- 1School of Science for Open and Environmental Systems, Graduate School of Science and Technology, Keio University, Yokohama, Japan
- 2Department of Precision Engineering, Graduate School of Engineering, The University of Tokyo, Tokyo, Japan
Recent advancements in cell culture have significantly impacted various fields, including drug discovery and regenerative medicine. Consequently, there is an increasing need to minimize the contamination risks and labor involved in cell culture processes. Traditional cell detachment methods typically employ proteolytic enzymes followed by centrifugation to remove these enzymes after cell detachment. This process often requires numerous manual interventions which can lead to potential contamination and deterioration of cell quality. In this study, we propose a novel cell detachment method that eliminates the need for centrifugation even with less trypsinization time. Our approach involves reducing the duration of trypsinization, collecting the trypsin before complete cell detachment, and subsequently detaching the cells using forced vibration within the culture medium. We conducted experiments to optimize the enzyme treatment time and vibration conditions. Our results demonstrated that this method achieved an 82.8% detachment rate of cells from the culture surface. These findings indicate that the proposed cell detachment technique is effective in removing cells from the culture substrate and the following subculture process without the need for centrifugation.
1 Introduction
Cell culture, a technique for maintaining or growing cells in an artificial environment, is a fundamental technology with broad applications across various fields, including regenerative medicine (Mani, 2023). Regenerative medicine is domain of research and clinical practice focused on replacing or regenerating human cells, tissues, or organs to restore or establish normal function (Mason and Dunnill, 2008). A prominent application within regenerative medicine is autologous cell therapy, which utilizes the patient’s own cells for treatment, thereby minimizing the risk of immune rejection and eliminating the need for external donors. In autologous cell therapy, cells are harvested from the patient, cultured, and subsequently utilized for therapeutic purposes (Kazmi et al., 2009; Li et al., 2021). Consequently, reducing the contamination risks and costs associated with cell culture can directly contribute to the quality of treatment and lowering the overall costs. Beyond regenerative medicine, cell culture plays a critical role in other domains such as drug discovery (Blay et al., 2020) and vaccine production (Rajaram et al., 2020). Therefore, minimizing risks and costs of cell culture is crucial for advancing these fields and enhancing their economic feasibility, leading to their dissemination.
Cells that are removed from organisms are initially isolated and cultured in primary culture, followed by proliferation through subculture (Freshney, 2015; Pastan and Jakoby, 1979). A common method for subculture involves the use of proteases, such as trypsin. In this process, cells adhering to the culture surface are detached by the enzyme, after which a medium is added to inhibit the enzyme’s activity. Subsequently, cells and enzyme are separated by centrifugation, and the enzyme is removed (Vanderbilt University, n.d.) (Thermo Fisher Scientific, n.d.). Although this traditional method has been extensively used for an extended period, it presents challenges, including the substantial operational burden due to the numerous and complex steps involved. More the steps, more the contamination risks (Imashiro et al., 2020). To address these issues, automated culture systems have been developed to enhance efficiency and to reduce the possible contamination due to manual procedure; however, these systems also face challenges related to reliability, cost, and spatial requirements (Doulgkeroglou et al., 2020). In addition to operational difficulties, the traditional method poses risks of cellular damage due to the enzyme. Alternative enzyme-free buffer detachment methods have been proposed; however, these methods encounter issues regarding safety and versatility (Heng et al., 2009). Cell scraping with a cell scraper offers a means to detach cells without the use of enzymes, but this method can lead to significant cell damage (Batista et al., 2010).Ultrasonic cell detachment offers a promising enzyme-free alternative; however, it still requires subsequent centrifugation during the subculture process, meaning that the necessary steps for subculture are maintained (Kurashina et al., 2017; Kurashina et al., 2019).
In this study, we propose a novel protocol for cell detachment in culture medium using controllable forced vibration. Since forced vibration alone is insufficient to detach cells, a brief exposure to trypsin is employed to weaken cell adhesion prior to the application of vibration. This approach contributes not only to automation but also to the simplification of the subculture process by eliminating the need for centrifugation, as cell detachment occurs directly within the culture medium. Additionally, the duration of trypsin treatment is reduced compared to conventional methods, potentially minimizing cellular damage. Furthermore, because cell detachment is achieved through forced vibration, the process can be easily controlled mechanically, which is anticipated to enhance efficiency by enabling simultaneous processing of multiple vessels through stacking and automation.
