
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 16 June 2020
Sec. Cancer Imaging and Image-directed Interventions
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00869
 Alessandro Rizzo1†
Alessandro Rizzo1† Maria Concetta Nigro1†
Maria Concetta Nigro1† Vania Ramponi2
Vania Ramponi2 Carmine Gallo3
Carmine Gallo3 Anna Myriam Perrone4
Anna Myriam Perrone4 Pierandrea De Iaco4
Pierandrea De Iaco4 Giovanni Frezza5
Giovanni Frezza5 Damiano Balestrini5
Damiano Balestrini5 Maika Di Benedetto6
Maika Di Benedetto6 Jarno Morbiducci7
Jarno Morbiducci7 Maria Abbondanza Pantaleo1,8†
Maria Abbondanza Pantaleo1,8† Margherita Nannini9*
Margherita Nannini9*Uterine leiomyosarcoma (uLMS) is a rare and aggressive malignancy with poor clinical outcomes. Even when localized, uLMS is associated with high rates of local and distant recurrences that are usually fatal. Common sites of recurrence are lung, liver, pelvic lymph nodes, and vertebral and long bones, though atypical patterns of recurrence have been described. Among them, intracranial recurrence appears as a rare finding, almost exceptional in skull and dura. We describe the case of a solitary skull metastasis from uLMS in a 39-year-old woman, which represents the third reported case of skull recurrence in literature. After multidisciplinary discussion, the patient underwent surgery and received adjuvant radiotherapy. After 4 months, she is currently alive, without evidence of extracranial disease. This case highlights the importance of suspecting and recognizing atypical and extremely rare metastasis to this region. We encourage the need for large case series in order to provide further information about cranial recurrences of uLMS taking into account the paucity of data currently available in literature and the frequently unpredictable behavior of this rare and highly lethal disease.
Uterine leiomyosarcoma (uLMS) is a rare malignancy accounting for 1–3% of all uterine cancers and approximately for 65% of all uterine sarcomas (US) (1). Most of the patients affected by uLMS present with advanced (metastatic or inoperable) disease, with poor prognosis, and short life expectancy (2). Currently, complete surgical resection is the only potentially curative treatment option for uLMS, where it represents the treatment of choice if the tumor is deemed resectable (3). Nevertheless, relapse rates are high in patients undergoing potentially curative resection. The risk of recurrence is between 50 and 70% and 5-year overall survival (OS) is <50% in early stages, even after radical resection (4). Although distant and local recurrences are a major issue after surgery, the role of postoperative treatment in uLMS remains controversial with no strong data currently supporting its use (5, 6). Lung, liver, peritoneal cavity, pelvic lymph nodes, vertebral and long bones are typically reported sites of recurrent uLMS (7). The array of tools currently available in imaging techniques and the prompt detection in high-risk patients has allowed the identification of more atypical patterns of uLMS recurrence, such as heart, parotid gland, gastrointestinal tract, brain, and skull (8–10).
We herein report a case of cranial recurrence of uLMS in a young woman, representing to our knowledge the third case of uLMS skull metastasis reported in literature.
In January 2018, a 39-year-old woman presented to her gynecologist with menorrhagia and pelvic pain; her past medical history included uterine multinodular fibromatosis. A transvaginal ultrasound showed the presence of an intrauterine mass, for which she underwent a total laparoscopic hysterectomy, bilateral adnexectomy, pelvic and paraaortic lymph node biopsies, and omentectomy. The pathology report described a 7 cm diameter, stage IB uterine mass consistent with grade 3 uLMS. Histology showed smooth muscle actin (SMA) positive and desmin positive spindle cells with marked cytological atypia and 15 mitoses per 10 high power fields (HPF). Tumor necrosis was below 50% and no tumoral invasion was found in the uterine serous and omentum. Postoperatively, the patient received adjuvant chemotherapy with epirubicine plus ifosfamide.
Clinical and imaging follow up showed no evidence of recurrence until June 2019, when a total body computed tomography (CT) scan revealed an osteolytic lesion in the right temporal-occipital region, 8 mm in diameter and positive to bone scintigraphy. Physical and neurological examination were normal. The subsequent cranial magnetic resonance imaging (MRI) confirmed the presence of the osteolytic temporal-occipital lesion, with epidural extension and partial compression of the underlying brain parenchyma (Figures 1A,B). MRI showed homogeneous signal on T2-weighted imaging and hypo-intensity on T1 weighted images; CT-PET was negative for other metastatic lesions.
