- Medical College of Acu-Moxi and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Mild cognitive impairment (MCI) is the early phase of Alzheimer's disease (AD). The aim of early intervention for MCI is to decrease the rate of conversion from MCI to AD. However, the efficacy of multiple interventions in MCI, and the optimal methods of delivery, remain controversial. We aimed to compare and rank the treatment methods for MCI in AD, in order to find an optimal intervention for MCI and a way to prevent or delay the occurrence of AD.
Methods: Pair-wise and network meta-analysis were conducted to integrate the treatment effectiveness through direct and indirect evidence. Four English databases and three Chinese databases were searched for international registers of eligible published, single or double blind, randomized controlled trials up to September 31st 2019. We included nine comparative interventions: pharmacological therapies which incorporated cholinesterase inhibitors (ChEI), ginkgo, nimodipine, and Chinese medicine; non-pharmacological therapies comprising of acupuncture, music therapy, exercise therapy, and nutrition therapy; and a placebo group. The primary outcome was the Mini-Mental State Examination (MMSE) score. The secondary outcome was the AD Assessment Scale-cognitive subscale (ADAS-cog).
Results: Twenty-eight trials were eligible, including 6,863 participants. In the direct meta-analysis, as for the Mini-Mental State Examination scale, the ChEIs (MD: −0.38; 95% CI: −0.74, −0.01), Chinese medicine (MD: −0.31; 95% CI: −0.75, 0.13), exercise therapy (MD: −0.50; 95% CI: −0.65, −0.35), music therapy (MD: −1.71; 95% CI: −4.49, 1.07), were statistically more efficient than placebo. For AD Assessment Scalecognitive subscale outcome, ChEIs (MD: 1.20; 95% CI: 0.73, 1.68), Acupuncture (MD: 1.36; 95% CI: 1.28, 1.44), Chinese medicine (MD: 0.61; 95% CI: 0.49, 0.73) and exercise (MD: 0.61; 95% CI: 0.49, 0.73) were better than placebo. In the network meta-analysis, the MMSE outcome ranked music therapy (59%) as the best and Acupuncture (26%) as second. Nutrition and Ginkgo treatment had the lowest rank among all interventions. For ADAS-cog outcome, acupuncture (52) ranked the best.
Conclusion: Among the nine treatments studied, music therapy appears to be the best treatment for MCI, followed by acupuncture. Our study provides new insights into potential clinical treatments for MCI due to AD, and may aid the development of guidelines for MCI in AD.
Introduction
Mild cognitive impairment (MCI) has been defined as a “transitional” state to describe individuals who are not cognitively “active” for their age, but who would not meet a clinical diagnosis of early dementia (Brendan and Kelley, 2015). It is an intermediate clinical condition with a number of sub-types and multiple pathologies (Petersen, 2004; Rountree et al., 2007).
MCI is currently an area of considerable clinical and research interest because a large proportion of patients with MCI develop Alzheimer's disease (AD). In order to delay the progress of AD, it is important to recognize the key neurobiological difference between MCI and AD. Several journal articles have demonstrated that MCI patients progress to AD at a higher rate (10–15% per year) than normal elderly patients (1–3% per year). Furthermore, two-thirds of patients with AD were previously recognized as having MCI (Rubin et al., 1989; Almkvist et al., 1998; Wolf et al., 1998; Kluger et al., 1999; Petersen et al., 1999, 2001; Collie and Maruff, 2000; Morris et al., 2001). Therefore, patients who have MCI are considered to be at a greater risk for AD. Unfortunately, treatment options for AD are currently suboptimal, especially in the advanced stage of AD where the brain damage is irreversible. Thus, it is important to start treatment for AD as early as possible, and intervention during the MCI stage may prevent or delay the occurrence of AD.
Several treatments have been used for MCI, including cholinesterase inhibitors (ChEIs), complementary and alternative medicine, lifestyle and nutrition interventions, and Chinese medicine. However, which of these interventions work, and to what extent, remains unknown.
Pairwise meta-analysis have previously been conducted to assess the efficacy of ChEIs, including donepezil, galantamine, and rivastigmine (Cooper et al., 2013; Tricco et al., 2013; Matsunaga et al., 2019). These studies suggested that ChEIs have a low efficacy in the treatment of MCI and many safety issues were raised. Therefore, before using ChEIs for MCI, other methods should be considered.
Other studies (Andreas et al., 2015; Liang et al., 2018) have suggested that physical exercise and computerized cognitive training could improve cognitive function and neuropsychiatric symptoms. Furthermore, non-pharmacological therapies might perform more effectively than pharmacological therapies. However, these studies were incomplete and provide insufficient data, because they were unable to introduce clear hierarchies among treatments and a number of interventions were not analyzed. Therefore, the aim of this article was to conduct a network meta-analysis to thoroughly compare and rank different treatments for MCI that help to improve cognitive function and prevent or delay the occurrence of AD.
Methods
Search Strategy
Four English databases (Medline [via Ovid], Embase [via Ovid], Cochrane Library [Central Register of Controlled Trials], and Web of Science [via Ovid]) and three Chinese databases (China Science Journal Citation Report [VIP], China National Knowledge Infrastructure [CNKI], and Wanfang) were searched for all relevant citations published from the date of the respective database onset to September 31st 2019. We established search strategies which combined subject word (keyword) and random words related to MCI, interventions of interest (drug therapy, diet/lifestyle therapy, physical activity/exercise, complementary therapies, sham/placebo) and randomized controlled trial (RCT). Furthermore, the reference lists of the included studies were manually reviewed to look for additional relevant manuscripts. The specific search strategies are shown in Supplementary Datasheet 1.
Selection and Exclusion Criteria
The RCTs which met the following criteria were included: (1) participants had mild cognitive impairment due to AD; (2) the interventions were pharmacological therapies including Cholinesterase inhibitors, Memantine, Ginkgo biloba, Huperzine A, Piracetam, Nimodipine, and Chinese medicine, or non-pharmacological therapies including acupuncture, music therapy, lifestyle therapy, exercise therapy, and nutrition therapy; (3) the comparisons were placebo, no intervention, usual care control, or other comparable interventions; (4) the study included at least one cognitive performance outcome measure in the form of either the MMSE (Mini-Mental State Examination) or ADAS-cog (AD Assessment Scale-cognitive subscale) and the efficacy of the studies must include mean changes from baseline to endpoint.
