- 1Department of Microbiology and Immunology, Tulane University School of Medicine, New Orleans, LA, United States
- 2Neuroscience Program, Tulane Brain Institute, School of Science and Engineering, Tulane University, New Orleans, LA, United States
- 3Division of Comparative Pathology, Tulane National Primate Research Center, Covington, LA, United States
- 4Tulane Brain Institute, Tulane University School of Medicine, New Orleans, LA, United States
The outbreak of the novel and highly infectious severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in hundreds of millions of infections and millions of deaths globally. Infected individuals that progress to coronavirus disease-19 (COVID-19) experience upper and lower respiratory complications that range in severity and may lead to wide-spread inflammation and generalized hypoxia or hypoxemia that impacts multiple organ systems, including the central and peripheral nervous systems. Since the SARS-CoV-2 outbreak, multiple reports continue to emerge that detail neurological symptoms, ranging from relatively mild (e.g., impaired taste and/or smell) to severe (e.g., stroke), suggesting SARS-CoV-2 may be neurotropic and/or contribute to nervous system injury through direct and/or indirect mechanisms. To gain insight into the types of neurological complications associated with SARS-CoV-2 infection and their possible relationship with age, sex, COVID-19 severity, and comorbidities, we performed a systematic review of case reports and series published in 2020 – April 4, 2021 of infected patients with neurological manifestations. Meta-analyses were conducted using individual patient data from reports where these data could be extracted. Here, we report neurological injury occurs across the lifespan in the context of infection, with and without known comorbidities, and with all disease severities, including asymptomatic patients. Older individuals, however, are more susceptible to developing life-threatening COVID-19 and cerebrovascular disease (CVD), such as stroke. A mild but inverse correlation with age was seen with CNS inflammatory diseases, such as encephalitis, as well as taste and/or smell disorders. When reported, increased age was also associated with comorbid cardiovascular risk factors, including hypertension, diabetes mellitus, and lipid disorders, but not with obesity. Obesity did correlate with development of critical COVID-19. Discussion into potential pathophysiological mechanisms by which neurological symptoms arise and long-term consequences of infection to the nervous system is also provided.
Introduction
Infectious disease, ranging in severity from symptoms of a mild cold to severe acute respiratory distress are attributed to coronaviruses (CoV)s. The majority of this large family of viruses are transmitted among non-human species, however, occasional zoonosis has resulted in seven known CoV strains that infect and cause disease in humans. Of these, three human CoVs (huCoVs) strains have emerged over the past two decades that can promote severe disease and even death. Severe acute respiratory coronavirus (SARS-CoV) and Middle East respiratory syndrome (MERS)-CoV, emerged in 2003 and 2012, respectively, causing significant global illness and mortality (Drosten et al., 2003; Memish et al., 2013). In December 2019, a novel CoV strain, now designated SARS-CoV-2, was first reported to infected humans and cause severe disease, termed CoV disease-19 (COVID-19). While most individuals with COVID-19 experience mild to moderate symptoms, others develop more severe disease, leading to death in a subset of these patients. Rapid transmission of the virus has resulted in a global pandemic resulting in hundreds of millions of infections and millions of deaths, worldwide, that remains on-going at the time of this review (Huang et al., 2020; WHO, 2021).
Although primarily considered a virus impacting the respiratory system, an increasing number of case studies have highlighted substantial neurological consequences of SARS-CoV-2 infection. Indeed, the Centers for Disease Control (CDC) lists new confusion or the inability to arouse as indicators of severe COVID-19 presentation, necessitating emergency medical attention (CDC, 2020). Early reports from Wuhan, China alerted the neuroinvasive potential of SARS-CoV-2, as multiple patients developed headache and dizziness, anosmia, and/or ageusia, which were often reported as initial symptoms of infection and disease (Chen N. et al., 2020; Huang et al., 2020; Mao et al., 2020; Yang et al., 2020). In addition, acute onset of more serious neurological symptoms, including altered mental status (encephalopathy), meningoencephalitis, demyelinating diseases, and stroke are increasingly reported in SARS-CoV-2 infected patients (Al-Olama et al., 2020; Farhadian et al., 2020; Lodigiani et al., 2020; Lu et al., 2020; Scullen et al., 2020; TunÇ et al., 2020). Many reports that reveal the age of the subjects studied suggest that patients older than 50 years are more likely to experience severe neurological complications, however, varying new onset neurological manifestations have also been reported among younger individuals and appear to be a common complication of COVID-19. As such, there is a critical need for investigating the impact of COVID-19 on the central nervous system (CNS). Here, we present evidence for a direct or indirect role of SARS-CoV-2 in promoting neurological disease in individuals across the lifespan via a systematic review of the literature and meta-analyses. We also discuss potential pathophysiology of SARS-CoV-2-associated CNS injury and the potential for long-term neurological complications of infection in recovered patients, including the potential impact of disease on pathological brain aging.
Methods
Search Strategy and Study Selections
A systematic review was conducted for the purposes of identifying the population of COVID-19 patients diagnosed with new onset neurological condition(s) during the disease course. This review was designed and organized in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines (Moher et al., 2009). The database PubMed-NCBI was systematically searched for peer-reviewed literature presenting original clinical data of COVID-19 patients diagnosed with a neurological condition. The search of PubMed-NCBI alone is considered comprehensive and reliable, as over 90% of MEDLINE is covered by this database, thus the search of additional databases was deemed unnecessary (Williamson and Minter, 2019). Manuscripts published from 2019 to April 4, 2021 were interrogated using the following search terms: (“COVID-19” OR “SARS-CoV-2”) AND (“Brain” OR “Neuro” OR “Stroke” OR “Seizure” OR “Anosmia” OR “Ageusia” OR “Guillain-Barré” OR “Headache” OR “Dizziness” OR “Confusion” OR “Impaired Consciousness” OR “Seizure” OR “Encephalopathy” OR “Meningitis”) NOT “review.” The search was restricted to full text peer-reviewed reports available in English containing original clinical data. Preprint articles were not included. The purpose of this systematic review and meta-analysis was to assess the type and incidence of neurological complications of COVID-19 in relation to age. As such, only published articles with original clinical data containing the following criteria were included: (1) age of patient(s) featured in the study, (2) a diagnosis of new onset neurological manifestations, and (3) laboratory-confirmed SARS-CoV-2 infection. Exclusion criteria included: (1) any known pre-existing neurological conditions, (2) known co-current viral or parasitic infection, and/or (3) opinions, viewpoints, personal anecdotes, and reviews. Seizure was reported in a SARS-CoV-2 positive 6-week-old male (Dugue et al., 2020), however, this case was excluded from analysis because a history of seizure could not be ruled out, due to the young age. An 80-year-old woman with Alzheimer’s dementia, who developed stroke (Xiong et al., 2020) and a 52-year-old HIV-infected woman with posterior reversible encephalitic syndrome (PRES) (Anand et al., 2020a) were also excluded from analyses, as these comorbidities could not be ruled-out as significant confounders to the development of neurological disease in the context of COVID-19.
Data Extraction and Synthesis
Articles vetted for inclusion were independently reviewed by BNS and TF, and the following information was extracted for analysis: age, gender, neurological manifestation, COVID-19 symptom severity, comorbidities, and presence of virus in CSF or autopsied brain. All data were captured and maintained in a Microsoft Excel workbook. Any disagreement regarding inclusion was resolved by discussion.
To reduce the effects of heterogeneity among the case reports, neurological diagnoses/symptoms were evaluated and categorized as cerebrovascular disease (CVD), peripheral neuropathy, encephalopathy, demyelinating disease, smell and/or taste disorder, and CNS inflammatory disease. The category “other” was included to capture patients who exhibited neurological symptoms, but the underlying cause was not determined or identified. This is expanded in Supplementary Table 1. Dichotomous outcomes were created for each category of CNS disease for statistical tests. Reported comorbidities were also reduced to dummy variables for assessing potential relationships with hypertension (HTN), diabetes mellitus (DM), lipid disorder, obesity, none, and other. This is expanded in Supplementary Table 2. For analyses, comorbidities were scaled based on their overall relationship with CVD, which had the strongest association with age and COVID-19 severity. Scores were designed as follows: None = 0, other = 1, obesity = 2, lipid disorder = 3, DM = 4, HTN = 5. This allowed for the inclusion of multiple reported comorbidities by synthesizing a “comorbidity score” for each patient equal to the sum of the individual scores. For example, a patient with HTN and DM would have a comorbidity score = 9. “None” includes only reports that specifically stated no comorbidities. Reports that did not include comorbidities were excluded from analyses that required these data. COVID-19 severity was converted to ordinal variables as follows: asymptomatic = 0, mild = 1, moderate = 2, severe = 3, critical = 4.
Statistical Analyses
Statistical tests were performed using Prism 9 for MacOS (v9.1.1) and the on-line statistics software, Intellectus Statistics (2021)1. Graphs were constructed with Prism and Microsoft Excel. Summary statistics for individual patient data were calculated for each variable. A general assessment of the relationship between age and each neurological disease category was conducted through simple linear regression using dummy coding (dichotomous outcomes) for the neurological disease category. Pearson correlation matrices were constructed to assess pair-wise relationships between variables. Cohen’s standard was used to evaluate the strength of the relationships, where coefficients between ±0.10 and ±0.29 represent a small effect size, coefficients between ±0.30 and ±0.49 represent a moderate effect size, and coefficients of ±0.50 and above indicate a large effect size (Cohen, 1998). The result of the correlations was examined using Holm corrections to adjust for multiple comparisons based on α = 0.05. Analysis of variance (ANOVA) was conducted to assess if there were significant differences in COVID-19 severity or comorbidities score between the levels of neurological disease category. Tukey pairwise comparisons were conducted for all significant effects based on α = 0.05. For each statistical test, only data from patients with all variables investigated were included. Cases with missing data were excluded from analysis. As such, the n for each test or summary is reported.
Results
Search Results and Population Characteristics
Of an initial 4,611 records retrieved, 4,372 were excluded as per our exclusion criteria (Figure 1). A total of 239 articles were included in an overall assessment for the prevalence of neurological conditions reported in all patients with confirmed COVID-19 (n = 2,307) (Figure 2). The prevalence of COVID-19 patients with neurological conditions were assessed by age (years) from a total of 230 articles (n = 584) in which individual ages could be extracted (Table 1). Patient diagnoses were categorized under the following general neurological conditions: cerebrovascular disease (CVD), peripheral neuropathies, encephalopathies, demyelinating diseases, smell and/or taste disorders, and CNS inflammatory diseases. An additional category of “other” was ascribed to patients who exhibited neurological symptoms, but the underlying cause was not determined or identified (Figure 2). Smell and/or taste disorders were the most prevalent neurological manifestation with 1,303 cases identified, or 56.5% of the total. CVD, including stroke and microhemorrhages, was seen less frequently, but impacted approximately one quarter of the total (n = 584). Each of the remaining neurological conditions comprised less than ten percent of the total reported with encephalopathy and “other” accounting for 5.3% (n = 122) and 6.8% (n = 156), respectively. Less prevalent, but nonetheless significant neurological conditions also reported include peripheral neuropathy [e.g., Guillain-Barré Syndrome (GBS) and critical illness neuromyopathy (CIM)], CNS inflammatory disease (e.g., encephalitis and myelitis), and demyelinating disease [e.g., multifocal demyelinating lesions and acute disseminated encephalomyelitis (ADEM)], constituting a respective 3.3% (n = 75), 2.1% (n = 49), and 0.8% (n = 18) of the total subjects.
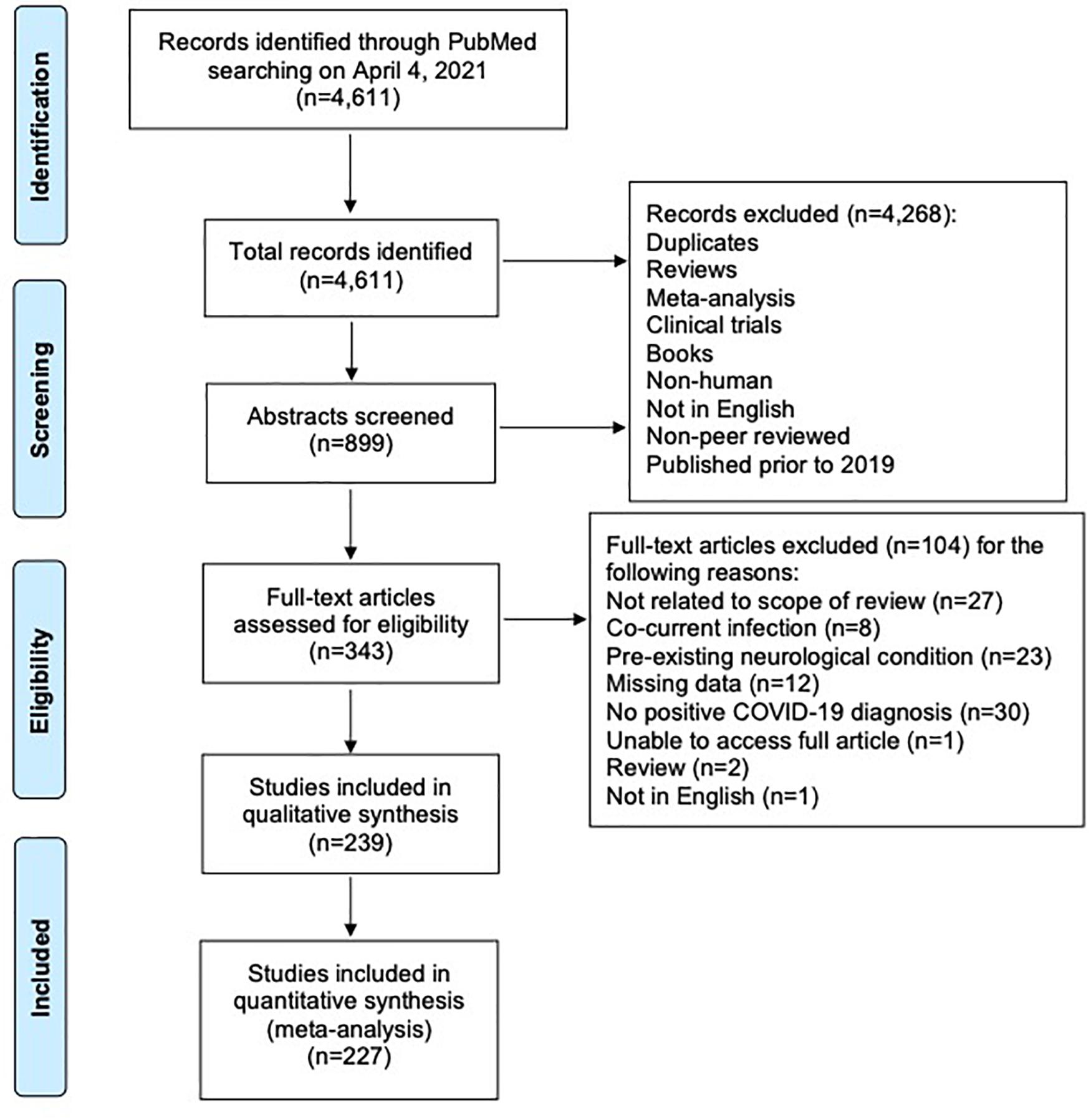
Figure 1. PRISMA flow diagram of systematic literature search and screening for studies of COVID-19 patients with neurological conditions.
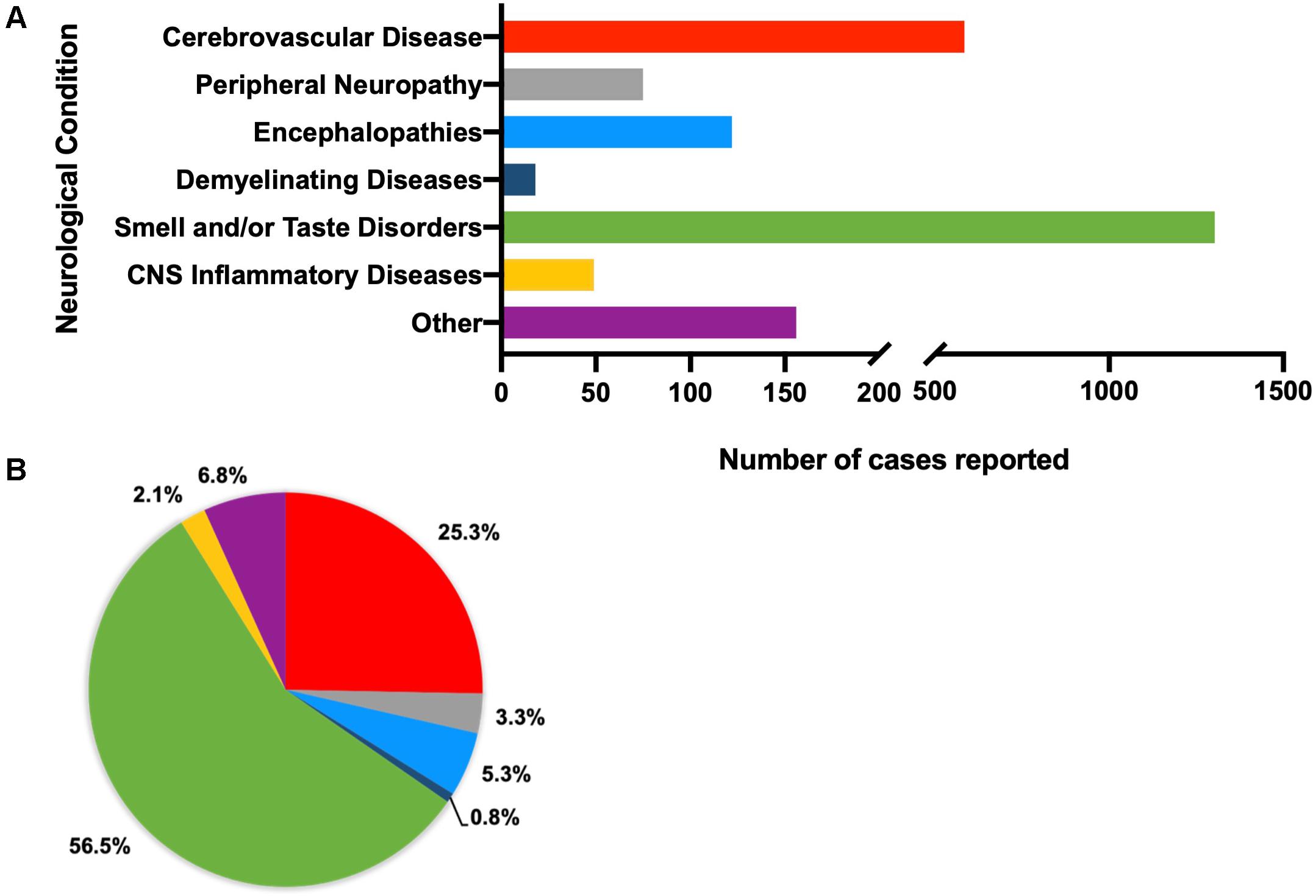
Figure 2. Total number (A) and percent of (B) reported neurological conditions occurring in patients, regardless of demographics (total n = 2,390). Individual diagnoses have been categorized as cerebrovascular diseases (n = 592), peripheral neuropathies (n = 75), encephalopathies (n = 175), demyelinating diseases (n = 23), smell and/or taste disorders (n = 1,303), CNS inflammatory diseases (n = 45), and other (neurological symptoms that cannot be attributed to a specific neurological condition, such as headache, seizure, ataxia, aphasia) (n = 177).
