
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 04 February 2021
Sec. Neurocognitive Aging and Behavior
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.578071
Background: More people with cognitive dysfunction and dementia also fall into the category of high vascular risk, for which aspirin is one of the most frequently used drugs. However, previous studies reporting that aspirin buffers against mild cognitive decline (MCI) and dementia remain controversial. We thus conducted an updated systematic review and meta-analysis to evaluate the association of aspirin use with the risk of MCI and dementia in older adults.
Methods: Data sources from PubMed, Embase, Web of Science, and the Cochrane Database for randomized controlled trails (RCTs) and cohort studies (published between January 1, 2000 and April 11, 2020). Relative risks (RRs) and 95% confidence intervals (95% CIs) were used to pool data on the occurrence of dementia and MCI with random-effects models.
Results: Of 3,193 identified articles, 15 studies (12 cohort studies and three RCTs) were eligible and were included in our analysis, which involved a total of 100,909 participants without cognitive dysfunctions or dementia at baseline. In pooled cohort studies, aspirin use did not reduce the incidence of MCI and dementia (the pooled RR = 0.97; 95% CI = 0.85–1.11; = 65%) compared with non-users. However, low-dose aspirin (75–100 mg/day) was associated with a decreased likelihood of developing dementia or MCI (the pooled RR = 0.75; 95% CI = 0.63–0.9; = 50.5%). This association existed in studies including all-cause dementia (the pooled RR = 0.82; 95% CI = 0.71–0.96) and Alzheimer's disease (AD) (the pooled RR = 0.54; 95% CI = 0.33–0.89), but not in MCI (the pooled RR = 0.58; 95% CI = 0.31–1.08). In RCTs, low-dose aspirin use was not significantly associated with less prevalence of dementia or MCI (RR = 0.94; 95% CI = 0.84–1.05; = 0.0%).
Conclusions: In cohort studies, we found that low-dose aspirin use had a higher likelihood of reducing the incidence of dementia, which was not supported by RCTs. The evidence was insufficient to fully evaluate the effect of aspirin on cognitive function and dementia. Well-designed studies and innovative approaches are therefore needed to clarify whether the use of aspirin improves cognitive function and reduces the risk of dementia.
The global prevalence of dementia was 50 million people worldwide in 2015, and the number of individuals with dementia could increase to 82 million in 2030 and 152 million by 2050, which will affect ~5–8% of people ≥60 years old (WHO, 2019). Alzheimer's disease (AD) is a multi-factorial, complex, and progressive neurodegenerative disorder and the most common form of dementia in the elderly, representing 50–75% of dementia patients (Aihw, 2012). After AD, vascular dementia (VaD) is the second leading cause of dementia. Previous population-based studies identified risk factors for vascular disease as targets for preventing dementia and cognitive dysfunction (Whalley and Mowat, 2007; Sanford, 2017). It is likely to involve a multi-factorial mechanism, for example, involving increasing the levels of cerebral amyloid-β (Aβ) peptide, changing the blood–brain barrier permeability, disrupting blood cholesterol homeostasis, altering brain insulin resistance, increasing oxidative stress, causing chronic inflammation, and damaging the dopamine cholinergic nervous system (Thal et al., 2008; Harrison et al., 2014; Melo, 2019).
Aspirin 75–300 mg daily has been widely used for secondary prevention of thrombotic cerebrovascular and cardiovascular disease over the past three decades. Low-dose aspirin is generally defined as 75–100 mg/day (Rands and Orrell, 2000; Jordan et al., 2020). Its biological actions involving anti-inflammatory and anti-thrombotic properties play an important role in several diseases (Marquis-Gravel et al., 2019; Zheng and Roddick, 2019). So far, two main mechanisms have concerned the potential protective effect of aspirin use on cognitive decline and dementia in older adults. One of the mechanisms suggests that aspirin reduces the related pathology effect on AD, such as degraded levels of cerebral Aβ peptide, decreased tau phosphorylation, and improved synaptic plasticity (Ferreira et al., 2014). The other mechanism indicates that aspirin may maintain cerebral blood flow and prevent stroke in patients with ischemic cerebrovascular disease and thus protect cognitive function via inhibiting cerebral damage of vascular origin (Patrono, 2015). A recent report has investigated the effects of low-dose aspirin to enhance astrocytic lysosome biogenesis and function, which is a newly studied pathway to reduce amyloid pathology in AD (Melo, 2019).
Several observational studies reported that the use of aspirin buffers against cognitive decline or dementia when it is identified in continuous low dosage for elderly women (Kang and Grodstein, 2003; Kern et al., 2012). A systematic review and meta-analysis including longitudinal studies and randomized controlled trails (RCTs) demonstrated no benefit of aspirin in preventing cognition decline and dementia (Veronese et al., 2017). Furthermore, data from two RCTs in 2020 suggested a contrary opinion on the use of aspirin for the prevention of dementia (Matsumoto et al., 2020; Ryan et al., 2020).
In clinical practice, there is an unresolved question as to whether treatment with aspirin benefits or harms cognitive function in older people. This study therefore analyzed the currently available evidence to assess the possible effects of aspirin on the development of dementia, as well as its effects on cognitive function or impairment.
