- 1College of Korean Medicine, Dongguk University, Goyang, South Korea
- 2Graduate School, Dongguk University, Seoul, South Korea
- 3Acupuncture and Meridian Science Research Center, Seoul, South Korea
- 4College of Korean Medicine, Kyung Hee University, Seoul, South Korea
Background: Acupuncture has been reported to have significant effects, not only in alleviating impaired motor function, but also rescuing dopaminergic neuron deficits in rodent models of Parkinson's disease (PD). However, a systemic analysis of these beneficial effects has yet to be performed.
Objective: To evaluate the neuroprotective effect of acupuncture in animal models of PD.
Methods: A literature search of the PubMed, MEDLINE, EMBASE, China National Knowledge Infrastructure, Research Information Service System, and Japan Society of Acupuncture and Moxibustion databases was performed to retrieve studies that investigated the effects of acupuncture on PD. The quality of each included study was evaluated using the 10-item checklist modified from the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies. RevMan version 5.3 (Foundation for Statistical Computing, Vienna, Austria) was used for meta-analysis.
Results: The 42 studies included scored between 2 and 7 points, with a mean score of 4.6. Outcome measures included tyrosine hydroxylase (TH) level and dopamine content. Meta-analysis results revealed statistically significant effects of acupuncture for increasing both TH levels (33.97 [95% CI 33.15–34.79]; p < 0.00001) and dopamine content (4.23 [95% CI 3.53–4.92]; p < 0.00001) compared with that observed in PD control groups. In addition, motor dysfunctions exhibited by model PD animals were also mitigated by acupuncture treatment.
Conclusions: Although there were limitations in the number and quality of the included studies, results of this analysis suggest that acupuncture exerts a protective effect on dopaminergic neurons in rodent models of PD.
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder first described by Dr. James Parkinson in 1817 as a “shaking palsy” (Demaagd and Philip, 2015). PD is characterized by motor symptoms, such as rigidity, resting tremors, and postural instability, and non-motor symptoms including sleep disturbance, hallucinations, and constipation (Demaagd and Philip, 2015). It has been reported that 1–2% of the global population >65 years of age is affected by PD (Alves et al., 2008). In terms of pathology, recent studies have suggested that PD is closely associated with the loss of dopaminergic (DA) neurons in the substantia nigra (SN) pars compacta of the brain caused by familial and/or sporadic factors (Zhou et al., 2008; Surmeier et al., 2010; Blesa et al., 2015). Levodopa has been widely used in recent decades for the management of PD; however, complications following the use of levodopa are considerable.
In East Asian countries, acupuncture has long been used to treat motor dysfunction and brain disorders such as PD (Joh et al., 2010). Moreover, in recent years, it has been shown that acupuncture improves motor function in rodent models of PD via mechanisms including anti-inflammatory and neurotrophic effects (Yu et al., 2010; Rui et al., 2013). Furthermore, the effect of acupuncture on PD has been demonstrated in clinical studies. Improvement in motor function in PD patients who underwent bee venom acupuncture treatment has been reported (Cho et al., 2012), and motor function-associated neural responses with acupuncture have also been shown in PD patients using functional magnetic resonance imaging fMRI (Chae et al., 2009; Yeo et al., 2012). Additionally, a systematic review of clinical studies of acupuncture involving PD patients demonstrated the potential effectiveness of acupuncture (Lam et al., 2008). Accordingly, acupuncture has been suggested as an integrative medicine treatment for PD.
As mentioned earlier, destruction and recovery of DA neurons in the SN, which play critical role in motor functions, is significant in terms of the pathology of PD. Thus, it is important to investigate the extent to which acupuncture treatment affects the recovery of DA neurons. Rodent models have been widely used in PD research because they can provide valuable information in terms of understanding pathogenic processes and developing effective therapies (Duty and Jenner, 2011; Blandini and Armentero, 2012).
Recent rodent-based studies have demonstrated that acupuncture recovered DA neurons in a mouse model of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD, and in a 6-hydroxydopamine (6-OHDA)-induced PD rat model (Kim et al., 2005; Jeon et al., 2008; Park et al., 2015). In contrast, it was also reported that acupuncture treatment did not demonstrate a neuroprotective effect in an MPTP mouse model (Yang et al., 2017).
Therefore, the present systematic review and meta-analysis aimed to assess the pre-clinical evidence supporting the neuroprotective effects of acupuncture in rodent models of PD.
Methods
Literature Search
English-language studies that examined the neuroprotective effect of acupuncture in PD rodent models were included in the present study. The PubMed, EMBASE, and MEDLINE, China National Knowledge Infrastructure, Research Information Service System, and Japan Society of Acupuncture and Moxibustion databases were searched from inception until June 2018 using the following search terms: “mouse (mice)” or “rat (rats),” “acupuncture (electroacupuncture),” and “Parkinson's disease.”
Inclusion/Exclusion Criteria
Studies were included based on the following criteria: subjects (rodent models of PD); intervention (acupuncture as the main intervention, but limited to manual acupuncture [MA] and electroacupuncture [EA]); and outcomes (tyrosine hydroxylase [TH] and DA neuron level were the main outcomes to evaluate the efficacy of acupuncture). Behavioral test data were the subsequent outcome to evaluate motor functions in PD rodent models. Studies not reporting exact outcome values and full-text articles not published in English were excluded.
Data Extraction
Two authors (Kim and Ko) extracted the data independently. Data extracted from the databases included the following: publication year, name of the first author, and type of rodent PD model; type of acupuncture; results of behavioral tests; and the outcome of treatment in acupuncture-treated groups. Three studies were excluded because exact outcome values were not reported; thus, 42 original research articles were selected for further analysis.
