
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 19 February 2021
Sec. Neuroinflammation and Neuropathy
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.628271
This article is part of the Research TopicCerebral Small Vessel Disease and Intracerebral HemorrhageView all 4 articles
Background: Uncertainty exists over the long-term prognostic significance of cerebral small vessel disease (CSVD) in primary intracerebral hemorrhage (ICH).
Methods: We performed a longitudinal analysis of CSVD and clinical outcomes in consecutive patients with primary ICH who had MRI. Baseline CSVD load (including white matter hyperintensities [WMH], cerebral microbleeds [CMBs], lacunes, and enlarged perivascular spaces [EPVS]) was evaluated. The cumulative CSVD score was calculated by combining the presence of each CSVD marker (range 0–4). We followed participants for poor functional outcome [modified Rankin scale [mRS] ≥ 4], stroke recurrence, and time-varying survival during a median follow-up of 4.9 [interquartile range [IQR] 3.1–6.0] years. Parsimonious and fuller multivariable logistic regression analysis and Cox-regression analysis were performed to estimate the association of CSVD markers, individually and collectively, with each outcome.
Results: A total of 153 patients were included in the analyses. CMBs ≥ 10 [adjusted OR [adOR] 3.252, 95% CI 1.181–8.956, p = 0.023] and periventricular WMH (PWMH) (adOR 2.053, 95% CI 1.220–3.456, p = 0.007) were significantly associated with poor functional outcome. PWMH (adOR 2.908, 95% CI 1.230–6.878, p = 0.015) and lobar CMB severity (adOR 1.811, 95% CI 1.039–3.157, p = 0.036) were associated with stroke recurrence. The cumulative CSVD score was associated with poor functional outcome (adOR 1.460, 95% CI 1.017–2.096) and stroke recurrence (adOR 2.258, 95% CI 1.080–4.723). Death occurred in 36.1% (13/36) of patients with CMBs ≥ 10 compared with 18.8% (22/117) in those with CMB < 10 (adjusted HR 2.669, 95% CI 1.248–5.707, p = 0.011). In addition, the cumulative CSVD score ≥ 2 was associated with a decreased survival rate (adjusted HR 3.140, 95% CI 1.066–9.250, p = 0.038).
Conclusions: Severe PWMH, CMB, or cumulative CSVD burden exert important influences on the long-term outcome of ICH.
Spontaneous primary intracerebral hemorrhage (ICH) is mainly caused by cerebral small vessel disease (CSVD), including hypertensive arteriopathy (HA) and cerebral amyloid angiopathy (CAA) (Charidimou et al., 2016; Hankey, 2017), and has both short- and long-term consequences of high case fatality, disability, and risk of recurrent serious vascular events (Poon et al., 2014). Both HA and CAA are associated with magnetic resonance imaging (MRI) markers of CSVD, including lacunes, white matter hyperintensities (WMH), cerebral microbleeds (CMBs), and enlarged perivascular spaces (EPVS) (Charidimou et al., 2016). These MRI markers could reflect the presence and severity of CSVD-related damage (Wardlaw et al., 2013) and rarely occur in isolation. Thus, several semiquantitative scales have been developed to assess the cumulative CSVD burden (Staals et al., 2014; Xu et al., 2018).
Numerous studies have investigated the predictors of mortality and functional outcome following ICH (Gregório et al., 2018), whereas few have evaluated the role of CSVD in clinical outcomes after ICH (Sykora et al., 2017; Lioutas et al., 2018). At day 90, the cumulative CSVD burden and the presence of CMB (Lioutas et al., 2018), as well as severe WMH and increasing lacune number (Sykora et al., 2017), are important determinants of poor outcomes. Another study has shown that severe leukoaraiosis on CT scan is significantly associated with a poor functional outcome at discharge and with less recovery in mRS from discharge to day 90 (Uniken Venema et al., 2019).
However, to our knowledge, the effect of the CSVD burden on MRI on the long-term prognosis after ICH (for instance, beyond 1 year) has not been studied. Insights into the longitudinal association between baseline CSVD burden, individually and collectively, and long-term prognosis could possibly lead to a better appraisal of ICH and aid in the development of new therapeutic and preventative strategies to relieve the disease burden.
To address this gap, we aimed to investigate the association between individual CSVD markers and cumulative CSVD burden and (1) poor functional outcome, (2) stroke recurrence, and (3) time-varying survival.
We analyzed consecutive patients with primary ICH with MRI susceptibility-weighted imaging (MRI-SWI), admitted to either the Department of Neurosurgery or the Department of Neurology, West China Hospital, Sichuan University from January 2012 to October 2017 in a prospectively collected database. Our study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. Informed consents were obtained from patients or their family members.
A total of 203 consecutive patients with primary ICH with MRI-SWI examination in our ongoing prospective cohort study were screened. For our present analysis, patients were eligible if they (1) meet the diagnosis of primary ICH and (2) had MRI with adequate sequences at baseline for the assessments of lacunes, CMBs, WMH, and EPVS. Patients were excluded if (1) the hemorrhage was considered to be primary intraventricular hemorrhage (IVH), (2) the time of onset was beyond 3 months of symptom onset, (3) they had hemorrhagic transformation after an ischemic stroke, (4) their images were of poor quality, or (5) the time of onset was unclear. Angiography was carried out when clinical characteristics were suggestive of a vascular malformation.
