- Department of Gastroenterology, The First Affiliated Hospital of Nanchang University, Nanchang, China
Helicobacter pylori (H. pylori) is a common gastrointestinal bacterial strain closely associated with the incidence of chronic gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer. A current research and clinical challenge is the increased rate of antibiotic resistance in H. pylori, which has led to a decreased H. pylori eradication rate. In this article, we review recent H. pylori infection and reinfection rates and H. pylori resistance to antibiotics, and we discuss the pertinent treatments. A PubMed literature search was performed using the following keywords: Helicobacter pylori, infection, reinfection, antibiotic resistance, bismuth, proton pump inhibitors, vonoprazan, susceptibility, quintuple therapy, dual therapy, and probiotic. The prevalence of H. pylori has remained high in some areas despite the decreasing trend of H. pylori prevalence observed over time. Additionally, the H. pylori reinfection rate has varied in different countries due to socioeconomic and hygienic conditions. Helicobacter pylori monoresistance to clarithromycin, metronidazole or levofloxacin was common in most countries. However, the prevalence of amoxicillin and tetracycline resistance has remained low. Because H. pylori infection and reinfection present serious challenges and because H. pylori resistance to clarithromycin, metronidazole or levofloxacin remains high in most countries, the selection of an efficient regimen to eradicate H. pylori is critical. Currently, bismuth-containing quadruple therapies still achieve high eradication rates. Moreover, susceptibility-based therapies are alternatives because they may avoid the use of unnecessary antibiotics. Novel regimens, e.g., vonoprazan-containing triple therapies, quintuple therapies, high-dose dual therapies, and standard triple therapies with probiotics, require further studies concerning their efficiency and safety for treating H. pylori.
Introduction
Helicobacter pylori (H. pylori) is a common human pathogen that has existed in the stomach since as early as 60,000 years ago (Moodley et al., 2012). In 1983, H. pylori, a Gram-negative flagellate and microaerophilic bacterium, was successfully isolated from the gastric antrum and cultivated in vitro (Marshall and Warren, 1984). The discovery of H. pylori remains a breakthrough in the field of gastroenterology because this bacterium is closely associated with chronic gastritis, peptic ulcers, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer (Wotherspoon et al., 1991; McColl, 2010; Wroblewski et al., 2010). The Maastricht V/Florence Consensus, the Kyoto Global Consensus and the Toronto Consensus reports have emphasized the importance of H. pylori in the pathogenesis of gastric diseases and recommended the eradication of H. pylori for preventing gastric cancer (Sugano et al., 2015; Fallone et al., 2016; Malfertheiner et al., 2016). Additionally, H. pylori eradication may rapidly decrease active inflammation in the gastric mucosa (Zhou et al., 2003), prevent progression toward precancerous lesions (Lee Y.-C. et al., 2013) and reverse gastric atrophy before the development of intestinal metaplasia (Wang et al., 2011; Ford et al., 2014). Undoubtedly, the earliest possible eradication of H. pylori is highly beneficial.
In the early 1990s, the eradication rate using legacy triple therapies (a proton pump inhibitor [PPI], clarithromycin and amoxicillin) was greater than 80%, which was acceptable (Misiewicz et al., 1997; Fennerty et al., 1998; Laine et al., 1998). Currently, this regimen is unacceptable as a first-line therapy for H. pylori (Graham and Fischbach, 2010). Several factors are involved in failed H. pylori eradication, including improper regimens, poor patient compliance, massive gastric bacterial loads, internalizing bacteria, high gastric acidity, gene polymorphisms (IL-1B and CYP2C19), antimicrobial washout and dilution, biofilm formation, and most importantly, resistance to antibiotics (Graham and Dore, 2016; Malfertheiner et al., 2016; Wang et al., 2017). During recent decades, the rate of antibiotic resistance (particularly to clarithromycin) has rapidly increased in most countries around the world (Thung et al., 2016). Empirical therapies should be based on the rate of change and status of H. pylori antibiotic resistance, and outcomes may be optimized when patient-, regional-, or population-specific susceptibility results are available. Researchers have also discovered and identified novel and effective therapeutic regimens for eradicating H. pylori, such as vonoprazan-containing triple therapies, susceptibility-based therapies, quintuple therapies, high-dose dual therapies, and standard triple therapies with probiotics (Pbs), which are reviewed here. We also focus on the prevalence of H. pylori infection, the reinfection rate of H. pylori in recent years, the trend and mechanisms of H. pylori resistance to antibiotics.
The Prevalence of H. pylori Infection
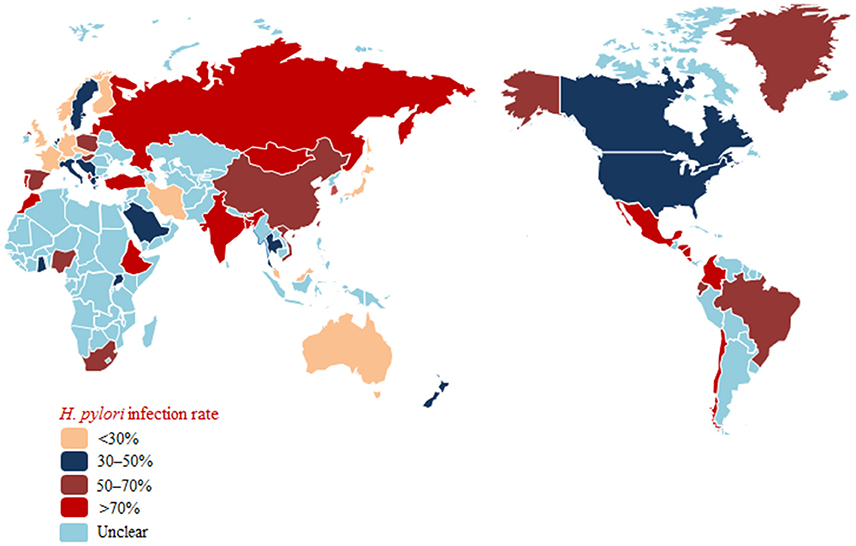
Tremendous differences in the prevalence of H. pylori infection exist worldwide because of prevailing variances in socioeconomic and hygienic conditions. In China, a developing country with a high prevalence of H. pylori infection and a high incidence of gastric cancer, the weighted mean prevalence of H. pylori infection was 55% (range: 28–82% [1983–2013]). The weighted mean prevalence of H. pylori infection was higher in rural Chinese populations (66%) compared with urban Chinese populations (47%). Additionally, a significant trend toward a decreasing prevalence of H. pylori infection was observed in studies that included only urban populations (Nagy et al., 2016). Recently, Pan et al. conducted a large community-based intervention trial in Linqu County that enrolled 184,786 residents aged 25–54 years. The total prevalence of H. pylori was 57.6% (Pan et al., 2016). Zhang et al. compared the H. pylori infection rate in Muping County (with a high incidence of gastric cancer) and Yanqing County (with a low incidence of gastric cancer) in adults and children. The results showed that the prevalence of H. pylori was higher in Muping County (50.95% in adults and 37.69% in children) than in Yanqing County (41.35% in adults and 25.58% in children). Moreover, a significant decrease in the H. pylori prevalence was observed in both regions (Zhang et al., 2009). Ding et al. conducted a prospective, cross-sectional, population-based study from 2009 to 2011 in three cities (Beijing, Guangzhou and Chengdu) in China. Of the included 3,491 healthy children, 237 (6.8%) were diagnosed with H. pylori, and the prevalence of H. pylori infection increased with age in all three cities (Ding et al., 2015). Japan is a country that actively screens for H. pylori and advocates eradication in H. pylori-infected individuals. In 2008, 21,144 healthy Japanese subjects were tested for H. pylori using serum anti-H. pylori antibody measures; 5,815 (27.5%) were H. pylori-positive, and the prevalence of H. pylori gradually decreased over the following 5 years (Hirayama et al., 2014). Similar trends in H. pylori infection rates over 40 years were also identified (Kamada et al., 2015). Overall, the prevalence of H. pylori infection was 74.7% (1970s), 53% (1990s) and 35.1% (2010s). The rapid decrease in H. pylori infection rates may be attributed to the westernization and improvements in economic and hygienic conditions that have occurred in Japan. A low prevalence of H. pylori infection (4.0–6.7%) existed in Japanese children in three different age groups (4, 7, and 10 years) (Naito et al., 2008). In 2011, a cross-sectional, nationwide, multicenter study surveyed anti-H. pylori IgG antibody levels in Korea, a country with a high prevalence of H. pylori infection and a high incidence of gastric cancer. The study found that the H. pylori infection rate was 54.4% (5,873/10,796), which was lower than that in 2005 (59.6%) and 1998 (66.9%) (Lim et al., 2013). In children, the overall prevalence of H. pylori infection was 7.4% (187/2,530) with low rates of H. pylori infection observed in children with recurrent abdominal pain from 2004 to 2007 (70/873, 8.0%), 2008 to 2010 (51/666, 7.7%), and 2011 to 2014 (66/991, 6.7%). However, no decreasing trend in the H. pylori infection rate was observed in Korean children (Jang et al., 2015). In Thailand, a country with a relatively low risk of developing gastric cancer, the H. pylori infection rate was 45.9% (710/1,546 patients from four regions, including 17 provinces) (Uchida et al., 2015). In the rest of the Asia-Pacific region, the prevalence of H. pylori infection varied from 15.5% to 94.3% due to the different geographical regions and ethnic groups (Goh et al., 2011).
In the USA, the weighted mean prevalence of H. pylori infection was 35% (range: 22–48% [1990–2006]), which was lower than that in China. As a developed country, no increasing or decreasing trends in H. pylori infection rate were observed over time in the USA (Nagy et al., 2016). In an interesting study conducted among 1,200 veterans, 347 (28.9%) were diagnosed with H. pylori infection, and even higher rates of H. pylori infection were observed in Blacks and Hispanics with lower education levels (Nguyen et al., 2015). Although socioeconomic and hygienic conditions have improved recently, disparities in the prevalence of this condition remain among different racial groups. Of 176 American children who were evaluated for H. pylori infection using the 13C–urea breath test (UBT), 48 were infected (Elitsur et al., 2009). Herrero et al. (Porras et al., 2013) investigated the prevalence of H. pylori infection in six Latin American countries, and the rate of H. pylori prevalence was 79.4% (range: 70.1–84.7%) among 1,852 eligible adults. Dattoli et al. investigated anti-H. pylori IgG antibody levels in 1,104 Brazilian children, and H. pylori was present in 28.7% (Dattoli et al., 2010).
Roberts et al. selected studies related to H. pylori prevalence conducted in Europe and analyzed the prevalence of H. pylori across 35 European countries and four European regions. Trends regarding H. pylori prevalence with respect to the incidence of gastric cancer were also analyzed. This investigation indicated that the prevalence of H. pylori infection ranged from 17% in Aarhus, Denmark to 88% in St. Petersburg, Russia. Compared with Southern or Eastern Europe, the prevalence of H. pylori was lower in Northern or Western Europe. A sharp decrease in the trend of H. pylori prevalence and gastric cancer incidence was observed throughout Europe (Roberts et al., 2016). In a group of 8,661 symptomatic children from Poland, H. pylori was identified in 1,390 (16.05%) children (Biernat et al., 2016). The H. pylori prevalence was lower in the year 2010 (8.90%) than in the year 2000 (23.06%). In Africa, an area with a high prevalence of H. pylori infection and a low incidence of gastric cancer, the H. pylori infection rate was high in both adults and children (Etukudo et al., 2012; Benajah et al., 2013; Tadesse et al., 2014), which may be attributed to poor socioeconomic and hygienic conditions. However, a retrospective study conducted in Ghana that compared the H. pylori infection rates in patients with upper gastrointestinal symptoms in 1999 and 2012 found a decreasing trend in the prevalence of H. pylori infection, i.e., 69.7% in 1999 and 45.2% in 2012 (Darko et al., 2015).
Taken together, these findings show decreasing rates of H. pylori infection with improvements in socioeconomic and hygienic conditions in most countries (Japan and Korea, among others). However, the H. pylori infection rate remains high in some areas (Russia, South Africa, and Africa, among others) (Figure 1).
