
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 13 March 2020
Sec. Alzheimer's Disease and Related Dementias
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.00044
This article is part of the Research Topic Translational Advances in Alzheimer's, Parkinson's, and other Neurodegenerative Dementias View all 70 articles
 Shan Wei1
Shan Wei1 Ling Gao1
Ling Gao1 Yu Jiang1
Yu Jiang1 Suhang Shang1
Suhang Shang1 Chen Chen1
Chen Chen1 Liangjun Dang1
Liangjun Dang1 Jin Wang1
Jin Wang1 Kang Huo1
Kang Huo1 Jingyi Wang2
Jingyi Wang2 Qiumin Qu1*
Qiumin Qu1*Objectives: Till now, the effect of serum lipid levels on cognitive function is still controversial. The apolipoprotein E (APOE) ε4 allele is the most critical genetic risk factor for Alzheimer’s disease (AD) and cognitive impairment. Additionally, APOE ε4 allele has a major impact on lipid metabolism. The aim of this study was to investigate the APOE genotype-dependent relationship between peripheral serum lipid levels and cognitive impairment.
Methods: A total of 1,273 subjects aged 40–86 years participated in this cross-sectional study. Serum lipid levels and the APOE genotype were detected. Mini-Mental State Examination was used to diagnose the cognitive impairment or not. Univariate and multivariate analyses were used to analyze the relationships between APOE genotype, serum lipid levels, and cognition function.
Results: After controlling for all possible covariates, a significant interaction between low serum high-density lipoprotein and the APOE ε4 allele on cognitive impairment (Wald’s χ2 = 4.269, df = 1, OR = 20.094, p = 0.039) was found in the total participants. In APOE ε4 carriers, low serum high-density lipoprotein was positively associated with cognitive impairment (Wald’s χ2 = 8.200, df = 1, OR = 60.335, p = 0.004) and serum high-density lipoprotein levels were positively correlated with Mini-Mental State Examination score (r = 0.217, df = 176, p = 0.004). There was no significant correlation between serum total cholesterol (TC), low-density lipoprotein, triglycerides (TG) levels, and cognitive impairment in either the total participants or APOE ε4 carriers/non-carriers.
Conclusions: APOE ε4 carriers, but not non-carriers, with lower serum high-density lipoprotein had a higher prevalence of cognitive impairment and a lower Mini-Mental State Examination score. These results suggest that the APOE ε4 allele may affect the relationship between serum lipid levels and cognitive impairment. However, the specific mechanism needs to be further elucidated.
Alzheimer’s disease (AD) is the most common type of dementia, and its prevalence has been increasing rapidly in China (Jia et al., 2014). A recent study revealed that, in China, the total annual socioeconomic costs of AD patients were US $167.74 billion in 2015 and were predicted to reach US $1.89 trillion in 2050, imposing an immense burden on patients and their families (Jia et al., 2018). Due to the complexity of the pathogenesis of AD, there are currently no unified and effective approaches for preventing or curing AD.
In the brain, amyloid-β (Aβ) accumulation and the formation of insoluble extracellular senile plaques are pathological hallmarks of AD. Aβ is produced by the endoproteolysis of amyloid precursor protein (APP). When cleaved by β-secretase and γ-secretase, APP is mainly hydrolyzed into the 38–43 amino acid residue Aβ peptide (De Felice and Ferreira, 2002), and senile plaques are mainly composed of amyloid Aβ40 and amyloid Aβ42 (Masters et al., 1985). Researchers have found that reducing cellular cholesterol levels appears to inhibit β-secretase and γ-secretase activity and, thus, decrease the amount of Aβ secreted by neurons (Wahrle et al., 2002; Subasinghe et al., 2003). The responsiveness of Aβ production to cholesterol levels suggests that cholesterol metabolism plays an essential role in the pathogenesis of AD (Hartmann et al., 2007). However, there are still no consistent results of epidemiological studies concerning the role of cholesterol as a risk factor for AD, although some studies have demonstrated that elevated cholesterol increases the risk of AD development, particularly in middle-aged individuals (Kivipelto et al., 2001; Whitmer et al., 2005). Other researchers have failed to confirm this result (Reitz et al., 2004; Tukiainen et al., 2012).
Apolipoprotein E (APOE) ε4 is the most important genetic risk factor for AD. APOE is a polymorphic protein involved in the development of late-onset AD, although the mechanism has not been fully elucidated (Siest et al., 2000). There are three alleles of APOE (E2, E3, and E4) that produce three homozygous (E2/2, E3/3, and E4/4) and three heterozygous (E2/3, E2/4, and E3/4) isoforms (Zannis and Breslow, 1981). Pathophysiological studies show that APOE immunoreactivity exists in senile plaques, indicating a significant role of APOE in the metabolism of Aβ (Kim et al., 2009; Castellano et al., 2011), which is thought to initiate toxic events and further have distinct functions in regulating tau hyperphosphorylation, synaptic plasticity, cell signaling, lipid transport and metabolism, and neuroinflammation (Yu et al., 2014; Giau et al., 2015). Additionally, APOE ε4 has a major impact on lipid metabolism (Mahley, 1988). The APOE gene modulates serum concentrations of lipid and lipoproteins according to its high affinity for binding to cell-surface lipoprotein receptors. Previous research has reported that carriers of APOE ε4 have a higher risk of hyperlipidemia (Dallongeville et al., 1992).
Considering that the APOE genotype plays an important role in both AD pathogenesis and lipid metabolism, we investigated the effects of the APOE genotype on the relationships between peripheral serum lipid levels and cognitive impairment in Chinese middle-aged and elderly subjects from Qubao village in the suburbs of Xi’an, northwest China.
