
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Adolesc. Med., 26 March 2025
Sec. Addiction in Adolescents
Volume 3 - 2025 | https://doi.org/10.3389/fradm.2025.1549771
This article is part of the Research TopicCannabis use and neurocognitive functioning in adolescentsView all 3 articles
Background: Chronic cannabis use (CU) can result in subtle deficits in cognitive performance that may be linked with alterations in underlying neural functioning. However, these network alterations are not well-characterized following monitored abstinence. Here, we evaluate differences in functional brain network activity associated with CU patterns in adolescents/young adults.
Methods: Functional connectomes were generated using resting-state fMRI data collected from 83 healthy young adults (44 male) following two weeks of monitored cannabis abstinence. Network topology metrics were calculated for each of the 7 Yeo 2011 intrinsic connectivity networks (ICNs) and on the whole-brain level. Multiple linear regressions were used to evaluate whether CU (regular-users, n = 35 vs. non-using controls, n = 40) was associated with network topology metric differences after controlling for past-year alcohol use, age, sex, and cotinine levels; moderation by sex was also investigated. Regressions were run within CU group to test for associations between cannabis use patterns (lifetime CU, age of CU initiation, and past-year CU) and network topology. Finally, a network-based statistic (NBS) approach was used to search for connectome subcomponents associated with CU group, CU*sex, and patterns of CU.
Results: No significant association between CU groups and ICN topology was observed. Sex moderation was observed; within male cannabis users, higher past-year CU was associated with significantly higher frontoparietal and ventral attention network (VAN) efficiency. Within female cannabis users, higher past-year CU was associated with significantly lower Default Mode Network assortativity. Within individuals who initiated CU before the age of 17, males had lower assortativity in the VAN and Somatomotor network. NBS analyses indicated that connectivity strength within a primarily right-lateralized subnetwork distributed throughout the connectome was significantly and reliably associated with past-year CU).
Conclusion: The present findings suggest that subtle differences in resting-state network topology associated with CU may persist after an extended period of abstinence in young adults, particularly males, especially those with heavier past-year use and those who initiated CU earlier in life. While further replication is required in larger samples, these findings suggest potential neuroimaging correlates underlying long-term changes in brain network topology associated with CU.
Cannabis is one of the most extensively used psychoactive drugs, with peak usage occurring during adolescence and young adulthood (1). Delta-9-tetrahydrocannabinol (delta-9-THC) is the primary psychoactive cannabinoid found in cannabis that is used for recreational purposes; delta-9-THC interacts with the endocannabinoid (eCB) system and acts as a partial agonist of the cannabinoid receptor 1 (CB1R) (2–4). CB1R are localized on neurons, astrocytes, microglia, and oligodendrocytes, and endogenous endocannabinoids (N-arachidonoylethanolamine, AEA; 2-arachidonoylglycerol, 2AG) are released in response to synaptic activity and regulate neurotransmitter release (5–8). The eCB system also contributes to numerous other processes linked with brain health, including cell differentiation, neurogenesis, energy metabolism, refinement of neuronal connections, pruning of dendrites, myelination of axons, as well as being involved in anti-inflammatory and immune functions (5–7, 9–14). Animal and human research has shown high density of CB1R and eCB signaling in the prefrontal cortex (PFC), parietal, cerebellar, limbic system, and ventral striatal regions (3, 4, 8, 15).
The eCB system demonstrates dynamic adolescent changes, with ligands (AEA, 2AG) increasing and CB1R expression peaking during the teenage years (15–20). These co-occur with other adolescent neurodevelopmental changes; subcortical regions (i.e., amygdala, hippocampus, nucleus accumbens) develop first in preadolescence and remain stable or slightly increase during later teen years (evidenced through volumetric and diffusion MRI investigations), while pruning of cortical (especially PFC and parietal cortices) gray matter and improved white matter integrity continues into early adulthood (21–29). These dynamic changes in the eCB system are hypothesized to at least partially underlie these adolescent neurodevelopmental processes, including changes in dendritic structure, synaptic pruning, fine-tuning of functional coupling, and enhancing white matter quality, particularly in the PFC, parietal, cerebellar, striatal, and limbic regions (3, 4, 8, 15, 30–32). Functional neuroimaging (fMRI) studies also demonstrate dynamic changes in the BOLD response during adolescence, with limbic and striatal response to salient and affective stimuli peaking resulting in increased “top-down” frontoparietal cognitive control and interhemispheric connectivity (25, 33–35). This has sparked interest in examining brain data using network-based statistics (i.e., “connectomes”) where specified regions are denoted as nodes and strength between nodes are evaluated (i.e., “edges”). These changes also correspond to a distributed reorganization of functional brain networks from “local” (i.e., within-network) to “distributed” (i.e., between-network) with increasing modularity, hierarchical organization, and greater specificity among within-network connections that mirror canonical adult resting-state networks (36–39).
Given the role of the eCB system during adolescent development, chronic exposure to cannabis may disrupt neuromaturation; there is evidence that repeated delta-9-THC may downregulate the eCB system (40–45) and alter glutamate-modulated synaptic refinement (46–49). Preclinical findings have suggested that repeated delta-9-THC dosing alters glucose uptake, dopamine, glutamate and eCB signaling, gene expression, microglial apoptosis, neuroplasticity, white matter quality, and functional coupling (50–56). However, the effects of delta-9-THC on distributed resting-state cortical networks in non-human animals, especially among adolescents and young adults, have not been well characterized.
Human longitudinal studies have collectively shown that chronic cannabis use (CU) during adolescence leads to modest cognitive decline, especially in the areas of executive functioning and verbal memory [for reviews see (57, 58)]. These cannabis-related deficits in cognitive function often correspond to differences in functional brain activity when both are assessed; for example, a recent meta-analysis focused on brain functional connectivity outcomes associated with CU found evidence for relatively widespread increased connectivity strength in late adolescent and adult (16–42 years old) cannabis users relative to controls, particularly in frontal-frontal, fronto-striatal, and fronto-temporal region pairings (59). Similarly, Hammond and colleagues conducted a meta-regression analysis on 1,216 cannabis using and 1,486 non-using adolescents and concluded that cannabis using youth demonstrate greater activation in rostral medial, ventral PFC, and ACC regions during executive control tasks, but blunted dorsal medial PFC and dorsal ACC response during affective processing tasks (60). Further, they found that extent of CUD, length of abstinence, as well as sex, were related to BOLD response in executive control, suggesting that severity of CU may be key indicators of neurobiological impacts of CU (60). Increased local connectivity in frontal and midbrain regions was also higher in individuals who met the criteria for CUD (n = 22) than non-using controls (n = 20), suggesting the pattern of hyperconnectivity may extend beyond cortical structures (61). However, not all seed-based analyses have indicated increased connectivity associated with CU.
Most studies highlighted in these reviews have utilized BOLD-response differences or seed-based functional connectivity analyses that focus solely on connectivity to a singular region, such as the anterior cingulate cortex (ACC) or insula. However, network-level patterns have emerged from these findings as many of the brain regions fall within the same set of brain networks: the Dorsal Attention Network (DAN; a set of brain regions associated with top-down, goal-directed processing of attention), the Ventral Attention Network (VAN; a set of brain regions associated with bottom-up, stimulus-driven processing of attention), the Frontoparietal Control Network [FPCN; a network commonly associated with executive function; (62)], and the DMN [a network commonly associated with self-referential thought; (63, 64)]. Evidence has been mixed regarding the links between adolescent and young adult CU and connectivity within the DMN. Some studies have found hypoactivity (65–67) while others indicate hyperactivity (68) among cannabis-using participants within the DMN using seed-based analyses. In terms of between-network connections, there is some evidence that hyperconnectivity between the DMN and VAN is associated with the acute feeling of being high (66). This association is further substantiated by the dose-dependent association between duration of CU and connectivity strength between key structures in the DMN and VAN (67). Harris et al. (69) identified decreased connectivity between the intraparietal sulcus (a key region in the DAN) and the insula (key region in the VAN) in abstinent cannabis users relative to healthy controls. Cannabis users have also demonstrated greater intra-network frontolimbic connectivity at rest compared to non-using youth (70). Ertl and colleagues (71) examined the impact of CU on connectivity in the frontoparietal control network (FPCN), they found greater seed-based functional connectivity in cannabis users vs. controls, though this association did not extend to other networks, suggesting some specificity of the effect. A data-driven approach supported this association, primarily indicating fronto-parietal brain regions as being hyper-active in heavy cannabis users (72). Therefore, to date, the FPCN, DMN, and DAN appear to be the most strongly associated with regular adolescent CU. Notably, these seed-based connectivity analyses typically do not quantify the degree in overlap between indicated brain regions and commonly found networks of brain regions which tend to coactivate with one another, or intrinsic connectivity networks (ICNs), as such, these results are difficult to place in a network context.
Given the complexity of cannabis effects on neuromodulation and glutamate-related functional coupling during development, the impact of CU may be better addressed by studying brain network topology. Analysis of brain activity on the level of network topology has the benefit of evaluating distributed functional brain activity using graph theory to represent the flow of information in the brain. This “network neuroscience” approach also reduces the issue of multiple comparisons in conventional fMRI analyses by allowing for analysis on multiple scales (e.g., network, module, or node level) and has proven to be informative at delineating specific network patterns and characteristics of interest within neuroscience investigations (73–76). Functional brain network characteristics have been reliably associated with dimensions of psychopathology, including substance use, and may provide a useful biomarker of adolescent neurodevelopment (35, 77–80). During adolescence, there is also evidence for age-associated increased integration of the VAN and FPCN with other specialized networks, suggesting that the developmental refinement of functional brain networks may not be uniformed in nature (81). Further, exposure to exogenous cannabis may differentially disrupt specific networks.
Few studies to date have examined network topology in adolescent and young adult recreational cannabis users. Global efficiency (Eglob) is a common network marker generally representing the global strength of connections, it is defined as the average of the inverse shortest path length and represents how quickly (in terms of number of steps) information can flow in a network. In a weighted graph, this is heavily influenced by edge strength (i.e., positive correlation strength between nodes or regions). Thus, this statistic leverages both edge strength values and the higher-level structure of the network. In one of the first studies using brain network topology in acutely-abstinent (∼12 h, n = 18) adolescents with CU disorder (CUD), Nestor and colleagues (82) found that individuals with CUD had reliably higher Eglob, demonstrating greater whole-brain connectivity strength, during a reward processing task than matched healthy controls (n = 18). Greater connectivity strength was positively associated with age of CU onset, supporting the idea that differential use trajectories may influence patterns of brain activity (82). Both of these findings were consistent across connectome thresholding levels, suggesting reliable and robust effects in adolescents with CUD. In another data-driven approach to identify differences in functional connectomes in a mixed sample of adolescents and adults (age 18–40), Ramaekers and colleagues (83) found hyperconnectivity across all major brain networks, but most prominently in the DMN, DAN, and VAN, in recently abstinent chronic cannabis users (∼24 h, n = 14) compared to abstinent occasional users (∼7 days, n = 12). Using a meta-analytic approach, Blest-Hopley and colleagues (84) identified hyperactivity within the FPCN and DMN structures in abstinent cannabis using (> = 25 days, n = 98) vs. non-using (n = 106) adolescents. Taken together, preliminary findings suggest general hyperconnectivity brain patterns in cannabis users, particularly among the FPCN, VAN, and DMN regions, with mixed findings within the DAN and DMN. Notably, most of these analyses have not tested for the impact of more nuanced CU patterns, such as dose-effects, age of regular use onset, length of abstinence, or sex of the user on differences in functional brain network activity (85).
