- 1Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, China
- 2Bamboo Research Institute, Nanjing Forestry University, Nanjing, China
- 3College of Biology and the Environment, Nanjing Forestry University, Nanjing, China
Bamboo is a perennial flowering plant with a distinctive life cycle: many bamboo species remain in the vegetative phase for decades, followed by mass synchronous flowering and subsequent death. The phenomenon of bamboo flowering is not fully understood, but its periodicity is a major research focus. Here, we collected information on bamboo flowering events by investigating historical documents and field studies at the Bamboo Research Institute of Nanjing Forestry University. We compiled information on more than 630 flowering events, 124 of which accurately recorded the flowering cycle time. We summarized the specific flowering cycles of 85 bamboo species, as well as four kinds of bamboo flowering habits in detail. We present a theory of the bamboo flowering cycle and discuss the reasons for the observed variations in bamboo flowering. This review also introduces two mechanisms by which bamboo forests are rejuvenated after flowering and explains the flowering phenomena of bamboo forests using the bamboo flowering cycle theory. Finally, we present suggestions for forest management strategies. Bamboo flowering is a normal physiological phenomenon, even though it has unique elements compared with flowering in other plants. The results presented here provide valuable reference material for understanding bamboo flowering and its periodicity.
Introduction
Bamboo (subfamily Bambusoideae) is a large clade of the family Poaceae. There are more than 88 genera and 1,642 species worldwide, of which 28 genera and more than 120 species are herbaceous bamboo (Vorontsova et al., 2016). Bamboo is widely distributed in the tropical, subtropical, and temperate regions of all continents, except Europe and Antarctica, from lowlands to ~4,000 m above sea level. China is a center of bamboo distribution, having more than 34 genera and 534 species (Chen et al., 2006). Bamboo species play important economic and ecological roles in many countries. Bamboo provides a wide range of products and has many uses for humans and other animals, while also having a major impact on the environment (Zhou, 1984).
Bamboo has attracted worldwide attention because of its distinctive life history. It is a perennial flowering plant, but many bamboo species remain in a vegetative phase for decades, or even a century, followed by mass synchronous flowering and subsequent death (Janzen, 1976). Thus, bamboo flowering has a negative effect on the livelihoods of people who depend on bamboo resources and could lead to famine among self-sufficient farmers. For example, Bambusa balcoa, B. tulda, Dendrocalamus hamiltonii, and Stapletonia arunachalensis all flowered in 2009 in Arunachal Pradesh, India. Subsequently, rodent outbreaks were reported in the flowering area, which caused severe damage to many crops (Kumawat et al., 2014). Consequently, people have begun to pay more attention to bamboo flowering for its scientific importance and its crucial role in many human communities.
All seed plants have similar life cycles, from seed germination, through a juvenile stage, vegetative growth phase, and reproductive period, including flowering and formation of seeds. Bamboo is similar, although it has its own unique flowering characteristics, which include: (i) it has a long vegetative phase; and (ii) its asexual reproductive capability is particularly strong, with a single clone possessing the ability to populate an entire bamboo forest. In addition, bamboo flowering has been linked to a number of poorly understood phenomena, such as bamboo groves bursting into bloom and dying, and sporadic flowering.
From more than 2,000 years ago to 1,721, many ancient Chinese books have recorded the phenomena of bamboo flowering and fruiting. However, most of these records mainly focus on the culinary and medicinal uses of bamboo fruit. The book Shan Hai Jing, written more than 2,000 years ago, states: “When bamboo flowers, it will wither. “The book Zhu Pu, written by Dai during the Jin Dynasty (from 317 to 420) states: “Bamboo flowering and seeding needs 60 years and bamboo can regenerate through seed in 6 years.” In 1,721, a local biography of Taizhou in Zhejiang province described the fruit of Fargesia sp., which could be used to treat dysentery (Zhou and Hu, 2000). From the mid-eighteenth to the early nineteenth century, researchers began to classify bamboo plants, but there were no reports of bamboo flowering. In fact, the first bamboo classification originates from Rumpf (1750), who divided the bamboos into eight classes, all with the name Arundo. Based on this division, in 1,753, Linnaeus used the name “Arundo bambos,” which included all the bamboos. Later, the genus name Bambusa was adopted (Holttum, 1956). In 1788, Retzius first established the genus of sympodial bamboo, using the name Bambusa (Jiang, 2007). In 1803, the first monopodial bamboo genus, Arundinaria Michaux, was established (Chao and Tang, 1993).
From 1,829 to the mid- nineteenth century, researchers paid closer attention to the reproductive organs, such as inflorescences and fruit, of bamboo plants. The earliest description of a bamboo inflorescence as a distinct structural unit was by Nees in 1829. This was quoted verbatim by Munro as a part of his characterization of the “Bambusaceae” (Munro, 1868; McClure, 1966). From the mid- nineteenth century to the early twentieth century, the amount of literature regarding bamboo flowering began to increase, and many reports used reproductive organs, such as inflorescences and fruits, as the main basis for classifying bamboo. For example, Munro used fruit characteristics as the main classification criterion, although the number of fruiting specimens was limited (Munro, 1868).
In the twentieth century, research on bamboo flowering greatly increased and inflorescence characteristics were widely used in the classification of bamboo. In addition, researchers comprehensively studied flowering-related events in bamboo, including flowering cycle, flowering habits, factors resulting in flowering, die-back and recovery, rejuvenation, and the effects of bamboo flowering (Lu, 1980; He et al., 1994; Chen et al., 1995; Cheng et al., 2014). In addition, McClure (1966) and Keng (1986) performed many detailed studies on inflorescences and the evolution of bamboo plants, and applied inflorescence characteristics to bamboo classification. Yao and Tan (2008) found that the fruits of only 72 species (out of 515 bamboo species) were described in the ninth volume of Chinese Flora. Since the beginning of the twenty-first century, studies on bamboo flowering have mainly focused on flowering biology (Qin, 1995; Zeng et al., 1998; Xing et al., 2005; Wang and Wu, 2009), molecular biology (Zhang et al., 2012; Gao et al., 2014, 2015; Wang et al., 2014), and the spatial and temporal distribution of flowering bamboo (Bhattacharya et al., 2006; Lin and Ding, 2007; Franklin, 2010; Zhang et al., 2012; de Carvalho et al., 2013; Gao et al., 2014; Wang et al., 2014; Crone et al., 2015).
Although bamboo flowering has been studied, many questions remain. Why do many sympodial bamboo species recorded in the literature often bloom sporadically? Why are differences in the flowering types of the same bamboo species observed in different areas? Why do the same bamboo species bloom in some areas and not in other areas? In this review, we address these questions by (I) summarizing and synthesizing information from many studies on the flowering cycle of bamboo, including more than 600 bamboo flowering events and 30 specific bamboo flowering events observed by researchers from the Bamboo Research Institute of Nanjing Forestry University. (II) Detailing the four types of bamboo flowering habits and summarizing the specific flowering cycles of 85 bamboo species. (III) Introducing a theory of the bamboo flowering cycle, which shows that each bamboo species and each clone has its own flowering cycle. In addition, we also introduce the flowering distribution and flowering wave phenomenon in bamboo, which explains why there are various flowering patterns in bamboo forests, and the reasons for bamboo flowering. (IV) Introducing two ways of rejuvenating flowering bamboo forests. We simplify the complicated flowering phenomena of various bamboo forests in nature using the bamboo flowering cycle theory to rationalize flowering phenomena.
Types of Bamboo Flowering
To classify the types of bamboo flowering, we first collected as many examples of bamboo flowering as we could find. Researchers at the Bamboo Research Institute of Nanjing Forestry University have observed and studied the flowering events of many bamboo species. Table 1 lists the flowering events of 30 species of bamboo, including details on the flowering time, site, and type. We observed that all the flowering culms died after flowering in many species such as Bambusa multiplex, Chimonobambusa sichuanensis, Phyllostachys edulis, Ph. glauca, Ph. nidularia, and Sasaella kogasensis “Aureostriatus.” There are also some differences among different flowering bamboo species, mainly in the seed setting rate and the mode of bamboo forest regeneration and rejuvenation. It is worth noting that the observed flowering habits of some bamboo species were not completely consistent with the historical literature, which may be related to a change in environment and/or whether they were wild or cultivated.