Between December 2016 and April 2017, 1,865 subjects were recruited from Qubao village in the suburbs of Xi’an, China. Inclusion criteria were as follows: being 40 years old or older; having lived in Qubao for more than 3 years; agreeing to participate in the study and completing the questionnaire; and having venous blood collected. The exclusion criteria were as follows: (1) participants who had used lipid-lowering drugs in the last 3 months; (2) participants who had severe liver, kidney, thyroid, and hematopoietic system diseases; (3) participants who had suffered from a clear history of acute cerebrovascular disease, including stroke; (4) participants who had suffered from severe nervous system diseases that can cause cognitive impairment, including infection, Parkinson’s disease, epilepsy, congenital intellectual disability, and craniocerebral operations; (5) participants who had suffered from other physical and chemical factors that led to cognitive impairment (drug poisoning, alcoholism, and carbon monoxide poisoning); (6) participants who had suffered from severe psychopathy, including schizophrenia, bipolar disorder, severe depression or anxiety; and (7) participants without a complete MMSE score, biomarkers, or covariates. Considering all the inclusion and exclusion criteria, 1,273 subjects were included in our study (Figure 1).
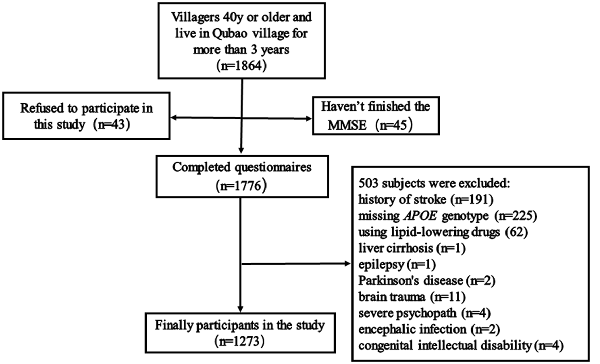
Figure 1. Flow chart of participant selection. Qubao village: the suburbs of Xi’an in northwest China. APOE, apolipoprotein E.
All of the participants were asked to complete the questionnaire survey in a structured in-person interview given by trained interviewers. The questionnaire included basic information (gender, age, education, and marital status), lifestyle (exercise, smoking, and alcohol consumption), and medical history (hypertension, diabetes, dyslipidemia, cerebrovascular disease, and heart disease). The definitions of hypertension and diabetes are consistent with those in our previous articles (Jia et al., 2018). According to the diagnostic criteria of Chinese adult dyslipidemia prevention guide (2007 edition; Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults, 2007), people who meet any of the following criteria can be defined as dyslipidemia: high total cholesterol (TC): TC ≥ 5.18 mmol/L; high low-density lipoprotein (LDL-c): LDL-c ≥ 3.37 mmol/L; low high-density lipoprotein (HDL-c): HDL-c < 1.04 mmol/L; high triglycerides (TG): TG ≥ 1.70 mmol/L. In addition, anthropometric measurements of participants were taken at the scene, including blood pressure, pulse rate, height, weight, and waist and hip circumference. The body mass index (BMI) was weight (kg) divided by the square of height in meters, and the waist–hip ratio was the waist circumference divided by the hip circumference.
The Mini-Mental State Examination (MMSE) questionnaire was completed by the participants to assess global cognitive function. The MMSE was chosen by Katzman et al. (1988) based on a Chinese version that had been shown to have good sensitivity and specificity. Participants with scores below the cutoff value were considered to have cognitive impairment. The cutoff values were as follows: scores ≤17 for illiteracy, scores ≤20 for participants who only finished primary school, and scores ≤24 for participants with junior high school or above level of education.
After completing the questionnaire, 10 ml of blood was extracted from the elbow vein of each participant under the condition of fasting for more than 8 h, which was put into a purple-top EDTA-anticoagulant tube and a red-top non-anticoagulant tube. The red-top tube blood samples were sent to the biochemical laboratory of the First Affiliated Hospital of Xi’an Jiaotong University for biochemical assessment [HDL-c, LDL-c, TG, TC, and fasting blood glucose (FBG)]. The concentrations of serum HDL-c, LDL-c, TC, TG, and FBG levels were tested by enzymatic method using an automated biochemical analyzer (C501, Roche, Sweden). The purple-top tube blood samples were centrifuged within 2 h after collection at a rate of 3,000 revolutions per second for 10 min, and all of the samples were stored in the refrigerator at −80°C for future analysis. An extraction kit (Tiangen Co. Beijing, China) was used to extract DNA from the frozen EDTA–anticoagulant blood according to the manufacturer’s protocol. Using human genome DNA as a template, the 244-bp length of the target DNA fragment that included two polymorphic sites at amino acid residues 112 and 158 (Mahley and Rall, 2000) was amplified by a PCR thermocycler. All PCR products were detected by Sanger sequencing (Sangon Company, Shanghai, China) to finally determine the APOE genotype.
SPSS 18.0 software (SPSS Inc., IBM, Chicago, IL, USA) was used to analyze all of the data. First, participants were divided into a dyslipidemia group and a non-dyslipidemia group. In addition, according to the APOE genotype, the participants could also be divided into APOE ε4 non-carriers (E2/2, E2/3, and E3/3) or APOE ε4 carriers (E2/4, E3/4, and E4/4). Unpaired Student’s t-tests and the mean ± SD were used for data that were approximately normally distributed; the Mann–Whitney U test and the median (quartiles) were used for skewed data distributions, and the Pearson χ2 test and percentages were used for categorical data. A p-value of <0.05 (two-tailed) was considered statistically significant.
