- 1Department of Dermatology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Institute of Dermatology, Shanghai Academy of Traditional Chinese Medicine, Shanghai, China
- 4Department of Dermatology, Songjiang Hospital Affiliated to Shanghai Jiao Tong University, Jiao Tong University School of Medicine, Shanghai, China
Background: Psoriasis and dementia are both inflammatory diseases. The association between psoriasis and dementia has rarely been investigated, and the existing results are conflicting. Thus, we conducted this study to evaluate whether an association exists between psoriasis and dementia.
Methods: We searched for studies from six databases from inception to July 30, 2020, using subject and free words. RevMan 5.4 was used to calculate the risk ratio (RR) of dementia in the subjects with psoriasis. When heterogeneity was present, a random-effects model was used. Subgroup, sensitivity, and meta-regression analyses were performed using Stata 15.1.
Results: Nine studies were identified and included in the study, of which seven that involved a total of 3,638,487 participants were included in the meta-analysis. We found that among the patients with psoriasis (RR: 1.14, 95% confidence interval [CI]: 1.06–1.24, p = 0.0009) and psoriatic arthritis (RR: 2.20, 95% CI: 1.29–3.78, p = 0.004), the proportions of those with non-vascular dementia (RR: 1.13, 95% CI: 1.11–1.15, p < 0.00001) and vascular dementia (RR: 1.41, 95% CI: 1.09–1.82, p = 0.009) were higher than that among the patients without psoriasis. Those with dementia were also more likely to develop psoriasis, and those with severe psoriasis were less likely to die from dementia (RR: 1.88, 95% CI: 0.72–4.90, p = 0.020). The meta-regression analysis did not show any significant sources of heterogeneity.
Conclusions: The patients with psoriasis and psoriatic arthritis show high prevalence of different types of dementia. Based on the findings of this study, dementia may not be considered a high-risk factor of death from severe psoriasis. However, identification of this potential risk allows for early intervention, thereby reducing comorbidities and deaths.
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin disease (Menter et al., 2019) that affects 0.5–11.4% of adults and 1.4% of children worldwide (Fu et al., 2018). It is characterized by recurrent erythema and scaly skin accompanied by varying degrees of pruritus (Li et al., 2015). This systemic inflammatory disease negatively affects patients by reducing their quality of life (Innamorati et al., 2016). Dementia, a neurological syndrome defined as a clinical symptom of progressive cognitive impairment, which mainly occurs in the elderly (Lin et al., 2019). Alzheimer's disease (AD) and vascular dementia are the two most common types of dementia. Currently, the pathogenesis of dementia is still unclear. Some studies indicate that its onset is related to aging, family history of the disease, inflammation, genetic susceptibility, immunization, and mild cognitive impairment (Forlenza et al., 2009; Zhang and Jiang, 2015; Yokoyama et al., 2016; Wotton and Goldacre, 2017).
Psoriasis and dementia are both inflammatory and immune diseases (Sardi et al., 2011). In psoriasis, CD4+ T cells differentiate into various types of T cells (including Th1, Th2, Th17, and Treg cells) after inflammatory stimulation (Stadhouders et al., 2018). Further, activated dendritic cells, a kind of antigen-presenting cell, secrete interleukin (IL) 23 and tumor necrosis factor alpha (TNF-α) to induce the activation of Th and Treg cells and the overexpression of inflammatory cytokines such as IL-17, IL-22, and interferon gamma (Jadali and Eslami, 2014; Lowes et al., 2014). These cytokines migrate to the epidermis and act on keratinocytes to participate in the pathogenesis of psoriasis. A study from Germany showed that the peripheral immune system of patients with dementia changed. Whether in Alzheimer's disease, vascular dementia, or frontotemporal dementia, the numbers of B and T cells are reduced (Busse et al., 2017). A recent cohort study compared the relationship between immune system imbalance and the risk of dementia (van der Willik et al., 2019). They used granulocytes and platelets to represent innate immunity and lymphocytes to represent adaptive immunity. The association between innate and adaptive immunities is reflected by three indicators, namely granulocyte-to-lymphocyte ratio (GLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII). Results showed that increased risk of dementia was associated with increased granulocyte and platelet counts, whereas decreased risk of dementia was associated with increased lymphocyte count. Increased GLR, PLR, and SII were also associated with increased risk of dementia (including AD and vascular dementia). After the immune system is activated, it secretes inflammatory factors to cause an inflammatory cascade. It is manifested as changes in the expression levels of IL-6, IL-12, IL-23, and other inflammatory cytokines in AD (Heppner et al., 2015) and IL-17A, IL-2, and IL-8 in Lewy body dementia (Surendranathan et al., 2018).
Recently, an increasing number of studies have found a relationship between psoriasis and dementia, with inconsistent results. Therefore, we conducted this research to review and analyze the current evidence, with the aim of investigating the correlation between the two diseases.
Materials and Methods
Eligibility Criteria
Articles were included when they met the following criteria: (1) observational research, including cohort, case-control, and cross-sectional studies; (2) any article reporting the association of psoriasis with dementia or dementia-related diseases, with the exact population or incidence/prevalence included; and (3) studies without restrictions according to country and sex.
Articles were excluded when they met any of the following criteria: (1) review articles, experimental studies, randomized controlled trials, or repeated articles; (2) studies without original data; and (3) studies with erroneous/incorrect statistical methods.
Information Sources and Search Strategy
The PubMed, Embase, China National Knowledge Infrastructure database, Wanfang Data Knowledge Service Platform, the Chinese Scientific Journals Full Text Database (CQVIP), and China Biology Medicine disc (CBMdisc) were searched from their date of inception to July 30, 2020. Subject words and free words, including “psoriasis,” “dementia,” “prevalence,” and “observational study,” were combined during study retrieval. These terms were translated into Chinese and then searched in the Chinese database. We also searched gray literature in the OpenGrey database (www.opengrey.eu).
