- Rotman Research Institute at Baycrest, University of Toronto, Toronto, ON, Canada
Older adults differ from their younger counterparts in the way they view faces. We assessed whether older adults can use past experience to mitigate these typical face-processing differences; that is, we examined whether there are age-related differences in the use of memory to support current processing. Eye movements of older and younger adults were monitored as they viewed faces that varied in the type/amount of prior exposure. Prior exposure was manipulated by including famous and novel faces, and by presenting faces up to five times. We expected that older adults may have difficulty quickly establishing new representations to aid in the processing of recently presented faces, but would be able to invoke face representations that have been stored in memory long ago to aid in the processing of famous faces. Indeed, younger adults displayed effects of recent exposure with a decrease in the total fixations to the faces and a gradual increase in the proportion of fixations to the eyes. These effects of recent exposure were largely absent in older adults. In contrast, the effect of fame, revealed by a subtle increase in fixations to the inner features of famous compared to non-famous faces, was similar for younger and older adults. Our results suggest that older adults’ current processing can benefit from lifetime experience, however the full benefit of recent experience on face processing is not realized in older adults.
Introduction
Along with typical age-related cognitive and memory decline (Small, 2001), there are marked age-related differences in the processing and remembering of faces. Using eye movement monitoring to examine face processing, previous work has shown that older adults make more eye fixations to, and more saccadic transitions between, the inner features of the face when compared to younger adults (Firestone et al., 2007). In spite of the increase in the amount of eye movements made by older adults to faces, numerous studies have found that older adults have relatively poor recognition memory for faces when compared to younger adults (Smith and Winograd, 1978; Bartlett et al., 1989; Crook and Larrabee, 1992; Boutet and Faubert, 2006). Older adults often confuse novel faces for faces they have previously viewed; especially if the novel face resembles someone they know (Smith and Winograd, 1978; Bartlett et al., 1989, 1991; Bartlett and Fulton, 1991; Crook and Larrabee, 1992; Boutet and Faubert, 2006). Older adults also have difficulty remembering exactly when a face was presented and may wrongly classify a previously viewed face as famous (Bartlett et al., 1991).
Although age-related changes in face processing and face recognition are robust (see Searcy et al., 1999, for review), certain faces are processed similarly by older and younger adults and tend to be accurately remembered by older and younger adults alike. In particular, age-related differences in processing and recognition are minimized for faces that may be most relevant to the daily activities of older adults, including familiar famous faces (Bäckman, 1991) and faces of people who belong to the same age cohort (Anastasi and Rhodes, 2003; Firestone et al., 2007). One explanation for this preserved processing and recognition with aging is that, in the case of familiar faces, older adults can use existing memories of those faces to support processing and minimize typical age-related differences. Similarly, older adults may have more daily interactions with same-aged adults than younger adults. By recruiting stored representations regarding faces of same-aged adults, older adults may receive the additional support that is needed for effective visual processing.
As noted above, eye movements are used as an index of the amount and pattern of processing of faces. If we consider that the purpose of eye movements is to extract new information from regions of a visual display (e.g., Parker, 1978), such as a face, then increasing the type/amount of past experience would gradually reduce the amount of new information available. Consequently, this would decrease the amount and scope of visual processing required to evaluate the stimulus and ultimately, increase the speed or efficiency by which processing occurs. Indeed, for younger adults, repeated exposure to faces results in a decrease in the overall amount of eye movements that are directed to faces (Heisz and Shore, 2008). Additionally, eye movements tend to be directed to highly informative face regions of previously viewed faces, such as the eyes (Althoff and Cohen, 1999; Heisz and Shore, 2008). This processing efficiency afforded by previously viewed faces is attributed to the retrieval of a stored representation of the face itself as well as any other associated information such as knowledge regarding when the face was previously encountered.