Then, a χ2 test or Fisher’s exact test was used to compare the differences in cognitive impairment between serum lipid groups in the total participants and in the subgroups according to APOE ε4 status. For multivariate analysis, binary logistic regression was used to correct for covariates, including gender, age, education years, smoking, drinking, physical activity, medical history, FBG, mean arterial pressure (MAP), pulse rate, and BMI.
Finally, partial correlation analysis was used to research the correlations between MMSE score and serum lipid levels in the subgroups according to the APOE ε4 status. The covariates included age, sex, education years, smoking, drinking, intensity of physical activity, BMI, log-transformed FBG, MAP, pulse rates, and heart disease.
A total of 1,273 subjects ranging from 40 to 86 years old (mean 57.1 ± 9.7 years) were included in the study, including 755 (59.3%) women. Participants with dyslipidemia comprised 59.2% (754) of the total population. Ninety-nine (7.8%) subjects met the diagnostic criteria of cognitive impairment, and a total of 189 people (14.8%) were APOE ε4 carriers. Table 1 shows the demographic characteristics of the total participants. There were significant differences in gender, education, smoking, hypertension, diabetes mellitus status, MAP, pulse rate, FBG level, BMI, and APOE ε4 carrier status between the dyslipidemia group and the normal lipids group.
As shown in Figure 2, in the total samples, the current prevalence of cognitive impairment was not significantly different between the normal serum lipids group and the dyslipidemia group. The stratified analyses according to APOE ε4 status showed that the current prevalence of cognitive impairment was still not significantly different between any of the serum lipid groups in APOE ε4 carriers or non-carriers.
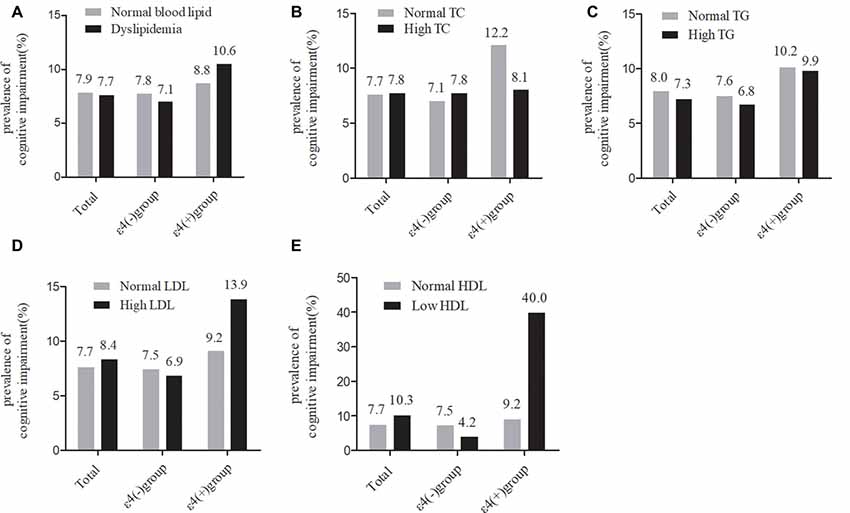
Figure 2. Comparison of the differences in cognitive impairment between serum lipid groups in the total participants and in subgroups according ApoEε4 status. TC, total cholesterol; LDL-c, low-density lipoprotein; HDL-c, high-density lipoprotein; TG, triglycerides; APOE, apolipoprotein E. (A–D) χ2 test. (E) Fisher’s exact test.
To eliminate the influence of covariates, binary logistic regression analysis was performed. In the total samples, after adjusting for age, gender, and education, no significant correlation was found between high TG, high TC, high LDL, low HDL levels, and cognitive impairment (Table 2, Model 1). When continuing to bring other covariates into the binary logistic regression models, the results were approximately the same (Table 2, Model 2). However, with the binary logistic regression analysis of the interaction between the serum lipids and APOE genotype on cognitive impairment, we found that the interaction between low HDL and APOE ε4 status was positively correlated with cognitive impairment (Wald’s χ2 = 4.269, df = 1, OR = 20.094, 95% CI = 1.167–346.056, p = 0.039), and the interaction between other serum lipid parameters (high TG, high TC, and high LDL levels) and APOE ε4 status had no significant effect on cognitive impairment (Table 2, Model 3).
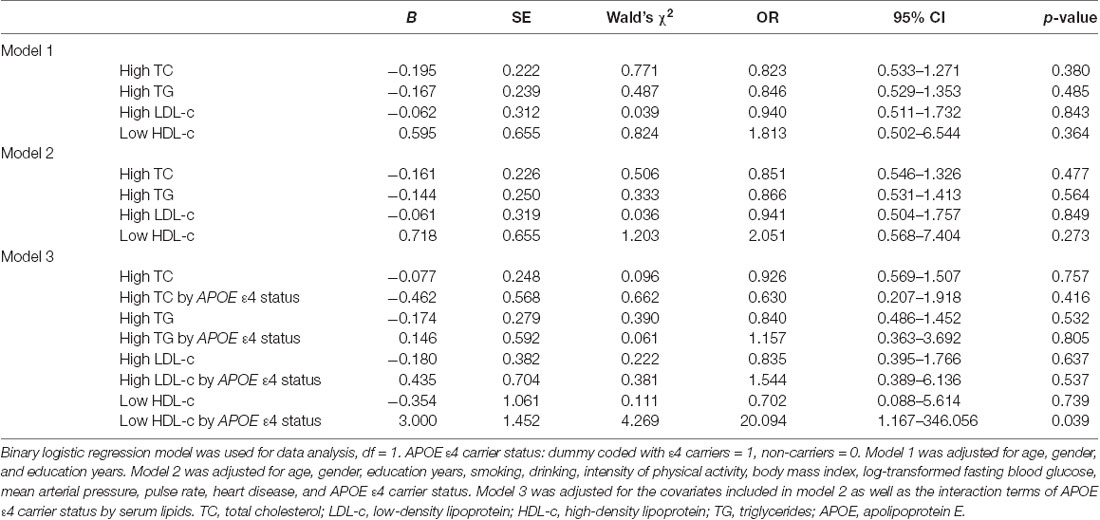
Table 2. The relationships between serum lipid parameters and cognitive impairment with binary logistic regression in the total samples.
