- 1Division of Pediatric Hematology and Oncology, Siteman Kids at St. Louis Children’s Hospital, St. Louis, MO, United States
- 2Department of Pediatric and Adolescent Gynecology, Washington University, St. Louis, MO, United States
- 3Division of Pediatric Hematology and Oncology, Washington University, St. Louis, MO, United States
Background: Despite ASCO and COG recommendations, increasing the rate of sperm-banking for newly diagnosed AYA males with cancer remains difficult. Multiple barriers to fertility preservation at pediatric institutions have been well described. Numerous logistical barriers can impede successful sperm cryopreservation. We analyzed our institution's experience in optimizing workflows to improve sperm-banking rates. Our processes improvement included: (1) striving for a fertility consult for each newly diagnosed male, (2) utilization of a member of the oncology provider team serving as a fertility navigator (FN) providing fertility consultation and counseling services.
Methods: Sperm banking rates at St. Louis Children's Hospital were reviewed and compared for three discrete calendar years spanning the implementation of these enhancements (2017, 2019, 2022). We plotted process changes over time to assess their impact on the rate of successful sperm-banking in eligible AYA males newly diagnosed with cancer.
Results: The rate of consults for sperm banking prior to therapy increased from 18% in 2017 to 91% in 2022 (p = .0003). Rates of sperm banking in eligible males increased from 18% to 82% (p = .0015) between 2017 and 2022.
Conclusion: Embedding a FN who is also a member of the primary oncology team increases not only the fertility consult rate but also the rate of successful sperm-banking in AYA males who are newly diagnosed with cancer at pediatric institutions.
Introduction
Advances in pediatric cancer treatment have increased the survival rate to more than 80% in those diagnosed under 20 years of age (1). An estimated 87,050 adolescents and young adults (AYAs) in the US were diagnosed with cancer in 2022 and the five-year relative survival rate for these patients is 85.5% (1). While treatment advances have increased survival rate, treatment for childhood cancer have been shown to have significant long-term sequelae, with most survivors having at least one chronic health condition before 40 years of age (inclusive of infertility or subfertility) (2). Fertility can be impaired by chemotherapy, radiotherapy, or the malignancy itself (3, 4). Although the impact of infertility after cancer treatment has been established, sperm-banking rates remain low with 18%–50% of AYA patients successfully participating (5–7). Fertility concerns in AYA cancer survivors negatively affect their sense of identity and are linked to symptoms of depression and anxiety. Semen analyses completed on survivors of pediatric and adolescent males with exposure to alkylating agents, but no radiation, identified that greater than 50% of males with an exposure of four grams or more of cyclophosphamide equivalent dose (CED) of alkylating agent had either azoospermia or oligospermia. Additionally, over 10% of males who would be considered low risk with under four grams of CED exposure had impaired spermatogenesis (3). A study by Green et al. (2010) demonstrated that male survivors of childhood cancer were 50% less likely to have a biological child than a healthy sibling. While this data were adjusted for race/ethnicity, education, and marital status between the survivor and sibling cohorts, data on lack of desire for pregnancy were not included (8). Male teenage patients with cancer have ranked having biological children as more valued than making money, owning a home, their faith, or their friends (9). Furthermore, the discussion with partners surrounding fertility and child-bearing potential has caused distress and impaired interpersonal relationships for survivors (10).
The American Society of Clinical Oncology (ASCO) recommends fertility counseling for all patients diagnosed with cancer regardless of age, sex, or other bias, However, there continue to be multiple barriers to implementation including but not limited to provider communication and comfort with counseling, patient and parent factors, institutional factors, and provider knowledge of cost and fertility options (10–13).
