- 1Department of Pediatrics, McMaster University, Hamilton, ON, Canada
- 2Global Health Program, McMaster University, Hamilton, ON, Canada
- 3Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
- 4Michael G. De Groote School of Medicine, McMaster University, Hamilton, ON, Canada
- 5Health Sciences Library, McMaster University, Hamilton, ON, Canada
- 6Department of Anesthesia, McMaster University, Hamilton, ON, Canada
- 7Centre for Evaluation of Medicines, St Joseph’s Health Care, Hamilton, ON, Canada
- 8Biostatistics Unit, St Joseph’s Healthcare, Hamilton, ON, Canada
- 9Department of Pediatrics, Queen’s University, Kingston, ON, Canada
- 10Division of Pediatric Endocrinology, Kingston Health Sciences Centre, Kingston, ON, Canada
Introduction: Type 2 diabetes mellitus (T2DM) is on the rise in the pediatric population. One of the main associations of T2DM is non-alcoholic fatty liver disease (NAFLD), yet the full burden of NAFLD in T2DM is unclear. This study aimed to estimate the prevalence of NAFLD and non-alcoholic steatohepatitis (NASH) in pediatric patients with T2DM. We also aimed to evaluate the association of sex, race/ethnicity, geographic location, NAFLD diagnostic methods, and glycemic control with NAFLD prevalence in this population.
Methods: Literature search was conducted in MEDLINE, Embase, CINAHL, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Web of Science Core Collection from database inception to 11 May 2023. This systematic review and meta-analysis has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42018091127). Observational studies with ≥10 participants reporting the prevalence of NAFLD in pediatric patients with T2DM were included. Four teams of two independent reviewers and one team with three reviewers screened articles and identified 26 papers fulfilling the eligibility criteria. Data extraction, risk of bias assessment, level of evidence assessment, and meta-analysis were performed.
Results: The pooled prevalence of NAFLD was 33.82% (95% CI: 24.23–44.11), and NASH prevalence was 0.28% (95% CI: 0.00–1.04). The Middle East had the highest NAFLD prevalence of 55.88% (95% CI: 45.2–66.29), and Europe had the lowest prevalence of 22.46% (95% CI: 9.33–38.97). The prevalence of NAFLD was 24.17% (95% CI, 17.26–31.81) when only liver function tests were used, but it increased to 48.85% (95% CI, 34.31–63.48) when the latter tests were combined with ultrasound. Studies reporting solely on an ultrasound-based diagnosis of NAFLD reported a prevalence of 40.61% (95% CI, 17.25–66.42) compared to 54.72% (95% CI, 34.76–73.95) in studies using magnetic resonance imaging/magnetic resonance spectroscopy. No differences in prevalence were noted based on glycemic control. Heterogeneity was high among studies.
Conclusion: NAFLD is a common comorbidity in pediatric T2DM. Further understanding of the optimal screening approaches for NAFLD diagnosis and evaluating its determinants and natural history are warranted to help establish its exact burden and to aid in the development of targeted screening, management, and prevention strategies for NAFLD in pediatric T2DM patients.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018091127, PROSPERO CRD42018091127.
1 Introduction
Type 2 diabetes mellitus (T2DM) accounts for more than 90% of cases of diabetes and is the most common endocrine non-communicable disease globally. It is estimated that close to 463 million adults have T2DM, and projections point to further surges in case numbers in the coming decades (1). T2DM is driven by complex interactions between genetic, epigenetic, and environmental factors that drive insulin resistance and pancreatic beta-cell dysfunction, which propagate hyperglycemia (2–4).
Low- and middle-income countries with the lowest socio-demographic indices are projected to have the greatest increase in T2DM in their population, including children—a global health challenge that requires urgent attention (5, 6). The emergence of T2DM in children has been linked mainly to the increased prevalence of obesity worldwide. The global prevalence of pediatric T2DM has yet to be established; however, it is estimated that 41,600 new cases are diagnosed annually (7).
Many of the pharmacotherapies used in diabetes treatment in adults are not approved or unavailable for children (8, 9). The global economic burden of diabetes in adults was estimated to be 1.32 trillion US dollars in 2015 and is projected to increase to 2.48 trillion by 2030; the emergence of pediatric T2DM poses additional high burdens on healthcare systems globally (10).
Youth living with T2DM are at an increased risk of early complications and comorbidities such as diabetic nephropathy, retinopathy, neuropathy, polycystic ovary syndrome, and non-alcoholic fatty liver disease (NAFLD) (11–14). While the relationship between NAFLD and T2DM is not fully understood, adult studies have demonstrated that T2DM and NAFLD are associated with increased cardiovascular risk and diabetes-related macro- and microvascular complications (15).
The gold standard for diagnosing NAFLD is a liver biopsy showing fat accumulation in over 5% of hepatocytes. Less invasive modalities are frequently used to screen for NAFLD including liver function tests (LFTs), such as alanine transaminase (ALT) and aspartate transferase (AST), and ultrasound and, less frequently, magnetic resonance imaging (MRI) (16). NAFLD can progress to non-alcoholic steatohepatitis (NASH) with associated liver inflammation, which can progress to liver fibrosis and cirrhosis that require liver transplantation (17, 18). While the relationship between T2DM and NAFLD is not fully understood, adult studies have demonstrated that T2DM and NAFLD are associated with increased cardiovascular risk, diabetes-related macro- and microvascular complications, and worsening hepatic outcomes (15). As the number of children with T2DM increases, it is crucial to understand the full scope of NAFLD in these patients to allow the design and resourcing of screening, prevention, and management options to improve NAFLD outcomes in pediatric T2DM patients.
The main aim of this systematic review was to estimate the prevalence of NAFLD in pediatric patients with T2DM. We also aimed to assess the prevalence of NASH and determine the impact of sex, race/ethnicity, geographical region, NAFLD screening modalities, and glycemic control on prevalence.
2 Methods
2.1 Systematic review protocol
This systematic review and meta-analysis has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; CRD42018091127) (19). The study did not need approval by an ethics review board because only anonymized data were aggregated. This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (Supplementary Table S1) and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines for systematic reviews and meta-analyses (Supplementary Table S2) (20, 21).
2.2 Search strategies and data sources
Search strategies were developed by a Senior Health Sciences Librarian (LB). Searches were conducted in MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Web of Science Core Collection from the inception of each database to 11 May 2023; no language restrictions were applied in the search strategy (Supplementary Tables S3–S7). Concepts related to pediatrics and T2DM were combined with terms of NAFLD, metabolic dysfunction-associated fatty liver disease (MAFLD), prevalence, and observational study design. References of included articles were also searched to identify potentially relevant studies. If a conference abstract was deemed eligible, the full-text publication was sought by searching for the paper and contacting the corresponding author if a published article could not be located.
The term MAFLD was recently proposed as an overarching name that encompasses NAFLD (22). However, the adoption of this term has been inconsistent, and there are concerns about its impact on disease awareness efforts, the lack of comprehensive understanding of the pathophysiological criteria associated with it, and the potential impact on clinical practice guideline development (23). Recognizing the potential use of the term, the search strategies were broadened to include search terms for MAFLD as a protocol deviation to attempt capturing studies that may have used this term to describe T2DM patients with fatty deposits in the liver.
2.3 Study selection and data abstraction
Two independent reviewers in four teams and three independent reviewers in one team (CH, MC, SR, AS, JD, AN, MH, YQ, SC, AR, and PT) screened titles, abstracts, and full-text articles and completed data abstraction, risk of bias evaluations, and level of evidence assessments. Reviewers resolved disagreements through discussion, and a third reviewer (MS) was available to resolve persistent disagreements.
Studies with observational designs, including retrospective and prospective cohort studies and cross-sectional studies, were included.
The eligibility criteria encompassed studies on human participants with a sample size of ≥10 reporting on the prevalence of NAFLD in T2DM patients who were ≤18 years of age. We included the report with the largest sample size for studies with serial data reporting. Studies reporting on participants with gestational or other types of diabetes were excluded.
The diagnosis of NAFLD was established according to criteria from international societies, blood tests for liver biomarkers, and imaging studies, including ultrasound, magnetic resonance imaging, or magnetic resonance spectroscopy (MRS). In some studies, the medical record reported the diagnosis, yet the exact diagnostic criteria were not always noted. We included these studies with this limitation in mind.