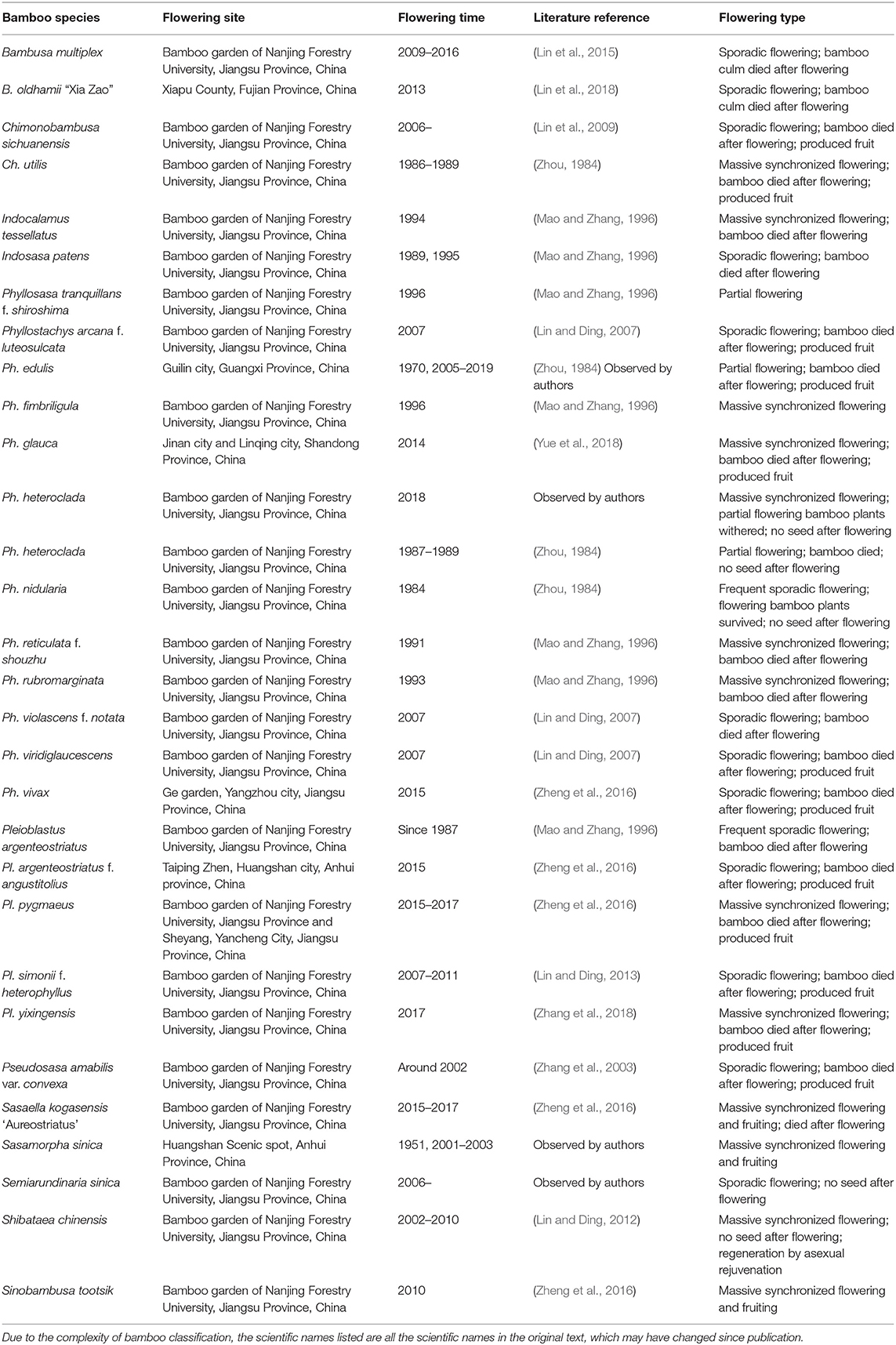
Table 1. Bamboo flowering events observed by the Bamboo Research Institute of Nanjing Forestry University.
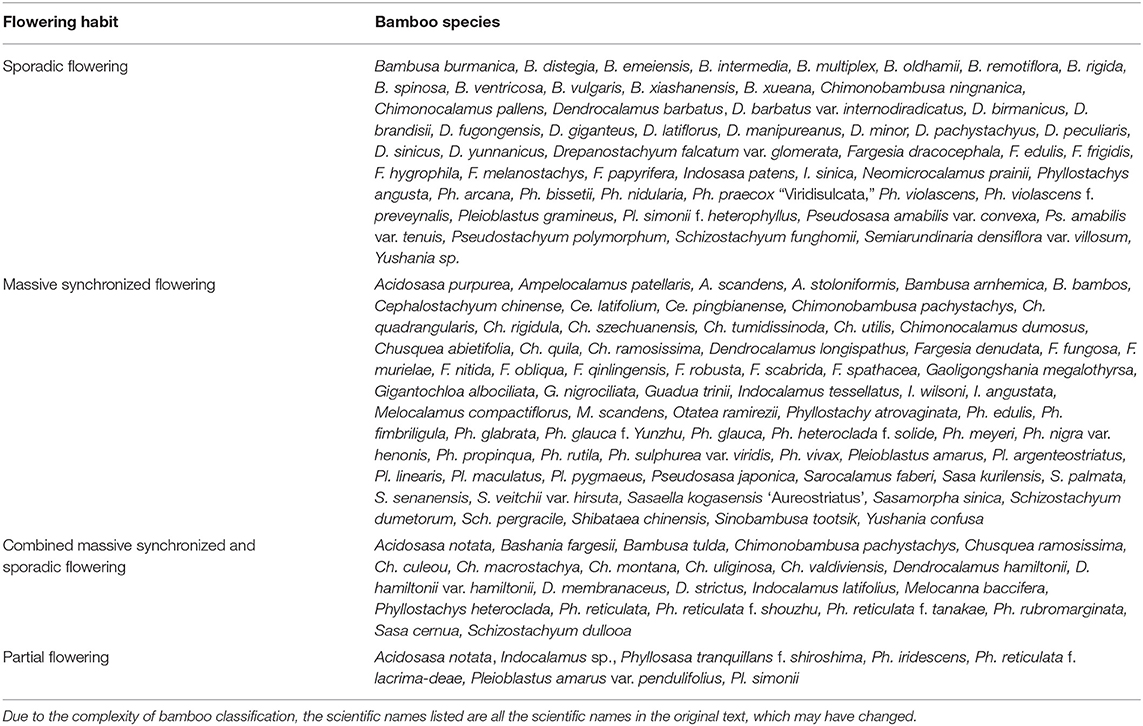
Based on the extensive examples collected, we found that the flowering habits of bamboo can be divided into four types: sporadic, massive synchronized, combined massive synchronized and sporadic, and partial flowering (McClure, 1966; Lin and Mao, 2007). The details are shown in Table 2. Classifying the flowering phenomenon is done here according to the proportion of bamboo flowering in bamboo forests, hoping to unify thereby the classification criteria.
In sporadic flowering, there are usually only 1–2 clusters or a small area of scattered bamboo flowering within a bamboo population. This flowering occurs randomly. As shown in Table 2, 53 bamboo species are listed as undergoing sporadic flowering, including common species such as Bambusa emeiensis, Dendrocalamus latiflorus, and Ph. violascens. According to the literature (Zhou, 1984; Du et al., 2000; Yuan et al., 2005, 2008, 2012; Franklin, 2010), sporadic flowering often occurs in cultivated or intensively managed bamboo species, which flower sporadically more often than wild species. Franklin (2010) suggested that the term “sporadic flowering” may imply random or other non-gregarious patterns of flowering, but application of the term has been variable and ill-defined, and there is no convincing evidence that any semelparous bamboo has a reproductive strategy that may be regarded as “not gregarious.” This paper holds that sporadic flowering is a random and irregular small number of flowering.
In massive synchronized flowering, also known as gregarious flowering, a large flowering area of >50% occurs within a bamboo population. Many bamboo plants show a cyclic pattern of gregarious flowering after a long vegetative period (Janzen, 1976; Du et al., 2000). As shown in Table 2, 70 bamboo species undergo massive synchronized flowering. Many researchers are concerned about this flowering type, because massive synchronized flowering of bamboo forests leads to large-scale bamboo death, which seriously affects the local economy and environment.
In the combined massive synchronized and sporadic flowering category, species may display sporadic and/or small areas of flowering before and after large areas undergo flowering. As shown in Table 2, 23 bamboo species, such as B. tulda, Chusquea culeou, C. montana, Melocanna baccifera, Ph. heteroclada, Ph. reticulata, and Sasa cernua, are in this category (Zhang W. Y. et al., 1992; Pearson et al., 1994; Du et al., 2000; Ramanayake and Weerawardene, 2003; Bhattacharya et al., 2006; Kitamura and Kawahara, 2009; Wang and Wu, 2009; Tagle et al., 2013).
In partial flowering, the degree of flowering in a bamboo forest is between sporadic and massive synchronized flowering and generally occurs in a patchy distribution. There are seven bamboo species listed as partial flowering in Table 2. For example, Pleioblastus simonii underwent partial flowering at Kew Gardens from 1892 to 1903 (Bean, 1907).
We investigated whether there is a relationship between the flowering habits of bamboo species and their taxonomic position at the genus level. We found that some genera, such as Arundinaria, Bambusa, Chimonobambusa, Dendrocalamus, Phyllostachys, Pleioblastus, and Schizostachyum, included sporadic, massive synchronized, and combined massive synchronized and sporadic flowering species (Bean, 1907; Jiao, 1956; Anonymous, 1961; Ram and Gopal, 1981; Zhou, 1984; Zhang and Ma, 1989; Pearson et al., 1994; Du et al., 2000; Li and Denich, 2004; Bhattacharya et al., 2006; Mao et al., 2008; Kitamura and Kawahara, 2009; Nath and Das, 2010; Sarma et al., 2010; Xu et al., 2012, 2014; Tagle et al., 2013; Inoue et al., 2014; Xie et al., 2016; Zheng et al., 2016). Some bamboo genera, such as Fargesia, Indosasa, Pseudosasa, and Yushania, included sporadic and massive synchronized flowering species (Zhang and Ma, 1989; Du et al., 2000; Li and Denich, 2004; Jiang, 2007). In some genera, such as Acidosasa, Ampelocalamus, Cephalostachyum, Drepanostachyum, Gaoligongshania, Gigantochloa, Melocalamus, Sasaella, Shibataea, and Sinobambusa, only massive synchronized flowering bamboo species have been observed (Du et al., 2000; Ramanayake and Weerawardene, 2003; Kumawat et al., 2014; Zheng et al., 2016). However, some bamboo genera, such as Neomicrocalamus, Pseudostachyum, and Semiarundinaria, contain only sporadic flowering species (Zhang and Ma, 1989; Du et al., 2000; Yuan et al., 2005). Obviously, there are some genera which only show one model, while others show more. This issue is more complicated and may be related to the number of species in the genus or the distribution range of species. Generally speaking, the genera with a large number of species are more widely distributed, and different flowering types are more likely to be seen. However, the differences of flowering types in different species within the same genus are mainly determined by the biological characteristics of the species. For example, it is a very common phenomenon that some species in Phyllostachys blossom sporadically, such as Phyllostachys nidularia, but some species usually show massive synchronized flowering, such as Ph. glauca, Ph. reticulata. Another example is that several flowering types can be observed in a bamboo species. Ph. heteroclada, a bamboo species widely distributed in China, massively bloomed in 1958 in Shennongjia, Hubei Province, China, and partially bloomed in 1987–1989 in Nanjing Forest University, China. In addition, it bloomed massively in Yaan, Sichuan Province, China in 2003–2007, and flowered sporadically in 1995 in Yiliang, Yunnan Province, China (Zhou, 1984; Du et al., 2000; Li and Denich, 2004; Wang and Wu, 2009). Therefore, bamboo flowering type can only be determined according to the specific flowering behavior of a bamboo population. There is no obvious relationship between the flowering habit of bamboo species and the taxonomic position at the genus level.