Data Extraction and Quality Assessment
The following data were extracted independently by two authors (YQ and XJD): first author, publication year, country of study, study design, diagnostic criteria of psoriasis and dementia, numbers of cases and controls, and mean age. Differences in the extracted data were resolved after discussion with a third author (XL). Two researchers (YL and YR) independently assessed the quality of all the observational studies. For the cohort and case-control studies, the Newcastle-Ottawa Scale (Wells et al., 2000) was applied to evaluate the selection, comparability, and outcome/exposure in each study. The scale score ranged from 0 to 9 points. Studies with scores of ≥ 7 points were considered to have a low risk; 4–6 points, moderate risk; and <3 points, high risk. For the cross-sectional studies, the Agency for Healthcare Research and Quality (AHRQ) tool (Viswanathan et al., 2012; Zeng et al., 2015) was used to assess the risk of bias by answering the 11-item questions with “Yes,” “No,” or “Unclear.” When the proportion of “Yes” answers was high among the items, the risk of bias of the study was considered low. When the two researchers had any disagreement, consensus was reached through discussion.
Data Analyses
A meta-analysis was performed using RevMan 5.4 and Stata 15.1. Risk ratios (RRs) with 95% confidence intervals (CIs) were calculated for dichotomous data, while mean differences with 95% CIs were summarized for continuous data. I2 values were used to analyze the extent of heterogeneity. An I2 value of <50% indicated homogeneity, and a fixed-effects model was applied. An I2 > 50% indicated substantial heterogeneity; thus, meta-regression, subgroup, and sensitivity analyses were performed to evaluate the possible sources of the heterogeneity. Egger's and Begg's linear regression tests were used to assess publication bias. A p < 0.10 in the funnel plot indicated a publication bias.
Results
Characteristics of the Pooled Studies
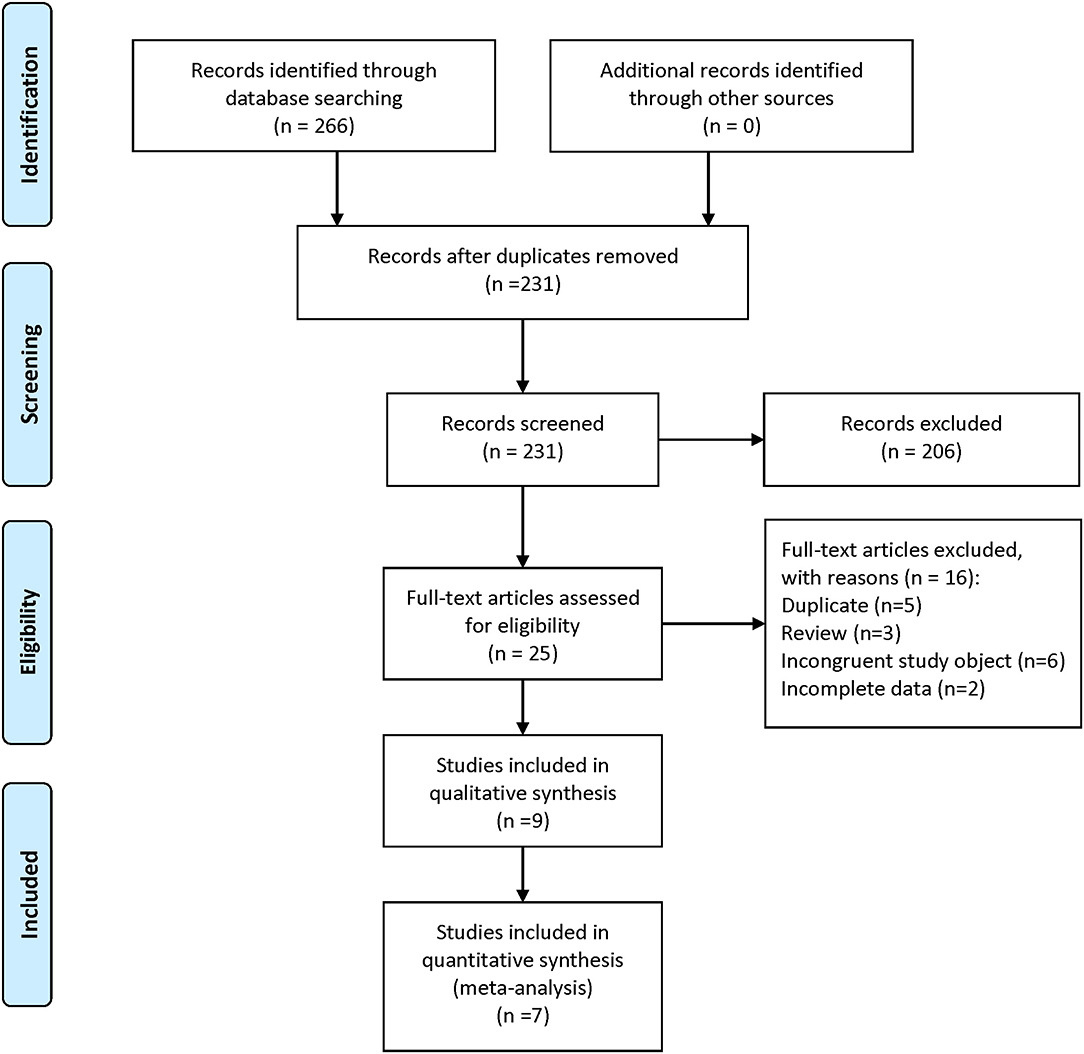
A total of 266 articles were retrieved in the initial search. After removing duplicates, 231 articles remained and were screened by their titles and abstracts. Thereafter, 25 studies were thought to be eligible and evaluated for full-text review. Sixteen studies were excluded, and the nine remaining studies (Abuabara et al., 2010; Feldman et al., 2015; Chen et al., 2018; Mitchell et al., 2018; Pezzolo et al., 2018; Huang et al., 2019; Leisner et al., 2019; Lin et al., 2019; Kim et al., 2020) were included. The search strategy and study selection are shown in Figure 1.
