- 1Physical Education and Sports Science (PESS) Academic Group, National Institute of Education, Nanyang Technological University, Singapore, Singapore
- 2Institute for Physical Activity and Nutrition (IPAN), School of Exercise and Nutrition Sciences, Deakin University, Geelong, VIC, Australia
Parkinson’s disease (PD) is characterized by motor and cognitive deficits that negatively impact on activities of daily living. While dopaminergic medications are used to attenuate motor symptoms, adjuvant therapies such as acoustic-based non-pharmacological interventions are used as a complement to standard drug treatments. At present, preliminary studies of acoustic-based interventions such as rhythmic-auditory stimulation (RAS) and vibroacoustic therapy (VAT) suggest two competing hypotheses: (1) RAS may recruit alternative motor networks that may bypass faulty spatiotemporal motor networks of movement in PD; or (2) the use of RAS enhances BG function through entrainment of beta oscillatory activities. In this mini review article, we discuss the mechanisms underlying the role of acoustic-based interventions and how it may serve to improve motor deficits such as gait impairments and tremors. We further provide suggestions for future work that may use a combination of RAS, VAT, and physical therapy to improve motor function in PD.
Introduction
Parkinson’s Disease (PD) is the second most ubiquitous neurodegenerative disorder that affects approximately 1% of individuals aged over 60 (Reeve et al., 2014). The prevalence of PD increases with age, and with an aging population, the number of individuals with PD is expected to double in the next decade (Rizek et al., 2016). Individuals with PD exhibit both motor (tremor; rigidity; bradykinesia) and non-motor related symptoms (cognitive; neurobehavioural abnormalities; and sleep disorders) that stems from dopamine depletion in the substantia nigra within the basal ganglia (BG; Chaudhuri et al., 2006; Sveinbjornsdottir, 2016). While dopaminergic medications can effectively manage symptoms, drug-resistance and levodopa-induced dyskinesias are common side-effects following long-term use (Vorovenci et al., 2016). Adjuvant physical therapies such as acoustic-based interventions may therefore be efficacious in promoting wellness and maintenance of physical and cognitive health in PD.
Music is an effective mood relaxer (Magee and Davidson, 2002), beneficial in relieving anxiety, pain, and stress (Nilsson, 2008) at a low cost. Recent advancements in brain imaging have allowed us to revise how our brains respond to music (Hunt, 2015). An established view is that music induces adaptable changes in the auditory-motor network, that may facilitate functional recovery of movements, thus may be harnessed in the treatment of motor-related disorders (Sihvonen et al., 2017). Neuroimaging studies have further reported the recruitment of sensorimotor areas during music listening in the absence of movement (Gordon et al., 2018), and enhanced motor connections in stroke patients during the musical intervention (Altenmüller et al., 2009). Furthermore, studies suggested a close connection between auditory, BG (Nombela et al., 2013), and cerebellar networks (Thaut et al., 2009). Since music can modulate neural activities that are implicated in PD, it is plausible that acoustic-based intervention may represent a low-cost yet effective treatment for people with PD.
Indeed, acoustic-based interventions have previously been shown to improve gait parameters (see Table 1 for studies using acoustic-based intervention in PD). A common method of acoustic-based intervention used in neurorehabilitation in PD is rhythmic auditory stimulation (RAS) in which external auditory cues (i.e., beat of a metronome) are applied to synchronize or provide timing to the movement (Ghai et al., 2018). A typical RAS procedure starts with presenting beats of a metronome that match an individual’s baseline cadence. The metronome beats are then gradually adjusted to an optimal pace, and individuals are tasked to synchronize their steps with the beats. This process requires the entrainment of movements to external auditory cues; the latter involves the coordination of timing and movement sequencing (Thaut, 2005). This mini-review will attempt to discuss the common types of acoustic-based interventions used to improve motor symptoms associated with PD. Additionally, we will provide suggestions for future investigations and plausible mechanisms underlying the role of acoustics in normalizing the aberrant neurophysiology of PD.
Mechanisms Underpinning RAS
In RAS, the application of external auditory cues serves as a time reference to regulate walking pace to external rhythm, allowing temporal expectations (anticipation of the next steps) to occur (Rohenkohl et al., 2012; Nombela et al., 2013). Motor symptoms in PD are likely due to faulty temporal and spatial mechanisms, necessary for coordinating and initiating structured movements (Jones et al., 2008). Therefore, auditory cues can act as an “internal clock” to stabilize aberrant internal rhythm in PD individuals via neural entrainment. An fMRI study by Grahn (2009) found that when the internal generation of beats was needed, the BG, specifically putamen activity was lowered with the presence of strong external beats. This suggests that the putamen may play a crucial role in mental beat prediction and generating accurate motor execution pace.
