
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 24 March 2022
Sec. Lipids in Cardiovascular Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.862999
This article is part of the Research Topic Lipids, Lipoproteins and COVID-19 View all 9 articles
 Vignesh Chidambaram1,2*
Vignesh Chidambaram1,2* Harinivaas Shanmugavel Geetha3
Harinivaas Shanmugavel Geetha3 Amudha Kumar2
Amudha Kumar2 Marie Gilbert Majella4
Marie Gilbert Majella4 Ranjith Kumar Sivakumar5
Ranjith Kumar Sivakumar5 Dinesh Voruganti6
Dinesh Voruganti6 Jawahar L. Mehta6,7†
Jawahar L. Mehta6,7† Petros C. Karakousis1,8*†
Petros C. Karakousis1,8*†
Background: Coronavirus disease 2019 (COVID-19) ranges from asymptomatic infection to severe illness. Cholesterol in the host cell plasma membrane plays an important role in the SARS-CoV-2 virus entry into cells. Serum lipids, especially low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), are in constant interaction with the lipid rafts in the host cell membranes and can modify the interaction of virus with host cells and the resultant disease severity. Recent studies on serum lipid levels and COVID-19 disease severity lack consistency.
Objectives: Our systematic review and meta-analysis compared the serum levels of total cholesterol (TC), LDL-C, HDL-C, and triglycerides (TG) between (1) COVID-19 patients vs. healthy controls; (2) severe vs. non-severe COVID-19 disease; (3) deceased vs. surviving COVID-19 patients.
Methods: PRISMA guidelines were followed. We included peer-reviewed articles on observational (case-control and cohort) studies from PubMed and Embase published from the database inception until September 1, 2021. We used random-effects meta-analysis for pooled mean-differences (pMD) in lipid levels (mg/dL) for the above groups.
Results: Among 441 articles identified, 29 articles (26 retrospective and 3 prospective cohorts), with an aggregate of 256,721 participants, were included. COVID-19 patients had lower TC (pMD-14.9, 95%CI-21.6 to −8.3) and HDL-C (pMD-6.9, 95%CI −10.2 to −3.7) levels (mg/dL). Severe COVID-19 patients had lower TC (pMD-10.4, 95%CI −18.7 to −2.2), LDL-C (pMD-4.4, 95%CI −8.4 to −0.42), and HDL-C (pMD-4.4, 95%CI −6.9 to −1.8) at admission compared to patients with non-severe disease. Deceased patients had lower TC (pMD-14.9, 95%CI −21.6 to −8.3), LDL-C (pMD-10.6, 95%CI −16.5 to −4.6) and HDL-C (pMD-2.5, 95%CI −3.9 to −1.0) at admission. TG levels did not differ based on COVID-19 severity or mortality. No publication bias was noted.
Conclusion: We demonstrated lower lipid levels in patients with COVID-19 infection and an association with disease severity and mortality. Their potential role in COVID-19 pathogenesis and their utility as prognostic factors require further investigation.
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, is currently the leading cause of death due to a single infectious agent (1). While the majority of patients infected with COVID-19 are in the asymptomatic or mild category, a considerable proportion develop severe illness, presenting with acute respiratory distress syndrome, renal or hepatic dysfunction, requiring respiratory and multiorgan support in critical care settings (2, 3). Cholesterol is an important component of the membranes of host cells and enveloped viruses and has been shown to play an important role in the entry of SARS-CoV-2 virus into cells (4). Serum lipids, especially low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). are in constant interaction with the lipid rafts in the host cell membranes (5, 6) and can modify the interaction of virus with host cells and the resultant disease severity.
Several studies have reported an association between COVID-19 severity and low serum levels of HDL-C (7, 8), but the magnitudes of the association between COVID-19 and total cholesterol (TC), LDL-C, and triglycerides (TG) are inconsistent. This finding is in agreement with similar observations in patients with other infections, such as HIV, dengue, and tuberculosis (9, 10). The precise mechanism underlying the relationship between COVID-19 infection and low serum lipid levels is unclear, but the low serum lipid levels have been hypothesized to reflect an acute phase response during inflammation (11). Increased secretory phospholipase and serum amyloid A (SAA), and decreased reverse cholesterol transport have been attributed to low HDL-C levels following inflammation (12–14).
Multiple cardiovascular and metabolic risk factors have been shown to be associated with the risk of severe COVID-19 disease (2). We hypothesized that serum lipid levels may serve as biomarkers predicting disease severity and mortality and provide insight into the pathogenesis of COVID-19. We performed a systematic review of the literature and meta-analysis to determine differences in serum lipid levels, such as TC, LDL-C, HDL-C, and TG, between patients with COVID-19 and healthy controls, and to assess the association of these lipid levels at admission with severity and mortality among patients with COVID-19.
The systematic review was performed following the PRISMA guidelines (15). A literature search was performed in the PubMed and Embase databases on April 20, 2021, and the last literature update was performed on September 1, 2021. The complete search strategy is described in the supplementary document (Appendix Section I). Only articles in the English language were included in this review. Observational studies, such as prospective and retrospective cohort studies, and case-control studies, were included. Authors VC and MM reviewed the study designs of the selected studies. We excluded cross-sectional studies, case reports, and case series from this review. No clinical trial data on COVID-19 were relevant to our research question.
We included studies that reported serum levels of lipids, such as TC, LDL-C, HDL-C, and TG, and at least one of the following: (1) differences in the lipid levels of patients with and without COVID-19; (2) direct comparison of lipid levels at admission among COVID-19 patients with and without severe disease (16, 17); or (3) direct comparison of lipid levels at admission among patients with COVID-19 who died and those who survived.
We used the COVIDENCE platform for the systematic review (18). After removing the duplicates, title and abstract screening was done by VC and AK and conflicts were resolved by MM. Full texts of the selected articles were independently screened by two authors (VC, AK) and conflicts were settled by MM. Two of the authors (VC, HN, RK) were independently involved in data extraction and quality assessment and conflicts were resolved by MM. Qualtrics platform was utilized for data extraction (19).