2 Materials and methods
2.1 Concept of the proposed method
Figure 1 illustrates our proposed and the conventional cell detachment method involved in the subculture process (Thermo Fisher Scientific, 2024; Vanderbilt University, 2024).
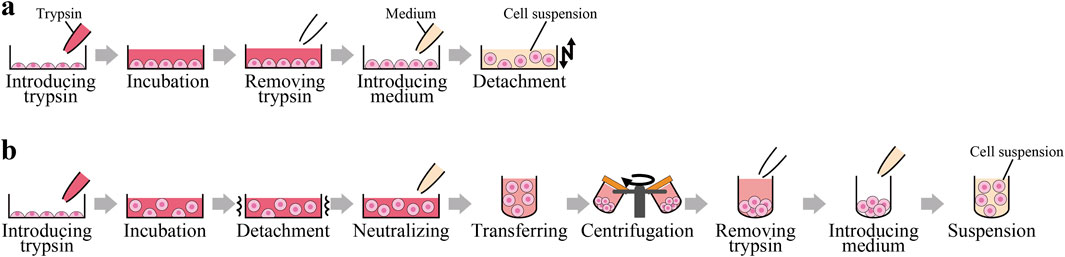
Figure 1. Processes of our proposed and the conventional cell detachment method. (A) Processes of our proposed cell detachment method. (B) Processes of the conventional cell detachment method.
In the proposed method, as illustrated in Figure 1A, trypsin is initially added to the cell culture vessel. The cells are then incubated briefly to partially degrade the proteins that mediate cell adhesion. Prior to complete cell detachment, trypsin is removed and fresh medium is introduced. This process results in the cells being suspended in the culture medium with reduced adhesion. Subsequently, the cells are detached through the application of a physical stimulus, specifically forced vibration of the culture vessel.
In the conventional method, as shown in Figure 1B, trypsin is added to the cell culture vessel. The cells are then incubated for several minutes to facilitate the degradation of proteins that mediate cell adhesion to the culture substrate. Complete detachment of the cells is achieved through tapping the culture vessel or pipetting. Following detachment, the enzymatic activity of trypsin is halted by the addition of culture medium. To remove trypsin, the cell suspension is transferred to a centrifuge tube and subjected to centrifugation. The supernatant is then replaced with fresh medium, and the resulting cell suspension is prepared for reseeding into a new culture vessel.
2.2 Experimental setup and condition of forced vibration
In this study, a 60-mm dish was utilized as a culture vessel, and cell detachment was achieved through forced vibration generated by a shaker (BT300 Vibration Speaker 36W, Adin) (Figure 2). Vibrations of 500 Hz, 1000 Hz, and 2000 Hz were employed during the experiments. Amplitudes at the center of the dish were measured using a laser Doppler vibrometer (Laser Vibrometer LV-1800, Ono Sokki). Given that the maximum amplitude achievable by the shaker at 2000 Hz was 2.08 µm, the vibration conditions for different frequencies were adjusted as outlined in Table 1 to ensure that the maximum acceleration at the center of the dish was approximately equal across all frequencies.
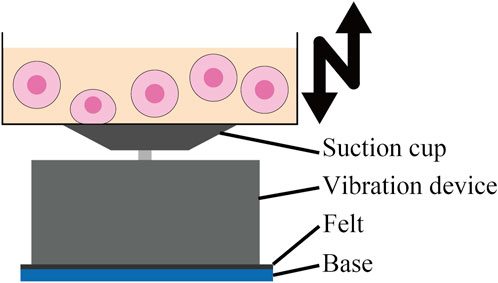
Figure 2. The system of applying forced vibration to 60-mm dish. Felt between the vibration device and the base absorbs vibration. The 60-mm dish is fixed to the vibration device by suction cups. Forced vibration is applied in the vertical direction.
