- 1Department of Neurosurgery, Ningbo Hospital, Zhejiang University School of Medicine, Ningbo, China
- 2Department of Neurosurgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Burrell College of Osteopathic Medicine, Las Cruces, NM, United States
DNA methylation at the gene promoter region is reportedly involved in the development of intracranial aneurysm (IA). This study aims to investigate the methylation levels of polypyrimidine tract-binding protein 1 (PTBP1) in IA, as well as its potential to predict IA. Forty-eight patients with IA and 48 age- and sex-matched healthy controls were recruited into this study. Methylation levels of CpG sites were determined via bisulfite pyrosequencing. The PTBP1 levels in the blood were determined using a real-time quantitative reverse transcription-polymerase chain reaction test. Significant differences were found between IAs and controls in CpG1 (p = 0.001), CpG2 (p < 0.001), CpG3 (p = 0.037), CpG4 (p = 0.003), CpG5 (p = 0.006), CpG6 (p = 0.02), and mean methylation (p < 0.001). The mRNA level of PTBP1 in the blood was much lower in IAs compared with controls (p = 0.002), and the PTBP1 expression was significantly associated with DNA methylation promoter levels in individuals (r = −0.73, p < 0.0001). In addition, stratification analysis comparing smokers and non-smokers revealed that tobacco smokers had significantly higher levels of DNA methylation in PTBP1 than non-smokers (p = 0.002). However, no statistical difference in PTBP1 methylation was found between ruptured and unruptured IA groups (p > 0.05). The ROC analyses of curves revealed that PTBP1 methylation may be a predictor of IA regardless of sex (both sexes, area under curve (AUC) = 0.78, p < 0.0001; male, AUC = 0.76, p = 0.002; female, AUC = 0.79, p < 0.0001). These findings suggest that long-term tobacco smoke exposure led to DNA methylation in the promoter region of the PTBP1 gene, which further decreased PTBP1 gene expression and participated in the pathogenesis of IA. The methylation of PTBP1 may be a potential predictive marker for the occurrence of IA.
Introduction
An intracranial aneurysm (IA) is a cystic pathological dilatation of the intracranial arteries. The walls of cerebral arteries are prone to rupture when they become too weak to resist hemodynamic pressure, leading to distention (Etminan et al., 2014). As a severe disease, IA has a prevalence and mortality of 7% and 30%, respectively, in ages 35–75 among the Chinese population (Chen et al., 2018). Previous studies have demonstrated that hemodynamic stress, vascular risk factors (i.e., hypertension, hyperlipidemia, arteriosclerosis, and smoking), and genetic susceptibility play an important role in the formation of IA (Kassam et al., 2004; Frosen et al., 2012, 2013). However, the mechanisms of IA pathogenesis are not fully understood.
DNA methylation is a common epigenetic modification that regulates gene expression by recruiting proteins involved in gene repression or by inhibiting the binding of transcription factors to DNA (Moore et al., 2013). The levels of DNA methylation can be altered by environmental shifts, such as a change in nutritional status or environmental exposures (e.g., smoking), which affects DNA conformation, stability, and its ability to interact with proteins (Tsaprouni et al., 2014). A previous study found that smoking can induce cytochrome-c oxidase subunit II aberrant methylation and apoptosis in human umbilical vascular endothelial cells (Yang et al., 2015). Tobacco smoking was a risk factor for cerebrovascular disease, including IA, which participated in the process of arterial vascular disease through epigenetic regulation of DNA methylation (Siemelink et al., 2018). Another study suggested that DNA methylation played an important role in the development of IA, involving the regulation of immune and inflammatory responses, cell functions, and cell signal transduction (Yu et al., 2017).
Polypyrimidine tract-binding protein (PTBP1), also known as hnRNP I, is a member of the heterogeneous nuclear ribonucleoprotein family, which has four RNA-binding domains, and plays a vital role in alternative splicing, mRNA stability, localization, and polyadenylation (Singh et al., 1995; Wollerton et al., 2004). Expression of PTBP1 positively correlates with the growth of various cancers, such as brain tumors (Cheung et al., 2009), breast cancers (He et al., 2014), primary colorectal tumors (Takahashi et al., 2015), and various malignant cell lines (Wang et al., 2008), thus indicating poor prognosis. PTBP1 is a main regulator of the enzyme, pyruvate kinase (PK), which plays an important role in the abnormal growth and enhanced glycolysis of pulmonary arterial endothelial cells (PAEC) in pulmonary hypertension (Caruso et al., 2017). Besides, it has also been proven that PTBP1 is expressed in vascular smooth muscle cells (VSMCs) (Gooding et al., 2003), which are involved in regulating the expression of endothelial nitric oxide synthase (Yi et al., 2015), promoting proliferation and dedifferentiation of VSMC to cause neointimal hyperplasia (Kang et al., 2013; Wang et al., 2019). Therefore, we hypothesized that the PTBP1 gene promotor of DNA methylation would be associated with the pathological development of IA.
In this study, we recruited 48 angiography-proven IA patients and 48 age- and sex-matched healthy individuals from Eastern China, and we performed a case-control test to validate the role of PTBP1 gene promotor of DNA methylation in increasing the risk of IA in our homogenous samples.
Materials and Methods
Sample and Clinical Data
This research was approved by the Ethics Committee of Ningbo First Hospital. A total of 48 IA (48.08 ± 0.82 years) cases and 48 age-/sex- matched controls (46.63 ± 0.87 years) were recruited from the Ningbo First Hospital between September 2015 and December 2016. All of the patients with IA were diagnosed according to standard classifications using 320 dynamic state volume computed tomography angiography or digital subtraction angiography. All of the healthy control subjects were excluded from cardiovascular diseases, cerebrovascular diseases, liver and kidney diseases, as well as other serious diseases, such as malignant tumors.
Clinical data, including age, sex, and plasma levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and total cholesterol (TC) were collected for analysis. Blood samples were collected from participants on the first day of hospitalization. The levels of HDL, LDL, TG, and TC were measured using standard enzymatic methods, and were assessed using the automatic biochemical analyzers (Olympus AU2700, Japan).