We searched PubMed, Embase, Web of Science, and the Cochrane Database between January 1, 2000 and April 11, 2020. The completed search for PubMed was as follows: “Aspirin” (MeSH terms) OR “Acetylsalicylic Acid” (title/abstract) OR “Acid, Acetylsalicylic” (title/abstract) OR “2-(Acetyloxy)benzoic Acid” (title/abstract) AND “Dementia” (title/abstract) OR “Alzheimer's” (title/abstract) OR “Alzheimer” (title/abstract) OR “Dementia, Vascular” (MeSH terms) OR “Dementias, Vascular” (title/abstract) OR “Vascular Dementias” (title/abstract) OR “Vascular Dementia” (title/abstract) OR “Cognitive” (title/abstract) OR “Cognition” (title/abstract) OR “Memory” (title/abstract) OR “Recall” (title/abstract) OR “MMSE” (title/abstract) OR “Mini mental state examination” (title/abstract) OR “Cognition” OR “Dementia” OR “Cognition Disorders” OR “Cognitive dysfunction” OR “Alzheimer disease” OR “Mental recall” (MeSH terms). The analysis was limited to humans, but we did not involve any language restriction. The investigators (HL and WL) independently examined titles and abstracts for eligibility. In case of disagreement, the full article was retrieved and the eligibility was discussed. We also reviewed cited references of the retrieved articles to identify additional published and unpublished studies. Reviews were also retrieved. In case of disagreement, the full article was retrieved and the eligibility was discussed.
The inclusion criteria for the study were as follows: cohort study and RCT design, population-based or community-based samples, reporting data on dementia and mild cognitive decline (MCI) diagnosed using validated criteria, and participants defined aspirin use in any dose and for any duration. Trials assessing the effect of aspirin vs. control (placebo in the RCTs or no intervention in cohort studies) were also included. Results, including odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) with corresponding 95% confidence intervals (CIs), or indirect data for the calculation of the risk estimates were noted.
The exclusion criteria for the study were as follows: participants who were diagnosed with MCI or dementia at baseline, use of active controls in the control group (non-placebo in the RCTs), studies with insufficient or overlapping data (the most recent and complete data were chosen), and the full text of relevant articles could not be obtained.
Collected data included general information (first author, publication year, study design, and region), participants' characteristics (age, sex, and sample size), dosage of aspirin, diagnostic criteria of dementia, MCI or cognitive change, follow-up period, results, and adjusted confounding factors. Two investigators independently extracted data and discussed any post-extraction discrepancies. When agreement could not be reached, a third reviewer was consulted.
Based on the extracted date, the Cochrane Risk of Bias Assessment Tool (Higgins et al., 2011) was used for RCTs that has five domains: randomization, deviation from intervention, missing data, measurement of outcome, and selective reporting. The nine-item Newcastle-Ottawa scale (Wells et al., 2000) was used for observational/non-randomized studies with domain selection, comparability, and exposure/outcome.
If available, pooled RRs, ORs, or HRs and 95% CIs were estimated as the ratio of the association between aspirin exposure and the risk of dementia and MCI. Because the absolute risk of dementia was low in all populations, the three measures were expected to be similar estimates of relative RRs (Greenland, 1987; Adami et al., 2008).
Meta-analysis was conducted using the Stata software, version 14.0 (Stata, College Station, TX, USA). The effect size (ES) of pooled RRs with 95% CIs was calculated to evaluate the effect of aspirin on cognitive function and dementia. Heterogeneity was assessed using the Cochran's Q statistic and quantified using the I2 tests with an I2 > 50% indicating moderate-to-high heterogeneity (Higgins et al., 2003). Publication bias was assessed by Egger's weighted regression test and Begg's rank correlation test (P < 0.05) (Begg and Mazumdar, 1994; Egger et al., 1997).
The searches returned 3,193 articles. After excluding 643 duplicates, two reviewers separately read titles and abstracts and excluded 2,550 irrelevant citations. Twenty-nine articles were examined for full-text review, and 15 studies were included in this meta-analysis (Figure 1). Twelve included studies were cohort studies (Zandi and Breitner, 2001; Jonker et al., 2003; Kang and Grodstein, 2003; Nilsson et al., 2003; Cornelius et al., 2004; Arvanitakis et al., 2008; Szekely et al., 2008; Kern et al., 2012; Kelley et al., 2015; Chang et al., 2016; Wichmann et al., 2016; Yang et al., 2020) and three RCTs (Kang et al., 2007; Matsumoto et al., 2020; Ryan et al., 2020). Studies were conducted in the US (Zandi and Breitner, 2001; Kang and Grodstein, 2003; Kang et al., 2007; Arvanitakis et al., 2008; Szekely et al., 2008; Kelley et al., 2015; Wichmann et al., 2016), Europe (Jonker et al., 2003; Nilsson et al., 2003; Cornelius et al., 2004; Kern et al., 2012), and Asia (Chang et al., 2016; Matsumoto et al., 2020; Yang et al., 2020). However, only one RCT (Ryan et al., 2020) included both American and Australian data. Two of the studies were from Taiwan (Chang et al., 2016; Yang et al., 2020) and extracted their data from the National Health Insurance Research Database, but the investigators used different study populations and separate cohorts.
This meta-analysis included 100,909 participants (62.9% females), and the mean age was >65 years. The sample sizes varied from 612 to 23,915. The follow-up period ranged from 2 to 15 years. Most of the studies adjusted for important confounders, such as age, sex, and education (Tables 1, 2). Critical appraisal of the included studies indicated that studies in most areas were rigorous, except that some cohort studies had potentially influential differences between groups at baseline and insufficient details on loss to follow-up (Supplementary Material).
