
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 21 August 2020
Sec. Multiple Sclerosis and Neuroimmunology
Volume 11 - 2020 | https://doi.org/10.3389/fneur.2020.00909
This article is part of the Research Topic COVID-19 in CNS and PNS: Basic and Clinical Focus on the Mechanisms of Infection and New Tools for the Therapeutic Approach View all 26 articles
 Antonio Zito1*
Antonio Zito1* Enrico Alfonsi2
Enrico Alfonsi2 Diego Franciotta3
Diego Franciotta3 Massimiliano Todisco1,2
Massimiliano Todisco1,2 Matteo Gastaldi3
Matteo Gastaldi3 Matteo Cotta Ramusino1,4
Matteo Cotta Ramusino1,4 Mauro Ceroni1,4
Mauro Ceroni1,4 Alfredo Costa1,4
Alfredo Costa1,4During the recent coronavirus disease 2019 (COVID-19) outbreak in Northern Italy, we observed a 57-year-old man developing acute motor-sensory axonal neuropathy, a variant of Guillain–Barré syndrome (GBS), 12 days after severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. Similarly to other bacterial and viral infections, dysregulation of the immune system due to post-infectious mechanisms, such as the molecular mimicry, could lead to an indirect damage of the peripheral nervous system related to SARS-CoV-2. GBS causes motor dysfunctions that are not easily recognizable in non-neurological settings or in patients requiring ventilatory assistance. Several reports also suggested that GBS and Miller Fisher syndrome (MFS) could be neurological complications of COVID-19. Therefore, we performed a review of the 29 articles so far published, describing 33 GBS cases and five MFS cases associated with SARS-CoV-2 infection. We recommend awareness of this rare, but treatable, neurological syndrome, which may also determine a sudden and otherwise unexplained respiratory deterioration in COVID-19 patients.
The coronavirus disease 2019 (COVID-19) outbreak started at the end of 2019 in Wuhan, the capital of Hubei province, in China. The novel coronavirus was designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). To date, millions of cases have been confirmed worldwide, and Italy has been one of the most affected countries.
Neurological manifestations have been described in one third of patients with COVID-19. Some of these neurological symptoms have proved to be quite specific, e.g., loss of smell or taste, but other ones are non-specific, e.g., headache, dizziness, or reduced level of consciousness (1). However, whether the neurological symptoms associated with SARS-CoV-2 are attributable to secondary mechanisms (i.e., multiorgan dysfunction or systemic inflammation), an abnormal immune response or the direct injury of the virus is still unknown.
Recently, several case reports have suggested a relationship between the occurrence of Guillain–Barré syndrome (GBS) and a previous SARS-CoV-2 infection, which preceded the GBS onset by up to 4 weeks. Therefore, a post-infectious dysregulation of the immune system, triggered by SARS-CoV2, appears to be the most probable cause.
Conversely, a recent pathological study on postmortem human brain tissues found that anosmia and dysgeusia, described in up to 20% of patients, are more likely due to the direct viral invasion of the olfactory nerve and bulb (2). In addition and in line with this observation, a COVID-19 patient with anosmia showed magnetic resonance imaging (MRI) abnormalities in the olfactory bulb and in the inferior frontal lobe, as a conceivable result of the direct invasion of SARS-CoV-2 through the olfactory pathway via trans-synaptic retrograde spreading (3).
Here we describe a patient with an axonal variant of GBS following COVID-19, and we review the available reports in the literature on other GBS cases related to SARS-CoV-2 infection.
A 57-year-old man developed dysgeusia, cough, and fever of up to 39°C lasting for 5 days. At 12 days after the resolution of the symptoms, he complained of numbness and tingling in the feet and, a few days later, also in the hands. Over 10 days, the patient developed distal limb weakness and severe gait impairment, so he was referred to the emergency department. A neurological examination showed weakness in the dorsiflexion of the foot and the extension of the toes [Medical Research Council (MRC) score: 3/5 on the right side and 4/5 on the left side], weakness in the extension of hand and fingers (MRC score: 4/5 bilaterally), gait ataxia, loss of touch and vibration sensation in the feet and ankles, weak tendon reflexes in the upper and the lower limbs, but absent ankle jerk reflex. The cranial nerves were spared. The chest radiography was negative for pneumonia, and a nasopharyngeal swab testing for SARS-CoV-2 with real-time polymerase chain reaction assay (RT-PCR) was negative, too.