Data were collected on the study characteristics, source of funding, type of lipid assessed (TC, LDL-C, HDL-C, and TG), year of publication, number of centers in each study, and study design. The primary outcomes were the pooled mean differences in lipid levels in the following groups: (a) subjects with and without COVID-19 infection; (b) patients with COVID-19 with and without severe disease; and (c) patients with COVID-19 who died and those who survived. Continuous data for the lipid levels were recorded as means. The median values for the lipid levels available from the studies were transformed into means (20). No secondary outcomes were considered for our systematic review.
Risk assessment for bias was performed independently by two of the authors (VC, MM or RK) using the Newcastle-Ottawa quality assessment scale (NOS) for observational studies (21). Conflicts related to the bias assessment were settled by AK.
We obtained pooled mean differences for the groups of interest using random-effects meta-analysis. We analyzed separately the mean levels of TC, LDL-C, HDL-C, and TG for each of the above comparisons. Statistical heterogeneity across the studies was evaluated by forest plots, I2 and Tau2 statistics. We assessed publication bias using funnel plot and Egger’s test. Meta regression analyses for the mean difference of lipid levels across comparison groups were performed against the mean difference in age or difference in proportions of the confounding parameters. Such as sex, diabetes, hypertension, and coronary artery disease across the groups. All analyses were carried out using the meta package in Stata (StataCorp, version 16) (22).
Among the 441 studies obtained from the PubMed and EMBASE databases. After removal of duplicates, we retrieved 354 articles. We assessed full texts of 117 studies and 29 studies met the inclusion criteria. The reasons for exclusion of studies are outlined in Figure 1. Among them, 7 studies compared lipid levels among patients with and without COVID-19, 18 studies reported lipid levels of patients with and without severe disease, and 9 studies reported lipid levels of patients who died compared to those who survived. Of the 29 studies included in the review, 26 were retrospective and 3 were prospective cohorts. In this systematic review, 23 reported TC levels, 26 reported LDL-C levels, 25 studies reported HDL-C levels, and 20 studies reported TG levels. Four articles were not included in the quantitative analysis, as they did not report quantitative differences in the lipid levels for the study outcomes of interest.
Of the studies that assessed lipid levels among patients with or without COVID-19, a total of 2,56,721 participants were available. There were 2,356 patients in studies comparing lipid levels in patients with vs. those without severe COVID-19 disease and 2,127 patients in studies comparing lipid levels in patients who died vs. those who survived. There were 17 studies from China, 3 from France, 2 from Spain, 2 from United Kingdom, and 1 each from United States, Mexico, Italy, Iran, and Turkey. The characteristics of the included studies are listed in Supplementary Table 1 (Appendix Section II). Quality assessment performed using the New-Castle Ottawa scale (NOS) revealed that one study (3.4%) scored 9, 17 studies (58.6%) scored 8, six studies (20.6%) scored 7, and five studies (17.2%) scored 6 or less (Supplementary Table 2—Appendix section II).
Figure 2 and Table 1 depict the pooled mean difference (pMD) of lipid parameters between different comparison groups in the study.
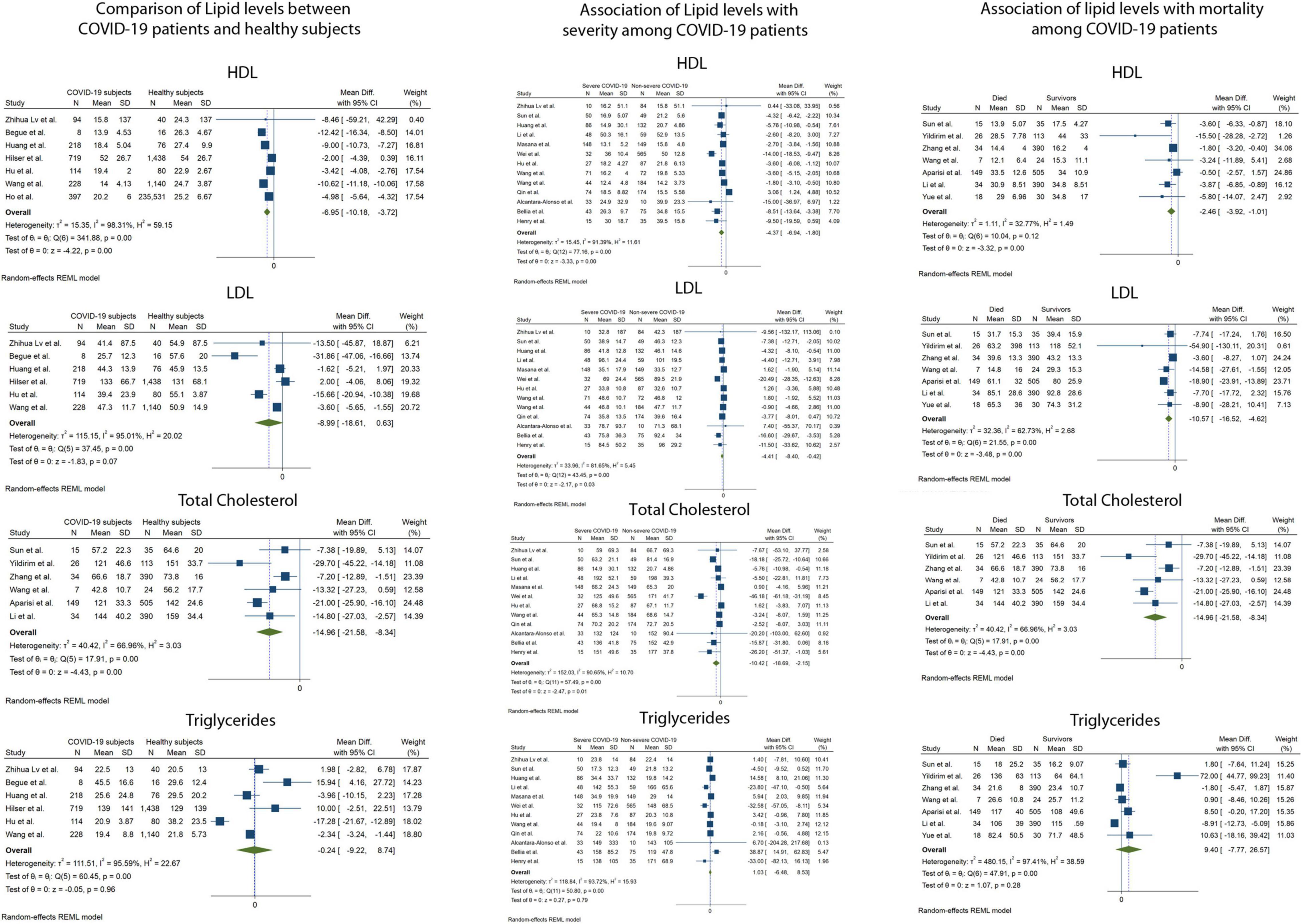
Figure 2. Forest plots showing the random effects meta-analysis for the association of lipid levels with COVID-19 infection, disease severity and mortality. LDL-C, Low density Lipoprotein, HDL-C, High Density Lipoprotein.