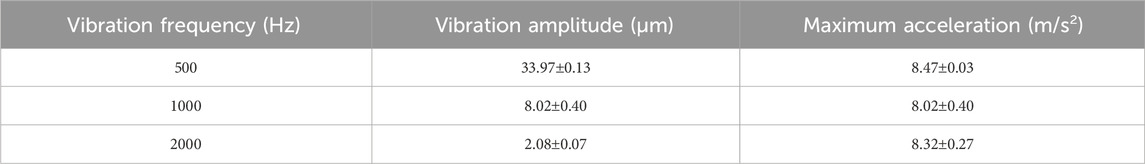
Table 1. Vibration amplitude and maximum acceleration at center of a 60-mm dish with different frequencies. Amplitudes for 500 Hz and 1000 Hz were adjusted to achieve maximum accelerations approximately equal to that at 2000 Hz.
2.3 Cell preparation
We utilized the Chinese hamster ovary-K1 (CHO-K1) cell line in this study. The CHO-K1 (RCB0285; Riken Bio Resource Center) were cultured in Ham’s F-12 Nutrient Mix (11,765,054; Gibco, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS) (2917354H; MP Biomedicals) and 2% Antibiotic-Antimycotic (100) (15,240,062; Gibco, Thermo Fisher Scientific) at
2.4 Trypsinization time
To determine the optimal trypsinization time required to weaken cell adhesion in the proposed method, we conducted experiments to identify the time at which cells begin to detach. Initially, the prepared cells were washed twice with 1 mL of phosphate-buffered saline (PBS). Subsequently, 1 mL of 0.05% trypsin-EDTA was added to the dish, and the cells were incubated at
This ratio was used to evaluate the cell detachment capability at each trypsinization time.
Figure 3 illustrates the values of R1 at various trypsinization times. Statistical analysis (cf. 2.7) revealed a significant difference only at a trypsinization time of 2 min. This result suggests that a substantial number of cells began to detach after 2 min of trypsinization. Given that trypsinization primarily affects cell adhesion and that trypsin-EDTA must be thoroughly removed before cell detachment in the proposed method, we selected a trypsinization time of 1.5 min. This time did not exhibit significant differences in detachment ratio against shorter trypsinization time, indicating its suitability for optimal cell detachment.
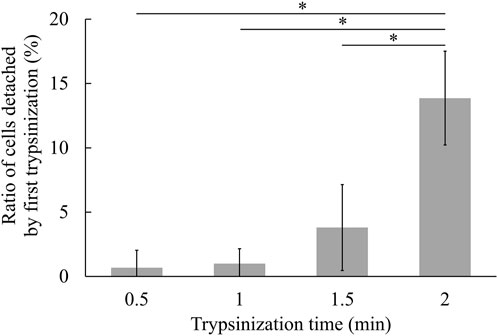
Figure 3. Cell detachment ratio at each trypsinization time (mean ± SD, n = 3, * p < 0.05). Cultured cells were trypsinized for the respective time, and the number of detached cells and remained cells were counted respectively.
2.5 Cell detachment with the proposed method
To evaluate the effectiveness of the proposed method in the centrifugation-free subculture procedure, CHO-K1 cells were detached using the proposed technique. Initially, cells prepared in a 60-mm dish were washed twice with 1 mL of phosphate-buffered saline (PBS). Subsequently, 1 mL of trypsin-EDTA was added, and the cells were incubated at
The cells were enumerated at each stage, and the following parameters were defined: the number of cells detached by trypsinization before the forced vibration (Nt), the number of cells detached by forced vibration (Nv), and the number of cells remaining on the dish surface after the forced vibration (Nr). The ratios of these quantities to the total number of cells (Nt + Nv + Nr) were calculated as Equations 1–4,
where, Rt, Rv, and Rr represent the proportions of cells detached by trypsinization, by forced vibration, and those remaining on the dish surface, respectively.