The present study assessed whether older adults, like younger adults, can use past experience to support current processing and thereby lessen the typical age-related differences in face processing. We manipulated past experience in two ways. First, participants were presented with famous and non-famous faces to examine the influence of pre-experimental exposure on current viewing. Second, famous and non-famous faces were repeated up to five times throughout the experiment to examine the influence of recent exposure on current viewing. Based on the findings from previous studies, we expected younger adults to make fewer fixations and direct a greater proportion of those fixations to the inner features of famous (Althoff and Cohen, 1999; Stacey et al., 2005; Barton et al., 2006) and experimentally repeated (Heisz and Shore, 2008) faces compared to novel faces. Furthermore, if memory continues to support visual processing across the lifespan, then older adults should show similar eye movements to famous faces, and even to experimentally familiarized faces, as younger adults. However, given that older adults experience a variety of changes in brain structure and function, there may be important age-related differences in the interplay between memory and visual processing. Older adults may be able to invoke face representations that have been stored in memory long ago to aid in the processing of famous faces but they may have difficulty quickly establishing new representations to aid in the processing of recently presented faces. In this case, the extent to which the eye movements of older adults resemble those of younger adults may depend on whether the face has recent or pre-experimental exposure. Ultimately, the current study will reveal the extent to which older adults can use recently acquired and previously established memory representations to support current visual processing of faces in a manner similar to that of younger adults.
Materials and Methods
Participants
Fourteen younger adults (five male, M = 25 years, range = 20–31 years) and 14 older adults (five male, M = 74 years, range = 65–83 years) from the Toronto community participated in exchange for monetary compensation. All procedures complied with the Canadian tri-council policy on ethics and were approved by the Baycrest Research Ethics Board. All participants were healthy with no reported memory complaints, prior head injuries, depression, learning disabilities, or psychiatric problems. All participants reported no visual problems and had normal or corrected-to-normal vision. Years of education did not significantly differ between younger (15.7 ± 1.3 years) and older adults (15.5 ± 3.9 years; p = 0.85).
Apparatus and Stimuli
A Dell Precision T3400 computer was connected to a ViewSonic G90fb 19″ CRT monitor for presentation of the stimuli. An additional Dell Optiplex 755 computer was used to collect eye movement data using the EyeLink II system (Version 2.22).
Stimuli consisted of 60 color pictures of Caucasian faces placed on a black background. Thirty famous (15 female) and 30 non-famous (15 female) were selected from a larger face database (Ryan et al., 2007). Famous faces consisted of high-profile movie actors and TV celebrities. All faces were front-viewing with direct gaze and either neutral or smiling expression. Faces were presented with hair but without neck or body, and positioned so that eye height was constant across all photos. Faces were judged by the authors to be of middle age (i.e., ∼35–60 years old). Faces were divided into three sets of 20, each with 10 famous faces (five male) and 10 non-famous faces (five male). Face Set A was presented in each of five blocks (five exposures), Face Set B was presented in each of the final three blocks (three exposures), and Face Set C was presented in the final block only (one exposure). Face order within a block was randomized and face sets were counterbalanced across participants.
Procedure
Across five blocks, participants were instructed to freely view faces that were presented singly, for 5 s each. Stimuli were presented at the center of the display. Faces were ∼9.5° by ∼6.3° of visual angle from a viewing distance of 90 cm. Blocks 1 and 2 each contained 20 trials; Blocks 3 and 4 each contained 40 trials; and Block 5 contained 60 trials. Each trial was initiated upon central fixation. Drift correction was performed in between trials when required.
Following viewing of the faces, participants were queried regarding their pre-experimental familiarity with the faces. For each face, participants answered the following question in a self-paced manner: is this face famous? (Possible answers: famous, unsure, or not famous). This question was used to identify which famous faces each participant recognized; only those famous faces that were accurately recognized as famous were included for analysis to keep this category of faces distinct from the repeated, non-famous faces. Younger adults recognized 90 ± 5% SEM of famous faces; older adults recognized 70 ± 5% SEM.
Analysis of Eye Movements
Analysis of eye movements focused on the fifth and final block, which consisted of the fifth presentation of Face Set A, the third presentation of Face Set B, and the first presentation of Face Set C. As a result of calibration error, an average of 1.4% (±0.7% SEM) of trials were excluded. Eye movement measures were analyzed with respect to the faces and to features within the faces. Fixations that began before face onset and continued after face offset were excluded. Fixations to the outside of the facial image, consisting of 0.2% of all fixations, were excluded from analysis. Inner features (eyes, nose, and mouth) were defined using non-overlapping rectangular sections. A unique area-of-interest template was used for each face (Figure 1). Interest areas (i.e., pixel number) defining the eyes, nose, and mouth of famous versus non-famous faces were not statistically different as revealed by independent t tests contrasting each feature area of each face type (all p’s > 0.1). The total number of fixations made to each face and the proportion of fixations made to the eyes, nose, and mouth were analyzed.

