
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 12 November 2021
Sec. Neuroinflammation and Neuropathy
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.741168
Objective: Ischemic stroke is an important cause of death and disability worldwide. Early reperfusion by thrombolysis or thrombectomy has improved the outcome of acute ischemic stroke. However, the therapeutic window for reperfusion therapy is narrow, and adjuvant therapy for neuroprotection is demanded. Electrical stimulation (ES) has been reported to be neuroprotective in many neurological diseases. In this study, the neuroprotective effect of early somatosensory cortical ES in the acute stage of ischemia/reperfusion injury was evaluated.
Methods: In this study, the rat model of transient middle cerebral artery occlusion was used to explore the neuroprotective effect and underlying mechanisms of direct primary somatosensory (S1) cortex ES with an electric current of 20 Hz, 2 ms biphasic pulse, 100 μA for 30 min, starting at 30 min after reperfusion.
Results: These results showed that S1 cortical ES after reperfusion decreased infarction volume and improved functional outcome. The number of activated microglia, astrocytes, and cleaved caspase-3 positive neurons after ischemia/reperfusion injury were reduced, demonstrating that S1 cortical ES alleviates inflammation and apoptosis. Brain-derived neurotrophic factor (BDNF) and phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway were upregulated in the penumbra area, suggesting that BDNF/TrkB signals and their downstream PI3K/Akt signaling pathway play roles in ES-related neuroprotection.
Conclusion: This study demonstrates that somatosensory cortical ES soon after reperfusion can attenuate ischemia/reperfusion injury and is a promising adjuvant therapy for thrombolytic treatment after acute ischemic stroke. Advanced techniques and devices for high-definition transcranial direct current stimulation still deserve further development in this regard.
Ischemic stroke is an important cause of death and disability worldwide (Katan and Luft, 2018). The development of rapid diagnosis and treatment with newer therapies such as intravenous tissue plasminogen activator and thrombectomy has effectively decreased mortality and disability of ischemic stroke over the past decade. However, only limited patients have the opportunity to receive therapy within a narrow therapeutic window. Failure to reperfuse before substantial irreversible injury occurs and results in permanent functional impairment or even death (Goyal et al., 2016). Adjuvant neuroprotective treatment for reperfusion intervention to protect brain tissue from reperfusion injury remains an important part of treatment for ischemic stroke (Shi et al., 2018).
Neuromodulation by electrical stimulation (ES) has been used in the treatment of many chronic central nervous system (CNS) diseases, such as Parkinson’s disease, epilepsy, and psychological disorders (Dougherty, 2018). In ischemic stroke, neuromodulatory strategies, such as transcranial magnetic stimulation, deep brain stimulation, and direct current cortical stimulation, are used for promoting recovery of function (Bao et al., 2020). ES can modulate neuro-regeneration and neuro-plasticity and thus, promote restoration of neurological function after brain injury (Henrich-Noack et al., 2017). Cortical activities induced by ES have been shown to improve functional recovery after ischemic stroke (Adkins et al., 2006; Koesler et al., 2009).
Neuroprotection by ES during the acute stage of ischemic injury has been reported in several studies. Direct ES of specific nuclei in the brain can attenuate ischemic injury as shown in several animal studies. Stimulation at the cerebellar fastigial nucleus was proposed to induce vasodilation, increase neuronal tolerance to depolarization, or decreased the metabolism of penumbra, and thus can reduce infarction area after middle cerebral artery occlusion (MCAO) (Wang et al., 2014). ES of the dorsal periaqueductal gray matter can induce hypertension and vasodilatation and protect the brain against ischemic injury (Glickstein et al., 2003). ES to the cortical surface overlying the ischemic boundary has antiapoptotic, angiogenic, and anti-inflammatory effects through phosphoinositide 3-kinase (PI3K)/Akt signaling pathway and thus can attenuate ischemic injury (Baba et al., 2009). Several studies also showed a neuroprotective effect of non-invasive transcranial direct current stimulation for ischemic stroke. Transcranial direct current stimulation modifies N-methyl-D-aspartate receptor expression and inhibits apoptosis and postischemic inflammation to attenuate ischemic injury (Peruzzotti-Jametti et al., 2013; Notturno et al., 2014). The above studies have demonstrated that ES applied in the acute stage of ischemic stroke is a promising adjuvant therapy to attenuate ischemia/reperfusion injury of the brain. However, optimal stimulation target and protocol remain to be explored. Optimal neural stimulation seeks to reduce the amount of charge needed to achieve the target effect and meanwhile reduces tissue damage caused by electric charge. Square biphasic pulse provides a charge-balanced, rectangular, electric pulse to reduce amplitude and charge per phase while preserving stimulation effectiveness and is an effective pattern of brain ES both in animal and human settings (Deprez et al., 2018; De Jesus et al., 2019). Current intensity, stimulation frequency, and pulse width are parameters related to stimulation effect and damage. Electric stimulation at current (0, 100, and 200 μA) and frequency (0, 2, 10, and 50 Hz) had been reported to be safe for rats (Baba et al., 2009). Short stimulus pulse width reduces charge injection and increases the safety range between therapeutic effects and side effects (Kuncel and Grill, 2004). Optimal stimulus parameters differ by therapeutic target and stimulator.
The neuroprotective potential of early sensory stimulation for the treatment of acute ischemic stroke has also been demonstrated in animal studies (von Bornstadt et al., 2018). Several studies support acute sensory stimulation to the affected limbs or whiskers as a potential therapy for ischemic stroke (Lay et al., 2011, 2013; Frostig et al., 2013). The Frostig group reported that mild sensory stimulation of the whiskers protected Sprague-Dawley rats from ischemic injury after MCAO. The proposed mechanism for neuroprotection by early peripheral sensory stimulation is an improvement of collateral circulation to the sensory cortex at the penumbra of the ischemic core by neuronal activities induced by sensory stimulation. Intact neurovascular unit and efficient cortical anastomoses are proposed to be the decisive factors influencing the neuroprotective effect of sensory stimulation. Inappropriate application of sensory stimulation after ischemic stroke may even be detrimental (von Bornstadt et al., 2018). Therefore, further studies to clarify the pros and cons of sensory stimulation are required.
Application of peripheral somatosensory stimulation in rodent model acute ischemic stroke is often confounded by extensive unintended sensory stimulation during experiments. ES of the primary somatosensory (S1) cortex for hand and tongue representations of S1 using intracranial electrodes showed a linear relationship between stimulation current intensity and intensity of sensation in a human study (Kirin et al., 2019). Direct ES of the S1 cortex is a more reliable method to study the neuroprotective effect by sensory stimulation.
Brain-derived neurotrophic factor (BDNF) is an important trophic factor that protects neuronal functions following ischemia, trauma, and toxic brain injury. BDNF was identified as an important mediator of the neuroprotective response to deep brain stimulation (DBS). ES induces the production of BDNF to promote the survival of neuronal cells. Subthalamic nucleus DBS induces the increase in BDNF to support the survival of the nigrostriatal system and promote the functionality of the basal ganglia-cortical circuitry in the parkinsonian brain (Fischer et al., 2017; Fischer and Sortwell, 2019). In an animal model of spinal cord injury, DBS induces the protein expression of BDNF and improves the hind limb motor function (Wang et al., 2020). Upregulation of PI3K/Akt/mammalian target of rapamycin (mTOR) has been reported to play a pivotal role in neuroprotection using pharmacological treatment (Zhou et al., 2010). Activation of BDNF/TrkB signal triggers the downstream PI3K/Akt/mTOR signaling pathway to regulate autophagy and inhibit translocation of Bax from the cytoplasm to mitochondria to support neuronal survival (Chen et al., 2013). The role of BDNF in cortical ES during the acute stage of ischemic stroke deserved further studies.
This study intends to examine the neuroprotective effect of somatosensory ES applied in the acute stage of ischemic stroke using a rodent animal model of transient ischemic stroke. After 90 min of temporal MCAO, rats received 30 min of ES targeting at S1 cortical area starting at 30 min after reperfusion. Neuroprotective effects were evaluated by examining infarction volume, functional outcome, neuronal survival, and apoptosis on post-MCAO day 3. Underlying molecular mechanisms about neuroprotection by ES after transient ischemic stroke were studied. Microglia are key players of inflammation after CNS injury. The impact of cortical ES on postischemic inflammation by the evaluation of migration, proliferation, and activation of microglia was evaluated by co-staining with the pan-microglial marker, ionized calcium-binding adapter molecule 1 (Iba1), and a marker of phagocytic activation, CD68 (Patel et al., 2013). The influence of S1 cortical ES on the BDNF/TrkB/PI3K/Akt/mTOR signaling pathway and autophagy was also studied. This study demonstrates that S1 cortical ES after revascularization is a promising strategy for the treatment of acute ischemic stroke.