Helicobacter pylori Recurrence
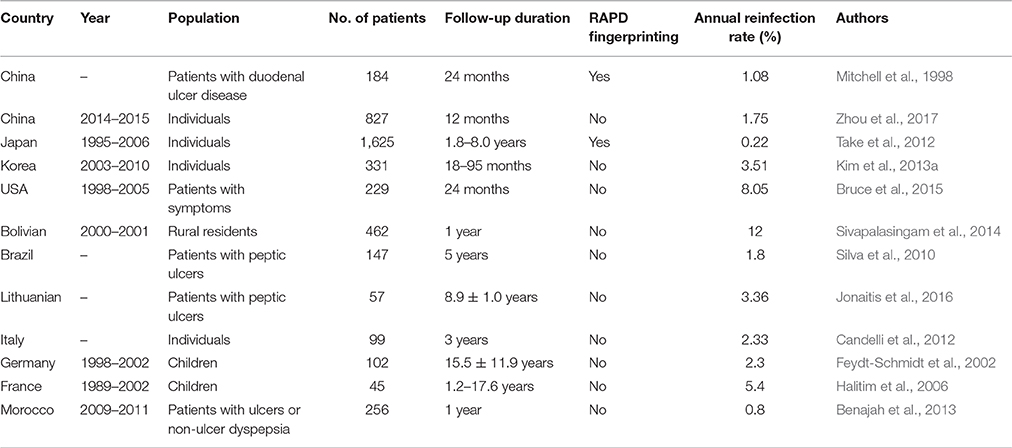
The recurrence of H. pylori remains a serious challenge worldwide, which is associated with living conditions and socioeconomic status. The annual recurrence risk was 3.4% (95% confidence interval [CI], 3.1–3.7%) in high–income countries and 8.7% (95% CI, 8.8–9.6%) in low-income countries (Gisbert, 2005). Yan et al. used the human development index to assess socioeconomic development and reported a significant and inverse correlation between the recurrence rate of H. pylori and the human development index (Yan et al., 2013).
Helicobacter pylori recurrence appears more frequent in developing countries. In 1998, Mitchell et al. investigated the recurrence rate of H. pylori in 184 Chinese patients followed-up by endoscopy and UBT for 24 months; of these, 4 patients became reinfected over 24 months (3 within 6 months and 1 within 24 months; average annual recurrence rate, 1.08%; Mitchell et al., 1998). Recurrence consisted of recrudescence and reinfection, and the true reinfection rate of H. pylori was 0.27% in this study, which is relatively low in comparison with other developing countries. A larger-sample, multicenter follow-up study was conducted in China from 2014 to 2015, which showed that the recurrence of H. pylori infection 1 year after eradication was low (1.75%) in urban population of China (Zhou et al., 2017). In Japan, a long-term prospective study (Take et al., 2012) (up to 12.5 years; mean, 4.7 years) was conducted in 1,625 patients; of these, 17 patients (reinfection rate, 0.22% per year) were strictly reinfected according to random amplification of polymorphic DNA (RAPD) fingerprinting, which indicated that the reinfection rate of H. pylori in the Japanese population was extremely low despite a high prevalence of infection. From 2003 to 2010, a total of 331 patients who maintained an H. pylori-eradicated state were followed-up for 18–95 months (mean, 37.1 months) in Korea, and the annual H. pylori reinfection rate was 3.51%, which was rather low. This study also showed an association between reinfection and both male gender and a low family income (Kim et al., 2013a). Interestingly, Kim et al. further identified the impact of first- and second-line eradication therapies on H. pylori recurrence (Kim et al., 2014). Annual recurrence rates within 2 years of follow-up were 9.3% (first-line therapy) and 4.5% (second-line therapy), and the rates after 2 years of follow-up were 2.0 and 2.9%, respectively. Additionally, Kim et al. compared the influence of a bismuth-containing quadruple therapy (EBMT) with that of a moxifloxacin-based triple therapy (MEA) on the H. pylori reinfection rate. The recrudescence rate of EBMT was 1.7%, and that of MEA was 3.3%; the annual reinfection rate of EBMT was 4.45%, and that of MEA 6.46% (Kim et al., 2013b). Both the recrudescence and reinfection rates of H. pylori showed no significant differences between the two groups, which indicated that reinfection was not an important factor affecting the choice of treatment.
In an Alaskan study that investigated the reinfection rate of H. pylori and the associated risk factors, three groups of patients (group 1: American Indian/Native Alaskan individuals living in urban communities; group 2: persons living in communities; group 3: urban non-Native Alaskans) were enrolled and followed-up for 24 months. An overall reinfection rate of 16.1% was observed (annual reinfection rate, 8.05%), and associations between reinfection and peptic ulcer disease, a low education level, and a higher proportion of household members infected with H. pylori were demonstrated (Bruce et al., 2015). However, this study was not a population-based study and did not distinguish reinfection from recrudescence. In rural Bolivia, 462 patients (including adults and children) diagnosed as H. pylori-positive and cured by triple therapy were followed-up for 1 year and tested for H. pylori reinfection; 12% (95% CI: 8–15) had recurrent infection and the reinfection rate was significantly higher in the children aged <5 years (odds ratios [OR] 2.7, 95% CI: 1.2–5.8) and 5–9 years (OR 2.7, 95% CI: 1.4–5.1) (Sivapalasingam et al., 2014). A similar study (Silva et al., 2010) was conducted in 147 Brazilian patients with peptic ulcer disease; a 5–year follow-up revealed that 10 patients were infected, with an annual reinfection rate of 1.8%, similar to the rates observed in developed countries.
Lithuania had a high prevalence of H. pylori infection; 57 peptic ulcer patients with successfully eradicated H. pylori-positivity were followed-up for 8.9 ± 1.0 years. Reinfection occurred in 17 patients (annual rate of infection, 3.36%; Jonaitis et al., 2016). Candelli et al. evaluated the reinfection rate of H. pylori 3 years after a standard eradication treatment in young diabetic patients and controls and found that the annual H. pylori reinfection rate was higher in young diabetic patients (8%) than in the controls (2.33%); additionally, age and socioeconomic status were confirmed to be independent factors associated with H. pylori reinfection (Candelli et al., 2012). In a population of German children, the calculated H. pylori reinfection rate was 2.3% per person per year (Feydt-Schmidt et al., 2002), which is relatively low. However, the H. pylori reinfection rate was up to 5.4% per patient-year in France (Halitim et al., 2006). A prospective study conducted in Morocco, a developing country in Africa, found that of 256 patients enrolled and followed-up for 12 months, 2 patients (0.8%) became reinfected with H. pylori (Benajah et al., 2013).