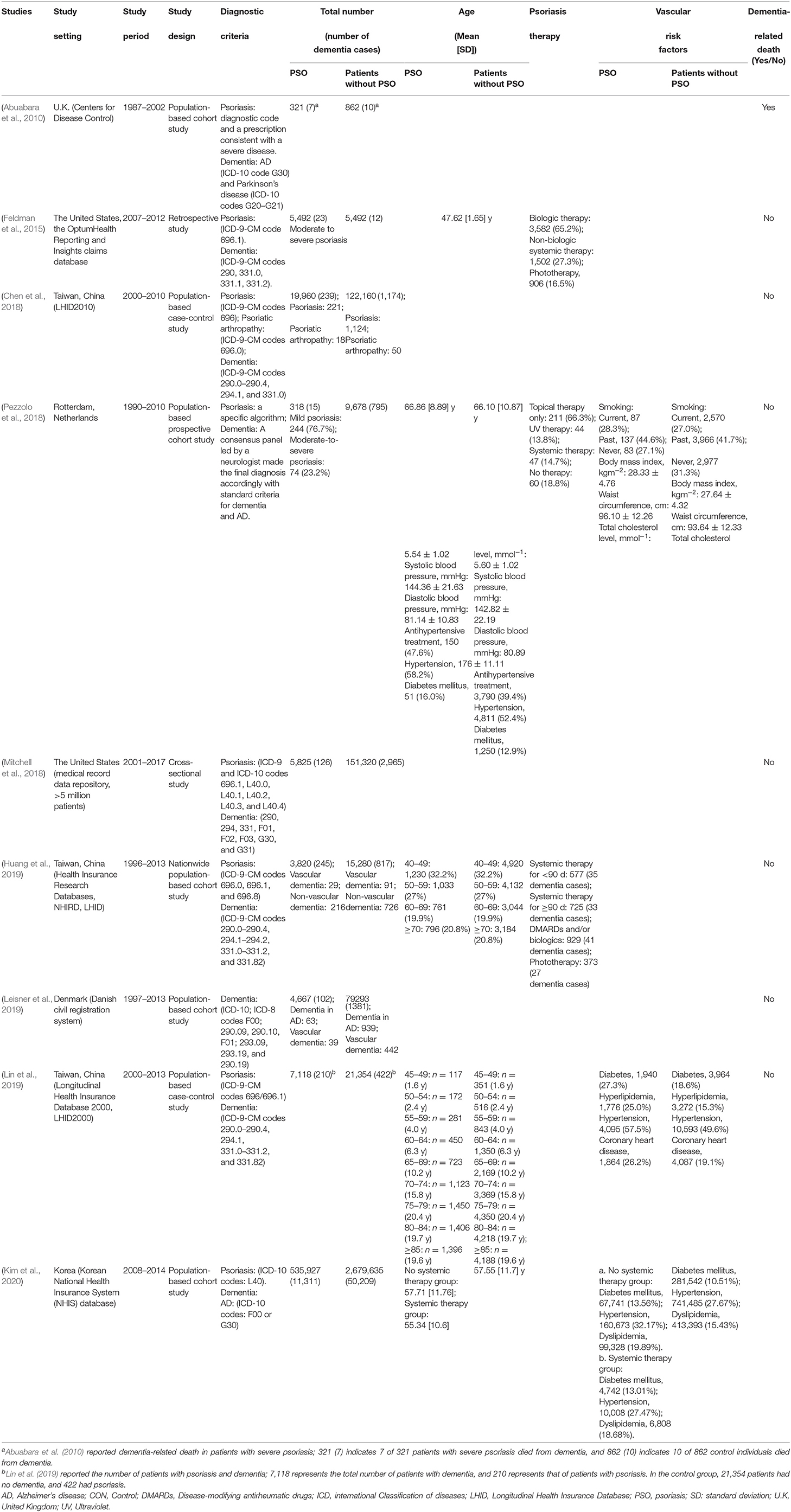
Of the nine studies, four were conducted in East Asia (Chen et al., 2018; Huang et al., 2019; Lin et al., 2019; Kim et al., 2020), three in Europe (Abuabara et al., 2010; Pezzolo et al., 2018; Leisner et al., 2019), and two in the USA (Feldman et al., 2015; Mitchell et al., 2018). Five were cohort studies (Abuabara et al., 2010; Pezzolo et al., 2018; Huang et al., 2019; Leisner et al., 2019; Kim et al., 2020); two were case-control studies (Chen et al., 2018; Lin et al., 2019); one was a cross-sectional study (Mitchell et al., 2018), and the remaining one was a retrospective study (Feldman et al., 2015). Almost all the studies used the codes of the International Statistical Classification of Diseases and Related Health Problems as a diagnostic basis. The detailed characteristics of these observational studies are shown in Table 1.
Risk of Bias
Only Chen et al. (2018) conducted a low-risk research with a score of 7 points. Three studies (Pezzolo et al., 2018; Huang et al., 2019; Lin et al., 2019; Kim et al., 2020) had a modest risk of bias. One study (Leisner et al., 2019) had a high risk with scores of 2 points (Table 2). Only one study had a cross-sectional design. We used the AHRQ tool to assess its risk of bias and found that this study fitted only the first item of the tool (i.e., clarification of the source of data; Table 3) and, therefore, was included/excluded.