Pyrosequencing Assay
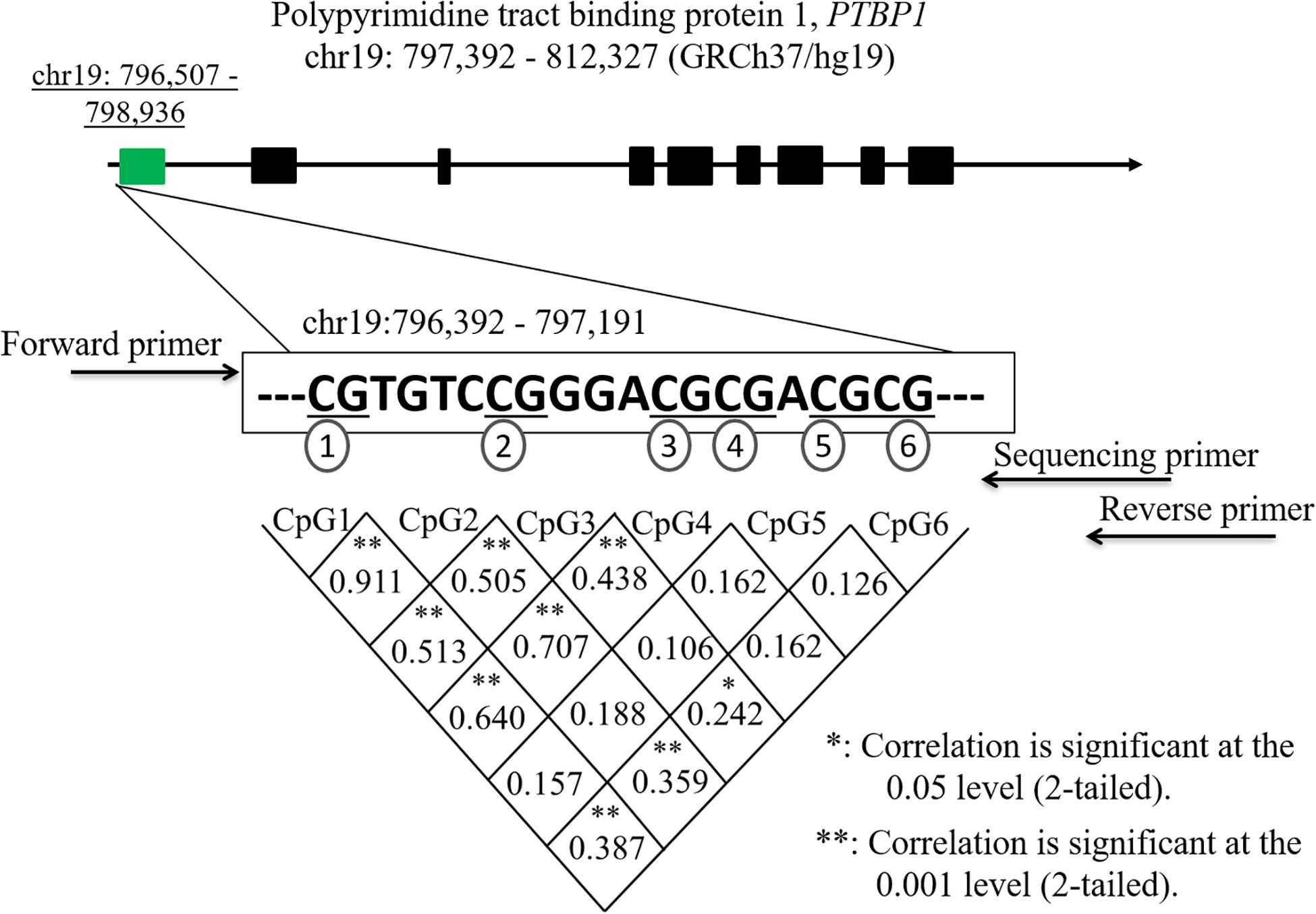
Human genomic DNA extraction was performed using an automatic nucleic acid extraction instrument (Lab-Aid 820, Xiamen City, China) using a magnetic bead isolation method. Six GpG dinucleotides on a fragment (chr19:796,392–797,191) within the promoter region of the PTBP1 gene were selected for DNA methylated pyrosequencing. The EpiTect Bisulfite kit (Qiagen, Hilden, Germany) was used for the sodium bisulfite DNA conversion. DNA methylation was performed on a PyroMark System (Qiagen, Hilden, Germany). The polymerase chain reaction (PCR) primers were designed using the PyroMark Assay Design software (Qiagen, Hilden, Germany). The sequences of the PCR primers for the six CpG regions of the PBTP1 gene are listed as follows: the forward primer, 5′-GTATTTGTGGTTATTGTGGAAATAGT-3′; the reverse primer, 5′-Biotin-AAAACCCTCAAAACTCCATATTAATT-3′; the sequencing primer, 5′-GGTTATTGTGGAAATAGTT-3′.
Quantitative Real-Time Polymerase Chain Reactions
The total RNA was extracted from the blood using TRIzol reagent (Invitrogen, CA, United States) according to the manufacturer’s detailed instructions. The quality of the RNA was assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, MA, United States). Real-time quantitative reverse transcription−polymerase chain reaction (qRT−PCR) was performed on the LightCycler 480 system (Roche, Mannheim, Germany) using the SYBR Green Kit (TaKaRa, Dalian, China). The GAPDH mRNA was used as the reference for quantification. The primers and PCR application conditions for GAPDH (forward primer, 5′-CAACGGATTTGGTCGTATTGG-3′ reverse primer, 5′-TGATGGCAACAATATCCACTTTACC-3′) and PTBP1 (forward primer, 5′-GCTCAGGATCATCGTGGAGAA-3′; reverse primer, 5′-ATCTTCAACACTGTGCCGAACTT-3′) were described in a previous study (Santiago and Potashkin, 2015).
Statistical Analyses
The DNA methylation levels and the clinical data between different groups were presented as means ± standard error (SE) or number. The correlation between PTBP1 methylation and clinical data was analyzed using Pearson’s correlation test. The normally distributed data were analyzed using the independent samples t-test, and the abnormally distributed data were analyzed via a non-parametric test. A receiver operating characteristic (ROC) curve was used to evaluate the sensitivity of the PTBP1 methylation for the diagnosis of IA. SPSS software version 23.0 (IBM Corp. Armonk, NY, United States) was used for statistical analyses, and GraphPad Prism V8.0 (La Jolla, CA, United States) was used for the figure analysis. Power analysis was performed using Power and Sample Size Calculation software (Nashville, TN, United States). The data were considered statistically significant at p < 0.05.
