1002/1873-3468.14205
Ravel-Godreuil, C., Massiani-Beaudoin, O., Mailly, P., Prochiantz, A., Joshi, R. L., and Fuchs, J. (2021a). Perturbed DNA methylation by Gadd45b induces chromatin disorganization, DNA strand breaks and dopaminergic neuron death. iScience 24:102756. doi: 10.1016/j.isci.2021.102756
Reddy, K., Cusack, C. L., Nnah, I. C., Khayati, K., Saqcena, C., Huynh, T. B., et al. (2016). Dysregulation of Nutrient Sensing and CLEARance in Presenilin Deficiency. Cell Rep. 14, 2166–2179. doi: 10.1016/j.celrep.2016.02.006
Redman, L. M., Smith, S. R., Burton, J. H., Martin, C. K., Il’yasova, D., and Ravussin, E. (2018). Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 27, 805–815.e4. doi: 10.1016/j.cmet.2018.02.019
Reilly, M. T., Faulkner, G. J., Dubnau, J., Ponomarev, I., and Gage, F. H. (2013). The role of transposable elements in health and diseases of the central nervous system. J. Neurosci. 33, 17577–17586. doi: 10.1523/JNEUROSCI.3369-13.2013
Rekaik, H., Blaudin de Thé, F.-X., Prochiantz, A., Fuchs, J., and Joshi, R. L. (2015). Dissecting the role of Engrailed in adult dopaminergic neurons - Insights into Parkinson disease pathogenesis. FEBS Lett. 589, 3786–3794. doi: 10.1016/j.febslet.2015.10.002
Ren, W., Fan, H., Grimm, S. A., Kim, J. J., Li, L., Guo, Y., et al. (2021). DNMT1 reads heterochromatic H4K20me3 to reinforce LINE-1 DNA methylation. Nat. Commun. 12:2490. doi: 10.1038/s41467-021-22665-4
Ribet, D., Harper, F., Dupressoir, A., Dewannieux, M., Pierron, G., and Heidmann, T. (2008). An infectious progenitor for the murine IAP retrotransposon: emergence of an intracellular genetic parasite from an ancient retrovirus. Genome Res. 18, 597–609. doi: 10.1101/gr.073486.107
Richardson, S. R., Morell, S., and Faulkner, G. J. (2014). L1 retrotransposons and somatic mosaicism in the brain. Annu. Rev. Genet. 48, 1–27. doi: 10.1146/annurev-genet-120213-092412
Rockwood, L. D., Felix, K., and Janz, S. (2004). Elevated presence of retrotransposons at sites of DNA double strand break repair in mouse models of metabolic oxidative stress and MYC-induced lymphoma. Mutat. Res. 548, 117–125. doi: 10.1016/j.mrfmmm.2004.01.005
Romanish, M. T., Cohen, C. J., and Mager, D. L. (2010). Potential mechanisms of endogenous retroviral-mediated genomic instability in human cancer. Semin. Cancer Biol. 20, 246–253. doi: 10.1016/j.semcancer.2010.05.005
Saito, K., and Siomi, M. C. (2010). Small RNA-mediated quiescence of transposable elements in animals. Dev. Cell 19, 687–697. doi: 10.1016/j.devcel.2010.10.011
Saleh, A., Macia, A., and Muotri, A. R. (2019). Transposable elements, inflammation, and neurological disease. Front. Neurol. 10:894. doi: 10.3389/fneur.2019.00894
Salloway, S. (2021). Repurposing Nucleoside Reverse Transcriptase Inhibitors for Treatment of AD. Available online at: https://clinicaltrials.gov/ct2/show/NCT04500847 (accessed July 29, 2021).
Sameri, S., Samadi, P., Dehghan, R., Salem, E., Fayazi, N., and Amini, R. (2020). Stem cell aging in lifespan and disease: a state-of-the-art review. Curr. Stem Cell Res. Ther. 15, 362–378. doi: 10.2174/1574888X15666200213105155
Satoh, A., Brace, C. S., Rensing, N., Cliften, P., Wozniak, D. F., Herzog, E. D., et al. (2013). Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 18, 416–430. doi: 10.1016/j.cmet.2013.07.013
Savage, A. L., Schumann, G. G., Breen, G., Bubb, V. J., Al-Chalabi, A., and Quinn, J. P. (2019). Retrotransposons in the development and progression of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 284–293.