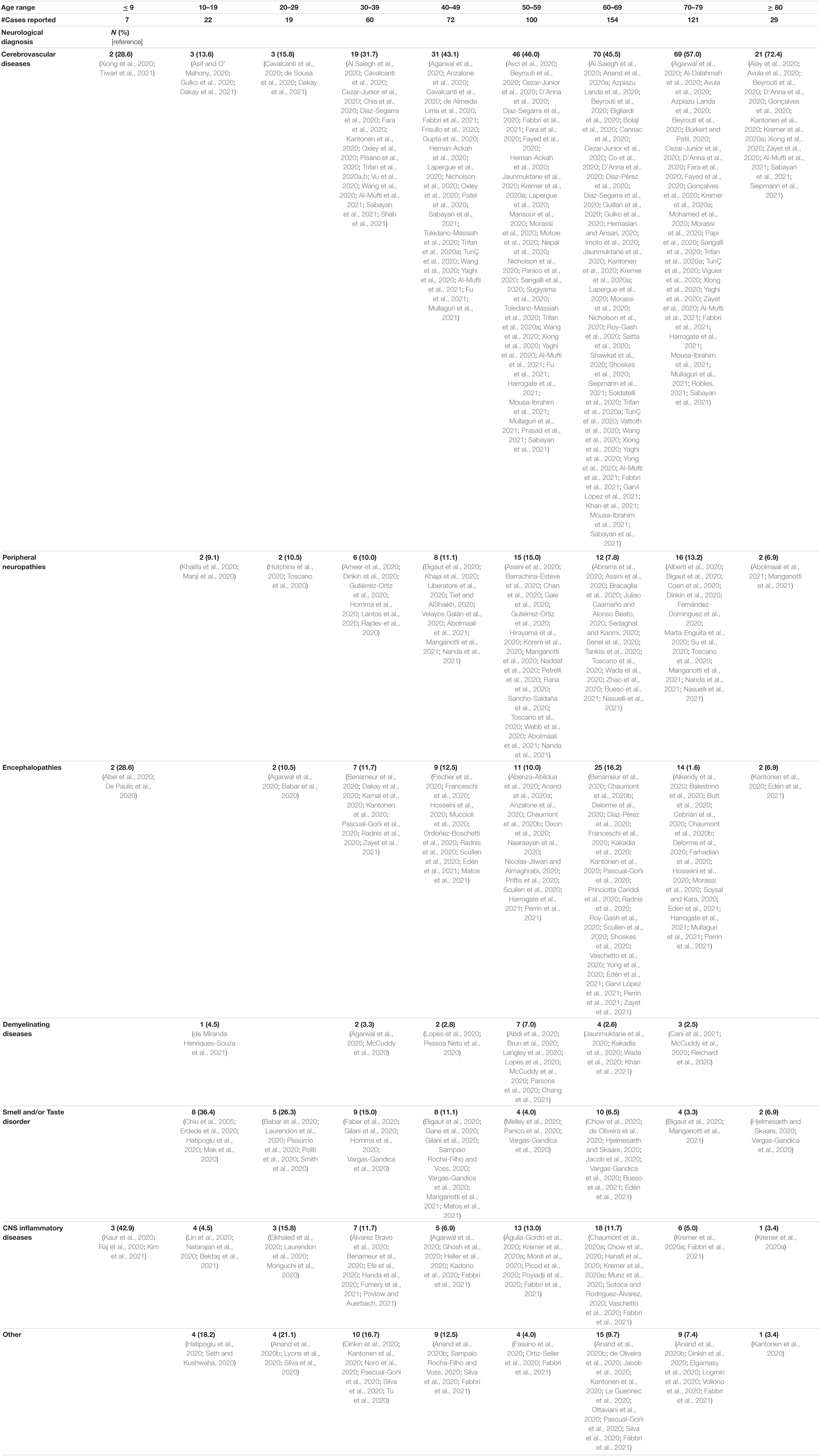
Table 1. Total number (n = 584) and percent of each neurological condition reported in patients diagnosed with COVID-19 per decade of age.
Summary of Individual Patient Data for Meta-Analyses
Data of 510 patients were extracted from 227 published reports, from which age and neurological complication could be matched. When possible, sex, COVID-19 severity, comorbidities, and the presence of detectable virus in cerebrospinal fluid (CSF) were also captured. As described above, neurological diagnoses were broadly categorized under CVD, CNS inflammatory disease, demyelinating disease, encephalopathy, peripheral neuropathy, taste/smell disorders, and other. Frequencies and percentages of individual diagnoses included under these categories are listed in Supplementary Table 1. The most frequently reported CVD was stroke of various types (n = 230, 89%). Among CNS inflammatory diseases, meningoencephalitis was most frequently observed (n = 22, 47%). Acute disseminated encephalomyelitis (ADEM) was the most frequently reported demyelinating disease (n = 9, 60%). Within the category of encephalopathy, a diagnosis of various types of encephalopathy were reported with the highest frequency (n = 47, 83%), while different manifestations of GBS were the most frequently observed peripheral neuropathy (n = 54, 84%). Loss of smell (anosmia) was the most frequent complication of taste/smell disorders (n = 11, 31%) and various manifestations of headache (n = 18, 49%) were the most frequently reported neurological manifestation categorized as “other,” which includes neurological manifestations for which the underlying cause was not identified.
Comorbidities were reported for 363 of the 510 patients for which individual patient data could be extracted. Risk factors for cardiovascular disease, including hypertension (HTN), diabetes mellitus (DM), obesity, and hyperlipidemia, were the most frequent comorbidities among COVID-19 patients with neurological manifestations (Supplementary Table 2). To assess the association of the stated comorbidities with neurological manifestations, comorbidities were limited to HTN, DM, obesity, and lipid disorders, which includes hyperlipidemia, dyslipidemia, and hypercholesterolemia. All other reported comorbidities were included as “other.”
Although the number of males outnumbered females, the mean age did not differ significantly between the two sexes, or from the mean age of a cohort of individuals for which sex was not specified (Table 2). Summary statistics for individual patient data were calculated for each variable, and frequencies and percentages were split by sex and reported in Table 3. Frequencies and percentages were only calculated on available data (n/a = not available excluded). This is reflected in the n reported for each variable. The most frequently observed neurological disease category for all patients regardless of sex, specified or not, was cerebrovascular disease (n = 257, 50% of total). Moderate COVID-19 severity was most often reported for all male and female subjects, however, patients for which sex was not specified (n = 57), severe COVID-19 was most frequently reported. Although no comorbidities (none) appear to be most frequently reported among all subjects (n = 131, 36%), when taken together, HTN, with and without additional comorbidities, is most frequently reported in toto (n = 165, 45%), as well as separately among males (n = 96, 46%) and persons for which sex was not specified (n = 27, 57%). No comorbidities (none) and HTN, with and without additional comorbidities, were equally reported among females (n = 42, 39%). CSF was assessed for detectable virus in 122 of the total 510 patients but only identified in four cases (Table 3), which included a 31-year-old male and a 74-year-old female with altered mental status (encephalopathy), a 24-year-old male with meningoencephalitis, and a 68-year-old male who developed stroke (Cebrián et al., 2020; Kamal et al., 2020; Moriguchi et al., 2020; Saitta et al., 2020).
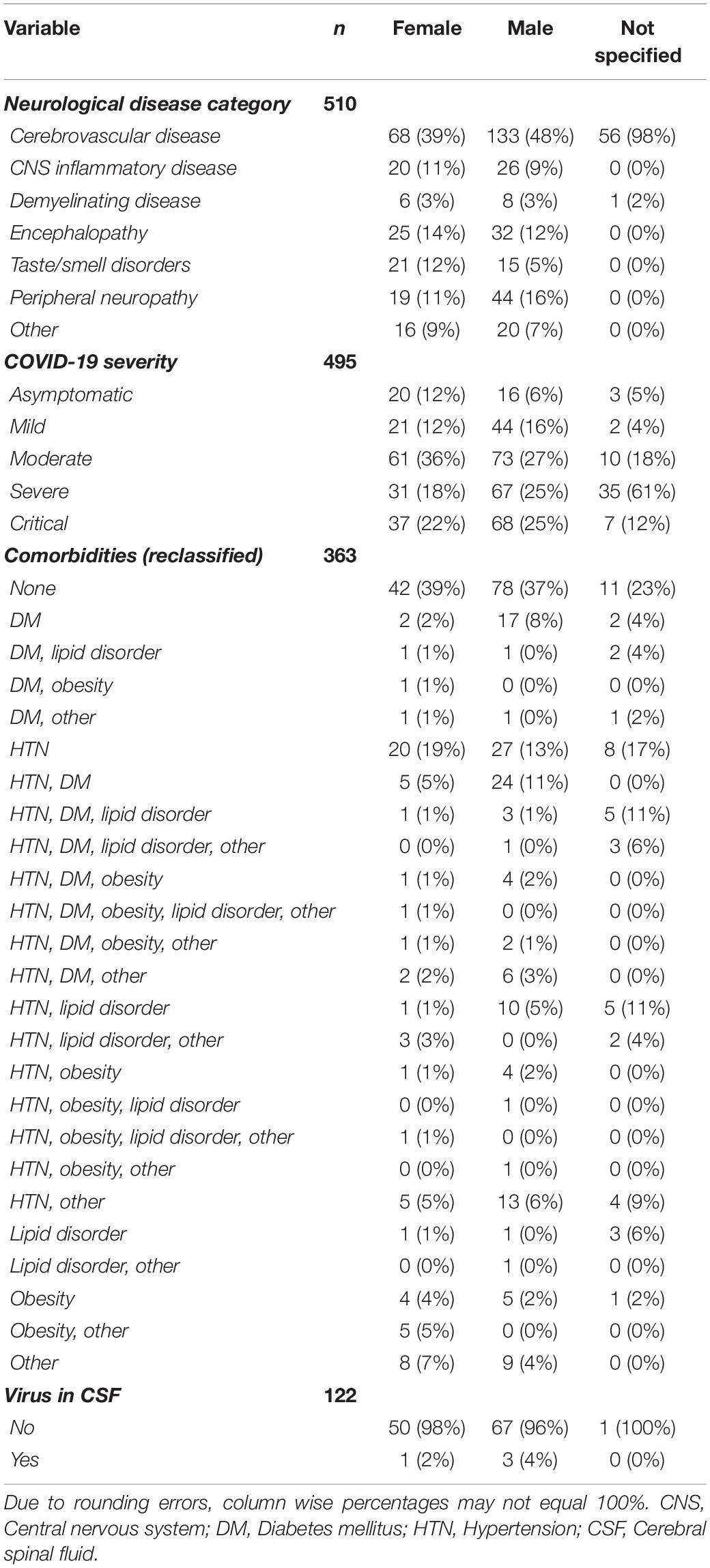
Table 3. Frequencies and percentages of neurological disease, COVID-19 severity, comorbidities, and detectable virus in CSF by sex.
Finally, frequencies and percentages were calculated for neurological disease category split by COVID-19 severity (n = 495; Table 4). Regardless of disease severity, CVD was the most frequently observed category of neurological disease, which may reflect a more serious injury, such as stroke, being more likely to prompt a case report. It is important to note, that CVD was reported in the context of SARS-COV-2 infection among individuals with few or no other symptoms typically associated with COVID-19.
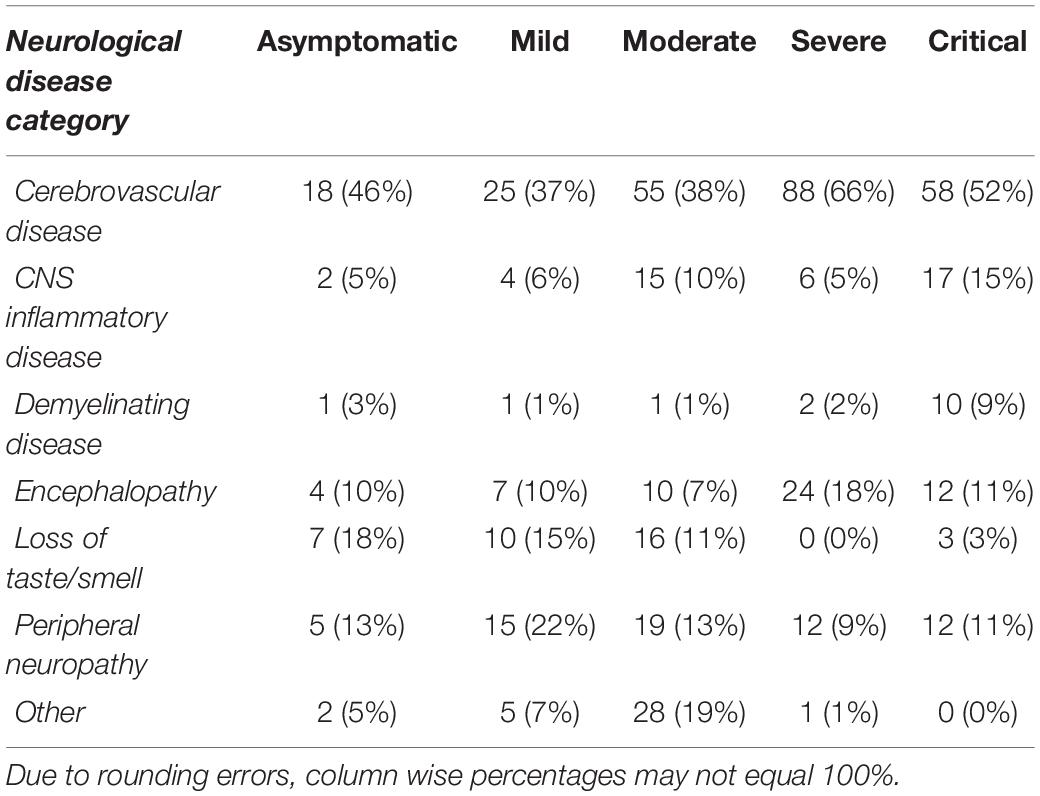
Table 4. Frequencies and percentages of observed neurological disease split by COVID-19 severity (n = 495).
Age-Associated Neurological Complications of COVID-19
Neurological conditions were evaluated by age, where the individual age or cohort age range was able to be determined and stratified to assess the overall frequency of specific types of neurological complications affecting children (<19 years), young adults (19–50 years), and older adults (>50) infected with SARS-CoV-2 (Figure 3). More specific details relating the number and percent of COVID-19 patients diagnosed with neurological conditions are stratified by decade of age and included in Table 1. Overall, patients 60–69 years showed the greatest population with neurological conditions (n = 154) and those less than or equal to 9 years of age had the least (n = 7) (Table 1 and Figure 4). Age and other available population characteristics of multicenter, retrospective, and observational studies with large cohorts reporting neurological conditions from which individual matched patient data could not be discerned is detailed in Supplementary Table 3. Instances where data were able to be extracted from these reports is detailed.
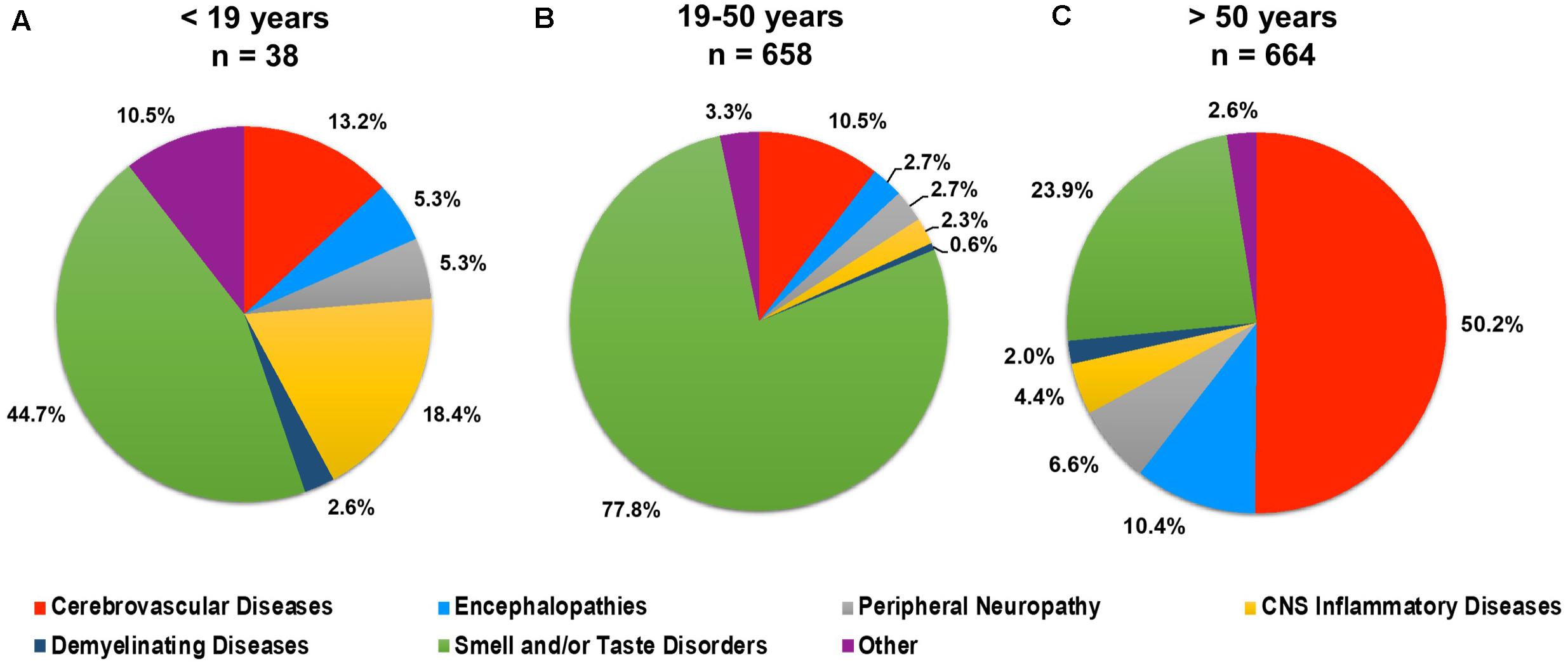
Figure 3. Percent of total neurological conditions (n = 1,360) occurring in (A) children (<19 years), (B) young adults (19–50 years), and (C) older adults (>50 years) diagnosed with COVID-19.
