- Liaoning Provincial Key Laboratory of Carbohydrates, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China
Colon cancer (CC) is the third common neoplasm worldwide, and it is still a big challenge for exploring new effective medicine for treating CC. Natural product promoting human health has become a hot topic and attracted many researchers recently. Pectin, a complex polysaccharide in plant cell wall, mainly consists of four major types of polysaccharides: homogalacturonan, xylogalacturonan, rhamnogalacturonan I and II, all of which can be degraded into various pectin oligosaccharides (POS) and may provide abundant resource for exploring potential anticancer drugs. POS have been regarded as a novel class of potential functional food with multiple health-promoting properties. POS have antibacterial activities against some aggressive and recurrent bacterial infection and exert beneficial immunomodulation for controlling CC risk. However, the molecular functional role of POS in the prevention of CC risk and progression remains doubtful. The review focuses on antioxidant and anti-inflammatory roles of POS for promoting human health by regulating some potential oxidative and inflammation-activated pathways, such as ATP-activated protein kinase (AMPK), nuclear factor erythroid-2-related factor-2 (Nrf2), and nuclear factor-κB (NF-κB) pathways. The activation of these signaling pathways increases the antioxidant and antiinflammatory activities, which will result in the apoptosis of CC cells or in the prevention of CC risk and progression. Thus, POS may inhibit CC development by affecting antioxidant and antiinflammatory signaling pathways AMPK, Nrf2, and NF-κB. However, POS also can activate signal transduction and transcriptional activator 1 and 3 signaling pathway, which will reduce antioxidant and anti-inflammatory properties and promote CC progression. Specific structural and structurally modified POS may be associated with their functions and should be deeply explored in the future. The present review paper lacks the important information for the linkage between the specific structure of POS and its function. To further explore the effects of prebiotic potential of POS and their derivatives on human immunomodulation in the prevention of CC, the specific POS with a certain degree of polymerization or purified polymers are highly demanded to be performed in clinical practice.
Introduction
Colon cancer (CC) is one of the third common cancers with more than 600,000 deaths worldwide yearly and causes a global burden (1). Chemotherapy and radiation therapy are the main treatments of CC with significant side effects. A dietary prebiotic improves glycemic indices, lipid profile (2, 3), antioxidant status (4), potential immunomodulatory benefits (5), and reduces cardiovascular disease risk (6). The common prebiotics are oligosaccharides while oligosaccharides are indigestible and pass through digestive tracts smoothly. The oligosaccharides produced in digestive tracts will promote the production of volatile fatty acids, which can release constipation, reduce serum blood glucose, improve mineral absorption and lipid metabolism, prevent colonic cancer, inhibit pathogen adhesion, and modulate immune activity. Pectin oligosaccharides (POS) belong to new potential prebiotics with various health-promoting effects (7, 8), such as against Shiga toxins (9) and pathogen binding (10), induction of apoptosis of human colonic adenocarcinoma cells (11), immunomodulation (12, 13), and cardiovascular protection (14). Long-term pectin consumption has been found to suppress weight gain and reduce obesity risk in an animal obesity model (15). Pectin is an efficient medication to repair wounds and an effective prophylaxis during surgery with antibacterial activities (16). POS exert antioxidant, anti-inflammatory, and antinociceptive effects. Grapefruit pectin (Citrus paradisi) can improve lipid profiles (17). In addition, POS are safe and non-mutagenic, and can be used in children food (18, 19).
Pectin oligosaccharides can stimulate apoptosis process in human colonic adenocarcinoma cells, show protective functions for cardiovascular tissues, reduce the damage caused by metals, and have anti-obesity effects, antitoxic, antibacterial, and antioxidant activities (20). Sweet potato pectin possesses anticancer activity and induces the apoptosis of CC cells and may be a cancer therapeutic drug (21). The pectin derivative with maleoyl groups also shows antitumor properties for CC (22).
Pectin oligosaccharides have also been used to treat gastrointestinal disorders (23), diabetes (24), and hypercholesterolemia (25). Specifically, POS consumption can increase probiotic flora in gastrointestinal tract, such as Lactobacillus Eubacterium, Faecalibacterium, and Roseburia (26). Similarly, POS increase bifidobacteria population but no change in Clostridium (27). Arabinose oligosaccharides can be selectively used by B. adolescentis, B. longum, B. vulgatus, and Lactobacillus (28). POS promote the growth of bifidobacteria in all population from younger adults to the elders, and increase their immunomodulatory capacity (29) while the increase of immunomodulation further promotes the apoptosis of CC (30).
Pectin oligosaccharides exert its antioxidant properties by significantly increasing the levels of antioxidant biomarkers while reducing oxidative biomarkers (31). The redox system may be regulated by POS (Figure 1). POS (as bioactive components of pectin) normalize the activity of glutathione reductase (GR) and glutathione peroxidase (GPx) (32), whereas GR catalyzes GSSG into reduced glutathione (GSH). GPx catalyzes H2O2 into H2O under the help from GSH. Furthermore, catalase (CAT) can be induced by POS (33) whereas CAT reduces H2O2 into H2O. POS also increase glutathione-S-transferase (GST) activity (31), while GST promotes the generation of plasma-reduced CysGly during GSH catabolism.
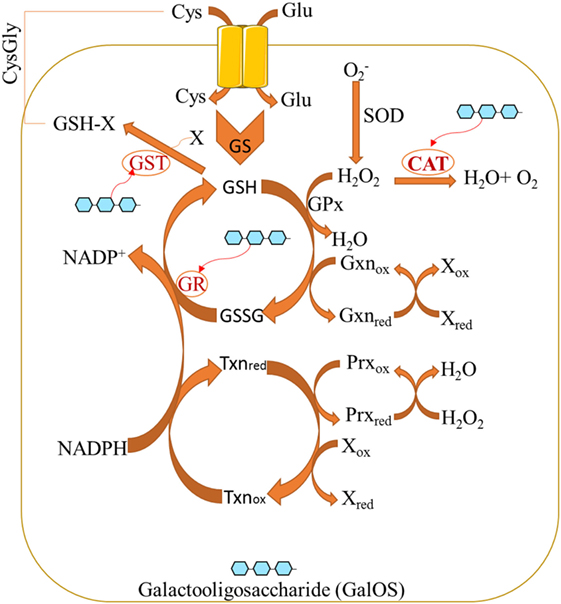
Figure 1. Pectin oligosaccharides regulate cellular antioxidant activities by affecting oxidative stress biomarkers.
The POS homogalacturonan (HG), isolated from green tea, shows phagocytosis-enhancing activity in HL-60 cells (34). Meanwhile, POS will increase natural killer bioactivity and the levels of anti-inflammatory cytokines (35) and reduce the levels of pro-inflammatory cytokines (Figure 2). POS can be developed as a beneficial dietary candidate for promoting gastrointestinal health and immune activities. Antioxidant and anti-inflammatory activities of functional foods will be beneficial in the prevention of the risk of colon carcinoma (36, 37). Nevertheless, the molecular mechanisms for POS function in human health remain doubtful. This work provides a new window for the possible effects of POS on antioxidant and anti-inflammatory signaling pathways.
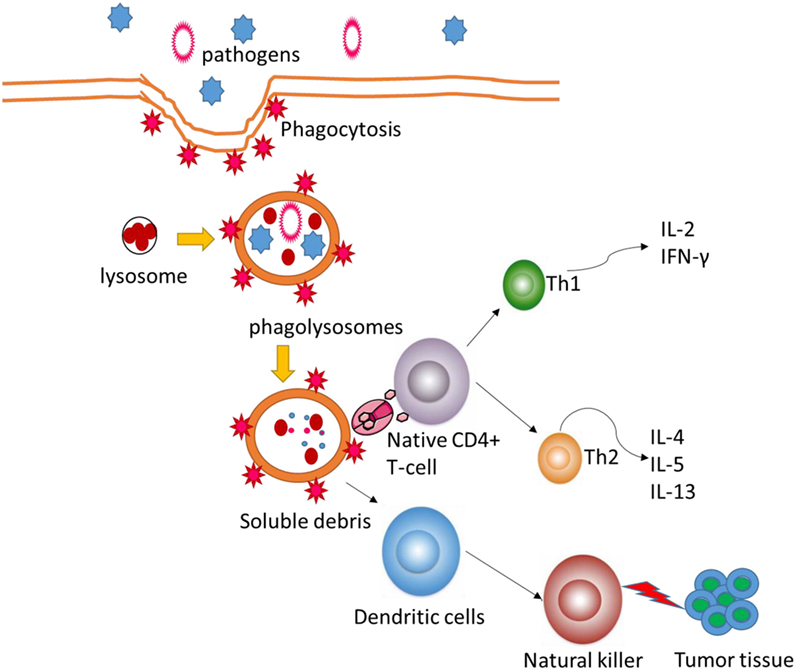
Figure 2. Pectin oligosaccharides regulate cellular autophagous activities by affecting natural killer.
POS Preparation
Pectin As a Source of POS
Pectin is the source of POS in natural products and mainly exists in citrus peel [it mainly consists of a homopolymer of 1–34-linked os-d-galactosyluronic acid with 85.7% methylated esterification and a rhamnogalacturonan I (RG-I) fragment] (38), sugar beet pulp (a high degree of acetylation and a relatively high neutral sugar content) (39), potato pulp (it has highly branched RG-I domain) (21, 40), and additional sources, etc. Pectin consists of fundamental units of α (1–4)-galacturonic acid, which is often acetylated and/or methylated. Figure 3 shows the complex structure of pectin, consisting of HG, a polymer with free or esterified carboxyl group; rough regions consists of RG-I with some units of rhamnose and galacturonic acid; and rhamnogalacturonan II (RG-II) with galacturonic acid units and multiple modification. All these regions can be degraded into POS. Various POS can be produced from pectin via de-polymerization (Figure 3).