Quality Assessment
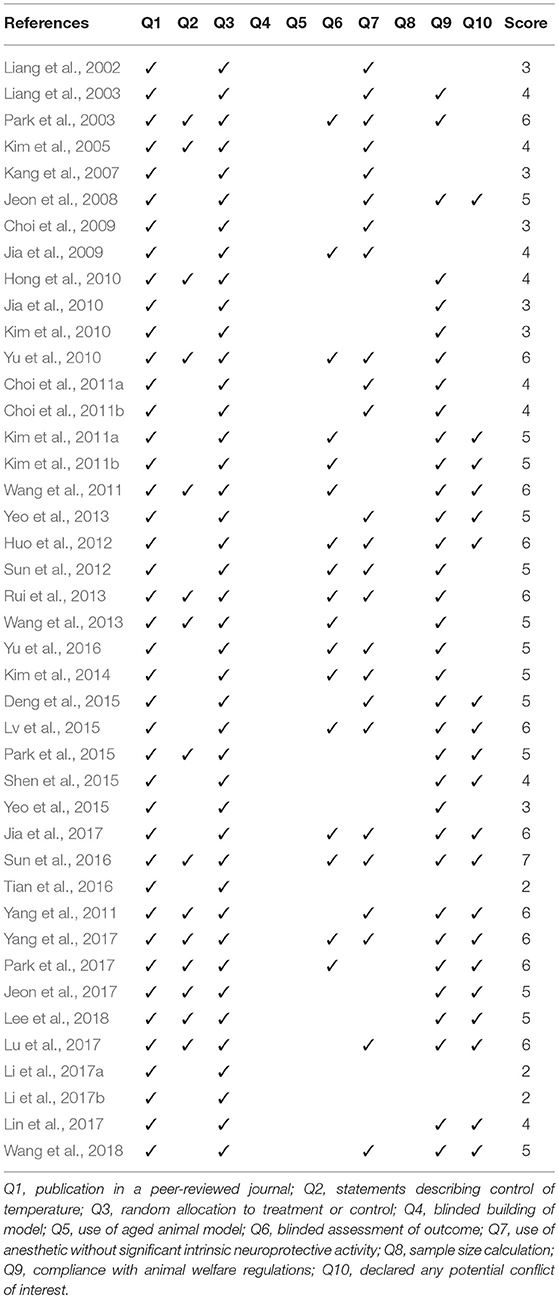
The methodological quality of each included study was assessed by two authors (Kim and Ko) using a 10-item checklist modified from the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies checklist (Sena et al., 2007): publication in a peer-reviewed journal; statements describing temperature control; random allocation to treatment or control; blinded building of the model; use of aged animal models; blinded assessment of outcome; use of anesthetic without significant intrinsic neuroprotective activity; sample size calculation; compliance with animal welfare regulations; and declarations of any potential conflicts of interest. A sum of the quality scores was recorded for each article, with a possible total score of 10 points.
Statistical Analysis
In each study, densitometry of TH-positive (TH+) staining or stereological cell counting results or dopamine content were considered as continuous data. Because the same comparison was used in the studies (i.e., compared with a control group), the mean differences for effect sizes were estimated based on a fixed-effects model. Publication bias was assessed using a funnel plot. To examine the influence of the type of rodent model on the outcome measures, specific subgroups were defined: MPTP-induced PD model; 6-OHDA-induced PD model; medial forebrain bundle (MFB)-axotomy-induced PD model; and an alpha-synuclein (α-syn) mutation PD model.
The meta-analysis was performed using RevMan version 5.3 (Foundation for Statistical Computing, Vienna, Austria). The confidence interval was established at 95%, and p < 0.05 was considered to be statistically significant. For the assessment of study heterogeneity, the chi-squared distribution and I2 statistic were used.
Results
Study Inclusion
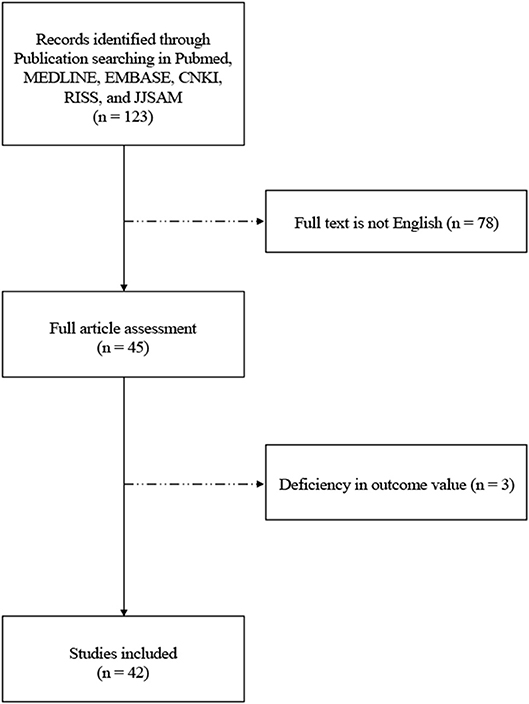
Among 123 initially identified studies, 78 were excluded because the full-texts were not available in English. Full-text screening was performed for the remaining 45 studies, of which 3 were excluded due to deficiency in exact outcome values. A total of 42 studies were, therefore, included in the present review. A flow diagram of the study selection process is shown in Figure 1.
Study Characteristics
Of the 42 studies, 23 used EA and 19 used MA. Of the PD models used in these studies, 21 used an MPTP-induced PD model, 15 used a 6-OHDA-induced PD model; and 5 other studies used an MFB lesion-induced PD model. One study used an A53T α-syn transgenic mouse model.
Quality Assessment
The quality assessment of the included studies is summarized in Table 1. The quality score of the included studies ranged from 2 to 7 of a total 10 points. One study scored 7, 11 scored 6, 13 scored 5, 8 scored 4, 6 scored 3, and 3 studies scored 2 points. All the 42 studies were peer-reviewed and included randomly allocated control and acupuncture groups. Fifteen studies included statements describing temperature control, 17 described blinded assessment of outcomes, and 25 reported use of anesthetic without significant intrinsic neuroprotective activity. Thirty-four studies reported compliance with animal welfare regulations and 20 declared potential conflicts of interest. No study conducted blind building of the model or sample size calculation. Finally, no study used aged animals.
Effect of Acupuncture on DA Neuron Protection
TH has a specific role in dopamine synthesis and is abundantly expressed in DA neurons; accordingly, it has been used as a dopamine neuronal marker in PD studies. Figure 2 shows the meta-analysis of studies with TH+ neurons in the SN of PD model animals. Twenty-nine studies adopted TH+ level as an outcome index. All of the studies reported a positive effect of acupuncture on increasing TH+ levels in the SN of acupuncture treated PD models compared with the PD control group except for two studies (Yang et al., 2017; Wang et al., 2018) (n = 426; standardized mean difference [SMD] 33.97; 95% CI 33.15–34.79; p < 0.00001; heterogeneity χ2 = 162.80, I2 = 98.2%, Figure 2). Each data point on the plot is shown with group comparisons (Figure 3). One study reported decreased TH+ cells in the acupuncture-treated group compared with the MPTP model (Figures 2, 3A,B), and one study demonstrated no difference in TH+ cells between acupuncture and 6-OHDA mouse models (Figures 2, 3C,D). However, TH+ cells were increased after acupuncture in almost all studies, except two that reported no significant difference (Figures 2, 3). Overall, integrated changes of TH+ neurons in PD models demonstrated 35.94% of normal brain and, interestingly, those of acupuncture treated improved these neuronal deficits by 70.43% (Figure 3). Additionally, a subgroup analysis of different PD models was performed to examine the effect of acupuncture on TH+ level. The result of the subgroup analysis indicated that acupuncture had a significant effect on MPTP models (SMD 56.2 [95% CI 37.53–39.73]; p < 0.00001), MFB models (SMD 25.88 [95% CI 23.47–28.30]; p < 0.00001), and 6-OHDA models (SMD 28.84 [95% CI 27.38–30.31]; p < 0.00001).