At this stage, the patient was admitted to our unit for further diagnostic workup. The nerve conduction studies, performed 4 weeks after the neurologic onset, showed reduced or absent compound muscle action potentials and sensory nerve action potentials in the lower limbs, absent F wave response in the lower limbs, and prolonged F wave response in the upper limbs. The electromyography showed very rich spontaneous activity (fibrillation potentials and positive sharp waves) in the lower limb muscles (Table 1). The cerebrospinal fluid (CSF) examination disclosed normal cell count and normal proteins, normal CSF/serum albumin ratio, and absence of oligoclonal banding. Serum SARS-CoV-2 IgG was detected (Maglumi, Snibe). Anti-GM1, anti-GD1b, and anti-GQ1b IgG and IgM were negative (ELISA, Bühlmann). The laboratory investigations demonstrated high C-reactive protein (18.9 mg/dl). The serological tests for HIV, syphilis, cytomegalovirus (CMV), Epstein–Barr virus (EBV), and Mycoplasma pneumoniae (MP) were negative, except for anti-EBV, anti-CMV, and anti-MP IgG. An intravenous immunoglobulin (IVIG) cycle at 0.4 g/kg/day over 5 days was started, leading to a significant improvement of the weakness in the upper limbs and the left foot but a poor benefit on the right foot and gait ataxic. The patient was then transferred to the rehabilitation unit. He slowly improved through physiotherapy and, after 1 month, he was able to walk without aid and was discharged. Figure 1 shows a timeline of the clinical milestones of the patient.
We reported a patient showing a stepwise progression of numbness, tingling, and weakness 12 days after the resolution of fever, cough, and dysgeusia. The clinical features and the electrophysiological findings along with the epidemiological context and the presence of IgG to SARS-CoV-2 supported the diagnosis of post-COVID-19 GBS. In particular, the neurophysiological examination was consistent with an acute motor-sensory axonal GBS (AMSAN) variant, with level 2 diagnostic certainty for GBS according to the Brighton Criteria (consistent clinical features and supporting nerve conduction study, but not CSF) (4, 5). Active SARS-CoV-2 infection was excluded by a complete recovery of the typical antecedent symptoms, absence of the viral genome in the nasopharyngeal swab, and negative chest radiography. The detection of serum IgG to SARS-CoV-2 is in line with the chronological profile of the antibody appearance. Indeed IgG seroconversion in COVID-19 patients is reached within a median of 13 days from the clinical onset (6).
More than half of GBS cases appear 1 to 2 weeks after an underlying infection. Campylobacter jejuni is the most frequent precipitant of GBS, but viral infections, including EBV,CMV, and Zika virus, are also frequently reported (7). The association of GBS with other coronaviruses was described only in two cases (8, 9). Recently, SARS-CoV-2 has also been related to GBS and Miller Fisher syndrome (MFS), wherein an autoimmune post-infectious mechanism, such as molecular mimicry or bystander activation, targeting self-ganglioside epitopes in spinal roots and peripheral nerves, might be involved, in analogy with all the other post-infectious cases.
We carried out a literature search in MEDLINE via PubMed for all articles published using the keywords or MeSH terms “COVID-19” or “SARS-CoV-2,” together with “Guillain–Barre syndrome,” “GBS,” “AIDP,” “AMAN,” “AMSAN,” “Miller Fisher syndrome,” or “MFS.” At the time of writing this manuscript, we found in literature 29 articles reporting 33 patients with GBS and five cases of MFS associated with SARS-CoV-2 infection, which are summarized in Table 2 (10–38).