The ICH subtypes (HA-ICH, CAA-ICH, mixed-location ICH, and undetermined-ICH) were coded according to the previous studies (Linn et al., 2010; Pasi et al., 2018). We defined individuals as having HA-ICH when patients had deep ICH, with or without deep CMBs, but no lobar ICH or lobar CMBs. Those with strictly lobar ICH (cerebellar hemorrhage allowed) without deep ICH or CMBs were coded as having CAA-ICH when they aged ≥55 years and as undetermined-ICH when they were younger than 55 years. ICH or CMBs in both deep and lobar regions were coded as mixed-location ICH.
We collected age, sex, vascular risk factors (including hypertension, diabetes mellitus, hyperlipidemia, cardiac disease, smoking, and alcohol consumption) (Valenti et al., 2016), a previous history of stroke, stroke severity [evaluated using Glasgow Coma Scale [GCS] score] on admission, serum factors (including blood glucose, albumin, cholesterol, high-density lipoprotein, low-density lipoprotein, and creatinine), and treatment (surgical hematoma evacuation vs. conservative treatment) for each enrolled participant at baseline. We also collected 10 prespecified complications recorded by trained neurologists, which included pneumonia, urinary tract infection, gastrointestinal bleeding, seizure, septic shock, electrolyte disturbances, deep vein thrombosis, other infections, depression, and anxiety. A list of complications and their definitions are provided in Supplementary Table 1. The definition of each complication was the same as that in the previous studies (Langhorne et al., 2000; Indredavik et al., 2008; Dellinger et al., 2013; Li et al., 2019). We did not include chronic medical complications such as chronic chest infection, since they were beyond the scope of this study.
Neurologists who were blinded to the clinical information of patients assessed the ICH location, the ICH volume, the presence of IVH, and midline shift. The ICH location was classified into lobar (hemorrhage originating at the cortex and subcortical junction), deep [hemorrhage located in the basal ganglia (BG), thalamus, or brain stem], or cerebellar ICH. We grouped cerebellar and brain stem ICH into infratentorial ICH. The ICH volume at baseline was assessed using the ABC/2 method (Kothari et al., 1996).
The median time from the onset to MRI examination was 6.4 [interquartile range [IQR] 3.9–15.8] days in our present study. All enrolled patients underwent MRI scanning, including T1- and T2-weighted, fluid-attenuated inversion recovery (FLAIR), and SWI sequences, with a magnetic field strength of 3 T. The details of MRI parameters of the four sequences were described in our previous studies (Xu et al., 2018, 2019).
A lacune of presumed vascular origin was defined as a small lesion (3–20 mm in diameter) and CSF-like intensity with hyperintensities on T2-weighted and hypointensities on T1-weighted images, with a perilesional halo on FLAIR images sometimes (Klarenbeek et al., 2013; Staals et al., 2014). WMH was assessed using the Fazekas scale, which was a simple scale rating separately for the deep and periventricular region on a 4-point scale ranging from 0 to 3 (Fazekas et al., 1987). We summed the deep WMH (DWMH) score and the periventricular WMH (PWMH) score to form a total WMH score (scored 0–6). The presence of WMH was defined as PWMH Fazekas 3 or/and DWMH Fazekas 2–3(Staals et al., 2014). A CMB lesion was defined as a small area of hypointense signal void on SWI sequences, round or ovoid, blooming, and at least partly surrounded by the brain parenchyma, in line with the current consensus criteria (Greenberg et al., 2009). We recorded the CMB number and their topographic distributions. The lobar CMB severity was defined as follows: 0 = absence of lobar CMB, 1 = 1–4 lobar CMBs, 2 = 5–9 lobar CMBs, 3 = 10–19 lobar CMBs, and 4 = equal to or more than 20 lobar CMBs. Deep CMB severity was defined as follows: 0 = absence of deep CMB, 1 = 1–4 deep CMBs, 2 = 5–9 deep CMBs, 3 = 10–19 deep CMBs, and 4 = equal to or more than 20 deep CMBs. CMB mimics, such as calcium and iron deposits, were excluded using CT scans (Greenberg et al., 2009). EPVS were defined as fluid-filled spaces, linear, round, or ovoid, of <3 mm in diameter and were evaluated on the axial T2-weighted images. The side of the slice with the highest EPVS number in BG and centrum semiovale (CSO) was recorded after reviewing all relevant slices for the two anatomical areas (Wardlaw et al., 2013).
The cumulative CSVD score was calculated using an ordinal 5-point scale ranging from 0 to 4 by combining the four individual CSVD markers, with one point allocated to each of the following: the presence of any lacune, the presence of any CMB, the presence of WMH, and BG EPVS > 10 according to the previous studies (Staals et al., 2014; Song et al., 2017). We defined severe cumulative CSVD burden when the CSVD score was equal to or greater than the median value.