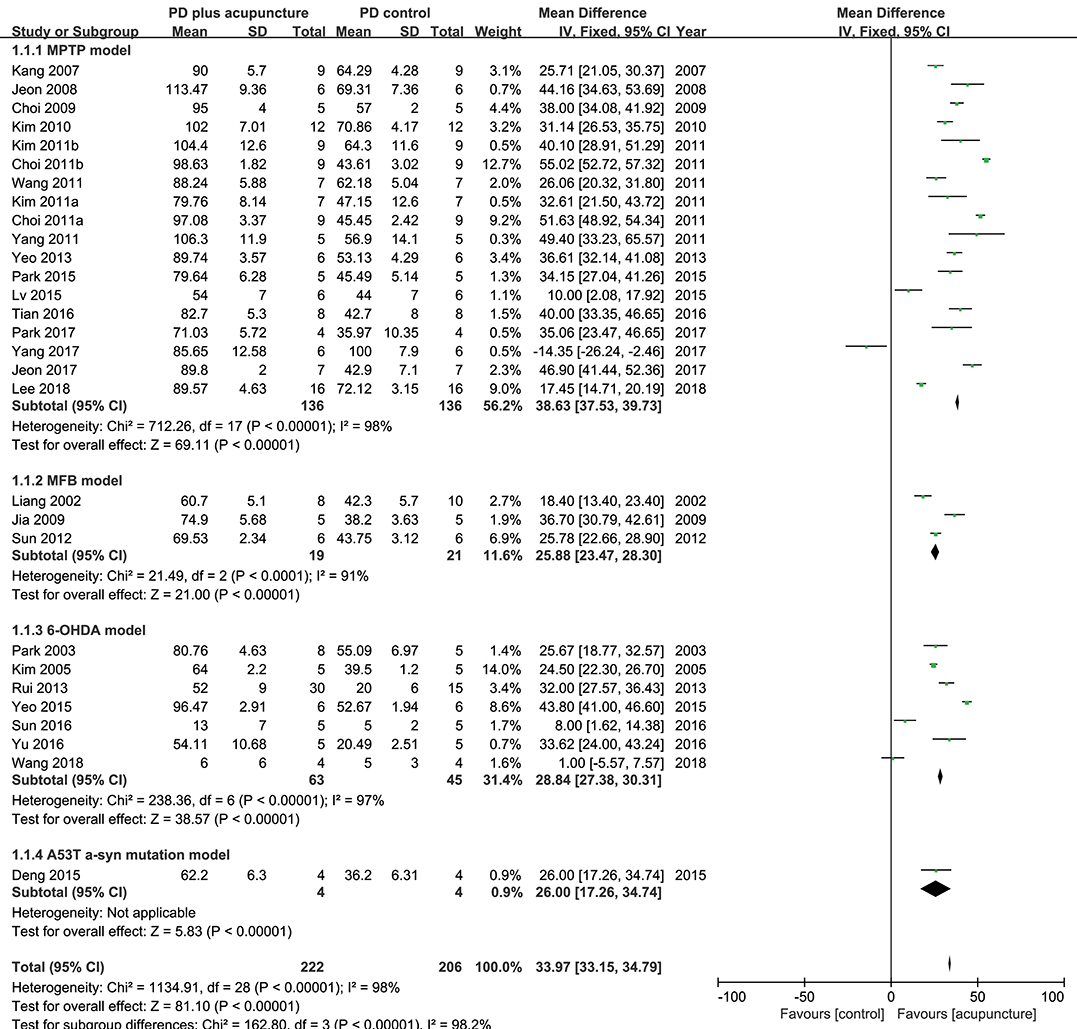
Figure 2. Comparison of tyrosine hydroxylase-positive neurons in substantia nigra with PD animal and acupuncture-treated PD animal.
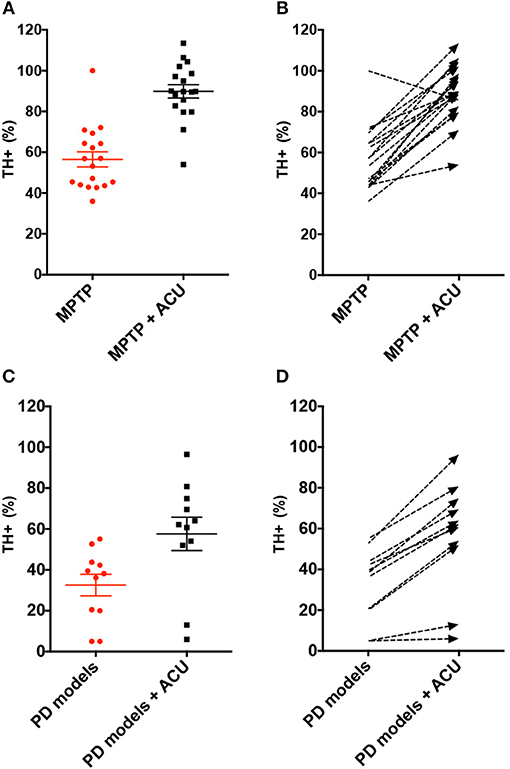
Figure 3. Analysis of difference in TH-positive level between PD rodent and acupuncture treatment group throughout studies. (A) Each TH+ level (% control) of MPTP-induced PD (MPTP) group and MPTP plus acupuncture (MPTP + ACU) group. Lines indicates the mean value and error. (B) Dashed arrows mean the slope of difference from MPTP to MPTP plus acupuncture group in each study. (C) Each TH+ level (% control) of 6-OHDA or MFB-axotomy or a-syn mutation-induced PD (PD models) group and acupuncture-treated PD (PD models + ACU) group. Lines indicates the mean value and error. (D) Dashed arrows mean the slope of difference between PD models to PD models plus acupuncture group in each study. Notice the difference of the TH+ level between acupuncture treated group compared to PD rodent model.
