
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 17 December 2021
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.758817
This article is part of the Research Topic Sex Differences in Aging: A Cognitive and Behavioral Perspective View all 8 articles
 Heidi Foo1*
Heidi Foo1* Anbupalam Thalamuthu1
Anbupalam Thalamuthu1 Jiyang Jiang1
Jiyang Jiang1 Forrest Koch1
Forrest Koch1 Karen A. Mather1,2
Karen A. Mather1,2 Wei Wen1
Wei Wen1 Perminder S. Sachdev1,3
Perminder S. Sachdev1,3Age and sex associated with changes in the functional brain network topology and cognition in large population of older adults have been poorly understood. We explored this question further by examining differences in 11 resting-state graph theory measures with respect to age, sex, and their relationships with cognitive performance in 17,127 United Kingdom Biobank participants (mean = 62.83 ± 7.41 years). Age was associated with an overall decrease in the effectiveness of network communication (i.e., integration) and loss of functional specialization (i.e., segregation) of specific brain regions. Sex differences were also observed, with women showing more efficient networks, which were less segregated than in men (FDR adjusted p < 0.05). The age-related changes were also more apparent in men than in women, which suggests that men may be more vulnerable to cognitive decline with age. Interestingly, while network segregation and strength of limbic network were only nominally associated with cognitive performance, the network measures collectively were significantly associated with cognition (FDR adjusted p ≤ 0.002). This may imply that individual measures may be inadequate to capture much of the variance in the neural activity or its output and need further refinement. The complexity of the organization of the functional brain may be shaped by the age and sex of an individual, which ultimately may influence the cognitive performance of older adults. Age and sex stratification may be used to inform clinical neuroscience research to identify older adults at risk of cognitive dysfunction.
The brain is topographically organized into distinct networks. In the recent years, neuroscientists have examined networks to understand the brain function in preference to the classic study of specific brain regions. There are several approaches to mapping these brain networks, with one approach being resting-state functional magnetic resonance imaging (rs-fMRI). The rs-fMRI measures the spontaneous brain activity as low-frequency fluctuations in bold oxygen level-dependent (BOLD) signals and is used to understand the brain function (Wang J. et al., 2010). In network models of rs-fMRI data, functional brain networks are summarized into a collection of nodes (i.e., brain regions) and edges (i.e., magnitude of temporal correlation in fMRI activity between regions) (Rubinov and Sporns, 2010; Bertolero et al., 2018). This network model can then be used to study the global and local properties of the functional brain networks (Table 1). There is evidence that adult human brains are organized into groups of specialized functional networks that are able to respond to various cognitive demands (Wang J. et al., 2010). Therefore, studying the organization of functional networks in the aging brain may allow us to understand age-associated cognitive changes, even in the absence of a brain disease (Burke and Barnes, 2006; Otte et al., 2015).
A reorganization of the functional networks in the brain has been observed with aging and is also associated with changes in cognition (Betzel et al., 2014; Chan et al., 2014; Song et al., 2014; Geerligs et al., 2015; Zhang et al., 2016). Age-related alterations have been associated with a less efficient global network, decreased modularity, longer path lengths, and higher clustering coefficient, which may suggest a shift to more local organization in older age (Achard and Bullmore, 2007; Wang L. et al., 2010; Geerligs et al., 2015; Zhang et al., 2016). These changes in the topological functional network occurred most pronouncedly in regions important for cognition. For instance, high clustering coefficients in some frontal, temporal, and parietal regions were related to a lower performance in verbal and visual (VIS) memory functions (Sala-Llonch et al., 2014). Declines in the default mode network (DMN), which comprises the medial and lateral parietal, medial prefrontal, and medial and lateral temporal cortices (Raichle, 2015), are reported in aging brains and have been associated with memory consolidation (Murman, 2015). In addition, it has been observed that age has a mediating role in the correlation between local clustering coefficients and verbal memory learning scores (Sala-Llonch et al., 2014). Similarly, another cross-sectional study found that the relationship between aging and general decline in cognition could be mediated by changes in the functional connectivity measures such as path length (Bagarinao et al., 2019).
Previous studies have also shown sex differences in the organization of brain functional networks using graph theory measures. Men showed network segregation (i.e., specialized processing of the brain at a local level), whereas women showed more network integration (i.e., how rapidly the brain can integrate specialized information at a global network level) (Zhang et al., 2016). Another study observed that men had a higher clustering coefficient in the right hemisphere than in the left hemisphere (Tian et al., 2011), suggesting that men had greater specialization of the right hemisphere. In addition, age-related differences in the reorganization of the functional connectivity may also differ by sex, with men showing increasing between-network connectivity (Goldstone et al., 2016) while women exhibiting less age-related decreases in the DMN and LIMB network (Scheinost et al., 2015). It is noteworthy that age-related changes in cognition also differ by sex. For instance, a recent study has observed that while women had significantly higher baseline global memory, executive function, and memory performance than men, they showed significantly faster declines in the global memory and executive function (Levine et al., 2021). Another study found that older men had steeper rates of decline on measures of perceptuomotor speed and integration as well as visuospatial abilities (McCarrey et al., 2016). Taken together, the findings show that sex may influence age-related functional reorganization in the brain and that improving our understanding of this may shed light onto why some cognitive abilities differ substantially by sex (Ritchie et al., 2018).
There is evidence to show that changes in cognition may be due to the changes in the functional network connectivity. Segregated functional networks, for instance, seemed to be associated with better long-term episodic memory and fluid processing (Wig, 2017). However, there have been mixed findings regarding how resting-state functional connectivity (RSFC) differences relate to cognitive performance. One longitudinal study found the age-related decline of within-network connectivity in the DMN and executive control network but without associations with cognitive decline, whereas an association of between-network connectivity of the DMN and executive control network with processing speed was also observed (Ng et al., 2016). In contrast, another longitudinal study showed positive associations between within-network connectivity of the DMN and memory performance (Persson et al., 2014).
One previous cross-sectional study has investigated the functional network architecture of older adults with respect to age, sex, and cognitive performance (e.g., attention, episodic and working memory, executive function, and language) in a cohort of 722 participants with ages between 55 and 85 years (mean age of 67.1 years) (Stumme et al., 2020). They found RSFC reorganization with respect to age, particularly in the VIS and sensorimotor networks, which may suggest that these networks may mediate age-related differences in cognitive performance. In addition, the authors observed that men showed higher network integration, whereas women showed more segregation, which may possibly facilitate sex-related differences in cognitive performance.
This study aims to extend previous work by first examining age, sex, and cognitive function in association with functional network properties but in a much larger sample of 17,127 United Kingdom Biobank participants. Additionally, a more extensive range of graph theory measures, which assess the global and local properties as well as the strength of the network, are examined as summarized in Table 1. These measures are global efficiency, characteristic path length, Louvain modularity, transitivity, strength of default, DAN, frontoparietal, LIMB, salience, somatomotor (SM), and VIS networks, which are typically found to change with aging (Song et al., 2014), and are involved in multiple neuropathological processes (Lebedev et al., 2014; Khazaee et al., 2015; Munilla et al., 2017).