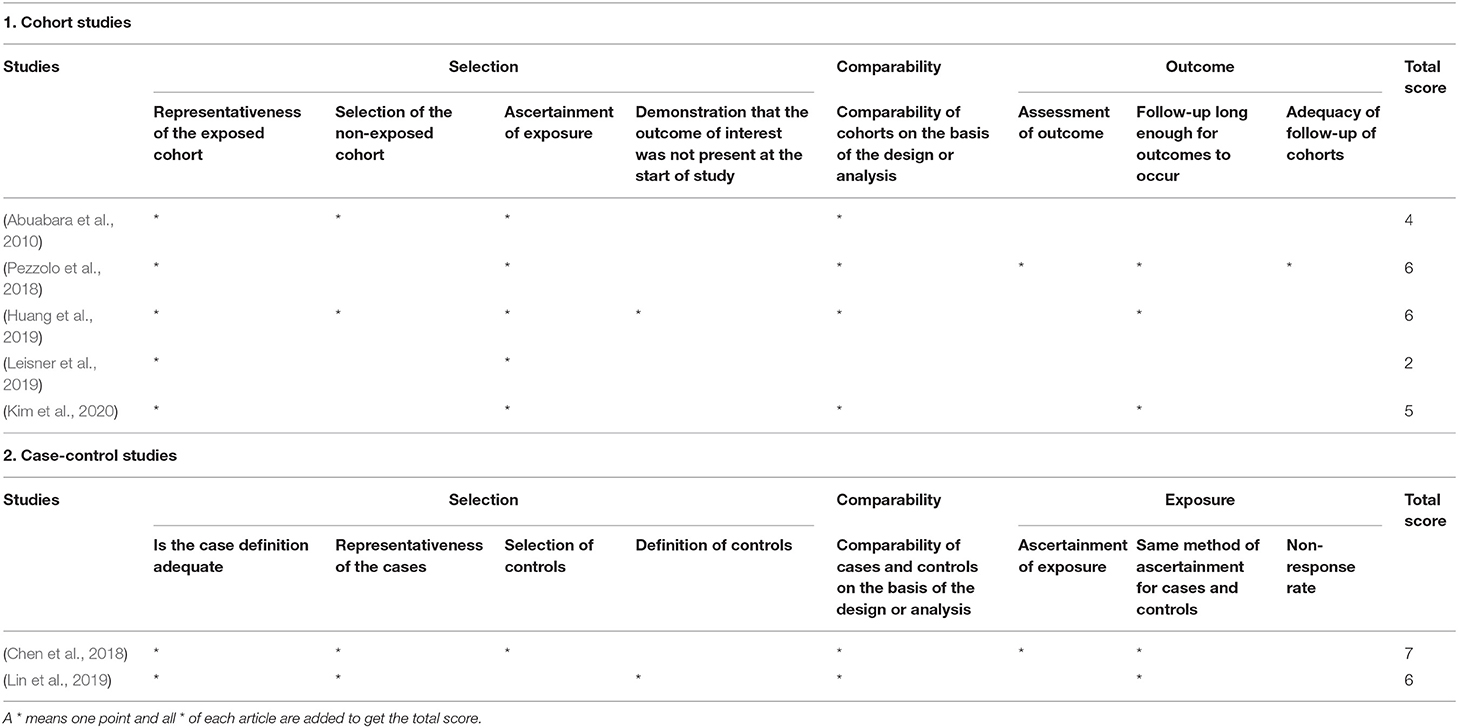
Table 2. Quality assessment of the cohort and case-control studies using the Newcastle-Ottawa Scale.

Table 3. Risk of bias of the cross-sectional studies assessed using the Agency for Healthcare Research and Quality tool.
Primary Outcomes
Seven studies (Feldman et al., 2015; Chen et al., 2018; Mitchell et al., 2018; Pezzolo et al., 2018; Huang et al., 2019; Leisner et al., 2019; Kim et al., 2020) that involved a total of 3,638,487 participants were included in the meta-analysis. We first analyzed the cases of dementia in all the patients, with and without psoriasis. As shown in Figure 2, a higher proportion of patients with psoriasis had dementia (RR: 1.16, 95% CI: 1.06–1.27, p = 0.001; random-effects model) than those without psoriasis.
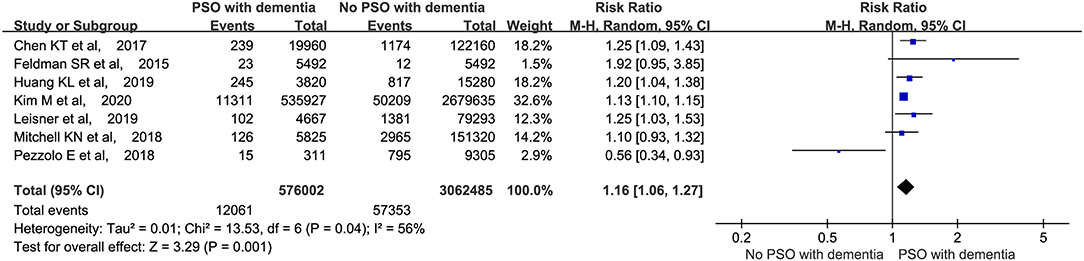
Figure 2. Meta-analysis of the incidence of dementia in patients with psoriasis with the corresponding 95% CI. CI, confidence interval.
Meanwhile, Lin et al. investigated the prevalence of psoriasis in patients with and without dementia. Finally, they found that the patients with dementia were at a higher risk of psoriasis (p < 0.001), which further confirms the comorbidity of the two disorders from another perspective.