Studies with the following characteristics were excluded: (1) participants with a diagnosis of MCI due to diseases other than AD; (2) irrelevant outcomes or deficient data; and (3) case reports, review articles, clinical protocols, conference abstracts, and animal experimental studies.
Data Extraction and Quality Assessment
Two investigators (XL and YL) screened the articles and extracted the data and related statistics independently. Basic information was organized into a standard table, including information on the characteristics of the population, intervention(s), comparison(s), treatment duration, event(s), and outcome. The Cochrane Risk of Bias Tool (Savovic et al., 2014) was used by two researchers (XL and YL) to assessed the risk of bias and quality of included trials. A third reviewer (HW) was consulted to recheck studies when the first two reviewers had disagreements and discrepancies.
Outcomes
Our network meta-analysis used Mini-Mental State Examination (MMSE) as the primary outcome to measure global cognition, with higher MMSE scores meaning better cognitive function. The second outcome was the AD Assessment Scale-cognitive subscale (ADAS-cog), in which lower scores mean better cognitive function. If the complete data on outcomes were not reported in the original article, we contacted authors via email to obtain the raw data, otherwise studies lacking such data were eliminated. The data were independently collected by two reviewers (XL and YL) and rechecked by a third investigator (HW).
Statistical Analysis
Firstly, a pair-wise meta-analysis was used to compare the compliance of different therapies. By using the Aggregate Data Drug Information System (ADDIS ver. 1.16.8, available at: https://drugis.org/software/addis/index) with a random-effects model, the odds ratio (OR) was measured for discontinuous outcomes along with 95% credible intervals (CI). Statistical heterogeneity was calculated in the pair-wise comparisons with an I2 statistic and the p-value.
Furthermore, we conducted a network meta-analysis. This network meta-analysis was set up with a Bayesian framework using the Aggregate Data Drug Information System (ADDIS ver. 1.16.8, available at: https://drugis.org/software/addis/index) to determine the cognitive outcomes of nine interventions. This software uses the Bayesian framework as a base combined with the Markov chain Monte Carlo method to evaluate research data. A random-effects model was used to evaluate the effect sizes in this network meta-analysis. Random-effect model attempted to generalize findings beyond the included studies by assuming that the selected studies are random samples from a larger population (Cheung et al., 2012). The mean difference (MD) was the effect size for continuous outcomes. The models used to estimate the effect size in ADDIS were “consistency” and “inconsistency.” The consistency model aims to evaluate effect sizes of the interventions, which was used to calculate the ranking probabilities for the whole group of interventions. Node-splitting analysis helps to determine the consistency test with an inconsistency model. For instance, a consistency model was chosen when the p-value of the node-splitting analysis was >0.05. If the p-value of the node-splitting analysis was <0.05, an inconsistency model was selected. In order to evaluate the convergence of the model, the potential scale reduction factor (PSRF) was used. If the PSRF value was close to 1, the convergence of the model was more desirable. If the PSRF value was <1.2, it was still considered as acceptable. For each intervention, the ranking probabilities were estimated for every treatment at every possible rank.
Results
Study Identification and Selection
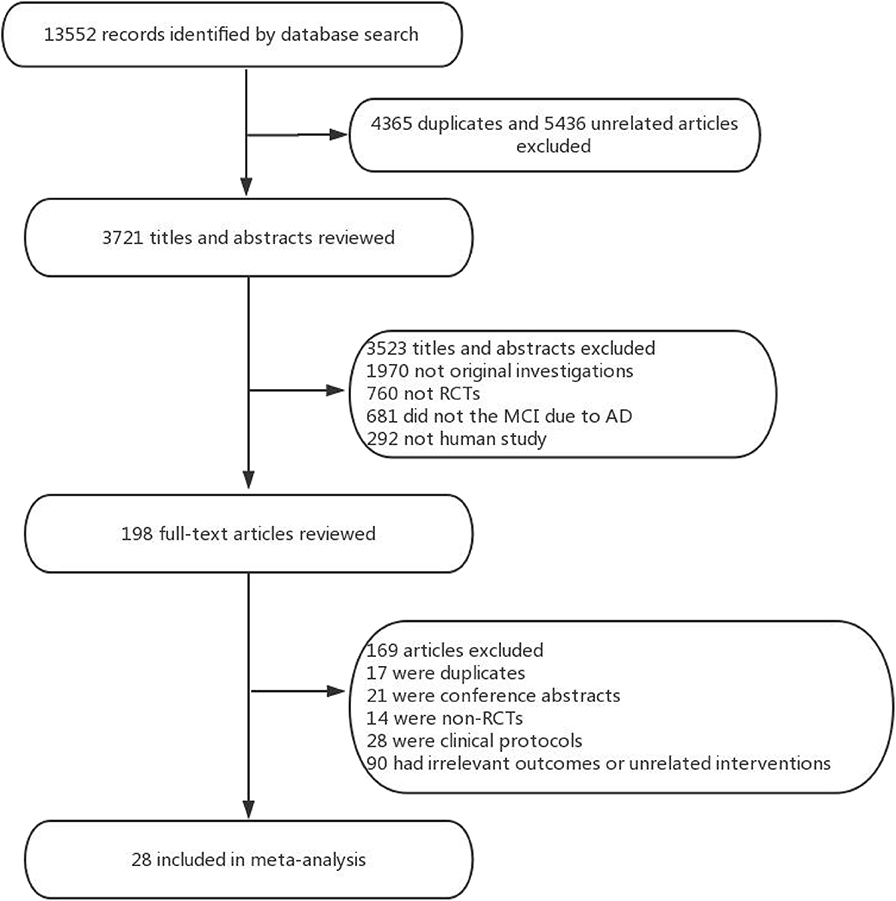
Overall, a total of 13,522 studies were identified from the seven electronic databases using the search strategy, and 198 relevant full-text articles were evaluated for eligibility. There were 169 citations excluded, including 17 duplicates, 21 conference abstracts, 28 clinical protocols, 14 non-RCTs, and 90 unrelated intervention or outcome articles. Ultimately, 28 studies including 6,863 patients were clinical eligible to be included in this network meta-analysis (Figure 1). The characteristics of the selected studies were listed in Table 1.
