- 1Respiratory Medicine, Department of Translational Medicine, University of Eastern Piedmont, Vercelli, Italy
- 2Respiratory Unit, Sant’Andrea Hospital, Vercelli, Italy
- 3Department of Medicine, Spedali Civili di Brescia, Brescia, Italy
- 4Department of Medical and Surgical Sciences, Section of Respiratory Diseases, University “Magna Græcia” of Catanzaro, Catanzaro, Italy
- 5Department of Emergency, Spedali Civili di Brescia, Brescia, Italy
Chronic obstructive pulmonary disease (COPD) is a common disabling disease characterized by progressive airflow obstruction. Great efforts were spent in the development of drugs able to improve symptoms, quality of life, reduce exacerbations, hospitalizations and the frequency of death of patients with COPD. The cornerstones of treatment are bronchodilator drugs of two different classes: beta agonists and muscarinic antagonists. Currently the Global initiative for COPD suggests the use of long acting beta agonists (LABAs) and long acting muscarinic antagonists (LAMAs) in combination for the majority of COPD patients, thus great interest is associated with the developing of LAMA/LABA fixed combination in the maintenance treatment of stable COPD. Many LAMA/LABA fixed dose combinations have been licensed in different countries and the clinical use of these drugs stimulated the performance of many clinical trials. The purpose of this review is a complete criticism of pharmacological and clinical aspects related to the use of LAMA/LABA single inhalers for the maintenance treatment of stable COPD, with particular mention to the most debated topics and future prospects in the field.
Introduction
Chronic obstructive pulmonary disease (COPD) is believed to be the third leading cause of death worldwide by 2020 (WHO, 2008).
Chronic obstructive pulmonary diseases is a common disease characterized by respiratory symptoms and progressive airflow obstruction due to alveolar and bronchial abnormalities and inflammation caused by exposition to noxious substances (Global initiative for chronic obstructive Lung disease [GOLD], 2018). COPD is associated to dyspnea, cough, and sputum with lung hyperinflation. COPD is a disabling disease with huge impact on normal daily activities and limiting quality of life, the disease abruptly worsen due to exacerbations inducing a step down of health conditions, following which the recovery of breath function and activities is gradually slower and more difficult leading to disability and death (Global initiative for chronic obstructive Lung disease [GOLD], 2018).
Following this series of reasons there is a great impulse in the development of drugs able to improve symptoms, quality of life, reduce exacerbations, hospitalizations and the frequency of death of patients with COPD. The cornerstones of treatment are bronchodilator drugs of different classes including beta agonists and muscarinic antagonists (Montuschi and Ciabattoni, 2015).
Currently the Global initiative for COPD (GOLD) promotes the “ABCD” assessment of COPD patients based on symptoms severity (assessed by questionnaire) and exacerbation risk (low risk consisting in no more than one moderate – severe exacerbation during the past year). GOLD group A includes patients with low symptom severity and low exacerbation risk. GOLD group B includes patients with high symptom severity and low exacerbation risk. GOLD group C includes patients with low symptom severity but high exacerbation risk. GOLD group D includes patients presenting high symptom severity and high exacerbation risk (Global initiative for chronic obstructive Lung disease [GOLD], 2018).
Global initiative for COPD suggests the use of long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) in combination for group B patients with persistent symptoms, group C patients with further exacerbations on LAMA treatment and for group D patients with or without the addition of inhaled corticosteroids (ICSs).
The administration of these two drugs simultaneously with the same device (single inhalator) should ensure a better adherence to the treatment and ad hoc dosages to produce a “synergistic effect between the two drugs respect to the administration of the two drugs separately (Tashkin and Ferguson, 2013).
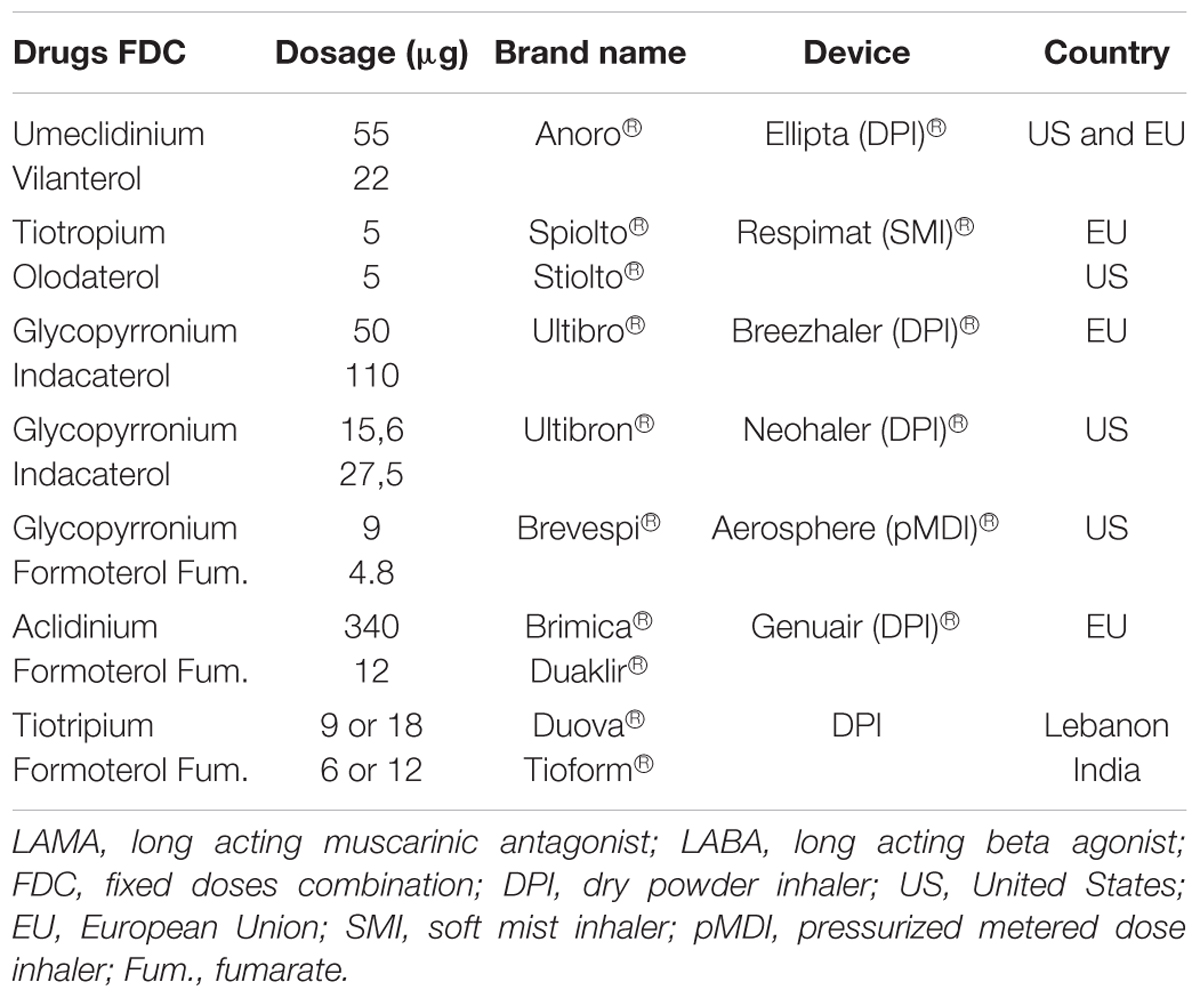
Currently the LAMA/LABA combination in single inhaler device disposable on the market include Umeclidinium/ Vilanterol (Anoro®), Tiotropium/Olodaterol (Stiolto®), Glycopyrrolate/Formoterol (Bevespi®) and Glycopyrronium/Indacaterol (Ultibron®) available in the United States. Glycopyrronium/Indacaterol (Ultibro®), Tiotropium bromide/Olodaterol (Spiolto®), Umeclidinium/Vilanterol (Anoro®), and Aclidinium/Formoterol (Duaklir® Genuair®, Brimica® Genuair®) are available in the European Union (Table 1).
Purpose of this review is providing a complete criticism of LAMA/LABA single inhaler treatment in COPD with particular mention to the most debated topics and future prospects in the field.
Pharmacology Mechanism of Action and Rationale for LAMA/LABA Fixed Dose Combinations in a Single Inhaler for COPD
Long acting beta agonist and LAMA are two major classes of bronchodilators and currently the principal medications for patients with COPD. LABA relax airway smooth muscle by linking with the beta2-adrenergic receptors. This linkage cause bronchodilation by inducing conformational changes in the post-synaptic β2 receptor on airway smooth muscle cells with consequent activation of a stimulatory guanosine-threephosphate-(GTP) binding protein (G2), increased adenylyl cyclase activity and cyclic adenosine monophosphate (cAMP) synthesis, Protein Kinase A activation and sequential intracellular events, including inhibition of myosin light chain kinase (MLCK), leading to reduction in smooth muscle airway contractility. This relaxation is also caused by activation of large conductance Ca2+ activated K+ channels via G2 which leads to plasma membrane hyperpolarization (Montuschi et al., 2016) (Figure 1).