Serum TC was reported in 23 of 29 included studies. The pMD in TC levels between COVID-19 patients and healthy controls was −14.9 mg/dL (95% Confidence Interval (CI), −21.6 to −8.3) based on 6 studies (I2 = 66.9). Patients with severe COVID-19 disease had lower mean TC levels at admission compared to patients with non-severe disease [pMD −10.4 mg/dL (95%CI −18.7 to −2.2)] based on 12 studies (I2 = 90.7%). Mean TC levels at admission in patients who died were lower than those of patients who survived [pMD −14.9 mg/dL (95%CI −21.6 to −8.3)] based on 6 studies (I2 = 66.9%).
Serum LDL-C was reported in 26 of 29 included studies. The pMD in LDL-C levels between COVID-19 patients and healthy controls was −8.9 mg/dL (95%CI, −18.6 to 0.6) based on 6 studies (I2 = 95.0%). Patients with severe COVID-19 disease had lower mean LDL-C levels at admission compared to patients with non-severe disease [pMD −4.4 mg/dL (95%CI −8.4 to −0.42)] based on 13 studies (I2 = 81.7%). Mean LDL-C levels at admission in patients who died were lower than those of patients who survived [pMD −10.6 mg/dL (95%CI −16.5 to −4.6)] based on 7 studies (I2 = 62.7%).
Serum HDL-C was reported in 25 of 29 included studies. The pMD in HDL-C levels between COVID-19 patients and healthy controls was −6.9 mg/dL (95%CI, −10.2 to −3.7) based on 7 studies (I2 = 98.3%). Patients with severe COVID-19 disease had lower mean HDL-C levels at admission compared to patients with non-severe disease [pMD −4.4 mg/dL (95%CI −6.9 to −1.8)] based on 13 studies (I2 = 91.4%). Mean HDL-C levels at admission in patients who died were lower than those of patients who survived [pMD −2.5 mg/dL (95%CI −3.9 to −1.0)] based on 7 studies (I2 = 32.8%).
Serum TG was reported in 20 of 29 included studies. The pMD in TG levels between COVID-19 patients and healthy controls was −0.2 mg/dL (95%CI, −9.2 to 8.4) based on 6 studies (I2 = 95.6%). Patients with severe COVID-19 disease had similar mean TG levels at admission compared to patients with non-severe disease [pMD −1.0 mg/dL (95%CI –6.5 to 8.5)] based on 12 studies (I2 = 93.7%). Mean TG levels at admission in patients who died were similar to those of patients who survived [pMD 9.4 mg/dL (95%CI −7.7 to 26.6)] based on 7 studies (I2 = 97.4%). None of the above analyses were found to have significant publication bias using Egger’s test and visual inspection of the funnel plots (Figure 3).
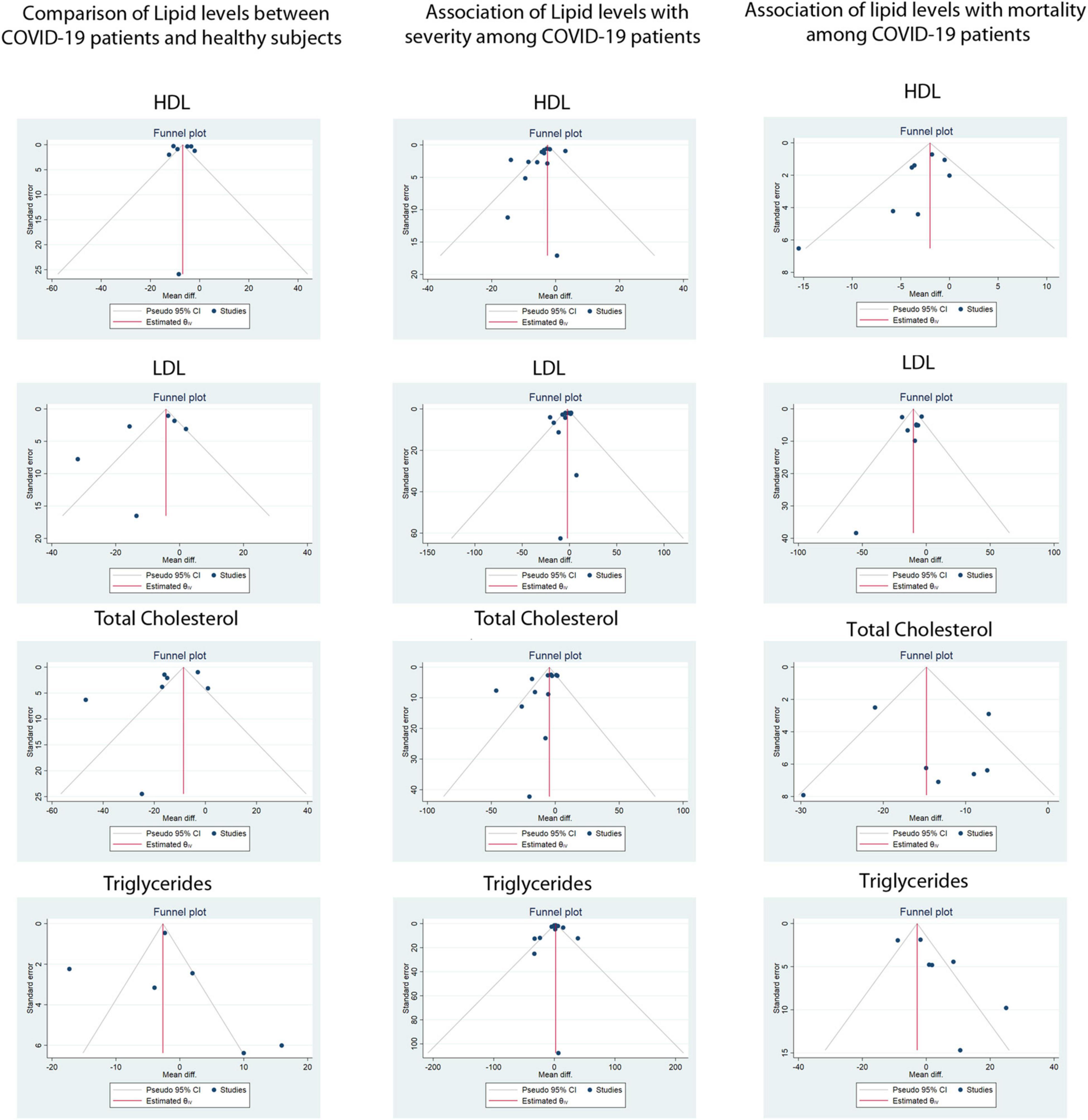
Figure 3. Funnel plots for assessment of publication bias for the association of lipid levels with COVID-19 infection, disease severity and mortality. LDL-C, Low density Lipoprotein, HDL-C, High Density Lipoprotein.