Effect of Acupuncture on Dopamine Content Alteration
DA neuronal deficit leads to decreases in dopamine content in striatal projections. The above analysis indicated that DA neurons in PD model rodents were recovered by acupuncture; thus, it was explored how dopamine content was changed by acupuncture in the studies (Figure 4). Twelve studies reported the effect of acupuncture on improving dopamine content in PD models compared with the control PD group. There was no remarkable increase in dopamine content, except in three studies with high increase (Jia et al., 2010; Tian et al., 2016; Yu et al., 2016) (n = 185; SMD 4.23 [95% CI 3.53–4.92]; p < 0.00001; heterogeneity χ2 = 86.92, I2 = 96.5%, Figure 4). While three studies reported large increases in the striatal dopamine by acupuncture, overall, studies showed that dopamine content was not significantly altered by acupuncture (Figures 4, 5). In the subgroup analysis of dopamine content, there was a significant effect of acupuncture was observed in MPTP models (SMD 5.83 [95% CI 4.96–6.70]; p < 0.00001), MFB models (SMD 16.03 [95% CI 11.24–20.82]; p < 0.00001), but no significant difference was found in the 6-OHDA models (SMD 0.18 [95% CI −1.02–1.37]; p = 0.77).
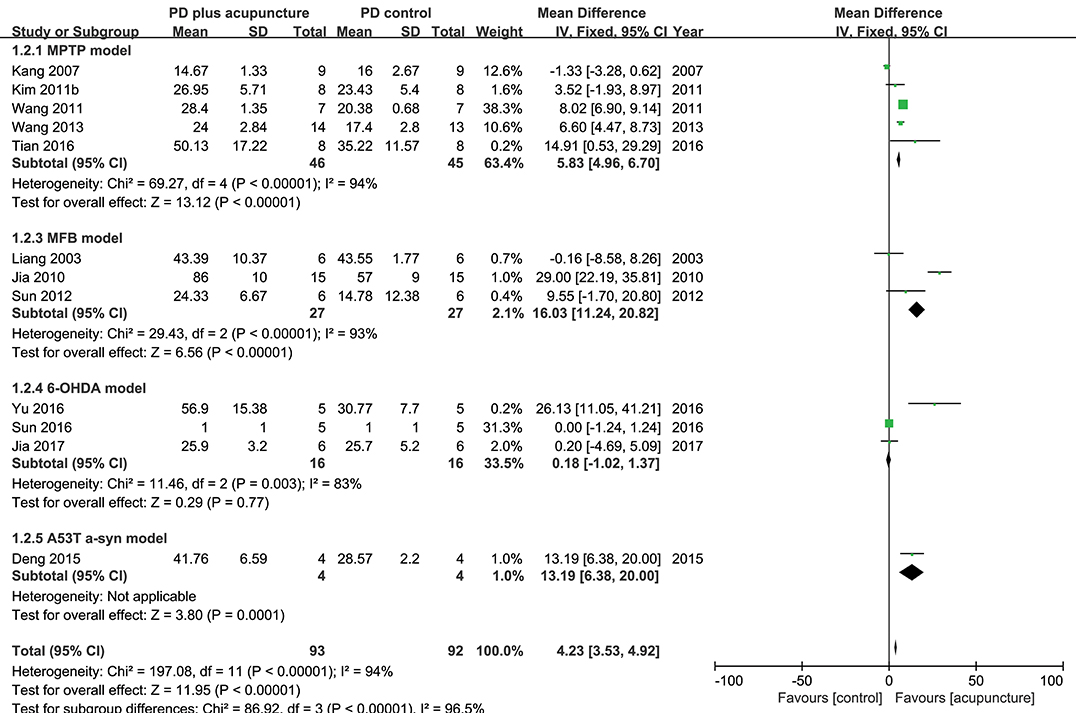
Figure 4. Comparison of dopamine content in striatum with PD animal and acupuncture-treated PD animal.
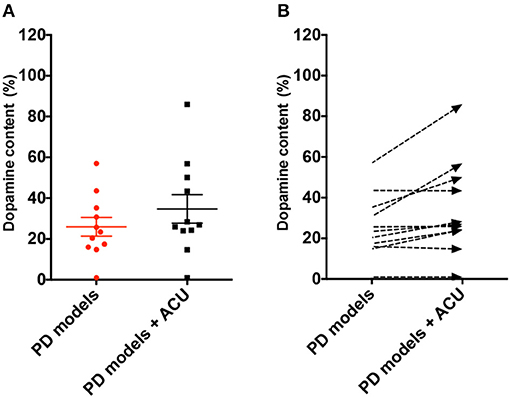
Figure 5. Analysis of difference in dopamine content level between PD rodent and acupuncture treatment group throughout studies. (A) Each dopamine content level (% control) of all the PD animal models (PD models) group and acupuncture-treated PD animal models (PD models + ACU) group. Lines indicates the mean value and error. (B) Dashed arrows mean the slope of difference from PD models to PD models plus acupuncture group in each study.