Bamboo flowering type is closely correlated with whether the bamboo forest is wild or cultivated. Du et al. (2000) studied the flowering phenomena and types of 61 bamboo species belonging to 23 genera in Yunnan, China. They divided bamboo flowering into massive synchronized and sporadic flowering, and showed that the flowering and fruiting characteristics of bamboo species were closely related to their status as wild or cultivated, as well as with their taxonomic relationship at the genus level.
The Flowering Cycle of Bamboo
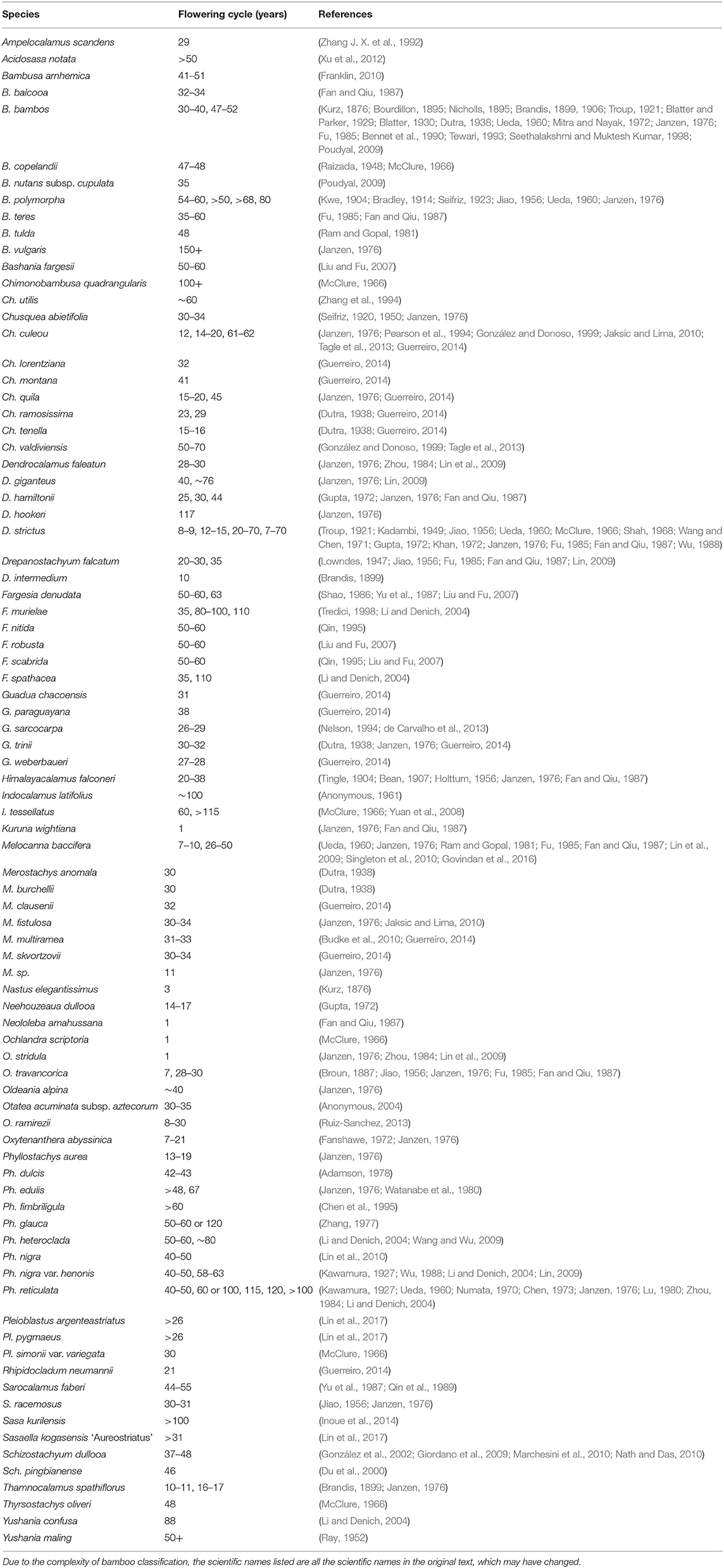
The period between two gregarious flowerings is generally called the flowering cycle, and the flowering cycles of different bamboo species are variable. The flowering cycle of bamboo ranged from 3 to 150 years (Kurz, 1876; Janzen, 1976) (Table 3). Brandis (1899) divided bamboo flowering habits into three types: annual, periodic, and uncertain.
In this review, we compiled more than 600 records of bamboo flowering events of 85 bamboo species from the literature, of which 187 had a defined flowering cycle. As shown in Table 3, some species that belong to apparently iteroparous bamboos, which grow to maturity and then flower and seed annually for many years, were termed annual flowering. This included Neololeba amahussana, Ochlandra scriptoria, O. stridula, and Kuruna wightiana (McClure, 1966; Janzen, 1976; Zhou, 1984; Fan and Qiu, 1987; Lin, 2009). However, the term annual flowering bamboo species in the literature remains to be discussed because it will take several years for some bamboo species from initial flowering to the end of flowering. For example, Pseudosasa amabilis blooms for 15 years, so it is easily mistaken for an annual flowering bamboo species (Lin and Mao, 2007). It is important to distinguish, in perennial bamboo species, annual flowering bamboo species from bamboos whose flowering periods last for a long time.
We found that the flowering cycles of different bamboo species, even those belonging to the same genus, fluctuate greatly. For example, the flowering cycle of Arundinaria varied from 10 to 60 years, Bambusa from 30 to 150+ years, Chusquea from 12 to 70 years, and Phyllostachys from 13 to 120 years (Brandis, 1899; Kawamura, 1927; Ueda, 1960; Numata, 1970; Chen, 1973; Janzen, 1976; Zhang, 1977; Lu, 1980; Zhou, 1984; Fu, 1985; Pearson et al., 1994; González and Donoso, 1999; Li and Denich, 2004; Liu and Fu, 2007; Jaksic and Lima, 2010; Tagle et al., 2013; Guerreiro, 2014). The flowering cycle in Guadua was less variable and relatively stable, being between 26 and 33 years (Dutra, 1938; Janzen, 1976; Nelson, 1994; de Carvalho et al., 2013; Guerreiro, 2014). In addition, for the same bamboo species, the flowering cycle observed by different researchers in different locations varied. For example, B. bambos flowered in 1804, 1836, 1868, and 1899 in Brazil, giving it a flowering cycle of 31–32 years (Dutra, 1938). In Dehra Dun, Uttar Pradesh, India, it flowered in 1836, 1881, and 1926, resulting in a flowering cycle of 45 years (Blatter and Parker, 1929). In Upper Weinganga Valley, Balaghat District, India, it flowered in 1818 and 1865–1870, giving it a flowering cycle of 47–52 years (Nicholls, 1895).
Janzen (1976) described the flowering cycles of 41 species of bamboo in detail. Guerreiro (2014) surveyed and estimated the flowering cycles of 16 woody bamboo species in Argentina and neighboring areas. Franklin (2010) recorded and summarized the flowering cycle of B. arnhemica. Their work revealed that the flowering cycle of the same bamboo species is not fixed and can be quite different. For example, the flowering cycle of Ph. edulis was generally believed to be 67 years (Watanabe et al., 1980), but according to other records, Ph. edulis has not flowered in Fenghua, Zhejiang Province, China for more than 200 years (Chai et al., 2006). This phenomenon may be caused by differences in flowering cycles in different clonal bamboo forests or the influence of environmental factors, especially those of managed plantations.
The synchronous flowering phenomenon of bamboo, which has attracted much attention, is unique. Janzen (1976) proposed a predator escape hypothesis to explain the synchronous flowering of bamboo. This hypothesis assumes that seed predators will eat up the seeds when bamboo forests blossom sporadically. Reproduction by seeds is only ensured when sufficient amounts of seeds are produced to overcome the loss via seed predators. Crone et al. (2015) also revealed that synchronous reproduction among conspecifics has several demonstrated fitness benefits, including enhanced rates of pollination, increased attraction of seed dispersers, and reduced seed predation. Veller et al. (2015) proposed a simple mathematical model that supported a two-step evolutionary process: first, an initial phase in which a mostly annual flowering population synchronizes to a small multi-year interval; and second, a phase of successive small multiplications of the initial synchronization interval, resulting in the extraordinary intervals seen today. Other researchers believe that many species have general synchronous flowering tendencies over large continental regions. Campbell (1985) noted a remarkable concentration of mean periods with multiples of 15–16 years among 20 taxa, most of them from southeastern Asia. Guerreiro (2014) showed that several species of woody bamboo native to southern South America undergo synchronous flowering, with flowering cycles of ~30 years.