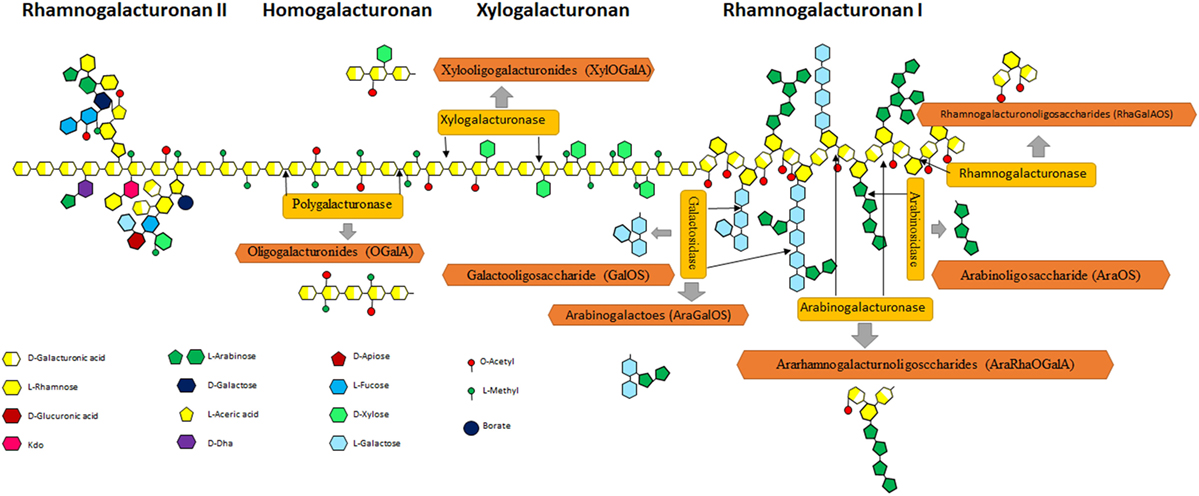
Figure 3. Schematic representation of pectin structure. Pectin consists of rhamnogalacturonan I (RG-I), homogalacturonan (HG), xylogalacturonan, and rhamnogalacturonan II regions. HG is a linear polymer consisting of a chain with an estimated length of 72–100 GalA units that represent, approximately, 60% of the total pectin (41). Xylogalacturonan is a chain of GalA residues partially substituted by d-xylose residues connected by β-(1,3) links at C-3 and/or C-2 positions. RG-I represents up to 7–14% of pectin and contains alternating units of α-(1,4)-galacturonosyl and α-(1,2)-rhamnosyl (42). In many cases, rhamnose residues show side chains as substituents on the O-4 position, made up of arabinan and/or arabinogalactan I and II, although xylose or glucose modification also exists (43). Rhamnogalacturonan II (RG-II) is a region characterized by a length of 7–9 GalA units, where complex branches made up of 12 types of monosaccharides (as a maximum) can exist, including some minority monomers such as apiose, fucose, acetic acid, DHA, or KDO (44).
POS Purification
Pectin oligosaccharides, as oligosaccharides, are often prepared by partial hydrolysis of pectin, which consists of complex heteropolysaccharides. There are three main methods for POS production, including bioenzymatic digestion (45), acid hydrolysis (46) or hydrothermal treatments, and high-pressure microfluidization (47). Many raw materials can be treated to obtain POS including orange, lemon, apple, beet pulp, and so on by using acids. There are some disadvantages for the chemical method: environmental contamination, simple products, and general toxicity. As an alternative, pectin can be degraded into peptic polymers by pectin enzymes. Although pectin has complex structures, which can be digested by a series of pectin enzymes, including hydrolases, lyase, and esterase (48–50). Since one enzyme generally targets only specific structure, and more defined oligosaccharides can be released when compared with chemical method. Finally, high-pressure microfluidization has been considered as a new method but most POS cannot be obtained by only using the physical techniques.
After production, purification processes are necessary to obtain food-grade final products. Membrane filtration is often used to purify specific POS. Diafiltration has been used to purify POS from the hydrolysis from lemon peel wastes and yields of target POS can reach 98 wt% of oligogalacturonides (2–18 DP) and AraOS (2–8 DP) (51). The similar work has been reported to achieve a refined POS with AraOS (3–21DP), GalOS (5–12 DP), and OGalA (2–12 DP) (52). Ultrafiltration and diafiltration have also been used to isolate AraOS, which can be further purified into specific POS by using a membrane with 1-kDa molecular weight cut-off (53). On the other hand, pectin can fulfill its function via its degraded products POS since pectin cannot be dissolved in water. In that case, POS are sometimes used to stand for pectin in subsequent introduction.
POS Affect Mitogen-Activated Protein Kinases (MAPK) Signaling Pathway
The MAPK signaling pathway plays an important role in most immune responses (54, 55). Downregulation of MAPK signaling pathway can inhibit the proliferation, invasion, and angiogenesis of CC (56), and promotes the apoptosis of CC (57). Larch Arabinogalactan (a kind of POS) has been reported to inhibit p38 phosphorylation in MAPK pathways (58). Thus, POS may prevent the risk or progression of CC by suppressing MAPK signaling pathway. However, there are still inverse reports for the effects of POS on MAPK/EKR signaling pathway. Mammalian cells respond to various extracellular stimuli by activating MAPK/extracellular signal-regulated kinase (ERK) signaling pathway. Typically, ERK activates phosphorylation events, which stimulate Ras gene after activating growth factor receptor. The activation of Rapidly Accelerated Fibrosarcoma (Raf) phosphorylates ERK. Some targets of ERK have been identified, such as p90RSK activation via Ser380 phosphorylation (59) (Figure 4). POS promotes the phosphorylation of ERK (60) and may also activate the phosphorylation of Raf, MEK, and p90RSK (Figure 4). Thus, POS may bind the receptor systems that activate Raf, MEK, and ERK since POS cannot transport across plasma membrane. ERK signaling pathways can be activated by POS, suggesting that there is an oligosaccharide receptor that transfers the information to the activated molecules (Figure 4). The final genetic identification of all components of the POS signals remains to be determined. Several evidence suggests that p90RSK is activated by MAPK (61). The activation of MAPK signaling pathway will increase antioxidant activities (62) properties. Furthermore, increasing antioxidant activity and activating MAPK signaling will result in the apoptosis of CC cells (63).
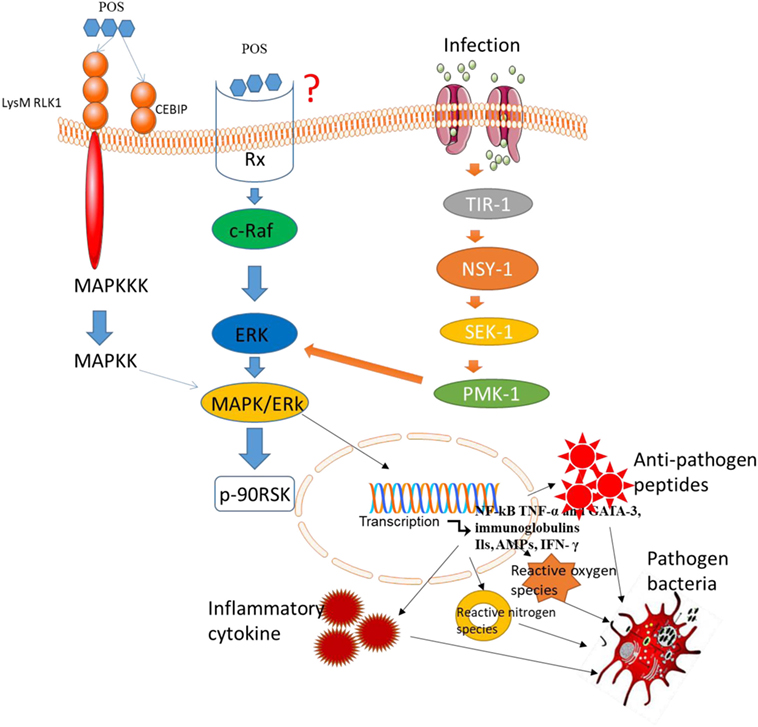
Figure 4. Pectin oligosaccharides binds potential membrane receptors in mitogen-activated protein kinases/ERK signaling pathway. LysM RLK1, chitin elicitor-binding protein, and RX are potential receptors in the pathway.
The lysin motif receptor-like kinase is necessary in the activation of chitin-induced signals (64). Furthermore, chitin elicitor-binding protein (CEBiP) has a LysM domain and is also a surface receptor for plant chitin (65). LysM domain-containing protein pectate lyase (66) suggests that POS has high affinity with LysM domain. Thus, LysM RLK1 and CEBiP may be potential receptors of POS (Figure 4). In general, POS binds potential membrane receptors and activates MAP3K, which activates MAP2K, resulting in the activation of MAPK, which can activate related transcription factors. Besides of these receptors, POS may interact with many membrane receptors. Capsaicin represents an important class of surface receptors (67, 68). Therefore, they cast light on how the cells regulate biological events.
POS Regulate STAT 1 and 3 Signaling via Leptin Receptor
Signal transduction and transcriptional activator 1 (STAT1) is encoded by STAT1 gene in human being. Specific expression of STAT1 can be mediated by some cytokines, such as IFN-α (69, 70), IFN-γ (71, 72), or IL-6 (73, 74). IFN-α binds receptor and triggers STAT signal via its phosphorylation and activation of STAT1 and STAT2. STAT binds ISGF3G/IRF-9 and forms a complex, which stimulates IFN-3 and IFN-9. STAT1 plays a key role in gene expression, cell survival, viability, or response to pathogens. In response to IFN-γ stimulation, STATl forms a homodimer or heterodimer with STAT3. The activation of STAT1 will improve the antitumor capability for CC (75). STAT1 deletion will change the interactions between tumor and fibroblast cells and contribute to CC progression, suggesting that STAT1 is an important link between intestinal inflammation and CC (76). In contrast, the activation of STAT3 signaling pathway regulates the pathogenesis of colon tumor (77).