Effect of Acupuncture on Motor Function in a PD Model
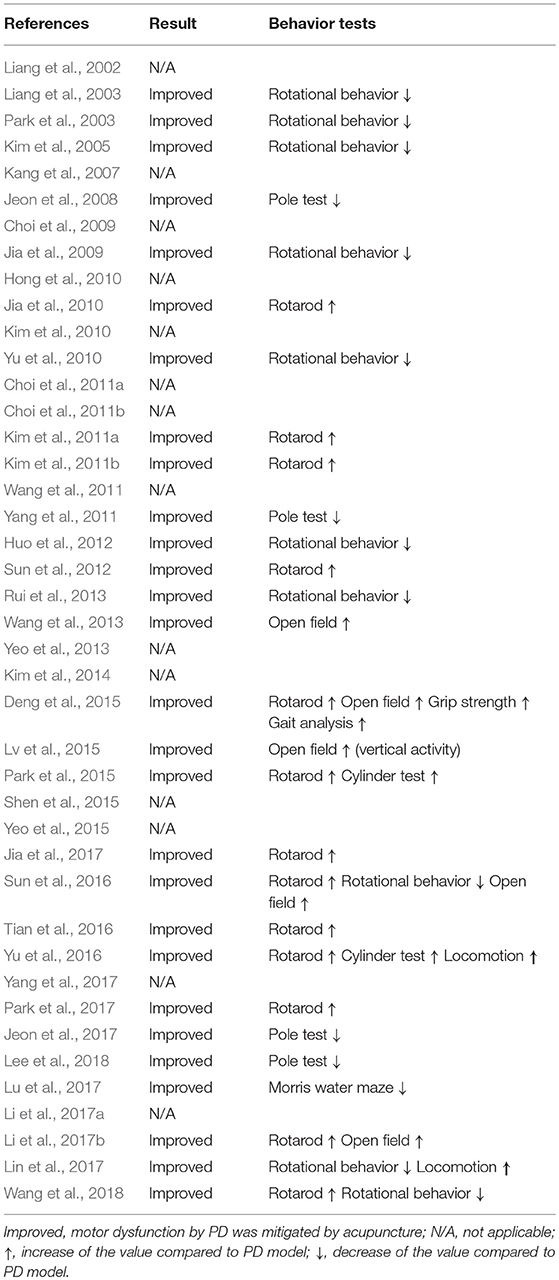
Table 2 shows how acupuncture affected motor function in PD models by examining the results of behavioral tests. Among the 42 studies, 13 conducted a rotarod test, 3 conducted a pole test, 5 performed the open field test, 11 examined rotational behavior, and the 5 remaining studies performed the Morris Water Maze test, grip strength test, gait analysis, cylinder test, and locomotor test, respectively. For studies that used the rotarod test, it was evident that motor dysfunctions were alleviated in the treatment group compared with the PD rodent model group. For the pole test result, descending time was shortened in the acupuncture-treated group in three studies. In studies that examined rotation behavior between model group and treatment group, acupuncture reduced rotational asymmetry induced by MFB-axotomy or on the side of neurotoxin injection. Based on most studies, it is believed that acupuncture alleviated motor dysfunction in PD rodent models.
Discussion
Summary of Evidence
In this review, we systematically analyzed 42 acupuncture studies that used rodent models of PD to determine whether acupuncture can improve PD symptoms and/or pathology. It is possible to study the progression of PD and therapeutic approaches by using rodent models. However, there is still no experimental rodent model that can perfectly phenocopy the disease (Jagmag et al., 2015). There was a broad range of experimental models used to study PD including MPTP mouse, 6-OHDA-lesioned rat, MFB-axotomy rodent, and an α-syn mutation mouse. In the studies, acupuncture treatment involved MA and EA. Overall, meta-analysis revealed that deficits of both TH+ levels and dopamine content in PD model animals were recovered by acupuncture. Acupuncture treatment in MPTP, MFB, and 6-OHDA models were also found to be effective according to subgroup analyses. Additionally, motor dysfunctions in those PD models were also alleviated by acupuncture.
Possible Mechanism of Neuroprotective Effects
Several mechanisms of acupuncture have been suggested to be involved in recovering DA neuronal deficits. First, the neurotrophic factor-induced cell proliferation pathway was suggested as a potential mechanism to explain the neuroprotective effect of acupuncture. For example, it was found that acupuncture increased brain-derived neurotrophic factor (BDNF) levels, followed by activation of TrkB-related cell proliferation cascade (Liang et al., 2002; Park et al., 2003; Sun et al., 2016). Glial cell-derived neurotrophic factor was also upregulated by acupuncture (Liang et al., 2003), and there was remarkable increase in cyclophilin A levels (Jeon et al., 2008). Additionally, it was found that acupuncture activates hypothalamic melanin-concentrating hormone (MCH), which is involved in neuronal protection by upregulating a downstream pathway related to neuroprotection in the SN of MPTP-induced and A53T α-syn mutant PD mice (Park et al., 2017). Moreover, various researchers have suggested possibilities that acupuncture helps PD patients recover from PD through biological processes such as anti-oxidant (Yu et al., 2010; Wang et al., 2011; Lv et al., 2015; Lee et al., 2018), anti-inflammation (Kang et al., 2007; Jeon et al., 2008; Deng et al., 2015), and regulation of autophagy (Tian et al., 2016). Although all of these processes need to be clinically verified, the scientific evidence revealed strong possibilities of a neuroprotective effect of acupuncture on PD.
Contradictory Results and Plausible Reasons
In contrast, we found that one study reported non-meaningful changes in TH+ levels (Jia et al., 2017) and contradicting results in another study (Yang et al., 2017), which reported that acupuncture is not effective in improving DA neuron protection. In a study by Yang et al., the authors reported that the number of TH+ neurons did not increase as a result of acupuncture treatment compared with PD models. However, this study did not have a normal control group and, as such, it is questionable whether a PD model was successfully established. Although C57/Bl6 mice are the most sensitive to MPTP, there are some cases in which models are not successfully induced (Jackson-Lewis and Przedborski, 2007). This illustrates why thorough controls, such as saline-injected normal mice, are needed for developing reliable rodent models. Therefore, it is doubtful whether their model would be appropriate to examine the effects of acupuncture in PD rodent models. In a study by Jia et al., changes in TH+ neurons were not meaningful but were partially restored. The study used 6-OHDA surgery-induced neuronal depletion model for development of PD. We found that the degree of neuronal deficit by 6-OHDA surgery was worse than neurotoxin injection (28.25 vs. 56.52% normal control TH+ value of 6-OHDA and MPTP, respectively) (Figure 5). Such differences among models may also lower the degree of recovery effect of acupuncture (52.33 vs. 89.83% normal control TH+ value of 6-OHDA+ACU and MPTP+ACU, respectively) (Figure 5). It also explains why the recent development of PD animal models remains arguable (Duty and Jenner, 2011; Blesa et al., 2012); therefore, it is important to carefully examine studies using various models to induce PD to draw the appropriate conclusions.