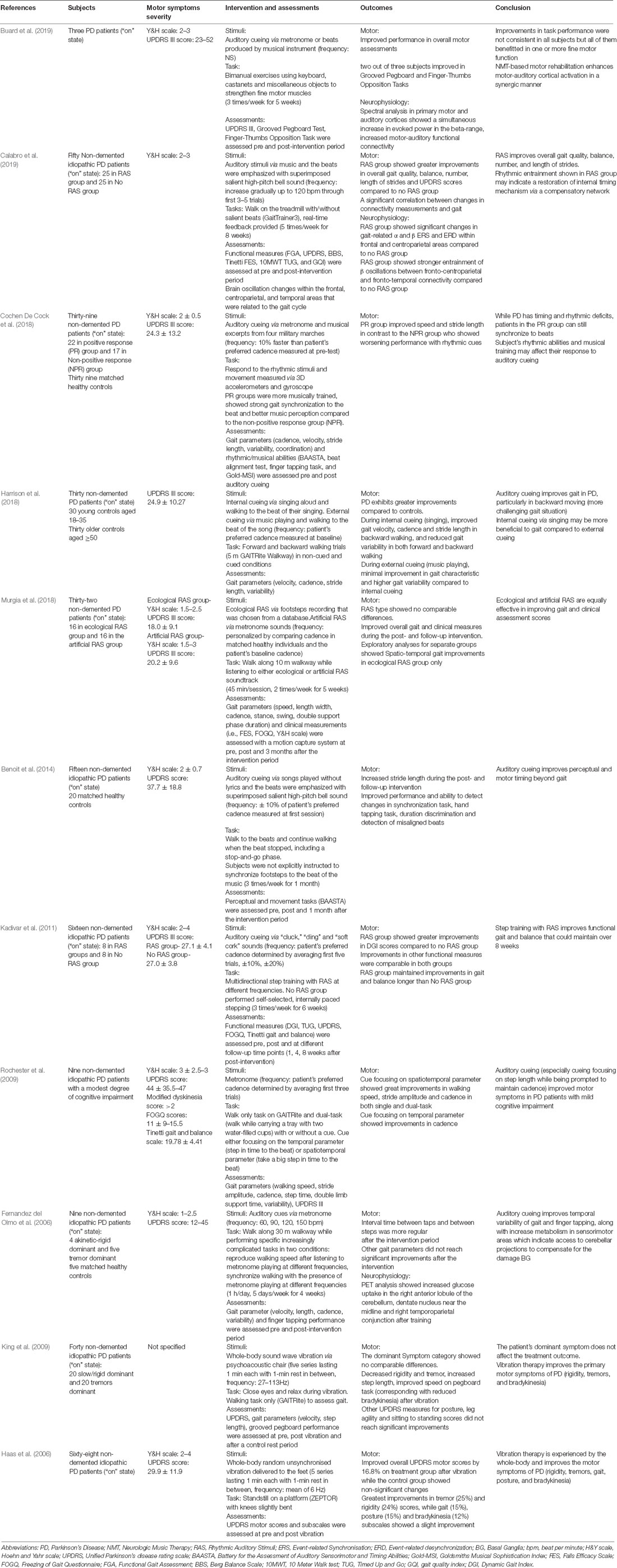
Besides its role in time perception to regulate movement, the BG mediates the interaction between auditory and motor systems during rhythm perception. This results in the engagement of motor areas (i.e., supplementary and premotor areas) when people attend to rhythms (Zatorre et al., 2007; Grahn, 2009; Grahn and Rowe, 2013; Ashoori et al., 2015; Raglio, 2015), which underpins the concept of RAS. Herein, we ask if RAS can potentially confer improvement when key brain areas required for rhythm and/or motor timing perception are impaired? The answer may be two-fold, with distinct hypotheses: Compensation and Restoration (Figure 1).
Figure 1. (A) An illustration of the striato-thalamocortical motor loop that is impaired in Parkinson’s disease (PD) due to dopamine depletion within the SNC of the basal ganglia (cross and dotted lines). (B) An illustration of The Compensation Hypothesis, which suggests recruitment of the cerebello-thalamocortical pathway that bypasses the BG (red lines). Alternatively, The Restoration Hypothesis suggests a facilitation effect on the striato-thalamocortical pathway, specifically facilitating dopaminergic function in the basal ganglia, (blue lines). M1, primary motor cortex; SMA, supplementary motor cortex; PMC, premotor cortex; PC, parietal cortex; SNC, substantia nigra pars compacta; GPe, external globus pallidus; GPi, internal globus pallidus; STN, subthalamic nucleus (adapted from Fujii and Wan, 2014).