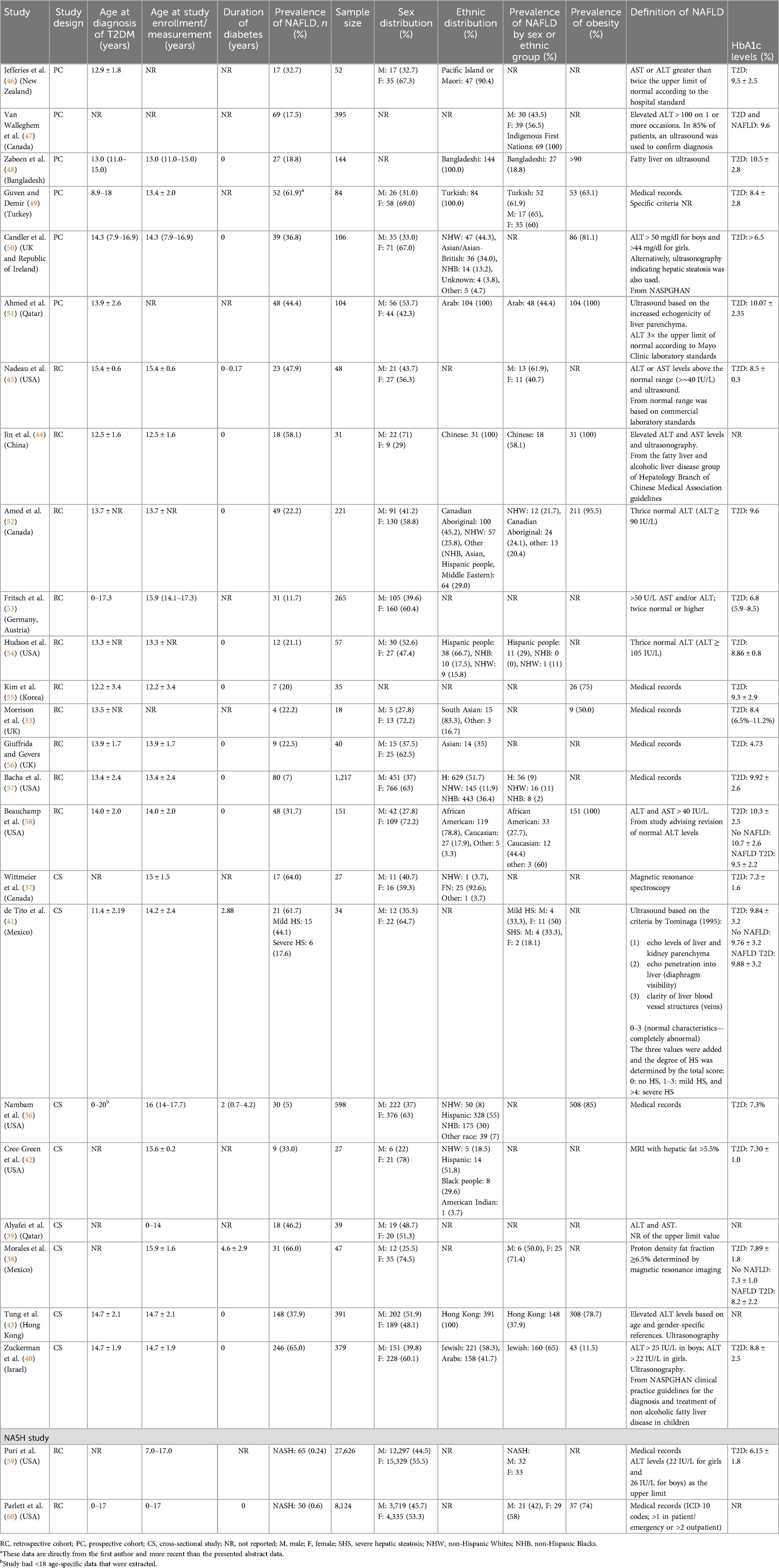
Data abstracted included the first author's name, country of study conduct, publication year, study design, age at T2DM diagnosis, duration of diabetes, age at study enrollment, sex, race/ethnicity, sample size, and prevalence of NAFLD. When reported, we also collected subgroup data on the prevalence of NAFLD by sex and race. We also attempted to collect data on the prevalence of obesity and the definition of NAFLD when reported.
The risk of bias analysis employed a validated tool to assess the internal and external validity of prevalence studies (24). The level of evidence analysis was evaluated using the Oxford Centre for Evidence-Based Medicine criteria (OCEBM) (25). Local and current random sample surveys were given a level of 1, while non-random surveys were given a level of 3 (corresponding to the highest and lowest levels of evidence used in this systematic review and meta-analysis, respectively). Studies were also rated lower based on imprecision, indirectness, and inconsistency.
2.4 Data analysis
Random-effects meta-analysis was performed if two or more studies reported data from similar populations and when using identical study designs, methods, analyses, and outcomes (26, 27). Prevalence was calculated by applying a study's weight, based on the random-effects model, to the reported proportion of patients with T2DM and NAFLD against the total sample of patients with T2DM for each study and then aggregating the weighted proportions to achieve a final pooled prevalence (28). The primary outcome was the pooled prevalence of NAFLD as a percentage (95% CI).
The data were transformed using the Freeman–Tukey double arcsine method to prevent the need to stabilize variances, and the results were transformed back to prevalence estimates for interpretation (29). To verify the results of the Freeman–Tukey double arcsine analysis and to control for sampling error and bias, an exploratory analysis was also conducted using the generalized linear mixed-effects logistic regression model, recognizing that the model does not account for study weights (29, 30).
Both inconsistency index (I2) and chi-squared (χ2) p-values were used to quantify heterogeneity. An I2 greater than 75% and a p-value threshold of 0.10 were set to indicate significant heterogeneity (28).
Subgroup analyses, meta-regression, sensitivity analyses, and small study effect evaluations were performed only if ≥10 studies were identified for a given outcome. Subgroup analyses were performed when two or more studies reported the prevalence of NAFLD by sex or race. The latter was classified using the National Institutes of Health definitions (31).
Sensitivity analyses were performed by removing studies reporting data from conference abstracts, studies that only used blood-based liver function tests for NAFLD screening, and studies with a sample size of <50 patients (32). A meta-regression was added to assess the association of glycemic control using the glycated hemoglobin A1c (HbA1c) level with NAFLD prevalence (28). The statistical significance of the regression coefficient for the association between each variable and NAFLD prevalence is reported. In addition, the mean difference in the HbA1c level for T2DM patients with and without NAFLD and the odds ratio for NAFLD diagnosis in males vs. females were calculated. The small study effect was assessed using a contour-enhanced funnel plot and Egger's test (33).
The statistical analyses were performed using the metafor package in RStudio software, version 1.1.383, using R language version 3.4.3 (R Foundation for Statistical Computing) (34, 35).
3 Results
3.1 Study details
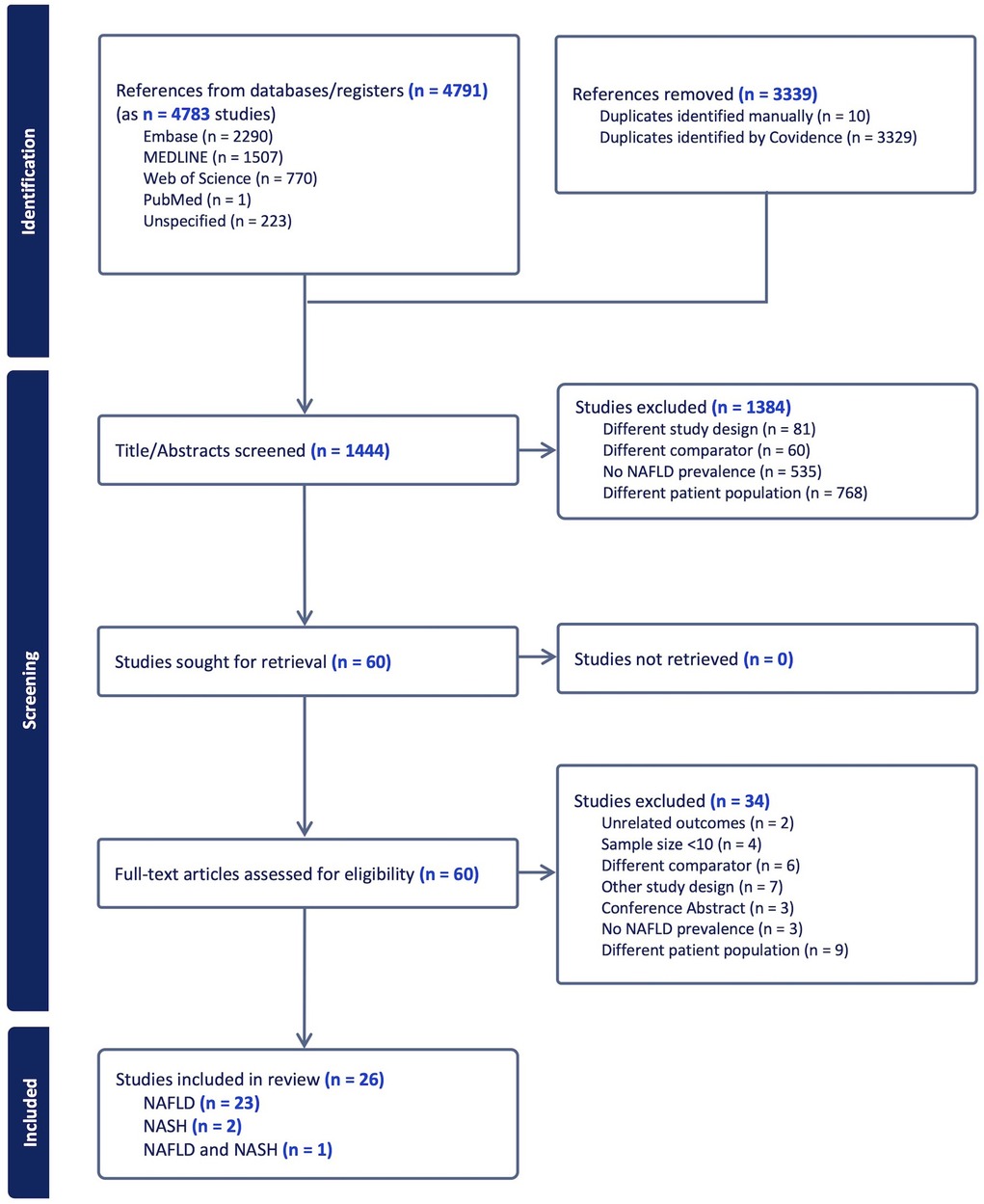
Figure 1 reports the PRISMA flowchart for study screening. Database searches yielded 1,444 unique records, and 26 eligible studies were considered for inclusion in the review, with 23 studies reporting on NAFLD, 2 reporting on NASH, and 1 reporting data on both NAFLD and NASH. The articles removed were either not relevant to the research question, reported on a NAFLD cohort with no T2DM, or reported data on adults with T2DM. The included studies encompassed 8 cross-sectional (36–43), 12 retrospective cohort, (44, 45), and 6 prospective cohort design (46–51) studies (Table 1).