Univariate analysis showed that compared with APOE ε4 non-carriers, serum TC, TG, and LDL levels were higher and HDL levels were lower in APOE ε4 carriers [TC: 5.36 ± 0.94 mmol/L vs. 5.15 ± 0.99 mmol/L, df = 1,271, p = 0.008; TG: 1.48 (1.09, 2.15) mmol/L vs. 1.29 (0.96, 1.80) mmol/L, p < 0.001; LDL: 2.82 ± 0.62 mmol/L vs. 2.61 ± 0.66 mmol/L, df = 1,271, p < 0.001; HDL: 1.53 ± 0.32 mmol/L vs. 1.60 ± 0.34 mmol/L, df = 1,271, p = 0.007], and the prevalence of dyslipidemia was also significantly higher in APOE ε4 carriers [132 (69.8%) vs. 622 (57.4%), df = 1, p = 0.001]. The current prevalence of cognitive impairment was not significantly different between APOE ε4 carriers and non-carriers. Other covariates (age, gender, degree of education, smoking, drinking, intensity of physical activity, hypertension, diabetes mellitus, coronary heart disease, MAP, and BMI) also showed no significant difference between the two groups (Table 3).
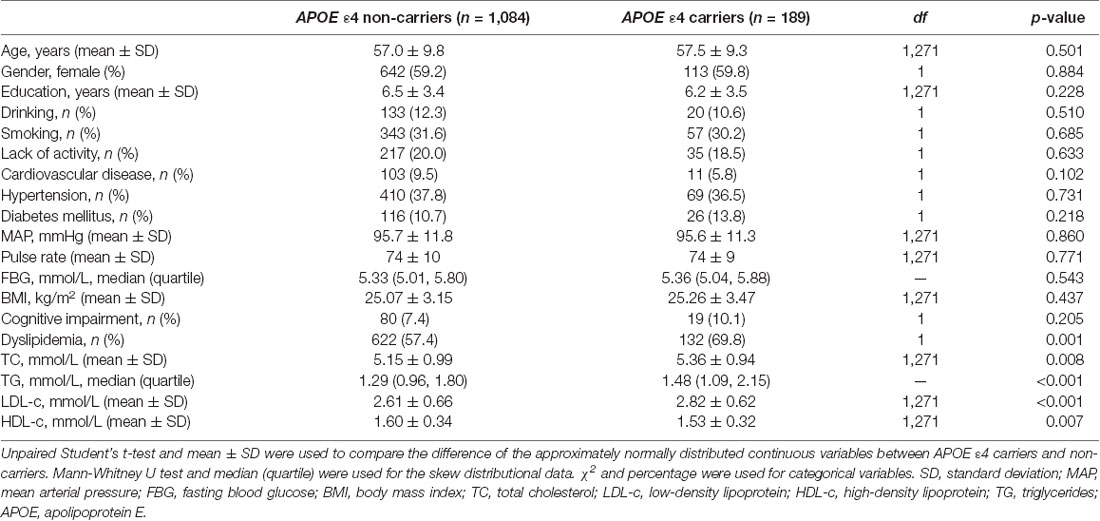
Table 3. Difference of the cognitive impairment, serum lipid levels, and other covariates between APOE ε4 carriers and non-carriers.
Because the APOE ε4 allele had effects on the relationship between serum lipid levels and cognitive impairment, stratified binary logistic regression analyses were performed according to APOE ε4 status. In APOE ε4 carriers, low HDL was positively correlated with cognitive impairment (Wald’s χ2 = 8.200, df = 1, OR = 60.335, 95% CI = 3.646–998.364, p = 0.004; Table 4, Model 5). However, such a correlation disappeared among APOE ε4 non-carriers (Wald’s χ2 = 0.057, df = 1, OR = 0.776, 95% CI = 0.097–6.221, p = 0.811; Table 4, Model 7). For other serum lipid parameters (high TC, high TG, and high LDL levels), there was no significant correlation with cognitive impairment in either APOE ε4 carriers or non-carriers (Table 3, Models 5 and 7).
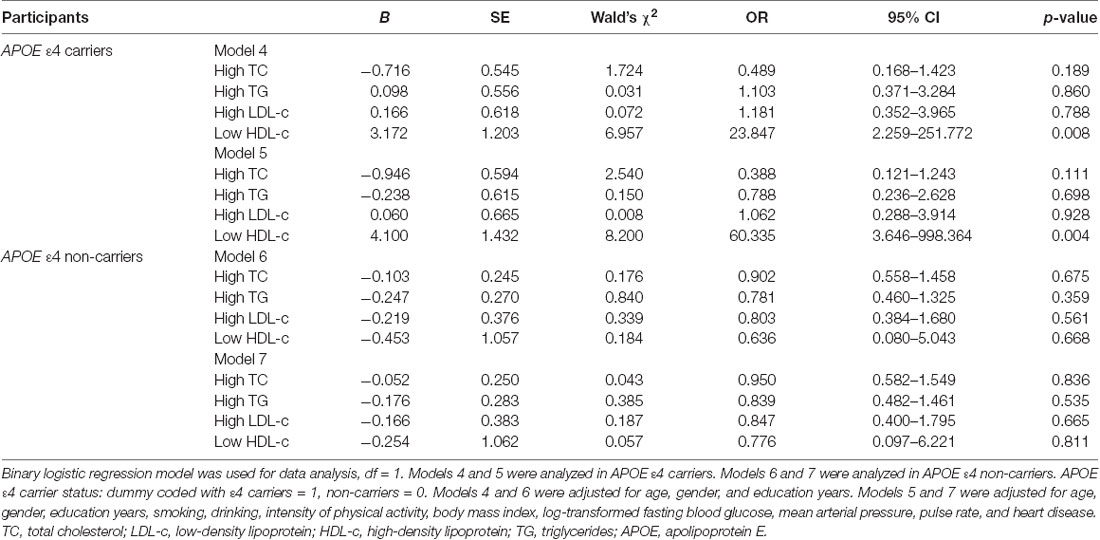
Table 4. The relationships between serum lipid parameters and cognitive impairment with binary logistic regression in the subgroups according APOEε4 status.