The Compensation Hypothesis
The compensation theory suggests that while the BG is impaired in PD, other cortical structures may execute the functions of rhythm perception typically performed by the BG. Acoustic rhythms may reinforce alternate networks, such as the cerebello-thalamocortical circuitry, to modulate neural entrainment and enhance motor functioning (Schwartze et al., 2011; Nombela et al., 2013). When movements were externally cued, studies have shown increased activation within the lateral premotor cortex (PMC), and cerebellar connections to motor cortices (Sen et al., 2010; Todd and Lee, 2015), which effectively bypasses the impaired striato-thalamocortical circuitry. Moreover, Fernandez del Olmo et al. (2006) reported elevated glucose uptake in the cerebellum of individuals with PD post-RAS training using positron emission tomography (PET) concomitant to reductions in the variability of gait and finger-tapping tasks. The enhanced cerebellar activity after a month of RAS may imply that cerebellar pathways have been recruited to compensate for the impaired BG-cortical circuitry.
The Restoration Hypothesis
In contrast, some reviews suggest that acoustic rhythms may facilitate impaired BG network in individuals with PD (Nombela et al., 2013; Raglio, 2015) rather than bypassing it as suggested by the Compensation Hypothesis. Beta activity is excessively synchronized in individuals with PD leading to motor dysfunctions (Little and Brown, 2012; Sharott et al., 2014). The mechanistic rationale of RAS in PD is therefore to normalize beta-band coupling of the motor network, which may provide similar effects to current treatments (i.e., deep brain stimulation and dopaminergic therapy) without surgery or medication (Ghai et al., 2018).
The Restoration Hypothesis is supported by a study conducted by Woerd et al. (2014), which suggested that RAS was beneficial only if there was an increase in modulation depth of beta oscillatory activity, defined as the difference between event-related synchronization and desynchronization (ERS/ERD). Their study showed that relative to controls, individuals with PD had lesser ERD before the cue, with greater ERD after cue, probably due to compensation. Therefore, increases in beta ERS reflected the involvement of the BG as a beat predictor in rhythm processing, whereas increases in beta ERD is likely to reflect compensatory mechanisms. The findings from Woerd et al. (2014) showed that gains in modulation depth of beta activity are comparable amongst patients and controls. Moreover, the gains are entirely due to increases in synchronization, conferring increased predictive movement-related beta power suppression. This indicates that the BG is involved in rhythm and motor timing perception in individuals with PD and that RAS facilitates this process.
Overall, both hypotheses have valid supporting evidence and at present, we have yet reached consensus about the neuroplastic changes associated with RAS. Future studies building on theory are thus encouraged to explore whether RAS facilitates movements by bypassing or/and facilitating the impaired BG network in PD.
Neurochemical Effects of RAS
On top of neural network reorganization, studies have highlighted the neurochemical effects of RAS (Chanda and Levitin, 2013; Sihvonen et al., 2017; Cochen De Cock et al., 2018). Music listening not only activates emotional networks but also stimulates dopamine production in the striatal system (Salimpoor et al., 2011). Emotions and physical movements are closely intertwined (Molnar-Szakacs and Overy, 2006), hence there are suggestions that motor improvements following music intervention may be a result of emotional reactions mediating the dopaminergic activity of the BG motor loop (Salimpoor et al., 2011; Morris et al., 2019).
A recent PET study by Koshimori et al. (2019) explored RAS-induced dopamine responses in the BG of healthy participants while they performed finger-tapping tasks. They reported that RAS significantly decreased the binding potential relative to the non-displaceable compartment (BPND) variability in the BG. Consequently, this led to a significant decrease in DA response in the left ventral striatum (VS), an area that plays a role in motivation and reward processing (Liljeholm and O’Doherty, 2012). The authors further explained that this decreased in DA response with RAS was associated with lesser motivational and attentional requirements while attending to the task, which could be beneficial in dopamine-deficient patients. This study, therefore, provided insights about the potential role of RAS to modulate dopamine responses in PD and warrant future studies with PD patients.
Morris et al. (2019) surveyed the music responses of people with and without PD. They observed that PD patients had a diminished connection between music and action, compared to controls who perceived music as a reward and felt motivated to sing or dance along. Motor responses during reward anticipation are mediated by the release of DA in the VS (Elliott et al., 2004), which is depleted in individuals with PD and may lack the motivation to act. If the results in Koshimori et al. (2019) study were taken into account, RAS may, therefore, be beneficial to individuals with PD as this intervention could lead to reduced DA response in the VS. Additionally, music mitigates motor impairments in PD by activating the emotional-limbic-network and inducing dopamine secretion within domains of reward and motivation (Chanda and Levitin, 2013), a process akin to taking dopamine medications. The reward centers remain connected to motor areas, whilst dopamine serves as reinforcement to motivate repeat behaviors individuals find pleasurable.