The H. pylori reinfection rate varied in different regions (Table 1), and several factors were closely associated with reinfection, including age, education level, proportion of household members infected with H. pylori and socioeconomic status. This study had many limitations, such as the small sample size, short-term duration, inability to distinguish reinfection vs. recrudescence and not being a population-based study. Therefore, long-term prospective studies using large sample sizes are crucial to conduct to investigate the H. pylori reinfection rates in national populations, particularly in developing countries.
Helicobacter pylori Resistance to Antibiotics
In most parts of the world, substantial concern has arisen regarding the increasing rate of antimicrobial resistance, leading to therapeutic regimen failures and low eradication rates. The prevalence of antimicrobial resistance varies in different geographical areas and appears to be changing over time.
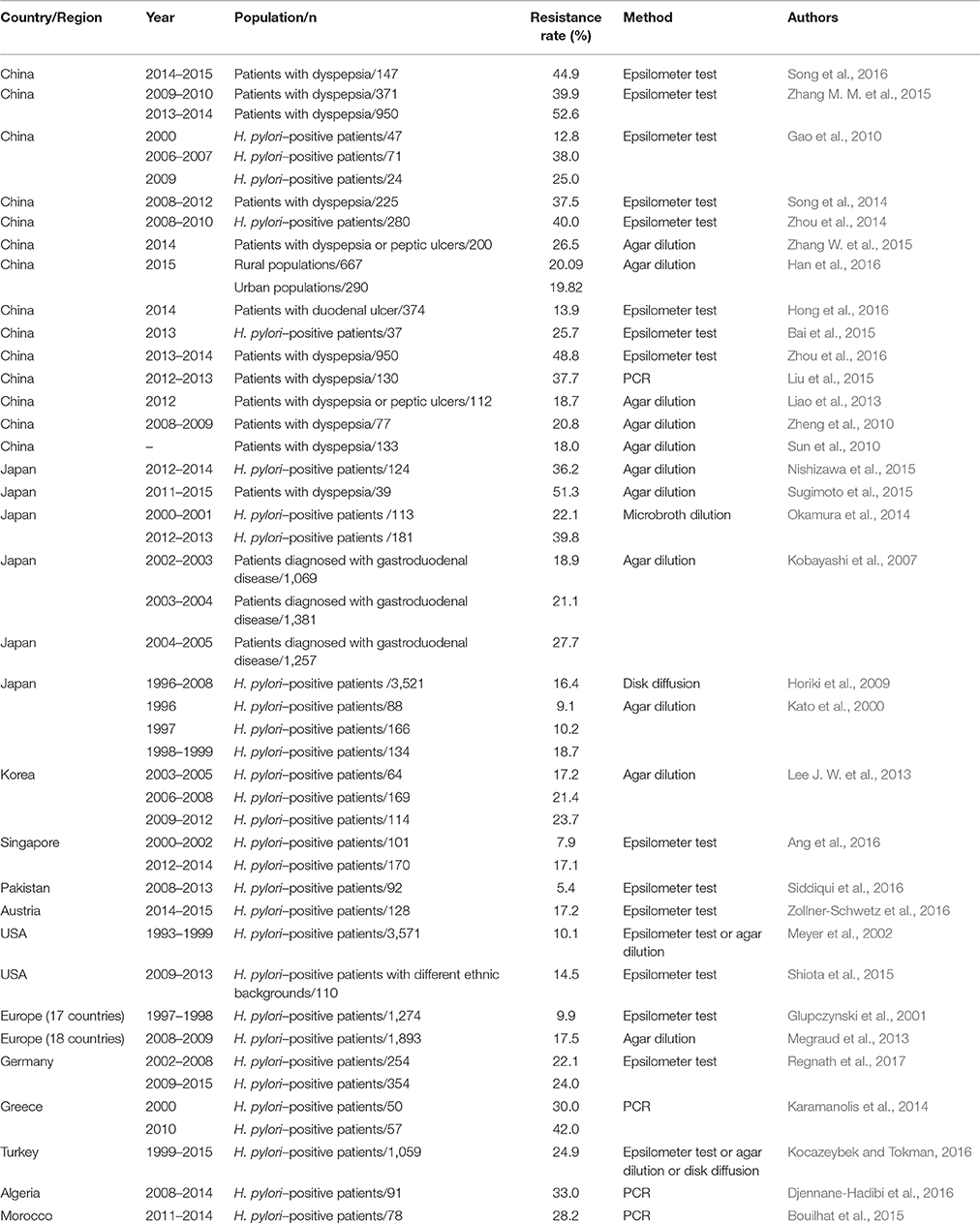
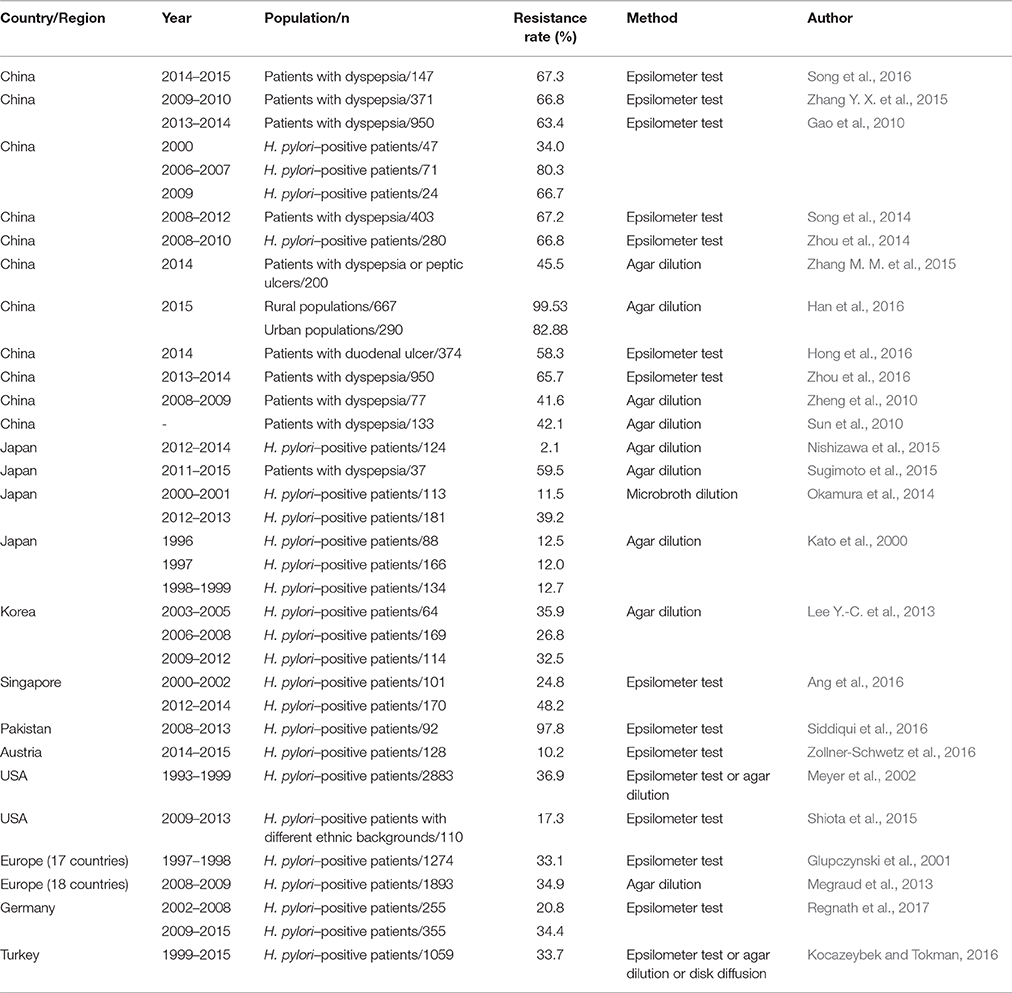
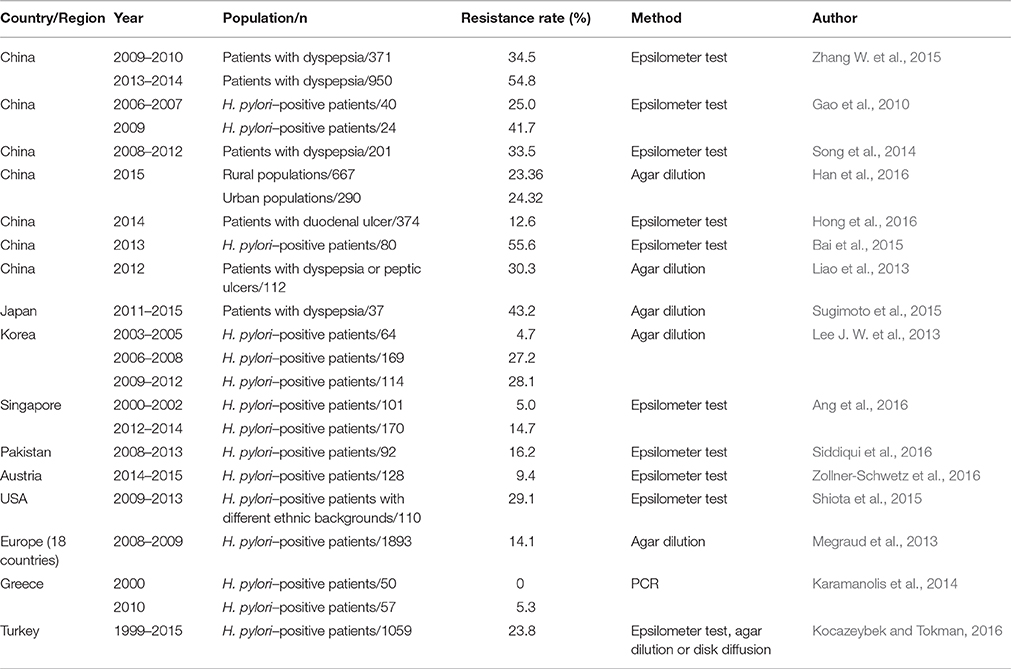
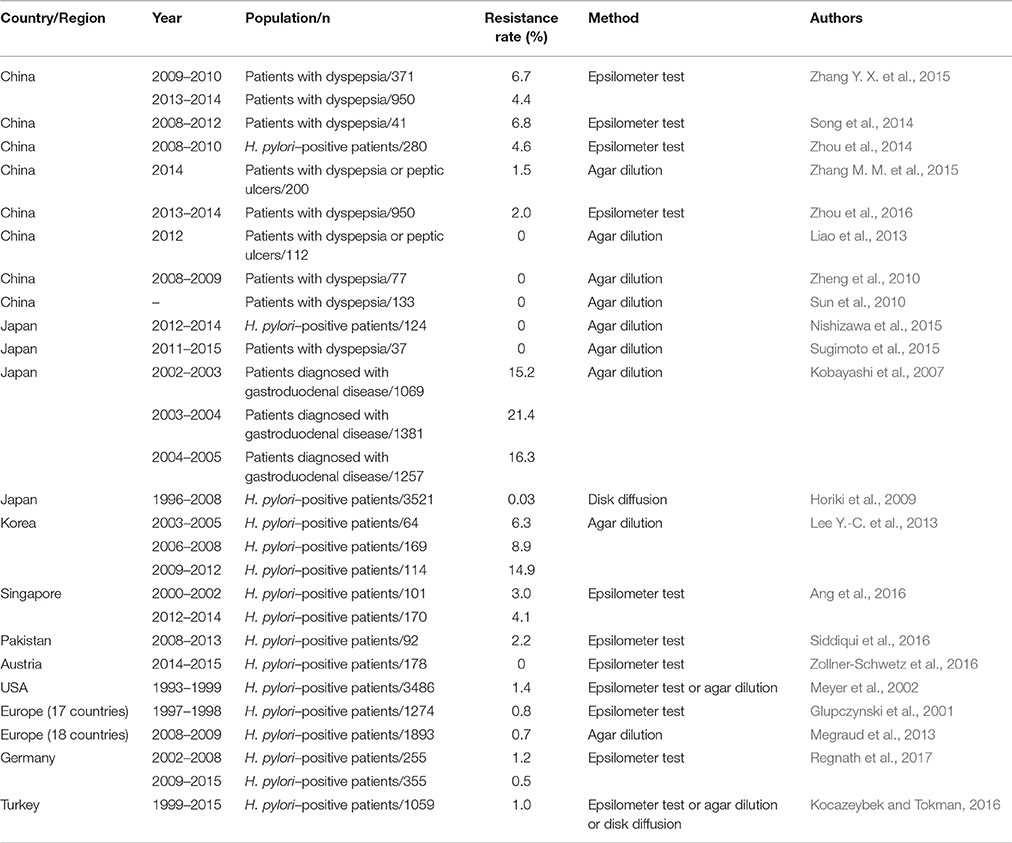
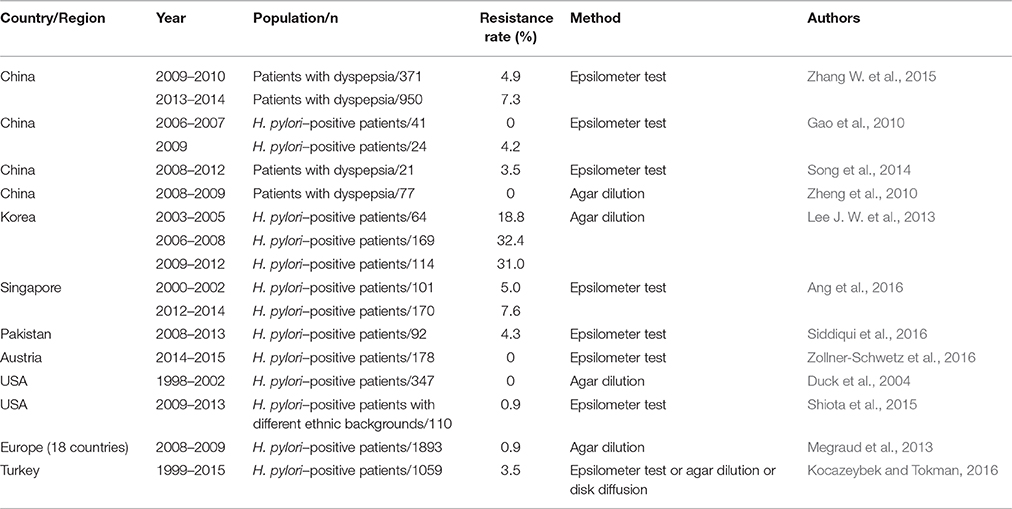
Considering the widespread and improper use of antibiotics in China, H. pylori resistance to antibiotics is more common in this country. Multiple studies have investigated antimicrobial resistance in different regions of China and its alterations over time. Because clarithromycin is a fundamental antibiotic used for eradicating H. pylori, clarithromycin resistance has an impact on the efficacy of regimens and the determination of first-line treatments for H. pylori (Malfertheiner et al., 2016). The resistance rate of clarithromycin is relatively high (13.9–52.6%) in China (Gao et al., 2010; Sun et al., 2010; Zheng et al., 2010; Liao et al., 2013; Su et al., 2013; Song et al., 2014, 2016; Zhou et al., 2014; Bai et al., 2015; Liu et al., 2015; Zhang W. et al., 2015; Zhang Y. X. et al., 2015; Han et al., 2016; Hong et al., 2016; Ji et al., 2016; Zhou et al., 2016), and similar resistance rates have also been observed for metronidazole (41.6–99.5%) (Gao et al., 2010; Sun et al., 2010; Zheng et al., 2010; Su et al., 2013; Song et al., 2014, 2016; Zhou et al., 2014; Bai et al., 2015; Zhang W. et al., 2015; Zhang Y. X. et al., 2015; Han et al., 2016; Hong et al., 2016; Ji et al., 2016), levofloxacin (12.6–54.8%) (Gao et al., 2010; Liao et al., 2013; Su et al., 2013; Song et al., 2014; Zhang Y. X. et al., 2015; Han et al., 2016; Hong et al., 2016; Ji et al., 2016), rifampicin (14.2–18.2%) (Song et al., 2014; Zhang Y. X. et al., 2015) and moxifloxacin (61.9%) (Gao et al., 2010). However, the resistance rates for amoxicillin (0–6.8%) (Sun et al., 2010; Zheng et al., 2010; Liao et al., 2013; Su et al., 2013; Song et al., 2014, 2016; Zhou et al., 2014; Bai et al., 2015; Zhang W. et al., 2015; Zhang Y. X. et al., 2015; Ji et al., 2016), tetracycline (0–7.3%) (Gao et al., 2010; Zheng et al., 2010; Song et al., 2014; Zhang, Y. C. et al., 2015) and furazolidone (0–0.1%) (Su et al., 2013; Ji et al., 2016) were relatively low. Moreover, the rates of clarithromycin, metronidazole and levofloxacin resistance have increased, whereas the rates of amoxicillin and tetracycline resistance have remained stable recently (Gao et al., 2010; Zhang Y. X. et al., 2015). Thus, the eradication rate of standard triple therapies clearly has gradually decreased from 88.54% (pre-2004) to 77.66% (2005–2009) and 71.13% (2010–2013) in China (Wang et al., 2014). In Japan, clarithromycin resistance remains high (16.4–81.1%) (Kobayashi et al., 2007; Horiki et al., 2009; Yamade et al., 2011; Okamura et al., 2014; Nishizawa et al., 2015; Sugimoto et al., 2015, 2017), as did levofloxacin resistance (42.3–43.2%) (Sugimoto et al., 2015, 2017), which was consistent with the rates observed in China. However, the rates of resistance to amoxicillin (0–0.03%) (Horiki et al., 2009; Nishizawa et al., 2015; Sugimoto et al., 2015, 2017) and sitafloxacin (3.1–4.8%) (Sugimoto et al., 2015, 2017) remain low and may be disregarded. Moreover, the resistance rate of metronidazole varied in different studies conducted in Japan (2.1–59.5%) (Okamura et al., 2014; Nishizawa et al., 2015; Sugimoto et al., 2015). Increased rates of clarithromycin and metronidazole resistance were observed despite the decreased prevalence of H. pylori (Kato et al., 2000; Okamura et al., 2014). Primary clarithromycin resistance was evaluated in 347 Korean patients from 2003 to 2005, 2006 to 2008, and 2009 to 2012; the resistance rates were 22.9, 25.5, and 37.0%, respectively, revealing a significantly increased rate of clarithromycin resistance (Lee J. W. et al., 2013), which was consistent with the findings of another study conducted in Korea (An et al., 2013). Similarly, an increasing trend of antibiotic resistance (e.g., to amoxicillin, tetracycline, levofloxacin, and moxifloxacin) was also observed (Lee J. W. et al., 2013). Therefore, the standard triple therapy regimen (consisting of a PPI, amoxicillin, and clarithromycin) was considered ineffective because of the high rate of clarithromycin resistance (Graham, 2015a). A 15-year study was conducted by Ang et al. regarding the changing profile of H. pylori antibiotic resistance in Singapore; in this study, a significant increase in the rates of H. pylori resistance to metronidazole, clarithromycin and levofloxacin was observed (Ang et al., 2016). Surprisingly, the rate of clarithromycin resistance was low (5.4%) in Pakistan, which is considered a nidus of drug-resistant bacteria (Siddiqui et al., 2016). Australia had a relatively low prevalence of H. pylori infection, and the primary rates of clarithromycin, levofloxacin and metronidazole resistance have also remained low (Zollner-Schwetz et al., 2016).