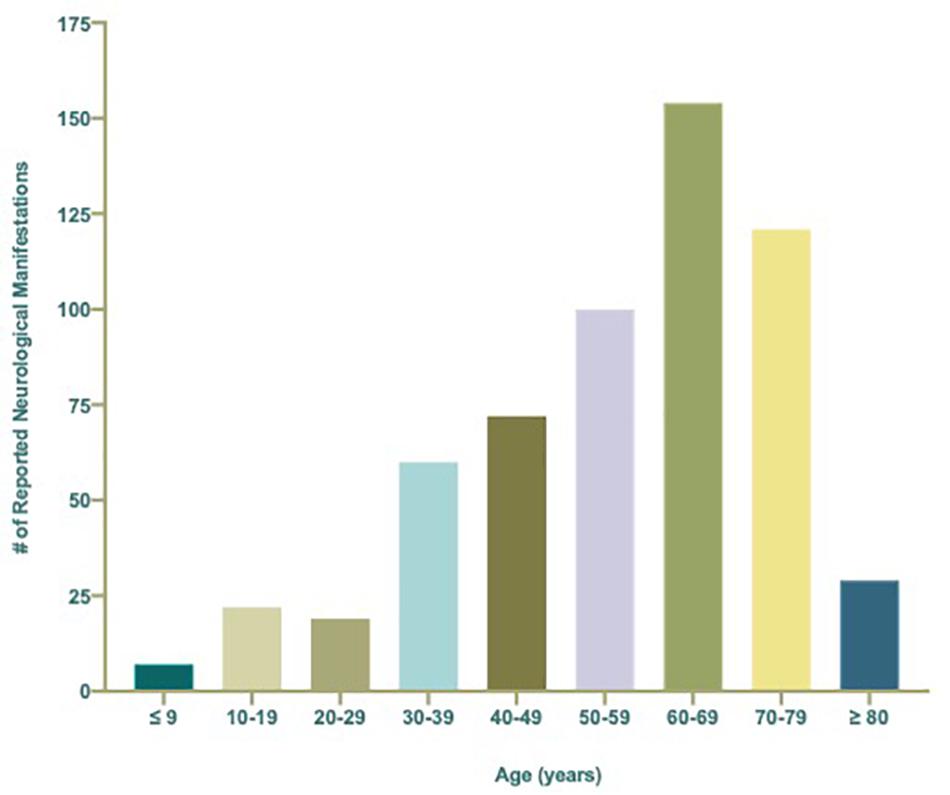
Figure 4. Total number (n = 584) of reported neurological manifestations occurring in patients with COVID-19 per decade ranging from ≤9 to ≥80 years of age.
Linear regression analyses were conducted to assess whether age significantly predicted any category of neurological complications of SARS-CoV-2 infection (Figure 5). The results of the linear regression model were significant for CVD, F(1,508) = 30.08, p < 0.001, r2 = 0.06, indicating that approximately 6% of the variance in CVD is explainable by increased age (Figure 5A). In contrast, taste/smell disorder was associated with decreased age, F(1,508) = 28.73, p < 0.001, r2 = 0.05, indicating that approximately 5% of the variance in this category is explainable by age (Figure 5F). A mild, but nonetheless statistically significant inverse relationship between age and CNS inflammatory disease or other was also observed. Approximately 1% of the variance in observation of COVID-19 patients with CNS inflammatory disease [F(1,508) = 7.19, p = 0.008, r2 = 0.01] or other [F(1,508) = 6.70, p = 0.01, r2 = 0.01] is also explainable by decreased age (Figures 5D,G). Age did not explain a significant proportion of variation in the observed frequencies of encephalopathy, peripheral neuropathy, or demyelinating disease (Figures 5B,C,E).
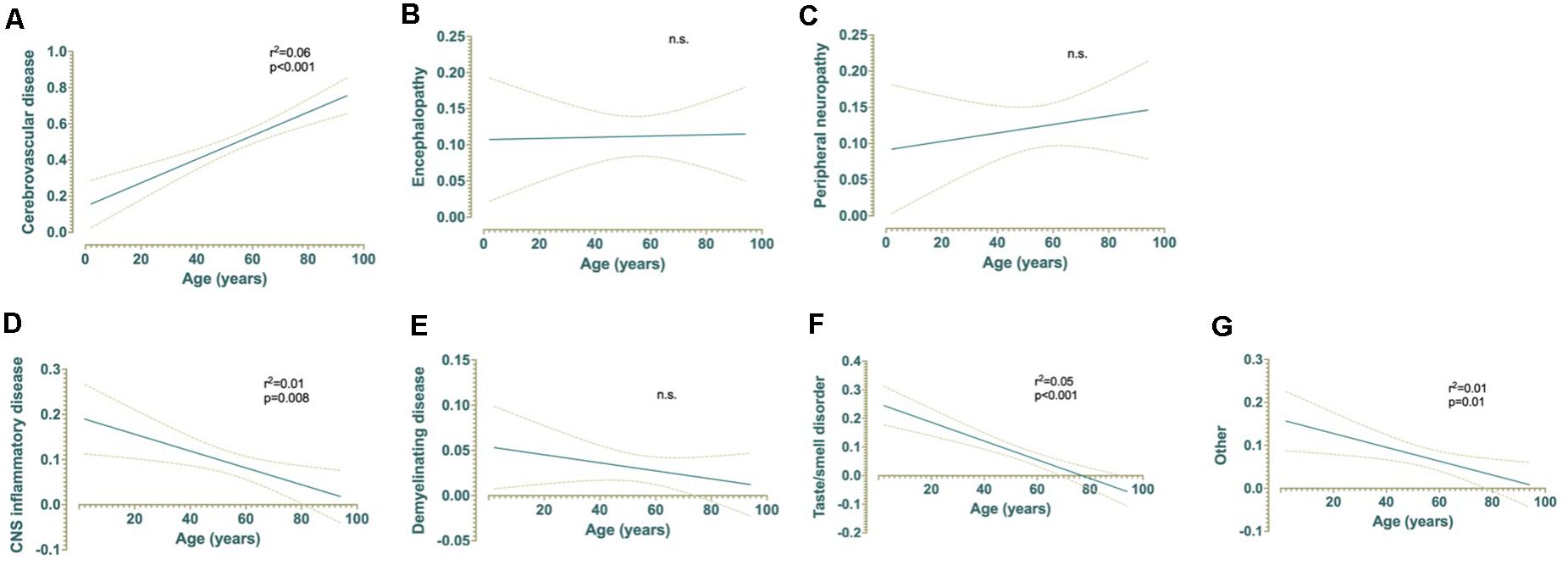
Figure 5. Simple linear regressions demonstrating the effect of age on (A) cerebrovascular disease, (B) encephalopathy, (C) peripheral neuropathy, (D) CNS inflammatory disease, (E) demyelinating disease, (F) taste and/or smell disorders, and (G) other non-specific neurological symptoms and their relationships with age (years) for patients with COVID-19 (n = 510).
Relationship of Age, COVID-19 Severity, and Comorbidities on Neurological Manifestations of COVID-19
A Pearson correlation analysis was conducted among age, sex, each category of neurological disease, COVID-19 severity, and individual comorbid factors to assess the relationships among these variables and displayed as heat maps based on the coefficient between variables (Figure 6). Cases with incomplete data for the variables being assessed were excluded from analysis, resulting in a different n for each analysis.
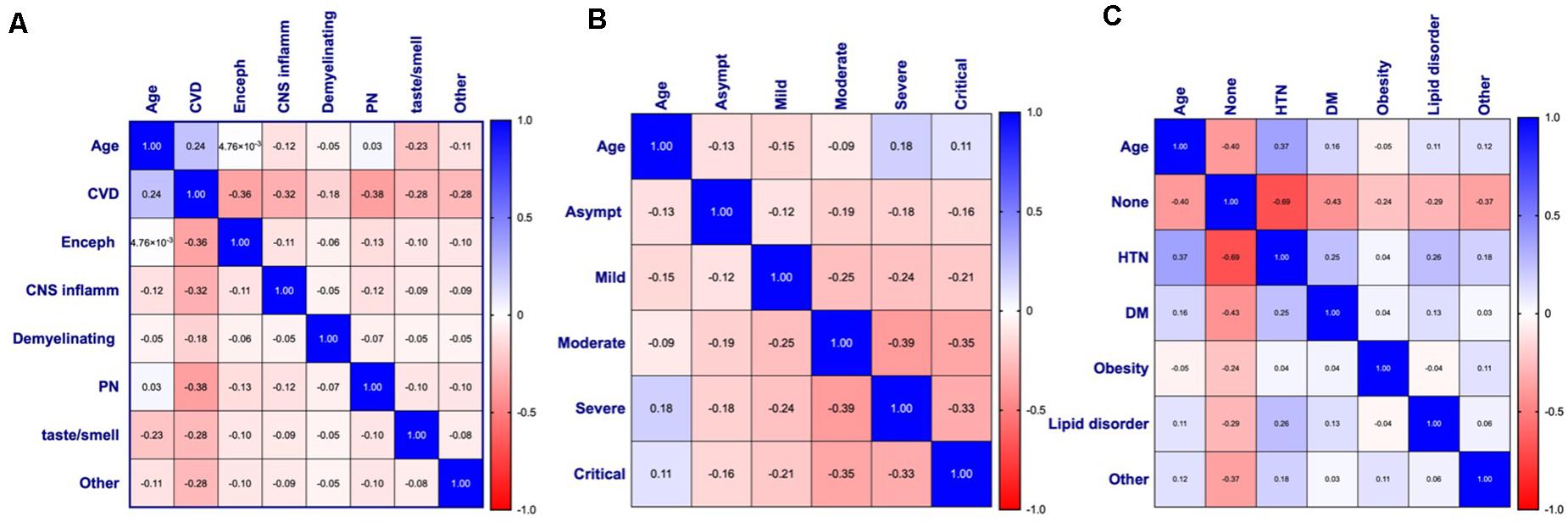
Figure 6. Pearson’s correlation matrices and heat maps for (A) age and neurological disease (n = 510), (B) age and COVID-19 symptom severity (n = 495), and (C) age and comorbidities (n = 363) of patients with COVID-19 and diagnosed with a neurological condition(s). Pearson’s correlation coefficients are displayed and assigned color based on the distance from zero, with blue representing positive and red representing negative correlations. CVD, Cerebrovascular disease; Enceph, Encephalopathy; CNS inflamm, Central nervous system inflammatory disease; PN, Peripheral neuropathy; Asympt, Asymptomatic; HTN, Hypertension; DM, Diabetes mellitus.
In agreement with the linear regression analyses, a significant positive correlation between age and CVD was seen (p < 0.001) with a coefficient (rp) of 0.24, indicating a small effect size (Figure 6A). Small effect size was also observed with age and CNS inflammatory disease (rp = −0.12, p = 0.008), smell and/or taste disorder (rp = −0.23, p < 0.001), and other (rp = −0.11, p = 0.01), all of which show a negative correlation with age (Figure 6A). The relationship between age and COVID-19 severity was statistically significant for all disease severities (Figure 6B). A negative relationship was observed between age and asymptomatic (rp = −0.13, p = 0.005), mild (rp = −0.15, p < 0.01), or moderate (rp = −0.09, p = 0.046) COVID-19 severity, while a positive correlation was seen between age and severe (rp = 0.18, p < 0.001) or critical (rp = 0.11, p = 0.013) disease (Figure 6B).
Apart from obesity, the relationship between age and comorbidities was statistically significant for all types examined (Figure 6C). A moderate effect size (rp = −0.40, p < 0.001) between age and stated no comorbidities (none) was observed, indicating that as age increases, the category of “none” tends to decrease. In contrast, the significant positive relationship between age and HTN (rp = 0.37, p < 0.001), DM (0 rp = 0.16, p = 0.002), or lipid disorders (rp = 0.11, p = 0.042), suggests comorbid cardiovascular risk factors may contribute to the increased risk for CVD and/or severe-critical COVID-19 observed. Although the relationship between age and “other” was found to be statistically significant with a small effect size (rp = 0.12, p = 0.023), the wide variety of conditions included in this category do not point to any one condition as being significant.
Relationships among all variables were also assessed and displayed in Figure 7. This revealed additional associations with neurological disease among patients for which all variables were available (n = 350). Age retained the strongest relationship with CVD, however, a significant positive correlation of CVD with HTN (rp = 0.16, p = 0.002), DM (rp = 0.13, p = 0.014), lipid disorders (rp = 0.19, p < 0.001), and severe COVID-19 (rp = 0.19, p < 0.001) were also seen. Encephalopathy correlated positively with severe COVID-19 (rp = 0.18, p = 0.001) and was seen most frequently among individuals with comorbid conditions categorized as “other” (rp = 0.11, p = 0.033). CNS inflammatory disease showed a positive correlation with moderate COVID-19 severity (rp = 0.17, p = 0.002), as well as patients without comorbid disease (rp = 0.17, p = 0.001). No significant relationship between comorbidities and demyelinating disease was observed, however, it did correlate with critical COVID-19 (rp = 0.22, p < 0.001). These results, however, may be less reliable due to a low number of patients within this neurological disease category (n = 12). There appears to be a small positive relationship between demyelinating disease and obesity, however, this did not reach statistical significance. Like demyelinating disease, obesity had a positive association with critical COVID-19 (rp = 0.30, p < 0.001). Peripheral neuropathy correlated with mild COVID-19 (rp = 0.19, p < 0.001) but not with any comorbid condition. Interestingly, impaired taste/smell only reached significant positive associations with asymptomatic COVID-19 (rp = 0.19, p < 0.001) and no comorbidities (rp = 0.24, p < 0.001). Although the reason for this is unclear, in the absence of more critical symptoms, impairments in taste and/or smell may be more discernable by patients.
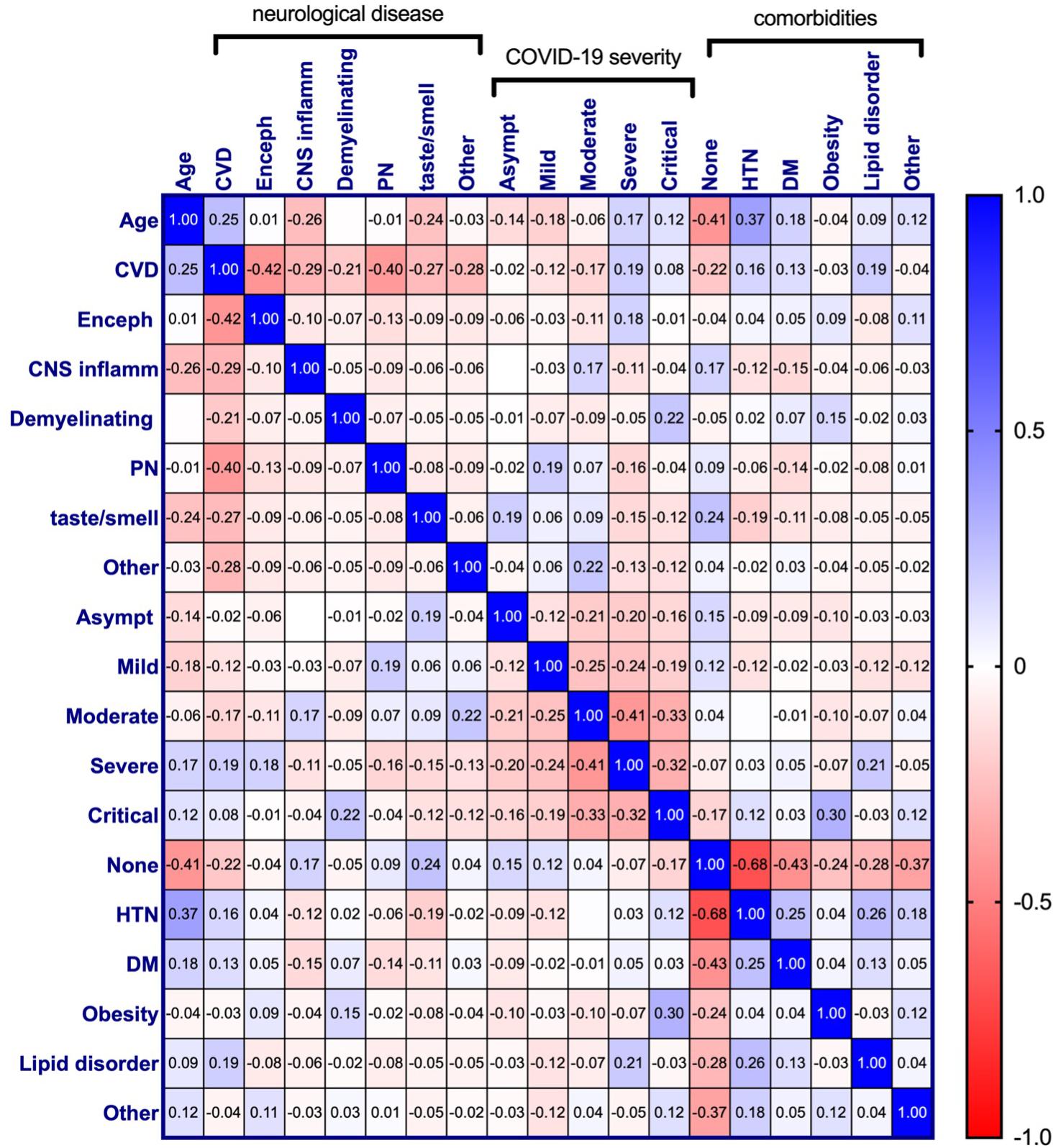
Figure 7. Pearson correlation matrix for age, neurological disease, COVID-19 symptom severity, and comorbidities of COVID-19 patients diagnosed with a neurological condition(s) (n = 303). Pearson’s correlation coefficients are displayed and assigned color based on the distance from zero, with blue representing positive and red representing negative correlations. CVD, Cerebrovascular disease; Enceph, Encephalopathy; CNS inflamm, Central nervous system inflammatory disease; PN, Peripheral neuropathy; Asympt, Asymptomatic; HTN, Hypertension; DM, Diabetes mellitus.
Effect of COVID-19 Severity and Comorbidities on Neurological Disease Outcome
In addition to age, COVID-19 severity and comorbidities appeared to associate with the observance of specific neurological disease (Figure 7). Multivariate analysis of covariance (MANCOVA) to assess if there were significant differences in the linear combination of COVID-19 severity and comorbidities score between the levels of neurological disease category after controlling for age were attempted, however, these tests failed assumptions of homogeneity of covariance and covariate-independent variable independence. As such, ANOVAs were performed separately to assess whether there were significant differences in COVID-19 severity or comorbidities score by neurological disease category. This demonstrated significant differences in the mean COVID-19 severity and comorbidities score among the different neurological disease categories (Figure 8). Demyelinating disease, CVD, and encephalopathy had the highest mean COVID-19 severity and comorbidities score, while loss of taste/smell had the lowest for both, demonstrating a relationship between disease severity and comorbid conditions. To aid viewing, neuronal disease categories were reordered by increasing mean of the two variables (Figure 8).