Researchers calculate the flowering cycle by subtracting two flowering times. This is only accurate if the later flowering bamboo is the offspring of the previously flowering bamboo. Thus, observing at least three generations is required for accurate prediction of a flowering cycle. However, the present data on bamboo flowering may be questionable. First, it is very easy to find data based on only a single recorded generation of flowering cycle. Second, several different flowering events are described in many bamboo species, but it is not known whether the bamboo forests in a flowering event were derived from the seeds produced from the last flowering event. Finally, the historical records of bamboo flowering are limited. For many bamboo species, only one mass flowering event has been recorded, but the historical flowering time of the bamboo forest and the subsequent seed flow are not described in detail. Although there is not a definitive way to address these issues, the historical data still provide an important reference.
Bamboo Flowering Cycle Theory
Based on the comprehensive historical documents and the field observations of the researchers at the Bamboo Research Institute of Nanjing Forestry University over the past decades, some conclusions about the bamboo flowering cycle theory are proposed and summarized.
First, a bamboo species has a certain flowering cycle, or rather, each ramet (that is, individuals with the same genotype from the same fertilized egg) of bamboo has a certain flowering cycle. In addition, differences in flowering cycles among different bamboo species are greater than those between different clones of the same species. For example, Phyllostachys glauca bloomed in 1909 to the west of Zhejiang, China, and partially bloomed in 1965 in Fuyang District, Zhejiang Province, China. In addition, Ph. glauca bloomed massively in Jinlu Village of Yinzhou District, Zhejiang Province, China since 1990, and also flowered in 2013–2016 in Linqing and Jinan Districts, Shandong Province, China (Anonymous, 1972; Xu and Chen, 1992). However, Ph. glauca did not blossom in other places, such as Nanjing, Jiangsu Province, China. This indicates that the Ph. glauca plants in these places were probably derived from different clones, with different specific flowering times and flowering cycles.
Clonal bamboo has very similar flowering cycles. For example, Sasaella kogasensis “Aureostriatus” was introduced from the Fuji Bamboo Garden in Japan to Nanjing Forestry University of China in 1984 by Professor Zhou. Subsequently, the bamboo was introduced from Nanjing Forestry University in all parts of China. Since 2015, forests of S. kogasensis “Aureostriatus” have been blooming one after another in China, indicating that the same clone keeps flowering synchronously. Nevertheless, changes in the local environment may result in slightly different flowering cycles. Thus, although the flowering cycle of the same clonal bamboo is theoretically stable, it is affected by the environment. If the clonal bamboo is susceptible to disturbances, then its flowering time will vary greatly in different environments. However, if clonal bamboo is not easily disturbed, then the flowering time is less varied. For example, Ph. glauca growing in the Royal Botanical Gardens of England and Japan, which were introduced from the west of Zhejiang, China, bloomed in 1907 (Cheng et al., 2014).
Second, flowering period may last several years in a bamboo forest and it generally goes through three phases: small areas of sporadic flowering, massive synchronized flowering, and small areas of sporadic flowering. This phenomenon is called the “bamboo flowering distribution”. Synchronous flowering refers to a flowering distribution in which the vast majority of clumps within a patch initiate flowering in a given year, with most of the remaining flowering the year before or after (Abe and Shibata, 2012). Suyama et al. (2010) proposed that sporadic flowering may occur as a result of mechanistic malfunction, and result in massive flowering, therefore this should be regarded as part of the normal massive-flowering schedule. For example, we observed that Pleioblastus pygmaeus began sporadic flowering in 2015 in the Bamboo Garden of Nanjing Forestry University in Jiangsu Province, China. By 2016, the number of flowering bamboo stems peaked at ~85%, but a few flowering clumps occurred until 2019. The proportion of clumps that flower during the peak period varies among different bamboo species, such as 80–90% for Sarocalamus faberi (Taylor and Qin, 1988), 95.7% for B. arnhemica (Franklin, 2010), and 96.5% for C. culeou (Marchesini et al., 2010).
Third, gregarious flowering occurs in patches over successive years, and this has been described as a “flowering wave.” These waves have been widely reported among bamboo species and are repeated in successive generations (Franklin, 2010). Franklin (2010) hypothesized that bamboo flowering waves were the product of environmentally induced increments in the flowering schedules in which the underlying periodicity was reset at germination by an endogenous biological clock. When part of a population is subjected to such an increment, and that part is sufficiently aggregated in time and space to maintain the viability derived from synchronicity, an offset (patch) is established that will be maintained across generations. Flowering waves are always temporally organized but are not necessarily spatially organized (Franklin, 2010; Abe and Shibata, 2012). This phenomenon represents the interaction of endogenous and exogenous factors. Bamboo is a special plant with a long vegetative growth period. Bud mutations occur frequently in bamboo plants, including Ph. edulis, Ph. edulis f. pachyloen, B. multiplex and its variant B. multiplex var. riviereorum. Bamboo forests are more likely to mutate during the period of sexual reproduction (Janzen, 1976; Franklin, 2010; Hanlon et al., 2019). Therefore, even if the plants in a bamboo forest all come from the same clone at the beginning, it is possible that there will be variation within the forest. In addition, the environment affects bamboo flowering. Generally, internal and external factors lead to different flowering cycles and form the flowering wave.
In accordance with the bamboo flowering references, four types of bamboo flowering have been summarized (Lin and Mao, 2007). Why are various flowering types observed? The reasons for these phenomena are as follows: On the one hand, the flowering events in bamboo forests generally form temporally structured but spatially chaotic flowering distributions, which undergo three phases: small areas of sporadic flowering, massive synchronized flowering, and small areas of sporadic flowering. Therefore, different flowering types can be obtained by observing the bamboo forest during different periods. On the other hand, it also depends on whether the bamboo forest is populated by the same clonal bamboo. The flowering cycles of different ramets of bamboo are different. Therefore, if the bamboo forest is composed of the same clone, then it will form a flowering distribution and complete the reproductive phase. However, if the bamboo forest is composed of different clones, then there will be different flowering times for the different clones. Thus, when one of the clones begins flowering, the other clones may still be in the vegetative stage. As a result, it is possible to form flowering waves, and researchers often observe sporadic flowering or partial flowering in a bamboo forest. Thus, a variety of flowering patterns are often observed in bamboo forests.
In the past, the causes of bamboo flowering remained controversial. Many theories have emerged, including growth cycle, nutrition, external cause, free radicals, pathology, individual variation, mutation, periodic aging, and rejuvenation (Janzen, 1976; Sharma, 1994; Keeley and Bond, 1999; Chai et al., 2006; Wang, 2013). Kawamura (1927) proposed that periodicity in bamboo must be the product of an endogenous mechanism that was relatively immune to environmental influences. Franklin (2010) offered hypotheses on the interactions of endogenous and exogenous cues to flowering that may lead to the development of flowering waves and other flowering patterns in bamboo. He also suggested that stressors on the plant might override or force the clock, such as triggering hormone production independently of the clock. It is possible that the threshold of physiological stress required to induce flowering decreases as the scheduled time of flowering approaches (Franklin, 2010). Recently, researchers have reached a consensus on the cause of bamboo flowering. They believe that bamboo flowering is modulated by environmental conditions, but the root cause is internal genetic factors and flowering occurs when bamboo grows to its physiologically mature age. External factors, such as climate and human disturbance, can advance, delay, or stop the occurrences of bamboo flowering events to a certain extent (Franklin, 2010). For example, the flowering time of bamboo can be delayed or advanced to a certain extent by applying exogenous hormones (Ding, 2007). However, the specific key factor that induces the initiation of flowering in bamboo is still unknown. We also hypothesize that endogenous hormones, which tend to be signaling molecules, may be key factors for initiation, but this requires further study.
In a word, the flowering cycle of bamboo is very important for predicting future bamboo flowering times. Janzen (1976), Franklin (2010), and Guerreiro (2014) have presented summaries of the flowering cycle of bamboo. Ma et al. (2017) found a negative correlation between the rate of molecular evolution and flowering cycle in Arundinarieae. Bamboo species with longer flowering cycles tend to evolve more slowly than those with shorter flowering cycles (Montti et al., 2011).
Rejuvenation and Regeneration of Flowering Bamboo Forests
Regeneration of a robust bamboo forest has major economic and ecological implications. There are generally two types of bamboo forest rejuvenation after flowering: sexual and asexual. The clonal composition of a bamboo forest will be changed after flowering and natural regeneration.
Regeneration Through Sexual Reproduction
Some bamboo species bloom and produce large amounts of seeds, which fall to the ground and germinate into seedlings. Seeds can grow into seedlings, and each seedling represents a clone. The flowering process of bamboo forests can last from several to more than 10 years, so the seedlings produced by the seeds may be of different ages. The bamboo species Chimonobambusa utilis (Zhang, 1985), Ch. pachystachys (Mao et al., 2008), Ch. tumidissinoda (Dong et al., 2001), Fargesia denudata (Shao, 1986), F. nitida (Yu et al., 1987), Melocanna arundina (Chai et al., 2006), Phyllostachys edulis (Qin, 2015), and Sarocalamus faberi (Qin et al., 1989; Taylor and Qin, 1993) belong to this type. S. faberi seedlings take ~15–20 years to develop into mature stands after a bamboo flowering event (Taylor and Qin, 1993). Thus, after flowering and rejuvenation, more clones can be formed in the bamboo forest.
