
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 10 December 2020
Sec. Neurocognitive Aging and Behavior
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.594810
This article is part of the Research Topic Behavioral and Cognitive Impairments Across the Life Span View all 34 articles
Motor control is associated with suppression of oscillatory activity in alpha (8–12 Hz) and beta (12–30 Hz) ranges and elevation of oxygenated hemoglobin levels in motor-cortical areas. Aging leads to changes in oscillatory and hemodynamic brain activity and impairments in motor control. However, the relationship between age-related changes in motor control and brain activity is not yet fully understood. Therefore, this study aimed to investigate age-related and task-complexity-related changes in grip force control and the underlying oscillatory and hemodynamic activity. Sixteen younger [age (mean ± SD) = 25.4 ± 1.9, 20–30 years] and 16 older (age = 56.7 ± 4.7, 50–70 years) healthy men were asked to use a power grip to perform six trials each of easy and complex force tracking tasks (FTTs) with their right dominant hand in a randomized within-subject design. Grip force control was assessed using a sensor-based device. Brain activity in premotor and primary motor areas of both hemispheres was assessed by electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS). Older adults showed significantly higher inaccuracies and higher hemodynamic activity in both FTTs than did young adults. Correlations between grip force control owing to task complexity and beta activity were different in the contralateral premotor cortex (PMC) between younger and older adults. Collectively, these findings suggest that aging leads to impairment of grip force control and an increase in hemodynamic activity independent of task complexity. EEG beta oscillations may represent a task-specific neurophysiological marker for age-related decline in complex grip force control and its underlying compensation strategies. Further EEG-fNIRS studies are necessary to determine neurophysiological markers of dysfunctions underlying age-related motor disabilities for the improvement of individual diagnosis and therapeutic approaches.
The ability to adapt our actions quickly to changes in the environment is a key function of human motor skills (Hermsdörfer et al., 2003). One of the most sophisticated characteristics of fine motor skills is the precise grip force regulation according to the physical requirements of a manipulated object, which is necessary for the successful performance of various everyday activities (Hermsdörfer et al., 2003; Voelcker-Rehage and Alberts, 2005; Parry et al., 2019). When gripping an object, radial forces are exerted that are large enough to prevent the object from slipping while ensuring that it does not break. Tangential forces are applied to move the object along the desired path (Flanagan and Wing, 1995; Haggard and Wing, 1995). Thus, regulation of precise grip force requires the coupling of radial and tangential grip forces (Hermsdörfer et al., 2000; Nowak et al., 2001; Rand et al., 2004), which is based on visuomotor transformation processes and the intact integration of sensory feedback within the motor-cortical network (Hermsdörfer et al., 2003; Prodoehl et al., 2009). Functional activity in motor-cortical areas can be quantified as cerebral hemodynamics using brain imaging techniques such as functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS; Agbangla et al., 2017) or as electrical signals using the electroencephalography (EEG; Hamacher et al., 2015). It is suggested that synchronous oscillatory activity in the alpha (8–12 Hz) and beta (12–30 Hz) ranges within the motor-cortical network represents the basic mechanism for functional communication underlying the motor control process (Davis et al., 2012; Wach et al., 2013; Pollok et al., 2015). The alpha rhythm reflects cortical processes during the awake and attentive-resting states, and the amplitude of the signal is suppressed by sensory stimulation or during the motor control process (Engel and Fries, 2010). Beta oscillations are attenuated during voluntary movements, which is known as movement-related beta desynchronization (MRBD; Zaepffel et al., 2013). After the completion of a movement, beta oscillations are more pronounced, which has been interpreted as increased inhibition to maintain the status quo of the motor system (Pfurtscheller et al., 2006; Koelewijn et al., 2008; Engel and Fries, 2010). Previous studies have shown that oscillatory activity and cerebral hemodynamics are inversely related during the motor control process, that is, hemodynamic activity increases with a decrease in alpha and beta oscillations (Lachert et al., 2017). Changes in brain activity during the motor control process have been observed in various cerebral structures (Zaepffel et al., 2013), mainly in sensorimotor areas with a contralateral predominance (Taniguchi et al., 2000). Concerning grip force tasks, as an example, young healthy people exhibited increased hemodynamic activity in the sensorimotor cortex (SMC) and in the premotor cortex (PMC; Ehrsson et al., 2000; Cramer et al., 2002; Wriessnegger et al., 2017). The SMC contralateral to the active hand showed the highest hemodynamic activity (Wriessnegger et al., 2017) confirming the involvement of the SMC during movement execution (Leff et al., 2011). Furthermore, the prefrontal cortex (PFC), supplementary motor area (SMA), and PMC bilaterally as well as the cerebellum were found to be involved in grip force execution (Dai et al., 2001). Thereby, the SMA represents a key structure controlling the motor-cortical network while driving the regulation of grip forces by promoting and suppressing its activity (Grefkes et al., 2008a). Alterations in brain functions within the motor-cortical network can be caused by neurological diseases (Schnitzler and Gross, 2005; Grefkes et al., 2008b; Engel and Fries, 2010; Kiyama et al., 2014). According to previous studies, reduced inter-and intra-hemispheric connectivity between the SMA and the primary motor cortex (Grefkes et al., 2008b), upregulation of the parieto-frontal network activity in the stroke-lesioned hemisphere (Bönstrup et al., 2018), and pathological increases in beta oscillations (Rossiter et al., 2014a) were correlated significantly with motor deficits (Grefkes et al., 2008b; Rossiter et al., 2014a; Bönstrup et al., 2018).
More than one billion people worldwide suffer from neurological diseases. The number will increase in the coming years because of the aging population (Semprini et al., 2018). Early signs of age-related diseases are altered brain structures and synaptic functions ranging from pathological oscillations and altered functional connectivity to reduced gray matter volume (Minati et al., 2007; Ishii et al., 2017; Xifra-Porxas et al., 2019). During grip force control, older adults exhibited higher beta power, greater (more negative) MRBD (Xifra-Porxas et al., 2019), and significantly higher hemodynamic activity in subcortical areas and PMC than did young healthy adults (Noble et al., 2011). Deficits in fine motor skills and behavioral declines are most often the consequences of these changes (Voelcker-Rehage and Alberts, 2005; Grefkes et al., 2008b; Engel and Fries, 2010; Kiyama et al., 2014). However, the nature of the relationship between age-related changes in brain activity patterns and declines in grip force regulation has not been fully elucidated. Previous studies concerning age-related deteriorations in motor functions used tasks such as finger tapping (Naccarato et al., 2006), key pressing (Mattay et al., 2002), index finger abductions/adductions, or wrist extensions/flexions (Hutchinson et al., 2002), thus focusing on repetitive movements that are limited in elderly individuals, when activities of everyday lives that require the regulation of grip forces must be performed (Diermayr et al., 2011; Bock and Steinberg, 2012). It is, therefore, important to investigate how the precise regulation of grip forces is related to brain activity and is influenced by the aging process.
In this study, EEG and fNIRS, which are well-established, promising electro/neurophysiological techniques that are affordable, easy to implement, and easy to integrate into the diagnosis of neurological conditions in patients of all age groups, were used (Makeig et al., 2009; Herold et al., 2017). Knowledge of how grip force regulation and hemodynamic/oscillatory brain activity are related and altered according to age provides crucial information for individualization of therapy protocols or advancement of interventions such as neurofeedback, brain-machine interfaces, or noninvasive brain stimulation (NIBS; Gassert and Dietz, 2018; Semprini et al., 2018; Berger et al., 2019a). More specifically, for example, identifying pathological hypo-/hyperactivity or changes in oscillatory activity while applying fine motor skills could guide future NIBS or training protocols to enable the administration of target-oriented and individualized interventions (Teo et al., 2016; Berger et al., 2018; Gassert and Dietz, 2018).
Therefore, the central purpose of this study was to investigate age- and task-related changes in hemodynamic and oscillatory activity during two visually guided force tracking tasks (FTTs) of different complexities. The easy FTT requires static grip force, whereas the complex FTT requires grip force-related characteristics necessary in everyday life settings (e.g., fine-tuning and quick adjustments of grip forces according to changing demands, such as when grasping objects to prevent slipping). As discussed above and based on the findings of previous studies that focused on task complexity (Holper et al., 2009; Wriessnegger et al., 2017) or age-related changes (Voelcker-Rehage and Alberts, 2005; Noble et al., 2011; Rossiter et al., 2014a,b; Xifra-Porxas et al., 2019), we hypothesized differences between the behavioral and neuronal factors associated with the two FTTs and between the younger and older adults (Noble et al., 2011) as follows: we expected: (1) that the complex FTT, which requires the regulation of grip force, results in higher inaccuracies in grip force control and greater brain activity in motor-cortical areas than does the easy FTT; and (2) that the older adults show greater deficits in precise grip force control and changes in sensorimotor activity during complex FTT than did younger adults.