Oxidative Stress and Inflammation Activates STAT 1/3 Pathways
STAT 1/3 signaling pathways participates in cellular responses to cytokines or growth factors. ROS activates STAT 1/3 pathways in the exterior membranes of basilar blood vessels (78). This pathway can cause morphological varies of the wall of blood vessels in brainy vasospasm (79). Oxidative stress is closely associated with the cell apoptosis and induces STAT activation (80). STAT1 and STAT3 inhibitors suppress TLR-induced TNF expression (81). Viral replication and inflammation are associated with STAT pathway. The result suggests that activation of STAT 1 and 3 signaling pathway will develop inflammation via the increase in IFN level. The inactivation of the STAT pathway can improve anti-inflammatory activities (82).
POS Regulate STAT-1 and -3 Signaling Pathways and Anti-Inflammatory Cytokine Secretion
Pectin oligosaccharides promotes the expression of cardiotropin-1, which upregulates JAK and STAT pathway (14) and delivers the signals to cardiomyocytes, resulting in transcriptional, differentiating, and immune activity (Figure 5) (14). PKC is activated by a variety of agonists, including biological macrophage chemokines (83) and modulates a variety of allogeneic megakaryocytes (84). Pectin consumption will induce the expression of PKC (85), which promotes STAT1 phosphorylation (86). Thus, POS may modulate STAT1 activation and also depends on PKC (Figure 5).
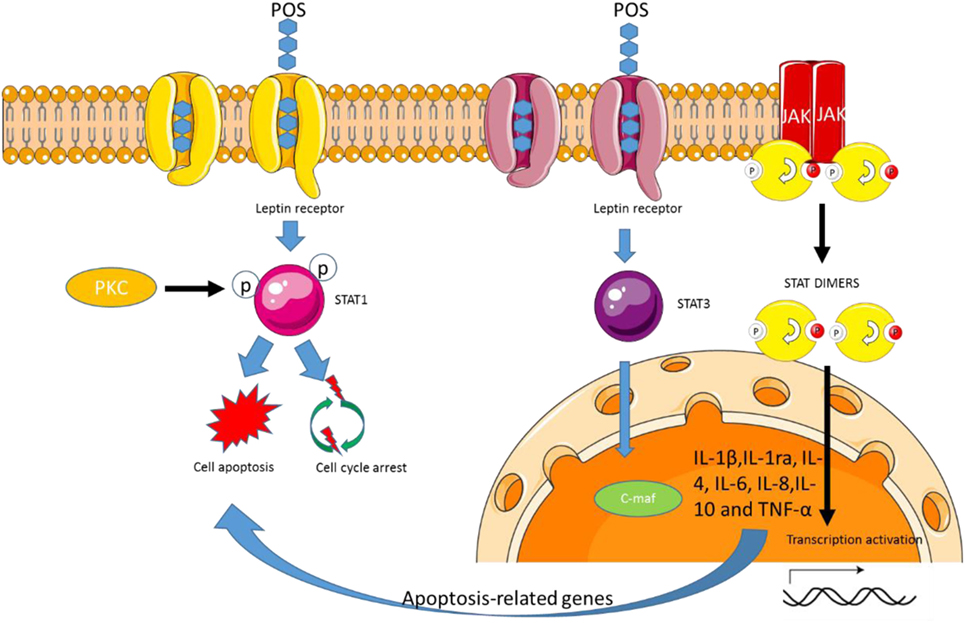
Figure 5. Pectin oligosaccharides (POS) regulates STAT 1 and 3 signaling pathway by the leptin receptor. POS-binding leptin receptor forms signal transduction and transcriptional activator 1 or STAT3 complex, which induces cell apoptosis or cell survival.
Pectin can regulate biological activities via the interaction with immune cells. Pectin treatment increases TNF-α, IL-1β, and IL-10 cytokines (Figure 5) (87). Further work showed that the degree of methyl esterification, molecular size, and the characteristics of pectin structure were closely associated with the regulation of cytokine. These data suggest that POS variety will affect macrophages releasing chemokines. On the other hand, all the cytokines can be secreted by activating STAT signaling pathway (88). All the cytokines can be inhibited by preventing the activity of STAT pathway in macrophages (89). Thus, POS may affect the release of cytokines by regulating STAT signaling pathway (Figure 5).
Pectin oligosaccharides treatment promotes IL-1ra and IL-10 secretion (90), which may be beneficial to cartilage reparation. IL-1ra can inhibit the activity of IL-1β, whereas IL-1β overexpression is associated with osteoarthritis progression (91). Thus, the release of IL-1ra by POS-stimulated may help to protect the synthetic metabolic environment of the natural cartilage during bone cartilage repair. POS activating STAT-1 and -3 signaling pathways will not be beneficial to CC control while the increase of anti-inflammatory cytokines will result in the prevention of CC (37, 92).
The Binding Between POS and Leptin Receptor
Pectin oligosaccharides has been regarded to have anti-obesity activities (15, 93). POS consumption increases leptin levels in adipose cells when compared to those without the treatment (P < 0.05). POS exerts anti-obesity properties via regulating appetite and satiety signals (94). An earlier report shows that POS can significantly decrease lipid accumulation by affecting lipid metabolism (95). POS from Hawthorn can reduce the concentrations of peroxisome proliferator-activated receptor γ, an important adipogenic regulating element (96). The POS tends to enhance TC level and to decrease sterol regulatory element-binding protein 2 and LDL receptor, suggesting that POS can be developed as a kind of functional food in improving lipid metabolism. Long-term pectin consumption can remarkably reduce lipid contents and decrease insulin and leptin resistance (97). Pectin diets can also reduce plasma leptin significantly by more than 60% in an obesity animal model (98). Leptin receptor (OB-R) can induce cardiac disorders (99) and also is linked with obesity development, which leads to obesity risk (100–102). Therefore, POS may affect these molecules by binding OB-R (Figure 5). Leptin regulates weight hemostasis (103, 104), reproduction (105), and possible hematopoiesis (106). Leptin receptor (OB-R) is produced in some alternating chunks of rodents (107) and humans (108). The activated JAK tyrosine kinase binds to ligands for rapid phosphorylation of STATS via the cytokine family of receptors (109). Gene transcription can be initiated by activating STATS homologous or heterologous fusion and migration to nuclear-binding STAT response elements such as GAS (IFN-gamma activation site). POS binding OB-R promotes the complex formation of STAT-1/3 (Figure 5). The low-level OB-R can activate STAT signal transduction pathway.
POS Regulates Nuclear Factor-Kappa B (NF-κB) Pathway via Toll-Like Receptor
POS Prevents Colonic Inflammation
The relationship between chronic intestinal inflammation and cancer has been widely reported (110, 111). The effect of POS on oral administration of colitis has been assessed by weight loss (112), disease activity index (DAI) (113), and bloody diarrhea events (114). DAI is associated with fecal consistency, fecal occult blood and weight loss. POS treatment significantly inhibits dextran sulfate sodium (DSS)-induced DAI (115). In addition, colon size is inversely proportional to the severity of DSS-induced colitis. These data indicate that POS can reduce intestinal inflammation in colitis mice. However, the related molecular mechanism remains widely unknown.
NF-κB Signaling Pathway Is Involved With Inflammatory and CC
Nuclear factor-kappa B regulates DNA transcription, cytokine generation, and cellular life activities. NF-κB is existed in most animal cells types and involves the responses to cytokines, ROS, bacterial and viral antigens (116). Regulation of NF-κB is closely associated with CC (117, 118), inflammation and autoimmune disorders (119), septic shock, viral infection, and dysfunctional immunological progression (120). NF-κB can be affected by cellular antioxidant activities. The ratio of GSSG/GSH can strongly affect NF-κB pathway (121). NF-κB is linked with diabetic neuropathy and promulgation of inflammatory activity (122). The signaling pathway has protective functions for neuroinflammation and oxidative stress. NF-κB can affect brain edema and infarct volume, and its expression will result in inflammatory response after cerebral ischemia–reperfusion (123).
POS Regulates NF-κB/TLR4/COX-2 Signaling Cascade
Ulcerative colitis (UC) is one common inflammatory bowel disorder and has high morbidity and prevalence throughout the world. UC is the main risk factor inducing CC (124). In UC patients, CC risk is higher than the average population (125, 126). The main feature of UC is the uncontrolled inflammation of the colon, causing acute abdominal pain, severe diarrhea, bloody stools, and reduced symptoms. The initiation and maintenance of colonic inflammation is characterized by the transmembrane invasion of leukocytes in the mucosa, the overproduction of inflammatory cytokines, etc., which are necessary for subsequent mucosal rupture and ulcers and involve in UC development, particularly in the early stages of disease (127, 128). Thus, UC therapy is mainly dependent on the drugs, which can inhibit colon inflammation and control symptoms.
However, conventional anti-inflammatory drug compounds generally have undesirable side effects, which may reduce patient compliance and degrade the condition. 5-aminosalicylic acid compounds and salazosulfa pyridine is considered first-line therapy for active UC therapy. However, side effects including abdominal pain, fever, diarrhea, cramps, rashes, and kidney failure limit their use. The lack of satisfactory treatment of UC has contributed to the study of alternative treatment strategies. Anti-inflammatory natural products or functional food from supplemental or alternative medicine represent a new class of drugs that are promising to UC therapy. Previous studies in vitro have found that POS can significantly and reliably attenuate lipopolysaccharide-induced inflammatory responses (129), demonstrating the potential medical utility of POS in controlling bowel disorder (130). The effect of oral POS on the prevention of inflammation has been proved, which shows a decrease in histological damage score and colonic PGE2 content in the mice with UC model and further confirmed the potential of POS for colitis therapy.
Apple POS has been proved to be effective to treat inflammatory and cancer diseases by affecting LPS/TLR4/NF-κB pathway (131). POS exerts beneficial effects on clinical colitis and carcinogenesis. Apple POS exhibit higher antibacterial effects on some pathogens than citrus POS (132). Staphylococcus has been reported to be isolated from the blood of the patients with cardiac disorder (133). The lipopolysaccharide derived from Escherichia coli and Pseudomonas aeruginosa induces cardiovascular damage (134). Apple POS prevent colon carcinogenesis that may partially depend on prostaglandin E, and POS types, which are associated with fecal enzyme function.
