- 1Division of Infectious Diseases, Department of Pediatrics, University of California, Los Angeles (UCLA) David Geffen School of Medicine, Los Angeles, CA, United States
- 2Division of Disease Prevention, Policy and Global Health, Department of Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, CA, United States
Chlamydial trachomatis infection has been associated with adverse pregnancy and neonatal outcomes such as premature rupture of membranes, preterm birth, low birth weight, conjunctivitis, and pneumonia in infants. This review evaluates existing literature to determine potential benefits of antenatal screening and treatment of C. trachomatis in preventing adverse outcomes. A literature search revealed 1824 studies with 156 full-text articles reviewed. Fifteen studies were selected after fulfilling inclusion criteria. Eight studies focused on chlamydial screening and treatment to prevent adverse pregnancy outcomes such as premature rupture of membranes, preterm birth, low birth weight, growth restriction leading to small for gestational age infants, and neonatal death. Seven studies focused on the effects of chlamydial screening and treatment on adverse infant outcomes such as chlamydial infection including positive mucosal cultures, pneumonia, and conjunctivitis. Given the heterogeneity of those studies, this focused review was exclusively qualitative in nature. When viewed collectively, 13 of 15 studies provided some degree of support that antenatal chlamydial screening and treatment interventions may lead to decreased adverse pregnancy and infant outcomes. However, notable limitations of these individual studies also highlight the need for further, updated research in this area, particularly from low and middle-income settings.
Introduction
Although Chlamydia trachomatis accounts for nearly 130 million new cases worldwide, it remains a perpetually overlooked global health issue, particularly in low and middle-income countries with limited resources (1–5). The consequences are exacerbated for pregnant women, where infection may be detrimental to the health of both mothers and their infants (3, 6).
Chlamydial infection in pregnancy has been associated with complications including fetal loss, premature rupture of membranes, preterm labor and delivery, and low birth weight among others (1, 3, 7–18). In particular, many studies, including a 12-study meta-analysis by Silva et al. (19), have found an association between chlamydial infection in pregnancy and increased risk for preterm labor, low birth weight, and/or perinatal mortality (12, 13, 15, 20). Some have suggested that untreated chlamydial infection in pregnancy may lead to as much as a two- to four-fold increased risk for preterm labor and delivery (12, 15, 20). The increased risk for preterm delivery with C. trachomatis infection is of particular concern given the high neonatal morbidity and mortality associated with premature birth (21). In addition, maternal infection with C. trachomatis may lead to neonatal infection including conjunctivitis and pneumonia due to high rates of vertical transmission, which some have estimated rates as high as 50–70% without treatment (22–25). It has been estimated that 30–50% of infants whose mothers have active, untreated C. trachomatis infection will develop conjunctivitis, and 10–20% of infants will develop pneumonia (11–13, 22).
Since Chlamydia trachomatis is an easily curable infection, antenatal screening programs that identify and treat infected mothers could potentially prevent many of these pregnancy and neonatal complications. The current U.S. Centers for Disease Control and Prevention (CDC) guidelines recommend a single dose azithromycin as first line treatment for chlamydia in pregnancy, with a course of amoxicillin or erythromycin listed as an alternative options (26, 27). Some have suggested that macrolides, such as erythromycin and azithromycin, may also be beneficial in preventing adverse pregnancy outcomes by suppressing tumor necrosis factor (TNF)-alpha, which has been implicated as an accomplice in preterm labor induction (28). Antenatal chlamydial screening and treatment for pregnant women, especially those considered high risk who are <25 years of age or with other risk factors, has been implemented in some countries such as the U.S, where it has been credited by some as the only effective means of preventing neonatal chlamydial infections (22, 29). While chlamydial screening and treatment of pregnant women was initially recommended by the CDC in the early 1980s, widespread implementation did not occur until the following decade (22). Some recent published studies have suggested the benefit of such interventions in the US by comparing rates of CT (pediatric seroprevalence and CT neonatal conjunctivitis) before and after routine implementation of CT screening and treatment in pregnant women in the US in 1993 (30, 31). These considerations are also important because studies have highlighted that standard neonatal ocular prophylaxis measures do not effectively prevent neonatal chlamydial conjunctivitis (32, 33).
Nevertheless, these initiatives remain controversial in many other countries (29). Both a 2016 Cochrane and 2014 USPSTF (US Preventive Services Task Force) systematic review of chlamydia screening have highlighted the lack of research investigating the benefits of chlamydia screening and treatment in pregnancy (34, 35). However, recent published studies from the US, Australia, and Netherlands have demonstrated that such interventions can be cost-effective in preventing morbidity associated with chlamydial infections, particularly among younger pregnant women in regions where chlamydia prevalence is high (36–38).
To comprehensively understand the potential benefits of an antenatal Chlamydia trachomatis screening and treatment intervention in pregnancy, a focused review of literature was performed. The specific objective was to review literature regarding the efficacy of screening and treatment interventions for Chlamydia trachomatis in pregnancy in preventing adverse pregnancy and neonatal outcomes.
Methods
Search Strategy and Selection Criteria
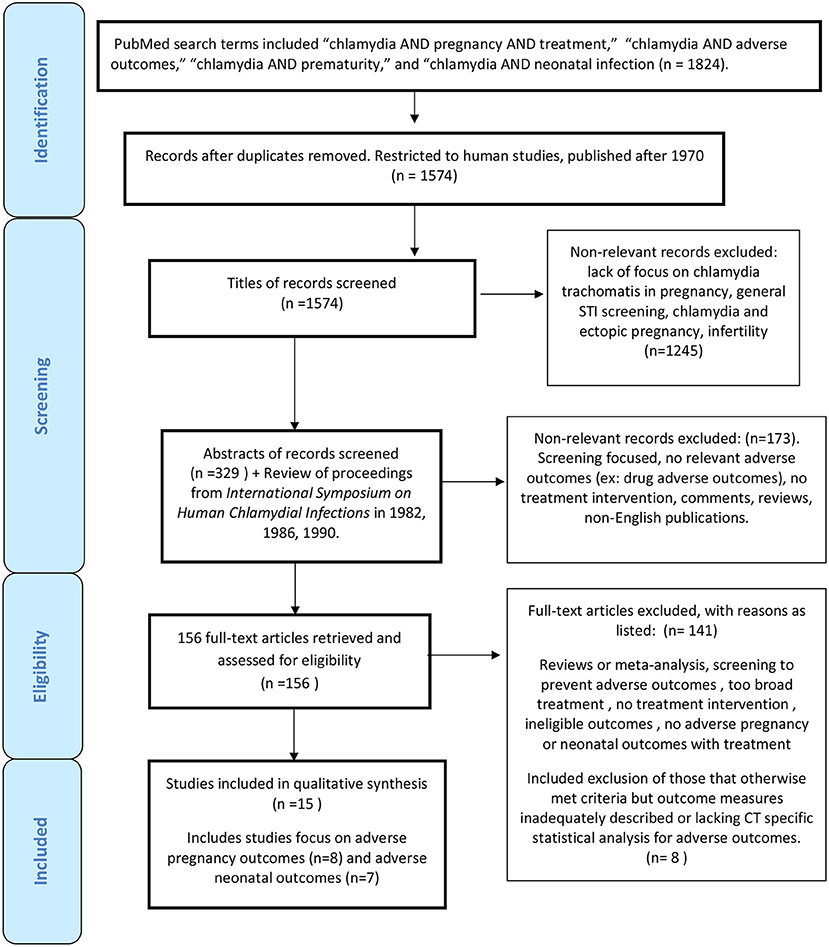
Primary searches using PubMed were initially completed on October 20, 2014 and subsequently updated three times on July 27, 2015, January 28, 2016, and July 30, 2016. An additional search was repeated in January 2020 to determine if additional published studies warranted inclusion. Searches were restricted to articles pertaining to humans, and articles published after 1970, which coincided with the first studies supporting vertical transmission of Chlamydia trachomatis from mothers to infants. We used broad search terms and many publications were ultimately excluded after review. We also assessed the reference lists of included studies and review articles for other relevant studies that may have been overlooked based on search criteria implemented. This process led to a review of all proceedings from the International Symposium on Human Chlamydial Infections in 1982, 1986, 1990 not available to PubMed, resulting in the inclusion of one additional study (39). PubMed search terms included “chlamydia AND pregnancy AND treatment,” “chlamydia AND adverse outcomes,” “chlamydia AND prematurity,” and “chlamydia AND neonatal infection,” which yielded a total of 1,824 articles. After restricting the search to only human studies and those published after 1970, 1,574 studies remained.
