
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 17 August 2021
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.670332
 Xue Du1†
Xue Du1† Yan Gao2†
Yan Gao2† Su Liu1
Su Liu1 Jingya Zhang1
Jingya Zhang1 Diksha Basnet1
Diksha Basnet1 Junjun Yang1
Junjun Yang1 Jiehui Liu1
Jiehui Liu1 Yijie Deng1
Yijie Deng1 Jiayong Hu1
Jiayong Hu1 Peijun Wang2
Peijun Wang2 Jianhui Liu1*
Jianhui Liu1*Background: Postoperative cognitive dysfunction (POCD) is a general complication following cardiac and major non-cardiac surgery amongst the elderly, yet its causes and mechanisms are still unknown. The present study aimed to detect whether regional cerebral blood flow (CBF) is altered in the brain before surgery in POCD patients compared with non-POCD (NPOCD) patients, thus, CBF variation may potentially predict the occurrence of early POCD.
Methods: Fifty patients scheduled for spinal stenosis surgery were enrolled in the study. All study participants completed a battery of neuropsychological tests (NPTs) by a well-trained neuropsychologist before the surgery and 1 week after the surgery. POCD was defined when the preoperative to postoperative difference of at least two of the NPTs’ |Z|-scores with reference to a control group exceeded 1.96. Pulsed arterial spin-labeling (ASL) MRI was scanned at least 1 day before surgery. The ASLtbx toolkit and SPM12 were applied to preprocess and correct the images, which were then normalized to the MNI brain template space to obtain standardized cerebral perfusion images.
Results: POCD was identified in 11 out of 50 patients (22%). The CBF of the right superior temporal lobe, right and left middle cingulate gyrus, and the right hippocampus, and parahippocampal gyrus in POCD group was lower than that in NPOCD group (P < 0.001). The CBF of the pars triangularis of inferior frontal gyrus in POCD group was higher than that in NPOCD group (P < 0.001).
Conclusions: These preliminary findings suggest that CBF premorbid alterations may happen in cognitively intact elderly patients that develop early POCD. Alterations of preoperative CBF might be a bio-marker for early POCD that can be detected by noninvasive MRI scans.
Surgical procedures and general anesthesia are correlated to a large number of complications, including postoperative cognitive dysfunction (POCD) or delayed neurocognitive recovery. Particularly in elderly patients, the POCD incidence after surgery is high, ranging from 10% to 62% (Chi et al., 2017; Glumac et al., 2019), and POCD seriously affects the mental health, social aspects, and quality of life of the aged patients (Spalletta et al., 2012; Needham et al., 2017). The main POCD susceptibility factors include aging combined with diabetes mellitus, hypertension, coronary heart disease, and other comorbidities, type and time of operation, anesthesia type, and anesthetic methods (Czyz-Szypenbejl et al., 2019). It can last for several days to several months (Rundshagen, 2014). POCD has a considerable impact on the healthcare system, which can lead to prolonged hospital admission, reduced quality of life, and increased dependency (Williams-Russo et al., 1995; Moller et al., 1998; Newman et al., 2001; Monk et al., 2008). To apply preventative measures and better balance the surgery risks and benefits, it is of great importance to identify more accurately preoperative patients at increased POCD risk. Neuropsychological tests (NPTs) are regarded to be salient among objective methods of cognition assessment. A complete cognitive function assessment is fairly difficult, and conducting a full neuropsychological examination is tedious and straining for patients. Therefore, it is urgent to search for an objective and effective tool to evaluate the POCD.
Advancements in neuro-imaging, especially in magnetic resonance imaging (MRI) have provided helpful tools to noninvasively measure neuronal activity as well as capture detailed human brain images (Alexopoulos et al., 2012). The present study tested the hypothesis that arterial spin-labeling (ASL) neuro-imaging might depict early and subtle changes in brain perfusion preoperatively in cognitively intact elderly patients and this information could be utilized to predict early phases of subsequent cognitive decline (Xekardaki et al., 2015). The major advantages of ASL MRI are its non-invasiveness without contrast agent injection and the lack of exposure to ionizing radiation (Riederer et al., 2018). MRI could be employed to establish neuropathological POCD aspects. MRI results relevant to POCD could help clinicians diagnose patients at high risk and reduce postoperative mortality (Kant et al., 2017). To date, there is still a lack of evidence of ASL-associated POCD diagnosis. The present study aimed to search and identify ASL findings associated with those cognitive deficits to help prediction and prevention of POCD patients at high risk.
A total of 103 patients scheduled for spinal stenosis surgery were admitted to Tongji Hospital from January 2017 to December 2019 and were screened for the study. Written informed consent was obtained from the patients. This investigation was approved by the Ethics Committee of Tongji Hospital affiliated with Tongji University (Approval Number K2017005). This study was registered before patient enrollment at http://www.chictr.org.cn (ChiCTR-DDD-17010762). We obtained informed consent from every participant after a full protocol explanation. The authors followed the Declaration of Helsinki principles. This manuscript adheres to the applicable Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Eligibility criteria were as follows: (1) age > 65 years; (2) American Society of Anesthesiologists (ASA) Physical Status Classification I–III; and (3) participants were undergoing spinal stenosis decompression surgery.