All patients were diagnosed with diabetes at ≤18 years of age. Diabetes duration ranged from the time of diabetes diagnosis to 4.6 years post-diagnosis (33, 36–58). Three studies providing data about NASH were included in a separate analysis (59–61). One study included in the pooled analysis used updated data provided directly by the first author, as the conference abstract preceded communication with the author (49). Heterogeneity was high across studies.
3.2 Prevalence of NAFLD in pediatric T2DM
The pooled prevalence of NAFLD across studies was 33.82% (95% CI, 24.23–44.11; I2 = 98%; p < 0.01; n = 1,053 of 4,510 subjects) (Figure 2; Supplementary Table S9) (33, 36–58). The NAFLD prevalence was 45.55% (95% CI, 21.95–70.22; I2 = 99%; p < 0.01; n = 520 of 1,542 subjects) in cross-sectional studies (36–43), 34.60% (95% CI, 20.99–49.60; I2 = 95%; p < 0.01; n = 252 of 885 subjects) in prospective cohort studies (46–51), and 24.38% (95% CI, 14.84–35.33; I2 = 95%; p < 0.01; n = 281 of 2,083 subjects) in retrospective cohort studies (33, 44, 45, 52–58).
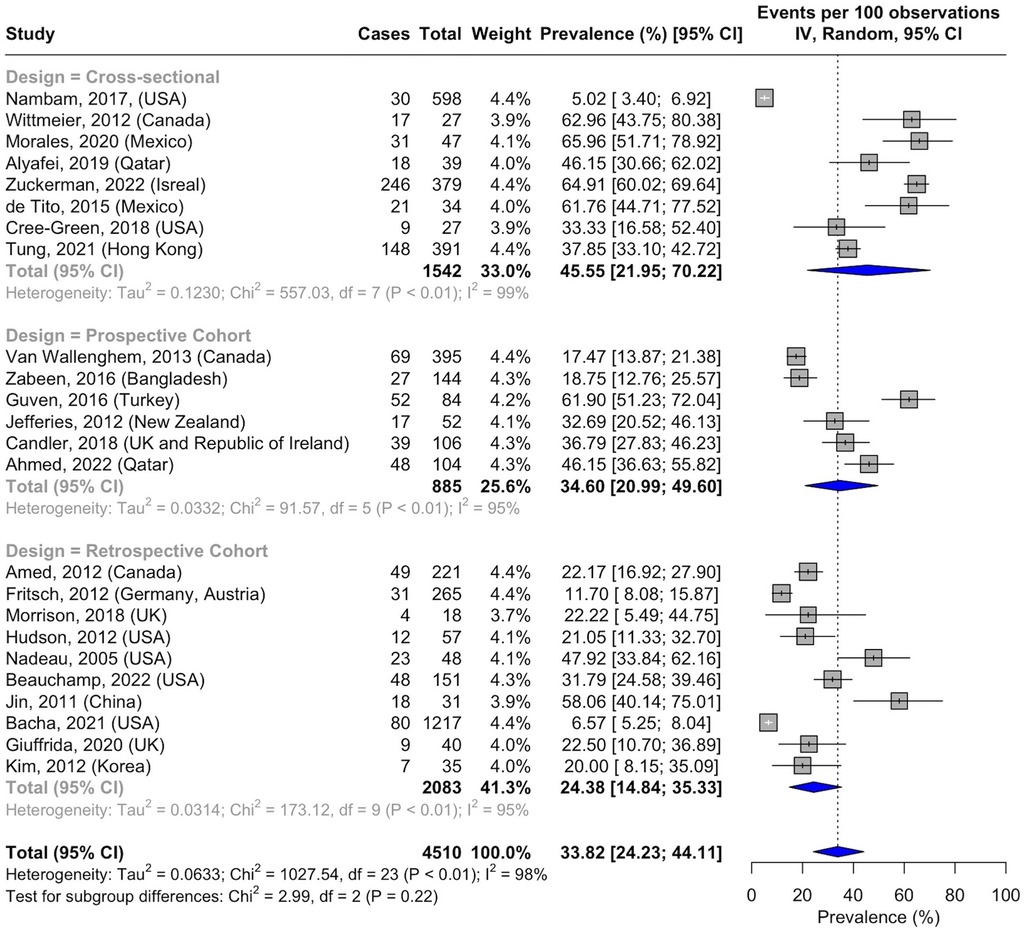
Figure 2. Forest plot of prevalence of non-alcoholic fatty liver disease in pediatric T2DM by study design.
3.3 Prevalence of NASH in pediatric T2DM
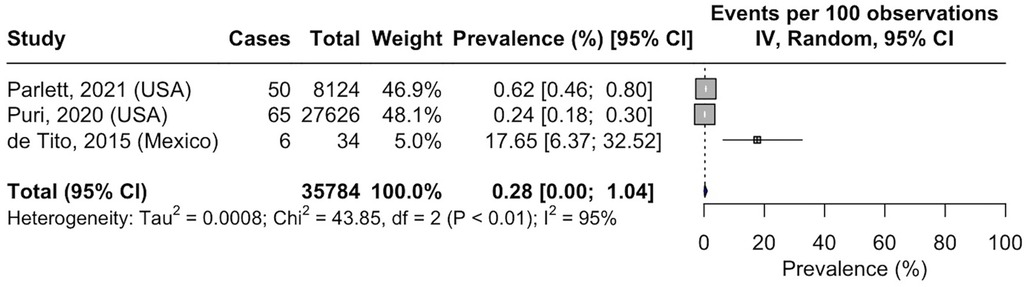
Three studies reported on NASH prevalence among the pediatric T2DM population. The calculated pooled prevalence was 0.28% (95% CI, 0.00–1.04; I2 = 95%; p < 0.01; n = 121 of 35,784 subjects) (Figure 3) (41, 59, 60).
3.4 Sex-based prevalence of NAFLD in T2DM
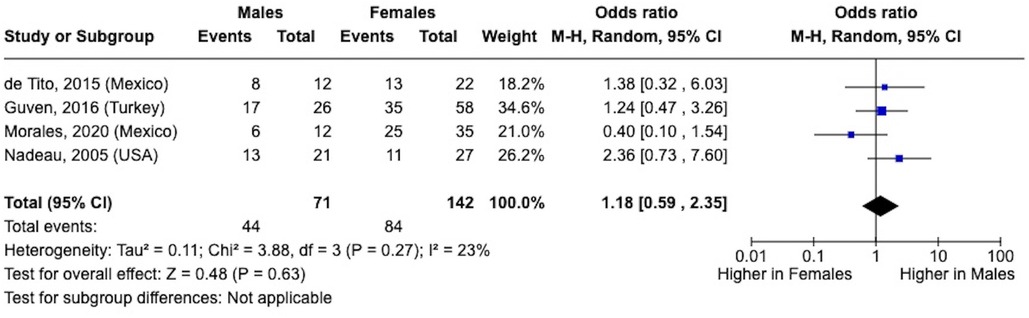
When calculating the sex differences in NAFLD prevalence, the sample sizes were quite small. The odds ratio trended higher in males vs. females (1.18; 95% CI, 0.59–2.35; I2 = 23%; p = 0.27; male: n = 44 of 71; female: n = 84 of 142) (Figure 4) (38, 41, 45, 49).
3.5 Race-based prevalence of NAFLD in T2DM
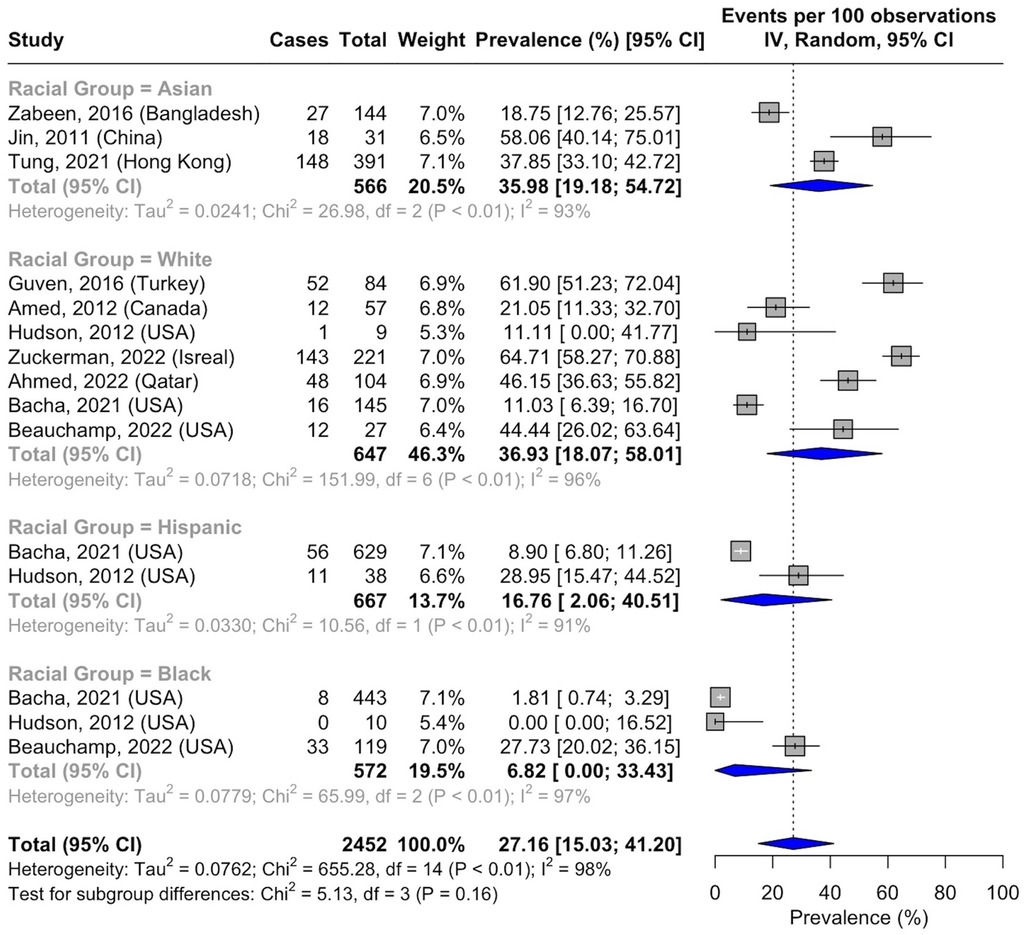
Race-based analysis included data collected from medical records or self-reported by participants. The pooled prevalence of NAFLD in Asians was 35.98% (95% CI, 19.18–54.71; I2 = 93%; p < 0.01; n = 193 of 566 subjects) (43, 44, 48), while a prevalence of 36.93% was reported in White patients (95% CI, 18.07–58.01; I2 = 96%; p < 0.01; n = 284 of 647 subjects) (40, 49, 51, 52, 54, 57, 58). The prevalence of NAFLD in Hispanic people was 16.76% (95% CI, 2.06–40.51; I2 = 91%; p < 0.01; n = 67 of 667 subjects) (54, 57), whereas a prevalence of 6.82% was reported in Black people (95% CI, 0.00–33.43; I2 = 97%; p < 0.01; n = 41 of 572 subjects) (54, 57, 58) (Figure 5). There were insufficient data to assess the pooled prevalence in other racial groups, including Indigenous populations.