Meta-regression analysis for COVID-19 disease severity did not result in a statistically significant association between the mean difference of either TC, LDL-C, HDL-C, or TG, and the mean difference in age, or difference in proportions of male sex, diabetes, hypertension, or coronary artery disease (Table 2). The bubble plots for the meta-regression analysis are shown in Supplementary Figures 1–5 (Appendix section III). Meta-regression analyses were not performed for the comparison between COVID-19 patients and healthy individuals, and the comparison between COVID-19 patients who died and survived, because of the small number of studies reporting the confounding parameters for these comparisons. The PRISMA checklist for our systematic review is available in Supplementary appendix section IV.
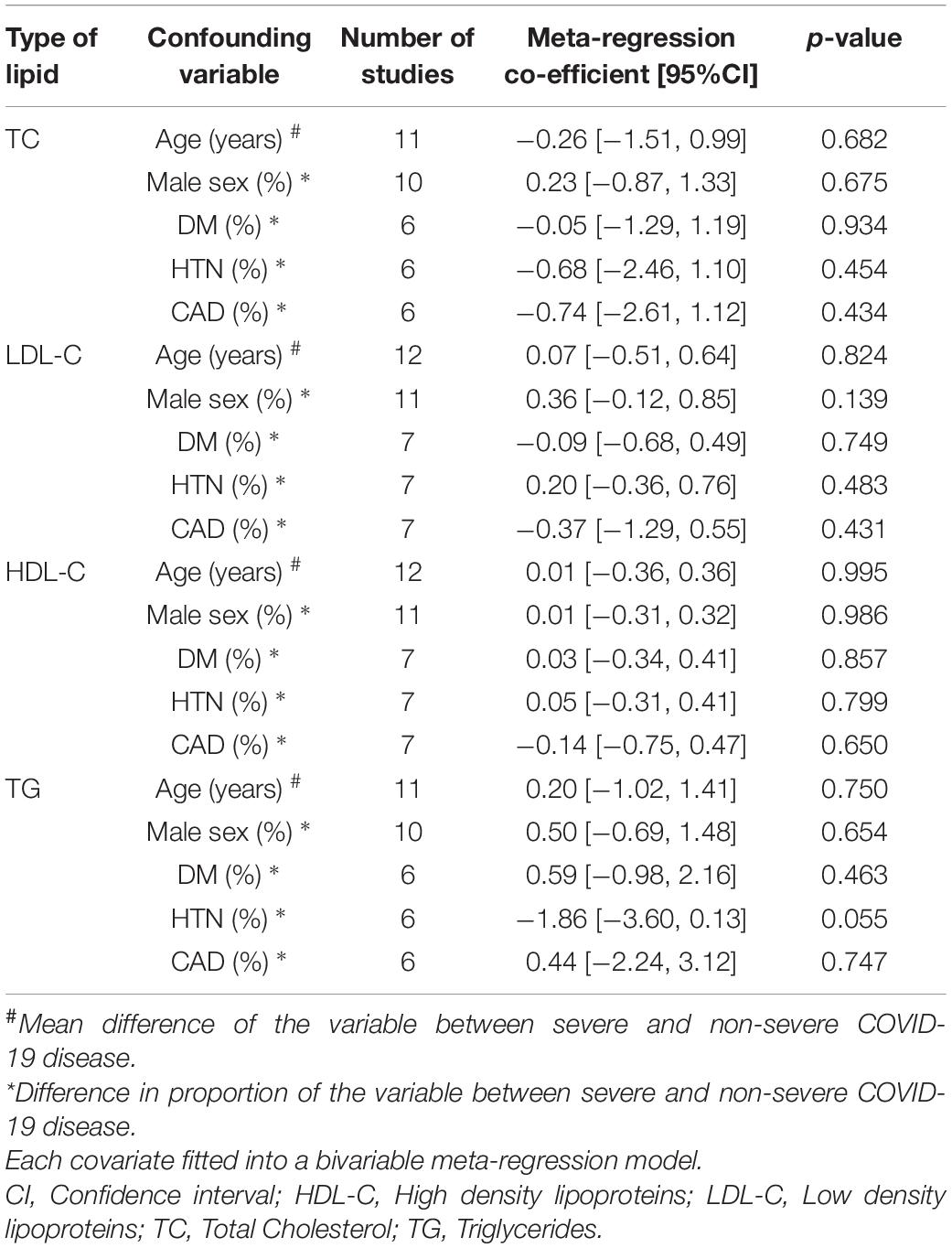
Table 2. Meta-regression analysis to assess the impact of the confounding variables on the mean difference of lipid levels (mg/dL) between patients with and without severe COVID-19 disease.