The exclusion criteria: (1) pre-existing mental and/or psychiatric disease; (2) Parkinson’s disease (PD); (3) audition impairment, vision or language troubles impeding communication; (4) situations unsuitable for an MRI (claustrophobia); (5) diagnosis of dementia or previous stroke; (6) inability to undergo preoperative and postoperative cognitive evaluation; and (7) preoperative Mini-Mental State Examination (MMSE) < 20.
Study-related processes included intraoperative data collection, preoperative (baseline) multimodal assessments, and postoperative multimodal assessments of the study participants postoperatively at 1 week. Patients were recruited 1–3 days before surgery. The MRI scan and the baseline Neuropsychological Tests (NPTs) were performed one day before the surgery. Post-operative NPTs were performed on the 7th day of the surgery. To generate the control group mean and standard deviation (SD), the volunteers were examined in the same intervals as the patient group and the mean difference was calculated. The demographics, MRI data, NPTs results, pre- and post-operative data were collected and analyzed. Figure 1 illustrates the workflow of the investigation assessments.
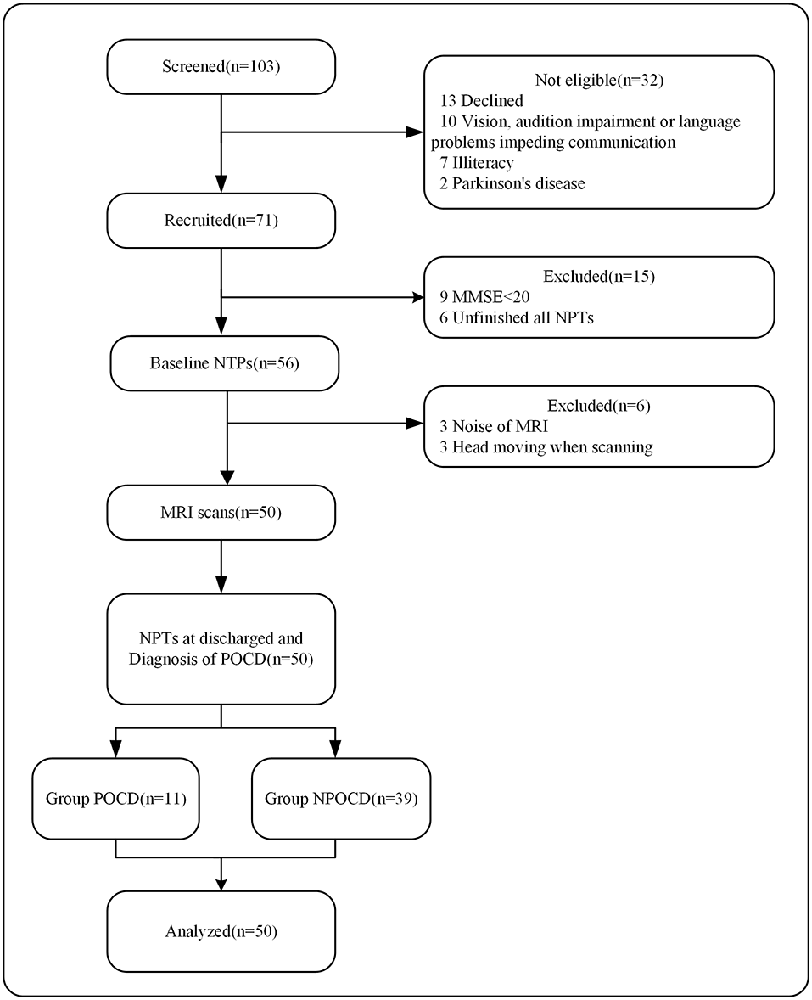
Figure 1. Flow diagram for study assessments. NPTs, neuropsychological tests; MRI, magnetic resonance imaging; POCD, postoperative cognitive dysfunction; NPOCD, non-POCD.
Neuropsychological evaluation was conducted by a well-trained neuropsychologist at two time points for patients and volunteers: (1) baseline: the day before surgery; and (2) 7 days postoperatively. In addition, subjects were first screened with MMSE and Montreal Cognitive Assessment (MoCA) to exclude subjects with cognitive impairment. Patients with an MMSE score <20 or who had Mild Cognitive Impairment (MCI) were not tested any further. Postoperative delirium was assessed daily by study members using either a disorder assessment (CAM) or a CAM-intensive care unit (CAM-ICU). Delirium assessments were completed daily until the 7th inclusive day of hospitalization.
The test battery consisted of 11 tests: the Rey Auditory Verbal Learning Test (AVLT immediate and delayed), Stroop Colour Word Test (STROOP), Digits Symbol Substitution Test (DSST), Judgment Of Line Orientation Test (JLOT), Brief Visuospatial Memory Test-revised (BVMT immediate and delayed), Trail Making Test (TMT), Semantic Fluency Test (SFT), and Forward And Backward Digit Span Test (DST forward and backward), which primarily focus on memory, attention, and executive function.