The age of the patients ranged between 23 and 77 years (mean ± standard deviation: 59 ± 12), with a male prevalence (63.2%). The severity of COVID-19 manifestations, defined as mild, severe, and critical, according to a previously described classification (39) was as follows: mild in 30/38 (78.9%), severe in 5/38 (13.2%), and critical in 3/38 (7.9%). The time elapsed from onset of the COVID-19 symptoms to the clinical GBS manifestations ranged between 3 and 28 days (mean 12 ± 6). Notably, the timing of the majority of cases was consistent with the parainfectious profile rather than a post-infectious paradigm. In two patients, the onset of GBS actually preceded by a few days the first manifestations of COVID-19, but an earlier presentation of COVID-19 characterized by very mild or even absent symptoms could be taken into account in both cases.
The main clinical, electrophysiological, and CSF features of the patients so far reported are summarized in Table 3. With regard to GBS subtypes, the main clinical variant was the classical sensory-motor GBS (30/38), the second phenotype was MFS (5/38), the third was featured by facial diplegia with sensory deficits (2/38), and in only one case the pharyngeal–cervical–brachial variant was observed.
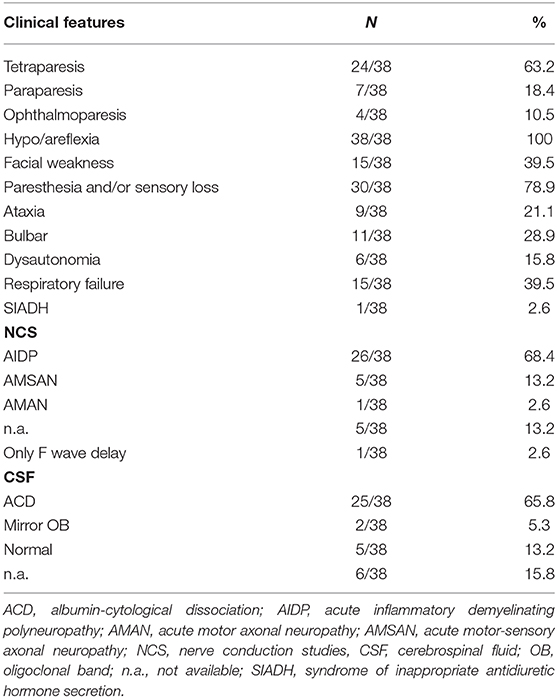
Table 3. Clinical, neurophysiological, and CSF features of Guillain–Barré syndrome/Miller Fisher syndrome cases.
Following the first reported cases of GBS related to SARS-CoV-2 (10), a more common axonal rather than demyelinating variant has been suggested. However, unlike the initial reports, the electrophysiological features in other cases did not show a higher prevalence of axonal variants in these patients. By contrast, the demyelinating and the mixed forms were more often observed.
The examination of CSF samples obtained from 32 patients showed an albumin-cytological dissociation in 68.4% of cases. All RT-PCRs for SARS-CoV-2 on CSF were negative, suggesting the lack of a direct causative role of the virus.
Anti-ganglioside antibodies were detected only in two cases of MFS, showing a borderline positivity for IgM anti-GM1 and a positivity for IgG anti-GD1b, respectively.
Brain and/or spinal cord MRI was performed in 20 patients and showed contrast enhancement of nerve roots at the level of the cauda equina, of brachial and lumbosacral plexus, and also of single or multiple cranial nerves. In addition, a brainstem and cervical leptomeningeal enhancement, which is an atypical feature in GBS, was seen in one case.
Almost all patients were treated with IVIG and/or plasma exchange, whereas one mild case received only symptomatic treatment. The recovery timing and the outcomes varied widely, but in 14/38 cases, a rapid improvement was reported. In 12/38 cases, the improvement was instead slower and required admission to rehabilitation facilities, whereas 6/38 patients had a poor outcome (prolonged stay in the intensive care unit and long-lasting severe disabilities). Two cases were fatal, and in 4/38 cases, the outcome was not available. Of relevance is the fact that it does not seem that COVID-19 severity at onset is correlated with GBS outcomes.