While the potential of RAS has been widely discussed, most studies do not specify the details of RAS, such as the type of acoustics employed and the intervention duration. Some studies presented metronome beats on their own (Dalla Bella et al., 2017; Rose et al., 2019), whereas others embedded beats within music selected by the patient (Thaut et al., 1996), or by therapists (McIntosh et al., 1997). Leow et al. (2014) proposed the use of familiar music/music with high beat salience during RAS therapy for better improvements in gait parameters, because less cognitive demand is required to entrain movement with familiar and strong beat structures. Furthermore, music enjoyment elicits an emotional response and serves as a good motivator during intervention programs. Acoustic-based intervention is also easier to incorporate into a patient’s lifestyle as opposed to other physical therapies. A suggestion for future investigation would be to explore how different genres of music affect movement and emotional states of PD patients. They may also investigate optimal duration (number of sessions) alongside long-term effects of RAS therapy when patients are off medication, to observe if therapeutic effects are retained.
Sound Vibration Reduces Tremors
The bulk of acoustic-based intervention studies on PD report improvements in bradykinesia and speech functions using RAS. However, tremors are often the first symptoms in PD to be noticed. These can affect the hands, mouth, and limbs (Jankovic, 2008), impacting activities of daily living (Bhidayasiri, 2005). Very limited studies to date have explored how acoustic-based interventions help reduce or control tremors.
To our best knowledge, tremor reduction is evidenced in studies applying Vibroacoustic therapy (VAT; see Table 1). VAT delivers passive low-frequency sound vibrations (20–100 Hz) in contrast to RAS, the latter, an active form of acoustic therapy. In clinical settings, VAT supports the idea that patients should not only listen to music in isolation but sense vibrations as well (Warth et al., 2015). The VAT involves transducers embedded into chairs or mattresses, which send vibrations to and through the body. According to Punkanen and Ala-Ruona (2012), the best combination for VAT is a combination of music, sound waves and therapeutic interaction, which allows therapists to address a patient’s mental and cognitive states, alongside motor function.
Skille and Wigram (1995) demonstrated that interventions involving music plus vibrations (40 and 55 Hz) are most effective in enhancing/supporting movement while reducing muscle tension in physically disabled persons, in contrast to music as a solo acoustic therapy, indicating the importance of vibrations in neurorehabilitation. Studies that have implemented a combination of music and vibration in their intervention procedures include Chesky and Michel (1991), who reported vibroacoustic frequencies between 60–600 Hz to provide optimal pain relief; while Wigram (1996) reported decreased muscle tension, resulting in movement improvements in patients with cerebral palsy, post-intervention. Zheng et al. (2009) only applied low-frequency sound wave stimulation to frail, elderly participants, who later showed enhanced mobility. Nonetheless, many aspects of VAT are not clearly defined, and no agreement has been established regarding the necessity for music during VAT procedures. As such, the following section aims to discuss the individual roles of vibration and music in VAT, highlight studies that have applied vibration to reduce tremors in PD, and clarify possible mechanisms involved.
The Relaxation Hypothesis
Studies that delivered vibrations to a single muscle (Jöbges et al., 2002) the entire body (Haas et al., 2006) or via physio-acoustic chair (King et al., 2009) have demonstrated some level of efficacy in reducing tremors in individuals with PD. These studies further suggest that vibrations applied to manipulate local sensory feedback to the muscles, which decrease tremor frequency. One hypothesis for its effect is related to relaxation, which is based on the concepts of resonance, such that every part of our body has its natural frequency (Punkanen and Ala-Ruona, 2012). VAT frequencies presented via vibroacoustic chair may cause entrainment of our natural frequencies to resonate with external frequencies, resulting in increased blood circulation, enhanced metabolism, and decreased tension in muscles (Zheng et al., 2009; Punkanen and Ala-Ruona, 2012).
Tremors in individuals with PD are exacerbated when they are mentally and emotionally stressed (Schlesinger et al., 2009; Buhmann et al., 2018), and conversely reduced during mental relaxation; disappearing in sleep (Poliaková and Králová, 2015). The key to curtailing tremors may be to provide patients with a relaxed state of mind (Punkanen and Ala-Ruona, 2012). Zach et al. (2017) measured tremor intensity and variability while PD patients were performing a mental arithmetic task in ON and OFF L-Dopa medication. They found that cognitive stress reduced the effects of the medication on resting tremors, which highlights the importance of relaxation. Since the purpose of VAT is to achieve mental and muscular relaxation, adding a component of music has the potential to enhance relaxation, whilst encouraging patients to receive therapy, providing the music to be chosen correctly. Therefore, the type of music employed in acoustic therapy should be carefully determined because certain musical genres may at times be distracting, causing irritation or triggering unpleasant memories (Punkanen and Ala-Ruona, 2012). Future studies should aim to investigate the role of music in VAT by comparing therapeutic effects between vibration only and vibration plus music. Also, neural factors accompanying VAT have not been extensively explored. It is debatable whether tremor reduction after VAT is merely due to a state of relaxation, or the entrainment of thalamic oscillations.
