- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Disease, Beijing, China
- 5The General Office of Stroke Prevention Project Committee, National Health Commission of the People’s Republic of China, Beijing, China
- 6Institute of Radiation Medicine, Chinese Academy of Medical Sciences, Peking Union Medical College, Tianjin, China
- 7Savaid Medical School, University of Chinese Academy of Sciences, Beijing, China
The main reasons for disability and death in aneurysmal subarachnoid hemorrhage (aSAH) may be early brain injury (EBI) and delayed cerebral ischemia (DCI). Despite studies reporting and progressing when DCI is well-treated clinically, the prognosis is not well-improved. According to the present situation, we regard EBI as the main target of future studies, and one of the key phenotype-oxidative stresses may be called for attention in EBI after laboratory subarachnoid hemorrhage (SAH). We summarized the research progress and updated the literature that has been published about the relationship between experimental and clinical SAH-induced EBI and oxidative stress (OS) in PubMed from January 2016 to June 2021. Many signaling pathways are related to the mechanism of OS in EBI after SAH. Several antioxidative stress drugs were studied and showed a protective response against EBI after SAH. The systematical study of antioxidative stress in EBI after laboratory and clinical SAH may supply us with new therapies about SAH.
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease, mainly induced by the rupture of an intracranial aneurysm and linked to high levels of morbidity and mortality (Bor et al., 2008; Connolly et al., 2012; Macdonald and Schweizer, 2017; Chao et al., 2021). Although we have progressed in treatment, 40% of aSAH survivors remain dependent as a consequence of neurological disability and behavioral and cognitive impairments (Brathwaite and Macdonald, 2014; Etminan and Macdonald, 2017). Clinical studies have shown that cerebral vasospasm (CVS) is not the single contributor to delayed cerebral ischemia (DCI) and poor prognosis in patients with aSAH (Naraoka et al., 2018; Mayer et al., 2019; Schupper et al., 2020; Post et al., 2021; Takeuchi et al., 2021). At present, amassing laboratory evidence demonstrates that early brain injury (EBI), which happens within 72 h after aSAH, causes subsequently pathophysiological changes and poor outcomes (Kusaka et al., 2004). The pathological changes and mechanisms of EBI collectively contain increased intracranial pressure (ICP), oxidative stress (OS), neuroinflammation, blood–brain barrier (BBB) disruption, brain edema, and cell death. Among them, the OS responses include a wide variety of active and inactive substances, which play a substantial role in EBI after aSAH and may be associated with DCI and long-term outcomes (Cahill et al., 2006; Rowland et al., 2012; Sehba et al., 2012; Shao et al., 2020). Therefore, we should pay more attention to strategies targeting cerebral redox responses to some extent. In this review, we update the impact of OS in the occurrence and development of SAH and several major oxidative pathways and biomarkers related to EBI after SAH. Additionally, we also take an overlook for the potential therapeutic drugs.
Although widely accepted than other pathogenic mechanisms, DCI has not reached further improvement clinically. By the same token, the failure of clazosentan, despite mitigating moderate and severe vasospasm, has shifted the focus of preclinical and clinical research from DCI to EBI toward a more multifactorial etiology in recent times (Macdonald et al., 2008, 2011; Cahill and Zhang, 2009; Caner et al., 2012).
Mechanisms of Early Brain Injury
The topic raised in 2004, EBI is a designation that refers to the damage occurring to the brain in the first 72 h ensuing the initial aneurysmal bleeding and preceding vasospasm, including the primary injury and its direct consequences (Kusaka et al., 2004). The mechanisms resulting in EBI after aSAH are multifactorial and complicated. Conventionally, they are partitioned into the following parts (Rowland et al., 2012): (1) mechanical: acute or chronic hydrocephalus (Asada et al., 2021; Toyota et al., 2021); (2) physiological: raised ICP, decreased cerebral perfusion pressure (CPP) and cerebral blood flow (CBF), impaired cerebral autoregulation (CA), vasoconstriction, global ischemia, and delayed edema; (3) ionic: impaired ionic homeostasis, Ca2++ overload, K+ efflux, and cortical spreading depression (CSD); (4) inflammatory: NO (nitric oxide)/NOS (nitric oxide synthase) pathway activation, endothelin-1 (ET-1) release, platelet activation; and (5) cell death: endothelial cells, neurons, and astrocytes.
Aside from the aforementioned classical mechanisms, atypical mechanisms are newly proposed. Diverse factors, such as micro-spasm rather than macro-spasm (Fumoto et al., 2019), microthrombosis (Fumoto et al., 2019), early cortical infarction (Hartings et al., 2017; Eriksen et al., 2019), white matter injury (WMI) (Wu Y. et al., 2017; Pang et al., 2019; Peng et al., 2019; Ru et al., 2021), endoplasmic reticulum (ER) stress (Xie et al., 2019; Xu et al., 2019; Xiong et al., 2020), and immune inflammation (Ju et al., 2020; Rubio-Perez et al., 2021; Zeyu et al., 2021), are involved in cell death-relevant mechanisms in EBI after aSAH. Admittedly, various mechanisms or contributors to early injury will consequently result in cell death of the catastrophic ictus. Nowadays, the cell death processes in study include apoptosis, necrosis, autophagy (Dou et al., 2017; Sun C. et al., 2019; Sun C. M. et al., 2019), necroptosis (Kooijman et al., 2014; Chen et al., 2017, 2018, 2019; Xie et al., 2017; Yang C. et al., 2018; Yuan et al., 2019; Fang et al., 2020; Xu H. et al., 2021), pyroptosis (Yuan et al., 2020; Xu P. et al., 2021; Zhang C. S. et al., 2021), and ferroptosis (Lee et al., 2010; Zhao et al., 2018; Fang et al., 2020; Sq et al., 2020; Cao et al., 2021; Li et al., 2021; Qu et al., 2021). All but the former two well-known cell death mechanisms remain novel types and cutting-edge hot topics. For example, autophagy is an important protective mechanism against apoptosis, and recombinant osteopontin (rOPN) inhibits neuronal apoptosis by activating autophagy and regulating autophagy-apoptosis interactions (Sun C. M. et al., 2019). Necroptosis, a caspase-independent mechanism, plays a crucial part in the pathophysiological process by reducing the number of abnormal cells in brain tissue. Recently, SAH-induced synaptic impairments mitigated by NEC-1 in the hippocampus that inhibits necroptosis in relation to the CREB-BDNF pathway were verified (Yang C. et al., 2018). Moreover, another type of cell death, SAH-induced neuronal pyroptosis, is ameliorated in part by postconditioning with hydrogen gas through the mitoKATP/ERK1/2/p38 MAPK signaling pathway (Zhang C. S. et al., 2021). Unlike other types of cell death, such as necrosis and apoptosis, ferroptosis is a regulated process caused by an imbalance of the redox system. Cell death results in damaged structure and function of vessels and nerves, causing ultimately post-SAH dysfunction.
Typically, the elementary changes after SAH can be segmented into two periods: EBI and DCI. Pathological changes occurring in the initial stage of hemorrhage propagate and lead to inflammation, OS, and apoptosis. Studies showed that OS plays a key role in the pathogenesis of EBI after SAH.
Oxidative Stress in Subarachnoid Hemorrhage
Although progress is made in cell death, the actual pathogenesis of EBI after SAH is still rarely understood. Several pieces of research demonstrate that OS is one of the basic drivers of EBI (Zhang et al., 2016c; Fumoto et al., 2019).
Relying on the activity of the producer and removal systems, OS has its yin and yang faces, but those covered in this review are the result of the dysregulation of reduction-oxidation (redox) reactions. Sies and Jones (2020) also defined the elevated constitution of various reactive oxygen species (ROS) resulting in all classes of molecular damage as “oxidative distress.” OS, a relative extra of ROS compared with antioxidants, has been associated with cardiovascular, neurodegenerative, and many other diseases. ROS is an overall term for derivatives of dioxygen not chemically precise, which serve as a normal character of aerobic life. Hydrogen peroxide (H2O2) and the O2•– are pivotal agents produced by greater than 40 enzymes (Murphy, 2009; Go et al., 2015). Additionally, many other reactive categories are contained in redox signaling, for example, hydrogen sulfide and oxidized lipids (Fujii et al., 2010; Yu et al., 2014; Jarocka-Karpowicz et al., 2020).
After SAH, exhibiting aberrant redox hemostasis, the production of oxidants mainly comes from the disruption of mitochondria (Yan et al., 2015; Fan et al., 2021; Xu W. et al., 2021), extravascular hemolyzed blood (Vecchione et al., 2009; Deng et al., 2018), and enzymatic sources of free radicals (Sies et al., 2017; Yang et al., 2017; Sies and Jones, 2020). Intrinsic antioxidant activity can be exhausted by excessive free radicals, resulting in lipid peroxidation, protein breakdown, and DNA damage. Mention must be made that beyond the biology of H2O2 and O2•–, a significant area of ROS research is lipid-derived ROS. Oxidative DNA damage has also been widely distinguished by mutagenesis, DNA methylation, and chromatin structure. Although substantiation is also gathered about oxidative damage to RNA, the underlying functional effect has not yet been fully illustrated.
Oxidative Stress in Early Brain Injury
For decades, the treatment of CVS and DCI has always been the focus of clinical practice. However, mounting evidence showed that even angiographic vasospasm is reversed, and clinical outcomes remain frustrating (Macdonald et al., 2008; Gomis et al., 2010). So far, nimodipine remains the only medication proven to lessen DCI and unfavorable outcomes. Therefore, new therapeutic regimens are promising (Hänggi et al., 2015).
Previously, the aspecific erasure of ROS using antioxidant compounds was unsuccessful in offsetting SAH initiation. However, regulating specific ROS-mediated signaling pathways offers a viewpoint, mainly containing enzymatic defense systems like those regulated by the nuclear factor erythroid 2 (NF-E2)-related factor 2 (Nrf2) (Zolnourian et al., 2019; Sies and Jones, 2020) and PI3K/Akt, the role of key molecules such as melatonin, sirtuins, and hydrogen sulfide, and the modifiable factors that are corporately thought as the exposome [by way of illustration, nutrition (Liu et al., 2015), lifestyle, and irradiation] (see overview in Table 1 and Figure 1). Discovering strategies for effectively detoxifying free radicals has become a theme of great interest from both practical and academic viewpoints.
