
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 26 November 2020
Sec. Ethnopharmacology
Volume 11 - 2020 | https://doi.org/10.3389/fphar.2020.593655
This article is part of the Research TopicFood/Diet Supplements from Natural Sources: Current Status and Future Challenges from a Pharmacological PerspectiveView all 17 articles
The fruits of Ziziphus jujuba, commonly known as jujube, red date or Chinese date, are taken as fresh or dried food, and as traditional medicine worldwide due to high nutritional and health values. Traditionally in China, jujube is considered as a medicinal fruit that is being used in treating blood deficiency. In this review, the beneficial effects of jujubes on the hematopoietic functions are summarized and discussed. As illustrated in cell and animal models, the application of jujube extract possessed beneficial effects, including regulation of erythropoiesis via activation of hypoxia inducible factor-induced erythropoietin, potential capacity in recycling heme iron during erythrophagocytosis and bi-directional regulation of immune response. Thus, the blood-nourishing function of jujube is being proposed here. Flavonoid, polysaccharide and triterpenoid within jujube could serve as the potential active ingredients accounting for the aforementioned health benefits. Taken together, these findings provide several lines of evidence for further development of jujube as supplementary products for prevention and/or treatment of anemia.
Jujube is usually called red date or Chinese date, which is the fruit of Ziziphus jujuba Mill. that belongs to Rhamnaceae family. Jujube is native to China, and which has been commonly consumed as food supplement and traditional Chinese medicine (TCM) for thousands of years (Figure 1). Today, jujube plant is distributed widely not only in China but also in other countries, e.g. Korea, India, Japan, Europe and the United States. In Huangdi Neijing (475–221 BC), a classic medical text from ancient China, jujube was recorded as one of extremely valuable fruits. According to Shennong Bencao Jing written between 300 BC and 200 AD, one of the earliest books specializing in Chinese medicine, jujube was regarded as one of the top-grade medicinal herbs that could extend one’s life expectancy by nourishing blood, increasing sleep quality and improving digestive system. Along with growing number of studies on jujube, various beneficial nutrients within jujube are being proposed, including carbohydrate, mineral, vitamin, sugar and amino acid. Thus, jujube is considered as a popular nutritious food, worldwide (Li et al., 2007; San and Yildirim, 2010; USDA, 2012; Guo et al., 2013; Reche et al., 2019). Being a Chinese herb or health food supplement, recent studies have indicated that jujube possesses a wide range of pharmacological activities in nervous system, cardiovascular system, as well as anti-oxidation and anti-cancer properties (Table 1).

FIGURE 1. The photo of Z. jujuba fruits. The photo of dried jujubes collected from Xinjiang province, one of major production regions of jujubes in China. Bar: 1 cm.
Clinically, blood deficiency is usually encountered in women due to the loss of menstrual blood, or in patient who has lost blood or suffered from chronic malnutrition. Jujube is a functional food, which is believed to possess robust effect in tonifying the blood, in order to prevent blood deficiency in human. According to the theory of TCM, blood deficiency shows similarity to anemia of individual in western medicine (Shi et al., 2019). In line to this notion, pharmacological studies have reported that jujube has potential hematopoietic functions both in vivo and in vitro (Xu et al., 2004; Chen et al., 2014b). Specific targets supporting the clinical usage of jujube in hematopoietic functions however remain unclear. Here, we are focusing on a discussion of jujube associating with hematopoietic functions, i.e., erythropoiesis, erythrophagocytosis and immune functions (Table 2). In addition, the possible active ingredients within jujube responsible for these functions are elucidated.
Jujube has a promising source of flavonoids, polysaccharides, terpenoids, saponins, nucleotides and others (Figure 2). Here, the trophic ingredients having potential beneficial effects on hematopoietic function are highlighted. At present, a variety of flavonoids were isolated and identified in jujube (Cheng et al., 2000; Pawlowska et al., 2009; Choi et al., 2011; Gao et al., 2013). Flavonoids from jujube have been found to stimulate the expression of erythropoietin (EPO), a hormone stimulating blood production (Zheng et al., 2011), and therefore we speculated that jujube flavonoid might be one of the active compounds that possessed the ability to induce the expression of EPO. Supporting this notion in the pHRE-Luc (the DNA promoter construct of EPO gene, hypoxia response element) transfected cultured HEKT293T cells, the application of kaempferol at 10 μM for 24 h could significantly induce the transcriptional activity of pHRE-Luc with 127% of increase, as compared to control group (Xu et al., 2018). Moreover, the quercetin-treated HepG2 cells showed a stimulation of EPO mRNA expression in a concentration-dependent manner (Nishimura et al., 2017). Similarly, the protein level of HIF-1α was markedly up regulated at the treatment of 10 μM quercetin (Nishimura et al., 2017). Indeed, kaempferol and quercetin derivatives, including kaempferol 3-O-rutinoside, quercetin, quercetin 3-O-rutinoside, quercetin 3-O-galactoside and quercetin 3-O-β-D-glucoside (Figures 3A), were identified in jujube (Gao et al., 2012; Chen et al., 2013). Therefore, jujube flavonoid can induce EPO expression, probably, through HIF-α protein accumulation. In addition, the combination of catalpol and puerarin with doses of 65.4 and 32.7 mg/kg, respectively, enhanced the expressions of EPO and EPO receptor in ischemic/reperfusion rats (Xue et al., 2016). In parallel, puerarin was identified in the seeds of jujube (Cheng et al., 2000). Flavonoids are common chemical presented in a wide range of plants. Plant extracts rich in aforesaid flavonoids are considered, therefore, potentially useful as therapeutic agents for anemia.
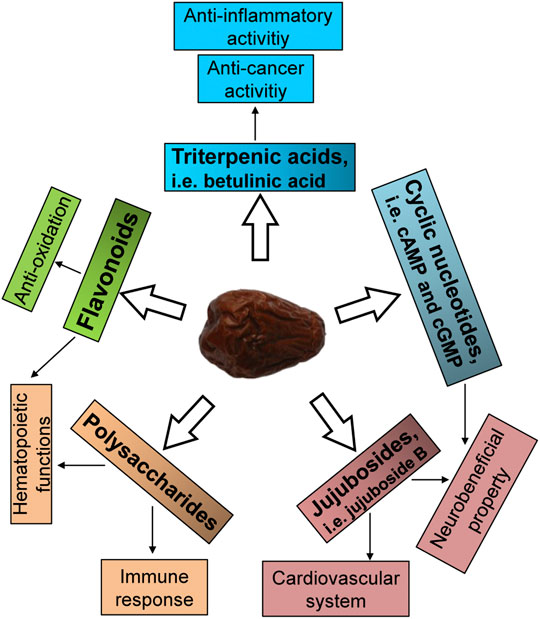
FIGURE 2. Active ingredients of jujube. The potential active ingredients relating to the activities of jujube are summarized.