Figure 1. An example of a non-famous face overlaid with its unique interest area template. All interest area templates captured features in the same manner depicted. During the experiment, stimuli were presented in color and without interest area template overlaid.
Statistical Analyses
Repeated-measures analyses of variance (ANOVAs) were done in R (http://www.r-project.org) using the package ezANOVA. Greenhouse–Geisser correction was applied to p values when necessary. All post hoc t tests were corrected for multiple comparisons using the Bonferroni method.
Results
Viewing to the Face
To examine the influence of pre-experimental and recent exposure the processing of faces (Figures 2 and 3), repeated-measures ANOVA was conducted on the number of fixations made to the faces with a between-subject factor of age (younger, older) and two within-subject factors of fame (famous, non-famous) and exposure (one, three, five). We observed significant main effects of age 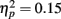 and exposure
and exposure 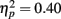 as well as a significant interaction of age with exposure
as well as a significant interaction of age with exposure 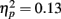 . Older adults made more fixations to the faces than younger adults. With increasing exposures, this age difference burgeoned as younger adults’ fixations decreased while older adults’ fixations remained relatively unchanged.
. Older adults made more fixations to the faces than younger adults. With increasing exposures, this age difference burgeoned as younger adults’ fixations decreased while older adults’ fixations remained relatively unchanged.
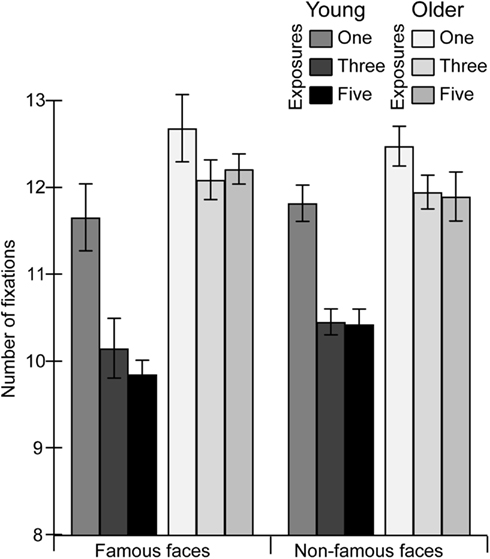
Figure 2. The total number of fixations to famous and non-famous faces by young and older adults as a function of prior exposure. Error bars represent SE of the mean corrected for repeated measures specific to the within-subject contrasts (see Masson and Loftus, 2003).
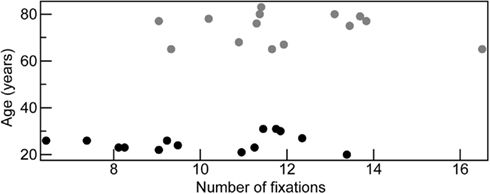
Figure 3. Participants’ age crossed with the total number of fixations (collapsed across fame) made across faces with five exposures.
When analyzed separately, younger adults showed a significant effect of exposure on the number of fixations 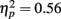 and a significant linear decreasing contrast [t(13) = 5.14, p < 0.001]; the three pair-wise comparisons of exposure revealed significant differences between one versus three exposures and one versus five exposures (ps < 0.01) but not between three versus five exposures (p = 0.5). The analysis for the older adults revealed a marginal but non-significant effect of exposure on the number of fixations [F(2,26) = 2.79, p = 0.08].
and a significant linear decreasing contrast [t(13) = 5.14, p < 0.001]; the three pair-wise comparisons of exposure revealed significant differences between one versus three exposures and one versus five exposures (ps < 0.01) but not between three versus five exposures (p = 0.5). The analysis for the older adults revealed a marginal but non-significant effect of exposure on the number of fixations [F(2,26) = 2.79, p = 0.08].