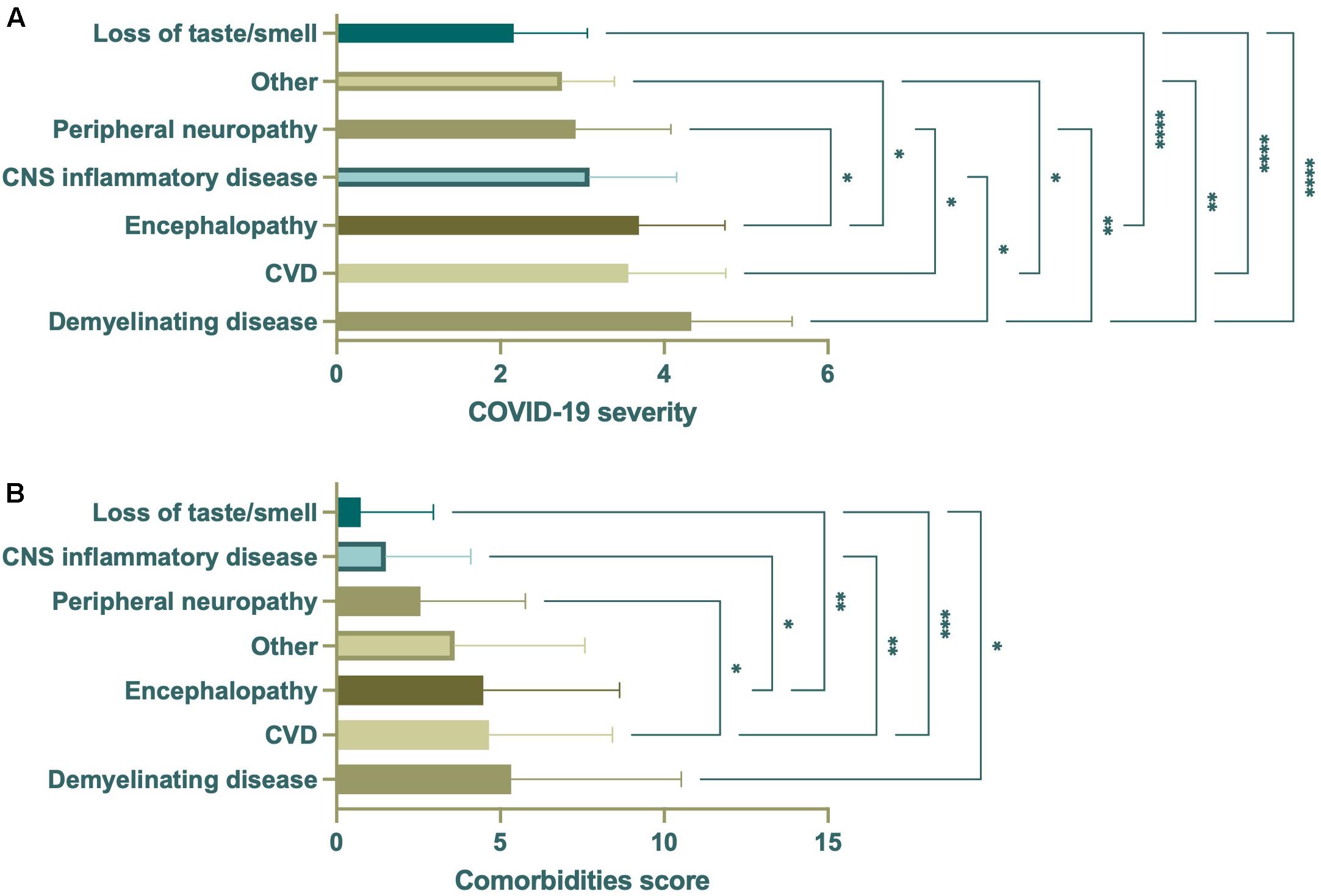
Figure 8. ANOVA of COVID-19 severity or comorbidities score by neurological disease category (n = 350). Significant differences in mean COVID-19 severity (A) were seen among all neurological disease categories. Demyelinating disease had the highest mean, with CVD and encephalopathy second and third. Similarly, demyelinating disease had the highest mean comorbidities score, with CVD and encephalopathy second and third (B). Loss of taste/smell had the lowest mean COVID-19 severity and comorbidities score. Data were derived from the same subjects. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
Neurological manifestations are a significant complication of SARS-CoV-2 infection and COVID-19. Although many anecdotal and case study reports have suggested relationships between neurological complications of disease with age, disease severity, and comorbid conditions, significant associations among these variables remain unclear. Through a systematic review of peer-reviewed, published patient reports spanning the entirety of 2020 through April 4, 2021, and meta-analyses, we report that while smell and/or taste disorders are the most common neurological manifestation of SARS-CoV-2 infection, CVD, manifesting almost entirely as stroke, is a major neurological complication of infection, affecting just over a quarter of individuals in this study. Other clinically significant CNS complications, broadly categorized as encephalopathy, CNS inflammatory disease, demyelinating disease, and peripheral neuropathy have been reported less frequently. Other symptoms, including headache, seizure, aphasia, and ataxia have also been reported in connection with infection without identification of the underlying cause and are categorized as “other” in this report.
When investigating a potential relationship between the type of neurological disorder and age, smell and/or taste disorders remained the most common neurological complication affecting infected individuals 50 years of age and younger. For infected individuals over 50, however, CVD became the most common neurological injury, where it was observed in over half of individuals in this age group. Linear regression analysis, however, suggests only 6% of the variance in CVD is explainable by age. Known risk factors for vascular disease, HTN and DM, as well as critical COVID-19, showed a positive correlation with CVD but with a small effect size. Additionally, stroke affected individuals across the lifespan, including individuals with reported no comorbidities and/or asymptomatic disease. Together, this suggests other factors, which may include virus and/or the host’s response to infection, contribute to the development of CVD.
Although there is currently no clear indicator as to which patients will suffer stroke, several risk factors for stroke in aged individuals have been reported in COVID-19 patients, such as coagulopathy, elevated D-dimer levels, and vascular endothelial dysfunction. A large retrospective study evaluating risk factors for mortality of COVID-19 patients found coagulopathy to be a significant indicator, affecting ∼50% of non-survivors (Zhou et al., 2020). Additionally, marked elevation (<0.5 μg/L) of D-dimer, a by-product of blood clotting that is often elevated in response to acute vascular disease, has been reported in COVID-19 patients and found to be predictive of severe disease and mortality (Gao et al., 2020; Han et al., 2020; Huang et al., 2020; Mao et al., 2020; Zhou et al., 2020).
Endothelial cell infection and/or injury may also contribute to increased risk for CVD with COVID-19. ACE2, the principal receptor used for viral entry, is reportedly expressed by endothelial cells throughout the body, including brain (Hamming et al., 2004; To and Lo, 2004), indicating the potential for viral infection in the endothelium in the CNS. In support of this notion, a post-mortem investigation reported the presence of endothelial cell infection and endotheliitis across the vascular beds of several organs (Varga et al., 2020). Although the brain was not evaluated in this study, the presence of endothelial infection of multiple organs reveals the potential for widespread disruption of vascular homeostasis, increasing the susceptibility of infected patients to CVD. Interestingly, endotheliopathy without evidence of infection has been reported in a cohort of COVID-19 patients (mean age = 62 years), which was associated with severe disease (Goshua et al., 2020). This suggests that the host response to infection may sufficiently promote endothelial cell inflammation and injury, without direct involvement of the virus.
In agreement with endotheliopathy in the CNS, several autopsy and neuroimaging reports demonstrate the presence of brain microvascular lesions and microhemorrhages in COVID-19 patients (Conklin et al., 2020; Fitsiori et al., 2020; Kremer et al., 2020b; Lee et al., 2020; Lin et al., 2020; Radmanesh et al., 2020; Shoskes et al., 2020). Autopsy findings reveal intact endothelium, suggesting that microbleeds may form due to inflammation of endothelial cells that allows for extravasation of red blood cells into the brain parenchyma (Goshua et al., 2020; Pugin et al., 2020). Cerebral microhemorrhages are associated with age and systemic disease, increasing the risk for microhemorrhage development in older patients with COVID-19. Moreover, the integrity of the blood-brain barrier (BBB) decreases with age and is posited to precede and contribute to the development of CVD (Li et al., 2018). The mechanisms of BBB dysfunction in aging are not completely clear; however, small atheromatous plaques, HTN, and endothelial cell inflammation are believed to play a prominent role and may help explain why individuals with underlying comorbidities, including DM and HTN, appear to be at greater risk for developing more severe COVID-19. Additionally, chronic, subclinical inflammation is a common feature of aging that increases the susceptibility of individuals to age-related disease (Franceschi et al., 2000). Chronic inflammation can induce cellular stress and injury that weakens tissues and reduces the ability of cells to counter additional insults. It is reasonable, therefore, that aging-associated inflammation promotes endotheliitis and endotheliopathy, leading to increased “leakiness” of the vasculature that is made more severe with COVID-19.
In addition to CVD, more frequent observations of clinically significant encephalopathy and peripheral neuropathy are also seen in patients over 50 years. Patients with encephalopathy, which is broadly characterized as disease or damage to the brain that affects brain function, present with altered mental status ranging from mild confusion to more severe dementia or coma. Several case reports detail infected patients presenting with acute encephalopathic episodes, irrespective of COVID-19 severity, including acute necrotizing encephalopathy and posterior reversible encephalopathy syndrome (PRES). Encephalopathy accounted for only 5.3% of the total population of COVID-19 patients with neurological manifestations and 10.4% of those over 50 years of age. It is highly probable, however, that due to the strong inflammatory response to infection in the periphery, which can negatively impact the CNS, encephalopathy among infected individuals occurs more frequently but not widely reported in case studies.
Peripheral neuropathy, including Guillain-Barré syndrome (GBS) and critical illness neuromyopathy, have emerged as one of the more serious neurological complications of COVID-19 infection. GBS is a neuromuscular disorder defined as an acute paralytic neuropathy, often preceded by an infection, and clinically characterized by symmetric weakness of the limbs (Yuki and Hartung, 2012). This disease is considered, primarily, to be an affliction of the peripheral nerves that has a 5% fatality rate and results in the severe disability of up 20% of GBS patients (Hughes et al., 2007; Yuki and Hartung, 2012). Critical illness neuromyopathy is characterized by muscle wasting and paralysis and often culminates into a severely disabling weakness of the muscles and/or paralysis (Latronico et al., 2007; Guarneri et al., 2008). In this review, we found that peripheral neuropathy was most frequent among older adults (6.6%, n = 44), with zero cases of critical illness neuromyopathy reported in patients younger than 60 years of age.
Less frequent observations of CNS inflammatory disease, demyelinating disease, and smell and/or taste disorders was seen among older adults, as compared to younger adults and patients under 19 years of age (children). CNS inflammatory disorders, which includes encephalitis, myelitis, and meningitis, was most frequently reported among patients under 19 years, while demyelinating disease was similar in frequency among the three age categories. Interestingly, the demyelinating disease, acute disseminated encephalomyelitis (ADEM), which is a rare but serious complication of viral infection most commonly affecting young children, was seen more frequently in older adults (72.8%, n = 8), as compared to younger adults (18.1%, n = 2) and children (9.1%, n = 1), in this review. Impaired smell and/or taste was seen at a high frequency in all age groupings but was the principal manifestation affecting children and young adults and the second most common complication among individuals over 50 years.
Pathophysiology of Neurological Involvement
How SARS-CoV-2 infection promotes the development of neurological complications is unclear and may involve several factors (Figure 9). Brain autopsy and CSF analyses seldom report detectable virus in the CNS compartment, however, the neuroinvasive character of other huCoVs suggest SARS-CoV-2 may also infect the CNS (Arbour et al., 2000) and has been demonstrated in a limited number of infected individuals (Filatov et al., 2020; Moriguchi et al., 2020; Poyiadji et al., 2020; Schurink et al., 2020). While the presence of virus in the CNS compartment and mechanism of entry is not fully elucidated, it is highly likely that SARS-CoV-2 is able to gain access to the brain through nasal epithelial cells. ACE2 is highly expressed in nasal epithelium, pointing to the olfactory bulb as a probable point of entry (Sungnak et al., 2020). Infected olfactory epithelial cells may then transfer virus to closely situated olfactory neurons, allowing for retrograde axonal transport into the CNS compartment. In support of this, unilateral oblation of the olfactory bulb prior to intranasal inoculation of a neurotropic coronavirus prevented CNS entry and viral spread in mouse brain (Perlman et al., 1990).
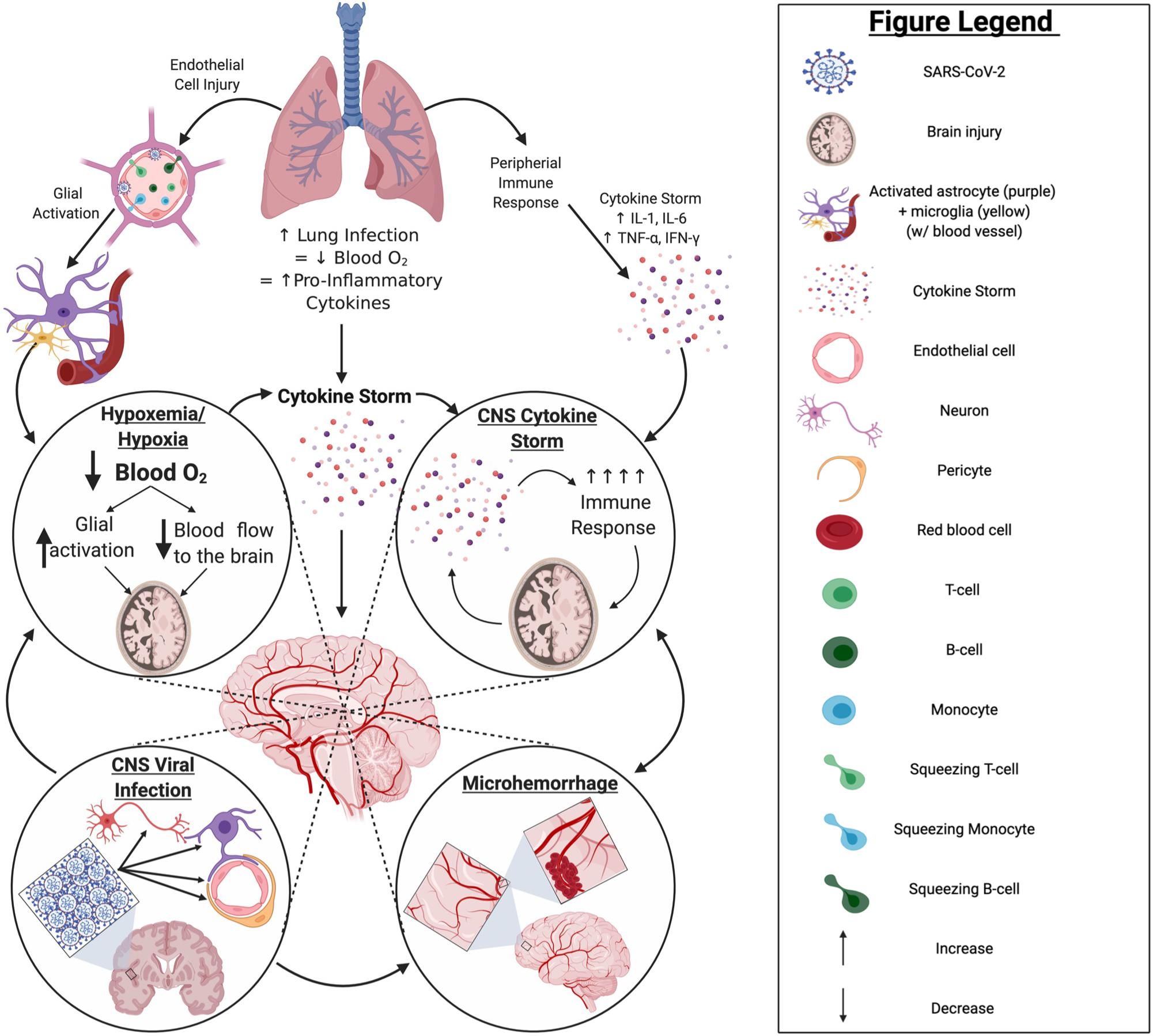
Figure 9. COVID-19 pathology in the CNS: primary impacts and potential mechanisms. Infection by SARS-CoV-2 primarily impacts the lungs, often leading to a hypoxic state and resulting in a robust increase in proinflammatory cytokine production. This may ultimately cause a “cytokine storm” and/or activate glia in the brain. Should such events occur, there is potential for hypoxemia or hypoxia, exacerbated “cytokine storm,” and/or infiltration of peripheral immune cells through endothelial cell infection to occur within the brain; any of which can result in significant brain injury to the infected patient. Although currently unclear, if SARS-CoV-2 can establish a productive or even non-productive infection within the CNS compartment, persistent inflammation and/or impaired cell function may result, increasing the potential for serious injury of the brain and/or pathological brain aging. Created with BioRender.com.
Hematological entry of virus into the CNS also cannot be ruled out. SARS-CoV-2 has been detected in endothelial cells throughout the body of infected subjects and, given the prevalence of endothelial ACE2 expression, SARS-CoV-2 may be found in brain endothelium (Hamming et al., 2004; Varga et al., 2020). Post-mortem analyses of human and non-human primate brain revealed hCoV-299E in brain endothelial cells (Cabirac et al., 1995). Viral infection of the endothelial cells by coronavirus has been found to cause inflammation of the endothelial cells which disrupts vascular homeostasis and coagulation, suggesting an increased risk for CVD, as a result (Cabirac et al., 1995; Varga et al., 2020).
Even in the absence of direct neuronal or neural cell infection, hypoxia/hypoxemia, coagulopathy, and uncontrolled inflammation or “cytokine storm” can also negatively impact the CNS and cognition (Figure 9). Indeed, most clinical evidence suggests neurological complications of COVID-19 are due to secondary effects of infection, including reduced O2 and hyperimmune responses, often referred to as “cytokine storm.” Serum levels of pro-inflammatory cytokines [e.g., interleukin (IL)-6, IL-8, tumor necrosis factor-α (TNF-α)] in COVID-19 patients are significantly predictive and/or correlative to the severity of infection and mortality (Chen T. et al., 2020; Gao et al., 2020; Huang et al., 2020; Zhou et al., 2020). Previously, SARS-CoV patients with severe disease were found to have elevated levels of pro-inflammatory cytokines and chemokines, and reduced levels of anti-inflammatory cytokines (IL-10), in comparison to patients with mild disease (Chien et al., 2006). Indeed, virus-associated diseases of the nervous system, such as acute necrotizing encephalopathy, are associated with high levels of pro-inflammatory cytokines in serum and CSF. As such, elevated pro-inflammatory cytokines in serum of severe COVID-19 patients may promote inflammation in brain and contribute to the neurological manifestations of disease (Kansagra and Gallentine, 2011; Sun et al., 2019).
Long-Term Impact of COVID-19 on the Nervous System
The long-term consequences of COVID-19-associated nervous system injury and/or dysfunction is currently unknown, however, reports continue to emerge describing persistent symptoms of disease months after resolution of infection, including impaired smell and/or taste, chronic fatigue, and impaired cognition. Long-term complications of infection are referred to as post-acute sequelae of COVID-19 (PASC) or Long COVID and evidence for this complication is seen with other viruses that induce neurological disease, including human immunodeficiency virus (HIV), West Nile virus, and multiple herpes- and picornaviruses. It is not entirely clear if SARS-CoV-2 directly infects neurons and/or non-neuronal cells of the CNS and, if so, whether virus is eradicated from these sites with recovery of COVID-19. The significance of this important consideration is seen in a single case report of a 78-year-old woman who recovered from COVID-19 but succumbed to a sudden cardiac arrest prior to hospital discharge (Yao et al., 2020). Although this individual had three consecutive SARS-CoV-2 PCR negative nasopharyngeal swabs, postmortem investigation of multiple tissues, excluding brain, revealed residual virus in lung (Yao et al., 2020). These findings suggest that the virus may not be completely cleared in some patients that appear to have recovered and raises the possibility that SARS-CoV-2 may evade immune surveillance, at least to some degree. It is important to note that replication-competency of virus found in lung of this patient was not determined and remains an important scientific and clinical question. This would have major implications for the brain if replication-competent virus persists in the CNS compartment after recovery. Even an abortive infection, if present, could negatively impact cell function and impair brain homeostasis. Alternatively, or in addition to direct viral involvement, chronic neuroinflammation can contribute to impaired brain homeostasis through production of soluble factors that directly and/or indirectly impair neuronal function. Clinical follow-up and prospective observational studies are critical for assessing long-term neurological outcomes of patients recovered from COVID-19. While this is a likely standard for follow-up of individuals who were diagnosed with serious neurological manifestations, functional and cognitive decline may continue after recovery among COVID-19 patients for whom neurological disease was not identified. There is significant evidence that supports the notion that these individuals, particularly those recovered from severe COVID-19, may have difficulty performing critical functions long after recovery, including reduced job performance and/or ability to attend to activities of daily living, that may worsen over time.