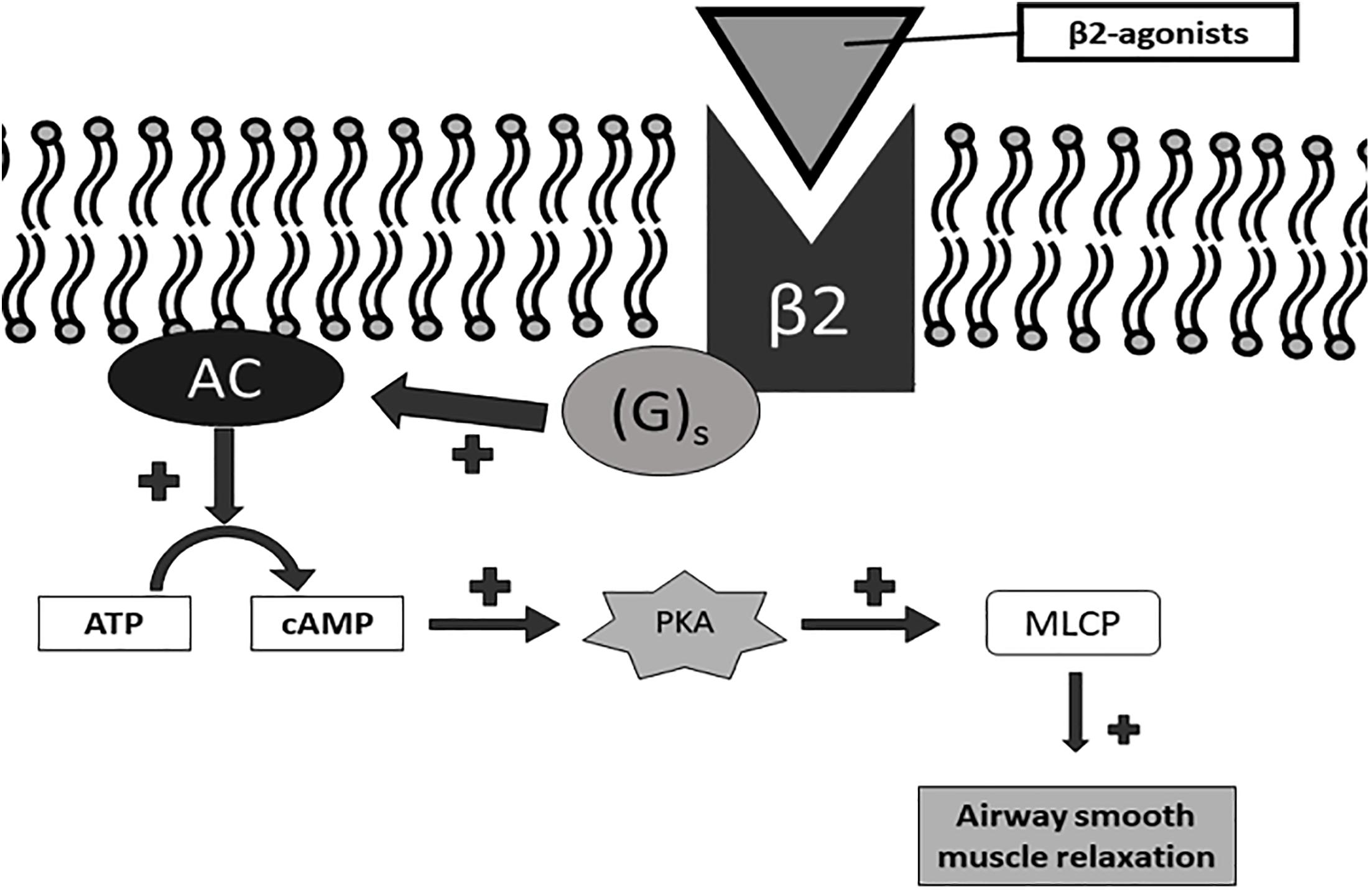
Figure 1. Parasympathic pathway involved in control of airway smooth muscle contraction. ACh, acetylcholine; M1, M1-muscarinic receptor; M2, M2-muscarinic receptor; M3, M3-muscarinic receptor.
Long acting beta agonist can be divided into once-daily and twice-daily LABA. Once-daily LABA are currently called ultra-LABA. Ultra-LABA are ultra-long acting and are dosed once a day such as indacaterol (IND), olodaterol (OLO), and vilanterol (VIL); they provides both the quick bronchodilation effect similar to short-acting beta agonists, and a 24-h bronchodilation effect permitting the once-daily administration (Horita et al., 2017). Twice-daily LABAs are formoterol fumarate (FF) or propionate (FP) and salmeterol (SAL). LAMA block the bronchoconstrinction effect of acetylcholine on M3 muscarinic receptors expressed in airway smooth muscle; they have prolonged binding to M3 muscarinic receptors with faster dissociation from M2 muscarinic receptors (Global initiative for chronic obstructive Lung disease [GOLD], 2018). M3 receptors are coupled (GTP) – binding protein (G2) which regulates intracellular calcium concentration and calcium-modulated proteins (Pera and Penn, 2014) (Figure 2).
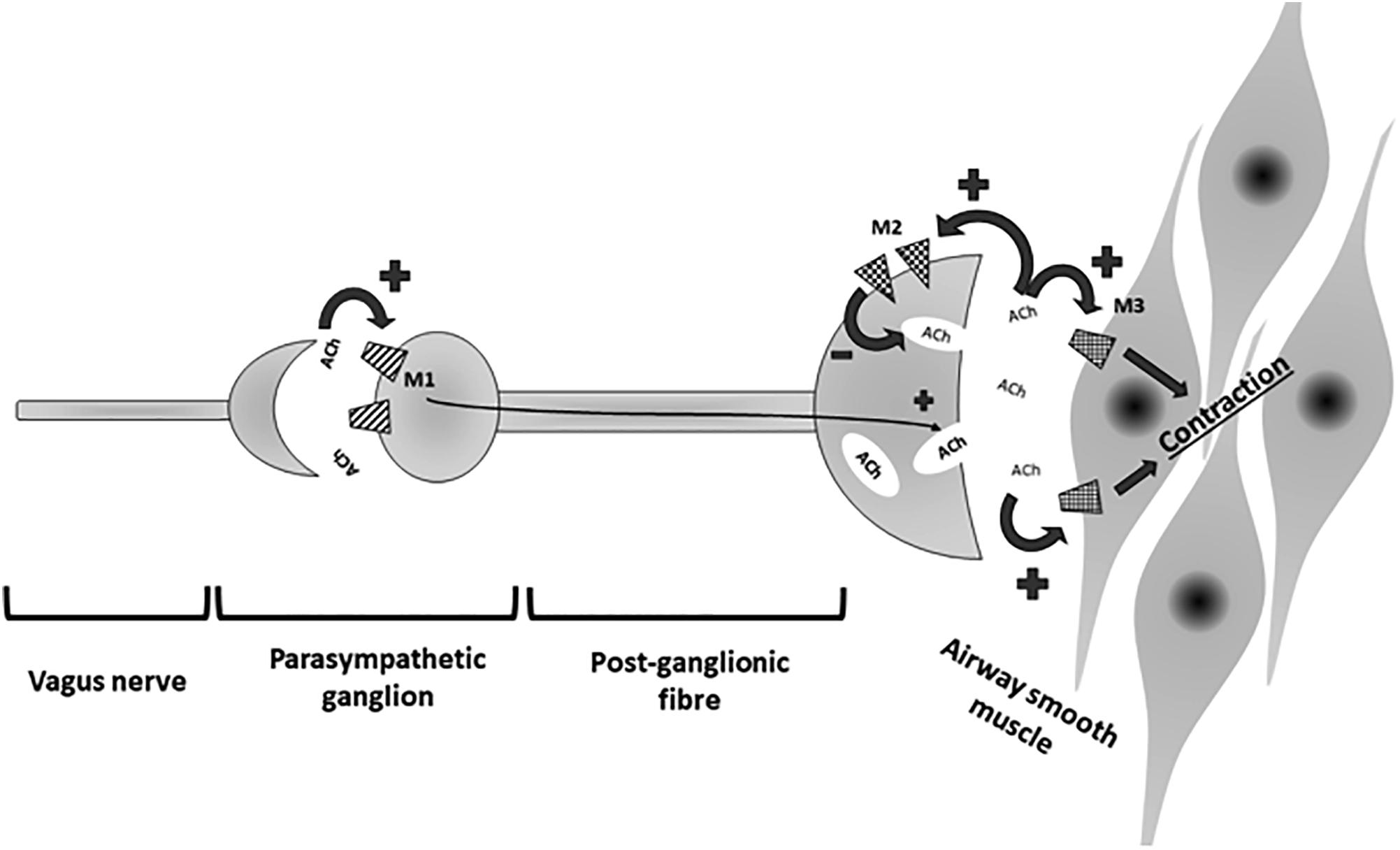
Figure 2. Signaling pathway of β2 receptor activation. Gs, Gs-protein; AC, adenylate cyclase; ATP, adenosine triphosphate; cAMP, cyclic AMP; PKA, protein kinase A; MLCP, myosin light chain phosphatase.
Long acting muscarinic antagonists such as tiotropium (TIO), umeclidinium (UMEC), and glycopyrronium (GLY) are long acting drugs and are dosed once a day, while aclidinium (ACL) is dosed twice a day.
Bronchodilator monotherapy is not always satisfactory for patients with advanced COPD. In that situation, a dual-bronchodilator therapy consisting of LAMA and LABA is a good option. LAMA/LABA fixed dose combinations (FDCs) have been shown to improve lung function, lung hyperinflation, exercise capacity, quality of life and exacerbation frequency thereby slowing disease progression in COPD (Global initiative for chronic obstructive Lung disease [GOLD], 2018). This combinations have a synergistic effect rather than just being additive one (Tashkin and Ferguson, 2013). Synergy is defined as the drug combination having a greater effect than would be expected from the mono-components alone. Synergy would be beneficial as a greater degree of bronchodilation could potentially be achieved at lower doses of the individual components thus minimizing side effects. LABAs activate pre-junctional β2-adrenoceptors and reduce acetylcholine release thereby prevent any functional competition by acetylcholine at post-junctional muscarinic receptors in the airways occupied by LAMAs. Thus LAMA/LABA combinations exploit both the adrenergic and cholinergic pathways in the airway smooth muscle to maximize bronchodilation (Lal and Strange, 2017) (Figure 3).