This study was performed following the ethical standards laid down in the Declaration of Helsinki. Experimental procedures were performed according to the recommendations of the Deutsche Gesellschaft für Psychologie (DGP) and approved by the local ethics committee of the Johannes Gutenberg-Universität Mainz. All participants were informed about the study-related contents before obtaining written informed consent before the initiation of the experiment.
Sixteen younger male participants aged 20–30 years (mean age: 25.44 ± 1.93 years) and sixteen older male participants aged 50–70 years (mean age: 56.75 ± 4.71 years) without any hand pathologies and extremity injuries were recruited to participate in this study. All participants were right-handed according to the Edinburg Handedness-scale (Oldfield, 1971), had no neurological or psychological disorders, and had a normal or corrected-to-normal vision. They were asked to disclose information on preexisting neurological, psychological, and medical conditions and on drug and alcohol/caffeine intake during the previous week.
The ability of precise grip force control was measured using the FTT, which is a well-known diagnostic tool used to investigate specific aspects of force control, that is, force generation and force release (Voelcker-Rehage and Alberts, 2005). It consists of a monitor and a force transducer for the dominant right hand (LIME medical GmbH, Germany). Four rails for the index, middle, ring, and little fingers guarantee a standardized position of the hand (see Figure 1A). The participants were instructed to use the power grip to match a curve representing the applied force of their dominant (right) hand to a predefined target route as accurately as possible. The participants and the target route were displayed on a screen at approximately 80 cm. The participants aimed to make as few deviations as possible. A deviation is defined as the absolute difference between the applied force and the optimal target force. All forces (the participant’s applied grip force; predefined, optimal target force; and the resulting deviation) were measured in Newton (N) and calculated with a sampling rate of 10 Hz.
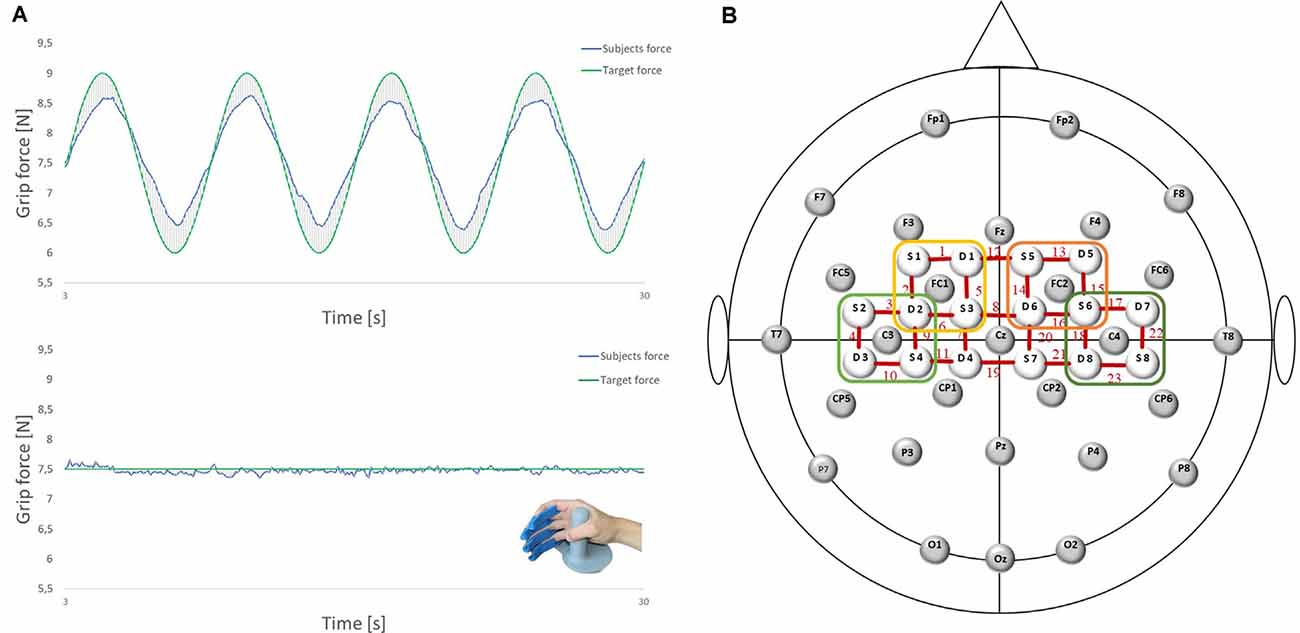
Figure 1. (A) Force tracking tasks (FTTs) and the force transducer for the right dominant hand (LIME medical GmbH, Germany): complex FTT (top); easy FTT (bottom); green: target force; blue: subjects force. (B) Electroencephalography (EEG)/functional near-infrared spectroscopy (fNIRS) Montage: 28 EEG electrodes and 16 fNIRS optodes placed according to the 5:10 EEG system. Regions of interest are marked. Left premotor cortex (PMC): FC1 and Channels 1, 2, 5, 6 (yellow); right PMC: FC2 and Channels 13, 14, 15, 16 (orange); left sensorimotor cortex (SMC): C3 and Channels 3, 4, 9, 10 (light green); right SMC: C4 and Channels 17, 18, 2, 23 (dark green).
EEG was recorded using Brain Vision Recorder 1.2 Brain Products, Germany equipped with 32 active Ag/AgCl electrodes, at a sampling rate of 1,000 Hz (notch filter at 50 Hz). Head dimensions were measured individually, and caps were adapted to the corresponding head sizes. Data were recorded using 28 electrodes placed on the scalp at Fp1, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, O1, Oz, and O2 according to the international 5:10 system (Jurcak et al., 2007; see Figure 1B). Vertical and horizontal electrooculograms were recorded with four additional electrodes placed next to the left and right eye and above/below the right eye to detect eye movements and eyelid artifacts. The reference and ground electrodes were placed at the nose tip and AFz location, respectively. Impedance was maintained below 5 kΩ by using a SuperViscTM electrode filled with gel (EASYCAP GmbH, Germany) for conductivity.
For fNIRS measurement, cerebral oxygenation changes were recorded using a near-infrared optical tomographic imaging device (NIRSport, NIRx, Germany, Wavelengths: 760 nm, 850 nm, sampling rate: 7.81 Hz). The methodology and underlying physiology are explained in detail elsewhere (Obrig and Villringer, 2003). A total of 16 optodes (eight emitters, eight detectors) were placed at 3 cm intervals above the motor cortex according to the international 5:10 system (Jurcak et al., 2007), resulting in 23 channels (source-detector pairs; see Figure 1B). Channel positions covered identical regions of both hemispheres, including the SMC [Brodmann Area (BA) 1–4] and SMA/PMC (BA6).
To control the maximum voluntary contraction (MVC) of the participants, a standardized Grip-Strength-Test was performed before the experiment. Participants sat upright in a standardized position on a height-adjustable chair (Massy-Westropp et al., 2011). The right upper arm was abducted, the elbow joint was flexed at 90°, the wrist was in a neutral zero position and the arm was held close to the body but not resting on the armrest of the chair. Using an electronic hand dynamometer (TL-LSC100, Trailite), three values of MVC were measured with the dominant right hand. The rest period in between was 1 min.
Participants performed two visually-guided grip-FTTs in a randomized order: in one task (easy FTT), participants had to follow a target force depicted as a straight line at 7.5 N by maintaining a constant grip force with the right dominant hand. In the other task (complex FTT), the target force changed over time in the form of a sinusoidal curve averaged to 7.5 N (6–9 N). Participants had to regulate and adapt their grip forces (N) continuously to the target value (see Figure 1A). Each FTT consisted of six task trials, with each trial lasting 30 s and alternating with six trials of rest (30 s; see Figure 2). During the trials of rest, participants were instructed to watch a fixation cross on the screen. Before and after each FTT, brain activity was recorded at rest for 30 s each with eyes closed and open. During the measurements, participants were instructed to avoid head movements and talking to reduce motion and physiological artifacts (Vitorio et al., 2017). For familiarization, two trials of the easy FTT and two trials of the complex FTT were performed before the experiment.