Another potential reason underlying inconsistent findings are potential unique sex effects on cannabis during neurodevelopment. There are significant sex-at-birth differences in typical adolescent brain development, with girls typically reaching structural maturity in limbic, striatal, and frontoparietal regions earlier than boys and demonstrating distinct patterns of functional connectivity maturation (29, 86–88). For example, males demonstrate progressively increases in putamen integration (in terms of participation coefficient) with age, but not females (89). On balance, animal studies have reported significant sex-variations in the eCB system, which plays a role in sexual dimorphism, including differences in CB1R receptor sensitivity, hormone-linked fluctuations in eCB signaling, and cannabis-related effects such as gene expression, brain morphometry, and neuroinflammation (50, 51, 56, 90–96). Human studies have also reported differential cognitive effects, with several studies finding increased male vulnerability (97–100) and some finding female vulnerability (97, 99, 101, 102). fMRI connectivity studies have also reported sex differences in the impact of cannabis on brain connectivity (50). However, not all analyses have indicated an interaction between cannabis use and sex (61, 65), and more studies examining sex differences are needed (59). Importantly, no studies to date have examined potential sex-differences in cannabis effects on network topology.
Prior literature has found that regular cannabis use can result in cognitive deficits and hyperconnectivity between brain areas, particularly ICNs associated with attentional processing and executive function, like the DAN, VAN, and FPCN (59, 83). These alterations in brain functioning may be due to the impact chronic cannabis use has on the eCB system via alterations in CB1 receptor density. Importantly, the eCB system is dynamic throughout the lifespan and is sexually dimorphic, which may explain sex differences in cannabis use outcomes. Very few studies have evaluated whether alterations in functional brain activity remain in regular users after prolonged abstinence. The current study aimed to examine markers of network connectivity in regular cannabis using adolescents and young adults after they completed a monitored three-week period of abstinence. Given the critical developmental time-period, we also sought to evaluate the hierarchical organization of brain networks as a marker of functional brain network maturation. Prior network-based analyses have quantified network organization through metrics like modularity, integration, and participation coefficient, all of which evaluate the degree to which subnetworks or modules are connected with one-another (82, 89). However, these metrics rely on one of many algorithms which allocate nodes into discrete modules (e.g., Louvain, Newman, etc.), resulting in a high number of researcher degrees of freedom. Alternatively, assortativity refers to the degree to which nodes are more likely to be neighbors with similar nodes (in terms of network characteristics like degree or clustering coefficient) and represents a metric of hierarchical organization free of any bias which might be introduced by module assignment. Assortativity can also be considered a metric of network robustness and resilience to damage (103). Thus, for our analyses we focused on both Egloband assortativity. We hypothesized that cannabis users would demonstrate significantly higher Eglob and lower assortativity. Further, we hypothesized that male users would demonstrate more robust relationships between CU and network topology compared to female users. Finally, we will examine the relationships between CU characteristics (age of regular use onset, past-year and lifetime use, and length of abstinence) and global and specific ICNs network outcomes. Sex differences will also be explored. We hypothesized that early CU initiation will be linked with less assortative networks, reflecting impaired maturation of the hierarchical structure of functional brain networks. We also predict that greater CU, and more recent use will be linked with hyperconnectivity, particularly in brain networks associated with executive functioning and attentional processing (FPCN, DAN & VAN). We predict these findings will be more robust in male users.
Ninety-four adolescents and young adults (42 female) were recruited through local advertising for the larger parent study (PI: Lisdahl, R01 DA030354). Inclusion criteria included being between the ages of 16 and 26 years old, being a fluent English speaker, and willingness to abstain from all alcohol or drug use (except nicotine) for three weeks. Exclusion criteria included being left-handed, major neurological and metabolic disorders, past-year co-morbid independent Axis-I mood, anxiety, attentional, and psychotic disorders, prenatal medical issues or premature gestation <35 weeks, prenatal alcohol (>4 drinks/day or >7 drinks/week) or illicit drugs (>10 uses) exposure (by parent or youth report), inability to complete VO2 maximum testing, being categorized as a “heavy drinker” (Cahalan Criteria), and excessive illicit drug use in lifetime (>50 uses of any drug category except nicotine, alcohol, or cannabis). Abstinence from all alcohol and drug use were confirmed through self-report and drug toxicology. Eleven participants (3 female) did not have sufficient neuroimaging data available and were excluded from the current analyses. For the purposes of between group comparisons, regular cannabis users (CAN) were defined as individuals who had engaged in at least 44 cannabis uses in the past-year and at least 100 lifetime cannabis uses. Control participants were defined as individuals who had engaged in no more than 5 past-year cannabis uses and no more than 20 lifetime cannabis uses (104). Other substance use (>50 lifetime occasions) was exclusionary in the current study. Eight participants (4 female) were excluded from group-comparison analyses because they fell outside of the criteria for being CAN or control subjects, resulting in a sample size of 75 for comparisons between CAN (n = 35) and control subjects (n = 40). For evaluation of the impact of cannabis use characteristics within cannabis users (characterized as individuals who had abstained for greater than 20 days and less than 100 days), 7 subjects were excluded due to missing data in critical covariates, resulting in a sample size 39 cannabis users. Of note, participants are comprised of illicit cannabis use due to lack of legal recreational or medical laws surrounding cannabis use in Wisconsin, USA (data was collected prior to the 2018 Farm Bill).
All study methods were approved by the UWM Institutional Review Board. Participants called in to a study line listed on the fliers posted around the community and local newspapers that highlighted a study recruiting active and sedentary participants with varying CU histories. Participant verbal consent, or verbal assent from minors, along with parent/guardian consent were obtained during a brief phone screen to assess for initial eligibility (i.e., age, MRI contraindications, and yes/no questions regarding psychiatric and substance use history). If the initial eligibility requirements were met, written consent/assent was received via mail and a 45 min, more detailed screening session was scheduled with both parents and youth. During this second session, more detailed demographic and medical history, physical health (to complete VO2 maximum testing), lifetime substance use [Customary Drinking and Drug Use Record; CDDR; (105, 106)] and psychiatric history (youth and parent versions of the Mini International Psychiatric Interview (MINI) or MINI-Kid if under 18 (107) were assessed. All participants and their parents were compensated $20 for the second screening session. Eligible participants were scheduled for study sessions. Ineligible participants were not informed of the specific reason for their exclusion to protect study integrity. Eligible youth completed five study sessions across 3.5–4 weeks [once a week for 3 weeks for the initial monitored abstinence period along with a mini-psychiatric and neuropsychological battery; see (108) for details]; during session 4, youth completed a detailed substance use interview, neuropsychological testing, and VO2 maximum testing. The fifth session was scheduled within 24–48 after session 4 and consisted of magnetic resonance imaging (MRI) scanning, TLFB update, and drug toxicology testing.
Substance Use Patterns. The CDDR (105) was used to measure lifetime CU episodes, age of regular CU initiation (defined as weekly), and symptoms of CUD. The Timeline Follow Back (TLFB) calendar interview was used to measure past-year substance use according to standard units [alcohol (standard drinks), nicotine (number of cigarettes, hits of chew/snuff/pipe/cigar/hookah), cannabis (smoked/vaped flower, concentrates, and edibles were measured and dosing was converted to joints based grams)], other drugs including ecstasy/MDMA (tablets), cocaine (grams), methamphetamine (mg), prescription sedatives (pills), prescription stimulants (pills), hallucinogens (hits), GHB (occasions), ketamine (occasions), heroin or opium (hits) and inhalants (hits). Other substance use was primarily utilized for exclusion criteria for the current sample. Total past-year cannabis use (in grams), lifetime cannabis use episodes, age of regular cannabis use onset (weekly), and length of abstinence (in days) were used in the current analyses. Past-year alcohol use (standard units) was used as a covariate.
Drug Toxicology. At each study session, drug toxicology urinalysis was completed screening for recent delta-9-THC, THCCOOH (delta-9-THC metabolite), amphetamines, barbiturates, benzodiazepines, cocaine, ecstasy/MDMA, methadone, methamphetamine, opiates, PCP (ACCUTEST SplitCup 10 Panel drug test) and cotinine concentrations (NicAlert). Cotinine was considered the most relevant covariate as a representation of acute nicotine use which has been known to influence BOLD signal. Past year nicotine use was not included in the modeling approach to prevent issues of multicollinearity. Participants were also administered the continuous sweat toxicology patch (PharmChek Drugs of Abuse Patch; PharmChem Inc., Fort Worth, TX, United States), which continuously monitored sweat toxicology and provided quantified values of THCCOOH (along with other drugs of abuse). Youth also completed breathalyzer screens for recent alcohol use at each session (Alco-Sensor IV; Inoximeters Inc., St. Louis, MO). If youth demonstrated increasing THCCOOH levels, indicating recent use, or new THCCOOH positive tests, they were offered up to one week extension to complete the monitored abstinence period; 95% of the youth completed the monitored abstinence period as scheduled. Cotinine level (day 5) was used as a covariate.
High-resolution anatomical images were collected using a T1-weighted (T1w) spoiled gradient-recalled at steady-state pulse sequence (TR = 8.2 ms, TE = 3.4 s, TI = 450, and flip angle of 12°). The in-plane resolution of the anatomical images was 256 × 256 with a square field of view (FOV) of 240 mm. One hundred fifty slices were acquired at 1 mm thickness. Echo planar images (EPI) were acquired for eight minutes during eyes-closed rest using T2 × weighted gradient-echo EPI pulse sequence (TR = 2000 ms, TE = 25 ms, FOV = 240 mm, matrix 64 × 64 voxels, slice thickness = 3.7 mm., flip angle = 77 degrees, 40 contiguous axial slices) with 240 TRs of volume data acquired per run.
Full MRI/fMRI preprocessing and functional connectome generation details can be found in the supplementary materials. Results included in this manuscript come from preprocessing performed using fMRIPrep 22.1.1 (109, 110); (RRID:SCR_016216), which is based on Nipype 1.8.5 (111, 112); (RRID:SCR_002502). Correction and registration was conducted using ANTs 2.3.3 (113), (RRID:SCR_004757) and images were normalized into standard space (MNI152NLin6Asym); (114). Functional images were skull-stripped, corrected for motion, and co-registered with the T1w-reference map Automatic removal of motion artifacts using independent component analysis (ICA-AROMA), (115) was performed on the preprocessed BOLD time-series after removal of non-steady state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6 mm FWHM (full-width half-maximum). Corresponding “non-aggressively” denoised runs were then censored to exclude volumes using the “basic scrubbing” procedure outlined in Parkes et al. (116) such that all volumes with FDPower > 0.2 mm or BOLD data variance (DVARS) >3% were excluded from subsequent analyses (117). After scrubbing, only one subject was excluded for having less than 4 min of viable data. The resulting smoothed and scrubbed data were used to generate functional connectomes.