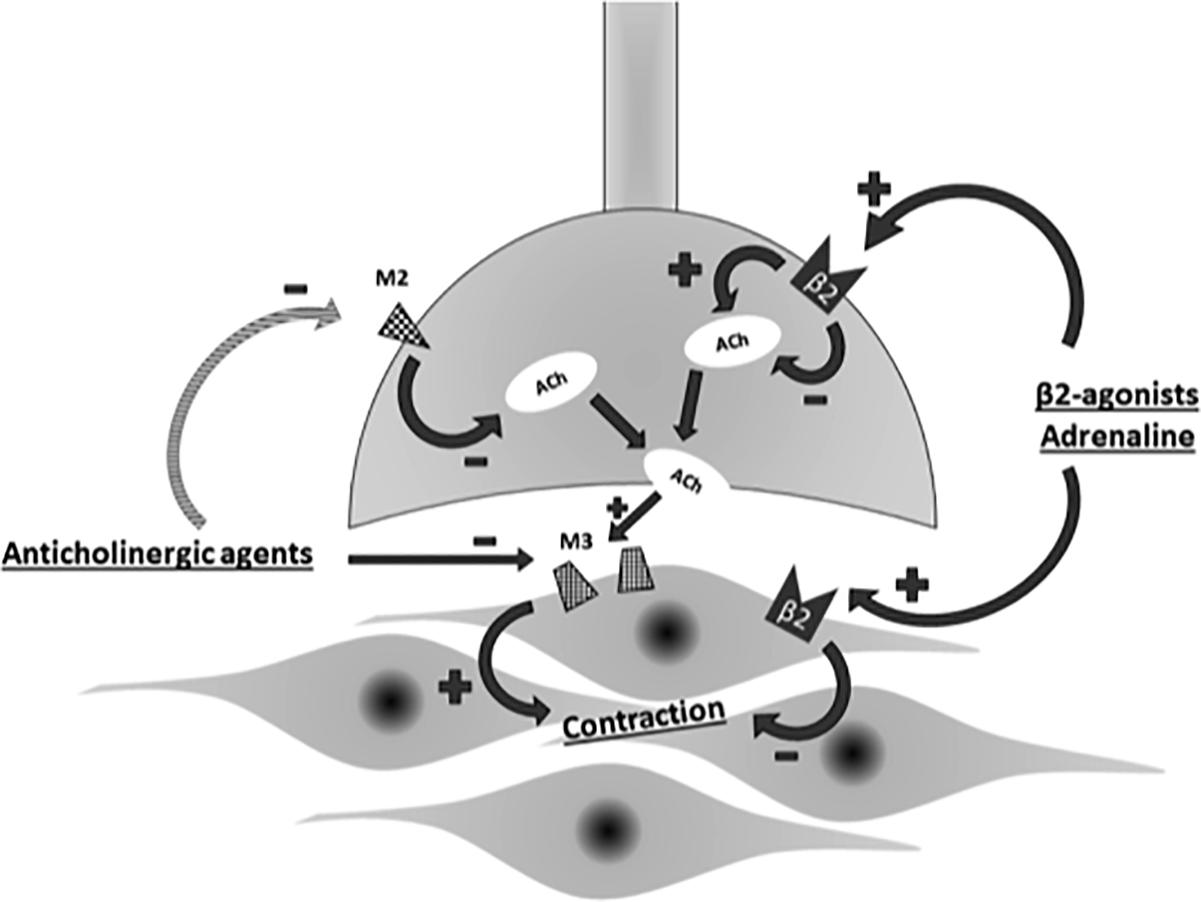
Figure 3. Interplay of adrenergic and cholinergic pathway on airway smooth muscle. ACh, acetylcholine; M2, M2-muscarinic receptor; M3,M3-muscarinic receptor.
LAMA/LABA Fixed Dose Combinations in a Single Inhaler for COPD: Evidences From Clinical Trials
LAMA/LABA combinations in a single inhaler with fixed dose are numerous. Here we resemble the principle international studies on efficacy and safety of LAMA/LABA combinations.
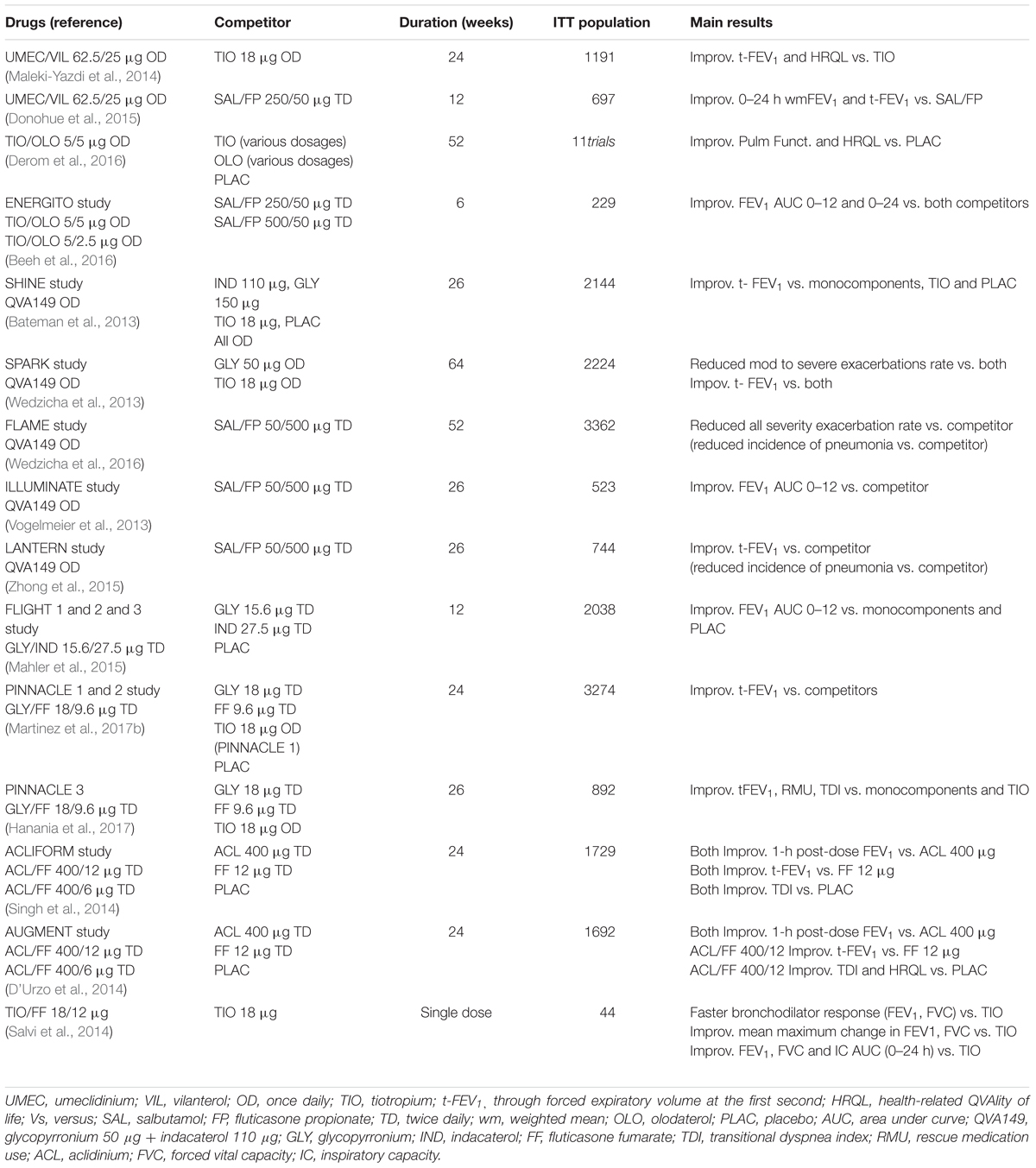
The main published studies on LAMA/LABA single inhaler treatments are summarized in Table 2.
Umeclidinium/Vilanterol
Numerous international clinical trials have evaluated the efficacy and safety of UMEC/VIL against placebo, monocomponents, TIO and associations between LABA and ICS (Malerba et al., 2016). We summarize the principal studies: a 24 weeks trial UMEC/VIL 125/25 μg once daily (OD) was tested vs. UMEC 125 μg, VIL 25 μg and placebo (OD) in more than 1000 COPD patients. Combined treatment was superior to placebo and monocomponents in improving difference from baseline of through forced expiratory volume at the first second (tFEV1), transitional dyspnea index (TDI) and rescue medication use (RMu) (Celli et al., 2014).
Decramer and colleagues conducted two 24 weeks trial in more than 800 COPD patients (each study) testing UMEC/VIL 125/25 μg, UMEC/VIL 62.5/25 μg, TIO 18 μg, VIL 25 μg or UMEC 125 μg (all OD). They reported that both associations treatments were superior to TIO, UMEC, and VIL alone in improving tFEV1 and RMu (Decramer et al., 2014). Another (Maleki-Yazdi et al., 2014) 24-week, multicenter, randomized, blinded, double-dummy, parallel-group study Phase III study compared UMEC/VIL 62.5/25 μg, to TIO 18 μg OD. Significant improvements in trough FEV1 was observed for UMEC/VIL versus TIO. Also, UMEC/VIL improved health-related quality of life (HRQL), and reduced the requirement for RMu compared with TIO.
Three 12-week trials enrolling more than 2000 patients with COPD compared UMEC/VIL 62.5/25 μg OD with Salmenterol/Fluticasone propionate (SAL/FP) 250/50 μg twice daily (TD) and 500/50 μg TD (Donohue et al., 2015; Singh et al., 2015). In these studies UMEC/VIL provided an improved of pulmonary function (mean change from baseline in tFEV1) in all trials. Only one study reported a reduction in RMu in favor of patients treated with UMEC/VIL (Donohue et al., 2015).
In summary the considered trials concluded that once-daily UMEC/VIL 62.5/25 μg OD, was well-tolerated, provided clinically-significant improvements in lung function and symptoms in COPD patients.
UMEC/VIL 55–22 μg is commercialized with the name of Anoro® and delivered by the Ellipta® dry powder inhaler (DPI).
Tiotropium/Olodaterol
The efficacy and safety of this combination was studied several randomized, double-blind, parallel-group, multicenter trials (Derom et al., 2016). In these studies, over 10,000 patients have been involved, for simplicity we consider only the dosage of 5/5 μg OD that is recommended and available on market. The majority of these studies involved patients with COPD GOLD II and III stage.
TIO/OLO 5/5 μg not only improved pulmonary function more than placebo but also resulted in statistically significant improvements on dyspnea, RMu, HRQL, and exercise endurance.
TIO/OLO 5/5 μg differed significantly from TIO 5 μg monotherapy in terms of pulmonary function [FEV1 area under curve (AUC)0–24, FEV1 AUC0–12, and FEV1 AUC12–24] and reducing symptoms of dyspnea (TDI and HRQ). Comparison between TIO/OLO 5/5 μg FDC OD and OLO 5 μg OD, was generally statistically but not clinically significant in favor of fixed dose association not reaching the minimal clinical important difference (MCID). In a 6-week trial that enrolled more than 200 patients with COPD, TIO/OLO 5/5 μg was significantly more effective than SAL/FP TD at improving pulmonary function (ENERGITO study) (Beeh et al., 2016).
TIO/OLO 5/5 mg is formulated in the Respimat®soft-mist inhaler (SMI) commercialized under the name of Spiolto®(EU) or Stiolto®(US).
Glycopyronium/Indacaterol
The fixed-dose combination of LAMA GLY 50 μg plus LABA IND 110 μg (QVA149) has been shown in a series of clinical trials to be more effective than placebo and the single components with regard to lung function, symptoms, and quality of life outcomes, being as safe as the single components and placebo. All the considered studies were randomized double-blind.
SHINE study (Bateman et al., 2013) is a multicenter, 26-week trial which evaluated the safety and efficacy in terms of tFEV 1 of QVA149 in comparison to IND, GLY, TIO, and placebo (OD) in patients with moderate-to-severe COPD. Trough FEV1 at week 26 was significantly improved with QVA149 compared to all other treatment arms, with a safety and tolerability profile similar to placebo.