The Surveillance of H. pylori Antimicrobial Resistance Partnership (SHARP) Study included 3,624 strains from clinical studies performed in the USA from 1993 to 1999 (Meyer et al., 2002). The results indicated that overall resistance to clarithromycin, metronidazole, and amoxicillin was 10.1, 36.9 and 1.4%, respectively. The subsequent H. pylori Antimicrobial Resistance Monitoring Program (HARP) study, which included 347 clinical H. pylori isolates collected from 1998 to 2002 (Duck et al., 2004), reported rates of clarithromycin, metronidazole, and amoxicillin resistance of 12.9, 25.1, and 0.9%, respectively. Recently, a multicenter, retrospective cohort study was conducted in different geographic regions in the USA regarding H. pylori clarithromycin resistance, and the overall prevalence of clarithromycin resistance was 31.3% (Park et al., 2016). The increasing trend of clarithromycin resistance was thus confirmed in the USA. Additionally, in a group of veteran patients, the prevalence of resistance to levofloxacin, metronidazole, clarithromycin, tetracycline and amoxicillin was 31.3, 20.3, 16.4, 0.8, and 0%, respectively. Clarithromycin resistance increased from 9.1% during 2009–2010 to 24.2% during 2011–2013 (Shiota et al., 2015). Camargo et al. conducted a systematic review in Latin America; this study included a total of 59 independent studies published up to October 2013 regarding H. pylori antibiotic resistance and found that the overall prevalence of antimicrobial primary resistance was 12% for clarithromycin (n = 35 studies), 53% for metronidazole (n = 34), 4% for amoxicillin (n = 28), 6% for tetracycline (n = 20), 3% for furazolidone (n = 6), and 15% for fluoroquinolones (n = 5) (Camargo et al., 2014).
In 1998, a multicenter survey was conducted in 22 European centers to assess the prevalence of in vitro H. pylori antibiotic resistance (Glupczynski et al., 2001). Among the 1,274 H. pylori isolates analyzed in total, the mean rates of resistance to clarithromycin, metronidazole, and amoxicillin were 9.9, 33.1, and 0.8%, respectively. A subsequent study was conducted in 18 European countries from 2008 to 2009 and reevaluated H. pylori resistance to antibiotics (Megraud et al., 2013). Resistance to clarithromycin increased to 17.5% with 34.9% resistance to metronidazole and 14.1% resistance to levofloxacin. Three antibiotics showed relatively low resistance rates, i.e., 0.7% for amoxicillin, 0.9% for tetracycline and 1.1% for rifabutin. Recently, multiple single-center studies were conducted in Poland, Germany and Greece, and these studies compared the resistance to antibiotics during two periods (Karamanolis et al., 2014; Karpinski et al., 2015; Regnath et al., 2017). Clarithromycin resistance increased in Poland and Greece and remained stable in Germany over time. Additionally, metronidazole resistance increased and amoxicillin resistance remained low in Poland and Germany over time. Kocazeybek et al. summarized the prevalence of primary H. pylori antimicrobial resistance in Turkey, and the results showed that the resistance rates were 24.86% for clarithromycin, 33.75% for metronidazole, 23.77% for levofloxacin, 0.97% for amoxicillin and 3.51% for tetracycline (Kocazeybek and Tokman, 2016). Low H. pylori antibiotic resistance was observed in the Netherlands despite the increasing trend of H. pylori resistance to antibiotics identified in most European countries (Mourad-Baars et al., 2015). In two prospective, multicenter molecular studies conducted in Algeria and Morocco regarding primary H. pylori resistance to clarithromycin (Bouilhat et al., 2015; Djennane-Hadibi et al., 2016), both studies reported high levels of H. pylori resistance to clarithromycin (33% in Algeria and 28.2% in Morocco), which indicated that standard clarithromycin-based triple therapies should not be considered as first-line treatments for H. pylori in these countries. However, low rates of clarithromycin and tetracycline resistance (1.7 and 2.5%, respectively) were observed in the Congo (Ngoyi et al., 2015).
Because antibiotic resistance is the most important factor leading to the failure of eradication regimens, a knowledge of regional antibiotic resistance patterns is crucial. In Japan, an area where metronidazole resistance is almost negligible (Nishizawa et al., 2015), triple therapy (PPI-metronidazole-amoxicillin) still achieves excellent cure rates. However, the circumstances differ in China, an area where high rates of H. pylori resistance to clarithromycin, metronidazole and levofloxacin exist. The eradication rate of standard triple therapies was less than 80%, which was unacceptable. Therefore, bismuth-containing quadruple therapy was recommended as a first-line treatment for H. pylori in China. Currently, primary H. pylori resistance to clarithromycin, metronidazole or levofloxacin is serious in most countries worldwide (Tables 2–4). However, the prevalence of amoxicillin and tetracycline resistance remains low (Tables 5, 6). The effect of clarithromycin and levofloxacin resistance is essentially all-or-none (Graham and Dore, 2016), that is, clarithromycin resistance undermines the efficacy of triple and sequential therapies (Malfertheiner et al., 2016). Resistance to standard metronidazole doses is also essentially all-or-none (Graham and Dore, 2016), considering that metronidazole resistance undermines the efficacy of sequential therapies (Malfertheiner et al., 2016). However, metronidazole resistance may be overcome by using traditional bismuth-containing quadruple therapies (BQTs) when metronidazole is administered at a dose of 1,500 or 1,600 mg per day (Zhang W. et al., 2015). Dual clarithromycin-metronidazole resistance also should be considered because it undermines the efficacy of sequential, hybrid and concomitant therapies (Malfertheiner et al., 2016). For example, the efficacy of a 14-day concomitant therapy (consisting of a PPI, clarithromycin, metronidazole, and amoxicillin, given twice a day) is less than 90% when the dual clarithromycin-metronidazole resistance is greater than 14.9% (Graham et al., 2014).
Antibiotic Resistance Mechanisms
Clarithromycin
Clarithromycin belongs to the macrolide group; its antibacterial action relies on its interaction with the peptidyl transferase loop of the V domain of the 23S ribosomal RNA molecule, which may inhibit bacterial protein synthesis. Point mutations in the V domain of the 23S ribosomal RNA may restrict the affinity between clarithromycin and the peptidyl transferase loop, leading to the inhibition of the interaction between clarithromycin and the 23S ribosomal RNA, which contributes to clarithromycin resistance (Stone et al., 1996). The 23S ribosomal RNA A2143G, A2142G, and A2142C mutations were the most frequent mutations, accounting for 80–90% of clarithromycin resistance (Mégraud, 2004). Moreover, the A2115G, G2141A, G2172T, T2182C, T2190C, C2195T, A2223G, G2224A, G2245T, G2254T, T2289C, and C2611A mutations were also implicated in primary or secondary clarithromycin resistance (Hao et al., 2004; Kim et al., 2008; Rimbara et al., 2008a; Zhu et al., 2013). Additionally, efflux pumps also play important roles in the clarithromycin resistance of H. pylori. Studies have found that Phe-Arg-β-naphthylamide, an efflux pump inhibitor, decreased the minimum inhibitory concentration of antibiotics (Hirata et al., 2010). PPIs have structural similarities with efflux pump inhibitors, which may restrict the efflux pump inhibitor of H. pylori, leading to H. pylori susceptibility to antibiotics (Zhang et al., 2010). Recently, Smiley et al. (2013) used comparative proteomic analyses of clarithromycin-susceptible and -resistant H. pylori strains to identify H. pylori outer membrane proteins (OMPs). Their results showed that iron-regulated membrane protein, urease B, elongation factor thermo unstable, and putative OMP were down-regulated, whereas HopT (BabB) transmembrane protein, HofC, and OMP31 were up-regulated in clarithromycin-resistant H. pylori. This study indicated that the OMP alterations may be involved in the H. pylori resistance to clarithromycin. However, the precise mechanisms remain unclear.
Metronidazole
Metronidazole is a synthetic nitroimidazole. It is activated through a transfer process of one or two electrons, which may induce nitro and superoxide radicals, nitroso derivatives and hydroxylamine, leading to the destruction of the DNA helical structure. This process is particularly active in anaerobic bacteria. Mutations of rdxA, a gene that encodes an oxygen-insensitive NADPH nitroreductase, were the main cause of H. pylori resistance to metronidazole (Goodwin et al., 1998). Additionally, the inactivation of frxA (encoding the NADPH flavin oxidoreductase) and fdxB (encoding the ferredoxin-like protein) may also induce H. pylori resistance to metronidazole (Kim et al., 2009). Recently, Mehrabadi et al. assessed the role of the resistance nodulation cell division (RND) family efflux pump in the metronidazole resistance of H. pylori using RT-PCR; they reported that excess amounts of metronidazole increased the gene expression levels of the outer-membrane protein (TolC) homologs of RND pumps (Mehrabadi et al., 2011). That study proposed that the RND family of efflux pumps may be involved in the metronidazole resistance of H. pylori clinical isolates.
Levofloxacin
Levofloxacin, a fluoroquinolone drug, exerts antibacterial effects through its interaction with DNA gyrase (encoded by gyrA and gyrB). The point mutations in the quinolone resistance-determining regions of gyrA may restrict this process, which contributes to the low- and high-level fluoroquinolone resistance of H. pylori (Tankovic et al., 2003). The most common mutations in the levofloxacin-resistant strains were those at positions 87, 88, 91, 97 of gyrA (Moore et al., 1995; Cattoir et al., 2007). Furthermore, Rimbara et al. proposed that a gyrB mutation at position 463 may also have contributed to fluoroquinolone resistance in H. pylori (Rimbara et al., 2012).
Amoxicillin
Amoxicillin, a beta-lactamase antibiotic, interacts tightly with penicillin-binding proteins (PBPs) and inhibits the synthesis of the cell wall, resulting in bacterial dissolution. To date, the expression of multiple PBPs have been reported in H. pylori. The most common mechanism leading to moderate or low-level amoxicillin resistance was the occurrence of point mutations in the pbp 1A gene (Okamoto et al., 2002). Additionally, pbp 2, pbp 3, hefC, hopC, and hofH mutations were also related to H. pylori resistance to amoxicillin (Rimbara et al., 2008b; Qureshi et al., 2014). Studies also reported that the production of beta-lactamase in H. pylori was associated with high-level amoxicillin resistance (Tseng et al., 2009). Moreover, factors affecting the efflux pump and decreased membrane permeability to amoxicillin may be novel mechanisms of high-level amoxicillin resistance (Godoy et al., 2007).
Tetracycline
Tetracycline, a macrolide antibiotic, restricts the linkage of codon and anticodon at the level of the 30S subunit of ribosomes, resulting in the inhibition of protein synthesis. Resistance to tetracycline was mainly caused by point mutations in tet-1 in 16S rRNA (Gerrits et al., 2002). The number of AGA (926–928) mutations was closely related to the level of tetracycline resistance. Single- and double-base-pair mutations mediated only low-level tetracycline resistance, whereas triple-base-pair 16S rDNA AGA (926–928) mutations mediated high-level resistance (Gerrits et al., 2003). Moreover, Anoushiravani et al. reported that in the presence of carbonyl cyanide m-chlorophenylhydrazone, an inhibitor of proton motive force, the tetracycline minimum inhibitory concentration decreased (Anoushiravani et al., 2009). This study indicated that proton motive force-dependent efflux mechanisms might be involved in the resistance of H. pylori clinical isolates to tetracycline. However, the exact mechanism of the efflux pump in H. pylori resistance to tetracycline warrants further study and characterization.
Rifabutin and Furazolidone
Rifabutin exerts bactericidal effects through its combination with DNA-dependent RNA polymerase and the inhibition of the transcription process. A point mutation of rpoB (coding beta subunits of RNA polymerase) was associated with rifabutin resistance (Heep et al., 2000). Furazolidone, a nitrofuran antibiotic, interferes with the activity of bacterial oxidoreductase, blocking bacterial metabolism. Mutations in the H. pylori porD and oorD genes were associated with furazolidone resistance (Su et al., 2006).