Regeneration Through Asexual Reproduction
Here, the bamboo forest is rejuvenated by the flowering bamboos rhizomes and the buds of culm bases. In a flowering bamboo forest, the buds on the rhizomes of the flowering bamboo can sprout and form dwarf and weak bamboo, which usually bloom in the same year and will grow new rhizomes underground. In the following years, the buds of the new rhizomes sprout and form dwarf and weak bamboos that coexist with flowers and leaves. Thus, the flowering bamboo forest can form normal non-flowering bamboo after several years (Xiong et al., 1979). During the process of asexual rejuvenation, the proportion of flowering bamboo generally first rises and then falls, while the proportion of non-flowering bamboo falls and then rises. At the end, the bamboo forest does not blossom at all. Some bamboo species renew their forests in this way, such as Pleioblastus amarus (Zheng and Huang, 1990), Pl. amarus var. pendulifolius (Zheng and Huang, 1990), Phyllostachys reticulata (Lu, 1980), Ph. atrovaginata (He et al., 1994), Ph. vivax (Xiong et al., 1979), Ph. praecox f. prevernalis (Chai et al., 2006), Ph. fimbriligula (Chai et al., 2006), and Shibataea chinensis (Lin and Ding, 2012).
The process of regeneration and rejuvenation of this type of flowering bamboo forest is slow. For example, Ph. reticulata requires 7–10 years (Lu, 1980), Ph. vivax requires 6–7 years (Xiong et al., 1979), and Pl. amarus and Pl. amarus var. pendulifolius require at least 10 years (Zheng and Huang, 1990). However, artificial measures can be used to improve the growth conditions of bamboo forests and accelerate regeneration. For example, it only took 5–6 years for Ph. atrovaginata to recover to the pre-flowering production level under intense management conditions (He et al., 1994). During the process of asexual rejuvenation, variation may also occur. For example, Pleioblastus pygmaeus flowered during the period from 2015 to 2018 in Baima Bamboo Resource Nursery of Nanjing Forestry University (119°07′42″E, 31°37′55″ N), Nanjing, Jiangsu Province, China. After flowering the authors of this paper observed that many bamboo clumps of Pl. pygmaeus with white-striped leaves appeared in the bamboo forest. Therefore, the clonal composition of a bamboo forest after asexual rejuvenation may not be identical to that of the bamboo forest before flowering.
There are three bamboo fruiting types that are characterized based on seed production: those that produce a large number of seeds, those that rarely produce seeds, and those having no seeds. A few bamboo species have no seeds and can only regenerate their forests through asexual rejuvenation. For example, Lin and Ding (2012) observed the flowering of Shibataea chinensis at Nanjing Forestry University from 2002 to 2010, and found it had no seeds. Some bamboo species have a low seed setting rate, such as Bambusa emeiensis, B. multiplex, and Ph. heteroclada, and some have high seed setting rate, such as Ph. edulis, Ph. vivax, Pl. pygmaeus, and Sasaella kogasensis “Aureostriatus.” Generally speaking, most bamboo species with few seeds or a large number of seeds regenerate their forests through sexual reproduction and asexual rejuvenation after flowering. For example, B. multiplex, Indocalamus tessellatus, Ph. glauca, Pl. pygmaeus, and S. kogasensis “Aureostriatus” can be renewed in either manner after flowering (Cheng et al., 2014). There may be a preferred approach, but the specific selection of the regeneration manner may be correlated with the growth environment. In addition, a few species can only be propagated through sexual reproduction, such as Fargesia murielae.
Explanations of Natural Phenomena Using the Bamboo Flowering Cycle Theory
The bamboo flowering cycle theory described in this review may explain many strange and complex phenomena associated with bamboo flowering.
Genetically similar bamboo of a range of different ages coexist in space and time, forming a continuous spectrum of clones. This age structure may eventually lead to sporadic or partial flowering in the same bamboo forest, with no obvious regularity in flowering.
The following examples illustrate the continuous spectrum of clones. First, we take Phyllostachys edulis, a scattered bamboo species, as an example. Ph. edulis, distributed in Guilin, Guangxi Province, China began to bloom and bear fruits in a large area in 1963, and from 1995 until the present it had small areas of continuous blooming every year, where a significant amount of breeding was carried out (Li and Gu, 2003). Because flowering time does not occur at the same time for a whole bamboo forest, and it probably maybe last many years, the age of the offspring produced by it will also be different, therefore, a continuous spectrum of clones has formed in this bamboo forest. When a certain clone reaches the flowering age, the other clones may be still in vegetative growth. So when this flowering clone renews and resumes vegetative growth, other clones may reach the flowering age and start blooming. Consequently, the bamboo forest will show continuous patchy flowering for many years. The other example is Chimonobambusa utilis, a species distributed in Tongzi, Guizhou Province, China. Due to many clones of different ages, bamboo forests of Ch. utilis have had patchy flowering phenomenon every year since 2006. Third, according to historical documents some bamboo species frequently blossom sporadically, such as Bambusa distegia, B. emeiensis, B. intermedia, B. oldhamii, B. multiplex, and Dendrocalamus latiflorus. These bamboo species belong to sympodial bamboos, in their bamboo forests, they form a series of clones. In the same time and space, there are bamboos of different ages in the bamboo forest, so they will bloom in different years (Supplementary Video 1).
Conclusion and Perspectives
Bamboo plays an important role in human life, especially in countries that have rich bamboo resources. Bamboo flowering and the subsequent death of entire forests results in huge economic losses and environmental problems. Therefore, it is necessary to understand the flowering cycle of economically important bamboo species. Bamboo flowering is a normal biological phenomenon, the transformation from vegetative to reproductive growth. It is the last stage of ontogeny and is an inevitable process of a biological organism's development (Wang, 2013).
The historical data that have been recorded lack many key details. Owing to the characteristic long lifespan of bamboo, it is very difficult to observe its full cycle in a generation (Guerreiro, 2014). The flowering cycle must be obtained by relying on the flowering events recorded in successive generations. In addition, with the advancement of civilization, many wild bamboo populations have disappeared, making it nearly impossible to observe flowering events in their original place. Consequently, this review suggests that seeds should be collected and seeded after the flowering and fruiting of bamboo plants. The seedlings can be separately planted in scientific research institutions that study bamboo, where they can be marked and managed. Similarly, data on bamboo forests that undergo asexual regeneration should be recorded. In this way, the next flowering times of bamboos in different regions, the variations of flowering in different clones, and the effects of the environment on bamboo flowering can be recorded.
Additionally, a database similar to the web-based Flora of China should be established. This would allow experts and non-experts to contribute to our understanding of bamboo flowering. It is necessary to record bamboo flowering events in detail, including information on the origin and whereabouts of the bamboo. At the same time, the identification of bamboo plants is difficult and error-prone. Therefore, it is essential to encourage scientific exchanges and validation. Finally, the website should contain information about the bamboo industry, technology, and other related aspects, so that it can become a useful resource for the bamboo industry.
The long flowering period of bamboo has long been a fascinating mystery. However, by examining historical data and generating accurate data going forward, we have begun to understand the flowering of this important and remarkable plant. Exploration of the molecular mechanisms setting the flowering time for bamboo, using knowledge leveraged from model plants, particularly other monocots, remains an exciting field for future research.
Author Contributions
XZ wrote the manuscript. YD and SL critically reviewed and added to the review. HF and YW contributions to acquisition of data, or analysis of data. All authors read and commented on this manuscript.
Funding
This work was financially supported by the National Natural Science Foundation for Scholars of China (31870595); and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We also thank Miranda Loney (http://abrc.sinica.edu.tw/editor/) for editing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00381/full#supplementary-material
Supplementary Video 1. Dynamic demonstation of flowering wave.
References
Abe, Y., and Shibata, S. (2012). Spatial and temporal flowering patterns of the monocarpic dwarf bamboo Sasa veitchii var. hirsuta. Ecol. Res. 27, 625–632. doi: 10.1007/s11284-012-0933-9
Adamson, W. C. (1978). Flowering interval of sweetshoot bamboo. Econ. Bot. 32, 360–362. doi: 10.2307/4253976
Anonymous (1961). A preliminary study on the cause of the flowering and fruiting of Indocalamus latifolius. J. Zhejiang Agric. Sci. 352–353. doi: 10.16178/j.issn.0528-9017.1961.07.014
Bean, W. J. (1907). The flowering of cultivated bamboos. Bull. Miscellan. Informat. 1907, 228–233. doi: 10.2307/4111870
Bennet, S. S. R., Gaur, R. C., and Sharma, P. N. (1990). Thirty-seven Bamboos Growing in India New Delhi. Controller of Publications, Government of India.
Bhattacharya, S., Das, M. R., and Pal, A. (2006). Morphological and molecular characterization of Bambusa tulda with a note on flowering. Ann. Bot. 98, 529–535. doi: 10.1093/aob/mcl143
Blatter, E. B., and Parker, R. N. (1929). The Indian bamboos brought up-to-date. Indian Forester 55, 541–562.
Bradley, J. W. (1914). Flowering of Kya-thaung bamboo (Bambusa polymorpha) in the Prome Division, Burma. Indian Forester 40, 526–529.