Study Quality
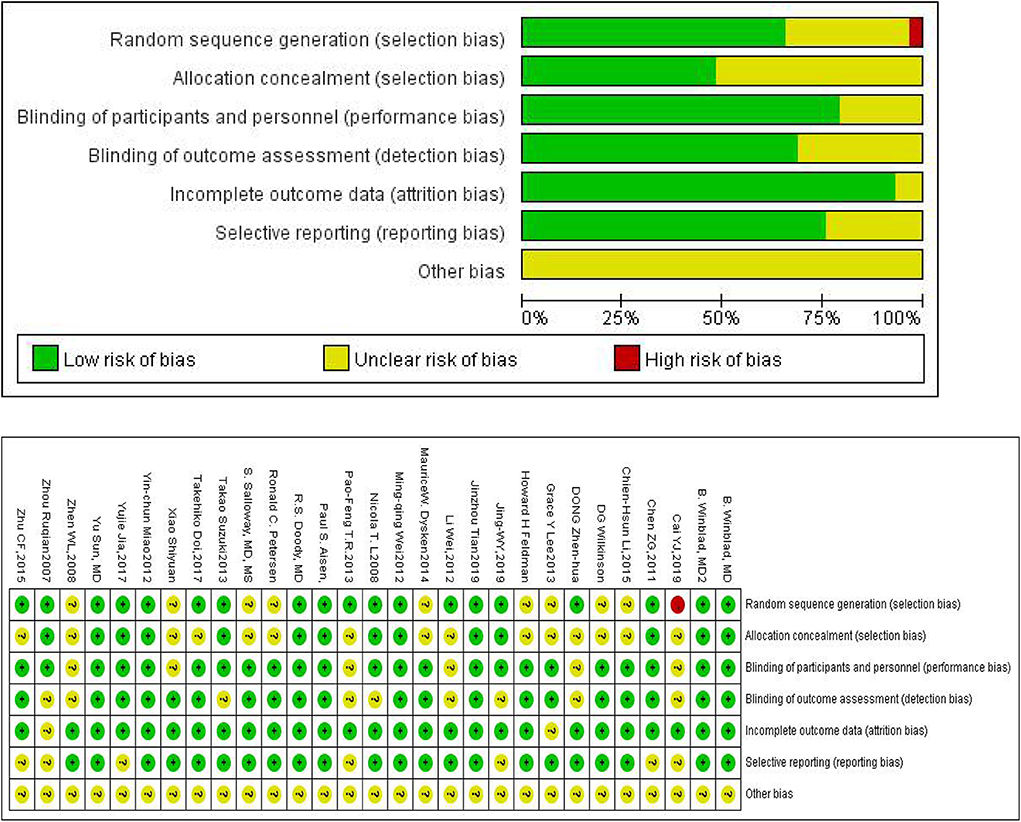
The Cochrane Risk of Bias Tool was used to assess the quality of all 28 trials included in our study (Figure 2 shows the summary risk of bias for selected studies). Among the 28 trials, 19 (66%) represented a random sequence generation process using a computer random number generator or a random number table. Fourteen trials (48.3%) described the use of allocation concealment methods, and 22 trials (75.9%) described the blinding methods for researchers and participants. Acupuncture, music therapy, and exercise therapy are non-pharmacologic therapies, therefore some participants and researchers involved in these studies were not able to be blinded. Six trials (21.4%) had an uncertain risk of outcome assessment and 25 trials (86.2%) had a low risk of attrition bias.
Pair-Wise Meta-Analysis
We conducted a classic pair-wise meta-analysis using a random-effects model to synthesize studies with the same pair of interventions. All interventions, except for nimodipine, had at least one placebo controlled trial. As for the MMSE outcome, the ChEIs (MD: −0.38; 95% CI: −0.74, −0.01), Chinese medicine (MD: −0.31; 95% CI: −0.75, 0.13), exercise therapy (MD: −0.50; 95% CI: −0.65, −0.35), music therapy (MD: −1.71; 95% CI: −4.49, 1.07), were worse than placebo,and other interventions were statistically more efficient than placebo. For ADAS-cog outcome, ChEIs (MD: 1.20; 95% CI: 0.73, 1.68), Acupuncture (MD: 1.36; 95% CI: 1.28, 1.44), Chinese medicine (MD: 0.61; 95% CI: 0.49, 0.73) and exercise (MD: 0.61; 95% CI: 0.49, 0.73) were better than placebo. The detailed results of the pair-wise meta-analysis are shown in Supplementary Datasheet 2.
Network Meta-Analysis
Primary Outcome: MMSE
We ran a network meta-analysis to thoroughly compare and assess different treatment rankings for MCI, the network of MMSE include 23 trails and 15 interventions,the network plot presented in Figure 3A. Node-splitting analysis was used to assess consistency, and all p-values between the direct and indirect effects were > 0.05. A PSRF value of 1 indicated that the model was convergent and the result was stable. Therefore, the consistency model was selected for the subsequent network analysis.