Preliminary screening was done based on titles of articles to exclude 1,245 articles that did not pertain to this review. Examples included a lack of focus on C. trachomatis in pregnancy, emphasis on other STIs or Chlamydia groups, and concentration on ectopic pregnancy and infertility complications. Three hundred twenty-nine abstracts were selected to be reviewed by one reviewer. Of those abstracts, 24 were excluded as they were not published in English. There were only two articles within available abstracts in English that could have potential relevance [Nishimura 1990 (Japanese) and Ottesen 1996 (Danish)] (40, 41). Other manuscripts excluded from full-text review included those focused only on screening, those without any adverse neonatal or pregnancy adverse outcomes reported, and those with only drug adverse outcomes reported such as potential congenital malformations. Because this review was not focused on discussing ectopic pregnancy, infertility, and other maternal adverse pregnancy outcomes such as chorioamnionitis, or post-partum endometritis potentially associated with chlamydial infection, articles that focused on these adverse pregnancy outcomes were also excluded.
Assessment and Data Extraction
One hundred and fifty-six documents were retrieved and evaluated in full-text review. Those that were considered for inclusion were classified as containing (1) treatment interventions in pregnancy with adverse pregnancy outcomes such as preterm birth, premature rupture of membranes, low birth weight, small for gestational age, or neonatal death; (2) treatment interventions in pregnancy with neonatal outcomes related to chlamydial infection such as positive chlamydial mucosal cultures, conjunctivitis or pneumonia. The 30 review articles were evaluated for other relevant articles that might have not been included in initial PubMed searches. Other articles did not meet study inclusion criteria due to lack of screening and treatment interventions or no neonatal or adverse pregnancy outcome measures with treatment intervention. Apart from the Cochrane Review (42), there were two articles (43, 44) that otherwise met inclusion criteria, but outcome measures were not adequately described. Another three articles lacked any CT specific analysis with respect to adverse outcomes (45–47). Two other recent studies evaluated rates of neonatal and pediatric CT pre- and post- routine implementation of CT screening and treatment in pregnancy in the US but were excluded given lack of information about specific maternal screening and treatment implemented in both and lack of CT specific neonatal outcomes in one, only positive CT serology in children under 10 years of age [(30, 31); Supplementary Table 1]. Our review was compliant with the PRISMA checklist for systematic reviews [(48); Figure 1].
Results
Antenatal Chlamydial Screening and Treatment to Prevent Adverse Pregnancy Outcomes and Neonatal Chlamydial Infection
While there is a large body of literature investigating the treatment of chlamydial infections in pregnancy, few of these studies provide meaningful data relevant to this review. The majority of those existing treatment studies focus on adverse treatment outcomes pertaining to drug safety and tolerability and test of cure (42).
Of the 15 studies that have been included in this review, eight of the studies provided at least some information on the chlamydial screening and treatment effect on adverse pregnancy outcomes such as low birth weight, preterm delivery, preterm labor, or premature rupture of membranes; the other seven studies provided at least some information on the chlamydial screening and treatment effect on neonatal chlamydial infections1. Of note, the Cochrane Review has been intentionally excluded from the 15 primary studies included in this review (42). While it was a meta-analysis of 11 randomized controlled trials for chlamydial treatment in pregnancy, few of the studies included evaluated adverse pregnancy or neonatal outcomes; it only featured 2 studies (Alary et al. and Bell et al.) discussed in our adverse neonatal outcomes section and 1 study (Martin et al.) discussed in the adverse pregnancy outcomes section [(39, 42, 49, 50); Refer to Supplementary Table 1 for further detail].
These two major outcome groups (pregnancy outcomes and neonatal outcomes) will be discussed separately in this review. Evaluation of the studies in both groups were not amenable to meta-analysis given the limited number of studies in each group as well as the different study designs, study objectives, variable study outcome measures employed. Thus, this paper exclusively focuses on a qualitative analysis. With regards to adverse pregnancy outcomes, focus is placed on those pertaining to infant health outcomes, specifically preterm delivery and other relevant measures including preterm premature rupture of membranes, premature rupture of membranes, preterm labor, and low birth weight.
Studies Preventing Adverse Pregnancy Outcomes
Study Characteristics
We evaluated eight studies (5, 28, 50–55) that provided information regarding the effect of chlamydial screening and treatment in preventing adverse pregnancy outcomes. All of these studies were written in English; six of the studies were conducted in the U.S., and the others took place in Uganda and in India (28, 53). All of the studies in the U.S. focused primarily on non-white, young women (5, 50–52, 54, 55). The studies included primarily young, black women (5, 50–52, 55). Four studies mentioned that women were of lower socioeconomic status (5, 51, 52, 55), but only four commented on either alcohol (50, 54), smoking (5, 50, 52, 54), and/or illegal substance use (54). Only one study focused on HIV-infected pregnant women (28). Studies ranged in publication from 1990 to 2014. Initial cohort sizes ranged markedly based on study objective, enrollment ranged from 229 to 13,750 women [(52, 54); Table 1].
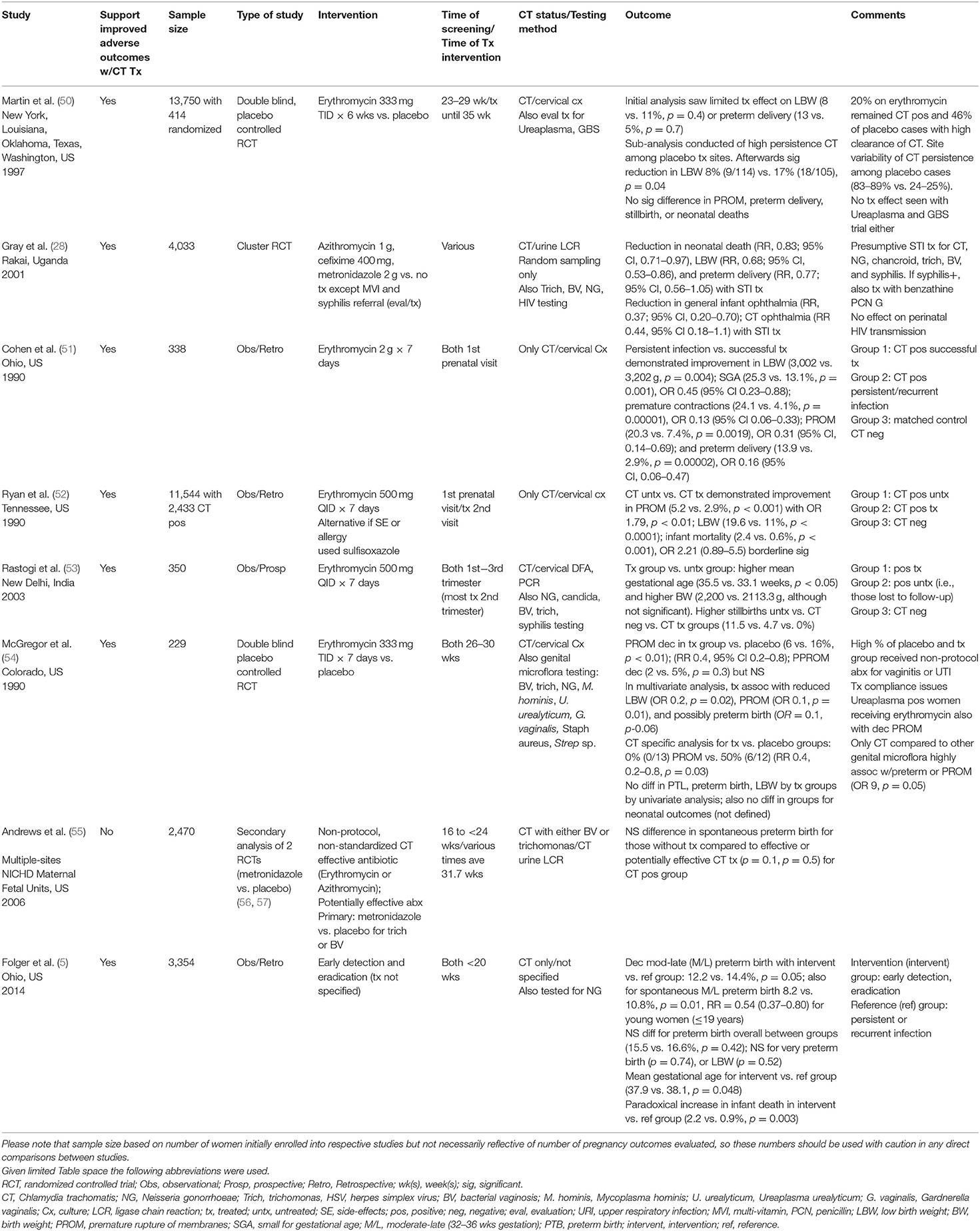
Table 1. Prevention of adverse pregnancy outcomes with antenatal chlamydial treatment (total studies N = 8).