Sedelnikova, O. A., Horikawa, I., Zimonjic, D. B., Popescu, N. C., Bonner, W. M., and Barrett, J. C. (2004). Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 6, 168–170. doi: 10.1038/ncb1095
Seifarth, W., Frank, O., Zeilfelder, U., Spiess, B., Greenwood, A. D., Hehlmann, R., et al. (2005). Comprehensive analysis of human endogenous retrovirus transcriptional activity in human tissues with a retrovirus-specific microarray. J. Virol. 79, 341–352. doi: 10.1128/JVI.79.1.341-352.2005
Sepe, S., Milanese, C., Gabriels, S., Derks, K. W. J., Payan-Gomez, C., van IJcken, W. F. J., et al. (2016). Inefficient DNA repair is an aging-related modifier of Parkinson’s disease. Cell Rep. 15, 1866–1875. doi: 10.1016/j.celrep.2016.04.071
Sgadò, P., Viaggi, C., Fantacci, C., and Corsini, G. U. (2008). Characterization of the Engrailed mutant mice as experimental models for Parkinson’s disease. Parkinsonism. Relat. Disord. 14, (Suppl. 2), S103–S106. doi: 10.1016/j.parkreldis.2008.04.011
Shanbhag, N. M., Evans, M. D., Mao, W., Nana, A. L., Seeley, W. W., Adame, A., et al. (2019). Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol. Commun. 7:77. doi: 10.1186/s40478-019-0723-5
Shaw, A. C., Joshi, S., Greenwood, H., Panda, A., and Lord, J. M. (2010). Aging of the Innate Immune System. Curr. Opin. Immunol 22, 507–513. doi: 10.1016/j.coi.2010.05.003
Shiloh, Y., and Ziv, Y. (2013). The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell Biol. 14, 197–210. doi: 10.1038/nrm3546
Simon, H. H., Saueressig, H., Wurst, W., Goulding, M. D., and O’Leary, D. D. (2001). Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 21, 3126–3134.
Simon, M., Van Meter, M., Ablaeva, J., Ke, Z., Gonzalez, R. S., Taguchi, T., et al. (2019). LINE1 Derepression in Aged Wild-Type and SIRT6-Deficient Mice Drives Inflammation. Cell Metab. 29, 871–885.e5. doi: 10.1016/j.cmet.2019.02.014
Sin, O., and Nollen, E. A. A. (2015). Regulation of protein homeostasis in neurodegenerative diseases: the role of coding and non-coding genes. Cell. Mol. Life Sci. 72, 4027–4047. doi: 10.1007/s00018-015-1985-0
Sliter, D. A., Martinez, J., Hao, L., Chen, X., Sun, N., Fischer, T. D., et al. (2018). Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262. doi: 10.1038/s41586-018-0448-9
Smith, C. L., Bolton, A., and Nguyen, G. (2010). Genomic and epigenomic instability, fragile sites, schizophrenia and autism. Curr. Genomics 11, 447–469. doi: 10.2174/138920210793176001
Sonnier, L., Le Pen, G., Hartmann, A., Bizot, J.-C., Trovero, F., Krebs, M.-O., et al. (2007). Progressive loss of dopaminergic neurons in the ventral midbrain of adult mice heterozygote for engrailed1. J. Neurosci. 27, 1063–1071. doi: 10.1523/JNEUROSCI.4583-06.2007
Sonninen, T.-M., Goldsteins, G., Laham-Karam, N., Koistinaho, J., and Lehtonen, Š. (2020). Proteostasis disturbances and inflammation in neurodegenerative diseases. Cells 9:E2183. doi: 10.3390/cells9102183
St. Laurent, G., Hammell, N., and McCaffrey, T. A. (2010). A LINE-1 component to human aging: Do LINE elements exact a longevity cost for evolutionary advantage? Mech. Ageing Dev. 131, 299–305. doi: 10.1016/j.mad.2010.03.008
Stekovic, S., Hofer, S. J., Tripolt, N., Aon, M. A., Royer, P., Pein, L., et al. (2019). Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 30, 462–476.e6. doi: 10.1016/j.cmet.2019.07.016
Stetson, D. B., Ko, J. S., Heidmann, T., and Medzhitov, R. (2008). Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598. doi: 10.1016/j.cell.2008.06.032
Suarez, N. A., Macia, A., and Muotri, A. R. (2018). LINE-1 retrotransposons in healthy and diseased human brain: L1 Activity in the Human Brain. Dev. Neurobiol. 78, 434–455. doi: 10.1002/dneu.22567
Suberbielle, E., Sanchez, P. E., Kravitz, A. V., Wang, X., Ho, K., Eilertson, K., et al. (2013). Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat. Neurosci. 16, 613–621. doi: 10.1038/nn.3356