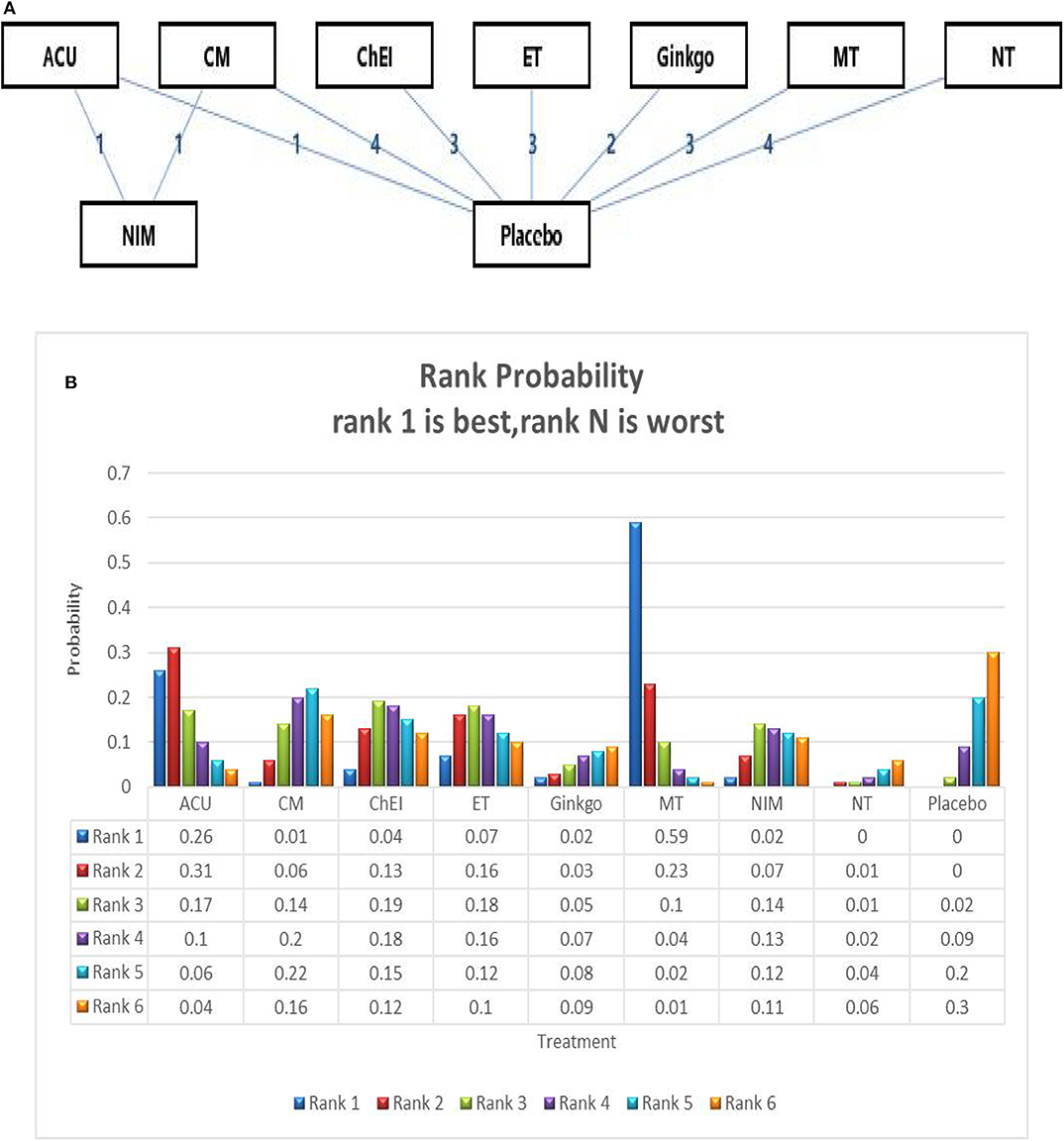
Figure 3. (A) The network structure of the analyzed treatment comparisons for the outcome of MMSE. (B) Rank probability of cognitive effects of MMSE. ACU, acupuncture; CM, Chinese medicine; CHEI, cholinesterase inhibitors; ET, exercise therapy; MT, music therapy; NIM, nimodipine; NT, nutrition therapy.
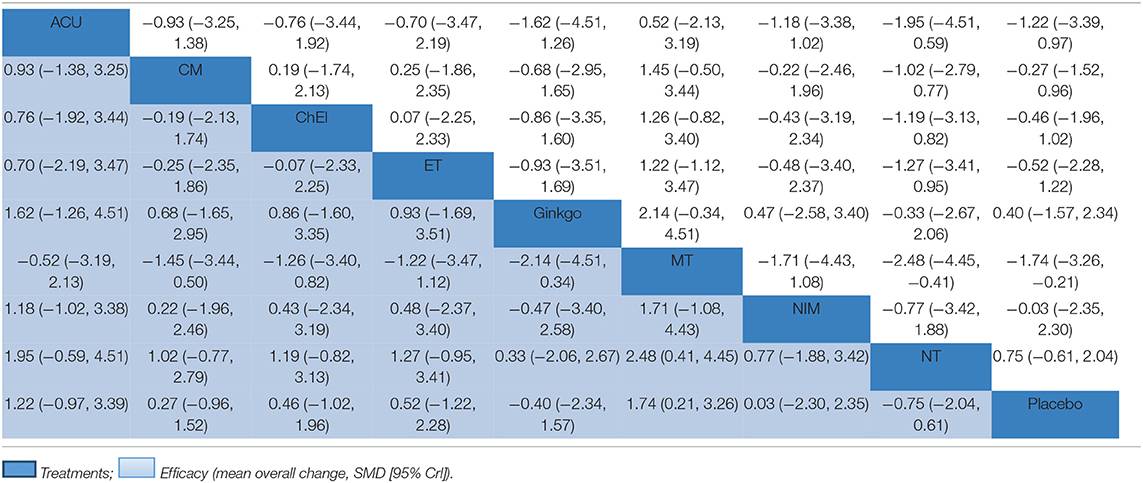
The network meta-analysis for the primary outcome (MMSE) is shown in Table 2. In terms of efficacy music therapy (MD: 1.74; 95% CI: 0.21, 3.26), Acupuncture (MD: 1.22; 95% CI:−0.97, 3.39), and exercise therapy (MD: 0.52; 95% CI: −1.22, 2.28) achieved better than placebo. Ginkgo (MD: −0.40; 95% CI: −2.34, 1.57) and nutrition therapy (MD: −0.75; 95% CI: −2.04, 0.61) were significantly less effective than other interventions and placebo. Other pharmacological therapies including Chinese medicine (MD: 0.27; 95% CI: −0.96, 1.52) and ChEIs (MD: 0.46; 95% CI: −1.02, 1.96) showed a slight improvement in MMSE scores; however, their efficacy in MCI needs further investigation. The ranking probability of MMSE is presented in Figure 3B, the results showed that music therapy had the highest probability (59%) of being the best treatment for MCI, followed by Acupuncture (26%) and then exercise (7%).
Secondary Outcome: ADAS-cog
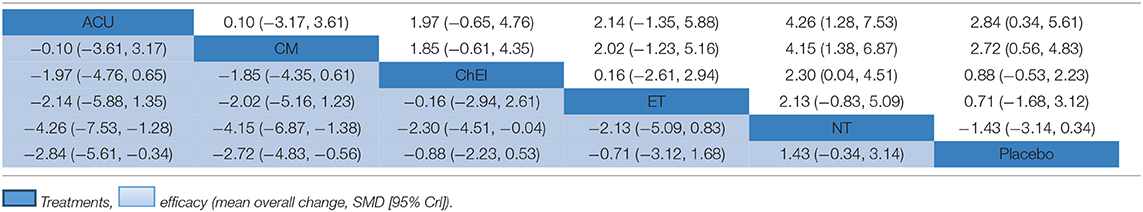
In relation to the secondary outcome (ADAS-cog score), 17 trails and 6 treatments were involved, 2 arms of Acupuncture, 6 arms of ChEIs, 3 arms of Chinese medicine, 2 arms of exercise therapy and 4 arms of nutrition therapy (presented in Figure 4A). After Node-splitting analysis,we adopted the consistency model to compare these different interventions. Chinese medicine (MD: −2.72; 95% CI: −4.83, −0.56), Acupuncture (MD: −2.84; 95% CI: −5.61, −0.34), exercise therapy (MD: −0.71; 95% CI: −3.12, 1.68), and ChEIs (MD: −0.88; 95% CI: −2.23, 0.53) were better than placebo. Nutrition therapy (MD: 1.43; 95% CI: −0.34, 3.14) was least effective compared to other interventions and placebo (shown in Table 3). The ranking probability of ADAS-cog is presented in Figure 3B. Acupuncture ranked the best (52%), it might be the most effective way to change the ADAS-cog score.