All experiments were conducted under the approval of the Institutional Animal Care and Use Committee (IACUC; Approval #108242) at National Chen Kung University. Adult male Sprague-Dawley rats purchased from the BioLASCO Taiwan Co., Ltd., and weighing 250–300 g were used for all experiments. A total of 60 rats were used in this study: 47 for transient MCAO (tMCAO) and 13 for the sham operation group. In the sham group (13 rats), 5 rats were used for immunohistological analysis and 8 rats were used for protein analysis. The experimental design is illustrated in Figure 1. Rats were randomly allocated to three groups and labeled as Sham (sham-operated), MCAO (tMCAO without treatment), and MCAO + ES (tMCAO with ES). Rats sustained 90 min of tMCAO or sham operation. After reperfusion, a pair of electrodes was stereotactically inserted into the S1 cortex. The ES-treated group received 30 min of ES starting at 30 min after reperfusion. The functional outcome was evaluated at post-tMCAO day 3, and rats were sacrificed for further histology and molecular biology evaluation. In the tMCAO group (47 rats), tMCAO was successfully conducted for 39 rats, whereas 3 of them failed to survive till post-tMCAO day 3. The three mortalities include two in the MCAO group and one in the MCAO + ES group. Thirty-six rats survived till post-tMCAO day 3. Notably, 20 rats (10 for each group) were used for immunohistological analysis and 16 rats (8 for each group) were used for protein analysis.
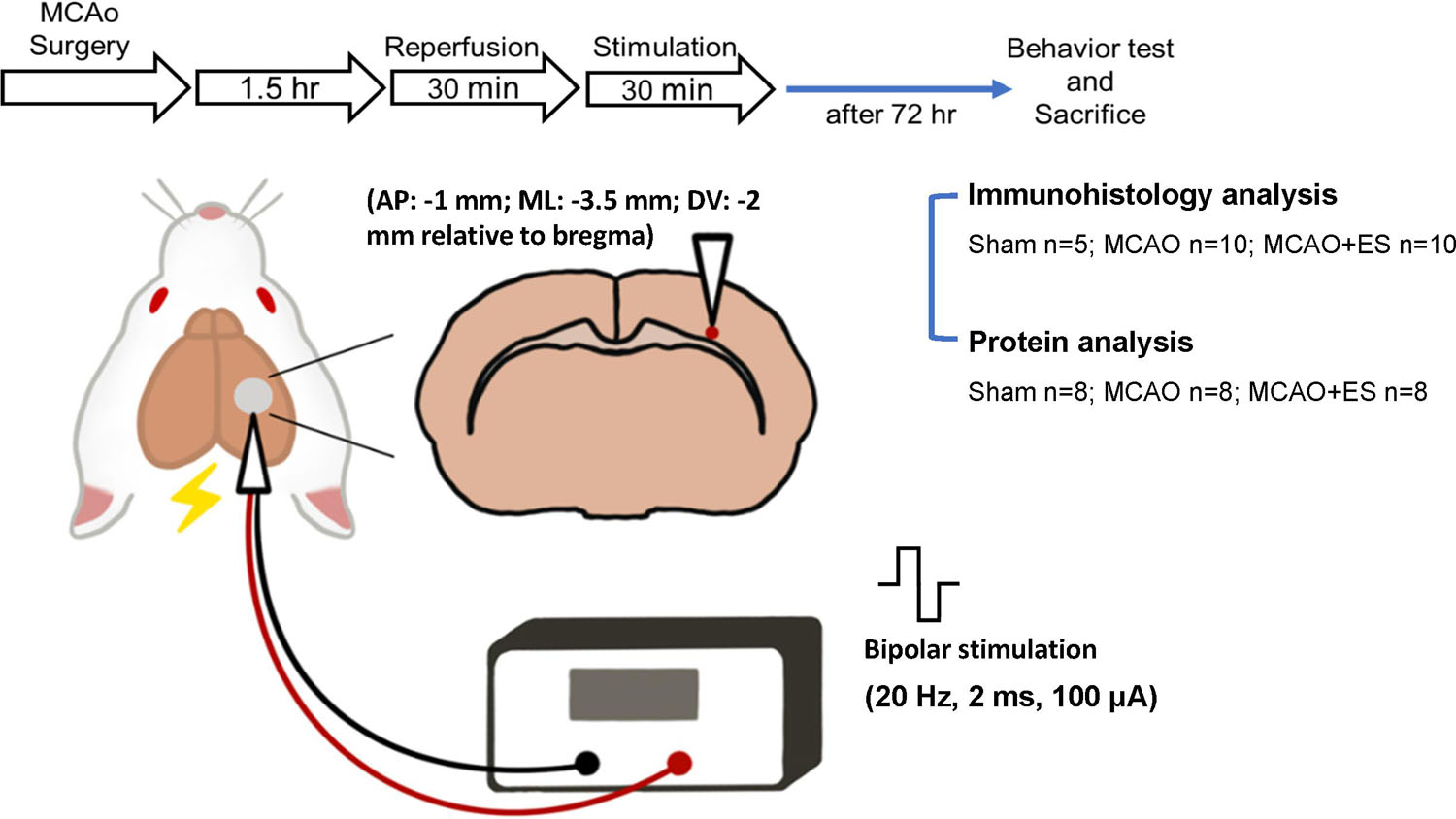
Figure 1. Schematic diagram of the primary somatosensory (S1) cortical electrical stimulation (ES) experimental design. Rats sustained 90 min of transient middle cerebral artery occlusion (tMCAO) or sham operation, and electrodes were implanted to the S1 cortical area. Rats were randomly allocated to three groups and labeled as Sham (sham-operated), MCAO (tMCAO without treatment), and MCAO + ES (tMCAO with ES). A pair of electrodes was stereotactically inserted into the S1 cortex (1 mm posterior to bregma, 3.5 mm lateral to midline, and 2 mm depth) in 30 min after reperfusion. The ES-treated group received 30 min of biphasic direct current ES (frequency, 20 Hz; pulse length, 2 ms; stimulating amplitude, 100 μA; biphasic pulse) after electrode implantation. Functional outcomes were evaluated 3 days after tMCAO, and animals were sacrificed for further studies.
The MCAO surgery with laser Doppler flowmetry (LDF) monitoring (OxyLab LDFTM; Oxford Optronix Ltd., Oxford, United Kingdom) of the cerebral blood flow was conducted as previously described (Boyko et al., 2010; Cao et al., 2017; Wu et al., 2018). Anesthesia was induced with 5% isoflurane mixed with 70% N2O and 30% O2 in an induction chamber. Surgical-depth anesthesia was maintained with 1–2% isoflurane mixed with 70% N2O and 30% O2 during surgery. In brief, an area of the skull bone 2 mm posterior and 6 mm lateral to the bregma was decorticated for LDF monitoring. The right common carotid artery and the complex of the internal and external carotid arteries were identified and dissected. The right internal carotid artery (ICA) was further ligated just distal to the bifurcation. The occluders were made of 22 mm long, 4-0 nylon treads. One end of the occluder was heat-treated to expand its diameter from 0.17 to 0.28 mm, thus creating a baseball bat-shaped occluder with a dilated segment of approximately 4 mm in length. The occluder was inserted into the arteriotomy on ICA, advanced until it reached the bifurcation of the anterior and middle cerebral arteries, and left in place for 90 min. A reduction in the cerebral blood flow under 30% of the baseline level was considered a successful occlusion of the middle cerebral artery. After removal of the occluder, a return of the cerebral blood flow to more than 60% of baseline as measured by LDF was considered adequate reperfusion from collateral circulation from the contralateral anterior cerebral artery. For sham surgery, all arteries were exposed for the normal duration of an MCAO operation but the filament was not inserted into the ICA.
Under general anesthesia using 1–2% isoflurane mixed with 70% N2O and 30% O2, a pair of twisted polyimide-coated stainless-steel bipolar electrodes (E363/3/SPC Invivo1) were stereotaxically positioned to right S1 primary sensory cortex (anteroposterior: −1 mm; mediolateral: −3.5 mm; dorsoventral: −2 mm relative to the bregma) within 30 min after reperfusion. Electrodes were connected to a linear insulation stimulator (WPI, A395), with data acquisition hardware (PowerLab, 8/35) to regulate the output of the stimulator. A 100 μA electric current setting to 20 Hz, 2 ms square biphasic pulse was applied to evaluate the therapeutic effect of short-term intense S1 cortical ES on the acute stage of transient MCAO. Rats received cortical stimulation for 30 min starting at 30 min after reperfusion. In this study, the stimulation intensity used was higher than generally used but 30 min of stimulation is a short one (Bahr Hosseini et al., 2019).
An 18-point focal scoring system for the modified neurological severity score (mNSS) was used to evaluate neurological outcomes of experimental rats on post-surgery day (PSD) 3 (Chen et al., 2001). Grade scores were assigned to each animal, with functional measures including motor (muscle status and abnormal movement), sensory (visual, tactile, and proprioceptive), reflex, and balance tests (Table 1).
Motor coordination was evaluated in MCAO rats using the rotarod test (Wu et al., 2018). During a training session, rats were placed on an accelerating rotarod (model number: LE8505 Rota-Rod, Panlab Harvard apparatus, Spain), and the running speed of the cylinder was increased slowly from 4 to 40 rpm within 5 min. The rats were trained for 3 days before MCAO. All rats received five separate test trials with 5-min intervals between each trial on PSD 3. The average time of five sessions was recorded for the assessment of motor function.