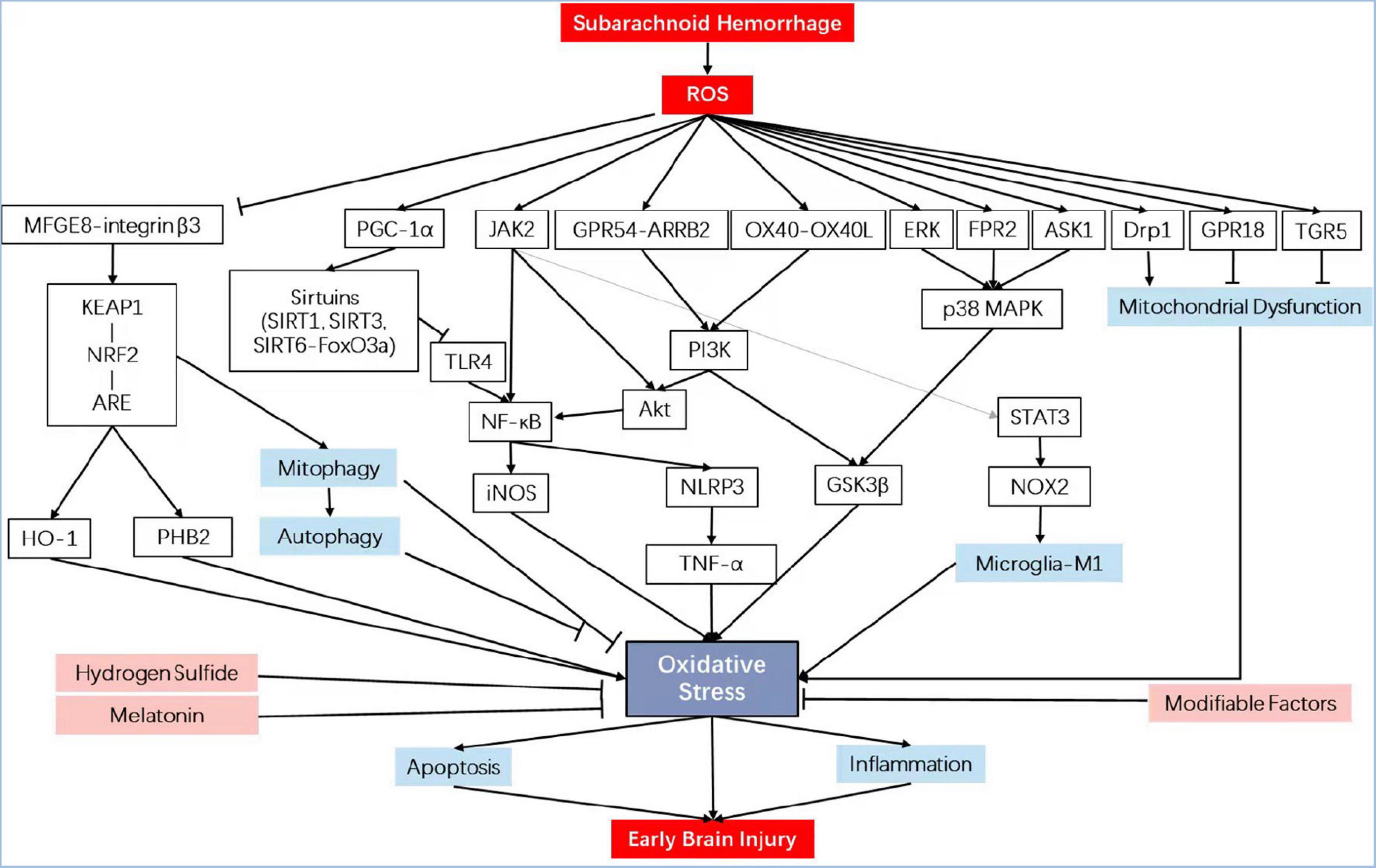
Figure 1. Schematic diagram illustrating the signaling pathways involved in oxidative stress in early brain injury. iNOS, inducible nitric oxide synthase; PHB2, prohibitin 2; NRF2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; NF-κB, nuclear factor kappa-B; KEAP1, Kelch-like epichlorohydrin-associated protein 1; ARE, antioxidant response element; MFGE8, milk fat globule–EGF factor-8; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; GPR18, G protein-coupled receptor 18; p38 MAPK, mitogen-activated protein kinase; FPR2, formyl peptide receptor 2; ASK1, apoptosis signal-regulating kinase 1; Drp1, dynamin-related protein 1; OX40L, OX40 cognate ligand-protein; GPR54, G protein-coupled receptor 54; ARRB2, β-arrestin 2; GSK3β, glycogen synthase kinase-3β; PI3K, phosphatidylinositol 3-kinase; TGR5, trans-membrane G protein-coupled receptor-5; PGC-1α, peroxisome proliferators-activated receptor-γ coactivator-1α; NLRP3, NLR family, pyrin domain containing 3.
KEAP1-NRF2-ARE Pathway
An important “sensors” protein that captures specific metabolic information after SAH and transforms it into an appropriate response is Kelch-like ECH-associated protein 1 (Keap1), which contains reactive cysteine residues that collectively respond to ROS resulting from heme. Covalent modification of Keap1 leads to reduce ubiquitination and accumulates Nrf2 (Bollong et al., 2018). It is combined with a given DNA site, the antioxidant response element, modulating the transcription of a series of antioxidant enzymes (Zolnourian et al., 2019). Lots of researches on stroke models have substantiated that Nrf2 levels lift soon after the stroke. It first appears within 3 h and peaks within 24 h post insult, with the peri-infarct area markedly increasing (Yang et al., 2009; Tanaka et al., 2011). The study indicated that Nrf2 expression is significantly activated in neurons, astrocytes, leukocytes, microglia, endothelin cells, smooth muscle cells, and adventitial cells after SAH-induced brain insult (Wang et al., 2010). Wang et al., found that the Nrf2-ARE pathway is upregulated in rat models with experimental SAH in the time course at 12, 24, and 48 h (Chen et al., 2011). Since then, lots of known activators of the Keap1-Nrf2-ARE pathway were overwhelmingly carried out in the study. Although controversial conclusions are sometimes achieved due to pleiotropic and primary mechanisms, more effective inducers with less crossing activation of other pathways are identified and reach favorable outcomes.
In addition to the agents summarized by former researchers, such as sulforaphane, astaxanthin, curcumin, lycopene, tetra-butyl hydroquinone, dimethyl fumarate, melatonin, and erythropoietin, there are also many other experimental studies conducted more recently. Efforts to target the Nrf2 signaling pathway therapeutically have largely focused on covalent small molecule agonists of Keap1. Mitoquinone (MitoQ), effective in the prevention of mitochondrial dysfunction, restrained OS-related neuronal death by stimulating mitophagy through Keap1/Nrf2/PHB2 pathway after SAH in rats (Zhang T. et al., 2019). In the endovascular perforation mice SAH model, MitoQ treatment reduced OS, both short term and long term. Another activator of Nrf2, RTA 408, a new second-generation semisynthetic oleanane triterpenoid, manifested an antioxidant and anti-inflammatory phenotype (Reisman et al., 2014). After administrated intraperitoneally, vasospasm was reversed by RTA 408 through growth in Nrf2 and reduction in NF-κB (Tsai et al., 2020).
Intriguingly, performing as a downstream molecule in the Keap1-Nrf2-ARE pathway, heme oxygenase-1 (HO-1), also known as HSP32, could be induced by upregulating expression of Nrf2 (Wang and Doré, 2007; Jiang et al., 2020). After SAH, vasospasm and lipid peroxidation can be weakened by HO-1 through the improvement of clearance. Post-hemorrhagic administration of Nrf2 activator, tetramethylpyrazine nitrone (Wu et al., 2019), aloperine (Song et al., 2018), milk fat globule-epidermal growth factor 8 (MFGE8) (Liu et al., 2014, 2015), tumor necrosis factor-alpha stimulated gene-6 (Li et al., 2020), ursolic acid (UA) (Zhang et al., 2014a,b; Ding et al., 2017), gastrodin (Wang et al., 2019), tert-Butylhydroquinone (Wang et al., 2014), promotes posttranscriptional augment of both Nrf2 and HO-1, attenuates OS, and then reduces early brain damage, including brain edema, BBB damage, and cognitive impairment following SAH in animal models. Moreover, an additional NRF family member, namely, Nrf1 (Qian et al., 2019), responsible for ROS detoxification, participates in an effective treatment to moderate SAH-elicited EBI. Researchers concluded that HSP22 played a part in neuroprotective effects by regulating TFAM/Nrf1-triggered mitochondrial biogenesis with positive feedback, further attenuating OS and EBI (Fan et al., 2021).
Mitochondrial Pathways
Shortly after the induction of SAH, EBI triggers mitochondrial disorder, in which many signaling molecules communicate with each other to control OS (Prentice et al., 2015). Thus, another pivotal key in alleviating EBI is discovering new options to keep normal mitochondrial activity by attenuating OS. The mechanisms for ROS generated by mitochondria are under the consensus that the production of ROS is maximal when the ingredients of the electron transport chains (ETCs) are superlatively impaired (Murphy et al., 1999; Moro et al., 2005). Particularly interacting with autophagy and apoptosis, activation of autophagic pathways attenuates EBI after SAH in rats (Jing et al., 2012; Shi et al., 2020; Xu W. et al., 2021). Among the mounts of antioxidant agents, docosahexaenoic acid (DHA), the so-called omega-3 fatty acid, reduces OS through enhancing mitochondrial dynamics in EBI (Zhang T. et al., 2018). Concretely, DHA reduced the number of ROC-positive cells, improved cell viability, attenuated malondialdehyde levels, and superoxide dismutase (SOD) stress. Furthermore, a metabolite of DHA, namely, resolvin D2 (RvD2), helps to defend EBI, especially in the cortex and hypothalamus (Zhang T. et al., 2021). The p38 mitogen-activated protein kinase (MAPK) is a major player in mitochondrial dysfunction after SAH (Sasaki et al., 2004; Yatsushige et al., 2007; Guo et al., 2016; Han et al., 2017a; Tomar et al., 2017; Lu et al., 2019). Recently, a p38 inhibitor, DJ-1, protects mitochondrial dysfunction by induction of translocation (Huang et al., 2018). Another selective inhibitor of Drp1, Mdivi-1, exerts neuroprotective effects against mitochondrial fission and OS (Fan et al., 2017; Wu P. et al., 2017). More recently, SS31 cell-membrane permeating mitochondria has been shown to exert potential neuroprotective effects (Petri et al., 2006). Via suppressing Bax translocation and cytochrome c release, SS31 ameliorated OS by inhibiting the mitochondrial pathway (Shen et al., 2020).
Other Pathways
PI3K/Akt Pathway
The PI3K/Akt pathway is one of the important pathways that inhibit cell apoptosis and, therefore, plays a protective role against SAH. In recent years, antiapoptosis agents targeting this pathway generated the cross-talk with antioxidative effect (Wu et al., 2020). For example, Aggf1, also known as an angiogenic factor with G, patch, and FHA domain 1, in a recombinant human form, reduces BBB disruption and neuroinflammation through PI3K/Akt/NF-κB pathway after SAH in rats by significantly decreasing the level of myeloperoxidase (Zhu et al., 2018). For the first time, John H. Zhang et al., found that KISS1 siRNA knockdown (KD) aggravated neurological deficits and the brain expression of markers for OS in rats both 24 h and 28 days after SAH, suggesting that KP54 attenuated OS through activating GPR54/ARRB2/AKT/GSK3β pathway after SAH in rats (Mead et al., 2007; Huang et al., 2021). Another frontier hotspot involved in the PI3K/Akt pathway is the microglial polarization-mediated WMI (Xue et al., 2021). Additionally proposed by the John H. Zhang research group, low-density lipoprotein receptor-related protein-1 (LRP1), a scavenger receptor of apolipoprotein E (apoE), is validated for microglia polarization toward pro-antioxidative M2 phenotypes via Shc1/PI3K/Akt pathway after SAH in rats (Wu Y. et al., 2017; Rojo et al., 2018; Peng et al., 2019). Uniformly, apoE and apoE-mimetic peptides possess whole-brain protective effects that may reduce EBI after mice SAH via M1 microglial quiescence through the attenuation of the JAK2/STAT3/NOX2 signaling pathway axis (Pang et al., 2018). A shred of direct evidence presented by Kuanyu Li and his colleagues is that pAkt effectively inhibits iron accumulation, defense against OS, and ameliorates EBI in a model of experimental SAH in the temporal lobe (Jo et al., 2012; Hao et al., 2016; Zhang et al., 2016a). Interestingly, SC79 is an absorptive permeability without reported side effects, indicating a novel and promising delivery drug in patients with EBI after SAH.