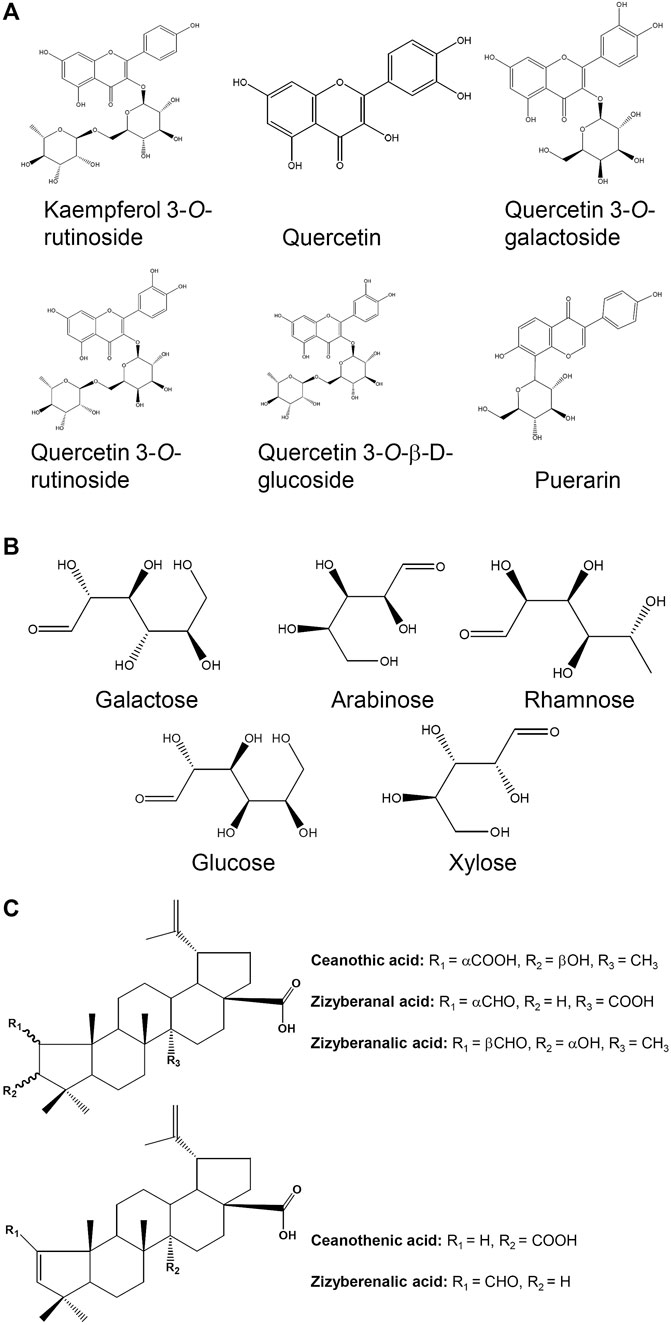
FIGURE 3. Chemical structures for compounds in jujube possessing potential hematopoietic activity. (A) The chemical structures of six flavonoids found in the fruits. (B) The composition in monosaccharide purified from jujube. (C) The chemical structures of varieties of triterpenic acids found in the fruit.
Polysaccharides from plant have been demonstrated to possess various bio-activities, e.g., anti-oxidation, anti-complementary and immunological activities (Li et al., 2003; Gao et al., 2007; Chen M. et al., 2016). Several polysaccharides have been isolated and purified from the jujube. The polysaccharides extracted from jujube usually consist of five monosaccharides, i.e., galactose, arabinose, rhamnose, glucose and xylose (Zhao et al., 2008) (Figures 3B). Fractions named as ZSP1, ZSP2, ZSP3 and ZSP4 with weight ratio of 29.3:17.6:37.2:15.9 have been purified from jujube (Li et al., 2011). The fractions of ZSP3 and ZSP4 at various concentrations (30–200 μg/ml) were applied onto peritoneal macrophages, and the cell proliferation was detected by MTT assay. These two fractions were found to dose-dependently induce proliferation of spleen lymphocyte, having the highest response under the treatment of jujube polysaccharide at 200 μg/ml. This finding suggests the immunological activity of jujube polysaccharide. In line with this, Ju-B-2, a molecular weight of over 2,000 kDa polysaccharide from jujube, was shown to have the immune activity. Application of Ju-B-2 at 10–100 μg/ml onto cultured spleen cells for 3 days induced cell proliferation. Furthermore, the authors proposed the structures of rhamnogalacturonan and its side chains of Ju-B-2 polysaccharide contributing to the immune response (Zhao et al., 2006). Although several reports support the beneficial effects of jujube polysaccharides in preventing anemia, its detail action mechanisms are still rather limit. Hence, possible signaling pathways involved in jujube polysaccharide-treated in vitro or in vivo models are needed for further investigation.
Triterpenic acids have been isolated and purified from jujube, including ceanothenic acid, zizyberanal acid, zizyberenalic acid, zizyberanalic acid, and ceanothic acid (Figures 3C) (Yu et al., 2012). These acids possessed notable inhibitory activity on the activated inflammatory cells, and which could be one of the main ingredients in supporting the anti-inflammatory activity of jujube (Yu et al., 2012). Besides, jujuboside and flavonoid in the fruit were also proposed to be active compounds, and which might responsible for anti-inflammatory effects (Goyal et al., 2011). Another animal study showed that jujube essential oil could inhibit the inflammatory responses of skin (Al-Reza et al., 2010).
In addition, jujube was reported to contain numerous minerals, e.g., iron and vitamin. About 0.48 mg iron and 69 mg vitamin C per 100 g of fresh fruit were reported (Li et al., 2007; USDA, 2012). Thus, the daily intake of jujube could increase our dietary iron and vitamin, as to prevent anemia due to deficiency of iron or vitamin C. Moreover, cAMP was found to have high abundance in jujube, and surprisingly this content was much higher than other horticultural fruits (Hanabusa et al., 1981). It is well accepted that increasing cAMP level can stimulate protein kinase A and, subsequently, which phosphorylates CREB (Argyrousi et al., 2020). Besides, jujube cAMP has been found to possess anti-melancholic effect in animal model of depression (Chi and Zhang, 2009). Thus, it is supposed that the cAMP in the jujube may account for its role on HIF (hypoxia inducible factor)-dependent EPO induction.
Erythropoiesis is considered to play critical roles in hematopoiesis, by which production of red blood cells (RBCs) is occurred. In this process, EPO, a RBC-specific hormone, is able to regulate erythropoiesis in bone marrow (Ascensao et al., 1991). EPO gene expression regulates primarily at the level of transcription, and which is further controlled by a number of transcriptional and post-transcriptional factors (Schuster et al., 1989; Goldberg et al., 1991). Failure to up regulate the circulating EPO under hypoxia thereafter leads to anemia (Jelkmann, 1992). Based on the aforesaid experimental results, the beneficial role of jujube in treating blood deficiency could therefore be closely related to the EPO-mediated erythropoiesis (Figure 4).
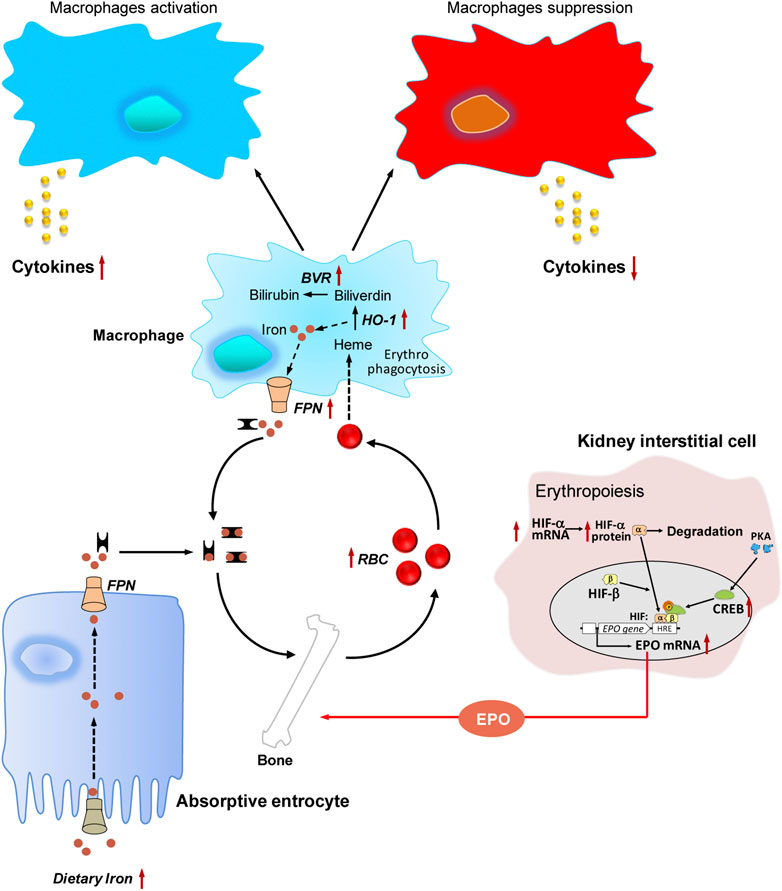
FIGURE 4. The hematopoietic functions of jujube. Jujube promotes erythropoiesis via activation of hypoxia inducible factor-induced erythropoietin, possesses potential capacity in recycling heme iron during erythrophagocytosis, exhibits bidirectional role in regulating immune response under different conditions, contains numerous minerals including iron. Red dots indicate iron, and yellow dots indicate cytokine. The arrow in red indicates the targets for jujube action. RBC, red blood cell; EPO, erythropoietin; HIF, hypoxia inducible factor; HRE, hypoxia response element; PKA, protein kinase A; CREB, cAMP response element-binding protein; FPN, ferroportin; BVR, biliverdin reductase; HO-1, heme oxygenase-1.