For assessing POCD, the difference between postoperative and preoperative test scores was used, instead of a single result. To generate a control group mean and SD, 20 healthy volunteers that were age and education-matched with the patients were tested in the same intervals as the patient group, and the mean difference was calculated. Improvement in the same repeated test scores is possible by subtracting the population mean from the observed differences in patient’s test scores to control the study effect. Healthy volunteers completed NPTs mentioned above at the same interval.
The difference in post- and pre-operative scores was termed x, and the counterpart in the volunteer group was μ (mean value for the difference in the volunteer group). The SD of the difference scores in volunteers was computed as δ, and the Z-score could be calculated as follows:
POCD was defined as at least two NPT |Z|-scores ≥ 1.96.
All the subjects underwent MRI at Tongji Hospital using a 3.0-T MRI (Verio, Siemens, Erlangen, Germany) scanner equipped with a 32-channel head coil. In the MRI data acquisition, we instructed participants to stay awake, relax with their eyes closed, and remain motionless. Participants fasted for at least 4 h prior to the scan and withheld any medications with vasoactive properties on the day of the scan. Pulsed ASL(PASL) images were acquired using an echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) 3,500 ms, echo time(TE) 15 ms, flip angle 90°, slice thickness 3.5 mm without gap, field of view (FOV) 224 mm × 224 mm, matrix size 128 × 128, inversion time (TI1 700 ms, TI2 1800 ms), number of excitations 1, number of slices 33, total scan time 5 min and 31 s. ASL imaging protocol acquired an M0 image and 45 control/label pairs. High-resolution T1-weighted images were acquired using a whole brain three-dimensional brain volume imaging sequence(MPRAGE)with the following parameters: TR 2530 ms, TE 2.98ms, inversion time 450 ms, flip angle 7°, slice thickness 1 mm, no gaps, FOV 256 mm ×256 mm, matrix size 256 × 256, voxel size 1 mm ×1 mm ×1 mm, number of slices 192, total scan time 6 min and 3 s. For each subject, all images were inspected during the collection of MRI data to ensure that no visible artifacts were found. The artifact appeared in three of our subjects’ MR images, so the relevant sequence was rescanned immediately to obtain a qualified MR image of each of these subjects.
Imaging data were processed using the ASL toolbox (ASL tbx1; Wang et al., 2008) and SPM12.The brain imaging toolkit DPABI2 was used for data pre-processing and analysis(Yan et al., 2016). The detailed procedures have been described in a previous study (Wang et al., 2008). Steps to process ASL images included motion correction, spatial smoothing, exclude out-of-brain voxels, CBF quantization, partial volume correction, and spatial registration to Montreal Neurological Institute (MNI) standard brain space. Spatial smoothing was performed with an isotropic Gaussian kernel with FWHM = 6 mm. Pre-processed ASL labels and control image pairs were then successively subtracted. CBF was then calculated by the equation based on the M0 as follows:
where f is regional CBF (in milliliters per 100 g per minute), λ is blood and tissue water partition coefficient which equals to 0.9 (in milliliters per gram), α is 95% inversion efficiency, M0 and ΔM are fully relaxed image intensity, and signal difference, respectively (control and label), TI1 and TI2 (in ms) are inversion times, and T1α is 1,500 ms at 3 T (longitudinal relaxation time of blood; Xekardaki et al., 2015). The mean ASL image was registered to the high-resolution structural T1 image using SPM 12. The corresponding registration transform was used to register the CBF maps to the structural MRI.
With the help of segmentation tools of SPM12, the high resolution T1-weighted MRI was segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). The images obtained were then projected into local ASL image space, based on the registration correspondence between the mean ASL control image and the structural image, which was later used to extract the CBF signal for partial volume correction. The Diffeomorphic Anatomical Registration Through Exponential Lie Algebra (DARTEL) procedure tool in SPM12 was used to prompt a probable map of GM and WM for all the subjects based on their segmented local templates. By applying a linear affine transformation, the local template was warped to the MNI standard space. The individual subject’s brain was mapped into MNI space with these transformations. On each voxel of GM, a partial volume correction was carried out. As mentioned above, with the help of the same registration transformation from the mean ASL control image to the structural image, the partial volume corrected CBF maps were subsequently warped to the MNI space.
SPM12 software package was used to conduct statistical modeling and model evaluation of standardized cerebral perfusion images obtained by the above steps in the POCD group and NPOCD group. CBF maps in the two groups were compared using two-sample t-tests. To exclude individual differences, age, education, and gender were included as covariates in the regression. To control the false positive rate and reduce type I error, Gaussian random field (Gauss random field, GRF) principle of FWE was used for multiple corrections (FWE cluster—level corrected, p < 0.05, voxel—p < 0.001). Then, we got the statistically significant brain regions level.
Cluster obtained from the difference analysis between ASL groups was selected as region of interest (ROI), and the CBF value of ROIs was extracted as the average CBF of each region.