Brandis, D. (1906). On some bamboos in Martaban south of Toungoo between the Salwin and Sitang River-II. Indian Forester. 32, 179–295.
Budke, J. C., Alberti, M. S., Zanardi, C., Baratto, C., and Zanin, E. M. (2010). Bamboo dieback and tree regeneration responses in a subtropical forest of south America. Forest Ecol. Manage. 260, 1345–1349. doi: 10.1016/j.foreco.2010.07.028
Campbell, J. J. N. (1985). Bamboo flowering patterns: a global view with special reference to east Asia. J. Am. Bamboo Soc. 6, 17–35.
Chai, Z. L., Qin, Y. C., Hua, X. Q., Wang, Z. J., and Wang, Q. (2006). Advance of studies on bamboo flowering causes. J. Zhejiang Forestr. Sci. Technol. 26, 53–57. doi: 10.3969/j.issn.1001-3776.2006.02.015
Chao, C., and Tang, G. G. (1993). The present status and problems of bamboo classification in China. J. Nanjing Forestr. Univ. 17, 1–8.
Chen, M. Y. (1973). Giant timber bamboo in Alabama. J. Forestr. 71:777. doi: 10.1515/hfsg.1973.27.6.214
Chen, S. L., Li, D. Z., Zhu, G. H., Wu, Z. L., Lu, S. L., Liu, L., et al. (2006). Flora of China, Vol. 22 (Poaceae). Beijing: Science Press.
Chen, Y. L., Ren, D. T., and Zhu, B. Y. (1995). Observations on flowering habit of Phyllostachys fimbriligura and its rejuvenation measures. J. Zhejiang Forestr. Sci. Technol. 15, 50–56.
Cheng, Y. L., Wang, H. L., Sun, Y., and Zhang, R. Y. (2014). Study on the phenomenon of centralized flowering and regeneration of Phyllostachys glauca in Jinan. Garden Sci. Technol. 44–46.
Crone, E. E., McIntire, E. J. B., and Brodie, J. (2015). What defines mast seeding? Spatio-temporal patterns of cone production by whitebark pine. J. Ecol. 99, 438–444. doi: 10.1111/j.1365-2745.2010.01790.x
de Carvalho, A. L., Nelson, B. W., Bianchini, M. C., Plagnol, D., Kuplich, T. M., and Daly, D. C. (2013). Bamboo-dominated forests of the southwest Amazon: detection, spatial extent, life cycle length and flowering waves. PLoS ONE 8:e54852. doi: 10.1371/journal.pone.0054852
Ding, X. C. (2007). Hormonal regulation mechanism of flowering of Phyllostachys violascens under mulching cultivation. Sci. Silvae Sinicae. 43, 10–15. doi: 10.3321/j.issn:1001-7488.2007.07.003
Dong, W. Y., Huang, B. L., Xie, Z. X., Xie, Z. H., and Liu, H. Y. (2001). Studies on the characteristics of blossoming and seed bearing of Qiongzhuea tumidinoda. J. Forestr. Univ. 25, 30–32. doi: 10.3969/j.issn.1000-2006.2001.06.008
Du, F., Xue, J. R., Yang, Y. M., Hui, C. M., and Wang, J. (2000). Study on flowering phenomenon and its type of bamboo in Yunnan in past fifteen years. Sci. Silvae Sinicae. 36, 57–68. doi: 10.11707/j.1001-7488.20000616
Fan, F. S., and Qiu, F. G. (1987). The bamboo production and scientific research in India. J. Bamboo Res. 6, 50–68.
Fanshawe, D. B. (1972). The bamboo, Oxytenanthera abyssinica - its ecology, silviculture and utilization. Kirkia 8, 157–166.
Franklin, D. C. (2010). Synchrony and asynchrony: observations and hypotheses for the flowering wave in a long-lived semelparous bamboo. J. Biogeogr. 31, 773–786. doi: 10.1111/j.1365-2699.2003.01057.x
Fu, Q. Y. (1985). Bamboo is a potential raw material for papermaking in tropical countries. Sichuan Papermaking. 34–41.
Gao, J., Ge, W., Zhang, Y., Cheng, Z., Li, L., Hou, D., et al. (2015). Identification and characterization of microRNAs at different flowering developmental stages in moso bamboo (Phyllostachys edulis) by high-throughput sequencing. Mol. Genet. Genomics 290, 2335–2353. doi: 10.1007/s00438-015-1069-8
Gao, J., Zhang, Y., Zhang, C., Qi, F., Li, X., Mu, S., et al. (2014). Characterization of the floral transcriptome of moso bamboo (Phyllostachys edulis) at different flowering developmental stages by transcriptome sequencing and RNA-Seq analysis. PLoS ONE 9:e98910. doi: 10.1371/journal.pone.0098910
Giordano, C. V., Rodolfo, A. S., and Austin, A. T. (2009). Gregarious bamboo flowering opens a window of opportunity for regeneration in a temperate forest of Patagonia. N. Phytol. 181, 880–889. doi: 10.1111/j.1469-8137.2008.02708.x
González, M. E., and Donoso, Y. C. (1999). Seed and litter fall in Chusquea quila (Poaceae: Bambusoideae), after synchronous flowering in south-central Chile. Rev. Chil Hist. Nat. 72, 169–180.
González, M. E., Veblen, T. T., Donoso, C., and Valeria, L. (2002). Tree regeneration responses in a lowland Nothofagus-dominated forest after bamboo dieback in south-central Chile. Plant Ecol. 161, 59–73. doi: 10.1023/A:1020378822847
Govindan, B., Johnson, A. J., Nair, S. N. A., Gopakumar, B., Mallampalli, K. S. L., Venkataraman, R., et al. (2016). Nutritional properties of the largest bamboo fruit Melocanna baccifera and its ecological significance. Sci. Rep. 6:26135. doi: 10.1038/srep26135
Guerreiro, C. (2014). Flowering cycles of woody bamboos native to southern south America. J. Plant Res. 127, 307–313. doi: 10.1007/s10265-013-0593-z
Gupta, K. K. (1972). Flowering in different species of bamboos in Cachar district of Assam in recent times. Indian Forest. 98, 83–85.
Hanlon, V. C. T., Otto, S. P., and Aitken, S. N. (2019). Somatic mutations substantially increase the per-generation mutation rate in the conifer Picea sitchensis. Evol. Lett. 3, 348–358. doi: 10.1002/evl3.121
He, Y. S., Huang, R. Q., and Wang, W. Y. (1994). Rejuvenation and regeneration technique of flowered Phyllostachys atrovaginata. J. Zhejiang Forestr. Sci. Technol. 14, 56–58.
Holttum, R. E. (1956). The typification of the generic name Bambusa and the status of the name Arundo bambos L. Taxon 5, 26–28. doi: 10.2307/1216941
Inoue, M., Ayaka, S., Ayumi, M., Yoshihisa, S., Ichirou, S. J., and Akifumi, M. (2014). Clonal structure, seed set, and self-pollination rate in mass-flowering bamboo species during off-year flowering events. PLoS ONE 9:e105051. doi: 10.1371/journal.pone.0105051
Jaksic, F. M., and Lima, M. (2010). Myths and facts on ratadas: bamboo blooms, rainfall peaks and rodent outbreaks in south America. Austr. Ecol. 28, 237–251. doi: 10.1046/j.1442-9993.2003.01271.x
Janzen, D. H. (1976). Why bamboos wait so long to flower. Annu. Rev. Ecol. Syst. 7, 347–391. doi: 10.2307/2096871
Jiao, Q. Y. (1956). Discussion on the flowering habit and stage development of perennial bamboo and sisal hemp. Plant Physiol. J. 13–19. doi: 10.13592/j.cnki.ppj.1956.02.004
Kadambi, K. (1949). On the ecology and silviculture of Dendrocalamus strictus in the bamboo forests of Bhadravati division, Mysore State, and comparative notes on the species Bambusa arundinacea, Oxytenanthera monostigma and Oxytenanthera stocksu. Indian Forester. 75, 289–299. doi: 10.2307/23117
Keeley, J. E., and Bond, W. J. (1999). Mast flowering and semelparity in bamboos: the bamboo fire cycle hypothesis. Am. Nat. 154, 383–391. doi: 10.2307/2463658
Keng, P. (1986). A preliminary study of the inflorescence type arising from bamboos and its variation. J. Wuhan Bot. Res. 4, 323–336.
Khan, M. A. W. (1972). Propagation of Bambusa vulgaris- its scope in forestry. Indian Forester. 359–362.