Meta-Regression Analysis
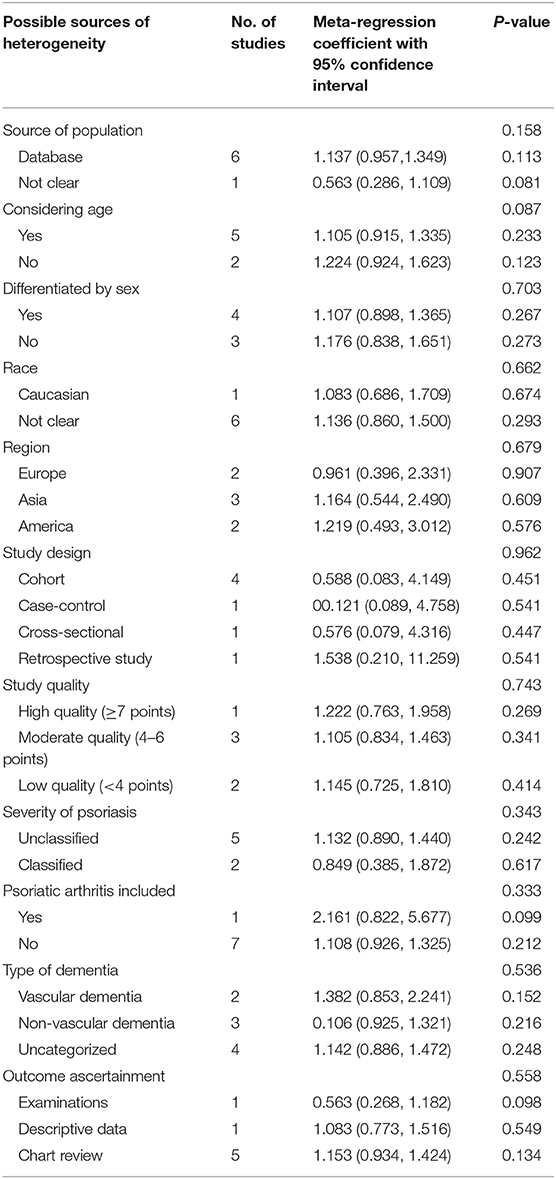
Owing to the moderate heterogeneity (I2 = 56%) of the primary outcome, we then performed a meta-regression analysis to examine the possible sources of heterogeneity. The analysis indicated that the variables we predicted were not the possible sources of heterogeneity with no statistical significance (p > 0.05; Table 4).
Secondary Outcomes
Different Types of Dementia
A meta-analysis was performed to assess the incidence of non-vascular (including AD) and vascular dementia. The patients with psoriasis had a high probability of developing both types of dementia (p < 0.01). The RRs for non-vascular and vascular dementia were 1.13 and 1.41, respectively (Figure 3).

Figure 3. Meta-analysis of the different types of dementia in patients with psoriasis. CI, confidence interval.
Comparison of Psoriasis and Psoriatic Arthritis
We performed a meta-analysis based on whether psoriatic arthritis was included in the studies. The analysis revealed that the patients with psoriasis (RR: 1.14, 95% CI: 1.06–1.24, p = 0.0009; random-effects model) and psoriatic arthritis (RR: 2.20, 95% CI: 1.29–3.78, p = 0.004; random-effects model) were both at a higher risk of developing dementia (Figure 4).
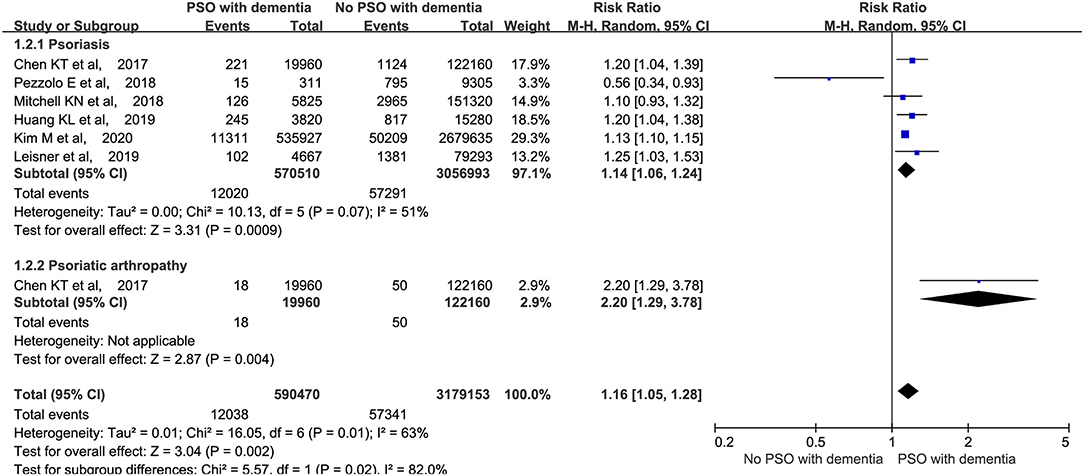
Figure 4. Meta-analysis of dementia in patients with psoriasis and psoriatic arthritis. CI, confidence interval.
Dementia-Related Death
Abuabara et al. (2010) reported no significant difference in the risk of dementia-related deaths among patients with and without psoriasis (p = 0.06), which was consistent with our results (p = 0.20, data not shown). However, after adjusting for age and sex, the patients with severe psoriasis were 3.64 times more likely to die from dementia than those without severe psoriasis (95% CI: 1.36–9.72).
Publication Bias
Egger's and Begg's regression tests revealed no publication bias in the pooled studies, with similar p values and 95% CIs (p = 0.685, 95% CI: −1.578 to 2.211).
Sensitivity Analysis
In the sensitivity analysis, the study by Pezzolo et al. (2018) might be a source of heterogeneity because when we excluded the study in the meta-analysis, the heterogeneity was reduced (I2 = 17%). However, the conclusion remained unchanged; that is, the patients with psoriasis had a higher risk of dementia than the patients without psoriasis (RR: 1.16, 95% CI: 1.10–1.21, p < 0.00001; Supplementary Figures 1, 2). The reduced heterogeneity can be explained by the fact that only Pezzolo et al. demonstrated that patients with psoriasis are at a lower risk of developing dementia.