Viewing to the Eyes, Nose, and Mouth
To examine the influence of pre-experimental and recent exposure on the processing of the inner face features (Table 1; Figure 4), repeated-measures ANOVA was conducted on the proportion of fixations with a between-subject factor of age (younger, older) and three within-subject factors of fame (famous, non-famous), exposure (one, three, five), and feature (eyes, nose, mouth). A significant main effect of face feature 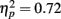 revealed that participants directed the greatest proportion of fixations to the eyes followed by the nose and then the mouth (all three pair-wise comparisons: ps < 0.001). The proportions of fixations made to the nose and mouth were higher for famous faces than non-famous faces (Table 1) as evidenced by a significant main effect of fame
revealed that participants directed the greatest proportion of fixations to the eyes followed by the nose and then the mouth (all three pair-wise comparisons: ps < 0.001). The proportions of fixations made to the nose and mouth were higher for famous faces than non-famous faces (Table 1) as evidenced by a significant main effect of fame 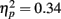 and a significant interaction of fame with feature
and a significant interaction of fame with feature 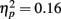 . There was a significant effect of fame (famous versus non-famous) on the proportion of fixations made to the nose and the mouth (ps < 0.016) but there was no significant effect of fame on the proportion of fixations directed to the eyes (p = 0.3). A significant two-way interaction of exposure with feature
. There was a significant effect of fame (famous versus non-famous) on the proportion of fixations made to the nose and the mouth (ps < 0.016) but there was no significant effect of fame on the proportion of fixations directed to the eyes (p = 0.3). A significant two-way interaction of exposure with feature 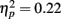 and a significant three-way interaction of exposure with feature and age
and a significant three-way interaction of exposure with feature and age 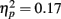 were observed. To assess the simple main effects of the three-way interaction, three two-way ANOVAs were conducted for the proportion of fixations directed to the eyes, nose, and mouth, separately, using a between-subject factor of age (younger, older) and a within-subject factor of exposure (one, three, five) for each analysis, below. The findings are depicted in Figure 4.
were observed. To assess the simple main effects of the three-way interaction, three two-way ANOVAs were conducted for the proportion of fixations directed to the eyes, nose, and mouth, separately, using a between-subject factor of age (younger, older) and a within-subject factor of exposure (one, three, five) for each analysis, below. The findings are depicted in Figure 4.
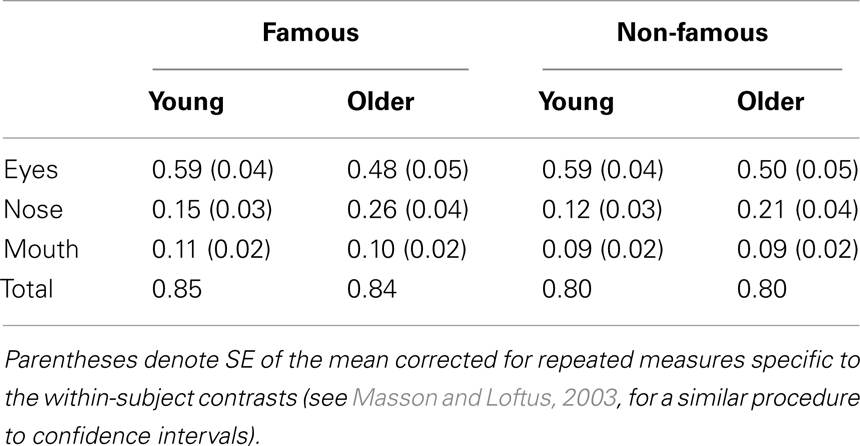
Table 1. The proportion of fixations made to the eyes, nose, and mouth of famous and non-famous faces across exposures.
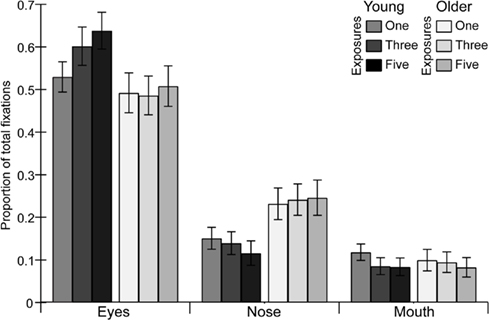
Figure 4. The proportion of fixations made to the eyes, nose, and mouth of faces by young and older adults as a function of prior exposure. Error bars represent SE of the mean corrected for repeated measures specific to the within-subject contrasts (see Masson and Loftus, 2003).
Eyes
With increasing exposures, the proportion of fixations to the eyes increased as revealed by a significant main effect of exposure 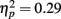 . However this effect was driven by the younger adults as evidenced by the significant interaction of exposure with age
. However this effect was driven by the younger adults as evidenced by the significant interaction of exposure with age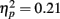 and a significant linear increase for younger adults [main effect of exposure,
and a significant linear increase for younger adults [main effect of exposure, 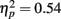 ; linear increasing contrast, t(1, 13) = 6.46, p < 0.001] but not for older adults [main effect of exposure, F(2, 26) = 0.60, p = 0.55].