Twelve cohort studies (10 prospective studies and two retrospective studies) investigated the association between aspirin use and the risk of dementia or MCI (Zandi and Breitner, 2001; Jonker et al., 2003; Kang and Grodstein, 2003; Nilsson et al., 2003; Cornelius et al., 2004; Arvanitakis et al., 2008; Szekely et al., 2008; Kern et al., 2012; Kelley et al., 2015; Chang et al., 2016; Wichmann et al., 2016; Yang et al., 2020). Overall, when adjusted for confounders, aspirin use showed no significant decreased risk of developing dementia or MCI (the pooled RR = 0.97; 95% CI = 0.85–1.11; = 65%) (Figure 2). There were methodological heterogeneities due to differences in the study type (prospective vs. retrospective studies), follow-up duration, and dosage.
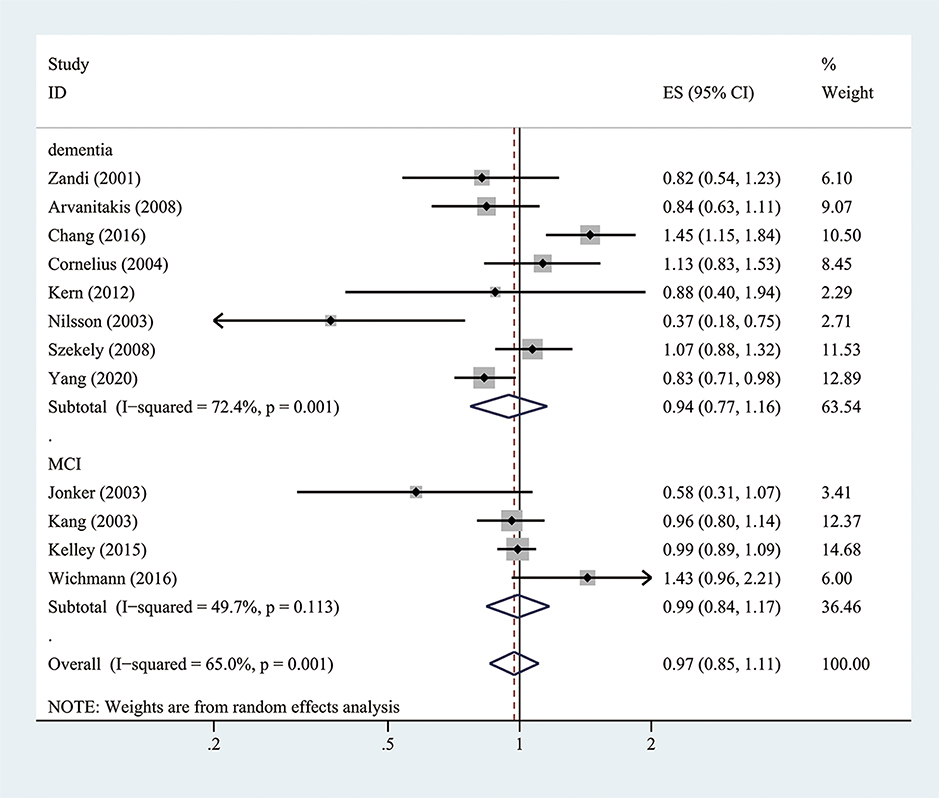
Figure 2. Association of aspirin use with incident dementia or mild cognitive decline in cohort studies.
However, after adjusting for low-dose aspirin, the results showed a protective effect against the risk of dementia and MCI (the pooled RR = 0.75; 95% CI = 0.63–0.9; = 50.5%) (Figure 3). For subgroup analysis stratified by the subtype of cognitive impairment, the results indicated that low-dose aspirin use had a protective effect against all-cause dementia (the pooled RR = 0.82; 95% CI = 0.71–0.96) and AD (the pooled RR = 0.54; 95% CI = 0.33–0.89), but not in MCI (the pooled RR = 0.58; 95% CI = 0.31–1.08) (Figure 3).
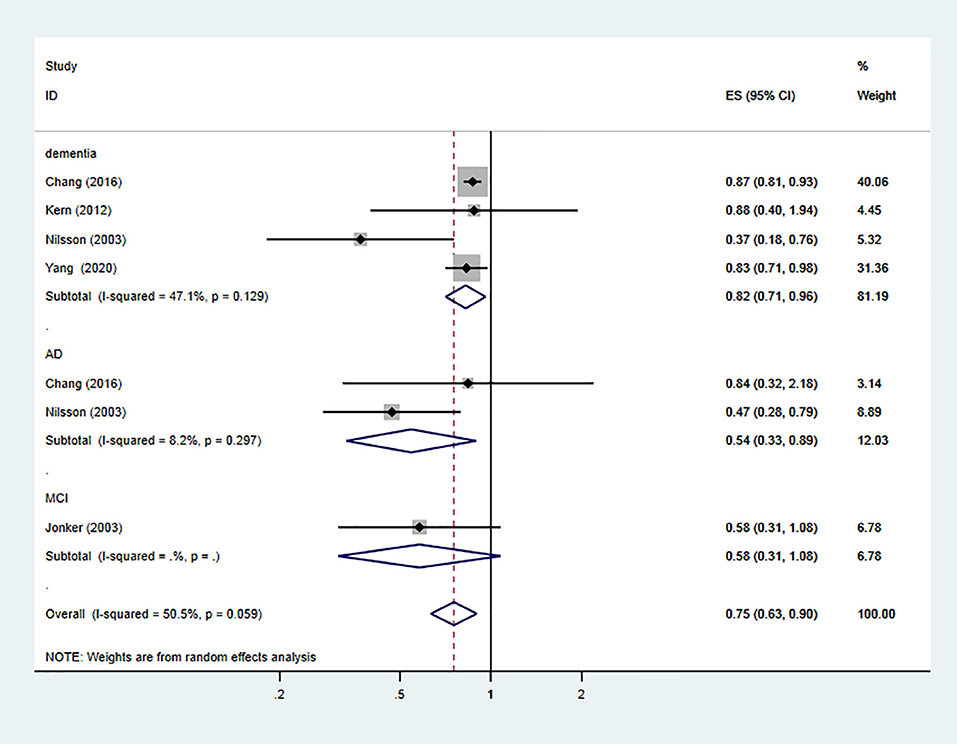
Figure 3. Association of low-dose aspirin use with incident dementia or mild cognitive decline in cohort studies.