Limited studies are available that investigate the long-term neurological consequences of COVID-19 and do not include neurological assessments but have relied on neuropsychological testing and self-reports. A large cross-sectional study involving cognitive assessments of subjects who had recovered from COVID-19 demonstrated impairment in a variety of cognitive domains and a lower global cognitive performance score, with worsening performance associated with the severity of respiratory disease (Hampshire et al., 2020). This may suggest irreversible injury to the brain due to reduced oxygen and/or chronic subclinical unresolved neuroinflammation, two factors that play a major role in pathologic brain aging. Importantly, minor deficits were even seen among individuals who had experienced only mild respiratory symptoms. Additional follow-up of these individuals is needed to assess the course of symptoms with increased recovery time. It is important to note that testing was performed on-line, rather than by a board-certified neuropsychologist. In a separate assessment of self-reports from subjects at 6-month post-infection, sleep difficulties, anxiety, and depression were the most commonly reported complaints, in addition to fatigue and muscle weakness (Huang et al., 2021).
Evidence of injury at the level of the CNS is very limited at this time, however, a functional imaging study of patients with persistent anosmia following recovery of SARS-CoV-2 infection displayed reduced metabolism in bilateral limbic cortices and the insular cortex of the left hemisphere, as compared to controls (Donegani et al., 2021). This suggests brain involvement in SARS-CoV-2-associated anosmia that may also impact cognitive function, as these brain regions are involved in multiple cognitive processes, including learning and memory, word, face, and body recognition, and consciousness. The insular cortices are also involved in taste, which is often impaired in SARS-CoV-2 infection, alone or concurrent with impaired smell, which may implicate injury within in this region among individuals suffering loss of taste and/or smell. With the potential for controlling the SARS-CoV-2 pandemic with the world-wide introduction of multiple vaccines, more comprehensive follow-up of recovered patients is likely to become an urgent public health concern, including neurological work-up and neuropsychological and/or psychiatric assessments.
This systematic review of the literature and meta-analysis has demonstrated that neurological manifestations are a common complication of SARS-CoV-2 infection and COVID-19 that effects individuals across the lifespan, with all severities of COVID-19, and with or without comorbidities. Consistently emerging case reports and retrospective studies detailing the neurological impact of COVID-19, point to the necessity for investigating the impact of hyperimmune responses and/or reduced oxygen more thoroughly on neuronal injury. In addition, the neuroinvasive potential of the virus should not be ruled outs, as neurological conditions may be seen as a presenting symptom of infection and arise in the absence of respiratory disease. Further, as SARS-CoV-2 infection can lead to devastating neurological diseases irrespective of age, sex, or comorbidities, targeted studies of the COVID-19 population are imperative to better understand and elucidate the true impact of COVID-19 on the CNS. COVID-19 patients need to be followed for potential long-term neurological sequelae after recovery from infection, including pathologic brain aging, that likely plays a key role in PASC. With multiple vaccines now available, we may continue to see a reduction in new cases and/or disease severity, however, the potential for CNS complications remains a major clinical and public health concern.
Author Contributions
BS and TF contributed to the writing of the article, figure and table preparation, and final assembly. Both authors contributed to the article and approved the submitted version.
Funding
TF was supported by P51OD011104 through the NIH, Office of Research Infrastructure Programs (ORIP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.653694/full#supplementary-material
Footnotes
References
Abdi, S., Ghorbani, A., and Fatehi, F. (2020). The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 416:117001. doi: 10.1016/j.jns.2020.117001
Abel, D., Shen, M. Y., Abid, Z., Hennigan, C., Boneparth, A., Miller, E. H., et al. (2020). Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology 95, 745–748. doi: 10.1212/wnl.0000000000010652
Abenza-Abildúa, M. J., Novo-Aparicio, S., Moreno-Zabaleta, R., Algarra-Lucas, M. C., Rojo Moreno-Arcones, B., Salvador-Maya, M., et al. (2020). Encephalopathy in severe SARS-CoV2 infection: Inflammatory or infectious? Int. J. Infect. Dis. 98, 398–400. doi: 10.1016/j.ijid.2020.07.020
Abolmaali, M., Heidari, M., Zeinali, M., Moghaddam, P., Ramezani Ghamsari, M., Jamshidi Makiani, M., et al. (2021). Guillain-Barré syndrome as a parainfectious manifestation of SARS-CoV-2 infection: A case series. J. Clin. Neurosci. 83, 119–122. doi: 10.1016/j.jocn.2020.11.013
Abrams, R. M. C., Kim, B. D., Markantone, D. M., Reilly, K., Paniz-Mondolfi, A. E., Gitman, M. R., et al. (2020). Severe rapidly progressive Guillain-Barré syndrome in the setting of acute COVID-19 disease. J. Neurovirol. 26, 797–799. doi: 10.1007/s13365-020-00884-7
Agarwal, A., Pinho, M., Raj, K., Yu, F. F., Bathla, G., Achilleos, M., et al. (2020). Neurological emergencies associated with COVID-19: stroke and beyond. Emerg. Radiol. 27, 747–754. doi: 10.1007/s10140-020-01837-7
Águila-Gordo, D., Manuel Flores-Barragán, J., Ferragut-Lloret, F., Portela-Gutierrez, J., LaRosa-Salas, B., Porras-Leal, L., et al. (2020). Acute myelitis and SARS-CoV-2 infection. A new etiology of myelitis? J. Clin. Neurosci. 80, 280–281. doi: 10.1016/j.jocn.2020.07.074
Al Saiegh, F., Ghosh, R., Leibold, A., Avery, M. B., Schmidt, R. F., Theofanis, T., et al. (2020). Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J. Neurol. Neurosurg. Psychiatry 91, 846–848.
Alay, H., Can, F. K., and Gözgeç, E. (2020). Cerebral Infarction in an Elderly Patient with Coronavirus Disease. Rev. Soc. Bras. Med. Trop. 53:e20200307.
Alberti, P., Beretta, S., Piatti, M., Karantzoulis, A., Piatti, M. L., Santoro, P., et al. (2020). Guillain-Barré syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 7:e741.
Al-Dalahmah, O., Thakur, K. T., Nordvig, A. S., Prust, M. L., Roth, W., Lignelli, A., et al. (2020). Neuronophagia and microglial nodules in a SARS-CoV-2 patient with cerebellar hemorrhage. Acta Neuropathol. Commun. 8:147.
Alkeridy, W. A. I, Almaghlouth, R., Alrashed, K., Alayed, K., Binkhamis, A., and Alsharidi, et al. (2020). A Unique Presentation of Delirium in a Patient with Otherwise Asymptomatic COVID-19. J. Am. Geriatr. Soc. 68, 1382–1384. doi: 10.1111/jgs.16536
Al-Mufti, F., Becker, C., Kamal, H., Alshammari, H., Dodson, V., Nuoman, R., et al. (2021). Acute Cerebrovascular Disorders and Vasculopathies Associated with Significant Mortality in SARS-CoV-2 Patients Admitted to The Intensive Care Unit in The New York Epicenter. J. Stroke Cerebrovasc. Dis. 30:105429. doi: 10.1016/j.jstrokecerebrovasdis.2020.105429
Al-Olama, M., Rashid, A., and Garozzo, D. (2020). COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochirurgica 162, 1495–1499. doi: 10.1007/s00701-020-04402-w
Álvarez Bravo, G., Ramió, L., and Torrentà, I. (2020). Anti-NMDA receptor encephalitis secondary to SARS-CoV-2 infection. Neurologia 35, 699–700. doi: 10.1016/j.nrleng.2020.07.011
Ameer, N., Shekhda, K. M., and Cheesman, A. (2020). Guillain-Barré syndrome presenting with COVID-19 infection. BMJ Case Rep. 13:e236978. doi: 10.1136/bcr-2020-236978
Anand, P., Lau, K. H. V., Chung, D. Y., Virmani, D., Cervantes-Arslanian, A. M., Mian, A. Z., et al. (2020a). Posterior Reversible Encephalopathy Syndrome in Patients with Coronavirus Disease 2019: Two Cases and A Review of The Literature. J. Stroke Cerebrovasc. Dis. 29:105212. doi: 10.1016/j.jstrokecerebrovasdis.2020.105212
Anand, P., Zakaria, A., Benameur, K., Ong, C., Putman, M., O’Shea, S., et al. (2020b). Myoclonus in Patients With Coronavirus Disease 2019: A Multicenter Case Series. Crit. Care Med. 48, 1664–1669. doi: 10.1097/ccm.0000000000004570
Anzalone, N., Castellano, A., Scotti, R., Scandroglio, A. M., Filippi, M., Ciceri, F., et al. (2020). Multifocal laminar cortical brain lesions: a consistent MRI finding in neuro-COVID-19 patients. J. Neurol. 267, 2806–2809. doi: 10.1007/s00415-020-09966-2
Arbour, N., Day, R., Newcombe, J., and Talbot, P. J. (2000). Neuroinvasion by human respiratory coronaviruses. J. Virol. 74, 8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000
Asif, R., and O’ Mahony, M. S. (2020). Rare complication of COVID-19 presenting as isolated headache. BMJ Case Rep. 13:e239275. doi: 10.1136/bcr-2020-239275
Assini, A., Benedetti, L., Di Maio, S., Schirinzi, E., and Del Sette, M. (2020). New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol. Sci. 41, 1657–1658. doi: 10.1007/s10072-020-04484-5
Avci, A., Yesiloglu, O., Avci, B. S., Sumbul, H. E., BugraYapici, S., Kuvvetli, A., et al. (2020). Spontaneous subarachnoidal hemorrhage in patients with Covid-19: case report. J. Neurovirol. 26, 802–804. doi: 10.1007/s13365-020-00888-3
Avula, A., Nalleballe, K., Narula, N., Sapozhnikov, S., Dandu, V., Toom, S., et al. (2020). COVID-19 presenting as stroke. Brain Behav. Immun. 87, 115–119.
Azpiazu Landa, N., Velasco Oficialdegui, C., Intxaurraga Fernández, K., Gonzalez Larrabe, I., Riaño Onaindia, S., and Telletxea Benguria, S. (2020). Ischemic-hemorrhagic stroke in patients with Covid-19. Rev. Esp. Anestesiol. Reanim. 67, 516–520.
Babar, A., Lewandowski, U., Capin, I., Khariton, M., Venkataraman, A., Okolo, N., et al. (2020). SARS-CoV-2 Encephalitis in a 20-year old Healthy Female. Pediatr. Infect. Dis. J. 39, e320–e321.
Balestrino, R., Rizzone, M., Zibetti, M., Romagnolo, A., Artusi, C. A., Montanaro, E., et al. (2020). Onset of Covid-19 with impaired consciousness and ataxia: a case report. J. Neurol. 267, 2797–2798. doi: 10.1007/s00415-020-09879-0
Barrachina-Esteve, O., Palau Domínguez, A., Hidalgo-Torrico, I., and Viguera Martínez, M. L. (2020). Guillain-Barré syndrome as the first manifestation of SARS-CoV-2 infection. Neurologia 35, 710–712. doi: 10.1016/j.nrleng.2020.07.002
Bektaş, G., Akçay, N., Boydað, K., and Şevketoðlu, E. (2021). Reversible splenial lesion syndrome associated with SARS-CoV-2 infection in two children. Brain Dev. 43, 230–233. doi: 10.1016/j.braindev.2020.10.002
Benameur, K., Agarwal, A., Auld, S. C., Butters, M. P., Webster, A. S., Ozturk, T., et al. (2020). Encephalopathy and Encephalitis Associated with Cerebrospinal Fluid Cytokine Alterations and Coronavirus Disease, Atlanta, Georgia, USA, 2020. Emerg. Infect. Dis. 26, 2016–2021. doi: 10.3201/eid2609.202122
Beyrouti, R., Adams, M. E., Benjamin, L., Cohen, H., Farmer, S. F., Goh, Y. Y., et al. (2020). Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 91, 889–891.
Bigaut, K., Mallaret, M., Baloglu, S., Nemoz, B., Morand, P., Baicry, F., et al. (2020). Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 7:e785.
Bigliardi, G., Ciolli, L., Giovannini, G., Vandelli, L., Dell’Acqua, M. L., Borzì, G. M., et al. (2020). Middle cerebral artery ischemic stroke and COVID-19: a case report. J. Neurovirol. 26, 967–969.
Bolaji, P., Kukoyi, B., Ahmad, N., and Wharton, C. (2020). Extensive cerebral venous sinus thrombosis: a potential complication in a patient with COVID-19 disease. BMJ Case Rep. 13:e236820. doi: 10.1136/bcr-2020-236820
Bracaglia, M., Naldi, I., Govoni, A., Brillanti Ventura, D., and De Massis, P. (2020). Acute inflammatory demyelinating polyneuritis in association with an asymptomatic infection by SARS-CoV-2. J. Neurol. 267, 3166–3168. doi: 10.1007/s00415-020-10014-2
Brun, G., Hak, J. F., Coze, S., Kaphan, E., Carvelli, J., Girard, N., et al. (2020). COVID-19-White matter and globus pallidum lesions: Demyelination or small-vessel vasculitis? Neurol. Neuroimmunol. Neuroinflamm. 7:e777. doi: 10.1212/nxi.0000000000000777
Bueso, T., Montalvan, V., Lee, J., Gomez, J., Ball, S., Shoustari, A., et al. (2021). Guillain-Barre Syndrome and COVID-19: A case report. Clin. Neurol. Neurosurg. 200:106413.
Burkert, J., and Patil, S. (2020). Acute cerebrovascular event in a COVID-19 positive patient immediately after commencing non-invasive ventilation. BMJ Case Rep. 13:e237737. doi: 10.1136/bcr-2020-237737
Butt, I., Sawlani, V., and Geberhiwot, T. (2020). Prolonged confusional state as first manifestation of COVID-19. Ann. Clin. Transl. Neurol. 7, 1450–1452. doi: 10.1002/acn3.51067
Cabirac, G. F., Murray, R. S., McLaughlin, L. B., Skolnick, D. M., Hogue, B., Dorovini-Zis, K., et al. (1995). In vitro interaction of coronaviruses with primate and human brain microvascular endothelial cells. Adv. Exp. Med. Biol. 380, 79–88. doi: 10.1007/978-1-4615-1899-0_11
Cagnazzo, F., Piotin, M., Escalard, S., Maier, B., Ribo, M., Requena, M., et al. (2021). European Multicenter Study of ET-COVID-19. Stroke 52, 31–39.
Cani, V. I, Barone, R. D., Angelo, L., Pisani, V., Allegri, L., and Spinardi, et al. (2021). Frontal encephalopathy related to hyperinflammation in COVID-19. J. Neurol. 268, 16–19. doi: 10.1007/s00415-020-10057-5
Cannac, O., Martinez-Almoyna, L., and Hraiech, S. (2020). Critical illness-associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology 95, 498–499. doi: 10.1212/wnl.0000000000010537
Cavalcanti, D. D., Raz, E., Shapiro, M., Dehkharghani, S., Yaghi, S., Lillemoe, K., et al. (2020). Cerebral Venous Thrombosis Associated with COVID-19. AJNR Am. J. Neuroradiol. 41, 1370–1376.
Cebrián, J., Gonzalez-Martinez, A., García-Blanco, M. J., Celdrán-Vivancos, D., Palacios, E. L., Reig-Roselló, G., et al. (2020). Headache and impaired consciousness level associated with SARS-CoV-2 in CSF: A case report. Neurology 95, 266–268. doi: 10.1212/wnl.0000000000010213
Cezar-Junior, A. B. I, Faquini, V., Silva, J. L. J., de Carvalho Junior, E. V., Lemos, L. E. A. S., Freire Filho, J. B. M., et al. (2020). Subarachnoid hemorrhage and COVID-19: Association or coincidence? Medicine 99:e23862. doi: 10.1097/md.0000000000023862
Chan, J. L., Ebadi, H., and Sarna, J. R. (2020). Guillain-Barré Syndrome with Facial Diplegia Related to SARS-CoV-2 Infection. Can. J. Neurol. Sci. 47, 852–854. doi: 10.1017/cjn.2020.106
Chang, P., Tasch, E. S., Rapoport, L. N., and Bakhadirov, K. (2021). A Post-Infectious Steroid-Responsive Brainstem Lesion Associated With COVID-19. Neurohospitalist 11, 152–155. doi: 10.1177/1941874420959544
Chaumont, H., Etienne, P., Roze, E., Couratier, C., Roger, P. M., and Lannuzel, A. (2020a). Acute meningoencephalitis in a patient with COVID-19. Rev. Neurol. 176, 519–521. doi: 10.1016/j.neurol.2020.04.014
Chaumont, H., San-Galli, A., Martino, F., Couratier, C., Joguet, G., Carles, M., et al. (2020b). Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection. J. Neurol. 267, 3121–3127. doi: 10.1007/s00415-020-09986-y
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. doi: 10.1016/s0140-6736(20)30211-7
Chen, T., Wu, D., Chen, H., Yan, W., Yang, D., Chen, G., et al. (2020). Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091. doi: 10.1136/bmj.m1091
Chia, K. X., Polakhare, S., and Bruno, S. D. (2020). Possible affective cognitive cerebellar syndrome in a young patient with COVID-19 CNS vasculopathy and stroke. BMJ Case Rep. 13:e237926. doi: 10.1136/bcr-2020-237926
Chien, J. Y., Hsueh, P. R., Cheng, W. C., Yu, C. J., and Yang, P. C. (2006). Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 11, 715–722. doi: 10.1111/j.1440-1843.2006.00942.x
Chiu, S. S., Chan, K. H., Chu, K. W., Kwan, S. W., Guan, Y., Poon, L. L., et al. (2005). Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong. China. Clin. Infect. Dis. 40, 1721–1729. doi: 10.1086/430301
Chow, C. C. N., Magnussen, J., Ip, J., and Su, Y. (2020). Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. 13:e236720. doi: 10.1136/bcr-2020-236720
Co, C. O. C., Yu, J. R. T., Laxamana, L. C., and David-Ona, D. I. A. (2020). Intravenous Thrombolysis for Stroke in a COVID-19 Positive Filipino Patient, a Case Report. J. Clin. Neurosci. 77, 234–236. doi: 10.1016/j.jocn.2020.05.006
Coen, M., Jeanson, G., Culebras Almeida, L. A., Hübers, A., Stierlin, F., Najjar, I., et al. (2020). Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav. Immun. 87, 111–112.