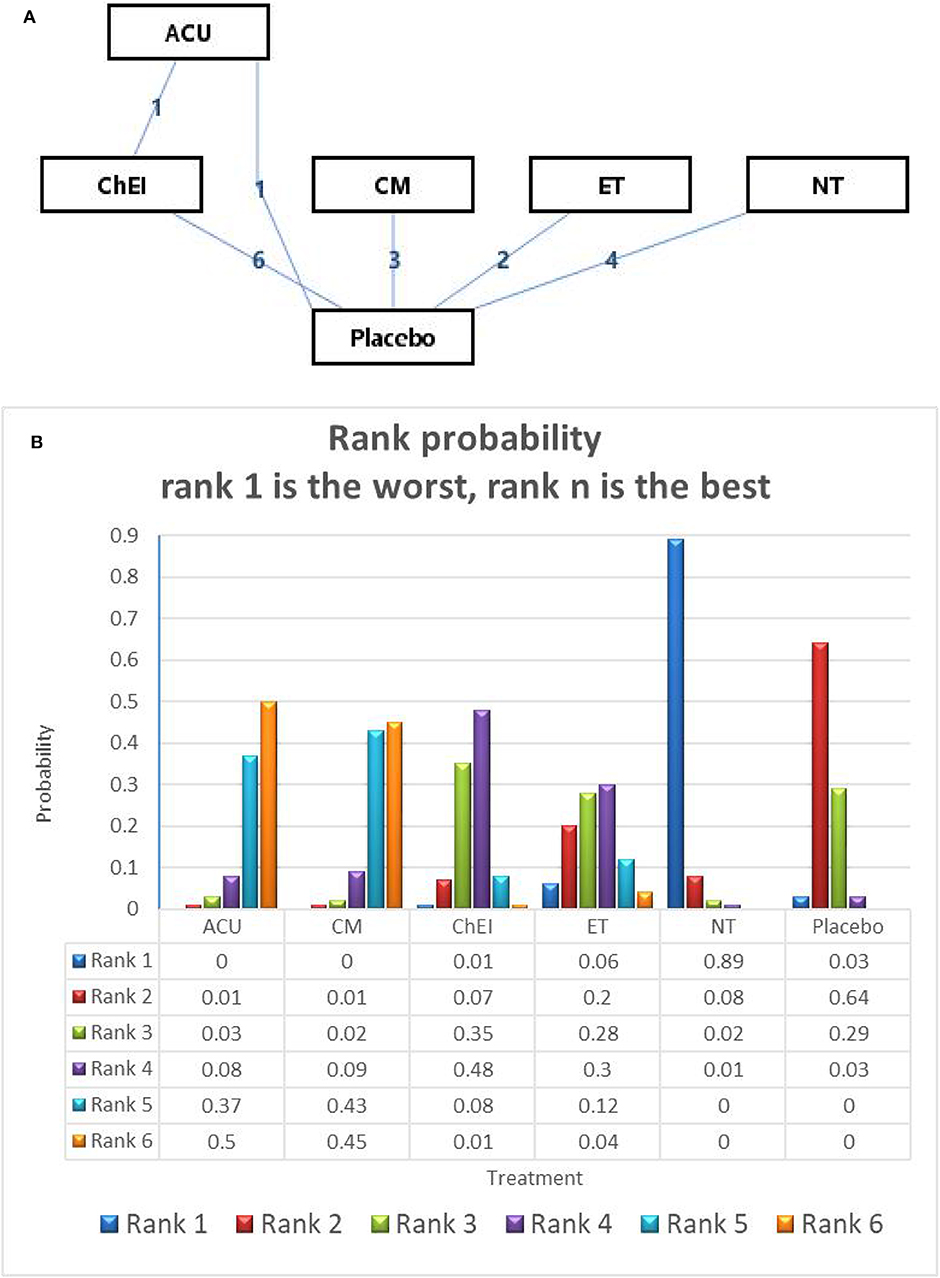
Figure 4. (A) The network structure of the analyzed treatment comparisons for the outcome of ADAS-cog. (B) Rank probability of cognitive effects of ADAS-cog.
Adverse Events
Out of 28 trials, 13 trails reported adverse events occurred, 14 trails did not mention whether there was adverse events and one trial stated no injures reported (Table 1). 9 out of 13 trials reported adverse events related to gastrointestinal discomforts such as diarrhea, nausea, vomiting and anorexia, these interventions were mainly ChEIs, nutrition therapy and Chinese medicine. 8 out of 13 trails reported insomnia as adverse effect which mainly related to ChEIs and nutrition interventions. 1 out of 13 trails reported cardiovascular problem due to exercises intervention and 1 out of 13 trials reported Acupuncture-fainting due to Acupuncture intervention.
Discussion
This study is the first network meta-analysis performed on potential pharmacological and non-pharmacological treatments for MCI, and has incorporated the most comprehensive data. The Bayesian statistical methods that we used allowed us to rank many different treatments by measuring comparable probability and then reporting them as the best, the second best, and so on. Through this method, we found that among all interventions, both pharmacological therapies (ChEIs, ginkgo, nimodipine, and Chinese medicine) and non-pharmacological therapies (acupuncture, music therapy, exercise, and nutrition therapy), music therapy and acupuncture were better than other treatments. Some pharmacological treatments like CHEIs such as donepezil, galantamine, and rivastigmine showed a slight efficacy to improve MMSE scores and cognitive function, but have many safety issues which require further clarification and study. In addition, ginkgo and nutrition therapy might be ineffective for MCI, which are thus not strongly recommended in clinical medicine.