Moreover, the clinical features of post-COVID-19 GBS did not differ from those of cases related to other viruses, with the notable exception of a remarkable respiratory involvement (Table 3). It is indeed crucial to highlight that respiratory failure was present in 15 /38 cases (39.5%) of GBS related to SARS-CoV-2, a percentage higher than that observed in previous GBS cohorts (ranging from 20 to 30%) (5). This suggests that COVID-19 pneumonia may overlap with GBS-associated respiratory muscle weakness and increase the number of cases needing a respiratory support. The reported observations indicate that physicians should always consider GBS in the differential diagnosis of a respiratory insufficiency in COVID-19 patients, especially in cases of normocapnic or hypercapnic respiratory failure (pointing to a restrictive respiratory pattern in contrast with the interstitial pattern of COVID-19 pneumonia) or when a discrepancy between chest imaging and respiratory parameters occurs. An additional explanation for this latter scenario is that the respiratory failure in GBS associated with COVID-19 may also be driven by a dysfunction of the cardiorespiratory centers in medulla oblongata directly induced by the virus (40) since the SARS-CoV-2 genome has also been detected in the human brainstem (2).
The early recognition of GBS symptoms is critical, given the associated high mortality as well as severe motor disabilities that may seriously limit the quality of life of these patients (41). Deficits induced by this neurological condition could be reasonably included among the post-COVID-19 sequelae, which requires an accurate evaluation by the neurologists, similarly to what has been suggested in the past outbreaks, for instance, in cases of post-polio syndrome (42).
Our case report and review of literature contribute to raise awareness of the possible association between GBS and SARS-CoV-2 infection. The underlying mechanism of injury could be an autoimmune reaction against peripheral nerve antigens, in light of the lack of a viral genome in the CSF.
The main clinical, electrophysiological, and CFS features of the patients so far reported proved to be similar to GBS cases related to other infectious diseases. Nevertheless, respiratory involvement is more frequent in GBS related to SARS-CoV2, and a reasonable explanation for this finding could be the coexistence of COVID-19 interstitial pneumonia and GBS respiratory muscle weakness since the majority of cases had a parainfectious profile.
However, the relationship between SARS-CoV-2 and GBS, actually described only in single case reports and small case series, should be confirmed in larger observational studies in order to evaluate the temporal correlation between GBS clusters and the COVID-19 epidemic curve in each affected country.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the patient for the publication of this case report, including any potentially identifiable images or data included in this article.
AZ, MC, and AC were involved in the work-up of the patient, planning and conducting investigations, and providing clinical care. AZ planned the case report and drafted the initial manuscript. EA and MT performed the electrophysiological investigation. DF and MG carried out the laboratory testing. AZ, MT, and MCo reviewed the literature. EA, DF, MG, MT, MCo, MC, and AC revised the manuscript. All the authors approved the final manuscript as submitted.
This paper is dedicated to the loving memory of our colleague, Prof. Arrigo Moglia, neurologist and neurophysiologist at our Institute, who recently died from COVID-19.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:1–9. doi: 10.1001/jamaneurol.2020.1127
2. Meinhardt J, Radke J, Dittmayer C, Mothes R, Franz J, Laue M, et al. Olfactory transmucosal SARS-CoV-2 invasion as port of central nervous system entry in COVID-19 patients. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.06.04.135012
3. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. (2020) 77:1028–29. doi: 10.1001/jamaneurol.2020.2125
4. Rajabally YA, Durand MC, Mitchell J, Orlikowski D, Nicolas G. Electrophysiological diagnosis of Guillain-Barré syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry. (2015) 86:115–9. doi: 10.1136/jnnp-2014-307815
5. Fokke C, Van Den Berg B, Drenthen J, Walgaard C, Antoon Van Doorn P, Jacobs BC. Diagnosis of guillain-barré syndrome and validation of brighton criteria. Brain. (2014) 137:33–43. doi: 10.1093/brain/awt285
6. Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. (2020) 26:845–8. doi: 10.1038/s41591-020-0897-1
7. Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, et al. Guillain–barré syndrome associated with zika virus infection in Colombia. N Engl J Med. (2016) 375:1513–23. doi: 10.1056/NEJMoa1605564
8. Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. (2017) 13:227–33. doi: 10.3988/jcn.2017.13.3.227
9. Sharma K, Tengsupakul S, Sanchez O, Phaltas R, Maertens P. Guillain–Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: a case report. SAGE Open Med Case Reports. (2019) 7:2050–313X. doi: 10.1177/2050313X19838750
10. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–barré syndrome associated with SARS-CoV-2. N Engl J Med. (2020) 382:2574–76. doi: 10.1056/NEJMc2009191
11. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 4422:2–3. doi: 10.1016/S1474-4422(20)30109-5
12. Riva N, Russo T, Falzone YM, Strollo M, Amadio S, Del Carro U, et al. Post-infectious guillain-barré syndrome related to SARS-CoV-2 infection: a case report. J Neurol. (2020) 26:1–3. doi: 10.1007/s00415-020-09907-z
13. Bigaut K, Mallaret M, Baloglu S, Nemoz B, Morand P, Baicry F, et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflammation. (2020) 7:e785. doi: 10.1212/NXI.0000000000000785
14. Su XW, Palka S V, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2 associated guillain-barre syndrome with dysautonomia. Muscle Nerve. (2020) 62:E48–9. doi: 10.1002/mus.26988
15. Assini A, Benedetti L, Di Maio S, Schirinzi E, Del Sette M. New clinical manifestation of COVID-19 related guillain-barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. (2020) 41:1657–58. doi: 10.21203/rs.3.rs-30354/v1
16. Sedaghat Z, Karimi N. Guillain barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. (2020) 76:233–5. doi: 10.1016/j.jocn.2020.04.062
17. Padroni M, Mastrangelo V, Asioli GM, Pavolucci L, Abu-Rumeileh S, Piscaglia MG, et al. Guillain-barré syndrome following COVID-19: new infection, old complication? J Neurol. (2020) 267:1877–9. doi: 10.1007/s00415-020-09849-6
18. Oguz-Akarsu E, Ozpar R, Mirzayev H, Acet-Ozturk NA, Hakyemez B, Ediger D, et al. Guillain–barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. (2020) 62:E54–E57. doi: 10.1002/mus.26992
19. Marta-Enguita J, Rubio-Baines I, Gastón-Zubimendi I. Fatal guillain-barre syndrome after infection with SARS-CoV-2. Neurologia. (2020) 35:265–7. doi: 10.1016/j.nrleng.2020.04.004
20. Lascano AM, Epiney J-B, Coen M, Serratrice J, Bernard-Valnet R, Lalive PH, et al. SARS-CoV-2 and guillain-barré syndrome: AIDP variant with favorable outcome. Eur J Neurol. (2020). doi: 10.1111/ene.14368. [Epub ahead of print].
21. Kilinc D, van de Pasch S, Doets AY, Jacobs BC, van Vliet J, Garssen MPJ. Guillain-barré syndrome after SARS-CoV-2 infection. Eur J Neurol. (2020). doi: 10.1111/ene.14398. [Epub ahead of print].
22. Virani A, Rabold E, Hanson T, Haag A, Elrufay R, Cheema T, et al. Guillain-barré syndrome associated with SARS-CoV-2 infection. IDCases. (2020) 20:e00771. doi: 10.1016/j.idcr.2020.e00771
23. Chan JL, Ebadi H, Sarna JR. Guillain-barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can J Neurol Sci. (2020). doi: 10.1017/cjn.2020.106. [Epub ahead of print].