The preprocessed, non-aggressively smoothed functional data was then parcellated using the NiftiLabelsMasker function in NiLearn v0.9.0 (171); (RRID:SCR_001362), using the 200 parcel 7 network atlas generated by Schaefer et al. (172). The parcel-wise mean BOLD signal was then subtracted from the values in the timeseries to center BOLD values for each parcel at a mean of 0. After this, every parcel was correlated with one another using the Pearson correlation to establish functional connectivity matrices for each session. The unique values from this matrix (i.e., the lower or upper diagonal) were extracted and the negative correlation values were set to zero. The resulting matrix was then normalized using a Fisher-z transformation and thresholded using estimated proportions of non-spurious correlations of .01, .1, .2, .35, and .5 in order to test the replicability of any findings across thresholds of network sparseness as typically used with network topology research (82, 118–120). While this approach inflates the number of comparisons, it also allows for greater reliability and comparability with other network-based analyses (36, 39, 81, 82). The resulting thresholded matrices were then treated as network objects in the form of weighted adjacency matrices.
Network-Based Statistics. To examine whether CU group status, age of initiation and CU patterns (past-year, lifetime use) impacted subnetwork connectivity across all connectomes and thresholding levels, we utilized the Network-Based Statistic (NBS) approach across the entire sample of available neuroimaging data (n = 83). This identifies links between CU markers and connectivity patterns including potentially unique subnetworks, while leveraging the network structure of connected sets of edges and controlling for the high family-wise error (FWE) [see Levakov et al. (121) for continuous NBS analyses]. Edges were thresholded based on the degree of correlation between edge strength and the CU markers of interest. Multiple thresholds (.3, .325, .35, and .375) were evalueated to ensure the robustness of results.
Network Topology. Global efficiency (Eglob) and weighted assortativity (122) were calculated for each functional connectome on a whole-brain level and on the level of each of the 7 Yeo ICNs (123) using brainconn V.0.0.2 in Python 3.10.11 across all thresholding levels. Next, we conducted and exploratory analysis of the associations between cannabis group and use characteristics and brain network outcomes (Eglob and assortativity) using multiple linear regression within the “stats” package in R 4.2.1 (124). All models included age, sex, past-year alcohol use, and session 5 cotinine concentrations as covariates. Separate models were created for each brain network characteristic on the whole brain level and on the level of each of the 7 ICNs. The first set of models included group membership (regular cannabis users vs. controls) as a predictor (n = 64, 32 female). The second set of models only included participants who endorsed CU (n = 31, 11 female), and included age, sex, past-year alcohol use, length of cannabis abstinence, age of regular CU onset (dummy coded variable indicating use was initiated at or prior to age 17), past-year CU, and interaction terms for sex*past-year use, sex*early CU initiation, and past-year use*early CU initiation to examine interaction effects within the same models. This resulted in a total of 80 regression models. Results for all loadings from regression analyses include uncorrected p-values as well as p-values corrected for false discovery rate (FDR; padj) within each thresholding level for the regressors of interest (heavy CU group and early initiation binary variables, past year CU, and interaction terms).
Table 1 shows the demographic distributions for all groups of participants. CU groups and age of initiation groups did not significantly differ in terms of age, sex, ethnic or racial identity and there were no significant correlations between any demographic variable and past-year CU, lifetime CU, or length of abstinence. Heavy cannabis users consumed more alcohol in the past-year and had higher cotinine levels than healthy control subjects; these were included as covariates. Neither framewise displacement nor number of volumes scrubbed were significantly associated with cannabis use characteristics or group membership.
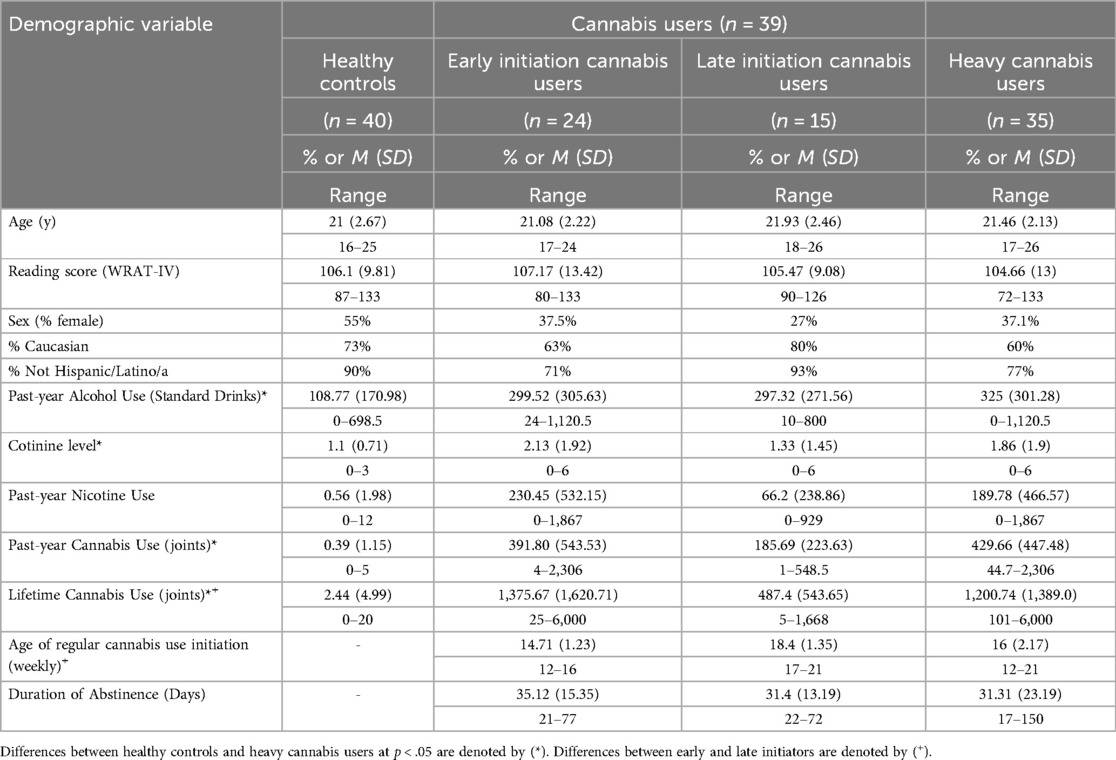
Table 1. Demographics for cannabis use group and early (prior to age 17) and late initiation cannabis users.
Network-Based Statistics: Cannabis Group Status. Across all thresholds, group-wise NBS analyses were unable to identify sub-networks which were significantly different between cannabis users and controls, nor between cannabis users who initiated CU early and those who initiated CU later in life.
Network-Based Statistics: Cannabis Use Patterns. The continuous NBS approach (121) analysis found that increased past-year CU was positively associated with a subnetwork of edges at multiple threshold-levels (thresholds of .3, .325, .35, and .375; all p's < .05); this subnetwork was comprised of brain regions from each of the ICN's, and was primarily comprised of between-network edges from the FPCN, DMN, Somatomotor Network, and VAN (Figure 1). Nodes in the ACC, Frontal Pole, and Precentral Gyrus were consistently identified across thresholds. No relationships were found with lifetime CU.
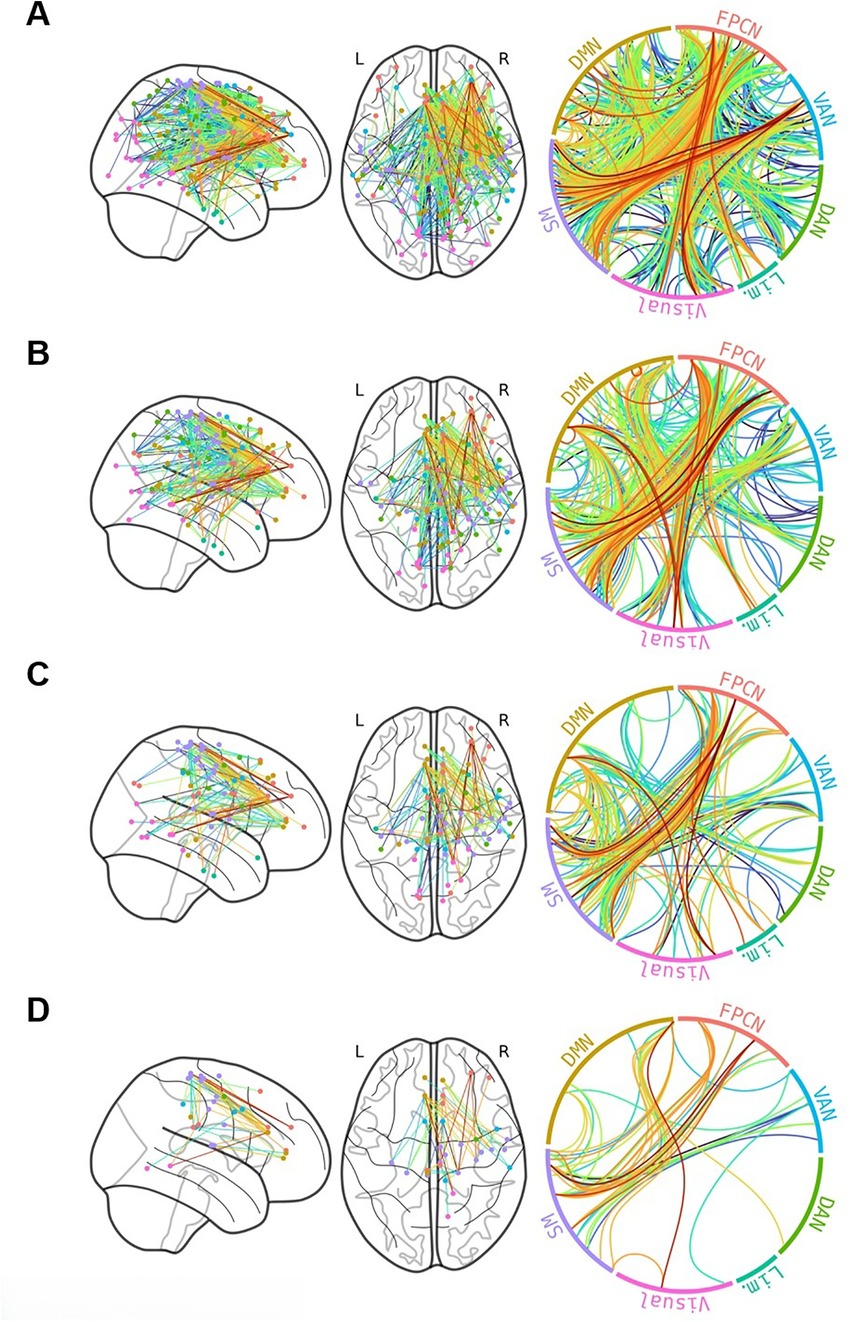
Figure 1. Network-based statistic subnetwork associated with past-year cannabis use among 79 adolescents at Pearson's r thresholds of 0.3 (A), 0.325 (B), 0.35 (C), and 3.75 (D) edge color corresponds to the correlation between edge strength and past-year cannabis use. Connections appear to be primarily right-lateralized and between rather than within networks. Frontoparietal Control Network (FPCN), Ventral Attention Network (VAN), Dorsal Attention Network (DAN), Limbic Network (Lim.), Somatomotor Network (SM), Default Mode Network (DMN).