Apple POS can modulate inflammatory activities by affecting NF-κB pathway (131). Normally, NF-κB forms a p65-p50 dimer, which enters into the nucleus and binds specific DNA sequence, and inhibits target gene expression. POS may inactivate NF-κB and affects the level of its downstream genes [cyclin D1 (135), TNF-α (136), and IL-6 (115)] have been tested in NF-κB signaling pathway. Some data show that POS are the most potent activators of NF-κB signaling (Figure 6) (137), whereas the activation of NF-κB signaling pathway will promote CC apoptosis (138).
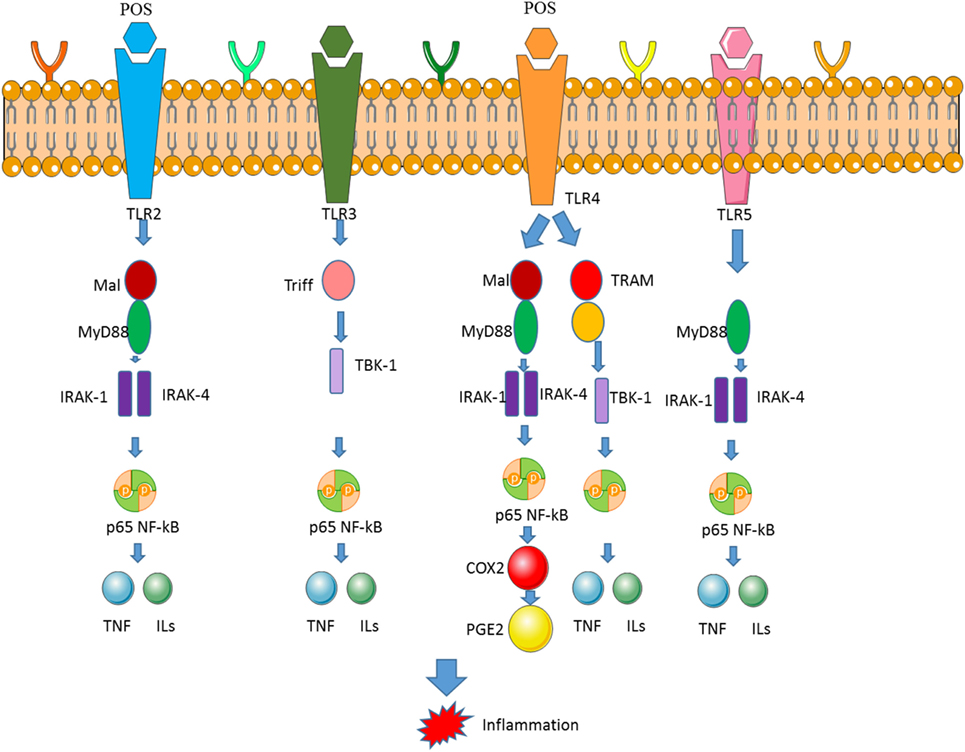
Figure 6. Pectin oligosaccharides (POS) downregulates the expression of nuclear factor-kappa B (NF-κB) and COX-2 by binding toll-like receptor 4 (TLR4). The binding between TLR4 and POS activates NF-κB and COX-2 signaling pathway, which is associated with inflammatory activities.
POS Bind Toll-Like Receptor
The oral administration of POS reduced the incidence of diarrhea and DAI, which shortens the length of colon caused by DSS. Importantly, it was found that POS showed an anti-colitis effect that appears to be related to its ability to downregulate COX-2 of TLR4/NF-κB pathway (129, 139). POS administration affects the activation of TLR4/NF-κB/COX-2 signaling cascade by binding TLR (Figure 6) (140). The level of TLR4 is associated with cardiac disorders and regarded as a clinical biomarker of heart disease (141).
COX-2 expression is closely related to TLR4/NF-κB pathway in the intestine, particularly in the setting of DSS colitis. As a key receptor in innate immunity, TLR4 has been found to be overexpressed in UC patients (142, 143). TLR4-modulated signaling further activates NF-κB, which is followed by expression of an array of subsequent genes participating in inflammatory signaling cascades that mediate the pathogenesis of colitis (Figure 6).
Understanding UC pathogenesis and progress has greatly accelerated the discovery of many therapeutic drugs targeting targeted inflammatory signaling, such as TLR4/NF-κB/COX-2 signaling pathway (Figure 6). COX-2 contributes to the production of inflammatory mediators of PGE2 (144). Consistent with the results of the POS anti-inflammatory mechanism obtained in other diseases, POS has been found to significantly downregulate COX-2 expression (145). Many therapeutic agents have been considered to eliminate intestinal inflammation in UC by blocking TLR4/NF-κB pathways. TLR4 is highly expressed in inflammatory mucosa of UC patients. As a pattern recognition receptor, TLR4 plays a key role in preventing intestinal pathogens. However, since TLR4 is considered to be the most important inflammatory inducer of all members of the TLR family, TLR4-mediated inflammation-related intestinal dysfunction further contributes to the development of UC. NF-κB can be stimulated by TLR4, which is a key transcription factor for inducing and regulating a series of inflammatory mediators. Apple POS has been found to significantly reduce the protein levels of TLR4 and NF-κB, suggesting that inhibition of TLR4/NF-κB pathway and its downstream COX-2 is associated with anti-inflammation properties of POS (Figure 6).
Controversies of the Present Review
The review focuses on antioxidant and anti-inflammatory roles of POS for promoting human health by regulating some potential oxidative and inflammation-activated ATP-activated protein kinase (AMPK), nuclear factor erythroid-2-related factor-2 (Nrf2), and NF-κB pathways. The activation of these signaling pathways increases the antioxidant and anti-inflammatory activities, which will result in the apoptosis of CC cells or in the prevention of CC risk and progression. Thus, POS may inhibit CC development by affecting antioxidant and anti-inflammatory signaling pathways AMPK, Nrf2, and NF-kB (Figure 7). However, POS also can activate STAT1 and 3 signaling pathway, which will reduce antioxidant and anti-inflammatory properties and promote CC progression (Figure 7). Furthermore, activation of AMPK and STAT also can promote CC progression (Figure 7). STAT signaling pathway also inhibits antioxidant and anti-inflammatory activities. All the results will be converse to the widely accepted antitumor properties of POS.
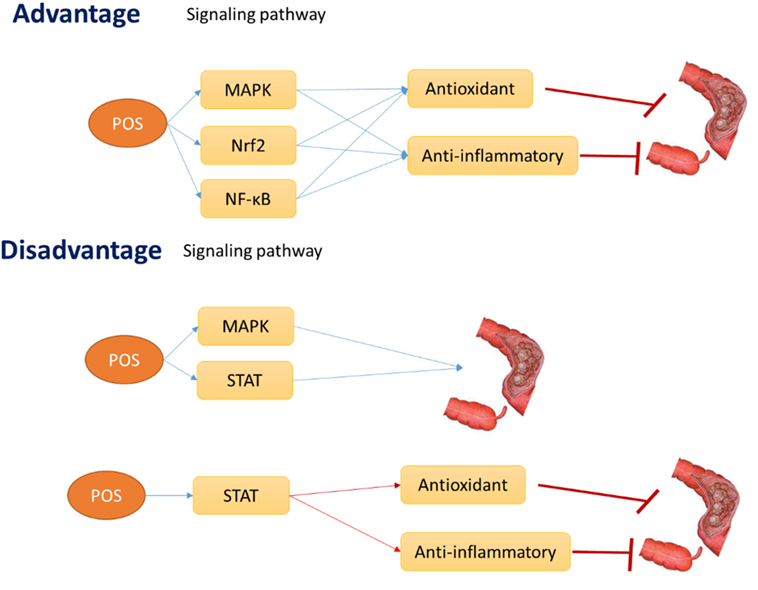
Figure 7. The hypothesis of the advantage and disadvantage effects of pectin oligosaccharides on colon cancer risk.
Conclusion
The present review provides signaling-pathway molecular mechanism of POS (the degraded products of pectin), which is different current widely accepted thoughts for pectin: pectin as a dietary fiber (146), pH-modified pectin (42), modified pectin to avoid chemoresistance (147), and pectin as a drug delivery system in tumor therapy (148).
Anti-inflammatory and antitumor effects for CC of natural products have been widely explored and studied. It is highly demanded for bio-materials used in functional food with few side effects and environmentally friendly properties. POS, as soluble dietary fibers with various health-promoting functions, have a good potential in controlling oxidant stress and inflammatory situation by affecting antioxidant and anti-inflammatory mediated signaling pathways, which contribute to antitumor effects on CC. Structurally modified POS may be associated with their functions and should be carefully selected. The present review paper lacks the important information for the linkage between the specific structure of POS and its function. To further understand the prebiotic role of POS and their derivatives effects on antitumor therapy, the specific POS with a certain degree of polymerization or purified polymers are highly demanded to be performed in clinical practice.
Author Contributions
HT collected all the literature. WC, GY, and QL analyzed all these data. KL wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very grateful to two reviewers’ for their important and strategical comments, which have significantly improved the quality of the present paper. This work was supported by National Key R&D Program of China (2017YFD0200900), Liaoning Provincial Natural Science Foundation (2015020690), Shanxi Provincial Key R&D Program (2017TSCXL-NY-02-05).
References
1. Wong TW, Colombo G, Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech (2011) 12(1):201–14. doi:10.1208/s12249-010-9564-z
2. Hashmi A, Naeem N, Farooq Z, Masood S, Iqbal S, Naseer R. Effect of prebiotic galacto-oligosaccharides on serum lipid profile of hypercholesterolemics. Probiotics Antimicrob Proteins (2016) 8(1):19–30. doi:10.1007/s12602-016-9206-1
3. Wong JM, Kendall CW, de Souza R, Emam A, Marchie A, Vidgen E, et al. The effect on the blood lipid profile of soy foods combined with a prebiotic: a randomized controlled trial. Metabolism (2010) 59(9):1331–40. doi:10.1016/j.metabol.2009.12.017
4. Halder SK, Adak A, Maity C, Jana A, Das A, Paul T, et al. Exploitation of fermented shrimp-shells hydrolysate as functional food: assessment of antioxidant, hypocholesterolemic and prebiotic activities. Indian J Exp Biol (2013) 51:924–34.