To prepare the tissue for immunostaining, rats were deeply anesthetized with 5% isoflurane and transcardially perfused with phosphate-buffered saline (PBS) (Marquardt et al., 2018; Shomer et al., 2020). The brain tissues were postfixed in 4% paraformaldehyde (PFA) overnight, dehydrated, and paraffinized. The sections were collected from the bregma +1 to −3 mm; the paraffinized tissues were sliced at 8 μm per section. For the cresyl violet staining, slides were placed into heated (50°C) 0.1% cresyl violet acetate for 20 min, and the excess stain was rinsed off in running tap water. Finally, the sections were mounted on coverslips with a mounting medium and dried overnight. The quantification of the infarction area was performed using the HistoQuest analysis software version 4.0 (TissueGnostics, Tarzana, CA, United States). The infarction area ratio with correction for edema was evaluated using the following method: infarction area ratio (%) = (contralateral hemisphere - non-infarcted area in the ipsilateral hemisphere) × 100/contralateral hemisphere (Swanson et al., 1990).
The detailed protocol for immunofluorescence (IF) staining has been described previously (Wu et al., 2018). Rats were deeply anesthetized with 5% isoflurane and transcardially perfused with PBS. The brain tissues were postfixed in 4% PFA overnight, dehydrated, and paraffinized. The sections were collected from the bregma +1 to −3 mm; the paraffinized tissues were sliced at 8 μm per section. For IF staining, antigen retrieval was performed with 0.01 M citrate acid at 100°C for 10–20 min. Then, the sections were blocked with 5% normal donkey serum (Millipore, Temecula, CA, United States) and 0.5% Triton X-100 (Sigma, United States) in PBS. IF staining was performed to detect with anti-NeuN (1:200; Millipore; MAB377), anti-cleaved-caspase-3 (1:200; Cell Signaling Technology, United States; 9661S), anti-Iba1 (1:200; Wako, Japan; 016-20001), anti-CD68 (1:200; Abcam, United Kingdom; AB31630), and anti-glial fibrillary acidic protein (GFAP) (1:300; Millipore; MAB3402). The Alexa Fluor 488-conjugated anti-rabbit secondary antibody (Invitrogen Life Technologies, United States) and Alexa Fluor 594-conjugated anti-mouse secondary antibody (Invitrogen Life Technologies) were used. Finally, the sections were incubated with DAPI and mounted on coverslips with the mounting medium (Dako, Denmark). Sections were examined by fluorescence microscopy (BX51, Olympus, Japan), and the images were captured using a camera coupled to a desktop computer. The number of positive cells and the total stained area were acquired using TissueFAXS and quantified using TissueQuest software version 4.0 (TissueGnostics, Vienna, Austria) as the intensity of immunoreactivity over the set threshold in a blinded manner. Each rat was randomly counted over six ipsilateral cerebral hemisphere views under 20× objective lens from coronal sections.
For protein analysis, MCAO rat tissues were prepared from the penumbra and core of the ipsilateral brain marked by 2,3,5-triphenyltetrazolium chloride (TTC) stain and homogenized in radio-immunoprecipitation assay (RIPA) lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)) containing protease inhibitor cocktail (Roche, Switzerland) and the mixture of phosphatase inhibitor cocktail 2/3 (Sigma Aldrich, United States). Then, the extracts were analyzed by 8–12% SDS PAGE, followed by blot hybridization with the following antibodies: Akt (1:3,000; Cell Signaling Technology; 2920), p-Akt (1:1,000; Cell Signaling Technology; 9271), extracellular signal-regulated kinase (ERK) (1:1,000; Millipore; 06-182), p-ERK (1:1,000; Millipore; 05-797), PI3K (1:1,000; Cell Signaling Technology; 4292), p-PI3K (1:1,000; Cell Signaling Technology; 4228), p62 (1:2,000; Abcam; ab56416), LC3B (1:500; GeneTex, United States; GTX127375), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5,000; GeneTex; GTX627408). After primary antibody binding, the blots were incubated with the appropriate secondary antibodies and Western Lightning Plus-ECL (PerkinElmer; Waltham, MA, United States) at room temperature. All data were repeated for at least three independent experiments. For quantitative analysis, relative intensities of the bands were normalized to the internal control protein.
For the detection and quantification of protein, rat brain tissues were homogenized in RIPA lysis buffer, and the total protein content was extracted. Notably, 40 μg of total protein from each sample was used to detect BDNF concentration using the specific BDNF ELISA Assay Kit (DBNT00; R&D Systems Inc., Minneapolis, MN, United States). ELISA assay was performed according to the instructions of the manufacturer. All data were repeated for at least three independent experiments.
All data are statistically analyzed and graphically represented using GraphPad Prism 8 (GraphPad Software, San Diego, CA, United States), which were normally distributed and are presented as the mean ± SE of mean (SEM). Independent experiments with multiple groups were compared with each other using one-way ANOVA followed by post hoc Tukey’s test calculated using Prism. The criteria for statistical significance were ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ****p < 0.0001.
Infarction volumes were evaluated using cresyl violet staining after tMCAO and 72 h reperfusion. These results demonstrated that S1 cortical ES significantly attenuated infarction volume compared with the control group. Infarction volume decreased from 51.24 ± 2.81% in the MCAO group to 36.79 ± 4.57% in the MCAO + ES group (p < 0.05) (Figure 2). Neurological outcomes were assessed by mNSS and the rotarod performance test in tMCAO rats. In line with decreased infarction volume, functional recovery after ischemia/reperfusion injury was also improved in the ES-treated group (Figure 2).
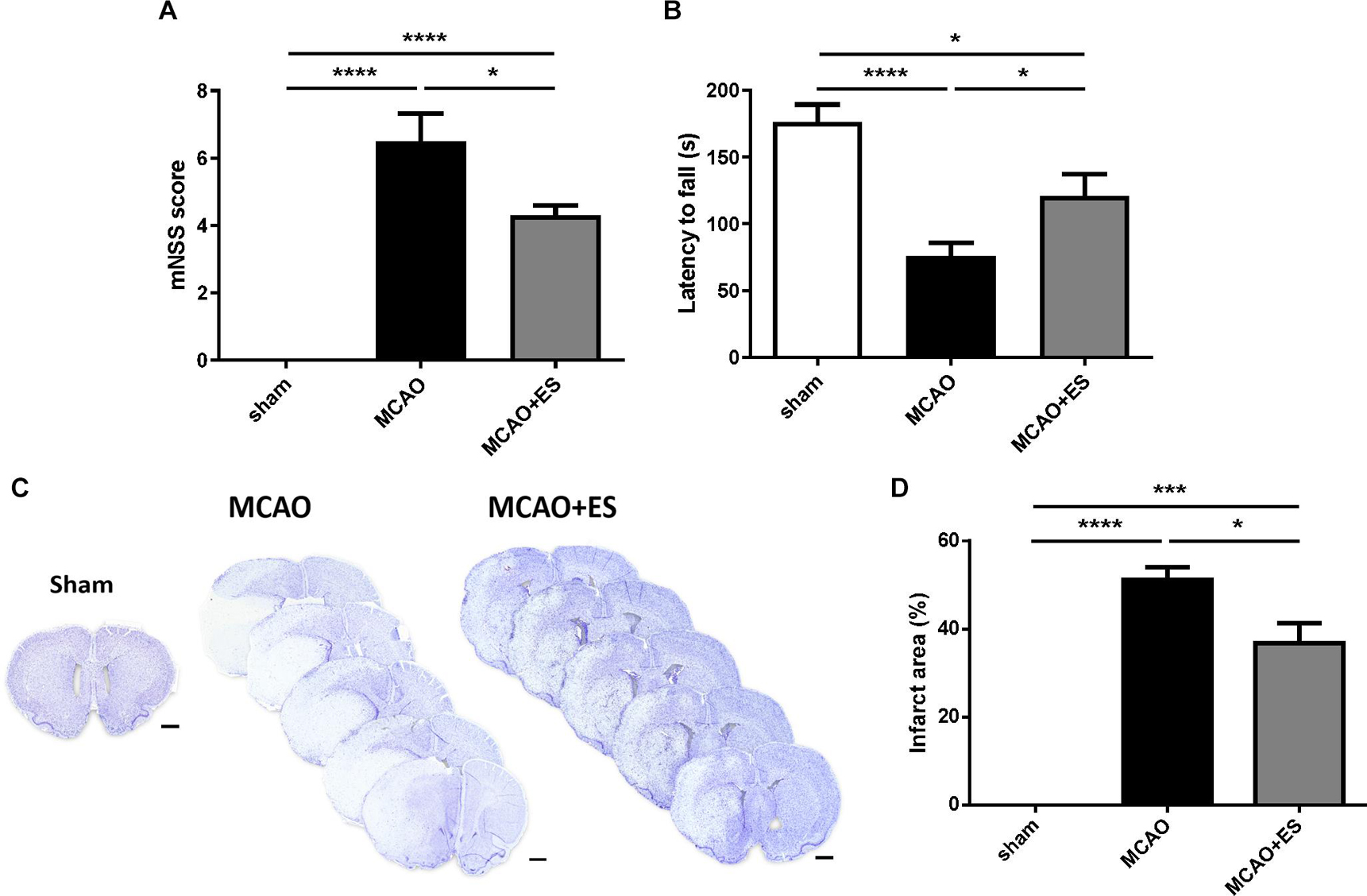
Figure 2. Middle cerebral artery occlusion rats perform better functional outcome recovery and showed reduced infarct area after ES treatment. (A) ES attenuates functional deficits as evaluated by mNSS after tMCAO (Sham n = 10; MCAO n = 10; and MCAO + ES n = 10). (B) ES improves rotarod performance after tMCAO (Sham n = 10; MCAO n = 10; and MCAO + ES n = 10). (C) Representative pictures of crystal violet staining. Scale bar = 1 mm. (D) ES significantly reduces infarction volume after tMCAO (Sham n = 5; MCAO n = 10; and MCAO + ES n = 10). *p < 0.05; ***p < 0.001; and ****p < 0.0001.