3.6 Pooled prevalence of NAFLD by geographical region
There were significant differences in the prevalence of NAFLD in T2DM based on geographical location. Many of the studies originated from North America, and the NAFLD prevalence was 30.54% (95% CI, 19.84–42.38; I2 = 97%; p < 0.01; n = 389 of 2,822 subjects) (36–38, 41, 42, 45, 47, 52, 54, 57, 58). The prevalence was 55.88% (95% CI, 45.2–66.29; I2 = 80%; p < 0.01; n = 364 of 606 subjects) in the Middle East (39, 40, 49, 51), 32.15% (95% CI, 18.34–47.70; I2 = 90%; p < 0.01; n = 200 of 601 subjects) in Asia (43, 44, 48, 55), and 22.46% (95% CI, 9.33–38.97; I2 = 89%; p < 0.01; n = 83 of 429 subjects) in Europe (33, 50, 53, 56) (Figure 6). The prevalence of NAFLD in Oceania could not be pooled, and data from one study reported a prevalence of 32.70% (95% CI, 20.52–46.13; n = 17 of 52 subjects) (46). No data from South America or Africa were available for inclusion (Figure 7).
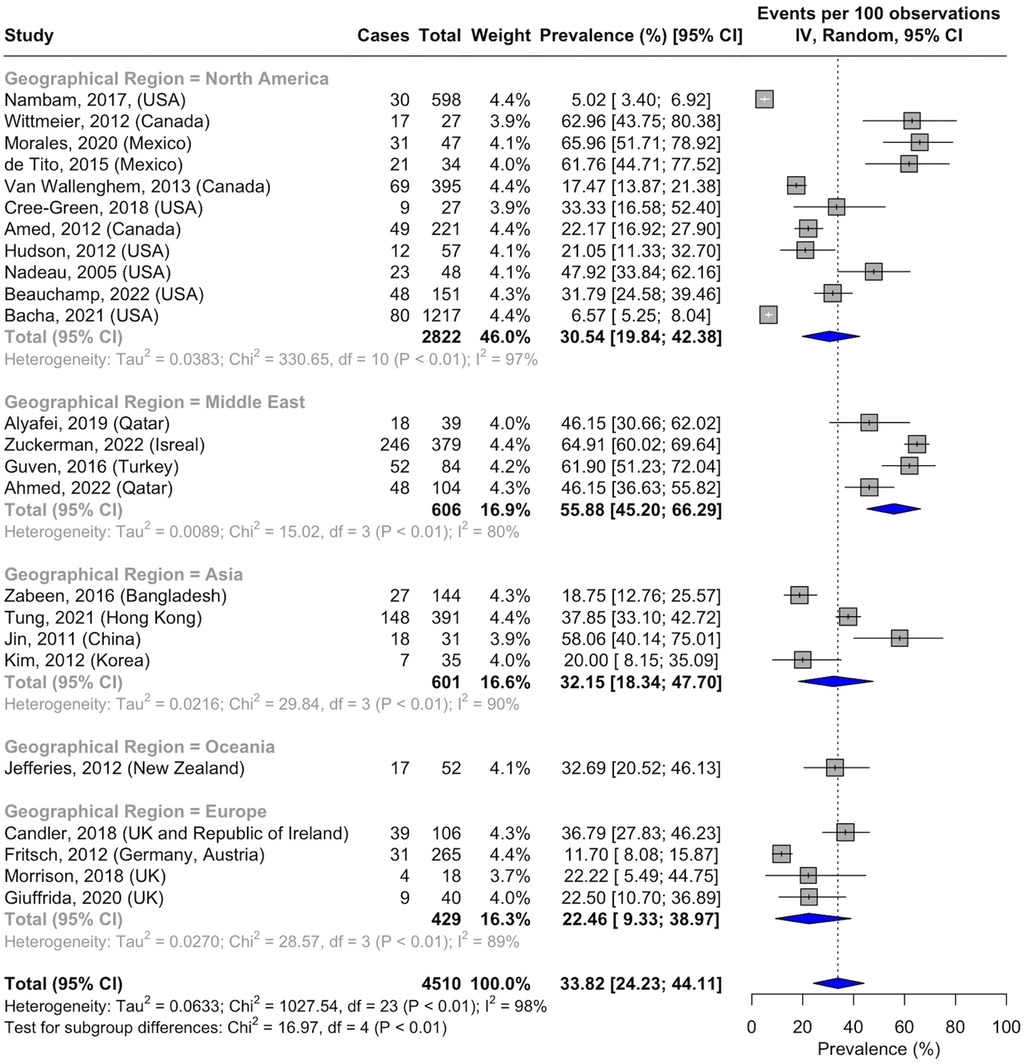
Figure 6. Forest plot of prevalence of non-alcoholic fatty liver disease in pediatric T2DM by geographical region.
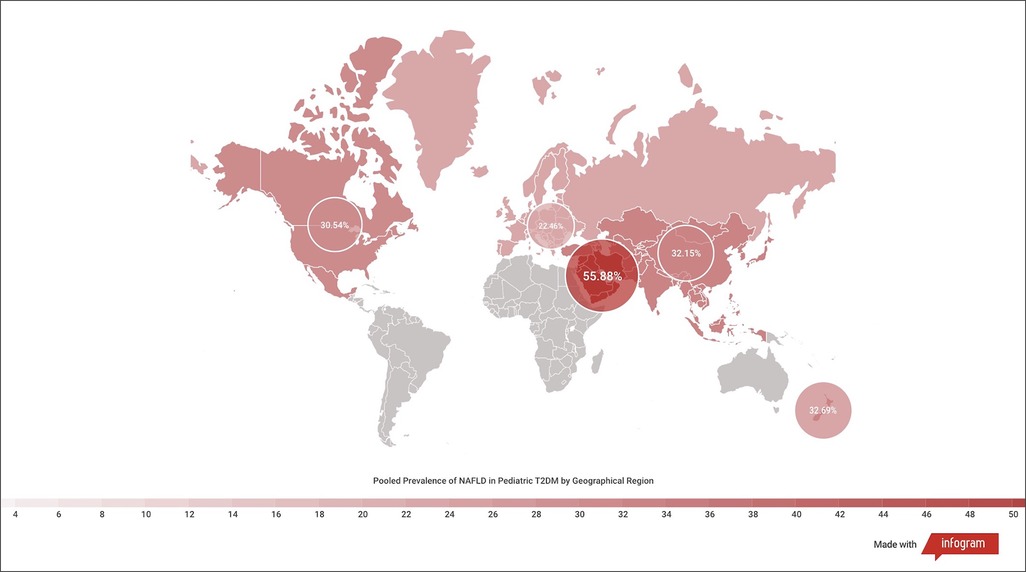
Figure 7. Heatmap of prevalence of non-alcoholic fatty liver disease in pediatric T2DM by geographical region.
3.7 Pooled prevalence by diagnostic modality of NAFLD
There were significant variations in NAFLD prevalence based on the screening criteria used to confirm the diagnosis. The NAFLD prevalence was 24.17% (95% CI, 17.26–31.81; I2 = 86%; p < 0.01; n = 244 of 1,180 subjects) when using blood-based LFTs alone (39, 46, 47, 52–54, 58) and 40.61% (95% CI, 17.25–66.42; I2 = 94%; p < 0.01; n = 96 of 282 subjects) when using ultrasound alone to diagnose NAFLD (41, 48, 51). The combination of LFTs and ultrasound was associated with a prevalence of 48.85% (95% CI, 34.31–63.48; I2 = 94%; p < 0.01; n = 474 of 955 subjects) (40, 43–45, 50). In a small number of subjects where MRI and MRS were used to screen for NAFLD, the prevalence was 54.72% (95% CI, 34.76–73.95; I2 = 75%; p < 0.01; n = 57 of 101 subjects) (Figure 8) (37, 38, 42).