The data from 20,598 participants of European ancestry with rs-fMRI scans from the United Kingdom Biobank (aged between 44 and 80 years) (Sudlow et al., 2015) were accessed in March 2019. The imaging assessment took place at three different assessment centers: Manchester, Newcastle, and Reading, United Kingdom. This project was approved by the National Health Service National Research Ethics Service (approval letter dated June 17, 2011, ref. 11/NW/0382), project 10279. All data and materials are available via United Kingdom Biobank1.
All participants underwent an rs-fMRI scan on a Siemens Skyra 3T scanner (Siemens Medical Solutions, Erlangen, Germany). The rs-fMRI that was obtained using a blood oxygen level-dependent (BOLD) sequence and an echo-planar imaging (EPI) sequence (TR = 0.735 s, TE = 39 ms, FoV = 88 × 88 × 64, voxel resolution 2.4 mm × 2.4 mm × 2.4 mm) lasted for ∼6 min (for more details, see2). We analyzed the rs-fMRI data that were previously preprocessed by the United Kingdom Biobank (Alfaro-Almagro et al., 2018). The preprocessing steps involved motion correction, intensity normalization, smoothing [i.e., Gaussian kernel of full width at half maximum (FWHM), 5 mm], high-pass temporal filtering, EPI unwarping, and gradient distortion correction. Independent Component Analysis (ICA) + FMRIB’s ICA-based X-noiseifier (FIX) processing (Beckmann and Smith, 2004; Griffanti et al., 2014; Salimi-Khorshidi et al., 2014) was then used to remove structural artifacts. Participants with motion of > 2 mm/degree of translation/rotation were removed. After the image preprocessing and quality control, 18,500 participants remained.
The regions of interest (ROIs) used to construct the network properties were selected from the Schaefer atlas (Schaefer et al., 2018), corresponding to 100 cortical regions classified into seven resting-state networks, namely, frontoparietal control, DMN, DAN, salience ventral attention, LIMB, SM, and VIS networks (Figure 1 and Supplementary Table 1). 3dNetCorr command from the analysis of functional neuroimaging (Cox, 1996) was used to produce the network adjacency matrix for each participant. The mean time-series for each region was correlated with the mean time-series for all other regions and extracted for each participant. Furthermore, these time courses are used to estimate the size of signal fluctuation in each node, as well as to estimate the connectivity between pairs of nodes using L2-regularization (rho = 0.5 for ridge regression option in FSLNets). More details can be found in the study by Miller et al. (2016). A partial correlation, r, between all pairs of signals was computed to form a 100-by-100 (i.e., Schaefer atlas) connectivity matrix, which was then Fisher z-transformed. Self-connections and negative correlations were set to zero. As rs-fMRI can vary across magnitude, the use of undirected weighted matrices may provide a more comprehensive picture of the functional brain networks. The stronger the weights, the stronger the connections between nodes. In addition, we used an undirected graph because the rs-fMRI data do not permit inferences about the possible direction of information flow. However, the undirected graph is useful as it allows us to identify existing connections between specific pairs of network nodes (Fornito et al., 2016). Therefore, we used weighted undirected matrices in our study.
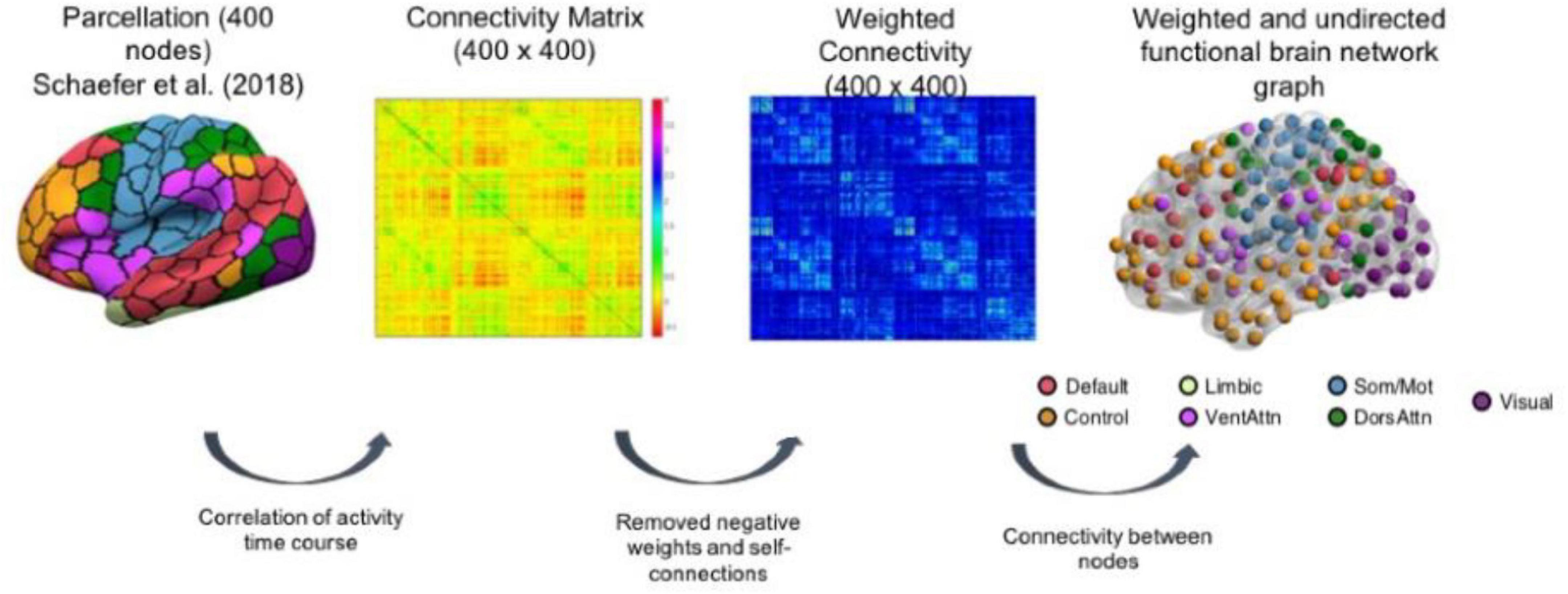
Figure 1. Schematic representation of brain network construction using Schaefer et al. (2018) parcellation to derive the weighted and undirected functional brain network graph. The figure is taken from Foo et al. (2021) https://www.nature.com/articles/s41598-021-94182-9.