The primary outcome in this study was a poor functional outcome [the Modified Rankin Scale [mRS] ≥ 4, which means severe functional dependence or death] at the end of the follow-up. Prespecified second outcomes included stroke recurrence and time-varying survival. We followed patients with telephone interviews at 3-months, 6-months, 1-year, and ≥3-years post-ICH. All patients were followed up from enrollment, until the occurrence of death, the last clinical documentation, or the end of the last follow-up (April 2020). A stroke recurrence (including either ischemic or hemorrhagic stroke) was confirmed by asking patients or their family members. We confirmed a stroke recurrence by asking if the patient went to medical institutions (both inpatient and outpatient settings) because of any episodes of new weakness or numbness in legs or/and arms, or new problems with speech or vision, with a new diagnosis of stroke (ischemic stroke or ICH) by specialists.
Interrater agreement of the CSVD markers was good or excellent, as previously described (Xu et al., 2019). We used the Pearson's chi-squared test or the Fisher's exact test for categorical variables, and the Student t-test or the Mann–Whitney U test for continuous variables, as appropriate. First, the association between risk factors and each outcome was estimated using the univariate analysis. Then, factors with p < 0.2 were forced into multivariable analyses as covariates to determine the association of each individual CSVD marker and the cumulative CSVD score with clinical outcomes. We used multivariable logistic regression analysis to determine the association between CSVD and poor functional outcome and stroke recurrence and multivariable Cox proportional hazard regression analysis to assess the relationship of CSVD with time-varying survival. Potential risk factors, which were entered into the multivariable analysis of the association between CSVD and poor functional outcome, included age (continuous variable), sex, hypertension, cardiac disease, the GCS score (continuous variable), serum albumin (continuous variable), and any complication. In addition, we adjusted for IVH and infratentorial ICH further since the two were the commonly used factors of ICH prognosis in a recent meta-analysis (Gregório et al., 2018). In the multivariable analysis of the association of CSVD with stroke recurrence, we only included patients who were first-ever ICH and had data of stroke recurrence, adjusting for age, sex, and ICH subtypes. We used the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) value for each MRI maker and the cumulative CSVD score to characterize the discrimination for poor functional outcome and stroke recurrence. DeLong et al. test was used to evaluate the differences between two AUC values for significance (DeLong et al., 1988). IBM SPSS Statistics (version 21) and MedCalc (version 19.1) were used for statistical analyses. p < 0.05 was considered to be significant.
A total of 203 patients with primary ICH and MRI-SWI examination were screened, and 35 out of 203 (17.2%) patients were excluded for various reasons (see Figure 1). Thus, 168 (82.8%; 61.0 ± 12.2 years, age range = 36–88 years) patients were enrolled in our present study. During a median follow-up of 4.9 (IQR 3.1–6.0) years, 15 participants did not complete any follow-up, leaving 153 patients who were included in the analyses (Figure 1). Of these, 111 (72.5%) were men; the most common ICH subtype was mixed-location ICH (43.1%), followed by HA-ICH (39.9%), and CAA-ICH (15.0%). Three patients younger than 55 years with strictly lobar (or cerebellar) ICH and without deep CMB were classified into the undetermined subgroup.
Comparing with those who were lost to follow-up, completers had a lower GCS score (p = 0.029) and a lower rate of diabetes mellitus (p = 0.025). Other clinical characteristics, serum factors, individual CSVD marker, and the cumulative CSVD score were comparable between the two groups (see Supplementary Table 2).
At the end of follow-up, 30.1% (46/153) developed poor functional outcome, older (p < 0.001), having lower GCS score (p = 0.004), and lower serum albumin levels (p = 0.001), as well as higher prevalence of any complication (p = 0.001) than those who did not develop poor functional outcome. The overall characteristics of the 153 patients in the poor functional outcome analysis were shown in Supplementary Table 3.
The univariate analysis showed that patients with poor functional outcome had a greater proportion of any lacune, more severe PWMH, DWMH, and total WMH score, as well as a greater proportion of CMB ≥ 10 and BG EPVS > 20, and expectedly higher cumulative CSVD score (all p < 0.05) compared with those without a poor functional outcome (see Table 1).