For the MVC, the mean of the three values was collected from each participant. Then, the arithmetic mean was calculated for the younger and older adults. Regarding the FTT, the participant’s applied grip force, the predefined, optimal target force, and their absolute deviations were measured for each FTT trial. Data from the first 3 s of each trial were excluded from further analysis considering the initial force adaptation at the onset. Then, the arithmetic mean of the absolute deviation values was computed, which represents the inaccuracy in grip force control (Gölz et al., 2018). Normal distribution was examined using the Shapiro–Wilk test (p ≥ 0.05). A two-way repeated-measures analysis of variance (rmANOVA) using the factors TASK (easy FTT, complex FTT) and GROUP (younger adults, older adults) was conducted to evaluate changes in grip force control owing to task complexity and aging. Bonferroni-adjusted post-hoc analyses were used to identify significant differences.
Electrophysiological data were preprocessed using the Brain Vision Analyzer 2.0 (Brain Products, Gilching, Germany) by applying a 0.5 Hz high-pass filter and a 50 Hz low-pass filter. Horizontal and vertical eye movements were corrected using the Gratton and Coles methods (Gratton et al., 1983). Any remaining artifacts were removed using a semiautomatic inspection tool by setting the maximum allowed gradient voltage step at 50 mV/ms and the maximally allowed amplitude at 100 mV. Further artifacts were visually identified and manually rejected so that only segments containing no artifacts were included for further processing. For the evaluation of changes in oscillatory activity over time, the two task conditions (easy FTT and complex FTT) and the rest condition were subdivided into six trials of 30 s. Each condition was segmented into 1 s epochs, a fast Fourier transformation [Maximum Resolution (0.977 Hz); Power (μV2); No window] was computed, and frequency spectra of the artifact-free segments were averaged for each condition. Then, oscillatory mean activities (mV) were calculated for the alpha (8–12 Hz) and beta (13–30 Hz) ranges. Since suppressed beta activity relative to rest is associated with movement execution (Xifra-Porxas et al., 2019), the MRBD was calculated in addition to the absolute power. Therefore, beta activity at rest as well as the difference of beta activity between each FTT and rest was calculated and statistically analyzed. Following the fNIRS channels and the corresponding regions of interest (ROI), the following EEG electrodes were used for further statistical analysis: left SMA/PMC, FC1; right SMA/PMC, FC2; left SMC, C3; right SMC: C4 (see Figure 1B).
Raw brain oxygenation data were preprocessed and analyzed using the time series analysis routine within the MATLAB-based NIRSlab analysis package (v2017.05, Nirx Medical Technologies, Glen Head, NY, USA; Xu et al., 2014) according to the current recommendations (Herold et al., 2017; Vitorio et al., 2017). Concentration changes of oxygenated hemoglobin (Hboxy) and deoxygenated hemoglobin (Hbdeoxy) were visually inspected concerning transient spikes and abrupt discontinuities representing the two most common forms of movement artifacts in fNIRS data. To achieve smoothening, epochs that contain discontinuities (or “jumps”) or long-term drifts were corrected, and spikes were replaced by the nearest signal (Xu et al., 2014). Then, a band-pass filter (0.01–0.2 Hz) was applied to remove slow drifts and high frequencies related to breathing, respiratory or cardiac rhythm, vasomotion, or other movement artifacts (Koenraadt et al., 2014). After preprocessing, parameters such as wavelengths (WL1 = 760 nm; WL2 = 850 nm), differential pathlength factors (7.25 for WL1; 6.38 for WL2), interoptode distances (3 cm), and background tissue values (totHb: 75 μM; MVO2Sat: 70%) were specified, and the time series of Hboxy/Hbdeoxy concentration changes (ΔHboxy/ΔHboxy) were computed using the modified Beer-Lambert law (Cope et al., 1988; Sassaroli and Fantini, 2004). Afterward, ΔHboxy data of the easy and complex FTT trials (30 s) were exported to analyze their hemodynamic responses. Averaged baseline concentration values of 15 s rest before each trial were subtracted from the task-evoked concentration measurements to account for time-dependent changes in cerebral oxygenation (Vitorio et al., 2017). ΔHboxy was calculated for ROI (left SMA/PMC: Channels 1, 2, 5, 6; right SMA/PMC: Channels 13, 14, 15, and 16; left SMC: Channels 3, 4, 9, and 10; right SMC: Channels: 17, 18, 22, 23) during the FTTs and was used as a marker for regional cortical activation because it is more sensitive to motor-related activities than is Hbdeoxy (Suzuki et al., 2004) and because it is an accurate indicator of hemodynamic activity (Strangman et al., 2002).
The statistical analysis of data corresponding to oscillatory and hemodynamic activity was conducted using SPSS 23 (IBM, Armonk, NY, USA). The normality of distribution was examined using the Shapiro–Wilk test (p ≥ 0.05). Oscillatory mean activities and averaged ΔHboxy values corresponding to both the FTTs were computed for each subject and ROI (Herold et al., 2017; Vitorio et al., 2017). A three-way rmANOVA with the factors TASK (easy FTT, complex FTT), ROI [ROI1: left SMA/PMC (BA6); ROI2: right SMA/PMC (BA6); ROI3: left SMC (BA1–4); ROI4: right SMC (BA1–3)], and GROUP (younger adults, older adults) was conducted to evaluate changes in oscillatory and hemodynamic activity owing to task complexity, brain regions, and between the groups. In case of significant effects, Bonferroni-adjusted post-hoc analyses were used to identify significant differences between the tasks, ROI, and groups.
The overall level of significance was set to p ≤ 0.05. Significant tendencies were defined as p ≤ 0.10. Mauchly’s test was used to check for any violations of sphericity. If a violation of sphericity was detected (p ≤ 0.05) and a Greenhouse–Geisser ε > 0.75 existed, Huynh-Feldt corrected p-values were reported. Otherwise (ε ≤ 0.75), a Greenhouse-Geisser correction was applied. Effect sizes were reported in the partial eta-square () for the significant main effects and interactions. According to Cohen (1988), we classified the effect sizes as follows: >0.01 for small, >0.06 for medium, and >0.14 for large effect sizes (Cohen, 1988).
To calculate the relationship between precise grip force control, oscillatory and hemodynamic activity, correlation analysis was performed for each task, region, and group. First, correlations were calculated for each task separately (e.g., correlation of inaccuracy and beta activity during the easy FTT). Second, differences between the easy FTT and the complex FTT were calculated. Then, difference values (Δ = complex FTT − easy FTT) were correlated (e.g., correlation of Δinaccuracy and Δβ activity) to analyze task complexity-related effects. Degrees of correlation between grip force control, oscillatory and hemodynamic activity were explored using the Pearson correlation coefficient: low, r > 0.1–0.3; medium, r > 0.3–0.5; strong, r > 0.5–0.7; very strong, r > 0.7–0.9; or perfect, r > 0.9. Furthermore, the correlations of younger and older adults were statistically compared (Eid et al., 2015).
Regarding the MVC, no differences between the two groups were presented (younger adults: 45.45 ± 4.43, younger adults: 45.59 ± 7.67, p = 0.951). For the FTT, the two-way repeated-measures ANOVA (rmANOVA) using the factors TASK (easy FTT, complex FTT) and GROUP (younger adults, older adults) showed a statistically significant main effect of TASK (F(1,30) = 184.10, p < 0.001, = 0.86) that indicates the incidence of a significantly higher inaccuracy during the complex FTT than during the easy FTT (p < 0.001). Furthermore, a significant main effect of GROUP (F(1,30) = 6.51, p = 0.02, = 0.18) emerged that presented higher inaccuracies in grip force regulation in older adults than in young adults (p = 0.02). No significant interaction of TASK × GROUP was observed (F(1,30) = 2.24, p = 0.15, = 0.07; see Figure 3).
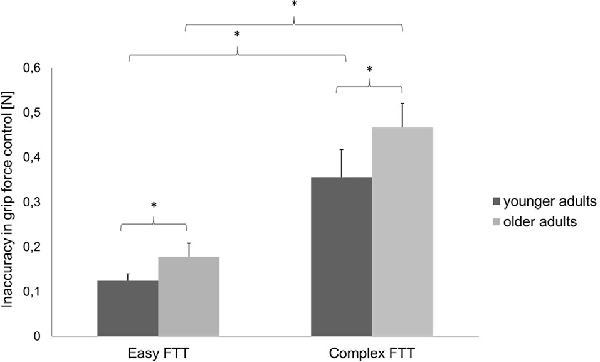
Figure 3. Precision in grip force control (mean ± SD) of the younger and older adults during the easy and complex FTTs; *p < 0.05.