Whether ES protected neuronal cells from death after tMCAO was further examined. Immunofluorescence staining of NeuN was used to evaluate the survival of neurons in penumbra on day 3 after tMCAO. These results showed the group that received ES after tMCAO had better neurons survival in penumbra as compared with the control group, suggesting that direct S1 cortical ES protects neurons against ischemic damage after tMCAO (Figure 3). Furthermore, cleaved caspase-3 immunofluorescence staining was performed to evaluate apoptosis after tMCAO with or without ES. The elevation of cleaved caspase-3 positive cell count after tMCAO was significantly reduced in the ES group (Figure 3). These data demonstrated that S1 cortical ES had neuroprotective effects and attenuated apoptosis after tMCAO.
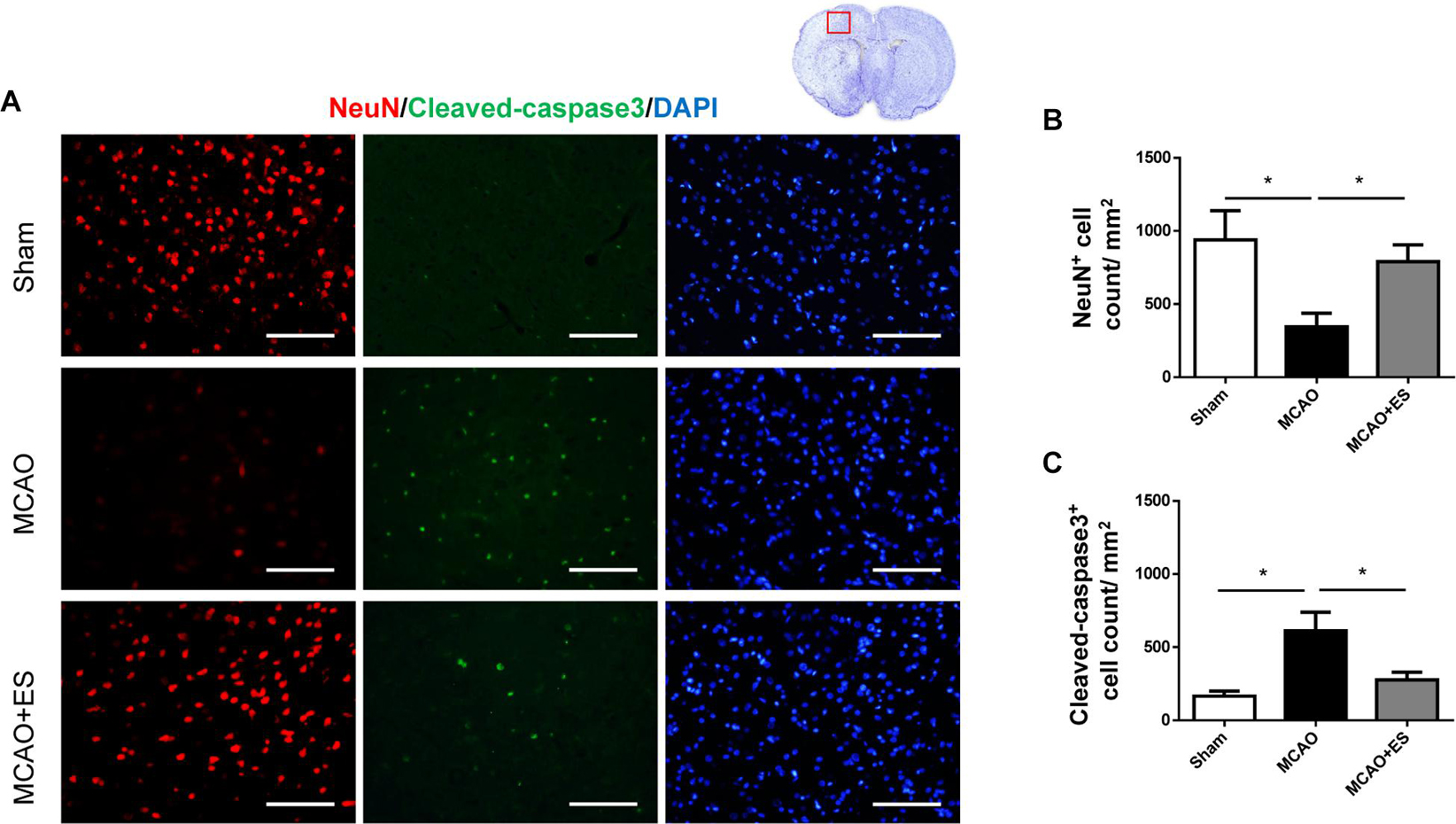
Figure 3. Electrical stimulation treatment inhibits neuronal cell death of the brain in the MCAO animal model. (A) Representative pictures of immunofluorescence stains for NeuN (red fluorescence), cleaved caspase-3 (green fluorescence), and DAPI (blue fluorescence). The red blocked box in the top-right panel represents views in the penumbral region. (B) Quantitative analysis for NeuN immunoreactive cells. The ES group shows significantly higher neurons survival than the control group. (C) Quantitative analysis shows a significantly reduced number of immunoreactive cells for cleaved caspase-3 in the ES group, demonstrating the antiapoptotic effect of ES (Sham n = 5; MCAO n = 10; and MCAO + ES n = 10; scale bar = 50 μm). *p < 0.05.
Activation of microglia/astrocytes plays an important role in the inflammatory response after ischemia/reperfusion insults. To investigate whether neuroinflammation was suppressed by S1 cortical ES, immunofluorescence stains of brain sections were used to evaluate the activation of microglia/astrocytes at 3 days after tMCAO. Activated microglia were identified by co-staining with the pan-microglial marker, Iba1, and a marker of microglial activation, CD68. The number of Iba1/CD68-positive cells in the ischemic penumbra of the cerebral cortex of tMCAO rats was significantly increased than that in the sham group (Figure 4). However, S1 cortical ES significantly reduces microglial activation and the total number of glial cells after tMCAO. Moreover, GFAP staining revealed that the number of astrocytes in the ipsilateral cerebral hemisphere was significantly reduced by ES (Figure 5). Together, S1 cortical ES attenuates proliferation and activation of microglial cells and astrocytes, suggesting the anti-inflammatory effect of ES.
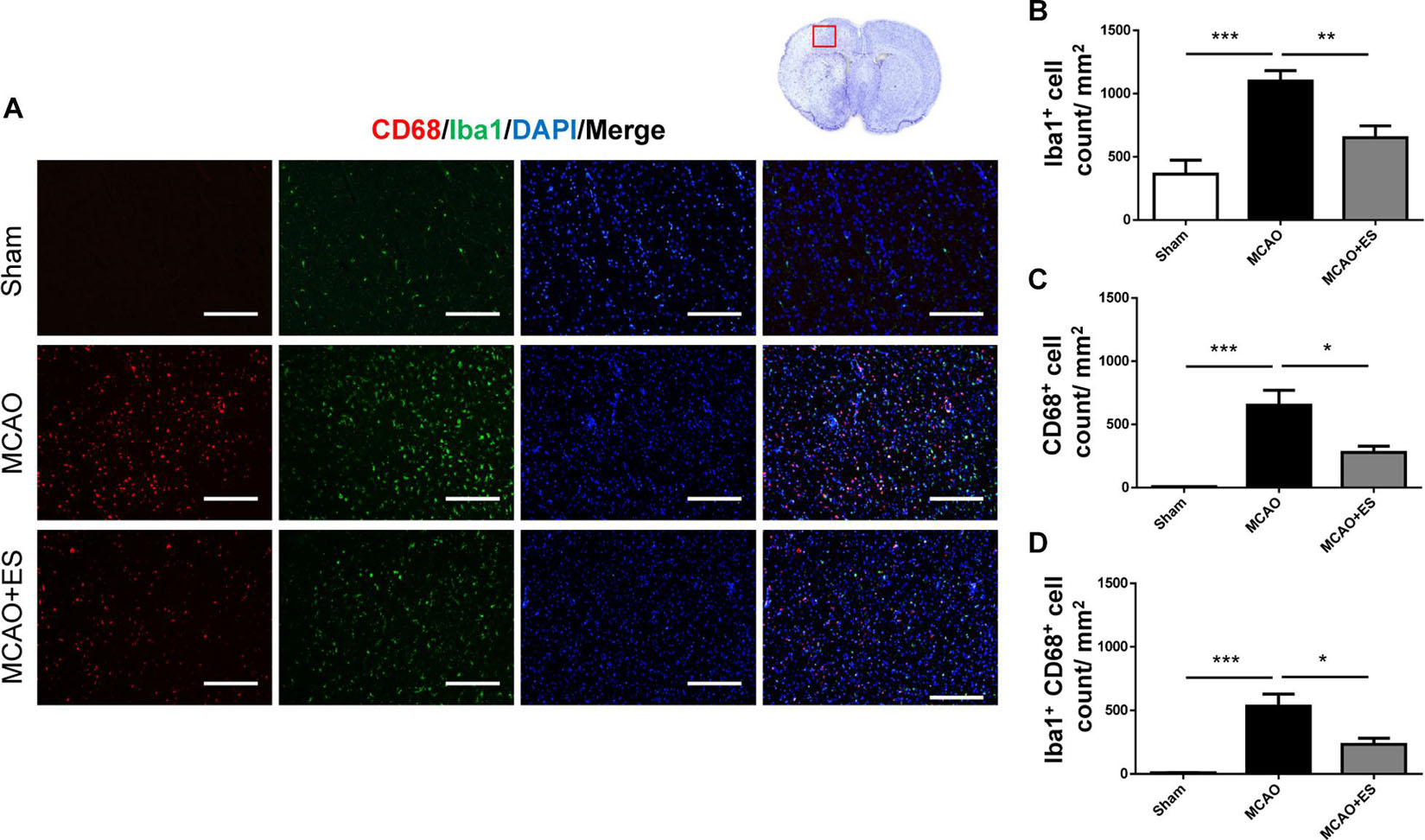
Figure 4. Electrical stimulation treatment affects the activation of microglia in the MCAO animal model. (A) Representative pictures of immunofluorescence stains for CD68 (red fluorescence), ionized calcium-binding adapter molecule 1 (Iba1) (green fluorescence), and DAPI (blue fluorescence). The red blocked box in the top-right panel represents views in the penumbral region. (B–D) Quantitative analysis of immunoreactive cells. ES treatment significantly reduced proliferation and activation of microglia after tMCAO. Suggesting possible anti-inflammatory effect of ES after tMCAO (Sham n = 5; MCAO n = 10; and MCAO + ES n = 10). Scale bar = 100 μm; *p < 0.05; **p < 0.01; and ***p < 0.001.