Kitamura, K., and Kawahara, T. (2009). Clonal identification by microsatellite loci in sporadic flowering of a dwarf bamboo species, Sasa cernua. J. Plant Res. 122, 299–304. doi: 10.1007/s10265-009-0220-1
Kumawat, M. M., Singh, K. M., Tripathi, R. S., Riba, T., Singh, S., and Sen, D. (2014). Rodent outbreak in relation to bamboo flowering in north-eastern region of India. Biol. Agric. Hortic. 30, 243–252. doi: 10.1080/01448765.2014.925828
Li, X. W., and Gu, D. Q. (2003). Discussion on afforestation of the seedling of Phyllostachys edulis in Guangdong. World Bamboo Rattan. 1, 32–34. doi: 10.3969/j.issn.1672-0431.2003.03.009
Li, Z., and Denich, M. (2004). Is Shennongjia a suitable site for reintroducing giant panda: an appraisal on food supply. Environmentalist. 24, 165–170. doi: 10.1007/s10669-005-6050-3
Lin, E. P. (2009). Functions of AP1/SQUA-?REV-?TB1-like genes and isolation and expression of microRNAs in Phyllostachys praecox (dissertation's thesis). Zhejiang University, Hangzhou, China
Lin, S. Y., and Ding, Y. L. (2007). Studies on the floral biological characteristics of three bamboo species of Phyllostachys. J. Forestr. Eng. 21, 52–55. doi: 10.3969/j.issn.1000-8101.2007.05.018
Lin, S. Y., and Ding, Y. L. (2012). Development of the male and female gametophytes in Shibataea chinensis (Bambusoideae). Acta Bot. Boreali-Occidentalia Sinica. 32, 907–914. doi: 10.3969/j.issn.1000-4025.2012.05.010
Lin, S. Y., and Ding, Y. L. (2013). Studies on the breeding system in Shibataea chinensis and Arundinaria simonii f. heterophylla. J. Nanjing Forestr. Univ. 37, 1–5. doi: 10.3969/j.issn.1000-2006.2013.03.001
Lin, S. Y., Fan, T. T., Jiang, M. Y., Zhang, L., Zheng, X., and Ding, Y. L. (2017). The revision of scientific names for three dwarf bamboo species (cultivar) based on the floral morphology. J. Nanjing Forestr. Univ. 41, 189–193. doi: 10.3969/j.issn.1000-2006.2017.01.029
Lin, S. Y., Hao, J. J., Hua, X., and Long, D. Y. (2009). The megasporogenesis, microsporogenesis and the development of their female and male gametophyte in Menstruocalamus sichuanensis. J. Nanjing Forest. Univ. 33, 9–12. doi: 10.3969/j.issn.1000-2006.2009.03.003
Lin, S. Y., Li, J., Zhao, R., Xu, Q., and Ding, Y. L. (2015). A research on the flowering biological characteristics of Bambusa multiplex in Nanjing city. J. Nanjing Forestr. Univ. 39, 52–56. doi: 10.3969/j.issn.1000-2006.2015.02.009
Lin, S. Y., and Mao, G. X. (2007). The habit and regeneration of bamboo flowering. Forestr. Sci. Technol. 32, 23–25. doi: 10.3969/j.issn.1001-9499.2007.05.008
Lin, S. Y., Shi, W. W., Miu, B. B., and Long, D. Y. (2010). Research advances in reproduction biology of bamboos. World Bamboo Rattan. 8, 1–6. doi: 10.3969/j.issn.1672-0431.2010.02.001
Lin, S. Y., Wan, Y. W., Fu, H. J., Zhang, L., Jiang, M. Y., Yin, Z. F., et al. (2018). Research on inflorescence establishment and revision of inflorescence type in bamboo plants. J. Nanjing Forestr. Univ. 42, 1–6.
Liu, Y. Y., and Fu, J. H. (2007). Bamboo in habitat of giant panda and its flowering phenomenon. World Bamboo Rattan. 5, 1–4. doi: 10.3969/j.issn.1672-0431.2007.01.001
Lu, J. L. (1980). Studies on flowering and regeneration of Phyllostachys reticulata. J. Henan Agric. Univ. 11–20. doi: 10.16445/j.cnki.1000-2340.1980.02.002
Ma, P. F., Vorontsova, M. S., Nanjarisoa, O. P., Razanatsoa, J., Guo, Z. H., Haevermans, T., et al. (2017). Negative correlation between rates of molecular evolution and flowering cycles in temperate woody bamboos revealed by plastid phylogenomics. BMC Plant Biol. 17, 260–275. doi: 10.1186/s12870-017-1199-8
Mao, G. X., and Zhang, C. X. (1996). The cause of bamboo flowering and its preventive measures. J. Forestr. Eng. 33–34. doi: 10.13360/j.issn.1000-8101.1996.04.016
Mao, W. J., Dong, W. Y., Zhao, J. F., Wang, L., and Chen, C. (2008). A study of growth and natural regeneration of blossoming Chimonobambusa pachystachys Stand. World Bamboo Rattan. 6, 25–28. doi: 10.3969/j.issn.1672-0431.2008.06.007
Marchesini, V. A., Sala, O. E., and Austin, A. T. (2010). Ecological consequences of a massive flowering event of bamboo (Chusquea culeou) in a temperate forest of Patagonia, Argentina. J. Veget. Sci. 20, 424–432. doi: 10.1111/j.1654-1103.2009.05768.x
Mitra, G. N., and Nayak, Y. (1972). Chemical composition of bamboo seeds (Bambusa arundinacea Willd). Indian Forest. 98, 479–481.
Montti, L., Campanello, P. I., and Goldstein, G. (2011). Flowering, die-back and recovery of a semelparous woody bamboo in the Atlantic forest. Acta Oecol. 37, 361–368. doi: 10.1016/j.actao.2011.04.004
Munro, W. (1868). A monograph of the Bambusaceae, including descriptions of all the species. Trans. Linnean Soc. London. 26, 1–157. doi: 10.1111/j.1096-3642.1968.tb00502.x
Nath, A. J., and Das, A. K. (2010). Gregarious flowering of a long-lived tropical semelparous bamboo Schizostachyum dullooa in Assam. Curr. Sci. 99, 154–155.
Nelson, B. W. (1994). Natural forest disturbance and change in the Brazilian Amazon. Remote Sens. Rev. 10, 105–125. doi: 10.1080/02757259409532239
Numata, M. (1970). Conservation implications of bamboo flowering and death in Japan. Biol. Conserv. 2, 227–229. doi: 10.1016/0006-3207(70)90120-5
Pearson, A. K., Pearson, O. P., and Gomez, I. A. (1994). Biology of the bamboo Chusquea culeou (Poaceae: Bambusoideae) in southern Argentina. Vegetatio. 111, 93–126. doi: 10.2307/20046407
Poudyal, P. P. (2009). Bamboo flowering in Sikkim and elsewhere in India. Mag. Am. Bamboo Soc. 30, 9–10.
Qin, Z. L. (2015). Flowering and seeding characteristics of Phyllostachys pubescens and need analysis of seedlings in Guilin. World Bamboo Rattan. 13, 29–31. doi: 10.13640/j.cnki.wbr.2015.02.008
Qin, Z. S. (1995). Study on reproductive characteristic of Bashania fangenia. Acta Bot. Boreali Occidentalia Sinica. 15, 229–233.
Qin, Z. S., Cai, X. S., and Huang, J. Y. (1989). Seed characteristics and natural regeneration of arrow bamboo (Bashania fangenia). J. Bamboo Res. 8, 1–12.
Raizada, M. B. (1948). A litter-known Burmese bamboo (Sibocalamus copelandi). Indian Forester 74, 7–10.
Ram, H. Y. M., and Gopal, B. H. (1981). Some observations on the flowering of bamboos in Mizoram. Curr. Sci. 50, 708–710.
Ramanayake, S. M. S. D., and Weerawardene, T. E. (2003). Flowering in a bamboo, Melocanna baccifera (Bambusoideae: Poaceae). Bot. J. Linn. Soc. 143, 287–291. doi: 10.1046/j.1095-8339.2003.00216.x
Ray, P. K. (1952). Gregarious flowering of a common hill bamboo Arundinaria maling Gamble. Indian Forester 78, 89–91.
Ruiz-Sanchez, E. (2013). Otatea ramirezii (Poaceae: Bambusoideae: Bambuseae) flower description and the importance of the Mexican national living bamboo collection. Phytotaxa 150, 54–60. doi: 10.11646/phytotaxa.150.1.4
Sarma, H., Sarma, A. M., Sarma, A., and Borah, S. (2010). A case of gregarious flowering in bamboo, dominated lowland forest of Assam, India: phenology, regeneration, impact on rural economy, and conservation. J. Forestr. Res. 21, 409–414. doi: 10.1007/s11676-010-0090-3
Seethalakshmi, K. K., and Muktesh Kumar, M. S. (1998). Bamboos of India: A Compendium. Beijing: International Network for Bamboo and Rattan.
Seifriz, W. (1920). The length of the life cycle of a climbing bamboo. A striking case of sexual periodicity in Chusquea abietifolia Griseb. Am. J. Bot. 7, 83–94. doi: 10.2307/2435166
Seifriz, W. (1923). Observations on the causes of gregarious flowering in plants. Am. J. Bot. 10, 93–112. doi: 10.2307/2435577
Shah, N. C. (1968). Flowering of the bamboo, Dendrocalamus hookerii and Dendrocalamus strictus in Assam and Bihar states. Indian Forester 94:717.
Shao, J. X. (1986). Preliminary survey on the ecological characteristics of Fargesia denudate. Chin. J. Ecol. 5, 41–44. doi: 10.13292/j.1000-4890.1986.0105
Sharma, M. L. (1994). “The flowering of bamboo: fallacies and facts,” in Proceedings of the Bamboo in Asia and the Pacific (Chiangmai), 68–70.
Singleton, G. R., Belmain, S. R., Brown, P. R., and Hardy, B. (2010). Rodent Outbreaks: Ecology and Impacts Los Banos. Philippines, PA: International Rice research Institute.
Suyama, Y., Suzuki, J., and Makita, A. (2010). For the comprehension of gregarious flowering in bamboos. Jpn. J. Ecol. Soc. 60, 97–106.