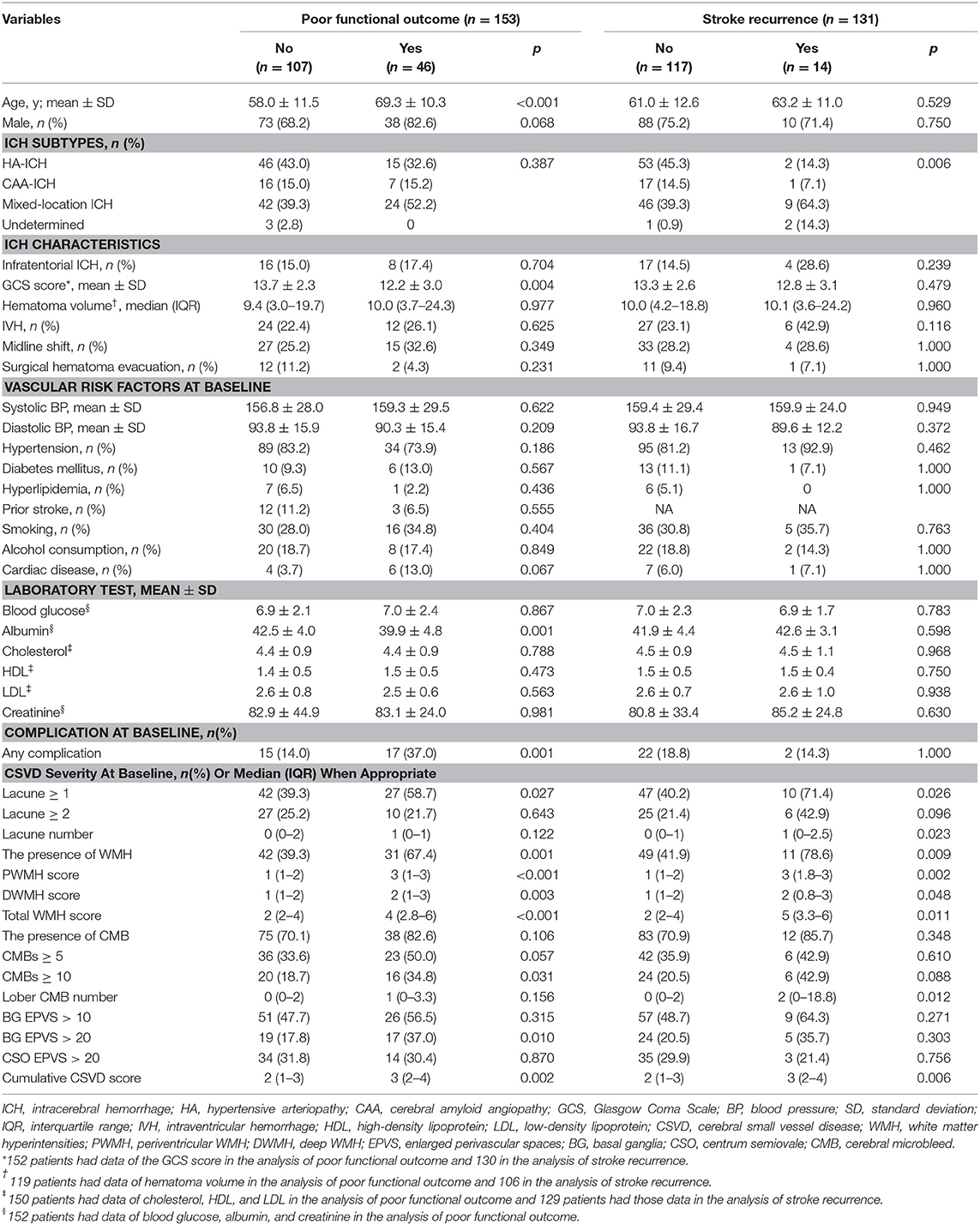
Table 1. Comparison between patients with and without poor functional outcome and between those with and without stroke recurrence after ICH.
After adjusting for the prespecified confounders higher PWMH score [adjusted OR[adOR] 2.085, 95% CI 1.255–3.462, p = 0.005], higher total WMH score (adOR 1.395, 95% CI 1.071–1.819, p = 0.014), CMB ≥ 5 (adOR 2.885, 95% CI 1.162–7.163, p = 0.022), CMB ≥ 10 (adOR 3.249, 95% CI 1.201–8.794, p = 0.020), and higher cumulative CSVD score (adOR 1.460, 95% CI 1.017–2.096, p = 0.040) were all significantly associated with poor functional outcome. The unfavorable prognostic effect of high CMB burden, PWMH severity on poor functional outcome was retained after adjusting for IVH and infratentorial ICH additionally (adOR 2.053, 95% CI 1.220–3.456, p = 0.007 for PWMH score; adOR 3.252, 95% CI 1.181–8.956, p = 0.023 for CMBs ≥ 10) (see Table 2).
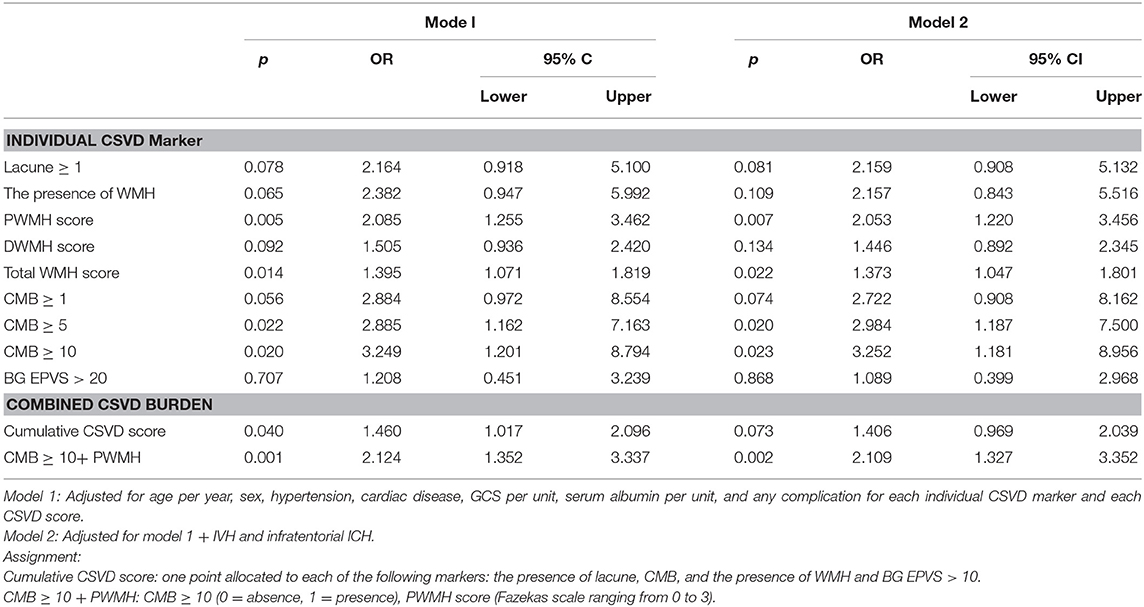
Table 2. Association of CSVD with poor functional outcome in the multivariable logistic regression analysis.