Cohen, J. (1998). Set Correlation and Contingency Tables. Appl. Psychol. Measurem. 12:014662168801200410. doi: 10.1177/014662168801200410
Conklin, J., Frosch, M., Mukerji, S., Rapalino, O., Maher, M., Schaefer, P., et al. (2020). Cerebral Microvascular Injury in Severe COVID-19. J. Neurol. Sci. 421:117308.
Dakay, K., Cooper, J., Bloomfield, J., Overby, P., Mayer, S. A., Nuoman, R., et al. (2021). Cerebral Venous Sinus Thrombosis in COVID-19 Infection: A Case Series and Review of The Literature. J. Stroke Cerebrovasc. Dis. 30:105434. doi: 10.1016/j.jstrokecerebrovasdis.2020.105434
Dakay, K., Kaur, G., Gulko, E., Santarelli, J., Bowers, C., Mayer, S. A., et al. (2020). Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J. Stroke Cerebrovasc. Dis. 29:105011. doi: 10.1016/j.jstrokecerebrovasdis.2020.105011
D’Anna, L., Kwan, J., Brown, Z., Halse, O., Jamil, S., Kalladka, D., et al. (2020). Characteristics and clinical course of Covid-19 patients admitted with acute stroke. J. Neurol. 267, 3161–3165. doi: 10.1007/s00415-020-10012-4
de Almeida Lima, A. N., Santos Leite Pessoa, M., Franco Costa Lima, C., Picasso de Araújo Coimbra, P., and Bezerra Holanda, J. L. (2020). Images in Vascular Medicine: Acute peripheral artery occlusion and ischemic stroke in a patient with COVID-19. Vasc. Med. 25, 482–483. doi: 10.1177/1358863x20945020
de Miranda Henriques-Souza, A. M., de Melo, A. C. M. G., de Aguiar Coelho Silva Madeiro, B., Freitas, L. F., Sampaio Rocha-Filho, P. A., and Gonçalves, F. G. (2021). Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology 63, 141–145.
de Oliveira, F. A. A., Palmeira, D. C. C., and Rocha-Filho, P. A. S. (2020). Headache and pleocytosis in CSF associated with COVID-19: case report. Neurol. Sci. 41, 3021–3022. doi: 10.1007/s10072-020-04694-x
De Paulis, M., Oliveira, D. B. L., Vieira, R. P. I, Pinto, C., Machado, R. R. G., Cavalcanti, M. P., et al. (2020). Multisystem Inflammatory Syndrome Associated With COVID-19 With Neurologic Manifestations in a Child: A Brief Report. Pediatr. Infect. Dis. J. 39, e321–e324.
de Sousa, G. C., de Sousa, T. C., Sakiyama, M. A. K., da Silva, J. S. N. L., and de Sousa, E. J. S. (2020). Vasculitis-related stroke in young as a presenting feature of novel coronavirus disease (COVID19) - Case report. J. Clin. Neurosci. 79, 169–171. doi: 10.1016/j.jocn.2020.07.034
Delorme, C., Paccoud, O., Kas, A., Hesters, A., Bombois, S., Shambrook, P., et al. (2020). COVID-19-related encephalopathy: a case series with brain FDG-positron-emission tomography/computed tomography findings. Eur. J. Neurol. 27, 2651–2657. doi: 10.1111/ene.14478
Díaz-Pérez, C., Ramos, C., ópez-Cruz, A. L., Muñoz Olmedo, J., ázaro González, J. L., De Vega-Ríos, E., et al. (2020). Acutely altered mental status as the main clinical presentation of multiple strokes in critically ill patients with COVID-19. Neurol. Sci. 41, 2681–2684. doi: 10.1007/s10072-020-04679-w
Diaz-Segarra, N., Edmond, A., Kunac, A., and Yonclas, P. (2020). COVID-19 Ischemic Strokes as an Emerging Rehabilitation Population: A Case Series. Am. J. Phys. Med. Rehabil. 99, 876–879. doi: 10.1097/phm.0000000000001532
Dinkin, M., Gao, V., Kahan, J., Bobker, S., Simonetto, M., Wechsler, P., et al. (2020). COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology 95, 221–223. doi: 10.1212/wnl.0000000000009700
Dixon, L., Varley, J., Gontsarova, A., Mallon, D., Tona, F., Muir, D., et al. (2020). COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol. Neuroimmunol. Neuroinflamm. 7:e789. doi: 10.1212/nxi.0000000000000789
Donegani, M., Miceli, A., Pardin, I. M., Bauckneht, M., Chiola, S., Pennone, M., et al. (2021). Brain Metabolic Correlates of Persistent Olfactory Dysfunction after SARS-Cov2 Infection. Biomedicines 9:287. doi: 10.3390/biomedicines9030287
Drosten, C., Günther, S., Preiser, W., van der Werf, S., Brodt, H. R., Becker, S., et al. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976.
Dugue, R., Cay-Martínez, K. C., Thakur, K. T., Garcia, J. A., Chauhan, L. V., Williams, S. H., et al. (2020). Neurologic manifestations in an infant with COVID-19. Neurology 94, 1100–1102.
Edén, A., Kanberg, N., Gostner, J., Fuchs, D., Hagberg, L., Andersson, L.-M., et al. (2021). CSF Biomarkers in Patients With COVID-19 and Neurologic Symptoms. Neurology 96, e294–e300.
Efe, I. E., Aydin, O. U., Alabulut, A., Celik, O., and Aydin, K. (2020). COVID-19-Associated Encephalitis Mimicking Glial Tumor. World Neurosurg. 140, 46–48. doi: 10.1016/j.wneu.2020.05.194
Elgamasy, S., Kamel, M. G., Ghozy, S., Khalil, A., Morra, M. E., and Islam, S. M. S. (2020). First case of focal epilepsy associated with SARS-coronavirus-2. J. Med. Virol. 92, 2238–2242. doi: 10.1002/jmv.26113
Elkhaled, W., Ben Abid, F., Akhtar, N., Abukamar, M. R., and Ibrahim, W. H. (2020). A 23-Year-Old Man with SARS-CoV-2 Infection Who Presented with Auditory Hallucinations and Imaging Findings of Cytotoxic Lesions of the Corpus Callosum (CLOCC). Am. J. Case Rep. 21:e928798.
Erdede, O., Sarı, E., Uygur Külcü, N., Uyur Yalçın, E., and Sezer Yamanel, R. G. (2020). An overview of smell and taste problems in paediatric COVID-19 patients. Acta Paediatr. 109, 2184–2186. doi: 10.1111/apa.15515
Fabbri, V. P., Foschini, M. P., Lazzarotto, T., Gabrielli, L., Cenacchi, G., Gallo, C., et al. (2021). Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. 31, 205–210. doi: 10.1111/bpa.12901
Faber, I., Brandão, P. R. P., Menegatti, F., de Carvalho Bispo, D. D., Maluf, F. B., and Cardoso, F. (2020). Coronavirus Disease 2019 and Parkinsonism: A Non-post-encephalitic Case. Mov. Disord. 35, 1721–1722. doi: 10.1002/mds.28277
Fara, M. G., Stein, L. K., Skliut, M., Morgello, S., Fifi, J. T., and Dhamoon, M. S. (2020). Macrothrombosis and stroke in patients with mild Covid-19 infection. J. Thromb. Haemost. 18, 2031–2033. doi: 10.1111/jth.14938
Farhadian, S., Glick, L. R., Vogels, C. B. F., Thomas, J., Chiarella, J., Casanovas-Massana, A., et al. (2020). Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 20:248. doi: 10.1186/s12883-020-01812-2
Fasano, A., Cavallieri, F., Canali, E., and Valzania, F. (2020). First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol. Sci. 41, 1651–1653. doi: 10.1007/s10072-020-04460-z
Fayed, I., Pivazyan, G., Conte, A. G., Chang, J., and Mai, J. C. (2020). Intracranial hemorrhage in critically ill patients hospitalized for COVID-19. J. Clin. Neurosci. 81, 192–195. doi: 10.1016/j.jocn.2020.08.026
Fernández-Domínguez, J., Ameijide-Sanluis, E., García-Cabo, C., García-Rodríguez, R., and Mateos, V. (2020). Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19). J. Neurol. 267, 2495–2496. doi: 10.1007/s00415-020-09912-2
Filatov, A., Sharma, P., Hindi, F., and Espinosa, P. S. (2020). Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 12:e7352.
Fischer, D., Threlkeld, Z. D., Bodien, Y. G., Kirsch, J. E., Huang, S. Y., Schaefer, P. W., et al. (2020). Intact Brain Network Function in an Unresponsive Patient with COVID-19. Ann. Neurol. 88, 851–854. doi: 10.1002/ana.25838
Fitsiori, A., Pugin, D., Thieffry, C., Lalive, P., and Vargas, M. (2020). COVID-19 is Associated with an Unusual Pattern of Brain Microbleeds in Critically Ill Patients. J. Neuroimaging 30, 593–597. doi: 10.1111/jon.12755
Franceschi, A. M., Ahmed, O., Giliberto, L., and Castillo, M. (2020). Hemorrhagic Posterior Reversible Encephalopathy Syndrome as a Manifestation of COVID-19 Infection. AJNR Am. J. Neuroradiol. 41, 1173–1176. doi: 10.3174/ajnr.a6595
Franceschi, C., Bonafè, M., Valensin, S., Olivieri, F., De Luca, M., Ottaviani, E., et al. (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. York Acad. Sci. 908, 244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x
Frisullo, G., Bellavia, S., Scala, I., Piano, C., Morosetti, R., Brunetti, V., et al. (2020). Stroke and COVID19: Not only a large-vessel disease. J. Stroke Cerebrovasc. Dis. 29:105074. doi: 10.1016/j.jstrokecerebrovasdis.2020.105074
Fu, B., Chen, Y., and Li, P. (2021). 2019 novel coronavirus disease with secondary ischemic stroke: two case reports. BMC Neurol. 21:4. doi: 10.1186/s12883-020-02033-3
Fumery, T., Baudar, C., Ossemann, M., and London, F. (2021). Longitudinally extensive transverse myelitis following acute COVID-19 infection. Mult. Scler. Relat. Disord. 48:102723. doi: 10.1016/j.msard.2020.102723
Gale, A., Sabaretnam, S., and Lewinsohn, A. (2020). Guillain-Barré syndrome and COVID-19: association or coincidence. BMJ Case Rep. 13:e239241. doi: 10.1136/bcr-2020-239241
Gane, S. B., Kelly, C., and Hopkins, C. (2020). Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 58, 299–301. doi: 10.4193/rhin20.114
Gao, Y., Li, T., Han, M., Li, X., Wu, D., Xu, Y., et al. (2020). Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 92, 791–796. doi: 10.1002/jmv.25770
Garví López, M., Tauler Redondo, M., and Tortajada Soler, J. (2021). Intracranial hemorrhages in critical COVID-19 patients: report of three cases. Med. Clin. 156, 38–39. doi: 10.1016/j.medcle.2020.08.002
Ghosh, R., Dubey, S., Finsterer, J., Chatterjee, S., and Ray, B. K. (2020). SARS-CoV-2-Associated Acute Hemorrhagic, Necrotizing Encephalitis (AHNE) Presenting with Cognitive Impairment in a 44-Year-Old Woman without Comorbidities: A Case Report. Am. J. Case Rep. 21:e925641.
Gilani, S., Roditi, R., and Naraghi, M. (2020). COVID-19 and anosmia in Tehran, Iran. Med. Hypotheses 141:109757. doi: 10.1016/j.mehy.2020.109757
Gonçalves, B., Righy, C., and Kurtz, P. (2020). Thrombotic and Hemorrhagic Neurological Complications in Critically Ill COVID-19 Patients. Neurocrit. Care 33, 587–590. doi: 10.1007/s12028-020-01078-z
Goshua, G., Pine, A., Meizlish, M., Chang, C., Zhang, H., Bahel, P., et al. (2020). Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 7, e575–e582.
Guarneri, B., Bertolini, G., and Latronico, N. (2008). Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J. Neurol. Neurosurg. Psychiatry 79, 838–841. doi: 10.1136/jnnp.2007.142430
Guillan, M., Villacieros-Alvarez, J., Bellido, S., Perez-Jorge Peremarch, C., Suarez-Vega, V. M., Aragones-Garcia, M., et al. (2020). Unusual simultaneous cerebral infarcts in multiple arterial territories in a COVID-19 patient. Thromb. Res. 193, 107–109. doi: 10.1016/j.thromres.2020.06.015
Gulko, E., Overby, P., Ali, S., Mehta, H., Al-Mufti, F., and Gomes, W. (2020). Vessel Wall Enhancement and Focal Cerebral Arteriopathy in a Pediatric Patient with Acute Infarct and COVID-19 Infection. AJNR Am. J. Neuroradiol. 41, 2348–2350. doi: 10.3174/ajnr.a6778
Gupta, N. A., Lien, C., and Iv, M. (2020). Critical illness-associated cerebral microbleeds in severe COVID-19 infection. Clin. Imaging 68, 239–241. doi: 10.1016/j.clinimag.2020.08.029
Gutiérrez-Ortiz, C., Méndez-Guerrero, A., Rodrigo-Rey, S., San Pedro-Murillo, E., Bermejo-Guerrero, L., Gordo-Mañas, R., et al. (2020). Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 95, e601–e605.
Hamming, I., Timens, W., Bulthuis, M. L., Lely, A. T., Navis, G., and van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637. doi: 10.1002/path.1570
Hampshire, A., Trender, W., Chamberlain, S. R., Jolly, A., Grant, J. E., Patrick, F., et al. (2020). Cognitive deficits in people who have recovered from COVID-19 relative to controls: An N=84,285 online study. [Preprint].
Han, H., Yang, L., Liu, R., Liu, F., Wu, K. L., Li, J., et al. (2020). Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 58, 1116–1120. doi: 10.1515/cclm-2020-0188
Hanafi, R., Roger, P. A., Perin, B., Kuchcinski, G., Deleval, N., Dallery, F., et al. (2020). COVID-19 Neurologic Complication with CNS Vasculitis-Like Pattern. AJNR Am. J. Neuroradiol. 41, 1384–1387. doi: 10.3174/ajnr.a6651
Handa, R., Nanda, S., Prasad, A., Anand, R., Zutshi, D., Dass, S. K., et al. (2020). Covid-19-associated acute haemorrhagic leukoencephalomyelitis. Neurol. Sci. 41, 3023–3026. doi: 10.1007/s10072-020-04703-z
Harrogate, S., Mortimer, A., Burrows, L., Fiddes, B., Thomas, I., and Rice, C. M. (2021). Non-aneurysmal subarachnoid haemorrhage in COVID-19. Neuroradiology 63, 149–152. doi: 10.1007/s00234-020-02535-4
Hatipoglu, N., Yazici, Z. M., Palabiyik, F., Gulustan, F., and Sayin, I. (2020). Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia in pediatric cases. Int. J. Pediatr. Otorhinolaryngol. 139:110469. doi: 10.1016/j.ijporl.2020.110469
Heller, H. M., Gonzalez, R. G., Edlow, B. L., Ard, K. L., and Gogakos, T. (2020). Case 40-2020: A 24-Year-Old Man with Headache and Covid-19. N. Engl. J. Med. 383, 2572–2580. doi: 10.1056/nejmcpc2027083
Heman-Ackah, S. M., Su, Y. S., Spadola, M., Petrov, D., Chen, H. I., Schuster, J., et al. (2020). Neurologically Devastating Intraparenchymal Hemorrhage in COVID-19 Patients on Extracorporeal Membrane Oxygenation: A Case Series. Neurosurgery 87, E147–E151.
Hemasian, H., and Ansari, B. (2020). First case of Covid-19 presented with cerebral venous thrombosis: A rare and dreaded case. Rev. Neurol. 176, 521–523. doi: 10.1016/j.neurol.2020.04.013
Hirayama, T., Hongo, Y., Kaida, K., and Kano, O. (2020). Guillain-Barré syndrome after COVID-19 in Japan. BMJ Case Rep. 13:e239218. doi: 10.1136/bcr-2020-239218
Hjelmesæth, J., and Skaare, D. (2020). Loss of smell or taste as the only symptom of COVID-19. Tidsskr Nor Laegeforen 140:0287.
Homma, Y., Watanabe, M., Inoue, K., and Moritaka, T. (2020). Coronavirus Disease-19 Pneumonia with Facial Nerve Palsy and Olfactory Disturbance. Intern. Med. 59, 1773–1775. doi: 10.2169/internalmedicine.5014-20
Hosseini, A. A., Shetty, A. K., Sprigg, N., Auer, D. P., and Constantinescu, C. S. (2020). Delirium as a presenting feature in COVID-19: Neuroinvasive infection or autoimmune encephalopathy? Brain Behav. Immun. 88, 68–70. doi: 10.1016/j.bbi.2020.06.012
Huang, C., Huang, L., Wang, Y., Li, X., Ren, L., and Gu, X. (2021). 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232.
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet 395, 497–506.
Hughes, R. A. C., Swan, A. V., Raphaël, J.-C., Annane, D., van Koningsveld, R., and van Doorn, P. A. (2007). Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain 130, 2245–2257. doi: 10.1093/brain/awm004
Hutchins, K. L., Jansen, J. H., Comer, A. D., Scheer, R. V., Zahn, G. S., Capps, A. E., et al. (2020). COVID-19-Associated Bifacial Weakness with Paresthesia Subtype of Guillain-Barré Syndrome. AJNR Am. J. Neuroradiol. 41, 1707–1711.
Imoto, W., Kaga, S., Noda, T., Oshima, K., Mizobata, Y., and Kakeya, H. (2020). Coronavirus disease with multiple infarctions. QJM 113, 907–908. doi: 10.1093/qjmed/hcaa240
Jacob, J., Flannery, W., and Mostert, C. (2020). Novel ENT triad of anosmia, ageusia and hearing impairment in COVID-19. Intern. Med. J. 50:1155. doi: 10.1111/imj.14880
Jaunmuktane, Z., Mahadeva, U., Green, A., Sekhawat, V., Barrett, N. A., Childs, L., et al. (2020). Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol. 140, 397–400. doi: 10.1007/s00401-020-02190-2
Ji, X. Y., Ma, Y., Shi, N. N., Liang, N., Chen, R. B., Liu, S. H., et al. (2021). Clinical characteristics and treatment outcome of COVID-19 patients with stroke in China: A multicenter retrospective study. Phytomedicine 81:153433.
Juliao Caamaño, D. S., and Alonso Beato, R. (2020). Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J. Clin. Neurosci. 77, 230–232. doi: 10.1016/j.jocn.2020.05.016
Kadono, Y., Nakamura, Y., Ogawa, Y., Yamamoto, S., Kajikawa, R., Nakajima, Y., et al. (2020). A case of COVID-19 infection presenting with a seizure following severe brain edema. Seizure 80, 53–55. doi: 10.1016/j.seizure.2020.06.015
Kakadia, B., Ahmed, J., Siegal, T., Jovin, T. G., and Thon, J. M. (2020). Mild encephalopathy with reversible splenium lesion (MERS) in a patient with COVID-19. J. Clin. Neurosci. 79, 272–274. doi: 10.1016/j.jocn.2020.07.009
Kamal, Y., Abdelmajid, Y., and Al Madani, A. (2020). Cerebrospinal fluid confirmed COVID-19-associated encephalitis treated successfully. BMJ Case Rep. 13:e237378. doi: 10.1136/bcr-2020-237378
Kansagra, S. M., and Gallentine, W. B. (2011). Cytokine Storm of Acute Necrotizing Encephalopathy. Pediatr. Neurol. 45, 400–402. doi: 10.1016/j.pediatrneurol.2011.09.007
Kantonen, J., Mahzabin, S., Mäyränpää, M. I., Tynninen, O., Paetau, A., Andersson, N., et al. (2020). Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 30, 1012–1016. doi: 10.1111/bpa.12889
Kaur, H., Mason, J. A., Bajracharya, M., McGee, J., Gunderson, M. D., Hart, B. L., et al. (2020). Transverse Myelitis in a Child With COVID-19. Pediatr. Neurol. 112, 5–6.