To further confirm our results, stratified partial correlation analysis was separately performed to research the correlations between serum lipids and MMSE score in APOE ε4 non-carriers and carriers. When adjusting for age, sex, education, smoking, drinking, intensity of physical activity, BMI, log-transformed FBG, MAP, pulse rates, and heart disease, MMSE scores positively correlated with serum HDL level (r = 0.217, df = 176, p = 0.004) but not with LDL, TG, or TC levels in APOE ε4 carriers. In APOE ε4 non-carriers, no correlations were found between MMSE scores and any of the serum lipids (Figure 3).
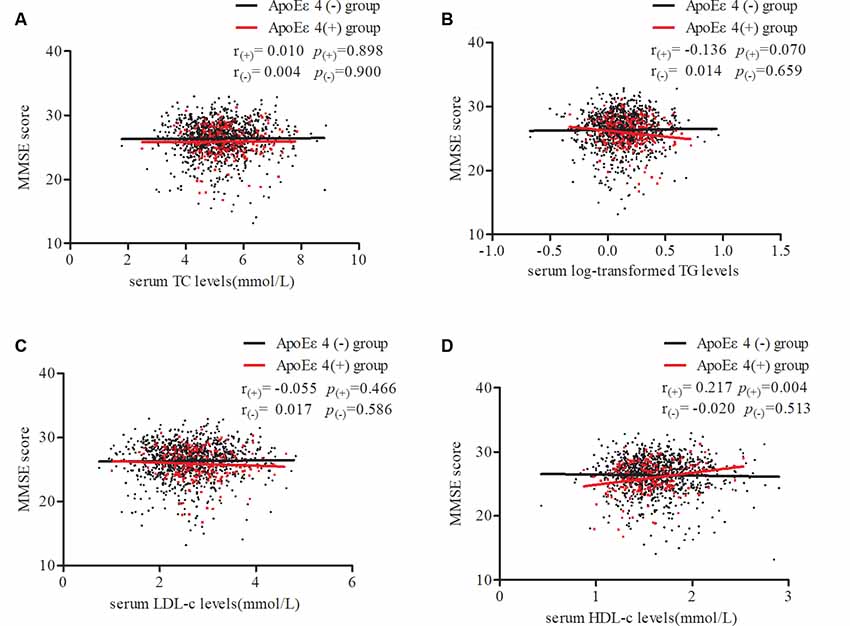
Figure 3. Partial correlations of MMSE score and serum lipid levels according APOE ε4 status. Participants were divided into two groups: APOE ε4 carriers and non-carriers, which are represented by red dots and black dots. Partial linear correlations between MMSE scores and serum TC (A), log-transformed TG (B), LDL-c (C), and HDL-c (D) levels are shown in the figure. r(+) and p(+) represent partial correlation coefficients in carriers, while r(-) and p(-) represent non-carriers. Adjusted for age, sex, education, smoking, drinking, intensity of physical activity, body mass index (BMI), log-transformed fasting blood glucose (FBG), mean arterial pressure, pulse rates, and heart disease. TC, total cholesterol; TG, triglycerides; LDL-c, low-density lipoprotein; HDL-c, high-density lipoprotein; APOE, apolipoprotein E.
In this cross-sectional study, we found a significant interaction effect between low HDL and the APOE ε4 allele on cognitive impairment in the total participants. In APOE ε4 carriers, low serum HDL-c levels were positively associated with cognitive impairment, and participants with higher HDL levels had higher MMSE scores. No correlation was found between cognitive impairment/MMSE score and any of the serum lipids in APOE ε4 non-carriers.
Although studies have shown that lipid metabolism is involved in the pathogenesis of AD (Hartmann et al., 2007; Anstey et al., 2008), and the effect of lipid levels on cognitive function has attracted the attention of many researchers, there is still no consistent conclusion. A previous study showed that high midlife TC levels can increase the risk of AD in older people (Anstey et al., 2017). In contrast, another study showed that higher TC levels were associated with better memory functioning in very elderly subjects without the APOE ε4 allele (West et al., 2008). One study showed that increased serum LDL levels were independently associated with AD (Chen et al., 2019), and one study showed that higher LDL levels were associated with better memory performance (Leritz et al., 2016). Katsumata et al. (2013) reported that HDL levels and lower TG/HDL-C ratios were associated with better memory performance, while other studies have shown no association between HDL and AD (Li et al., 2005). Therefore, to study the relationship between lipids and cognition, stratified analysis was separately performed according to the APOE genotype. Our study found that low HDL was associated with cognitive impairment in APOE ε4 allele carriers, while TC, LDL, and TG levels were not associated with cognitive function.