SPARK (Wedzicha et al., 2013) is a 64-week, parallel-group, active controlled study to evaluated the effect of QVA149 vs. GLY 50 μg and TIO 18 μg (OD) in 2224 patients with severe-to-very severe COPD (GOLD COPD stages III and IV and one or more moderate COPD exacerbation in the past year). QVA149 was found to significantly reduce the rate of moderate to severe COPD exacerbations by 12% compared to GLY, and by 10% compared to TIO; however, with not significant differences. QVA149 also significantly improved tFEV 1 and quality of life as compared to GLY and TIO without any increase in adverse events.
FLAME A 52-week multi-center, parallel-group, active controlled study to compare the effect of QVA149 OD with SAL/FP TD on the rate of exacerbations in 3332 patients with moderate-to-very severe COPD. QVA149 reduced the annual rate of all COPD exacerbations (the rate was 11% lower in the QVA149 group than in the SAL/FP group) (Wedzicha et al., 2016).
ILLUMINATE study (Vogelmeier et al., 2013) is a 26 weeks parallel-group study, in which 523 patients with moderate to severe COPD were assigned to OD QVA149 or TD SAL/FP 500/50 μg. At week 26 FEV1 AUC 0–12 h was significantly higher with QVA149 than with SAL/FP, with a similar incidence of serious adverse events.
LANTERN is a 26-week parallel-group study conducted to assess the efficacy and safety of OD QVA149 compared to TD SAL/FP 500/50 μg in 744 patients with moderate-to-severe stable COPD with or without exacerbations in the previous year. QVA149 had significant superiority to SAL/FP for tFEV1 and for the standardized AUC from 0 to 4 h. QVA149 showed similar improvements in TDI focal score, St George Respiratory Questionnaire total score, and RMu compared to the other active arm. However, QVA149 significantly reduced the rate of moderate or severe exacerbations by 31% (p = 0.048) over SAL/FP. The incidence of pneumonia was threefold lower with QVA149 (0.8%) (Zhong et al., 2015).
The FDA approved dose for GLY/IND in the US is 15.6/27.5 μg TD based on the FLIGHT1 and FLIGHT2 studies (Mahler et al., 2015) while in the European Union the approved dose for GLY/IND is 50/110 μg OD. Studies have shown that the two formulations have similar effects. The EXPEDITION program consisted of FLIGHT 1, 2, and 3 studies which tested GLY/IND 15.6/27.5 μg TD in US. It has shown a significant improvement in lung function for the FDC compared to monocomponents and in HRQL and RMu compared to placebo (Mahler et al., 2015).
GLY/IND association 50–110 μg is marketed in EU with the brand name of Ultibro®, delivered by Neohaler®DPI.
GLY/IND association 15,6–27,5 μg is marketed in United States with the brand name of Ultibron®, delivered by the Breezhaler®DPI.
Glycopyrrolate/Formoterol
Two 24-weeks, randomized, double blind, and placebo controlled phase III trials: PINNACLE-1 and PINNACLE-2 assessed the clinical efficacy and safety of GLY/FF 18/9.6 μg fixed dose association in patients with moderate and very severe COPD (Martinez et al., 2017b). Patients received GLY/FF, GLY 18 μg, FF 9.6 μg, or placebo (all TD), or TIO 18 μg. At the end of the trials patients were randomized to prosecute the trial for 52 w: PINNACLE-3 (Hanania et al., 2017). At week 24, significant differences in change from baseline in the trough FEV1 for GLY/FF vs. placebo, GP, and FF were seen in both PINNACLE-1 and PINNACLE-2. The incidence of adverse events was similar between all treatment arms. Improvements in efficacy endpoints were also sustained over 52 weeks.
GLY/FF is delivered by a Metered Dose Inhaler Formulated Using Co-Suspension Delivery Technology and commercialized with the name of Bevespi® (available only in US market).
Aclidinium/Formoterol
Two 6-month, multicentre, randomized studies (ACLIFORM and AUGMENT) have evaluated the safety and efficacy of ACL/FF FDC. The first study (Singh et al., 2014) compared the 400/6 μg and 400/12 μg combinations of ACL/FF with ACL 400 μg, FF 12 μg and placebo (all TD). Both combinations demonstrated statistically significant improvements to placebo in from baseline in 1-h post-dose FEV1, tFEV1 in the changes in TDI. At week 24, both combinations showed significant improvements from baseline in 1-h post-dose FEV1 versus ACL and tFEV1 versus FF.
AUGMENT study (D’Urzo et al., 2014) was conducted in 1692 stable COPD patients comparing ACL/FF 400/12 μg, ACL/FF 400/6 μg, ACL 400 μg, FF 12 μg, or placebo (all TD).
Statistically significant improvements at week 24 were observed in the co-primary endpoints of FEV1 1-h post-dose, by the 400/12 μg combination of ACL/FF, versus 400 μg ACL and for tFEV1 versus FF 12 μg. The 400/6 μg combination produced statistically significant improvements in (FEV1) 1-h post-dose versus ACL, but for the change from baseline tFEV1 did not reach significance as compared to FF 12 μg. The 400/12 μg combination induced a Peak FEV1 improvement at 24 weeks by 285 ml versus placebo (p < 0.0001) and the 400/6 μg combination by 259 ml with versus placebo (p < 0.0001).
A pooled analysis of these two trials (Bateman et al., 2015) revealed that 400/12 combination improved TDI focal score, HQRL, overall night-time and early-morning symptom severity versus placebo and both monotherapies at Week 24 (all p < 0.05).
The rate of moderate or severe exacerbations was significantly reduced with ACL/FF 400/12 μg compared with placebo (p < 0.05) but not monotherapies. 400/12 μg ACL/FF combination was the most effective dose and was found well-tolerated and safe.
ACL/FF 400/12 is commercialized with the names of Duaklir® and Brimica®, delivered by the Genuair® dry powder inhaler (available only in European market).
Tiotropium/Formoterol
A single study enrolled 44 COPD subjects in a randomized, double-blind, multi-center, cross-over study. On two separate days 18 μg TIO and 18 μg TIO plus 12 μg FF (single dose) were administered via pressurized metered dose inhalers. The results showed that the combination of TIO plus FF showed a faster onset of bronchodilator response (p < 0.01 for FEV1 and forced vital capacity FVC), a greater mean maximum change in FEV1 (p = 0.01) and FVC (p = 0.008) and greater AUC0–24 h values for FEV1, FVC and inspiratory capacity (IC) compared with TIO alone. In the combination treated group Trough FEV1 and FVC values were also improved (Salvi et al., 2014).
TIO/FF is commercialized only in few countries with the name of DUOVA® (Lebanon) with different dosages (9/6 mcg; 9/12 mcg and 18/12 mcg) and TIOFORM® (India) 18/12 mcg; 9/6 mcg.
The Role of LAMA/LABA Fixed Dose Combinations in a Single Inhaler for COPD Therapy in Global Initiative for Obstructive Lung Disease Recommendations
The rationale for fixed combination bronchodilator therapy in COPD is based on the increased bronchodilation and reduced side-effects compared to the single bronchodilators effects (Cazzola and Molimard, 2010). Since 2011, and after their last revision, GOLD recommendations introduced ABCD assessment tool to classify patients in four groups depending on symptoms and their history of exacerbations. Together with clinical and functional evaluation, ABCD assessment is crucial for stratification of prognosis and to decide which is the better therapy for patients (Vanfleteren et al., 2013; Soriano et al., 2015). In the management of stable COPD patients, the identification and reduction of exposure to risk factors are fundamental. The aim of pharmacological treatment is to reduce symptoms, the risk and severity of exacerbations in addition to improve health status and tolerance to exercise (Global initiative for chronic obstructive Lung disease [GOLD], 2018). Therapeutic decisions are strictly dependent to availability of medications and patient’s response or preference; nevertheless, since drugs are delivered with inhalators, a proper inhaler technique is required to take correctly medicaments.
Global initiative for COPD recommendations provide a model for initiation of the treatment and for subsequent adjustments such as escalation and/or de-escalation according to following evaluation of symptoms control and risk of exacerbations. The combined therapy with LABA and LAMA plays an important role in therapeutic strategies three out of four ABCD groups:
Group B: in group B, characterized by patients with high symptoms burden but low number of moderate/severe exacerbations, the initial therapy should be based on a long acting bronchodilator, LABA or LAMA (Barr et al., 2005; Appleton et al., 2006). In this group of patients, when there is the persistence of dyspnoea despite a monotherapy with a bronchodilator, it’s recommended to add a second bronchodilator (Karner and Cates, 2012). This approach originates from the results of a meta-analysis that included four studies (Aaron et al., 2007; Vogelmeier et al., 2008; Tashkin et al., 2009; Mahler et al., 2012).
Aaron et al. (2007) investigated SAL and TIO, Vogelmeier FF and TIO; both studies demonstrated a significant reduction of St George’s Respiratory Questionnaire (SGRQ) score, therefore an improvement of quality of life, for the group of patients in treatment with combined therapy LABA and TIO. Same studies did not report a reduction of hospital admission for any cause, neither for exacerbations. Taking into account the lung function, all the studies included in the meta-analysis demonstrated an improvement in pre-bronchodilator FEV1 at the end of the study for the LABA + TIO groups.
Recently Calzetta et al. (2016) published a review and meta-analysis on duration of treatment of LABA and LAMA combinations in COPD patients; they included 14 studies that reported results of 20 randomized controlled trials. In these studies the drugs used for LAMA/LABA therapy were: GLY/IND, UMEC/VIL, TIO/OLO, ACL/FF with different regimens of administration (once or twice daily). LAMA/LABA significantly improved trough FEV1, SGRQ and TDI after 3, 6, and 12 months of treatment, when compared with monocomponents. Again Rodrigo et al. (2017) in a recent meta analysis demonstrated the effectiveness of dual broncodilation respect monocomponents, in particular for changes of through FEV1 when compared to LABA. The stability of the effect of combined therapy on FEV1 lasts for 12 months and remain stable and superior for the whole period of observation.
For those patients in group B that complain severe breathless, GOLD recommendations suggest to start with combination therapy. This approach originates from a study conduced by Martinez et al. (2017a) that evaluated post hoc analysis of pooled data from PINNACLE-1 and PINNACLE-2 phase III studies, evaluating GLY/FF combination versus single components, on lung function, exacerbation and baseline symptom burden (measured with COPD Assessment Test, CAT) (Martinez et al., 2017a). The significant improvement of SGRQ score obtained by combination therapy respect to monotherapies was greater in those patients with baseline CAT score ≥ 20 points. Similar results have been obtained considering other clinical parameters such as function of baseline symptom burden.
The GOLD Guidelines authors recommended that if the addition of the second bronchodilator did not reduce symptoms, the treatment could be stepped down to a single bronchodilator (LABA or LAMA). In this case it’s mandatory to evaluate or re-evaluate comorbidities that could be responsible of the poor symptoms’ control and affect the patient’s prognosis (Lange et al., 2012; Agustí, 2013).