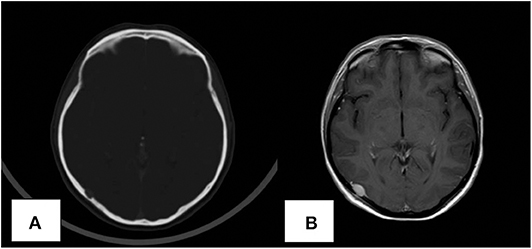
Figure 1. Computed tomography (CT) scan revealing the presence of the skull lesion (A). MRI image of the solitary skull metastatic lesion (B).
In October 2019, she underwent a right craniotomy with removal of the lesion and the involved scalp; epidural extension of the tumor was confirmed. Although surgery resulted in removal of all macroscopic disease, microscopic margins were positive (R1). Histological exam showed the presence of spindle cells with moderate pleomorphism, 15 mitotic figures per 10 HPF (Figure 2A), and strong positivity for smooth muscle actin (SMA) (Figure 2B), vimentin, and desmin in immunohistochemistry (Figure 2C). All these findings were consistent with metastatic uLMS. Given the extreme rarity of skull recurrence of uLMS, a multidisciplinary approach was followed. In absence of other metastatic foci and given the R1 resection and the overall limited benefit of chemotherapy as systemic treatment in a patient who had already undergone anthracycline treatment reaching a high cumulative dose, radiation treatment was performed (3,500 centigray—cGy—in 5 fractions of 700 cGy). The patient is currently alive, without evidence of extracranial disease.
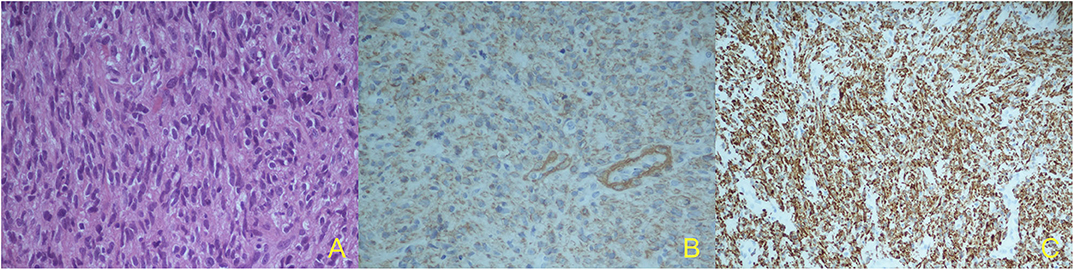
Figure 2. Histological specimen: marked cellular pleomorphism, nuclear atypia, mitotic figures. Hematoxylin and eosin (H&E stain), X40 (A); Immunohistochemical findings: strong cell positivity for alfa-smooth muscle actin (a-SMA), X40 (B) and strong cell positivity for vimentin, X20 (C).
Although hysterectomy offers the best chance of prolonged survival and it is considered as the standard treatment for uLMS, post-operative recurrence rate ranges from 50 to 70%, with a very poor prognosis (11). Lungs represent the most common site of recurrence, followed by peritoneum, liver, vertebral and long bones. However, a heightened awareness of atypical patterns of recurrence and the recent progress in imaging techniques have resulted in a marked increase in the detection of unusual and rare sites of uLMS relapse (12–15). These findings are not exclusively limited to case reports, with retrospective analysis suggesting the presence of less common sites of metastases whose knowledge could reduce diagnostic delay and improve survival (14). With regards to atypical sites of recurrence, a wide range of time intervals between surgical resection of the primary tumor and metastasis onset has been reported, highlighting the need for long-term follow-up and adequate diagnostic confirmation (15).
To the best of our knowledge, two cases of skull recurrence of uLMS have been reported in literature to date (16, 17) (Table 1). In the first case, a 54-year-old woman presented with a cranial mass 5 years after radical hysterectomy for uLMS. No additional sites of metastasis were reported. Although radical surgery was performed, the patient developed disease progression after 1 year, with multiple bone and lung metastases; she died of pulmonary dysfunction after 12 months. In the other one, a 63-year-old woman presented a bulging mass over her left parietal region 14 months after radical surgery for uLMS. Despite the patient undergoing total tumor resection, she expired after 4 months.