Some possible mechanisms might explain the positive correlation between HDL levels and cognitive function. First, it has been shown that ApoA-I, the main component of HDL, is highly efficient at cholesterol efflux in the CSF (Demeester et al., 2000), resulting in increased membrane fluidity that could enhance the α-secretase cleavage of APP at the cell membrane. As mentioned above, increased α-secretase activity can reduce the production of Aβ. Second, apoA-I/HDL can bind to Aβ and make the clearance of Aβ by astrocytes and/or microglia more efficient (Sagare et al., 2012), leading to a decrease in Aβ aggregation and cytotoxicity. Third, it is well established that the antioxidant and anti-inflammatory effects of HDL play an important role in cardiovascular diseases (Barter et al., 2007), so the same disease mechanism may also affect neurodegenerative diseases (Lewis et al., 2010). Finally, the main role of HDL is lipid metabolism, which can reverse lipoprotein transport from the arterial wall of the brain (Mulder and Terwel, 1998), leading to a decrease in ischemic lesions that are involved in the development of cognitive decline and dementia (Kalaria, 2000).
However, the difference in the relationship between HDL and cognitive function between APOE ε4 carriers and APOE ε4 non-carriers remains to be addressed. We hypothesized that in APOE ε4 carriers, there may be two reasons for the positive association between cognitive function and HDL levels. First, this study showed lower levels of HDL in APOE ε4 carriers, suggesting that APOE exerts isoform-specific effects on HDL metabolism in humans. Using C57BL/6 APOE−/− mice, Hopkins et al. (2002) reported that compared with APOE ε3, the presence of APOE ε4 was less efficient at transferring ApoA-I from chylomicron remnants to HDL, resulting in lower plasma HDL levels and smaller HDL volumes. The reduced HDL levels may be associated with eventual cognitive decline, which has been confirmed by some previous studies (van Exel et al., 2002; Zuliani et al., 2010). However, pathophysiological studies have shown that APOE ε4 can directly lead to cognitive decline without HDL involvement by affecting Aβ clearance, tau hyperphosphorylation, synaptic plasticity, cell signaling, and neuroinflammation (Yu et al., 2014; Giau et al., 2015). The combination of these two mechanisms may lead to the emergence of a positive relationship between HDL levels and cognitive function in APOE ε4 carriers. However, in APOE ε4 non-carriers, without APOE ε4 as a risk factor, the effect of HDL alone on cognitive function might not be sufficiently apparent, leading to the result that there was no significant relationship between HDL and cognitive function. Of course, the abovementioned ideas are only our reasonable speculation, and the specific mechanism remains to be further studied.
Consistent with our findings, one community-based study with 2,356 participants showed no correlation between TC levels and the risk of dementia or AD (Elias et al., 2005). In contrast, the 3C study (Schilling et al., 2017) and another 14-year cohort study (Toro et al., 2014) found a positive correlation, and a meta-analysis (Anstey et al., 2017) also showed that high midlife TC levels increase the risk of late-life AD. However, the lipid measurements in these studies were not from cross-sectional studies and were performed significantly earlier than the onset of AD by at least 13 years, and some of the cholesterol levels were measured in midlife and not late life. A previous study of 444 men from the Finnish cohorts of the Seven Countries (Notkola et al., 1998) showed that the TC levels of men in midlife who subsequently developed AD were significantly higher than those of normal men. However, because cholesterol levels decline with age, in the preclinical manifestations of AD stage, the TC levels of those eventually developing AD decreased more rapidly and were eventually lower in late life than those of normal men. This study was a cross-sectional study in Chinese middle-aged and elderly subjects, and all lipid measurements were clustered around the time of the study, which may explain the lack of correlation between TC levels and cognitive impairment.
Some limitations should be mentioned. First, the study is a cross-sectional study, so it can only explain the correlation between blood lipids and cognition; however, the causal relationship between them is difficult to explain, and randomized controlled trial is necessary in the future to see if increasing HDL-c plasma levels can prevent cognitive decline in the at-risk population of APOE ε4 carriers and thus clarify the causal relationship. Second, the serum lipids were only detected at a single time point, not allowing for an evaluation of the dynamic changes. In addition, our study focused on elderly individuals in the Chinese Han population in Northwest China, and the diagnosis of cognitive impairment was based on the Chinese version of the MMSE, so the generalizability of our findings may be limited in other ethnic or age groups, and multi-center and large population studies need to be performed to validate our results. Third, with the numbers of patients with cerebrovascular diseases increasing, the incidence rate of vascular dementia (VaD) rises year by year. VaD has become the second leading cause of dementia after AD itself (Lobo et al., 2000). In this study, we have excluded people with a clear history of acute cerebrovascular disease, including stroke. However, owing to lack of standard diagnostic procedures and biomarkers simultaneously (Formichi et al., 2010), it is still hard to rule out the possibility that some cases with cognitive impairment may be vascular dementia or mixed dementia (AD+VaD; Jellinger, 2005). So, further studies involving AD biomarkers need to be performed to elaborate the conclusion more deeply and accurately.
In summary, by measuring serum lipids and cognitive function, this cross-sectional study found that in APOE ε4 carriers, but not APOE ε4 non-carriers, low serum HDL-c levels were positively associated with cognitive impairment and that those with higher HDL levels had higher MMSE scores. These data indicated that the APOE ε4 allele may affect the relationship between serum lipid levels and cognitive impairment. However, this relationship needs to be further elucidated.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
This study and its protocol were approved by the Medical Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. All participants were required to sign a written informed consent form before participating in the study.
SW participated in the questionnaire survey and biochemical assessment, conducted the results analysis, and wrote the manuscript. LG and YJ participated in the questionnaire survey, sample collection, and biochemical assessment. SS designed this study and participated in the questionnaire survey and sample collection. CC, LD, JinW, KH, and JingW participated in the questionnaire survey and sample collection. QQ coordinated and supervised all stages of the project. All authors have read and approved the final version of the manuscript.