Cannabis Group & Network Topology: After controlling for age, sex, alcohol use and cotinine levels, CU group status was not significantly related to Eglob or assortativity in any of the ICNs or on the whole-brain level (all p's > .05). This null finding was consistent across thresholding levels. Cannabis Group*Sex. There were no significant cannabis group-by-sex interactions in predicting global or ICN network outcomes at all thresholds.
Cannabis Use Characteristics & Network Topology: Within cannabis users, a the most stringent threshold (.01), whole-brain assortativity was significantly associated with duration of cannabis abstinence and the interaction term between early regular CU and past-year CU [F(10,28) = 2.55, p = .02, R2Adj = .29]. Specifically, shorter duration of abstinence was associated with increased whole-brain assortativity [t(28) = 2.41, b = .003, p = .02, padj = .48, f2 = .32] and within late regular CU onset individuals, past-year CU was associated with higher whole brain assortativity [t(28) = −2.52, b = −.15, p = .02, padj = .48, f2 = .23]. There was also an observed interaction between past-year CU and early regular CU initiation for whole-brain Eglob [F(10,28) = 2.33, p = .038, R2Adj = .26; t(28) = −2.08, b = −.009, p = .05, padj = .89, f2 = .16]. post-hoc evaluation revealed non-significant findings. At less stringent thresholds, shorter duration of abstinence was associated with increased assortativity in the VAN [0.35: F(10, 28) = 2.26, p = .04, R2Adj = .25, t(28) = 2.08, b = .003, p = .05, padj = .67, f2 = .28; 0.5: F[10, 28] = 2.32, p = .04, R2Adj = .26, t(28) = 2.05, b = .003, p = .05, padj = .80, f2 = .29]. Greater past-year use was also associated with decreased Eglob in the FPCN at the threshold of.35 [F(10, 28) = 2.59, p = .02, R2Adj = .3, t(28) = −2.22, β = −.08, p = .03, padj = .54, f2 = .11]. Effect size ranges for these findings were generally small to medium (f2 range .11–.32).
Cannabis Use Characteristics*Sex & Network Topology: Within cannabis users, there were significant interactions between sex and past-year CU in association with Eglob in the FPCN at thresholds of .35 [F(10,28) = 2.59, p = .02, R2Adj = .3; t(28) = 3.16, b = −.13, p = .004, padj = .18, f2 = .37] and .5 [F(10,28) = 3.29, p = .006, b = −.13, R2Adj = .38; t(28) = 3.26, p = .003, padj = .14, f2 = .41]. Within males, past-year cannabis use was positively associated with Eglob at both thresholds [.35: F(7, 18) = 3.13, p = .02, R2Adj = .37; t(18) = 3.07, β = .06, p = .007, f2 = .43;.5: F[7, 18] = 4.79, p = .003, R2Adj = .51; t(18) = 4.24, β = .07, p < .001, f2 = .74], while there was no significant association within females. Similarly, there were significant interactions between sex and past-year cannabis use on assortativity within the Somatomotor network at thresholds of .2 [F(10,28) = 3.37, p = .005, R2Adj = .38; t(28) = 2.38, b = .136, p = .02, padj = .58, f2 = .21] and .35 [F(10,28) = 3.29, p = .006, R2Adj = .38; t(28) = 2.94, b = .16, p = .007, padj = .31, f2 = .34]; within males only, increased pass year cannabis use was associated with increased Somatomotor assortativity [.2: F(7, 18) = 5.79, p = .001, R2Adj = .57; t(18) = 4.32, β = .1, p < .001, f2 = 1.29;.5: F(7, 18) = 4.08, p = .007, R2Adj = .46; t(18) = 4.3, β = .1, p < .001, f2 = .99]. Effect size ranges for these findings were medium to large (f2 range .21–1.29).
Interaction terms between sex and early regular CU onset accounted for a significant portion of variance in assortativity in the Somatomotor network [1: F(10,28) = 2.49, p = .028, R2Adj = .28; t(28) = 2.72, b = .31, p = .01, padj = .18, f2 = .24;.2: F(10,28) = 3.37, p = .005, R2Adj = .38; t(28) = .2.75, b = .24, p = .01, padj = .49, f2 = .18] and VAN [35: F(10,28) = 2.26, p = .04, R2Adj = .25; t(28) = 2.39, b = .29, p = .02, padj = .57, f2 = .21;.5: F(10,28) = 2.32, p = .04, R2Adj = .26; t(28) = 2.83, b = .29, p = .009, padj = .41, f2 = .25]. For both networks, post-hoc regressions indicated that within males only, early regular CU onset was associated with decreased assortativity [Somatomotor.1: F(7, 18) = 2.99, p = .029, R2Adj = .36; t(18) = −2.99, b = −.22, p = .008, f2 = .21; Somatomotor .2: F(7, 18) = 5.79, p = .001, R2Adj = .57; t(18) = −3.69, b = −.17, p = .002, f2 = .18; VAN .35: F(7, 18) = 2.73, p = .04, R2Adj = .33; t(18) = −2.47, b = −.19, p = .02, f2 = .13; VAN .5: F(7, 18) = 3.12, p = .02, R2Adj = .37; t(18) = −2.93, b = −.18, p = .009, f2 = .27]. Effect size ranges for these findings spanned small to medium (f2 range .13–.27). A list of whole model statistics relevant to the results reported here can be found in Table 2 and a summary of significant findings can be found in Table 3.
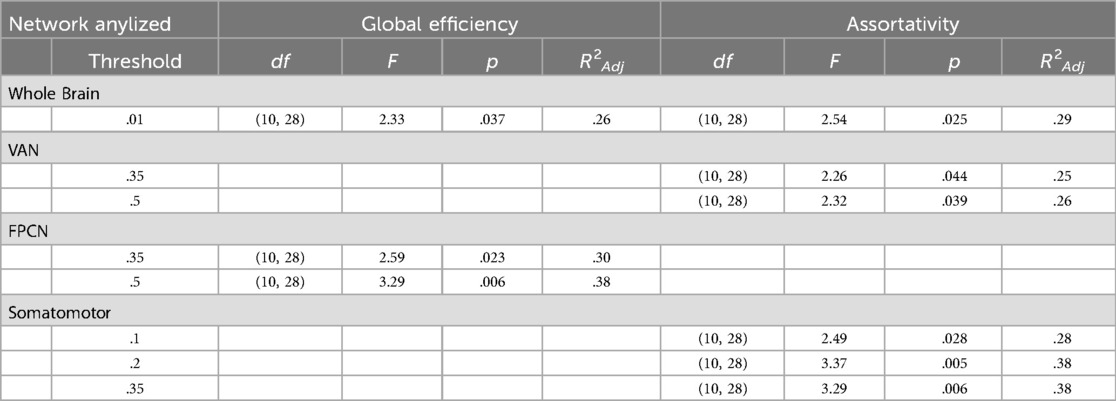
Table 2. Whole model statistics for significant models in which CU characteristics within cannabis users accounted for a significant portion of variance in network topology metrics of interest.
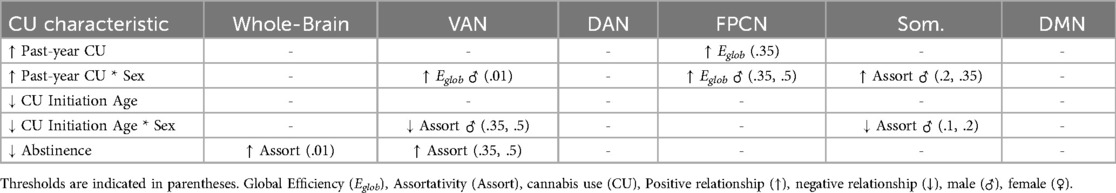
Table 3. Cannabis Use Characteristics & Network Topology. Significant associations between brain network topology and cannabis use characteristics are summarized here.
CU peaks during late adolescence into young adulthood, a period of significant ongoing neurodevelopment. Prior work has suggested subtle effects of chronic CU on functional connectivity during adolescence, especially in the VAN, FPCN, DMN, and DAN, though seed-based findings have been inconsistent across networks. Our study aimed to expand upon this work by examining the impact of regular CU on brain network connectivity, utilizing both network based statistics (NBS) and network topology approaches, in adolescents and young adult cannabis users after a period of three weeks of monitored abstinence. We also examined whether sex moderated these findings.
Using an NBS approach, we found that increased past-year CU was linked with increased subnetwork of edges at multiple thresholds (.3, .325, .35, .375), and these primarily included between-network edges from the VAN, DMN, Somatomotor Network, and FPCN. This finding is consistent with the general pattern observed in the network topology analysis, finding subtle relationships between CU patterns and network outcomes in the VAN, Somatomotor network, and FPCN, and less so in the DMN. The subnetwork consistently comprised primarily of right-hemisphere structures, representing the first evidenced lateralization of the impact of CU on brain connectivity. Though we could not examine sex differences in the NBS approach, the exploratory regression analyses demonstrated primary male vulnerability in the VAN, FPCN and Somatomotor networks. This hyperconnectivity, especially in the right hemisphere, may suggest cannabis-related subtle differences in sex-related hemispheric connectivity asymmetry during middle to late adolescence (125). These differences may underlie cannabis-related reduced cognitive performance in right-hemisphere associated tasks that require visuospatial and visuo-motor integration, including visuospatial function (126), psychomotor sequencing ability (98, 127–129), spatial working memory (127, 130–135), complex visuospatial attention (98, 129, 130, 135–141), and motor-based cognitive control (98, 132, 133, 137, 138, 140, 142–144). Further, hyperconnectivity between the VAN and the other networks could represent a higher-likelihood of switching between network states (145), a process which is known to be associated with cognitive task performance (146). On the other hand, the increased connectivity associated with past-year cannabis use might represent a more disorganized connectome as a result of dysregulation of CB1 receptor density. This is supported by the high representation of the ACC in the subnetwork of hyperconnectivity associated with past-year CU. The high density of CB1 receptors in the ACC may make it particularly prone to dysregulation through heavy cannabis use, which is supported by prior research that has demonstrated reduced rACC volume linked with reduced emotional discrimination (173), blunted BOLD response during fearful face processing (174), and hyperconnectivity between bilateral rACC, left amygdala and left insula at rest (70) in regular cannabis using adolescents and young adults. Thus, additional research is needed to specifically examine the impact of regular cannabis use on ACC development during the critical stage of adolescent development.