Results
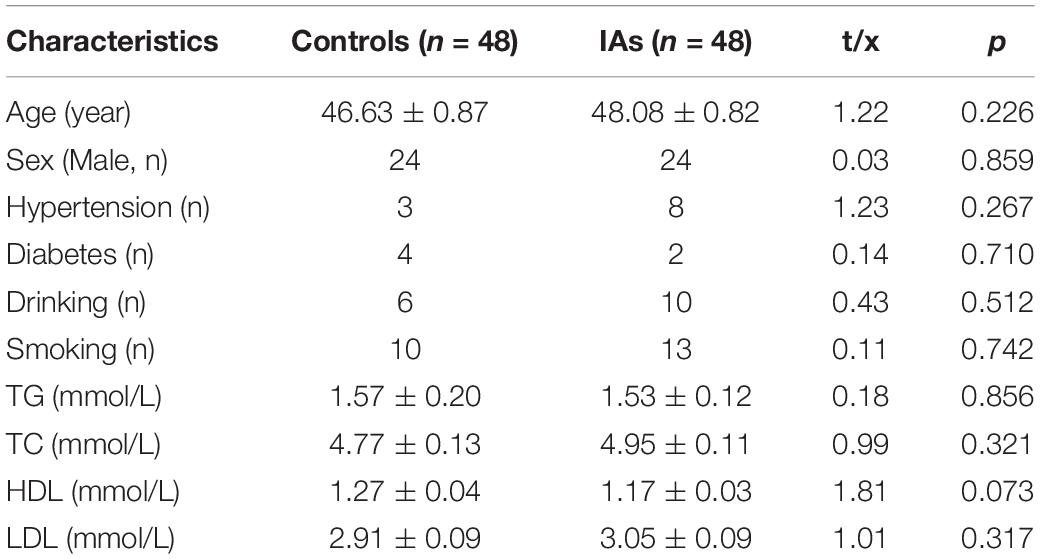
The clinical characteristics between IA patients and controls are shown in Table 1. No significant differences were observed between the two groups regarding clinical data, such as age, sex, hypertension, diabetes, drinking, smoking, TG, TC, HDL, and LDL (p > 0.05). Six CpG dinucleotides in the promoter region (hg19, chr19:796,392–797,191) of the PTBP1 gene were selected to measure DNA methylation levels (Figure 1). Significant correlations were found among the levels of CpG1, CpG2, CpG3, CpG4, and CpG6 methylation (p < 0.05).
As shown in Table 2, significant differences were found in CpG1 (IAs vs. Controls: 3.27 ± 0.26 vs. 1.89 ± 0.31, p = 0.001), CpG2 (IAs vs. Controls: 4.32 ± 0.28 vs. 2.59 ± 0.36, p < 0.001), CpG3 (IAs vs. Controls: 2.22 ± 0.21 vs. 1.20 ± 0.48, p = 0.037), CpG4 (IAs vs. Controls: 1.72 ± 0.24 vs. 0.74 ± 0.22, p = 0.003), CpG5 (IAs vs. Controls: 1.94 ± 0.59 vs. 0.22 ± 0.16, p = 0.006), and CpG6 (IAs vs. Controls: 1.37 ± 0.40 vs. 0.32 ± 0.18, p = 0.02). Therefore, the mean DNA methylation level in IA patients (2.48 ± 0.20) was much higher than that of control subjects (1.16 ± 0.22, p < 0.001). Power calculation showed that our study had great power (100%) to detect the significant association of PTBP1 methylation based on the nominal type I error rate of 0.01.
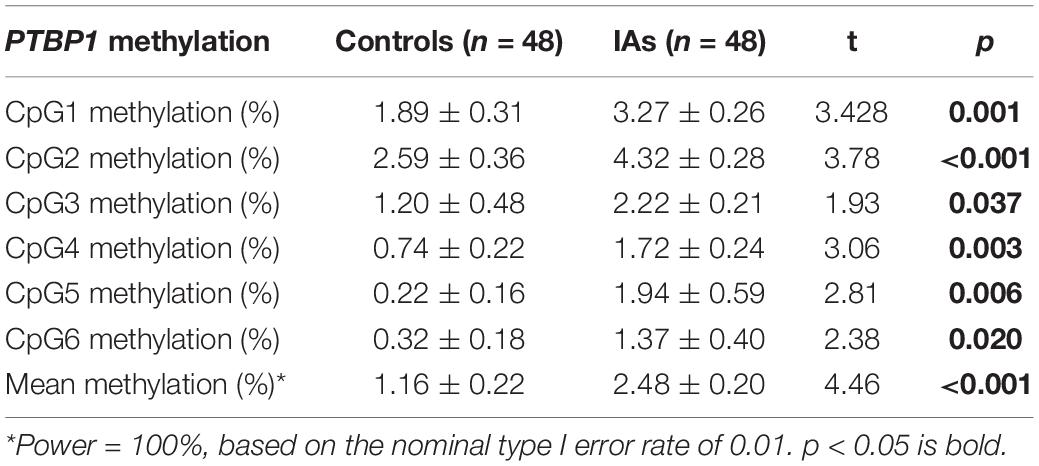
Table 2. DNA methylation difference of six CpG dinucleotides comparison within the PTBP1 between IAs and Controls.
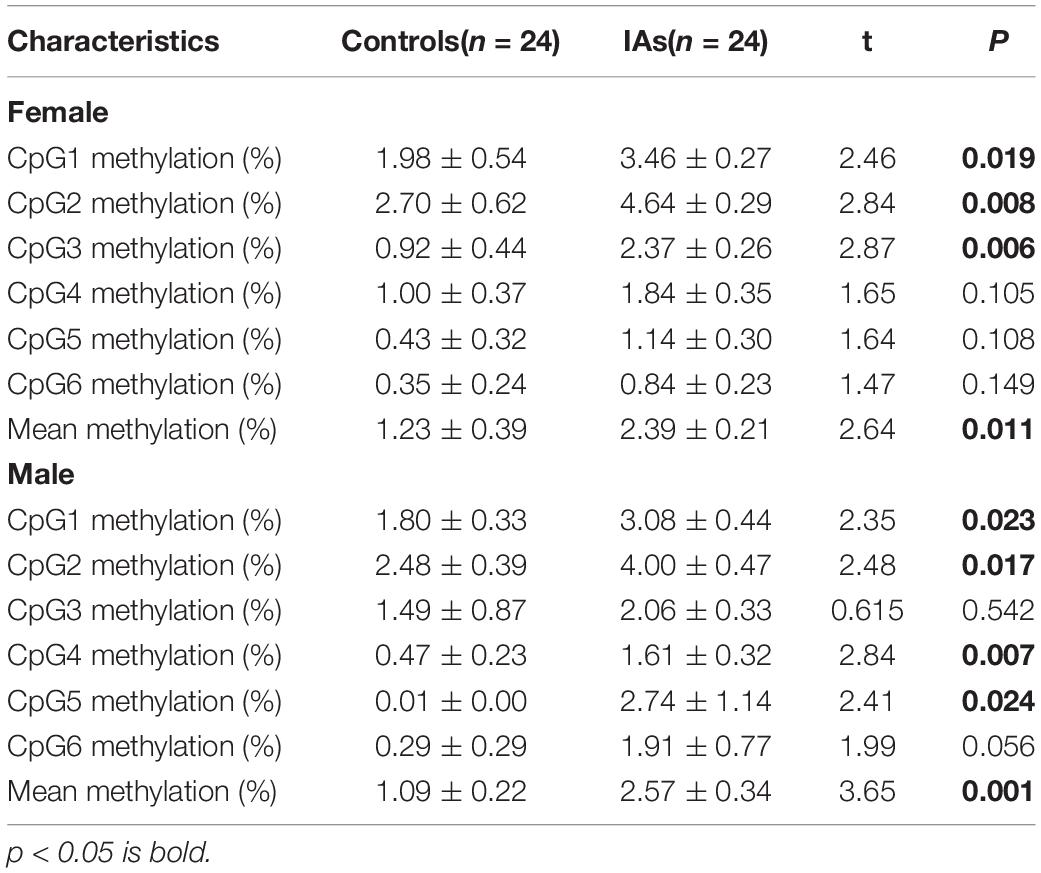
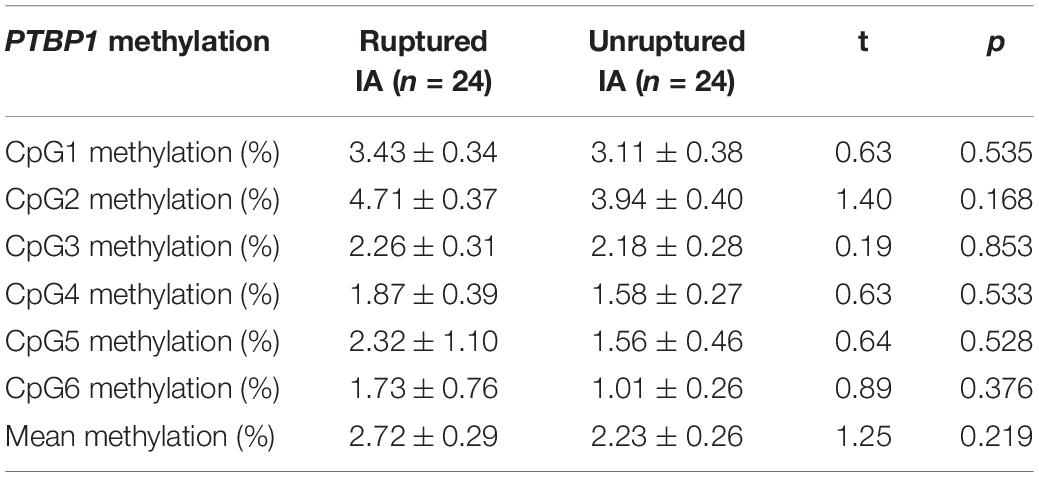