Music therapy is one of the non-pharmacological therapies which incorporates active and passive therapy. Active music therapy can be defined as therapeutic music activities which involve active participation by clients, such as singing, dancing with music, playing an instrument, composing, and discussing clients' thoughts and feelings in regards to music-related activities in order to reach the optimal treatment effect. Passive music therapy, on the other hand, means achieving the treatment effect by listening and enjoying music. Passive music therapy is also called receptive music therapy and is widely used (Chang et al., 2015; Han et al., 2017; Claudia et al., 2019). Previous research has shown that music can activate some brain regions that govern cognitive function, affective function, and motor skills, and activate neurological stimulation which may develop new neural networks (Raglio et al., 2014; Mofredj et al., 2016). Evidence has shown that, in the context of patients with cognitive function decline, music therapy intervention increases cerebral blood flow and pre-frontal cortex activity (Shimizu et al., 2018).
Acupuncture has been shown to be a good management option in neurological diseases, with studies demonstrating acupuncture is associated with activation in motor-related brain regions as shown in functional magnetic resonance imaging (fMRI) (Chen et al., 2008, 2012; Chae et al., 2009). Other studies supplemented that Acupuncture is able to regulate the emotional components of the pain matrix, and inducing brain activation which provides a neurobiological basis of acupuncture (Shangjie et al., 2014; Shan et al., 2018). Research also suggests that acupuncture can activate resting brain networks, which incorporate anti-nociceptive, memory, and emotion brain regions. Our network meta-analysis suggests that music therapy is the optimal intervention to treat MCI patients and improve their cognitive function, whereas acupuncture was the second best option.Further research is required to assess other interventions not included in this meta-analysis. A recent study found that mindfulness therapy improved inflammatory biomarkers in patients with MCI (Ng et al., 2020). This provides an interesting perspective on the current management paradigms for MCI.
The adverse events reports showed that some participants experienced gastrointestinal reactions and insomnia due to ChEIs, nutrition and Chinese medicine interventions. However, none of these events was related to cognitive distress. Other small samples such as exercise and Acupuncture reported therapy related adverse effects such as cardiovascular problems and Acupuncture fainting respectively. Music therapy didn't report any adverse events.
There were limitations to our study. Firstly, a few RCTs showed potential bias because of the small number of participants and elective reporting. Fortunately, there was no obvious inconsistency or heterogeneity shown in this network meta-analysis, but there is a possibility that some included articles might have overestimated the effectiveness of treatments, and this might have influenced our results. Secondly, a few included reports were non-pharmaceutical therapies which cannot be blinded to participants, especially acupuncture. However, blinding of outcome assessment and single-blind methodologies should be used where possible to reduce the potential for any bias. Thirdly, we have excluded some drug interventions because of our selection criterion of outcome measures, which may influence the strength of evidence.
Conclusion
The findings of this comprehensive network meta-analysis provide some evidence that music therapy and acupuncture might improve the cognitive function of patients with MCI. Our results indicate that music therapy and, to a lesser extent, acupuncture may be the preferred options for treatment of MCI. Ginkgo and nutrition therapy do not seem to be adequate as regular treatment options. Our study provides new insights into the clinical treatments available for MCI, and may help the development of guidelines for the management of MCI.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
CT and LL designed the study. XL, YL, and HW collected the data. XL performed all analysis. XL, YL, and HW wrote the manuscript. All authors contributed to writing of this manuscript.
Funding
This work was funded by grants from National Natural Science Foundation of China (No. 81873375).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.00121/full#supplementary-material
References
Aisen, P. S., Schneider, L. S., Sano, M., Diaz-Arrastia, R., Van, C. H., Weiner, M. F., et al. (2008). High-dose B vitamin supplementation and cognitive decline in Alzheimer's disease: a randomized controlled trial. JAMA 300, 1774–1783. doi: 10.1001/jama.300.15.1774
Almkvist, O., Basun, H., Backman, L., Lannfelt, L., Small, B., Viitanen, M., et al. (1998). Mild cognitive impairment—an early stage of Alzheimer's disease. J. Neural Transm. Suppl. 54, 21–29. doi: 10.1007/978-3-7091-7508-8_3
Andreas, S., Dietlinde, K. S., Florian, S., Nina, F., Theresa, S., Rainer, H., et al. (2015). Exercise Treatment of Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am. J. Geriatric Psychiatry 23, 1234–1249. doi: 10.1016/j.jagp.2015.07.007
Brendan, J., and Kelley, M. D. (2015). Treatment of mild cognitive impairment. Curr Treat Options Neurol. 17:40. doi: 10.1007/s11940-015-0372-3
Cai, Y. J., Xu, H. L., Cao, J. Q., Cao, Y. F., and Yuan, S. (2019). Effect analysis of neuromuscular therapy in young people with amnestic mild cognitive impairment. Sci. Technol. Wind 232–236. doi: 10.19392/j.cnki.16717341.201922202
Chae, Y. Y., Lee, H. J., Kim, H. J., Sohn, H. J., Park, J. H., and Park, H. J. (2009). The neural substrates of verum acupuncture compared to non-penetrating placebo needle: an fMRI study. Neurosci. Lett. 450, 80–84. doi: 10.1016/j.neulet.2008.11.048
Chang, Y. S., Chu, H., Yang, C. Y., Tsai, J. C., Chung, M. H., Liao, Y. M., et al. (2015). The efficacy of music therapy for people with dementia: a meta-analysis of randomised controlled trials. J. Clin. Nurs. 24, 3425–3440. doi: 10.1111/jocn.12976
Chen, S. J., Liu, B., Fu, W., Wu, S., Chen, J., and Ran, P. (2008). A fMRI observation on different cererbral regions activated by acupuncture of Shenmen (HT 7) and Yanglao (SI 6). Zhen Ci Yan Jiu. 33, 267–271. doi: 10.1007/s11726-008-0242-6
Chen, S. J., Meng, L., Yan, H., Bai, L., Wang, F., Huang, Y., et al. (2012). Functional organization of complex brain networks modulated by acupuncture at different acupoints belonging to the same anatomic segment. Chin. Med. J. 125, 2694–2700. doi: 10.3760/cma.j.issn.0366-6999.2012.15.009
Chen, Z. G. (2011). Multi-Center Randomized Controlled Study of Electroacupuncture on Acupoints for Treatment of Mild Cognitive Impairment. Chengdu University of Traditional Chinese Medicine.