; linear increasing contrast, t(1, 13) = 6.46, p < 0.001] but not for older adults [main effect of exposure, F(2, 26) = 0.60, p = 0.55].
Nose
Older adults made a greater proportion of fixations to the nose than younger adults as revealed by a significant effect of age 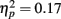 .
.
Mouth
The proportion of fixations directed to the mouth decreased across exposures as revealed a significant main effect 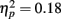 and linear decreasing contrast [t(1, 27) = 3.10, p < 0.01] of exposure.
and linear decreasing contrast [t(1, 27) = 3.10, p < 0.01] of exposure.
Discussion
Eye movements serve to gather new information from the visual world, as well as information that was not sufficiently encoded during previous exposures (e.g., Parker, 1978). Therefore, eye movements reveal the interplay between memory and visual processing. Repeated exposures to the same faces gradually reduces the amount of new information available and consequently, decreases the amount and scope of visual processing required to evaluate the face. By examining the eye movements of younger and older adults, the present study assessed whether older adults, like younger adults, can use past experience to support current processing.
For the younger adults, the influence of memory on visual processing was evidenced here, as in previous work, with a decrease in the number of fixations directed to the faces and a subtle change in the distribution of those fixations (Althoff and Cohen, 1999; Heisz and Shore, 2008). Regardless of whether experience was garnered in the real world (as with famous faces) or in the laboratory (as with non-famous faces), previously stored representations of faces influenced subsequent viewing for younger adults.
A greater proportion of fixations were directed to the inner features of familiar famous compared to non-famous faces, though this difference was small. In addition, with repeated exposure to the faces in the laboratory, younger adults exhibited a decrease in the total fixations to the faces. Within the faces, younger adults exhibited a subtle increase in the proportion of fixations to the eyes and a corresponding decrease in the proportion of fixations to the mouth. Thus, prior experience influenced processing of faces for younger adults, with the type of prior experience exerting distinct effects.
However, this influence of memory on current processing was less evident for older adults. Like younger adults, older adults showed similar subtle differences in fixations to the features of famous versus non-famous faces. However, in contrast to younger adults, older adults did not show an effect of exposure on the total number of fixations directed to the faces and there was no change in the distribution of fixations to the eyes, even with 25 s worth of encoding time (5 s by five exposures). These findings suggest that memory for prior experiences may support visual processing in older adults but the full benefit of recent experience on face processing is not realized to the same extent in older as in younger adults.
Since the full realization of these repetition-based changes in younger adults has been linked to face learning (Heisz and Shore, 2008), the current findings suggest that the perceived novelty of a face may be slower to wane with repetition in older adults than in younger adults; that is, older adults may have difficulty developing new face representations within memory. Moreover, given that older adults have more lifetime experience with faces and thus more instances of faces stored in memory, it may require extra effort or time for older adults to match a presented face with one stored in memory due to increased competition. Indeed, one finding in particular suggests that older adults may require additional efforts to develop representations of faces in memory. Previous studies have revealed that the number of fixations made to visual displays, including faces, is predictive of subsequent recognition memory (Chan et al., 2011). Overall, older adults made more fixations to the faces than younger adults; thus older adults may require more eye movements than younger adults in order to develop stable representations of faces that can support recognition memory, even if, ultimately, the level of recognition success is still not at that of younger adults (Firestone et al., 2007). Though somewhat tangential, similar findings are also observed with visual search paradigms; despite increased eye movements, older adults are less successful at target detection than younger adults (Scialfa et al., 1987; Watson et al., 2005).