Jujube polysaccharide has been reported to improve hematological parameters in anemic animal models. Mice of blood deficiency model were induced by releasing blood and injection of cyclophosphamide. The levels of RBC, hemoglobin and hematocrit were decreased, and the level of platelet was increased in model mice, as compared to control group. Compared with model mice, the decreased levels of RBC, hemoglobin and hematocrit were reversed by treatment with jujube extract; while the increased level of platelet however was down regulated in jujube-treated mice (Xu et al., 2004). In another investigation, the activities of dietary jujube on the levels of RBC, hemoglobin and hematocrit were analyzed in colitis-associated colon cancer mice. These hematopoietic parameters in cancer mice were significantly decreased, as compared to control group; however, which were markedly increased in the jujube-treated mice (Periasamy et al., 2020), suggesting possible beneficial effects of this fruit on cancer patients suffering from anemia. Besides, the extract of jujube possessed the ability to stimulate the activity of ATPase, i.e., Na+-K+-ATPase, Ca2+-ATPase and Ca2+-Mg2+-ATPase, in erythrocyte, and therefore which was shown to promote bone marrow nuclear proliferation and to inhibit atrophy thymus and spleen in blood deficient animals (Miao et al., 2006; Miao et al., 2010). Moreover, the intake of jujube extract (100–400 mg/kg) was found to increase the level of EPO in blood circulation, which suggested that jujube might promote RBC level through up regulation of EPO production. In support of this notion, the applied jujube extract at concentrations of 0.75–3 mg/ml in cultured Hep3B cells for 48 h increased the expression of EPO transcription, and the increase was shown to be in a dose-dependent manner (Chen et al., 2014b). In parallel, the applied jujube extract was able to stimulate the protein expression of EPO, giving ∼50% increase of the total protein (Lam et al., 2016). The circulating EPO is produced by adult kidney cells; while kidney dysfunction contributes to inadequate amount of EPO production and renal anemia. In ibuprofen-induced nephrotoxicity rats, the intake of jujube extract (500 mg/kg) improved kidney function by declining the levels of creatinine and urea, and this treatment could prevent histopathological damages of kidney (Awad et al., 2014). On the other hand, the intake of Jian-Pi-Yi-Shen, a Chinese herbal decoction comprising of Astragali Radix, Atractylodis Macrocephalae Rhizoma, Dioscoreae Rhizoma, Cistanches Herba, and other four herbs, was able to improve renal function and kidney injury in anemia rats suffering from chronic kidney disease (Chen et al., 2019). Jian-Pi-Yi-Shen improved the hematological parameters and stimulated EPO production (Chen et al., 2019). Massive EPO-producing cells are identified in the renal interstitium. The occurrence of renal interstitial fibrosis is accompanied by decrease of fibroblasts, which impairs the production of EPO. Moreover, Jian-Pi-Yi-Shen was believed to ameliorate renal interstitial fibrosis, and the renal recovery might be related to improvement of EPO production. Thus, the function of jujube in promoting EPO expression in renal anemia patients was in line to that of Jian-Pi-Yi-Shen (Chen et al., 2019). This assumption requires further studies as to confirm the ability of jujube to prevent/treat renal anemia through regulation of EPO production.
The promoter of EPO gene contains HRE, and thus the activation of hypoxia-mediated signaling pathway is leading to activation of EPO expression (Post and Van Meir, 2001). Cultured Hep3B cells were transfected with HRE promoter fragment (i.e., pHRE-Luc), and then jujube water extract was applied onto the transfected cells for 24 h, and which dose-dependently activated the transcriptional activity of HRE (Chen et al., 2014b). To account for the possible mechanism of HIF signaling in jujube-induced HRE activation, the expression of HIF-1α was determined. The jujube extract at various concentrations (0.75–3 mg/ml) was applied onto cultured Hep3B cells for 6 h, and then total RNA was harvested from the cultures for PCR analysis. Jujube extract stimulated the expression of HIF-1α mRNA, and the induced expression was demonstrated to be in a dose-dependent manner, having the highest effect by ∼80% of increase. In parallel, the protein level of HIF-1α in the cultures turned to increase after 2 h, and subsequently HIF-1α protein was induced by ∼150% at 6 h after the treatment (Chen et al., 2014b). These results support the effect of jujube on HIF-1α expression in both mRNA and protein levels. In addition, Xu et al. (2014) reported that CREB-binding protein was required for HIF-α acetylation and efficient HIF-mediated EPO production during hypoxic stress. In consistent with this, Chen et al. (2014c) found that jujube extract (2 mg/ml) induced CREB phosphorylation in cultured cells, and this effect was fully blocked by H89, a cyclic AMP-dependent protein kinase A inhibitor. These results indicate that CREB-binding protein/HIF signaling can be involved in jujube-induced EPO production.
Erythrophagocytosis is a process, where the senescent RBCs are phagocytosed by macrophage (Klei et al., 2017). Within the macrophage, the senescent RBC undergoes hemolysis, and the components, such as heme iron, are being recycled. The reused iron will be carried back to bone marrow for erythropoiesis (Gottlieb et al., 2012). Thus, the disorders in iron recycling can result in anemia (Batchelor et al., 2020). Here, the potential effects of jujube on erythrophagocytosis were summarized (Figure 4).