Taken together, numerous studies have reported point mutations as related to antibiotic resistance and novel mechanisms (efflux pump, alterations of the OMPs, membrane permeability, etc.) in the development of H. pylori resistance to antibiotics (Figure 2). However, further research is required to elucidate the roles of the pertinent genes and mutations governing antibiotic resistance. Moreover, the genes and mutations involved in the alterations of the efflux pump and membrane permeability remain unclear. Furthermore, the rapid, efficient and comprehensive analysis of multiple antibiotic resistance remains uncertain.
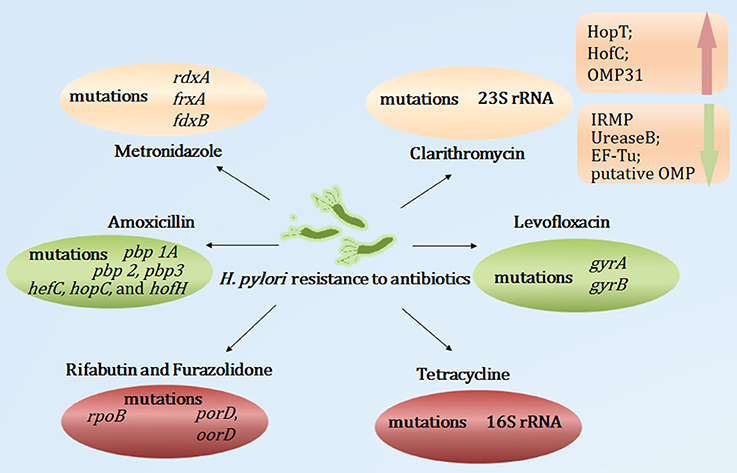
Figure 2. Summary of mutations and novel mechanisms involved in H. pylori antibiotic resistance. OMP, outer membrane protein; IRMP, iron-regulated membrane protein; EF-Tu, elongation factor thermo unstable.
Treatment
The treatment outcome in a population is based on the following formula: (% success with susceptible strains) + (% success with resistant strains) = overall eradication rate (Dore et al., 2016). The eradication rates of different regimens (14-day triple therapies, sequential therapies, concomitant therapies, BQTs, levofloxacin triple therapies or 7-day vonoprazan triple therapies) are greater than 95% in subpopulations with susceptible infections. However, the eradication rates of different regimens varied in subpopulations with resistant infections (Graham and Dore, 2016). Therefore, any improvements in eradication rates should be derived from the effects of regimens on subpopulations with resistant infections.
The Importance of Applying BQTs
Classic BQTs consist of bismuth, a PPI, metronidazole, and tetracycline (Borody et al., 1995), and this type of regimen remains effective in an era of increasing antibiotic resistance (Malfertheiner et al., 2011). Regarding classic BQTs consisting of bismuth, a PPI and two antibiotics, numerous studies have tested different antibiotic combinations, including clarithromycin and amoxicillin (Sun et al., 2010), amoxicillin and furazolidone (Xie et al., 2014), amoxicillin and metronidazole (Zhang, W. et al., 2015), amoxicillin and tetracycline (Liang et al., 2013), and metronidazole and furazolidone (Liang et al., 2013), among others. According to a report card grading of H. pylori therapy (Graham et al., 2007; Graham and Dore, 2016), the efficacy of BQTs is good or fair in most studies conducted in China and Taiwan (Sun et al., 2010; Liang et al., 2013; Xie et al., 2014; Zhang W. et al., 2015; Liou et al., 2016), with the intention-to-treat (ITT) analysis showing an efficacy above 85% and the per protocol (PP) analysis showing an efficacy above 90%. However, some studies in Iran and Turkey reported variable results with triple therapies plus bismuth (Sezgin et al., 2006; Uygun et al., 2008; Minakari et al., 2010; Vafaeimanesh et al., 2013). This discrepancy might be explained by differences in the prevalence of resistance, the cure rate of resistant infections or both. Recently, Graham and Dore conducted a review focused on the role of bismuth in improving H. pylori eradication with triple therapies, and they indicated that the addition of bismuth (i.e., a 14-day triple therapy plus bismuth) might improve cure rates despite a high prevalence of antimicrobial resistance; the major effect of bismuth was a 30–40% increase in the success rate with resistant infections (Graham and Dore, 2016). Comparing the advantages of BQTs (consisting of a PPI plus amoxicillin, bismuth and one antibiotic) and non-BQTs (consisting of a PPI plus amoxicillin and two antibiotics), H. pylori showed only slight resistance to amoxicillin plus bismuth, although H. pylori might potentially be resistant to the antibiotics (except amoxicillin) used in both regimens. Thus, BQTs are superior to non-BQTs in an era of increasing antibiotic resistance, particularly regarding dual resistance because such resistance might undermine the efficacy of non-BQTs. Compared with non-BQTs, BQT regimens include one fewer antibiotic with a wider range of antibiotics available after the failure of a first-line eradication therapy. Additionally, the short-term application of bismuth in the treatment of H. pylori infection is safe (Ford et al., 2008). Thus, BQTs are recommended as first-line treatments for H. pylori infections (Liu et al., 2013; Fallone et al., 2016; Malfertheiner et al., 2016), particularly in areas of high antibiotic resistance.
Selecting Efficient PPIs for a Regimen
The main role of PPIs in the treatment of H. pylori infections is to elevate the gastric pH, leading to an increase in the population of dividing H. pylori. Subsequently, the bacteria become more susceptible to antibiotics such as amoxicillin and clarithromycin (Scott et al., 1998; Graham and Fischbach, 2010). Maintaining the gastric microenvironment at a higher pH (above 6) is a key factor contributing to the efficacy of H. pylori eradication. Lind et al. randomized H. pylori-positive patients (n = 539) with a history of duodenal ulcers into 4 groups: the OAC group (omeprazole, amoxicillin and clarithromycin), the OMC group (omeprazole, metronidazole and clarithromycin), the AC group (amoxicillin and clarithromycin) and the MC group (metronidazole and clarithromycin). The reported treatment success was ~68% higher in the OAC group than in the AC group and 18% higher in the OMC group than in the MC group. In the subpopulation with metronidazole-resistant strains, the H. pylori eradication rate was higher in the OMC group (76%) than in the MC group (43%) (Lind et al., 1999). This study indicated that the addition of a PPI to a regimen improved the eradication rate and reduced the impact of primary resistance. Moreover, the effect of a PPI on acid secretion is dose-independent; thus, a higher PPI dose might increase the gastric pH and improve the cure rate of the regimen (Labenz, 2001; Anagnostopoulos et al., 2004). Gene polymorphisms of CYP2C19 (the principal enzyme implicated in the metabolism of PPIs) also played an important role in the efficiency of H. pylori eradication (Padol et al., 2006). The frequency of CYP2C19 polymorphisms varied in different regions; Caucasian populations had a higher proportion of homozygous extensive metabolizers (70%) than did Asian populations (30–40%) (Kuo et al., 2014). Homozygous extensive metabolizers produce abundant amounts of the enzyme that metabolizes PPI, which is thus metabolized at high rates, leading to a low intragastric pH compared with that of heterozygous extensive metabolizers and poor metabolizers. Sahara et al. compared the acid-inhibitory effects of four PPIs (i.e., omeprazole, lansoprazole, rabeprazole, and esomeprazole). Dosed twice daily, these four PPIs produced sufficient acid suppression in intermediate and rapid CYP2C19 metabolizers. However, 20 mg of esomeprazole twice daily caused the strongest inhibition in rapid CYP2C19 metabolizers. The median pH levels after treatment with esomeprazole, omeprazole, rabeprazole and lansoprazole were 5.4, 5.0, 4.8, and 4.7, respectively (Sahara et al., 2013). Hong et al. conducted an open-label, randomized, single-center clinical trial to evaluate the effect of CYP2C19 polymorphisms on the efficiency of H. pylori eradication in China. The results showed that the H. pylori eradication rate in the group of intermediate and poor metabolizers (89.6%) was higher than that in the group of rapid metabolizers (72.8%) when the patients were administered omeprazole (20 mg), amoxicillin (1,000 mg), clarithromycin (500 mg) and metronidazole (400 mg) for 10 days (all drugs, twice daily). However, no significant differences in the eradication rate between the two groups were observed when the patients were administered esomeprazole (20 mg), amoxicillin (1,000 mg), clarithromycin (500 mg) and metronidazole (400 mg) for 10 days (all drugs, twice daily) (Hong et al., 2016). This study confirmed the effect of CYP2C19 polymorphisms on H. pylori eradication and found esomeprazole to be less influenced by CYP2C19 polymorphisms than omeprazole. Thus, the PPIs less influenced by CYP2C19 polymorphisms, such as esomeprazole or rabeprazole, are recommended for use in H. pylori eradication regimens, particularly in areas with a high proportion of rapid metabolizers, such as Europe and North America (Malfertheiner et al., 2016).
Vonoprazan, a novel potassium-competitive acid blocker, is a potentially important addition to gastroenterological treatments. In animal models, vonoprazan was reported to produce more potent and sustained acid-inhibitory effects and to increase gastric pH levels more than lansoprazole because of its ability to accumulate in high concentrations and be slowly cleared from gastric glands (Hori et al., 2010, 2011; Shin et al., 2011). Recently, a randomized, double-blind, multicenter, parallel-group study (Murakami et al., 2016) was conducted in Japan to assess the efficacy, safety and tolerability of vonoprazan as a component of H. pylori eradication therapy. Helicobacter pylori-positive patients with gastric or duodenal ulcers were divided into four groups: two groups were treated with vonoprazan (20 mg), amoxicillin (750 mg) and clarithromycin (200 or 400 mg) for 7 days; the other two groups were treated with lansoprazole (30 mg), amoxicillin (750 mg) and clarithromycin (200 or 400 mg) for 7 days. Overall, the eradication rate of H. pylori was 92.6% (95% CI, 89.2–95.2%) with vonoprazan vs. 75.9% (95% CI, 70.9–80.5%) with lansoprazole. In the subpopulation with clarithromycin-susceptible strains, a vonoprazan triple therapy was not superior to a lansoprazole triple therapy (eradication rates, 97.6 vs. 97.3%). However, a higher eradication rate was observed with the vonoprazan triple therapy than the lansoprazole triple therapy in the subpopulation with clarithromycin-resistant strains (82.0 vs. 40.0%). Additionally, both the first-line and second-line vonoprazan triple therapies were well tolerated. However, this study raised some concerns. First, the vonoprazan triple therapy cure rate in the clarithromycin-resistant patients was 82%, which was unacceptably low. Second, this therapy appeared unable to maintain the gastric pH above 6 because vonoprazan-amoxicillin failed to achieve >90% cure rates. Finally, the therapy duration was too brief, and the antimicrobial doses were insufficient (Graham, 2017). Further studies regarding the efficacy of vonoprazan-based therapies with higher doses and longer durations are required.
Applying Bacterial Susceptibility-Based Therapy to Avoid Unnecessary Antibiotics
As stated in the Kyoto Global Consensus report on Helicobacter pylori gastritis, H. pylori gastritis should be defined as an infectious disease (Sugano et al., 2015). Any therapy of an infectious disease is largely based on the results of susceptibility testing, and the outcomes of H. pylori eradication are optimized when patient-, regional- or population-specific susceptibility results are available. Theoretically, susceptibility-based therapy is superior to empirical therapy should antibiotic resistance exist in any population (Graham, 2015b). Recently, a meta-analysis was conducted by Chen et al. to compare tailored therapies (based on susceptibility test results and/or CYP2C19 polymorphisms) with empirically chosen regimens; a total of 13 controlled clinical trials with 3,512 participants were included. The results showed that the tailored therapies were superior to the 7-day standard triple therapies (RR = 1.22; 95% CI, 1.16–1.29) and the BQTs (RR = 1.14; 95% CI, 1.07–1.22) in terms of eradication rates. Furthermore, the tailored therapies were superior to the empirical treatments in both Asia and Europe, and the first-line tailored therapies achieved higher eradication rates than the empirical regimens (pooled RR = 1.18; 95% CI, 1.14–1.22). No significant differences in eradication rates were observed between the tailored and empirical rescue regimens (pooled RR = 1.16; 95% CI, 0.96–1.39; Chen et al., 2016). This study had some limitations. First, side and cost effects were not analyzed. Second, the meta-analysis included clinical trials with small sample sizes, which might restrict the application of its findings. Third, because the eradication rate of the 7-day standard triple therapies was less than 80%, it might be unethical to compare the tailored and 7-day standard triple therapies (Graham, 2015b).