Signs and symptoms of skull metastases appears as highly non-specific, depending on tumor extension. This may lead to differential diagnosis, especially after several years of disease-free survival (18). In this context, clinical suspicion and complete physical examination are necessary but not sufficient tools. Another key element is the imaging technique chosen during follow-up of completely resected uLMS. Current guidelines recommend chest, abdomen, and pelvis CT scan during postoperative follow-up, however, a cranial CT scan is rarely included (19).
If metastatic uLMS is certainly associated with poor prognosis, from a theoretical point of view solitary metastasis may show better clinical outcomes. All the patients reported in literature to date, including our case, showed solitary skull recurrence in the absence of other metastatic foci of uMLS. Given the rarity of metastasis to this region, there is a lack of evidence supporting a specific treatment of intracranial metastases from uLMS, whether metastatic site is brain or skull (20). Management should therefore be personalized depending on clinical condition, site, and size of metastasis (9). Maximum treatment should be given to patients with good performance status and who are suitable for surgery, chemotherapy, and/or radiotherapy. The heterogeneity of recurrence patterns, the lack of an established treatment in this setting, and the prognostic burden of metastatic uLMS justify the need for a multidisciplinary team (MDT) in order to provide quicker time to treatment after diagnosis and to translate MDT approach into better survival outcomes. However, given the rarity of intracranial recurrence, it is worth it to underline that the optimal MDT modalities, sequence, and timing of treatments are all yet to be defined.
Diagnosis of skull recurrence of uLMS requires a high index of suspicion. Its early recognition can be extremely difficult, especially in asymptomatic, healthy patients in which cranial CT scan is often not performed during post-surgery follow-up. There is lack of evidence for choosing the best therapeutic strategy in these patients and appropriate treatment of intracranial recurrences is yet to be defined.
The presented report is the third documented case of skull metastasis from uLMS suggesting the importance of improving awareness of this extremely rare occurrence.
Therefore, we encourage the collection of larger series in order to provide further information about cranial recurrences of uLMS, taking into account the paucity of data currently available in literature and the frequently unpredictable behavior of this rare and highly lethal disease.
All datasets generated for this study are included in the article/supplementary material.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AR and MCN have made substantial contributions to conception of the study, interpreted the patient data, and drafted the manuscript. VR performed surgery and has been involved in drafting the manuscript. CG performed the histological and pathologic examination of the surgical tissue and has been involved in drafting figures. AP and PD have been involved in drafting and revising the manuscript. GF, DB, and MD performed radiotherapy and have been involved in drafting and revising the manuscript. JM performed imaging and has been involved in drafting the manuscript. MP has made substantial contributions to conception of the study, interpreted the patient data, and drafted the manuscript. MN was the major contributor in concept and designed and revised the manuscript. All authors read and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
cGy, centigray; CT, computed tomography; HPF, high power fields; MDT, multidisciplinary team; MRI, magnetic resonance imaging; OS, overall survival; uLMS, uterine leiomyosarcoma; US, uterine sarcoma.