In addition, stratification analysis by sex showed that the mean methylation levels of PTBP1 in both male (2.57 ± 0.34 vs. 1.09 ± 0.22, p = 0.001) and female (2.39 ± 0.21 vs. 1.23 ± 0.39, p = 0.011, Table 3) patients with IA were higher than the control group. However, the CpG3 methylation was significantly higher in female IA patients (IAs vs. Controls: 2.37 ± 0.26 vs. 0.92 ± 0.44, p = 0.006), but not in males (IAs vs. Controls: 2.06 ± 0.33 vs. 1.49 ± 0.87, p = 0.542, Figure 2A). The CpG4 and CpG5 methylation levels were significant higher in male IA patients (IAs vs. Controls: CpG4, 1.61 ± 0.32 vs. 0.47 ± 0.32, p = 0.007; CpG5, 2.74 ± 1.14 vs. 0.01 ± 0.00, p = 0.024), but not in females (IAs vs. Controls: CpG4, 1.84 ± 0.35 vs. 1.00 ± 0.37, p = 0.105; CpG5, 1.14 ± 0.30 vs. 0.43 ± 0.32, p = 0.108, Figures 2B,C). We also performed subgroup analysis stratified by smoking and non-smoking in all participants. Tobacco smokers had significantly higher levels of DNA methylation in PTBP1 than non-smokers (2.92 ± 0.35 vs. 1.49 ± 0.17, p = 0.002, Figure 3A). The mean methylation levels of PTBP1 in smoke IAs (3.74 ± 0.44) were significantly higher than that in smoke controls (1.76 ± 0.20, p = 0.005, Figure 3B), non-smoking IAs (2.29 ± 0.19, p = 0.007, Figure 3B), and non-smoker controls (0.97 ± 0.25, p < 0.001, Figure 3B). A similar result was also shown between non-smoker IAs and non-smoker controls (p = 0.002, Figure 3B). The PTBP1 mRNA levels in the blood were much lower in IAs compared with controls (p = 0.002, Figure 3C). Moreover, the mRNA expression of PTBP1 was significantly associated with the level of DNA methylation promotion in individuals (r = −0.73, p < 0.0001, Figure 3D). However, the following subgroup analysis stratified by ruptured and unruptured aneurysms in patients revealed no statistical difference in PTBP1 methylation between ruptured IA and unruptured IA groups (Table 4, p > 0.05).