Study Objectives and Interventions
Seven studies described how maternal chlamydial infection was diagnosed. Five used cervical specimens, with four using only cultures (50–52, 54) and one using both direct fluorescent antibody (DFA) and polymerase chain reaction (PCR) (53). The other remaining two used maternal urine samples sent for nucleic acid detection with ligase chain reaction (28, 55). For the seven studies (28, 50–55) reporting maternal chlamydial prevalence rates, the mean was 11.1% with a range of 1.1–2.7% to 21.1% (28, 52). Enrollment and chlamydial screening occurred at various times depending on the study with two at the 1st prenatal visit (51, 52), one at 16 to <24 weeks (55), one at <20 weeks (5), one at 23–29 weeks (50), one at 26–30 weeks (54), and two with non-specific enrollment between first to third trimesters [(28, 53); Table 1].
All studies provided information regarding the effect of chlamydial treatment on adverse pregnancy outcomes as one of the major outcome measures. Five of the studies (5, 50–53) focused primarily on the effects of Chlamydia trachomatis and pregnancy outcomes, although some included an evaluation for additional organisms: Ureaplasma urealyticum and Group B Streptococcus (GBS) in one (50) and candida, syphilis, bacterial vaginosis, trichomonas, and gonorrhea (5) in others (53). The other studies evaluated C. trachomatis as one of several genital infections of equal importance in various combinations including bacterial vaginosis, Trichomonas, and N. gonorrhoeae among others (28, 54, 55). Only one study also evaluated treatment of sexually transmitted infections with respect to HIV perinatal transmission [(28); Table 1].
Five studies used erythromycin as the primary treatment intervention (50–54). Another, which was an STI treatment study, used empiric combination treatment with cefixime, metronidazole, and azithromycin in place of erythromycin (28). One study evaluated the effects of antibiotics with anti-chlamydial activity such as erythromycin and azithromycin as well as those with potential activity such as penicillin, amoxicillin, and ampicillin (55). In contrast, the antibiotics used in one of the studies was not stated but eradication was implied (5). Treatment protocols using erythromycin varied per study with dosing ranging from 333 mg orally three times a day for 1 week (54) to 6 weeks (50). The other three studies used higher dosing of 500 mg orally four times a day for 7 days [(51–53); Table 1].
Timing of treatment in pregnancy differed in these studies with two occurring after the first antenatal visit with chlamydial infection diagnosis (51, 52), three at various times during pregnancy (28, 53, 55), one later during pregnancy sometime between 26 and 30th weeks (54), another for a prolonged period of at least 6 weeks between 23–29th weeks until the 35th week (50), and the last unspecified (5). Only three studies recommended partner treatment (28, 50, 53), and only two of these treated partners directly [(28, 53); Table 1].
Three studies (28, 50, 54) were designed as randomized controlled trials, two (50, 54) of which were double-blinded evaluations of erythromycin vs. placebo. The other (28) was a cluster randomization study designed to evaluate interventions of empiric STI combination treatment vs. prenatal vitamins, which also included syphilis evaluation and treatment, on HIV transmission and pregnancy outcome. Of note, both of the other randomized controlled trials evaluated the effect of the erythromycin treatment intervention with respect to chlamydial infections and other genital flora/infections and pregnancy outcomes (50, 54). The remaining five studies were observational studies (5, 51–53, 55).
These studies varied in design and objective. One study, which used case-matched controls for sociodemographic factors, included three groups that compared those with chlamydial infection responsive to treatment with erythromycin, those unresponsive to treatment with persistent infection, and those that were uninfected (51). Another compared pregnant women initially screened for chlamydia and untreated (even if positive) with those that were later screened and treated with erythromycin if they had tested positive (52). Another was a prospective study evaluating the effects of erythromycin on chlamydial infection in pregnant women, and the group that was infected and untreated was composed of women initially lost to follow-up (53).
In contrast, one of the studies was a secondary analysis of several parent studies (56, 57) that were randomized controlled trials evaluating the effects of metronidazole for bacterial vaginosis and trichomonas on adverse birth outcomes; the study analyzed the impact of antibiotics on chlamydial infection and infant outcomes as a secondary aim (55). The last one was a retrospective cohort using linked public health databases to evaluate birth outcomes for pregnant women with early chlamydial infection and unspecified treatment eradication vs. those with recurrent and persistent infection [(5); Table 1].
Adverse Pregnancy Outcomes
Seven of eight studies provided some support regarding the potential benefits of chlamydial screening and treatment during pregnancy to prevent adverse pregnancy outcomes such as preterm delivery, premature rupture of membranes, and low birth weight (5, 28, 50–54). Five of these studies provided direct support of the benefit of treatment with erythromycin in reduction of adverse outcomes [(50–54); Tables 1, 2]. In contrast, the study by Andrews et al., found that chlamydia-infected pregnant women treated with effective or potentially effective antibiotics against chlamydia did not show differences in rates of preterm delivery from those of untreated women (p = 0.11 and p = 0.5); no other adverse pregnancy outcomes were evaluated (55).