Dopamine Content and Possible Mechanisms for Improvements in Motor Function
Studies have shown that dopamine content is recovered, although not as much as neuronal deficits, by acupuncture treatment (Figures 4, 5). These results were unexpected because most studies reported that motor dysfunctions in PD animals were significantly altered after acupuncture (Table 2). Studies have reported that partial recovery of DA neurons is not sufficient to rescue neuronal function completely in terms of dopamine secretion. Instead, studies have suggested that acupuncture treatment has another effect on PD animals, which is improvement of synaptic function in addition to neuronal protection (Jia et al., 2009; Kim et al., 2011b; Yang et al., 2011; Sun et al., 2012; Rui et al., 2013; Yu et al., 2016). Notably, single-photon emission computed tomography imaging has demonstrated that neurotransmission was increased by acupuncture (Yang et al., 2011). Moreover, analysis of synaptic changes showed that dopamine use was enhanced via regulating the D1 dopamine receptor and dopamine transporter (Rui et al., 2013). Additionally, in vivo microdialysis results have shown that dopamine availability (Kim et al., 2011b) and other neurotransmitters, such as acetylcholine and glutamate, were mitigated by acupuncture (Sun et al., 2012). Furthermore, postsynaptic cortico-striatal pathway alteration was found after acupuncture (Wang et al., 2018). Based on these results, it is possible to hypothesize that acupuncture can modulate DA synaptic pathways via alteration of neuronal plasticity; however, it rescues less striatal dopamine content itself in PD animal models.
Limitations and Future Direction
There were several limitations in terms of drawing definitive conclusions based on the included studies. First, it is difficult to perform an accurate analysis by including studies using a variety of PD models and acupuncture methods. Nevertheless, it is meaningful that the potential neuroprotective effect of acupuncture treatment was evident in most of the included studies. Second, the number of available studies was not conducive to a thorough systematic review, which reflects the limited research investigating effects of acupuncture. In other words, more studies will enable us to draw more accurate conclusions in the future.
Conclusion
Results of the present review and analysis suggest that acupuncture treatment potentially protected DA neurons through various beneficial mechanisms. Nevertheless, resolving the low quality of studies and further research investigating the efficacy of different acupuncture treatment methods in PD rodent models will be needed.
Author Contributions
JK analyzed the data. S-NK and H-JP supervised the project. JK, HL, S-NK, and H-JP wrote the paper.
Funding
This work was supported by National Research Foundation of Korea funded by the Korea government (MSIT) (NRF-2017R1C1B5018061, NRF-2015M3A9E3052338, and 2017R1A2B4009963) and from the Korea Institute of Oriental Medicine (grant K18182).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alves, G., Forsaa, E. B., Pedersen, K. F., Dreetz Gjerstad, M., and Larsen, J. P. (2008). Epidemiology of Parkinson's disease. J. Neurol. 255 (Suppl. 5), 18–32. doi: 10.1007/s00415-008-5004-3
Blandini, F., and Armentero, M. T. (2012). Animal models of Parkinson's disease. FEBS J. 279, 1156–1166. doi: 10.1111/j.1742-4658.2012.08491.x
Blesa, J., Phani, S., Jackson-Lewis, V., and Przedborski, S. (2012). Classic and new animal models of Parkinson's disease. J. Biomed. Biotechnol. 2012:845618. doi: 10.1155/2012/845618
Blesa, J., Trigo-Damas, I., Quiroga-Varela, A., and Jackson-Lewis, V. R. (2015). Oxidative stress and Parkinson's disease. Front. Neuroanat. 9:91. doi: 10.3389/fnana.2015.00091
Chae, Y., Lee, H., Kim, H., Kim, C. H., Chang, D. I., Kim, K. M., et al. (2009). Parsing brain activity associated with acupuncture treatment in Parkinson's diseases. Mov. Disord. 24, 1794–1802. doi: 10.1002/mds.22673
Cho, S. Y., Shim, S. R., Rhee, H. Y., Park, H. J., Jung, W. S., Moon, S. K., et al. (2012). Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson's disease. Parkinsonism Relat. Disord. 18, 948–952. doi: 10.1016/j.parkreldis.2012.04.030
Choi, Y. G., Park, J. H., and Lim, S. (2009). Acupuncture inhibits ferric iron deposition and ferritin-heavy chain reduction in an MPTP-induced parkinsonism model. Neurosci. Lett. 450, 92–96. doi: 10.1016/j.neulet.2008.11.049
Choi, Y. G., Yeo, S., Hong, Y. M., Kim, S. H., and Lim, S. (2011a). Changes of gene expression profiles in the cervical spinal cord by acupuncture in an MPTP-intoxicated mouse model: microarray analysis. Gene 481, 7–16. doi: 10.1016/j.gene.2011.03.006
Choi, Y. G., Yeo, S., Hong, Y. M., and Lim, S. (2011b). neuroprotective changes of striatal degeneration-related gene expression by acupuncture in an mptp mouse model of parkinsonism: microarray analysis. Cell. Mol. Neurobiol. 31, 377–391. doi: 10.1007/s10571-010-9629-2
Demaagd, G., and Philip, A. (2015). Parkinson's disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T 40, 504–532.