Group C: in group C, characterized by patients with low symptoms burden but high number of moderate/severe exacerbations, the initial therapy should be based on a single long acting bronchodilator, LABA or LAMA. The two studies that investigated this approach concluded that LAMA was superior to LABA in prevention of exacerbations and the GOLD authors recommend to start with a LAMA in this group of patients (Vogelmeier et al., 2011; Decramer et al., 2013).
In those patients with persistent exacerbations, the addition of a second long acting bronchodilator or a combination of a LABA and an ICS may be beneficial (Miravitlles et al., 2017). In SPARK study, the annual rates of moderate or severe exacerbations and all exacerbations were lower in the group of patients treated with GLY/IND combination versus GLY alone (Wedzicha et al., 2013).
Due to an increase of the risk of development of pneumonia in some patients related to ICS, the first choice of combination therapy is LAMA/LABA even if not resulting from studies complaining patients in group C (Zhong et al., 2015; Wedzicha et al., 2016).
Group D: in group D, characterized by patients with high symptoms burden and high number of moderate/severe exacerbations, the initial therapy should be based on a combination bronchodilator therapy LAMA/LABA. This assumption is mainly based on results of Wedzicha et al. (2013, 2016) previously mentioned studies on combined LAMA/LABA versus monotherapies and LAMA/LABA versus ICS/LABA. Same results have been obtained in DYNAGITO trial with TIO/OLO dual therapy versus TIO alone (Calverley et al., 2018). Patients belonging to group D could be naive patients (at first diagnosis), or could be shifted to this group coming from group B, in case of increased number of exacerbations during the previous year, or from group C, in case of increase of symptoms’ burden. Those patients in treatment with combined LAMA/LABA bronchodilation, could be stepped up to triple-therapy LAMA/LABA/ICS in case of further exacerbations.
LAMA/LABA Single Inhaler: Devices
Bronchodilators consist in a complex and technological drug delivery system composed by a specific drug formulation and delivery device that made inhalable the drug inside. The devices are substantially divided in: active devices, where the energy needed to create the aerosol is generated by the same inhaler through a complex technological system, and passive devices where the aerosolization of the drug is dependent from the inhaled air stream.
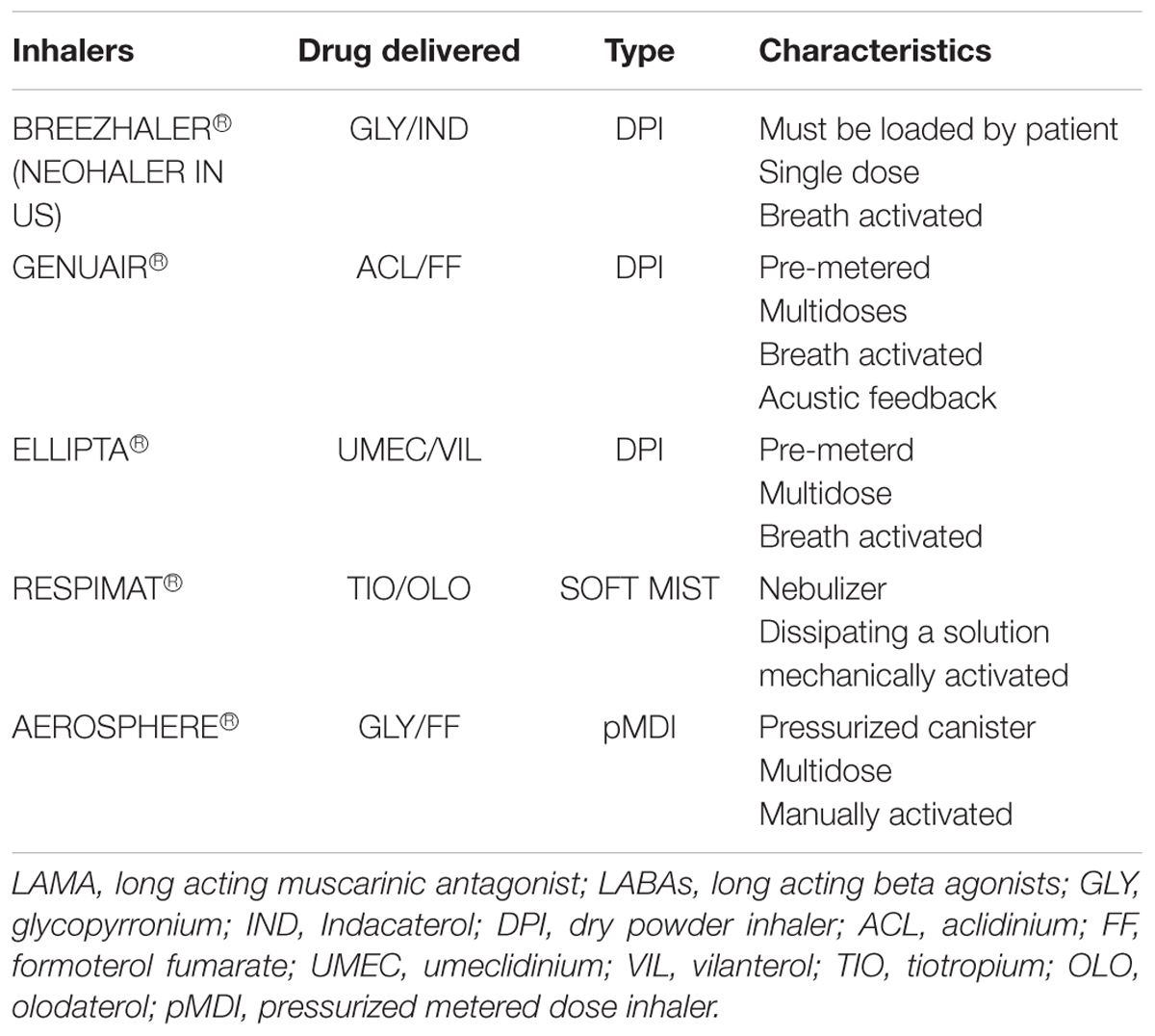
Inhalation devices can be distinguished in three types: pressurized metered dose inhalers (pMDIs), dry powder inhalers (DPIs) and, recently, soft mist inhaler (SMI, Boehringer Ingelheim). A comparison among inhaler devices in LABA/LAMA single inhaler treatment is reported in Table 3.
Pressurized Metered Dose Inhalers
In pMDIs the active drugs are dissolved or suspended in a propellant, a mixture of propellants, or a mixture of solvents and they are delivered via a compact pressurized aerosol dispenser (canister) by pushing the canister down into the holder. The canister contains several 100s metered doses of the medication. PMDIs are therefore not breath-activated and require the user to coordinate pressing down the canister and inhaling the medication.
Dry Powder Inhalers
Dry powder inhalers contain the drug in dry state; the devices are usually constituted by a powder formulation, a dose metering mechanism that contains or measures a single dose of the drug, a powder de-agglomeration principle and a mouthpiece (Frijlink and De Boer, 2004). The vast majority of DPIs are breath-actuated devices, using a passive mechanism, without the need of patient-device coordination; for a proper dose release the patient is required to make a minimal inspiratory effort.
The delivery of the formulation is consequent an active process usually initiated by an inhalation maneuvre of the patient; then the drug formulation is de-agglomerated or dispersed in an aerosol of small and inhalable particles of active agent and excipients. Each of these processes is dependent from several factors such as the powder formulation, the DPI used and patient’ inspiratory effort. Indeed, in DPIs the inhalation maneuver is crucial and reflects the deposition rate of active principle in the respiratory tract; each DPI device has a different inhalation maneuver and the ability to perform a correct process is dependent from patient’s characteristics such as age and clinical condition (Brocklebank et al., 2001; Lavorini et al., 2008; Pedersen et al., 2010; Inhaler Error Steering Committee et al., 2013; Lexmond et al., 2014).
DPIs up to now used in the delivery of LAMA/LABA drugs for COPD patients are:
Breezhaler® (Novartis, A.G., Basel, Switzerland) is a single-dose, breath-actuated DPI that releases a dry powder contained a pierced gelatin capsule loaded in the device by the patient before the inhalation. Brochodilators combination used is GLY/IND.
Genuair® (AstraZeneca, Cambridge, United Kingdom) is a pre-metered multidose, breath-actuated, medium-resistance DPI; it provides both visual and acoustic feedback to indicate the correct inhalation of the dose. Bronchodilators combination used is ACLI/FF (van der Palen et al., 2013).
Ellipta® (GlaxoSmithKline, Brentford, United Kingdom) is a pre-metered multidose, breath-actuated DPI; bronchodilators combinations used is UMEC/VIL (Komase et al., 2014).
Some studies investigated satisfaction, preference, and error occurrence of these DPIs. The preference of the patients and his skills in using the inhaler device can affect the effectiveness of the treatment. The ease of use is one of the most important characteristics the a device must have, in particular for elderly because they represent a huge part of patients affected by COPD (Man et al., 2018). The recent study conducted by Man et al. (2018) compared these three devices and evaluated satisfaction, error occurrence during use of DPIs (evaluating critical and non-critical errors). Authors demonstrated that Breezhaler® had the highest score for “comfort”; this may be related to the shape, size of the device in particular for female population, but Breezhaler® had a significantly lower score for “ease of dose preparation.” When authors investigated the “clarity to indicate correct dose preparation” and “clarity of indicate correct inhalation” Genuair® received higher scores and this is due to the devices feedback mechanism that informs the patient to the correct preparation and inhalation of the loaded dose. Indeed, adherence to therapy is strictly related to the patient’s confidence of regular inhalation of the drug (Rajan and Gogtay, 2014). Satisfaction varies with the age of patients; younger patients gave higher scores to Ellipta® than Breezhaler® for “ease of operation” and “handling time,” and this results suggest that handling procedures of Ellipta®could be difficult for the older population (Svedsater et al., 2013; Komase et al., 2014).
Comparing pMDIs and DPIs
Pressurized metered dose inhaler and DPIs have their advantages and disadvantages. PMDIs require the user to coordinate pressing down the canister and inhaling the medication while DPIs are activated by breath; however, the inspiratory flow rate is a disadvantage of DPIs. The rate required to deliver the medication in pMDI inhalers is low (about 30 L/min) while the rate required for DPIs is higher (differs based on the build of the inhaler). This would make it more difficult for some patients to be able to deliver the medication properly. DPIs also require the patient not to disperse medication via exhalation into the device prior to using. Another disadvantage of pMDIs is the propellant; previously chlorofluorocarbon (CFC) was used now substituted by hydrofluoroalkane (HFA) and this can cause the inhaler to be more expensive.