Khaja, M., Gomez, G. P. R., Santana, Y., Hernandez, N., Haider, A., Lara, J. L. P., et al. (2020). A 44-Year-Old Hispanic Man with Loss of Taste and Bilateral Facial Weakness Diagnosed with Guillain-Barré Syndrome and Bell’s Palsy Associated with SARS-CoV-2 Infection Treated with Intravenous Immunoglobulin. Am. J. Case Rep. 21:e927956.
Khalifa, M., Zakaria, F., Ragab, Y., Saad, A., Bamaga, A., Emad, Y., et al. (2020). Guillain-Barré Syndrome Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Detection and Coronavirus Disease 2019 in a Child. J. Pediatric. Infect. Dis. Soc. 9, 510–513. doi: 10.1093/jpids/piaa086
Khan, A. W., Ullah, I., and Khan, K. S. (2021). Ischemic stroke leading to bilateral vision loss in COVID-19 patient-A rare case report. J. Med. Virol. 93, 683–685. doi: 10.1002/jmv.26484
Kihira, S., Schefflein, J., Mahmoudi, K., Rigney, B., Delman, B. N., Mocco, J., et al. (2021). Association of Coronavirus Disease (COVID-19) With Large Vessel Occlusion Strokes: A Case-Control Study. AJR Am. J. Roentgenol. 216, 150–156. doi: 10.2214/ajr.20.23847
Kim, M. G., Stein, A. A., Overby, P., Kleinman, G., Nuoman, R., Gulko, E., et al. (2021). Fatal Cerebral Edema in a Child With COVID-19. Pediatr. Neurol. 114, 77–78. doi: 10.1016/j.pediatrneurol.2020.10.005
Korem, S., Gandhi, H., and Dayag, D. B. (2020). Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. 13:e237215. doi: 10.1136/bcr-2020-237215
Kremer, S., Lersy, F., Anheim, M., Merdji, H., Schenck, M., Oesterlé, H., et al. (2020a). Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology 95, e1868–e1882.
Kremer, S., Lersy, F., de Sèz, E. J., Ferré, J., Maamar, A., Carsin-Nicol, B., et al. (2020b). Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology 297, E242–E251.
Langley, L., Zeicu, C., Whitton, L., and Pauls, M. (2020). Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. 13:e239597. doi: 10.1136/bcr-2020-239597
Lantos, J. E., Strauss, S. B., and Lin, E. (2020). COVID-19-Associated Miller Fisher Syndrome: MRI Findings. AJNR Am. J. Neuroradiol. 41, 1184–1186. doi: 10.3174/ajnr.a6609
Lapergue, B., Lyoubi, A., Meseguer, E., Avram, I., Denier, C., Venditti, L., et al. (2020). Large vessel stroke in six patients following SARS-CoV-2 infection: a retrospective case study series of acute thrombotic complications on stable underlying atherosclerotic disease. Eur. J. Neurol. 27, 2308–2311. doi: 10.1111/ene.14466
Latronico, N., Bertolini, G., Guarneri, B., Botteri, M., Peli, E., Andreoletti, S., et al. (2007). Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: the Italian multi-centre CRIMYNE study. Crit. Care 11:R11.
Laurendon, T., Radulesco, T., Mugnier, J., Gérault, M., Chagnaud, C., El Ahmadi, A. A., et al. (2020). Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 95, 224–225. doi: 10.1212/wnl.0000000000009850
Le Guennec, L., Devianne, J., Jalin, L., Cao, A., Galanaud, D., Navarro, V., et al. (2020). Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia 61, e90–e94.
Lechien, J. R., Chiesa-Estomba, C. M., De Siati, D. R., Horoi, M., Le Bon, S. D., Rodriguez, A., et al. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 277, 2251–2261. doi: 10.1007/s00405-020-05965-1
Lee, M.-H., Perl, D. P., Nair, G., Li, W., Maric, D., Murray, H., et al. (2020). Microvascular Injury in the Brains of Patients with Covid-19. N. Engl. J. Med. 384, 481–483. doi: 10.1056/nejmc2033369
Li, Y., Li, M., Zuo, L., Shi, Q., Qin, W., Yang, L., et al. (2018). Compromised Blood-Brain Barrier Integrity Is Associated With Total Magnetic Resonance Imaging Burden of Cerebral Small Vessel Disease. Front. Neurol. 9:221. doi: 10.3389/fneur.2018.00221
Liberatore, G., De Santis, T., Doneddu, P. E., Gentile, F., Albanese, A., and Nobile-Orazio, E. (2020). Clinical Reasoning: A case of COVID-19-associated pharyngeal-cervical-brachial variant of Guillain-Barré syndrome. Neurology 95, 978–983. doi: 10.1212/wnl.0000000000010817
Lin, E., Lantos, J., Strauss, S., Phillips, C., Campion, T., Navi, B., et al. (2020). Brain Imaging of Patients with COVID-19: Findings at an Academic Institution during the Height of the Outbreak in New York City. AJNR Am. J. Neuroradiol. 41, 2001–2008. doi: 10.3174/ajnr.a6793
Lodigiani, C., Iapichino, G., Carenzo, L., Cecconi, M., Ferrazzi, P., Sebastian, T., et al. (2020). Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis. Res. 191, 9–14. doi: 10.1016/j.thromres.2020.04.024
Logmin, K., Karam, M., Schichel, T., Harmel, J., and Wojtecki, L. (2020). Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19. J. Neurol. 267, 2490–2491. doi: 10.1007/s00415-020-09904-2
Lopes, C. C. B., Brucki, S. M. D., Passos Neto, C. E. B., Corazza, L. A., Baima, J. P. S., Fiorentino, M. D., et al. (2020). Acute Disseminated Encephalomyelitis in COVID-19: presentation of two cases and review of the literature. Arq. Neuropsiquiatr. 78, 805–810. doi: 10.1590/0004-282x20200186
Lu, L., Xiong, W., Liu, D., Liu, J., Yang, D., Li, N., et al. (2020). New-onset acute symptomatic seizure and risk factors in Corona Virus Disease 2019: A Retrospective Multicenter Study. Epilepsia 61, e49–e53.
Lyons, S., O’Kelly, B., Woods, S., Rowan, C., Brady, D., Sheehan, G., et al. (2020). Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure 80, 113–114. doi: 10.1016/j.seizure.2020.06.010
Mak, P. Q., Chung, K. S., Wong, J. S., Shek, C. C., and Kwan, M. Y. (2020). Anosmia and Ageusia: Not an Uncommon Presentation of COVID-19 Infection in Children and Adolescents. Pediatr. Infect. Dis. J. 39, e199–e200.
Manganotti, P., Bellavita, G., D’Acunto, L., Tommasini, V., Fabris, M., Sartori, A., et al. (2021). Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: A case series. J. Med. Virol. 93, 766–774. doi: 10.1002/jmv.26289
Manganotti, P., Pesavento, V., Buoite Stella, A., Bonzi, L., Campagnolo, E., Bellavita, G., et al. (2020). Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J. Neurovirol. 26, 605–606. doi: 10.1007/s13365-020-00858-9
Manji, H., Carr, A. S., Brownlee, W. J., and Lunn, M. P. (2020). Neurology in the time of covid-19. J. Neurol. Neurosurg. Psychiatry 91, 568–570.
Mansour, O. Y., Malik, A. M., and Linfante, I. (2020). Mechanical Thrombectomy of COVID-19 positive acute ischemic stroke patient: a case report and call for preparedness. BMC Neurol. 20:358. doi: 10.1186/s12883-020-01930-x
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., et al. (2020). Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 683–690. doi: 10.1001/jamaneurol.2020.1127
Marta-Enguita, J., Rubio-Baines, I., and Gastón-Zubimendi, I. (2020). Fatal Guillain-Barre syndrome after infection with SARS-CoV-2. Neurologia 35, 265–267. doi: 10.1016/j.nrleng.2020.04.004
Matos, A. R., Quintas-Neves, M., Oliveira, A. I., Dias, L., Marques, S., Carvalho, R., et al. (2021). COVID-19 Associated Central Nervous System Vasculopathy. Can. J. Neurol. Sci. 48, 139–140. doi: 10.1017/cjn.2020.109
McCuddy, M., Kelkar, P., Zhao, Y., and Wicklund, D. (2020). Acute Demyelinating Encephalomyelitis (ADEM) in COVID-19 Infection: A Case Series. Neurol. India 68, 1192–1195.
Melley, L. E., Bress, E., and Polan, E. (2020). Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Rep. 13:e236080. doi: 10.1136/bcr-2020-236080
Memish, Z. A., Zumla, A. I., Al-Hakeem, R. F., Al-Rabeeah, A. A., and Stephens, G. M. (2013). Family cluster of Middle East respiratory syndrome coronavirus infections. N. Engl. J. Med. 368, 2487–2494.
Mohamed, I. Z. B., Balson, L., and Madathil, S. (2020). Massive bilateral stroke in a COVID-19 patient. BMJ Case Rep. 13:e236254. doi: 10.1136/bcr-2020-236254
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097
Monti, G., Giovannini, G., Marudi, A., Bedin, R., Melegari, A., Simone, A. M., et al. (2020). Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure 81, 18–20. doi: 10.1016/j.seizure.2020.07.006
Morassi, M., Bagatto, D., Cobelli, M., D’Agostini, S., Gigli, G. L., Bnà, C., et al. (2020). Stroke in patients with SARS-CoV-2 infection: case series. J. Neurol. 267, 2185–2192. doi: 10.1007/s00415-020-09885-2
Moriguchi, T., Harii, N., Goto, J., Harada, D., Sugawara, H., Takamino, J., et al. (2020). A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 94, 55–58.
Motoie, R., Akai, M., Kitahara, T., Imamura, H., Tanabe, T., Sarazawa, K., et al. (2020). Coronavirus Disease 2019 Complicated by Multiple Simultaneous Intracerebral Hemorrhages. Intern. Med. 59, 2597–2600. doi: 10.2169/internalmedicine.5697-20
Mousa-Ibrahim, F., Berg, S., Od TPDetola, O., Teitcher, M., and Ruland, S. (2021). Intracranial Hemorrhage in Hospitalized SARS-CoV-2 Patients: A Case Series. J. Stroke Cerebrovasc. Dis. 30:105428. doi: 10.1016/j.jstrokecerebrovasdis.2020.105428
Mowla, A., Shakibajahromi, B., Shahjouei, S., Borhani-Haghighi, A., Rahimian, N., Baharvahdat, H., et al. (2020). Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J. Neurol. Sci. 419:117183.
Muccioli, L., Pensato, U., Cani, I., Guerra, L., Provini, F., Bordin, G., et al. (2020). COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J. Neuroimmunol. 349:577400. doi: 10.1016/j.jneuroim.2020.577400
Mullaguri, N., Hepburn, M., Gebel, J. M., Itrat, A., George, P., and Newey, C. R. (2021). COVID-19 Disease and Hypercoagulability Leading to Acute Ischemic Stroke. Neurohospitalist 11, 131–136. doi: 10.1177/1941874420960324
Munz, M., Wessendorf, S., Koretsis, G., Tewald, F., Baegi, R., Krämer, S., et al. (2020). Acute transverse myelitis after COVID-19 pneumonia. J. Neurol. 267, 2196–2197. doi: 10.1007/s00415-020-09934-w
Naaraayan, A., Pant, S., and Jesmajian, S. (2020). Severe Hyponatremic Encephalopathy in a Patient With COVID-19. Mayo Clin. Proc. 95, 2285–2286. doi: 10.1016/j.mayocp.2020.06.033
Naddaf, E., Laughlin, R. S., Klein, C. J., Toledano, M., Theel, E. S., Binnicker, M. J., et al. (2020). Guillain-Barré Syndrome in a Patient With Evidence of Recent SARS-CoV-2 Infection. Mayo Clin. Proc. 95, 1799–1801. doi: 10.1016/j.mayocp.2020.05.029
Nanda, S., Handa, R., Prasad, A., Anand, R., Zutshi, D., Dass, S. K., et al. (2021). Covid-19 associated Guillain-Barre Syndrome: Contrasting tale of four patients from a tertiary care centre in India. Am. J. Emerg. Med. 39, 125–128. doi: 10.1016/j.ajem.2020.09.029
Nasuelli, N. A., Pettinaroli, R., Godi, L., Savoini, C., De Marchi, F., Mazzini, L., et al. (2021). Critical illness neuro-myopathy (CINM) and focal amyotrophy in intensive care unit (ICU) patients with SARS-CoV-2: a case series. Neurol. Sci. 42, 1119–1121. doi: 10.1007/s10072-020-04820-9
Natarajan, S., Ganesh, R., Palaniappan, N., and Kannan, L. (2020). SARS-CoV- 2 Encephalitis in an Adolescent Girl. Indian Pediatr. 57, 1186–1187. doi: 10.1007/s13312-020-2080-7
Nepal, P., Batchala, P. P., Songmen, S., Parashar, K., and Sapire, J. (2020). An unresponsive COVID-19 patient. Emerg. Radiol. 27, 755–759. doi: 10.1007/s10140-020-01799-w
Nicholson, P., Alshafai, L., and Krings, T. (2020). Neuroimaging Findings in Patients with COVID-19. AJNR Am. J. Neuroradiol. 41, 1380–1383. doi: 10.3174/ajnr.a6630
Nicolas-Jilwan, M., and Almaghrabi, R. S. (2020). Diffuse necrotising leukoencephalopathy with microhaemorrhages in a patient with severe COVID-19 disease. Neuroradiol. J. 33, 528–531. doi: 10.1177/1971400920959324
Noro, F., Cardoso, F. M., and Marchiori, E. (2020). COVID-19 and benign intracranial hypertension: A case report. Rev. Soc. Bras. Med. Trop. 53:e20200325.
Ordoñez-Boschetti, L., Torres-Romero, C. M., and Ortiz de Leo, M. J. (2020). Associated posterior reversible encephalopathy syndrome (PRES) to SARS-CoV-2. Case report. Neurologia 35, 696–698. doi: 10.1016/j.nrleng.2020.08.006
Ortiz-Seller, A., Martínez Costa, L., Hernández-Pons, A., Valls Pascual, E., Solves Alemany, A., and Albert-Fort, M. (2020). Ophthalmic and Neuro-ophthalmic Manifestations of Coronavirus Disease 2019 (COVID-19). Ocul. Immunol. Inflamm. 28, 1285–1289. doi: 10.1080/09273948.2020.1817497
Ottaviani, D., Boso, F., Tranquillini, E., Gapeni, I., Pedrotti, G., Cozzio, S., et al. (2020). Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol. Sci. 41, 1351–1354. doi: 10.1007/s10072-020-04449-8
Oxley, T. J., Mocco, J., Majidi, S., Kellner, C. P., Shoirah, H. I, Singh, P., et al. (2020). Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 382:e60. doi: 10.1056/nejmc2009787
Panico, F., Arini, A., Cantone, P., Crisci, C., and Trojano, L. (2020). Balint-Holmes syndrome due to stroke following SARS-CoV-2 infection: a single-case report. Neurol. Sci. 41, 3487–3489. doi: 10.1007/s10072-020-04860-1
Papi, C., Spagni, G., Alexandre, A., Calabresi, P., Della Marca, G., and Broccolini, A. (2020). Unprotected stroke management in an undiagnosed case of Severe Acute Respiratory Syndrome Coronavirus 2 infection. J. Stroke Cerebrovasc. Dis. 29:104981. doi: 10.1016/j.jstrokecerebrovasdis.2020.104981
Parsons, T., Banks, S., Bae, C., Gelber, J., Alahmadi, H., and Tichauer, M. (2020). COVID-19-associated acute disseminated encephalomyelitis (ADEM). J. Neurol. 267, 2799–2802. doi: 10.1007/s00415-020-09951-9
Pascual-Goñi, E., Fortea, J., Martínez-Domeño, A., Rabella, N., Tecame, M., ómez-Oliva, C. G., et al. (2020). COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurol. Neuroimmunol. Neuroinflamm. 7:e823. doi: 10.1212/nxi.0000000000000823
Patel, S. D., Kollar, R., Troy, P., Song, X., Khaled, M., Parra, A., et al. (2020). Malignant Cerebral Ischemia in A COVID-19 Infected Patient: Case Review and Histopathological Findings. J. Stroke Cerebrovasc. Dis. 29:105231. doi: 10.1016/j.jstrokecerebrovasdis.2020.105231
Perlman, S., Evans, G., and Afifi, A. (1990). Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J. Exp. Med. 172, 1127–1132. doi: 10.1084/jem.172.4.1127
Perrin, P., Collongues, N., Baloglu, S., Bedo, D., Bassand, X., Lavaux, T., et al. (2021). Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 28, 248–258. doi: 10.1111/ene.14491
Pessoa Neto, A. D., Alves, M. D. M., Brito, P. S. M., Moreira Neto, M., Silva, R. A. E., Teixeira Dourado, M. E., et al. (2020). Possible acute multifocal demyelinating lesions in a COVID-19 patient. Arq. Neuropsiquiatr. 78:666. doi: 10.1590/0004-282x20200126
Petrelli, C., Scendoni, R., Paglioriti, M., and Logullo, F. O. (2020). Acute Motor Axonal Neuropathy Related to COVID-19 Infection: A New Diagnostic Overview. J. Clin. Neuromuscul. Dis. 22, 120–121. doi: 10.1097/cnd.0000000000000322
Picod, A., Dinkelacker, V., Savatovsky, J., Trouiller, P., Guéguen, A., and Engrand, N. (2020). SARS-CoV-2-associated encephalitis: arguments for a post-infectious mechanism. Crit. Care 24:658.
Pisano, T. J., Hakkinen, I., and Rybinnik, I. (2020). Large Vessel Occlusion Secondary to COVID-19 Hypercoagulability in a Young Patient: A Case Report and Literature Review. J. Stroke Cerebrovasc. Dis. 29:105307. doi: 10.1016/j.jstrokecerebrovasdis.2020.105307
Pissurno, N. S. C. A., Lichs, G. G. C., Santos, E. J. L. D., Druzian, A. F., Oliveira, S. M. D. V., and Paniago, A. M. M. (2020). Anosmia in the course of COVID-19: A case report. Medicine 99:e21280.