Deng, J., Lv, E., Yang, J., Gong, X., Zhang, W., Liang, X., et al. (2015). Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic alpha-synuclein mutant mouse model. J. Neuroinflammation 12:103. doi: 10.1186/s12974-015-0302-z
Duty, S., and Jenner, P. (2011). Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 164, 1357–1391. doi: 10.1111/j.1476-5381.2011.01426.x
Hong, M. S., Park, H. K., Yang, J. S., Park, H. J., Kim, S. T., Kim, S. N., et al. (2010). Gene expression profile of acupuncture treatment in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease model. Neurol. Res. 32, 74–78. doi: 10.1179/016164109X12537002794165
Huo, L. R., Liang, X. B., Li, B., Liang, J. T., He, Y., Jia, Y. J., et al. (2012). The cortical and striatal gene expression profile of 100 Hz electroacupuncture treatment in 6-hydroxydopamine-induced Parkinson's disease model. Evid. Based Complement. Alternat. Med. 2012:908439. doi: 10.1155/2012/908439
Jackson-Lewis, V., and Przedborski, S. (2007). Protocol for the MPTP mouse model of Parkinson's disease. Nat. Protoc. 2, 141–151. doi: 10.1038/nprot.2006.342
Jagmag, S. A., Tripathi, N., Shukla, S. D., Maiti, S., and Khurana, S. (2015). Evaluation of models of Parkinson's disease. Front. Neurosci. 9:503. doi: 10.3389/fnins.2015.00503
Jeon, H., Ryu, S., Kim, D., Koo, S., Ha, K. T., and Kim, S. (2017). Acupuncture stimulation at GB34 restores MPTP-induced neurogenesis impairment in the subventricular zone of mice. Evid. Based Complement. Alternat. Med. 2017:3971675. doi: 10.1155/2017/3971675
Jeon, S., Kim, Y. J., Kim, S. T., Moon, W., Chae, Y., Kang, M., et al. (2008). Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson's disease mouse model. Proteomics 8, 4822–4832. doi: 10.1002/pmic.200700955
Jia, J., Li, B., Sun, Z. L., Yu, F., Wang, X., and Wang, X. M. (2010). Electro-acupuncture stimulation acts on the basal ganglia output pathway to ameliorate motor impairment in Parkinsonian model rats. Behav. Neurosci. 124, 305–310. doi: 10.1037/a0018931
Jia, J., Sun, Z., Li, B., Pan, Y., Wang, H., Wang, X., et al. (2009). Electro-acupuncture stimulation improves motor disorders in Parkinsonian rats. Behav. Brain Res. 205, 214–218. doi: 10.1016/j.bbr.2009.06.024
Jia, Y. J., Deng, J. H., Zhang, W. Z., Sun, Z. L., Yang, J., Yu, Y., et al. (2017). The role of group II metabotropic glutamate receptors in the striatum in electroacupuncture treatment of Parkinsonian rats. CNS Neurosci. Ther. 23, 23–32. doi: 10.1111/cns.12587
Joh, T. H., Park, H. J., Kim, S. N., and Lee, H. (2010). Recent development of acupuncture on Parkinson's disease. Neurol. Res. 32(Suppl. 1), 5–9. doi: 10.1179/016164109X12537002793643
Kang, J. M., Park, H. J., Choi, Y. G., Choe, I. H., Park, J. H., Kim, Y. S., et al. (2007). Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 1131, 211–219. doi: 10.1016/j.brainres.2006.10.089
Kim, S. N., Doo, A. R., Park, J. Y., Bae, H., Chae, Y., Shim, I., et al. (2011b). Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson's disease. PLoS ONE 6:e27566. doi: 10.1371/journal.pone.0027566
Kim, S. N., Doo, A. R., Park, J. Y., Choo, H. J., Shim, I., Park, J. J., et al. (2014). Combined treatment with acupuncture reduces effective dose and alleviates adverse effect of L-dopa by normalizing Parkinson's disease-induced neurochemical imbalance. Brain Res. 1544, 33–44. doi: 10.1016/j.brainres.2013.11.028
Kim, S. N., Kim, S. T., Doo, A. R., Park, J. Y., Moon, W., Chae, Y., et al. (2011a). Phosphatidylinositol 3-kinase/akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson's disease. Int. J. Neurosci. 121, 562–569. doi: 10.3109/00207454.2011.591515
Kim, S. T., Moon, W., Chae, Y., Kim, Y. J., Lee, H., and Park, H. J. (2010). The effect of electroaucpuncture for 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced proteomic changes in the mouse striatum. J. Physiol. Sci. 60, 27–34. doi: 10.1007/s12576-009-0061-7
Kim, Y. K., Lim, H. H., Song, Y. K., Lee, H. H., Lim, S., Han, S., et al. (2005). Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci. Lett. 384, 133–138. doi: 10.1016/j.neulet.2005.04.068
Lam, Y. C., Kum, W. F., Durairajan, S. S., Lu, J. H., Man, S. C., Xu, M., et al. (2008). Efficacy and safety of acupuncture for idiopathic Parkinson's disease: a systematic review. J. Altern. Complement. Med. 14, 663–671. doi: 10.1089/acm.2007.0011
Lee, Y., Choi, G., Jeon, H., Kim, D., Ryu, S., Koo, S., et al. (2018). Acupuncture stimulation at GB34 suppresses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative stress in the striatum of mice. J. Physiol. Sci. 68, 455–462. doi: 10.1007/s12576-017-0547-7
Li, M., Li, L., Wang, K., Su, W., and Jia, J. (2017a). The effect of electroacupuncture on proteomic changes in the motor cortex of 6-OHDA Parkinsonian rats. Brain Res. 1673, 52–63. doi: 10.1016/j.brainres.2017.07.027
Li, M., Wang, K., Su, W. T., Jia, J., and Wang, X. M. (2017b). Effects of electroacupuncture on metabolic changes in motor cortex and striatum of 6-hydroxydopamine-induced Parkinsonian rats. Chin. J. Integr. Med. doi: 10.1007/s11655-017-2975-x. [Epub ahead of print].
Liang, X. B., Liu, X. Y., Li, F. Q., Luo, Y., Lu, J., Zhang, W. M., et al. (2002). Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Res. Mol. Brain Res. 108, 51–59. doi: 10.1016/S0169-328X(02)00513-2
Liang, X. B., Luo, Y., Liu, X. Y., Lu, J., Li, F. Q., Wang, Q., et al. (2003). Electro-acupuncture improves behavior and upregulates GDNF mRNA in MFB transected rats. Neuroreport 14, 1177–1181. doi: 10.1097/00001756-200306110-00015
Lin, J. G., Chen, C. J., Yang, H. B., Chen, Y. H., and Hung, S. Y. (2017). electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of Parkinson's disease. Int. J. Mol. Sci. 18:1846. doi: 10.3390/ijms18091846
Lu, K. W., Yang, J., Hsieh, C. L., Hsu, Y. C., and Lin, Y. W. (2017). Electroacupuncture restores spatial learning and downregulates phosphorylated N-methyl-D-aspartate receptors in a mouse model of Parkinson's disease. Acupunct. Med. 35, 133–141. doi: 10.1136/acupmed-2015-011041
Lv, E., Deng, J., Yu, Y., Wang, Y., Gong, X., Jia, J., et al. (2015). Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson's disease. Free Radic. Res. 49, 1296–1307. doi: 10.3109/10715762.2015.1067696
Park, H. J., Lim, S., Joo, W. S., Yin, C. S., Lee, H. S., Lee, H. J., et al. (2003). Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson's disease model. Exp. Neurol. 180, 93–98. doi: 10.1016/S0014-4886(02)00031-6
Park, J. Y., Choi, H., Baek, S., Jang, J., Lee, A., Jeon, S., et al. (2015). p53 signalling mediates acupuncture-induced neuroprotection in Parkinson's disease. Biochem. Biophys. Res. Commun. 460, 772–779. doi: 10.1016/j.bbrc.2015.03.105
Park, J. Y., Kim, S. N., Yoo, J., Jang, J., Lee, A., Oh, J. Y., et al. (2017). Novel neuroprotective effects of melanin-concentrating hormone in Parkinson's disease. Mol. Neurobiol. 54, 7706–7721. doi: 10.1007/s12035-016-0258-8
Rui, G., Guangjian, Z., Yong, W., Jie, F., Yanchao, C., Xi, J., et al. (2013). High frequency electro-acupuncture enhances striatum DAT and D1 receptor expression, but decreases D2 receptor level in 6-OHDA lesioned rats. Behav. Brain Res. 237, 263–269. doi: 10.1016/j.bbr.2012.09.047
Sena, E., Van Der Worp, H. B., Howells, D., and Macleod, M. (2007). How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 30, 433–439. doi: 10.1016/j.tins.2007.06.009
Shen, X., Xie, Y. Y., Chen, C., and Wang, X. P. (2015). Effects of electroacupuncture on cognitive function in rats with Parkinson's disease. Int. J. Physiol. Pathophysiol. Pharmacol. 7, 145–151.