Statistical analysis of data on relative Hboxy concentration changes revealed a significant main effect of GROUP (F(1,30) = 4.36, p = 0.04, = 0.13), indicating a significantly higher hemodynamic activity in the older adults than in the young adults. The significant main effect of ROI (F(3,90) = 3.56, p = 0.02, = 0.11) showed significant differences between the cortical areas. Bonferroni-corrected post-hoc tests revealed significantly higher hemodynamic activity in the ipsilateral SMC than in the ipsilateral PMC (p = 0.04) and contralateral SMC (p = 0.01; see Figure 4). Otherwise, no further main effects or interactions were observed (all p > 0.13).
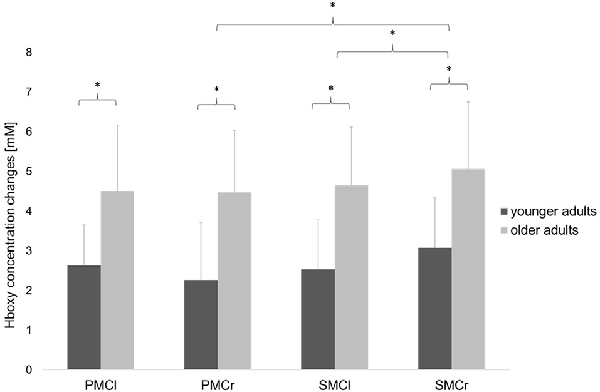
Figure 4. Relative concentration changes of Hboxy (mean ± SD) in the four regions of interest averaged over the easy FTT and the complex FTT for the younger and older adults. *p < 0.05.
Statistical analysis of data on oscillatory alpha activity (8–12 Hz) using a three-way rmANOVA with the factors TASK, ROI, and GROUP revealed neither main effects nor interaction effects (all p > 0.10). Concerning beta activity (13–30 Hz), the three-way rmANOVA showed a significant main effect of TASK (F(1,30) = 11.23, p < 0.001, = 0.27), indicating a significantly higher beta activity during the complex FTT than during the easy FTT. The significant main effect ROI (F(3,90) = 5.65, p = 0.02, = 0.16) revealed significantly higher beta activity in the left SMC than in the PMC and bilateral SMC. Furthermore, a statistically significant trend for the interaction of TASK × GROUP (F(1,30) = 3.10, p = 0.08, = 0.09) emerged. Bonferroni-corrected post-hoc tests indicated significantly higher beta activity during the complex FTT than during the easy FTT in the group of older adults (p = 0.001; see Figure 5). Furthermore, analysis of data on beta activity during rest, the two-way rmANOVA with the factors ROI, and GROUP revealed neither main effects nor interaction effects (all p > 0.10). Regarding the MRBD, the three-way rmANOVA showed a significant main effect of TASK (F(1,30) = 11.04, p < 0.002, = 0.27), indicating a significantly greater (more negative) MRBD during the easy FTT than during the complex FTT. The significant main effect ROI (F(3,90) = 5.41, p = 0.01, = 0.15) revealed significantly higher beta suppression in the left SMC than in the left PMC. Furthermore, a statistically significant trend for the interaction of TASK × GROUP (F(1,30) = 3.11, p = 0.08, = 0.09) and TASK × ROI × GROUP (F(3,90) = 2.78, p = 0.08, = 0.09) emerged. Bonferroni-corrected post-hoc tests indicated that older adults exhibited significantly greater MRBD on the SMC bilaterally during the easy FTT compared to the younger adults (left SMC p = 0.04, right SMC p = 0.03; see Figure 6).
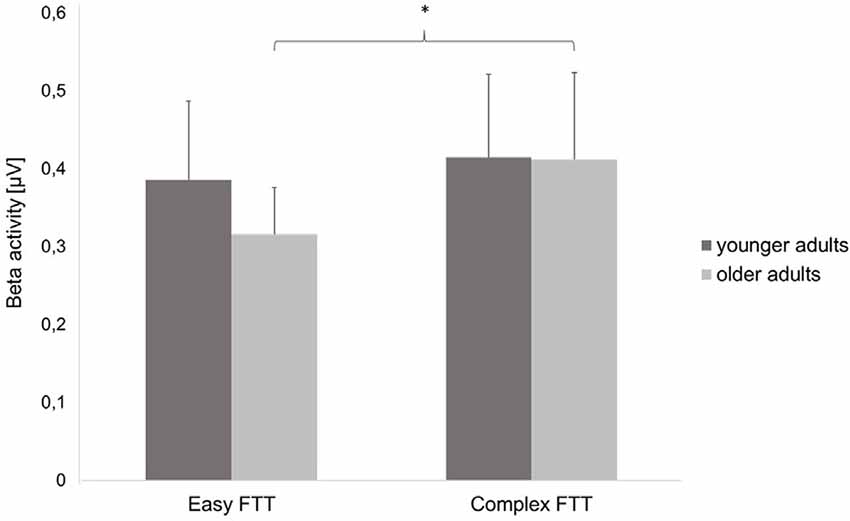
Figure 5. Beta activity (mean ± SD) of the younger and older adults during the easy and complex FTT, *p < 0.05.
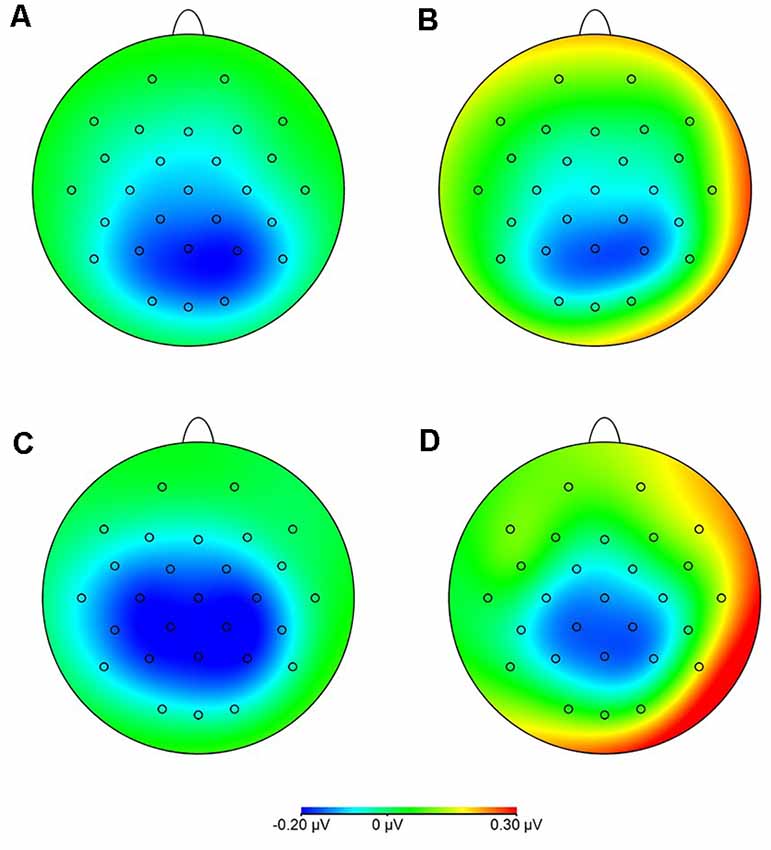
Figure 6. Movement-related beta desynchronization (MRBD) (A) younger adults, easy FTT (B) younger adults, complex FTT (C) older adults, easy FTT (D) older adults, complex FTT.