Soft Mist Inhaler
Soft mist inhaler Respimat® is one of the more recently inhalers available; it’s a nebuliser dissipating a solution composed by fine droplets of active agents. SMI is different from traditional nebuliser since it is portable and doesn’t require a power source to work because it’s activated mechanically. As pMDIs, the formation of the aerosol is instantaneous but for a correct inhalation it’s required a higher coordination (Lavorini, 2013). Nevertheless, even if SMI takes a longer time to generate an entire aerosol, the nebulization is emitted in a slow-moving mist fashion, allowing a higher lung deposition (Dalby et al., 2004). Due to the combination of smaller particle size, lower velocity and longer duration of the nebulization, a smaller dose of bronchodilators ensure the same level of efficacy and safety than metered dose inhalers. An higher lung deposition of nebulized drugs with SMI was demonstrated with a radio labeled drug particles (Newman et al., 1996, 1998; Steed et al., 1997; Pitcairn et al., 2005; Anderson, 2006).
LAMA/LABA combination administered with Respimat® is TIO/OLO Dal Negro and Povero (2016) compared some devices in terms of patients’ preference and acceptability; Respimat® and Genuair® were preferred to Breezhaler® reporting less difficulties in understanding maneouvers for activate and correctly practicing the inhalation. Respimat® has been judged the easiest to use, the less problematic and the most easy learned device at first attempt of use (Dal Negro and Povero, 2016).
Critical Issues for LABA/LAMA Combinations in a Single Inhaler Therapy for COPD
Dual bronchodilation treatment with LAMA/LABA FDC has demonstrated to be able to improve quality of life in COPD patients in terms of symptoms scores, rescue medication use (van Noord et al., 2005) and lung hyperinflation when compared to placebo. In comparison to in several trials (Bateman et al., 2013; Celli et al., 2014; Decramer et al., 2014; Mahler et al., 2015; Derom et al., 2016; Martinez et al., 2017b) as exercise tolerance (Berton et al., 2010). Moreover LAMA/LABA combinations have been noted to be superior compared to LABA/ICS combined treatment for COPD with at least one exacerbation in the previous year (Wedzicha et al., 2016). A meta-analysis of heterogeneous studies comparing LAMA/LABA with LABA/ICS (Horita et al., 2017) concluded that LAMA/LABA has fewer exacerbations, a larger improvement of FEV1, a lower risk of pneumonia, and more frequent improvement in quality of life indicators.
Clearly none of the pharmacological treatments have ever shown a significant impact on COPD mortality, however we believe that the effects of the LABA/LAMA combination treatment observed improving patient quality of life represent main issues in the treatment of COPD.
Trials Limits
Concerns have been raised about the ability of large trials to actually identify the potential benefits of the investigated treatments. In particular, classical trials focused on the benefits of lung function (FEV1 changes in particular) as a primary endpoint and this could lead to an underestimation of the impact of the treatments studied on the improvement of endurance symptoms, frequency of exacerbations and hospitalizations and quality of life in general. It has also emerged how focusing on the change in FEV1 is deeply influenced by the choice of patients enrolled and may not translate into real life in an equally effective treatment, or vice versa fails to highlight an effective clinical impact in patients who, however, qualitatively improve quality of life performance. For these reasons clinical trials aimed at the ability to detect different endpoints capturing different aspects of COPD pathobiology such as real life clinical improvement are claimed. It is important to underline that the results of the different meta-analysis reported need to be taken with caution because of differences in the studied population and in the outcomes. This heterogeneity may result in difficulties to translate results in real life. Moreover LAMA/LABA direct comparison in head-to-head trials would be suggested.
FDCs Are Better Than Monocomponent Treatment
The administration of two drugs simultaneously with the same device should ensure a better adherence to the treatment (Tashkin and Ferguson, 2013) Using together two bronchodilators with different mechanism of action can overcome specific patient dissimilarities in the response to different treatments (Cazzola and Molimard, 2010), which may be due to different distribution of different receptors (beta agonists and muscarinic) in the lungs (Ikeda et al., 2012; Cazzola et al., 2015). However, the impossibility of change in the individual dosages of the two components may lead to potential problems in relation to individual side effects to one of the components. In the clinical trials these problems have never been observed and all LAMA/LABA FDC have shown optimal tolerability profiles. However, only the observation in real life clinical setting can reassure us about this aspect. Until now, the experiences reported in the daily clinical practice have given encouraging results, but we should keep the guard high in the detection of possible tolerance problems, especially in complex patients with multiple associated diseases usually excluded from clinical trials.
Dosing and Administration
It is necessary to underline that the various LAMA/LABA combinations available on the market are provided in a single fixed dose regimen, which allows poor treatment flexibility and the impossibility of administering different doses to different patients (e.g., in the presence of relevant differences in BMI in patients routinely excluded from large clinical trials).
Some other aspects may generate uncertainties in the interpretation of data received from trials and in the prescription or dispensation of the drug: some formulations require one puff/administration some others two consecutive puffs/administration. Some of the LAMA/LABA combinations are provided in regimen of two administrations per day (ACL/FF disposable in US and EU, GLY/IND 15,6/27,5 μg disposable only in US, GLY/FF disposable only in US), others in OD formulations (UMEC/VIL and TIO/OLO both disposable in US and EU and GLY/IND 50/110 μg disposable only in EU). In particular It should be noted that the GLY/IND associations in the US is available only at a lower dosage than that used in clinical trials (Vogelmeier et al., 2013; Zhong et al., 2015; Wedzicha et al., 2016) in relation to the differences in the US-approved IND dosage compared to the one approved in the EU.
All these differences in the regimens of administration must be kept in mind and could disorient the less experienced prescribers and the patients switching from one treatment to another.
A separate chapter deals with the treatment of the differences related to the different delivery systems of the different LABA/LAMA combinations. Most of the inhalers for the LABA/LAMA associations are DPI except SMI for Stiolto® and pMDI for Bevespi®.
As before discussed each inhaler type has pros and cons that must be considered in the selection of a device for a particular patient. The DPIs, SMI and pMDIs present therefore differences in the size of the particles carrying the drug and therefore in the ability to reach the lung tissue more or less deep. The flow rate able to activate the different inhalers, the need for greater or less coordination of the patient and the preferences of the patients regarding the “taste” of the different formulations are all elements that will play a fundamental role not yet studied in the daily clinical application of these formulations.
Future Directions
Future studies should indicate clinicians the results of head-to-head comparisons among the different LABA/LAMA associations in order to make less empirical the choice of the combination to be used. Since none of the available LAMA/LABA FDCs have been compared in head-to-head trials, they can only be weighed against each other by indirect comparisons as meta-analysis. Conclusions of that meta-analysis should be interpreted with great caution because of differences in population studied, and differences in primary and co-primary outcomes.
A recent comparison meta-analysis was not conclusive due to the heterogeneity of phase III studies and the few number of studies for some of the investigated drugs (ACL/FF and GLY/FF in particular), but confirmed that dual bronchodilation was always more effective than LABA or LAMA alone, both in lung function and quality of life indices (Calzetta et al., 2016). This meta-analysis suggested that among the different FDCs may exist a gradient of effectiveness, UMEC/VIL slightly improving the effects of GLY/IND (-12 mL), TIO/OLO (-30 mL), and ACL/FF (-49 mL), though statistically significant differences were not observed.
Recently Kerwin et al. (2017) provided data about two cross-over trials comparing efficacy and safety of GLY/IND twice-daily 15.6/27.5 μg versus once-daily UMEC/VIL 62.5/25 μg. Both treatments provided clinically meaningful and comparable bronchodilation. Non-inferiority of GLY/IND to UMEC/VIL could not be declared although between-treatment differences were not clinically relevant (Kerwin et al., 2017).
Kalberg et al. (2016) published data from a 12 weeks’ treatment study with UMEC/VIL 62.5/25 μg that provided similar (non-inferior) improvements in lung function, symptoms, and health status, as well as comparable safety profiles to those achieved with free combinations of a LABA plus LAMA (TIO 18 μg + IND 150 μg).
In order to provide safety and efficacy data to treating physicians, further head-to-head comparative studies applying appropriate non-inferiority limits should be conducted.
Triple Therapy
It is necessary spending few words on the role of adding ICS to the LABA/LAMA combinations in some particular COPD patient categories. The so-called “Triple therapy” has demonstrated to produce benefits in COPD patients in GOLD group D (Singh et al., 2016) FF 6 μg, GLY bromide 12.5 μg, and beclomethasone 100 μg was compared to FF and beclomethasone in reducing exacerbation frequency providing a -12% of exacerbation frequency. Moreover, TRINITY study compared FF 6 μg, GLY 12.5 μg, and beclomethasone 100 μg to TIO and found that the triple combination therapy resulted in a 20% reduction in the rate of moderate-to-severe COPD exacerbations, improvement in pre-dose FEV1 in COPD patients with frequent exacerbations (Vestbo et al., 2017). However, the results of head to head comparison between LAMA/LABA and LAMA/LABA/ICS combinations are still pending. Recently the TRIBUTE study (Papi et al., 2018) compared single-inhaler triple combination of beclometasone, FF, and GLY (87 μg/5 μg/9 μg) 2 inhalations twice per day versus a single-inhaler dual bronchodilator combination of GLY/IND (43 μg/85 μg) one inhalation daily in a group of symptomatic COPD with severe or very severe airflow limitation and at least one moderate or severe exacerbation in the previous year. Authors reported that triple therapy significantly reduced the rate of moderate-to-severe exacerbations compared with GLY/IND, without increasing the risk of pneumonia. Confirming the possible importance of triple treatment in more severe COPD patients. Currently another study is comparing TIO/OLO with ICS/LABA/LAMA triple therapy but results are still waited (Clinical Trials Registry, 2017).
Lipson et al. (2018) recently we compared 52 weeks of a OD combination of FF/UMEC/VIL 100/62.5/25 μg with FF/VIL 100/25 μg and UMEC/VIL 62.5/25 μg. Authors observed that Triple therapy with FF/UMEC/VIL resulted in a lower rate of moderate or severe COPD exacerbations than the other dual combinations in this population. Triple therapy also resulted in a lower rate of hospitalization due to COPD than UMEC/VIL association (Lipson et al., 2018).
Treatment (IMPACT) study will evaluate the efficacy and safety of FF/UMEC/VIL 100/62.5/25 μg versus FF/VIL 100/25 μg or UMEC/VIL 62.5/25 μg, over a 52-week treatment period on COPD D patients. The study was designed to assess not only the value of triple therapy compared to dual therapy, but also the relative comparative advantages of the two dual therapies (ICS/LABA and LABA/LAMA) (Pascoe et al., 2016).