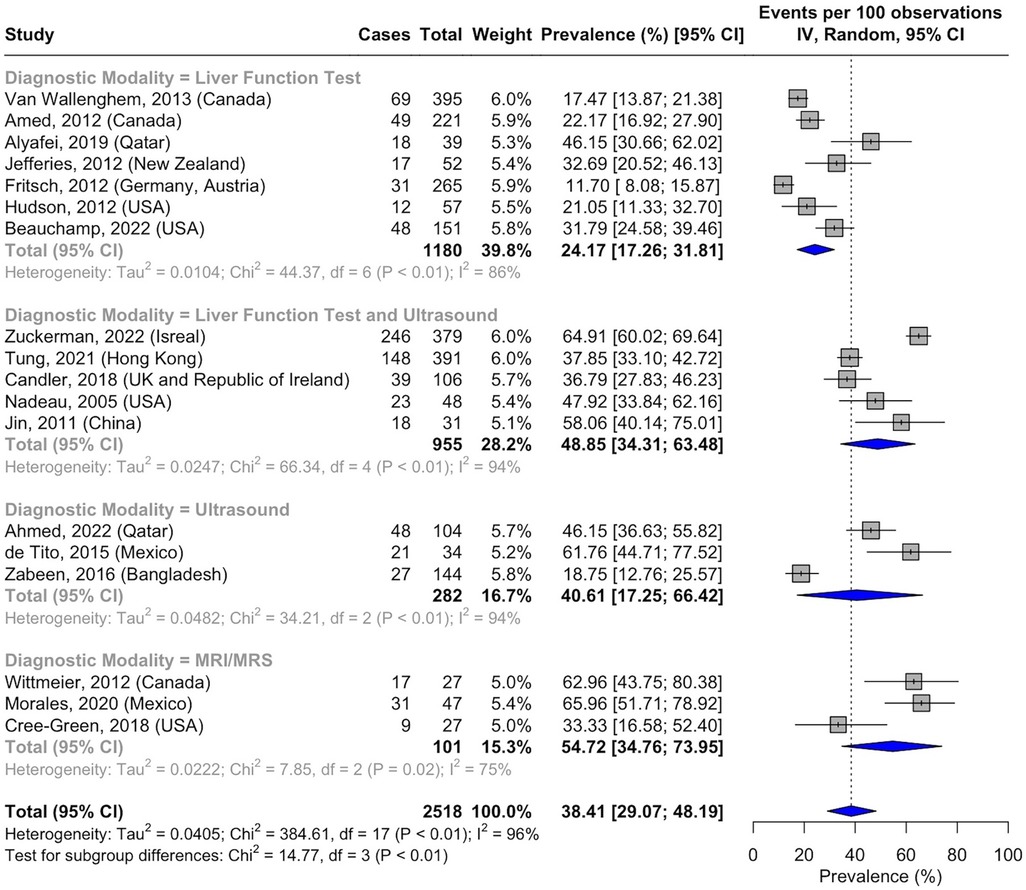
Figure 8. Forest plot of prevalence of non-alcoholic fatty liver disease in pediatric T2DM by diagnostic modality.
3.8 Association of glycemic control with NAFLD prevalence in T2DM
There was no significant association between HbA1c levels and NAFLD prevalence [mean HbA1c difference, 0.10 (95% CI, −1.49 to 1.69); I2 = 83%] (Figure 9) (38, 58, 62).
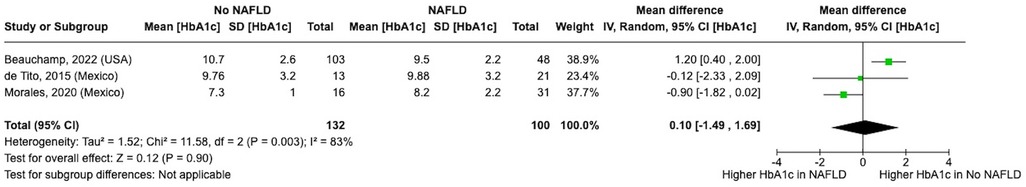
Figure 9. Forest plot showing mean difference in HbA1c in participants with and without non-alcoholic fatty liver disease.
3.9 Assessment of the risk of bias and level of evidence
Eleven studies had a low risk of bias (36, 38, 40, 43, 44, 46, 47, 50, 51, 53, 59), with 15 studies having a moderate risk of bias (33, 37, 39, 41, 42, 45, 48, 49, 52, 54–58, 60) (Supplementary Table S8).
The risk of bias increased when the patients were from a single clinic or city and not from a nationally representative sample, which limits generalizability. Some studies did not use a representative sampling framework, while others neither conducted a census nor randomly selected patients.
The risk of bias also increased if the definition of NAFLD or the assessment method was not described.
Fourteen studies (36, 40, 43, 46–48, 50–54, 57–59) had the highest level of evidence assessment (level 1), 8 studies (33, 38, 39, 41, 44, 45, 55, 56) had level 2 evidence, and 4 studies (37, 42, 49, 60) had level 3 evidence (Supplementary Table S8). The level of evidence downgraded if random sampling or census (37, 42, 49, 60) was not used during the recruitment process and if the study had a small sample size (33, 38, 39, 41, 44, 45, 55, 56).
3.10 Sensitivity analyses
Sensitivity analyses excluding the studies that only used LFTs as the diagnostic modality for NAFLD led to a pooled NAFLD prevalence estimate of 35.82% (95% CI 23.15–49.53, I2 = 98%; p < 0.01). Removing conference abstracts yielded a pooled NAFLD prevalence of 37.73% (95% CI 24.40–52.05, I2 = 98%; p < 0.01), and removing studies with a sample size of <50 was associated with a prevalence of 27.75% (95% CI 16.82–40.20, I2 = 99%; p < 0.01).
The exploratory analysis using the generalized linear mixed-effects logistic regression model was completed to control for sampling error and bias. The results were compared with the reported Freeman–Tukey double arcsine analysis and were consistent with overlapping 95% CIs (Supplementary Table S10). The pooled overall NAFLD prevalence for the generalized linear mixed-effect model was 34.92% (95% CI, 27.49–42.36). By study design, the prevalence was 47.01% (95% CI, 23.60–70.42) in cross-sectional studies, 35.23% (95% CI, 21.79–48.67) in prospective cohort studies, and 24.91% (95% CI, 16.85–32.97) in retrospective cohort studies. The prevalence was 30.71% (95% CI, 23.48–37.94) in North America, 32.52% (95% CI 18.18–46.86) in Asia, 55.80% (95% CI, 45.36–66.24) in the Middle East, 23.09% (95% CI, 8.88–37.29) in Europe, and 32.69% (95% CI, 19.94–45.44) in Oceania. Using LFTs to diagnose NAFLD yielded a prevalence of 24.20% (95% CI, 17.52–30.87), 41.41% (95% CI, 16.52–66.30) with ultrasound, 48.87% (95% CI, 34.28–63.46) with combined LFTs and ultrasound, and 54.51% (95% CI, 34.52–74.49) with MRI/MRS.
3.11 Small study effect
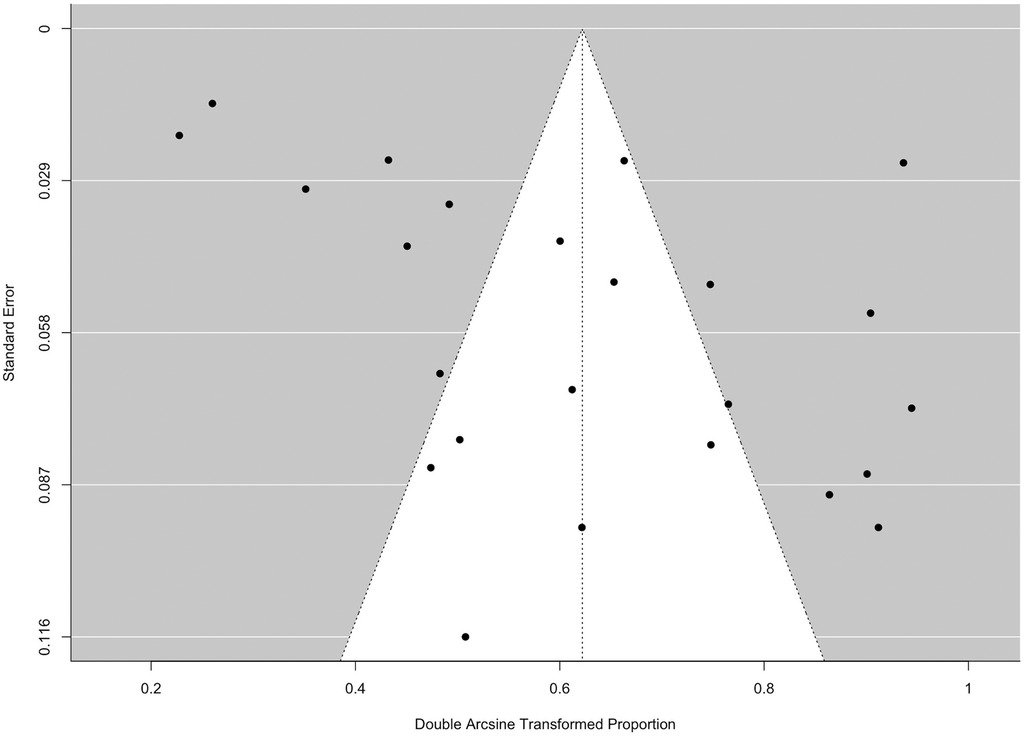
The potential presence of the small study effect was identified based on the funnel plot and Egger's test (p = 0.028) (Figure 10).