2.6 Cell proliferation in the following subculture process
To assess the viability of cells detached using the proposed method, we performed a subculture procedure. Cells were detached under the following conditions: a trypsinization duration of 1.5 min, a forced vibration frequency of 500 Hz, and a vibration duration of 5 min. Following detachment,
2.7 Statistical analysis
Values are expressed as means
3 Results and discussion
3.1 Cells detached by the proposed method
Figure 4 illustrates the proportions of cells detached via pre-trypsinization (Rt), by forced vibration (Rv), and those remaining adherent to the dish surface (Rr), at frequencies of 0 Hz, 500 Hz, 1000 Hz, and 2000 Hz, respectively. At 0 Hz, indicating no applied mechanical stimulation, approximately 30% of cells were detached. This detachment is attributed to minor medium flows associated with procedural operations, such as the addition or removal of liquids, or the handling of the dish when placing it in or removing it from the incubator. At frequencies of 500 Hz and 1000 Hz, a higher proportion of cells were detached compared to 0 Hz, suggesting that forced vibration contributed to cell detachment. Conversely, at 2000 Hz, the detachment levels were comparable to those observed at 0 Hz, indicating that the forced vibration at this frequency did not significantly influence cell detachment. Comparing the results at 500 Hz and 1000 Hz, where cell detachment was observed, it is evident that more cells were detached at 500 Hz. These findings imply that the maximum acceleration of forced vibration is not the primary factor influencing cell detachment; rather, the frequency, amplitude of the vibration, and the resultant medium flow are crucial factors.It has been shown that cell detachment is predominantly influenced by the shear flow of the surrounding medium (Kurashina et al., 2019). Additionally, Goldasteh et al. investigated particle removal from a substrate under fluid flow, demonstrating that the drag force magnitude depends on the shear velocity, which is, in turn, proportional to frequency and amplitude (Goldasteh et al., 2013). Based on this understanding, the shear velocity is likely maximized at 500 Hz, decreasing to half and one-quarter of this value at 1000 Hz and 2000 Hz, respectively. From this perspective, the cell detachment results presented in Figure 4 appear to be reasonable.
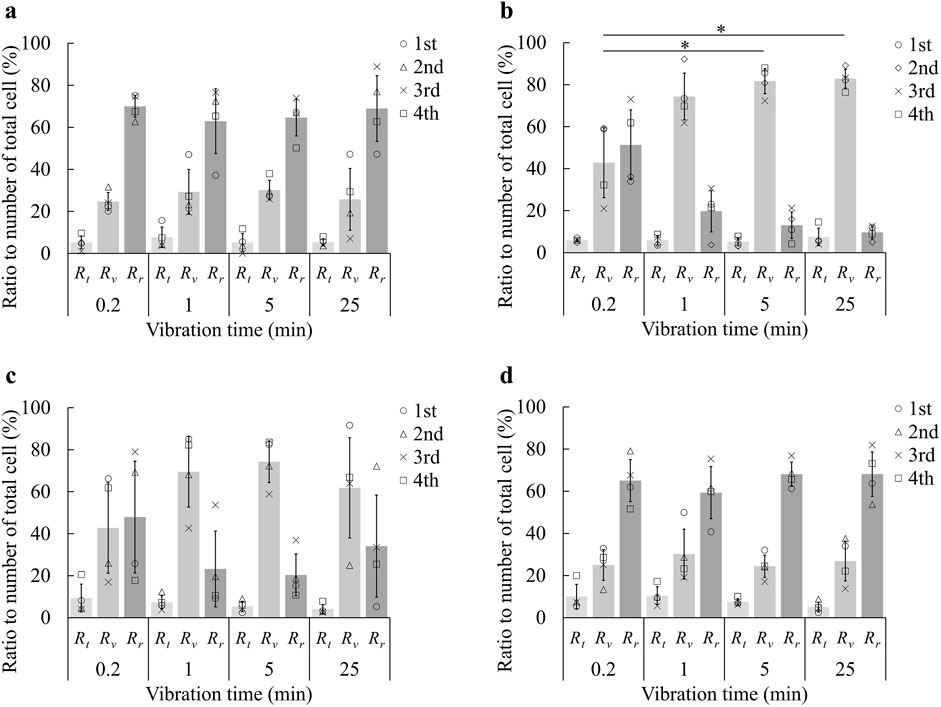
Figure 4. Ratio of number of cells detached by each step in cell detachment using forced vibration at forced vibration frequency of (A) 0 Hz, (B) 500 Hz, (C) 1000 Hz, and (D) 2000 Hz (mean±SD, n = 4, * p < 0.05). The first trypsin treatment to reduce cell adhesion was performed for 1.5 min. The plots represent the results from four trials. Rt, Rr, and Rv are the proportions of cells detached via pre-trypsinization, those by forced vibration, and those remaining adherent to the dish surface, respectively. Since Rt is common among (A–D), and Rr is related to Rv, statistical evaluation was only conducted about Rv.