Going ahead, a more personalized approach to therapy is required since we have understood that with the term COPD we define a disease characterized by persistent airways obstruction but at the basis of this has been demonstrated the presence of different phenotypes (Montuschi et al., 2014).
In fact, if we consider asthma COPD overlap syndrome (ACOS), it is clear that identification of these patients will make it possible to get more benefit from the use of ICS compared to patients with other phenotypes. Moreover, the identification of COPD patients with elevated eosinophil count in the sputum may guide the physician in the choice of treatment adding ICS to bronchodilators. These examples allow us to understand the importance of the knowledge of the physio-pathological bases of COPD that will allow in the future identifying treatments more and more tailored beyond the standardized classifications of COPD based on spirometry and frequency of exacerbations.
Conclusion
LAMA/LABA combinations are recommended in COPD with persistent symptoms or with further exacerbations treated with monotherapy. LAMA/LABA combinations have a synergistic effect rather than just being additive one and have been shown to improve lung function, lung hyperinflation, exercise tolerance, exacerbations frequency and quality of life in COPD. However, a number of questions is still pending and under debate. It is now more and important identifying different phenotypes, so future data are warrant to make clear which combination is more favorable to use, in which patient and at which stadium of the disease, tailoring the treatment to be more and more personalized.
Author Contributions
MM and AR conceived the idea of the manuscript. AR, VF, FP, and PP contributed to various parts of the text and wrote the manuscript. MN prepared the figures and bibliography. CP, MM, and AR edited the manuscript. All the authors revised and commented on the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BR declared a shared affiliation, with no collaboration, with the authors MN and AR to the handling Editor at the time of review.
References
Aaron, S. D., Vandemheen, K. L., Fergusson, D., Maltais, F., Bourbeau, J., Goldstein, R., et al. (2007). Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann. Intern. Med. 146, 545–555. doi: 10.7326/0003-4819-146-8-200704170-00152
Agustí, A. (2013). Phenotypes and disease characterization in chronic obstructive pulmonary disease. Toward the extinction of phenotypes?. Ann. Am. Thorac. Soc. 10(Suppl.), S125–S130. doi: 10.1513/AnnalsATS.201303-055AW
Anderson, P. (2006). Use of Respimat Soft Mist inhaler in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 1, 251–259.
Appleton, S., Poole, P., Smith, B., Veale, A., Lasserson, T. J., and Chan, M. M. (2006). Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 19:CD001104. doi: 10.1002/14651858.CD001104.pub2
Barr, R. G., Bourbeau, J., Camargo, C. A., and Ram, F. S. F. (2005). Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 4:CD002876. doi: 10.1002/14651858.CD002876.pub2
Bateman, E. D., Chapman, K. R., Singh, D., D’Urzo, A. D., Molins, E., Leselbaum, A., et al. (2015). Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir. Res. 16:92. doi: 10.1186/s12931-015-0250-2
Bateman, E. D., Ferguson, G. T., Barnes, N., Gallagher, N., Green, Y., Henley, M., et al. (2013). Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur. Respir. J. 42, 1484–1494. doi: 10.1183/09031936.00200212
Beeh, K.-M., Derom, E., Echave-Sustaeta, J., Grönke, L., Hamilton, A., Zhai, D., et al. (2016). The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study). Int. J. Chron. Obstruct. Pulmon. Dis. 11, 193–205. doi: 10.2147/COPD.S95055
Berton, D. C., Reis, M., Siqueira, A. C. B., Barroco, A. C., Takara, L. S., Bravo, D. M., et al. (2010). Effects of tiotropium and formoterol on dynamic hyperinflation and exercise endurance in COPD. Respir. Med. 104, 1288–1296. doi: 10.1016/j.rmed.2010.05.017
Brocklebank, D., Ram, F., Wright, J., Barry, P., Cates, C., Davies, L., et al. (2001). Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol. Assess. 5, 1–149. doi: 10.3310/hta5260
Calverley, P. M. A., Anzueto, A. R., Carter, K., Grönke, L., Hallmann, C., Jenkins, C., et al. (2018). Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir. Med. 6, 337–344. doi: 10.1016/S2213-2600(18)30102-4
Calzetta, L., Rogliani, P., Matera, M. G., and Cazzola, M. (2016). A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 149, 1181–1196. doi: 10.1016/j.chest.2016.02.646
Cazzola, M., Calzetta, L., Ora, J., Puxeddu, E., Rogliani, P., and Matera, M. G. (2015). Searching for the synergistic effect between aclidinium and formoterol: from bench to bedside. Respir. Med. 109, 1305–1311. doi: 10.1016/j.rmed.2015.08.005
Cazzola, M., and Molimard, M. (2010). The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm. Pharmacol. Ther. 23, 257–267. doi: 10.1016/j.pupt.2010.03.003
Celli, B., Crater, G., Kilbride, S., Mehta, R., Tabberer, M., Kalberg, C. J., et al. (2014). Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 145, 981–991. doi: 10.1378/chest.13-1579
Clinical Trials Registry (2017). Assessment in a Real World Setting of the Effect of Inhaled Steroid-Based Triple Therapy Versus the Combination of Tiotropium and Olodaterol on Reducing Chronic Obstructive Pulmonary Disease (COPD) Exacerbations [AIRWISE]. Available at: https://clinicaltrials.gov/ct2/show/NCT03265145?term=NCT03265145&rank=1 (accessed October 10, 2018).
Dal Negro, R. W., and Povero, M. (2016). Acceptability and preference of three inhalation devices assessed by the Handling Questionnaire in asthma and COPD patients. Multidiscip. Respir. Med. 11:7. doi: 10.1186/s40248-016-0044-5
Dalby, R., Spallek, M., and Voshaar, T. (2004). A review of the development of respimat soft mist inhaler. Int. J. Pharm. 283, 1–9. doi: 10.1016/j.ijpharm.2004.06.018
Decramer, M., Anzueto, A., Kerwin, E., Kaelin, T., Richard, N., Crater, G., et al. (2014). Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir. Med. 2, 472–486. doi: 10.1016/S2213-2600(14)70065-7
Decramer, M. L., Chapman, K. R., Dahl, R., Frith, P., Devouassoux, G., Fritscher, C., et al. (2013). Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir. Med. 1, 524–533. doi: 10.1016/S2213-2600(13)70158-9
Derom, E., Brusselle, G. G., and Joos, G. F. (2016). Efficacy of tiotropium-olodaterol fixed-dose combination in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 11, 3163–3177. doi: 10.2147/COPD.S92840
Donohue, J. F., Worsley, S., Zhu, C.-Q., Hardaker, L., and Church, A. (2015). Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir. Med. 109, 870–881. doi: 10.1016/j.rmed.2015.04.018
D’Urzo, A. D., Rennard, S. I., Kerwin, E. M., Mergel, V., Leselbaum, A. R., and Caracta, C. F. (2014). Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir. Res. 15:123. doi: 10.1186/s12931-014-0123-0
Frijlink, H. W., and De Boer, A. H. (2004). Dry powder inhalers for pulmonary drug delivery. Expert Opin. Drug Deliv. 1, 67–86. doi: 10.1517/17425247.1.1.67
Global initiative for chronic obstructive Lung disease [GOLD] (2018). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Available at: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf (accessed November 20, 2018).
Hanania, N. A., Tashkin, D. P., Kerwin, E. M., Donohue, J. F., Denenberg, M., O’Donnell, D. E., et al. (2017). Long-term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co-SuspensionTM delivery technology in patients with chronic obstructive pulmonary disease. Respir. Med. 126, 105–115. doi: 10.1016/j.rmed.2017.03.015
Horita, N., Goto, A., Shibata, Y., Ota, E., Nakashima, K., Nagai, K., et al. (2017). Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2:CD012066. doi: 10.1002/14651858.CD012066.pub2
Ikeda, T., Anisuzzaman, A. S. M., Yoshiki, H., Sasaki, M., Koshiji, T., Uwada, J., et al. (2012). Regional quantification of muscarinic acetylcholine receptors and β-adrenoceptors in human airways. Br. J. Pharmacol. 166, 1804–1814. doi: 10.1111/j.1476-5381.2012.01881.x
Inhaler Error Steering Committee, Price, D., Bosnic-Anticevich, S., Briggs, A., Chrystyn, H., Rand, C., et al. (2013). Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir. Med. 107, 37–46. doi: 10.1016/j.rmed.2012.09.017
Kalberg, C., O’Dell, D., Galkin, D., Newlands, A., and Fahy, W. A. (2016). Dual bronchodilator therapy with umeclidinium/vilanterol versus tiotropium plus indacaterol in chronic obstructive pulmonary disease: a randomized controlled trial. Drugs R. D. 16, 217–227. doi: 10.1007/s40268-016-0131-2
Karner, C., and Cates, C. J. (2012). Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 4:CD008989. doi: 10.1002/14651858.CD008989.pub2
Kerwin, E., Ferguson, G. T., Sanjar, S., Goodin, T., Yadao, A., Fogel, R., et al. (2017). Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung 195, 739–747. doi: 10.1007/s00408-017-0055-9
Komase, Y., Asako, A., Kobayashi, A., and Sharma, R. (2014). Ease-of-use preference for the ELLIPTA® dry powder inhaler over a commonly used single-dose capsule dry powder inhaler by inhalation device-naïve Japanese volunteers aged 40 years or older. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 1365–1375. doi: 10.2147/COPD.S72762
Lal, C., and Strange, C. (2017). A review of current and developing fixed-dose LABA/LAMA combinations for treating COPD. Expert Opin. Pharmacother. 18, 1833–1843. doi: 10.1080/14656566.2017.1403583
Lange, P., Marott, J. L., Vestbo, J., Olsen, K. R., Ingebrigtsen, T. S., Dahl, M., et al. (2012). Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am. J. Respir. Crit. Care Med. 186, 975–981. doi: 10.1164/rccm.201207-1299OC
Lavorini, F. (2013). The challenge of delivering therapeutic aerosols to asthma patients. ISRN Allergy 2013:102418. doi: 10.1155/2013/102418
Lavorini, F., Magnan, A., Dubus, J. C., Voshaar, T., Corbetta, L., Broeders, M., et al. (2008). Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir. Med. 102, 593–604. doi: 10.1016/j.rmed.2007.11.003
Lexmond, A. J., Kruizinga, T. J., Hagedoorn, P., Rottier, B. L., Frijlink, H. W., and de Boer, A. H. (2014). Effect of inhaler design variables on paediatric use of dry powder inhalers. PLoS One 9:e99304. doi: 10.1371/journal.pone.0099304
Lipson, D. A., Barnhart, F., Brealey, N., Brooks, J., Criner, G. J., Day, N. C., et al. (2018). Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med. 378, 1671–1680. doi: 10.1056/NEJMoa1713901
Mahler, D. A., D’Urzo, A., Bateman, E. D., Özkan, S. A., White, T., Peckitt, C., et al. (2012). Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax 67, 781–788.