Sun, W., Samimi, H., Gamez, M., Zare, H., and Frost, B. (2018). Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 21, 1038–1048. doi: 10.1038/s41593-018-0194-1
Sur, D., Kustwar, R. K., Budania, S., Mahadevan, A., Hancks, D. C., Yadav, V., et al. (2017). Detection of the LINE-1 retrotransposon RNA-binding protein ORF1p in different anatomical regions of the human brain. Mob. DNA 8:17. doi: 10.1186/s13100-017-0101-4
Surmeier, D. J., Obeso, J. A., and Halliday, G. M. (2017). Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 18, 101–113. doi: 10.1038/nrn.2016.178
Tam, O. H., Ostrow, L. W., and Gale Hammell, M. (2019a). Diseases of the nERVous system: retrotransposon activity in neurodegenerative disease. Mob. DNA 10:32. doi: 10.1186/s13100-019-0176-1
Tam, O. H., Rozhkov, N. V., Shaw, R., Kim, D., Hubbard, I., Fennessey, S., et al. (2019b). Postmortem cortex samples identify distinct molecular subtypes of ALS: retrotransposon activation, oxidative stress, and activated glia. Cell Rep. 29, 1164–1177.e5. doi: 10.1016/j.celrep.2019.09.066
Tang, M., Tang, H., Tu, B., and Zhu, W.-G. (2021). SIRT7: a sentinel of genome stability. Open Biol. 11:210047. doi: 10.1098/rsob.210047
Thomas, C. A., Tejwani, L., Trujillo, C. A., Negraes, P. D., Herai, R. H., Mesci, P., et al. (2017). Modeling of TREX1-dependent autoimmune disease using human stem cells highlights L1 accumulation as a source of neuroinflammation. Cell Stem Cell 21, 319–331.e8. doi: 10.1016/j.stem.2017.07.009
Tristem, M. (2000). Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J. Virol. 74, 3715–3730. doi: 10.1128/jvi.74.8.3715-3730.2000
Turner, G., Barbulescu, M., Su, M., Jensen-Seaman, M. I., Kidd, K. K., and Lenz, J. (2001). Insertional polymorphisms of full-length endogenous retroviruses in humans. Curr. Biol. 11, 1531–1535. doi: 10.1016/s0960-9822(01)00455-9
Tyagi, R., Li, W., Parades, D., Bianchet, M. A., and Nath, A. (2017). Inhibition of human endogenous retrovirus-K by antiretroviral drugs. Retrovirology 14:21. doi: 10.1186/s12977-017-0347-4
Van Bulck, M., Sierra-Magro, A., Alarcon-Gil, J., Perez-Castillo, A., and Morales-Garcia, J. (2019). Novel approaches for the treatment of Alzheimer’s and Parkinson’s disease. IJMS 20:719. doi: 10.3390/ijms20030719
Van Meter, M., Kashyap, M., Rezazadeh, S., Geneva, A. J., Morello, T. D., Seluanov, A., et al. (2014). SIRT6 represses LINE1 retrotransposons by ribosylating KAP1 but this repression fails with stress and age. Nat. Commun. 5:5011. doi: 10.1038/ncomms6011
Vance, C., Rogelj, B., Hortobágyi, T., De Vos, K. J., Nishimura, A. L., Sreedharan, J., et al. (2009). Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211. doi: 10.1126/science.1165942
Vaquero, A., Scher, M., Erdjument-Bromage, H., Tempst, P., Serrano, L., and Reinberg, D. (2007). SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 450, 440–444. doi: 10.1038/nature06268
Vaquero, A., Scher, M., Lee, D., Erdjument-Bromage, H., Tempst, P., and Reinberg, D. (2004). Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 16, 93–105. doi: 10.1016/j.molcel.2004.08.031
Vazquez, B. N., Thackray, J. K., Simonet, N. G., Chahar, S., Kane-Goldsmith, N., Newkirk, S. J., et al. (2019). SIRT7 mediates L1 elements transcriptional repression and their association with the nuclear lamina. Nucleic Acids Res. 47, 7870–7885. doi: 10.1093/nar/gkz519
Vermeij, W. P., Dollé, M. E. T., Reiling, E., Jaarsma, D., Payan-Gomez, C., Bombardieri, C. R., et al. (2016). Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 537, 427–431. doi: 10.1038/nature19329
Vernier, P. (2004). The degeneration of dopamine neurons in Parkinson’s disease: insights from embryology and evolution of the mesostriatocortical system. Ann. N. Y. Acad. Sci. 1035, 231–249. doi: 10.1196/annals.1332.015
Villesen, P., Aagaard, L., Wiuf, C., and Pedersen, F. S. (2004). Identification of endogenous retroviral reading frames in the human genome. Retrovirology 1:32.