In an exploratory follow-up analyses, we also aimed to examine effects of more nuanced cannabis use characteristics and potential sex differences on brain network topology in the whole brain, VAN, DAN, FPCN, Somatosensory network and DMN in adolescents and young adults. Notably, due to the large number of statistical tests, none of the comparisons of interest survived FDR correction for multiple comparisons despite medium to large effect. Still, we believe the pattern of results, emphasizing threshold values and effect sizes, are worth reporting in order to support replication. We found that at the group level, CU was not significantly linked with network outcomes. However, more nuanced exploratory analyses found preliminary evidence that increased past-year CU was associated with greater Eglob in the FPCN (.35 threshold), though this was a small effect size (f2 = .11). Shorter abstinence from CU was associated with greater assortativity on the whole-brain level (.01 threshold, f2 = .32) and within the VAN (.35 and .5 thresholds, f2 = .28, .29). Further, in later onset cannabis users, increased past year use was linked with increased assortativity at the whole brain level (.01, f2 = .32). Preliminary sex-dependent findings were also revealed; increased past year CU was associated with Eglob in the FPCN in males only (.35 and .5, f2 = .43, .74). We also found links between increased past-year CU and greater assortativity in the Somatomotor network in males across two thresholds (.2, .35, f2 = .43, .74). Interestingly, we found that earlier regular CU onset was associated with decreased assortativity in the VAN (thresholds of.35 and .5; f2 = .13, .27) and Somatomotor network (thresholds of .1 and .2; f2 = .21, .18) in male cannabis users, suggesting reduced connectivity in this subset of male users. While the directionality of these effects were consistent across thresholding levels, the findings were not consistently significant across thresholding levels. This is in contrast to findings such as those found by Nestor et al. (82), which were global in nature and were consistent across thresholding levels. This differential finding may be due to characteristics of the sample, as their study was almost entirely comprised of male participants and exclusively included youth being treated for CUD following roughly 12 h of abstinence, while ours included a community sample of recreational users with a range of use (weekly to daily) and following a minimum of three weeks of monitored abstinence (with average >30 days).
Still, taken together, these preliminary findings generally support the main NBS findings demonstrating patterns of hyperconnectivity associated with increased severity of use, especially in male user and early onset users. The VAN underlies stimulus-driven attention processes, many of which are negatively associated with cannabis use (83, 147, 148). The NBS analyses in which significant associations between CU frequency and brain network activity were identified were primarily driven by past-year use, rather than lifetime use. This is fairly consistent with other attention based neurocognitive dose-dependent findings (67, 83, 99). Interestingly, in the exploratory analyses, at more lenient thresholds (.35 and .5) and when accounting for total past-year use and earlier age of onset, shorter periods of abstinence in this case were linked with greater assortativity in the VAN. Thus, acute recovery of function reported in other analyses (73, 108, 149–151) may not explain the reduced assortativity in the male early onset users observed here. Prior analyses have not strongly associated the Somatomotor network with CU, but not many analyses have tested for sex-dependent effects and included relatively small sample sizes. The increased VAN and FPCN connectivity strength and lower assortativity in the Somatomotor network in males with risky cannabis use patterns may suggest male vulnerability to cannabis-effects, especially in these frontal-parietal and temporal-frontal networks (99). These findings suggest that nuanced cannabis markers may be linked with whole brain, VAN, FPCN, and Somatomotor network connectivity strength and hierarchical structure and results were dependent upon dose of exposure, age of onset, and sex of the user. As stated above, results did not survive correction for multiple comparisons; given the low power of this subset of participants, additional studies are needed with larger sample sizes that include a range of cannabis characteristics in order to replicate findings. Additionally, future studies conducted in a large, sex-balanced sample utilizing repeated neuroimaging during a period of monitored abstinence are needed to further characterize these cannabis-related sex differences in network topology recovery of function.
The preliminary evidence of sex differences in associations between CU patterns and ICN global efficiency and assortativity may be due to sex differences in brain maturation rates. As males develop later than females, they may be particularly sensitive to heavy CU during middle adolescence in terms of functional frontoparietal, frontotemporal and Somatomotor development and synaptic refinement. The eCB system dynamically shifts in function throughout development, with some processes like presynaptic signal modulation from the PFC to the hippocampus and amygdala do not mature until late adolescence, especially in boys (152). CB1 receptor density tends to be at its highest prior to this shift (153). Early CU initiation may also interrupt the eCB system's other functions (e.g., synaptic pruning & myelination) which may have more long-term impacts on the organizational structure of brain networks. The network specificity may represent a particular sensitivity of certain networks to eCB system modulation or may be due to sex differences in terms of which networks underwent this refinement during a person's peak CU. The lower assortativity in early vs. late CU males in the Somatomotor network and the VAN may indicate that eCB functioning in these networks had already shifted from the more long-term organizational effects to more neuromodulatory effects. The result is that early CU males display less organized brain network structures, similar to what would be expected from younger individuals. However, it is important to note that the low number of female participants in the late CU initiation group may have prevented meaningful comparisons between females in the early and late CU initiation groups and between males and females who initiated CU later in life. Thus, longitudinal studies examining the relationships between endogenous eCB circulating levels (154), detailed cannabis use patterns, and brain network topology development are needed to further characterize the impact of escalating use on functional activity patterns across development in boys and girls.
These findings should be considered carefully in the context of our sample characteristics and potential limitations. Our participants were healthy young adults who were willing to abstain from CU for at least 2 weeks, limiting the generalizability to clinical populations, younger adolescents, or older adults. The resting-state scan was also only eight minutes which is lower than some recommend (155), but still long enough to attain replicability (156); still, longer scan time collection may result in more robust or reproducible findings. These analyses were cross-sectional in nature and cannot account for causality; though dose-dependent findings were reported, premorbid differences may predict more severe cannabis use trajectories. Prospective, longitudinal studies will need to replicate findings. Further, given the relatively small sample size of cannabis users and resulting low statistical power, future studies should expand the network-based analytical framework with a large sample of cannabis users with diverse use characteristics to evaluate the generalizability of these findings. The current sample participants had a range of abstinence; repeated, longitudinal MRI studies examining recovery of function over a more controlled range are needed. Because of the large shift in availability of cannabis products in recent years, more nuanced characterizations of CU (e.g., method of consumption, cannabinoid content, potency) will be necessary to address additional predictors of cannabis-related effects (157–160). Particular care should be taken to include female participants and evaluate sex differences, as females are typically underrepresented in CU research (161) and demonstrate differential cannabis use patterns. Future studies should further examine the impacts of sex steroid hormones, particularly estradiol, should also be considered given their interaction with CB1 receptors (92, 162, 163), eCB levels (164–166), and CU outcomes (167–170).
Primary results revealed that, as a group, healthy cannabis-using adolescents and young adults who had abstained from CU for at least 2 weeks did not differ from non-using youth. The most consistent finding was that increased past-year cannabis use was associated with a distributed pattern of hyperconnectivity, particularly with regions with high CB1 receptor density like the ACC, and exploratory analyses revealed that effects may also be dependent upon age of regular cannabis use onset length of cannabis abstinence, and sex of the user. However, network topology results did not emerge consistently across all thresholding levels evaluated and were no longer significant after FDR correction for multiple comparisons, suggesting a larger sample size is needed to replicate findings related to cannabis use characteristics. In male users, age of regular CU initiation appeared to be associated with hierarchical brain network structure, a potential index of functional brain development. This could indicate that CU during adolescence could have subtle impacts on brain function, especially in male early onset, heavy cannabis users. These findings reinforce the need to restrict access and discourage use among developing adolescents. Further, these findings highlight the critical need to attend to sex differences in the eCB system, its role in adolescent neurodevelopment, and CU outcomes. Large-scale longitudinal studies are needed to further characterize the impact of nuanced cannabis use patterns on brain development of functional networks across development. These findings add to the growing literature that cannabis-related effects are likely nuanced, complex, and require large samples with diverse cannabis characteristics in order to examine interactions between factors [see (85)].
The data analyzed in this study is subject to the following licenses/restrictions: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Krista M. Lisdahl, bWVkaW5ha0B1d20uZWR1.
The studies involving humans were approved by University of Wisconsin-Milwaukee Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
KB: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. RS: Writing – review & editing. CS: Writing – review & editing. KL: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Institute on Drug Abuse (NIDA) award R01DA030354 and the National Institute of Health (NIH) award U01DA041025 (PI: Lisdahl, K. M. and Larson, C. L.). Sullivan, R. M. was supported by T32AA013525 (PI: Riley/Spadoni to Sullivan).
We thank the study participants, BraIN lab team, and our funding agencies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fradm.2025.1549771/full#supplementary-material
1. Miech RA, Johnston LD, Patrick ME, O’Malley PM, Bachman JG. Monitoring the Future National Survey Results on Drug Use, 1975–2023: Overview and Detailed Results for Secondary School Students (2024). Monitoring the Future Monograph Series.