This work was supported by the Key Research & Development Programs of Shaanxi Province (Grant No. 2018ZDXM-SF-052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are thankful for the cooperation of all participants in our study.
Anstey, K. J., Ashby-Mitchell, K., and Peters, R. (2017). Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J. Alzheimers Dis. 56, 215–228. doi: 10.3233/jad-160826
Anstey, K. J., Lipnicki, D. M., and Low, L. F. (2008). Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am. J. Geriatr. Psychiatry 16, 343–354. doi: 10.1097/JGP.0b013e31816b72d4
Barter, P. J., Puranik, R., and Rye, K. A. (2007). New insights into the role of HDL as an anti-inflammatory agent in the prevention of cardiovascular disease. Curr. Cardiol. Rep. 9, 493–498. doi: 10.1007/bf02938394
Castellano, J. M., Kim, J., Stewart, F. R., Jiang, H., DeMattos, R. B., Patterson, B. W., et al. (2011). Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3:89ra57. doi: 10.1126/scitranslmed.3002156
Chen, H., Du, Y., Liu, S., Ge, B., Ji, Y., and Huang, G. (2019). Association between serum cholesterol levels and Alzheimer’s disease in China: a case-control study. Int. J. Food Sci. Nutr. 70, 405–411. doi: 10.1080/09637486.2018.1508426
Dallongeville, J., Lussier-Cacan, S., and Davignon, J. (1992). Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. J. Lipid Res. 33, 447–454.
De Felice, F. G., and Ferreira, S. T. (2002). β-amyloid production, aggregation, and clearance as targets for therapy in Alzheimer’s disease. Cell. Mol. Neurobiol. 22, 545–563. doi: 10.1023/a:1021832302524
Demeester, N., Castro, G., Desrumaux, C., De Geitere, C., Fruchart, J. C., Santens, P., et al. (2000). Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J. Lipid Res. 41, 963–974.
Elias, P. K., Elias, M. F., D’Agostino, R. B., Sullivan, L. M., and Wolf, P. A. (2005). Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom. Med. 67, 24–30. doi: 10.1097/01.psy.0000151745.67285.c2
Formichi, P., Parnetti, L., Radi, E., Cevenini, G., Dotti, M. T., and Federico, A. (2010). CSF biomarkers profile in CADASIL—a model of pure vascular dementia: usefulness in differential diagnosis in the dementia disorder. Int. J. Alzheimers Dis. 2010:959257. doi: 10.4061/2010/959257
Giau, V. V., Bagyinszky, E., An, S. S. A., and Sang, Y. K. (2015). Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 11, 1723–1737. doi: 10.2147/ndt.s84266
Hartmann, T., Kuchenbecker, J., and Grimm, M. O. (2007). Alzheimer’s disease: the lipid connection. J. Neurochem. 103, 159–170. doi: 10.1111/j.1471-4159.2007.04715.x
Hopkins, P. C. R., Huang, Y., McGuire, J. G., and Pitas, R. E. (2002). Evidence for differential effects of apoE3 and apoE4 on HDL metabolism. J. Lipid Res. 43, 1881–1889. doi: 10.1194/jlr.m200172-jlr200
Jellinger, K. A. (2005). Understanding the pathology of vascular cognitive impairment. J. Neurol. Sci. 229–230, 57–63. doi: 10.1016/j.jns.2004.11.029
Jia, J., Wang, F., Wei, C., Zhou, A., Jia, X., Li, F., et al. (2014). The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 10, 1–9. doi: 10.1016/j.jalz.2013.01.012
Jia, J., Wei, C., Chen, S., Li, F., Tang, Y., Qin, W., et al. (2018). The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 14, 483–491. doi: 10.1016/j.jalz.2017.12.006
Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. (2007). Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 35, 390–419. doi: 10.3760/j.issn:0253-3758.2007.05.003
Kalaria, R. N. (2000). The role of cerebral ischemia in Alzheimer’s disease. Neurobiol. Aging 21, 321–330. doi: 10.1016/s0197-4580(00)00125-1
Katsumata, Y., Todoriki, H., Higashiuesato, Y., Yasura, S., Ohya, Y., Willcox, D. C., et al. (2013). Very old adults with better memory function have higher low-density lipoprotein cholesterol levels and lower triglyceride to high-density lipoprotein cholesterol ratios: KOCOA Project. J. Alzheimers Dis. 34, 273–279. doi: 10.3233/jad-121138
Katzman, R., Zhang, M. Y., Ouang-Ya-Qu, Wang, Z. Y., Liu, W. T., Yu, E., et al. (1988). A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 41, 971–978. doi: 10.1016/0895-4356(88)90034-0
Kim, J., Basak, J. M., and Holtzman, D. M. (2009). The role of apolipoprotein E in Alzheimer’s disease. Neuron 63, 287–303. doi: 10.1016/j.neuron.2009.06.026
Kivipelto, M., Helkala, E. L., Hänninen, T., Laakso, M. P., Hallikainen, M., Alhainen, K., et al. (2001). Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology 56, 1683–1689. doi: 10.1212/wnl.56.12.1683
Leritz, E. C., McGlinchey, R. E., Salat, D. H., and Milberg, W. P. (2016). Elevated levels of serum cholesterol are associated with better performance on tasks of episodic memory. Metab. Brain Dis. 31, 465–473. doi: 10.1007/s11011-016-9797-y
Lewis, T. L., Cao, D., Lu, H., Mans, R. A., Su, Y. R., Lisa, J., et al. (2010). Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J. Biol. Chem. 285, 36958–36968. doi: 10.1074/jbc.m110.127829
Lobo, A., Launer, A. L., Fratiglioni, L., Andersen, K., Di Carlo, W. A., Breteler, M. M. B., et al. (2000). Prevalence of dementia and major subtypes in europe: a collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology 54, S4–S9.