Focusing on the effect of vibration time, it was observed at 500 Hz that the proportion of cells detached by forced vibration (Rv) increased with longer vibration times. However, the rate of increase in Rv diminished as vibration time continued to increase. At 1000 Hz, Rv also increased with vibration time from 0.2 min to 5 min, similar to the trend observed at 500 Hz. However, Rv decreased at 25 min. This decrease can be attributed to an anomalously low experimental value at 25 min, which caused a reduction in the mean value and an increase in the standard deviation. When considering this outlier, the mean Rv at 25 min is 74.1%, with a standard deviation of 12.4%. The trend at 1000 Hz shows that the increase in Rv with longer vibration times becomes less pronounced over time. These findings suggest that while increasing vibration time generally leads to greater cell detachment, there is an upper limit to the detachment ratio that depends on the vibration frequency.
The optimal results were achieved with a pre-trypsinization time of 1.5 min, a vibration frequency of 500 Hz, and a vibration duration of 5 min. Under these conditions, cells were exposed to 0.05% trypsin-EDTA for only 1.5 minutes—half the duration typically required for conventional trypsinization—while the cell detachment ratio was effectively controlled by the forced vibration. This approach may offer an additional advantage by potentially eliminating the need for centrifugation in the subculture process. Consequently, it could mitigate the adverse effects of trypsin on cell viability and function (Danika et al., 2006).
3.2 Cell proliferation assay
Figure 5 presents the cell counts following incubation in the subsequent subculture for cells detached using both methods. A reader may doubt the negative effect of forced vibration on cell viability; however, no statistically significant difference in cell numbers was observed between cells detached by the proposed method and those detached by the conventional method. These results suggest that the proposed method does not have a detrimental effect on cell proliferation, suggesting that the proposed method is compatible for cell passage.

Figure 5. The number of cells detached by both methods after 24, 48, and 72 h of culture (mean±SD, n = 6, * p < 0.05).
4 Conclusion
In this study, we propose a novel cell detachment method utilizing forced vibration to detach cells from the culture medium, potentially eliminating the need for centrifugation in the subsequent subculture process. This may reduce the risk of potential contamination, as well as the associated economic and labor costs, and the necessary space required for the system. We conducted experiments to investigate the relationship between trypsinization time and cell detachment ratio. Based on these results, we identified the optimal pre-trypsinization time that weakens cell adhesion without fully detaching the cells. Subsequently, we performed cell detachment experiments using the proposed method at various vibration frequencies and duration, preceded by pre-trypsinization and replacement with fresh medium.
Our results demonstrated that, for CHO-K1 cells, significant detachment occurred when the trypsinization time exceeded 2 min with 0.05% trypsin-EDTA. Applying the proposed method with a pre-trypsinization time of 1.5 min, we observed effective cell detachment at vibration frequencies of 500 Hz and 1000 Hz. The detachment ratio increased with longer vibration times, though it approached a maximum limit. The optimization of trypsinization and vibration conditions across different cell types and culture conditions requires comprehensive parametric studies for practical applications. Nevertheless, this study primarily demonstrates the feasibility of in-medium cell detachment and provides an initial framework for determining optimal trypsinization and vibration conditions.