2. Di Marzo V, Bisogno T, De Petrocellis L. The biosynthesis, fate and pharmacological properties of endocannabinoids. In: Pertwee RG, editor. Cannabinoids. Berlin, Heidelberg: Springer (2005). p. 147–85. doi: 10.1007/3-540-26573-2_5
3. Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. (2007) 17:175–91. doi: 10.1093/cercor/bhj136
4. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci. (1990) 87:1932–6. doi: 10.1073/pnas.87.5.1932
5. Benarroch EE. Synaptic effects of cannabinoids: complexity, behavioral effects, and potential clinical implications. Neurology. (2014) 83:1958–67. doi: 10.1212/WNL.0000000000001013
6. Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. (2009) 89:309–80. doi: 10.1152/physrev.00019.2008
7. Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. (2005) 168:299–325. doi: 10.1007/3-540-26573-2_10
8. Viveros M-P, Marco E-M, Llorente R, López-Gallardo M. Endocannabinoid system and synaptic plasticity: implications for emotional responses. Neural Plast. (2007) 2007:52908. doi: 10.1155/2007/52908
9. Díaz-Alonso J, Guzmán M, Galve-Roperh I. Endocannabinoids via CB₁ receptors act as neurogenic niche cues during cortical development. Philos Trans R Soc Lond B Biol Sci. (2012) 367:3229–41. doi: 10.1098/rstb.2011.0385
10. Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. (2008) 14:923–30. doi: 10.1038/nm.f.1869
11. Lago-Fernandez A, Zarzo-Arias S, Jagerovic N, Morales P. Relevance of peroxisome proliferator activated receptors in multitarget paradigm associated with the endocannabinoid system. Int J Mol Sci. (2021) 22:1001. doi: 10.3390/ijms22031001
12. Lee TT-Y, Gorzalka BB. Timing is everything: evidence for a role of corticolimbic endocannabinoids in modulating hypothalamic-pituitary-adrenal axis activity across developmental periods. Neuroscience. (2012) 204:17–30. doi: 10.1016/j.neuroscience.2011.10.006
13. Manterola A, Bernal-Chico A, Cipriani R, Canedo-Antelo M, Moreno-García Á, Martín-Fontecha M, et al. Deregulation of the endocannabinoid system and therapeutic potential of ABHD6 blockade in the cuprizone model of demyelination. Biochem Pharmacol. (2018) 157:189–201. doi: 10.1016/j.bcp.2018.07.042
14. O’Sullivan SE. An update on PPAR activation by cannabinoids. Br J Pharmacol. (2016) 173:1899–910. doi: 10.1111/bph.13497
15. Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. (2011a) 65:278–86. doi: 10.1002/syn.20844
16. Bernal-Chico A, Tepavcevic V, Manterola A, Utrilla C, Matute C, Mato S. Endocannabinoid signaling in brain diseases: emerging relevance of glial cells. Glia. (2023) 71:103–26. doi: 10.1002/glia.24172
17. Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. (2008) 18:826–34. doi: 10.1016/j.euroneuro.2008.06.009
18. Lee TT-Y, Hill MN, Hillard CJ, Gorzalka BB. Temporal changes in N-acylethanolamine content and metabolism throughout the peri-adolescent period. Synapse. (2013) 67:4–10. doi: 10.1002/syn.21609
19. Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. (2003) 17:1747–54. doi: 10.1046/j.1460-9568.2003.02599.x
20. Terry GE, Liow J-S, Zoghbi SS, Hirvonen J, Farris AG, Lerner A, et al. Quantitation of cannabinoid CB1 receptors in healthy human brain using positron emission tomography and an inverse agonist radioligand. Neuroimage. (2009) 48:362–70. doi: 10.1016/j.neuroimage.2009.06.059
21. Backhausen LL, Fröhner JH, Lemaître H, Artiges E, Martinot MP, Herting MM, et al. Adolescent to young adult longitudinal development of subcortical volumes in two European sites with four waves. Hum Brain Mapp. (2024) 45:e26574. doi: 10.1002/hbm.26574
22. Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, et al. Neuroanatomical assessment of biological maturity. Curr Biol. (2012) 22:1693–8. doi: 10.1016/j.cub.2012.07.002
23. Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. (2008) 28:62–77. doi: 10.1016/j.dr.2007.08.003
24. Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. (2006) 26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006
25. Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. (2008) 63:927–34. doi: 10.1016/j.biopsych.2008.03.015
26. Mills KL, Lalonde F, Clasen LS, Giedd JN, Blakemore S-J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc Cogn Affect Neurosci. (2014b) 9:123–31. doi: 10.1093/scan/nss113
27. Mills KL, Goddings A-L, Clasen LS, Giedd JN, Blakemore S-J. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. (2014a) 36:147–60. doi: 10.1159/000362328
28. Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, et al. Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cereb Cortex. (2016) 26:4101–21. doi: 10.1093/cercor/bhv205
29. Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci. (2014) 111:1592–7. doi: 10.1073/pnas.1316911111
30. Manterola A, Chara JC, Aguado T, Palazuelos J, Matute C, Mato S. Cannabinoid CB1 receptor expression in oligodendrocyte progenitors of the hippocampus revealed by the NG2-EYFP-knockin mouse. Front Neuroanat. (2022) 16:1030060. doi: 10.3389/fnana.2022.1030060
31. Oltrabella F, Melgoza A, Nguyen B, Guo S. Role of the endocannabinoid system in vertebrates: emphasis on the zebrafish model. Dev Growth Differ. (2017) 59:194–210. doi: 10.1111/dgd.12351
32. Sisk LM, Rapuano KM, Conley MI, Greene AS, Horien C, Rosenberg MD, et al. Genetic variation in endocannabinoid signaling is associated with differential network-level functional connectivity in youth. J Neurosci Res. (2022) 100:731–43. doi: 10.1002/jnr.24946
33. Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. (2010) 329:1358–61. doi: 10.1126/science.1194144
34. Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms in adolescents versus adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. (2013) 37(5):976–91. doi: 10.1016/j.neubiorev.2013.03.004
35. Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. (2013) 64:240–56. doi: 10.1016/j.neuroimage.2012.08.052
36. Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. (2009) 5:e1000381. doi: 10.1371/journal.pcbi.1000381
37. Lopez KC, Kandala S, Marek S, Barch DM. Development of network topology and functional connectivity of the prefrontal cortex. Cerebral Cortex. (2020) 30:2489–505. doi: 10.1093/cercor/bhz255
38. Stevens MC. The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci Biobehav Rev Adolesc Brain. (2016) 70:13–32. doi: 10.1016/j.neubiorev.2016.07.027
39. Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. (2009) 7:e1000157. doi: 10.1371/journal.pbio.1000157
40. Hirvonen J, Goodwin R, Li C-T, Terry G, Zoghbi S, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. (2012) 17:642–9. doi: 10.1038/mp.2011.82
41. Martin EL, Baker NL, Sempio C, Christians U, Klawitter J, McRae-Clark AL. Sex differences in endocannabinoid tone in a pilot study of cannabis use disorder and acute cannabis abstinence. Addict Biol. (2023) 28:e13337. doi: 10.1111/adb.13337
42. Morgan CJA, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. (2013) 202:381–2. doi: 10.1192/bjp.bp.112.121178
43. Sloan ME, Grant CW, Gowin JL, Ramchandani VA, Le Foll B. Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies. Acta Pharmacol Sin. (2019) 40:342–50. doi: 10.1038/s41401-018-0081-z
44. Spindle TR, Kuwabara H, Eversole A, Nandi A, Vandrey R, Antoine DG, et al. Brain imaging of cannabinoid type I (CB1) receptors in women with cannabis use disorder and male and female healthy controls. Addict Biol. (2021) 26:e13061. doi: 10.1111/adb.13061
45. Thieme U, Schelling G, Hauer D, Greif R, Dame T, Laubender RP, et al. Quantification of anandamide and 2-arachidonoylglycerol plasma levels to examine potential influences of tetrahydrocannabinol application on the endocannabinoid system in humans. Drug Test Anal. (2014) 6:17–23. doi: 10.1002/dta.1561
46. Chowdhury KU, Holden ME, Wiley MT, Suppiramaniam V, Reed MN. Effects of cannabis on glutamatergic neurotransmission: the interplay between cannabinoids and glutamate. Cells. (2024) 13:1130. doi: 10.3390/cells13131130
47. Zuo CS, Davis KA, Kuppe MK, Dahlgren MK, Gruber S, Fitzmaurice GM, et al. Elevated striatal glutamate+glutamine in recreational cannabis users during abstinence. J Psychiatr Res. (2022a) 146:192–200. doi: 10.1016/j.jpsychires.2021.12.041
48. Zuo CS, Davis KA, Lukas SE. Lower dACC glutamate in cannabis users during early phase abstinence. Neuropsychopharmacol. (2022b) 47:1969–75. doi: 10.1038/s41386-022-01321-5
49. Zuo CS, Lukas SE. Chronic cannabis use alters dACC-striatal glutamatergic balance. Pharmacol Biochem Behav. (2023) 225:173544. doi: 10.1016/j.pbb.2023.173544
50. Coleman JR, Madularu D, Ortiz RJ, Athanassiou M, Knudsen A, Alkislar I, et al. Changes in brain structure and function following chronic exposure to inhaled vaporised cannabis during periadolescence in female and male mice: a multimodal MRI study. Addict Biol. (2022) 27:e13169. doi: 10.1111/adb.13169
51. Ginder DE, Wright HR, McLaughlin RJ. The stoned age: sex differences in the effects of adolescent cannabinoid exposure on prefrontal cortex structure and function in animal models. Int Rev Neurobiol. (2021) 161:121–45. doi: 10.1016/bs.irn.2021.07.005
52. Hasegawa Y, Kim J, Ursini G, Jouroukhin Y, Zhu X, Miyahara Y, et al. Synergistic impact of microglia-mediated adverse effect of adolescent THC exposure and 16p11.2 duplication on prefrontal cortex maturation and social memory. Neuropsychopharmacology. (2023) 48:365–365.
53. Miederer I, Uebbing K, Röhrich J, Maus S, Bausbacher N, Krauter K, et al. Effects of tetrahydrocannabinol on glucose uptake in the rat brain. Neuropharmacology. (2017) 117:273–81. doi: 10.1016/j.neuropharm.2017.02.011
54. Poulia N, Delis F, Brakatselos C, Polissidis A, Koutmani Y, Kokras N, et al. Detrimental effects of adolescent escalating low-dose Δ9-tetrahydrocannabinol leads to a specific bio-behavioural profile in adult male rats. Br J Pharmacol. (2021) 178:1722–36. doi: 10.1111/bph.15394
55. Stringfield SJ, Torregrossa MM. Intravenous self-administration of delta-9-THC in adolescent rats produces long-lasting alterations in behavior and receptor protein expression. Psychopharmacology. (2021) 238:305–19. doi: 10.1007/s00213-020-05684-9
56. Zuo Y, Iemolo A, Montilla-Perez P, Li H-R, Yang X, Telese F. Chronic adolescent exposure to cannabis in mice leads to sex-biased changes in gene expression networks across brain regions. Neuropsychopharmacol. (2022) 47:2071–80. doi: 10.1038/s41386-022-01413-2