More Recently Progressed Pathway
In addition to the Keap-Nrf2-ARE pathway and PI3K/Akt pathway, there are many other pathways that are oxidative related and proved to be effective. For instance, Scutellarin, a flavonoid from the Chinese herb Erigeron breviscapus, reduces vasospasm via the Erk5-KLF2-eNOS pathway after SAH (Li Q. et al., 2016). An agonist of the Shh co-receptor plays a part in neuroprotection against SAH-induced damage, mediated in part by antioxidant mechanisms, upregulating phospho-ERK levels, and mediating Shh signaling molecules in the prefrontal cortex (Hu et al., 2016). Benefitting from a broad distribution in neurons, astrocytes, and microglia, activation of TGR5 with INT-777 significantly attenuates OS through cAMP/PKCε/ALDH2 pathway after SAH in rats (Zuo G. et al., 2019). Recognized as a new component of the brain renin-angiotensin system, Mas is selectively target-activated by AVE 0991 and reduces OS through Mas/PKA/CREB/UCP-2 pathway (Mo et al., 2019). Moreover, 12/15-LOX is overexpressed in macrophages after SAH in mice, and restraint of the pathway attenuates brain injury and ameliorates unfavorable neurological outcomes. Progressing data support that various pathways may participate in the redox balance in EBI after SAH and needs to be added with a new insight of other underlying pathways.
Melatonin
The number of data accumulated till now concerning the protective action of melatonin against OS is preponderant (Galano et al., 2011; Wu H. J. et al., 2017; Luo et al., 2019; Shao et al., 2020). Melatonin, a lipophilic amino acid that originated from tryptophan, N-acetyl-5-methoxytryptamine, is synthesized in the pineal gland and other organs and exhibits both direct and indirect antioxidant effects. Melatonin first reported the antioxidative function in preventing focal regions of injury via inducing HO-1 expression following a rat SAH model in 2002 (Martinez-Cruz et al., 2002). Before the post-clazosentan era, studies regarding the EBI experiments were still broadly focused on delayed brain injury, such as setting the assessment point at day 5 (Fang et al., 2009). Although melatonin shows no improvement in neurologic scores, the phenomenon is settled by large doses with immensely lessened mortality (Ayer et al., 2008a,b). However, the latest study shows that patients with higher serum melatonin concentrations are more likely to have a poor prognosis (Zhan et al., 2021). The increased concentrations of serum melatonin correlate with admission WFNS scores and mFS and serum melatonin appears as an independent predictor for poor 6-month prognosis after aSAH, with a high discriminatory ability for the risk of the poor outcome under the ROC curve, indicating that serum melatonin might serve as a promising prognostic biomarker for aSAH (Zhan et al., 2021). In vivo experiments exhibit that melatonin supplied protection from the effects of EBI after SAH by adjusting the expression of murine SIRT3 (one of the members of the sirtuin family) (Yang S. et al., 2018). Another two recent studies also denoted that melatonin plays a neuroprotective role by increasing the expression of NRF2-mitophagy and ER stress via inducing antioxidant, LC3-II/LC3-I, and Atg 5-mediated autophagy, NLRP3 inflammasome-mediated anti-inflammatory effects, and detoxifying enzymes post-SAH (Wang et al., 2012; Dong et al., 2016; Wu H. J. et al., 2017; Sun et al., 2018). Melatonin may reduce neurobehavioral dysfunction in the SAH model through the TRL4 pathway (Wang et al., 2013). Furthermore, melatonin reduces the EBI by influencing NLRP3 inflammasome-associated apoptosis (Dong et al., 2016) and inhibiting NF-κB activation and attenuating HO-1, NQO-1, and c-GCLC expressions (Jumnongprakhon et al., 2015). The mechanism of these antiapoptosis effects was linked to the improvement of autophagy through a mitochondrial pathway (Chen et al., 2014b). Melatonin inhibits the disruption of tight junction proteins possibly linked to the adjustment of proinflammatory cytokines (Chen et al., 2014a). Taken together, these results demonstrate that regulation of melatonin attenuates symptomatic dysfunction (Chen et al., 2015).
Sirtuins
Sirtuins (SIRTs), including the seven SIRTs identified, are a family of deacetylases with homology. Lines of studies showed that SIRTs could modulate diverse biological functions, Sirtuin 1 (SIRT1) with antioxidative properties particularly. Demonstrating that sequential inhibition of forkhead transcription factors of the O class-, NF-κ B-, and p53-induced oxidative pathways, SIRT1 enhanced the neuroprotective role against EBI in rats (Zhang et al., 2016). A well-recognized antioxidant, astaxanthin, mitigates SAH-induced EBI by increasing SIRT1 and suppressing the TLR4 signaling pathway (Wu et al., 2014; Zhang et al., 2014; Zhang X. et al., 2019). Interestingly, derived from seaweeds, fucoxanthin (Fx) mitigates SAH-induced oxidative damage via the SIRT1-dependent pathway (Zhang X. S. et al., 2020). The activation of melanocortin 1 receptor with BMS-470539 immensely reduced EBI after SAH by restraining OS, apoptosis, and mitochondrial fission via the AMPK/SIRT1/PGC-1α signaling pathway (Xu W. et al., 2021). Modulated by SIRT1 activation, salvianolic acid B protects against SAH-triggered oxidative damage by upregulating the Nrf2 antioxidant signaling pathway (Zhang X. et al., 2018). Salvianolic acid homolog A also presented antioxidative, antiapoptotic, and anti-inflammatory properties (Gu et al., 2017; Zhang X. et al., 2018).
Other members of the SIRT family are increasingly studied recently. SIRT3, a type of NAD-dependent deacetylase, remarkedly activated in vivo and in vitro following SAH, is involved in the PGC-1a/SIRT3 pathway attenuating OS (Zhang K. et al., 2020). Drawing on the successful experience of the SIRT6 protective role of the heart from I/R injury via upregulating antioxidants and suppressing OS, the activation of RXR ameliorated neurological deficits after SAH at least partially via adjusting the PPARγ/SIRT6/FoxO3a pathway (Zuo Y. et al., 2019).
Hydrogen Sulfide
Hydrogen sulfide (H2S), a neuromodulator, which can be generated in the CNS from L-cysteine by cystathionine-β-synthase (CBS), may prove protective effects in experimental SAH (Xiong et al., 2020). The hypothesis that signaling through hydrogen sulfide may mediate protection from DCI clinically in patients with SAH was proposed in 2011 (Grobelny et al., 2011) and further demonstrated by Yu et al. (2014). Afterward, hydrogen sulfide attenuated brain edema formation and promoted the secretion of inflammatory cytokines (Cui et al., 2016). Soon after the proinflammation demonstrated in EBI after SAH, exogenous hydrogen sulfide functioning as an antioxidant and antiapoptotic mediator, donated by NaSH and L-cysteine, could significantly reduce EBI (Cui et al., 2016; Li et al., 2017; Xiong et al., 2020). Inspired by inhaled hydrogen gas markedly decreasing OS on ischemia/reperfusion injury and stroke in rats (Ohsawa et al., 2007), hydrogen gas therapy was conducted, and the rate of survival and neurological deficits were improved in a pilot study as expected (Camara et al., 2019). Mechanistically, the above advantageous effects might be linked to the suppression of the ROS/NLRP3 axis (Zhuang et al., 2019). Similarly, hydrogen-rich saline exhibited the satisfying outcome of alleviating EBI through alleviating OS following experimental SAH in both rabbit and rat models (Zhuang et al., 2012; Shao et al., 2016). Furthermore, sodium/hydrogen exchanger 1 participates in EBI activated by SAH via mediating OS (Song et al., 2019). As abovementioned earlier, a recent study shows that postconditioning with hydrogen gas ameliorated SAH-induced neuronal pyroptosis (Zhang C. S. et al., 2021). Produced endogenously through HO, carbon oxide (CO) minimizes neurobehavioral deficits, indicating that posttreatment with CO gas or CO-donors can be further tested as a potential therapy against SAH (Kamat et al., 2019).
Modifiable Factors
Peaking onset age between 50 and 60 years, many patients with aSAH have modifiable hypertension, dyslipidemia, diabetes mellitus, cardiovascular diseases, and so on (de Rooij et al., 2007; Zhang L. et al., 2017; Macdonald and Schweizer, 2017). To control the clinical status, they are recommended with antihypertensive drugs, statins, and so on, part of whom demonstrate the antioxidative effect in the laboratory, for example, telmisartan, ameliorates OS, and SAH-induced CVS (Erdi et al., 2016). One of the L-type calcium channel blockers, verapamil, can inhibit vasospasm, OS, and apoptosis following experimental SAH (Akkaya et al., 2019). Rosuvastatin, commonly used clinically, ameliorates EBI after SAH through restraining SOD formation and NF-κB activation in rats. Moreover, 3,4-dihydroxyphenylethanol may be a powerful agent in the treatment of EBI after SAH because of its free radical scavenging capacity and modulating the Akt and NF-κB signaling pathway (Zhong et al., 2016; Fu and Hu, 2016). In addition to the intervening abovementioned diseases, other drugs commonly used clinically, such as heparin (Hayman et al., 2017), albumin (Deng et al., 2021), and propofol (Zhang H. B. et al., 2019), all projected an antioxidative role. Some authors believe that preconditioning would provide the greatest chance of benefit but is obviously not effective (Mayor et al., 2013; Zolnourian et al., 2019; Ruan et al., 2020; Otsuka et al., 2021). Given the sophistication of brain damage after aSAH, therapeutic multimodality is promising. Supported by the evidence shown above, we suppose that the regular drug taken with high adherence may benefit favorable outcomes after aneurysm rupture than those who do not.
Perspectives and Limitations
Shifted from phenotype research on OS in EBI to pathway-related research, the mechanisms become evident than at any time in the past (Zhang L. et al., 2017; Ye et al., 2018; Pan et al., 2021). However, since the term EBI was coined from an angle of preclinical mechanism insight, there is still a long way to apply clinically. Preclinical and clinical studies should proceed hand in hand, and none of the multi-omics and clinical trials should stop (Xu et al., 2017; Xu W. et al., 2018).
In addition to the traditional markers, such as malondialdehyde (MDA), SOD, reduced/oxidized glutathione (GSH/GSSG) ratio, and myeloperoxidase (MPO), new approaches that measure energetics and metabolomics of cells should be explored, such as the bioenergetic health index (BHI) (Chacko et al., 2016), to further guide the development of therapies. A lot of conducted studies showed that drugs introduced into the area of EBI after aSAH previously drew on the strength of the fields of ischemic stroke or traumatic brain injury. Furthermore, a close tracing of novel antioxidants is necessary, even in other disciplines with mutual adoption, promotion, and advancement, especially traditional Chinese medicine and nutrition (Wang et al., 2015; Zhang et al., 2015; Li Q. et al., 2016; Han et al., 2017b; Liu et al., 2017, 2020; Shao et al., 2019; Du et al., 2020; Wang T. et al., 2020). As the OS-related mechanisms and pathways have surfaced and matured, more modern technologies should emphasize their parts, such as designing a metabolite-derived protein alteration integrating glycolysis with Keap1-Nrf2 signaling directly (Bollong et al., 2018) or recombinant human drugs (Xie et al., 2018; Sun C. et al., 2019; Sun C. M. et al., 2019; Wang J. et al., 2020; Wu et al., 2020; Tu et al., 2021). Effective drugs validated in a laboratory should be carefully compared and put into clinical use properly. Typical drug pharmaceutical effects combined with evolving drug-loaded methods may be promising in future exploration. For example, the conventional antioxidant agent, curcumin, loaded into a nanosized PLGA-encapsulated the therapeutic potential was enhanced for precision medicine in downregulating the NF-κB pathway and preventing OS in EBI (Li X. et al., 2016; Zhang et al., 2016b; Cai et al., 2017; Zhang Z. Y. et al., 2017).