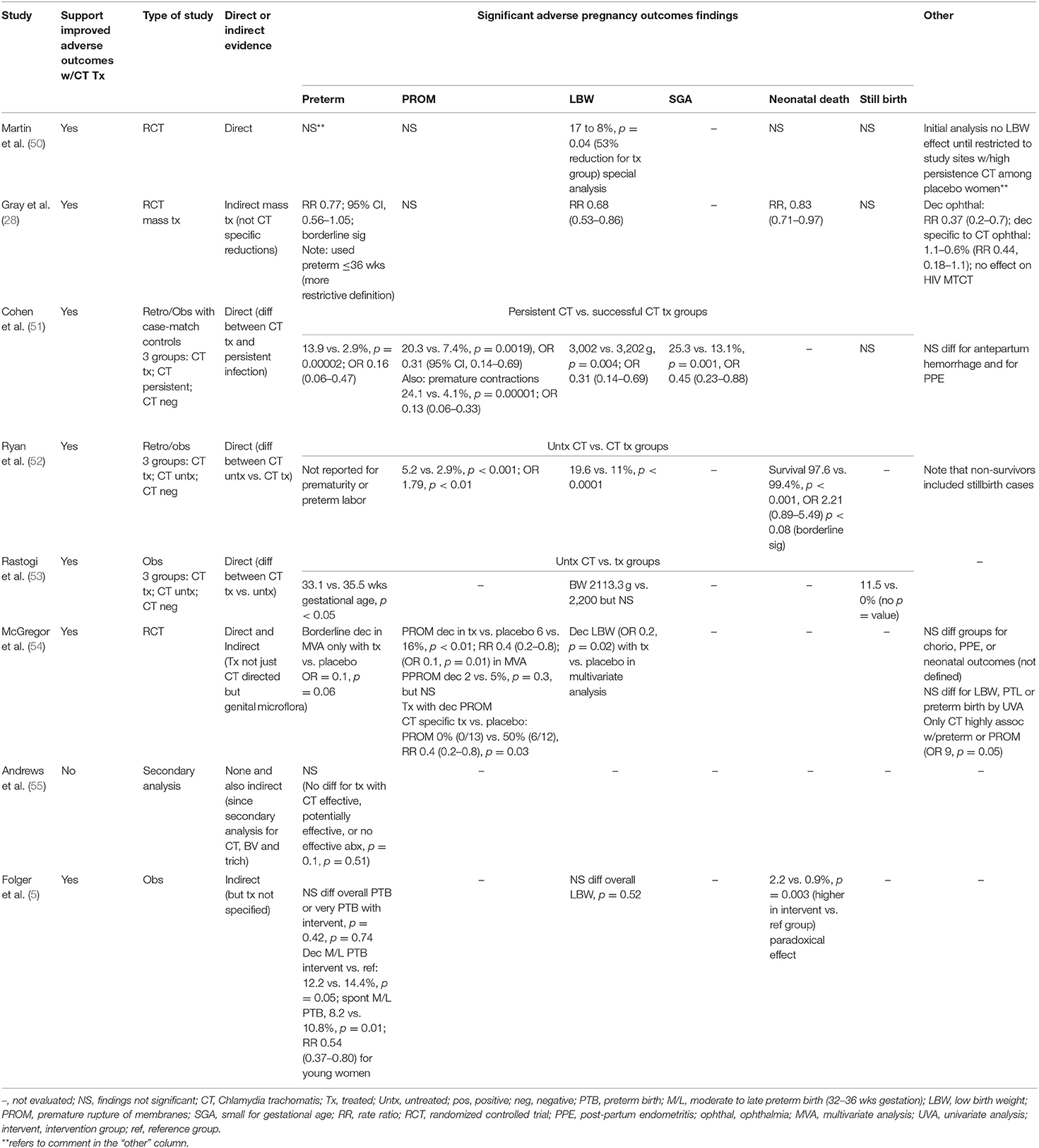
Table 2. Studies of prevention of adverse pregnancy outcomes with antenatal chlamydial treatment (N = 8).
Premature Rupture of Membranes, Preterm Delivery, Low Birth Weight, Small for Gestational Age, and Neonatal Death
Three of five studies (28, 50–52, 54), evaluating premature rupture of membranes after a treatment intervention, reported reduction in this specific outcome. A reduction in premature rupture of membranes was seen in some of the studies when comparing untreated or persistently infected vs. treated women: 5.2 to 2.9% (OR 0.56, 95% CI 0.37–0.85) (52), 20.3 to 7.4% (OR 0.31, 95% CI 0.14–0.69) (51), and 50 to 0% (RR 0.4, 95% CI 0.2–0.8) [(54); Table 2].
Seven (5, 28, 50, 51, 53–55) of eight studies, evaluated the effect of maternal treatment on preterm delivery, and all but two studies (50, 55) suggested a possible benefit in preventing this outcome. The strongest evidence reported a significant reduction from 13.9 to 2.9% (p = 0.00002) in preterm births for 244 chlamydia-infected women receiving treatment compared to 79 of those persistently infected, including a significantly decreased odds of delivering a preterm infant if treated (OR 0.16, 95% CI, 0.06–0.47) (51). Another study suggested higher mean gestational ages by more than 2 weeks (35.5 vs. 33.1 weeks, p < 0.05) for women treated for chlamydia compared to untreated women (53). The remaining studies provided either more indirect evidence of the benefits of treatment for preterm delivery (54), mixed results depending on the specific analysis (5), or evidence of borderline significance [(28); Table 2].
Seven studies evaluated the impact of chlamydial treatment in pregnancy on infant birth weights (5, 28, 50–54). Three studies provided more direct support for improvement in birth weights or reduction in low birth weight infants with chlamydial treatment in pregnancy (50–52). One of these studies reported a significant increase in mean birth weight by 200 g [p = 0.0041; (51)]. Two other studies found significant reductions in low birth weight infants with maternal treatment: 17 to 8% [p = 0.04; (50)] and 19.6 to 11% [p < 0.0001; (52)]. Other studies found indirect support of the benefit of these interventions (28, 54), whereas two studies did not find significant differences in the number of low birth weight infants (5, 53). Of note, one of the studies that found reductions in preterm delivery and low birth weight infants also noted that treated women were also less likely to deliver infants who were small for gestational age (OR 0.45, 95% CI 0.23–0·88) [(51); Table 2].
Only four studies evaluated neonatal survival following treatment during pregnancy (5, 28, 50, 52). Findings were mixed with one study (5), which showed a paradoxical increase in neonatal deaths from 0.9 to 2.2% (p = 0.003) following a treatment intervention, while other studies suggested a possible decline or no significant difference in neonatal mortality (50). In contrast, four studies (28, 50, 51, 53) evaluated differences in stillbirth rates, but only one found a decrease in this outcome with maternal chlamydial treatment [(53); Table 2].
Study Quality
These studies analyzed varied widely with respect to study design, specimens collected, method of testing, timing of testing, evaluation for other STIs, and type of antibiotic and regimen used for treatment. As a result, each had strengths and limitations regarding study quality. Since only 3 studies (28, 50, 54) were randomized, selection bias may have been a factor that impacted the other study results (5, 51–53, 55), particularly since many of the adverse pregnancy outcomes in question may be influenced by multiple factors beyond infectious etiologies such as C. trachomatis.
Other issues included heterogeneity of methods used to test for C. trachomatis, a limited number of studies which also involved treatment of partners (28, 53), and only some employed repeat testing or test of cure after treatment to evaluate for treatment failure (50–54). Consistent with the practices of the time these studies were conducted, the majority used culture to diagnose chlamydial infection, which are less sensitive that nucleic acid amplification testing (NAAT) methods currently employed. Although one study employed directly observed antibiotic therapy (28), the impact of screening and treatment in other studies may have also been influenced by patient follow-up and treatment non-compliance, which were high in certain studies (50, 53, 54). Other issues included persistence of chlamydial infection in spite of treatment and spontaneous clearance of infection in placebo cases in one study (50). Few studies (28, 55) also commented on whether other antibiotics were taken by women for other reasons during pregnancy (50, 54), which may also have had an impact on C. trachomatis clearance and pregnancy outcomes.
Studies Preventing Neonatal Chlamydial Infection
Study Characteristics
Only seven studies (39, 49, 58–62) provided data regarding the effect of screening and treatment in preventing neonatal chlamydial infection. All seven studies were written in English, and all studies occurred in the U.S., with the exception of one from Quebec, Canada (49). Two studies reported that their cohorts consisted primarily of young black women (59, 61), and two others were mainly composed of young, Hispanic and black women (58, 60). None of the studies were published in the last 15 years, ranging in publication date from 1982 to 1994. Cohort sizes varied based on study objectives, ranging from 21 to 1,082 women [(39, 62); Table 3].