In the current study, we found that patients with COVID-19 had lower mean serum TC and HDL-C levels at the time of testing compared to healthy controls (Figure 2 and Table 1). There was no difference in mean LDL-C and TG levels among all patients with and without COVID-19. Lower serum TC, LDL-C, and HDL-C levels at admission were observed in patients with severe COVID-19, as well as in those who died relative to the respective comparison groups. Serum TG levels showed no relationship with COVID-19 severity or mortality. We did not find statistically significant publication bias for any of the analyses.
Lipids play a significant role in respiratory infections. The composition of pulmonary surfactant, which is an integral part of the innate and adaptive immune systems (23, 24), is 90% lipids (25). Moreover, more than 80% of total lung cholesterol is derived from the plasma (26). Of note, lower HDL-C was associated with COVID-19 infection, severity, and mortality in our meta-analysis. HDL-C plays a significant role in host resistance to bacterial, viral and parasitic infections (27, 28) and exhibits multiple properties, such as anti-inflammatory, anti-thrombotic, and anti-oxidative activities (28, 29). HDL-C and its structural protein, apoA-1, have been shown in pre-clinical studies to directly exert anti-inflammatory effects (30, 31) by neutralizing lipopolysaccharide and lipoteichoic acid, and preventing activation of peripheral blood monocytes, thereby decreasing TNF-α and IL-1β synthesis and secretion (32–34).
Oster et al. reported one of the first associations of hypocholesterolemia with increased in-hospital mortality (35). Multiple recent studies have found that patients with various infections, such as cytomegalovirus (36), Epstein-Barr virus (37), and tuberculosis (38), had lower levels of serum lipoprotein compared to non-infected controls. Several studies have established that plasma lipid levels are inversely associated with infection-related and all-cause mortality (10, 39), and have defined the role of lipid levels in acute infectious processes (40). In line with these reports, our meta-analysis highlights the association between COVID-19 infection and lower TC, LDL-C and HDL-C levels.
Recent systematic reviews have also noted findings similar to ours (41, 42), but they have not compared the lipid levels between infected patients and uninfected controls. Additionally, these reviews (41, 42) have only included studies up to January, 2021 while our study included articles up to September, 2021. Meta-regression analysis for the cofounder variables was not performed in the review by Mahat et al. (41), and thus did not account adequately for these variables. In the review by Zinellu et al. (42), the outcomes, namely severe disease and mortality, were combined into a composite outcome, thus the control group for the patients who survived may also contain patients with severe disease leading to a higher degree of heterogeneity in the analysis. Consistent with our findings, the meta-analysis by Ulloque-Badaracco et al. (43) found that serum levels of apoproteins Apo-A1 and Apo-B, which are major apoproteins of HDL-C and LDL-C, respectively, were lower in patients with severe COVID-19.
Several potential mechanisms explaining the altered serum lipid levels observed during COVID-19 infection have been postulated (Figure 4). Firstly, increased secretory phospholipase and SAA, together with decreased reverse cholesterol transport, have been attributed to low HDL-C levels following inflammation (12–14, 23). SAA-enriched HDL-C is known to be cleared more rapidly from circulation than regular HDL-C through scavenging mechanisms (23). Secondly, the liver plays a critical role in lipid metabolism, and liver dysfunction occurs frequently (in up to 53% cases) in severely ill patients with COVID-19 (44). Furthermore, severe COVID-19 is associated with excessive release of pro-inflammatory cytokines, such as IL-1, IL-6, IFN-γ, and TNF-α, which were shown to decrease the synthesis and secretion of apolipoproteins in hepatic cell lines in a dose-dependent manner (11, 28, 45). Thirdly, severe inflammation can cause leakage of lipoproteins and apolipoproteins in the capillaries (46). Lower serum levels of HDL-C result in a decrease in the anti-inflammatory activities of this molecule, leading to cytokine overproduction and further reduction of HDL-C levels (23). The net effect of this vicious cycle is a more profound depletion of serum HDL-C in the setting of inflammation (8, 34, 47). In vitro studies have reported that SARS-CoV-2 can bind to HDL particles via the spike protein of the virus (48), but whether this is an important mechanism for lowering HDL cholesterol that is specific to COVID-19 is unclear. Additionally, there are no currently available studies directly comparing the effect of COVID-19 and non-COVID-19 acute respiratory illness on serum lipid levels.