There are several limitations in this study. First, at present, there remains a translational cleft between experimental SAH and clinical SAH, especially aSAH. Although injection and endovascular perforation models are well established and invaluable, no model, even the in vivo cerebral aneurysmal models, could perfectly replicate the actual rupture of an aneurysm in human beings. Second, the unequivocal definition of EBI could not be circumvented in animal models with the variation assessment points in 3, 12, 24, or 72 h. Clear evaluation time is still up for debate. Third, based on a multifactorial pathophysiology after EBI, OS plays a pivotal part but is still only one of the considerable phenotypes with promising therapeutic strategies covering as many pathways as possible.
Conclusion
Management of EBI after aSAH remains not only a challenge but also an opportunity. With in-depth understanding of oxidative pathophysiology of EBI, the way ahead becomes gradually clearer. Despite initial experimental studies demonstrating the effectiveness of the abovementioned antioxidants in EBI, these studies are comparatively rudimentary, with further translational medicine demanded to prove the utility of all of them clinically.
Author Contributions
FL, RL, W-JT, KW, and YC contributed to the search and assessment of the available literature. FL and RL wrote the manuscript. XC and JZ helped to revise the manuscript to the final form. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (Grant No. 2020YFC2004701).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akkaya, E., Evran, Ş, Çalış, F., Çevik, S., Hanımoğlu, H., Seyithanoğlu, M. H., et al. (2019). Effects of intrathecal verapamil on cerebral vasospasm in experimental rat study. World Neurosurg. 127, e1104–e1111. doi: 10.1016/j.wneu.2019.04.050
Asada, R., Nakatsuka, Y., Kanamaru, H., Kawakita, F., Fujimoto, M., Miura, Y., et al. (2021). Higher plasma osteopontin concentrations associated with subsequent development of chronic shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Transl. Stroke Res. 12, 808–816. doi: 10.1007/s12975-020-00886-x
Ayer, R. E., Sugawara, T., Chen, W., Tong, W., and Zhang, J. H. (2008a). Melatonin decreases mortality following severe subarachnoid hemorrhage. J. Pineal Res. 44, 197–204. doi: 10.1111/j.1600-079X.2007.00508.x
Ayer, R. E., Sugawara, T., and Zhang, J. H. (2008b). Effects of melatonin in early brain injury following subarachnoid hemorrhage. Acta Neurochir. Suppl. 102, 327–330. doi: 10.1007/978-3-211-85578-2_62
Bollong, M. J., Lee, G., Coukos, J. S., Yun, H., Zambaldo, C., Chang, J. W., et al. (2018). A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature 562, 600–604. doi: 10.1038/s41586-018-0622-0
Bor, A., Rinkel, G., Adami, J., Koffijberg, H., Ekbom, A., Buskens, E., et al. (2008). Risk of subarachnoid haemorrhage according to number of affected relatives: a population based case-control study. Brain 131, 2662–2665. doi: 10.1093/brain/awn187
Brathwaite, S., and Macdonald, R. L. (2014). Current management of delayed cerebral ischemia: update from results of recent clinical trials. Transl. Stroke Res. 5, 207–226. doi: 10.1007/s12975-013-0316-8
Cahill, J., Calvert, J. W., and Zhang, J. H. (2006). Mechanisms of early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 26, 1341–1353. doi: 10.1038/sj.jcbfm.9600283
Cahill, J., and Zhang, J. H. (2009). Subarachnoid hemorrhage: is it time for a new direction? Stroke 40, S86–S87. doi: 10.1161/strokeaha.108.533315
Cai, J., Xu, D., Bai, X., Pan, R., Wang, B., Sun, S., et al. (2017). Curcumin mitigates cerebral vasospasm and early brain injury following subarachnoid hemorrhage via inhibiting cerebral inflammation. Brain Behav. 7:e00790. doi: 10.1002/brb3.790
Camara, R., Matei, N., Camara, J., Enkhjargal, B., Tang, J., and Zhang, J. H. (2019). Hydrogen gas therapy improves survival rate and neurological deficits in subarachnoid hemorrhage rats: a pilot study. Med. Gas Res. 9, 74–79. doi: 10.4103/2045-9912.260648
Caner, B., Hou, J., Altay, O., Fujii, M., and Zhang, J. H. (2012). Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J. Neurochem. 123(Suppl. 2), 12–21. doi: 10.1111/j.1471-4159.2012.07939.x
Cao, Y., Li, Y., He, C., Yan, F., Li, J. R., Xu, H. Z., et al. (2021). Selective ferroptosis inhibitor liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neurosci. Bull. 37, 535–549. doi: 10.1007/s12264-020-00620-5
Chacko, B. K., Zhi, D., Darley-Usmar, V. M., and Mitchell, T. (2016). The bioenergetic health index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 8, 43–50. doi: 10.1016/j.redox.2015.12.008
Chao, B. H., Yan, F., Hua, Y., Liu, J. M., Yang, Y., Ji, X. M., et al. (2021). Stroke prevention and control system in China: CSPPC-stroke program. Int. J. Stroke 16, 265–272. doi: 10.1177/1747493020913557
Chen, F., Su, X., Lin, Z., Lin, Y., Yu, L., Cai, J., et al. (2017). Necrostatin-1 attenuates early brain injury after subarachnoid hemorrhage in rats by inhibiting necroptosis. Neuropsychiatr. Dis. Treat. 13, 1771–1782. doi: 10.2147/NDT.S140801
Chen, G., Fang, Q., Zhang, J., Zhou, D., and Wang, Z. (2011). Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J. Neurosci. Res. 89, 515–523. doi: 10.1002/jnr.22577
Chen, J., Wang, L., Wu, C., Hu, Q., Gu, C., Yan, F., et al. (2014b). Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J. Pineal Res. 56, 12–19. doi: 10.1111/jpi.12086
Chen, J., Chen, G., Li, J., Qian, C., Mo, H., Gu, C., et al. (2014a). Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: a possible role for the regulation of pro-inflammatory cytokines. J. Pineal Res. 57, 340–347. doi: 10.1111/jpi.12173
Chen, J., Jin, H., Xu, H., Peng, Y., Jie, L., Xu, D., et al. (2019). The neuroprotective effects of necrostatin-1 on subarachnoid hemorrhage in rats are possibly mediated by preventing blood-brain barrier disruption and RIP3-mediated necroptosis. Cell Transplant. 28, 1358–1372. doi: 10.1177/0963689719867285
Chen, J., Qian, C., Duan, H., Cao, S., Yu, X., Li, J., et al. (2015). Melatonin attenuates neurogenic pulmonary edema via the regulation of inflammation and apoptosis after subarachnoid hemorrhage in rats. J. Pineal Res. 59, 469–477. doi: 10.1111/jpi.12278
Chen, T., Pan, H., Li, J., Xu, H., Jin, H., Qian, C., et al. (2018). Inhibiting of RIPK3 attenuates early brain injury following subarachnoid hemorrhage: possibly through alleviating necroptosis. Biomed. Pharmacother. 107, 563–570. doi: 10.1016/j.biopha.2018.08.056
Connolly, E. S. Jr., Rabinstein, A. A., Carhuapoma, J. R., Derdeyn, C. P., Dion, J., Higashida, R. T., et al. (2012). Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 43, 1711–1737. doi: 10.1161/STR.0b013e3182587839
Cui, Y., Duan, X., Li, H., Dang, B., Yin, J., Wang, Y., et al. (2016). Hydrogen sulfide ameliorates early brain injury following subarachnoid hemorrhage in rats. Mol. Neurobiol. 53, 3646–3657. doi: 10.1007/s12035-015-9304-1
de Rooij, N. K., Linn, F. H., van der Plas, J. A., Algra, A., and Rinkel, G. J. (2007). Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 78, 1365–1372. doi: 10.1136/jnnp.2007.117655
Deng, S., Liu, S., Jin, P., Feng, S., Tian, M., Wei, P., et al. (2021). Albumin reduces oxidative stress and neuronal apoptosis via the ERK/Nrf2/HO-1 pathway after intracerebral hemorrhage in rats. Oxid. Med. Cell. Longev. 2021:8891373. doi: 10.1155/2021/8891373
Deng, W., Kandhi, S., Zhang, B., Huang, A., Koller, A., and Sun, D. (2018). Extravascular blood augments myogenic constriction of cerebral arterioles: implications for hemorrhage-induced vasospasm. J. Am. Heart Assoc. 7:e008623. doi: 10.1161/jaha.118.008623
Ding, H., Wang, H., Zhu, L., and Wei, W. (2017). Ursolic acid ameliorates early brain injury after experimental traumatic brain injury in mice by activating the Nrf2 pathway. Neurochem. Res. 42, 337–346. doi: 10.1007/s11064-016-2077-8
Dong, Y., Fan, C., Hu, W., Jiang, S., Ma, Z., Yan, X., et al. (2016). Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J. Pineal Res. 60, 253–262. doi: 10.1111/jpi.12300
Dou, Y., Shen, H., Feng, D., Li, H., Tian, X., Zhang, J., et al. (2017). Tumor necrosis factor receptor-associated factor 6 participates in early brain injury after subarachnoid hemorrhage in rats through inhibiting autophagy and promoting oxidative stress. J. Neurochem. 142, 478–492. doi: 10.1111/jnc.14075
Du, C., Xi, C., Wu, C., Sha, J., Zhang, J., and Li, C. (2020). Ginkgo biloba extract protects early brain injury after subarachnoid hemorrhage via inhibiting thioredoxin interacting protein/NLRP3 signaling pathway. Iran. J. Basic Med. Sci. 23, 1340–1345. doi: 10.22038/ijbms.2020.42834.10090
Erdi, F., Keskin, F., Esen, H., Kaya, B., Feyzioglu, B., Kilinc, I., et al. (2016). Telmisartan ameliorates oxidative stress and subarachnoid haemorrhage-induced cerebral vasospasm. Neurol. Res. 38, 224–231. doi: 10.1080/01616412.2015.1105626
Eriksen, N., Rostrup, E., Fabricius, M., Scheel, M., Major, S., Winkler, M. K. L., et al. (2019). Early focal brain injury after subarachnoid hemorrhage correlates with spreading depolarizations. Neurology 92, e326–e341. doi: 10.1212/wnl.0000000000006814
Etminan, N., and Macdonald, R. L. (2017). Management of aneurysmal subarachnoid hemorrhage. Handb. Clin. Neurol. 140, 195–228. doi: 10.1016/B978-0-444-63600-3.00012-X
Fan, H., Ding, R., Liu, W., Zhang, X., Li, R., Wei, B., et al. (2021). Heat shock protein 22 modulates NRF1/TFAM-dependent mitochondrial biogenesis and DRP1-sparked mitochondrial apoptosis through AMPK-PGC1α signaling pathway to alleviate the early brain injury of subarachnoid hemorrhage in rats. Redox Biol. 40:101856. doi: 10.1016/j.redox.2021.101856
Fan, L. F., He, P. Y., Peng, Y. C., Du, Q. H., Ma, Y. J., Jin, J. X., et al. (2017). Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic. Biol. Med. 112, 336–349. doi: 10.1016/j.freeradbiomed.2017.08.003
Fang, Q., Chen, G., Zhu, W., Dong, W., and Wang, Z. (2009). Influence of melatonin on cerebrovascular proinflammatory mediators expression and oxidative stress following subarachnoid hemorrhage in rabbits. Mediators Inflamm. 2009:426346. doi: 10.1155/2009/426346
Fang, Y., Gao, S., Wang, X., Cao, Y., Lu, J., Chen, S., et al. (2020). Programmed cell deaths and potential crosstalk with blood-brain barrier dysfunction after hemorrhagic stroke. Front. Cell. Neurosci. 14:68. doi: 10.3389/fncel.2020.00068
Fu, P., and Hu, Q. (2016). 3,4-Dihydroxyphenylethanol alleviates early brain injury by modulating oxidative stress and Akt and nuclear factor-κB pathways in a rat model of subarachnoid hemorrhage. Exp. Ther. Med. 11, 1999–2004. doi: 10.3892/etm.2016.3101
Fujii, S., Sawa, T., Ihara, H., Tong, K. I., Ida, T., Okamoto, T., et al. (2010). The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J. Biol. Chem. 285, 23970–23984. doi: 10.1074/jbc.M110.145441
Fumoto, T., Naraoka, M., Katagai, T., Li, Y., Shimamura, N., and Ohkuma, H. (2019). The role of oxidative stress in microvascular disturbances after experimental subarachnoid hemorrhage. Transl. Stroke Res. 10, 684–694. doi: 10.1007/s12975-018-0685-0
Galano, A., Tan, D. X., and Reiter, R. J. (2011). Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51, 1–16. doi: 10.1111/j.1600-079X.2011.00916.x
Go, Y. M., Chandler, J. D., and Jones, D. P. (2015). The cysteine proteome. Free Radic. Biol. Med. 84, 227–245. doi: 10.1016/j.freeradbiomed.2015.03.022
Gomis, P., Graftieaux, J. P., Sercombe, R., Hettler, D., Scherpereel, B., and Rousseaux, P. (2010). Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J. Neurosurg. 112, 681–688. doi: 10.3171/2009.4.JNS081377
Grobelny, B. T., Ducruet, A. F., DeRosa, P. A., Kotchetkov, I. S., Zacharia, B. E., Hickman, Z. L., et al. (2011). Gain-of-function polymorphisms of cystathionine beta-synthase and delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J. Neurosurg. 115, 101–107. doi: 10.3171/2011.2.JNS101414
Gu, X., Zheng, C., Zheng, Q., Chen, S., Li, W., Shang, Z., et al. (2017). Salvianolic acid A attenuates early brain injury after subarachnoid hemorrhage in rats by regulating ERK/P38/Nrf2 signaling. Am. J. Transl. Res. 9, 5643–5652.