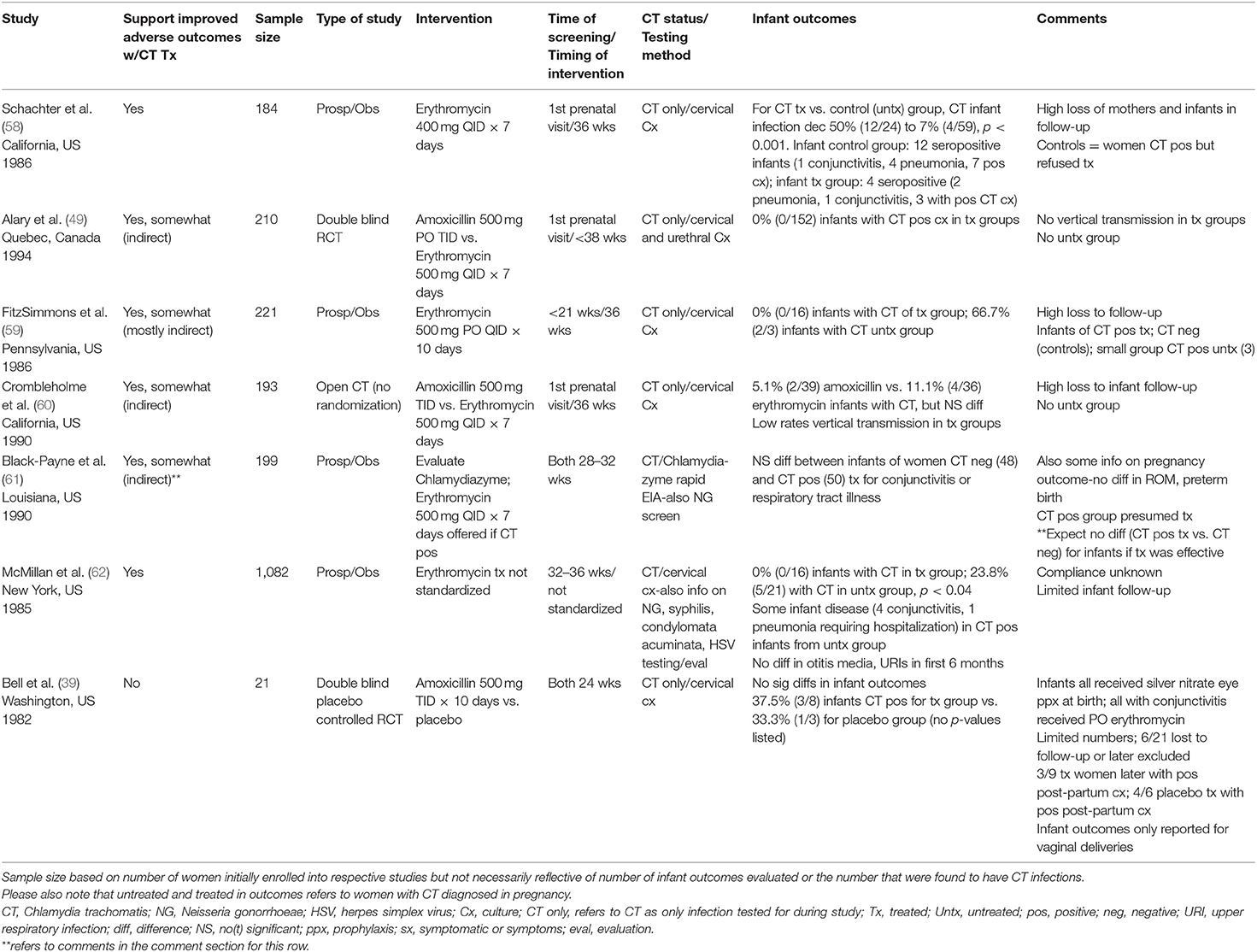
Table 3. Prevention of adverse neonatal outcomes with antenatal chlamydial treatment (total studies N = 7).
Study Objectives and Interventions
The primary focus of all of these studies was Chlamydia trachomatis as opposed to other sexually transmitted infections or genital infections. Only two studies collected some information on other STIs apart from chlamydia (61, 62). All seven studies used cervical specimens to screen for Chlamydia trachomatis infection in their cohorts of pregnant women. Six (39, 49, 58–60, 62) of seven studies used cervical cultures, of which one also used urethral swabs for culture (49). One of the studies used a chlamydia rapid enzyme immunoassay antigen detection assay (Chlamydiazyme) to assess for chlamydial infection (61). For the four studies reporting chlamydial prevalence rates, findings ranged broadly from 1.7 to 26% (49, 59, 61, 62). Three studies (49, 58, 60) conducted chlamydial testing of pregnant women at their first prenatal visit, while others provided screening later [<21 weeks gestation (59), 24 weeks (39), 28–32 weeks (61), and 32–36 weeks (62); Table 3].
Erythromycin was used as either the main therapeutic intervention or one of the interventions in six of seven studies (49, 58–62). In contrast, one study used only amoxicillin as treatment (39). The dosage of erythromycin varied from 400 mg given orally four times a day for 7 days (58) to 500 mg orally four times a day for seven (49, 60, 61) to 10 days (59). Information regarding the dosage of erythromycin was not discussed in one study due to lack of standardization, which was based upon the discretion of individual practitioners (62).
Four studies were prospective with erythromycin used as the therapeutic intervention (58, 59, 61, 62). Two studies were treatment trials comparing amoxicillin to erythromycin, which was the standard of care at the time (49, 60). One of these studies was a double blind, randomized trial of amoxicillin vs. erythromycin (49). The other was an open trial of amoxicillin and erythromycin, where all participants were offered amoxicillin as an alternate treatment option to erythromycin (60). While the main objective of these two studies was to provide a comparison of side-effects between treatment groups, perinatal chlamydial infection was one of the outcome measures evaluated (49, 60). The focus of the last study differed from the others; it was a double blind, randomized study of amoxicillin vs. placebo (39). In each of the studies, treatment occurred at different time points during pregnancy. Treatment in three studies occurred at 36 weeks (58–60), but occurred at various times in the other studies: 24 weeks (39), 28–32 weeks (61), <38 weeks (49), or not specified [(62); Table 3].
Infant Outcomes
Only three of the studies primarily focused on determining the potential of chlamydial treatment to prevent perinatal chlamydial infection by comparing infants of women with chlamydial infections, who were either treated or untreated (39, 58, 62). As a result, these studies were able to provide more direct information about the potential to reduce perinatal chlamydial infections with maternal treatment during pregnancy. Of note, another study also provided information on perinatal chlamydial infection in untreated infants, but the number was too small (only three infants) to draw any definitive conclusions (59). In one of the studies, 32 women refused treatment after all women with chlamydial infection were offered erythromycin; 24 infants of untreated women served as controls for the 59 infants of treated women (58). In contrast, the decision to treat or not treat in another study was based on provider discretion; they followed 21 infant outcomes from a group of 47 chlamydia-infected, untreated women and outcomes of 16 infants from 38 women treated with erythromycin (62). The last study, which sought an alternative to erythromycin for treatment of chlamydia, randomized 21 women to treatment with either amoxicillin or placebo and followed infant outcomes [(39); Table 3].
Infant chlamydia infection was detected by different methods in the studies. Two studies were the most comprehensive using a combination of methods including persistence of chlamydia IgG antibody in first year of life, chlamydia mucosal cultures (nasopharynx, conjunctiva, rectum), and evaluation for symptomatic infection such as pneumonia and conjunctivitis (58, 60). A similar strategy was also used in another study that tested infant tears and blood for chlamydia antibody and obtained chlamydia cultures (nasopharynx, oropharynx, conjunctiva, rectum, genitalia) (39). Two other studies used a combination of infant mucosal cultures (nasopharynx and conjunctiva) taken from 2 to 11 or 12 weeks along with evaluation for symptomatic disease such as conjunctivitis and pneumonia among others (59, 62). For the remaining studies, one used only surface cultures collected 1 week after birth (49), and another evaluated exclusively for symptomatic disease in the first 6–8 weeks of life [(61); Table 3].
Two studies found significantly decreased rates of infant chlamydial infection among those born to women receiving treatment with erythromycin as opposed to no treatment (58, 62). A decrease in infant chlamydial infection from 50% (12/24) to 7% (4/59) (p < 0.001) and 23.8% (5/21) to 0% (0/16) (p < 0.04) were noted with treatment (58, 62). Symptomatic chlamydia infection with conjunctivitis and pneumonia were more frequently seen among infants of untreated women (58, 62). Two other studies reported observations on small numbers of infants, who were born to untreated women: 2 of 3 infants had positive cultures (59), and 1 of 2 infants had conjunctivitis among women who refused completion of erythromycin [(60); Table 3].