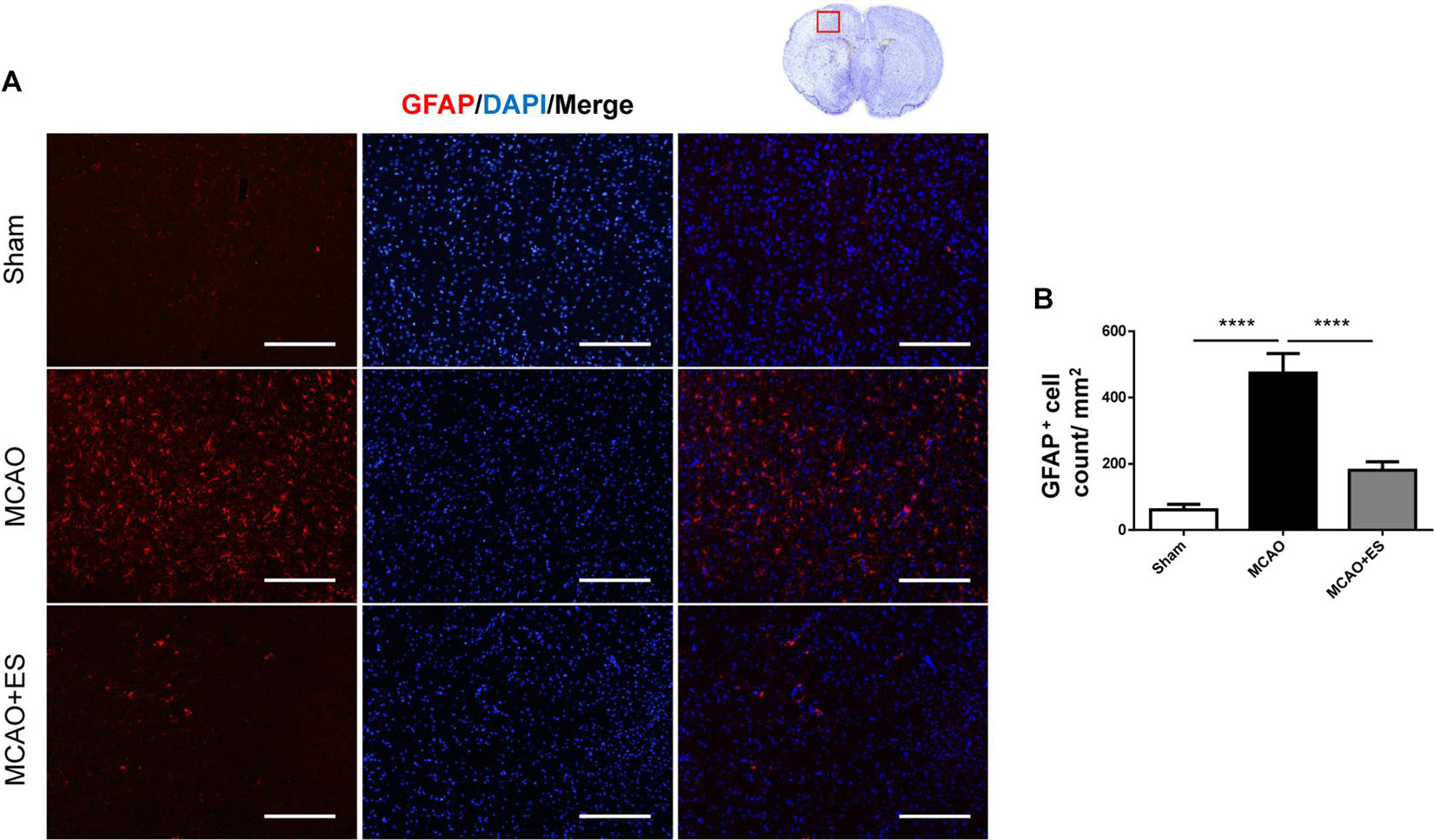
Figure 5. Electrical stimulation treatment reduces the count of astrocytes in the MCAO animal model. Glial fibrillary acidic protein (GFAP) staining demonstrated that proliferation of astrocytes was suppressed by ES. (A) Immunofluorescence stains for GFAP (red fluorescence) and DAPI (blue fluorescence). The red blocked box in the top-right panel represents views in the penumbral region. (B) Quantitative analysis of immunoreactive cells (Sham n = 5; MCAO n = 10; and MCAO + ES n = 10). Scale bar = 100 μm; ****p < 0.0001.
The BDNF has been reported to play an important role in ES-related neuroprotection. ELISA was used to evaluate the expression of BDNF in penumbra after tMCAO (Figure 6). The data revealed that ES significantly upregulated the expression of BDNF after tMCAO, suggesting that BDNF and its downstream signal pathway play a role in neuroprotection in this ES paradigm.
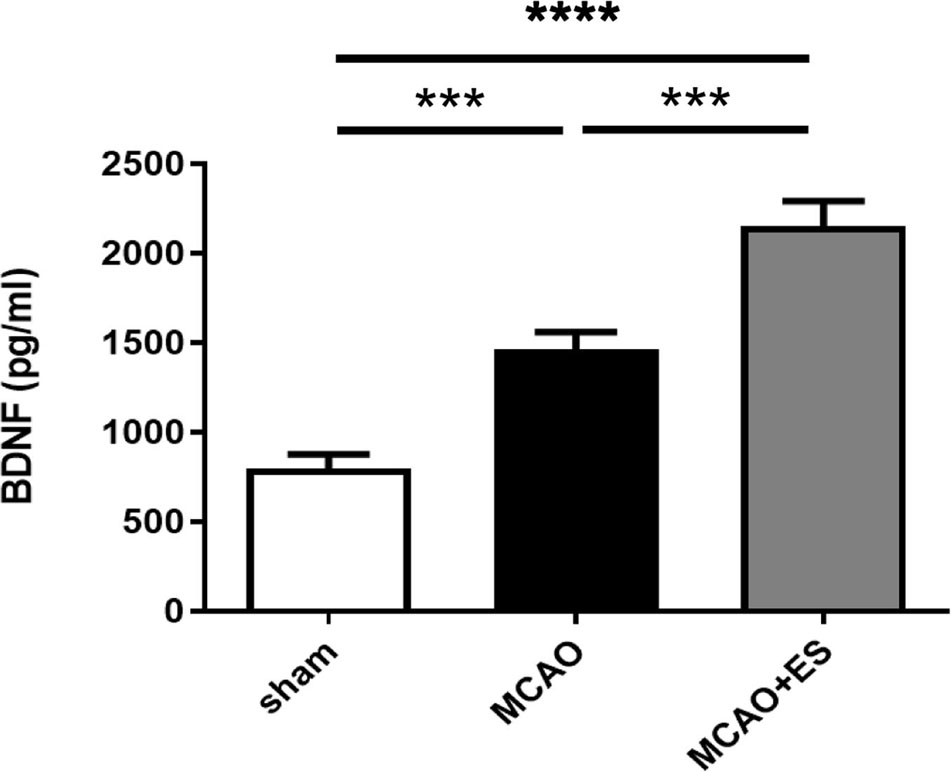
Figure 6. Electrical stimulation treatment enhances the expression of brain-derived neurotrophic factor (BDNF) after tMCAO. Expression of BDNF in penumbra was evaluated by ELISA (Sham n = 8; MCAO n = 8; and MCAO + ES n = 8). ***p ≤ 0.001; ****p ≤ 0.0001.