Recent literature has reported possible interventions to improve sperm-banking consultation rates in pediatric and AYA cancer patients. Interventions included addressing: (1) patient-level barriers such as decisional pressure; (2) provider-level barriers including limited knowledge and training; and (3) system-level barriers such as perceived lack of time to engage in fertility preservation discussions (12–16). It has been established that providing accurate information on risk, counseling on fertility preservation options, and referral to banking services improved quality of life while also reducing regret and fertility concerns (17–19). However, the majority of survivors of pediatric and AYA cancers still report a desire for more information about the effect of cancer directed therapy on their fertility (20). Available resources such as educational websites regarding AYA fertility are of low quality and written at high reading levels which further limits AYA access to reliable fertility preservation information (21). Investigators examined the barriers to fertility preservation at multiple levels and provided suggestions for improvement including creation of a specialized counseling role (fertility navigator, FN) to mitigate professional and organizational level barriers (22).
This manuscript describes our institution’s experience in implementing process improvements to increase the rate of successful sperm banking.
Methods and materials
Study design
A retrospective chart review was completed as part of a quality improvement project to calculate the percentage of AYA male patients with a new oncologic diagnosis who were subsequently referred for fertility counseling and ultimately opted for sperm cryopreservation. Patients eligible to be included in the chart review were identified from a division list of newly diagnosed patients managed by the division research team. This list is kept for study reporting and research tracking for the Children's Oncology Group (COG). Male patients who were at least 14 years of age at the time of cancer diagnosis from a single institution were reviewed. Charts of these identified patients were then accessed in the electronic medical record (EMR). The record was then searched for fertility consultation notes and subsequently results of the post-thaw semen analysis completed at the time of sperm banking and entered in the EMR. The patients were segregating into three groups based on calendar year that corresponded to different implementation processes. No additional demographic or personal information was recorded or included. Gonadotoxic risk was not evaluated given recommendations that all post-pubertal males be offered sperm-bank prior to initiation of therapy regardless of risk category (19). Eligible charts were identified from a division list of all newly diagnosed patients with cancer who present to the institution. Inclusion criteria were (1) male sex at birth; (2) age 14 or older at the time of diagnosis; (3) patients projected to receive any chemotherapy or radiation therapy as part of cancer-directed treatment; (4) diagnosed with cancer at the institution in years of implementation processes (2017, 2019, or 2022). These timepoints were chosen as 2017 was the last full calendar year prior to establishment of a fertility preservation program when no specialized service were in place, the first full calendar year with a FN (2019), and the first full calendar year with an APN navigator working within the oncology division (2022).
Setting
This study was conducted at an academic pediatric oncology program in the United States. This institution sees between 175 and 200 new pediatric and AYA cancer diagnosis each year, although this study only looked at adolescent males. The time points to evaluate were established for the three discrete calendar years (2017, 2019, 2022) during which this institution established a dedicated fertility preservation program and established processes to improve workflows. Timeframe 1, (2017) no formal fertility preservation program was in existence. Timeframe 2 (2019), formal fertility program established and staffed in non-oncology division. Timeframe 3 (2022), a billable FN from the oncology service was added to the established fertility program.
During Timeframe 1, AYA male patients were referred to the adult reproductive endocrinology division at this institution for fertility counseling and sperm-banking at patient request if they were interested in proceeding with fertility preservation. In 2018, a formal fertility preservation program (FPP) was established with the goal to provide counseling to all patients with a new oncologic diagnosis. The FPP initially consisted of one gynecology/fertility physician expert and one fertility navigator (FN) who was a non-advanced practice trained RN, both working out of the Pediatric and Adolescent Gynecology Division. The year 2019 (Timeframe 2) was chosen for this analysis as it was the first full year the FPP was in place and was available for consult on all patients newly diagnosed with cancer. In 2021, the FN position was restructured to include an advanced practice nurse who was serving as a clinician in the pediatric oncology division. This nurse was licensed in family medicine, allowing them to see patients of all ages. The years of 2020 and 2021 were not utilized for evaluation secondary to mitigating factors from the worldwide Covid pandemic. The year 2022 was chosen for the third time point for analysis as it was the first full year the FPP had a FN housed within the pediatric oncology division.
Fischer’s exact tests were performed to determine if there was a significant association between (1) rates of consultation and sperm-banking before and after the establishment of a formal FPP; (2) rates of referral for fertility preservation consultation and sperm-banking before and after a FN was housed within pediatric oncology.