Guo, Z., Hu, Q., Xu, L., Guo, Z. N., Ou, Y., He, Y., et al. (2016). Lipoxin A4 reduces inflammation through formyl peptide receptor 2/p38 MAPK signaling pathway in subarachnoid hemorrhage rats. Stroke 47, 490–497. doi: 10.1161/STROKEAHA.115.011223
Han, Y., Su, J., Liu, X., Zhao, Y., Wang, C., and Li, X. (2017a). Naringin alleviates early brain injury after experimental subarachnoid hemorrhage by reducing oxidative stress and inhibiting apoptosis. Brain Res. Bull. 133, 42–50. doi: 10.1016/j.brainresbull.2016.12.008
Han, Y., Zhang, T., Su, J., Zhao, Y., and Chenchen, Wang, et al. (2017b). Apigenin attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage. J. Clin. Neurosci. 40, 157–162. doi: 10.1016/j.jocn.2017.03.003
Hänggi, D., Etminan, N., Macdonald, R., Steiger, H., Mayer, S., Aldrich, F., et al. (2015). NEWTON: nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage. Neurocrit. Care 23, 274–284. doi: 10.1007/s12028-015-0112-2
Hao, S., Song, C., Shang, L., Yu, J., Qiao, T., and Li, K. (2016). Phosphorylation of Akt by SC79 prevents iron accumulation and ameliorates early brain injury in a model of experimental subarachnoid hemorrhage. Molecules 21:325. doi: 10.3390/molecules21030325
Hartings, J. A., York, J., Carroll, C. P., Hinzman, J. M., Mahoney, E., Krueger, B., et al. (2017). Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain 140, 2673–2690. doi: 10.1093/brain/awx214
Hayman, E. G., Patel, A. P., James, R. F., and Simard, J. M. (2017). Heparin and heparin-derivatives in post-subarachnoid hemorrhage brain injury: a multimodal therapy for a multimodal disease. Molecules 22:724. doi: 10.3390/molecules22050724
Hu, Q., Li, T., Wang, L., Xie, Y., Liu, S., Bai, X., et al. (2016). Neuroprotective effects of a smoothened receptor agonist against early brain injury after experimental subarachnoid hemorrhage in rats. Front. Cell. Neurosci. 10:306. doi: 10.3389/fncel.2016.00306
Huang, L., Hou, Y., Wang, L., Xu, X., Guan, Q., Li, X., et al. (2018). p38 inhibitor protects mitochondrial dysfunction by induction of DJ-1 mitochondrial translocation after subarachnoid hemorrhage. J. Mol. Neurosci. 66, 163–171. doi: 10.1007/s12031-018-1131-1
Huang, Y., Guo, Y., Huang, L., Fang, Y., Li, D., Liu, R., et al. (2021). Kisspeptin-54 attenuates oxidative stress and neuronal apoptosis in early brain injury after subarachnoid hemorrhage in rats via GPR54/ARRB2/AKT/GSK3β signaling pathway. Free Radic. Biol. Med. 171, 99–111. doi: 10.1016/j.freeradbiomed.2021.05.012
Jarocka-Karpowicz, I., Syta-Krzyżanowska, A., Kochanowicz, J., and Mariak, Z. D. (2020). Clinical prognosis for SAH Consistent with redox imbalance and lipid peroxidation. Molecules 25:1921. doi: 10.3390/molecules25081921
Jiang, W. C., Chen, C. M., Hamdin, C. D., Orekhov, A. N., Sobenin, I. A., Layne, M. D., et al. (2020). Therapeutic potential of heme oxygenase-1 in aneurysmal diseases. Antioxidants (Basel) 9:1150. doi: 10.3390/antiox9111150
Jing, C. H., Wang, L., Liu, P. P., Wu, C., Ruan, D., and Chen, G. (2012). Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience 213, 144–153. doi: 10.1016/j.neuroscience.2012.03.055
Jo, H., Mondal, S., Tan, D., Nagata, E., Takizawa, S., Sharma, A. K., et al. (2012). Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. U.S.A. 109, 10581–10586. doi: 10.1073/pnas.1202810109
Ju, Q., Li, X., Zhang, H., Yan, S., Li, Y., and Zhao, Y. (2020). NFE2L2 is a potential prognostic biomarker and is correlated with immune infiltration in brain lower grade glioma: a pan-cancer analysis. Oxid. Med. Cell. Longev. 2020:3580719. doi: 10.1155/2020/3580719
Jumnongprakhon, P., Govitrapong, P., Tocharus, C., Pinkaew, D., and Tocharus, J. (2015). Melatonin protects methamphetamine-induced neuroinflammation through NF-κB and Nrf2 pathways in glioma cell line. Neurochem. Res. 40, 1448–1456. doi: 10.1007/s11064-015-1613-2
Kamat, P. K., Ahmad, A. S., and Doré, S. (2019). Carbon monoxide attenuates vasospasm and improves neurobehavioral function after subarachnoid hemorrhage. Arch Biochem. Biophys. 676:108117. doi: 10.1016/j.abb.2019.108117
Kooijman, E., Nijboer, C. H., van Velthoven, C. T., Kavelaars, A., Kesecioglu, J., and Heijnen, C. J. (2014). The rodent endovascular puncture model of subarachnoid hemorrhage: mechanisms of brain damage and therapeutic strategies. J. Neuroinflammation 11:2. doi: 10.1186/1742-2094-11-2
Kusaka, G., Ishikawa, M., Nanda, A., Granger, D. N., and Zhang, J. H. (2004). Signaling pathways for early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 24, 916–925. doi: 10.1097/01.Wcb.0000125886.48838.7e
Lee, J. Y., Keep, R. F., He, Y., Sagher, O., Hua, Y., and Xi, G. (2010). Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J. Cereb. Blood Flow Metab. 30, 1793–1803. doi: 10.1038/jcbfm.2010.137
Li, Q., Chen, Y., Zhang, X., Zuo, S., Ge, H., Chen, Y., et al. (2016). Scutellarin attenuates vasospasm through the Erk5-KLF2-eNOS pathway after subarachnoid hemorrhage in rats. J. Clin. Neurosci. 34, 264–270. doi: 10.1016/j.jocn.2016.09.028
Li, T., Wang, L., Hu, Q., Liu, S., Bai, X., Xie, Y., et al. (2017). Neuroprotective roles of l-Cysteine in attenuating early brain injury and improving synaptic density via the CBS/H(2)S pathway following subarachnoid hemorrhage in rats. Front. Neurol. 8:176. doi: 10.3389/fneur.2017.00176
Li, X., Liu, W., Li, R., Guo, S., Fan, H., Wei, B., et al. (2020). TSG-6 attenuates oxidative stress-induced early brain injury in subarachnoid hemorrhage partly by the HO-1 and Nox2 Pathways. J. Stroke Cerebrovasc. Dis. 29:104986. doi: 10.1016/j.jstrokecerebrovasdis.2020.104986
Li, X., Zhao, L., Yue, L., Liu, H., Yang, X., Wang, X., et al. (2016). Evidence for the protective effects of curcumin against oxyhemoglobin-induced injury in rat cortical neurons. Brain Res. Bull. 120, 34–40. doi: 10.1016/j.brainresbull.2015.11.006
Li, Y., Liu, Y., Wu, P., Tian, Y., Liu, B., Wang, J., et al. (2021). Inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo via reduction of lipid peroxidation. Cell. Mol. Neurobiol. 41, 263–278. doi: 10.1007/s10571-020-00850-1
Liu, F., Chen, Y., Hu, Q., Li, B., Tang, J., He, Y., et al. (2015). MFGE8/Integrin β3 pathway alleviates apoptosis and inflammation in early brain injury after subarachnoid hemorrhage in rats. Exp. Neurol. 272, 120–127. doi: 10.1016/j.expneurol.2015.04.016
Liu, F., Hu, Q., Li, B., Manaenko, A., Chen, Y., Tang, J., et al. (2014). Recombinant milk fat globule-EGF factor-8 reduces oxidative stress via integrin β3/nuclear factor erythroid 2-related factor 2/heme oxygenase pathway in subarachnoid hemorrhage rats. Stroke 45, 3691–3697. doi: 10.1161/strokeaha.114.006635
Liu, H., Guo, W., Guo, H., Zhao, L., Yue, L., Li, X., et al. (2020). Bakuchiol attenuates oxidative stress and neuron damage by regulating Trx1/TXNIP and the phosphorylation of AMPK after subarachnoid hemorrhage in mice. Front. Pharmacol. 11:712. doi: 10.3389/fphar.2020.00712
Liu, H., Zhao, L., Yue, L., Wang, B., Li, X., Guo, H., et al. (2017). Pterostilbene attenuates early brain injury following subarachnoid hemorrhage via inhibition of the NLRP3 inflammasome and Nox2-related oxidative stress. Mol. Neurobiol. 54, 5928–5940. doi: 10.1007/s12035-016-0108-8
Lu, Y., Zhang, X. S., Zhou, X. M., Gao, Y. Y., Chen, C. L., Liu, J. P., et al. (2019). Peroxiredoxin 1/2 protects brain against H(2)O(2)-induced apoptosis after subarachnoid hemorrhage. FASEB J. 33, 3051–3062. doi: 10.1096/fj.201801150R
Luo, C., Yang, Q., Liu, Y., Zhou, S., Jiang, J., Reiter, R. J., et al. (2019). The multiple protective roles and molecular mechanisms of melatonin and its precursor N-acetylserotonin in targeting brain injury and liver damage and in maintaining bone health. Free Radic. Biol. Med. 130, 215–233. doi: 10.1016/j.freeradbiomed.2018.10.402
Macdonald, R., Kassell, N., Mayer, S., Ruefenacht, D., Schmiedek, P., Weidauer, S., et al. (2008). Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39, 3015–3021. doi: 10.1161/strokeaha.108.519942
Macdonald, R. L., Higashida, R. T., Keller, E., Mayer, S. A., Molyneux, A., Raabe, A., et al. (2011). Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 10, 618–625. doi: 10.1016/S1474-4422(11)70108-9
Macdonald, R. L., and Schweizer, T. A. (2017). Spontaneous subarachnoid haemorrhage. Lancet 389, 655–666. doi: 10.1016/s0140-6736(16)30668-7
Martinez-Cruz, F., Espinar, A., Pozo, D., Osuna, C., and Guerrero, J. M. (2002). Melatonin prevents focal rat cerebellum injury as assessed by induction of heat shock protein (HO-1) following subarachnoid injections of lysed blood. Neurosci. Lett. 331, 208–210. doi: 10.1016/s0304-3940(02)00884-4
Mayer, S., Aldrich, E., Bruder, N., Hmissi, A., Macdonald, R., Viarasilpa, T., et al. (2019). Thick and diffuse subarachnoid blood as a treatment effect modifier of clazosentan after subarachnoid hemorrhage. Stroke 50, 2738–2744. doi: 10.1161/strokeaha.119.025682