4 Discussion
The surge in T2DM pediatric patients poses a significant challenge to healthcare systems globally; it is a serious disease with multiple comorbidities and complications emerging early in life, and one of these comorbid conditions is NAFLD.
This systematic review demonstrated that a significant proportion of pediatric patients with T2DM have NAFLD and that a smaller group will progress to NASH. The pooled prevalence varied across geographical locations, with T2DM youth in the Middle East having the highest and those in Europe having the lowest prevalence of NAFLD. No sex or glycemic control differences were noted, and heterogeneity among studies was high.
A recent study reported a prevalence of NAFLD of 7.4% in the general pediatric population compared to its prevalence in children living with obesity of 52.49% (63). There were insufficient data from this systematic review to associate obesity with NAFLD in T2DM, which limits data comparisons with those from the general pediatric population (64, 65).
Importantly, it is unclear whether NAFLD is driven by factors unrelated to obesity in T2DM, so the association between obesity and NAFLD in pediatric T2DM requires further study. The treatment and prevention of obesity and T2DM will likely be crucial in reducing the overall risk of developing NAFLD and NASH. However, further studies are needed to address this question.
A small number of studies reported data on NASH in pediatric T2DM patients, and the small number of events limits the certainty about the prevalence of NASH in pediatric T2DM. The estimated prevalence of NASH is 37.33% in adult T2DM patients and 33.67% in adults living with obesity, which is higher than the prevalence reported in pediatric T2DM (66, 67). It is possible that the duration of diabetes may impact the development of NASH, as per the studies reported on patients who were included within a few years post-diabetes diagnosis. Adequately powered cohort studies are needed to assess the natural history of NAFLD in T2DM patients, including its potential progression to NASH.
There are limited sex-based data on NAFLD risk in T2DM (61, 62). Visceral abdominal adiposity positively correlates with insulin resistance and NAFLD risk, which may be more frequent in males (68). Previous reports demonstrated a higher NAFLD risk in boys compared to girls, which is congruent with the trends observed in our analysis (69). One potential explanation for the lower risk in females comes from animal data suggesting a protective role of estrogen against steatosis and insulin resistance (70). While the data in this review suggested a trend for higher risk of NAFLD in males, the small sample size precluded firm conclusions about the relationship between sex and NAFLD risk in pediatric T2DM.
While the data from race-based analyses for NAFLD in T2DM exhibited high heterogeneity, the studies suggested lower NAFLD prevalence in Hispanic people and Black people than in other groups. Importantly, these groups have a high risk of obesity and T2DM. Further studies are needed to assess ethnic variations in NAFLD risk in pediatric T2DM patients.
The Middle East is projected to have one of the greatest increases in T2DM in the coming decade. This expansion in case numbers will very likely include children and may increase the risk of NAFLD (1) (71, 72). Regional variations in obesity and T2DM prevalence will likely drive NAFLD risk, and there is an urgent need to assess these trends globally.
A key global health equity consideration is where pediatric T2DM research is being conducted. There were no data for NAFLD prevalence in T2DM for South America and Africa, and very limited data were available from Oceania. The latter regions are among the “Global South,” which refers to areas historically viewed as underdeveloped and encompass many low-income countries (73). Most of the studies included in this review originated from North America, a high-income and developed region of the world (74). The data demonstrate regional variations in the longitudinal tracking of NAFLD in T2DM.
Global health equity efforts need to expand to bridge the knowledge gaps in relation to NAFLD prevalence and determinants in pediatric T2DM patients. There is a need to provide resources, funding, training, and governmental support to track obesity-driven diseases including T2DM and NAFLD (75).
Moreover, the diagnosis of NAFLD relies on access to technology such as laboratories for blood testing and advanced imaging technologies and the need for resources to occasionally perform a liver biopsy–the gold standard test in diagnosing NAFLD (32). There is a crucial need to ensure equitable access to medical technologies to assess patients and have the resources to drive clinical care and research efforts for NAFLD care in pediatric T2DM.
A crucial consideration from the studies is the need to choose screening tests to identify NAFLD. While screening for NAFLD in T2DM is needed at diabetes diagnosis and regular intervals afterward, not all guidelines endorse this approach (76).
Recent clinical practice guidelines from several pediatric diabetes organizations provided a comprehensive platform for T2DM care in pediatric patients (77–83). However, only some of these guidelines highlighted the need for NAFLD screening in T2DM (77, 78, 80, 81). Some guidelines recommended screening for NAFLD at diabetes diagnosis and annually thereafter, while other guidelines (79, 82, 83) did not address screening needs (Supplementary Table S11).
In addition, NAFLD screening recommendations from international liver health organizations suggest different test combinations for screening (Supplementary Table S12).
While a liver biopsy is a gold standard for NAFLD diagnosis, it is recommended only for assessing the severity of NAFLD and confirming the diagnosis when initial screening tests do not confirm the diagnosis (16). The studies included in this review relied on LFTs as the most commonly used tests (Supplementary Table S13). LFTs are more accessible, relatively inexpensive, and have short turnaround times. However, one-time results are sometimes unreliable and require repeated tests and additional testing modalities to confirm the diagnosis (32). Ultrasounds, although more readily available than MRI/MRS, are not universally accessible and do not quantify steatosis severity (84).
A comparison of clinical screening guidelines for pediatric NAFLD (Supplementary Table S14) from liver health agencies indicated conflicting reports about ultrasound use to screen for NAFLD. While the North American Society For Pediatric Gastroenterology, Hepatology & Nutrition does not recommend ultrasound scanning for NAFLD screening, the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition recommends ultrasound scanning in obese children with elevated ALT (85). The American Association for the Study of Liver Disease also recommends ultrasound use with limitations in children with milder degrees of steatosis (16).
Given the differences in screening recommendations, research into the best test or test combinations and novel diagnostic tools is warranted.
4.1 Glycemic control and NAFLD
The HbA1c provides information about a patient's average glucose level over 3 months and is the standard of care for assessing diabetes control (86). There were no differences in HbA1c levels in pediatric T2DM patients with NAFLD vs. those without NAFLD (87). Improved glycemic control and lifestyle interventions may mitigate NAFLD risk or progression to NASH and cirrhosis. Further research is required to assess whether optimal glycemic control alters the natural history of NAFLD or NASH in these patients.
4.2 Strengths and limitations
The strengths of this study included the robust methodology used to conduct the review and the overall high level of evidence included in the analysis. The data allowed conducting meta-analyses for some of the outcomes. This study also captured a wide range of data worldwide, providing key insight into the global scale of NAFLD in pediatric T2DM patients.
This study has several limitations. The heterogeneity was high across studies. Some studies did not report the NAFLD screening method used to define NAFLD and relied only on medical record mention of the diagnosis (88). Data on obesity specific to NAFLD in T2DM patients were limited; thus, analysis regarding the combined impact of obesity and T2DM in NAFLD risk could not be performed.
Future research needs to focus on defining the best diagnostic modalities to accurately diagnose NAFLD that considers resource availability globally. In addition, the definition of therapies that can mitigate NAFLD risk and progression to NASH is a priority.
5 Conclusions
The findings of this systematic review and meta-analysis suggest that NAFLD is a significant comorbidity in children with T2DM. The pathogenesis of NAFLD in pediatric T2DM is not fully understood, yet its high prevalence raises concerns about the emergence of T2DM-related comorbidities as pediatric diseases within a few years of diabetes diagnosis. Current clinical practice guidelines for screening for NAFLD are inconsistent and warrant further efforts to determine the best screening approach for NAFLD in T2DM patients. Reaching a consensus regarding the most efficient and effective screening modalities is necessary for improving early detection, treatment, and prevention of NAFLD in an ever-growing pediatric T2DM population.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: this systematic review included anonymized aggregate data that were published already, with one study providing data separately. The search strategies are included in the Supplementary Materials. Request for access to the data set will be granted upon request with reasonable justification. All data analyzed were anonymized aggregate data.
Author contributions
CH: Writing – review & editing, Writing – original draft, Visualization, Software, Project administration, Formal Analysis, Data curation, Conceptualization. MC: Writing – review & editing, Writing – original draft, Visualization, Software, Project administration, Methodology, Formal Analysis, Data curation, Conceptualization. AS: Writing – review & editing, Formal Analysis, Data curation. SR: Writing – review & editing, Validation, Methodology, Formal Analysis, Data curation. JD: Writing – review & editing, Validation, Investigation, Formal Analysis, Data curation. AN: Writing – review & editing, Validation, Formal Analysis, Data curation. MH: Writing – review & editing, Validation, Formal Analysis, Data curation. YQ: Writing – review & editing, Validation, Formal Analysis, Data curation. SC: Writing – review & editing, Validation, Investigation, Formal Analysis, Data curation. AR: Writing – review & editing, Validation, Formal Analysis, Data curation. PP: Writing – review & editing, Validation, Formal Analysis, Data curation. LB: Writing – review & editing, Validation, Software, Methodology, Formal Analysis, Data curation, Conceptualization. LT: Writing – review & editing, Validation, Resources, Methodology, Formal Analysis, Data curation, Conceptualization. MS: Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fradm.2024.1303375/full#supplementary-material.
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels, Belgium (2021). Available online at: https://www.diabetesatlas.org (Accessed July 29, 2024).