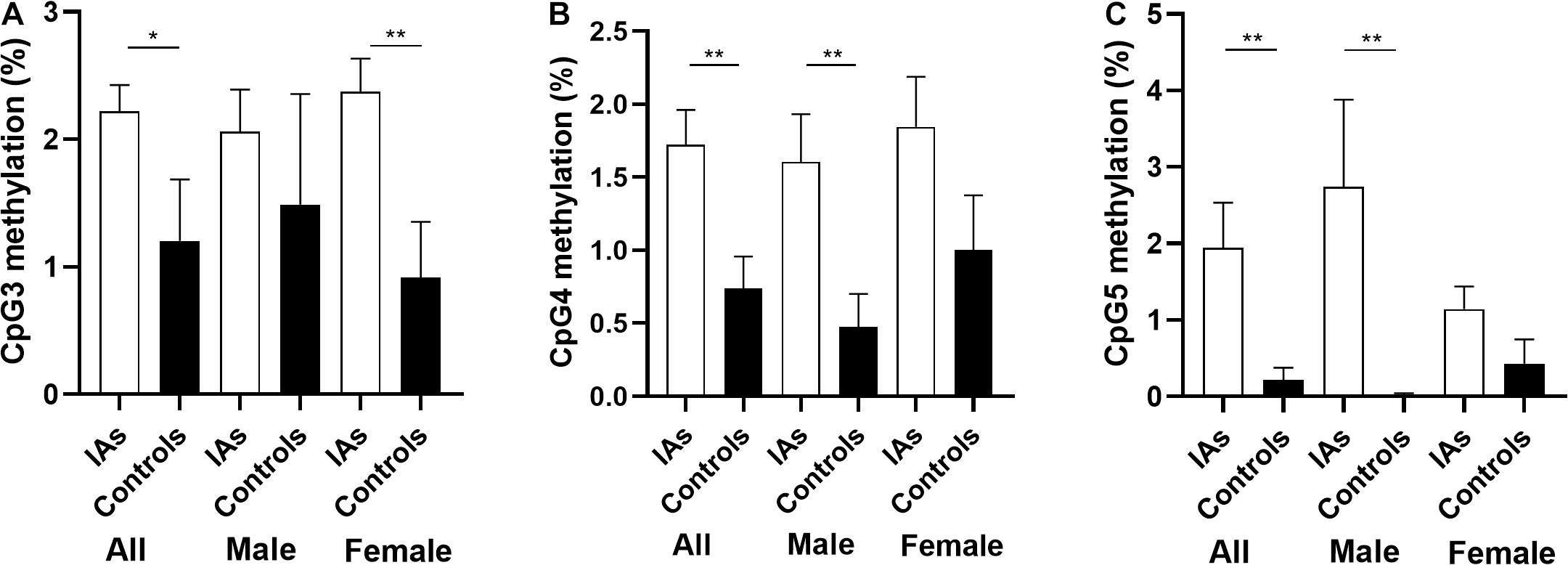
Figure 2. Comparison of PTBP1 promoter CpG3, CpG4, CpG5 methylation between IAs and controls in different sex. (A) The comparison of CpG3 methylation among groups. (B) The comparison of CpG4 methylation among groups. (C) The comparison of CpG5 methylation among groups. *p < 0.05, **p < 0.001.
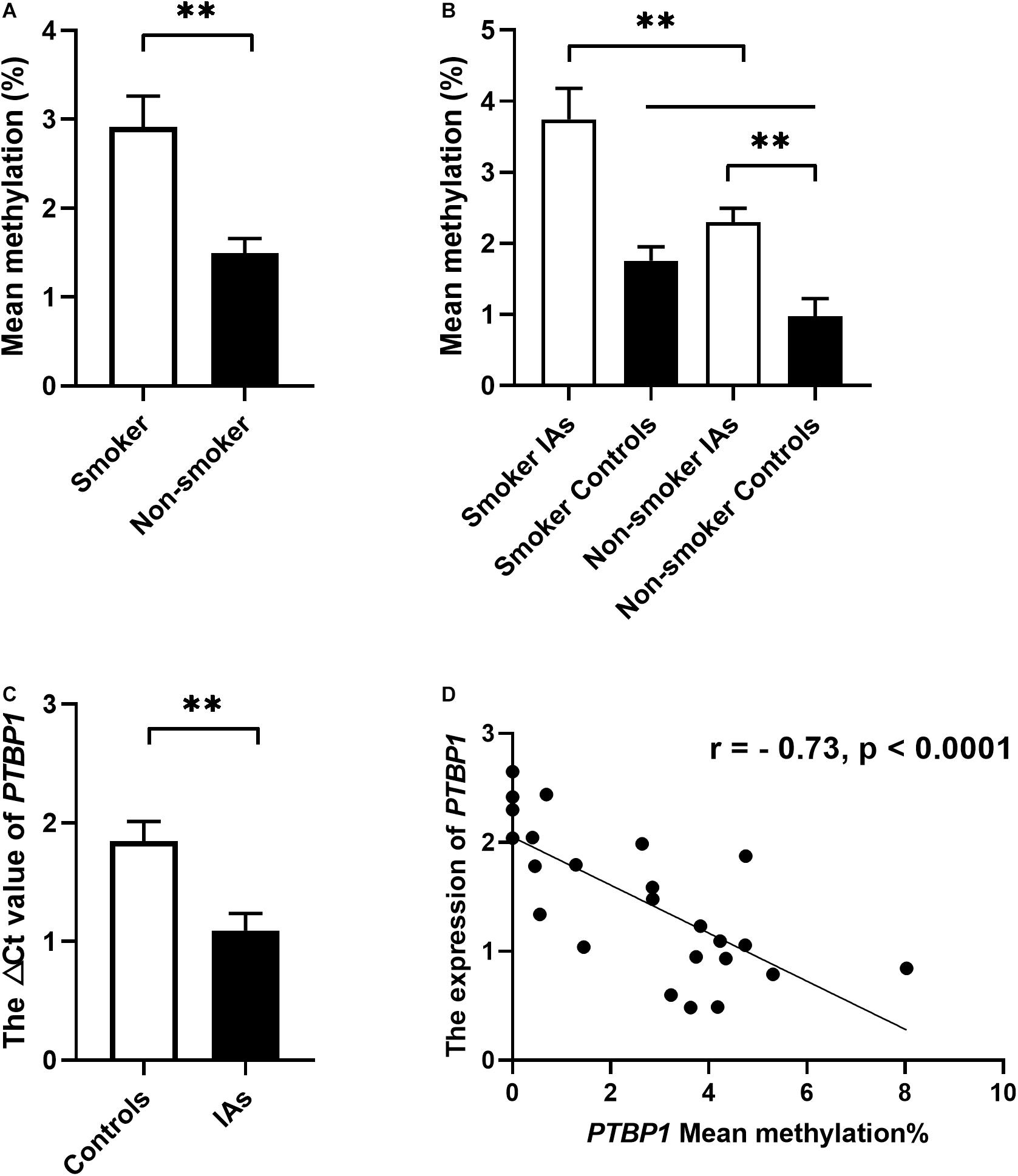
Figure 3. The relationship between PTBP1 promoter DNA methylation, smoking, and gene expression in the individuals. (A) PTBP1 DNA methylation was much higher in smokers than in non-smokers. (B) PTBP1 DNA methylation was much higher in smoker IAs than other groups. (C) PTBP1 mRNA expression was much lower in IAs than in healthy controls. (D) The PTBP1 expression was significantly associated with DNA methylation in all individuals. **p < 0.001.
The ROC analyses of curves revealed that PTBP1 methylation may be a predictor of IA regardless of sex (both sex, area under curve (AUC) = 0.78, p < 0.0001; male, AUC = 0.76, p = 0.002; female, AUC = 0.79, p < 0.0001, Figure 4).