It has been reported that BDNF modulates autophagy through the PI3K/Akt/mTOR pathway (Nikoletopoulou et al., 2017). Activation of the PI3K/Akt pathway has been reported to promote cell survival and inhibit caspase-3-dependent apoptosis. We hypothesized that S1 ES protects neurons from ischemic/reperfusion injury by the upregulating BDNF/PI3K/Akt signal. To investigate the effect of ES on the PI3K/Akt pathway following an ischemic stroke, Western blotting was performed to examine the expression levels of phosphorylated PI3K, Akt, and ERK in the ischemic brain. Results showed that ES upregulated the levels of phosphorylated PI3K and Akt following tMCAO. However, the change of phosphorylated ERK was not significant (Figure 7). The markers indicating the level of autophagy, p62, and LC3B level were also examined. There was a significant elevation of P62 and reduction of LC3B, suggesting suppression of autophagy by cortical ES. These results demonstrated that ES activated the PI3K/Akt pathway and suppressed autophagy to protect the brain from ischemia/reperfusion injury.
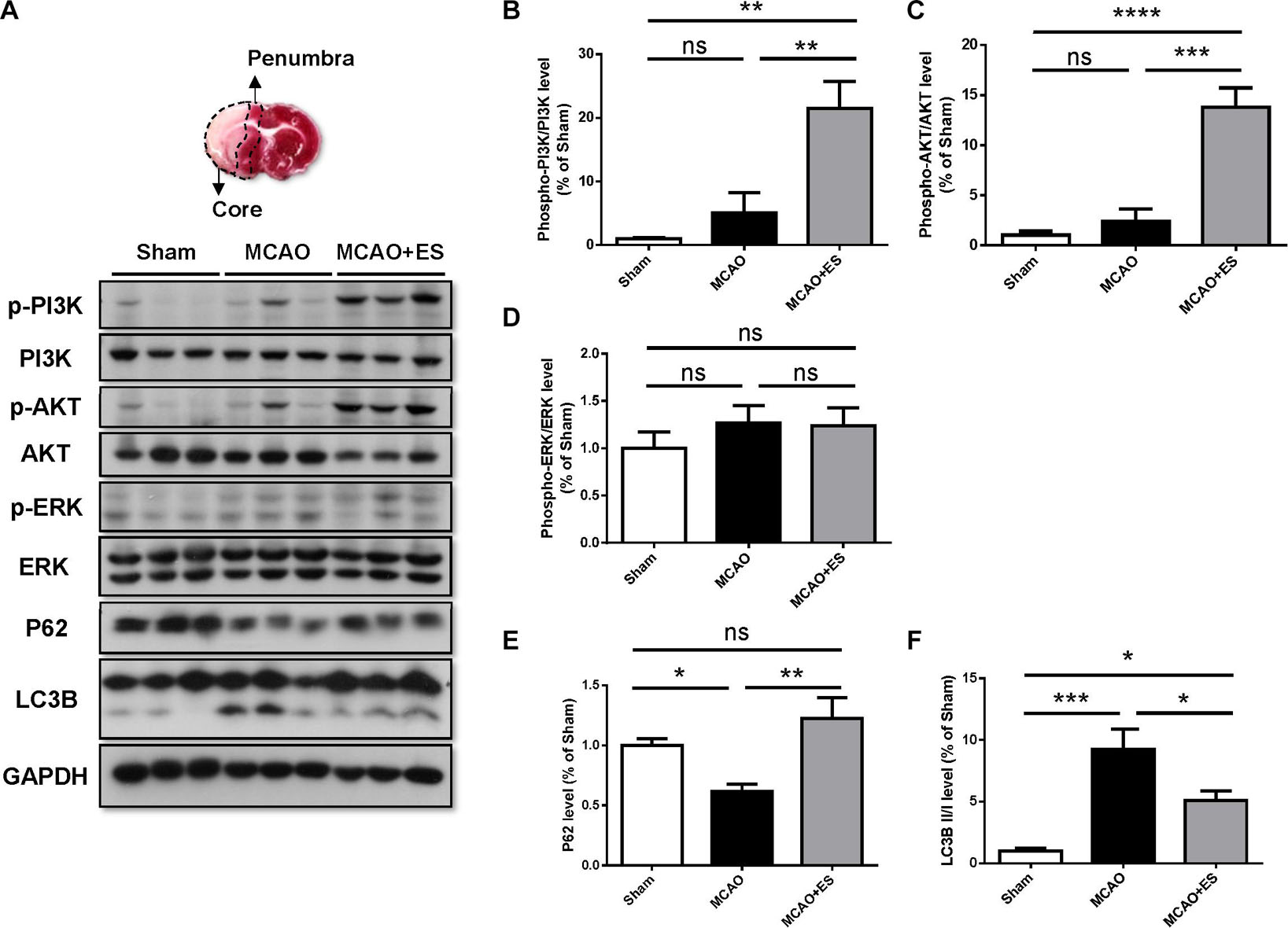
Figure 7. Cortical ES treatment activated the PI3K/Akt pathway and suppressed autophagy to protect the brain from ischemia/reperfusion injury after tMCAO. Western blotting for PI3K, Akt, extracellular signal-regulated kinase (ERK), P62, and LC3B. (A) Representative pictures of Western blot. The upper panel represents the 2,3,5-triphenyltetrazolium chloride (TTC)-stained view of demarking core and penumbral region. (B,C) Phosphorylation of PI3K and Akt were significantly upregulated in ES groups. (D) There was no significant change in the phosphorylation of ERK. (E,F) In the ES group, there were increased P62 and reduced LC3B, indicating suppression of autophagy by ES (n = 5 for each group). *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
This study demonstrates that 30 min of square biphasic pulse ES at the S1 cortex soon after reperfusion following MCAO attenuates ischemic/reperfusion injury. S1 cortical ES significantly reduced infarction volume, improved functional outcome, and decreased neuronal death and apoptosis. Cortical ES decreased neuronal death and apoptosis. BDNF, PI3K, and Akt were upregulated after ES, demonstrating that BDNF/TrkB and its downstream PI3K/Akt/mTOR signal pathway play a role in neuroprotection by cortical ES.
The neuroprotection effects of CNS ES in acute ischemic stroke have been shown in several preclinical studies. Several strategies for ES have been proposed. In non-invasive transcranial direct current stimulation, a low-voltage electrical current is delivered to the brain via scalp electrodes. Despite its easier clinical application, the delivery of electric current is less focused and reliable (Karabanov et al., 2019). Electrical current can also be delivered by electrodes implanted in the brain to provide precise ES. Targets for ES are the fastigial nucleus (Yamamoto et al., 1993), subthalamic vasodilator area (Glickstein et al., 2001), and dorsal periaqueductal gray matter (Glickstein et al., 2003). ES of these deep brain nuclei demands precise targeting and carries the risk of damaging vital structures. Clinical application of these deep brain nucleus stimulation strategies in the acute stage of ischemic stroke is difficult. Cortical ES has the advantage of easier application with less risk. This study demonstrates that cortical ES applied concomitantly with reperfusion could attenuate ischemia/reperfusion injury. The recent development of the high-definition transcranial direct current stimulation technique will make early cortical stimulation a promising treatment for acute ischemic stroke.
Sensory stimulation has been proposed as a potential therapy for ischemic stroke. Ron Frostig’s group from the University of California Irvine reported the therapeutic effect of whisker stimulation after permanent MCAO (pMCAO) (Frostig et al., 2013). Mild sensory stimulation of the whisker(s) protected Sprague-Dawley rats from neuronal damage and functional loss after pMCAO (Lay et al., 2011, 2012). It was hypothesized that early sensory stimulation induces cortical activation, which plays a key role in sensory stimulation-related neuroprotection (Lay et al., 2010). After acute vascular occlusion, collateral blood flow through anastomoses between cerebral arteries is important to rescue the tissue from ischemic injury (Cook et al., 2012). Under physiological conditions, increased neuronal activity induces regional vasodilation according to a process of neurovascular coupling that increases the delivery of oxygen and nutrients. In the penumbra area of ischemia where blood supply is decreased but the neurovascular coupling is still relatively preserved, the increased neural activity may attenuate ischemic injury by inducing vasodilatation and blood perfusion. However, in areas where the neurovascular coupling is lost, the increased neural activity is detrimental due to increased energy consumption without corresponding incremental blood flow (Krainik et al., 2005). The timing of stimulation, the vascular anatomy, and especially efficient cortical anastomoses between the cerebral arteries was proposed to be the decisive factors influencing the neuroprotective effect of sensory stimulation (von Bornstadt et al., 2018). In this study, the animal model of tMCAO was used by ligation of ipsilesional ICA, used heat-shaped filament to occlude the origin of middle cerebral artery to minimize collateral blood supply, and then stimulated directly on the cortical S1 area after removal of the occluder for reperfusion. This study design minimized the impact of stimulation time and collateral circulation on the neuroprotective effect. Neurovascular coupling in penumbra is also an important factor for the spread of ischemic injury. Depolarizing events during focal ischemia, such as anoxic depolarization and peri-infarct spreading depolarizations, may result in the vasoconstrictive form of neurovascular coupling and deteriorated blood flow in penumbra (Shin et al., 2006). In the rat model of stroke, cathodal transcranial direct current stimulation in the rat stroke model had been reported to decrease the number of depolarization and attenuate infraction volume (Notturno et al., 2014). Vagus nerve stimulation can also reduce spreading depolarization burden and cortical infarct volume (Lindemann et al., 2020). This study applies ES to the penumbra S1 cortex during the acute stage of tMCAO. Whether S1 cortical ES protects ischemia/reperfusion by influencing postischemic depolarization and neurovascular coupling remained to be explored. The proposed influence of vasodilation induced by ES cannot be excluded in these experiments.