Results
The total number of eligible patient charts identified for review were 17, 30, and 11 for time points 1, 2, and 3 respectively. Identified charts were screened for referral of fertility consultation and attempted sperm-banking. Rates of sperm-banking increased from 18% to 37% (p = 0.2037) between 2017 and 2019. The rate of sperm-banking in 2022 was 82% (Table 1). This was a statistically significant increase in rates of sperm-banking between 2019 and 2022 (p = 0.0148) (Table 2) as well as between 2017 and 2022 (p = .0015) (Table 2). Referrals of newly diagnosed AYA males for fertility preservation consultation between the years 2017, 2019 and 2022 were 18%, 64%, and 83% respectively. There was a statistically significant increase in referral for consult between 2017 and 2019 (p = .0054), between 2019 and 2022 (p = .0036) (Table 2), as well as between 2017 and 2022 (p = .0003) (Table 2, Chart 1).
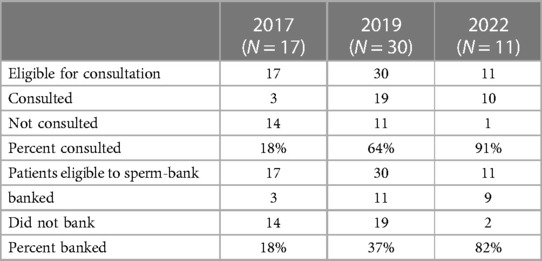
Table 1. Changes in rates of consultation and subsequent sperm-banking between time point 1 (2017), time point 2 (2019), and time point 3 (2022).

Table 2. Significance of change in rates of consultation and subsequent sperm-banking between time point 1 (2017), time point 2 (2019), and time point 3 (2022).
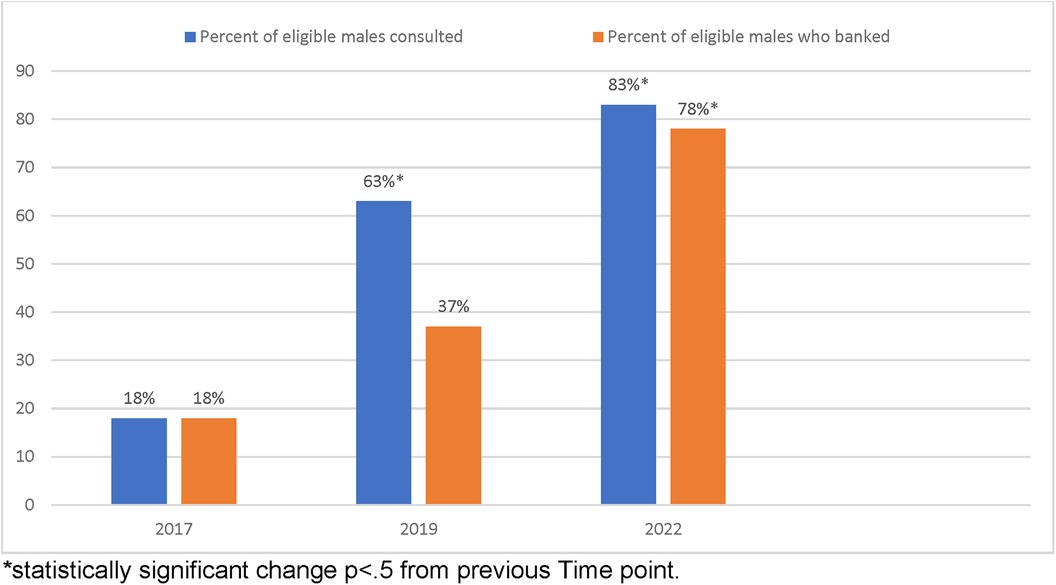
Chart 1. Percentage of males eligible for fertility consult and subsequent sperm-banking by reviewed year. *Statistically significant change p < .5 from previous time point.