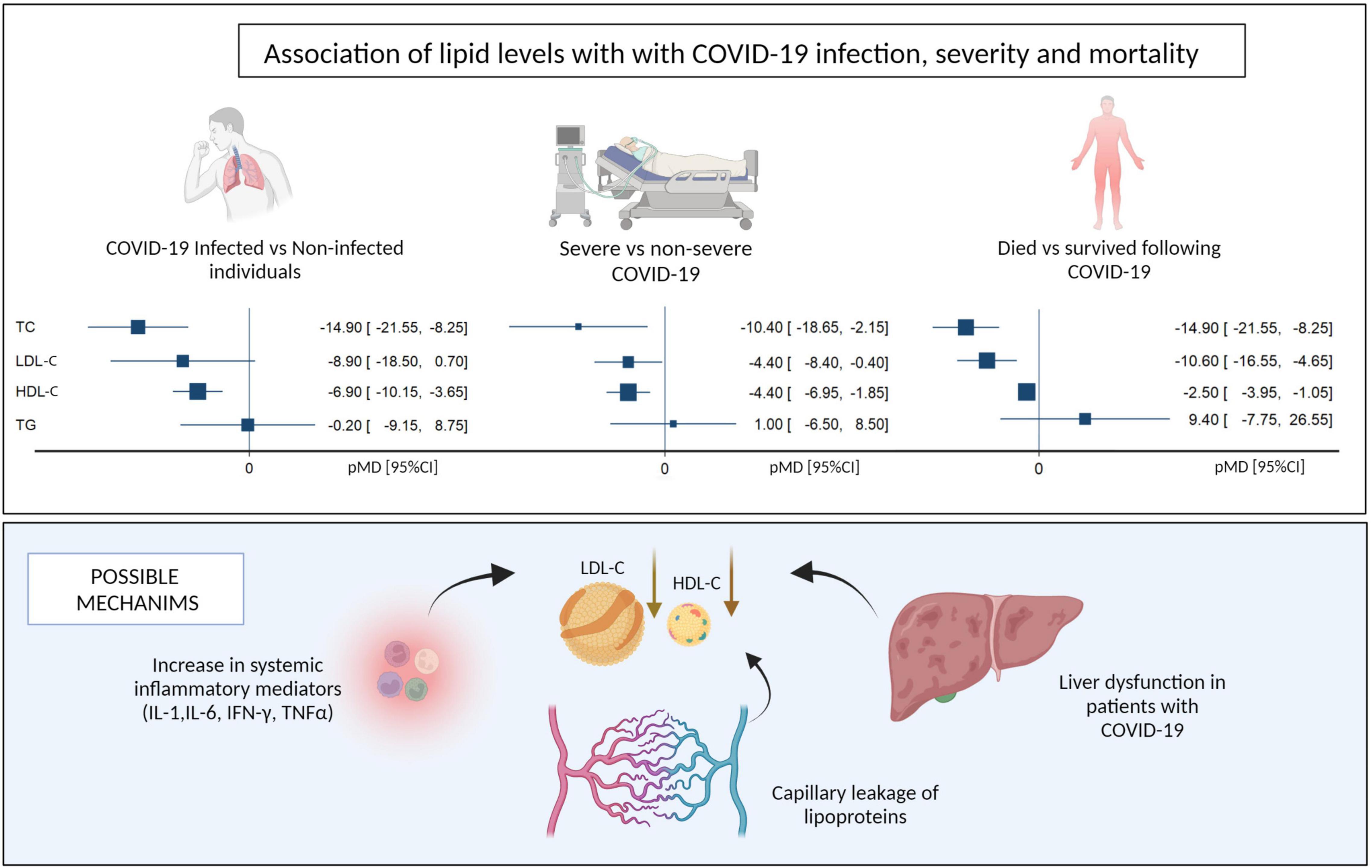
Figure 4. Summary of pooled mean differences and possible mechanisms for the association of lipid levels with COVID-19 infection, disease severity and mortality. LDL-C, Low density Lipoprotein, HDL-C, High Density Lipoprotein (Created with BioRender.com).
Although LDL-C levels appear to correlate with disease activity in other infections, such as dengue (49) and tuberculosis (10), a similar consistent correlation has not been observed with COVID-19 (50). The studies reporting low LDL-C levels attribute this finding to COVID-19-induced liver injury (44), and increased uptake by monocyte-derived macrophages in patients with COVID-19 (51). We observed a significant association between low serum LDL-C levels and severe COVID-19 infection and mortality. A systematic review noted that patients with dyslipidemia or hyperlipidemia were associated with higher risk of COVID-19 severity and mortality, but the study did not report the precise numerical values of plasma lipids (52).
Although some studies have reported an association between elevated serum TG levels and mortality in COVID-19 (53, 54), our meta-analysis showed that TG levels were not associated with COVID-19 infection, or disease severity or mortality. Of note, TG and its breakdown products, namely fatty acids, activate the NF-κB pathway, with increased expression of pro-inflammatory cytokines, including TNFα, IL-1β, IL-6, and MCP-1 (monocyte chemoattractant protein-1) (55). Also, TG-rich particles in the serum can increase local inflammation, activate the complement pathway and promote endothelial dysfunction (56, 57).
Our study has several strengths. This is the first systematic review to examine the association between lipid levels and the presence, severity, and mortality in COVID-19 infections and assess the robustness of our conclusions through meta-regression for common confounding variables, such as age, sex, diabetes, hypertension, and coronary artery disease. Our systematic review of studies from nine countries with variable healthcare access, allow for generalizability of our results. Our study also has some limitations. First, the information on COVID-19 is highly dynamic, and this is a summary of the currently published articles. Although a pooled analysis of adjusted estimates is desirable, most studies do not give uniform estimates adjusted for similar parameters. Time points of assessment of lipid levels were taken at admission to maintain uniformity but the lipid levels were observed to be dynamic during the time of hospital admission in multiple studies. The included studies did not clearly report whether these lipid levels were taken in the fasting or non-fasting state. The use of lipid modifying agents by the patients in the cohorts were not recorded well in most included studies. Lasty, very few of the studies that assessed changes in serum lipid levels presented data on inflammation and liver injury markers.
Our analysis demonstrated lower lipid levels in patients with COVID-19 compared to healthy controls. Among COVID-19 patients, lower TC, LDL-C and HDL-C levels at admission were associated with disease severity and mortality. Our results might spur further interest in probing the association and interaction of lipid levels and inflammation in atherosclerotic cardiovascular diseases. Current evidence suggests that reduced lipoprotein levels are secondary to systemic inflammation and hepatic dysfunction, but the causal relationship between circulating lipid levels and COVID-19 disease requires further study. Finally, whether low lipid levels prior to hospital admission contribute directly to increased COVID-19 incidence and severity requires additional study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
VC and PK conceived the idea for the review. VC, AK, MM, and PK designed and undertook the literature review. VC, HS, MM, AK, and RS screened the articles and extracted the data. VC and MM performed the statistical analysis, figures, and appendix and analyzed and interpreted the data. VC, HS, AK, MM, RS, DV, JM, and PK wrote the first draft of the manuscript. VC, AK, HS, DV, JM, and PK revised the subsequent drafts of the manuscript. All authors reviewed the final draft of the manuscript.