The BDNF plays an important mediator of the neuroprotective response by DBS. Neuron, astrocyte, and microglia are all possible sources of BDNF. BDNF mRNA is expressed by numerous nuclei in the basal ganglia, and the secretion of BDNF was reported to play an important role in neuroprotection by subthalamic nucleus DBS (Fischer and Sortwell, 2019). The abundance of BDNF transcripts can be found in both glutamatergic and GABAergic neurons from the hippocampus and cortex (Barreda Tomás et al., 2020). However, changes in BDNF expression under pathological conditions are also not fully understood. ES can induce the release of native BDNF, and high-frequency ES (25 and 100 Hz) is more effective at releasing BDNF than low-frequency stimulation (5 and 10 Hz) (Balkowiec and Katz, 2002). This study shows that 30 min of short-term somatosensory cortical ES at a frequency of 20 Hz soon after reperfusion increases the secretion of BDNF. Downstream signal pathways of phosphorylated PI3K and Akt were also upregulated. These results suggested that the BDNF/TrkB signal pathway also plays a role in ES-related neuroprotection during acute ischemia/reperfusion injury.
Autophagy has been proposed as a “double-edged sword” during ischemic injury. Acute and severe ischemia causes excessive autophagy and promotes neuronal damage. Chronic and mild ischemia triggers moderate autophagy to remove damaged proteins and supports neuronal survival (Morselli et al., 2008). Beclin 1, LC3-phosphatidylethanolamine conjugates (LC3-II), and P62 are the three major proteins indicating the level of autophagy. This experiment also showed cortical ES upregulated P62 and suppressed LC3B expression, suggesting that the suppression of autophagy plays a role in cortical ES-related neuroprotection. The detailed mechanisms for the change of BDNF were not studied in these experiments.
Inflammation and autophagy play an important role in the survival of nerve cells and the recovery of neural tissue after ischemia/reperfusion injuries (Mo et al., 2020). Anti-inflammation was also proposed as a possible mechanism of neuroprotection by cortical ES (Baba et al., 2009). Microglia are key players of inflammation after CNS injury. In this study, the activation of phagocytic microglia after ischemia was suppressed by cortical ES, as presented by the decreased number of Iba1/CD68-positive cells in the ischemic penumbra of the cerebral cortex. The result suggested the anti-inflammatory effect of cortical ES. However, the impact of cortical ES on postischemic neuroinflammation was not studied in detail. Activated microglia and infiltrating macrophage are the main phagocytic cells that remove dead cells. In this study, decreased microglial activation may just be a reflection of decreased neuronal death by neuroprotection after ES.
Early revascularization therapy, such as thrombolysis or thrombectomy, is the current treatment of choice for acute ischemic stroke. However, the narrow therapeutic window limits the outcome of these therapies. Adjuvant therapies are required to protect the brain tissue and prolong the therapeutic window. Several preclinical studies demonstrated that CNS ES is a potential acute neuroprotective intervention for ischemic stroke. This study demonstrates the neuroprotective effect of sensory cortical ES applied at the time of reperfusion after occlusion of the middle cerebral artery. These results showed the potential of ES as adjuvant therapy for revascularization treatment after acute ischemic stroke. However, implantation of electrodes is an invasive procedure that may be an obstacle during the clinical application of ES in the acute stage of stroke. The recent development of non-invasive methods for ES, such as high-definition transcranial direct current stimulation, will help the application of early ES during the acute stage of ischemic stroke. The results of this study provide a possible neuroprotective strategy concomitant with revascularization therapies to improve the outcome of acute ischemic stroke.
This study demonstrates that S1 cortical ES is a promising adjuvant neuroprotective therapy for revascularization treatment during the acute stage of ischemic stroke. Advanced techniques and devices for high-definition transcranial direct current stimulation still deserve further development in this regard.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at National Chen Kung University.
K-JT and L-CW: conceptualization, investigation, funding acquisition, project administration, resources, supervision, and writing – review and editing. W-YW, P-CH, and P-YW: data curation. W-YW, K-JT, and L-CW: methodology. W-YW, P-CH, K-JT, and L-CW: validation. L-CW, Y-PC, and W-YW: writing – original draft. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST-108-2321-B-006-024-MY2 and MOST-106-2628-B-006-001-MY4) and National Cheng Kung University Hospital, Taiwan (NCKUH-10909027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to Ya-Chun Hsiao for the services of image acquiring and analyzing from the FACS-like Tissue Cytometry in “Bioimaging Core Facility of the National Core Facility for Biopharmaceuticals, Ministry of Science and Technology, Taiwan.”
Akt, protein kinase B; BDNF, brain-derived neurotrophic factor; DBS, deep brain stimulation; ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal-regulated kinase; ES, electrical stimulation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; Iba1, ionized calcium-binding adapter molecule 1; ICA, internal carotid artery; LC3-II, LC3-phosphatidylethanolamine conjugates; LDF, laser Doppler flowmetry; mTOR, the mammalian target of rapamycin; mNSS, modified neurological severity score; PBS, phosphate-buffered saline; PFA, paraformaldehyde; PI3K, phosphoinositide 3-kinase; PSD, post-surgery day; RIPA, radio-immunoprecipitation assay; SDS, sodium dodecyl sulfate; SEM, standard error of mean; tMCAO, transient intraluminal occlusion of middle cerebral artery; TTC, 2,3,5-triphenyltetrazolium chloride.
Adkins, D. L., Campos, P., Quach, D., Borromeo, M., Schallert, K., and Jones, T. A. (2006). Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp. Neurol. 200, 356–370. doi: 10.1016/j.expneurol.2006.02.131
Baba, T., Kameda, M., Yasuhara, T., Morimoto, T., Kondo, A., Shingo, T., et al. (2009). Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke 40, e598–e605. doi: 10.1161/STROKEAHA.109.563627
Bahr Hosseini, M., Hou, J., Bikson, M., Iacoboni, M., Gornbein, J., and Saver, J. L. (2019). Central nervous system electrical stimulation for neuroprotection in acute cerebral ischemia: meta-analysis of preclinical studies. Stroke 50, 2892–2901. doi: 10.1161/STROKEAHA.119.025364
Balkowiec, A., and Katz, D. M. (2002). Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 22, 10399–10407. doi: 10.1523/jneurosci.22-23-10399.2002
Bao, S. C., Khan, A., Song, R., and Kai-Yu Tong, R. (2020). Rewiring the lesioned brain: electrical stimulation for post-stroke motor restoration. J. Stroke 22, 47–63. doi: 10.5853/jos.2019.03027
Barreda Tomás, F. J., Turko, P., Heilmann, H., Trimbuch, T., Yanagawa, Y., Vida, I., et al. (2020). BDNF expression in cortical GABAergic interneurons. Int. J. Mol. Sci. 21:1567. doi: 10.3390/ijms21051567
Boyko, M., Zlotnik, A., Gruenbaum, B. F., Gruenbaum, S. E., Ohayon, S., Goldsmith, T., et al. (2010). An experimental model of focal ischemia using an internal carotid artery approach. J. Neurosci. Methods 193, 246–253. doi: 10.1016/j.jneumeth.2010.08.026
Cao, S., Zhang, J., Ma, L., Hsia, C. J. C., and Koehler, R. C. (2017). Transfusion of polynitroxylated pegylated hemoglobin stabilizes pial arterial dilation and decreases infarct volume after transient middle cerebral artery occlusion. J. Am. Heart Assoc. 6:e006505. doi: 10.1161/JAHA.117.006505
Chen, A., Xiong, L. J., Tong, Y., and Mao, M. (2013). Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 8, 1011–1016. doi: 10.3892/mmr.2013.1628
Chen, J., Sanberg, P. R., Li, Y., Wang, L., Lu, M., Willing, A. E., et al. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688. doi: 10.1161/hs1101.098367
Cook, D. J., Teves, L., and Tymianski, M. (2012). Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature 483, 213–217. doi: 10.1038/nature10841
De Jesus, S., Okun, M. S., Foote, K. D., Martinez-Ramirez, D., Roper, J. A., Hass, C. J., et al. (2019). Square biphasic pulse deep brain stimulation for parkinson’s disease: the BiP-PD study. Front. Hum. Neurosci. 13:368. doi: 10.3389/fnhum.2019.00368
Deprez, M., Luyck, K., Luyten, L., Tambuyzer, T., Nuttin, B., and Mc Laughlin, M. (2018). An evaluation of the effect of pulse-shape on grey and white matter stimulation in the rat brain. Sci. Rep. 8:752. doi: 10.1038/s41598-017-19023-0
Dougherty, D. D. (2018). Deep brain stimulation: clinical applications. Psychiatr. Clin. North Am. 41, 385–394.