5. Pretorius R, Prescott SL, Palmer DJ. Taking a prebiotic approach to early immunomodulation for allergy prevention. Expert Rev Clin Immunol (2018) 14(1):43–51. doi:10.1080/1744666X.2018.1411191
6. Aliasgharzadeh A, Khalili M, Mirtaheri E, Pourghassem Gargari B, Tavakoli F, Abbasalizad Farhangi M, et al. A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: a randomized controlled clinical trial. Adv Pharm Bull (2015) 5(4):507–14. doi:10.15171/apb.2015.069
7. Chung WSF, Meijerink M, Zeuner B, Holck J, Louis P, Meyer AS, et al. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol (2017) 93. doi:10.1093/femsec/fix127
8. Babbar N, Dejonghe W, Gatti M, Sforza S, Elst K. Pectic oligosaccharides from agricultural by-products: production, characterization and health benefits. Crit Rev Biotechnol (2016) 36(4):594–606. doi:10.3109/07388551.2014.996732
9. Olano-Martin E, Williams MR, Gibson GR, Rastall RA. Pectins and pectic-oligosaccharides inhibit Escherichia coli O157:H7 Shiga toxin as directed towards the human colonic cell line HT29. FEMS Microbiol Lett (2003) 218:101–5. doi:10.1111/j.1574-6968.2003.tb11504.x
10. Di R, Vakkalanka MS, Onumpai C, Chau HK, White A, Rastall RA, et al. Pectic oligosaccharide structure-function relationships: prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem (2017) 227:245–54. doi:10.1016/j.foodchem.2017.01.100
11. Delphi L, Sepehri H, Khorramizadeh MR, Mansoori F. Pectic-oligoshaccharides from apples induce apoptosis and cell cycle arrest in MDA-MB-231 cells, a model of human breast cancer. Asian Pac J Cancer Prev (2015) 16:5265–71. doi:10.7314/APJCP.2015.16.13.5265
12. Venkateshaiah SU, Eswaraiah MS, Annaiah HN, Dharmesh SM. Antimetastatic pectic polysaccharide from Decalepis hamiltonii; galectin-3 inhibition and immune-modulation. Clin Exp Metastasis (2017) 34(2):141–54. doi:10.1007/s10585-017-9836-z
13. Expert D, Patrit O, Shevchik VE, Perino C, Boucher V, Creze C, et al. Dickeya dadantii pectic enzymes necessary for virulence are also responsible for activation of the Arabidopsis thaliana innate immune system. Mol Plant Pathol (2016) 19(2):313–27. doi:10.1111/mpp.12522
14. Sibiya N, Ngubane P, Mabandla M. Cardioprotective effects of pectin-insulin patch in streptozotocin-induced diabetic rats. J Diabetes (2017) 9(12):1073–81. doi:10.1111/1753-0407.12538
15. Sefcikova Z, Racek L. Effect of pectin feeding on obesity development and duodenal alkaline phosphatase activity in Sprague-Dawley rats fed with high-fat/high-energy diet. Physiol Int (2016) 103(2):183–90. doi:10.1556/036.103.2016.2.5
16. Pallavicini P, Arciola CR, Bertoglio F, Curtosi S, Dacarro G, D’Agostino A, et al. Silver nanoparticles synthesized and coated with pectin: an ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J Colloid Interface Sci (2017) 498:271–81. doi:10.1016/j.jcis.2017.03.062
17. Cerda JJ, Robbins FL, Burgin CW, Baumgartner TG, Rice RW. The effects of grapefruit pectin on patients at risk for coronary heart disease without altering diet or lifestyle. Clin Cardiol (1988) 11:589–94. doi:10.1002/clc.4960110902
18. Szu SC, Lin KF, Hunt S, Chu C, Thinh ND. Phase I clinical trial of O-acetylated pectin conjugate, a plant polysaccharide based typhoid vaccine. Vaccine (2014) 32(22):2618–22. doi:10.1016/j.vaccine.2014.03.023
19. Kumada T, Mikuni T, Kimura N, Miyajima T, Fujii T. [Viscosity regulating pectin solution and calcium lactate increase the viscosity of milk and decrease the severity of gastroesophageal reflex disease in children with severe motor and intellectual disabilities]. No To Hattatsu (2008) 40:487–9.
20. Gullon B, Gomez B, Martinez-Sabajanes M, Yanez R, Parajo JC, Alonso JL. Pectic oligosaccharides: manufacture and functional properties. Trends Food Sci Tech (2013) 30:153–61. doi:10.1016/j.tifs.2013.01.006
21. Ogutu FO, Mu TH, Sun H, Zhang M. Ultrasonic modified sweet potato pectin induces apoptosis like cell death in colon cancer (HT-29) cell line. Nutr Cancer (2018) 70(1):136–45. doi:10.1080/01635581.2018.1406123
22. Almeida EA, Facchi SP, Martins AF, Nocchi S, Schuquel IT, Nakamura CV, et al. Synthesis and characterization of pectin derivative with antitumor property against Caco-2 colon cancer cells. Carbohydr Polym (2015) 115:139–45. doi:10.1016/j.carbpol.2014.08.085
23. Tian LM, Bruggeman G, van den Berg M, Borewicz K, Scheurink AJW, Bruininx E, et al. Effects of pectin on fermentation characteristics, carbohydrate utilization, and microbial community composition in the gastrointestinal tract of weaning pigs. Mol Nutr Food Res (2017) 61. doi:10.1002/mnfr.201600186
24. Chen QL, Zhu L, Tang Y, Zhao ZZ, Yi T, Chen HB. Preparation-related structural diversity and medical potential in the treatment of diabetes mellitus with ginseng pectins. Ann Ny Acad Sci (2017) 1401(1):75–89. doi:10.1111/nyas.13424
25. Hosobuchi C, Rutanassee L, Bassin SL, Wong ND. Efficacy of acacia, pectin, and guar gum-based fiber supplementation in the control of hypercholesterolemia. Nutr Res (1999) 19:643–9. doi:10.1016/S0271-5317(99)00029-9
26. Al-Tamimi MA, Palframan RJ, Cooper JM, Gibson GR, Rastall RA. In vitro fermentation of sugar beet arabinan and arabino-oligosaccharides by the human gut microflora. J Appl Microbiol (2006) 100:407–14. doi:10.1111/j.1365-2672.2005.02780.x
27. Holck J, Lorentzen A, Vigsnaes LK, Licht TR, Mikkelsen JD, Meyer AS. Feruloylated and nonferuloylated arabino-oligosaccharides from sugar beet pectin selectively stimulate the growth of Bifidobacterium spp. in human fecal in vitro fermentations. J Agric Food Chem (2011) 59(12):6511–9. doi:10.1021/jf200996h
28. Gullon B, Gullon P, Sanz Y, Alonso JL, Parajo JC. Prebiotic potential of a refined product containing pectic oligosaccharides. Lwt-Food Sci Technol (2011) 44:1687–96. doi:10.1016/j.lwt.2011.03.006
29. Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr (2008) 88:1438–46.
30. Zhang H, Zhang M, Tao Y, Wang G, Xia B. Madecassic acid inhibits the mouse colon cancer growth by inducing apoptosis and immunomodulation. J BUON (2014) 19:372–6.