All graph theory measures were derived using the Brain Connectivity Toolbox (Rubinov and Sporns, 2010). Functional integration can be assessed by global efficiency, which refers to the transmission of information at a global level, and characteristic path length, which is the average shortest distance between any two nodes in the network. To assess network segregation, which characterizes the specialized processing of the brain at a local level, we calculated the Louvain modularity and transitivity. Louvain modularity is a community detection method, which iteratively transforms the network into a set of communities, each consisting of a group of nodes. Higher modularity values indicate denser within-modular connections but sparer connections between nodes that are in different modules. Transitivity refers to the sum of all the clustering coefficients around each node in the network and is normalized collectively. Finally, strength (i.e., weighted degree) is described as the sum of all neighboring edge weights. High connectivity strength indicates a stronger connectivity between the regions, which provides an estimation of functional importance of each network. Subsequently, we averaged the two hemispheres to derive a value for each node and averaged within each network to derive a value for each of the 7 networks for strength measures. A total of eleven graph theory measures were used in the current study.
Cognitive assessments were administered on a touchscreen computer and were acquired at the imaging visit (instance 2). Seven tests from the United Kingdom Biobank battery of tests were selected to represent three cognitive domains (Kendall et al., 2017; Cox et al., 2019), namely, processing speed, memory, and executive function, in this study. All test scores were first z-transformed and then averaged to form domain scores. Processing speed domain included the following tests: “Reaction Time” (i.e., average time to correctly identify matches in a “snap”-like card game task), “Trail Making A” (i.e., time taken to complete a numeric path), and “Symbol Digit Substitution” (i.e., number of correct symbol number matches within the time limit). “numeric memory” (i.e., maximum number of digits remembered correctly) and “pairs matching” (i.e., number of incorrect visual matching) represented the memory domain, whereas “Trail Making B” (i.e., time taken to complete an alphanumeric path) and “fluid intelligence” (i.e., total number of questions that required logic and reasoning correctly answered) formed the executive function domain. Global cognition was computed by averaging the domain scores and z-transformed. After including those with cognition and graph theory data, the final sample in this study was 17,127 United Kingdom Biobank participants.
Statistical analyses were performed using the R software (version 4.0.0) (R Core Team, 2020). The graph theory measures were normalized using ranked transformed within the “rntransform” function in R from the GeneABEL package (Karssen et al., 2016), and age was z-transformed for the regression analysis. In line with the previous studies (Elliott et al., 2018), we controlled for imaging covariates, including head size, head motion from rs-fMRI, and volumetric scaling factor needed to normalize for head size, as well as scanning site and education. The network measures were residualized for imaging covariates and assessment center and used in all subsequent analyses.
To explore age effects and sex-related changes in the networks, a multiple linear regression that modeled the targeted property of networks as the dependent variable and age, age2, sex (female = 0, male = 1), years of education, and age-by-sex and age2-by-sex interactions as predictors was undertaken. In addition, separate multiple linear regressions were performed to study whether the network measures influenced cognitive functions (i.e., dependent variable) with covariates as in the previous model.
The multivariate analysis was carried out to further examine the joint effect of the network measures on cognitive functions after accounting for the same set of covariates in the univariate model. Since the network measures are correlated, we used the penalized regression analysis using the glmnet algorithm as implemented in the r package caret (Kuhn, 2015). The glmnet uses two penalty functions with tuning parameters to shrink the beta coefficients in the generalized linear model (glm). We used the elastic net glm model with default options to identify the optimum tuning parameter estimates. Network measures and the covariates with non-zero regression co-efficient in the training step was fit with the linear regression model. Likelihood ratio tests, p-values, and the incremental r-square were computed by comparing the model with network measures (i.e., full model), again a model with only the covariates (i.e., base model). False discovery rate-adjusted p-values were obtained by using Benjamini and Hochberg (1995) procedure as implemented in the R function p. adjust.
The current sample of 17,127 participants is a group of generally healthy middle-aged and older adults (range = 45.17–80.67 years, mean age = 62.83 ± 7.41 years) after including only samples with cognition and graph theory data. Of this sample, 9,037 were women and 8,090 were men, with an overall mean of 15.73 ± 4.74 years of education. Significant differences were observed for the demographics, graph theory measures, and memory scores between men and women (Table 2). Figure 2 shows the significant correlations between the network measures, except for transitivity, which was not significantly associated with any other measures.
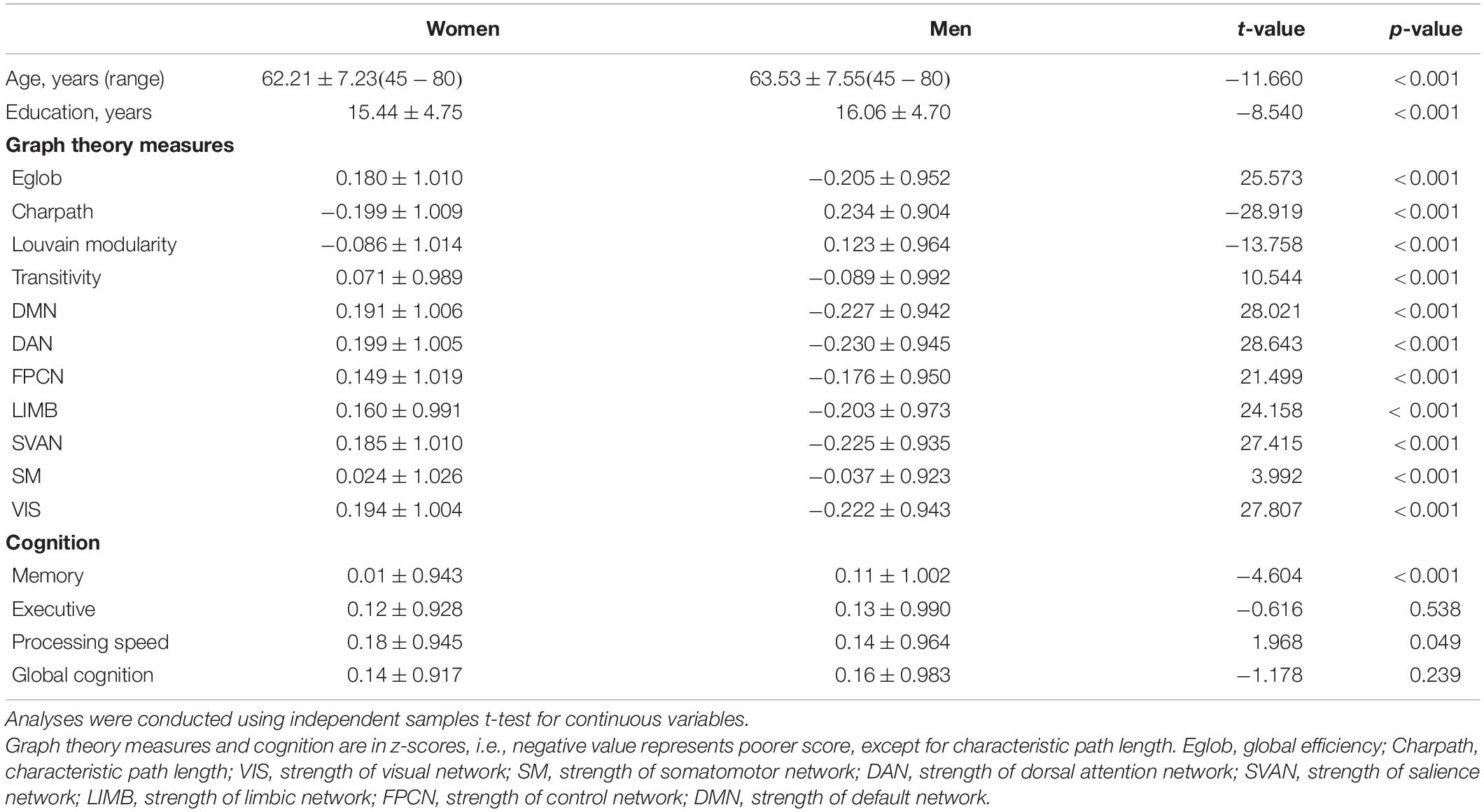
Table 2. United Kingdom Biobank sample characteristics and descriptive statistics (mean ± standard deviation) of graph theory measures and cognition measures in women and men.