Mayor, F., Bilgin-Freiert, A., Connolly, M., Katsnelson, M., Dusick, J. R., Vespa, P., et al. (2013). Effects of remote ischemic preconditioning on the coagulation profile of patients with aneurysmal subarachnoid hemorrhage: a case-control study. Neurosurgery 73, 808–815; discussion 815. doi: 10.1227/NEU.0000000000000098
Mead, E. J., Maguire, J. J., Kuc, R. E., and Davenport, A. P. (2007). Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. Br. J. Pharmacol. 151, 1143–1153. doi: 10.1038/sj.bjp.0707295
Mo, J., Enkhjargal, B., Travis, Z. D., Zhou, K., Wu, P., Zhang, G., et al. (2019). AVE 0991 attenuates oxidative stress and neuronal apoptosis via Mas/PKA/CREB/UCP-2 pathway after subarachnoid hemorrhage in rats. Redox Biol. 20, 75–86. doi: 10.1016/j.redox.2018.09.022
Moro, M. A., Almeida, A., Bolanos, J. P., and Lizasoain, I. (2005). Mitochondrial respiratory chain and free radical generation in stroke. Free Radic. Biol. Med. 39, 1291–1304. doi: 10.1016/j.freeradbiomed.2005.07.010
Murphy, A. N., Fiskum, G., and Beal, M. F. (1999). Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J. Cereb. Blood Flow Metab. 19, 231–245. doi: 10.1097/00004647-199903000-00001
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. doi: 10.1042/BJ20081386
Naraoka, M., Matsuda, N., Shimamura, N., Asano, K., Akasaka, K., Takemura, A., et al. (2018). Long-acting statin for aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled trial. J. Cereb. Blood Flow Metab. 38, 1190–1198. doi: 10.1177/0271678X17724682
Ohsawa, I., Ishikawa, M., Takahashi, K., Watanabe, M., Nishimaki, K., Yamagata, K., et al. (2007). Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 13, 688–694. doi: 10.1038/nm1577
Otsuka, S., Setoyama, K., Takada, S., Nakanishi, K., Terashi, T., Norimatsu, K., et al. (2021). Preconditioning exercise in rats attenuates early brain injury resulting from subarachnoid hemorrhage by reducing oxidative stress, inflammation, and neuronal apoptosis. Mol. Neurobiol. 58, 5602–5617. doi: 10.1007/s12035-021-02506-7
Pan, J., Lao, L., Shen, J., Huang, S., Zhang, T., Fan, W., et al. (2021). Utility of serum NOX4 as a potential prognostic biomarker for aneurysmal subarachnoid hemorrhage. Clin. Chim. Acta 517, 9–14. doi: 10.1016/j.cca.2021.02.007
Pang, J., Peng, J., Matei, N., Yang, P., Kuai, L., Wu, Y., et al. (2018). Apolipoprotein E exerts a whole-brain protective property by promoting M1? Microglia quiescence after experimental subarachnoid hemorrhage in mice. Transl. Stroke Res. 9, 654–668. doi: 10.1007/s12975-018-0665-4
Pang, J., Peng, J., Yang, P., Kuai, L., Chen, L., Zhang, J. H., et al. (2019). White Matter injury in early brain injury after subarachnoid hemorrhage. Cell Transplant. 28, 26–35. doi: 10.1177/0963689718812054
Peng, J., Pang, J., Huang, L., Enkhjargal, B., Zhang, T., Mo, J., et al. (2019). LRP1 activation attenuates white matter injury by modulating microglial polarization through Shc1/PI3K/Akt pathway after subarachnoid hemorrhage in rats. Redox Biol. 21:101121. doi: 10.1016/j.redox.2019.101121
Petri, S., Kiaei, M., Damiano, M., Hiller, A., Wille, E., Manfredi, G., et al. (2006). Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J. Neurochem. 98, 1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x
Post, R., Germans, M. R., Tjerkstra, M. A., Vergouwen, M. D. I., Jellema, K., Koot, R. W., et al. (2021). Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet 397, 112–118. doi: 10.1016/S0140-6736(20)32518-6
Prentice, H., Modi, J. P., and Wu, J. Y. (2015). Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid. Med. Cell. Longev. 2015:964518. doi: 10.1155/2015/964518
Qian, X., Li, X., Shi, Z., Bai, X., Xia, Y., Zheng, Y., et al. (2019). KDM3A senses oxygen availability to regulate PGC-1alpha-mediated mitochondrial biogenesis. Mol. Cell 76, 885–895.e7. doi: 10.1016/j.molcel.2019.09.019
Qu, X. F., Liang, T. Y., Wu, D. G., Lai, N. S., Deng, R. M., Ma, C., et al. (2021). Acyl-CoA synthetase long chain family member 4 plays detrimental role in early brain injury after subarachnoid hemorrhage in rats by inducing ferroptosis. CNS Neurosci. Ther. 27, 449–463. doi: 10.1111/cns.13548
Reisman, S. A., Lee, C. Y., Meyer, C. J., Proksch, J. W., Sonis, S. T., and Ward, K. W. (2014). Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat. Res. 181, 512–520. doi: 10.1667/RR13578.1
Rojo, A. I., Pajares, M., Garcia-Yague, A. J., Buendia, I., Van Leuven, F., Yamamoto, M., et al. (2018). Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 18, 173–180. doi: 10.1016/j.redox.2018.07.006
Rowland, M. J., Hadjipavlou, G., Kelly, M., Westbrook, J., and Pattinson, K. T. (2012). Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br. J. Anaesth. 109, 315–329. doi: 10.1093/bja/aes264
Ru, X., Qu, J., Li, Q., Zhou, J., Huang, S., Li, W., et al. (2021). MiR-706 alleviates white matter injury via downregulating PKCα/MST1/NF-κB pathway after subarachnoid hemorrhage in mice. Exp. Neurol. 341:113688. doi: 10.1016/j.expneurol.2021.113688
Ruan, W., Hu, J., Zhou, H., Li, Y., Xu, C., Luo, Y., et al. (2020). Intranasal wnt-3a alleviates neuronal apoptosis in early brain injury post subarachnoid hemorrhage via the regulation of wnt target PPAN mediated by the moonlighting role of aldolase C. Neurochem. Int. 134:104656. doi: 10.1016/j.neuint.2019.104656
Rubio-Perez, C., Planas-Rigol, E., Trincado, J., Bonfill-Teixidor, E., Arias, A., Marchese, D., et al. (2021). Immune cell profiling of the cerebrospinal fluid enables the characterization of the brain metastasis microenvironment. Nat. Commun. 12:1503. doi: 10.1038/s41467-021-21789-x
Sasaki, T., Kasuya, H., Onda, H., Sasahara, A., Goto, S., Hori, T., et al. (2004). Role of p38 mitogen-activated protein kinase on cerebral vasospasm after subarachnoid hemorrhage. Stroke 35, 1466–1470. doi: 10.1161/01.STR.0000127425.47266.20
Schupper, A., Eagles, M., Neifert, S., Mocco, J., and Macdonald, R. (2020). Lessons from the CONSCIOUS-1 Study. J. Clin. Med. 9:2970. doi: 10.3390/jcm9092970
Sehba, F. A., Hou, J., Pluta, R. M., and Zhang, J. H. (2012). The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 97, 14–37. doi: 10.1016/j.pneurobio.2012.02.003
Shao, A., Lin, D., Wang, L., Tu, S., Lenahan, C., and Zhang, J. (2020). Oxidative stress at the crossroads of aging, stroke and depression. Aging Dis. 11, 1537–1566. doi: 10.14336/ad.2020.0225
Shao, A., Wu, H., Hong, Y., Tu, S., Sun, X., Wu, Q., et al. (2016). Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol. Neurobiol. 53, 3462–3476. doi: 10.1007/s12035-015-9242-y
Shao, J., Wu, Q., Lv, S. Y., Zhou, X. M., Zhang, X. S., Wen, L. L., et al. (2019). Allicin attenuates early brain injury after experimental subarachnoid hemorrhage in rats. J. Clin. Neurosci. 63, 202–208. doi: 10.1016/j.jocn.2019.01.024
Shen, R., Zhou, J., Li, G., Chen, W., Zhong, W., and Chen, Z. (2020). SS31 attenuates oxidative stress and neuronal apoptosis in early brain injury following subarachnoid hemorrhage possibly by the mitochondrial pathway. Neurosci. Lett. 717:134654. doi: 10.1016/j.neulet.2019.134654
Shi, G., Cui, L., Chen, R., Liang, S., Wang, C., and Wu, P. (2020). TT01001 attenuates oxidative stress and neuronal apoptosis by preventing mitoNEET-mediated mitochondrial dysfunction after subarachnoid hemorrhage in rats. Neuroreport 31, 845–850. doi: 10.1097/wnr.0000000000001492
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037
Sies, H., and Jones, D. P. (2020). Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383. doi: 10.1038/s41580-020-0230-3
Song, H., Yuan, S., Zhang, Z., Zhang, J., Zhang, P., Cao, J., et al. (2019). Sodium/hydrogen exchanger 1 participates in early brain injury after subarachnoid hemorrhage both in vivo and in vitro via promoting neuronal apoptosis. Cell Transplant. 28, 985–1001. doi: 10.1177/0963689719834873
Song, S., Chen, Y., Han, F., Dong, M., Xiang, X., Sui, J., et al. (2018). Aloperine activates the Nrf2-ARE pathway when ameliorating early brain injury in a subarachnoid hemorrhage model. Exp. Ther. Med. 15, 3847–3855. doi: 10.3892/etm.2018.5896
Sq, G., Jq, L., Yl, H., Qz, D., Wd, Z., Hj, D., et al. (2020). Neuroprotective role of glutathione peroxidase 4 in experimental subarachnoid hemorrhage models. Life Sci. 257:118050. doi: 10.1016/j.lfs.2020.118050
Sun, B., Yang, S., Li, S., and Hang, C. (2018). Melatonin upregulates nuclear factor erythroid-2 related factor 2 (Nrf2) and mediates mitophagy to protect against early brain injury after subarachnoid hemorrhage. Med. Sci. Monit. 24, 6422–6430. doi: 10.12659/msm.909221
Sun, C., Enkhjargal, B., Reis, C., Zhang, T., Zhu, Q., Zhou, K., et al. (2019). Osteopontin-enhanced autophagy attenuates early brain injury via FAK-ERK pathway and improves long-term outcome after subarachnoid hemorrhage in rats. Cells 8:980. doi: 10.3390/cells8090980
Sun, C. M., Enkhjargal, B., Reis, C., Zhou, K. R., Xie, Z. Y., Wu, L. Y., et al. (2019). Osteopontin attenuates early brain injury through regulating autophagy-apoptosis interaction after subarachnoid hemorrhage in rats. CNS Neurosci. Ther. 25, 1162–1172. doi: 10.1111/cns.13199