Besides the relaxation hypothesis, Punkanen and Ala-Ruona (2012) have provided two other theories to explain the possible mechanisms underlying VAT. The first suggests low-frequency sound stimulates pain inhibitory mechanisms (i.e., Pacinian corpuscle), which mitigates pain impulse to the brain. In other words, VAT may be an effective pain reliever because inhibitory interneurons are activated in the process (Janzen et al., 2019). The second hypothesis is Jindrak postulate. Since the brain does not have a lymphatic drainage system, vibrations through it may assist the removal of waste molecules via diffusion through intercellular spaces (Skille and Wigram, 1995). However, these hypotheses do not explain how VAT is beneficial for relieving PD symptoms. A recent paper by Janzen et al. (2019) suggests vibroacoustic stimuli mimic brain stimulation (e.g., during transcranial electrical stimulation, wherein brain oscillations are induced), which in turn regulate thalamocortical dysrhythmias and enhance functional connectivity of the pain network in fibromyalgia patients. Based on this concept of neural entrainment, we may speculate that the delivery of rhythmic entrainment and sound vibration achieved with VAT can regulate abnormal neural oscillatory activity in PD (Teo et al., 2017). This hypothesis warrants further investigation to elaborate on the effects of vibroacoustic stimuli on neural oscillation and network connectivity.
Conclusion
In summary, there is growing evidence to support the beneficial effects of acoustic-based interventions in the form of music; rhythmic auditory cues; sound vibrations, or a combination of the three, in people with PD. Different PD symptoms can thus be treated with varying procedures such as RAS, which motivate the speed of movements, while sound vibration reduces involuntary shaking.
Herein, we propose directions for future research. If music serves as a relaxation tool to relieve symptoms, can individuals with PD habituate this form of relaxation to control their tremors through practice? As discussed, music plus vibration is far beneficial than either element alone; albeit systematic evidence to support this approach is necessary. Finally, the long-term effects of acoustic-mediated therapy in PD should be explored concerning its parameters (i.e., treatment duration; frequency; and type of music, where applicable) within each intervention. Systematic, longitudinal studies will greatly advance our understanding of music’s applicability in neurorehabilitation.
Author Contributions
JL: manuscript conceptualization, writing, and editing. LL and W-PT: manuscript writing and editing.
Funding
JL is supported by Nanyang Technological University (NTU) Research Scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Altenmüller, E., Marco-Pallares, J., Münte, T. F., and Schneider, S. (2009). Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy. Ann. N Y Acad. Sci. 1169, 395–405. doi: 10.1111/j.1749-6632.2009.04580.x
Ashoori, A., Eagleman, D. M., and Jankovic, J. (2015). Effects of auditory rhythm and music on gait disturbances in Parkinson’s disease. Front. Neurol. 6:234. doi: 10.3389/fneur.2015.00234
Benoit, C. E., Dalla Bella, S., Farrugia, N., Obrig, H., Mainka, S., and Kotz, S. A. (2014). Musically cued gait-training improves both perceptual and motor timing in Parkinson’s disease. Front. Hum. Neurosci. 8:494. doi: 10.3389/fnhum.2014.00494
Bhidayasiri, R. (2005). Differential diagnosis of common tremor syndromes. Postgrad. Med. J. 81, 756–762. doi: 10.1136/pgmj.2005.032979
Buard, I., Dewispelaere, W. B., Thaut, M., and Kluger, B. M. (2019). Preliminary neurophysiological evidence of altered cortical activity and connectivity with neurologic music therapy in Parkinson’s disease. Front. Neurosci. 13:105. doi: 10.3389/fnins.2019.00105
Buhmann, C., Jungnickel, D., and Lehmann, E. (2018). Stress management training (SMT) improves coping of tremor-boosting psychosocial stressors and depression in patients with Parkinson’s disease: a controlled prospective study. Parkinsons Dis. 2018:4240178. doi: 10.1155/2018/4240178
Calabro, R. S., Naro, A., Filoni, S., Pullia, M., Billeri, L., Tomasello, P., et al. (2019). Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson’s disease. J. Neuroeng. Rehabil. 16:68. doi: 10.1186/s12984-019-0533-9
Chanda, M. L., and Levitin, D. J. (2013). The neurochemistry of music. Trends Cogn. Sci. 17, 179–193. doi: 10.1016/j.tics.2013.02.007
Chaudhuri, K. R., Healy, D. G., and Schapira, A. H. (2006). Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 5, 235–245. doi: 10.1016/S1474-4422(06)70373-8