Heme oxygenase-1 (HO-1) has a vital role in metabolizing heme to biliverdin, carbon monoxide and free iron. Biliverdin is immediately conversed to bilirubin, as catalyzed by biliverdin reductase containing two isozymes, i.e., biliverdin reductase A and B. Free iron is released to blood circulation by ferroportin and further carried to bone marrow (Kovtunovych et al., 2010). Therefore, HO-1, biliverdin reductase and ferropotin are considered as the main target enzymes in determining the iron recycling in macrophages. Jujube extract at different concentration (0–3.0 mg/ml) was applied onto cultured macrophages for 24 h. The applied jujube extract stimulated the mRNA expressions of HO-1, biliverdin reductase A and B, and ferropotin in dose-dependent manners, giving the highest response by ∼2.0, 2.0, 3.0, and 4.0 folds, respectively (Chen J. et al., 2016). In good agreement with this finding, Yang et al. (2016) reported that the intake of jujube extract showed an improvement in iron deficiency anemia rats. In parallel, the extract of jujube significantly increased serum iron, iron saturation, total iron binding capacity in anemia rats, indicating the supply of circulation iron for erythropoiesis. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor, was found to regulate HO-1 expression (Motohashi and Yamamoto, 2004). Nrf2 directly binds to anti-oxidant response element (ARE) in the promoter region of HO-1 gene resulting in the transcription. In pARE-Luc-expressed cells treated with jujube water extract, the luciferase assay was activated in a dose-dependent manner (Chen J. et al., 2016). The activation by two folds was confirmed under application of jujube extract at 3.0 mg/ml (Chen J. et al., 2016). This result suggests the involvement of Nrf2/HO-1 signaling in jujube-treated cells. In support of this notion, Almeer et al. (2018) revealed the effects of jujube extract on gene expressions of Nrf2 and HO-1 in colitis rats, as induced by treating intrarectally with acetic acid. Compared with model group, the pre-treatment with jujube extract at different doses (100, 200, and 400 mg/kg/day) in colon of rats for 5 days by oral gavage significantly induced mRNA expressions of Nrf2 and HO-1, giving the highest response by seven and two folds, respectively (Almeer et al., 2018). These studies however need further confirmation as no observation has been found in a physiology model of iron recycling. For instance, a cellular model of erythrophagocytosis using artificially-aged RBCs and macrophages may be designed to investigate mRNA and protein expressions of target enzymes relating to iron recycling.
The immune response is impaired in anemia condition. Here, the immune-modulatory properties of jujube under different scenarios are summarized (Figure 4). The intake of jujube extracts at concentrations of 150 and 250 mg/kg/day significantly stimulated thymus and spleen indices in mice, which indicated obviously strengthening the non-specific immunity in jujube-treated mice (Li et al., 2011). Furthermore, the authors described that jujube at various concentrations (30–200 μg/ml) showed a significant dose-dependent promotion of splenocyte proliferation, with the highest response at ∼100% increase. The effect of jujube extract on anti-complementary activity was also reported. Jujube extracts (25 and 125 μg/ml) exhibited ability to interact with the complement cascade (Li et al., 2011). This finding indicates the activation of innate immune system in jujube-treated cells. In support of this notion, the water-soluble polysaccharide isolated from jujube stimulated proliferation of lymphocyte. In particular, the application of jujube polysaccharide at various concentrations (10–100 μg/ml) onto cultured lymphocytes for 3 days demonstrated an enlarging cell volume and an increasing cell number (Zhao et al., 2008). Moreover, jujube extracts at different concentrations of 0–3 mg/ml were applied onto cultured RAW 264.7 cells for 24 h. The extract dose-dependently stimulated the expressions of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, and the highest effect was induced at ∼7-fold, ∼9-fold, and 4-fold, respectively (Chen et al., 2014a).
The hydroalcoholic extract of jujube at 200–400 mg/kg was applied onto acute and chronic rat models of inflammation. The study revealed that jujube extract markedly declined granuloma tissue formation, as compared with model rat. Serum nitrite/nitrate level was notably up regulated in inflammatory rat; while pre-treatment with jujube significantly reduced the increased level of nitrite/nitrate (Goyal et al., 2011). These findings suggest the anti-inflammatory effects of jujube. In line with this, the applied jujube extract at 100–500 μg/ml inhibited nitric oxide production and splenocyte proliferation on the inflammatory activated cells (Yu et al., 2012). The excessive induction of pro-inflammatory cytokines, i.e., IL-1β and IL-6, also contributes to chronic inflammation. In the lipopolysaccharide (LPS)-induced macrophages, the pre-treatment with jujube water extract repressed the expressions of IL-1β and IL-6 (Chen et al., 2014a). Besides, the triterpene acid fraction of jujube at a dose higher than 10 μg/ml was able to inhibit the production TNF-α (Yu et al., 2012). NF-κB is one of main transcription factors that has vital role in controlling pro-inflammatory cytokine production (Du et al., 2013). The treatment with jujube extract slightly inhibited NF-κB activity. On the other hand, the pre-treatment with jujube water extract (0–3 mg/ml) for 3 h in cultured macrophages, before the addition of LPS at 1 μg/ml for 24 h, dose-dependently repressed the activation of NF-κB activity. The reduction at 60% was observed under the pre-treatment of extract at 3 mg/ml (Chen et al., 2014a). In parallel, the anti-inflammatory property of jujube was shown in colitis-associated colon cancer mice (Periasamy et al., 2020). In this study, mice were injected with azoxymethane followed by three cycles of dextran sulfate sodium, as to induce colitis-associated colon, before the intake of jujube extract for 70 days. The results showed that dietary jujube intake could attenuate inflammation in model mice. Moreover, the extract of jujube markedly inhibited the protein expressions of IL-6, NF-κB, JAK1, and STAT3 in colon tissues, as compared with model group. This result suggests that jujube can suppress the stimulation of NF-κB/IL-6/JAK1/STAT3 signaling (Periasamy et al., 2020).
Blood is circulating within the blood vessels, and the effects of jujube in blood circulation are summarized here. The pre-incubation of jujube extract (30, 100, and 300 mg/ml) for 5 min at 37°C in platelet-rich plasma was reported to suppress collagen (2 mg/ml)-, thrombin (0.4 U/ml)-, and arachidonic acid (100 mM)-induced aggregation of platelets (Seo et al., 2013). Jujuboside B from jujube markedly inhibited platelet aggregation, and which was considered as one of the active ingredients in processing anti-platelet effect (Seo et al., 2013). In animal model, angiotensin II was intravenously injected into rats to induce acute hypertension, which characterized by cardiovascular parameters, i.e., notably elevated systolic blood pressure and mean arterial pressure, as well as the decline of heart rate (HR) compared with control group. Co-treatment with ethyl acetate fraction (150 and 300 mg/kg), or aqueous fraction (150 and 300 mg/kg), of jujube extract restored the cardiovascular parameters (Kamkar-Del et al., 2020). In line with this notion, Mohebbati et al. (2018) reported protective effect of jujube extract on hypertensive rats. The rats were treated with hydroalcoholic extracts of jujube at various concentrations (from 100 to 400 mg/kg) for four weeks, and then L-NAME (10 mg/kg) was injected intravenously into rats to induce hypertension. The results showed that jujube extract attenuated blood pressure and mean arterial pressure in L-NAME-induced hypertensive rats (Mohebbati et al., 2018). Betulinic acid, found in Zizyphi Spinosi Semen and jujube, possessed combined properties of inducing endothelial nitric oxide synthase and decreasing nicotinamide adenine dinucleotide phosphate oxidase. In human endothelial cells treated with betulinic acid, the endothelial nitric oxide synthase expression and nitric oxide production were robustly increased (Steinkamp-Fenske et al., 2007). In addition, a triple-masked randomized controlled clinical trial revealed that jujube was well tolerated in general, and which might possess potential beneficial roles on serum lipid profile (Sabzghabaee et al., 2013).