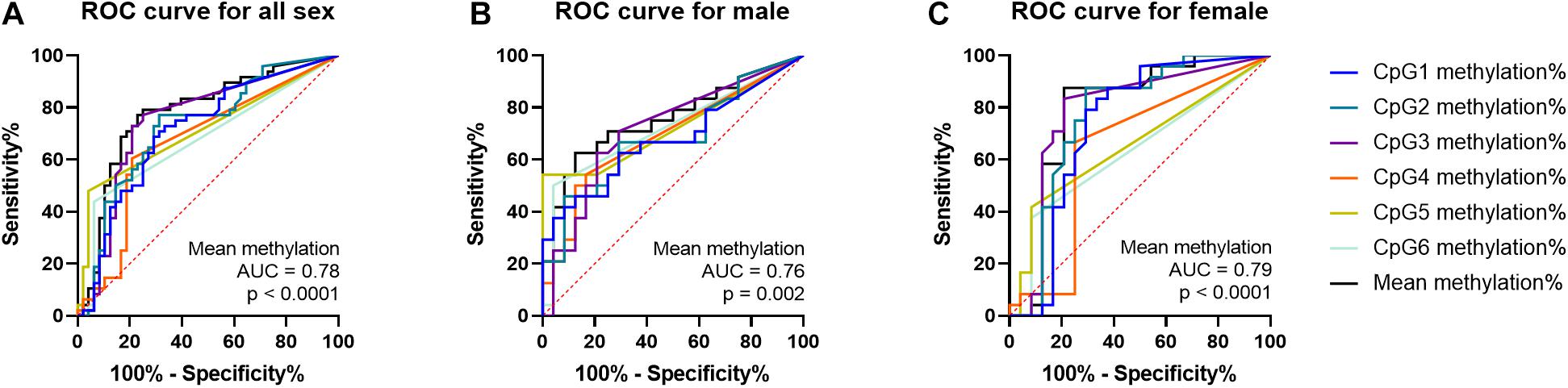
Figure 4. ROC curves of PTBP1 DNA methylation in all sexes, male and female samples. (A) ROC curve for PTBP1 DNA methylation in all sexes. (B) ROC curve for PTBP1 DNA methylation in males. (C) ROC curve for PTBP1 DNA methylation in females.
Discussion
IA formation was reportedly affected by the complex interaction between genetic and environmental risk factors (Vlak et al., 2013). The goal of this study was to explore the relationship between PTBP1 gene methylation and the risk of IA in the Chinese Han population. Our results found that tobacco smoking could increase the level of PTBP1 DNA methylation. The PTBP1 methylation levels in IA patients were much higher than those found in healthy controls, whereas blood PTBP1 expression in IAs was much lower than controls. The PTBP1 methylation in CpG3, CpG4, and CpG5 showed a sex-dependent effect between IAs and controls.
PTBP1 is widely expressed in the vasculature, including endothelial cells, VSMCs, and adventitial fibroblasts, and is involved in the pathophysiological changes of blood vessels (Caruso et al., 2017; Zhang et al., 2017). PTBP1 regulates VSMC proliferation and neointimal hyperplasia during the development of vascular restenosis (Wang et al., 2019). PTBP1 inhibition could attenuate the VSMC proliferation and suppress neointimal hyperplasia after vascular injury (Wang et al., 2019). Current studies believe that the main feature of IA pathogenesis is derived from the decreased extracellular matrix and the number of VSMCs in the cerebral vessel wall (Miyata et al., 2020). DNA methylation in the promoter region negatively correlates with gene expression, and is involved in the pathological process of cerebrovascular disease (Deng et al., 2019). Abnormal methylation of gene promoter participated in the regulation of VSMC (Han et al., 2014) proliferation and intimal hyperplasia (Wang et al., 2021) after vascular injury by affecting the expression of vascular-related genes. Such as, VSMCs mitochondrial D-loop methylation can lead to impaired mitochondrial function and loss of contractile phenotype in vascular disease (Liu et al., 2020). In the current study, our results showed that blood PTBP1 levels in IA patients were much lower than that of healthy controls, which correlated with the higher level of DNA methylation in the PTBP1 promotor region in IAs compared to controls. However, no significant difference was found in the methylation level of the PTBP1 gene promoter region between ruptured and unruptured IAs. Therefore, DNA methylation of the PTBP1 promotor may participate in the pathophysiological formation of IA by attenuating PTBP1 expression in patients.
Long-term heavy smoking abuse is an important factor for epigenetic modifications, including DNA methylation in human diseases (Hillemacher et al., 2019; Lee and Park, 2020). Cigarette smoke can promote cerebrovascular disease in an important pathway by inducing the inflammatory/matrix remodeling phenotype in cerebral VSMCs (Starke et al., 2013). It was also suggested that smoking tobacco affects the regulation of gene expression by affecting DNA methylation, thereby increasing the risk of cardiovascular disease (Haase et al., 2018; Siemelink et al., 2018). Studies have shown that long-term smoking could increase or decrease the level of DNA methylation in the promoter region of genes, further alter the level of gene expression, and participate in the pathological process of smoking-related diseases (Huan et al., 2016). Our previous study proved that regular tobacco smoking could increase the methylation of nitric oxide synthase 1 adaptor protein, and could increase the risk of IA and cerebral arteriovenous malformations in Han Chinese (Wang et al., 2016). In the current study, our results showed that smoking could increase the level of PTBP1 promotor methylation in Han Chinese individuals. Therefore, tobacco smoking may be involved in the pathophysiological formation of IA by increasing the level of DNA methylation in the PTBP1 promotor and by attenuating PTBP1 expression in the patients.
Sex-specific differences have been reported in IA outcomes (Vlak et al., 2011) and many gene DNA methylation rates (Bain et al., 2021). Compared with males, the females had a much incidence of unruptured aneurysms, as well as a much higher risk of rupture (Yanagisawa et al., 2020). Besides, males and females will also have different methylation reactions to changes in the external environment. For example, when people are exposed to polybrominated biphenyls (Curtis et al., 2020) or perinatally exposed to lead (Wang et al., 2020), they can induce tissue- and sex-specific DNA methylation changes. In the present study, PTBP1 CpG3 methylation levels were much higher in female IAs than in the controls, but higher levels of CpG4 and CpG5 methylation were found in male IAs. However, the average methylation of PTBP1 promotor between the case and control groups is consistent in male and female groups. Moreover, The ROC analyses revealed that PTBP1 methylation may be a predictor of IA regardless of sex.
Study Limitations
Some limitations remain in our work. Firstly, although our study has excellent statistical power, the sample size was relatively small, and more sample tests, including various ages of smokers, will be needed to confirm our findings in future studies. Secondly, only six GpG dinucleotides on a fragment (chr19:796,392–797,191) were selected to represent the entire promoter of the PTBP1 gene. More CpGs should be included in future studies. Thirdly, this was a candidate study, but the mechanism research was likely not as thorough as it should have been. Animal models and cell experiments are necessary to further verify and validate future results.