Then, we combined CMB ≥ 10 (0 = absence, 1 = presence) and PWMH score (Fazekas scale ranging 0–3) into a single score. With every point increase in this score, the risk of poor functional outcome increased by 2.1 times (adOR 95% CI 1.327–3.352, p = 0.002) (Table 2). The predictive value of CMB ≥ 10+ PWMH for poor functional outcome performed better than the individual marker of CMB ≥ 10 (CMB ≥ 10+ PWMH vs. CMB ≥ 10, AUC 0.704 vs. 0.580, p = 0.003) or CMB ≥ 5 (CMB ≥ 10+ PWMH vs. CMB ≥ 5, AUC 0.704 vs. 0.582, p = 0.007) (Supplementary Table 4).
Of the 153 patients with mRS data, 15 had a previous history of stroke, and seven had missing data on recurrence, leaving 131 first-ever ICH patients who were included in the stroke recurrence analysis. During a median follow-up of 4.9 years, 14 patients experienced recurrent stroke (16 recurrent events overall; seven ICHs, six ischemic strokes, and three unclear stroke subtypes). The recurrence rate in HA-ICH, CAA-ICH, and mixed-location ICH was 3.5% (2/57), 5.6% (1/18), and 17.0% (9/53), respectively. The risk of stroke recurrence varied by ICH subtypes (p = 0.006, Fisher's exact test).
Compared to patients without stroke recurrence, recurrent patients were more likely to present with lacunes, WMH, lobar CMB, and a higher cumulative CSVD score in the univariate analysis (all p <0.05) (Table 1). After adjusting for age, sex, and ICH subtypes, the unfavorable prognostic effect of PWMH (adOR 2.908, 95% CI 1.230–6.878; p = 0.015), total WMH (adOR 1.602, 95% CI 1.046–2.455; p = 0.030), lobar CMB severity (adOR 1.811, 95% CI 1.039–3.157; p = 0.036), and the cumulative CSVD score (adOR 2.258, 95% CI 1.080–4.723; p = 0.030) on stroke recurrence was retained.
Similarly, we combined PWMH (Fazekas scale ranging from 0 to 3) and lobar CMB severity into a single score. The association between this score and stroke recurrence was substantially significant (adOR 1.847, 95% CI 1.194–2.858, p = 0.006) (Table 3). The predictive value of the combined CSVD burden (cumulative CSVD score or PWMH + lobar CMB severity) for stroke recurrence was not significantly different from that of an individual marker (Supplementary Table 4).
The median value of the cumulative CSVD score was 2 (1–3); so, the prespecified high cumulative CSVD burden was defined as the cumulative CSVD score ≥ 2. The Cox proportional hazards regression analysis showed that only CMBs ≥ 10 was significantly related to mortality (adjusted HR 2.669, 95% CI 1.248–5.707; p = 0.011) after adjusting for age, sex, hypertension, cardiac disease, GCS, albumin, and any complication. Additionally, a higher combined CSVD burden was associated with a decreased survival rate (adjusted HR 3.140, 95% CI 1.066–9.250, p = 0.038 for cumulative CSVD score ≥ 2; adjusted HR 1.457, 95% CI 1.049–2.024, p = 0.025 for CMB ≥ 10 + PWMH) (Figure 2). We found no statistically significant differences in survival, stratified by lacune, PWMH, DWMH, or EPVS.