Cheung, M. W., Roger, C. M., Yonghao, L. I. M., and Anselm, M. A. K. (2012). Conducting a meta-analysis: basics and good practices. Int. J. Rheum. Dis. 15, 129–135. doi: 10.1111/j.1756-185X.2012.01712.x
Claudia, J. F., Murrock, C. J., Guerrero, P. I. C., and Salazar-González, B. C. (2019). Music therapy intervention in community-dwelling older adults with mild cognitive impairment: a pilot study. Geriatric Nurs. 40, 614–619. doi: 10.1016/j.gerinurse.2019.06.004
Collie, A., and Maruff, P. (2000). The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neurosci. Biobehav. Rev. 24, 365–374. doi: 10.1016/S0149-7634(00)00012-9
Cooper, C., Li, R., Lyketsos, C., and Livingston, G. (2013). Treatment for mild cognitive impairment: systematic review. Br. J. Psychiatry. 203, 255–264. doi: 10.1192/bjp.bp.113.127811
Doi, T., Verghese, J., Makizako, H., Tsutsumimoto, K., Hotta, R., Nakakubo, S., et al. (2017). Effects of cognitive leisure activity on cognition in mild cognitive impairment: results of a randomized controlled trial. J Am Med Dir Assoc. 18, 686–691. doi: 10.1016/j.jamda.2017.02.013
Dong, Z. H., Zhang, C. Y., and Pu, B. H. (2012). Effects of ginkgo biloba tablet in treating mild cognitive impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi 32, 1208–1211. doi: 10.3736/jcim20120605
Doody, R. S., Ferris, S. H., Salloway, S., Sun, Y., Goldman, R., Watkins, W. E., et al. (2009). Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology 72, 1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03
Dysken, M. W., Sano, M., Asthana, S., Vertrees, J. E., Pallaki, M., Llorente, M., et al. (2014). Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 311, 33–44. doi: 10.1001/jama.2013.282834
Grace, Y. L., Calvin, C. K. Y., Edwin, C. S., and David, W. K. (2013). Evaluation of a computer assisted errorless learning based memory training program for patients with early Alzheimer's disease in Hong Kong: a pilot study. Clin. Interv. Aging 8, 623–633. doi: 10.2147/CIA.S45726
Han, J. W., Lee, H., Hong, J. W., Kim, K., Kim, T., Byun, H. L., et al. (2017). Multimodal cognitive enhancement therapy for patients with mild cognitive impairment and mild dementia: a multi- center, randomized, controlled, double-blind, crossover trial. J. Alzheimers Dis. 55, 787–796. doi: 10.3233/JAD-160619
Howard, H. F., and Roger, L. (2007). Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer's disease. J. Neurol. Psychiatry 78, 1056–1063. doi: 10.1136/jnnp.2006.099424
Jia, Y. J., Zhang, X. Z., Yu, J., Han, J., Yu, T., Shi, J., et al. (2017). Acupuncture for patients with mild to moderate Alzheimer's disease: a randomized controlled trial. BMC Complement. Altern. Med. 17:556. doi: 10.1186/s12906-017-2064-x
Kluger, A., Ferris, S. H., Golomb, J., Mittelman, M. S., and Reisberg, B. (1999). Neuropsychological prediction of decline to dementia in nondemented elderly. J. Geriatr. Psychiatry Neurol. 12, 168–179. doi: 10.1177/089198879901200402
Lautenschlager, N. T., Cox, K. L., Flicker, L., Foster, J. K., van Bockxmeer, F. M., Xiao, J., et al. (2008). Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 300, 1027–1037. doi: 10.1001/jama.300.9.1027
Li, C. H., Liu, C. K., Yang, Y. H., Chou, M. C., and CH Lai, C. L. Adjunct effect of music therapy on cognition in Alzheimer's disease in Taiwan: a pilot study. Neuropsych Dis Treat. (2015) 11. doi: 10.2147/NDT.S73928
Liang, J. H., Xu, Y., Lin, L., Jia, R. X., Zhang, H. B., and Hang, L. (2018). Comparison of multiple interventions for older adults with Alzheimer's disease or mild cognitive impairment: a PRISMA-compliant network meta-analysis. Medicine 97:e10744. doi: 10.1097/MD.0000000000010744
Matsunaga, S., Fujishiro, H., and Takechi, H. (2019). Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta-analysis. J. Alzheimers Dis. 71, 513–523. doi: 10.3233/JAD-190546
Miao, Y., Tian, J., Shi, J., and Mao, M. (2012). Effects of Chinese medicine for tonifying the kidney and resolving phlegm and blood stasis in treating patients with amnestic mild cognitive impairment: a randomized, double-blind and parallel-controlled trial. Zhong Xi Yi Jie He Xue Bao 10, 390–397. doi: 10.3736/jcim20120407
Mofredj, A., Alaya, S., Tassaioust, K., Bahloul, H., and Mrabet, A. (2016). Music therapy, a review of the potential therapeutic benefits for the critically ill. J. Crit. Care 35, 195–199. doi: 10.1016/j.jcrc.2016.05.021
Morris, J. C., Storandt, M., Miller, J. P., McKeel, D. W., Price, J. L., Rubin, E. H., et al. (2001). Mild cognitive impairment represents early-stage Alzheimer's disease. Arch. Neurol. 58, 397–405. doi: 10.1001/archneur.58.3.397
Ng, T. K. S., Fam, J., Feng, L., Cheah, I. K., Tan, C. T., Nur, F., et al. (2020). Mindfulness improves inflammatory biomarker levels in older adults with mild cognitive impairment: a randomized controlled trial. Transl Psychiatry. 10:21. doi: 10.1038/s41398-020-0696-y
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194. doi: 10.1111/j.1365-2796.2004.01388.x
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Petersen, R. C., Thomas, R. G., Grundman, M., Bennett, D., Doody, R., Ferris, S., et al. (2005). Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 352, 2379–2388. doi: 10.1056/NEJMoa050151
Raglio, A., Filippi, S., Bellandi, D., and Stramba-Badiale, M. (2014). Global music approach to persons with dementia: evidence and practice. Clin. Interv. Aging 9, 1669–1676. doi: 10.2147/CIA.S71388