For the separate analysis of the tasks, correlations between precise grip force control and oscillatory activity as well as precise grip force control and hemodynamic activity were statistically not significant. Regarding the relation of oscillatory beta activity and hemodynamic activity, three significant tendencies (p < 0.08) and one significant correlation of beta activity in the right SMC and hemodynamic activity in the left SMC emerged during the easy FTT for the younger adults (r = 0.55, p = 0.03). For the older adults, correlation analysis showed nine significant tendencies (p < 0.08) and one significant correlation of beta activity in the right PMC and hemodynamic activity in the right SMC during the easy FTT (r = 0.50, p = 0.05; see Table 1). During the complex FTT, no significant tendencies or correlations were presented. Concerning complexity-based differences (Δ = complex FTT − easy FTT), older adults showed a positive association with a trend between inaccuracy in precise grip force regulation and oscillatory beta activity in the left PMC (r = 0.46, p = 0.07), whereas young adults showed a non-significant negative association (r = − 0.32, p = 0.22; see Figure 7). However, these correlations differed significantly between younger and older adults (p = 0.03). Thus, complexity-related deteriorations are accompanied by different motor cognitive processes in young and older adults.
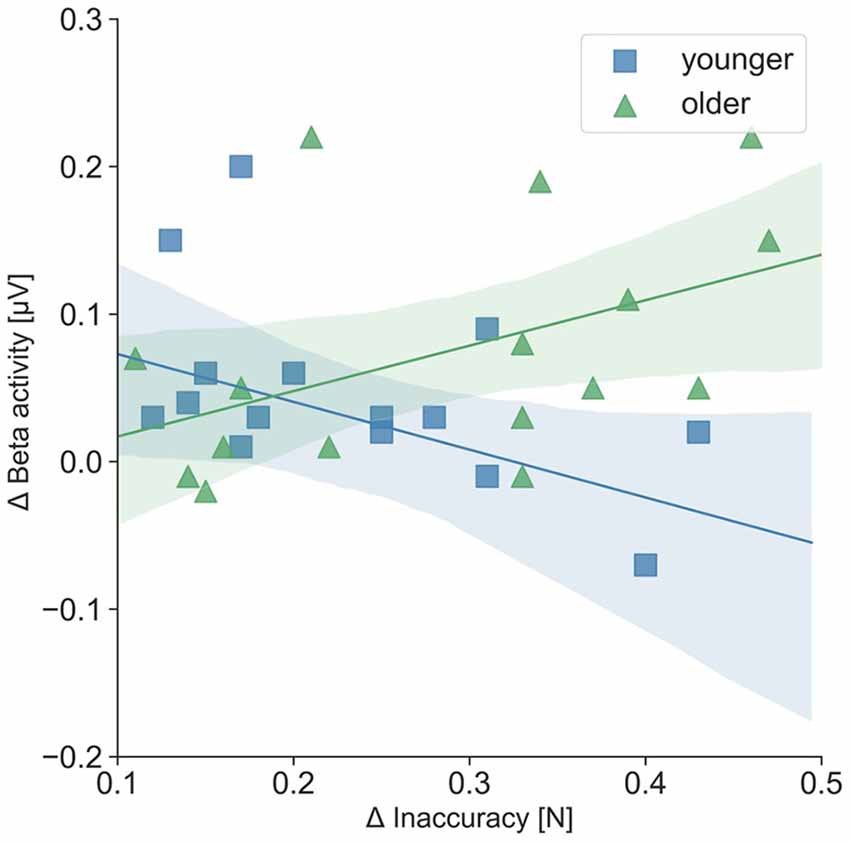
Figure 7. Correlation of Δ inaccuracy in precise grip force regulation and Δ left PMC beta activity (μV); Δ = complex FTT − easy FTT; younger adults: r = − 0.32; older adults: r = 0.46. The correlations differed significantly between younger and older adults (p = 0.03).
This study aimed to investigate age-related changes in grip force regulation and associated changes in oscillatory and hemodynamic brain activity. The following is the summary of our findings: (1) age-related deteriorations in grip force control and increases in hemodynamic activity in motor-cortical areas occurred independently of task complexity and (2) deteriorations in grip force control owing to the task complexity was positively associated with left PMC beta activity in older adults and negatively associated in younger adults.
First, the older adults showed significantly greater inaccuracies in grip force control and significantly higher hemodynamic brain activity in all motor-cortical areas during the easy and complex FTTs than did the younger adults. These results confirm the existence of expected age-related deteriorations in grip force control (Voelcker-Rehage and Alberts, 2005) and increases in hemodynamic activity (Mattay et al., 2002; Naccarato et al., 2006; Kim et al., 2010; Noble et al., 2011). On one hand, aging is associated with diminished tactile sensations that reduce hand sensibilities, which lead to declines in fine motor skills (Cole, 1991; Bennett and Castiello, 1994; Voelcker-Rehage and Alberts, 2005). On the other hand, physiological brain aging is characterized by a loss of synaptic contacts and neuronal cell death that provokes age-dependent declines of sensory processing and motor control (Rossini et al., 2007; Boisgontier et al., 2012; Xifra-Porxas et al., 2019). Thus, it may be possible that age-related decrease in tactile sensibility and the ability to process sensory feedback (Cole et al., 1999) lead to declines in grip force control and increased brain activity within the motor-cortical network regardless of the task complexity. Following the compensation theory of aging (Reuter-Lorenz and Park, 2010), over-recruitment of cortical resources with aging appears to reflect a greater computational effort and oxygenation owing to compensatory patterns such as reorganization and redistribution of functional networks to counteract age-related structural and neurochemical changes in motor control systems (Bennett and Castiello, 1994; Mattay et al., 2002; Ward, 2006; Noble et al., 2011; Larivière et al., 2019). Nevertheless, the results of deteriorations in grip force control and higher hemodynamic brain activity in older subjects cannot only be associated with the grip force tasks. As we did not include an additional control task such as a pinch force task or similar, increased brain activity in healthy aging during movements may be a general result that might also occur in other motor tasks and not only during the FTT. Furthermore, we identified decreases in beta power during both FTT relative to the resting period, known as MRBD, which showed the typical attenuation of beta activity during voluntary movements. After completion, beta power is pronounced, which has been interpreted as increased inhibition to maintain the status quo of the motor system (Pfurtscheller et al., 2006; Koelewijn et al., 2008; Engel and Fries, 2010). However, previous studies showed that older adults exhibited significantly higher beta oscillations at rest as well as greater MRBD during dynamic muscle contractions than did younger controls (Xifra-Porxas et al., 2019). However, our results did not show any significant differences in baseline beta power between the two groups. Additionally, older adults exhibited significantly greater MRBD in SMC bilaterally during the easy static FTT rather than during the complex variable FTT.
Second, all participants showed significantly higher inaccuracies in grip force control during the complex FTT than during the easy FTT. While the performance of grip force control was affected by task complexity, the respective hemodynamic activity seems to be unaffected. This is in line with the findings of previous studies, which showed that hemodynamic responses for all motor-cortical regions and two different grip force execution tasks (20% and 40% of the maximum grip strength) were similar (Wriessnegger et al., 2017). In contrast, significant differences in hemodynamic activity owing to task complexity were detected during finger tapping (Holper et al., 2009) and hand squeezing tasks in young healthy participants (Cramer et al., 2002). Thus, task-specific factors other than task complexity seem to affect hemodynamic responses. Furthermore, we identified that complexity-related deteriorations in grip force control were correlated with increased left PMC beta activity in older adults, which differ significantly (p = 0.03) from the negative correlation of the younger adults. Therefore, the processing of visuomotor transformation and the integration of sensory feedback that underly precise grip force regulation (Hermsdörfer et al., 2003; Prodoehl et al., 2009) may be different between younger and older adults. In previous studies, older adults showed significantly higher absolute beta activity during movements than did younger adults (Heinrichs-Graham and Wilson, 2016) that correlated with the degree of motor deficits during a visually guided grip task (Rossiter et al., 2014a). Due to the close relationship of beta activity and motor-cortical GABAergic inhibition (Rossiter et al., 2014b; Xifra-Porxas et al., 2019), it is suggested that age-related increases in beta activity may lead to a reduced ability to control the inhibition within the motor control network during movements. In the complex FTT, where the grip force must be regulated and adapted continuously, high cognitive effort in motor planning is required. Thus, older adults might show increased use of the PMC/SMA (Leff et al., 2011) which represents a key structure in the motor-cortical network driving manual movements by promoting and suppressing brain activity (Grefkes et al., 2008a; Bönstrup et al., 2016).