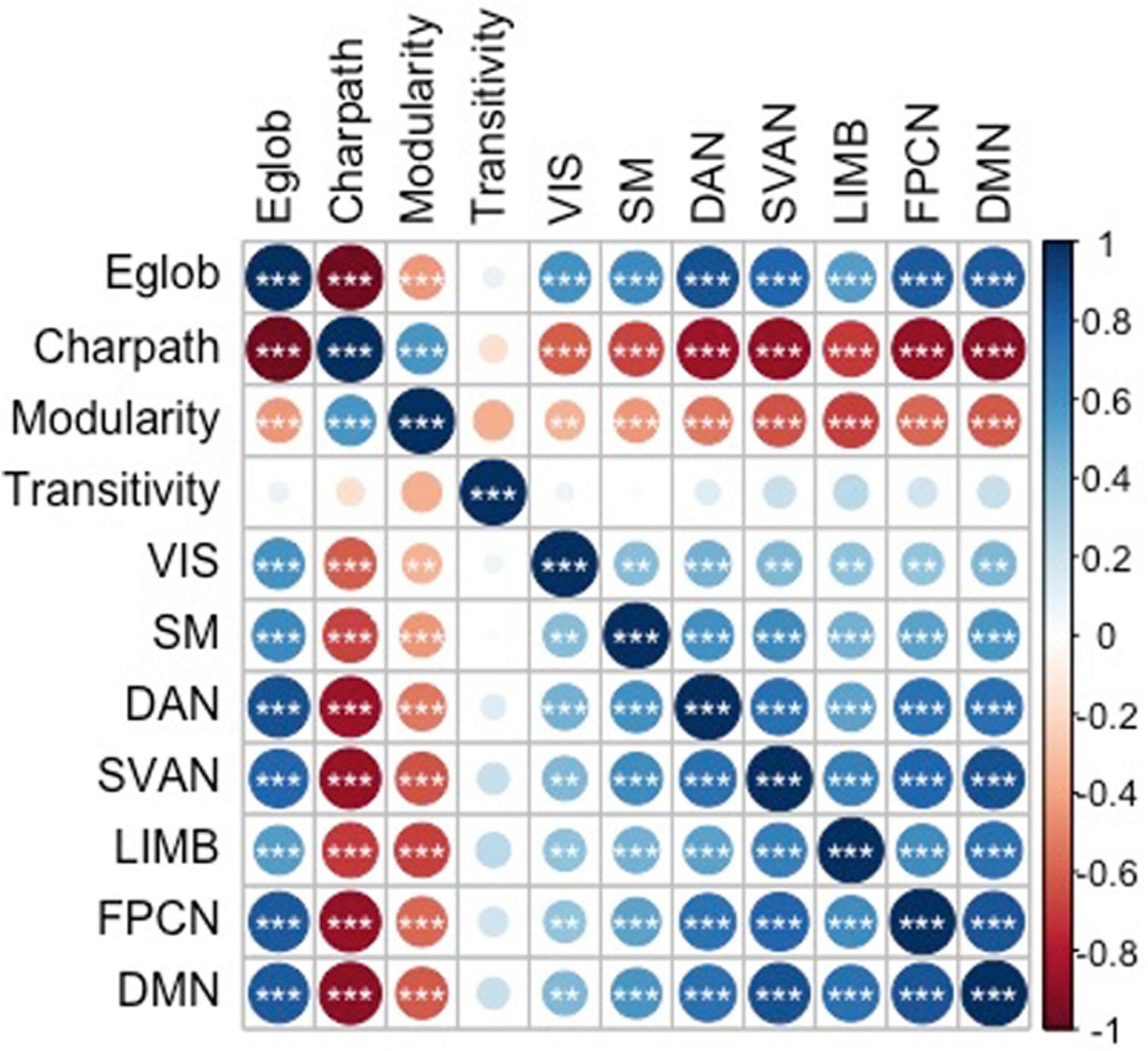
Figure 2. Correlations between the graph theory measures. * p < 0.05, ** p < 0.01, and *** p < 0.001. Eglob, global efficiency; Charpath, characteristic path length; VIS, strength of visual network; SM, strength of somatomotor network; DAN, strength of dorsal attention network; SVAN, strength of salience network; LIMB, strength of limbic network; FPCN, strength of control network; DMN, strength of default network. The figure is taken from Foo et al. (2021) https://www.nature.com/articles/s41598-021-94182-9.
Figure 3 and Table 3 summarize the results of age- and sex-related differences in the graph theory measures. Global efficiency, Louvain modularity, and strength of the networks decreased significantly with age, whereas characteristic path length and transitivity increased significantly with age. The only exceptions were that strength of default and salience networks were not significantly associated with age.