Currently, non-BQTs will likely expose most subjects to one or more antibiotics, e.g., patients receiving concomitant therapies would clearly receive at least one unnecessary antibiotic, a situation that not only provides no benefits but also potentially furthers global resistance. As such, susceptibility-based therapies are an alternative. However, the side and cost effects also should be considered when deciding whether to use a susceptibility-based therapy.
Novel Regimens in an Era of Increasing Antibiotic Resistance
Quintuple Therapies
To the best of our knowledge, the first study of a quintuple therapy was performed in Italy via the addition of lactoferrin (bLf) and Pbs to a standard triple therapy (de Bortoli et al., 2007). This study randomized 206 H. pylori-positive patients into two groups: 101 patients (group A), who were administered a standard triple therapy (esomeprazole, clarithromycin, amoxicillin) for 7 days; and 105 patients, who were administered a standard triple therapy plus bLf and Pbs (group B). The results showed a higher eradication rate in group B than in group A (ITT: 88.6 vs. 72.5%, PP: 92.1 vs. 76.0%). Furthermore, significant differences were observed in the side effects between groups A (40.6%) and B (9.5%). This study indicated that the addition of bLf and Pbs to a standard triple therapy improved the efficiency of H. pylori eradication and reduced side effects. However, the reported side effects were too high in the standard triple therapy group (40.6%), and the effects of cost were not considered (Zullo et al., 2007). Moreover, this study failed to identify whether the improved eradication rate and reduced side effects were attributable to the bLf or Pbs. In Turkey, a country with a high prevalence of clarithromycin resistance (24.9%) (Kocazeybek and Tokman, 2016), a prospective study (including 144 patients with H. pylori) was conducted to assess the efficiency, tolerability, and patient compliance of a 5-day concomitant BQT as a first-line treatment for H. pylori; the results showed that the eradication rate of this regimen was greater than 93.1% in the ITT analysis and 95.7% in the PP analysis. Good compliance (97.2%) and a relatively low rate of side effects (8.5%) were also observed in this study (Dolapcioglu et al., 2016). This study did not show that the quintuple therapy was superior to other regimens because it lacked regimens for comparison and because the effects of the quintuple therapy on the subpopulation with resistant strains were unclear. Another study was conducted in Iran to evaluate the efficiency of 7 days of a quintuple therapy as a rescue treatment for H. pylori; the results showed a fair eradication rate and a high rate of side effects (Mansour-Ghanaei et al., 2015).
Theoretically, the eradication rate of a quintuple therapy is expected to be high due to the addition of bismuth to a concomitant quintuple therapy. Some questions remain concerning this type of regimen. First, the side and cost effects should be considered because the regimen involves five drugs. Second, the effects of a quintuple therapy on subpopulations with resistant strains are unclear. Third, the efficacy of the different durations of this regimen (e.g., 5, 7, 10, or 14 days) are unknown. Taken together, these findings indicate that quintuple therapies are a new type of treatment for H. pylori, and further studies are required to identify their efficiency.
High-Dose Dual Therapy
In the late 1980s, dual therapies (a PPI and amoxicillin) were introduced to eradicate H. pylori. Dual therapies achieved low eradication rates because they were unable to maintain the intragastric pH above 6, which influenced the effect of amoxicillin on H. pylori (Graham et al., 2014). Subsequently, researchers focused on the effects of high-dose dual therapies on H. pylori eradication. The results varied in different countries. In Taiwan, a high-dose dual therapy (rabeprazole 20 mg qid and amoxicillin 750 mg qid) for 14 days achieved a 95.3% success rate as a first-line treatment for H. pylori eradication and an 89.3% success rate as a second-line treatment (Yang et al., 2015). Similarly, Zullo et al. showed that a 10-day high-dose dual therapy (esomeprazole 40 mg tid and amoxicillin 1 g tid) was effective and safe as a first-line treatment for H. pylori infection in Italy (Zullo et al., 2015). Furthermore, a 14-day high-dose PPI-amoxicillin dual therapy (rabeprazole 20 mg tid and amoxicillin 1 g tid) followed by a PPI-amoxicillin-levofloxacin triple therapy achieved high H. pylori eradication rates after the failures of other treatments (Goh et al., 2012). However, in the USA, a poor eradication rate was observed when H. pylori-positive individuals were administered a high-dose dual therapy for 14 days (Graham et al., 2010; Attumi and Graham, 2014), which was consistent with results from Korea (Kwack et al., 2016). The failure of these high-dose dual therapies may be attributed to their inability to maintain the intragastric pH above 6. Additionally, the proportion of rapid CYP2C19 metabolizers is also a factor that influences the efficiency of high-dose dual therapies, which may restrict its application in Western countries, where the proportion of rapid CYP2C19 metabolizers is high (Kuo et al., 2014).
The Addition of Pbs to Standard Triple Therapy
Pbs are defined as living bacteria that confer health benefits to the host when administered in sufficient quantities. Pbs have been applied in the prevention and treatment of gastrointestinal diseases (Sarowska et al., 2013), including diarrhea, inflammatory bowel disease, and irritable bowel syndrome. The pertinent mechanisms of Pbs include their beneficial effects on the microbiota, their anti-allergenic effects and their immune system enhancement, among others. Diarrhea, nausea, vomiting, bloating, and abdominal pain may occur in the process of H. pylori eradication because of the antibiotics used in the regimens. Numerous meta-analyses have implicated the importance of Pbs (mainly Lactobacillus or Saccharomyces boulardii or Bacillus clausii) in improving H. pylori-related side effects (Nista et al., 2004; Tong et al., 2007; Zou et al., 2009; Szajewska et al., 2010, 2015; Wang et al., 2013; Dang et al., 2014; Zhang M. M. et al., 2015; McFarland et al., 2016). One meta-analysis (Zou et al., 2009) proposed that certain types of multi-strain probiotic mixtures might reduce adverse events and antibiotic-associated diarrhea; the results showed that five mixtures (L. acidophilus/B. animalis, L. acidophilus/B. bifidum, L. helveticus/L. rhamnosus, L. acidophilus/B. longum/E. faecalis and the eight-strain mixture) administered with standard triple therapies significantly reduced the incidence of adverse events and that three multi-strain Pbs (L. acidophilus/B. animalis, L. acidophilus/B. bifidum, and the eight-strain mixture) were effective in reducing the incidence of antibiotic-associated diarrhea. Additionally, Emara et al. focused on the emerging role of Pbs from a histopathological perspective and found that Pbs lowered H. pylori density on the luminal side of the epithelium, thereby improving the histological inflammatory and activity scores in the gastric corpus and antrum over an extended period (Emara et al., 2016). Moreover, H. pylori eradication influenced the gut microbiota (Jakobsson et al., 2010; Malfertheiner et al., 2016); the addition of Pbs (e.g., Streptococcus faecium and Bacillus subtilis) to standard triple therapies may restrict the growth of antibiotic-resistant bacteria and reduce the fluctuation of the gut microbiota (Oh et al., 2016).
Multiple meta-analyses have also shown the important role of Pbs (mainly Lactobacillus or Saccharomyces boulardii or Bacillus clausii) in improving the H. pylori eradication rate (Nista et al., 2004; Tong et al., 2007; Zou et al., 2009; Szajewska et al., 2010, 2015; Wang et al., 2013; Dang et al., 2014; Zhang M. M. et al., 2015; McFarland et al., 2016). The inhibitory effect of Pbs on H. pylori may be attributed to their abilities to produce antioxidants and antimicrobial substances, alter local pH, and affect H. pylori colonization and adherence to gastric cells (Ruggiero, 2014). McFarland et al. proposed that four probiotic mixtures (L. acidophilus/B. animalis, L. helveticus/L. rhamnosus, L. acidophilus/B. longum/E. faecalis and the eight-strain mixture) were significantly effective (eradication rates higher than 90%) as an adjunct treatment for H. pylori eradication; in this study, Pbs were used at high doses (greater than 1010 CFU/day) and for long durations (3–5 weeks), and the multi-strain Pbs achieved >90% eradication rates (McFarland et al., 2016).
Pbs have been implicated in improving the H. pylori eradication rate, reducing the side effects of regimens and the fluctuations of the gut microbiota. However, the role of specific Pbs strains, dosages, and treatment durations concerning these three effects remain uncertain; thus, further research is required.
Conclusions
A decreasing trend in H. pylori prevalence was observed in most countries partly due to improvements in socioeconomic and hygienic conditions. However, the prevalence of H. pylori infection has remained high (more than 50%) in some regions, such as China, Korea, Mongolia, Russia, Latin America, and African countries. Additionally, H. pylori reinfection varies in different countries and presents a serious challenge, which should be considered. Currently, the data concerning H. pylori reinfection are insufficient, and further long-term, population-based prospective studies should be conducted to characterize the alterations and trends in H. pylori reinfection.
A standard triple therapy regimen is not recommended as a first-line treatment for H. pylori because it achieves a relatively low eradication rate. The most important factor contributing to the inefficiency of a standard triple therapy was antibiotic resistance. Helicobacter pylori monoresistance to clarithromycin is common in most countries (e.g., China, Japan, Korea, Germany, and Turkey), with rates higher than 20%, which restricts the application of clarithromycin in the treatment of H. pylori in these areas. Similar results were also observed concerning metronidazole and levofloxacin. However, resistance to metronidazole may be overcome by increasing the dose to 1,500 or 1,600 mg per day. The rates of resistance to amoxicillin and tetracycline were negligible. Multiple mechanisms are involved in the resistance of H. pylori to antibiotics, including point mutations, the efflux pump, alterations of the OMPs, and altered membrane permeability, among others. However, the exact mechanisms of antibiotic resistance remain unclear.
With the changing profile of H. pylori antibiotic resistance, empirical therapies are being challenged and are not as effective as they once were. The use of unnecessary antibiotics in regimens should be avoided because such use may influence the regimen efficacy and may further worsen resistance circumstances. BQTs are still effective and safe in most countries; the addition of bismuth to triple therapies led to 30–40% improvements in the cure rates of subpopulations with resistant strains. Additionally, effective PPIs should be selected to maintain the intragastric pH above 6. Currently, although 7-day vonoprazan-containing triple therapies achieve >90% cure rates and are well tolerated, further studies are warranted regarding the efficacy of vonoprazan-containing therapies at higher doses and for longer durations. Furthermore, susceptibility-based therapies are alternatives in an era of increasing antibiotic resistance because they avoid the use of unnecessary antibiotics. Some studies continue to focus on the efficacy and safety of novel regimens for treating H. pylori infections, including quintuple therapies, high-dose dual therapies, and standard triple therapies with Pbs. Although some promising results have been reported, the evidence is insufficient.
In conclusion, H. pylori infection and reinfection remain serious challenges, H. pylori resistance to clarithromycin, metronidazole or levofloxacin is common, and H. pylori resistance to amoxicillin and tetracycline is negligible. The importance of applying BQTs and susceptibility-based therapies, selecting efficient PPIs for regimens and identifying novel and effective regimens for treating H. pylori infections should not be underestimated.
Author Contributions
YH wrote the manuscript; YZ and NL revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81270479, 81460116, 81470832 and 81670507), the Graduate Innovation Fund of Jiangxi Province (YC2016–B025), grants from the Jiangxi Province Talent 555 Project, and the National Science and Technology Major Projects for “Major New Drug Innovation and Development” of China (No. 2011ZX09302–007–03).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor David Y. Graham at Baylor College of Medicine for providing suggestions for this review.