31. Koriem KM, Arbid MS, Emam KR. Therapeutic effect of pectin on octylphenol induced kidney dysfunction, oxidative stress and apoptosis in rats. Environ Toxicol Pharmacol (2014) 38:14–23. doi:10.1016/j.etap.2014.04.029
32. Khasina E, Kolenchenko E, Sgrebneva M, Kovalev V, Khotimchenko YS. Antioxidant activities of a low etherified pectin from the seagrass Zostera marina. Russ J Mar Biol (2003) 29:259–61. doi:10.1023/A:1025493128327
33. Ko H-S, Fujiwara H, Yokoyama Y, Ohno N, Amachi S, Shinoyama H, et al. Inducible production of alcohol oxidase and catalase in a pectin medium by Thermoascus aurantiacus IFO 31693. J Biosci Bioeng (2005) 99:290–2. doi:10.1263/jbb.99.290
34. Wang H, Wei G, Liu F, Banerjee G, Joshi M, Bligh SW, et al. Characterization of two homogalacturonan pectins with immunomodulatory activity from green tea. Int J Mol Sci (2014) 15:9963–78. doi:10.3390/ijms15069963
35. Ye MB, Lim BO. Dietary pectin regulates the levels of inflammatory cytokines and immunoglobulins in interleukin-10 knockout mice. J Agric Food Chem (2010) 58:11281–6. doi:10.1021/jf103262s
36. Jimenez S, Gascon S, Luquin A, Laguna M, Ancin-Azpilicueta C, Rodriguez-Yoldi MJ. Rosa canina extracts have antiproliferative and antioxidant effects on caco-2 human colon cancer. PLoS One (2016) 11(7):e0159136. doi:10.1371/journal.pone.0159136
37. Hu Q, Yuan B, Xiao H, Zhao L, Wu X, Rakariyatham K, et al. Polyphenols-rich extract from Pleurotus eryngii with growth inhibitory of HCT116 colon cancer cells and anti-inflammatory function in RAW264.7 cells. Food Funct (2018). doi:10.1039/c7fo01794d
38. Colodel C, Vriesmann LC, Teofilo RF, de Oliveira Petkowicz CL. Extraction of pectin from ponkan (Citrus reticulata Blanco cv. Ponkan) peel: optimization and structural characterization. Int J Biol Macromol (2018) 117:385–91. doi:10.1016/j.ijbiomac.2018.05.048
39. Hou JJ, Guo J, Wang JM, Yang XQ. Effect of interfacial composition and crumbliness on aroma release in soy protein/sugar beet pectin mixed emulsion gels. J Sci Food Agric (2016) 96:4449–56. doi:10.1002/jsfa.7656
40. Yang JS, Mu TH, Ma MM. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem (2018) 244:197–205. doi:10.1016/j.foodchem.2017.10.059
41. Voragen AGJ, Coenen GJ, Verhoef RP, Schols HA. Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem (2009) 20:263–75. doi:10.1007/s11224-009-9442-z
42. Jackson CL, Dreaden TM, Theobald LK, Tran NM, Beal TL, Eid M, et al. Pectin induces apoptosis in human prostate cancer cells: correlation of apoptotic function with pectin structure. Glycobiology (2007) 17:805–19. doi:10.1093/glycob/cwm054
43. Ele-Ekouna JP, Pau-Roblot C, Courtois B, Courtois J. Chemical characterization of pectin from green tea (Camellia sinensis). Carbohyd Polym (2011) 83:1232–9. doi:10.1016/j.carbpol.2010.09.028
45. Lara-Marquez A, Oyama K, Zavala-Paramo MG, Villa-Rivera MG, Conejo-Saucedo U, Cano-Camacho H. Evolutionary analysis of pectin lyases of the genus colletotrichum. J Mol Evol (2017) 85:120–36. doi:10.1007/s00239-017-9812-x
46. Zhang S, Hu H, Wang L, Liu F, Pan S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem (2018) 244:232–7. doi:10.1016/j.foodchem.2017.10.071
47. Chen J, Liang R-h, Liu W, Li T, Liu C-m, Wu S-s, et al. Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties. Carbohydr Polym (2013) 91:175–82. doi:10.1016/j.carbpol.2012.08.021
48. Tayi L, Maku RV, Patel HK, Sonti RV. Identification of pectin degrading enzymes secreted by Xanthomonas oryzae pv. oryzae and determination of their role in virulence on rice. PLoS One (2016) 11:e0166396. doi:10.1371/journal.pone.0166396
49. Senechal F, Mareck A, Marcelo P, Lerouge P, Pelloux J. Arabidopsis PME17 activity can be controlled by pectin methylesterase inhibitor4. Plant Signal Behav (2015) 10. doi:10.4161/15592324.2014.983351
50. Xu SX, Qin X, Liu B, Zhang DQ, Zhang W, Wu K, et al. An acidic pectin lyase from Aspergillus niger with favourable efficiency in fruit juice clarification. Lett Appl Microbiol (2015) 60.
51. Gomez B, Gullon B, Yanez R, Parajo JC, Alonso JL. Pectic oligosacharides from lemon peel wastes: production, purification, and chemical characterization. J Agric Food Chem (2013) 61:10043–53. doi:10.1021/jf402559p
52. Gomez B, Gullon B, Remoroza C, Schols HA, Parajo JC, Alonso JL. Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. J Agric Food Chem (2014) 62:9769–82. doi:10.1021/jf503475b
53. Gavlighi HA, Michalak M, Meyer AS, Mikkelsen JD. Enzymatic depolymerization of gum tragacanth: bifidogenic potential of low molecular weight oligosaccharides. J Agric Food Chem (2013) 61:1272–8. doi:10.1021/jf304795f
54. Zhang Y, Mei S, Zhou Y, Yang D, Pan T, Chen Z, et al. TIPE2 negatively regulates mycoplasma pneumonia-triggered immune response via MAPK signaling pathway. Sci Rep (2017) 7:13319. doi:10.1038/s41598-017-13825-y
55. Kakavand H, Rawson RV, Pupo GM, Yang JYH, Menzies AM, Carlino MS, et al. PD-L1 expression and immune escape in melanoma resistance to MAPK inhibitors. Clin Cancer Res (2017) 23:6054–61. doi:10.1158/1078-0432.CCR-16-1688
56. Ma J, Su H, Yu B, Guo T, Gong Z, Qi J, et al. CXCL12 gene silencing down-regulates metastatic potential via blockage of MAPK/PI3K/AP-1 signaling pathway in colon cancer. Clin Transl Oncol (2018). doi:10.1007/s12094-017-1821-0
57. Daaboul HE, Daher CF, Bodman-Smith K, Taleb RI, Shebaby WN, Boulos J, et al. Antitumor activity of beta-2-himachalen-6-ol in colon cancer is mediated through its inhibition of the PI3K and MAPK pathways. Chem Biol Interact (2017) 275. doi:10.1016/j.cbi.2017.08.003
58. Lim SH. Larch Arabinogalactan attenuates myocardial injury by inhibiting apoptotic cascades in a rat model of ischemia-reperfusion. J Med Food (2017) 20:691–9. doi:10.1089/jmf.2016.3886
59. Chatterjee S, Huang EH, Christie I, Kurland BF, Burns TF. Acquired resistance to the Hsp90 inhibitor, ganetespib, in KRAS-mutant NSCLC is mediated via reactivation of the ERK-p90RSK-mTOR signaling network. Mol Cancer Ther (2017) 16:793–804. doi:10.1158/1535-7163.MCT-16-0677
60. Nishida M, Murata K, Kanamaru Y, Yabe T. Pectin of Prunus domestica L. alters sulfated structure of cell-surface heparan sulfate in differentiated Caco-2 cells through stimulation of heparan sulfate 6-O-endosulfatase-2. Biosci Biotechnol Biochem (2014) 78:635–43. doi:10.1080/09168451.2014.891937
61. Di Agostino S, Rossi P, Geremia R, Sette C. The MAPK pathway triggers activation of Nek2 during chromosome condensation in mouse spermatocytes. Development (2002) 129:1715–27.
62. Choi SI, Lee JH, Kim JM, Jung TD, Cho BY, Choi SH, et al. Ulmus macrocarpa hance extracts attenuated H(2)O(2) and UVB-induced skin photo-aging by activating antioxidant enzymes and inhibiting MAPK pathways. Int J Mol Sci (2017) 18. doi:10.3390/ijms18061200
63. Ajayi BO, Adedara IA, Farombi EO. Benzo(a)pyrene induces oxidative stress, pro-inflammatory cytokines, expression of nuclear factor-kappa B and deregulation of wnt/beta-catenin signaling in colons of BALB/c mice. Food Chem Toxicol (2016) 95:42–51. doi:10.1016/j.fct.2016.06.019
64. Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, et al. LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell (2008) 20. doi:10.1105/tpc.107.056754
65. Kishimoto K, Kouzai Y, Kaku H, Shibuya N, Minami E, Nishizawa Y. Perception of the chitin oligosaccharides contributes to disease resistance to blast fungus Magnaporthe oryzae in rice. Plant J (2010) 64:343–54. doi:10.1111/j.1365-313X.2010.04328.x
66. Qiu D, Wilson IW, Gan S, Washusen R, Moran GF, Southerton SG. Gene expression in Eucalyptus branch wood with marked variation in cellulose microfibril orientation and lacking G-layers. New Phytologist (2008) 179:94–103. doi:10.1111/j.1469-8137.2008.02439.x
67. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature (1997) 389.
68. Alpizar YA, Gees M, Boonen B, Sanchez A, Nilius B, Voets T, et al. Activation and sensitization of the capsaicin receptor TRPV1 by allyl isothiocyanate. Biophys J (2014) 106:337a. doi:10.1016/j.bpj.2013.11.1930
69. Hsu KS, Zhao X, Cheng X, Guan D, Mahabeleshwar GH, Liu Y, et al. Dual regulation of Stat1 and Stat3 by the tumor suppressor protein PML contributes to interferon alpha-mediated inhibition of angiogenesis. J Biol Chem (2017) 292. doi:10.1074/jbc.M116.771071
70. Yamauchi S, Takeuchi K, Chihara K, Honjoh C, Kato Y, Yoshiki H, et al. STAT1 is essential for the inhibition of hepatitis C virus replication by interferon-lambda but not by interferon-alpha. Sci Rep (2016) 6. doi:10.1038/srep38336
71. Madapura HS, Nagy N, Ujvari D, Kallas T, Krohnke MC, Amu S, et al. Interferon gamma is a STAT1-dependent direct inducer of BCL6 expression in imatinib-treated chronic myeloid leukemia cells. Oncogene (2017). doi:10.1038/onc.2017.85
72. Kulkarni A, Scully TJ, O’Donnell LA. The antiviral cytokine interferon-gamma restricts neural stem/progenitor cell proliferation through activation of STAT1 and modulation of retinoblastoma protein phosphorylation. J Neurosci Res (2017) 95:1582–601. doi:10.1002/jnr.23987
73. Chung SS, Wu Y, Okobi Q, Adekoya D, Atefi M, Clarke O, et al. Proinflammatory cytokines IL-6 and TNF-alpha increased telomerase activity through NF-kappaB/STAT1/STAT3 activation, and withaferin A inhibited the signaling in colorectal cancer cells. Mediators Inflamm (2017) 2017.
74. Huang Q, Duan I, Qian X, Fan J, Lv Z, Zhang X, et al. Corrigendum: IL-17 promotes angiogenic factors IL-6, IL-8, and Vegf production via Stat1 in lung adenocarcinoma. Sci Rep (2017) 7.
75. Chen Y, Fang L, Li G, Zhang J, Li C, Ma M, et al. Synergistic inhibition of colon cancer growth by the combination of methylglyoxal and silencing of glyoxalase I mediated by the STAT1 pathway. Oncotarget (2017) 8:54838–57. doi:10.18632/oncotarget.18601
76. Kaler P, Owusu BY, Augenlicht L, Klampfer L. The role of STAT1 for crosstalk between fibroblasts and colon cancer cells. Front Oncol (2014) 4:88. doi:10.3389/fonc.2014.00088
77. Li W, Lee MR, Kim T, Kim YW, Cho MY. Activated STAT3 may participate in tumor progression through increasing CD133/survivin expression in early stage of colon cancer. Biochem Biophys Res Commun (2018) 497:354–61. doi:10.1016/j.bbrc.2018.02.084
78. Hirashima Y, Endo S, Kato R, Takaku A. Prevention of cerebrovasospasm following subarachnoid hemorrhage in rabbits by the platelet-activating factor antagonist, E5880. J Neurosurg (1996) 84:826–30. doi:10.3171/jns.1996.84.5.0826
79. Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Wakabayashi T, Takayasu M. Oxidative stress activates STAT1 in basilar arteries after subarachnoid hemorrhage. Brain Res (2010) 1332:12–9. doi:10.1016/j.brainres.2010.03.046
80. Burova EB, Gonchar IV, Nikol’skii NN. STAT1 and STAT3 activation by oxidative stress in A431 cells involves Src-dependent EGF receptor transactivation. Tsitologiia (2003) 45:466–77.