Discussion
To our knowledge, the association between psoriasis and dementia has rarely been investigated, and the existing results are conflicting. Recently, Lam et al. (2020) conducted a systematic review on this topic. They included eight observational studies and found an association between psoriasis and vascular dementia, which was consistent with our findings. Furthermore, we also performed another subgroup analysis, which revealed that the patients with psoriasis and psoriatic arthritis were more susceptible to dementia, regardless of the accompanying type of dementia. The incidence of psoriasis in those with dementia was higher than in those without dementia. Although two studies divided dementia into vascular and non-vascular dementia, they did not report the risk factors related to vascular dementia, including diabetes, hypertension, and metabolic syndrome, which are also risk factors of psoriasis (Li et al., 2016). An interesting result we obtained was that one study reported that dementia-related deaths accounted for most deaths among patients who died from severe psoriasis. However, the meta-analysis revealed no significant difference in the prevalence of severe psoriasis among the patients who died from dementia.
To date, only a few studies have investigated the mechanism of vascular dementia; thus, we can only obtain possible links between AD, one of the most common diseases of non-vascular dementia, and psoriasis from published studies. Moreover, the correlation between them can be explained from three aspects, namely genetics, immunity, and inflammation. A study that analyzed the genetic association of inflammatory diseases with AD revealed a strong relationship between rs2516049, a single-nucleotide polymorphism in the psoriasis gene, and AD, which indicates a genetic overlap between AD and psoriasis, and suggests that the onset of AD is related to the immune process (Yokoyama et al., 2016). Apolipoprotein E (APOE) is the main cholesterol carrier, which is closely related to lipoprotein metabolism, immune regulation, and neural tissue repair (Liu et al., 2013). Several research studies indicated that high expression levels of APOE genes might be an independent risk factor for the occurrence of psoriasis (Al Harthi et al., 2014; Shih et al., 2018). Similarly, the APOE genotypes in AD greatly affect the amyloid beta (Aβ) deposition to form senile plaques and result in cerebral amyloid angiopathy (Liu et al., 2013). APOE4 increases tau phosphorylation and exacerbates the tau pathology in mouse models (Shi and Holtzman, 2018). Therefore, explaining the correlation between the two diseases at a genetic level provides new directions for future research.
Psoriasis and AD are both inflammatory and immune diseases. Colgecen et al. found that patients with psoriasis have impaired visuospatial working memory and executive functions involving the prefrontal cortex because of the underlying ongoing proinflammatory pathology (Colgecen et al., 2016). The IL-23/IL-17 axis plays a significant role in psoriasis. In AD, the IL-12 and IL-23 receptor (p40) expressions on astrocytes and microglia lead to exacerbation of the AD pathology (Vom Berg et al., 2012; Heppner et al., 2015). Mohammadi Shahrokhi et al. (2018) verified that IL-17A expression deteriorates the AD condition through the induction of Aβ and indicated the important roles of the IL-23/IL-17A axis in the AD pathogenesis. Anti-inflammatory drugs used for prolonged periods have been reported to be retained in the body for longer periods, which can retard the progression of AD and delay neurodegeneration (McGeer et al., 2016). TNF-α is a key proinflammatory factor; several pharmacological studies indicated that TNF-α signaling exacerbates both Aβ peptides and tau protein pathologies in vivo, which are neuropathological hallmarks of AD (Decourt et al., 2017). Etanercept is a disease-modifying antirheumatic drug (DMARDs) that targets TNF-α. A study showed that etanercept rapidly improved the cognitive function of patients with AD by perispinal administration (Tobinick and Gross, 2008; Decourt et al., 2017). The results of the study by Huang et al. were consistent with the finding that patients with psoriasis who received anti-inflammatory systemic therapy for at least 90 days had a lower risk of dementia, especially those treated with DMARDs and biologics (Huang et al., 2019). Further studies are required to assess the impact of systemic therapy on psoriasis accompanied with dementia.
This study has some limitations. Firstly, only a few articles reported on lifestyle habits and risk factors related to psoriasis and dementia, which affected our comprehensive analysis of the links between the two disorders. Secondly, only one article reported on patients with mild to moderate psoriasis. Therefore, we cannot subdivide the severity of psoriasis and evaluate its relationship with dementia. Thirdly, as only one study reported dementia-related deaths in patients with severe psoriasis, we could not draw a certain conclusion. Therefore, additional similar studies are needed to guide clinical practice in the future. Lastly, the low quality of the involved studies and the fact that only one prospective cohort study was included resulted in weak evidence being included in the meta-analysis and may have biased the results to a certain degree. Further similar analysis-reports including high-level prospective and stratified studies that control confounding factors are required.
Conclusion
In summary, patients with psoriasis and psoriatic arthritis are at a high risk of developing both non-vascular and vascular dementia. Those with severe psoriasis may not have a higher risk of death from dementia. Clinicians should pay attention to this comorbidity as the identification of this potential risk allows for early intervention to reduce comorbidities and deaths. This study provides a feasible reference for further research on whether the use of preventive medication can reduce the risk of dementia-related death.
Author Contributions
XL and BL proposed and designed the study. BL obtained funding support. LL, S-tC, MX, and X-yS retrieved and selected the data. YQ and X-jD extracted the data. YL and YR assessed the quality of all the studies. LL, H-jL, LK, and Y-qZ performed all the statistical analyses. LL and S-tC drafted the manuscript and XL revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81874470 and 82074427) and the National Key Research and Development Program of China (grant no. 2018YFC1705301). It was also supported by the National Natural Science Foundation of Shanghai (grant no. 19ZR1458700).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.570992/full#supplementary-material
Supplementary Figure 1. Sensitivity analysis of the prevalence of dementia in patients with psoriasis, performed using RevMan 5.4. CI, confidence interval.
Supplementary Figure 2. Sensitivity analysis performed using Stata 15.1. CI, confidence interval.
Supplementary File 1. MOOSE Checklist. MOOSE, Meta-analyses Of Observational Studies in Epidemiology.