1. Koivisto-Korander R, Martinsen JI, Weiderpass E, Leminen A, Pukkala E. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas. (2012) 72:56–60. doi: 10.1016/j.maturitas.2012.01.021
2. Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynaecol Obstet. (2018) 143 Suppl 2:51–58. doi: 10.1002/ijgo.12613
3. Roberts ME, Aynardi JT, Chu CS. Uterine leiomyosarcoma: a review of the literature and update on management options. Gynecol Oncol. (2018) 151:562–72. doi: 10.1016/j.ygyno.2018.09.010
4. Cui RR, Wright JD, Hou JY. Uterine leiomyosarcoma: a review of recent advances in molecular biology, clinical management and outcome. BJOG. (2017) 124:1028–37. doi: 10.1111/1471-0528.14579
5. Rizzo A, Pantaleo MA, Saponara M, Nannini M. Current status of the adjuvant therapy in uterine sarcoma: a literature review. World J Clin Cases. (2019) 7:1753–63. doi: 10.12998/wjcc.v7.i14.1753
6. Bogani G, Fucà G, Maltese G, Ditto A, Martinelli F, Signorelli M, et al. Efficacy of adjuvant chemotherapy in early stage uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. (2016) 143:443–7. doi: 10.1016/j.ygyno.2016.07.110
7. Lusby K, Savannah KB, Demicco EG, Zhang Y, Ghadimi MP, Young ED, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol. (2013) 20:2364–72. doi: 10.1245/s10434-012-2834-0
8. Tunio MA, Alasiri M, Saleh RM, Akbar SA, Ali NM, Senosy Hassan MA. Obstructive small bowel metastasis from uterine leiomyosarcoma: a case report. Case Rep Obstet Gynecol. (2014) 2014:603097. doi: 10.1155/2014/603097
9. Ahuja A, Agarwal P, Sardana R, Bhaskar S. Extensively metastasizing leiomyosarcoma: a diagnostic challenge. J Midlife Health. (2017) 8:148–50. doi: 10.4103/jmh.JMH_60_17
10. Sandruck J, Escobar P, Lurain J, Fishman D. Uterine leiomyosarcoma metastatic to the sphenoid sinus: a case report and review of the literature. Gynecol Oncol. (2004) 92:701–4. doi: 10.1016/j.ygyno.2003.10.043
11. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:170–99. doi: 10.6004/jnccn.2018.0006
12. Yamada S, Yamada SM, Nakaguchi H, Murakami M, Hoya K, Matsuno A. A case of multiple brain metastases of uterine leiomyosarcoma with a literature review. Surg Oncol. (2011) 20:e127–31. doi: 10.1016/j.suronc.2011.04.001
13. Bernstein-Molho R, Grisaro D, Soyfer V, Safra T, Merimsky O. Metastatic uterine leiomyosarcomas: a single-institution experience. Int J Gynecol Cancer. (2010) 20:255–60. doi: 10.1111/IGC.0b013e3181c9e289
14. Tirumani SH, Deaver P, Shinagare AB, Tirumani H, Hornick JL, George S, et al. Metastatic pattern of uterine leiomyosarcoma: retrospective analysis of the predictors and outcome in 113 patients. J Gynecol Oncol. (2014) 25:306–12. doi: 10.3802/jgo.2014.25.4.306
15. Bartosch C, Afonso M, Pires-Luís AS, Galaghar A, Guimarães M, Antunes L, et al. Distant metastases in uterine leiomyosarcomas: the wide variety of body sites and time intervals to metastatic relapse. Int J Gynecol Pathol. (2017) 36:31–41. doi: 10.1097/PGP.0000000000000284
16. Uchino M, Endo G, Shibata I, Terao H, Kuramitsu T, Kushida Y, et al. Uterine leiomyosarcoma metastasis to the skull–case report. Neurol Med Chir (Tokyo). (1996) 36:469–71. doi: 10.2176/nmc.36.469
17. Yip CM, Yang KC, Lo YS, Liao WC, Chen JY, Hsu SS. Skull metastasis from uterine leiomyosarcoma: a case report. Acta Neurol Taiwan. (2006) 15:109–113.
18. Honeybul S, Ha T. Leiomyosarcoma of the uterus metastatic to the brain: a case report. Arch Gynecol Obstet. (2009) 279:391–3. doi: 10.1007/s00404-008-0717-1
19. Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv51–67. doi: 10.1093/annonc/mdy096
Keywords: uterine leiomyosarcoma, uterine sarcoma, skull metastasis, uterine cancer, uterine sarcoma, intracranial recurrence
Citation: Rizzo A, Nigro MC, Ramponi V, Gallo C, Perrone AM, De Iaco P, Frezza G, Balestrini D, Di Benedetto M, Morbiducci J, Pantaleo MA and Nannini M (2020) Skull Metastasis From Uterine Leiomyosarcoma, a Rare Presentation for a Rare Tumor: A Case Report and Review of the Literature. Front. Oncol. 10:869. doi: 10.3389/fonc.2020.00869
Received: 17 February 2020; Accepted: 04 May 2020;
Published: 16 June 2020.
Edited by:
Cicero Matthew R. Habito, Harvard Medical School, United StatesReviewed by:
Johanna Patricia Adevoso Canal, University of the Philippines Manila, PhilippinesCopyright © 2020 Rizzo, Nigro, Ramponi, Gallo, Perrone, De Iaco, Frezza, Balestrini, Di Benedetto, Morbiducci, Pantaleo and Nannini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Margherita Nannini, bWFyZ2hlcml0YS5uYW5uaW5pQHVuaWJvLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.