3. Punthakee Z, Goldenberg R, Definition KP. Classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. (2018) 42:S10–5. doi: 10.1016/j.jcjd.2017.10.003
4. Smith K, Deutsch AJ, McGrail C, Kim H, Hsu S, Huerta-Chagoya A, et al. Multi-ancestry polygenic mechanisms of type 2 diabetes. Nat Med. (2024) 30:1065–74. doi: 10.1038/s41591-024-02865-3
5. Safiri S, Karamzad N, Kaufman JS, Bell AW, Nejadghaderi SA, Sullman MJM, et al. Prevalence, deaths and disability-adjusted-life-years (DALYs) due to type 2 diabetes and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. Front Endocrinol (Lausanne). (2022) 13:838027. doi: 10.3389/fendo.2022.838027
6. Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab Syndr Obes Targets Ther. (2021) 14:3567–602. doi: 10.2147/DMSO.S319895
7. Wu H, Patterson CC, Zhang X, Ghani RBA, Magliano DJ, Boyko EJ, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. (2022) 185:109785. doi: 10.1016/j.diabres.2022.109785
8. D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. (2011) 34(Suppl. 2):S161–5. doi: 10.2337/dc11-s212
9. Fagot-Campagna A, Narayan KMV, Imperatore G. Type 2 diabetes in children. Br Med J. (2001) 322(7283):377–8. doi: 10.1136/bmj.322.7283.377
10. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. (2018) 41(5):963–70. doi: 10.2337/dc17-1962
11. Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, et al. Global prevalence of diabetic retinopathy in pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. (2023) 6(3):e231887. doi: 10.1001/jamanetworkopen.2023.1887
12. Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, et al. The prevalence of obesity among children with type 2 diabetes. JAMA Netw Open. (2022) 5(12):e2247186. doi: 10.1001/jamanetworkopen.2022.47186
13. Cioana M, Deng J, Hou M, Nadarajah A, Qiu Y, Chen SSJ, et al. Prevalence of hypertension and albuminuria in pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. (2021) 4(4):e216069. doi: 10.1001/jamanetworkopen.2021.6069
14. Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, et al. Prevalence of polycystic ovary syndrome in patients with pediatric type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. (2022) 5(2):e2147454. doi: 10.1001/jamanetworkopen.2021.47454
15. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metab Clin Exp. (2016) 65(8):1096–108. doi: 10.1016/j.metabol.2016.01.001
16. Hunter AK, Lin HC. Review of clinical guidelines in the diagnosis of pediatric nonalcoholic fatty liver disease. Clin Liver Dis. (2021) 18(1):40–4. doi: 10.1002/cld.1094
17. Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clin Med. (2018) 18(3):245–50. doi: 10.7861/clinmedicine.18-3-245
18. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. (2005) 115(5):1343–51. doi: 10.1172/JCI23621
19. Cioana M, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, et al. The prevalence of obesity among children with type 2 diabetes: a systematic review and meta-analysis. JAMA Netw Open. (2022) 5(12):e2247186. doi: 10.1001/jamanetworkopen.2022.47186
20. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
22. Eslam M, Sanyal AJ, George J, Sanyal A, Neuschwander-Tetri B, Tiribelli C, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
23. García-Compeán D, Jiménez-Rodríguez AR. NAFLD vs MAFLD. The evidence-based debate has come. Time to change? Ann Hepatol. (2022) 27(6):1–4. doi: 10.1016/j.aohep.2022.100765
24. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. (2012) 65(9):934–9. doi: 10.1016/j.jclinepi.2011.11.014
25. OCEBM Levels of Evidence. Centre for Evidence-Based Medicine (CEBM). UK: University of Oxford (2011). Available online at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (Accessed May 31, 2023).
26. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1(2):97–111. doi: 10.1002/jrsm.12
27. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. (2013) 67(11):974–8. doi: 10.1136/jech-2013-203104
28. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023).
29. Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. (1978) 32(4):138–138. doi: 10.1080/00031305.1978.10479283
30. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. (1950) 21(4):607–11. doi: 10.1214/aoms/1177729756
31. NOT-OD-15-089: Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Available online at: https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html (Accessed May 31, 2023).
32. Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN hepatology committee. J Pediatr Gastroenterol Nutr. (2012) 54(5):700–13. doi: 10.1097/MPG.0b013e318252a13f
33. Morrison A, Chatterjee S, Greening J, James J, Higgins K, Lawrence I, et al. Phenotype and burden of comorbidities in adolescents with type 2 diabetes in a multiethnic population. Diabetes Med. (2018) 35:107–8.
34. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
35. RStudio 1.1.383. Available online at: https://www.npackd.org/p/rstudio/1.1.383 (Accessed May 31, 2023).
36. Nambam B, Silverstein J, Cheng P, Ruedy KJ, Beck RW, Paul Wadwa R, et al. A cross-sectional view of the current state of treatment of youth with type 2 diabetes in the USA: enrollment data from the pediatric diabetes consortium type 2 diabetes registry. Pediatr Diabetes. (2017) 18(3):222–9. doi: 10.1111/pedi.12377
37. Wittmeier KDM, Wicklow BA, MacIntosh AC, Sellers EAC, Ryner LN, Serrai H, et al. Hepatic steatosis and low cardiorespiratory fitness in youth with type 2 diabetes. Obes Silver Spring Md. (2012) 20(5):1034–40. doi: 10.1038/oby.2011.379
38. Morales JAO, Tamayo MT, Suarez PD, Urrutia AM, Bravo PGM. Effect of metabolic control on the presence of nonalcoholic fatty liver disease (NAFLD) in adolescents with type 2 diabetes. Horm Res Paediatr. (2021) 94(Suppl 1):237–8. Available online at: https://abstracts.eurospe.org/hrp/0094/hrp0094p2-120
39. Alyafei F, Soliman A, Sabt A, Aldarsy N. “Familial versus non-familial type-2 diabetes mellitus in children and adolescents: clinical and biochemical data.” In: ESPE Abstracts. UK: European Society for Paediatric Endocrinology (2019). p. 1. Available online at: https://abstracts.eurospe.org/hrp/0092/hrp0092p1-313 (Accessed May 31, 2023).
40. Zuckerman Levin N, Cohen M, Phillip M, Tenenbaum A, Koren I, Tenenbaum-Rakover Y, et al. Youth-onset type 2 diabetes in Israel: a national cohort. Pediatr Diabetes. (2022) 23(6):649–59. doi: 10.1111/pedi.13351
41. de Tito ACH, García HM, Klünder MK, Reyes MTV, Huang F, Díaz MM, et al. Type 4 retinol binding protein as a marker of hepatic steatosis in children and adolescents with type 2 diabetes. Int J Pediatr Neonatal Care. (2015) 1:110–4. doi: 10.15344/2455-2364/2015/110
42. Cree-Green M, Wiromrat P, Stuppy JJ, Thurston J, Bergman BC, Baumgartner AD, et al. Youth with type 2 diabetes have hepatic, peripheral, and adipose insulin resistance. Am J Physiol Endocrinol Metab. (2019) 316(2):E186–95. doi: 10.1152/ajpendo.00258.2018
43. Tung JYL, Kwan EYW, But BWM, Wong WHS, Fu ACC, Pang G, et al. Incidence and clinical characteristics of pediatric-onset type 2 diabetes in Hong Kong: the Hong Kong childhood diabetes registry 2008 to 2017. Pediatr Diabetes. (2022) 23(5):556–61. doi: 10.1111/pedi.13231
44. Jin YY, Liang L, Fu JF, Wang XM. The prevalence of type 2 diabetes mellitus and prediabetes in children. Zhongguo Dang Dai Er Ke Za Zhi Chin J Contemp Pediatr. (2011) 13(2):138–40. Available online at: http://www.zgddek.com/EN/Y2011/V13/I2/138
45. Nadeau KJ, Klingensmith G, Zeitler P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J Pediatr Gastroenterol Nutr. (2005) 41(1):94–8. doi: 10.1097/01.MPG.0000164698.03164.E5
46. Jefferies C, Carter P, Reed PW, Cutfield W, Mouat F, Hofman PL, et al. The incidence, clinical features, and treatment of type 2 diabetes in children <15yr in a population-based cohort from Auckland, New Zealand, 1995–2007. Pediatr Diabetes. (2012) 13(4):294–300. doi: 10.1111/j.1399-5448.2011.00851.x
47. Van Walleghem N, Dean HJ, Sellers E. Natural history of elevated alanine aminotransferase (ALT) levels in youth with type 2 diabetes (T2D). Diabetes. (2013) 62(Suppl. 1):A341–2. doi: 10.2337/db13-859-1394
48. Zabeen B, Nahar J, Tayyeb S, Nhar N, Azad K. Type 2 diabetes in Bangladeshi children and adolescents-an emerging problem. Pediatr Diabetes. (2016) 17(Supplement 24):80–1. doi: 10.1111/pedi.12451
49. Guven A, Demir EG. “Cardiovascular risk and long term follow-up of Turkish children with type 2 diabetes: single center experience”. In: ESPE Abstracts. Switzerland: Bioscientifica (2016). p. 159. Available online at: https://abstracts.eurospe.org/hrp/0086/hrp0086p2-p304 (Accessed May 31, 2023).
50. Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med. (2018) 35(6):737–44. doi: 10.1111/dme.13609
51. Ahmed SM, Haris B, Saraswathi S, Elawwa A, Khalifa A, AlMaadheed M, et al. The epidemiology, clinical, biochemical, immunological and radiological features of youth onset type 2 diabetes mellitus in the state of Qatar. Diabetol Int. (2022) 13(2):381–6. doi: 10.1007/s13340-021-00548-9
52. Amed S, Hamilton JK, Sellers EAC, Panagiotopoulos C, Hadjiyannakis S, Shah BR, et al. Differing clinical features in aboriginal vs. non-aboriginal children presenting with type 2 diabetes. Pediatr Diabetes. (2012) 13(6):470–5. doi: 10.1111/j.1399-5448.2012.00859.x
53. Fritsch MJ, Schober E, Grulich-Henn J, Meissner T, Kapellen T, Dunstheimer D, et al. Prevalence of increased liver enzymes in 15466 children and adolescents with type 1 diabetes mellitus and type 2 diabetes mellitus in the DPV cohort. Diabetologia. (2012) 55(Suppl. 1):S369. doi: 10.1007/s00125-012-2688-9
54. Hudson OD, Nunez M, Shaibi GQ. Ethnicity and elevated liver transaminases among newly diagnosed children with type 2 diabetes. BMC Pediatr. (2012) 12(100967804):174. doi: 10.1186/1471-2431-12-174
55. Kim YJ, Lee HS, Hwang JS. Clinical characteristics and laboratory findings of children and adolescents who were newly diagnosed with diabetes mellitus. Int J Pediatr Endocrinol. (2013) 2013(Suppl 1):16. doi: 10.1186/1687-9856-2013-S1-P16
56. Giuffrida A, Gevers E. Paediatric type 2 diabetes in a single centre in East London in the period 2009–2018. Pediatr Diabetes. (2019) 20(Supplement 28):249. doi: 10.1210/jendso/bvaa046
57. Bacha F, Cheng P, Gal RL, Beaulieu LC, Kollman C, Adolph A, et al. Racial and ethnic disparities in comorbidities in youth with type 2 diabetes in the pediatric diabetes consortium (PDC). Diabetes Care. (2021) 44:dc210143. doi: 10.2337/dc21-0143
58. Beauchamp G, Barr MM, Vergara A, Ashraf A, Bril F. Treatment of hyperglycemia not associated with NAFLD improvement in children with type 2 diabetes mellitus. Int J Pediatr Adolesc Med. (2022) 9(2):83–8. doi: 10.1016/j.ijpam.2021.02.007
59. Puri M, Lizzi-Ansok K, Filozof C, Goldstein B. Labcorp data in >19,000 children with a diagnosis code for T2D show predominantly normal liver function tests: implications for identifying nonalcoholic fatty liver disease or steatohepatitis (NAFLD/NASH). Diabetes. (2020) 69. doi: 10.2337/db20-1260-P
60. Parlett L, Ma Q, Shi Q, Crawford G, Herrera Scott L, Willey V, et al. Burden of non-alcoholic steatohepatitis among children with type 2 diabetes mellitus. Diabetol Metab Syndr. (2021) 13(1):47. doi: 10.1186/s13098-021-00665-0
61. Newfield RS, Graves CL, Newbury RO, Schwimmer JB, Proudfoot JA, Say DS, et al. Non-alcoholic fatty liver disease in pediatric type 2 diabetes: metabolic and histologic characteristics in 38 subjects. Pediatr Diabetes. (2018) 20:41–7. doi: 10.1111/pedi.12798
62. Medina-Bravo P, Hill De Titto AC, Marrodan Garcia HGLI, Molina-Diaz M, Valadez-Reyes MT, Klunder-Klunder M, et al. Type 4 retinol binding protein as a marker of hepatic steatosis in adolescents with type 2 diabetes. Horm Res Paediatr. (2012) 78(Suppl. 2):16. doi: 10.15344/2455-2364/2015/110
63. Li J, Ha A, Rui F, Zou B, Yang H, Xue Q, et al. Meta-analysis: global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000–2021. Aliment Pharmacol Ther. (2022) 56(3):396–406. doi: 10.1111/apt.17096
64. Centers for Disease Control and Prevention. About Child and Teen BMI (2022). Available online at: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html (Accessed June 14, 2023).
65. Giorgio V, Prono F, Graziano F, Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. (2013) 13:40. doi: 10.1186/1471-2431-13-40
66. Younossi Z, Golabi P, Deavila L, Paik J, Srishord M, Fukui N, et al. The global epidemiology on non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Hepatol. (2019) 70(1 Supplement):e349–50. doi: 10.1016/S0618-8278(19)30684-X
67. Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2023) 8(1):20–30. doi: 10.1016/S2468-1253(22)00317-X
68. Scapaticci S, D’Adamo E, Mohn A, Chiarelli F, Giannini C. Non-alcoholic fatty liver disease in obese youth with insulin resistance and type 2 diabetes. Front Endocrinol. (2021) 12(101555782):639548. doi: 10.3389/fendo.2021.639548
69. Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. (2015) 10(10):e0140908. doi: 10.1371/journal.pone.0140908
70. Varlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol. (2015) 5:241. doi: 10.3389/fendo.2014.00241
71. Abuyassin B, Laher I. Diabetes epidemic sweeping the Arab world. World J Diabetes. (2016) 7(8):165–74. doi: 10.4239/wjd.v7.i8.165
72. Al-Rubeaan K. National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population-based study (SAUDI-DM). J Epidemiol Community Health. (2015) 69(11):1045–51. doi: 10.1136/jech-2015-205710
73. Dados N, Connell R. The global south. Contexts. (2012) 11(1):12–3. doi: 10.1177/1536504212436479
74. Braff L, Nelson K. “Chapter 15: The Global North: Introducing the Region”. In: Gendered Lives. US: milnepublishing (2022). Available online at: https://milnepublishing.geneseo.edu/genderedlives/chapter/chapter-15-the-global-north-introducing-the-region/ (Accessed June 23, 2023).
75. Abouzeid M, Muthanna A, Nuwayhid I, El-Jardali F, Connors P, Habib RR, et al. Barriers to sustainable health research leadership in the global south: time for a grand bargain on localization of research leadership? Health Res Policy Syst. (2022) 20(1):136. doi: 10.1186/s12961-022-00910-6
76. Bhatt M, Nahari A, Wang PW, Kearsley E, Falzone N, Chen S, et al. The quality of clinical practice guidelines for management of pediatric type 2 diabetes mellitus: a systematic review using the AGREE II instrument. Syst Rev. (2018) 7(1):193. doi: 10.1186/s13643-018-0843-1
77. Koren D, Levitsky LL. Type 2 diabetes mellitus in childhood and adolescence. Pediatr Rev. (2021) 42(4):167–79. doi: 10.1542/pir.2019-0236
78. Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, et al. ISPAD clinical practice consensus guidelines 2022: type 2 diabetes in children and adolescents. Pediatr Diabetes. (2022) 23(7):872–902. doi: 10.1111/pedi.13409
79. Guidelines. Available online at: https://www.idf.org/e-library/guidelines/89-pocketbook-for-management-of-diabetes-in-childhood-and-adolescence-in-under-resourced-countries-2nd-edition.html (Accessed May 31, 2023).
80. American Diabetes Association Professional Practice Committee. 14. Children and adolescents: standards of medical care in diabetes-2022. Diabetes Care. (2022) 45(Suppl 1):S208–31. doi: 10.2337/dc22-s014
81. Panagiotopoulos C, Hadjiyannakis S, Henderson M. Type 2 Diabetes in Children and Adolescents. DiabetesCanadaWebsite (2018). Available online at: https://www.diabetes.ca/health-care-providers/clinical-practice-guidelines/chapter-35 (Accessed May 31, 2023).
82. SIGN. Management of Diabetes. (2010). Available online at: https://testing36.scot.nhs.uk (Accessed May 31, 2023).
83. Recommendations | Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management | Guidance | NICE. UK: NICE (2015).
84. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67(1):328. doi: 10.1002/hep.29367
85. Shah J, Okubote T, Alkhouri N. Overview of updated practice guidelines for pediatric nonalcoholic fatty liver disease. Gastroenterol Hepatol. (2018) 14(7):407–14. PMC6111502.
86. Eyth E, Naik R. “Hemoglobin A1C”. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2023). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK549816/ (Accessed August 10, 2023).
87. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. (2020) 81(5). doi: 10.4088/JCP.20f13681
88. Page MJ, Higgins JPT, Sterne JAC. Chapter 13: assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023). Available online at: https://www.training.cochrane.org/handbook (Accessed July 29, 2024).
Keywords: type 2 diabetes mellitus, adolescent, pediatric, NAFLD, systematic review, meta-analysis
Citation: Hu C, Cioana M, Saini A, Ragganandan S, Deng J, Nadarajah A, Hou M, Qiu Y, Chen SSJ, Rivas A, Pal Toor P, Banfield L, Thabane L and Samaan MC (2024) The prevalence of non-alcoholic fatty liver disease in pediatric type 2 diabetes: a systematic review and meta-analysis. Front. Adolesc. Med. 2:1303375. doi: 10.3389/fradm.2024.1303375
Received: 27 September 2023; Accepted: 16 July 2024;
Published: 20 August 2024.
Edited by:
Andrea Enzo Scaramuzza, Istituti Ospitalieri di Cremona, ItalyReviewed by:
Ömer Tarim, Bursa Uludağ University, TürkiyeNavoda Atapattu, Lady Ridgeway Hospital for Children, Sri Lanka
Joseph M. Pappachan, Lancashire Teaching Hospitals NHS Foundation Trust, United Kingdom
Copyright: © 2024 Hu, Cioana, Saini, Ragganandan, Deng, Nadarajah, Hou, Qiu, Chen, Rivas, Pal Toor, Banfield, Thabane and Samaan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Constantine Samaan, Y29uc3RhbnRpbmUuc2FtYWFuQHF1ZWVuc3UuY2E=
†These authors have contributed equally to this work
 Catherine Hu
Catherine Hu Milena Cioana1,†
Milena Cioana1,† Stephanie Ragganandan
Stephanie Ragganandan Jiawen Deng
Jiawen Deng Maggie Hou
Maggie Hou Sondra Song Jie Chen
Sondra Song Jie Chen Lehana Thabane
Lehana Thabane M. Constantine Samaan
M. Constantine Samaan