The three RCT (Kang et al., 2007; Matsumoto et al., 2020; Ryan et al., 2020) showed that low-dose aspirin use did not have a positive effect on reducing MCI or dementia (the pooled RR = 0.94; 95% CI = 0.84–1.05; = 0.0%) (Figure 4). However, the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) trial (Matsumoto et al., 2020) showed that use of low-dose aspirin resulted in a 42% lower risk of dementia compared to the placebo group in females with diabetes (P = 0.03).
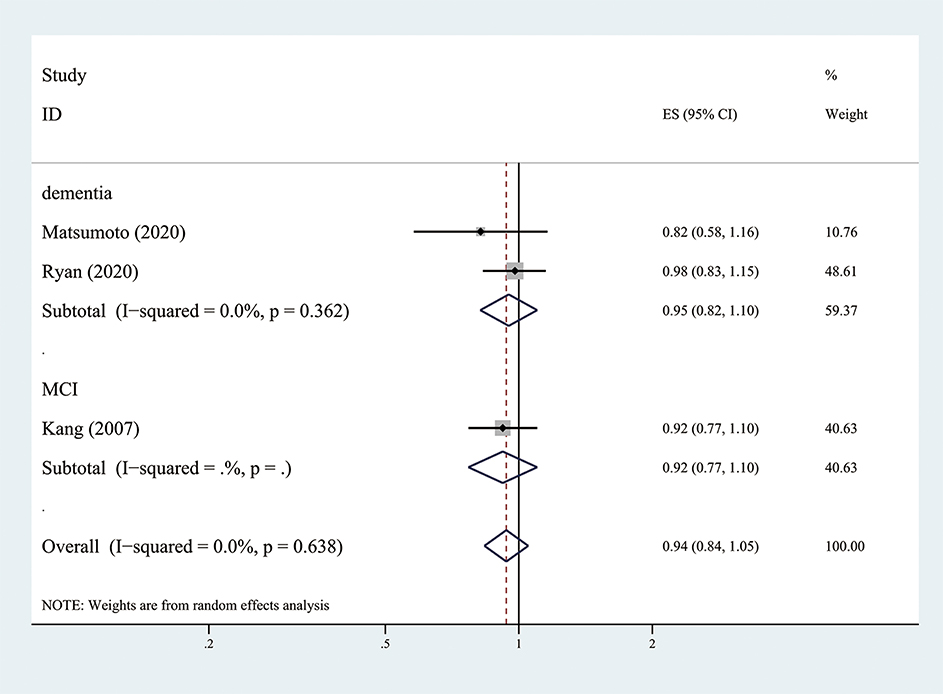
Figure 4. Association of low-dose aspirin use with incident dementia or mild cognitive decline in randomized controlled trails.
Twelve cohort studies (P = 0.837 and P = 0.923) and three RCTs (P = 0.296 and P = 0.280) according to Begg's and Egger's tests, respectively (Figures 5A,B), suggested no evidence of publication bias in all publications.
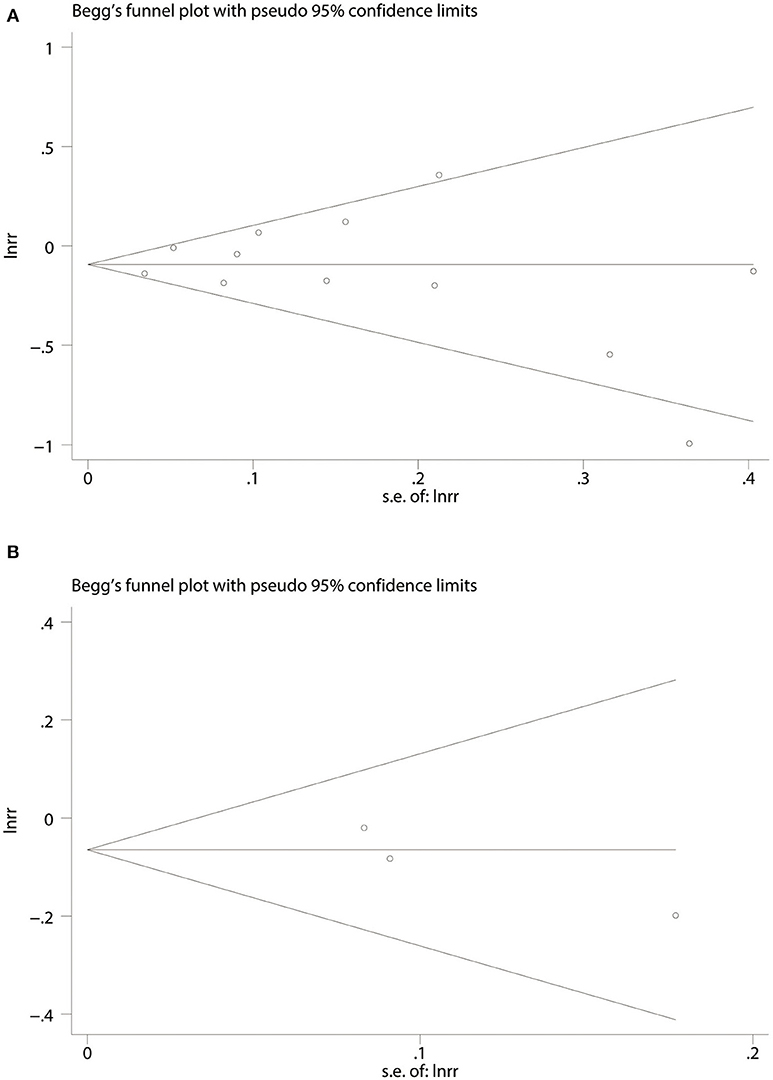
Figure 5. Begg's funnel plot for association of aspirin use with incident dementia or mild cognitive decline in twelve cohort studies (A). Three randomized controlled trails (B). RR, relative risk; lnrr, the Napierian logarithm of relative risk; s.e. of lnrr, standard error of lnrr.