After obtaining written consent, standard anesthesia was provided. We induced anesthesia by sufentanil, etomidate, and cis-atracurium, followed by endotracheal intubation. We mechanically ventilated patients and held the end-tidal CO2 constant at 35 ± 5 mmHg. Following general anesthesia induction, we maintained patients with 1–1.2 minimal alveolar concentration sevoflurane with remifentanil and propofol TCI until the end of the surgery. All the patients were infused with cis-atracurium intermittently as required. During the entire surgical procedure, we rigorously monitored the electrocardiogram, arterial blood pressure, and oxygen saturation. We kept the bispectral index between 60 and 40 to guarantee appropriate anesthesia depth. We provided postoperative analgesia through patient-controlled analgesia.We delivered sufentanil at a rate of 1.5 μg/h, with a 1.5–2 μg bolus and lockout interval of 15 min for breakthrough pain. We assessed pain after surgery using the visual analog scale (VAS) score (0 = no pain and 10 = worst pain imaginable).
We performed statistical analysis using SPSS 21.0 (IBM, USA). Descriptive statistics of variables were examined in patients with and without POCD. Normally distributed data are presented as mean and standard deviation (SD), whereas non-normally distributed data are presented as median and inter-quartile range (IQR). Categorical data were expressed as the number and percentage. Standard Chi-square statistics or Fisher’s exact test was used to analyze categorical variables. Quantitative data were analyzed either using a two-sample t-test or Wilcoxon rank sum test when data deviated from the normal distribution.
A total of 71 patients were included. The trial details are shown in Figure 1. Fifty patients completed both the preoperative MRI scan and NPTs follow-up. The study group included 27 females and 23 males. We identified POCD in 11 of the 50 patients (22%) on the 7th day after surgery. No positive CAM scores occurred on the postoperative assessment. Demographic and operative data for these patients are presented in Tables 1, 2, respectively based on the POCD presence or absence. The mean POCD patient age was 68 ± 2.4 years, while the mean NPOCD patient age was 71 ± 5.5 years. There was no significant difference in other clinical characteristics.
Table 3 shows the pre- and post-operative NPTs scores between the POCD group and the NPOCD group.
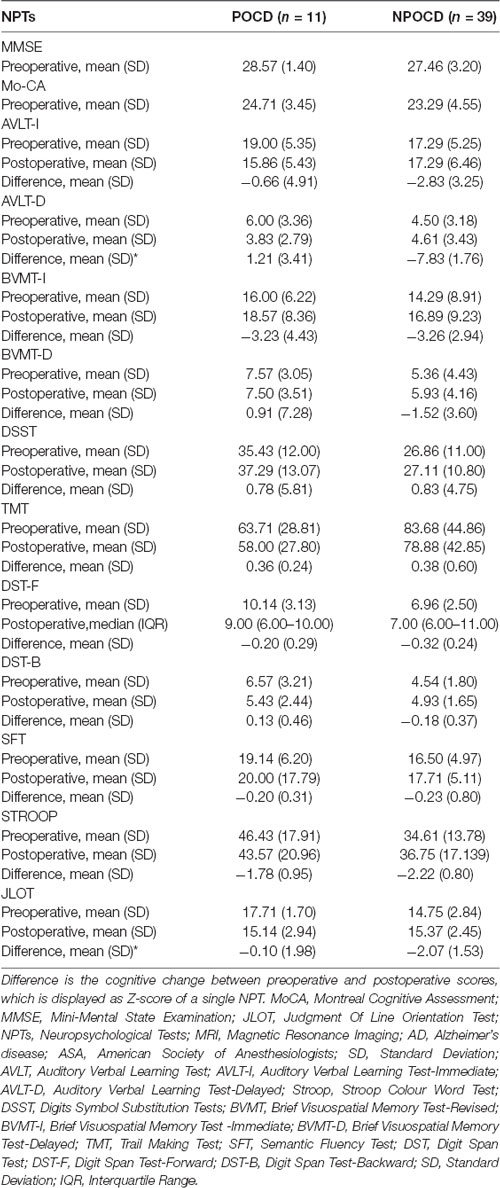
Table 3. Summary statistics of patients with and without POCD in different cognitive function tests.
After FWE multiple comparison correction, we found significant CBF differences in brain areas including the frontal gyrus (the pars triangularis of inferior frontal gyrus), the temporal lobe (right superior temporal gyrus), the right hippocampus and the parahippocampal gyrus, and the cingulate gyrus (right and left medial gyrus). Hypoperfusion was observed in the right superior temporal gyrus of the temporal lobe, right and left middle cingulate gyrus, the right hippocampus, and parahippocampal gyrus in the POCD group. In addition, hyperperfusion in the pars triangularis of the inferior frontal gyrus in POCD patients were also found (Figure 2 and Table 4).