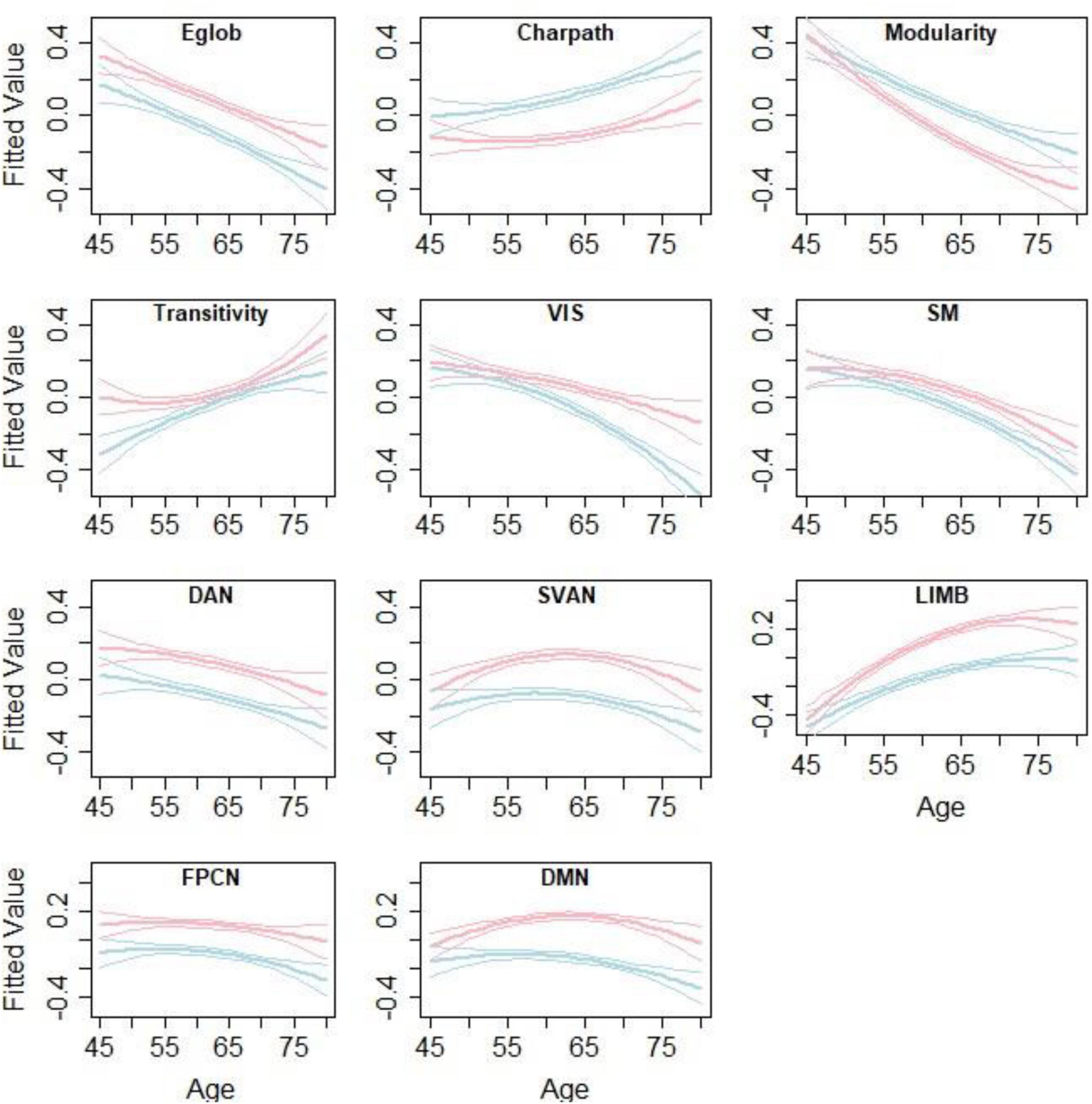
Figure 3. Age- and sex-related differences in the graph theory measures. Lines represent the fitted values for men (blue) and women (red) separately. The middle line shows the fitted equation evaluated at the mean value of education for each sex, while the top and lower lines represent confidence bands. The figure is taken from Foo et al. (2021) https://www.nature.com/articles/s41598-021-94182-9.
Sex was significantly associated with all measures, except for transitivity. Men appeared to have lower global efficiency, transitivity, and strengths of all the networks, as well as longer characteristic path lengths compared with those in women. Men showed increased Louvain modularity compared to women.
Age and sex interaction were negatively associated with Louvain modularity and strength of visual, limbic, and default networks. This implies that age-related changes in these measures were more apparent in males than females.
We examined the network influence on cognition after controlling for age, age2, sex, and education. Although none of these results would survive correction for multiple testing, we reported the results that were nominally significant. Louvain modularity showed positive associations with global cognition, whereas transitivity was negatively associated with memory. The strength of limbic network also showed negative associations with global cognition and memory (Supplementary Table 2).
We further examined this relationship to see if it was moderated by age and sex. However, none of the interaction effects between network measures and age or sex on cognition was significant (Supplementary Table 3).
Given the significant correlations between the network measures, we further investigated whether the joint effect of the network measures contributed to cognition after controlling for age, sex, and education variables. A summary of the results of each of the examined models are presented in Supplementary Table 4. We observed that while the R2 difference between the base model (age, age2, sex, and education) and the full model with the network measures was small, the joint effect of the network measures still significantly contributed to cognition (Table 4).
Changes to resting-state networks due to aging arguably reflect more fundamental alterations or adaptations at the general level of brain function (Jockwitz and Caspers, 2021). Graph theoretical approaches may be the most integrative way to investigate the RSFC as it studies the connectivity at both nodal and systems levels (Jockwitz and Caspers, 2021). Therefore, in this study, we examined the topological age and sex relationship with functional brain networks, using graph theory measures, and cognition. We observed that most functional brain network measures showed a decreasing strength of connectivity as well as reduced efficiency of communication and specialization between the networks with aging. However, the default mode and salience networks were an exception to this finding, with no significant results observed. In addition, there were significant sex differences in brain functional network topology where women showed greater efficiency of networks and network strength but less modularity than men. Further, age-related changes were more apparent in men than in women. Finally, the collective effect of the network measures contributed significantly to cognitive performance, with the highest correlation being with processing speed. However, no one network measure was significant after multiple testing adjustment.
We observed that global efficiency correlated negatively with age, whereas characteristic path length correlated positively with age, which was similar to a previous study (Sala-Llonch et al., 2014). This suggests an overall age-related decrease in the effectiveness of the communication between brain regions. In addition, the finding that modularity decreases with age has also been reported previously (Song et al., 2014). This implies that increasing age is associated with a less differentiated functional modular structure, which may be either due to the increase in between-network connections or the decrease in within-network connections or both (Chan et al., 2014; Song et al., 2014). At younger ages, functional brain networks are more segregated with every network being relatively specialized for distinct mental processes (Chan et al., 2014). The data suggest that there is some loss of functional specialization of specific brain networks as the brain ages (Goh, 2011), which may be important for cognitive reserve and compensation in older adults. Furthermore, we showed age-related decline in all of the other network strengths, excluding the DMN and salience network. However, results for other networks from previous studies are more complex. For instance, Betzel et al. (2014) found a within-network decline for higher order control and attention networks but stability for visual and somatomotor networks, while another study (Song et al., 2014) showed an increased global and local efficiency in the sensorimotor network in older compared with that in younger adults. Taken together, our data and others suggest age-related vulnerability in global network measures as well as specific network strengths.