Takeuchi, S., Kumagai, K., Toyooka, T., Otani, N., Wada, K., and Mori, K. (2021). Intravenous hydrogen therapy with intracisternal magnesium sulfate infusion in severe aneurysmal subarachnoid hemorrhage. Stroke 52, 20–27. doi: 10.1161/STROKEAHA.120.031260
Tanaka, N., Ikeda, Y., Ohta, Y., Deguchi, K., Tian, F., Shang, J., et al. (2011). Expression of Keap1-Nrf2 system and antioxidative proteins in mouse brain after transient middle cerebral artery occlusion. Brain Res. 1370, 246–253. doi: 10.1016/j.brainres.2010.11.010
Tomar, A., Vasisth, S., Khan, S. I., Malik, S., Nag, T. C., Arya, D. S., et al. (2017). Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine 34, 154–161. doi: 10.1016/j.phymed.2017.05.007
Toyota, Y., Shishido, H., Ye, F., Koch, L. G., Britton, S. L., Garton, H. J. L., et al. (2021). Hydrocephalus following experimental subarachnoid hemorrhage in rats with different aerobic capacity. Int. J. Mol. Sci. 22:4489. doi: 10.3390/ijms22094489
Tsai, T. H., Lin, S. H., Wu, C. H., Tsai, Y. C., Yang, S. F., and Lin, C. L. (2020). Mechanisms and therapeutic implications of RTA 408, an activator of Nrf2, in subarachnoid hemorrhage-induced delayed cerebral vasospasm and secondary brain injury. PLoS One 15:e0240122. doi: 10.1371/journal.pone.0240122
Tu, T., Yin, S., Pang, J., Zhang, X., Zhang, L., Zhang, Y., et al. (2021). Irisin contributes to neuroprotection by promoting mitochondrial biogenesis after experimental subarachnoid hemorrhage. Front. Aging Neurosci. 13:640215. doi: 10.3389/fnagi.2021.640215
Vecchione, C., Frati, A., Di Pardo, A., Cifelli, G., Carnevale, D., Gentile, M. T., et al. (2009). Tumor necrosis factor-alpha mediates hemolysis-induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension 54, 150–156. doi: 10.1161/hypertensionaha.108.128124
Wang, J., and Doré, S. (2007). Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 130, 1643–1652. doi: 10.1093/brain/awm095
Wang, J., Zuo, Y., Zhuang, K., Luo, K., Yan, X., Li, J., et al. (2020). Recombinant human milk fat globule-epidermal growth factor 8 attenuates microthrombosis after subarachnoid hemorrhage in rats. J. Stroke Cerebrovasc. Dis. 29:104536. doi: 10.1016/j.jstrokecerebrovasdis.2019.104536
Wang, T., Xu, L., Gao, L., Zhao, L., Liu, X. H., Chang, Y. Y., et al. (2020). Paeoniflorin attenuates early brain injury through reducing oxidative stress and neuronal apoptosis after subarachnoid hemorrhage in rats. Metab. Brain Dis. 35, 959–970. doi: 10.1007/s11011-020-00571-w
Wang, X., Li, S., Ma, J., Wang, C., Chen, A., Xin, Z., et al. (2019). Effect of gastrodin on early brain injury and neurological outcome after subarachnoid hemorrhage in rats. Neurosci. Bull. 35, 461–470. doi: 10.1007/s12264-018-00333-w
Wang, Y., Gao, A., Xu, X., Dang, B., You, W., Li, H., et al. (2015). The neuroprotection of lysosomotropic agents in experimental subarachnoid hemorrhage probably involving the apoptosis pathway triggering by cathepsins via chelating intralysosomal iron. Mol. Neurobiol. 52, 64–77. doi: 10.1007/s12035-014-8846-y
Wang, Z., Chen, G., Zhu, W. W., and Zhou, D. (2010). Activation of nuclear factor-erythroid 2-related factor 2 (Nrf2) in the basilar artery after subarachnoid hemorrhage in rats. Ann. Clin. Lab. Sci. 40, 233–239.
Wang, Z., Ji, C., Wu, L., Qiu, J., Li, Q., Shao, Z., et al. (2014). Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS One 9:e97685. doi: 10.1371/journal.pone.0097685
Wang, Z., Ma, C., Meng, C. J., Zhu, G. Q., Sun, X. B., Huo, L., et al. (2012). Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J. Pineal Res. 53, 129–137. doi: 10.1111/j.1600-079X.2012.00978.x
Wang, Z., Wu, L., You, W., Ji, C., and Chen, G. (2013). Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4-mediated inflammatory pathway. J. Pineal Res. 55, 399–408. doi: 10.1111/jpi.12087
Wu, H. J., Wu, C., Niu, H. J., Wang, K., Mo, L. J., Shao, A. W., et al. (2017). Neuroprotective mechanisms of melatonin in hemorrhagic stroke. Cell. Mol. Neurobiol. 37, 1173–1185. doi: 10.1007/s10571-017-0461-9
Wu, L., Su, Z., Zha, L., Zhu, Z., Liu, W., Sun, Y., et al. (2019). Tetramethylpyrazine nitrone reduces oxidative stress to alleviate cerebral vasospasm in experimental subarachnoid hemorrhage models. Neuromolecular Med. 21, 262–274. doi: 10.1007/s12017-019-08543-9
Wu, L. Y., Enkhjargal, B., Xie, Z. Y., Travis, Z. D., Sun, C. M., Zhou, K. R., et al. (2020). Recombinant OX40 attenuates neuronal apoptosis through OX40-OX40L/PI3K/AKT signaling pathway following subarachnoid hemorrhage in rats. Exp. Neurol. 326:113179. doi: 10.1016/j.expneurol.2020.113179
Wu, P., Li, Y., Zhu, S., Wang, C., Dai, J., Zhang, G., et al. (2017). Mdivi-1 alleviates early brain injury after experimental subarachnoid hemorrhage in rats, possibly via inhibition of Drp1-activated mitochondrial fission and oxidative stress. Neurochem. Res. 42, 1449–1458. doi: 10.1007/s11064-017-2201-4
Wu, Q., Zhang, X. S., Wang, H. D., Zhang, X., Yu, Q., Li, W., et al. (2014). Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar. Drugs 12, 6125–6141. doi: 10.3390/md12126125
Wu, Y., Peng, J., Pang, J., Sun, X., and Jiang, Y. (2017). Potential mechanisms of white matter injury in the acute phase of experimental subarachnoid haemorrhage. Brain 140:e36. doi: 10.1093/brain/awx084
Xie, Y., Guo, H., Wang, L., Xu, L., Zhang, X., Yu, L., et al. (2017). Human albumin attenuates excessive innate immunity via inhibition of microglial Mincle/Syk signaling in subarachnoid hemorrhage. Brain Behav. Immun. 60, 346–360. doi: 10.1016/j.bbi.2016.11.004
Xie, Y. K., Zhou, X., Yuan, H. T., Qiu, J., Xin, D. Q., Chu, X. L., et al. (2019). Resveratrol reduces brain injury after subarachnoid hemorrhage by inhibiting oxidative stress and endoplasmic reticulum stress. Neural Regen. Res. 14, 1734–1742. doi: 10.4103/1673-5374.257529
Xie, Z., Huang, L., Enkhjargal, B., Reis, C., Wan, W., Tang, J., et al. (2018). Recombinant Netrin-1 binding UNC5B receptor attenuates neuroinflammation and brain injury via PPARγ/NFκB signaling pathway after subarachnoid hemorrhage in rats. Brain Behav. Immun. 69, 190–202. doi: 10.1016/j.bbi.2017.11.012
Xiong, Y., Xin, D. Q., Hu, Q., Wang, L. X., Qiu, J., Yuan, H. T., et al. (2020). Neuroprotective mechanism of L-cysteine after subarachnoid hemorrhage. Neural Regen. Res. 15, 1920–1930. doi: 10.4103/1673-5374.280321
Xu, H., Cai, Y., Yu, M., Sun, J., Cai, J., Li, J., et al. (2021). Celastrol protects against early brain injury after subarachnoid hemorrhage in rats through alleviating blood-brain barrier disruption and blocking necroptosis. Aging (Albany NY) 13, 16816–16833. doi: 10.18632/aging.203221
Xu, P., Tao, C., Zhu, Y., Wang, G., Kong, L., Li, W., et al. (2021). TAK1 mediates neuronal pyroptosis in early brain injury after subarachnoid hemorrhage. J. Neuroinflammation 18:188. doi: 10.1186/s12974-021-02226-8
Xu, W., Gao, L., Zheng, J., Li, T., Shao, A., Reis, C., et al. (2018). The roles of MicroRNAs in stroke: possible therapeutic targets. Cell Transplant. 27, 1778–1788. doi: 10.1177/0963689718773361
Xu, W., Li, F., Liu, Z., Xu, Z., Sun, B., Cao, J., et al. (2017). MicroRNA-27b inhibition promotes Nrf2/ARE pathway activation and alleviates intracerebral hemorrhage-induced brain injury. Oncotarget 8, 70669–70684. doi: 10.18632/oncotarget.19974
Xu, W., Li, T., Gao, L., Zheng, J., Yan, J., Zhang, J., et al. (2019). Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J. Neuroinflammation 16:247. doi: 10.1186/s12974-019-1620-3
Xu, W., Yan, J., Ocak, U., Lenahan, C., Shao, A., Tang, J., et al. (2021). Melanocortin 1 receptor attenuates early brain injury following subarachnoid hemorrhage by controlling mitochondrial metabolism via AMPK/SIRT1/PGC-1α pathway in rats. Theranostics 11, 522–539. doi: 10.7150/thno.49426
Xue, Y., Nie, D., Wang, L. J., Qiu, H. C., Ma, L., Dong, M. X., et al. (2021). Microglial polarization: novel therapeutic strategy against ischemic stroke. Aging Dis. 12, 466–479. doi: 10.14336/ad.2020.0701
Yan, H., Zhang, D., Hao, S., Li, K., and Hang, C. H. (2015). Role of mitochondrial calcium uniporter in early brain injury after experimental subarachnoid hemorrhage. Mol. Neurobiol. 52, 1637–1647. doi: 10.1007/s12035-014-8942-z
Yang, C., Li, T., Xue, H., Wang, L., Deng, L., Xie, Y., et al. (2018). Inhibition of necroptosis rescues SAH-induced synaptic impairments in hippocampus via CREB-BDNF pathway. Front. Neurosci. 12:990. doi: 10.3389/fnins.2018.00990
Yang, C., Zhang, X., Fan, H., and Liu, Y. (2009). Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 1282, 133–141. doi: 10.1016/j.brainres.2009.05.009
Yang, S., Chen, X., Li, S., Sun, B., and Hang, C. (2018). Melatonin treatment regulates SIRT3 expression in early brain injury (EBI) due to reactive oxygen species (ROS) in a mouse model of subarachnoid hemorrhage (SAH). Med. Sci. Monit. 24, 3804–3814. doi: 10.12659/msm.907734
Yang, Y., Chen, S., and Zhang, J. M. (2017). The updated role of oxidative stress in subarachnoid hemorrhage. Curr. Drug Deliv. 14, 832–842. doi: 10.2174/1567201813666161025115531