References
An, B., Moon, B. S., Kim, H., Lim, H. C., Lee, Y. C., Lee, G., et al. (2013). Antibiotic resistance in Helicobacter pylori strains and its effect on H. pylori eradication rates in a single center in Korea. Ann. Lab. Med. 33, 415–419. doi: 10.3343/alm.2013.33.6.415
Anagnostopoulos, G. K., Tsiakos, S., Margantinis, G., Kostopoulos, P., and Arvanitidis, D. (2004). Esomeprazole versus omeprazole for the eradication of Helicobacter pylori infection: results of a randomized controlled study. J. Clin. Gastroenterol. 38, 503–506. doi: 10.1097/01.mcg.0000129061.54277.c6
Ang, T. L., Fock, K. M., Ang, D., Kwek, A. B., Teo, E. K., and Dhamodaran, S. (2016). The changing profile of Helicobacter pylori antibiotic resistance in Singapore: a 15-year study. Helicobacter 21, 261–265. doi: 10.1111/hel.12291
Anoushiravani, M., Falsafi, T., and Niknam, V. (2009). Proton motive force-dependent efflux of tetracycline in clinical isolates of Helicobacter pylori. J. Med. Microbiol. 58, 1309–1313. doi: 10.1099/jmm.0.010876-0
Attumi, T. A., and Graham, D. Y. (2014). High-dose extended-release lansoprazole (dexlansoprazole) and amoxicillin dual therapy for Helicobacter pylori infections. Helicobacter 19, 319–322. doi: 10.1111/hel.12126
Bai, P., Zhou, L. Y., Xiao, X. M., Luo, Y., and Ding, Y. (2015). Susceptibility of Helicobacter pylori to antibiotics in Chinese patients. J. Dig. Dis. 16, 464–470. doi: 10.1111/1751-2980.12271
Benajah, D. A., Lahbabi, M., Alaoui, S., El Rhazi, K., El Abkari, M., Nejjari, C., et al. (2013). Prevalence of Helicobacter pylori and its recurrence after successful eradication in a developing nation (Morocco). Clin. Res. Hepatol. Gastroenterol. 37, 519–526. doi: 10.1016/j.clinre.2013.02.003
Biernat, M. M., Iwanczak, B., Binkowska, A., Grabinska, J., and Gosciniak, G. (2016). The prevalence of Helicobacter pylori infection in symptomatic children: a 13-year observational study in the lower Silesian region. Adv. Clin. Exp. Med. 25, 303–308. doi: 10.17219/acem/44372
Borody, T. J., Andrews, P., Fracchia, G., Brandl, S., Shortis, N. P., and Bae, H. (1995). Omeprazole enhances efficacy of triple therapy in eradicating Helicobacter pylori. Gut 37, 477–481. doi: 10.1136/gut.37.4.477
Bouilhat, N., Burucoa, C., Benkirane, A., El Idrissi-Lamghari, A., Al Bouzidi, A., El Feydi, A., et al. (2015). High-level primary clarithromycin resistance of Helicobacter pylori in Morocco: a prospective multicenter molecular study. Helicobacter 20, 422–423. doi: 10.1111/hel.12219
Bruce, M. G., Bruden, D. L., Morris, J. M., Reasonover, A. L., Sacco, F., Hurlburt, D., et al. (2015). Reinfection after successful eradication of Helicobacter pylori in three different populations in Alaska. Epidemiol. Infect. 143, 1236–1246. doi: 10.1017/S0950268814001770
Camargo, M. C., Garcia, A., Riquelme, A., Otero, W., Camargo, C. A., Hernandez-Garcia, T., et al. (2014). The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am. J. Gastroenterol. 109, 485–495. doi: 10.1038/ajg.2014.24
Candelli, M., Rigante, D., Schiavino, A., Gabrielli, M., Crea, F., Minguell, D. L. L., et al. (2012). High reinfection rate of Helicobacter pylori in young type 1 diabetic patients: a three-year follow-up study. Eur. Rev. Med. Pharmacol. Sci. 16, 1468–1472.
Cattoir, V., Nectoux, J., Lascols, C., Deforges, L., Delchier, J. C., Megraud, F., et al. (2007). Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int. J. Antimicrob. Agents 29, 389–396. doi: 10.1016/j.ijantimicag.2006.11.007
Chen, H., Dang, Y., Zhou, X., Liu, B., Liu, S., and Zhang, G. (2016). Tailored therapy versus empiric chosen treatment for Helicobacter pylori eradication: a meta-analysis. Medicine (Baltimore) 95:e2750. doi: 10.1097/md.0000000000002750
Dang, Y., Reinhardt, J. D., Zhou, X., and Zhang, G. (2014). The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS ONE 9:e111030. doi: 10.1371/journal.pone.0111030
Darko, R., Yawson, A. E., Osei, V., Owusu-Ansah, J., and Aluze-Ele, S. (2015). Changing Patterns of the prevalence of Helicobacter pylori among patients at a corporate hospital in Ghana. Ghana Med. J. 49, 147–153. doi: 10.4314/gmj.v49i3.4
Dattoli, V. C., Veiga, R. V., da Cunha, S. S., Pontes-de-Carvalho, L. C., Barreto, M. L., and Alcantara-Neves, N. M. (2010). Seroprevalence and potential risk factors for Helicobacter pylori infection in Brazilian children. Helicobacter 15, 273–278. doi: 10.1111/j.1523-5378.2010.00766.x
de Bortoli, N., Leonardi, G., Ciancia, E., Merlo, A., Bellini, M., Costa, F., et al. (2007). Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am. J. Gastroenterol. 102, 951–956. doi: 10.1111/j.1572-0241.2007.01085.x
Ding, Z., Zhao, S., Gong, S., Li, Z., Mao, M., Xu, X., et al. (2015). Prevalence and risk factors of Helicobacter pylori infection in asymptomatic Chinese children: a prospective, cross-sectional, population-based study. Aliment. Pharmacol. Ther. 42, 1019–1026. doi: 10.1111/apt.13364
Djennane-Hadibi, F., Bachtarzi, M., Layaida, K., Arous, N. A., Nakmouche, M., Saadi, B., et al. (2016). High-level primary clarithromycin resistance of Helicobacter pylori in Algiers, Algeria: a prospective multicenter molecular study. Microb. Drug Resist. 22, 223–226. doi: 10.1089/mdr.2015.0209
Dolapcioglu, C., Sayiner, M., Akkus, E. E., Kural, A., Dolapcioglu, H., Dabak, R., et al. (2016). First-line bismuth-containing five-day concomitant quintuple therapy for Helicobacter pylori eradication. Helicobacter 21, 100–105. doi: 10.1111/hel.12241
Dore, M. P., Lu, H., and Graham, D. Y. (2016). Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 65, 870–878. doi: 10.1136/gutjnl-2015-311019
Duck, W. M., Sobel, J., Pruckler, J. M., Song, Q., Swerdlow, D., Friedman, C., et al. (2004). Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg. Infect. Dis. 10, 1088–1094. doi: 10.3201/eid1006.030744
Elitsur, Y., Tolia, V., Gilger, M. A., Reeves-Garcia, J., Schmidt-Sommerfeld, E., Opekun, A. R., et al. (2009). Urea breath test in children: the United States prospective, multicenter study. Helicobacter 14, 134–140. doi: 10.1111/j.1523-5378.2009.00670.x
Emara, M. H., Elhawari, S. A., Yousef, S., Radwan, M. I., and Abdel-Aziz, H. R. (2016). Emerging role of probiotics in the management of Helicobacter pylori infection: histopathologic perspectives. Helicobacter 21, 3–10. doi: 10.1111/hel.12237
Etukudo, O. M., Ikpeme, E. E., and Ekanem, E. E. (2012). Seroepidemiology of Helicobacter pylori infection among children seen in a tertiary hospital in Uyo, southern Nigeria. Pan. Afr. Med. J. 12, 39.
Fallone, C. A., Chiba, N., van Zanten, S. V., Fischbach, L., Gisbert, J. P., Hunt, R. H., et al. (2016). The toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151, 51-69.e14. doi: 10.1053/j.gastro.2016.04.006
Fennerty, M. B., Kovacs, T. O., Krause, R., Haber, M., Weissfeld, A., Siepman, N., et al. (1998). A comparison of 10 and 14 days of lansoprazole triple therapy for eradication of Helicobacter pylori. Arch. Intern. Med. 158, 1651–1656. doi: 10.1001/archinte.158.15.1651
Feydt-Schmidt, A., Kindermann, A., Konstantopoulos, N., Demmelmair, H., Ballauff, A., Findeisen, A., et al. (2002). Reinfection rate in children after successful Helicobacter pylori eradication. Eur. J. Gastroenterol. Hepatol. 14, 1119–1123. doi: 10.1097/00042737-200210000-00013
Ford, A. C., Forman, D., Hunt, R. H., Yuan, Y., and Moayyedi, P. (2014). Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 348:g3174. doi: 10.1136/bmj.g3174
Ford, A. C., Malfertheiner, P., Giguere, M., Santana, J., Khan, M., and Moayyedi, P. (2008). Adverse events with bismuth salts for Helicobacter pylori eradication: systematic review and meta-analysis. World J. Gastroenterol. 14, 7361–7370. doi: 10.3748/wjg.14.7361
Gao, W., Cheng, H., Hu, F., Li, J., Wang, L., Yang, G., et al. (2010). The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter 15, 460–466. doi: 10.1111/j.1523-5378.2010.00788.x
Gerrits, M. M., Berning, M., Van Vliet, A. H., Kuipers, E. J., and Kusters, J. G. (2003). Effects of 16S rRNA gene mutations on tetracycline resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 47, 2984–2986. doi: 10.1128/AAC.47.9.2984-2986.2003
Gerrits, M. M., de Zoete, M. R., Arents, N. L. A., Kuipers, E. J., and Kusters, J. G. (2002). 16S rRNA mutation-mediated tetracycline resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 46, 2996–3000. doi: 10.1128/AAC.46.9.2996-3000.2002
Gisbert, J. P. (2005). The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review. Am. J. Gastroenterol. 100, 2083–2099. doi: 10.1111/j.1572-0241.2005.50043.x
Glupczynski, Y., Megraud, F., Lopez-Brea, M., and Andersen, L. P. (2001). European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 20, 820–823. doi: 10.1007/s100960100611
Godoy, A. P. O., Reis, F. C., Ferraz, L. F. C., Gerrits, M. M., Mendonça, S., Kusters, J. G., et al. (2007). Differentially expressed genes in response to amoxicillin in Helicobacter pylori analyzed by RNA arbitrarily primed PCR. FEMS Immunol. Med. Microbiol. 50, 226–230. doi: 10.1111/j.1574-695X.2006.00209.x
Goh, K. L., Chan, W. K., Shiota, S., and Yamaoka, Y. (2011). Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 16, 1–9. doi: 10.1111/j.1523-5378.2011.00874.x
Goh, K. L., Manikam, J., and Qua, C. S. (2012). High-dose rabeprazole-amoxicillin dual therapy and rabeprazole triple therapy with amoxicillin and levofloxacin for 2 weeks as first and second line rescue therapies for Helicobacter pylori treatment failures. Aliment. Pharmacol. Ther. 35, 1097–1102. doi: 10.1111/j.1365-2036.2012.05054.x
Goodwin, A., Kersulyte, D., Sisson, G., Veldhuyzen van Zanten, S. J., Berg, D. E., and Hoffman, P. S. (1998). Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28, 383–393. doi: 10.1046/j.1365-2958.1998.00806.x
Graham, D. Y. (2015a). Roadmap for elimination of gastric cancer in Korea. Korean J. Intern. Med. 30, 133–139. doi: 10.3904/kjim.2015.30.2.133
Graham, D. Y. (2015b). Editorial-avoiding unethical Helicobacter pylori clinical trials: susceptibility-based studies and probiotics as adjuvants. Helicobacter 20, 321–325. doi: 10.1111/hel.12244
Graham, D. Y. (2017). Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 66, 384–386. doi: 10.1136/gutjnl-2016-311796
Graham, D. Y., and Dore, M. P. (2016). Helicobacter pylori therapy: a paradigm shift. Expert Rev. Anti Infect. Ther. 14, 577–585. doi: 10.1080/14787210.2016.1178065
Graham, D. Y., and Fischbach, L. (2010). Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59, 1143–1153. doi: 10.1136/gut.2009.192757
Graham, D. Y., Javed, S. U., Keihanian, S., Abudayyeh, S., and Opekun, A. R. (2010). Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: studies from the United States. J. Gastroenterol. 45, 816–820. doi: 10.1007/s00535-010-0220-x
Graham, D. Y., Lee, Y. C., and Wu, M. S. (2014). Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin. Gastroenterol. Hepatol. 12, 177–186.e173; Discussion e112-173. doi: 10.1016/j.cgh.2013.05.028
Graham, D. Y., Lu, H., and Yamaoka, Y. (2007). A report card to grade Helicobacter pylori therapy. Helicobacter 12, 275–278. doi: 10.1111/j.1523-5378.2007.00518.x
Halitim, F., Vincent, P., Michaud, L., Kalach, N., Guimber, D., Boman, F., et al. (2006). High rate of Helicobacter pylori reinfection in children and adolescents. Helicobacter 11, 168–172. doi: 10.1111/j.1523-5378.2006.00396.x