Discussion
This paper examined the impact of process improvement enhancements for a fertility preservation program on rates of referral for fertility preservation and sperm-banking in male AYA patients newly diagnosed with cancer. Our results are consistent with prior publications showing that a separate team of fertility experts available for counseling and coordination of care improves rates of consultation and fertility preservation. The inclusion of an advanced practice nurse who was also a working member of the oncology service provided further increases in fertility consultation referrals and sperm-banking attempts. We hypothesize that this is secondary to ease of access for consulting services, patients and the fertility provider.
Rates of sperm-banking at our institution prior to the establishment of the FPP (17%) were comparable to rates of AYA sperm-banking in the literature of 18%–30% (9, 22, 23). Establishment and integration of a dedicated fertility preservation service in pediatric hospitals has been found to increase rates of fertility counseling and fertility preservation at pediatric institutions (24). A formal fertility consultation service addresses known barriers to fertility preservation in the pediatric setting. These barriers include but are not limited to lack of provider knowledge, the oncologist underestimation of gonadotoxic risks, and oncologist's lack of comfort with fertility discussions with AYA patients (12, 13, 25). The impact of the establishment of a formal in-house fertility counseling service is reinforced by the continued increase in males who sperm-bank at diagnosis. A FN is crucial member for fertility teams to have success. The FN becomes a single point of contact for the patient as they move through a complex medical system at diagnosis. Key tasks of the FN include completion of timely consultation, individualized fertility risk assessment, coordination of any fertility preservation procedures, and continued engagement with the patient and multidisciplinary team to ensure decisions are made quickly if needed (26, 27).
The addition of a dedicated fertility team with APN navigator has been shown to increase referrals for fertility counseling both before therapy and in survivorship (28). Inclusion of a fertility navigator in the multidisciplinary approach to children and adolescents diagnosed with has demonstrated an increase in both patient and provider satisfaction as well as improved outcomes by connecting multiple teams with different priorities across the healthcare system (29). When the FN role is occupied by an APN there are multiple streams of revenue that could be beneficial to oncology programs to be considered. Billable services provided by an APN acting as a fertility navigator would include consultation prior to the initiation of therapy for all newly diagnosed patients, consultation for patients in survivorship, management of menstrual suppression, and contraception management for AYA patients undergoing active therapy. Additionally, revenue from tissue cryopreservation in both male and female patients are also sources of revenue in institutions that offer these services to pre- and post-pubertal patients. The addition of an APN FN also increases referrals to fertility consultation services for non-oncology patients including those undergoing stem cell transplant for benign bone marrow disorders such as Sickle Cell Disease (28).
While there were consistent increases in banking with the establishment a dedicated fertility team, these increases were not significant until the inclusion of an APRN who was a working member of the oncology program to serve as FN. This addition addresses system level barriers such as (1) seamless access to the FN by the oncology team once a new patient is identified, (2) comfort in communication with a known colleague who has a well-established working relationship with the oncology team, (3) coordination of care with procedures with a knowledge of the oncology workflows and lines of communication, (4) knowledge of the urgency of initiation of cancer-directed therapy (12). A FN who has knowledge of chemotherapy protocols, diagnosis, planned procedures, and oncology workflow can ensure timely consultation as well as alternatives for fertility preservation timing that may be non-traditional. Additionally, the inclusion of an APRN acting as FN expands the number of billable providers on an FPP team, which can increase program sustainability. Given the substantial impact that these process improvements had on successful sperm banking rates, programs should consider comparable changes to their fertility preservation program to optimize their success. Limitations of this analysis include small sample sizes and some expected increases in consultation and sperm-banking as the FPP continued to grow (even without the changes to the FN position).
Conclusion
The establishment of a dedicated fertility preservation team with a FN who is a working member of the primary oncology team can result in improved rates of fertility preservation consultation, and successful sperm banking rates. Programs should consider investing resources in comparable ways to provide the best opportunity for patients to have biological children once their therapy for cancer has been completed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Washington University institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
TS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. KS: Project administration, Writing – review & editing. RH: Conceptualization, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. HH: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Partial funding for this project was provided by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. SEER. Cancer Among Adolescents and Young Adults (AYAs) - Cancer Stat Facts. Available online at: https://seer.cancer.gov/statfacts/html/aya.html (cited May 8, 2022).
2. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. (2014) 14(1):61–70. doi: 10.1038/nrc3634
3. Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the st jude lifetime cohort study. Lancet Oncol. (2014) 15(11):1215–23. doi: 10.1016/S1470-2045(14)70408-5
4. Jahnukainen K, Ehmcke J, Hou M, Schlatt S. Testicular function and fertility preservation in male cancer patients. Best Pract Res Clin Endocrinol Metab. (2011) 25(2):287–302. doi: 10.1016/j.beem.2010.09.007
5. Crawshaw MA, Sloper P. ’Swimming against the tide’–the influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care (Engl). (2010) 19(5):610–20. doi: 10.1111/j.1365-2354.2009.01118.x
6. Klosky JL, Randolph ME, Navid F, Gamble HL, Spunt SL, Metzger ML, et al. Sperm cryopreservation practices among adolescent cancer patients at risk for infertility. Pediatr Hematol Oncol. (2009) 26(4):252–60. doi: 10.1080/08880010902901294
7. Saraf AJ, Stanek J, Audino A, DaJusta D, Hansen-Moore J, McCracken K, et al. Examining predictors and outcomes of fertility consults among children, adolescents, and young adults with cancer. Pediatr Blood Cancer. (2018) 65(12):e27409. doi: 10.1002/pbc.27409
8. Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of male survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. (2010) 28(2):332–9. doi: 10.1200/JCO.2009.24.9037
9. Klosky JL, Simmons JL, Russell KM, Foster RH, Sabbatini GM, Canavera KE, et al. A qualitative study of the experiences of teenagers and young adults when faced with possible or actual fertility impairment following cancer treatment. Support Care Cancer. (2015) 23(2):333–41.25082365
10. Benedict C, Shuk E, Ford JS. Fertility issues in adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. (2016) 5(1):48–57. doi: 10.1089/jayao.2015.0024
11. Vadaparampil S, Quinn G, King L, Wilson C, Nieder M. Barriers to fertility preservation among pediatric oncologists. Patient Educ Couns. (2008) 72(3):402–10. doi: 10.1016/j.pec.2008.05.013
12. Anazodo A, Laws P, Logan S, Saunders C, Travaglia J, Gerstl B, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. (2019) 25(2):159–79. doi: 10.1093/humupd/dmy038
13. Daly C, Micic S, Facey M, Speller B, Yee S, Kennedy ED, et al. A review of factors affecting patient fertility preservation discussions & decision-making from the perspectives of patients and providers. Eur J Cancer Care (Engl). (2019) 28(1):e12945. doi: 10.1111/ecc.12945
14. Hendershot E, Maloney AM, Fawcett S, Sarvanantham S, McMahon E, Gupta A, et al. Advanced practice nurses: improving access to fertility preservation for oncology patients. Can Oncol Nurs J. (2016) 26(1):40–5. doi: 10.5737/236880762614045
15. Klosky JL, Anderson LE, Russell KM, Huang L, Zhang H, Schover LR, et al. Provider influences on sperm banking outcomes among adolescent males newly diagnosed with cancer. J Adolesc Health. (2017) 60(3):277–83. doi: 10.1016/j.jadohealth.2016.10.020
16. Lewin J, Ma JMZ, Mitchell L, Tam S, Puri N, Stephens D, et al. The positive effect of a dedicated adolescent and young adult fertility program on the rates of documentation of therapy-associated infertility risk and fertility preservation options. Support Care Cancer. (2017) 25(6):1915–22. doi: 10.1007/s00520-017-3597
17. Benedict C, Thom B, Friedman DN, Diotallevi D, Pottenger EM, Raghunathan NJ, et al. Young adult female cancer survivors’ unmet information needs and reproductive concerns contribute to decisional conflict regarding posttreatment fertility preservation. Cancer. (2016) 122(13):2101–9. doi: 10.1002/cncr.29917