81. Appelberg KS, Wallet MA, Taylor JP, Cash MN, Sleasman JW, Goodenow MM. HIV-1 infection primes macrophages through STAT signaling to promote enhanced inflammation and viral replication. AIDS Res Hum Retroviruses (2017) 33:690–702. doi:10.1089/aid.2016.0273
82. Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct (2017) 8:387–96. doi:10.1039/c6fo01529h
83. Kamdar SJ, Fuller JA, Nishikawa SI, Evans R. Priming of mouse macrophages with the macrophage colony-stimulating factor (CSF-1) induces a variety of pathways that regulate expression of the interleukin 6 (Il6) and granulocyte-macrophage colony-stimulating factor (Csfgm) genes. Exp Cell Res (1997) 235:108–16. doi:10.1006/excr.1997.3632
84. Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nat Med (1996) 2:561–6. doi:10.1038/nm0596-561
85. Davidson LA, Lupton JR, Jiang Y-H, Chang W-C, Aukema HM, Chapkin RS. Dietary fat and fiber alter rat colonic protein kinase C isozyme expression. J Nutr (1995) 125:49.
86. Sakhalkar SP, Patterson EB, Khan MM. Involvement of histamine H1 and H2 receptors in the regulation of STAT-1 phosphorylation: inverse agonism exhibited by the receptor antagonists. Int Immunopharmacol (2005) 5:1299–309. doi:10.1016/j.intimp.2005.03.019
87. do Nascimento GE, Winnischofer SMB, Ramirez MI, Iacomini M, Cordeiro LMC. The influence of sweet pepper pectin structural characteristics on cytokine secretion by THP-1 macrophages. Food Res Int (2017) 102:588–94. doi:10.1016/j.foodres.2017.09.037
88. Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis (2016) 75:311–5. doi:10.1136/annrheumdis-2014-207201
89. Lee SB, Lee WS, Shin JS, Jang DS, Lee KT. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-alpha, and IL-6 via AP-1, NF-kappaB, and JAK-STAT inactivation in RAW 264.7 macrophages. Int Immunopharmacol (2017) 49:21–9. doi:10.1016/j.intimp.2017.05.021
90. Salman H, Bergman M, Djaldetti M, Orlin J, Bessler H. Citrus pectin affects cytokine production by human peripheral blood mononuclear cells. Biomed Pharmacother (2008) 62:579–82. doi:10.1016/j.biopha.2008.07.058
91. Zeng L, Wang W, Rong XF, Zhong Y, Jia P, Zhou GQ, et al. Chondroprotective effects and multi-target mechanisms of Icariin in IL-1 beta-induced human SW 1353 chondrosarcoma cells and a rat osteoarthritis model. Int Immunopharmacol (2014) 18:175–81. doi:10.1016/j.intimp.2013.11.021
92. Sesarman A, Tefas L, Sylvester B, Licarete E, Rauca V, Luput L, et al. Anti-angiogenic and anti-inflammatory effects of long-circulating liposomes co-encapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharmacol Rep (2018) 70:331–9. doi:10.1016/j.pharep.2017.10.004
93. Jiang T, Gao X, Wu C, Tian F, Lei Q, Bi J, et al. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients (2016) 8:126. doi:10.3390/nu8030126
94. Pelkman CL, Navia JL, Miller AE, Pohle RJ. Novel calcium-gelled, alginate-pectin beverage reduced energy intake in nondieting overweight and obese women: interactions with dietary restraint status. Am J Clin Nutr (2007) 86:1595–602. doi:10.1093/ajcn/86.5.1595
95. Story JA. Dietary Fiber and Lipid Metabolism: An Update, Medical Aspects of Dietary Fiber. Purdue: Springer (1980). p. 137–52.
96. Li T-p, Zhu R-g, Dong Y-p, Liu Y-h, Li S-h, Chen G. Effects of pectin pentaoligosaccharide from Hawthorn (Crataegus pinnatifida Bunge. var. Major) on the activity and mRNA levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. J Agric Food Chem (2013) 61:7599–605. doi:10.1021/jf400283w
97. Palou M, Sanchez J, Garcia-Carrizo F, Palou A, Pico C. Pectin supplementation in rats mitigates age-related impairment in insulin and leptin sensitivity independently of reducing food intake. Mol Nutr Food Res (2015) 59:2022–33. doi:10.1002/mnfr.201500292
98. Adam CL, Gratz SW, Peinado DI, Thomson LM, Garden KE, Williams PA, et al. Effects of dietary fibre (Pectin) and/or increased protein (casein or pea) on satiety, body weight, adiposity and caecal fermentation in high fat diet-induced obese rats. PLoS One (2016) 11:e0155871. doi:10.1371/journal.pone.0155871
99. Wu L, Sun D. Leptin receptor gene polymorphism and the risk of cardiovascular disease: a systemic review and meta-analysis. Int J Environ Res Public Health (2017) 14:1–12. doi:10.3390/ijerph14040375
100. Olza J, Ruperez AI, Gil-Campos M, Leis R, Canete R, Tojo R, et al. Leptin receptor gene variant rs11804091 is associated with BMI and insulin resistance in Spanish female obese children: a case-control study. Int J Mol Sci (2017) 18:1–14. doi:10.3390/ijms18081690
101. Olczyk P, Koprowski R, Komosinska-Vassev K, Jura-Poltorak A, Winsz-Szczotka K, Kuznik-Trocha K, et al. Adiponectin, leptin, and leptin receptor in obese patients with type 2 diabetes treated with insulin detemir. Molecules (2017) 22:1–15. doi:10.3390/molecules22081274
102. Gajewska J, Kurylowicz A, Mierzejewska E, Ambroszkiewicz J, Chelchowska M, Weker H, et al. Complementary effects of genetic variations in LEPR on body composition and soluble leptin receptor concentration after 3-month lifestyle intervention in prepubertal obese children. Nutrients (2016) 8:1–16. doi:10.3390/nu8060328
103. Bravo C, Cataldo LR, Galgani J, Parada J, Santos JL. Leptin/adiponectin ratios using either total or high-molecular-weight adiponectin as biomarkers of systemic insulin sensitivity in normoglycemic women. J Diabetes Res (2017) 2017. doi:10.1155/2017/9031079
104. Hartl A, Sieper J, Syrbe U, Listing J, Hermann KG, Rudwaleit M, et al. Serum levels of leptin and high molecular weight adiponectin are inversely associated with radiographic spinal progression in patients with ankylosing spondylitis: results from the ENRADAS trial. Arthritis Res Ther (2017) 19:140. doi:10.1186/s13075-017-1350-9
105. Chehab FF. 20 years of leptin: leptin and reproduction: past milestones, present undertakings, and future endeavors. J Endocrinol (2014) 223:T37–48. doi:10.1530/JOE-14-0413
106. Zhang J, Wang N. Leptin in chronic kidney disease: a link between hematopoiesis, bone metabolism, and nutrition. Int Urol Nephrol (2014) 46:1169–74. doi:10.1007/s11255-013-0623-8
107. Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience (2002) 113:607–15. doi:10.1016/S0306-4522(02)00162-8
108. Han G, Li Y, Cao Y, Yue Z, Zhang Y, Wang L, et al. Overexpression of leptin receptor in human glioblastoma: correlation with vasculogenic mimicry and poor prognosis. Oncotarget (2017) 8(35):58163–71. doi:10.18632/oncotarget.17344
109. Xu N, Yuan H, Liu W, Li S, Liu Y, Wan J, et al. Activation of RAW264.7 mouse macrophage cells in vitro through treatment with recombinant ricin toxin-binding subunit B: involvement of protein tyrosine, NF-kappaB and JAK-STAT kinase signaling pathways. Int J Mol Med (2013) 32:729–35. doi:10.3892/ijmm.2013.1426
110. Zhang X, Wei L, Wang J, Qin Z, Wang J, Lu Y, et al. Suppression colitis and colitis-associated colon cancer by anti-S100a9 antibody in mice. Front Immunol (2017) 8:1774. doi:10.3389/fimmu.2017.01774
111. Ochoa-Callejero L, Garcia-Sanmartin J, Martinez-Herrero S, Rubio-Mediavilla S, Narro-Iniguez J, Martinez A. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci Rep (2017) 7:17488. doi:10.1038/s41598-017-17573-x
112. Conforti F, Zinck J. Hydrocolloid-lipid coating affect on weight loss, pectin content, and textural quality of green bell peppers. J Food Sci (2002) 67:1360–3. doi:10.1111/j.1365-2621.2002.tb10289.x
113. Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D, et al. Failure of synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci (2007) 52:385–9. doi:10.1007/s10620-006-9549-7
114. Sethi S, Khurana RK, Kamboj S, Sharma R, Singh A, Rana V. Investigating the potential of Tamarindus indica pectin-chitosan conjugate for reducing recovery period in TNBS induced colitis. Int J Biol Macromol (2017) 98:739–47. doi:10.1016/j.ijbiomac.2017.01.135
115. Lim BO, Lee SH, Park DK, Choue RW. Effect of dietary pectin on the production of immunoglobulins and cytokines by mesenteric lymph node lymphocytes in mouse colitis induced with dextran sulfate sodium. Biosci Biotechnol Biochem (2003) 67:1706–12. doi:10.1271/bbb.67.1706
116. Hoover T, Mikovits J, Court D, Liu YL, Kung HF, Raziuddin. A nuclear matrix-specific factor that binds a specific segment of the negative regulatory element (NRE) of HIV-1 LTR and inhibits NF-kappa(B) activity. Nucleic Acids Res (1996) 24:1895–900. doi:10.1093/nar/24.10.1895
117. El-Deeb NM, Yassin AM, Al-Madboly LA, El-Hawiet A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-kappaB inflammatory pathways in human colon cancer. Microb Cell Fact (2018) 17:1–15. doi:10.1186/s12934-018-0877-z
118. Wang Q, Gao X, Yu T, Yuan L, Dai J, Wang W, et al. REGgamma controls Hippo signaling and reciprocal NF-kappaB-YAP regulation to promote colon cancer. Clin Cancer Res (2018). doi:10.1158/1078-0432.CCR-17-2986
119. El-Agamy DS. Pirfenidone ameliorates concanavalin A-induced hepatitis in mice via modulation of reactive oxygen species/nuclear factor kappa B signalling pathways. J Pharm Pharmacol (2016) 68:1559–66. doi:10.1111/jphp.12651
120. Zhang LD, Ma L, Zhang L, Dai JG, Chang LG, Huang PL, et al. Hyperbaric oxygen and Ginkgo Biloba extract ameliorate cognitive and memory impairment via nuclear factor Kappa-B pathway in rat model of Alzheimer’s disease. Chin Med J (Engl) (2015) 128:3088–93. doi:10.4103/0366-6999.169105
121. Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol (1996) 157:1630–7.