This manuscript was supported by the NIAID/NIH UH3 AI122309 and K24AI143447 grants to PK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.862999/full#supplementary-material
1. Ahmad FB, Cisewski JA, Miniño A, Anderson RN. Provisional mortality data — United States, 2020. MMWR Morb Mortal Wkly Rep. (2021) 70:519–22. doi: 10.15585/mmwr.mm7014e1
2. Del Sole F, Farcomeni A, Loffredo L, Carnevale R, Menichelli D, Vicario T, et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. (2020) 50:e13378. doi: 10.1111/eci.13378
3. Chidambaram V, Tun NL, Haque WZ, Gilbert Majella M, Kumar Sivakumar R, Kumar A, et al. Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and meta-analysis. PLoS One. (2020) 15:e0241541. doi: 10.1371/journal.pone.0241541
4. Sun X, Whittaker GR. Role for influenza virus envelope cholesterol in virus entry and infection. J Virol. (2003) 77:12543. doi: 10.1128/JVI.77.23.12543-12551.2003
5. Song J, Ping LY, Duong DM, Gao XY, He CY, Wei L, et al. Native low density lipoprotein promotes lipid raft formation in macrophages. Mol Med Rep. (2016) 13:2087. doi: 10.3892/MMR.2016.4781
6. Peng Y, Akmentin W, Connelly MA, Lund-Katz S, Phillips MC, Williams DL. Scavenger receptor BI (SR-BI) clustered on microvillar extensions suggests that this plasma membrane domain is a way station for cholesterol trafficking between cells and high-density lipoprotein. Mol Biol Cell. (2004) 15:384–96. doi: 10.1091/MBC.E03-06-0445
7. Feingold KR. Lipid and Lipoprotein Levels in Patients With COVID-19 Infections. Endotext. (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK564657/ (accessed October 16, 2021).
8. Sun JT, Chen Z, Nie P, Ge H, Shen L, Yang F, et al. Lipid profile features and their associations with disease severity and mortality in patients with COVID-19. Front Cardiovasc Med. (2020) 7:584987. doi: 10.3389/FCVM.2020.584987
9. Feingold KR. Lipid and Lipoprotein Levels in Patients With COVID-19 Infections. MDText.com, Inc. (2000). Available online at: http://www.ncbi.nlm.nih.gov/pubmed/33237691 (accessed April 25, 2021).
10. Chidambaram V, Zhou L, Castillo JR, Kumar A, Ayeh SK, Gupte A, et al. Higher serum cholesterol levels are associated with reduced systemic inflammation and mortality during tuberculosis treatment independent of body mass index. Front Cardiovasc Med. (2021) 8:583. doi: 10.3389/FCVM.2021.696517
11. Ettinger WH, Harris T, Verdery RB, Tracy R, Kouba E. Evidence for inflammation as a cause of hypocholesterolemia in older people. J Am Geriatr Soc. (1995) 43:264–6. doi: 10.1111/j.1532-5415.1995.tb07334.x
12. Tietge UJF, Maugeais C, Lund-Katz S, Grass D, DeBeer FC, Rader DJ. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and ApoA-I in response to inflammation in human ApoA-I transgenic mice. Arterioscler Thromb Vasc Biol. (2002) 22:1213–8. doi: 10.1161/01.ATV.0000023228.90866.29
13. Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler Thromb Vasc Biol. (2000) 20:763–72. doi: 10.1161/01.ATV.20.3.763
14. Yin K, Liao DF, Tang CK. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med. (2010) 16:438–49. doi: 10.2119/molmed.2010.00004
15. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev Esp Nutr Hum Diet. (2016) 20:148–60. doi: 10.1186/2046-4053-4-1
16. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. (2019) 200:E45–67. doi: 10.1164/RCCM.201908-1581ST
17. Lin L, Li TS. Interpretation of “guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (Trial Version 5)”. Zhonghua Yi Xue Za Zhi. (2020) 100:E001. doi: 10.3760/CMA.J.ISSN.0376-2491.2020.0001
18. McMaster University. Covidence - Better Systematic Review Management. (2021). Available online at: https://www.covidence.org/ (accessed November 18, 2021).
19. Qualtrics. QualtricsXM//The Leading Experience Management Software. (2021). Available online at: https://www.qualtrics.com/au/?rid=ip&prevsite=en&newsite=au&geo=IN&geomatch=au (accessed November 18, 2021).
20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
21. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2021). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 21, 2021).
22. StataCorp. Stata Statistical Software: Release 16. College Station TSL. Stata | StataCorp LLC. (2019). Available online at: https://www.stata.com/company/ (accessed April 25, 2021).
23. Wendel M, Paul R, Heller AR. Lipoproteins in inflammation and sepsis. II. Clinical aspects. Intensive Care Med. (2006) 33:25–35. doi: 10.1007/S00134-006-0433-X
24. Mb F, Rs S. Surfactant lipids at the host-environment interface. Metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am J Respir Cell Mol Biol. (2016) 54:624–35. doi: 10.1165/RCMB.2016-0011PS
25. Han S, Mallampalli RK. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc. (2015) 12:765. doi: 10.1513/ANNALSATS.201411-507FR
26. Turley SD, Andersen JM, Dietschy’ JM. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. (1981) 22:551–69. doi: 10.1016/S0022-2275(20)37366-1
27. Singh IP, Chopra AK, Coppenhaver DH, Ananatharamaiah GM, Baron S. Lipoproteins account for part of the broad non-specific antiviral activity of human serum. Antiviral Res. (1999) 42:211–8. doi: 10.1016/S0166-3542(99)00032-7
28. Tanaka S, Couret D, Tran-Dinh A, Duranteau J, Montravers P, Schwendeman A, et al. High-density lipoproteins during sepsis: from bench to bedside. Crit Care. (2020) 24:1–11. doi: 10.1186/S13054-020-02860-3/FIGURES/3
29. Georgila K, Vyrla D, Drakos E. Apolipoprotein A-I (ApoA-I), immunity, inflammation and cancer. Cancers. (2019) 11:1097. doi: 10.3390/CANCERS11081097
30. Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, et al. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. (2004) 110:3252–8. doi: 10.1161/01.CIR.0000147232.75456.B3
31. Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-Density Lipoprotein Loses Its Anti-Inflammatory Properties During Acute Influenza A Infection. (2001). Available online at: http://www.circulationaha.org (accessed October 11, 2021).