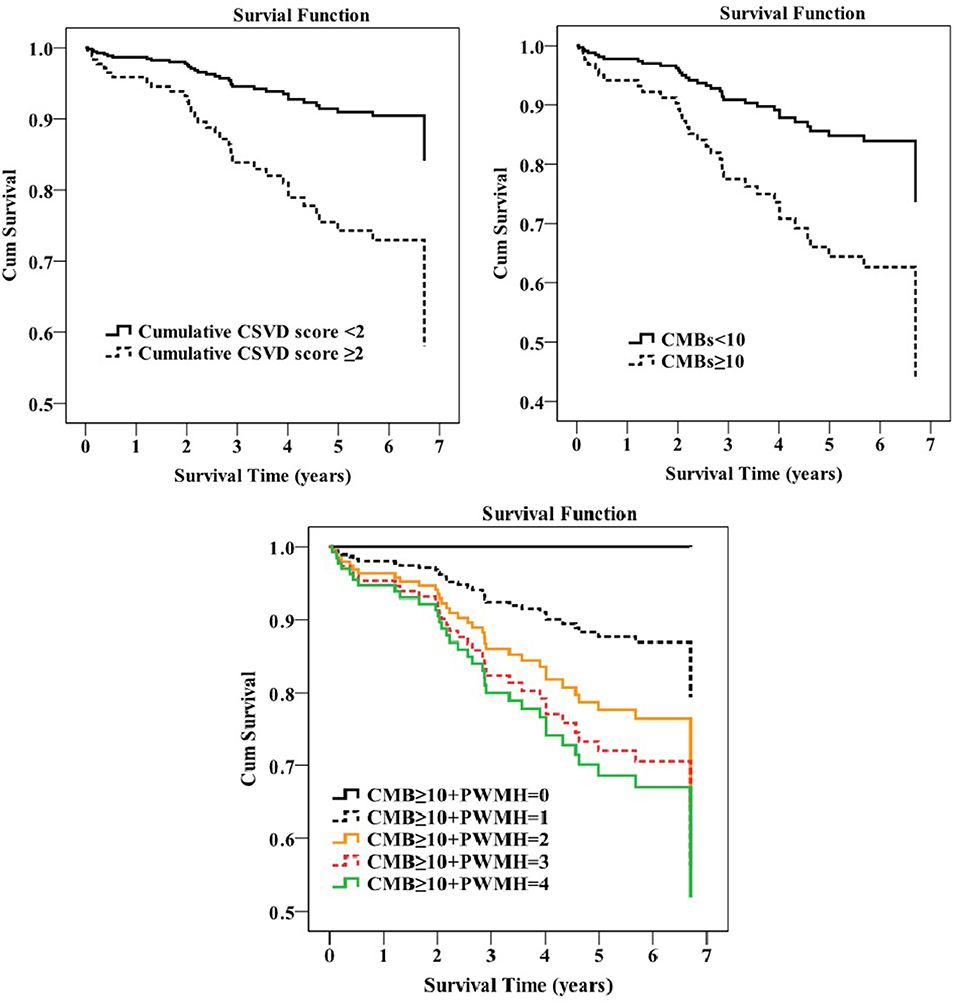
Figure 2. Survival plot of how survival was influenced by the cerebral small vessel disease (CSVD) burden in the Cox regression analysis.
The key findings of this study are that severe PWMH and CMB were independent predictors of poor functional outcome. There was a substantial risk of stroke recurrence in patients with greater PWMH or lobar CMB burden in patients with ICH. As expected, cumulative CSVD burden altered the occurrence risk of each of the three prespecified outcomes in long-term follow-up, mainly because of the comprehensive effect of WMH and CMB. The predictive value of the combined score (CMB ≥ 10 + PWMH) for poor functional outcome performed better than that of the individual marker of CMB ≥ 10 or CMB ≥ 5.
In our present study, severe PWMH were significantly associated with poor functional outcome and stroke recurrence, independent of age and other potential confounders. Patients with a high PWMH score were older (p < 0.001, ANOVA) and more likely to have hypertension (p < 0.05, the chi-squared test) than those with a low PWMH score. Severe PWMH was significantly associated with the presence of mixed-location ICH (p < 0.001, the chi-squared test) in this study. As shown in the previous literature, mixed ICHs were more likely to have pronounced classic vascular risk factor burden (hypertension, diabetes mellitus, and creatinine) and high WMH volume compared to HA-ICH or CAA-ICH (p < 0.001) (Pasi et al., 2018), suggesting that the burden of classic vascular risk factor could be a critical player in the development of a severe arteriopathy in the brain. The mechanism underlying why PWMH, but not DWMH, was associated with a poor outcome is not clear. Perhaps, the vascular architecture in periventricular white matter is more susceptible to damage than that in other areas (Pantoni and Garcia, 1997). Anatomically, there is a high density of long association fibers connecting the cortex with distant brain territories and subcortical nuclei, while the subcortical region has a high density of short-looped U-fibers, which connect adjacent regions (Filley, 1998; De Groot et al., 2002). Lesions affecting long association fibers may have more influence on function than lesions affecting connectivity between neighboring brain areas because of possibly reserve mechanisms of the latter (De Groot et al., 2002).
The strong association of CMB with the long-term poor functional outcome is a novel observation. Lioutas et al. (2018) reported a significant detrimental effect of CMB on 90-days functional independence (mRS ≤ 2) (Lioutas et al., 2018). Our study extended the follow-up to a median of 5 years, verifying the detrimental effect of CMB on long-term prognosis, and further found that PWMH, not DWMH, was closely associated with a poor outcome and stroke recurrence.
The association between the lobar CMB severity and the stroke recurrence is expected. Histopathologically, CMB corresponds with the focal deposition of hemosiderin surrounding small vessels (Greenberg et al., 2009). Those lobar CMBs, which might be due to CAA (Greenberg et al., 2009), are correlated to a greater beta-amyloid burden than deep CMBs (Tsai et al., 2017). A previous study showed that the lobar CMB number was associated with the recurrence of lobar ICH during a median follow-up of 34.3 months (Biffi et al., 2010). A pooled analysis of 10 studies also found that multiple baseline CMBs were associated with the recurrence during follow-up (Charidimou et al., 2017).
First, we used 3-T SWI to detect CMB. Second, although recurrence was more common in the lobar ICH than in the deep ICH as per the previous systematic reviews (Bailey et al., 2001; Poon et al., 2014), probably because of the type and severity of the underlying microangiopathies (Pantoni, 2010), few studies have systematically addressed the phenotype of the underlying small vessel disease type (Bailey et al., 2001; Poon et al., 2014). In our present study, we used MRI which is the most useful and non-invasive method to phenotype microangiopathies (Charidimou et al., 2017) to directly control the association between CSVD burden and stroke recurrence. Third, our follow-up was relatively long. As reported in a recent study, management in the stroke unit or neurointensive care unit might influence stroke prognosis (Pilato et al., 2020). In our study, we included eligible patients who were admitted to either the Department of Neurosurgery or the Department of Neurology in West China Hospital; both departments are high-volume stroke centers, as well as diagnosis and treatment centers for complicated and severe neurological diseases in Southwest China. All the included patients were treated according to current guidelines.