Li, G., Shofer, J. B., Kukull, W. A., Peskind, E. R., Tsuang, D. W., Breitner, J. C., et al. (2005). Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology 65, 1045–1050. doi: 10.1212/01.wnl.0000178989.87072.11
Mahley, R. W. (1988). Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630. doi: 10.1126/science.3283935
Mahley, R. W., and Rall, S. C. Jr. (2000). Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537. doi: 10.1146/annurev.genom.1.1.507
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L., and Beyreuther, K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U S A 82, 4245–4249. doi: 10.1073/pnas.82.12.4245
Mulder, M., and Terwel, D. (1998). Possible link between lipid metabolism and cerebral amyloid angiopathy in Alzheimer’s disease: a role for high-density lipoproteins? Haemostasis 28, 174–194. doi: 10.1159/000022429
Notkola, I. L., Sulkava, R., Pekkanen, J., Erkinjuntti, T., Ehnholm, C., Kivinen, P., et al. (1998). Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 17, 14–20. doi: 10.1159/000026149
Reitz, C., Tang, M.-X., Luchsinger, J., and Mayeux, R. (2004). Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neuro. 61, 705–714. doi: 10.1001/archneur.61.5.705
Sagare, A. P., Bell, R. D., and Zlokovic, B. V. (2012). Neurovascular dysfunction and faulty amyloid β-peptide clearance in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2:a011452. doi: 10.1101/cshperspect.a011452
Schilling, S., Tzourio, C., Soumaré, A., Kaffashian, S., Dartigues, J. F., Ancelin, M. L., et al. (2017). Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C study: a longitudinal, population-based prospective cohort study. PLoS Med. 14:e1002265. doi: 10.1371/journal.pmed.1002265
Siest, G., Bertrand, P., Qin, B., Herbeth, B., Serot, J. M., Masana, L., et al. (2000). Apolipoprotein E polymorphism and serum concentration in Alzheimer’s disease in nine European centres: the ApoEurope study. ApoEurope group. Clin. Chem. Lab. Med. 38, 721–730. doi: 10.1515/cclm.2000.102
Subasinghe, S., Unabia, S., Barrow, C. J., Mok, S. S., Aguilar, M. I., and Small, D. H. (2003). Cholesterol is necessary both for the toxic effect of Aβ peptides on vascular smooth muscle cells and for Aβ binding to vascular smooth muscle cell membranes. J. Neurochem. 84, 471–479. doi: 10.1046/j.1471-4159.2003.01552.x
Toro, P., Degen, C., Pierer, M., Gustafson, D., Schröder, J., and Schönknecht, P. (2014). Cholesterol in mild cognitive impairment and Alzheimer’s disease in a birth cohort over 14 years. Eur. Arch. Psychiatry Clin. Neurosci. 264, 485–492. doi: 10.1007/s00406-013-0468-2
Tukiainen, T., Jylänki, P., Mäkinen, V. P., Gröhn, O., Hallikainen, M., Soininen, H., et al. (2012). Mild cognitive impairment associates with concurrent decreases in serum cholesterol and cholesterol-related lipoprotein subclasses. J. Nutr. Health Aging 16, 631–635. doi: 10.1007/s12603-011-0341-9
van Exel, E., de Craen, A. J., Gussekloo, J., Houx, P., Bootsma-van der Wiel, A., Macfarlane, P. W., et al. (2002). Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann. Neurol. 51, 716–721. doi: 10.1002/ana.10220
Wahrle, S., Das, P., Nyborg, A. C., McLendon, C., Shoji, M., Kawarabayashi, T., et al. (2002). Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9, 11–23. doi: 10.1006/nbdi.2001.0470
West, R., Beeri, M. S., Schmeidler, J., Hannigan, C. M., Angelo, G., Grossman, H. T., et al. (2008). Better memory functioning associated with higher total and low-density lipoprotein cholesterol levels in very elderly subjects without the apolipoprotein e4 allele. Am. J. Geriatr. Psychiatry 16, 781–785. doi: 10.1097/jgp.0b013e3181812790
Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C., and Yaffe, K. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. doi: 10.1212/01.wnl.0000149519.47454.f2
Yu, J. T., Tan, L., and Hardy, J. (2014). Apolipoprotein E in Alzheimer’s disease: an update. Annu. Rev. Neurosci. 37, 79–100. doi: 10.1146/annurev-neuro-071013-014300
Zannis, V. I., and Breslow, J. L. (1981). Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry 20, 1033–1041. doi: 10.1021/bi00507a059
Keywords: Alzheimer’s disease, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglyceride, apolipoprotein E, risk factors
Citation: Wei S, Gao L, Jiang Y, Shang S, Chen C, Dang L, Wang J, Huo K, Wang J and Qu Q (2020) The Apolipoprotein E ε4 Allele-Dependent Relationship Between Serum Lipid Levels and Cognitive Function: A Population-Based Cross-sectional Study. Front. Aging Neurosci. 12:44. doi: 10.3389/fnagi.2020.00044
Received: 12 November 2019; Accepted: 10 February 2020;
Published: 13 March 2020.
Edited by:
Jiehui Jiang, Shanghai University, ChinaReviewed by:
Chongren Tang, University of Washington, United StatesCopyright © 2020 Wei, Gao, Jiang, Shang, Chen, Dang, Wang, Huo, Wang and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiumin Qu, cXVxaXVtaW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.