Age-related differences in face processing could result from older adults’ difficulty processing faces holistically. A holistic face representation is thought to reflect the identity of the face, with features bound into a coherent whole or gestalt (Sergent, 1984; Farah et al., 1998). Convincing evidence for holistic face processing is garnered from the composite face effect (Young et al., 1987; Hole, 1994). When the top half of one person’s face is aligned with the bottom half of another person’s face, the resultant composite face produces the strong perception of a completely novel face. In contrast, when the two halves are misaligned via slight horizontal displacement, the composite face illusion disappears and the two halves can be recognized separately. Boutet and Faubert (2006) measured the composite face illusion in younger and older adults who viewed previously unfamiliar faces. At test, participants were presented composites of studied faces and composites of new faces and were required to judge whether the top half of each composite was previously studied or new. Younger adults were more likely to recognize the top half of misaligned composites compared to aligned composites, thus illustrating the strong tendency for holistic possessing of typically aligned faces. In contrast, older adults’ recognition was not affected by composite alignment, suggesting a weaker tendency to engage in holistic processing. For older adults, this reduced ability to integrate facial features into a whole may make it more difficult to encode and represent the structural details of a novel face in memory. As a result, older adults may need to continually revisit face features across exposures to develop a sufficient representation of the face that can subsequently support recognition. In turn, the eye movements of older adults may reflect the continued attempt to develop a holistic face representation and this may be why older adults spend a greater proportion of time viewing the nose (i.e., the center of the face) than younger adults.
A similar interpretation of the current findings (that is not mutually exclusive to the idea of age-related differences in holistic processing) is that age-related differences in face processing may reflect a binding deficit that underlies not only deficits in face recognition but also other classes of memory performance. For instance, Firestone et al. (2007) observed heightened fixations and transitions by older adults during encoding that did not translate into better recognition performance at test. The authors suggested that this increase in transitional behavior, coupled with poorer recognition performance, might indicate an age-related deficit in binding that would otherwise associate the distinct features of the face in memory. A binding deficit has also been invoked to account for age-related deficits in memory for associations within and between various stimulus categories, including word-pairs (Castel and Craik, 2003), picture-pairs (Naveh-Benjamin et al., 2003), or face–name associations (Naveh-Benjamin et al., 2004). The reduced ability to bind the features of a face and/or a face with the encoding context (in this case, the experimental session) may have made it more difficult for older adults to maintain a representation of the face in memory that could otherwise influence processing, thereby causing older adults to view previously studied faces as if they were novel.
Although the current findings point to potential difficulties for older adults in establishing representations of faces that can be used to support processing, face learning in the real world is a much richer experience than that of the laboratory. In the current experiment, a single photograph was used to represent each individual, whereas in the real world, faces can be encoded from many angles and under various viewing conditions. A richer real-world experience may provide additional support for face processing recognition. Furthermore, there may also be an influence of associated information (e.g., a person’s name, occupation) that is used to support processing and recognition. In that case, it may not only be the perceptual information that is retrieved but the retrieval of associated information that influences eye movements. Consistent with this idea is the finding that older and younger adults scanned famous faces similarly. However, it is not clear whether this display of youthful scanning by older adults is a result of learning that occurred within the experimental session or a result of learning that took place when the participant was younger, and perhaps prior to the onset of structural and functional age-related changes that may otherwise impact processing. This may be an important distinction for future research; nevertheless, the current work demonstrates that some classes of memory representations can be used to efficiently guide eye movement processing in younger and older adults alike.
In conclusion, younger and older adults differ in the way they can use past experience (memory) to guide current face processing. Although both younger and older adults show similar support for the processing of familiar famous faces acquired in the real world, only younger adults benefited from the effects of recent exposure on face processing in the laboratory setting. Our results suggest that older adults’ current processing can benefit from lifetime experience, however the full benefit of recent experience on face processing is not realized in older adults.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The Natural Sciences and Engineering Research Council of Canada, the Canada Research Chairs Program, and the Canadian Foundation for Innovation supported this research.
References
Althoff, R. R., and Cohen, N. J. (1999). Eye-movement-based memory effect: a reprocessing effect in face perception. J. Exp. Psychol. Learn. Mem. Cogn. 25, 997–1010.
Anastasi, J. S., and Rhodes, M. G. (2003). An own-age bias in face recognition for children and older adults. Psychon. Bull. Rev. 12, 1043–1047.
Bäckman, L. (1991). Recognition memory across the adult life span. The role of prior knowledge. Mem. Cognit. 19, 63–71.
Bartlett, J. C., and Fulton, A. (1991). Familiarity and recognition of faces in old age. Mem. Cognit. 19, 229–238.
Bartlett, J. C., Leslie, J. E., Tubbs, A., and Fulton, A. (1989). Aging and memory for pictures of faces. Psychol. Aging 4, 276–283.