Mahler, D. A., Kerwin, E., Ayers, T., FowlerTaylor, A., Maitra, S., Thach, C., et al. (2015). FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (Indacaterol/Glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 192, 1068–1079. doi: 10.1164/rccm.201505-1048OC
Maleki-Yazdi, M. R., Kaelin, T., Richard, N., Zvarich, M., and Church, A. (2014). Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir. Med. 108, 1752–1760. doi: 10.1016/j.rmed.2014.10.002
Malerba, M., Radaeli, A., Montuschi, P., and Morjaria, J. B. (2016). Vilanterol trifenatate for the treatment of COPD. Expert Rev. Respir. Med. 10, 719–731. doi: 10.1080/17476348.2016.1184976
Man, K. N., Tian, Z., Lam, D. C.-L., Wan, J. M. F., and Tan-Un, K. C. (2018). Satisfaction, preference and error occurrence of three dry powder inhalers as assessed by a cohort naïve to inhaler operation. Int. J. Chron. Obstruct. Pulmon. Dis. 13, 1949–1963. doi: 10.2147/COPD.S152285
Martinez, F. J., Fabbri, L. M., Ferguson, G. T., Orevillo, C., Darken, P., Martin, U. J., et al. (2017a). Baseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPD. Chest 152, 1169–1178. doi: 10.1016/j.chest.2017.07.007
Martinez, F. J., Rabe, K. F., Ferguson, G. T., Fabbri, L. M., Rennard, S., Feldman, G. J., et al. (2017b). Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest 151, 340–357. doi: 10.1016/j.chest.2016.11.028
Miravitlles, M., Anzueto, A., and Jardim, J. R. (2017). Optimizing bronchodilation in the prevention of COPD exacerbations. Respir. Res. 18:125. doi: 10.1186/s12931-017-0601-2
Montuschi, P., and Ciabattoni, G. (2015). Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. J. Med. Chem. 58, 4131–4164. doi: 10.1021/jm5013227
Montuschi, P., Malerba, M., Macis, G., Mores, N., and Santini, G. (2016). Triple inhaled therapy for chronic obstructive pulmonary disease. Drug Discov. Today 21, 1820–1827. doi: 10.1016/j.drudis.2016.07.009
Montuschi, P., Malerba, M., Santini, G., and Miravitlles, M. (2014). Pharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotyping. Drug Discov. Today 19, 1928–1935. doi: 10.1016/j.drudis.2014.08.004
Newman, S. P., Brown, J., Steed, K. P., Reader, S. J., and Kladders, H. (1998). Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest 113, 957–963.
Newman, S. P., Steed, K. P., Reader, S. J., Hooper, G., and Zierenberg, B. (1996). Efficient delivery to the lungs of flunisolide aerosol from a new portable hand-held multidose nebulizer. J. Pharm. Sci. 85, 960–964. doi: 10.1021/js950522q
Papi, A., Vestbo, J., Fabbri, L., Corradi, M., Prunier, H., Cohuet, G., et al. (2018). Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet 391, 1076–1084. doi: 10.1016/S0140-6736(18)30206-X
Pascoe, S. J., Lipson, D. A., Locantore, N., Barnacle, H., Brealey, N., Mohindra, R., et al. (2016). A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur. Respir. J. 48, 320–330. doi: 10.1183/13993003.02165-2015
Pedersen, S., Dubus, J. C., Crompton, G. K., and ADMIT Working Group (2010). The ADMIT series–issues in inhalation therapy. 5) Inhaler selection in children with asthma. Prim. Care Respir. J. 19, 209–216. doi: 10.4104/pcrj.2010.00043
Pera, T., and Penn, R. B. (2014). Crosstalk between beta-2-adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr. Opin. Pharmacol. 16, 72–81. doi: 10.1016/j.coph.2014.03.005
Pitcairn, G., Reader, S., Pavia, D., and Newman, S. (2005). Deposition of corticosteroid aerosol in the human lung by respimat soft mist inhaler compared to deposition by metered dose inhaler or by turbuhaler dry powder inhaler. J. Aerosol. Med. 18, 264–272. doi: 10.1089/jam.2005.18.264
Rajan, S. K., and Gogtay, J. A. (2014). Ease-of-use, preference, confidence, and satisfaction with Revolizer(®), a novel dry powder inhaler, in an Indian population. Lung India 31, 366–374. doi: 10.4103/0970-2113.142122
Rodrigo, G. J., Price, D., Anzueto, A., Singh, D., Altman, P., Bader, G., et al. (2017). LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 907–922. doi: 10.2147/COPD.S130482
Salvi, S., Brashier, B., Gothi, D., Karkhanis, V., Madas, S., Gogtay, J., et al. (2014). Bronchodilator efficacy of tiotropium-formoterol via single pressurized meter dose inhaler (pMDI) versus tiotropium alone in COPD. Pulm. Pharmacol. Ther. 27, 90–95. doi: 10.1016/j.pupt.2013.05.010
Singh, D., Jones, P. W., Bateman, E. D., Korn, S., Serra, C., Molins, E., et al. (2014). Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm. Med. 14:178. doi: 10.1186/1471-2466-14-178
Singh, D., Papi, A., Corradi, M., Pavlišová, I., Montagna, I., Francisco, C., et al. (2016). Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 388, 963–973. doi: 10.1016/S0140-6736(16)31354-X
Singh, D., Worsley, S., Zhu, C.-Q., Hardaker, L., and Church, A. (2015). Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm. Med. 15:91. doi: 10.1186/s12890-015-0092-1
Soriano, J. B., Lamprecht, B., Ramírez, A. S., Martinez-Camblor, P., Kaiser, B., Alfageme, I., et al. (2015). Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet. Respir. Med. 3, 443–450. doi: 10.1016/S2213-2600(15)00157-5
Steed, K., Towse, L., Freund, B., and Newman, S. (1997). Lung and oropharyngeal depositions of fenoterol hydrobromide delivered from the prototype III hand-held multidose Respimat nebuliser. Eur. J. Pharm. Sci. 5, 55–61. doi: 10.1016/S0928-0987(96)00016-4
Svedsater, H., Dale, P., Garrill, K., Walker, R., and Woepse, M. W. (2013). Qualitative assessment of attributes and ease of use of the ELLIPTATM dry powder inhaler for delivery of maintenance therapy for asthma and COPD. BMC Pulm. Med. 13:72. doi: 10.1186/1471-2466-13-72
Tashkin, D. P., and Ferguson, G. T. (2013). Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir. Res. 14:49. doi: 10.1186/1465-9921-14-49
Tashkin, D. P., Pearle, J., Iezzoni, D., and Varghese, S. T. (2009). Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD 6, 17–25. doi: 10.1080/15412550902724073
van der Palen, J., Ginko, T., Kroker, A., van der Valk, P., Goosens, M., Padullés, L., et al. (2013). Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin. Drug Deliv. 10, 1023–1031. doi: 10.1517/17425247.2013.808186
van Noord, J. A., Aumann, J.-L., Janssens, E., Smeets, J. J., Verhaert, J., Disse, B., et al. (2005). Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur. Respir. J. 26, 214–222. doi: 10.1183/09031936.05.00140404
Vanfleteren, L. E., Spruit, M. A., Groenen, M., Gaffron, S., van Empel, V. P. M., Bruijnzeel, P. L. B., et al. (2013). Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 728–735. doi: 10.1164/rccm.201209-1665OC
Vestbo, J., Papi, A., Corradi, M., Blazhko, V., Montagna, I., Francisco, C., et al. (2017). Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 389, 1919–1929. doi: 10.1016/S0140-6736(17)30188-5
Vogelmeier, C., Hederer, B., Glaab, T., Schmidt, H., Rutten-van Mölken, M. P., Beeh, K. M., et al. (2011). Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N. Engl. J. Med. 364, 1093–1103. doi: 10.1056/NEJMoa1008378
Vogelmeier, C., Kardos, P., Harari, S., Gans, S. J. M., Stenglein, S., and Thirlwell, J. (2008). Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir. Med. 102, 1511–1520. doi: 10.1016/j.rmed.2008.07.020
Vogelmeier, C. F., Bateman, E. D., Pallante, J., Alagappan, V. K. T., D’Andrea, P., Chen, H., et al. (2013). Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet. Respir. Med. 1, 51–60. doi: 10.1016/S2213-2600(12)70052-8
Wedzicha, J. A., Banerji, D., Chapman, K. R., Vestbo, J., Roche, N., Ayers, R. T., et al. (2016). Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N. Engl. J. Med. 374, 2222–2234. doi: 10.1056/NEJMoa1516385
Wedzicha, J. A., Decramer, M., Ficker, J. H., Niewoehner, D. E., Sandström, T., Taylor, A. F., et al. (2013). Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet. Respir. Med. 1, 199–209. doi: 10.1016/S2213-2600(13)70052-3
Zhong, N., Wang, C., Zhou, X., Zhang, N., Humphries, M., Wang, L., et al. (2015). lanTern: a randomized study of QVa149 versus salmeterol/fluticasone combination in patients with COPD background: the current Global initiative for chronic obstructive lung disease (GOLD). Int. J. COPD 10, 1015–1026. doi: 10.2147/COPD.S84436
Keywords: chronic obstructive pulmonary disease (COPD), LABA, LAMA, maintenance treatment, fixed dose combination (FDC)
Citation: Malerba M, Foci V, Patrucco F, Pochetti P, Nardin M, Pelaia C and Radaeli A (2019) Single Inhaler LABA/LAMA for COPD. Front. Pharmacol. 10:390. doi: 10.3389/fphar.2019.00390
Received: 18 October 2018; Accepted: 29 March 2019;
Published: 25 April 2019.
Edited by:
Paolo Montuschi, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Beatrice Ragnoli, Azienda Socio Sanitaria Territoriale of the Spedali Civili of Brescia, ItalyTimothy E. Albertson, UC Davis Medical Center, United States
Copyright © 2019 Malerba, Foci, Patrucco, Pochetti, Nardin, Pelaia and Radaeli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Malerba, bWFyaW8ubWFsZXJiYUB1bml1cG8uaXQ=
 Mario Malerba
Mario Malerba Valentina Foci
Valentina Foci Filippo Patrucco
Filippo Patrucco Patrizia Pochetti
Patrizia Pochetti Matteo Nardin3
Matteo Nardin3 Corrado Pelaia
Corrado Pelaia Alessandro Radaeli
Alessandro Radaeli