In addition to the age-related and task-complexity effects on grip force control and brain activity, correlations of oxygenated hemoglobin and oscillatory beta activity were found. During the easy FTT, increases in oscillatory beta activity were related to increases in oxygenated hemoglobin. However, in a previous study, increases in hemodynamic activity were accompanied by decreases of alpha and beta activity during a motor task (Lachert et al., 2017). Also, increases of alpha power following 10 and 20 Hz tACS were accompanied by decreases in oxygenated hemoglobin (Berger et al., 2018). Although it is well known that increases in oxygenated hemoglobin (Strangman et al., 2002) and suppressions in beta activity (Rossiter et al., 2014b; Xifra-Porxas et al., 2019) represent the neural correlated underlying movements, the relationship between the two parameters and how they change depending on motor tasks and complexities is not yet fully understood. Thus, numerous questions regarding the correlation of electrical signals and vascular changes (e.g., co-localization/time lag of the correlated changes, consistency of changes in connectivity) remain.
In summary, the older adults showed significant deterioration in grip force control and greater hemodynamic activity during both the FTTs than did the young adults, suggesting a greater computational effort and oxygen supply during the motor tasks (Ward, 2006) which represent a general age-related compensatory mechanism of changes in the sensorimotor network (Noble et al., 2011; Larivière et al., 2019). In contrast, increases in left PMC beta activity that are related to deteriorations in grip force regulation in older adults are task-complexity-dependent, thus representing an increased cognitive effort during motor planning or a compensatory mechanism of deficits in processing the sensory feedback within the sensorimotor network. Nevertheless, the age-related changes in oscillatory and hemodynamic activity as well as their correlation do not show clear results that allow an unequivocal conclusion. Understanding the relationship between motor control and brain activity as well as between motor recovery and its underlying neuroplasticity, remain major challenges in neurorehabilitation (Bönstrup et al., 2018). Thus, uncovering the link between age- and disease-related impairments of motor control and changes in brain activity to identify objective biomarkers for neuroplastic changes and motor recovery is a future goal in neurorehabilitation (Bönstrup et al., 2018; Coscia et al., 2019). Based on this, individualized training protocols and interventions with new technologies such as BMI and NIBS could be developed, which enable sustainable rehabilitation and optimization of the outcome and effectiveness of motor recovery (Semprini et al., 2018; Steinberg et al., 2019; Berger et al., 2019a,b).
Although our findings regarding age- and task complexity-related changes in precise grip force regulation and brain activity were interesting, three limitations must be discussed and considered while designing future studies. First, the small number of subjects (n = 32) limits a clear and general conclusion. Referring to other neurophysiological studies (Noble et al., 2011; Xifra-Porxas et al., 2019), we focused on the methodological challenge of a combined EEG-fNIRS measurement during a motor task. In future studies, however, a higher sample size should be considered. Second, all participants were required to match the same target force in the visually guided FTT. Although our two groups do not differ in terms of MVC, previous studies have shown that grip strength declines with increasing age (Zammit et al., 2019) and after neurological diseases (Hermsdörfer et al., 2003). Thus, individual target forces depending on their own individual MVC can be considered in future FTT tasks to ensure that an optimal balance of support and challenge for each patient is achieved. However, different percentual forces ranging from 5 to 60% of the MVC were used in previous studies (Voelcker-Rehage and Alberts, 2005; Wriessnegger et al., 2017). Third, we had to focus on specific motor-cortical ROI because of the limited number of fNIRS channels and limited spatial resolution. Based on the knowledge that skilled grip force control depends on the intact communication of the entire sensorimotor network (Hermsdörfer et al., 2003) and because previous studies investigated motor-cortical areas using fNIRS during handgrip tasks (Wriessnegger et al., 2017), premotor and sensorimotor areas were chosen for investigation. Nevertheless, daily activities requiring visuomotor and sensorimotor integration (Vingerhoets, 2014) are based on a large neural network in which further cortical and subcortical areas are also involved (Vaillancourt et al., 2007; Wasson et al., 2010). Also, further analyses such as the coherence analysis between ROI to evaluate network communication and other recordings such as EMG on the performing arm to control muscle activity and to more precisely describe possible causes for age-related changes in grip force control would have added valuable further insights.
In this study, we found evidence for age-related changes in grip force control and hemodynamic activity as well as correlations between grip force control owing to task complexity and beta activity that were different in the left PMC between younger and older adults. Assessing both motor precision and brain activity is essential for understanding how motor control, oscillatory, and hemodynamic activity within the motor-cortical network are related. This knowledge could contribute to the development of new therapeutic approaches to individualize and sustainably improve the treatment of motor impairments.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Local ethics committee of the Johannes Gutenberg-Universität Mainz. The participants provided their written informed consent to participate in this study.
AB: conception and execution of the research project, data acquisition, statistical analysis, interpretation, and manuscript writing. AB acts as the corresponding author. FS, FT, and MD: conception and execution of the research project and review and critique of the manuscript. All authors have read and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the company LIME medical GmbH for the implementation of the FTT and Dominik Endert and Michèle Völcker for assisting with data collection.
Agbangla, N. F., Audiffren, M., and Albinet, C. T. (2017). Use of near-infrared spectroscopy in the investigation of brain activation during cognitive aging: a systematic review of an emerging area of research. Ageing Res. Rev. 38, 52–66. doi: 10.1016/j.arr.2017.07.003
Bönstrup, M., Schulz, R., Feldheim, J., Hummel, F. C., and Gerloff, C. (2016). Dynamic causal modelling of EEG and fMRI to characterize network architectures in a simple motor task. NeuroImage 124, 498–508. doi: 10.1016/j.neuroimage.2015.08.052
Bönstrup, M., Schulz, R., Schön, G., Cheng, B., Feldheim, J., Thomalla, G., et al. (2018). Parietofrontal network upregulation after motor stroke. NeuroImage Clin. 18, 720–729. doi: 10.1016/j.nicl.2018.03.006
Bennett, K. M., and Castiello, U. (1994). Reach to grasp: changes with age. J. Gerontol. 49, P1–P7. doi: 10.1093/geronj/49.1.p1
Berger, A., Horst, F., Müller, S., Steinberg, F., and Doppelmayr, M. (2019a). Current state and future prospects of EEG and fNIRS in robot-assisted gait rehabilitation: a brief review. Front. Hum. Neurosci. 13:172. doi: 10.3389/fnhum.2019.00172
Berger, A., Horst, F., Steinberg, F., Thomas, F., Müller-Eising, C., Schöllhorn, W. I., et al. (2019b). Increased gait variability during robot-assisted walking is accompanied by increased sensorimotor brain activity in healthy people. J. Neuroeng. Rehabil. 16:161. doi: 10.1186/s12984-019-0636-3
Berger, A., Pixa, N. H., Steinberg, F., and Doppelmayr, M. (2018). Brain oscillatory and hemodynamic activity in a bimanual coordination task following transcranial alternating current stimulation (tACS): a combined EEG-fNIRS study. Front. Behav. Neurosci. 12:67. doi: 10.3389/fnbeh.2018.00067
Bock, O., and Steinberg, F. (2012). Age-related deficits of manual grasping in a laboratory versus in an everyday-like setting. Ageing Res. 3:e7. doi: 10.4081/ar.2012.e7
Boisgontier, M. P., Olivier, I., Chenu, O., and Nougier, V. (2012). Presbypropria: the effects of physiological ageing on proprioceptive control. Age 34, 1179–1194. doi: 10.1007/s11357-011-9300-y
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. 2nd Edn. Hillsdale, NJ: L. Erlbaum Associates.