122. Kumar A, Negi G, Sharma SS. JSH-23 targets nuclear factor-kappa B and reverses various deficits in experimental diabetic neuropathy: effect on neuroinflammation and antioxidant defence. Diabetes Obes Metab (2011) 13:750–8. doi:10.1111/j.1463-1326.2011.01402.x
123. Wang H, Zhang K, Zhao L, Tang J, Gao L, Wei Z. Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa B and tumor necrosis factor-alpha in a rat model of cerebral ischemia-reperfusion injury. Neurosci Lett (2014) 566:247–51. doi:10.1016/j.neulet.2014.02.045
124. Setia S, Nehru B, Sanyal SN. Upregulation of MAPK/Erk and PI3K/Akt pathways in ulcerative colitis-associated colon cancer. Biomed Pharmacother (2014) 68:1023–9. doi:10.1016/j.biopha.2014.09.006
125. Tanaka T, Kobunai T, Yamamoto Y, Murono K, Otani K, Yasuda K, et al. Increased copy number variation of mtDNA in an array-based digital PCR assay predicts ulcerative colitis-associated colorectal cancer. In Vivo (2017) 31:713–8. doi:10.21873/invivo.11119
126. Altobelli E, Latella G, Morroni M, Licini C, Tossetta G, Mazzucchelli R, et al. Low HtrA1 expression in patients with longstanding ulcerative colitis and colorectal cancer. Oncol Rep (2017) 38:237–44. doi:10.3892/or.2017.5700
127. Kamiya T, Ando T, Watanabe O, Nakamura M, Yamamura T, Miyahara R, et al. Suitability of surveillance colonoscopy for patients with ulcerative colitis to detect colorectal cancer: current guidelines miss some early-stage cases. Nagoya J Med Sci (2015) 77:237–44.
128. Zhang Y, Lin L, Xu Y, Lin Y, Jin Y, Zheng C. 1H NMR-based spectroscopy detects metabolic alterations in serum of patients with early-stage ulcerative colitis. Biochem Biophys Res Commun (2013) 433:547–51. doi:10.1016/j.bbrc.2013.03.012
129. Chanput W, Mes J, Vreeburg RA, Savelkoul HF, Wichers HJ. Transcription profiles of LPS-stimulated THP-1 monocytes and macrophages: a tool to study inflammation modulating effects of food-derived compounds. Food Funct (2010) 1:254–61. doi:10.1039/c0fo00113a
130. Rose DJ, DeMeo MT, Keshavarzian A, Hamaker BR. Influence of dietary fiber on inflammatory bowel disease and colon cancer: importance of fermentation pattern. Nutr Rev (2007) 65:51–62. doi:10.1111/j.1753-4887.2007.tb00282.x
131. Liu L, Li YH, Niu YB, Sun Y, Guo ZJ, Li Q, et al. An apple oligogalactan prevents against inflammation and carcinogenesis by targeting LPS/TLR4/NF-kappaB pathway in a mouse model of colitis-associated colon cancer. Carcinogenesis (2010) 31:1822–32. doi:10.1093/carcin/bgq070
132. Tazawa K, Okami H, Yamashita I, Ohnishi Y, Kobashi K, Fujimaki M. Anticarcinogenic action of apple pectin on fecal enzyme activities and mucosal or portal prostaglandin E2 levels in experimental rat colon carcinogenesis. J Exp Clin Cancer Res (1997) 16:33–8.
133. Dinakaran V, Shankar M, Jayashree S, Rathinavel A, Gunasekaran P, Rajendhran J. Genome sequence of Staphylococcus arlettae strain CVD059, isolated from the blood of a cardiovascular disease patient. J Bacteriol (2012) 194:6615–6. doi:10.1128/JB.01732-12
134. Matsushita A, Iwase M, Kato Y, Ichihara S, Ichihara G, Kimata H, et al. Differential cardiovascular effects of endotoxin derived from Escherichia coli or Pseudomonas aeruginosa. Exp Anim (2007) 56:339–48. doi:10.1538/expanim.56.339
135. Umar S, Morris A, Kourouma F, Sellin J. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif (2003) 36:361–75. doi:10.1046/j.1365-2184.2003.00291.x
136. Silva DC, Freitas AL, Pessoa CD, Paula RC, Mesquita JX, Leal LK, et al. Pectin from Passiflora edulis shows anti-inflammatory action as well as hypoglycemic and hypotriglyceridemic properties in diabetic rats. J Med Food (2011) 14:1118–26. doi:10.1089/jmf.2010.0220
137. Shin M-S, Lee H, Hong H-D, Shin K-S. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki Thumb (Persimmon). J Funct Foods (2016) 26:319–29. doi:10.1016/j.jff.2016.07.025
138. Luan Y, Li Y, Zhu L, Zheng S, Mao D, Chen Z, et al. Codonopis bulleynana forest ex Diels inhibits autophagy and induces apoptosis of colon cancer cells by activating the NF-kappaB signaling pathway. Int J Mol Med (2018) 41.
139. Li X, Jiang J, Shi S, Bligh SA, Li Y, Jiang Y, et al. A RG-II type polysaccharide purified from Aconitum coreanum alleviates lipopolysaccharide-induced inflammation by inhibiting the NF-κB signal pathway. PLoS One (2014) 9:e99697. doi:10.1371/journal.pone.0099697
140. Ishisono K, Yabe T, Kitaguchi K. Citrus pectin attenuates endotoxin shock via suppression of toll-like receptor signaling in Peyer’s patch myeloid cells. J Nutr Biochem (2017) 50:38–45. doi:10.1016/j.jnutbio.2017.07.016
141. Shao L, Zhang P, Zhang Y, Lu Q, Ma A. TLR3 and TLR4 as potential clinically biomarkers of cardiovascular risk in coronary artery disease (CAD) patients. Heart Vessels (2014) 29:690–8. doi:10.1007/s00380-013-0421-3
142. Rafa H, Benkhelifa S, AitYounes S, Saoula H, Belhadef S, Belkhelfa M, et al. All-trans retinoic acid modulates TLR4/NF-kappaB signaling pathway targeting TNF-alpha and nitric oxide synthase 2 expression in colonic mucosa during ulcerative colitis and colitis associated cancer. Mediators Inflamm (2017) 2017. doi:10.1155/2017/7353252
143. Gupta RA, Motiwala MN, Dumore NG, Danao KR, Ganjare AB. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J Ethnopharmacol (2015) 164:239–46. doi:10.1016/j.jep.2015.01.039
144. Lee CW, Lin ZC, Hu SC, Chiang YC, Hsu LF, Lin YC, et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci Rep (2016) 6:27995. doi:10.1038/srep27995
145. Chen C-H, Sheu M-T, Chen T-F, Wang Y-C, Hou W-C, Liu D-Z, et al. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem Pharmacol (2006) 72:1001–9. doi:10.1016/j.bcp.2006.07.001
146. Triff K, McLean MW, Callaway E, Goldsby J, Ivanov I, Chapkin RS. Dietary fat and fiber interact to uniquely modify global histone post-translational epigenetic programming in a rat colon cancer progression model. Int J Cancer (2018). doi:10.1002/ijc.31525
147. Hossein G, Keshavarz M, Ahmadi S, Naderi N. Synergistic effects of PectaSol-C modified citrus pectin an inhibitor of Galectin-3 and paclitaxel on apoptosis of human SKOV-3 ovarian cancer cells. Asian Pac J Cancer Prev (2013) 14:7561–8. doi:10.7314/APJCP.2013.14.12.7561
Keywords: immunomodulation, colon cancer, pectin oligosaccharides, signaling pathway, prebiotics
Citation: Tan H, Chen W, Liu Q, Yang G and Li K (2018) Pectin Oligosaccharides Ameliorate Colon Cancer by Regulating Oxidative Stress- and Inflammation-Activated Signaling Pathways. Front. Immunol. 9:1504. doi: 10.3389/fimmu.2018.01504
Received: 08 March 2018; Accepted: 18 June 2018;
Published: 27 June 2018
Edited by:
Sandra Gessani, Istituto Superiore di Sanità, ItalyReviewed by:
Zhenquan Jia, University of North Carolina at Greensboro, United StatesJang-gi Choi, Korea Institute of Oriental Medicine, South Korea
Copyright: © 2018 Tan, Chen, Liu, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haidong Tan, aGR0JiN4MDAwNDA7ZGljcC5hYy5jbg==
 Haidong Tan
Haidong Tan Wei Chen
Wei Chen