Rountree, S. D., Waring, S. C., Chan, W. C., Lupo, P. J., Darby, E. J., and Doody, R. S. (2007). Importance of subtle amnestic and nonamnestic deficits in mild cognitive impairment: prognosis and conversion to dementia. Dement. Geriatr. Cogn. Disord. 24, 476–482. doi: 10.1159/000110800
Rubin, E., Morris, J., Grant, E., and Vendegna, T. (1989). Very mild senile dementia of the Alzheimer type. I. Clinical assessment. Arch. Neurol. 46, 379–382. doi: 10.1001/archneur.1989.00520400033016
Salloway, S., Ferris, S., Kluger, A., Goldman, R., Griesing, T., Kumar, D., et al. (2004). Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 63, 651–657. doi: 10.1212/01.WNL.0000134664.80320.92
Savovic, J., Weeks, L., Sterne, J. A., Turner, L., Altman, D. G., Moher, D., et al. (2014). Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst. Rev. 3:37. doi: 10.1186/2046-4053-3-37
Shan, Y., Wang, J., Wang, Z., Zhao, Z., Zhang, M., Xu, J., et al. (2018). Neuronal specificity of acupuncture in Alzheimer's disease and mild cognitive impairment patients: a functional MRI study. Evid. Based Complement. Altern. Med. 2018:7619197. doi: 10.1155/2018/7619197
Shangjie, C., Maosheng, X., Hong, L., Jiuping, L., Liang, Y., Xia, L., et al. (2014). Acupuncture at the Taixi(KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment. Neural Regener. Res. 9, 1163–1168. doi: 10.4103/1673-5374.135319
Shimizu, N., Umemura, T., Matsunaga, M., and Hirai, T. (2018). Effects of movement music therapy with a percussion instrument on physical and frontal lobe function in older adults with mild cognitive impairment: a randomized controlled trial. Aging Ment. Health 22, 1614–1626. doi: 10.1080/13607863.2017.1379048
Sun, Y., Lu, C. J., Chien, K. L., Chen, S. T., and Chen, R. C. (2007). Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin. Ther. 29, 2204–2214. doi: 10.1016/j.clinthera.2007.10.012
Suzuki, T., Shimada, H., Makizako, H., Doi, T., Yoshida, D., Ito, K., et al. (2013). Randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE 8:e61483. doi: 10.1371/journal.pone.0061483
Tian, J., Shi, J., Wei, M., Ni, J., Fang, Z., Gao, J., et al. (2019). Chinese herbal medicine Qinggongshoutao for the treatment of amnestic mild cognitive impairment: a 52-week randomized controlled trial. Alzheimers Dement. 5, 441–449. doi: 10.1016/j.trci.2019.03.001
Tricco, A. C., Soobiah, C., Berliner, S., Ho, J. M., Ng, C. H., Ashoor, H. M., et al. (2013). Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. Can. Med. Assoc. J. 185, 1393–1401. doi: 10.1503/cmaj.130451
Tsai, P. F., Chang, J. Y., Beck, C., Kuo, Y. F., and Keefe, F. J. (2013). A pilot cluster-randomized trial of a 20-week tai chi program in elders with cognitive impairment and osteoarthritic knee: effects on pain and other health outcomes. J. Pain Symptom. Manage. 45, 660–669. doi: 10.1016/j.jpainsymman.2012.04.009
Wei, M. Q., Tian, J. Z., Shi, J., Ma, F., Miao, Y., and Wang, Y. (2012). Effects of Chinese medicine for promoting blood circulation and removing blood stasis in treating patients with mild to moderate vascular dementia: a randomized, double-blind and parallel-controlled trial. Zhong Xi Yi Jie He Xue Bao 10, 1240–1246. doi: 10.3736/jcim20121107
Wilkinson, D. G., Passmore, A. P., Bullock, R., Hopker, S. W., Smith, R., Potocnik, F. C. V., et al. (2002). A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. Int. J. Clin. Pract. 56, 441–446. doi: 10.1175/1520-0450(1995)0342.0.CO;2
Winblad, B., Gauthier, S., Scinto, L., Feldman, H., Wilcock, G. K., Truyen, L., et al. (2008). Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 70, 2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26
Wolf, H., Grunwald, M., Ecke, G., Zedlick, D., Bettin, S., Dannenberg, C., et al. (1998). The prognosis of mild cognitive impairment in the elderly. J. Neural Transm. Suppl. 54, 31–50. doi: 10.1007/978-3-7091-7508-8_4
Xiao, S., Zhang, C., Li, J., Liu, Y., and Zhao, M. (2011). Clinical study of Ginkgo biloba G (Styron) in the treatment of mild cognitive impairment. Tradit. Chinese Patent Med. 33, 751–754. doi: 10.3969/j.issn.1001-1528.2011.05.006
Yang, J., Shi, G., Zhang, S., Tu, J., Wang, L., Yan, C., et al. (2019). Effectiveness of acupuncture for vascular cognitive impairment no dementia: a randomized controlled trial. Clin. Rehabilit. 33, 642–652. doi: 10.1177/0269215518819050
Zheng, W., Zhang, D., and Liu, Y. (2008). Clinical study on Yiqihuoxue therapy for mild cognitive dysfunction. Xinjiang Traditional Chinese Medicine 5–7. doi: 10.3969/j.issn.1009-3931.2008.03.004
Zhou, R., Lin, S., and Yuan, Q. (2007). Clinical study of Shenyin Oral Liquid in treating mild cognitive impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi 27, 793–795.
Keywords: acupuncture, Alzheimer's disease, mild cognitive impairment, multiple interventions, music therapy, network meta-analysis
Citation: Lai X, Wen H, Li Y, Lu L and Tang C (2020) The Comparative Efficacy of Multiple Interventions for Mild Cognitive Impairment in Alzheimer's Disease: A Bayesian Network Meta-Analysis. Front. Aging Neurosci. 12:121. doi: 10.3389/fnagi.2020.00121
Received: 17 December 2019; Accepted: 09 April 2020;
Published: 05 June 2020.
Edited by:
Woon-Man Kung, Chinese Culture University, TaiwanReviewed by:
Tae-Hun Kim, Kyung Hee University, South KoreaRoger C. Ho, National University of Singapore, Singapore
Dafin F. Muresanu, Iuliu Haţieganu University of Medicine and Pharmacy, Romania
Copyright © 2020 Lai, Wen, Li, Lu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunzhi Tang, am9yZGFuNjY0QDE2My5jb20=
 Xin Lai
Xin Lai Hao Wen
Hao Wen Chunzhi Tang
Chunzhi Tang