Cole, K. J. (1991). Grasp force control in older adults. J. Mot. Behav. 23, 251–258. doi: 10.1080/00222895.1991.9942036
Cole, K. J., Rotella, D. L., and Harper, J. G. (1999). Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J. Neurosci. 19, 3238–3247. doi: 10.1523/JNEUROSCI.19-08-03238.1999
Cope, M., Delpy, D. T., Reynolds, E. O., Wray, S., Wyatt, J., and van der Zee, P. (1988). Methods of quantitating cerebral near infrared spectroscopy data. Adv. Exp. Med. Biol. 222, 183–189. doi: 10.1007/978-1-4615-9510-6_21
Coscia, M., Wessel, M. J., Chaudary, U., Millán, J. D. R., Micera, S., Guggisberg, A., et al. (2019). Neurotechnology-aided interventions for upper limb motor rehabilitation in severe chronic stroke. Brain 142, 2182–2197. doi: 10.1093/brain/awz181
Cramer, S. C., Weisskoff, R. M., Schaechter, J. D., Nelles, G., Foley, M., Finklestein, S. P., et al. (2002). Motor cortex activation is related to force of squeezing. Hum. Brain Mapp. 16, 197–205. doi: 10.1002/hbm.10040
Dai, T. H., Liu, J. Z., Sahgal, V., Brown, R. W., and Yue, G. H. (2001). Relationship between muscle output and functional MRI-measured brain activation. Exp. Brain Res. 140, 290–300. doi: 10.1007/s002210100815
Davis, N. J., Tomlinson, S. P., and Morgan, H. M. (2012). The role of β-frequency neural oscillations in motor control. J. Neurosci. 32, 403–404. doi: 10.1523/JNEUROSCI.5106-11.2012
Diermayr, G., McIsaac, T. L., and Gordon, A. M. (2011). Finger force coordination underlying object manipulation in the elderly—a mini-review. Gerontology 57, 217–227. doi: 10.1159/000295921
Ehrsson, H. H., Fagergren, A., Jonsson, T., Westling, G., Johansson, R. S., and Forssberg, H. (2000). Cortical activity in precision- versus power-grip tasks: an fMRI study. J. Neurophysiol. 83, 528–536. doi: 10.1152/jn.2000.83.1.528
Eid, M., Gollwitzer, M., and Schmitt, M. (2015). Statistik und Forschungsmethoden: Mit Online-Materialien 5. korrigierte auflage. Weinheim: Beltz.
Engel, A. K., and Fries, P. (2010). Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Flanagan, J. R., and Wing, A. M. (1995). The stability of precision grip forces during cyclic arm movements with a hand-held load. Exp. Brain Res. 105, 455–464. doi: 10.1007/BF00233045
Gölz, C., Voelcker-Rehage, C., Mora, K., Reuter, E.-M., Godde, B., Dellnitz, M., et al. (2018). Improved neural control of movements manifests in expertise-related differences in force output and brain network dynamics. Front. Physiol. 9:1540. doi: 10.3389/fphys.2018.01540
Gassert, R., and Dietz, V. (2018). Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J. Neuroeng. Rehabil. 15:46. doi: 10.1186/s12984-018-0383-x
Gratton, G., Coles, M. G. H., and Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 55, 468–484. doi: 10.1016/0013-4694(83)90135-9
Grefkes, C., Eickhoff, S. B., Nowak, D. A., Dafotakis, M., and Fink, G. R. (2008a). Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage 41, 1382–1394. doi: 10.1016/j.neuroimage.2008.03.048
Grefkes, C., Nowak, D. A., Eickhoff, S. B., Dafotakis, M., Küst, J., Karbe, H., et al. (2008b). Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 63, 236–246. doi: 10.1002/ana.21228
Haggard, P., and Wing, A. (1995). Coordinated responses following mechanical perturbation of the arm during prehension. Exp. Brain Res. 102, 483–494. doi: 10.1007/BF00230652
Hamacher, D., Herold, F., Wiegel, P., Hamacher, D., and Schega, L. (2015). Brain activity during walking: a systematic review. Neurosci. Biobehav. Rev. 57, 310–327. doi: 10.1016/j.neubiorev.2015.08.002
Heinrichs-Graham, E., and Wilson, T. W. (2016). Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage 134, 514–521. doi: 10.1016/j.neuroimage.2016.04.032
Hermsdörfer, J., Hagl, E., Nowak, D. A., and Marquardt, C. (2003). Grip force control during object manipulation in cerebral stroke. Clin. Neurophysiol. 114, 915–929. doi: 10.1016/s1388-2457(03)00042-7
Hermsdörfer, J., Marquardt, C., Philipp, J., Zierdt, A., Nowak, D., Glasauer, S., et al. (2000). Moving weightless objects. Grip force control during microgravity. Exp. Brain Res. 132, 52–64. doi: 10.1007/s002219900325
Herold, F., Wiegel, P., Scholkmann, F., Thiers, A., Hamacher, D., and Schega, L. (2017). Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics 4:041403. doi: 10.1117/1.NPh.4.4.041403
Holper, L., Biallas, M., and Wolf, M. (2009). Task complexity relates to activation of cortical motor areas during uni- and bimanual performance: a functional NIRS study. NeuroImage 46, 1105–1113. doi: 10.1016/j.neuroimage.2009.03.027
Hutchinson, S., Kobayashi, M., Horkan, C. M., Pascual-Leone, A., Alexander, M. P., and Schlaug, G. (2002). Age-related differences in movement representation. NeuroImage 17, 1720–1728. doi: 10.1006/nimg.2002.1309
Ishii, R., Canuet, L., Aoki, Y., Hata, M., Iwase, M., Ikeda, S., et al. (2017). Healthy and pathological brain aging: from the perspective of oscillations, functional connectivity and signal complexity. Neuropsychobiology 75, 151–161. doi: 10.1159/000486870
Jurcak, V., Tsuzuki, D., and Dan, I. (2007). 10/20, 10/10 and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage 34, 1600–1611. doi: 10.1016/j.neuroimage.2006.09.024
Kim, J.-H., Lee, Y.-S., Lee, J.-J., Song, H.-J., Yoo, D.-S., Lee, H. J., et al. (2010). Functional magnetic resonance imaging reveals age-related alterations to motor networks in weighted elbow flexion-extension movement. Neurol. Res. 32, 995–1001. doi: 10.1179/016164110X12670144737693
Kiyama, S., Kunimi, M., Iidaka, T., and Nakai, T. (2014). Distant functional connectivity for bimanual finger coordination declines with aging: an fMRI and SEM exploration. Front. Hum. Neurosci. 8:251. doi: 10.3389/fnhum.2014.00251
Koelewijn, T., van Schie, H. T., Bekkering, H., Oostenveld, R., and Jensen, O. (2008). Motor-cortical beta oscillations are modulated by correctness of observed action. NeuroImage 40, 767–775. doi: 10.1016/j.neuroimage.2007.12.018
Koenraadt, K. L. M., Roelofsen, E. G. J., Duysens, J., and Keijsers, N. L. W. (2014). Cortical control of normal gait and precision stepping: an fNIRS study. NeuroImage 85, 415–422. doi: 10.1016/j.neuroimage.2013.04.070
Lachert, P., Janusek, D., Pulawski, P., Liebert, A., Milej, D., and Blinowska, K. J. (2017). Coupling of oxy- and deoxyhemoglobin concentrations with EEG rhythms during motor task. Sci. Rep. 7:15414. doi: 10.1038/s41598-017-15770-2
Larivière, S., Xifra-Porxas, A., Kassinopoulos, M., Niso, G., Baillet, S., Mitsis, G. D., et al. (2019). Functional and effective reorganization of the aging brain during unimanual and bimanual hand movements. Hum. Brain Mapp. 40, 3027–3040. doi: 10.1002/hbm.24578
Leff, D. R., Orihuela-Espina, F., Elwell, C. E., Athanasiou, T., Delpy, D. T., Darzi, A. W., et al. (2011). Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. NeuroImage 54, 2922–2936. doi: 10.1016/j.neuroimage.2010.10.058
Makeig, S., Gramann, K., Jung, T.-P., Sejnowski, T. J., and Poizner, H. (2009). Linking brain, mind and behavior. Int. J. Psychophysiol. 73, 95–100. doi: 10.1016/j.ijpsycho.2008.11.008
Massy-Westropp, N. M., Gill, T. K., Taylor, A. W., Bohannon, R. W., and Hill, C. L. (2011). Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res. Notes 4:127. doi: 10.1186/1756-0500-4-127
Mattay, V. S., Fera, F., Tessitore, A., Hariri, A. R., Das, S., Callicott, J. H., et al. (2002). Neurophysiological correlates of age-related changes in human motor function. Neurology 58, 630–635. doi: 10.1212/wnl.58.4.630
Minati, L., Grisoli, M., and Bruzzone, M. G. (2007). MR spectroscopy, functional MRI and diffusion-tensor imaging in the aging brain: a conceptual review. J. Geriatr. Psychiatry Neurol. 20, 3–21. doi: 10.1177/0891988706297089