References
Abuabara, K., Azfar, R. S., Shin, D. B., Neimann, A. L., Troxel, A. B., and Gelfand, J. M. (2010). Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br. J. Dermatol. 163, 586–592. doi: 10.1111/j.1365-2133.2010.09941.x
Al Harthi, F., Huraib, G. B., Zouman, A., Arfin, M., Tariq, M., and Al-Asmari, A. (2014). Apolipoprotein E gene polymorphism and serum lipid profile in Saudi patients with psoriasis. Dis. Markers 2014:239645. doi: 10.1155/2014/239645
Busse, M., Michler, E., von Hoff, F., Dobrowolny, H., Hartig, R., Frodl, T., et al. (2017). Alterations in the peripheral immune system in Dementia. J. Alzheimers. Dis. 58, 1303–1313. doi: 10.3233/JAD-161304
Chen, K. T., Chen, Y. C., Fan, Y. H., Lin, W. X., Lin, W. C., Wang, Y. H., et al. (2018). Rheumatic diseases are associated with a higher risk of dementia: a nation-wide, population-based, case-control study. Int. J. Rheum. Dis. 21, 373–380. doi: 10.1111/1756-185X.13246
Colgecen, E., Celikbilek, A., and Keskin, D. T. (2016). Cognitive impairment in patients with psoriasis: a cross-sectional study using the montreal cognitive assessment. Am. J. Clin. Dermatol. 17, 413–419. doi: 10.1007/s40257-016-0187-3
Decourt, B., Lahiri, D. K., and Sabbagh, M. N. (2017). Targeting tumor necrosis factor alpha for Alzheimer's disease. Curr. Alzheimer Res. 14, 412–425. doi: 10.2174/1567205013666160930110551
Feldman, S. R., Zhao, Y., Shi, L., and Tran, M. H. (2015). Economic and comorbidity burden among patients with moderate-to-severe psoriasis. J. Manag. Care Special. Pharm. 21, 874–888. doi: 10.18553/jmcp.2015.21.10.874
Forlenza, O. V., Diniz, B. S., Nunes, P. V., Memória, C. M., Yassuda, M. S., and Gattaz, W. F. (2009). Diagnostic transitions in mild cognitive impairment subtypes. Int. Psychogeriatr. 21, 1088–1095. doi: 10.1017/S1041610209990792
Fu, Y., Lee, C. H., and Chi, C. C. (2018). Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 154, 1417–1423. doi: 10.1001/jamadermatol.2018.3631
Heppner, F. L., Ransohoff, R. M., and Becher, B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16, 358–372. doi: 10.1038/nrn3880
Huang, K. L., Yeh, C. C., Wu, S. I., Huang, K. Y., Yang, Y. H., Kuo, T. Y., et al. (2019). Risk of dementia among individuals with psoriasis: a nationwide population-based cohort study in Taiwan. J. Clin. Psychiatr. 80:18m12462. doi: 10.4088/JCP.18m12462
Innamorati, M., Quinto, R. M., Imperatori, C., Lora, V., Graceffa, D., Fabbricatore, M., et al. (2016). Health-related quality of life and its association with alexithymia and difficulties in emotion regulation in patients with psoriasis. Compr. Psychiatr. 70, 200–208. doi: 10.1016/j.comppsych.2016.08.001
Jadali, Z., and Eslami, M. B. (2014). T cell immune responses in psoriasis. Iran J. Allergy Asthma Immunol. 13, 220–230.
Kim, M., Park, H. E., Lee, S. H., Han, K., and Lee, J. H. (2020). Increased risk of Alzheimer's disease in patients with psoriasis: a nationwide population-based cohort study. Sci. Rep. 10:6454. doi: 10.1038/s41598-020-63550-2
Lam, M., Khorvash, R., and Drucker, A. M. (2020). Association between psoriasis and risk of dementia: a systematic review and meta-analysis. J. Am. Acad. Dermatol. doi: 10.1016/j.jaad.2020.05.091. [Epub ahead of print].
Leisner, M. Z., Riis, J. L., Schwartz, S., Iversen, L., Østergaard, S. D., and Olsen, M. S. (2019). Psoriasis and risk of mental disorders in Denmark. JAMA Dermatol. 155, 745–747. doi: 10.1001/jamadermatol.2019.0039
Li, X., Kong, L., Li, F., Chen, C., Xu, R., Wang, H., et al. (2015). Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS ONE 10:e0145221. doi: 10.1371/journal.pone.0145221
Li, X., Miao, X., Wang, H., Wang, Y., Li, F., Yang, Q., et al. (2016). Association of serum uric acid levels in psoriasis: a systematic review and meta-analysis. Medicine 95:e3676. doi: 10.1097/MD.0000000000003676
Lin, C. C., Lin, H. C., and Chiu, H. W. (2019). Association between psoriasis and dementia: a population-based case-control study. Am. J. Clin. Dermatol. 20, 457–463. doi: 10.1007/s40257-018-00420-8
Liu, C.-C., Liu, C.-C., Kanekiyo, T., Xu, H., and Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118. doi: 10.1038/nrneurol.2012.263
Lowes, M. A., Suarez-Farinas, M., and Krueger, J. G. (2014). Immunology of psoriasis. Annu. Rev. Immunol. 32, 227–255. doi: 10.1146/annurev-immunol-032713-120225
McGeer, P. L., Rogers, J., and McGeer, E. G. (2016). Inflammation, Antiinflammatory Agents, and Alzheimer's Disease: The Last 22 Years. J. Alzheimers Dis. 54, 853–857. doi: 10.3233/JAD-160488
Menter, A., Strober, B. E., Kaplan, D. H., Kivelevitch, D., Prater, E. F., Stoff, B., et al. (2019). Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J. Am. Acad. Dermatol. 80, 1029–1072. doi: 10.1016/j.jaad.2018.11.057
Mitchell, K. N., Ali, Y., Yousif, R., Ramsey, K., Amin, S., Laumann, A. E., et al. (2018). Prevalence of dementia in psoriasis patients: a cross-sectional study in a large US population. J. Invest. Dermatol. 138:S41. doi: 10.1016/j.jid.2018.03.247
Mohammadi Shahrokhi, V., Ravari, A., Mirzaei, T., Zare-Bidaki, M., Asadikaram, G., and Arababadi, M. K. (2018). IL-17A and IL-23: plausible risk factors to induce age-associated inflammation in Alzheimer's disease. Immunol. Invest. 47, 812–822. doi: 10.1080/08820139.2018.1504300
Pezzolo, E., Mutlu, U., Vernooij, M. W., Dowlatshahi, E. A., Gisondi, P., Girolomoni, G., et al. (2018). Psoriasis is not associated with cognition, brain imaging markers and risk of dementia: the rotterdam study. J. Am. Acad. Dermatol. doi: 10.1016/j.jaad.2018.07.046. [Epub ahead of print].