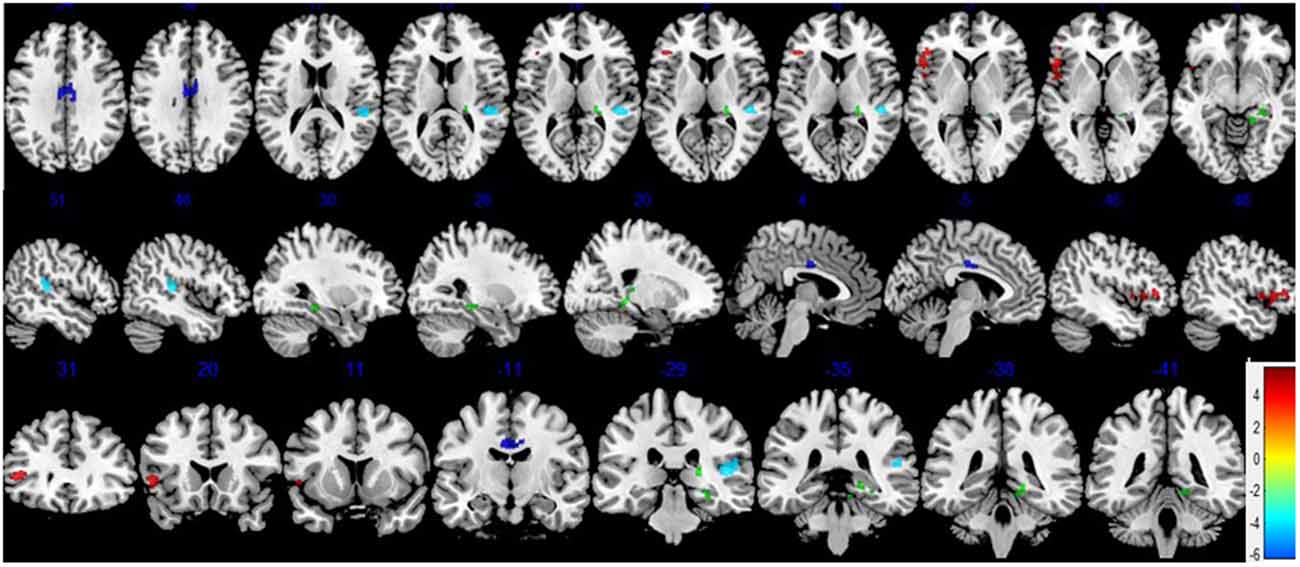
Figure 2. Differences in cerebral blood flow (CBF) between the POCD and NPOCD groups. Voxelwise comparison of the partial volume corrected ASL revealed decreased CBF in the right superior temporal gyrus of the temporal lobe, right and left middle cingulate gyrus, and the right hippocampus and the parahippocampal gyrus, and increased CBF in the pars triangularis of inferior frontal gyrus in POCD patients compared with NPOCD patients. Voxel-based comparison superimposed onto Montreal Neurologic Institute standard brain in the coronal, axial, and sagittal planes. Due to the large cluster of brain regions corrected by multiple comparisons, space is limited to show all sections of brain regions, and only some brain regions with statistical differences are shown. There was no significant difference in the overlapping brain regions. Blue to red color indicates low to high blood flow.
Cerebrovascular imaging has a great appeal in understanding neurological diseases. Among the various MRI parameters, ASL provides an indicator of tissue perfusion (Alsop et al., 2000, 2015; Johnson et al., 2005; Dai et al., 2009). Because MRI is a part of routine screening for cognitive decline in many centers, ASL is a cost-effective and operator-independent tool for assessing early cognitive decline that simply extends the existed scanning session by a few minutes. However, there have been limited studies using ASL MRI to investigate POCD. The primary goal of the current study was to explore the association between ASL-derived measures of brain CBF and POCD incidence. We found a significant association between CBF measures and POCD incidence in our study.
A large number of studies have shown that decreased CBF is a sensitive marker of early cognitive impairment (Scheff et al., 2015). We observed that the POCD subjects with normal performance evaluated by neuropsychological tests preoperatively displayed obvious decreased CBF both in a part of the temporal lobe, the cingulate cortex, the hippocampus, and parahippocampal gyrus, which might be interpreted as a neurocognitive reserve (CR; Tomlinson et al., 1968). Previous studies have found cognitive reserve in patients with AD, MCI, and Parkinson’s disease (PD; Bosch et al., 2010; Xekardaki et al., 2015). Cognitive reserve (CR) means individuals with a higher reserve are able to cope with brain pathology (i.e., decreased CBF in our study) through some form of active compensatory strategy better than those with lower reserve. CR can be defined as the ability to use alternate cognitive strategies, in order to optimize or maximize performance on cognitive tasks (Baldivia et al., 2008). The above so-called active compensatory strategy or alternative cognitive strategies include the individual predisposition, education, and social integration (Xekardaki et al., 2015). That is why some individuals may maintain normal cognitive function longer than other individuals. Therefore, CR provides a possible explanation for the current discrepancy between the evidence of brain damage and the absence of clinical symptoms (Stern, 2002). CR is hypothesized to moderate the association between brain pathology and the expression of the pathology rather than protecting the brain against the development of brain pathology (Brickman et al., 2011; Singh-Manoux et al., 2011). In other words, greater CR allows individuals to cope better with the cognitive changes associated with aging with reductive CBF in our study.Some individuals may maintain normal cognitive function longer than others due to their predisposition, education, and social integration. Subjects with POCD may initially be able to compensate for these changes of CBF and maintain normal cognitive status on account of the above reasons. Therefore, CBF may be a potential bio-marker of associated POCD. These may also indicate that surgery and anesthesia may accelerate the depletion of cognitive reserve in POCD patients, thus, cognitive symptoms may become apparent.