Other studies provided indirect evidence about the potential to prevent adverse neonatal outcomes through maternal treatment, as low rates of infant chlamydial infection and symptomatic infant disease were observed among treated women. These studies primarily evaluated infant outcomes for treated women (49, 59, 60), but one study compared differences in infant outcomes for treated, chlamydia-infected women vs. chlamydia-uninfected women (61).
In infants of treated women, they found low rates of infant chlamydial infection based on either chlamydial mucosal cultures or persistent antibody levels ranging from 0% (0/152, 0/16) to 11% (4/36) (49, 59, 60); another study found no significant differences in symptomatic disease among infants born to women without chlamydial infection vs. those with treated chlamydial infection, which would be the expected outcome with effective chlamydial treatment [(61); Table 3]. In contrast to the other six studies, one small study did not find significant differences in the number of neonatal chlamydial infections among women treated with amoxicillin vs. placebo [3/8 (37.5%) vs. 1/3 (33.3%)] (39).
Study Quality
Some factors that could have impacted the quality of results included test of cure to ensure the therapeutic intervention had eradicated chlamydial infection, maternal compliance with the treatment regimen, loss of follow-up, and cohort sample size. In five of seven studies, information regarding effectiveness of treatment was documented by test of cure (i.e., repeat cultures after treatment), which showed no evidence of continued infection in most treated women (92–99.5%) (49, 58, 60, 61). The exception was one study that used amoxicillin, which had much higher failure rates of 33.3% (3/9) (39).
Information regarding non-compliance with the study such as maternal follow-up and treatment recommendations also varied considerably from 5.2% to as high as 41.8% (39, 49, 58–61). These findings were particularly striking for the limited degree of infant follow-up achieved by these studies, which ranged from 38.9 to 72.4% (39, 49, 58–61). Other possible confounders that may have impacted infant outcomes included limited information on testing of sexual partners, which was only done in one study (49), and treatment of sexual partners, which was offered in five of the studies, but no information was available in any of the studies regarding the percentage of partners that actually received treatment (39, 49, 58, 60, 61). While delivery method (caesarian section vs. vaginal delivery) could also impact the likelihood of chlamydia vertical transmission, few of the studies excluded infants that were born via caesarian section without prior rupture of membranes (39, 60). Other factors that could have impacted results included the reliance on infant chlamydia serology along with cultures to diagnose chlamydial infection (39, 58, 60). which were the main methods used at the time these studies were conducted but lack the sensitivity and specificity of molecular testing methods (NAATs) currently used. In addition, one of the studies (53) also used Chlamydiazyme, an enzyme immunoassay, to identify maternal chlamydial infections. This method of testing has been reported to have decreased sensitivity in the detection of chlamydial infections compared to culture (63). There was also a lack of information regarding how symptomatic infections such as pneumonia and conjunctivitis were determined to be due to chlamydia as opposed to other etiologies (58).
Discussion
When viewed comprehensively, this review provides fair to moderate support from thirteen of fifteen studies that chlamydial screening and treatment in pregnancy may lead to improved pregnancy and infant outcomes. However, the heterogeneity of those studies precluded any combined quantitative assessments such as meta-analyses of the effects of treatment on those outcomes. Overall, the strength of the evidence was limited by only a handful of studies directly comparing pregnancy or neonatal outcomes between treated and untreated chlamydia-infected mothers. As with any focused review, there was likely some degree of publication bias given the inherent difficulty in locating studies with negative findings (Tables 1–3).
Three studies, which included two retrospective/observational studies and one double-blind randomized placebo controlled trial, provided the strongest evidence within the group suggesting that chlamydial treatment with erythromycin may lead to improved pregnancy outcomes such as reduction in preterm birth, premature rupture of membranes, and/or low birth weight infants (50–52). These studies also had important limitations. This included: (1) The regimen of erythromycin used in one (50); (2) Significant findings only after adjustment of one of the study's sample size because of spontaneous clearance of infection in placebo cases (50); (3) Evaluation of the effect of treatment on other infections such as Ureaplasma and Group B Streptococcus apart from just C. trachomatis (50); (4) Use of persistent or recurrent infection as opposed to untreated patients in one study (51); (5) Lack of information regarding use of other antibiotics during pregnancy with possible effects on chlamydial infection (50–52).
In contrast, the strongest evidence that antenatal chlamydial treatment with erythromycin may decrease neonatal infection came from two observational studies in the mid-1980s (58, 62). Both studies, however, had several limitations including significant losses to follow-up and use of a non-standardized treatment regimen in one study [(58, 62); Tables 1–3].
Viewed collectively, our review of these studies also highlights serious gaps in existing knowledge. While the study by Martin et al. came the closest, none of the randomized controlled trials focused exclusively on the effect of early screening and treatment of chlamydial infections by comparing treated and untreated groups with respect to adverse pregnancy and neonatal outcomes (50). Furthermore, only a few studies used sensitive molecular methods such as PCR to detect C. trachomatis infection, and none of the studies focused exclusively on evaluating more easily administered antimicrobials such as single dose azithromycin as the only intervention. Additional gaps include the lack of interventional research in regions of the world outside of the US, where STIs like C. trachomatis are most prevalent and the burden of problems such as preterm birth and adverse neonatal and pregnancy outcomes are the highest (3, 4, 29). In fact, only two of the studies meeting selection criteria for review were in such countries [(28, 53); Tables 1–3].
Although countries such as the U.S. have implemented chlamydial screening for pregnant women, particularly for those at risk for the past few decades, many countries around the world, particularly resource-limited countries have continued to rely on the WHO-endorsed “syndromic approach” to symptomatic STIs in pregnancy (1, 3, 29, 64–66). Few studies have evaluated the prevalence of Chlamydia trachomatis in pregnancy in high-risk regions of the world, and the extent of the morbidity associated, particularly infant morbidity, is largely unknown (3, 4). Given the greater recognition in recent years of the association of preterm birth with worldwide morbidity and mortality, the possible role that chlamydial infections in pregnancy may play and the potential to treat these infections assumes even greater importance (3, 67).
Conclusion
This focused review has found fair to moderate evidence with a consistent trend supporting a potential beneficial role for screening and treating Chlamydia trachomatis in pregnancy in order to reduce adverse pregnancy outcomes such as premature rupture of membranes, premature, and low birth weight infants and neonatal chlamydial infection. However, further research, is needed to optimize understanding and benefits of such interventions, particularly with regards to adverse pregnancy outcomes in regions of the world most at risk.
Author Contributions
KA and JK have collaborated to perform the literature search, article selection, and the writing and development of this manuscript. KN-S has assisted with the analysis, writing, development, and editing of this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.531073/full#supplementary-material
Footnotes
1. ^Please note that Gray et al. (28), study also discusses neonatal ophthalmia but has been included in adverse pregnancy outcomes section because it primarily deals with adverse pregnancy outcomes as opposed to neonatal outcomes.
References
1. World Health Organization. Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections−2008. Geneva: World Health Organization (2012).
2. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012. Based on systematic review and global reporting. PLoS ONE. (2015) 10:e0143304. doi: 10.1371/journal.pone.0143304
3. Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis infection in pregnancy: the global challenge of preventing adverse pregnancy and infant outcomes in Sub-Saharan Africa and Asia. BioMed Res Inte. (2016) 2016:9315757. doi: 10.1155/2016/9315757
4. Joseph Davey DL, Shull HI, Billings JD, Wang D, Adachi K, Klausner JD. Prevalence of curable sexually transmitted infections in pregnant women in low- and middle-income countries from 2010 to 2015: a systematic review. Sex Transm Dis. (2016) 43:450–8. doi: 10.1097/OLQ.0000000000000460
5. Folger AT. Maternal Chlamydia trachomatis infections and preterm birth: the impact of early detection and eradication during pregnancy. Maternal Child Health J. (2014) 18:1795–802. doi: 10.1007/s10995-013-1423-6
6. Adachi K, Klausner JD, Xu J, Ank B, Bristow CC, Morgado MG, et al. Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected pregnant women and adverse infant outcomes. Pediatr Infect Dis J. (2016) 35:894–900. doi: 10.1097/INF.0000000000001199
7. Silveira MF, Ghanem KG, Erbelding EJ, Burke AE, Johnson HL, Singh RH, et al. Chlamydia trachomatis infection during pregnancy and the risk of preterm birth: a case-control study. Int J STD AIDS. (2009) 20:465–9. doi: 10.1258/ijsa.2008.008388
8. Silveira MF, Erbelding EJ, Ghanem KG, Johnson HL, Burke AE, Zenilman JM. Risk of Chlamydia trachomatis infection during pregnancy: effectiveness of guidelines-based screening in identifying cases. Int J STD AIDS. (2010) 21:367–70. doi: 10.1258/ijsa.2010.009559
9. Claman P, Toye B, Peeling RW, Jessamine P, Belcher J. Serologic evidence of Chlamydia trachomatis infection and risk of preterm birth. CMAJ. (1995) 153:259–62.
10. Gencay M, Koskiniemi M, Ammala P, Fellman V, Narvanen A, Wahlstrom T, et al. Chlamydia trachomatis seropositivity is associated both with stillbirth and preterm delivery. APMIS. (2000) 108:584–8. doi: 10.1034/j.1600-0463.2000.d01-101.x
11. Bekler C, Kultursay N, Ozacar T, Sayiner A, Yalaz M, Akisu M. Chlamydial infections in term and preterm neonates. Jap J Infect Dis. (2012) 65:1–6.
12. Rours GI, Duijts L, Moll HA, Arends LR, de Groot R, Jaddoe VW, et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol. (2011) 26:493–502. doi: 10.1007/s10654-011-9586-1
13. Rours GI, de Krijger RR, Ott A, Willemse HF, de Groot R, Zimmermann LJ, et al. Chlamydia trachomatis and placental inflammation in early preterm delivery. Eur J Epidemiol. (2011) 26:421–8. doi: 10.1007/s10654-011-9569-2
14. Baud D, Goy G, Jaton K, Osterheld MC, Blumer S, Borel N, et al. Role of Chlamydia trachomatis in miscarriage. Emerg Infect Dis. (2011) 17:1630–5. doi: 10.3201/eid1709.100865
15. Mardh PA. Influence of infection with Chlamydia trachomatis on pregnancy outcome, infant health and life-long sequelae in infected offspring. Best Pract Res Clin Obstetr Gynaecol. (2002) 16:847–64. doi: 10.1053/beog.2002.0329
16. McGregor JA, French JI, Lawellin D, Todd JK. Preterm birth and infection: pathogenic possibilities. Am J Reproduct Immunol Microbiol. (1988) 16:123–32. doi: 10.1111/j.1600-0897.1988.tb00181.x
17. Baud D, Regan L, Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis. (2008) 21:70–6. doi: 10.1097/QCO.0b013e3282f3e6a5
18. Locksmith G, Duff P. Infection, antibiotics, and preterm delivery. Semin Perinatol. (2001) 25:295–309. doi: 10.1053/sper.2001.27163
19. Silva MJ, Florencio GL, Gabiatti JR, Amaral RL, Eleuterio Junior J, Goncalves AK. Perinatal morbidity and mortality associated with chlamydial infection: a meta-analysis study. Brazil J Infect Dis. (2011) 15:533–9. doi: 10.1590/S1413-86702011000600006
20. Gravett MG, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA. (1986) 256:1899–903. doi: 10.1001/jama.256.14.1899
21. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. (2014) 385:430–40. doi: 10.1016/S0140-6736(14)61698-6
22. Hammerschlag MR. Chlamydial and gonococcal infections in infants and children. Clin Infect Dis. (2011) 53(Suppl. 3):S99–102. doi: 10.1093/cid/cir699
23. Hammerschlag MR, Chandler JW, Alexander ER, English M, Koutsky L. Longitudinal studies on chlamydial infections in the first year of life. Pediatr Infect Dis. (1982) 1:395–401. doi: 10.1097/00006454-198211000-00007
24. Schachter J, Grossman M, Sweet RL, Holt J, Jordan C, Bishop E. Prospective study of perinatal transmission of Chlamydia trachomatis. JAMA. (1986) 255:3374–7. doi: 10.1001/jama.255.24.3374
25. Zar HJ. Neonatal chlamydial infections: prevention and treatment. Paediatr Drugs. (2005) 7:103–10. doi: 10.2165/00148581-200507020-00003
26. Workowski KA, Berman SM. Centers for disease control and prevention sexually transmitted disease treatment guidelines. Clin Infect Dis. (2011) 53(Suppl. 3):S59–63. doi: 10.1093/cid/cir694
27. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. (2015) 64:1–137.
28. Gray RH, Wabwire-Mangen F, Kigozi G, Sewankambo NK, Serwadda D, Moulton LH, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstetr Gynecol. (2001) 185:1209–17. doi: 10.1067/mob.2001.118158
29. Medline A, Joseph Davey D, Klausner JD. Lost opportunity to save newborn lives: variable national antenatal screening policies for Neisseria gonorrhoeae and Chlamydia trachomatis. Int J STD AIDS. (2016) 28:660–6. doi: 10.1177/0956462416660483
30. Banniettis N, Wisecup K, Boland L, Watanabe I, Hammerschlag MR, Kohlhoff S. Association of routine Chlamydia trachomatis screening during pregnancy and seroprevalence of chlamydial infection in children, 1991-2015. J Pediatr Infect Dis Soc. (2021) 10:172–4. doi: 10.1093/jpids/piaa002
31. Kohlhoff S, Roblin PM, Clement S, Banniettis N, Hammerschlag MR. Universal prenatal screening and testing and Chlamydia trachomatis conjunctivitis in infants. Sex Transm Dis. (2020). doi: 10.1097/OLQ.0000000000001344. [Epub ahead of print].
32. Hammerschlag MR, Cummings C, Roblin PM, Williams TH, Delke I. Efficacy of neonatal ocular prophylaxis for the prevention of chlamydial and gonococcal conjunctivitis. N Engl J Med. (1989) 320:769–72. doi: 10.1056/NEJM198903233201204
33. Smith-Norowitz TA, Ukaegbu C, Kohlhoff S, Hammerschlag MR. Neonatal prophylaxis with antibiotic containing ointments does not reduce incidence of chlamydial conjunctivitis in newborns. BMC Infect Dis. (2021) 21:270. doi: 10.1186/s12879-021-05974-3
34. Low N, Redmond S, Uuskula A, van Bergen J, Ward H, Andersen B, et al. Screening for genital chlamydia infection. Cochrane Database Syst Rev. (2016) 9:CD010866. doi: 10.1002/14651858.CD010866.pub2
35. Zakher B, Cantor AG, Pappas M, Daeges M, Nelson HD. Screening for gonorrhea and Chlamydia: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. (2014) 161:884–93. doi: 10.7326/M14-1022
36. Ditkowsky J, Shah KH, Hammerschlag MR, Kohlhoff S, Smith-Norowitz TA. Cost-benefit analysis of Chlamydia trachomatis screening in pregnant women in a high burden setting in the United States. BMC Infect Dis. (2017) 17:155. doi: 10.1186/s12879-017-2248-5
37. Rours GI, Smith-Norowitz TA, Ditkowsky J, Hammerschlag MR, Verkooyen RP, de Groot R, et al. Cost-effectiveness analysis of Chlamydia trachomatis screening in Dutch pregnant women. Pathog Glob Health. (2016) 110:292–302. doi: 10.1080/20477724.2016.1258162
38. Ong JJ, Chen M, Hocking J, Fairley CK, Carter R, Bulfone L, et al. Chlamydia screening for pregnant women aged 16-25 years attending an antenatal service: a cost-effectiveness study. BJOG. (2016) 123:1194–202. doi: 10.1111/1471-0528.13567
39. Bell TA, Sandstrom IK, Eschenbach DA, Hummel D, Kuo C, Wang S, et al. Treatment of Chlamydia trachomatis in Pregnancy with Amoxicillin. New York, NY: Elsevier Biomedical Press (1982).