57. Debenham J, Birrell L, Champion K, Lees B, Yücel M, Newton N. Neuropsychological and neurophysiological predictors and consequences of cannabis and illicit substance use during neurodevelopment: a systematic review of longitudinal studies. Lancet Child Adolesc Health. (2021) 5:589–604. doi: 10.1016/S2352-4642(21)00051-1
58. Gonzalez R, Pacheco-Colón I, Duperrouzel JC, Hawes SW. Does cannabis use cause declines in neuropsychological functioning? A review of longitudinal studies. J Int Neuropsychol Soc. (2017) 23:893–902. doi: 10.1017/S1355617717000789
59. Thomson H, Labuschagne I, Greenwood L-M, Robinson E, Sehl H, Suo C, et al. Is resting-state functional connectivity altered in regular cannabis users? A systematic review of the literature. Psychopharmacology. (2022) 239:1191–209. doi: 10.1007/s00213-021-05938-0
60. Hammond C, Allick A, Park G, Rizwan B, Kim K, Lebo R, et al. A meta-analysis of fMRI studies of youth cannabis use: alterations in executive control, social cognition/emotion processing, and reward processing in cannabis using youth. Brain Sci. (2022) 12:1281. doi: 10.3390/brainsci12101281
61. Manza P, Tomasi D, Volkow ND. Subcortical local functional hyperconnectivity in cannabis dependence. Biol Psychiatry. (2018) 3:285–93. doi: 10.1016/j.bpsc.2017.11.004
62. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
63. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Ann N Y Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
64. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. (2007) 315:393–5. doi: 10.1126/science.1131295
65. Ritchay MM, Huggins AA, Wallace AL, Larson CL, Lisdahl KM. Resting state functional connectivity in the default mode network: relationships between cannabis use, gender, and cognition in adolescents and young adults. NeuroImage: Clinical. (2021) 30:102664. doi: 10.1016/j.nicl.2021.102664
66. Wall MB, Pope R, Freeman TP, Kowalczyk OS, Demetriou L, Mokrysz C, et al. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J Psychopharmacol. (2019) 33:822–30. doi: 10.1177/0269881119841568
67. Wetherill RR, Fang Z, Jagannathan K, Childress AR, Rao H, Franklin TR. Cannabis, cigarettes, and their co-occurring use: disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. (2015) 153:116–23. doi: 10.1016/j.drugalcdep.2015.05.046
68. Pujol J, Blanco-Hinojo L, Batalla A, López-Solà M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. (2014) 51:68–78. doi: 10.1016/j.jpsychires.2013.12.008
69. Harris JC, Wallace AL, Thomas AM, Wirtz HG, Kaiver CM, Lisdahl KM. Disrupted resting state attentional network connectivity in adolescent and young adult cannabis users following two-weeks of monitored abstinence. Brain Sci. (2022) 12:287. doi: 10.3390/brainsci12020287
70. Shollenbarger S, Thomas AM, Wade NE, Gruber SA, Tapert SF, Filbey FM, et al. Intrinsic frontolimbic connectivity and mood symptoms in young adult cannabis users. Front Public Health. (2019) 7:311. doi: 10.3389/fpubh.2019.00311
71. Ertl N, Lawn W, Mokrysz C, Freeman TP, Alnagger N, Borissova A, et al. Associations between regular cannabis use and brain resting-state functional connectivity in adolescents and adults. J Psychopharmacol. (2023) 37:904–19. doi: 10.1177/02698811231189441
72. Hirjak D, Schmitgen MM, Werler F, Wittemann M, Kubera KM, Wolf ND, et al. Multimodal MRI data fusion reveals distinct structural, functional and neurochemical correlates of heavy cannabis use. Addict Biol. (2022) 27:e13113. doi: 10.1111/adb.13113
73. Barbey AK. Network neuroscience theory of human intelligence. Trends Cogn Sci (Regul Ed). (2018) 22:8–20. doi: 10.1016/j.tics.2017.10.001
74. Bassett DS, Sporns O. Network neuroscience. Nat Neurosci. (2017) 20:353–64. doi: 10.1038/nn.4502
75. Douw L, van Dellen E, Gouw AA, Griffa A, de Haan W, van den Heuvel M, et al. The road ahead in clinical network neuroscience. Network Neurosci. (2019) 3:969–93. doi: 10.1162/netn_a_00103
76. Morgan SE, White SR, Bullmore ET, Vértes PE. A network neuroscience approach to typical and atypical brain development. Biol Psychiatry. (2018) 3:754–66. doi: 10.1016/j.bpsc.2018.03.003
77. Dennis EL, Thompson PM. Typical and atypical brain development: a review of neuroimaging studies. Dialogues Clin Neurosci. (2013) 15:359–84. doi: 10.31887/DCNS.2013.15.3/edennis
78. Marchitelli R, Paillère Martinot M-L, Trouvé A, Banaschewski T, Bokde ALW, Desrivières S, et al. Coupled changes between ruminating thoughts and resting-state brain networks during the transition into adulthood. Mol Psychiatry. (2024) 29:3769–78. doi: 10.1038/s41380-024-02610-9
79. Saha R, Saha DK, Rahaman MA, Fu Z, Liu J, Calhoun VD. A method to estimate longitudinal change patterns in functional network connectivity of the developing brain relevant to psychiatric problems, cognition, and age. Brain Connect. (2024) 14:130–40. doi: 10.1089/brain.2023.0040
80. Sato JR, Biazoli CE Jr, Salum GA, Gadelha A, Crossley N, Satterthwaite TD, et al. Temporal stability of network centrality in control and default mode networks: specific associations with externalizing psychopathology in children and adolescents. Hum Brain Mapp. (2015) 36:4926–37. doi: 10.1002/hbm.22985
81. Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. (2015) 13:e1002328. doi: 10.1371/journal.pbio.1002328
82. Nestor LJ, Behan B, Suckling J, Garavan H. Cannabis-dependent adolescents show differences in global reward-associated network topology: a functional connectomics approach. Addict Biol. (2020) 25:e12752. doi: 10.1111/adb.12752
83. Ramaekers JG, Mason NL, Toennes SW, Theunissen EL, Amico E. Functional brain connectomes reflect acute and chronic cannabis use. Sci Rep. (2022) 12:2449. doi: 10.1038/s41598-022-06509-9
84. Blest-Hopley G, Giampietro V, Bhattacharyya S. Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: results from a complementary meta-analysis. Neurosci Biobehav Rev. (2019) 96:45–55. doi: 10.1016/j.neubiorev.2018.10.026
85. Lisdahl KM, Filbey FM, Gruber SA. Introduction to JINS special issue: clarifying the complexities of cannabis and cognition. J Int Neuropsychol Soc. (2021) 27:515–9. doi: 10.1017/S1355617721000874
86. Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Dif. (2012) 3:19. doi: 10.1186/2042-6410-3-19
87. Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. (2010) 72:46–55. doi: 10.1016/j.bandc.2009.10.008
88. Zuo X-N, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. (2010) 30:15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010
89. Sanders AFP, Harms MP, Kandala S, Marek S, Somerville LH, Bookheimer SY, et al. Age-related differences in resting-state functional connectivity from childhood to adolescence. Cerebral Cortex. (2023) 33:6928–42. doi: 10.1093/cercor/bhad011
90. Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure. Br J Pharmacol. (2010) 161:103–12. doi: 10.1111/j.1476-5381.2010.00870.x
91. Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology. (2018) 43:34–51. doi: 10.1038/npp.2017.140
92. Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, et al. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. (2019a) 194:20–7. doi: 10.1016/j.drugalcdep.2018.09.018
93. Laurikainen H, Tuominen L, Tikka M, Merisaari H, Armio R-L, Sormunen E, et al. Sex difference in brain CB1 receptor availability in man. NeuroImage. (2019) 184:834–42. doi: 10.1016/j.neuroimage.2018.10.013
94. Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. (2008) 33:2760–71. doi: 10.1038/sj.npp.1301664
95. Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis. (2015) 73:60–9. doi: 10.1016/j.nbd.2014.09.015
96. Zamberletti E, Gabaglio M, Prini P, Rubino T, Parolaro D. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. Eur Neuropsychopharmacol. (2015) 25:2404–15. doi: 10.1016/j.euroneuro.2015.09.021
97. Crane NA, Schuster RM, Gonzalez R. Preliminary evidence for a sex-specific relationship between amount of cannabis use and neurocognitive performance in young adult cannabis users. J Int Neuropsychol Soc. (2013) 19:1009–15. doi: 10.1017/S135561771300088X
98. Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. (2012) 18:678–88. doi: 10.1017/S1355617712000276
99. Savulich G, Rychik N, Lamberth E, Hareli M, Evins AE, Sahakian BJ, et al. Sex differences in neuropsychological functioning are domain-specific in adolescent and young adult regular cannabis users. J Int Neuropsychol Soc. (2021) 27:592–606. doi: 10.1017/S1355617720001435
100. Schnakenberg Martin AM, D’Souza DC, Newman SD, Hetrick WP, O’Donnell BF. Differential cognitive performance in females and males with regular cannabis use. J Int Neuropsychol Soc. (2021) 27:570–80. doi: 10.1017/S1355617721000606
101. Banks PJ, Bennett PJ, Sekuler AB, Gruber AJ. Cannabis use is associated with sexually dimorphic changes in executive control of visuospatial decision-making. Front Integr Neurosci. (2022) 16:884080. doi: 10.3389/fnint.2022.884080
102. Wright NE, Scerpella D, Lisdahl KM. Marijuana use is associated with behavioral approach and depressive symptoms in adolescents and emerging adults. PLoS One. (2016) 11:e0166005. doi: 10.1371/journal.pone.0166005
103. Lim S, Radicchi F, van den Heuvel MP, Sporns O. Discordant attributes of structural and functional brain connectivity in a two-layer multiplex network. Sci Rep. (2019) 9:2885. doi: 10.1038/s41598-019-39243-w
104. Wallace AL, Wade NE, Hatcher KF, Lisdahl KM. Effects of cannabis use and subclinical ADHD symptomology on attention based tasks in adolescents and young adults. Arch Clin Neuropsychol. (2019) 34:700–5. doi: 10.1093/arclin/acy080
105. Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. (1998) 59:427–38. doi: 10.15288/jsa.1998.59.427
106. Stewart DG, Brown SA. Withdrawal and dependency symptoms among adolescent alcohol and drug abusers. Addiction. (1995) 90:627–35. doi: 10.1046/j.1360-0443.1995.9056274.x
107. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl 20):22–33.;quiz 34-57.9881538
108. Wallace AL, Wade NE, Lisdahl KM. Impact of 2 weeks of monitored abstinence on cognition in adolescent and young adult cannabis users. J Int Neuropsychol Soc. (2020) 26:776–84. doi: 10.1017/S1355617720000260
109. Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. (2019) 16:111–6. doi: 10.1038/s41592-018-0235-4
110. Markiewicz CJ, Esteban O, Goncalves M, Provins C, Salo T, Kent JD, et al. fMRIPrep: A Robust Preprocessing Pipeline for Functional MRI (2024). Available at: https://doi.org/10.5281/ZENODO.852659 (Accessed December 9, 2024).
111. Esteban O, Markiewicz CJ, Burns C, Goncalves M, Jarecka D, Ziegler E, et al. nipy/nipype: 1.8.3 (2022). Available at: https://doi.org/10.5281/ZENODO.596855 (Accessed December 9, 2024).