However, this present study has several limitations. There might have been selection bias because not all patients had an MRI; thus, the findings could only be generalized to patients with ICH who undergo MRI in clinical practice. Since the included sample size was relatively small, we were unable to test the influence of individual CMB markers and the cumulative CSVD score on this long-term prognosis in each of the ICH subtypes. Finally, we followed after discharge, and stroke recurrence was confirmed by asking patients or their family members, so the possibility of recall bias could not be excluded. In future, large studies are needed to verify our results.
We concluded that the increasing burden of PWMH, CMB, and combined CSVD burden exerts important influence on poor functional outcome and the stroke recurrence in patients with ICH. CMBs ≥ 10 and the combined CSVD burden are significantly related to time-varying survival. Our findings are important to stratify patients with ICH who are more prone to poor clinical outcomes. Our data demonstrated that MRI is valuable in assessing prognosis in patients with primary ICH.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
MX and ML designed the study. MX, BL, DZ, YC, and QW enrolled and followed subjects. MX and YC reviewed clinical head CTs and MRIs for radiologic grading. MX carried out data analysis and wrote the manuscript. Important data analysis suggestions and manuscript revisions were made by BW, ML, ShuZ, and ShiZ. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key R&D Program of China, Ministry of Science and Technology of China (2016YFC1300500-505), the National Natural Science Foundation of China (Grant No. 82001250), the China Postdoctoral Science Foundation (Grant No. 2020M683322), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYGD18009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.628271/full#supplementary-material
CSVD, cerebral small vessel disease; ICH, intracerebral hemorrhage; WMH, white matter hyperintensities; CMB, cerebral microbleed; HA, hypertensive arteriopathy; CAA, cerebral amyloid angiopathy; EPVS, enlarged perivascular spaces; IVH, intraventricular hemorrhage; GCS, Glasgow Coma Scale; DWMH, deep WMH; PWMH, periventricular WMH; BG, basal ganglia; CSO, centrum semiovale; SWI, susceptibility-weighted imaging; IQR, interquartile range; FLAIR, fluid-attenuated inversion recovery; adOR, adjusted OR; mRS, Modified Rankin Scale; ROC, receiver operating characteristic; AUC, area under the ROC curve.
Bailey, R. D., Hart, R. G., Benavente, O., and Pearce, L. A. (2001). Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 56, 773–777. doi: 10.1212/WNL.56.6.773
Biffi, A., Halpin, A., Towfighi, A., Gilson, A., Busl, K., Rost, N., et al. (2010). Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 75, 693–698. doi: 10.1212/WNL.0b013e3181eee40f
Charidimou, A., Boulouis, G., Haley, K., Auriel, E., van Etten, E. S., Fotiadis, P., et al. (2016). White matter hyperintensity patterns in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 86, 505–511. doi: 10.1212/WNL.0000000000002362
Charidimou, A., Imaizumi, T., Moulin, S., Biffi, A., Samarasekera, N., Yakushiji, Y., et al. (2017). Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology 89, 820–829. doi: 10.1212/WNL.0000000000004259
De Groot, J. C., De Leeuw, F. E., Oudkerk, M., Van Gijn, J., Hofman, A., Jolles, J., et al. (2002). Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann. Neurol. 52, 335–341. doi: 10.1002/ana.10294
Dellinger, R. P., Levy, M. M., Rhodes, A., Annane, D., Gerlach, H., Opal, S. M., et al. (2013). Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637. doi: 10.1097/CCM.0b013e31827e83af
DeLong, E. R., DeLong, D. M., and Clarke-Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845. doi: 10.2307/2531595
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR 149, 351–356. doi: 10.2214/ajr.149.2.351
Filley, C. M. (1998). The behavioral neurology of cerebral white matter. Neurology 50, 1535–1540. doi: 10.1212/WNL.50.6.1535
Greenberg, S. M., Vernooij, M. W., Cordonnier, C., Viswanathan, A., Al-Shahi Salman, R., Warach, S., et al. (2009). Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 8, 165–174. doi: 10.1016/S1474-4422(09)70013-4
Gregório, T., Pipa, S., Cavaleiro, P., Atanásio, G., Albuquerque, I., Chaves, P. C., et al. (2018). Prognostic models for intracerebral hemorrhage: systematic review and meta-analysis. BMC Med. Res. Methodol. 18:145. doi: 10.1186/s12874-018-0613-8
Indredavik, B., Rohweder, G., Naalsund, E., and Lydersen, S. (2008). Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke 39, 414–420. doi: 10.1161/STROKEAHA.107.489294