Chesky, K. S., and Michel, D. E. (1991). The music vibration table (MVT™): developing a technology and conceptual model for pain relief. Music Ther. Perspect. 9, 32–38. doi: 10.1093/mtp/9.1.32
Cochen De Cock, V., Dotov, D. G., Ihalainen, P., Bégel, V., Galtier, F., Lebrun, C., et al. (2018). Rhythmic abilities and musical training in Parkinson’s disease: do they help? NPJ Parkinsons Dis. 4:8. doi: 10.1038/s41531-018-0043-7
Dalla Bella, S., Benoit, C.-E., Farrugia, N., Keller, P. E., Obrig, H., Mainka, S., et al. (2017). Gait improvement via rhythmic stimulation in Parkinson’s disease is linked to rhythmic skills. Sci. Rep. 7:42005. doi: 10.1038/srep42005
Elliott, R., Newman, J. L., Longe, O. A., and William Deakin, J. F. (2004). Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. NeuroImage 21, 984–990. doi: 10.1016/j.neuroimage.2003.10.010
Fernandez del Olmo, M., Arias, P., Furio, M., Pozo, M., and Cudeiro, J. (2006). Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients—a combined motor and [18F]-FDG PET study. Parkinsonism Relat. Disord. 12, 155–164. doi: 10.1016/j.parkreldis.2005.11.002
Fujii, S., and Wan, C. Y. (2014). The role of rhythm in speech and language rehabilitation: the SEP hypothesis. Front. Hum. Neurosci. 8:777. doi: 10.3389/fnhum.2014.00777
Ghai, S., Ghai, I., Schmitz, G., and Effenberg, A. O. (2018). Effect of rhythmic auditory cueing on parkinsonian gait: a systematic review and meta-analysis. Sci. Rep. 8:506. doi: 10.1038/s41598-017-16232-5
Gordon, C. L., Cobb, P. R., and Balasubramaniam, R. (2018). Recruitment of the motor system during music listening: an ALE meta-analysis of fMRI data. PLoS One 13:e0207213. doi: 10.1371/journal.pone.0207213
Grahn, J. A. (2009). The role of the basal ganglia in beat perception: neuroimaging and neuropsychological investigations. Ann. N Y Acad. Sci. 1169, 35–45. doi: 10.1111/j.1749-6632.2009.04553.x
Grahn, J. A., and Rowe, J. B. (2013). Finding and feeling the musical beat: striatal dissociations between detection and prediction of regularity. Cereb. Cortex 23, 913–921. doi: 10.1093/cercor/bhs083
Haas, C. T., Turbanski, S., Kessler, K., and Schmidtbleicher, D. (2006). The effects of random whole-body-vibration on motor symptoms in Parkinson’s disease. NeuroRehabilitation 21, 29–36. doi: 10.3233/nre-2006-21105
Harrison, E. C., Horin, A. P., and Earhart, G. M. (2018). Internal cueing improves gait more than external cueing in healthy adults and people with Parkinson disease. Scienti. Rep. 8, 1–9.
Hunt, A. M. (2015). Boundaries and potentials of traditional and alternative neuroscience research methods in music therapy research. Front. Hum. Neurosci. 9:342. doi: 10.3389/fnhum.2015.00342
Jankovic, J. (2008). Parkinson’s disease: clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 79, 368–376. doi: 10.1136/jnnp.2007.131045
Janzen, T. B., Paneduro, D., Picard, L., Gordon, A., and Bartel, L. R. (2019). A parallel randomized controlled trial examining the effects of rhythmic sensory stimulation on fibromyalgia symptoms. PLoS One 14:e0212021. doi: 10.1371/journal.pone.0212021
Jöbges, E., Elek, J., Rollnik, J., Dengler, R., and Wolf, W. (2002). Vibratory proprioceptive stimulation affects Parkinsonian tremor. Parkinsonism Relat. Disord. 8, 171–176. doi: 10.1016/s1353-8020(01)00016-5
Jones, C. R., Malone, T. J., Dirnberger, G., Edwards, M., and Jahanshahi, M. (2008). Basal ganglia, dopamine and temporal processing: performance on three timing tasks on and off medication in Parkinson’s disease. Brain Cogn. 68, 30–41. doi: 10.1016/j.bandc.2008.02.121
Kadivar, Z., Corcos, D. M., Foto, J., and Hondzinski, J. M. (2011). Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neuro. Neu. Repair 25, 626–635. doi: 10.1177/1545968311401627
King, L. K., Almeida, Q. J., and Ahonen, H. (2009). Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. NeuroRehabilitation 25, 297–306. doi: 10.3233/NRE-2009-0528
Koshimori, Y., Strafella, A. P., Valli, M., Sharma, V., Cho, S.-S., Houle, S., et al. (2019). Motor synchronization to rhythmic auditory stimulation (RAS) attenuates dopaminergic responses in ventral striatum in young healthy adults:[11C]-(+)-PHNO PET study. Front. Neurosci. 13:106. doi: 10.3389/fnins.2019.00106
Leow, L. A., Parrott, T., and Grahn, J. A. (2014). Individual differences in beat perception affect gait responses to low- and high-groove music. Front. Hum. Neurosci. 8:811. doi: 10.3389/fnhum.2014.00811
Liljeholm, M., and O’Doherty, J. P. (2012). Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn. Sci. 16, 467–475. doi: 10.1016/j.tics.2012.07.007