Vyjayanti, V. N., and Rao, K. S. (2006). DNA double strand break repair in brain: reduced NHEJ activity in aging rat neurons. Neurosci. Lett. 393, 18–22. doi: 10.1016/j.neulet.2005.09.053
Wallace, A. D., Wendt, G. A., Barcellos, L. F., de Smith, A. J., Walsh, K. M., Metayer, C., et al. (2018). To ERV is human: a phenotype-wide scan linking polymorphic human endogenous retrovirus-K insertions to complex phenotypes. Front. Genet. 9:298. doi: 10.3389/fgene.2018.00298
Wallace, N. A., Belancio, V. P., and Deininger, P. L. (2008). L1 mobile element expression causes multiple types of toxicity. Gene 419, 75–81. doi: 10.1016/j.gene.2008.04.013
Wang, W.-Y., Pan, L., Su, S. C., Quinn, E. J., Sasaki, M., Jimenez, J. C., et al. (2013). Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 16, 1383–1391. doi: 10.1038/nn.3514
Wang, Y., Xu, E., Musich, P. R., and Lin, F. (2019). Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 25, 816–824. doi: 10.1111/cns.13116
Wątroba, M., and Szukiewicz, D. (2016). The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 61, 52–62. doi: 10.1016/j.advms.2015.09.003
Xue, B., Sechi, L. A., and Kelvin, D. J. (2020). Human endogenous retrovirus K (HML-2) in health and disease. Front. Microbiol. 11:1690. doi: 10.3389/fmicb.2020.01690
Yang, P., Wang, Y., and Macfarlan, T. S. (2017). The Role of KRAB-ZFPs in transposable element repression and mammalian evolution. Trends Genet. 33, 871–881. doi: 10.1016/j.tig.2017.08.006
Yang, W., Hamilton, J. L., Kopil, C., Beck, J. C., Tanner, C. M., Albin, R. L., et al. (2020). Current and projected future economic burden of Parkinson’s disease in the U.S. NPJ Parkinsons Dis. 6:15.
Zamudio, N., and Bourc’his, D. (2010). Transposable elements in the mammalian germline: a comfortable niche or a deadly trap? Heredity 105, 92–104. doi: 10.1038/hdy.2010.53
Zhong, L., D’Urso, A., Toiber, D., Sebastian, C., Henry, R. E., Vadysirisack, D. D., et al. (2010). The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293. doi: 10.1016/j.cell.2009.12.041
Keywords: transposable elements (TEs), genomic instability, LINE-1, DNA damage, neurodegenerative diseases, Parkinson’s disease, Alzheimer’s disease, aging
Citation: Peze-Heidsieck E, Bonnifet T, Znaidi R, Ravel-Godreuil C, Massiani-Beaudoin O, Joshi RL and Fuchs J (2022) Retrotransposons as a Source of DNA Damage in Neurodegeneration. Front. Aging Neurosci. 13:786897. doi: 10.3389/fnagi.2021.786897
Received: 30 September 2021; Accepted: 30 November 2021;
Published: 04 January 2022.
Edited by:
Christian Neri, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Steve Finkbeiner, University of California, San Francisco, United StatesCopyright © 2022 Peze-Heidsieck, Bonnifet, Znaidi, Ravel-Godreuil, Massiani-Beaudoin, Joshi and Fuchs. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia Fuchs, SnVsaWEuZnVjaHNAY29sbGVnZS1kZS1mcmFuY2UuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.