32. Trinder M, Genga KR, Kong HJ, Blauw LL, Lo C, Li X, et al. Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am J Respir Crit Care Med. (2019) 199:854–62. doi: 10.1164/RCCM.201806-1157OC
33. Casas AT, Hubsch AP, Rogers BC, Doran JE. Reconstituted high-density lipoprotein reduces LPS-stimulated TNFα. J Surg Res. (1995) 59:544–52. doi: 10.1006/JSRE.1995.1204
34. Thompson PA, Tobias PS, Viriyakosol S, Kirkland TN, Kitchens RL. Lipopolysaccharide (LPS)-binding protein inhibits responses to cell-bound LPS *. J Biol Chem. (2003) 278:28367–71. doi: 10.1074/JBC.M302921200
35. Oster P, Muchowski H, Heuck CC, Schlierf G. The prognostic significance of hypocholesterolemia in hospitalized patients. Klin Wochenschr. (1981) 59:857–60. doi: 10.1007/BF01721056
36. Fleck-Derderian S, McClellan W, Wojcicki JM. The association between cytomegalovirus infection, obesity, and metabolic syndrome in U.S. adult females. Obesity (Silver Spring). (2017) 25:626–33. doi: 10.1002/OBY.21764
37. Apostolou F, Gazi IF, Lagos K, Tellis CC, Tselepis AD, Liberopoulos EN, et al. Acute infection with Epstein-Barr virus is associated with atherogenic lipid changes. Atherosclerosis. (2010) 212:607–13. doi: 10.1016/J.ATHEROSCLEROSIS.2010.06.006
38. Sahin F, Yildiz P. Distinctive biochemical changes in pulmonary tuberculosis and pneumonia. Arch Med Sci. (2013) 9:656–61. doi: 10.5114/aoms.2013.34403
39. Kaysen GA, Ye X, Raimann JG, Wang Y, Topping A, Usvyat LA, et al. Lipid levels are inversely associated with infectious and all-cause mortality: international MONDO study results1. J Lipid Res. (2018) 59:1519–28. doi: 10.1194/JLR.P084277
40. Filippas-Ntekouan S, Liberopoulos E, Elisaf M. Lipid testing in infectious diseases: possible role in diagnosis and prognosis. Infect. (2017) 45:575–88. doi: 10.1007/S15010-017-1022-3
41. Mahat RK, Rathore V, Singh N, Singh N, Singh SK, Shah RK, et al. Lipid profile as an indicator of COVID-19 severity: a systematic review and meta-analysis. Clin Nutr ESPEN. (2021) 45:91–101. doi: 10.1016/j.clnesp.2021.07.023
42. Zinellu A, Paliogiannis P, Fois AG, Solidoro P, Carru C, Mangoni AA. Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: a systematic review and meta-analysis with meta-regression. Front Public Health. (2021) 9:705916. doi: 10.3389/fpubh.2021.705916
43. Ulloque-Badaracco JR, Hernandez-Bustamante EA, Herrera-Añazco P, Benites-Zapata VA. Prognostic value of apolipoproteins in COVID-19 patients: a systematic review and meta-analysis. Travel Med Infect Dis. (2021) 44:102200. doi: 10.1016/j.tmaid.2021.102200
44. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. (2020) 73:1231–40. doi: 10.1016/J.JHEP.2020.06.006
45. Pirillo A, Catapano AL, Norata GD. HDL in infectious diseases and sepsis. Handb Exp Pharmacol. (2015) 224:483–508. doi: 10.1007/978-3-319-09665-0_15
46. Van Leeuwen HJ, Heezius ECJM, Dallinga GM, Van Strijp JAG, Verhoef J, Van Kessel KPM. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. (2003) 31:1359–66. doi: 10.1097/01.CCM.0000059724.08290.51
47. Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, et al. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. (2009) 29:261–7. doi: 10.1161/ATVBAHA.108.178681
48. Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. (2020) 2:1391–400. doi: 10.1038/s42255-020-00324-0
49. Biswas HH, Gordon A, Nuñez A, Perez MA, Balmaseda A, Harris E. Lower low-density lipoprotein cholesterol levels are associated with severe dengue outcome. PLoS Negl Trop Dis. (2015) 9:e0003904. doi: 10.1371/JOURNAL.PNTD.0003904
50. Wang G, Zhang Q, Zhao X, Dong H, Wu C, Wu F, et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: an observational study. Lipids Health Dis. (2020) 19:204. doi: 10.1186/S12944-020-01382-9
51. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. (2015) 15:104. doi: 10.1038/NRI3793
52. Liu Y, Pan Y, Yin Y, Chen W, Li X. Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): a meta-analysis. Virol J. (2021) 18:157. doi: 10.1186/s12985-021-01604-1
53. Zhong P, Wang Z, Du Z. Serum triglyceride levels and related factors as prognostic indicators in COVID-19 patients: a retrospective study. Immunity Inflamm Dis. (2021) 9:1055–60. doi: 10.1002/IID3.469
54. Golucci APBS, Marson FAL, Ribeiro AF, Nogueira RJN. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition. (2018) 55–56:7–14. doi: 10.1016/J.NUT.2018.04.007
55. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. (2011) 18:139–43. doi: 10.1097/MED.0B013E3283444B09
56. Twickler TB, Dallinga-Thie GM, Cohn JS, Chapman MJ. Elevated remnant-like particle cholesterol concentration. Circulation. (2004) 109:1918–25. doi: 10.1161/01.CIR.0000125278.58527.F3
Keywords: HDL cholesterol, LDL cholesterol, meta-regression, COVID-19, severity, mortality
Citation: Chidambaram V, Shanmugavel Geetha H, Kumar A, Majella MG, Sivakumar RK, Voruganti D, Mehta JL and Karakousis PC (2022) Association of Lipid Levels With COVID-19 Infection, Disease Severity and Mortality: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:862999. doi: 10.3389/fcvm.2022.862999
Received: 26 January 2022; Accepted: 25 February 2022;
Published: 24 March 2022.
Edited by:
Hanrui Zhang, Columbia University, United StatesReviewed by:
Danilo Menichelli, Sapienza University of Rome, ItalyCopyright © 2022 Chidambaram, Shanmugavel Geetha, Kumar, Majella, Sivakumar, Voruganti, Mehta and Karakousis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petros C. Karakousis, cGV0cm9zQGpobWkuZWR1; Vignesh Chidambaram, dmNoaWRhbWJhcmFtQHVhbXMuZWR1
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.