Conclusion
Collectively, these findings suggest that long-term tobacco smoke exposure led to DNA methylation in the promoter region of the PTBP1 gene, which further decreased PTBP1 gene expression, and participated in the pathogenesis of IA. PTBP1 methylation may be a potential predictive marker for the occurrence of IA.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Ningbo First Hospital.
Author Contributions
YH, ZL, and GC contributed to the conception and design of the study. ZW, SZ, JZ, SN, JS, and XG organized the database and experiments. SZ and YH performed the statistical analysis. ZW and YH wrote the first draft of the manuscript. YH and CL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the grants from the Zhejiang Provincial Natural Science Foundation of China (LQ18H090002), the Medicine and Health Science and Technology Projects of Zhejiang Province (2018KY665, 2019KY565, 2019KY573, and 2019KY160), the Ningbo Health Branding Subject Fund (PPXK2018-04), and the Ningbo Science and Technology Innovation 2025 Major Project (2019B10105).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bain, S. A., Marshall, H., De La Filia, A. G., Laetsch, D. R., Husnik, F., and Ross, L. (2021). Sex-specific expression and DNA methylation in a species with extreme sexual dimorphism and paternal genome elimination. Mol. Ecol. 2021:15842. doi: 10.1111/mec.15842
Caruso, P., Dunmore, B. J., Schlosser, K., Schoors, S., Dos Santos, C., Perez-Iratxeta, C., et al. (2017). Identification of MicroRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 136, 2451–2467. doi: 10.1161/circulationaha.117.028034
Chen, J., Liu, J., Zhang, Y., Tian, Z., Wang, K., Zhang, Y., et al. (2018). China Intracranial Aneurysm Project (CIAP): protocol for a registry study on a multidimensional prediction model for rupture risk of unruptured intracranial aneurysms. J. Transl. Med. 16:263.
Cheung, H. C., Hai, T., Zhu, W., Baggerly, K. A., Tsavachidis, S., Krahe, R., et al. (2009). Splicing factors PTBP1 and PTBP2 promote proliferation and migration of glioma cell lines. Brain 132, 2277–2288. doi: 10.1093/brain/awp153
Curtis, S. W., Gerkowicz, S. A., Cobb, D. O., Kilaru, V., Terrell, M. L., Marder, M. E., et al. (2020). Sex-specific DNA methylation differences in people exposed to polybrominated biphenyl. Epigenomics 12, 757–770. doi: 10.2217/epi-2019-0179
Deng, G. X., Xu, N., Huang, Q., Tan, J. Y., Zhang, Z., Li, X. F., et al. (2019). Association between promoter DNA methylation and gene expression in the pathogenesis of ischemic stroke. Aging 11, 7663–7677. doi: 10.18632/aging.102278
Etminan, N., Buchholz, B. A., Dreier, R., Bruckner, P., Torner, J. C., Steiger, H. J., et al. (2014). Cerebral aneurysms: formation, progression, and developmental chronology. Transl. Stroke Res. 5, 167–173. doi: 10.1007/s12975-013-0294-x
Frosen, J., Tulamo, R., Heikura, T., Sammalkorpi, S., Niemela, M., Hernesniemi, J., et al. (2013). Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol. Commun. 1:71.
Frosen, J., Tulamo, R., Paetau, A., Laaksamo, E., Korja, M., Laakso, A., et al. (2012). Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol. 123, 773–786. doi: 10.1007/s00401-011-0939-3
Gooding, C., Kemp, P., and Smith, C. W. (2003). A novel polypyrimidine tract-binding protein paralog expressed in smooth muscle cells. J. Biol. Chem. 278, 15201–15207. doi: 10.1074/jbc.m210131200
Haase, T., Muller, C., Krause, J., Rothemeier, C., Stenzig, J., Kunze, S., et al. (2018). Novel DNA methylation sites influence GPR15 expression in relation to smoking. Biomolecules 8:74. doi: 10.3390/biom8030074
Han, X. B., Zhang, H. P., Cao, C. J., Wang, Y. H., Tian, J., Yang, X. L., et al. (2014). Aberrant DNA methylation of the PDGF gene in homocysteinemediated VSMC proliferation and its underlying mechanism. Mol. Med. Rep. 10, 947–954. doi: 10.3892/mmr.2014.2249
He, X., Arslan, A. D., Ho, T. T., Yuan, C., Stampfer, M. R., and Beck, W. T. (2014). Involvement of polypyrimidine tract-binding protein (PTBP1) in maintaining breast cancer cell growth and malignant properties. Oncogenesis 3:e84. doi: 10.1038/oncsis.2013.47
Hillemacher, T., Rhein, M., Burkert, A., Heberlein, A., Wilhelm, J., Glahn, A., et al. (2019). DNA-methylation of the dopamin receptor 2 gene is altered during alcohol withdrawal. Eur. Neuropsychopharmacol. 29, 1250–1257. doi: 10.1016/j.euroneuro.2019.09.002
Huan, T., Joehanes, R., Schurmann, C., Schramm, K., Pilling, L. C., Peters, M. J., et al. (2016). A whole-blood transcriptome meta-analysis identifies gene expression signatures of cigarette smoking. Hum. Mol. Genet. 25, 4611–4623.
Kang, K., Peng, X., Zhang, X., Wang, Y., Zhang, L., Gao, L., et al. (2013). MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J. Biol. Chem. 288, 25414–25427. doi: 10.1074/jbc.m113.460287
Kassam, A., Horowitz, M., Chang, Y. F., and Peters, D. (2004). Altered arterial homeostasis and cerebral aneurysms: a review of the literature and justification for a search of molecular biomarkers. Neurosurgery 54, 1199–1211. doi: 10.1227/01.neu.0000119708.26886.55
Lee, H. S., and Park, T. (2020). The influences of DNA methylation and epigenetic clocks, on metabolic disease, in middle-aged Koreans. Clin. Epigenet. 12:148.
Liu, Y. F., Zhu, J. J., Yu Tian, X., Liu, H., Zhang, T., Zhang, Y. P., et al. (2020). Hypermethylation of mitochondrial DNA in vascular smooth muscle cells impairs cell contractility. Cell Death Dis. 11:35.
Miyata, T., Minami, M., Kataoka, H., Hayashi, K., Ikedo, T., Yang, T., et al. (2020). Osteoprotegerin prevents intracranial aneurysm progression by promoting collagen biosynthesis and vascular smooth muscle cell proliferation. J. Am. Heart Assoc. 9:e015731.