Importantly, we did not observe any age-related decline in the DMN and salience network. Prior works suggest that within-network posterior DMN connectivity, including the angular gyrus, anterior cingulate cortex (ACC), precuneus, dorsal prefrontal, and inferior parietal lobe, decreases with age (Betzel et al., 2014; Chan et al., 2014; Song et al., 2014; Geerligs et al., 2015; Stumme et al., 2020). In contrast, within the older adult population, DMN as a whole remains relatively stable (Jones et al., 2011; Stumme et al., 2020). This finding is important as it shows that anterior-posterior DMN has differential vulnerability to age-related changes. Moreover, the salience network seems to remain relatively stable throughout the lifespan (Chan et al., 2014; Varangis et al., 2019) as well as in older age (Siman-Tov et al., 2017; Stumme et al., 2020). Interestingly, the DMN and salience network have also been implicated in age-related diseases such as Alzheimer’s disease (AD) and depression. One study observed that individuals with AD showed a moderate decrease of within-network DMN between the posterior cingulate cortex and right hippocampus as compared with healthy controls, but no differences were evident for whole-network DMN (Grieder et al., 2018). Further, compared with older adult controls, individuals with AD showed a significantly decreased within-network functional connectivity in the frontoinsular cortices and increased FC in medial prefrontal cortex in the salience network (He et al., 2014). Similarly, older adults with depression demonstrated higher within-network DMN in the left precuneus, subgenual ACC, ventromedial prefrontal cortex, and lateral parietal regions than controls (Alexopoulos et al., 2012). In addition, regarding the salience network, within-network bilateral anterior insula showed a decreased connectivity, but bilateral ACC showed an increased connectivity in middle-aged adults with depression compared with controls (Manoliu et al., 2014). These findings suggest that while the whole DMN may be preserved, within-network posterior DMN may be vulnerable to aging and aging-related diseases.
The topology of functional brain networks differed by sex. We detected significant sex effects on all the assessed graph theory measures. Consistent with results from Zhang et al. (2016), showing that female brains facilitated functional integration in young adults, we found that in older individuals, women indeed had a higher global efficiency and shorter characteristic path length than men. Similarly, congruent with previous findings, we also observed women had higher normalized clustering coefficients (i.e., transitivity) than men (Zhang et al., 2016). However, men exhibited stronger Louvain modularity, which suggests that there may be sex differences even within network segregation. It has previously been reported that women tend to exhibit overall higher within-network RSFC (Allen et al., 2011), which is consistent with our finding that women had higher network strengths than men. Similarly, consistent with previous findings that women show less age-related decreases in RSFC in the default and limbic network (Scheinost et al., 2015), we found that age-related changes in strengths of the limbic and default networks in addition to Louvain modularity and strength of visual network were more apparent in males than females. This suggests that aging-related changes in the functional brain network are different in the two sexes and that this difference may in part account for the differential vulnerability in cognitive decline between men and women.
The functional connectivity architecture in the brain has been associated with cognitive performance in older adults independent of age, sex, and education in this study. We observed that decreased Louvain modularity was nominally associated with a decline in global cognition. Individuals with less segregated networks exhibited poorest memory ability after controlling for age, which may suggest that network segregation may be an age-invariant marker of individual differences in cognition (Chan et al., 2014). However, we also saw that decreased transitivity was nominally associated with better memory performance. It is possible that different markers of network segregation have varying degrees and direction of influence on cognition. In addition, prior evidence from cognitive training interventions has shown that higher modularity at baseline in older adults was associated with greater cognitive training improvements, especially in sensory-motor processing (Gallen et al., 2016). Furthermore, given that the limbic network derived from the Schaefer parcellation comprises the orbitofrontal cortex and temporal pole, and these regions are associated with memory formation (Petrides et al., 2002) and executive function (Robinson et al., 2014), it supports our finding that the strength of the limbic network showed negative associations with memory and executive function. While there is nominal significance between individual network measures and cognition, the joint effect of all the network measures contributed significantly to cognition after accounting for age, sex, and education. This suggests that cognitive decline observed in older adults may be partially explained by independent changes in brain functional network organization. It also implies that individual network measures may be inadequate to capture much of the variance in neural activity and the functional output. Future studies are needed to combine various strategies to more holistically understand the network topology in relation to cognition.
The strengths of this study include a well-characterized large middle- and older-aged cohort, uniform imaging methods, the inclusion of a range of network measures associated with age and aging-related diseases, and the examination of a number of cognitive domains. This is the largest study of its kind thus far. However, limitations should also be considered. First, this study is cross-sectional, which precludes the ability to detect subtle changes in the functional brain topology over time within individuals. Second, while using weighted undirected matrix circumvents issues surrounding filtering/thresholding the connectivity matrix to maintain significant edge weights represented in a binary matrix, there are inherent difficulties associated with the interpretation of the results. As brain signals recorded from resting-state fMRI are typically noisy, it is possible that edge weights may be affected by non-neural contributions (De Vico Fallani et al., 2014). Despite this, with careful denoising of the resting-state fMRI data (Power et al., 2017; Parkes et al., 2018) and covarying for motion, it is possible to minimize the noise in the data. Given that we have performed motion correction and included it as a covariate as well as performed regularization on the imaging data, we are confident that the estimation of the partial correlation matrix derived for the subsequent analysis of the graph theory measures is valid. Moreover, while we were only interested in investigating the whole network functional connectivity, given the findings from DMN and salience network, it may be beneficial to look at individual nodes within the network to more holistically capture the nodal topology. One such way is to examine centrality measures including within-module degree and participation coefficient to understand the contribution of within/between network connections to network modularity and strength. Additionally, given that hemispheric lateralization may be important to the functional specialization in humans, future studies may investigate this further. Finally, given the principles of neurobiology, we assumed that network properties influence cognition and not the other way around. This question needs to be examined longitudinally to confirm the directionality of the relationship.
In conclusion, in this large population-based study, age was associated with decreased overall network integrity and specialized processing of the brain at a local level. Women had better functional network topology properties than men, with men tending to have denser within-network connections but sparser between-network connections. This work demonstrates the complexity of functional brain organization that is shaped by age, sex, and other factors, which ultimately may influence the cognitive performance of older adults. Given this significance, future fMRI studies should account for age and sex in the covariates. This work may also potentially distinguish between normal and pathological aging in the sexes.
The datasets presented in this article are not readily available because United Kingdom Biobank restriction. Requests to access the datasets should be directed to YWNjZXNzQHVrYmlvYmFuay5hYy51aw==.
The studies involving human participants were reviewed and approved by United Kingdom Biobank. The patients/participants provided their written informed consent to participate in this study.