24. Helbok R, Beer R, Löscher W, Boesch S, Reindl M, Hornung R, et al. Guillain-barré syndrome in a patient with antibodies against SARS-COV-2. Eur J Neurol. (2020) doi: 10.1111/ene.14388
25. Webb S, Wallace VCJ, Martin-Lopez D, Yogarajah M. Guillain-Barré syndrome following COVID-19: a newly emerging post-infectious complication. BMJ Case Rep. (2020) 13:e236182. doi: 10.1136/bcr-2020-236182
26. Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce guillain-barré syndrome. Rev Neurol. (2020) 176:516–8. doi: 10.1016/j.neurol.2020.04.003
27. El Otmani H, El Moutawakil B, Rafai M-A, El Benna N, El Kettani C, Soussi M, et al. Covid-19 and guillain-barré syndrome: more than a coincidence! Rev Neurol. (2020) 176:518–9. doi: 10.1016/j.neurol.2020.04.007
28. Velayos Galán A, del Saz Saucedo P, Peinado Postigo F, Botia Paniagua E. Guillain-barré syndrome associated with SARS-CoV-2 infection. Neurologia. (2020) 35:e00771 doi: 10.1016/j.nrleng.2020.04.006
29. Coen M, Jeanson G, Culebras Almeida LA, Hübers A, Stierlin F, Najjar I, et al. Guillain-barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. (2020) 87:111–2. doi: 10.1016/j.bbi.2020.04.074
30. Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflammation. (2020) 7:e741. doi: 10.1212/NXI.0000000000000741
31. Arnaud S, Budowski C, Ng Wing Tin S, Degos B. Post SARS-CoV-2 Guillain-Barré syndrome. Clin Neurophysiol. (2020) 131:1652–4. doi: 10.1016/j.clinph.2020.05.003
32. Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. (2020) 25:204–7. doi: 10.1111/jns.12382
33. Ottaviani D, Boso F, Tranquillini E, Gapeni I, Pedrotti G, Cozzio S, et al. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. (2020) 41:1351–54. doi: 10.21203/rs.3.rs-24886/v1
34. Esteban Molina A, Mata Martínez M, Sánchez Chueca P, Carrillo López A, Sancho Val I, Sanjuan-Villarreal TA. Síndrome de guillain-barré asociado a infección por Covid-19. Med Intensiva. (2020) 95:e601–e605. doi: 10.1016/j.medin.2020.04.015
35. Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. (2020) 95. doi: 10.1212/WNL.0000000000009619
36. Lantos JE, Strauss SB, Lin E. COVID-19–associated miller fisher syndrome: MRI findings. Am J Neuroradiol. (2020) 41:1184–86. doi: 10.3174/ajnr.A6609
37. Rana S, Lima AA, Chandra R, Valeriano J, Desai T, Freiberg W, et al. Novel coronavirus (COVID-19)-associated guillain-barré syndrome: case report. J Clin Neuromuscul Dis. (2020) 21:240–2. doi: 10.1097/CND.0000000000000309
38. Reyes-Bueno JA, García-Trujillo L, Urbaneja P, Ciano-Petersen NL, Postigo-Pozo MJ, Martínez-Tomás C, et al. Miller-fisher syndrome after SARS-CoV-2 infection. Eur J Neurol. (2020). doi: 10.1111/ene.14383
39. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
40. Tassorelli C, Mojoli F, Baldanti F, Bruno R, Benazzo M. COVID-19: what if the brain had a role in causing the deaths? Eur J Neurol. (2020). doi: 10.1111/ene.14275
41. Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, et al. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. (2020) 19:255–65. doi: 10.1016/S1474-4422(19)30411-9
Keywords: guillain–barré syndrome, miller fisher syndrome, COVID-19, SARS-CoV-2, AMSAN, post-infectious
Citation: Zito A, Alfonsi E, Franciotta D, Todisco M, Gastaldi M, Cotta Ramusino M, Ceroni M and Costa A (2020) COVID-19 and Guillain–Barré Syndrome: A Case Report and Review of Literature. Front. Neurol. 11:909. doi: 10.3389/fneur.2020.00909
Received: 11 May 2020; Accepted: 14 July 2020;
Published: 21 August 2020.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Jordi A. Matias-Guiu, Hospital Clínico San Carlos, SpainCopyright © 2020 Zito, Alfonsi, Franciotta, Todisco, Gastaldi, Cotta Ramusino, Ceroni and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Zito, YW50b25pby56aXRvMDFAdW5pdmVyc2l0YWRpcGF2aWEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.