In our study, we found that POCD patients showed distinct decreased CBF preoperatively in the temporal lobe, the cingulate cortex, the hippocampus, and parahippocampal gyrus. The temporal lobe mainly functions as sensory information processor, turning it into meaningful memories, language, and emotions (Aminoff et al., 2013; Pauli et al., 2017). Previous studies have demonstrated hypoperfusion in the temporal lobe in early cognitive impairment patients (Pantoni, 2010; Goto et al., 2011; Maekawa et al., 2014). The cingulate cortex is involved in many brain diseases because of its diverse structure and behavioral functions, as well as its extensive connections to many different cortical regions involved in a variety of behaviors. This region is the center of emotion, behavior, and memory (Scheff et al., 2015). Yoshida et al. (2011) reported that in the early stage of AD, cingulate gyrus CBF decreased significantly. Xekardaki et al. (2015) demonstrated reduced ASL in the posterior cingulate cortex at baseline is associated with the development of subsequent subtle neuropsychological deficits in healthy elderly control subjects. Accumulating studies indicate that deterioration in cognitive function is associated with a reduction in local CBF in the hippocampus. Recently, a research demonstrated that CBF in the hippocampus decreases with the progression of AD (Li et al., 2020). Meanwhile, Another research found the strongest decrease in CBF in the left hippocampal region in the MCI in their recent study (Duan et al., 2020). These studies indicated that decreased cognitive function is associated with decreased regional CBF in the temporal lobe, the cingulate gyrus, the hippocampus, and parahippocampal gyrus, which may underline the role of decreased CBF in cognitive impairment, consistent with our results.
In contrast with the above, our results also showed that, compared with the NPOCD group, preoperative MRI in the POCD group indicated increased CBF in the frontal gyrus. Previous studies have demonstrated hypoperfusion in the frontal gyrus in patients with impaired cognitive function (Bangen et al., 2014; Ding et al., 2014; Zou et al., 2014; Zhang et al., 2021). There have also been some studies about hyperperfusion in the frontal gyrus in patients with impaired cognitive function which is consistent with our results (Duan et al., 2020). It was also found that reduced CBF can coexist with increased CBF in the early stages of a neurodegenerative process. It was suggested that hyperperfusion is a compensatory increase in neural activity (Alsop et al., 2008). Thus, we speculate that hyperperfusion in the frontal gyrus may occur in the POCD as a result of mechanisms in the brain that compensate for altered metabolism. Hypoperfusion occurs when these compensatory mechanisms fail at later stages of the disease process. This may provide further evidence for the existence of compensatory mechanisms in POCD patients at an early age.
Based upon the former and current results, we speculated that these patients have no clinically detectable cognitive symptoms due to the cognitive reserve and/or compensatory mechanism, whereas cognitive symptoms would become apparently deteriorated after stressors such as surgery and anesthesia. Therefore, we can identify high-risk patients with POCD earlier through preoperative MRI results for early prediction and prevention.
More interestingly, we found that patients in the POCD group were even less elderly than those in the NPOCD group, inconsistent with previous studies (Czyz-Szypenbejl et al., 2019), further indicating that MRI detection was more sensitive and accurate than the age prediction of POCD.
The main limitation of the current study was the small sample size. The difficulty of patient recruitment may have led to a relatively moderate sample size, which could lead to potential type II errors. The second limitation was that we had only 7 days of follow-up. We could not conduct longer postoperative follow-up to help us determine the long-term prognosis of patients. Therefore, the correlation between preoperative MRI examination and long-term cognitive impairment needs to be further elucidated. Finally, the current study measured regional CBF using PASL which has lower signal- to-noise ratio than the recommended pseudo-continuous ASL with background suppression. With the improvement of equipment and research technology, we will provide better experimental results in further studies.
The current study showed the difference in preoperative CBF in the frontal gyrus (the pars triangularis of inferior frontal gyrus), the temporal lobe (right superior temporal gyrus), the right hippocampus and parahippocampal gyrus, and the cingulate gyrus (right and left medial gyrus) in POCD compared with NPOCD patients. These results suggested that pre-operative CBF might be a more sensitive bio-marker of patients who developed early POCD after major non-cardiac surgery. Based upon former and present results, we speculated that POCD patients would have subtle cognitive decline and MRI alterations before surgery. Thus, we can diagnose patients at high risk of POCD with preoperative MRI results for early prediction and prevention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Tongji Hospital affiliated with Tongji University (Approval Number K2017005). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XD drafted the manuscript. YG performed the MRI scan and ASL data analysis. Jianhui Liu designed the protocol. PW, XD, SL, JY, YD, JH, JZ, and Jiehui Liu acquired the data. XD, YG, and Jianhui Liu analyzed the data. DB refined the language. All authors contributed to the interpretation of the data and revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (No.81974155 to Jianhui Liu), the Shanghai Medicine Guidance Project (No. 16411967700 to Jianhui Liu), and the Pujiang Talent Programme (No. 2019PJD049 to Jianhui Liu).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We express our deepest gratitude to the researchers and co-workers working on the project from the Departments of MRI, Spinal Surgery, and Anesthesiology, Tongji Hospital, affiliated with Tongji University (Shanghai, China).