Yatsushige, H., Ostrowski, R. P., Tsubokawa, T., Colohan, A., and Zhang, J. H. (2007). Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J. Neurosci. Res. 85, 1436–1448. doi: 10.1002/jnr.21281
Ye, Z. N., Wu, L. Y., Liu, J. P., Chen, Q., Zhang, X. S., Lu, Y., et al. (2018). Inhibition of leukotriene B4 synthesis protects against early brain injury possibly via reducing the neutrophil-generated inflammatory response and oxidative stress after subarachnoid hemorrhage in rats. Behav. Brain Res. 339, 19–27. doi: 10.1016/j.bbr.2017.11.011
Yu, Y. P., Chi, X. L., and Liu, L. J. (2014). A hypothesis: hydrogen sulfide might be neuroprotective against subarachnoid hemorrhage induced brain injury. ScientificWorldJournal 2014:432318. doi: 10.1155/2014/432318
Yuan, B., Zhou, X. M., You, Z. Q., Xu, W. D., Fan, J. M., Chen, S. J., et al. (2020). Inhibition of AIM2 inflammasome activation alleviates GSDMD-induced pyroptosis in early brain injury after subarachnoid haemorrhage. Cell Death Dis. 11:76. doi: 10.1038/s41419-020-2248-z
Yuan, S., Yu, Z., Zhang, Z., Zhang, J., Zhang, P., Li, X., et al. (2019). RIP3 participates in early brain injury after experimental subarachnoid hemorrhage in rats by inducing necroptosis. Neurobiol. Dis. 129, 144–158. doi: 10.1016/j.nbd.2019.05.004
Zeyu, Z., Yuanjian, F., Cameron, L., and Sheng, C. (2021). The role of immune inflammation in aneurysmal subarachnoid hemorrhage. Exp. Neurol. 336:113535. doi: 10.1016/j.expneurol.2020.113535
Zhan, C. P., Zhuge, C. J., Yan, X. J., Dai, W. M., and Yu, G. F. (2021). Measuring serum melatonin concentrations to predict clinical outcome after aneurysmal subarachnoid hemorrhage. Clin. Chim. Acta 513, 1–5. doi: 10.1016/j.cca.2020.12.006
Zhang, C. S., Han, Q., Song, Z. W., Jia, H. Y., Shao, T. P., and Chen, Y. P. (2021). Hydrogen gas post-conditioning attenuates early neuronal pyroptosis in a rat model of subarachnoid hemorrhage through the mitoKATP signaling pathway. Exp. Ther. Med. 22:836. doi: 10.3892/etm.2021.10268
Zhang, L., Wu, J., Duan, X., Tian, X., Shen, H., Sun, Q., et al. (2016c). Oxidase: a potential target for treatment of stroke. Oxid. Med. Cell. Longev. 2016:5026984. doi: 10.1155/2016/5026984
Zhang, D., Zhang, H., Hao, S., Yan, H., Zhang, Z., Hu, Y., et al. (2016a). Akt specific activator SC79 protects against early brain injury following subarachnoid hemorrhage. ACS Chem. Neurosci. 7, 710–718. doi: 10.1021/acschemneuro.5b00306
Zhang, L., Kong, X. J., Wang, Z. Q., Xu, F. S., and Zhu, Y. T. A. (2016b). Study on neuroprotective effects of curcumin on the diabetic rat brain. J. Nutr. Health Aging 20, 835–840. doi: 10.1007/s12603-016-0723-0
Zhang, H. B., Tu, X. K., Chen, Q., and Shi, S. S. (2019). Propofol reduces inflammatory brain injury after subarachnoid hemorrhage: involvement of PI3K/Akt pathway. J. Stroke Cerebrovasc. Dis. 28:104375. doi: 10.1016/j.jstrokecerebrovasdis.2019.104375
Zhang, K., Cheng, H., Song, L., and Wei, W. (2020). Inhibition of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)/sirtuin 3 (SIRT3) pathway aggravates oxidative stress after experimental subarachnoid hemorrhage. Med. Sci. Monit. 26:e923688. doi: 10.12659/msm.923688
Zhang, L., Li, Z., Feng, D., Shen, H., Tian, X., Li, H., et al. (2017). Involvement of Nox2 and Nox4 NADPH oxidases in early brain injury after subarachnoid hemorrhage. Free Radic. Res. 51, 316–328. doi: 10.1080/10715762.2017.1311015
Zhang, T., Su, J., Guo, B., Zhu, T., Wang, K., and Li, X. (2014a). Ursolic acid alleviates early brain injury after experimental subarachnoid hemorrhage by suppressing TLR4-mediated inflammatory pathway. Int. Immunopharmacol. 23, 585–591. doi: 10.1016/j.intimp.2014.10.009
Zhang, T., Su, J., Wang, K., Zhu, T., and Li, X. (2014b). Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci. Lett. 579, 12–17. doi: 10.1016/j.neulet.2014.07.005
Zhang, T., Wu, P., Budbazar, E., Zhu, Q., Sun, C., Mo, J., et al. (2019). Mitophagy reduces oxidative stress via Keap1 (kelch-like epichlorohydrin-associated protein 1)/Nrf2 (nuclear factor-E2-related factor 2)/PHB2 (prohibitin 2) pathway after subarachnoid hemorrhage in rats. Stroke 50, 978–988. doi: 10.1161/strokeaha.118.021590
Zhang, T., Wu, P., Zhang, J. H., Li, Y., Xu, S., Wang, C., et al. (2018). Docosahexaenoic acid alleviates oxidative stress-based apoptosis via improving mitochondrial dynamics in early brain injury after subarachnoid hemorrhage. Cell. Mol. Neurobiol. 38, 1413–1423. doi: 10.1007/s10571-018-0608-3
Zhang, T., Zuo, G., and Zhang, H. (2021). GPR18 agonist resolvin D2 reduces early brain injury in a rat model of subarachnoid hemorrhage by multiple protective mechanisms. Cell. Mol. Neurobiol. doi: 10.1007/s10571-021-01114-2 [Epub ahead of print].
Zhang, X., Lu, Y., Wu, Q., Dai, H., Li, W., Lv, S., et al. (2019). Astaxanthin mitigates subarachnoid hemorrhage injury primarily by increasing sirtuin 1 and inhibiting the Toll-like receptor 4 signaling pathway. FASEB J. 33, 722–737. doi: 10.1096/fj.201800642RR
Zhang, X., Wu, Q., Lu, Y., Wan, J., Dai, H., Zhou, X., et al. (2018). Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radic. Biol. Med. 124, 504–516. doi: 10.1016/j.freeradbiomed.2018.06.035
Zhang, X. S., Lu, Y., Tao, T., Wang, H., Liu, G. J., Liu, X. Z., et al. (2020). Fucoxanthin mitigates subarachnoid hemorrhage-induced oxidative damage via sirtuin 1-dependent pathway. Mol. Neurobiol. 57, 5286–5298. doi: 10.1007/s12035-020-02095-x
Zhang, X. S., Wu, Q., Wu, L. Y., Ye, Z. N., Jiang, T. W., Li, W., et al. (2016). Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 7:e2416. doi: 10.1038/cddis.2016.292
Zhang, X. S., Zhang, X., Zhou, M. L., Zhou, X. M., Li, N., Li, W., et al. (2014). Amelioration of oxidative stress and protection against early brain injury by astaxanthin after experimental subarachnoid hemorrhage. J. Neurosurg. 121, 42–54. doi: 10.3171/2014.2.Jns13730
Zhang, Z. Y., Jiang, M., Fang, J., Yang, M. F., Zhang, S., Yin, Y. X., et al. (2017). Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. Mol. Neurobiol. 54, 1–14. doi: 10.1007/s12035-015-9635-y
Zhang, Z. Y., Yang, M. F., Wang, T., Li, D. W., Liu, Y. L., Zhang, J. H., et al. (2015). Cysteamine alleviates early brain injury via reducing oxidative stress and apoptosis in a rat experimental subarachnoid hemorrhage model. Cell. Mol. Neurobiol. 35, 543–553. doi: 10.1007/s10571-014-0150-x
Zhao, H., Chen, Y., and Feng, H. (2018). P2X7 receptor-associated programmed cell death in the pathophysiology of hemorrhagic stroke. Curr. Neuropharmacol. 16, 1282–1295. doi: 10.2174/1570159X16666180516094500
Zhong, Y. W., Wu, J., Hu, H. L., Li, W. X., and Zhong, Y. (2016). Protective effect 3,4-dihydroxyphenylethanol in subarachnoid hemorrhage provoked oxidative neuropathy. Exp. Ther. Med. 12, 1908–1914. doi: 10.3892/etm.2016.3526
Zhu, Q., Enkhjargal, B., Huang, L., Zhang, T., Sun, C., Xie, Z., et al. (2018). Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-κB pathway after subarachnoid hemorrhage in rats. J. Neuroinflammation 15:178. doi: 10.1186/s12974-018-1211-8
Zhuang, K., Zuo, Y. C., Sherchan, P., Wang, J. K., Yan, X. X., and Liu, F. (2019). Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front. Neurosci. 13:1441. doi: 10.3389/fnins.2019.01441
Zhuang, Z., Zhou, M. L., You, W. C., Zhu, L., Ma, C. Y., Sun, X. J., et al. (2012). Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 13:47. doi: 10.1186/1471-2202-13-47
Zolnourian, A., Galea, I., and Bulters, D. (2019). Neuroprotective role of the Nrf2 pathway in subarachnoid haemorrhage and its therapeutic potential. Oxid. Med. Cell. Longev. 2019:6218239. doi: 10.1155/2019/6218239
Zuo, G., Zhang, T., Huang, L., Araujo, C., Peng, J., Travis, Z., et al. (2019). Activation of TGR5 with INT-777 attenuates oxidative stress and neuronal apoptosis via cAMP/PKCε/ALDH2 pathway after subarachnoid hemorrhage in rats. Free Radic. Biol. Med. 143, 441–453. doi: 10.1016/j.freeradbiomed.2019.09.002
Keywords: oxidative stress, subarachnoid hemorrhage, early brain injury, delayed cerebral ischemia, experimental – animal models
Citation: Lin F, Li R, Tu W-J, Chen Y, Wang K, Chen X and Zhao J (2021) An Update on Antioxidative Stress Therapy Research for Early Brain Injury After Subarachnoid Hemorrhage. Front. Aging Neurosci. 13:772036. doi: 10.3389/fnagi.2021.772036
Received: 07 September 2021; Accepted: 08 November 2021;
Published: 06 December 2021.
Edited by:
Li Li, Capital Medical University, ChinaReviewed by:
Christian Scheiwe, University Hospital Freiburg, GermanyMing Feng, Peking Union Medical College Hospital (CAMS), China
Zongjian Liu, Capital Medical University, China
Copyright © 2021 Lin, Li, Tu, Chen, Wang, Chen and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Chen, Y3hsX2JqdHRoQDE2My5jb20=; Jizong Zhao, emhhb2ppem9uZ0BianR0aC5vcmc=
†These authors have contributed equally to this work
 Fa Lin
Fa Lin Runting Li
Runting Li Wen-Jun Tu
Wen-Jun Tu Yu Chen
Yu Chen Ke Wang
Ke Wang Xiaolin Chen
Xiaolin Chen Jizong Zhao
Jizong Zhao