Jujube is not only consumed as daily food, but also prescribed as a tonic TCM for blood nourishment in a formulated decoction. Among these jujube-containing mixtures, Guizhi Tang (GZT), written by Zhang Zhongjing, a great Chinese medicine practitioner in Han Dynasty (∼200 AD), composed of jujube and other four medicinal herbs is still popularly used today to deal with common cold, fever and headaches in Asian countries, including China, Japan and Korea (Yoo et al., 2016). GZT belongs to exterior-releasing formula that is able to dispel pathogenic factors from superficies of body, and the effect of jujube within this formula is believed to tonify “Qi” and to replenish “Blood” of the body resulting from corresponding pathological changes. Yoo et al. (2016) reported that application of GZT extract in cultured RAW264.7 macrophages showed anti-inflammatory activity, which involved in blocking ERK and NF-κB signaling pathways. The cells were pre-treated with GZT (31.25–1,000 μg/ml) for 4 h prior to application of LPS for an additional 20 h. The treatment with GZT extract enhanced the expression of HO-1 and significantly inhibited pro-inflammatory cytokines, e.g., TNF-α and IL-6, in LPS-induced macrophages. Besides, GZT extract prevented ERK phosphorylation and NF-κB translocation in LPS-treated macrophages (Yoo et al., 2016). In another experiment, Lam et al. (2016) investigated the inductive roles of GZT on EPO expression in cultures. Cultured Hep3B cells were treated with GZT extracts (0.5–4.0 mg/ml) for 24 h. Applied GZT was able to stimulate the mRNA and protein expressions of EPO. In addition, GZT stimulated the transcriptional activity of HRE in a dose-dependent manner (Lam et al., 2016). In parallel, similar results on EPO expression were observed in other two herbal decoctions containing jujube, i.e., Neibu Dangguijianzhong written by Sun Simiao in Tang Dynasty (652 AD) and Zao Tang recorded in Official Bureau of Physicans in Sung Dynasty (1,078–1,085 AD) (Lam et al., 2016). In Zhigancao Tang, jujube combining with ginseng was used to tonify the “Qi.” Besides, jujube was proposed to regulate the relationship between protective and nutritive “Qi.” Nevertheless, jujube showed similar function with other herbal formulae in treating “Qi” and “Blood” deficiency, e.g., Renshen Yangying Tang, Bazhen Tang and Xiangbei Yangying Tang.
In addition to formulated decoction, jujube is also commonly supplemented with other foods to achieve health benefits. Jeong and Kim (2019) investigated the effect of jujube and chokeberry diet in high-fat and high-fructose diet-induced dyslipidemia in animal studies. Jujube (0.5%) and chokeberry powder (0.5%) were mixed to animal diet. After 10 weeks of dietary treatment, jujube and chokeberry significantly ameliorated high-fat and high-fructose diet-induced dyslipidemia and improved insulin resistance (Jeong and Kim, 2019). In support of this finding, the consumption of mixed jujube, almond and rice in healthy human showed a significantly lower glucose level, as compared to those with rice as reference (Zhu et al., 2018).
Herbal cuisine is a practice in achieving the therapeutic functions by using natural herbs, especially the edible and medicinal dual-purpose herbs as materials during cooking processes. Jujube has been considered as a favorite fruit in daily life for its health properties spanning thousands of years. In practice of herbal cuisine, jujube is one of common materials that is considered as boosting or nourishing type of food. It can be taken into decoction for daily consumption, or which can be taken together with other foods to prepare delicious soup. According to the aforesaid cellular and animal findings, jujube has a promising potential in developing medicinal food and supplement for prevention against anemia, cancer, inflammation and iron/vitamin deficiency.
Jujube-containing herbal decoctions are routinely recorded in Jingui Yaolue by Zhang Zhongjing, which are prescribed to address various ailments. One-sixth of prescriptions described in this ancient classic book contain jujube, in which jujube is commonly served as assistant or courier medicinal herb with a herbal formulated decoction, according to the theory of TCM. The intake of jujube is believed to increase blood supply to the spleen meridian that further improves nutrient uptake and strengthens the immune system. Clinically, several controlled trials have revealed that jujube is a safe and effective herb for human consumption (Naftali et al., 2008; Ebrahimimd et al., 2011; Sabzghabaee et al., 2013); however, there is currently no human study on the blood deficiency effects. In addition, there are no known toxicity and drug interaction being reported clinically for consumption of jujube.
Apart from the medicinal application, fresh immature jujubes are widely consumed as fruits, and the dried fruits are also eaten as a snack, or with tea. In China, jujube has been made into a wide range of products, i.e., juice, vinegar and wine. In southern part of India, jujube is mixed with tamarind, jaggery, salt and chilies, and then pounded into cakes. In Lebanon and Persia, jujube is used as digestive aid being consumed with the desserts. In Morocco, the honey obtained from jujube extract is believed to be beneficial to sore throats (Lim, 2013). Additional of natural herbal extracts to dairy products has increasingly popular because of their health benefits. Feng et al. (2019) demonstrated a new goat dairy product adding of jujube pulp as ingredients with satisfactory nutritional quality and sensory property, which provided alternative approach in developing a unique goat dairy product with high nutritional properties. The water extract of immature jujube extract showed better activity in stimulating transcriptional activity of HRE than that of mature jujube (Chen et al., 2015). These findings indicate the maturity of jujube should therefore be taken into consideration when it is being prepared for health food supplements.
In folk medicine, three pieces of jujube are recommended to be consumed daily, about 15 g of dried weight in total. The content of benefit ingredients within jujube could be changed robustly between fresh jujubes and dried ones (Guo et al., 2015; USDA, 2012; Chen et al., 2013). The dietary nutrient facts of main ingredients in fresh and dried jujubes are summarized in Supplementary Table S1, which provides an appropriate recommendation for selection of different forms of jujube for certain health benefits. In addition, it has been reported that the content of bio-active ingredients, including nucleotide, flavonoid and polysaccharide, varied among different jujube cultivars (Chen et al., 2013), as indicated in Supplementary Table S2. Jujubes from Shanxi, Shaanxi, Hebei, Xinjiang, Shandong, Ningxia provinces of China had higher chemical amounts, which might contribute better biological functions and could be a good choice of selection.
JC and KT: Concept, design, literature search and manuscript review. JC: acquisition of data, drafting the manuscript. All authors have read and approved the manuscript.