Klarenbeek, P., van Oostenbrugge, R. J., Rouhl, R. P., Knottnerus, I. L., and Staals, J. (2013). Ambulatory blood pressure in patients with lacunar stroke: association with total MRI burden of cerebral small vessel disease. Stroke 44, 2995–2999. doi: 10.1161/STROKEAHA.113.002545
Kothari, R. U., Brott, T., Broderick, J. P., Barsan, W. G., Sauerbeck, L. R., Zuccarello, M., et al. (1996). The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27, 1304–1305. doi: 10.1161/01.STR.27.8.1304
Langhorne, P., Stott, D. J., Robertson, L., MacDonald, J., Jones, L., McAlpine, C., et al. (2000). Medical complications after stroke: a multicenter study. Stroke 31, 1223–1229. doi: 10.1161/01.STR.31.6.1223
Li, J., Zhang, P., Wu, S., Wang, Y., Zhou, J., Yi, X., et al. (2019). Stroke-related complications in large hemisphere infarction: incidence and influence on unfavorable outcome. Ther. Adv. Neurol. Disord. 12:1756286419873264. doi: 10.1177/1756286419873264
Linn, J., Halpin, A., Demaerel, P., Ruhland, J., Giese, A. D., Dichgans, M., et al. (2010). Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 74, 1346–1350. doi: 10.1212/WNL.0b013e3181dad605
Lioutas, V. A., Wu, B., Norton, C., Helenius, J., Modak, J., and Selim, M. (2018). Cerebral small vessel disease burden and functional and radiographic outcomes in intracerebral hemorrhage. J. Neurol. 265, 2803–2814. doi: 10.1007/s00415-018-9059-5
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Pantoni, L., and Garcia, J. H. (1997). Pathogenesis of leukoaraiosis: a review. Stroke 28, 652–659. doi: 10.1161/01.STR.28.3.652
Pasi, M., Charidimou, A., Boulouis, G., Auriel, E., Ayres, A., Schwab, K. M., et al. (2018). Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology 90:e119–e26. doi: 10.1212/WNL.0000000000004797
Pilato, F., Silva, S., Valente, I., Distefano, M., Broccolini, A., Brunetti, V., et al. (2020). Predicting factors of functional outcome in patients with acute ischemic stroke admitted to neuro-intensive care unit-a prospective cohort study. Brain Sci. 10:911. doi: 10.3390/brainsci10120911
Poon, M. T., Fonville, A. F., and Al-Shahi Salman, R. (2014). Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatr. 85, 660–667. doi: 10.1136/jnnp-2013-306476
Song, T. J., Kim, J., Song, D., Yoo, J., Lee, H. S., Kim, Y. J., et al. (2017). Total cerebral small-vessel disease score is associated with mortality during follow-up after acute ischemic stroke. J. Clin. Neurol. 13, 187–195. doi: 10.3988/jcn.2017.13.2.187
Staals, J., Makin, S. D., Doubal, F. N., Dennis, M. S., and Wardlaw, J. M. (2014). Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83, 1228–1234. doi: 10.1212/WNL.0000000000000837
Sykora, M., Herweh, C., and Steiner, T. (2017). The association between leukoaraiosis and poor outcome in intracerebral hemorrhage is not mediated by hematoma growth. J. Stroke Cerebrovasc. Dis. 26, 1328–1333. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.003
Tsai, H. H., Tsai, L. K., Chen, Y. F., Tang, S. C., Lee, B. C., Yen, R. F., et al. (2017). Correlation of cerebral microbleed distribution to amyloid burden in patients with primary intracerebral hemorrhage. Sci. Rep. 7:44715. doi: 10.1038/srep44715
Uniken Venema, S. M., Marini, S., Lena, U. K., Morotti, A., Jessel, M., Moomaw, C. J., et al. (2019). Impact of cerebral small vessel disease on functional recovery after intracerebral hemorrhage. Stroke 50, 2722–2728. doi: 10.1161/STROKEAHA.119.025061
Valenti, R., Del Bene, A., Poggesi, A., Ginestroni, A., Salvadori, E., Pracucci, G., et al. (2016). Cerebral microbleeds in patients with mild cognitive impairment and small vessel disease: The Vascular Mild Cognitive Impairment (VMCI)-Tuscany study. J. Neurol. Sci. 368, 195–202. doi: 10.1016/j.jns.2016.07.018
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Xu, M., Cheng, Y., Song, Q., Yuan, R., Zhang, S., Hao, Z., et al. (2019). Total burden of cerebral small vessel disease in recurrent ich versus first-ever ICH. Aging Dis. 10, 570–577. doi: 10.14336/AD.2018.0804
Keywords: cerebral small vessel disease, long-term follow-up, functional outcome, stroke recurrence, cerebral microbleeds
Citation: Xu M, Li B, Zhong D, Cheng Y, Wu Q, Zhang S, Zhang S, Wu B and Liu M (2021) Cerebral Small Vessel Disease Load Predicts Functional Outcome and Stroke Recurrence After Intracerebral Hemorrhage: A Median Follow-Up of 5 Years. Front. Aging Neurosci. 13:628271. doi: 10.3389/fnagi.2021.628271
Received: 11 November 2020; Accepted: 26 January 2021;
Published: 19 February 2021.
Edited by:
Guo-Yuan Yang, Shanghai Jiao Tong University, ChinaReviewed by:
Daojun Hong, The First Affiliated Hospital of Nanchang University, ChinaCopyright © 2021 Xu, Li, Zhong, Cheng, Wu, Zhang, Zhang, Wu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Liu, d3lwbG1oQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.