Naccarato, M., Calautti, C., Jones, P. S., Day, D. J., Carpenter, T. A., and Baron, J.-C. (2006). Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? an fMRI study. NeuroImage 32, 1250–1256. doi: 10.1016/j.neuroimage.2006.05.003
Noble, J. W., Eng, J. J., Kokotilo, K. J., and Boyd, L. A. (2011). Aging effects on the control of grip force magnitude: an fMRI study. Exp. Gerontol. 46, 453–461. doi: 10.1016/j.exger.2011.01.004
Nowak, D. A., Hermsdörfer, J., Philipp, J., Marquardt, C., Glasauer, S., and Mai, N. (2001). Effects of changing gravity on anticipatory grip force control during point-to-point movements of a hand-held object. Motor Control 5, 231–253. doi: 10.1123/mcj.5.3.231
Obrig, H., and Villringer, A. (2003). Beyond the visible—imaging the human brain with light. J. Cereb. Blood Flow Metab. 23, 1–18. doi: 10.1097/01.WCB.0000043472.45775.29
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Parry, R., Macias Soria, S., Pradat-Diehl, P., Marchand-Pauvert, V., Jarrassé, N., and Roby-Brami, A. (2019). Effects of hand configuration on the grasping, holding and placement of an instrumented object in patients with hemiparesis. Front. Neurol. 10:240. doi: 10.3389/fneur.2019.00240
Pfurtscheller, G., Leeb, R., Keinrath, C., Friedman, D., Neuper, C., Guger, C., et al. (2006). Walking from thought. Brain Res. 1071, 145–152. doi: 10.1016/j.brainres.2005.11.083
Pollok, B., Boysen, A.-C., and Krause, V. (2015). The effect of transcranial alternating current stimulation (tACS) at alpha and beta frequency on motor learning. Behav. Brain Res. 293, 234–240. doi: 10.1016/j.bbr.2015.07.049
Prodoehl, J., Corcos, D. M., and Vaillancourt, D. E. (2009). Basal ganglia mechanisms underlying precision grip force control. Neurosci. Biobehav. Rev. 33, 900–908. doi: 10.1016/j.neubiorev.2009.03.004
Rand, M. K., Shimansky, Y., Stelmach, G. E., and Bloedel, J. R. (2004). Adaptation of reach-to-grasp movement in response to force perturbations. Exp. Brain Res. 154, 50–65. doi: 10.1007/s00221-003-1637-8
Reuter-Lorenz, P. A., and Park, D. C. (2010). Human neuroscience and the aging mind: a new look at old problems. J. Gerontol. B Psychol. Sci. Soc. Sci. 65, 405–415. doi: 10.1093/geronb/gbq035
Rossini, P. M., Rossi, S., Babiloni, C., and Polich, J. (2007). Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog. Neurobiol. 83, 375–400. doi: 10.1016/j.pneurobio.2007.07.010
Rossiter, H. E., Boudrias, M.-H., and Ward, N. S. (2014a). Do movement-related beta oscillations change after stroke? J. Neurophysiol. 112, 2053–2058. doi: 10.1152/jn.00345.2014
Rossiter, H. E., Davis, E. M., Clark, E. V., Boudrias, M.-H., and Ward, N. S. (2014b). Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage 91, 360–365. doi: 10.1016/j.neuroimage.2014.01.012
Sassaroli, A., and Fantini, S. (2004). Comment on the modified Beer-Lambert law for scattering media. Phys. Med. Biol. 49, N255–N257. doi: 10.1088/0031-9155/49/14/n07
Schnitzler, A., and Gross, J. (2005). Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 6, 285–296. doi: 10.1038/nrn1650
Semprini, M., Laffranchi, M., Sanguineti, V., Avanzino, L., De Icco, R., De Michieli, L., et al. (2018). Technological approaches for neurorehabilitation: from robotic devices to brain stimulation and beyond. Front. Neurol. 9:212. doi: 10.3389/fneur.2018.00212
Steinberg, F., Pixa, N. H., and Fregni, F. (2019). A review of acute aerobic exercise and transcranial direct current stimulation effects on cognitive functions and their potential synergies. Front. Hum. Neurosci. 12:534. doi: 10.3389/fnhum.2018.00534
Strangman, G., Culver, J. P., Thompson, J. H., and Boas, D. A. (2002). A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage 17, 719–731. doi: 10.1006/nimg.2002.1227
Suzuki, M., Miyai, I., Ono, T., Oda, I., Konishi, I., Kochiyama, T., et al. (2004). Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. NeuroImage 23, 1020–1026. doi: 10.1016/j.neuroimage.2004.07.002
Taniguchi, M., Kato, A., Fujita, N., Hirata, M., Tanaka, H., Kihara, T., et al. (2000). Movement-related desynchronization of the cerebral cortex studied with spatially filtered magnetoencephalography. NeuroImage 12, 298–306. doi: 10.1006/nimg.2000.0611
Teo, W.-P., Muthalib, M., Yamin, S., Hendy, A. M., Bramstedt, K., Kotsopoulos, E., et al. (2016). Does a combination of virtual reality, neuromodulation and neuroimaging provide a comprehensive platform for neurorehabilitation?—A narrative review of the literature. Front. Hum. Neurosci. 10:284. doi: 10.3389/fnhum.2016.00284
Vaillancourt, D. E., Yu, H., Mayka, M. A., and Corcos, D. M. (2007). Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. NeuroImage 36, 793–803. doi: 10.1016/j.neuroimage.2007.03.002
Vingerhoets, G. (2014). Contribution of the posterior parietal cortex in reaching, grasping and using objects and tools. Front. Psychol. 5:151. doi: 10.3389/fpsyg.2014.00151
Vitorio, R., Stuart, S., Rochester, L., Alcock, L., and Pantall, A. (2017). fNIRS response during walking—artefact or cortical activity? A systematic review. Neurosci. Biobehav. Rev. 83, 160–172. doi: 10.1016/j.neubiorev.2017.10.002
Voelcker-Rehage, C., and Alberts, J. L. (2005). Age-related changes in grasping force modulation. Exp. Brain Res. 166, 61–70. doi: 10.1007/s00221-005-2342-6
Wach, C., Krause, V., Moliadze, V., Paulus, W., Schnitzler, A., and Pollok, B. (2013). The effect of 10 Hz transcranial alternating current stimulation (tACS) on corticomuscular coherence. Front. Hum. Neurosci. 7:511. doi: 10.3389/fnhum.2013.00511
Ward, N. S. (2006). Compensatory mechanisms in the aging motor system. Ageing Res. Rev. 5, 239–254. doi: 10.1016/j.arr.2006.04.003
Wasson, P., Prodoehl, J., Coombes, S. A., Corcos, D. M., and Vaillancourt, D. E. (2010). Predicting grip force amplitude involves circuits in the anterior basal ganglia. NeuroImage 49, 3230–3238. doi: 10.1016/j.neuroimage.2009.11.047
Wriessnegger, S. C., Kirchmeyr, D., Bauernfeind, G., and Müller-Putz, G. R. (2017). Force related hemodynamic responses during execution and imagery of a hand grip task: a functional near infrared spectroscopy study. Brain Cogn. 117, 108–116. doi: 10.1016/j.bandc.2017.06.010
Xifra-Porxas, A., Niso, G., Larivière, S., Kassinopoulos, M., Baillet, S., Mitsis, G. D., et al. (2019). Older adults exhibit a more pronounced modulation of beta oscillations when performing sustained and dynamic handgrips. NeuroImage 201:116037. doi: 10.1016/j.neuroimage.2019.116037
Xu, Y., Graber, H. L., and Barbour, R. L. (2014). “nirsLAB: a computing environment for fNIRS neuroimaging data analysis,” in Proceedings Biomedical Optics 2014 (Miami, FL: Optical Society of America), Paper BM3A.1.
Zaepffel, M., Trachel, R., Kilavik, B. E., and Brochier, T. (2013). Modulations of EEG beta power during planning and execution of grasping movements. PLoS One 8:e60060. doi: 10.1371/journal.pone.0060060
Keywords: electroencephalography, functional near-infrared spectroscopy, aging, motor control, motor recovery, neuroplasticity
Citation: Berger A, Steinberg F, Thomas F and Doppelmayr M (2020) Neural Correlates of Age-Related Changes in Precise Grip Force Regulation: A Combined EEG-fNIRS Study. Front. Aging Neurosci. 12:594810. doi: 10.3389/fnagi.2020.594810
Received: 14 August 2020; Accepted: 11 November 2020;
Published: 10 December 2020.
Edited by:
Franca Rosa Guerini, Fondazione Don Carlo Gnocchi Onlus (IRCCS), ItalyReviewed by:
Francesco Mattia Noè, University of Helsinki, FinlandCopyright © 2020 Berger, Steinberg, Thomas and Doppelmayr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alisa Berger, YWxpc2EuYmVyZ2VyQHVuaS1tYWluei5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.