Our results of 15 pooled studies indicated that the use of any dose of aspirin was not associated with a lower risk of developing dementia or MCI. When restricted to adjusting the dosage for low-dose aspirin, it was associated with a 25% (95% CI = 10–37) reduction in the risk of incident dementia and MCI in five cohort studies. Nevertheless, across RCTs, the results did not support the use of low-dose aspirin to improve general cognitive test results or dementia incidence among the elderly population.
In cohort studies, discrepancies regarding the effects of different aspirin dosages suggested that low-dose aspirin might have a protective effect against dementia and cognitive decline through two main mechanisms: (i) effect on AD-related pathology and (ii) protection against vascular accidents that may subsequently generate VaD or vascular cognitive impairment (VCI).
Aspirin reduces the related pathology effect on AD. First, in animal models, because platelets are the main source of systemic Aβ and amyloid precursor protein (APP), low-dose aspirin might directly reduce the amount of circulating Aβ derived from platelets (Bush et al., 1990; Inyushin et al., 2019). Chandra et al. (2018) reported a new function of low-dose aspirin in mediating peroxisome proliferator-activated receptor alpha (PPARα), which upregulated transcription factor EB, leading to increased astrocytic lysosome biogenesis and functioning in the brain, leading to enhanced clearance of Aβ. Second, Khezrian et al. (2020) and Sato et al. (2004) reported that low-dose aspirin use might slow the progression or occurrence of white matter lesions (WMLs) and might help to preserve cognition in the elderly population. WMLs are common findings in AD (Richard et al., 2010), and Ferrari et al. (2018) reported that low-dose aspirin therapy slowed AD progression, especially among non-apolipoprotein E allele carriers via probable modulation of the WML burden on AD patients.
Aspirin has a protective effect against vascular accidents that may lead to VaD or vascular cognitive decline. First, experimental data showed that low-dose aspirin mainly resulted in an anti-platelet effect, which might play an important role in the protection of cognitive function by enhancing the cerebral blood flow in the cognitive area, to prevent transient cerebral ischemic attacks and maintain blood–brain barrier integrity (Ridker et al., 1996; Patrono, 2015). In addition, the limited anti-inflammatory action of low-dose aspirin may help reduce neurodegenerative diseases, which does not necessarily require inhibition of COX-2 in inflammatory cells, which occurs with high daily doses of aspirin, but it may simply reflect its anti-platelet effect (Patrignani and Patrono, 2015). Furthermore, aspirin-triggered lipoxins reduce the impact of neuroinflammation on the development of cerebral small vessel disease (cSVD) by mitigating the neurotoxicity and stimulating alternative activation of microglia (Kaiser et al., 2014; Patrono, 2015). Finally, translational models indicated aspirin may maintain cerebral blood flow and prevent stroke in patients with ischemic cerebrovascular disease, thereby protecting against VCI and VaD by inhibiting vasogenic brain damage (Hainsworth et al., 2017).
Importantly, previous studies have shown that the pathology of typical dementia occurs 20 years before the clinical onset (Reiman et al., 2012), so low-dose aspirin may produce a sustained protective effect in participants only if they have taken aspirin for a sufficient duration, which probably explains the negative effect of pooled RCTs on the prevention of dementia and cognitive dysfunction. One of the RCTs, the ASPREE trial (Ryan et al., 2020), found that low-dose aspirin use showed no significant decrease in the risk of developing dementia, with a median follow-up period of 4.7 years, just 5 months ahead of schedule. However, the JPAD trial reported a positive effect in females with a median follow-up of 11.4 years, and Matsumoto et al. (2020) reported that the cumulative incidence of dementia increased significantly during a follow-up of 8 years. These findings contribute to the existing literature that has minimized biases about low-dose aspirin therapy because of insufficient treatment durations, facilitating stronger conclusions about the specificity of low-dose aspirin for incident dementia and cognitive changes.
Furthermore, some important confounding and potential risk factors should be considered. For example, previous studies have shown that poor socioeconomic status (Prince et al., 2012), social relationships (Kuiper et al., 2015), and behavioral lifestyle (Kivipelto et al., 2018) might affect the development of dementia and cognitive decline. Recently, the Harvard Aging Brain Study (Biddle et al., 2020) suggested that widowhood was an underrecognized risk factor associated with AD-related cognitive decline and impairment. In addition, a large nationwide case-control study (Savolainen-Peltonen et al., 2019) and RCT (Shumaker et al., 2004) indicated that postmenopausal hormone therapy could be a potential risk factor for cognitive change in elderly females. The actual effects of aspirin might therefore be understated or overstated by these potential confounding or underrecognized risk factors.