While further biological assays are necessary for a comprehensive evaluation, our findings suggest that the proposed method is effective for cell detachment within the medium, eliminating the need for centrifugation. This method simplifies culture operations by removing the need for centrifugation equipment and manual cell transfer. Additionally, it avoids the necessity of manual tapping or pipetting, with cell detachment achieved through easily controllable forced vibration, thereby contributing to the automation of the culture process.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
HS: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing–original draft, Writing–review and editing. CI: Conceptualization, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. KT: Conceptualization, Funding acquisition, Methodology, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by JSPS KAKENHI Grant Numbers 22H01390 and 23K22661.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors used ChatGPT 4o to edit this manuscript to improve grammar and expression.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Batista, U., Garvas, M., Nemec, M., Schara, M., Veranič, P., and Koklic, T. (2010). Effects of different detachment procedures on viability, nitroxide reduction kinetics and plasma membrane heterogeneity of v-79 cells. Cell Biol. Int. 34, 663–668. doi:10.1042/cbi20090276
Blay, V., Tolani, B., Ho, S. P., and Arkin, M. R. (2020). High-throughput screening: today’s biochemical and cell-based approaches. Drug Discov. Today 25, 1807–1821. doi:10.1016/j.drudis.2020.07.024
Danika, M., Hayman, C. C. S. K. A. A., Blumberg, T. J., and Athanasiou, K. A. (2006). The effects of isolation on chondrocyte gene expression. Tissue Eng. 12, 2573–2581. doi:10.1089/ten.2006.12.2573
Doulgkeroglou, M.-N., Di Nubila, A., Niessing, B., König, N., Schmitt, R. H., Damen, J., et al. (2020). Automation, monitoring, and standardization of cell product manufacturing. Front. Bioeng. Biotechnol. 8, 811. doi:10.3389/fbioe.2020.00811
Freshney, R. I. (2015). Culture of animal cells: a manual of basic technique and specialized applications. John Wiley & Sons.
Goldasteh, I., Ahmadi, G., and Ferro, A. R. (2013). Monte Carlo simulation of micron size spherical particle removal and resuspension from substrate under fluid flows. J. Aerosol Sci. 66, 62–71. doi:10.1016/j.jaerosci.2013.07.012
Heng, B. C., Cowan, C. M., and Basu, S. (2009). Comparison of enzymatic and non-enzymatic means of dissociating adherent monolayers of mesenchymal stem cells. Biol. Proced. online 11, 161. doi:10.1007/s12575-009-9001-4
Imashiro, C., Tokuoka, Y., Kikuhara, K., Yamada, T. G., Takemura, K., and Funahashi, A. (2020). Direct cell counting using macro-scale smartphone images of cell aggregates. IEEE Access 8, 170033–170043. doi:10.1109/access.2020.3024100
Kazmi, B., Inglefield, C., and Lewis, M. (2009). Autologous cell therapy: current treatments and future prospects. Wounds 21, 234–242.
Kurashina, Y., Hirano, M., Imashiro, C., Totani, K., Komotori, J., and Takemura, K. (2017). Enzyme-free cell detachment mediated by resonance vibration with temperature modulation. Biotechnol. Bioeng. 114, 2279–2288. doi:10.1002/bit.26361
Kurashina, Y., Imashiro, C., Hirano, M., Kueibara, T., Totani, K., Ohmura Kiyoshiand Friend, J., et al. (2019). Enzyme-free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves. Commun. Biol. 2, 393. doi:10.1038/s42003-019-0638-5
Li, C., Zhao, H., Cheng, L., and Wang, B. (2021). Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell & Biosci. 11, 187. doi:10.1186/s13578-021-00698-y
Mason, C., and Dunnill, P. (2008). A brief definition of regenerative medicine. Regenerative Medicine, 1–5. doi:10.2217/17460751.3.1.1
Rajaram, S., Boikos, C., Gelone, D. K., and Gandhi, A. (2020). Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther. Adv. vaccines Immunother. 8, 2515135520908121. doi:10.1177/2515135520908121
Keywords: cell detachment, forced vibration, centrifugal-free, automation, cell suspension
Citation: Shimoguchi H, Imashiro C and Takemura K (2024) Detaching cells in culture medium using forced vibration for removing a centrifugation from culture process. Front. Acoust. 2:1502136. doi: 10.3389/facou.2024.1502136
Received: 26 September 2024; Accepted: 14 November 2024;
Published: 29 November 2024.
Edited by:
James Friend, University of California, San Diego, United StatesReviewed by:
Tuncay Alan, Monash University, AustraliaYuyang Gu, Binghamton University, United States
Aditya Vasan, University of California, San Diego, United States
Copyright © 2024 Shimoguchi, Imashiro and Takemura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenjiro Takemura, dGFrZW11cmFAbWVjaC5rZWlvLmFjLmpw
 Hayato Shimoguchi
Hayato Shimoguchi Chikahiro Imashiro
Chikahiro Imashiro Kenjiro Takemura
Kenjiro Takemura