Politi, L. S., Salsano, E., and Grimaldi, M. (2020). Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 77, 1028–1029. doi: 10.1001/jamaneurol.2020.2125
Porta-Etessam, J., Matías-Guiu, J. A., González-García, N., ómez Iglesias, P. G., Santos-Bueso, E., Arriola-Villalobos, P., et al. (2020). Spectrum of Headaches Associated With SARS-CoV-2 Infection: Study of Healthcare Professionals. Headache 60, 1697–1704. doi: 10.1111/head.13902
Povlow, A., and Auerbach, A. J. (2021). Acute Cerebellar Ataxia in COVID-19 Infection: A Case Report. J. Emerg. Med. 60, 73–76. doi: 10.1016/j.jemermed.2020.10.010
Poyiadji, N., Shahin, G., Noujaim, D., Stone, M., Patel, S., and Griffith, B. (2020). COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology 296, E119–E120.
Prasad, A., Kataria, S., Srivastava, S., Lakhani, D. A., and Sriwastava, S. (2021). Multiple embolic stroke on magnetic resonance imaging of the brain in a COVID-19 case with persistent encephalopathy. Clin. Imaging 69, 285–288. doi: 10.1016/j.clinimag.2020.09.010
Priftis, K., Algeri, L., Villella, S., and Spada, M. S. (2020). COVID-19 presenting with agraphia and conduction aphasia in a patient with left-hemisphere ischemic stroke. Neurol. Sci. 41, 3381–3384. doi: 10.1007/s10072-020-04768-w
Princiotta Cariddi, L., Tabaee Damavandi, P., Carimati, F., Banfi, P., Clemenzi, A., Marelli, M., et al. (2020). Reversible Encephalopathy Syndrome (PRES) in a COVID-19 patient. J. Neurol. 267, 3157–3160.
Pugin, D., Vargas, M., Thieffry, C., Schibler, M., Grosgurin, O., Pugin, J., et al. (2020). COVID-19-related encephalopathy responsive to high-dose glucocorticoids. Neurology 95, 543–546. doi: 10.1212/wnl.0000000000010354
Qiu, C., Cui, C., Hautefort, C., Haehner, A., Zhao, J., Yao, Q., et al. (2020). Olfactory and Gustatory Dysfunction as an Early Identifier of COVID-19 in Adults and Children: An International Multicenter Study. Otolaryngol. Head Neck Surg. 163, 714–721. doi: 10.1177/0194599820934376
Radmanesh, A., Derman, A., Lui, Y., Raz, E., Loh, J., Hagiwara, M., et al. (2020). COVID-19-associated Diffuse Leukoencephalopathy and Microhemorrhages. Radiology 297, E223–E227.
Radnis, C., Qiu, S., Jhaveri, M., Da Silva, I., Szewka, A., and Koffman, L. (2020). Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J. Neurol. Sci. 418:117119. doi: 10.1016/j.jns.2020.117119
Raj, S. L., Vasanthi, T., Baineni, R., and Sivabalan, S. (2020). Neurological Manifestations of COVID-19 in Children. Indian Pediatr. 57, 1185–1186.
Rajdev, K., Victor, N., Buckholtz, E. S., Hariharan, P., Saeed, M. A., Hershberger, D. M., et al. (2020). A Case of Guillain-Barré Syndrome Associated With COVID-19. J. Investig. Med. High Impact. Case Rep. 8:2324709620961198.
Rana, S., Lima, A. A., Chandra, R., Valeriano, J., Desai, T., Freiberg, W., et al. (2020). Novel Coronavirus (COVID-19)-Associated Guillain-Barré Syndrome: Case Report. J. Clin. Neuromuscul. Dis. 21, 240–242. doi: 10.1097/cnd.0000000000000309
Reichard, R. R., Kashani, K. B., Boire, N. A., Constantopoulos, E., Guo, Y., and Lucchinetti, C. F. (2020). Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 140, 1–6. doi: 10.1007/s00401-020-02166-2
Robles, L. A. (2021). Bilateral Large Vessel Occlusion Causing Massive Ischemic Stroke in a COVID-19 Patient. J. Stroke Cerebrovasc. Dis. 30:105609. doi: 10.1016/j.jstrokecerebrovasdis.2021.105609
Roy-Gash, F., De Mesmay, M., Devys, J. M., Vespignani, H., Blanc, R., and Engrand, N. (2020). COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit. Care 24:419.
Sabayan, B., Moghadami, M., Assarzadegan, F., Komachali, S. H., Poorsaadat, L., Babaeepour, Z., et al. (2021). COVID-19 Respiratory Illness and Subsequent Cerebrovascular Events, the Initial Iranian Experience. J. Stroke Cerebrovasc. Dis. 30:105454. doi: 10.1016/j.jstrokecerebrovasdis.2020.105454
Saitta, L., Molin, A., Villani, F., Insorsi, A., Roccatagliata, L., Inglese, M., et al. (2020). Brain microvascular occlusive disorder in COVID-19: a case report. Neurol. Sci. 41, 3401–3404. doi: 10.1007/s10072-020-04795-7
Sampaio Rocha-Filho, P. A., and Voss, L. (2020). Persistent Headache and Persistent Anosmia Associated With COVID-19. Headache 60, 1797–1799. doi: 10.1111/head.13941
Sancho-Saldaña, A., Lambea-Gil, Á, Liesa, J. L. C., Caballo, M. R. B., Garay, M. H., Celada, D. R., et al. (2020). Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin. Med. 20, e93–e94.
Sangalli, D., Polonia, V., Colombo, D., Mantero, V., Filizzolo, M., Scaccabarozzi, C., et al. (2020). A single-centre experience of intravenous thrombolysis for stroke in COVID-19 patients. Neurol. Sci. 41, 2325–2329. doi: 10.1007/s10072-020-04591-3
Schurink, B., Roos, E., Radonic, T., Barbe, E., Bouman, C., de Boer, H., et al. (2020). Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 1, e290–e299.
Scullen, T., Keen, J., Mathkour, M., Dumont, A. S., and Kahn, L. (2020). Coronavirus 2019 (COVID-19)-Associated Encephalopathies and Cerebrovascular Disease: The New Orleans Experience. World Neurosurg. 141, e437–e446.
Sedaghat, Z., and Karimi, N. (2020). Guillain Barre syndrome associated with COVID-19 infection: A case report. J. Clin. Neurosci. 76, 233–235. doi: 10.1016/j.jocn.2020.04.062
Senel, M., Abu-Rumeileh, S., Michel, D., Garibashvili, T., Althaus, K., Kassubek, J., et al. (2020). Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur. J. Neurol. 27, 2378–2380. doi: 10.1111/ene.14473
Seth, V., and Kushwaha, S. (2020). Headache Due to COVID-19: A Disabling Combination. Headache 60, 2618–2621. doi: 10.1111/head.14006
Shah, H., Iyer, A., Zaghlol, R., and Raparla, S. (2021). Case Report: Multiple Strokes and Digital Ischemia in a Young COVID-19 Patient. Am. J. Trop. Med. Hyg. 104, 60–62. doi: 10.4269/ajtmh.20-1101
Shawkat, A., Merrell, E. T., Fadel, G. A., Amzuta, I., Amin, H., Shah, A. J., et al. (2020). Multiple Thrombotic Events in a 67-Year-Old Man 2 Weeks After Testing Positive for SARS-CoV-2: A Case Report. Am. J. Case Rep. 21: e925786.
Shoskes, A., Migdady, I., Fernandez, A., Ruggieri, P., and Rae-Grant, A. (2020). Cerebral Microhemorrhage and Purpuric Rash in COVID-19: The Case for a Secondary Microangiopathy. J. Stroke Cerebrovasc. Dis. 29:105111. doi: 10.1016/j.jstrokecerebrovasdis.2020.105111
Siepmann, T., Sedghi, A., Simon, E., Winzer, S., Barlinn, J., de With, K., et al. (2021). Increased risk of acute stroke among patients with severe COVID-19: a multicenter study and meta-analysis. Eur. J. Neurol. 28, 238–247. doi: 10.1111/ene.14535
Silva, M. T. T., Lima, M. A., Torezani, G., Soares, C. N., Dantas, C., Brandão, C. O., et al. (2020). Isolated intracranial hypertension associated with COVID-19. Cephalalgia 40, 1452–1458. doi: 10.1177/0333102420965963
Smith, L. T. A. C., Hodges, C. D. R. J., Pratt, M., and Porter, I. M. (2020). Case Report: COVID-19 Patient With Chief Complaint of Anosmia and Ageusia; a Unique Perspective on Atypical Symptomatology and Management in the Military. Mil. Med. 185, e2176–e2179.
Soldatelli, M. D., Amaral, L. F. D., Veiga, V. C., Rojas, S. S. O., Omar, S., and Marussi, V. H. R. (2020). Neurovascular and perfusion imaging findings in coronavirus disease 2019: Case report and literature review. Neuroradiol. J. 33, 368–373. doi: 10.1177/1971400920941652
Sotoca, J., and Rodríguez-Álvarez, Y. (2020). COVID-19-associated acute necrotizing myelitis. Neurol. Neuroimmunol. Neuroinflamm. 7:e803. doi: 10.1212/nxi.0000000000000803
Soysal, P., and Kara, O. (2020). Delirium as the first clinical presentation of the coronavirus disease 2019 in an older adult. Psychogeriatrics 20, 763–765. doi: 10.1111/psyg.12587
Su, X. W., Palka, S. V., Rao, R. R., Chen, F. S., Brackney, C. R., and Cambi, F. (2020). SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia. Muscle Nerve 62, E48–E49.
Sugiyama, Y., Tsuchiya, T., Tanaka, R., Ouchi, A., Motoyama, A., Takamoto, T., et al. (2020). Cerebral venous thrombosis in COVID-19-associated coagulopathy: A case report. J. Clin. Neurosci. 79, 30–32. doi: 10.1016/j.jocn.2020.07.038
Sun, T., Chen, X., Shi, S., Liu, Q., and Cheng, Y. (2019). Peripheral Blood and Cerebrospinal Fluid Cytokine Levels in Guillain Barré Syndrome: A Systematic Review and Meta-Analysis. Front. Neurosci. 13:717. doi: 10.3389/fnins.2019.00717
Sungnak, W., Huang, N., Bécavin, C., Berg, M., Queen, R., Litvinukova, M., et al. (2020). SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26, 681–687. doi: 10.1038/s41591-020-0868-6
Tankisi, H., Tankisi, A., Harbo, T., Markvardsen, L. K., Andersen, H., and Pedersen, T. H. (2020). Critical illness myopathy as a consequence of Covid-19 infection. Clin. Neurophysiol. 131, 1931–1932. doi: 10.1016/j.clinph.2020.06.003
Tiet, M. Y., and AlShaikh, N. (2020). Guillain-Barré syndrome associated with COVID-19 infection: a case from the UK. BMJ Case Rep. 13:e236536. doi: 10.1136/bcr-2020-236536
Tiwari, L., Shekhar, S., Bansal, A., and Kumar, S. (2021). COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: a case report. Lancet Child Adolesc. Health 5, 88–90. doi: 10.1016/s2352-4642(20)30314-x
To, K. F., and Lo, A. W. (2004). Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J. Pathol. 203, 740–743. doi: 10.1002/path.1597
Toledano-Massiah, S., Badat, N., Leberre, A., Bruel, C., Ray, A., Gerber, S., et al. (2020). Unusual Brain MRI Pattern in 2 Patients with COVID-19 Acute Respiratory Distress Syndrome. AJNR Am. J. Neuroradiol. 41, 2204–2205. doi: 10.3174/ajnr.a6817
Toscano, G., Palmerini, F., Ravaglia, S., Ruiz, L., Invernizzi, P., Cuzzoni, M. G., et al. (2020). Guillain-Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 382, 2574–2576.
Trifan, G., Goldenberg, F. D., Caprio, F. Z., Biller, J., Schneck, M., Khaja, A., et al. (2020b). Characteristics of a Diverse Cohort of Stroke Patients with SARS-CoV-2 and Outcome by Sex. J. Stroke Cerebrovasc. Dis. 29:105314. doi: 10.1016/j.jstrokecerebrovasdis.2020.105314
Trifan, G., Hillmann, M., and Testai, F. D. (2020a). Acute Stroke as the Presenting Symptom of SARS-CoV-2 Infection in a Young Patient with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. J. Stroke Cerebrovasc. Dis. 29:105167. doi: 10.1016/j.jstrokecerebrovasdis.2020.105167
Tu, T. M., Goh, C., Tan, Y. K., Leow, A. S., Pang, Y. Z., Chien, J., et al. (2020). Cerebral Venous Thrombosis in Patients with COVID-19 Infection: a Case Series and Systematic Review. J. Stroke Cerebrovasc. Dis. 29:105379. doi: 10.1016/j.jstrokecerebrovasdis.2020.105379
TunÇ, A., ÜnlÜbaŞ, Y., Alemdar, M., and AkyÜz, E. (2020). Coexistence of COVID-19 and acute ischemic stroke report of four cases. J. Clin. Neurosci 77, 227–229. doi: 10.1016/j.jocn.2020.05.018
Uginet, M., Breville, G., Assal, F., Lövblad, K. O., Vargas, M. I., Pugin, J., et al. (2021). COVID-19 encephalopathy: clinical and neurobiological features. J. Med. Virol. 93, 4374–4381. doi: 10.1002/jmv.26973
Vaira, L. A., Hopkins, C., Petrocelli, M., Lechien, J. R., Chiesa-Estomba, C. M., Salzano, G., et al. (2020a). Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J. Laryngol. Otol. 134, 703–709. doi: 10.1017/s0022215120001826
Vaira, L. A., Hopkins, C., Petrocelli, M., Lechien, J. R., Soma, D., Giovanditto, F., et al. (2020b). Do olfactory and gustatory psychophysical scores have prognostic value in COVID-19 patients? A prospective study of 106 patients. J. Otolaryngol. Head Neck Surg. 49:56.
Vaira, L. A., Hopkins, C., Salzano, G., Petrocelli, M., Melis, A., Cucurullo, M., et al. (2020c). Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 42, 1560–1569. doi: 10.1002/hed.26269
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A. S., et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418. doi: 10.1016/s0140-6736(20)30937-5
Vargas-Gandica, J., Winter, D., Schnippe, R., Rodriguez-Morales, A. G., Mondragon, J., Escalera-Antezana, J. P., et al. (2020). Ageusia and anosmia, a common sign of COVID-19? A case series from four countries. J. Neurovirol. 26, 785–789. doi: 10.1007/s13365-020-00875-8
Vaschetto, R., Cena, T., Sainaghi, P. P., Meneghetti, G., Bazzano, S., Vecchio, D., et al. (2020). Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J. Clin. Neurosci. 79, 71–73. doi: 10.1016/j.jocn.2020.07.032
Vattoth, S., Abdelhady, M., Alsoub, H., Own, A., and Elsotouhy, A. (2020). Critical illness-associated cerebral microbleeds in COVID-19. Neuroradiol. J. 33, 374–376. doi: 10.1177/1971400920939229
Velayos Galán, A., Del Saz Saucedo, P., Peinado Postigo, F., and Botia Paniagua, E. (2020). Guillain-Barré syndrome associated with SARS-CoV-2 infection. Neurologia 35, 268–269. doi: 10.1016/j.nrleng.2020.04.006
Viguier, A., Delamarre, L., Duplantier, J., Olivot, J. M., and Bonneville, F. (2020). Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J. Neuroradiol. 47, 393–394. doi: 10.1016/j.neurad.2020.04.003
Vollono, C., Rollo, E., Romozzi, M., Frisullo, G., Servidei, S., Borghetti, A., et al. (2020). Focal status epilepticus as unique clinical feature of COVID-19: A case report. Seizure 78, 109–112. doi: 10.1016/j.seizure.2020.04.009
Vu, D., Ruggiero, M., Choi, W. S., Masri, D., Flyer, M., Shyknevsky, I., et al. (2020). Three unsuspected CT diagnoses of COVID-19. Emerg. Radiol. 27, 229–232. doi: 10.1007/s10140-020-01775-4
Wada, S., Nagasaki, Y., Arimizu, Y., Shimo, M., Matsukuma, Y., Okamoto, M., et al. (2020). Neurological Disorders Identified during Treatment of a SARS-CoV-2 Infection. Intern. Med. 59, 2187–2189. doi: 10.2169/internalmedicine.5447-20
Wang, A., Mandigo, G. K., Yim, P. D., Meyers, P. M., and Lavine, S. D. (2020). Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J. Neurointerv. Surg. 12, 648–653. doi: 10.1136/neurintsurg-2020-016220
Webb, S., Wallace, V. C., Martin-Lopez, D., and Yogarajah, M. (2020). Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. 13:e236182. doi: 10.1136/bcr-2020-236182
Williamson, P. O., and Minter, C. I. J. (2019). Exploring PubMed as a reliable resource for scholarly communications services. J. Med. Libr. Assoc. 107, 16–29.
Xiong, W., Mu, J., Guo, J., Lu, L., Liu, D., Luo, J., et al. (2020). New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology 95, e1479–e1487.
Yaghi, S., Ishida, K., Torres, J., Mac Grory, B., Raz, E., Humbert, K., et al. (2020). SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke 51, 2002–2011. doi: 10.1161/strokeaha.120.030335
Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J., Liu, H., et al. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 475–481. doi: 10.1016/s2213-2600(20)30079-5
Yao, X. H., He, Z. C., Li, T. Y., Zhang, H. R., Wang, Y., Mou, H., et al. (2020). Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 30, 541–543. doi: 10.1038/s41422-020-0318-5
Yong, M. H., Chan, Y. F. Z., Liu, J., Sanamandra, S. K., Kheok, S. W., Lim, K. C., et al. (2020). A Rare Case of Acute Hemorrhagic Leukoencephalitis in a COVID-19 Patient. J. Neurol. Sci. 416:117035. doi: 10.1016/j.jns.2020.117035
Yuki, N., and Hartung, H.-P. (2012). Guillain-Barre syndrome.(Disease/Disorder overview). N. Engl. J. Med. 366:2294.
Zayet, S., Ben Abdallah, Y., Royer, P. Y., Toko, L., Gendrin, V., and Klopfenstein, T. (2021). Encephalopathy in patients with COVID-19: “Causality or coincidence?”. J. Med. Virol. 93:1193. doi: 10.1002/jmv.26027
Zayet, S., Klopfenstein, T., Kovács, R., Stancescu, S., and Hagenkötter, B. (2020). Acute Cerebral Stroke with Multiple Infarctions and COVID-19, France. Emerg. Infect. Dis. 26, 2258–2260. doi: 10.3201/eid2609.201791
Zhao, H., Shen, D., Zhou, H., Liu, J., and Chen, S. (2020). Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 19, 383–384. doi: 10.1016/s1474-4422(20)30109-5
Keywords: brain, COVID-19, cerebrovascular events, SARS-CoV-2, encephalopathy, aging brain
Citation: Sullivan BN and Fischer T (2021) Age-Associated Neurological Complications of COVID-19: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 13:653694. doi: 10.3389/fnagi.2021.653694
Received: 15 January 2021; Accepted: 08 June 2021;
Published: 02 August 2021.
Edited by:
Agustín Ruiz Laza, Fundació ACE, SpainReviewed by:
Jukka Jolkkonen, University of Eastern Finland, FinlandMiguel Calero, Instituto de Salud Carlos III (ISCIII), Spain
Copyright © 2021 Sullivan and Fischer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tracy Fischer, dGZpc2NoZXIxQHR1bGFuZS5lZHU=
 Brianne N. Sullivan
Brianne N. Sullivan Tracy Fischer
Tracy Fischer