40. Ottesen M, Sahl I, Herbstman MM, Friis HM, Philipsen T. [Chlamydia trachomatis in pregnant women in the county of Vestsjaelland. Prevalence, prevention of perinatal transmission and cost-effectiveness of screening]. Ugeskr Laeger. (1996) 158:756–8.
41. Nishimura M, Kumamoto Y, Koroku M, Tsunekawa T, Hiroe T, Hayashi K, et al. [Epidemiological study on Chlamydia trachomatis infection in pregnant housewives and investigation on its influence on outcome of pregnancy and on their newborns]. Kansenshogaku Zasshi. (1990) 64:179–87. doi: 10.11150/kansenshogakuzasshi1970.64.179
42. Brocklehurst P, Rooney G. Interventions for treating genital Chlamydia trachomatis infection in pregnancy. Cochrane Database Syst Rev. (2000) 1998:CD000054. doi: 10.1002/14651858.CD000054
43. Jain S. Perinatally acquired Chlamydia trachomatis associated morbidity in young infants. J Matern Fetal Med. (1999) 8:130–3. doi: 10.1002/(SICI)1520-6661(199905/06)8:3 <130::AID-MFM11>3.0.CO;2-X
44. Nadafi M, Abdali KH, Parsanejad ME, Rajaee-Fard AR, Kaviani M. A comparison of amoxicillin and erythromycin for asymptomatic Chlamydia trachomatis infection in pregnancy. Int J Gynaecol Obstetr. (2005) 90:142–3. doi: 10.1016/j.ijgo.2005.02.016
45. French JI, McGregor JA, Parker R. Readily treatable reproductive tract infections and preterm birth among black women. Am J Obstetr Gynecol. (2006) 194:1717–26; discussion: 1726–17. doi: 10.1016/j.ajog.2006.03.004
46. Kovacs L, Nagy E, Berbik I, Meszaros G, Deak J, Nyari T. The frequency and the role of Chlamydia trachomatis infection in premature labor. Int J Gynaecol Obstetr. (1998) 62:47–54. doi: 10.1016/S0020-7292(98)00075-7
47. Rivlin ME, Morrison JC, Grossman JH III. Comparison of pregnancy outcome between treated and untreated women with chlamydial cervicitis. J Miss State Med Assoc. (1997) 38:404–7.
48. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
49. Alary M, Joly JR, Moutquin JM, Mondor M, Boucher M, Fortier A, et al. Randomised comparison of amoxycillin and erythromycin in treatment of genital chlamydial infection in pregnancy. Lancet. (1994) 344:1461–5. doi: 10.1016/S0140-6736(94)90288-7
50. Martin DH, Eschenbach DA, Cotch MF, Nugent RP, Rao AV, Klebanoff MA, et al. Double-blind placebo-controlled treatment trial of Chlamydia trachomatis endocervical infections in pregnant women. Infect Dis Obstetr Gynecol. (1997) 5:10–17. doi: 10.1002/(SICI)1098-0997(1997)5:1<10::AID-IDOG5>3.0.CO;2-I
51. Cohen I, Veille JC, Calkins BM. Improved pregnancy outcome following successful treatment of chlamydial infection. JAMA. (1990) 263:3160–3. doi: 10.1001/jama.263.23.3160
52. Ryan GM Jr., Abdella TN, McNeeley SG, Baselski VS, Drummond DE. Chlamydia trachomatis infection in pregnancy and effect of treatment on outcome. Am J Obstetr Gynecol. (1990) 162:34–9. doi: 10.1016/0002-9378(90)90815-O
53. Rastogi S, Das B, Salhan S, Mittal A. Effect of treatment for Chlamydia trachomatis during pregnancy. Int J Gynaecol Obstetr. (2003) 80:129–37. doi: 10.1016/S0020-7292(02)00371-5
54. McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double-blind, placebo-controlled trial of erythromycin treatment. Am J Obstetr Gynecol. (1990) 163(5 Pt 1):1580–91. doi: 10.1016/0002-9378(90)90632-H
55. Andrews WW, Klebanoff MA, Thom EA, Hauth JC, Carey JC, Meis PJ, et al. Midpregnancy genitourinary tract infection with Chlamydia trachomatis: association with subsequent preterm delivery in women with bacterial vaginosis and Trichomonas vaginalis. Am J Obstetr Gynecol. (2006) 194:493–500. doi: 10.1016/j.ajog.2005.08.054
56. Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. (2000) 342:534–40. doi: 10.1056/NEJM200002243420802
57. Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. (2001) 345:487–93. doi: 10.1056/NEJMoa003329
58. Schachter J, Sweet RL, Grossman M, Landers D, Robbie M, Bishop E. Experience with the routine use of erythromycin for chlamydial infections in pregnancy. N Engl J Med. (1986) 314:276–9. doi: 10.1056/NEJM198601303140503
59. FitzSimmons J, Callahan C, Shanahan B, Jungkind D. Chlamydial infections in pregnancy. J Reproduct Med. (1986) 31:19–22.
60. Crombleholme WR, Schachter J, Grossman M, Landers DV, Sweet RL. Amoxicillin therapy for Chlamydia trachomatis in pregnancy. Obstetr Gynecol. (1990) 75:752–6.
61. Black-Payne C, Ahrabi MM, Bocchini JA Jr., Ridenour CR, Brouillette RM. Treatment of Chlamydia trachomatis identified with Chlamydiazyme during pregnancy. Impact on perinatal complications and infants. J Reproduct Med. (1990) 35:362–7.
62. McMillan JA, Weiner LB, Lamberson HV, Hagen JH, Aubry RH, Abdul-Karim RW, et al. Efficacy of maternal screening and therapy in the prevention of chlamydia infection of the newborn. Infection. (1985) 13:263–6. doi: 10.1007/BF01645435
63. Newhall WJ, Johnson RE, DeLisle S, Fine D, Hadgu A, Matsuda B, et al. Head-to-head evaluation of five chlamydia tests relative to a quality-assured culture standard. J Clin Microbiol. (1999) 37:681–5. doi: 10.1128/JCM.37.3.681-685.1999
64. Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. (2010) 59:1–110.
65. Meyers D, Wolff T, Gregory K, Marion L, Moyer V, Nelson H, et al. USPSTF recommendations for STI screening. Am Family Phys. (2008) 77:819–24.
66. WHO. Global Strategy for Prevention and Control of Sexually Transmitted Infections: 2006-2105. Geneva: World Health Organization (2006).
Keywords: Chlamydia trachomatis, sexually transmitted infections, pregnancy, infant outcomes, adverse pregnancy outcomes
Citation: Adachi KN, Nielsen-Saines K and Klausner JD (2021) Chlamydia trachomatis Screening and Treatment in Pregnancy to Reduce Adverse Pregnancy and Neonatal Outcomes: A Review. Front. Public Health 9:531073. doi: 10.3389/fpubh.2021.531073
Received: 04 February 2020; Accepted: 19 April 2021;
Published: 10 June 2021.
Edited by:
Diamantis Plachouras, European Centre for Disease Prevention and Control (ECDC), SwedenReviewed by:
Margaret Hammerschlag, SUNY Downstate Medical Center, United StatesStephan Kohlhoff, SUNY Downstate Medical Center, United States
Copyright © 2021 Adachi, Nielsen-Saines and Klausner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karin Nielsen-Saines, a25pZWxzZW5AbWVkbmV0LnVjbGEuZWR1
 Kristina N. Adachi
Kristina N. Adachi Karin Nielsen-Saines
Karin Nielsen-Saines Jeffrey D. Klausner2
Jeffrey D. Klausner2