Fischer, D. L., Kemp, C. J., Cole-Strauss, A., Polinski, N. K., Paumier, K. L., Lipton, J. W., et al. (2017). Subthalamic nucleus deep brain stimulation employs trkB signaling for neuroprotection and functional restoration. J. Neurosci. 37, 6786–6796. doi: 10.1523/JNEUROSCI.2060-16.2017
Fischer, D. L., and Sortwell, C. E. (2019). BDNF provides many routes toward STN DBS-mediated disease modification. Mov. Disord. 34, 22–34. doi: 10.1002/mds.27535
Frostig, R. D., Lay, C. C., and Davis, M. F. (2013). A rat’s whiskers point the way toward a novel stimulus-dependent, protective stroke therapy. Neuroscientist 19, 313–328. doi: 10.1177/1073858412462607
Glickstein, S. B., Ilch, C. P., and Golanov, E. V. (2003). Electrical stimulation of the dorsal periaqueductal gray decreases volume of the brain infarction independently of accompanying hypertension and cerebrovasodilation. Brain Res. 994, 135–145. doi: 10.1016/j.brainres.2003.08.001
Glickstein, S. B., Ilch, C. P., Reis, D. J., and Golanov, E. V. (2001). Stimulation of the subthalamic vasodilator area and fastigial nucleus independently protects the brain against focal ischemia. Brain Res. 912, 47–59. doi: 10.1016/s0006-8993(01)02602-6
Goyal, M., Menon, B. K., Van Zwam, W. H., Dippel, D. W., Mitchell, P. J., Demchuk, A. M., et al. (2016). Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731. doi: 10.1016/S0140-6736(16)00163-X
Henrich-Noack, P., Sergeeva, E. G., and Sabel, B. A. (2017). Non-invasive electrical brain stimulation: from acute to late-stage treatment of central nervous system damage. Neural Regen. Res. 12, 1590–1594. doi: 10.4103/1673-5374.217322
Karabanov, A. N., Saturnino, G. B., Thielscher, A., and Siebner, H. R. (2019). Can transcranial electrical stimulation localize brain function? Front. Psychol. 10:213. doi: 10.3389/fpsyg.2019.00213
Kirin, S. C., Yanagisawa, T., Oshino, S., Edakawa, K., Tanaka, M., Kishima, H., et al. (2019). Somatosensation evoked by cortical surface stimulation of the human primary somatosensory cortex. Front. Neurosci. 13:1019. doi: 10.3389/fnins.2019.01019
Koesler, I. B., Dafotakis, M., Ameli, M., Fink, G. R., and Nowak, D. A. (2009). Electrical somatosensory stimulation improves movement kinematics of the affected hand following stroke. J. Neurol. Neurosurg. Psychiatry 80, 614–619. doi: 10.1136/jnnp.2008.161117
Krainik, A., Hund-Georgiadis, M., Zysset, S., and Von Cramon, D. Y. (2005). Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 36, 1146–1152. doi: 10.1161/01.str.0000166178.40973.a7
Kuncel, A. M., and Grill, W. M. (2004). Selection of stimulus parameters for deep brain stimulation. Clin. Neurophysiol. 115, 2431–2441. doi: 10.1016/j.clinph.2004.05.031
Lay, C. C., Davis, M. F., Chen-Bee, C. H., and Frostig, R. D. (2010). Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS One 5:e11270. doi: 10.1371/journal.pone.0011270
Lay, C. C., Davis, M. F., Chen-Bee, C. H., and Frostig, R. D. (2011). Mild sensory stimulation reestablishes cortical function during the acute phase of ischemia. J. Neurosci. 31, 11495–11504. doi: 10.1523/JNEUROSCI.1741-11.2011
Lay, C. C., Davis, M. F., Chen-Bee, C. H., and Frostig, R. D. (2012). Mild sensory stimulation protects the aged rodent from cortical ischemic stroke after permanent middle cerebral artery occlusion. J. Am. Heart Assoc. 1:e001255. doi: 10.1161/JAHA.112.001255
Lay, C. C., Jacobs, N., Hancock, A. M., Zhou, Y., and Frostig, R. D. (2013). Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia. Eur. J. Neurosci. 38, 2445–2452. doi: 10.1111/ejn.12217
Lindemann, J., Rakers, C., Matuskova, H., Simon, B. J., Kinfe, T., and Petzold, G. C. (2020). Vagus nerve stimulation reduces spreading depolarization burden and cortical infarct volume in a rat model of stroke. PLoS One 15:e0236444. doi: 10.1371/journal.pone.0236444
Marquardt, N., Feja, M., Hünigen, H., Plendl, J., Menken, L., Fink, H., et al. (2018). Euthanasia of laboratory mice: are isoflurane and sevoflurane real alternatives to carbon dioxide? PLoS One 13:e0203793. doi: 10.1371/journal.pone.0203793
Mo, Y., Sun, Y. Y., and Liu, K. Y. (2020). Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 15, 1388–1396. doi: 10.4103/1673-5374.274331
Morselli, E., Tasdemir, E., Maiuri, M. C., Galluzzi, L., Kepp, O., Criollo, A., et al. (2008). Mutant p53 protein localized in the cytoplasm inhibits autophagy. Cell Cycle 7, 3056–3061. doi: 10.4161/cc.7.19.6751
Nikoletopoulou, V., Sidiropoulou, K., Kallergi, E., Dalezios, Y., and Tavernarakis, N. (2017). Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab. 26:e235. doi: 10.1016/j.cmet.2017.06.005
Notturno, F., Pace, M., Zappasodi, F., Cam, E., Bassetti, C. L., and Uncini, A. (2014). Neuroprotective effect of cathodal transcranial direct current stimulation in a rat stroke model. J. Neurol. Sci. 342, 146–151. doi: 10.1016/j.jns.2014.05.017
Patel, A. R., Ritzel, R., Mccullough, L. D., and Liu, F. (2013). Microglia and ischemic stroke: a double-edged sword. Int. J. Physiol. Pathophysiol. Pharmacol. 5, 73–90.
Peruzzotti-Jametti, L., Cambiaghi, M., Bacigaluppi, M., Gallizioli, M., Gaude, E., Mari, S., et al. (2013). Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke 44, 3166–3174. doi: 10.1161/strokeaha.113.001687
Shi, L., Rocha, M., Leak, R. K., Zhao, J., Bhatia, T. N., Mu, H., et al. (2018). A new era for stroke therapy: integrating neurovascular protection with optimal reperfusion. J. Cereb. Blood Flow Metab. 38, 2073–2091. doi: 10.1177/0271678x18798162
Shin, H. K., Dunn, A. K., Jones, P. B., Boas, D. A., Moskowitz, M. A., and Ayata, C. (2006). Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J. Cereb. Blood Flow Metab. 26, 1018–1030. doi: 10.1038/sj.jcbfm.9600252
Shomer, N. H., Allen-Worthington, K. H., Hickman, D. L., Jonnalagadda, M., Newsome, J. T., Slate, A. R., et al. (2020). Review of rodent euthanasia methods. J. Am. Assoc. Lab. Anim. Sci. 59, 242–253. doi: 10.30802/aalas-jaalas-19-000084
Swanson, R. A., Morton, M. T., Tsao-Wu, G., Savalos, R. A., Davidson, C., and Sharp, F. R. (1990). A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 10, 290–293. doi: 10.1038/jcbfm.1990.47
von Bornstadt, D., Gertz, K., Lagumersindez Denis, N., Seners, P., Baron, J. C., and Endres, M. (2018). Sensory stimulation in acute stroke therapy. J. Cereb. Blood Flow Metab. 38, 1682–1689.
Wang, J., Dong, W. W., Zhang, W. H., Zheng, J., and Wang, X. (2014). Electrical stimulation of cerebellar fastigial nucleus: mechanism of neuroprotection and prospects for clinical application against cerebral ischemia. CNS Neurosci. Ther. 20, 710–716. doi: 10.1111/cns.12288
Wang, M., Jia, L., Wu, X., Sun, Z., Xu, Z., Kong, C., et al. (2020). Deep brain stimulation improves motor function in rats with spinal cord injury by increasing synaptic plasticity. World Neurosurg. 140, e294–e303. doi: 10.1016/j.wneu.2020.05.029
Wu, C. C., Wang, L. C., Su, Y. T., Wei, W. Y., and Tsai, K. J. (2018). Synthetic α5β1 integrin ligand PHSRN is proangiogenic and neuroprotective in cerebral ischemic stroke. Biomaterials 185, 142–154. doi: 10.1016/j.biomaterials.2018.09.014
Yamamoto, S., Golanov, E. V., and Reis, D. J. (1993). Reductions in focal ischemic infarctions elicited from cerebellar fastigial nucleus do not result from elevations in cerebral blood flow. J. Cereb. Blood Flow Metab. 13, 1020–1024. doi: 10.1038/jcbfm.1993.128
Zhou, Y., Lekic, T., Fathali, N., Ostrowski, R. P., Martin, R. D., Tang, J., et al. (2010). Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke 41, 1521–1527. doi: 10.1161/STROKEAHA.110.583757
Keywords: cortical electrical stimulation, neuromodulation, ischemic stroke, BDNF, PI3K – PKB/Akt signaling pathway
Citation: Wang L-C, Wei W-Y, Ho P-C, Wu P-Y, Chu Y-P and Tsai K-J (2021) Somatosensory Cortical Electrical Stimulation After Reperfusion Attenuates Ischemia/Reperfusion Injury of Rat Brain. Front. Aging Neurosci. 13:741168. doi: 10.3389/fnagi.2021.741168
Received: 14 July 2021; Accepted: 11 October 2021;
Published: 12 November 2021.
Edited by:
Poornima Venkat, Henry Ford Health System, United StatesReviewed by:
Zhongwu Liu, Henry Ford Hospital, United StatesCopyright © 2021 Wang, Wei, Ho, Wu, Chu and Tsai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuen-Jer Tsai, a2p0c2FpQG1haWwubmNrdS5lZHUudHc=; Liang-Chao Wang, bGlhbmdjaGFAbWFpbC5uY2t1LmVkdS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.