HF devised the study. HF and AT jointly designed the analyses, analyzed the data, interpreted and wrote the manuscript with technical support and guidance with data interpretation from KM, WW, and PS. HF, AT, JJ, and FK contributed to the statistical concepts. PS supervised the work. All authors commented on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank UNSW Scientia Scholarship Programme for their support to HF. The authors report no biomedical financial interests or potential conflicts of interest. The authors gratefully acknowledge that this research has been conducted using the United Kingdom Biobank Resource (Application Number: 45262).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.758817/full#supplementary-material
Achard, S., and Bullmore, E. (2007). Efficiency and Cost of Economical Brain Functional Networks. PLoS Computat. Biol. 3:e17. doi: 10.1371/journal.pcbi.0030017
Alexopoulos, G. S., Hoptman, M. J., Kanellopoulos, D., Murphy, C. F., Lim, K. O., and Gunning, F. M. (2012). Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 139, 56–65. doi: 10.1016/j.jad.2011.12.002
Alfaro-Almagro, F., Jenkinson, M., Bangerter, N. K., Andersson, J. L. R., Griffanti, L., Douaud, G., et al. (2018). Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424. doi: 10.1016/j.neuroimage.2017.10.034
Allen, E., Erhardt, E., Damaraju, E., Gruner, W., Segall, J., Silva, R., et al. (2011). A Baseline for the Multivariate Comparison of Resting-State Networks. Front. Syst. Neurosci. 5:2. doi: 10.3389/fnsys.2011.00002
Bagarinao, E., Watanabe, H., Maesawa, S., Mori, D., Hara, K., Kawabata, K., et al. (2019). Reorganization of brain networks and its association with general cognitive performance over the adult lifespan. Sci. Rep. 9:11352. doi: 10.1038/s41598-019-47922-x
Beckmann, C. F., and Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152. doi: 10.1109/tmi.2003.822821
Benjamini, Y., and Hochberg, Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Statist. Soc. Ser. B 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bertolero, M. A., Yeo, B. T. T., Bassett, D. S., and D’Esposito, M. (2018). A mechanistic model of connector hubs, modularity and cognition. Nat. Hum. Behav. 2, 765–777. doi: 10.1038/s41562-018-0420-6
Betzel, R. F., Byrge, L., He, Y., Goni, J., Zuo, X. N., and Sporns, O. (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage 102(Pt 2), 345–357. doi: 10.1016/j.neuroimage.2014.07.067
Burke, S. N., and Barnes, C. A. (2006). Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40. doi: 10.1038/nrn1809
Chan, M. Y., Park, D. C., Savalia, N. K., Petersen, S. E., and Wig, G. S. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proc. Natl. Acad. Sci. 111:E4997. doi: 10.1073/pnas.1415122111
Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173. doi: 10.1006/cbmr.1996.0014
Cox, S. R., Ritchie, S. J., Fawns-Ritchie, C., Tucker-Drob, E. M., and Deary, I. J. (2019). Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence 76:101376. doi: 10.1016/j.intell.2019.101376
De Vico Fallani, F., Richiardi, J., Chavez, M., and Achard, S. (2014). Graph analysis of functional brain networks: practical issues in translational neuroscience. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369:0521. doi: 10.1098/rstb.2013.0521
Elliott, L. T., Sharp, K., Alfaro-Almagro, F., Shi, S., Miller, K. L., Douaud, G., et al. (2018). Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–216. doi: 10.1038/s41586-018-0571-7
Foo, H., Thalamuthu, A., Jiang, J. Koch F. C., Mather, K. A., Wen, W., et al. (2021). Novel genetic variants associated with brain functional networks in 18,445 adults from the UK Biobank. Sci. Rep. 11:14633. doi: 10.1038/s41598-021-94182-9
Fornito, A., Zalesky, A., and Bullmore, E. (2016). “Connectivity Matrices and Brain Graphs,” in Fundamentals of Brain Network Analysis, Chap. 3, eds A. Fornito, A. Zalesky, and E. T. Bullmore (San Diego: Academic Press), 89–113. doi: 10.1016/b978-0-12-407908-3.00003-0
Gallen, C. L., Baniqued, P. L., Chapman, S. B., Aslan, S., Keebler, M., Didehbani, N., et al. (2016). Modular Brain Network Organization Predicts Response to Cognitive Training in Older Adults. PLoS One 11:e0169015–e0169015. doi: 10.1371/journal.pone.0169015
Geerligs, L., Renken, R. J., Saliasi, E., Maurits, N. M., and Lorist, M. M. (2015). A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb. Cortex 25, 1987–1999. doi: 10.1093/cercor/bhu012
Goh, J. O. (2011). Functional Dedifferentiation and Altered Connectivity in Older Adults: Neural Accounts of Cognitive Aging. Aging Dis. 2, 30–48.
Goldstone, A., Mayhew, S. D., Przezdzik, I., Wilson, R. S., Hale, J. R., and Bagshaw, A. P. (2016). Gender Specific Re-organization of Resting-State Networks in Older Age. Front. Aging Neurosci. 8:285–285. doi: 10.3389/fnagi.2016.00285
Grieder, M., Wang, D. J. J., Dierks, T., Wahlund, L.-O., and Jann, K. (2018). Default Mode Network Complexity and Cognitive Decline in Mild Alzheimer’s Disease. Front. Neurosci. 12:770–770. doi: 10.3389/fnins.2018.00770
Griffanti, L., Salimi-Khorshidi, G., Beckmann, C. F., Auerbach, E. J., Douaud, G., Sexton, C. E., et al. (2014). ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage 95, 232–247. doi: 10.1016/j.neuroimage.2014.03.034
He, X., Qin, W., Liu, Y., Zhang, X., Duan, Y., Song, J., et al. (2014). Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 35, 3446–3464. doi: 10.1002/hbm.22414
Jockwitz, C., and Caspers, S. (2021). Resting-state networks in the course of aging—differential insights from studies across the lifespan vs. amongst the old. Pflügers Archiv Eur. J. Physiol. 2021:7. doi: 10.1007/s00424-021-02520-7
Jones, D. T., Machulda, M. M., Vemuri, P., McDade, E. M., Zeng, G., Senjem, M. L., et al. (2011). Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77, 1524–1531. doi: 10.1212/WNL.0b013e318233b33d
Karssen, L. C., van Duijn, C. M., and Aulchenko, Y. S. (2016). The GenABEL Project for statistical genomics. F1000Research 5, 914–914. doi: 10.12688/f1000research.8733.1
Kendall, K. M., Rees, E., Escott-Price, V., Einon, M., Thomas, R., Hewitt, J., et al. (2017). Cognitive Performance Among Carriers of Pathogenic Copy Number Variants: Analysis of 152,000 UK Biobank Subjects. Biol. psychiatry 82, 103–110. doi: 10.1016/j.biopsych.2016.08.014
Khazaee, A., Ebrahimzadeh, A., and Babajani-Feremi, A. (2015). Identifying patients with Alzheimer’s disease using resting-state fMRI and graph theory. Clin. Neurophysiol. 126, 2132–2141. doi: 10.1016/j.clinph.2015.02.060
Lebedev, A., Westman, E., Simmons, A., Lebedeva, A., Siepel, F., Pereira, J., et al. (2014). Large-scale resting state network correlates of cognitive impairment in Parkinson’s disease and related dopaminergic deficits. Front. Syst. Neurosci. 8:45. doi: 10.3389/fnsys.2014.00045