Tagle, L., Roberto, M., Mireya, B., Rene, M., and Xavier, L. (2013). Determination of minimal age of five species of Chusquea bamboos through rhizome analysis as a tool to predict the flowering in southern Chile. Rev. Chil. Hist. Nat. 86, 423–432. doi: 10.4067/S0716-078X2013000400004
Taylor, A. H., and Qin, Z. S. (1988). Regeneration from seed of Sinarundinaria fangiana, a bamboo, in the Wolong giant panda reserve, Sichuan, China. Am. J. Bot. 75, 1065–1073. doi: 10.2307/2443774
Taylor, A. H., and Qin, Z. S. (1993). Bamboo regeneration after flowering in the Wolong giant panda reserve, China. Biol. Conserv. 63, 231–234. doi: 10.1016/0006-3207(93)90717-F
Ueda, K. (1960). Studies on the physiology of bamboo: with reference to practical application. Bull. Tokyo Univ. Forests. 30, 1–167.
Veller, C., Nowak, M. A., and Davis, C. C. (2015). Extended flowering intervals of bamboos evolved by discrete multiplication. Ecol. Lett. 18, 653–659. doi: 10.1111/ele.12442
Vorontsova, M. S., Clark, L. G., Dransfield, J., Govaerts, R., and Baker, W. J. (2016). World Checklist of Bamboos and Rattans. Beijing: Science Press.
Wang, T. T., and Chen, M. Y. (1971). Studies on bamboo flowering in Taiwan. Taipei Nat. Taiwan Univ. Forest Exp. Sta Tech. Bull. 87, 27.
Wang, X., Zhang, X., Zhao, L., and Guo, Z. (2014). Morphology and quantitative monitoring of gene expression patterns during floral induction and early flower development in Dendrocalamus latiflorus. Int. J. Mol. Sci. 15, 12074–12093. doi: 10.3390/ijms150712074
Wang, X. H. (2013). An overview of bamboo flowering research. J. Forestry Eng. 27, 10–14. doi: 10.3969/j.issn.1000-8101.2013.05.003
Wang, X. H., and Wu, H. M. (2009). Biological characteristics study of Phyllostachys Heteroclada's flowering. J. Chengdu Univ. 28, 195–198. doi: 10.3969/j.issn.1004-5422.2009.03.003
Watanabe, M., Ueda, K., Manabe, I., and Akai, T. (1980). Flowering, seeding, germination, and flowering periodicity of Phyllostachys pubescens. Jpn. Forestr. Soc. 64, 107–111.
Wu, G. M. (1988). Reproduction of bamboo species of Phyllostachys- I. Flowering of bamboo species of Phyllostachys. J. Nanjing Forestry Univ. 60–67.
Xie, N., Chen, L. N., Wong, K. M., Cui, Y. Z., and Yang, H. Q. (2016). Seed set and natural regeneration of Dendrocalamus membranaceus Munro after mass and sporadic flowering in Yunnan, China. PLoS ONE 11:e0153845. doi: 10.1371/journal
Xing, X. T., Fu, M. Y., and Xiao, X. T. (2005). Biological characteristics of flowering and controlled pollination of Dendrocalamus latiflorus Munro. J. Beijing Forestr. Univ. 27, 103–107. doi: 10.3321/j.issn:1000-1522.2005.06.020
Xiong, W. Y., Wu, G. M., Shen, H. J., Zhang, Z. Y., and Lu, X. H. (1979). An investigation on flowering and rejuvenation of Phyllostachys vivax Stands. J. Nanjing Forestr. Univ. 14–21.
Xu, C. T., and Chen, W. G. (1992). Large area of Phyllostachys glauca blossoms in Yinzhou District. J. Bamboo Res. 97.
Xu, Y. K., Li, Q., Zhang, S. H., Xu, X. W., and Peng, X. K. (2012). Textual research on the flowering history of Pleioblastus intermedius and analysis of death. For. By-Product Speciality China. 86–87. doi: 10.3969/j.issn.1001-6902.2012.01.042
Xu, Z. G., Guo, Q. R., Huang, D. Y., and Li, L. J. (2014). The floral biological characteristics of five sympodial bamboo species. Forestr. Sci. Technol. 28, 74–77. doi: 10.13360/j.issn.1000-8101.2014.02.019
Yao, L. G., and Tan, H. C. (2008). Observation and determination of bamboos fruits from and quality. Forestr. Invent. Plan. 33, 36–39. doi: 10.3969/j.issn.1671-3168.2008.05.011
Yu, Q. Z., Wu, M., Zhao, B. H., and Zhang, X. P. (1987). A preliminary study on flowering habits of staple food bamboo of giant pandas. Sichuan Forestr. Sci. Technol. 8, 49–54. doi: 10.16779/j.cnki.1003-5508.1987.01.008
Yuan, J. L., Fu, M. Y., Zhuang, J. K., Xiao, X. T., and Wang, P. H. (2005). The characteristics of flowering and pollinating of several sympodial bamboos and elementary selection of Dendrocalamus latiflorus seedlings. J. Bamboo Res. 24, 9–13. doi: 10.3969/j.issn.1000-6567.2005.03.003
Yuan, J. L., Guo, G. P., Yue, J. J., Wu, X. L., and Gu, X. P. (2012). Featres of DNA methylation during the flowering process of Bambusa multiplex. Acta Bot. Boreali Occidentalia Sinica 32, 60–66. doi: 10.3969/j.issn.1000-4025.2012.01.010
Yuan, X. L., Huang, Q. C., and Peng, H. Z. (2008). Correct understanding of bamboo flowering. Zhejiang Forestr. 32–33.
Yue, X. H., Zhao, R., and Lin, S. Y. (2018). Flowering biological characteristics of Phyllostachy glauca. Jiangsu Agric. Sci. 46, 117–122. doi: 10.15889/j.issn.1002-1302.2018.10.031
Zeng, L., Ren, P., Li, Z. Q., Yang, D. S., Mou, X. W., Chen, Z. Y., et al. (1998). Report of biological features of Phyllostachys nidularia. Econ. Forest Res. 16, 9–73. doi: 10.14067/j.cnki.1003-8981.1998.04.003
Zhang, C. X., Xie, Y. F., and Ding, Y. L. (2003). The studies of leaf senescence of Pseudosasa amabilis var. convexa during flowering and seeding stage. J. Nanjing Forestr. Univ. 27, 59–61. doi: 10.3969/j.issn.1000-2006.2003.02.014
Zhang, H., Zou, J. Y., and Wang, Y. Y. (1994). Inquiry to flowering and seed bearing of Chimonobambusa quadrangularis Makino in Jinfoshan of Tongzi county. J. Bamboo Res. 13, 66–69.
Zhang, J. X. (1985). Investigations on the flowering, fruiting, and regeneration behavior in Orecalamus utilis Keng. J. Bamboo Res. 4, 86–88.
Zhang, J. X., Luo, W., and Ming, Y. (1992). A study on flowering and fruitage of Ampelocalamus scandens. J. Bamboo Res. 11, 97–99.
Zhang, S. S. (1977). Methods of flowering withered and restored in bamboo forest. Sichuan Forestr. Sci. Technol. 19–21. doi: 10.16779/j.cnki.1003-5508.1978.01.008
Zhang, W. Y., and Ma, N. X. (1989). Biological characteristics of bamboo plants in flowering stage. Forest Res. 2, 596–600.
Zhang, W. Y., Ma, N. X., Wu, L. L., Huang, S. T., and Zhang, J. W. (1992). A study of flowering and fruiting of Phyllostachys bambusoides. J. Bamboo Res. 11, 15–25.
Zhang, X. M., Zhao, L., Larsonrabin, Z., Li, D. Z., and Guo, Z. H. (2012). De novo sequencing and characterization of the floral transcriptome of Dendrocalamus latiflorus (Poaceae: Bambusoideae). PLoS ONE 7:e42082. doi: 10.1371/journal.pone.0042082
Zhang, Y., Zhang, L., Lan, F. R., Zhu, Z. Y., Chen, X. D., and Guo, Q. R. (2018). The first record of flowering and bearing about Pleioblastus yixingensis (Bambusoideae: Poaceae). J. Trop. Subtrop. Bot. 26, 171–177. doi: 10.11926/jtsb.3792
Zheng, J. G., and Huang, W. F. (1990). Rejuvenescence technique of flowering Pleiblastus. J. Bamboo Res. 9, 61–72.
Zheng, X., Jiang, M. Y., Zhang, L., Lin, S. Y., and Ding, Y. L. (2016). Fruit morphological characteristics of thirteen bamboo species. J. Plant Resour. Environ. 25, 96–103. doi: 10.3969/j.issn.1674-7895.2016.04.12
Keywords: Bambusoideae, bamboo flowering events, theory of bamboo flowering cycle, rejuvenation, flowering diversity
Citation: Zheng X, Lin S, Fu H, Wan Y and Ding Y (2020) The Bamboo Flowering Cycle Sheds Light on Flowering Diversity. Front. Plant Sci. 11:381. doi: 10.3389/fpls.2020.00381
Received: 04 September 2019; Accepted: 17 March 2020;
Published: 17 April 2020.
Edited by:
Gerald Matthias Schneeweiss, University of Vienna, AustriaReviewed by:
Eduardo Ruiz-Sanchez, Universidad de Guadalajara, MexicoKeisuke Nagai, Nagoya University, Japan
Copyright © 2020 Zheng, Lin, Fu, Wan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulong Ding, eWxkaW5nQHZpcC4xNjMuY29t
†These authors have contributed equally to this work
 Xiao Zheng
Xiao Zheng Shuyan Lin
Shuyan Lin Huajun Fu1,2,3
Huajun Fu1,2,3 Yulong Ding
Yulong Ding