112. Gorgolewski K, Burns CD, Madison C, Clark D, Halchenko YO, Waskom ML, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. (2011) 5:13. doi: 10.3389/fninf.2011.00013
113. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. (2008) 12:26–41. doi: 10.1016/j.media.2007.06.004
114. Evans AC, Janke AL, Collins DL, Baillet S. Brain templates and atlases. NeuroImage. (2012) 62:911–22. doi: 10.1016/j.neuroimage.2012.01.024
115. Pruim RHR, Mennes M, Van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. (2015) 112:267–77. doi: 10.1016/j.neuroimage.2015.02.064
116. Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage. (2018) 171:415–36. doi: 10.1016/j.neuroimage.2017.12.073
117. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to carp. NeuroImage. (2013) 76:439–41. doi: 10.1016/j.neuroimage.2012.03.017
118. Ginestet CE, Nichols TE, Bullmore ET, Simmons A. Brain network analysis: separating cost from topology using cost-integration. PLoS One. (2011) 6:e21570. doi: 10.1371/journal.pone.0021570
119. Jiang C, He Y, Betzel RF, Wang Y-S, Xing X-X, Zuo X-N. Optimizing network neuroscience computation of individual differences in human spontaneous brain activity for test-retest reliability. Network Neurosci. (2023) 7:1080–108. doi: 10.1162/netn_a_00315
120. Viering T, Hoekstra PJ, Philipsen A, Naaijen J, Dietrich A, Hartman CA, et al. Emotion dysregulation and integration of emotion-related brain networks affect intraindividual change in ADHD severity throughout late adolescence. NeuroImage. (2021) 245:118729. doi: 10.1016/j.neuroimage.2021.118729
121. Levakov G, Kaplan A, Yaskolka Meir A, Rinott E, Tsaban G, Zelicha H, et al. Neural correlates of future weight loss reveal a possible role for brain-gastric interactions. NeuroImage. (2021) 224:117403. doi: 10.1016/j.neuroimage.2020.117403
122. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. (2010) 52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003
123. Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. (2011) 106:1125–65. doi: 10.1152/jn.00338.2011
125. Rogers LJ. Brain lateralization and cognitive capacity. Animals (Basel). (2021) 11:1996. doi: 10.3390/ani11071996
126. Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, et al. Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: a three-year longitudinal study. Neuropsychology. (2015) 29:829–43. doi: 10.1037/neu0000203
127. Becker MP, Collins PF, Luciana M. Neurocognition in college-aged daily marijuana users. J Clin Exp Neuropsychol. (2014) 36:379–98. doi: 10.1080/13803395.2014.893996
128. Grant JE, Odlaug BL, Mooney ME. Telescoping phenomenon in pathological gambling: association with gender and comorbidities. J Nerv Mental Dis. (2012) 200:996–8. doi: 10.1097/NMD.0b013e3182718a4d
129. Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Inter Neuropsych Soc. (2007) 13: 807–820. doi: 10.1017/S1355617707071032
130. Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. (2007) 26:309–19. doi: 10.1080/09595230701247772
131. Selamoglu A, Langley C, Crean R, Savulich G, Cormack F, Sahakian BJ, et al. Neuropsychological performance in young adults with cannabis use disorder. J Psychopharmacol. (2021) 35:1349–55. doi: 10.1177/02698811211050548
132. Wade NE, Wallace AL, Huestis MA, Lisdahl KM, Sullivan RM, Tapert SF. Cannabis use and neurocognitive performance at 13–14 years-old: optimizing assessment with hair toxicology in the adolescent brain cognitive development (ABCD) study. Addict Behav. (2024b) 150:107930. doi: 10.1016/j.addbeh.2023.107930
133. Wade NE, Courtney KE, Wallace AL, Hatz L, Jacobus J. Investigating sex differences and age of onset in emotion regulation, executive functioning, and cannabis use in adolescents and young adults. J Cannabis Res. (2024a) 6:20. doi: 10.1186/s42238-024-00225-z
134. Wade NE, Wallace AL, Swartz AM, Lisdahl KM. Aerobic fitness level moderates the association between cannabis use and executive functioning and psychomotor speed following abstinence in adolescents and young adults. J Int Neuropsychol Soc. (2019) 25:134–45. doi: 10.1017/S1355617718000966
135. Winward JL, Hanson KL, Tapert SF, Brown SA. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. (2014) 20:784–95. doi: 10.1017/S1355617714000666
136. Bechtel S, Lazar V, Albuisson E, Schwan R, Laprevote V, Bernardin F, et al. Assessment of neuropsychological impairments in regular cannabis users. Enceph-Rev Psychiatr Clin Biol Ther. (2022) 48:132–8. doi: 10.1016/j.encep.2021.02.013
137. Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, et al. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. (2013) 226:307–19. doi: 10.1007/s00213-012-2908-5
138. Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Frontal assessment battery (FAB) is a simple tool for detecting executive deficits in chronic cannabis users. J Clin Exp Neuropsychol. (2011) 33:523–31. doi: 10.1080/13803395.2010.535505
139. Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. (2010) 35:970–6. doi: 10.1016/j.addbeh.2010.06.012
140. Mathias CW, Blumenthal TD, Dawes MA, Liguori A, Richard DM, Bray B, et al. Failure to sustain prepulse inhibition in adolescent marijuana users. Drug Alcohol Depend. (2011) 116:110–6. doi: 10.1016/j.drugalcdep.2010.11.020
141. McHale S, Hunt N. Executive function deficits in short-term abstinent cannabis users. Hum Psychopharmacol Clin Exp. (2008) 23:409–15. doi: 10.1002/hup.941
142. Cousijn J, Watson P, Koenders L, Vingerhoets WAM, Goudriaan AE, Wiers RW. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. (2013) 38:2825–32. doi: 10.1016/j.addbeh.2013.08.011
143. Moreno M, Estevez AF, Zaldivar F, Montes JMG, Gutiérrez-Ferre VE, Esteban L, et al. Impulsivity differences in recreational cannabis users and binge drinkers in a university population. Drug Alcohol Depend. (2012) 124:355–62. doi: 10.1016/j.drugalcdep.2012.02.011
144. Sagar KA, Dahlgren MK, Gönenç A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cogn Neurosci. (2015) 16:84–92. doi: 10.1016/j.dcn.2015.03.003
145. Sidlauskaite J, Wiersema JR, Roeyers H, Krebs RM, Vassena E, Fias W, et al. Anticipatory processes in brain state switching — evidence from a novel cued-switching task implicating default mode and salience networks. NeuroImage. (2014) 98:359–65. doi: 10.1016/j.neuroimage.2014.05.010
146. Pedersen M, Zalesky A, Omidvarnia A, Jackson GD. Multilayer network switching rate predicts brain performance. Proc Natl Acad Sci USA. (2018) 115:13376–81. doi: 10.1073/pnas.1814785115
147. D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, et al. Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Δ-9-tetrahydrocannabinol in humans. Psychopharmacology. (2008) 198:587–603. doi: 10.1007/s00213-007-1042-2
148. Hunault CC, Mensinga TT, Böcker KBE, Schipper CMA, Kruidenier M, Leenders MEC, et al. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC). Psychopharmacology. (2009) 204:85–94. doi: 10.1007/s00213-008-1440-0
149. Fried P, Watkinson B, Gray R. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol. (2005) 27:231–9. doi: 10.1016/j.ntt.2004.11.003
150. Scott JC, Wolf DH, Calkins ME, Bach EC, Weidner J, Ruparel K, et al. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia neurodevelopmental cohort. Psychol Addict Behav. (2017) 31:423–34. doi: 10.1037/adb0000268
151. Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. (2011) 106:2195–203. doi: 10.1111/j.1360-0443.2011.03574.x
152. Molla HM, Miguelez Fernández AMM, Tseng KY. Late-adolescent onset of prefrontal endocannabinoid control of hippocampal and amygdalar inputs and its impact on trace-fear conditioning behavior. Neuropsychopharmacol. (2024) 43: 21–33. doi: 10.1038/s41386-024-01844-z
153. Meyer HC, Lee FS, Gee DG. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacol. (2018) 43:21–33. doi: 10.1038/npp.2017.143
154. Ramirez CEM, Ruiz-Perez G, Stollenwerk TM, Behlke C, Doherty A, Hillard CJ. Endocannabinoid signaling in the central nervous system. Glia. (2023) 71:5–35. doi: 10.1002/glia.24280
155. Abdul Wahab NS, Yahya N, Yusoff AN, Zakaria R, Thanabalan J, Othman E, et al. Effects of different scan duration on brain effective connectivity among default mode network nodes. Diagnostics (Basel). (2022) 12:1277. doi: 10.3390/diagnostics12051277
156. Chou Y-h, Panych LP, Dickey CC, Petrella JR, Chen N-k. Investigation of long-term reproducibility of intrinsic connectivity network mapping: a resting-state fMRI study. AJNR Am J Neuroradiol. (2012) 33:833–8. doi: 10.3174/ajnr.A2894
157. Ewusi Boisvert E, Bae D, Pang RD, Davis JP, Kelley-Quon LI, Barrington-Trimis JL, et al. Subjective effects of combustible, vaporized, and edible cannabis: results from a survey of adolescent cannabis users. Drug Alcohol Depend. (2020) 206:107716. doi: 10.1016/j.drugalcdep.2019.107716
158. Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use—basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. (2018) 52:87–96. doi: 10.1016/j.drugpo.2017.11.008
159. Schlienz NJ, Spindle TR, Cone EJ, Herrmann ES, Bigelow GE, Mitchell JM, et al. Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug Alcohol Depend. (2020) 211:107969. doi: 10.1016/j.drugalcdep.2020.107969
160. Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychol. (2019) 30:98–102. doi: 10.1016/j.copsyc.2019.04.002
161. Schlienz NJ, Budney AJ, Lee DC, Vandrey R. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr Addict Rep. (2017) 4:75–81. doi: 10.1007/s40429-017-0143-1
162. Chang H-A, Dai W, Hu SS-J. Sex differences in cocaine-associated memory: the interplay between CB1, mGluR5, and estradiol. Psychoneuroendocrinology. (2021) 133:105366. doi: 10.1016/j.psyneuen.2021.105366
163. Potier M, Maitre M, Leste-Lasserre T, Marsicano G, Chaouloff F, Marighetto A. Age-dependent effects of estradiol on temporal memory: a role for the type 1 cannabinoid receptor? Psychoneuroendocrinology. (2023) 148:106002. doi: 10.1016/j.psyneuen.2022.106002
164. de Fonseca FR, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ. Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. (1994) 54:159–70. doi: 10.1016/0024-3205(94)00585-0
165. El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril. (2010) 93:1989–96. doi: 10.1016/j.fertnstert.2008.12.033
166. Kim HJJ, Zagzoog A, Black T, Baccetto SL, Laprairie RB. Molecular and cellular mechanisms underlying brain region-specific endocannabinoid system modulation by estradiol across the rodent estrus cycle. Prog Mol Biol Transl Sci. (2023) 195:27–45. doi: 10.1016/bs.pmbts.2022.06.010
167. Chahkandi M, Sepehri G, Komeili G, Hadad MK, Haghparast E, Chahkandi M. The different role of G-protein-coupled receptor 30 (GPR30) in the interaction effects of marijuana and estradiol on spatial learning and memory at different ages. Brain Res Bull. (2022) 178:155–63. doi: 10.1016/j.brainresbull.2021.11.006
168. Fattore L, Spano MS, Altea S, Fadda P, Fratta W. Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol. (2010) 160:724–35. doi: 10.1111/j.1476-5381.2010.00734.x
169. Macatee RJ, Cannon MJ, Schermitzler BS, Preston TJ, Afshar K. Biological sex and hormonal contraceptive associations with drug cue reactivity in cannabis use disorder. J Psychiatr Res. (2024) 174:121–8. doi: 10.1016/j.jpsychires.2024.04.016
170. Marusich JA, Craft RM, Lefever TW, Wiley JL. The impact of gonadal hormones on cannabinoid dependence. Exp Clin Psychopharmacol. (2015) 23:206–16. doi: 10.1037/pha0000027
171. Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. (2014) 8:14. doi: 10.3389/fninf.2014.00014
172. Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. (2018) 28:3095–114. doi: 10.1093/cercor/bhx179
173. Maple KE, Thomas AM, Kangiser MM, Lisdahl KM. Anterior cingulate volume reductions in abstinent adolescent and young adult cannabis users: association with affective processing deficits. Psychiatry Res Neuroimaging. (2019) 288:51–9. doi: 10.1016/j.pscychresns.2019.04.011
Keywords: cannabis, networks, fMRI, young adult, abstinence
Citation: Baacke KA, Sullivan RM, Shankula CA and Lisdahl KM (2025) Network topology and cannabis use following two weeks of monitored abstinence: moderation of sex and patterns of use findings. Front. Adolesc. Med. 3:1549771. doi: 10.3389/fradm.2025.1549771
Received: 21 December 2024; Accepted: 11 March 2025;
Published: 26 March 2025.
Edited by:
Anita Cservenka, Oregon State University, United StatesReviewed by:
Lucy J Troup, University of the West of Scotland, United KingdomCopyright: © 2025 Baacke, Sullivan, Shankula and Lisdahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krista M. Lisdahl, bWVkaW5ha0B1d20uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.