Levine, D. A., Gross, A. L., Briceño, E. M., Tilton, N., Giordani, B. J., Sussman, J. B., et al. (2021). Sex Differences in Cognitive Decline Among US Adults. JAMA Netw. Open 4:e210169. doi: 10.1001/jamanetworkopen.2021.0169
Manoliu, A., Meng, C., Brandl, F., Doll, A., Tahmasian, M., Scherr, M., et al. (2014). Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 7:930–930. doi: 10.3389/fnhum.2013.00930
McCarrey, A. C., An, Y., Kitner-Triolo, M. H., Ferrucci, L., and Resnick, S. M. (2016). Sex differences in cognitive trajectories in clinically normal older adults. Psychol. Aging 31, 166–175. doi: 10.1037/pag0000070
Miller, K. L., Alfaro-Almagro, F., Bangerter, N. K., Thomas, D. L., Yacoub, E., Xu, J., et al. (2016). Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 19, 1523–1536. doi: 10.1038/nn.4393
Munilla, J., Ortiz, A., Górriz, J. M., Ramírez, J., Weiner, M. W., and Ravdin, L. (2017). Construction and Analysis of Weighted Brain Networks from SICE for the Study of Alzheimer’s Disease. Front. Neuroinformat. 11:19. doi: 10.3389/fninf.2017.00019
Murman, D. L. (2015). The Impact of Age on Cognition. Semin. Hearing 36, 111–121. doi: 10.1055/s-0035-1555115
Ng, K. K., Lo, J. C., Lim, J. K. W., Chee, M. W. L., and Zhou, J. (2016). Reduced functional segregation between the default mode network and the executive control network in healthy older adults: A longitudinal study. NeuroImage 133, 321–330. doi: 10.1016/j.neuroimage.2016.03.029
Otte, W. M., van Diessen, E., Paul, S., Ramaswamy, R., Subramanyam Rallabandi, V. P., Stam, C. J., et al. (2015). Aging alterations in whole-brain networks during adulthood mapped with the minimum spanning tree indices: the interplay of density, connectivity cost and life-time trajectory. Neuroimage 109, 171–189. doi: 10.1016/j.neuroimage.2015.01.011
Parkes, L., Fulcher, B., Yücel, M., and Fornito, A. (2018). An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage 171, 415–436. doi: 10.1016/j.neuroimage.2017.12.073
Persson, J., Pudas, S., Nilsson, L. G., and Nyberg, L. (2014). Longitudinal assessment of default-mode brain function in aging. Neurobiol. Aging 35, 2107–2117. doi: 10.1016/j.neurobiolaging.2014.03.012
Petrides, M., Alivisatos, B., and Frey, S. (2002). Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc. Natl. Acad. Sci. 99:5649. doi: 10.1073/pnas.072092299
Power, J. D., Plitt, M., Laumann, T. O., and Martin, A. (2017). Sources and implications of whole-brain fMRI signals in humans. NeuroImage 146, 609–625. doi: 10.1016/j.neuroimage.2016.09.038
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Raichle, M. E. (2015). The Brain’s Default Mode Network. Annu. Rev. Neurosci. 38, 433–447. doi: 10.1146/annurev-neuro-071013-014030
Ritchie, S. J., Cox, S. R., Shen, X., Lombardo, M. V., Reus, L. M., Alloza, C., et al. (2018). Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 28, 2959–2975. doi: 10.1093/cercor/bhy109
Robinson, H., Calamia, M., Gläscher, J., Bruss, J., and Tranel, D. (2014). Neuroanatomical correlates of executive functions: a neuropsychological approach using the EXAMINER battery. J. Int. Neuropsychol. Soc. JINS 20, 52–63. doi: 10.1017/S135561771300060X
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Sala-Llonch, R., Junqué, C., Arenaza-Urquijo, E. M., Vidal-Piñeiro, D., Valls-Pedret, C., Palacios, E. M., et al. (2014). Changes in whole-brain functional networks and memory performance in aging. Neurobiol. Aging 35, 2193–2202. doi: 10.1016/j.neurobiolaging.2014.04.007
Salimi-Khorshidi, G., Douaud, G., Beckmann, C. F., Glasser, M. F., Griffanti, L., and Smith, S. M. (2014). Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468. doi: 10.1016/j.neuroimage.2013.11.046
Schaefer, A., Kong, R., Gordon, E. M., Laumann, T. O., Zuo, X. N., Holmes, A. J., et al. (2018). Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb. Cortex 28, 3095–3114. doi: 10.1093/cercor/bhx179
Scheinost, D., Finn, E. S., Tokoglu, F., Shen, X., Papademetris, X., Hampson, M., et al. (2015). Sex differences in normal age trajectories of functional brain networks. Hum. Brain Mapp. 36, 1524–1535. doi: 10.1002/hbm.22720
Siman-Tov, T., Bosak, N., Sprecher, E., Paz, R., Eran, A., Aharon-Peretz, J., et al. (2017). Early Age-Related Functional Connectivity Decline in High-Order Cognitive Networks. Front. Aging Neurosci. 8:330. doi: 10.3389/fnagi.2016.00330
Song, J., Birn, R. M., Boly, M., Meier, T. B., Nair, V. A., Meyerand, M. E., et al. (2014). Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connect. 4, 662–676. doi: 10.1089/brain.2014.0286
Stumme, J., Jockwitz, C., Hoffstaedter, F., Amunts, K., and Caspers, S. (2020). Functional network reorganization in older adults: Graph-theoretical analyses of age, cognition and sex. NeuroImage 214:116756. doi: 10.1016/j.neuroimage.2020.116756
Sudlow, C., Gallacher, J., Allen, N., Beral, V., Burton, P., Danesh, J., et al. (2015). UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12:e1001779. doi: 10.1371/journal.pmed.1001779
Supekar, K., Menon, V., Rubin, D., Musen, M., and Greicius, M. D. (2008). Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput. Biol. 4:e1000100. doi: 10.1371/journal.pcbi.1000100
Tian, L., Wang, J., Yan, C., and He, Y. (2011). Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. NeuroImage 54, 191–202. doi: 10.1016/j.neuroimage.2010.07.066
Varangis, E., Habeck, C. G., Razlighi, Q. R., and Stern, Y. (2019). The Effect of Aging on Resting State Connectivity of Predefined Networks in the Brain. Front. Aging Neurosci. 11:234. doi: 10.3389/fnagi.2019.00234
Wang, J., Zuo, X., and He, Y. (2010). Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 4:16. doi: 10.3389/fnsys.2010.00016
Wang, L., Li, Y., Metzak, P., He, Y., and Woodward, T. S. (2010). Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage 50, 862–872. doi: 10.1016/j.neuroimage.2010.01.044
Wig, G. S. (2017). Segregated Systems of Human Brain Networks. Trends Cognit. Sci. 21, 981–996. doi: 10.1016/j.tics.2017.09.006
Keywords: resting-state fMRI, graph theory, age, sex, cognition
Citation: Foo H, Thalamuthu A, Jiang J, Koch F, Mather KA, Wen W and Sachdev PS (2021) Age- and Sex-Related Topological Organization of Human Brain Functional Networks and Their Relationship to Cognition. Front. Aging Neurosci. 13:758817. doi: 10.3389/fnagi.2021.758817
Received: 15 August 2021; Accepted: 25 November 2021;
Published: 17 December 2021.
Edited by:
Annalena Venneri, Brunel University London, United KingdomReviewed by:
Laura Marie Wright, Newcastle University, United KingdomCopyright © 2021 Foo, Thalamuthu, Jiang, Koch, Mather, Wen and Sachdev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi Foo, aGVpZGkuZm9vQHN0dWRlbnQudW5zdy5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.