Sun, M., Wang, K., Yu, Y., Su, W. T., Jiang, X. X., Yang, J., et al. (2016). Electroacupuncture alleviates depressive-like symptoms and modulates BDNF signaling in 6-hydroxydopamine rats. Evid. Based Complement. Alternat. Med. 2016:7842362. doi: 10.1155/2016/7842362
Sun, Z., Jia, J., Gong, X., Jia, Y., Deng, J., Wang, X., et al. (2012). Inhibition of glutamate and acetylcholine release in behavioral improvement induced by electroacupuncture in parkinsonian rats. Neurosci. Lett. 520, 32–37. doi: 10.1016/j.neulet.2012.05.021
Surmeier, D. J., Guzman, J. N., Sanchez-Padilla, J., and Goldberg, J. A. (2010). What causes the death of dopaminergic neurons in Parkinson's disease? Prog. Brain Res. 183, 59–77. doi: 10.1016/S0079-6123(10)83004-3
Tian, T., Sun, Y., Wu, H., Pei, J., Zhang, J., Zhang, Y., et al. (2016). Acupuncture promotes mTOR-independent autophagic clearance of aggregation-prone proteins in mouse brain. Sci. Rep. 6:19714. doi: 10.1038/srep19714
Wang, H., Liang, X., Wang, X., Luo, D., Jia, J., and Wang, X. (2013). Electro-acupuncture stimulation improves spontaneous locomotor hyperactivity in MPTP intoxicated mice. PLoS ONE 8:e64403. doi: 10.1371/journal.pone.0064403
Wang, H., Pan, Y., Xue, B., Wang, X., Zhao, F., Jia, J., et al. (2011). The antioxidative effect of electro-acupuncture in a mouse model of Parkinson's disease. PLoS One 6:e19790. doi: 10.1371/journal.pone.0019790
Wang, Y., Wang, Y., Liu, J., and Wang, X. (2018). Electroacupuncture alleviates motor symptoms and Up-regulates vesicular glutamatergic transporter 1 expression in the subthalamic nucleus in a unilateral 6-hydroxydopamine-lesioned hemi-Parkinsonian rat model. Neurosci. Bull. 34, 476–484. doi: 10.1007/s12264-018-0213-y
Yang, H. J., Gao, Y., Yun, J. Y., Kim, Y. E., Ehm, G., Lee, J. Y., et al. (2017). Acupuncture does not protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage of dopaminergic neurons in a preclinical mouse model of Parkinson's disease. Neuroreport 28, 50–55. doi: 10.1097/WNR.0000000000000709
Yang, J. L., Chen, J. S., Yang, Y. F., Chen, J. C., Lin, C. H., Chang, R. S., et al. (2011). Neuroprotection effects of retained acupuncture in neurotoxin-induced Parkinson's disease mice. Brain Behav. Immun. 25, 1452–1459. doi: 10.1016/j.bbi.2011.05.012
Yeo, S., An, K. S., Hong, Y. M., Choi, Y. G., Rosen, B., Kim, S. H., et al. (2015). Neuroprotective changes in degeneration-related gene expression in the substantia nigra following acupuncture in an MPTP mouse model of Parkinsonism: microarray analysis. Genet. Mol. Biol. 38, 115–127. doi: 10.1590/S1415-475738120140137
Yeo, S., Choi, Y. G., Hong, Y. M., and Lim, S. (2013). Neuroprotective changes of thalamic degeneration-related gene expression by acupuncture in an MPTP mouse model of Parkinsonism: microarray analysis. Gene 515, 329–338. doi: 10.1016/j.gene.2012.12.002
Yeo, S., Lim, S., Choe, I. H., Choi, Y. G., Chung, K. C., Jahng, G. H., et al. (2012). Acupuncture stimulation on GB34 activates neural responses associated with Parkinson's disease. CNS Neurosci. Ther. 18, 781–790. doi: 10.1111/j.1755-5949.2012.00363.x
Yu, Y., Wang, K., Deng, J., Sun, M., Jia, J., and Wang, X. (2016). Electroacupuncture produces the sustained motor improvement in 6-hydroxydopamine-lesioned mice. PLoS ONE 11:e0149111. doi: 10.1371/journal.pone.0149111
Yu, Y. P., Ju, W. P., Li, Z. G., Wang, D. Z., Wang, Y. C., and Xie, A. M. (2010). Acupuncture inhibits oxidative stress and rotational behavior in 6-hydroxydopamine lesioned rat. Brain Res. 1336, 58–65. doi: 10.1016/j.brainres.2010.04.020
Keywords: acupuncture, dopamine neuroprotection, meta analysis, Parkinson's disease, rodent model, systematic review
Citation: Ko JH, Lee H, Kim S-N and Park H-J (2019) Does Acupuncture Protect Dopamine Neurons in Parkinson's Disease Rodent Model?: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 11:102. doi: 10.3389/fnagi.2019.00102
Received: 28 December 2018; Accepted: 16 April 2019;
Published: 08 May 2019.
Edited by:
Allison B. Reiss, Winthrop University Hospital, United StatesReviewed by:
Peggy Bosch, Radboud University Nijmegen, NetherlandsZhe Zhang, Shandong University of Traditional Chinese Medicine, China
Copyright © 2019 Ko, Lee, Kim and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung-Nam Kim, c25raW1AZG9uZ2d1ay5lZHU=
Hi-Joon Park, YWN1ZmluZEBraHUuYWMua3I=
 Jade Heejae Ko1,2
Jade Heejae Ko1,2 Hyangsook Lee
Hyangsook Lee Seung-Nam Kim
Seung-Nam Kim Hi-Joon Park
Hi-Joon Park