Alexopoulos, P., Sorg, C., Förschler, A., Grimmer, T., Skokou, M., Wohlschläger, A., et al. (2012). Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur. Arch. Psychiatry Clin. Neurosci. 262, 69–77. doi: 10.1007/s00406-011-0226-2
Alsop, D., Casement, M., de Bazelaire, C., Fong, T., and Press, D. (2008). Hippocampal hyperperfusion in Alzheimer’s disease. NeuroImage 42, 1267–1274. doi: 10.1016/j.neuroimage.2008.06.006
Alsop, D., Detre, J., and Grossman, M. (2000). Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann. Neurol. 47, 93–100.
Alsop, D., Detre, J., Golay, X., Günther, M., Hendrikse, J., Hernandez-Garcia, L., et al. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the european consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116. doi: 10.1002/mrm.25197
Aminoff, E., Kveraga, K., and Bar, M. (2013). The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17, 379–390. doi: 10.1016/j.tics.2013.06.009
Baldivia, B., Andrade, V., and Bueno, O. (2008). Contribution of education, occupation and cognitively stimulating activities to the formation of cognitive reserve. Dement. Neuropsychol. 2, 173–182. doi: 10.1590/S1980-57642009DN20300003
Bangen, K., Nation, D., Clark, L., Harmell, A., Wierenga, C., Dev, S., et al. (2014). Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front. Aging Neurosci. 6:159. doi: 10.3389/fnagi.2014.00159
Bosch, B., Bartrés-Faz, D., Rami, L., Arenaza-Urquijo, E., Fernández-Espejo, D., Junqué, C., et al. (2010). Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex 46, 451–461. doi: 10.1016/j.cortex.2009.05.006
Brickman, A., Siedlecki, K., Muraskin, J., Manly, J., Luchsinger, J., Yeung, L., et al. (2011). White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol. Aging 32, 1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013
Chi, Y., Li, Z., Lin, C., Wang, Q., and Zhou, Y. (2017). Evaluation of the postoperative cognitive dysfunction in elderly patients with general anesthesia. Eur. Rev. Med. Pharmacol. Sci. 21, 1346–1354.
Czyz-Szypenbejl, K., Medrzycka-Dabrowska, W., Kwiecien-Jagus, K., and Lewandowska, K. (2019). The occurrence of postoperative cognitive dysfunction (pocd) - systematic review. Psychiatr. Pol. 53, 145–160. doi: 10.12740/PP/90648
Dai, W., Lopez, O., Carmichael, O., Becker, J., Kuller, L., and Gach, H. (2009). Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250, 856–866. doi: 10.1148/radiol.2503080751
Ding, B., Ling, H., Zhang, Y., Huang, J., Zhang, H., Wang, T., et al. (2014). Pattern of cerebral hyperperfusion in Alzheimer’s disease and amnestic mild cognitive impairment using voxel-based analysis of 3D arterial spin-labeling imaging: initial experience. Clin. Interv. Aging 9, 493–500. doi: 10.2147/CIA.S58879
Duan, W., Sehrawat, P., Balachandrasekaran, A., Bhumkar, A., Boraste, P., Becker, J., et al. (2020). Cerebral blood flow is associated with diagnostic class and cognitive decline in Alzheimer’s disease. J. Alzheimer’s Dis. 76, 1103–1120. doi: 10.3233/JAD-200034
Glumac, S., Kardum, G., and Karanovic, N. (2019). Postoperative cognitive decline after cardiac surgery: a narrative review of current knowledge in 2019. Med. Sci. Monit. 25, 3262–3270. doi: 10.12659/MSM.914435
Goto, N., Yoshimura, R., Kakeda, S., Moriya, J., Hayashi, K., Ikenouchi-Sugita, A., et al. (2011). Comparison of brain N-acetylaspartate levels and serum brain-derived neurotrophic factor (BDNF) levels between patients with first-episode schizophrenia psychosis and healthy controls. Eur. Psychiatry 26, 57–63. doi: 10.1016/j.eurpsy.2009.10.001
Johnson, N., Jahng, G., Weiner, M., Miller, B., Chui, H., Jagust, W., et al. (2005). Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234, 851–859. doi: 10.1148/radiol.2343040197
Kant, I. M. J., de Bresser, J., van Montfort, S. J. T., Slooter, A. J. C., and Hendrikse, J. (2017). Mri markers of neurodegenerative and neurovascular changes in relation to postoperative delirium and postoperative cognitive decline. Am. J. Geriatr. Psychiatry 25, 1048–1061. doi: 10.1016/j.jagp.2017.06.016
Li, D., Liu, Y., Zeng, X., Xiong, Z., Yao, Y., Liang, D., et al. (2020). Quantitative study of the changes in cerebral blood flow and iron deposition during progression of Alzheimer’s disease. J. Alzheimer’s Dis. 78, 439–452. doi: 10.3233/JAD-200843
Maekawa, K., Baba, T., Otomo, S., Morishita, S., and Tamura, N. (2014). Low pre-existing gray matter volume in the medial temporal lobe and white matter lesions are associated with postoperative cognitive dysfunction after cardiac surgery. PLoS One 9:e87375. doi: 10.1371/journal.pone.0087375