To the best of our knowledge, this meta-analysis is the most comprehensive one in the field of evidence-based medicine (EMB). In 2000, Rands and Orrell (2000) failed to select the eligible RCTs for an intervention review between aspirin and VaD. In 2015, Wang et al. (2015) conducted a meta-analysis of eight cohort studies and three case-control studies characterizing the association of aspirin use and AD risk, which concluded that there was a significant reduction in the risk of AD. In 2017, Veronese et al. (2017) summarized the data from five cohort studies and three RCTs, to show no protective effect of low-dose aspirin on cognitive function; however, only limited studies were involved. Compared to previous studies, our updated meta-analysis did not include case-control studies because they were less adept at showing a causal relationship than cohort studies or RCTs, and case-control studies were more prone to bias, especially when reporting bias and recall bias. Furthermore, the meta-analysis provides more comprehensive evidence, including a relatively larger number of studies (n = 15), which were not included in previous studies. Additionally, according to the Cochrane handbook, we conducted a rigorous and extensive literature search for all eligible studies in large databases, including PubMed, Embase, Web of Science, and the Cochrane Database. Finally, we did not use any language restriction in retrieval processing, so it is unlikely that we missed important articles.
However, there are several potential limitations, which have to be addressed in interpreting the results of our meta-analysis. First, the possibility of selection bias in observational studies included the fact that a percentage of participants that replaced aspirin with non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs), or other active drugs, might have created another important bias. Second, in cohort studies, although we conducted a subgroup analysis stratified by the subtypes of dementia (all-cause dementia and AD), it failed to analyze VaD, so the limited number of studies of each outcome and the effects of aspirin might be different depending on the subtype. Third, variability in diagnostic criteria of dementia and cognitive decline between data sets may have affected our results. Finally, although the important potential confounders were adjusted in the pooled analyses, statistical adjustment for the studies may not entirely resolve these problems. For example, the indications for aspirin may be different across countries, other comorbidities, and other medications, including anti-hyperglycemic drugs, antidepressants, and non-aspirin NSAIDs, which may influence the conclusions.
Our meta-analysis of observational studies and a single RCT indicated that low-dose aspirin use over long periods might protect against the occurrence of dementia and MCI. However, all pooled RCTs did not support a preventive effect. Taking into account the controversial results between observational studies and RCTs, well-designed studies and innovative approaches are required in future long-term studies to determine whether low-dose aspirin has the ability to decrease the prevalence of MCI and dementia.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
HL drafted the rough manuscript. R-WZ and WL designed the study. Literature search and selection were conducted by XZ and X-CM. All authors have read and approved the final manuscript.
This study was supported by the National Key R&D Program of China (grant no. 2018YFC1311600) and the Liaoning Revitalization Talents Program (grant no. XLYC1808036).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.578071/full#supplementary-material
Adami, H. O., Hunter, D., and Trichopoulos, D. (2008). Textbook of Cancer Epidemiology. New York, NY: Oxford University Press. doi: 10.1093/acprof:oso/9780195311174.001.0001
Aihw (2012). Improving Dementia Data in Australia: Supplement to Dementia in Australia 2012. Available online at: https://www.aihw.gov.au/reports/dementia/improving-dementia-data-in-australia-supplement-to-dementia-in-australia-2012/formats (accessed February 18, 2020).
Arvanitakis, Z., Grodstein, F., Bienias, J. L., Schneider, J. A., Wilson, R. S., Kelly, J. F., et al. (2008). Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology 70, 2219–2225. doi: 10.1212/01.wnl.0000313813.48505.86
Begg, C. B., and Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. doi: 10.2307/2533446
Biddle, K. D., Jacobs, H. I. L., D'oleire Uquillas, F., Zide, B. S., Kirn, D. R., Properzi, M. R., et al. (2020). Associations of widowhood and beta-amyloid with cognitive decline in cognitively unimpaired older adults. JAMA Netw. Open 3:e200121. doi: 10.1001/jamanetworkopen.2020.0121
Bush, A. I., Martins, R. N., Rumble, B., Moir, R., Fuller, S., Milward, E., et al. (1990). The amyloid precursor protein of Alzheimer's disease is released by human platelets. J. Biol. Chem. 265, 15977–15983.