Moore, L. D., Le, T., and Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38. doi: 10.1038/npp.2012.112
Santiago, J. A., and Potashkin, J. A. (2015). Network-based metaanalysis identifies HNF4A and PTBP1 as longitudinally dynamic biomarkers for Parkinson’s disease. Proc. Natl. Acad. Sci. U S A 112, 2257–2262. doi: 10.1073/pnas.1423573112
Siemelink, M. A., Van Der Laan, S. W., Haitjema, S., Van Koeverden, I. D., Schaap, J., Wesseling, M., et al. (2018). Smoking is associated to DNA methylation in atherosclerotic carotid lesions. Circ. Genom. Precis Med. 11:e002030.
Singh, R., Valcarcel, J., and Green, M. R. (1995). Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science 268, 1173–1176. doi: 10.1126/science.7761834
Starke, R. M., Ali, M. S., Jabbour, P. M., Tjoumakaris, S. I., Gonzalez, F., Hasan, D. M., et al. (2013). Cigarette smoke modulates vascular smooth muscle phenotype: implications for carotid and cerebrovascular disease. PLoS One 8:e71954. doi: 10.1371/journal.pone.0071954
Takahashi, H., Nishimura, J., Kagawa, Y., Kano, Y., Takahashi, Y., Wu, X., et al. (2015). Significance of polypyrimidine tract-binding Protein 1 expression in colorectal cancer. Mol. Cancer Ther. 14, 1705–1716. doi: 10.1158/1535-7163.mct-14-0142
Tsaprouni, L. G., Yang, T. P., Bell, J., Dick, K. J., Kanoni, S., Nisbet, J., et al. (2014). Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics 9, 1382–1396. doi: 10.4161/15592294.2014.969637
Vlak, M. H., Algra, A., Brandenburg, R., and Rinkel, G. J. (2011). Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 10, 626–636. doi: 10.1016/s1474-4422(11)70109-0
Vlak, M. H., Rinkel, G. J., Greebe, P., and Algra, A. (2013). Independent risk factors for intracranial aneurysms and their joint effect: a case-control study. Stroke 44, 984–987. doi: 10.1161/strokeaha.111.000329
Wang, C., Norton, J. T., Ghosh, S., Kim, J., Fushimi, K., Wu, J. Y., et al. (2008). Polypyrimidine tract-binding protein (PTB) differentially affects malignancy in a cell line-dependent manner. J. Biol. Chem. 283, 20277–20287. doi: 10.1074/jbc.m803682200
Wang, K., Liu, S., Svoboda, L. K., Rygiel, C. A., Neier, K., Jones, T. R., et al. (2020). Tissue- and Sex-Specific DNA methylation changes in mice perinatally exposed to lead (Pb). Front. Genet. 11:840. doi: 10.3389/fgene.2020.00840
Wang, Y., Xu, Y., Yan, S., Cao, K., Zeng, X., Zhou, Y., et al. (2021). Adenosine kinase is critical for neointima formation after vascular injury by inducing aberrant DNA hypermethylation. Cardiovasc. Res. 117, 561–575. doi: 10.1093/cvr/cvaa040
Wang, Z., Gan, X., Qiu, C., Yang, D., Sun, X., and Zeng, Z. (2019). Role of polypyrimidine tract-binding protein 1/yin yang 2 signaling in regulating vascular smooth muscle cell proliferation and neointima hyperplasia. Toxicol. Appl. Pharmacol. 383:114747. doi: 10.1016/j.taap.2019.114747
Wang, Z., Zhao, J., Sun, J., Nie, S., Li, K., Gao, F., et al. (2016). Sex-dichotomous effects of NOS1AP promoter DNA methylation on intracranial aneurysm and brain arteriovenous malformation. Neurosci. Lett. 621, 47–53. doi: 10.1016/j.neulet.2016.04.016
Wollerton, M. C., Gooding, C., Wagner, E. J., Garcia-Blanco, M. A., and Smith, C. W. (2004). Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol. Cell. 13, 91–100. doi: 10.1016/s1097-2765(03)00502-1
Yanagisawa, T., Zhang, H., Suzuki, T., Kamio, Y., Takizawa, T., Morais, A., et al. (2020). Sex and Genetic background effects on the outcome of experimental intracranial aneurysms. Stroke 51, 3083–3094. doi: 10.1161/strokeaha.120.029651
Yang, M., Chen, P., Peng, H., Zhang, H., Chen, Y., Cai, S., et al. (2015). Cigarette smoke extract induces aberrant cytochrome-c oxidase subunit II methylation and apoptosis in human umbilical vascular endothelial cells. Am. J. Physiol. Cell Physiol. 308, C378–C384.
Yi, B., Ozerova, M., Zhang, G. X., Yan, G., Huang, S., and Sun, J. (2015). Post-transcriptional regulation of endothelial nitric oxide synthase expression by polypyrimidine tract-binding Protein 1. Arterioscler. Thromb. Vasc. Biol. 35, 2153–2160. doi: 10.1161/atvbaha.115.305750
Yu, L., Wang, J., Wang, S., Zhang, D., Zhao, Y., Wang, R., et al. (2017). DNA Methylation regulates gene expression in intracranial aneurysms. World Neurosurg. 105, 28–36. doi: 10.1016/j.wneu.2017.04.064
Zhang, H., Wang, D., Li, M., Plecita-Hlavata, L., D’alessandro, A., Tauber, J., et al. (2017). Metabolic and Proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a MicroRNA-124/PTBP1 (Polypyrimidine tract binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 136, 2468–2485. doi: 10.1161/circulationaha.117.028069
Keywords: intracranal aneurysm, DNA Methylation, polypyrimidine tract binding protein 1, tobacco smoking, CpG island
Citation: Wang Z, Zhou S, Zhao J, Nie S, Sun J, Gao X, Lenahan C, Lin Z, Huang Y and Chen G (2021) Tobacco Smoking Increases Methylation of Polypyrimidine Tract Binding Protein 1 Promoter in Intracranial Aneurysms. Front. Aging Neurosci. 13:688179. doi: 10.3389/fnagi.2021.688179
Received: 30 March 2021; Accepted: 14 June 2021;
Published: 06 July 2021.
Edited by:
Gang Chen, First Affiliated Hospital of Soochow University, ChinaReviewed by:
Yuyun Xiong, Affiliated Hospital of Jiangsu University, ChinaYang Wang, Anhui Provincial Hospital, China
Copyright © 2021 Wang, Zhou, Zhao, Nie, Sun, Gao, Lenahan, Lin, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqin Lin, bmJ5eWx6cUBhbGl5dW4uY29t; Yi Huang, aHVhbmd5MTAyQGdtYWlsLmNvbQ==; Gao Chen, ZC1jaGVuZ2FvQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Zhepei Wang1,2†
Zhepei Wang1,2† Cameron Lenahan
Cameron Lenahan Yi Huang
Yi Huang Gao Chen
Gao Chen