This work is supported by Natural Science Foundation of Guangdong Province (2018A030313305), Natural Science Foundation of China (81804052), Shenzhen Science and Technology Plan Project (JSGG20191129102216637 and ZDSYS201606081515458), Traditional Chinese Medicine Bureau of Guangdong Province (20201320) to JC. Shenzhen Science and Technology Innovation Committee (ZDSYS201707281432317; JCYJ20170413173747440; JCYJ20180306174903174), China Post-doctoral Science Foundation (2019M653087), Zhongshan Municipal Bureau of Science and Technology (ZSST20SC03); Guangzhou Science and Technology Committee Research Grant (GZSTI16SC02; GZSTI17SC02); Hong Kong RGC Theme-based Research Scheme (T13-605/18-W); Hong Kong Innovation Technology Fund (UIM/340, UIM/385, ITS/500/18FP; TCPD/17-9); TUYF19SC02, PD18SC01 and HMRF18SC06 to KT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.593655/full#supplementary-material
Al-Reza, S. M., Yoon, J. I., Kim, H. J., Kim, J. S., and Kang, S. C. (2010). Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food Chem. Toxicol. 48, 639–643. doi:10.1016/j.fct.2009.11.045 | PubMed Abstract
Almeer, R. S., Mahmoud, S. M., Amin, H. K., and Abdel, M. A. (2018). Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol. 115, 49–62. doi:10.1016/j.fct.2018.03.002 | PubMed Abstract
Argyrousi, E. K., Heckman, P., and Prickaerts, J. (2020). Role of cyclic nucleotides and their downstream signaling cascades in memory function: being at the right time at the right spot. Neurosci. Biobehav. Rev. 113, 12–38. doi:10.1016/j.neubiorev.2020.02.004 | PubMed Abstract
Ascensao, J. L., Bilgrami, S., and Zanjani, E. D. (1991). Erythropoietin. Biology and clinical applications. Am. J. Pediatr. Hematol. Oncol. 13, 376–387. doi:10.1097/00043426-199124000-00002PubMed Abstract
Awad, D. S., Ali, R. M., Mhaidat, N. M., and Shotar, A. M. (2014). Zizyphus jujuba protects against ibuprofen-induced nephrotoxicity in rats. Pharm. Biol. 52, 182–186. doi:10.3109/13880209.2013.821665 | PubMed Abstract
Batchelor, E. K., Kapitsinou, P., Pergola, P. E., Kovesdy, C. P., and Jalal, D. I. (2020). Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J. Am. Soc. Nephrol. 31, 456–468. doi:10.1681/ASN.2019020213PubMed Abstract
Chen, J., Chan, P. H., Lam, C. T., Li, Z., Lam, K. Y., Yao, P., et al. (2015). Fruit of Ziziphus jujuba (Jujube) at two stages of maturity: distinction by metabolic profiling and biological assessment. J. Agric. Food Chem. 63, 739–744. doi:10.1021/jf5041564 | PubMed Abstract
Chen, J., Du, C. Y., Lam, K. Y., Zhang, W. L., Lam, C. T., Yan, A. L., et al. (2014a). The standardized extract of Ziziphus jujuba fruit (jujube) regulates pro-inflammatory cytokine expression in cultured murine macrophages: suppression of lipopolysaccharide-stimulated NF-kappaB activity. Phytother Res. 28, 1527–1532. doi:10.1002/ptr.5160
Chen, J., Lam, C. T., Kong, A. Y., Zhang, W. L., Zhan, J. Y. X., Bi, C. W. C., et al. (2014b). The extract of Ziziphus jujuba fruit (jujube) induces expression of erythropoietin via hypoxia-inducible factor-1alpha in cultured Hep3B cells. Planta Med. 80, 1622–1627. doi:10.1055/s-0034-1383049 | PubMed Abstract
Chen, J., Maiwulanjiang, M., Lam, K. Y., Zhang, W. L., Zhan, J. Y., Lam, C. T. W., et al. (2014c). A standardized extract of the fruit of Ziziphus jujuba (Jujube) induces neuronal differentiation of cultured PC12 cells: a signaling mediated by protein kinase a. J. Agric. Food Chem. 62, 1890–1897. doi:10.1021/jf405093f | PubMed Abstract
Chen, J., Lam, C. T., Li, Z., Yao, P., Lin, H., Dong, T. T. X., et al. (2016). Extract of Ziziphus jujuba fruit (Jujube) stimulates expression of enzymes responsible for heme recycle via anti-oxidant response element in cultured murine macrophages. Phytother Res. 30, 267–271. doi:10.1002/ptr.5526
Chen, M., Wu, J., Shi, S., Chen, Y., Wang, H., Fan, H., et al. (2016). Structure analysis of a heteropolysaccharide from Taraxacum mongolicum Hand.-Mazz. and anticomplementary activity of its sulfated derivatives. Carbohydr. Polym. 152, 241–252. doi:10.1016/j.carbpol.2016.06.110 | PubMed Abstract
Chen, J., Li, Z., Maiwulanjiang, M., Zhang, W. L., Zhan, J. Y., Lam, C. T. W., et al. (2013). Chemical and biological assessment of Ziziphus jujuba fruits from China: different geographical sources and developmental stages. J. Agric. Food Chem. 61, 7315–7324. doi:10.1021/jf402379u | PubMed Abstract
Chen, J., Wang, F., Huang, S., Liu, X., Li, Z., Qi, A., et al. (2019). Jian-Pi-Yi-Shen decoction relieves renal anemia in 5/6 nephrectomized rats: production of erythropoietin via hypoxia inducible factor signaling. Evid. Based Complement. Alternat. Med. 2019 (12), 1–8. doi:10.1155/2019/2807926 | PubMed Abstract
Cheng, G., Bai, Y., Zhao, Y., Tao, J., Liu, Y., Tu, G., et al. (2000). Flavonoids from Ziziphus jujuba Mill var. Spinosa. Tetrahedron 56, 8915–8920. doi:10.1016/S0040-4020(00cxv)00842-5
Chi, Y. F., and Zhang, Z. (2009). Antimelancholic medicine prepared from jujube cAMP materials. Canada CA2707192A1
Choi, S. H., Ahn, J. B., Kozukue, N., Levin, C. E., and Friedman, M. (2011). Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of Jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 59, 6594–6604. doi:10.1021/jf200371r
Du, C. Y., Choi, R. C., Zheng, K. Y., Dong, T. T., Lau, D. T., and Tsim, K. W. K. (2013). Yu ping feng san, an ancient Chinese herbal decoction containing Astragali Radix, Atractylodis Macrocephalae rhizoma and saposhnikoviae Radix, regulates the release of cytokines in murine macrophages. PLoS One 8, e78622. doi:10.1371/journal.pone.0078622 | PubMed Abstract
Ebrahimimd, S., Ashkani-Esfahani, S., and Poormahmudibs, A. (2011). Investigating the efficacy of Zizyphus jujuba on neonatal jaundice. Iran J. Pediatr. 21, 320–324.
Fazio, A., La Torre, C., Caroleo, M. C., Caputo, P., Plastina, P., and Cione, E. (2020). Isolation and purification of glucans from an Italian cultivar of Ziziphus jujuba Mill. and in vitro effect on skin repair. Molecules 25, 968. doi:10.3390/molecules25040968 | PubMed Abstract
Feng, C., Wang, B., Zhao, A., Wei, L., Shao, Y., and Wang, Y. (2019). Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 277, 238–245. doi:10.1016/j.foodchem.2018.10.104 | PubMed Abstract
Gao, Q. H., Wu, C. S., and Wang, M. (2013). The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 61, 3351–3363. doi:10.1021/jf4007032 | PubMed Abstract
Gao, Q. H., Wu, C. S., Yu, J. G., Wang, M., Ma, Y. J., and Li, C.-L. (2012). Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J. Food Sci. 77, C1218–C1225. doi:10.1111/j.1750-3841.2012.02946.x
Gao, Q. T., Cheung, J. K., Li, J., Jiang, Z. Y., Chu, G. K., and Duan, R. (2007). A Chinese herbal decoction, Danggui Buxue Tang, activates extracellular signal-regulated kinase in cultured T-lymphocytes. FEBS Lett. 581, 5087–5093. doi:10.1016/j.febslet.2007.09.053 | PubMed Abstract
Goldberg, M. A., Gaut, C. C., Schapira, L., Antin, J. H., Ransil, B. J., and Antman, K. H. (1991). Erythropoietin gene regulation: from the laboratory to the bedside. Contrib. Nephrol. 88 (35-45), 46–47. doi:10.1159/000419514 | PubMed Abstract
Gottlieb, Y., Topaz, O., Cohen, L. A., Yakov, L. D., Haber, T., and Morgenstern, A. (2012). Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro. Haematologica 97, 994–1002. doi:10.3324/haematol.2011.057620 | PubMed Abstract
Goyal, R., Sharma, P. L., and Singh, M. (2011). Possible attenuation of nitric oxide expression in anti-inflammatory effect of Ziziphus jujuba in rat. J. Nat. Med. 65, 514–518. doi:10.1007/s11418-011-0531-0 | PubMed Abstract
Guo, S., Duan, J. A., Qian, D., Tang, Y., Qian, Y., and Wu, D. (2013). Rapid determination of amino acids in fruits of Ziziphus jujuba by hydrophilic interaction ultra-high-performance liquid chromatography coupled with triple-quadrupole mass spectrometry. J. Agric. Food Chem. 61, 2709–2719. doi:10.1021/jf305497r