18. Letourneau JM, Smith JF, Ebbel EE, Craig A, Katz PP, Cedars MI, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. (2012) 118(18):4579–88. doi: 10.1002/cncr.26649
19. Oktay K, Harvey BE, Loren AW. Fertility preservation in patients with cancer: aSCO clinical practice guideline update summary. J Oncol Pract. (2018) 14(6):381–5. doi: 10.1200/JOP.18.00160
20. Sandheinrich T, Wondmeneh SB, Mohrmann C, Gettinger K, Henry J, Hayashi RJ. Knowledge and perceptions of infertility in female cancer survivors and their parents. Support Care Cancer. (2018) 26(7):2433–9. doi: 10.1007/s00520-018-4080-x
21. Ruiz S, Mintz R, Sijecic A, Eggers M, Hoffman A, Woodard T, et al. Websites about, not for, adolescents? A systematic analysis of online fertility preservation information for adolescent and young adult cancer patients. Res Sq. (2023):rs.3.rs-2587513. doi: 10.1007/s11764-023-01386-1
22. Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. (2002) 20(7):1880–9. doi: 10.1200/JCO.2002.07.175
23. Klosky JL, Lehmann V, Flynn JS, Su Y, Zhang H, Russell KM, et al. Patient factors associated with sperm cryopreservation among at-risk adolescents newly diagnosed with cancer. Cancer. (2018) 124(17):3567–75. doi: 10.1002/cncr.31596
24. Carlson CA, Kolon TF, Mattei P, Hobbie W, Gracia CR, Ogle S, et al. Developing a hospital-wide fertility preservation service for pediatric and young adult patients. J Adolesc Health. (2017) 61(5):571–6. doi: 10.1016/j.jadohealth.2017.07.008
25. Halpern JA, Das A, Faw CA, Brannigan RE. Oncofertility in adult and pediatric populations: options and barriers. Transl Androl Urol. (2020) 9(Suppl 2):S227–38. doi: 10.21037/tau.2019.09.27
26. Moravek MB, Appiah LC, Anazodo A, Burns KC, Gomez-Lobo V, Hoefgen HR, et al. Development of a pediatric fertility preservation program: a report from the pediatric initiative network of the oncofertility consortium. J Adolesc Health. (2019) 64(5):563–73. doi: 10.1016/j.jadohealth.2018.10.297
27. Smith K, Efymow B, Gracia C. Patient navigation and coordination of care for the oncofertility patient: a practical guide. In: Gracia C, Woodruff TK, editors. Oncofertility Medical Practice: Clinical Issues and Implementation. New York, NY: Springer (2012). p. 175–85. doi: 10.1007/978-1-4419-9425-7_13
28. Wright ML, Theroux CI, Olsavsky AL, DaJusta D, McCracken KA, Hansen-Moore J, et al. The impact of hiring a full-time fertility navigator on fertility-related care and fertility preservation at a pediatric institution. Pediatr Blood Cancer. (2022) 69(9):e29857. doi: 10.1002/pbc.29857
Keywords: AYA, oncofertility, sperm-banking, fertility, cancer
Citation: Sandheinrich T, Schultz K, Hayashi RJ and Hoefgen H (2024) Process improvement to increase rates of sperm-banking in AYA patients newly diagnosed with cancer: an institutional experience. Front. Adolesc. Med. 2:1302642. doi: 10.3389/fradm.2024.1302642
Received: 26 September 2023; Accepted: 4 March 2024;
Published: 18 March 2024.
Edited by:
Michael W. Bishop, St. Jude Children’s Research Hospital, United StatesReviewed by:
Asher Marks, Yale University, United StatesJessica Sheth Bhutada, Children’s Hospital of Los Angeles, United States
© 2024 Sandheinrich, Schultz, Hayashi and Hoefgen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taryn Sandheinrich c2FuZGhlaW5yaWNoLnRAd3VzdGwuZWR1
 Taryn Sandheinrich
Taryn Sandheinrich Katie Schultz3
Katie Schultz3 Robert J. Hayashi
Robert J. Hayashi Holly Hoefgen
Holly Hoefgen