Bartlett, J. C., Strater, L., and Fulton, A. (1991). False recency and false fame of faces in young adulthood and old age. Mem. Cognit. 19, 177–188.
Barton, J. J. S., Radcliffe, N., Cherkasova, M. V., Edelman, J., and Intriligator, J. M. (2006). Information processing during face recognition: the effects of familiarity, inversion, and morphing on scanning fixations. Perception 35, 1089–1105.
Boutet, I., and Faubert, J. (2006). Recognition of faces and complex objects in younger and older adults. Mem. Cognit. 34, 854–864.
Castel, A. D., and Craik, F. I. M. (2003). The effects of aging and divided attention on memory for item and associative information. Psychol. Aging 18, 873–885.
Chan, J. P. K., Kamino, D., Binns, M., and Ryan, J. D. (2011). Can changes in eye movement scanning alter the age-related deficit in recognition memory? Front. Psychol. 2:92.
Crook, T. H., and Larrabee, G. J. (1992). Changes in facial recognition memory across the adult life span. J. Gerontol. 47, 138–141.
Farah, M. J., Wilson, K. D., Drain, M., and Tanaka, J. W. (1998). What is “special” about face perception? Psychol. Rev. 105, 482–498.
Firestone, A., Turk-Browne, N. B., and Ryan, J. D. (2007). Age-related deficits in face recognition are related to underlying changes in scanning behavior. Aging Neuropsychol. Cogn. 14, 594–607.
Masson, M. E. J., and Loftus, G. R. (2003). Using confidence intervals for graphically based data interpretation. Can. J. Exp. Psychol. 57, 203–220.
Naveh-Benjamin, M., Guez, J., Kilb, A., and Reedy, S. (2004). The associative memory deficit of older adults: further support using face-name associations. Psychol. Aging 19. 541–546.
Naveh-Benjamin, M., Hussain, Z., Guez, J., and Bar-On, M. (2003). Adult age difference in episodic memory: further support for an associative-deficit hypothesis. J. Exp. Psychol. Learn. Mem. Cogn. 29, 826–837.
Parker, R. E. (1978). Picture processing during recognition. J. Exp. Psychol. Hum. Percept. Perform. 4, 284–293.
Ryan, J. D., Hannula, D. E., and Cohen, N. J. (2007). The obligatory effects of memory on eye movements. Memory 15, 508–525.
Scialfa, C. T., Klien, D. W., and Lyman, B. J. (1987). Age differences in target identification as a function of retinal location and noise level: examination of the useful field of view. Psychol. Aging 2, 14–19.
Searcy, J. H., Bartlett, J. C., and Memon, A. (1999). Age difference in accuracy and choosing in eyewitness identification and face recognition. Mem. Cognit. 27, 538–552.
Sergent, J. (1984). An investigation into component and configural processes underlying face perception. Br. J. Psychol. 75, 221–242.
Small, S. A. (2001). Age-related memory decline: current concepts and future directions. Arch. Neurol. 58, 360–364.
Smith, A. D., and Winograd, E. (1978). Adult age difference in remembering faces. Dev. Psychol. 14, 443–444.
Stacey, P. C., Walker, S., and Underwood, D. M. (2005). Face processing and familiarity: evidence from eye-movement data. Br. J. Psychol. 96, 407–422.
Watson, D. G., Maylor, E. A., and Bruce, L. A. M. (2005). Search, Enumeration, and aging: eye movement requirements cause age-equivalent performance in enumeration but not in search tasks. Psychol. Aging 20, 226–240.
Keywords: aging, face processing, prior exposure, eye movements, memory
Citation: Heisz JJ and Ryan JD (2011) The effects of prior exposure on face processing in younger and older adults. Front. Ag. Neurosci. 3:15. doi: 10.3389/fnagi.2011.00015
Received: 03 April 2011; Accepted: 19 September 2011;
Published online: 05 October 2011.
Edited by:
Jean Mariani, Universite Pierre et Marie Curie, FranceReviewed by:
Rongqiao He, Chinese Academy of Sciences, ChinaJ. Arturo García-Horsman, University of Helsinki, Finland
Copyright: © 2011 Heisz and Ryan. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Jennifer J. Heisz, Rotman Research Institute at Baycrest, 3560 Bathurst Street, Toronto, ON, Canada M6A 2E1. e-mail: jheisz@rotman-baycrest.on.ca