Guo, S., Duan, J. A., Zhang, Y., Qian, D., Tang, Y., Zhu, Z., et al. (2015). Contents changes of triterpenic acids, nucleosides, nucleobases, and saccharides in jujube (Ziziphus jujuba) fruit during the drying and steaming process. Molecules 12 (20), 22329–22340. doi:10.3390/molecules201219852
Hanabusa, K., Cyong, J., and Takahashi, M. (1981). High-level of cyclic AMP in the jujube plum. Planta Med. 42, 380–384. doi:10.1055/s-2007-971659 | PubMed Abstract
Heo, H. J., Park, Y. J., Suh, Y. M., Choi, S. J., Kim, M. J., and Cho, H.-Y. (2003). Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci. Biotechnol. Biochem. 67, 1284–1291. doi:10.1271/bbb.67.1284 | PubMed Abstract
Huang, X., Kojima-Yuasa, A., Norikura, T., Kennedy, D. O., Hasuma, T., and Matsui-Yuasa, I. (2007). Mechanism of the anti-cancer activity of Zizyphus jujuba in HepG2 cells. Am. J. Chin. Med. 35, 517–532. doi:10.1142/S0192415X0700503X | PubMed Abstract
Jelkmann, W. (1992). Erythropoietin: structure, control of production, and function. Physiol. Rev. 72, 449–489. doi:10.1152/physrev.1992.72.2.449
Jeong, O., and Kim, H. S. (2019). Dietary chokeberry and dried jujube fruit attenuates high-fat and high-fructose diet-induced dyslipidemia and insulin resistance via activation of the IRS-1/PI3K/Akt pathway in C57BL/6 J mice. Nutr. Metab. 16, 38. doi:10.1186/s12986-019-0364-5 | PubMed Abstract
Ji, X., Hou, C., Yan, Y., Shi, M., and Liu, Y. (2020). Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 149, 1008–1018. doi:10.1016/j.ijbiomac.2020.02.018 | PubMed Abstract
Kamkar-Del, Y., Mohebbati, R., Hosseini, M., Khajavirad, A., Shafei, M. N., and Rakhshandeh, H. (2020). Ethyl acetate and aqueous fractions of Ziziphus jujuba prevent acute hypertension induced by angiotensin II in rats. Cardiovasc. Hematol. Disord. Drug Targets 20, 108–115. doi:10.2174/1871529X19666191119141400 | PubMed Abstract
Klei, T. R., Meinderts, S. M., van den Berg, T. K., and van Bruggen, R. (2017). From the cradle to the grave: the role of macrophages in erythropoiesis and erythrophagocytosis. Front. Immunol. 8, 73. doi:10.3389/fimmu.2017.00073 | PubMed Abstract
Kovtunovych, G., Eckhaus, M. A., Ghosh, M. C., Ollivierre-Wilson, H., and Rouault, T. A. (2010). Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood 116, 6054–6062. doi:10.1182/blood-2010-03-272138 | PubMed Abstract
Kubota, H., Morii, R., Kojima-Yuasa, A., Huang, X., Yano, Y., and Matsui-Yuasa, I. (2009). Effect of Zizyphus jujuba extract on the inhibition of adipogenesis in 3T3-L1 preadipocytes. Am. J. Chin. Med. 37, 597–608. doi:10.1142/S0192415X09007089
Lam, C., Chan, P. H., Lee, P., Lau, K. M., Kong, A., and Gong, A. G. W. (2016). Chemical and biological assessment of jujube (Ziziphus jujuba)-containing herbal decoctions: induction of erythropoietin expression in cultures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1026, 254–262. doi:10.1016/j.jchromb.2015.09.021
Li, B., Wang, L., Liu, Y., Chen, Y., Zhang, Z., and Zhang, J. (2013). Jujube promotes learning and memory in a rat model by increasing estrogen levels in the blood and nitric oxide and acetylcholine levels in the brain. Exp Ther Med 5, 1755–1759. doi:10.3892/etm.2013.1063 | PubMed Abstract
Li, J., Ding, S., and Ding, X. (2005). Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochem. 40, 3607–3613. doi:10.1016/j.procbio.2005.03.005
Li, J., Fan, L., Ding, S., and Ding, X. (2007). Nutritional composition of five cultivars of Chinese jujube. Food Chem. 103, 454–460. doi:10.1016/j.foodchem.2006.08.016
Li, J., Shan, L., Liu, Y., Fan, L., and Ai, L. (2011). Screening of a functional polysaccharide from Zizyphus jujuba cv. jinsixiaozao and its property. Int. J. Biol. Macromol. 49, 255–259. doi:10.1016/j.ijbiomac.2011.04.006 | PubMed Abstract
Li, S. P., Zhao, K. J., Ji, Z. N., Song, Z. H., Dong, T. T., and Lo, C. K. (2003). A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 73, 2503–2513. doi:10.1016/s0024-3205(03)00652-0
Lim, T. K. (2013). Edible medicinal and non-medicinal plants. Dordrecht, the Netherlands: Springer Science+Business Media
Miao, M., Miao, Y., and Fang, X. (2010). Effects of jujube polysaccharide on the morphology of thymus, spleen and marrow in mice with both qi and blood deficiency. Pharmacol. Clin. Chin. Mater. Med. 26, 42–46.
Miao, M., Miao, Y., and Sun, Y. (2006). Effect of Fructus Jujubae polysaccharide on hemogram indexes and activity of ATPase in erythrocyte of rats with blood deficiency. Chin. J. Clin. Rehabil. 10, 97–99.
Mitsuhashi, Y., Furusawa, Y., Aradate, T., Zhao, Q. L., Moniruzzaman, R., and Kanamori, M. (2017). 3-O-trans-p-coumaroyl-alphitolic acid, a triterpenoid from Zizyphus jujuba, leads to apoptotic cell death in human leukemia cells through reactive oxygen species production and activation of the unfolded protein response. PLoS One 12, e183712. doi:10.1371/journal.pone.0183712
Mohebbati, R., Bavarsad, K., Rahimi, M., Rakhshandeh, H., Khajavi, R. A., and Shafei, M. N. (2018). Protective effects of long-term administration of Ziziphus jujuba fruit extract on cardiovascular responses in L-NAME hypertensive rats. Avicenna J. Phytomed. 8, 143–151.
Motohashi, H., and Yamamoto, M. (2004). Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 10, 549–557. doi:10.1016/j.molmed.2004.09.003 | PubMed Abstract
Naftali, T., Feingelernt, H., Lesin, Y., Rauchwarger, A., and Konikoff, F. M. (2008). Ziziphus jujuba extract for the treatment of chronic idiopathic constipation: a controlled clinical trial. Digestion 78, 224–228. doi:10.1159/000190975 | PubMed Abstract
Ninave, P. B., and Patil, S. D. (2019). Antiasthmatic potential of Zizyphus jujuba Mill. and Jujuboside B. - possible role in the treatment of asthma. Respir. Physiol. Neurobiol. 260, 28–36. doi:10.1016/j.resp.2018.12.001 | PubMed Abstract
Nishimura, K., Matsumoto, R., Yonezawa, Y., and Nakagawa, H. (2017). Effect of quercetin on cell protection via erythropoietin and cell injury of HepG2 cells. Arch. Biochem. Biophys. 636, 11–16. doi:10.1016/j.abb.2017.10.013 | PubMed Abstract
Pahuja, M., Mehla, J., Reeta, K. H., Joshi, S., and Gupta, Y. K. (2011). Hydroalcoholic extract of Zizyphus jujuba ameliorates seizures, oxidative stress, and cognitive impairment in experimental models of epilepsy in rats. Epilepsy Behav. 21, 356–363. doi:10.1016/j.yebeh.2011.05.013
Pawlowska, A. M., Camangi, F., Bader, A., and Braca, A. (2009). Flavonoids of Zizyphus jujuba L. And Zizyphus spina-christi (L.) willd (Rhamnaceae) fruits. Food Chem. 112, 858–862. doi:10.1016/j.foodchem.2008.06.053 | PubMed Abstract
Periasamy, S., Wu, W. H., Chien, S. P., Liu, C. T., and Liu, M. Y. (2020). Dietary Ziziphus jujuba fruit attenuates colitis-associated tumorigenesis: a pivotal role of the NF-kappaB/IL-6/JAK1/STAT3 pathway. Nutr Cancer 72, 120–132. doi:10.1080/01635581.2019.1615515 | PubMed Abstract
Post, D. E., and Van Meir, E. G. (2001). Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 8, 1801–1807. doi:10.1038/sj.gt.3301605 | PubMed Abstract
Reche, J., Almansa, M. S., Hernandez, F., Carbonell-Barrachina, A. A., Legua, P., and Amorós, A. (2019). Fatty acid profile of peel and pulp of Spanish jujube (Ziziphus jujuba Mill.) fruit. Food Chem. 295, 247–253. doi:10.1016/j.foodchem.2019.05.147 | PubMed Abstract
Sabzghabaee, A. M., Khayam, I., Kelishadi, R., Ghannadi, A., Soltani, R., and Badri, S. (2013). Effect of Zizyphus jujuba fruits on dyslipidemia in obese adolescents: a triple-masked randomized controlled clinical trial. Med. Arch. 67, 156–159. doi:10.5455/medarh.2013.67.156-160
San, B., and Yildirim, A. N. (2010). Phenolic, alpha-tocopherol, beta-carotene and fatty acid composition of four promising jujube (Ziziphus jujuba Miller) selections. J. Food Compost. Anal. 23, 706–710. doi:10.1016/j.jfca.2010.02.008
Schuster, S. J., Badiavas, E. V., Costa-Giomi, P., Weinmann, R., Erslev, A. J., and Caro, J. (1989). Stimulation of erythropoietin gene transcription during hypoxia and cobalt exposure. Blood 73, 13–16.