Moller, J., Cluitmans, P., Rasmussen, L., Houx, P., Rasmussen, H., Canet, J., et al. (1998). Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. international study of post-operative cognitive dysfunction. Lancet 351, 857–861. doi: 10.1016/s0140-6736(97)07382-0
Monk, T., Weldon, B., Garvan, C., Dede, D., van der Aa, M., Heilman, K., et al. (2008). Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108, 18–30. doi: 10.1097/01.anes.0000296071.19434.1e
Needham, M., Webb, C., and Bryden, D. (2017). Postoperative cognitive dysfunction and dementia: what we need to know and do. Br. J. Anaesth. 119, i115–i125. doi: 10.1093/bja/aex354
Newman, M., Kirchner, J., Phillips-Bute, B., Gaver, V., Grocott, H., Jones, R., et al. (2001). Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N. Engl. J. Med. 344, 395–402. doi: 10.1056/NEJM200102083440601
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Pauli, C., de Oliveira Thais, M. E. R., Guarnieri, R., Schwarzbold, M. L., Diaz, A. P., Ben, J., et al. (2017). Decline in word-finding: the objective cognitive finding most relevant to patients after mesial temporal lobe epilepsy surgery. Epilepsy Behav. 75, 218–224. doi: 10.1016/j.yebeh.2017.08.012
Riederer, I., Bohn, K., Preibisch, C., Wiedemann, E., Zimmer, C., Alexopoulos, P., et al. (2018). Alzheimer disease and mild cognitive impairment: integrated pulsed arterial spin-labeling MRI and F-FDG PET. Radiology 288, 198–206. doi: 10.1148/radiol.2018170575
Rundshagen, I. (2014). Postoperative cognitive dysfunction. Dtsch. Arztebl. Int. 111, 119–125. doi: 10.3238/arztebl.2014.0119
Scheff, S. W., Price, D. A., Ansari, M. A., Roberts, K. N., Schmitt, F. A., Ikonomovic, M. D., et al. (2015). Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J. Alzheimers Dis. 43, 1073–1090. doi: 10.3233/JAD-141518
Singh-Manoux, A., Marmot, M., Glymour, M., Sabia, S., Kivimäki, M., and Dugravot, A. (2011). Does cognitive reserve shape cognitive decline. Ann. Neurol. 70, 296–304. doi: 10.1002/ana.22391
Spalletta, G., Girardi, P., Caltagirone, C., and Orfei, M. (2012). Anosognosia and neuropsychiatric symptoms and disorders in mild Alzheimer disease and mild cognitive impairment. J. Alzheimer’s Dis. 29, 761–772. doi: 10.3233/JAD-2012-111886
Stern, Y. (2002). What is cognitive reserve? theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460.
Tomlinson, B., Blessed, G., and Roth, M. (1968). Observations on the brains of non-demented old people. J. Neurol. Sci. 7, 331–356.
Wang, Z., Aguirre, G., Rao, H., Wang, J., Fernández-Seara, M., Childress, A., et al. (2008). Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 26, 261–269. doi: 10.1016/j.mri.2007.07.003
Williams-Russo, P., Sharrock, N., Mattis, S., Szatrowski, T., and Charlson, M. (1995). Cognitive effects after epidural vs. general anesthesia in older adults. a randomized trial. JAMA 274, 44–50.
Xekardaki, A., Rodriguez, C., Montandon, M., Toma, S., Tombeur, E., Herrmann, F., et al. (2015). Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 274, 490–499. doi: 10.1148/radiol.14140680
Yan, C., Wang, X., Zuo, X., and Zang, Y. (2016). DPABI: data processing analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Yoshida, T., Kazui, H., Tokunaga, H., Kito, Y., Kubo, Y., Kimura, N., et al. (2011). Protein synthesis in the posterior cingulate cortex in Alzheimer’s disease. Psychogeriatrics 11, 40–45. doi: 10.1111/j.1479-8301.2010.00350.x
Zhang, Q., Wang, Q., He, C., Fan, D., Zhu, Y., Zang, F., et al. (2021). Altered regional cerebral blood flow and brain function across the Alzheimer’s disease spectrum: a potential biomarker. Front. Aging Neurosci. 13:630382. doi: 10.3389/fnagi.2021.630382
Keywords: arterial spin labeling, magnetic resonance imaging, postoperative cognitive dysfunction, cerebral blood flow, pre-operative changes in brain
Citation: Du X, Gao Y, Liu S, Zhang J, Basnet D, Yang J, Liu j, Deng Y, Hu J, Wang P and Liu J (2021) Early Warning Value of ASL-MRI to Estimate Premorbid Variations in Patients With Early Postoperative Cognitive Dysfunctions. Front. Aging Neurosci. 13:670332. doi: 10.3389/fnagi.2021.670332
Received: 21 February 2021; Accepted: 13 July 2021;
Published: 17 August 2021.
Edited by:
Denis Gris, Université de Sherbrooke, CanadaReviewed by:
Sudipto Dolui, University of Pennsylvania, United StatesCopyright © 2021 Du, Gao, Liu, Zhang, Basnet, Yang, Liu, Deng, Hu, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhui Liu, amlhbmh1aWxpdV8xMjQ2QDE2My5jb20=
† These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.