Little, S., and Brown, P. (2012). What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann. N Y Acad. Sci. 1265, 9–24. doi: 10.1111/j.1749-6632.2012.06650.x
Magee, W. L., and Davidson, J. W. (2002). The effect of music therapy on mood states in neurological patients: a pilot study. J. Music Ther. 39, 20–29. doi: 10.1093/jmt/39.1.20
McIntosh, G. C., Brown, S. H., Rice, R. R., and Thaut, M. H. (1997). Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 62, 22–26. doi: 10.1136/jnnp.62.1.22
Molnar-Szakacs, I., and Overy, K. (2006). Music and mirror neurons: from motion to ‘e’motion. Soc. Cogn. Affect. Neurosci. 1, 235–241. doi: 10.1093/scan/nsl029
Morris, I. B., Vasudevan, E., Schedel, M., Weymouth, D., Loomis, J., Pinkhasov, T., et al. (2019). Music to one’s ears: familiarity and music engagement in people with Parkinson’s disease. Front. Neurosci. 13:661. doi: 10.3389/fnins.2019.00661
Murgia, M., Pili, R., Corona, F., Sors, F., Agostini, T. A., Bernardis, P., et al. (2018). The Use of Footstep Sounds as Rhythmic Auditory Stimulation for Gait Rehabilitation in Parkinson’s Disease: A Randomized Controlled Trial. Front. Neurol. 9:348. doi: 10.3389/fneur.2018.00348
Nilsson, U. (2008). The anxiety- and pain-reducing effects of music interventions: a systematic review. AORN J. 87, 780–807. doi: 10.1016/j.aorn.2007.09.013
Nombela, C., Hughes, L. E., Owen, A. M., and Grahn, J. A. (2013). Into the groove: can rhythm influence Parkinson’s disease? Neurosci. Biobehav. Rev. 37, 2564–2570. doi: 10.1016/j.neubiorev.2013.08.003
Poliaková, N., and Králová, E. (2015). 22. Potency of music and movement stimulation in the health care of patients with Parkinson’s disease. Rev. Art. Educ. 9, 164–175.
Punkanen, M., and Ala-Ruona, E. (2012). Contemporary vibroacoustic therapy: perspectives on clinical practice, research, and training. Music Med. 4, 128–135. doi: 10.1177/1943862112445324
Raglio, A. (2015). Music therapy interventions in Parkinson’s disease: the state-of-the-art. Front. Neurol. 6:185. doi: 10.3389/fneur.2015.00185
Reeve, A., Simcox, E., and Turnbull, D. (2014). Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 14, 19–30. doi: 10.1016/j.arr.2014.01.004
Rizek, P., Kumar, N., and Jog, M. S. (2016). An update on the diagnosis and treatment of Parkinson disease. CMAJ 188, 1157–1165. doi: 10.1503/cmaj.151179
Rochester, L., Burn, D. J., Woods, G., Godwin, J., and Nieuwboer, A. (2009). Does auditory rhythmical cueing improve gait in people with Parkinson’s disease and cognitive impairment? A feasibility study. Mov. Disord. 24, 839–845. doi: 10.1002/mds.22400
Rohenkohl, G., Cravo, A. M., Wyart, V., and Nobre, A. C. (2012). Temporal expectation improves the quality of sensory information. J. Neurosci. 32, 8424–8428. doi: 10.1523/JNEUROSCI.0804-12.2012
Rose, D., Delevoye-Turrell, Y., Ott, L., Annett, L. E., and Lovatt, P. J. (2019). Music and metronomes differentially impact motor timing in people with and without Parkinson’s disease: effects of slow, medium, and fast tempi on entrainment and synchronization performances in finger tapping, toe tapping, and stepping on the spot tasks. Parkinsons Dis. 2019:6530838. doi: 10.1155/2019/6530838
Salimpoor, V. N., Benovoy, M., Larcher, K., Dagher, A., and Zatorre, R. J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 14, 257–262. doi: 10.1038/nn.2726
Schlesinger, I., Benyakov, O., Erikh, I., Suraiya, S., and Schiller, Y. (2009). Parkinson’s disease tremor is diminished with relaxation guided imagery. Mov. Disord. 24, 2059–2062. doi: 10.1002/mds.22671
Schwartze, M., Keller, P. E., Patel, A. D., and Kotz, S. A. (2011). The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo and the detection of tempo changes. Behav. Brain Res. 216, 685–691. doi: 10.1016/j.bbr.2010.09.015
Sen, S., Kawaguchi, A., Truong, Y., Lewis, M. M., and Huang, X. (2010). Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson’s disease. Neuroscience 166, 712–719. doi: 10.1016/j.neuroscience.2009.12.036
Sharott, A., Gulberti, A., Zittel, S., Tudor Jones, A. A., Fickel, U., Munchau, A., et al. (2014). Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in Parkinson’s disease. J. Neurosci. 34, 6273–6285. doi: 10.1523/JNEUROSCI.1803-13.2014
Sihvonen, A. J., Särkämö, T., Leo, V., Tervaniemi, M., Altenmüller, E., and Soinila, S. (2017). Music-based interventions in neurological rehabilitation. Lancet Neurol. 16, 648–660. doi: 10.1016/S1474-4422(17)30168-0
Skille, O., and Wigram, T. (1995). “The effect of music, vocalisation and vibration on brain and muscle tissue: studies in vibroacoustic therapy,” in The Art and Science of Music Therapy: A Handbook, eds T. Wigram, B. Saperston and R. West (London: Harwood Academic), 23–57.