Seo, E. J., Lee, S. Y., Kang, S. S., and Jung, Y. S. (2013). Zizyphus jujuba and its active component jujuboside B inhibit platelet aggregation. Phytother Res. 27, 829–834. doi:10.1002/ptr.4809 | PubMed Abstract
Shi, X. Q., Yue, S. J., Tang, Y. P., Chen, Y. Y., Zhou, G. S., and Zhang, J. (2019). A network pharmacology approach to investigate the blood enriching mechanism of Danggui buxue Decoction. J. Ethnopharmacol. 235, 227–242. doi:10.1016/j.jep.2019.01.027 | PubMed Abstract
Steinkamp-Fenske, K., Bollinger, L., Xu, H., Yao, Y., Horke, S., and Förstermann, U. (2007). Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J. Pharmacol. Exp. Therapeut. 322, 836–842. doi:10.1124/jpet. 107.123356 | PubMed Abstract
USDA (2012). USDA nutrient database for standard reference. Riverdale, MD: USDA, Nutrient Data Laboratory, Agricultural Research Service
Xu, M., Nagati, J. S., Xie, J., Li, J., Walters, H., and Moon, Y.-A. (2014). An acetate switch regulates stress erythropoiesis. Nat. Med. 20, 1018–1026. doi:10.1038/nm.3587 | PubMed Abstract
Xu, Y.-L., Miao, M.-S., Sun, Y.-H., and Miao, Y.-Y. (2004). Effect of Fructus Jujubae polysaccharide on the hematopoietic function in mice model of both qi and blood deficiencies. Chin. J. Clin. Rehabil. 8, 5050–5051.
Xu, Y., Tao, Z., Jin, Y., Yuan, Y., Dong, T., and Tsim, K. W. K. (2018). Flavonoids, a potential new insight of leucaena leucocephala foliage in ruminant health. J. Agric. Food Chem. 66, 7616–7626. doi:10.1021/acs.jafc.8b02739 | PubMed Abstract
Xue, Q., Liu, Y., He, R., Yang, S., Tong, J., and Li, X. (2016). Lyophilized powder of catalpol and puerarin protects neurovascular unit from stroke. Int. J. Biol. Sci. 12, 367–380. doi:10.7150/ijbs.14059 | PubMed Abstract
Yang, Q., Li, Y., Chen, Y., Weng, X., Cai, W. Y., Qi, L., et al. (2016). Protective effect of extract of jujubae fructus on iron deficiency anemia in rats. Chin. J. Experi. Trad. Med. Formul. 23, 102–109.
Yazdanpanah, Z., Ghadiri-Anari, A., Mehrjardi, A. V., Dehghani, A., Zardini, H. Z., and Nadjarzadeh, A. (2017). Effect of Ziziphus jujube fruit infusion on lipid profiles, glycaemic index and antioxidant status in type 2 diabetic patients: a randomized controlled clinical trial. Phytother Res. 31, 755–762. doi:10.1002/ptr.5796
Yoo, S. R., Kim, Y., Lee, M. Y., Kim, O. S., Seo, C. S., Shin, H.-K., et al. (2016). Gyeji-tang water extract exerts anti-inflammatory activity through inhibition of ERK and NF-κB pathways in lipopolysaccharide-stimulated RAW 264.7 cells. BMC Compl. Alternative Med. 16, 390. doi:10.1186/s12906-016-1366-8 | PubMed Abstract
Yoon, J. I., Al-Reza, S. M., and Kang, S. C. (2010). Hair growth promoting effect of Zizyphus jujuba essential oil. Food Chem. Toxicol. 48, 1350–1354. doi:10.1016/j.fct.2010.02.036
Yu, L., Jiang, B. P., Luo, D., Shen, X. C., Guo, S., Duan, J. A., et al. (2012). Bioactive components in the fruits of Ziziphus jujuba Mill. Against the inflammatory irritant action of Euphorbia plants. Phytomedicine 19, 239–244. doi:10.1016/j.phymed.2011.09.071 | PubMed Abstract
Zhao, Z., Li, J., Wu, X., Dai, H., Gao, X., Liu, M., et al. (2006). Structures and immunological activities of two pectic polysaccharides from the fruits of Ziziphus jujuba Mill. cv. jinsixiaozao Hort. Food Res. Int. 39, 917–923. doi:10.1016/j.foodres.2006.05.006
Zhao, Z., Liu, M., and Tu, P. (2008). Characterization of water soluble polysaccharides from organs of Chinese jujube (Ziziphus jujuba Mill. cv. dongzao). Eur. Food Res. Technol. 226, 985–989. doi:10.1007/s00217-007-0620-1
Zheng, K. Y., Choi, R. C., Cheung, A. W., Guo, A. J., Bi, C. W., Zhu, K. Y., et al. (2011). Flavonoids from Radix Astragali induce the expression of erythropoietin in cultured cells: a signaling mediated via the accumulation of hypoxia-inducible factor-1α. J. Agric. Food Chem. 59, 1697–1704. doi:10.1021/jf104018u | PubMed Abstract
Zhu, R., Fan, Z., Dong, Y., Liu, M., Wang, L., and Pan, H. (2018). Postprandial glycaemic responses of dried fruit-containing meals in healthy adults: results from a randomised trial. Nutrients 10, 694. doi:10.3390/nu10060694 | PubMed Abstract
Keywords: Ziziphus jujuba, Rhamnaceae, blood deficiency, bio-active ingredient, food supplement
Citation: Chen J and Tsim KWK (2020) A Review of Edible Jujube, the Ziziphus jujuba Fruit: A Heath Food Supplement for Anemia Prevalence. Front. Pharmacol. 11:593655. doi: 10.3389/fphar.2020.593655
Received: 11 August 2020; Accepted: 08 October 2020;
Published: 26 November 2020.
Edited by:
Marcello Locatelli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Jian Huang, Northwest A and F University, ChinaCopyright © 2020 Chen and Tsim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Chen, bHljanBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.