Chandra, S., Jana, M., and Pahan, K. (2018). Aspirin induces lysosomal biogenesis and attenuates amyloid plaque pathology in a mouse model of Alzheimer's disease via PPARalpha. J. Neurosci. 38, 6682–6699. doi: 10.1523/JNEUROSCI.0054-18.2018
Chang, C. W., Horng, J. T., Hsu, C. C., and Chen, J. M. (2016). Mean daily dosage of aspirin and the risk of incident Alzheimer's dementia in patients with Type 2 diabetes mellitus: a nationwide retrospective cohort study in Taiwan. J. Diabetes Res. 2016:9027484. doi: 10.1155/2016/9027484
Cornelius, C., Fastbom, J., Winblad, B., and Viitanen, M. (2004). Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology 23, 135–143. doi: 10.1159/000075957
Egger, M., Smith, G. D., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Ferrari, C., Lombardi, G., Polito, C., Lucidi, G., Bagnoli, S., Piaceri, I., et al. (2018). Alzheimer's disease progression: factors influencing cognitive decline. J. Alzheimers. Dis. 61, 785–791. doi: 10.3233/JAD-170665
Ferreira, S. T., Clarke, J. R., Bomfim, T. R., and De Felice, F. G. (2014). Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer's disease. Alzheimer's Dementia. 10, S76–S83. doi: 10.1016/j.jalz.2013.12.010
Greenland, S. (1987). Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 9, 1–30. doi: 10.1093/oxfordjournals.epirev.a036298
Hainsworth, A. H., Allan, S. M., Boltze, J., Cunningham, C., Farris, C., Head, E., et al. (2017). Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 15:16. doi: 10.1186/s12916-017-0793-9
Harrison, S. L., Ding, J., Tang, E. Y., Siervo, M., Robinson, L., Jagger, C., et al. (2014). Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS ONE 9:e114431. doi: 10.1371/journal.pone.0114431
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327:557. doi: 10.1136/bmj.327.7414.557
Inyushin, M., Zayas-Santiago, A., Rojas, L., Kucheryavykh, Y., and Kucheryavykh, L. (2019). Platelet-generated amyloid beta peptides in Alzheimer's disease and glaucoma. Histol. Histopathol. 34, 843–856. doi: 10.14670/HH-18-111
Jonker, C., Comijs, H. C., and Smit, J. H. (2003). Does aspirin or other NSAIDs reduce the risk of cognitive decline in elderly persons? Results from a population-based study. Neurobiol. Aging 24, 583–588. doi: 10.1016/S0197-4580(02)00188-4
Jordan, F., Quinn, T. J., Mcguinness, B., Passmore, P., Kelly, J. P., Tudur Smith, C., et al. (2020). Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst. Rev. 4:CD011459. doi: 10.1002/14651858.CD011459.pub2
Kaiser, D., Weise, G., Möller, K., Scheibe, J., Pösel, C., Baasch, S., et al. (2014). Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol. Commun. 2:169. doi: 10.1186/s40478-014-0169-8
Kang, J. H., Cook, N., Manson, J., Buring, J. E., and Grodstein, F. (2007). Low dose aspirin and cognitive function in the women's health study cognitive cohort. Br. Med. J. 334, 987–990. doi: 10.1136/bmj.39166.597836.BE
Kang, J. H., and Grodstein, F. (2003). Regular use of nonsteroidal anti-inflammatory drugs and cognitive function in aging women. Neurology 60, 1591–1597. doi: 10.1212/01.WNL.0000065980.33594.B7
Kelley, B. J., Mcclure, L. A., Unverzagt, F. W., Kissela, B., Kleindorfer, D., Howard, G., et al. (2015). Regular aspirin use does not reduce risk of cognitive decline. J. Am. Geriatr. Soc. 63, 390–392. doi: 10.1111/jgs.13271
Kern, S., Skoog, I., Östling, S., Kern, J., and Börjesson-Hanson, A. (2012). Does low-dose acetylsalicylic acid prevent cognitive decline in women with high cardiovascular risk? A 5-year follow-up of a non-demented population-based cohort of Swedish elderly women. BMJ Open 2:e001288. doi: 10.1136/bmjopen-2012-001288
Khezrian, M., Waymont, J. M. J., Myint, P. K., Mcneil, C. J., Whalley, L. J., Staff, R., et al. (2020). Aspirin moderates the association between cardiovascular risk, brain white matter hyperintensity total lesion volume and processing speed in normal ageing. Maturitas 133, 49–53. doi: 10.1016/j.maturitas.2020.01.001
Kivipelto, M., Mangialasche, F., and Ngandu, T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666. doi: 10.1038/s41582-018-0070-3
Kuiper, J. S., Zuidersma, M., Oude Voshaar, R. C., Zuidema, S. U., Van Den Heuvel, E. R., Stolk, R. P., et al. (2015). Social relationships and risk of dementia: a systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 22, 39–57. doi: 10.1016/j.arr.2015.04.006
Marquis-Gravel, G., Roe, M. T., Harrington, R. A., Munoz, D., Hernandez, A. F., and Jones, W. S. (2019). Revisiting the role of aspirin for the primary prevention of cardiovascular disease. Circulation 140, 1115–1124. doi: 10.1161/CIRCULATIONAHA.119.040205
Matsumoto, C., Ogawa, H., Saito, Y., Okada, S., Soejima, H., Sakuma, M., et al. (2020). Sex difference in effects of low-dose aspirin on prevention of dementia in patients with Type 2 Diabetes: a long-term follow-up study of a randomized clinical trial. Diabetes Care 43, 314–320. doi: 10.2337/dc19-1188
Melo, H. M. (2019). Potential effects of aspirin on lysosomal biogenesis and amyloid-beta clearance: an old drug and novel insights in Alzheimer's Disease therapy. J. Neurosci. 39, 197–198. doi: 10.1523/JNEUROSCI.2283-18.2018
Nilsson, S. E., Johansson, B., Takkinen, S., Berg, S., Zarit, S., Mcclearn, G., et al. (2003). Does aspirin protect against Alzheimer's dementia? A study in a Swedish population-based sample aged ≥80 years. Eur. J. Clin. Pharmacol. 59, 313–319. doi: 10.1007/s00228-003-0618-y