Sveinbjornsdottir, S. (2016). The clinical symptoms of Parkinson’s disease. J. Neurochem. 139, 318–324. doi: 10.1111/jnc.13691
Teo, W. P., Hendy, A. M., Goodwill, A. M., and Loftus, A. M. (2017). Transcranial alternating current stimulation: a potential modulator for pathological oscillations in Parkinson’s disease? Front. Neurol. 8:185. doi: 10.3389/fneur.2017.00185
Thaut, T. H. (2005). The future of music in therapy and medicine. Ann. N Y Acad. Sci. 1060, 303–308. doi: 10.1196/annals.1360.023
Thaut, M. H., McIntosh, G. C., Rice, R. R., Miller, R. A., Rathbun, J., and Brault, J. M. (1996). Rhythmic auditory stimulation in gait training for Parkinson’s disease patients. Mov. Disord. 11, 193–200. doi: 10.1002/mds.870110213
Thaut, M. H., Stephan, K. M., Wunderlich, G., Schicks, W., Tellmann, L., Herzog, H., et al. (2009). Distinct cortico-cerebellar activations in rhythmic auditory motor synchronization. Cortex 45, 44–53. doi: 10.1016/j.cortex.2007.09.009
Todd, N. P., and Lee, C. S. (2015). The sensory-motor theory of rhythm and beat induction 20 years on: a new synthesis and future perspectives. Front. Hum. Neurosci. 9:444. doi: 10.3389/fnhum.2015.00444
Vorovenci, R. J., Biundo, R., and Antonini, A. (2016). Therapy-resistant symptoms in Parkinson’s disease. J. Neural Transm. 123, 19–30. doi: 10.1007/s00702-015-1463-8
Warth, M., Kessler, J., Kotz, S., Hillecke, T. K., and Bardenheuer, H. J. (2015). Effects of vibroacoustic stimulation in music therapy for palliative care patients: a feasibility study. BMC Complement. Altern. Med. 15:436. doi: 10.1186/s12906-015-0933-8
Wigram, A. L. (1996). The Effects of Vibroacoustic Therapy on Clinical and Non-Clinical Populations. London: University of London.
Woerd, E. S., Oostenveld, R., de Lange, F. P., and Praamstra, P. (2014). A shift from prospective to reactive modulation of β-band oscillations in Parkinson’s disease. NeuroImage 100, 507–519. doi: 10.1016/j.neuroimage.2014.06.039
Zach, H., Dirkx, M. F., Pasman, J. W., Bloem, B. R., and Helmich, R. C. (2017). Cognitive stress reduces the effect of levodopa on Parkinson’s resting tremor. CNS Neurosci. Ther. 23, 209–215. doi: 10.1111/cns.12670
Zatorre, R. J., Chen, J. L., and Penhune, V. B. (2007). When the brain plays music: auditory-motor interactions in music perception and production. Nat. Rev. Neurosci. 8, 547–558. doi: 10.1038/nrn2152
Keywords: music therapy (MT), Parkinson’s disease, motor symptom (MDS-UPDRS-III), rhythm entrainment, acoustic therapy
Citation: Leuk JSP, Low LLN and Teo W-P (2020) An Overview of Acoustic-Based Interventions to Improve Motor Symptoms in Parkinson’s Disease. Front. Aging Neurosci. 12:243. doi: 10.3389/fnagi.2020.00243
Received: 05 March 2020; Accepted: 13 July 2020;
Published: 14 August 2020.
Edited by:
Adam J. Woods, University of Florida, United StatesReviewed by:
Nabin Koirala, Haskins Laboratories, United StatesHeidemarie Zach, Medical University of Vienna, Austria
Copyright © 2020 Leuk, Low and Teo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Peng Teo, d2VpcGVuZy50ZW9AbmllLmVkdS5zZw==
 Jessie Siew Pin Leuk
Jessie Siew Pin Leuk Linette Li Neng Low1
Linette Li Neng Low1 Wei-Peng Teo
Wei-Peng Teo