Sardi, F., Fassina, L., Venturini, L., Inguscio, M., Guerriero, F., Rolfo, E., et al. (2011). Alzheimer's disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun. Rev. 11, 149–153. doi: 10.1016/j.autrev.2011.09.005
Shi, Y., and Holtzman, D. M. (2018). Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 18, 759–772. doi: 10.1038/s41577-018-0051-1
Shih, C. M., Huang, C. Y., Wang, K. H., Huang, C. Y., Wei, P. L., Chang, Y. J., et al. (2018). Oxidized low-density lipoprotein-deteriorated psoriasis is associated with the upregulation of lox-1 receptor and il-23 expression in vivo and in vitro. Int. J. Mol. Sci. 19:2610. doi: 10.3390/ijms19092610
Stadhouders, R., Lubberts, E., and Hendriks, R. W. (2018). A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 87, 1–15. doi: 10.1016/j.jaut.2017.12.007
Surendranathan, A., Su, L., Mak, E., Passamonti, L., Hong, Y. T., Arnold, R., et al. (2018). Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain 141, 3415–3427. doi: 10.1093/brain/awy265
Tobinick, E. L., and Gross, H. (2008). Rapid cognitive improvement in Alzheimer's disease following perispinal etanercept administration. J. Neuroinflammation 5:2. doi: 10.1186/1742-2094-5-2
van der Willik, K. D., Fani, L., Rizopoulos, D., Licher, S., Fest, J., Schagen, S. B., et al. (2019). Balance between innate versus adaptive immune system and the risk of dementia: a population-based cohort study. J. Neuroinflammation 16:68. doi: 10.1186/s12974-019-1454-z
Viswanathan, M., Ansari, M. T., Berkman, N. D., Chang, S., Hartling, L., McPheeters, L. M., et al. (2012). “Assessing the risk of bias of individual studies in systematic reviews of health care interventions,” in Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews, (Rockville, MD: Agency for Healthcare Research and Quality US).
Vom Berg, J., Prokop, S., Miller, K. R., Obst, J., Kälin, R. E., Lopategui-Cabezas, I., et al. (2012). Inhibition of IL-12/IL-23 signaling reduces Alzheimer's disease-like pathology and cognitive decline. Nat. Med. 18, 1812–1819. doi: 10.1038/nm.2965
Wells, G. A., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., et al. (2000). The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed December 24, 2019).
Wotton, C. J., and Goldacre, M. J. (2017). Associations between specific autoimmune diseases and subsequent dementia: retrospective record-linkage cohort study, UK. J. Epidemiol. Community Health. 71, 576–583. doi: 10.1136/jech-2016-207809
Yokoyama, J. S., Wang, Y., Schork, A. J., Thompson, W. K., Karch, C. M., Cruchaga, C., et al. (2016). Association between genetic traits for immune-mediated diseases and alzheimer disease. JAMA Neurol. 73, 691–697. doi: 10.1001/jamaneurol.2016.0150
Zeng, X., Zhang, Y., Kwong, J. S. W., Zhang, C., Li, S., Sun, F., et al. (2015). The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based Med. 8, 2–10. doi: 10.1111/jebm.12141
Keywords: psoriasis, dementia, systematic review, meta-analysis, psoriatic arthritis
Citation: Liu L, Chen S-t, Li H-j, Qiang Y, Sun X-y, Zhou Y-q, Xing M, Luo Y, Ru Y, Ding X-j, Kuai L, Li B and Li X (2020) Association Between Psoriasis and Dementia: Current Evidence. Front. Aging Neurosci. 12:570992. doi: 10.3389/fnagi.2020.570992
Received: 09 June 2020; Accepted: 12 August 2020;
Published: 22 October 2020.
Edited by:
Panteleimon Giannakopoulos, Université de Genève, SwitzerlandReviewed by:
Joshua D. Grill, University of California, Irvine, United StatesPaolo Gisondi, University of Verona, Italy
Christian Salazar, University of California, Irvine, United States, in collaboration with reviewer JG
Copyright © 2020 Liu, Chen, Li, Qiang, Sun, Zhou, Xing, Luo, Ru, Ding, Kuai, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Li, MTM2NjE5NTYzMjZAMTYzLmNvbQ==; Bin Li, MTg5MzA1NjgxMjlAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Liu Liu
Liu Liu Si-ting Chen
Si-ting Chen Hong-jin Li
Hong-jin Li Yan Qiang
Yan Qiang Xiao-ying Sun
Xiao-ying Sun Ya-qiong Zhou
Ya-qiong Zhou Meng Xing
Meng Xing Ying Luo
Ying Luo Yi Ru
Yi Ru Xiao-jie Ding
Xiao-jie Ding Le Kuai
Le Kuai Bin Li
Bin Li Xin Li
Xin Li