Patrignani, P., and Patrono, C. (2015). Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim. Biophys. Acta 1851, 422–432. doi: 10.1016/j.bbalip.2014.09.016
Patrono, C. (2015). The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J. Am. Coll. Cardiol. 66, 74–85. doi: 10.1016/j.jacc.2015.05.012
Prince, M., Acosta, D., Ferri, C. P., Guerra, M., Huang, Y., Rodriguez, J. J. L., et al. (2012). Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet 380, 50–58. doi: 10.1016/S0140-6736(12)60399-7
Rands, G., and Orrell, M. (2000). Aspirin for vascular dementia. Cochrane Database Syst. Rev. 4:CD001296. doi: 10.1002/14651858.CD001296
Reiman, E. M., Quiroz, Y. T., Fleisher, A. S., Chen, K., Velez-Pardo, C., Jimenez-Del-Rio, M., et al. (2012). Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 11, 1048–1056. doi: 10.1016/S1474-4422(12)70228-4
Richard, E., Gouw, A. A., Scheltens, P., and Van Gool, W. A. (2010). Vascular care in patients with Alzheimer disease with cerebrovascular lesions slows progression of white matter lesions on MRI: the evaluation of vascular care in Alzheimer's disease (EVA) study. Stroke 41, 554–556. doi: 10.1161/STROKEAHA.109.571281
Ridker, P. M., Hennekens, C. H., Tofler, G. H., Lipinska, I., and Buring, J. E. (1996). Anti-platelet effects of 100 mg alternate day oral aspirin: a randomized, double-blind, placebo-controlled trial of regular and enteric coated formulations in men and women. J. Cardiovasc. Risk 3, 209–212. doi: 10.1097/00043798-199604000-00013
Ryan, J., Storey, E., Murray, A. M., Woods, R. L., Wolfe, R., Reid, C. M., et al. (2020). Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. 95, 320–331. doi: 10.1212/WNL.0000000000009277
Sanford, A. M. (2017). Mild cognitive impairment. Clin. Geriatr. Med. 33, 325–337. doi: 10.1016/j.cger.2017.02.005
Sato, H., Koretsune, Y., Fukunami, M., Kodama, K., Yamada, Y., Fujii, K., et al. (2004). Aspirin attenuates the incidence of silent brain lesions in patients with nonvalvular atrial fibrillation. Circ. J. 68, 410–416. doi: 10.1253/circj.68.410
Savolainen-Peltonen, H., Rahkola-Soisalo, P., Hoti, F., Vattulainen, P., Gissler, M., Ylikorkala, O., et al. (2019). Use of postmenopausal hormone therapy and risk of Alzheimer's disease in Finland: nationwide case-control study. BMJ 364:l665. doi: 10.1136/bmj.l665
Shumaker, S. A., Legault, C., and Kuller, L. H. (2004). Women's health initiative memory study. conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. JAMA 291, 2947–2958. doi: 10.1001/jama.291.24.2947
Szekely, C. A., Breitner, J. C. S., Fitzpatrick, A. L., Rea, T. D., Psaty, B. M., Kuller, L. H., et al. (2008). NSAID use and dementia risk in the cardiovascular health study: role of APOE and NSAID type. Neurology 70, 17–24. doi: 10.1212/01.wnl.0000284596.95156.48
Thal, D. R., Griffin, W. S., and Braak, H. (2008). Parenchymal and vascular Abeta-deposition and its effects on the degeneration of neurons and cognition in Alzheimer's disease. J. Cell. Mol. Med. 12, 1848–1862. doi: 10.1111/j.1582-4934.2008.00411.x
Veronese, N., Stubbs, B., Maggi, S., Thompson, T., Schofield, P., Muller, C., et al. (2017). Low-dose aspirin use and cognitive function in older age: a systematic review and meta-analysis. J. Am. Geriatr. Soc. 65, 1763–1768. doi: 10.1111/jgs.14883
Wang, J., Tan, L., Wang, H.-F., Tan, C.-C., Meng, X.-F., Wang, C., et al. (2015). Anti-inflammatory drugs and risk of Alzheimer's disease: an updated systematic review and meta-analysis. J. Alzheimers Dis. 44, 385–396. doi: 10.3233/JAD-141506
Wells, G. A., Shea, B., O'connell, D., Peterson, J., Welch, V., Losos, M., et al. (2000). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed April 15, 2020).
Whalley, L. J., and Mowat, D. H. (2007). Aspirin and cognitive function. BMJ 334, 961–962. doi: 10.1136/bmj.39204.473252.80
WHO (2019). World Alzheimer Report 2019: Risk Reduction of Cognitive Decline and Dementia. Available online at: https://www.who.int/publications/i/item/risk-reduction-of-cognitive-decline-and-dementia (accessed March 21, 2019).
Wichmann, M. A., Cruickshanks, K. J., Carlsson, C. M., Chappell, R., Fischer, M. E., Klein, B. E., et al. (2016). nsaid use and incident cognitive impairment in a population-based cohort. Alzheimer Dis. Assoc. Disord. 30, 105–112. doi: 10.1097/WAD.0000000000000098
Yang, Y. H., Chiu, C. C., Teng, H. W., Huang, C. T., Liu, C. Y., and Huang, L. J. (2020). Aspirin and risk of dementia in patients with late-onset depression: a population-based cohort study. Biomed Res. Int. 2020:1704879. doi: 10.1155/2020/1704879
Zandi, P. P., and Breitner, J. C. S. (2001). Do NSAIDs prevent Alzheimer's disease? And, if so, why? The epidemiological evidence. Neurobiol Aging 22, 811–817. doi: 10.1016/S0197-4580(01)00297-4
Keywords: aspirin, dementia, mild cognitive decline, meta-analysis, association
Citation: Li H, Li W, Zhang X, Ma X-C and Zhang R-W (2021) Aspirin Use on Incident Dementia and Mild Cognitive Decline: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 12:578071. doi: 10.3389/fnagi.2020.578071
Received: 30 June 2020; Accepted: 27 October 2020;
Published: 04 February 2021.
Edited by:
Lakshmi Rajagopal, Northwestern University, United StatesReviewed by:
Anna Villar-Piqué, Institut d'Investigacio Biomedica de Bellvitge (IDIBELL), SpainCopyright © 2021 Li, Li, Zhang, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong-Wei Zhang, cm9uZ3dlaXpoYW5nQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.