Han, R., Lu, H., Jiang, M. W., Tan, K. W., Peng, Z., Hu, J. L., et al. (2016). Multicenter study of antibiotic resistance profile of H. pylori and distribution of CYP2C19 gene polymorphism in rural population of chongqing, China. Gastroenterol. Res. Pract. 2016:8547686. doi: 10.1155/2016/8547686
Hao, Q., Li, Y., Zhang, Z. J., Liu, Y., and Gao, H. (2004). New mutation points in 23S rRNA gene associated with Helicobacter pylori resistance to clarithromycin in northeast China. World J. Gastroenterol. 10, 1075–1077. doi: 10.3748/wjg.v10.i7.1075
Heep, M., Rieger, U., Beck, D., and Lehn, N. (2000). Mutations in the beginning of the rpoB gene can induce resistance to rifamycins in both Helicobacter pylori and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44, 1075–1077. doi: 10.1128/AAC.44.4.1075-1077.2000
Hirata, K., Suzuki, H., Nishizawa, T., Tsugawa, H., Muraoka, H., Saito, Y., et al. (2010). Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J. Gastroenterol. Hepatol. 25, S75–S79. doi: 10.1111/j.1440-1746.2009.06220.x
Hirayama, Y., Kawai, T., Otaki, J., Kawakami, K., and Harada, Y. (2014). Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J. Gastroenterol. Hepatol. 29, 16–19. doi: 10.1111/jgh.12795
Hong, J., Shu, X., Liu, D., Zhu, Y., Xie, C., Xie, Y., et al. (2016). Antibiotic resistance and CYP2C19 polymorphisms affect the efficacy of concomitant therapies for Helicobacter pylori infection: an open-label, randomized, single-centre clinical trial. J. Antimicrob. Chemother. 71, 2280–2285. doi: 10.1093/jac/dkw118
Hori, Y., Imanishi, A., Matsukawa, J., Tsukimi, Y., Nishida, H., Arikawa, Y., et al. (2010). 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin e monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J. Pharmacol. Exp. Ther. 335, 231–238. doi: 10.1124/jpet.110.170274
Hori, Y., Matsukawa, J., Takeuchi, T., Nishida, H., Kajino, M., and Inatomi, N. (2011). A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J. Pharmacol. Exp. Ther. 337, 797–804. doi: 10.1124/jpet.111.179556
Horiki, N., Omata, F., Uemura, M., Suzuki, S., Ishii, N., Iizuka, Y., et al. (2009). Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter 14, 86–90. doi: 10.1111/j.1523-5378.2009.00714.x
Jakobsson, H. E., Jernberg, C., Andersson, A. F., Sjolund-Karlsson, M., Jansson, J. K., and Engstrand, L. (2010). Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One 5:e9836. doi: 10.1371/journal.pone.0009836
Jang, K. M., Choe, B.-H., Choe, J. Y., Hong, S. J., Park, H. J., Chu, M. A., et al. (2015). Changing prevalence of Helicobacter pylori infections in Korean children with recurrent abdominal pain. Pediatr. Gastroenterol. Hepatol. Nutr. 18, 10–16. doi: 10.5223/pghn.2015.18.1.10
Ji, Z., Han, F., Meng, F., Tu, M., Yang, N., and Zhang, J. (2016). The association of age and antibiotic resistance of Helicobacter Pylori: a study in Jiaxing City, Zhejiang province, China. Medicine (Baltimore) 95:e2831. doi: 10.1097/MD.0000000000002831
Jonaitis, L., Kiudelis, G., Slepavicius, P., and Kupcinskas, L. (2016). High rate of Helicobacter pylori reinfection in Lithuanian peptic ulcer patients. World J. Gastrointest. Pathophysiol. 7, 181–185. doi: 10.4291/wjgp.v7.i1.181
Kamada, T., Haruma, K., Ito, M., Inoue, K., Manabe, N., Matsumoto, H., et al. (2015). Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter 20, 192–198. doi: 10.1111/hel.12193
Karamanolis, G. P., Daikos, G. L., Xouris, D., Goukos, D., Delladetsima, I., and Ladas, S. D. (2014). The evolution of Helicobacter pylori antibiotics resistance over 10 years in Greece. Digestion 90, 229–231. doi: 10.1159/000369898
Karpinski, T. M., Andrzejewska, E., Eder, P., Linke, K., and Szkaradkiewicz, A. (2015). Evaluation of antimicrobial resistance of Helicobacter pylori in the last 15 years in West Poland. Acta Microbiol. Immunol. Hung. 62, 287–293. doi: 10.1556/030.62.2015.3.6
Kato, M., Yamaoka, Y., Kim, J. J., Reddy, R., Asaka, M., Kashima, K., et al. (2000). Regional differences in metronidazole resistance and increasing clarithromycin resistance among Helicobacter pylori isolates from Japan. Antimicrob. Agents Chemother. 44, 2214–2216. doi: 10.1128/AAC.44.8.2214-2216.2000
Kim, J. M., Kim, J. S., Kim, N., Kim, Y. J., Kim, I. Y., Chee, Y. J., et al. (2008). Gene mutations of 23S rRNA associated with clarithromycin resistance in Helicobacter pylori strains isolated from Korean patients. J. Microbiol. Biotechnol. 18, 1584–1589.
Kim, M. S., Kim, N., Kim, S. E., Jo, H. J., Shin, C. M., Lee, S. H., et al. (2013a). Long-term follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter 18, 135–142. doi: 10.1111/hel.12018
Kim, M. S., Kim, N., Kim, S. E., Jo, H. J., Shin, C. M., Park, Y. S., et al. (2013b). Long-term follow up Helicobacter pylori reinfection rate after second-line treatment: bismuth-containing quadruple therapy versus moxifloxacin-based triple therapy. BMC Gastroenterol. 13:138. doi: 10.1186/1471-230X-13-138
Kim, S. Y., Hyun, J. J., Jung, S. W., Koo, J. S., Yim, H. J., and Lee, S. W. (2014). Helicobacter pylori recurrence after first- and second-line eradication therapy in Korea: the problem of recrudescence or reinfection. Helicobacter 19, 202–206. doi: 10.1111/hel.12117
Kim, S. Y., Joo, Y. M., Lee, H. S., Chung, I. S., Yoo, Y. J., Merrell, D. S., et al. (2009). Genetic analysis of Helicobacter pylori clinical isolates suggests resistance to metronidazole can occur without the loss of functional rdxA. J. Antibiot. 62, 43–50. doi: 10.1038/ja.2008.6
Kobayashi, I., Murakami, K., Kato, M., Kato, S., Azuma, T., Takahashi, S., et al. (2007). Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J. Clin. Microbiol. 45, 4006–4010. doi: 10.1128/JCM.00740-07
Kocazeybek, B., and Tokman, H. B. (2016). Prevalence of primary antimicrobial resistance of H. pylori in Turkey: a systematic review. Helicobacter 21, 251–260. doi: 10.1111/hel.12272
Kuo, C. H., Lu, C. Y., Shih, H. Y., Liu, C. J., Wu, M. C., Hu, H. M., et al. (2014). CYP2C19 polymorphism influences Helicobacter pylori eradication. World J. Gastroenterol. 20, 16029–16036. doi: 10.3748/wjg.v20.i43.16029
Kwack, W., Lim, Y., Lim, C., and Graham, D. Y. (2016). High dose ilaprazole/amoxicillin as first-line regimen for Helicobacter pylori infection in Korea. Gastroenterol. Res. Pract. 2016, 1–7. doi: 10.1155/2016/1648047
Labenz, J. (2001). Current role of acid suppressants in Helicobacter pylori eradication therapy. Best Pract. Res. Clin. Gastroenterol. 15, 413–431. doi: 10.1053/bega.2001.0188
Laine, L., Suchower, L., Frantz, J., Connors, A., and Neil, G. (1998). Twice-daily, 10-day triple therapy with omeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in duodenal ulcer disease: results of three multicenter, double-blind, United States trials. Am. J. Gastroenterol. 93, 2106–2112. doi: 10.1111/j.1572-0241.1998.00602.x
Lee, J. W., Kim, N., Kim, J. M., Nam, R. H., Chang, H., Kim, J. Y., et al. (2013). Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter 18, 206–214. doi: 10.1111/hel.12031
Lee, Y.-C., Chen, T. H., Chiu, H. M., Shun, C. T., Chiang, H., Liu, T. Y., et al. (2013). The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 62, 676–682. doi: 10.1136/gutjnl-2012-302240
Liang, X., Xu, X., Zheng, Q., Zhang, W., Sun, Q., Liu, W., et al. (2013). Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin. Gastroenterol. Hepatol. 11, 802–807. doi: 10.1016/j.cgh.2013.01.008
Liao, J., Zheng, Q., Liang, X., Zhang, W., Sun, Q., Liu, W., et al. (2013). Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter 18, 373–377. doi: 10.1111/hel.12052
Lim, S. H., Kwon, J. W., Kim, N., Kim, G. H., Kang, J. M., Park, M. J., et al. (2013). Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 13:104. doi: 10.1186/1471-230X-13-104
Lind, T., Megraud, F., Unge, P., Bayerdorffer, E., O'Morain, C., Spiller, R., et al. (1999). The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology 116, 248–253. doi: 10.1016/S0016-5085(99)70119-8
Liou, J. M., Fang, Y. J., Chen, C. C., Bair, M. J., Chang, C. Y., Lee, Y. C., et al. (2016). Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 388, 2355–2365. doi: 10.1016/S0140-6736(16)31409-X
Liu, Q., Qi, D., Kang, J., Jin, Y., Liu, W., Gao, W., et al. (2015). Efficacy of real-time PCR-based detection of Helicobacter pylori infection and genotypic resistance-guided quadruple therapy as the first-line treatment for functional dyspepsia with Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 27, 221–225. doi: 10.1097/MEG.0000000000000186
Liu, W. Z., Xie, Y., Cheng, H., Lu, N. H., Hu, F. L., Zhang, W. D., et al. (2013). Fourth Chinese national consensus report on the management of Helicobacter pylori infection. J. Dig. Dis. 14, 211–221. doi: 10.1111/1751-2980.12034
Malfertheiner, P., Bazzoli, F., Delchier, J. C., Celinski, K., Giguere, M., Riviere, M., et al. (2011). Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 377, 905–913. doi: 10.1016/S0140-6736(11)60020-2
Malfertheiner, P., Megraud, F., O'Morain, C., Gisbert, J., Kuipers, E., Axon, A., et al. (2016). Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Mansour-Ghanaei, F., Joukar, F., Naghipour, M. R., Forouhari, A., and Saadat, S. M. (2015). Seven-day quintuple regimen as a rescue therapy for Helicobacter pylori eradication. World J. Gastroenterol. 21, 661–666. doi: 10.3748/wjg.v21.i2.661
Marshall, B. J., and Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315. doi: 10.1016/S0140-6736(84)91816-6
McColl, K. E. (2010). Helicobacter pylori infection. N Engl. J. Med. 362, 1597–1604. doi: 10.1056/NEJMcp1001110
McFarland, L. V., Huang, Y., Wang, L., and Malfertheiner, P. (2016). Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol. J. 4, 546–561. doi: 10.1177/2050640615617358
Mégraud, F. (2004). H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53, 1374–1384. doi: 10.1136/gut.2003.022111
Megraud, F., Coenen, S., Versporten, A., Kist, M., Lopez-Brea, M., Hirschl, A. M., et al. (2013). Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 62, 34–42. doi: 10.1136/gutjnl-2012-302254
Mehrabadi, J. F., Sirous, M., Daryani, N. E., Eshraghi, S., Akbari, B., and Shirazi, M. H. (2011). Assessing the role of the RND efflux pump in metronidazole resistance of Helicobacter pylori by RT-PCR assay. J. Infect. Dev. Ctries. 5, 88–93. doi: 10.3855/jidc.1187
Meyer, J. M., Silliman, N. P., Wang, W., Siepman, N. Y., Sugg, J. E., Morris, D., et al. (2002). Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993-1999. Ann. Intern. Med. 136, 13–24. doi: 10.7326/0003-4819-136-1-200201010-00008
Minakari, M., Davarpanah Jazi, A. H., Shavakhi, A., Moghareabed, N., and Fatahi, F. (2010). A randomized controlled trial: efficacy and safety of azithromycin, ofloxacin, bismuth, and omeprazole compared with amoxicillin, clarithromycin, bismuth, and omeprazole as second-line therapy in patients with Helicobacter pylori infection. Helicobacter 15, 154–159. doi: 10.1111/j.1523-5378.2009.00739.x
Misiewicz, J., Harris, A., Bardhan, K., Levi, S., O'Morain, C., Cooper, B., et al. (1997). One week triple therapy for Helicobacter pylori: a multicentre comparative study. Gut 41, 735–739. doi: 10.1136/gut.41.6.735
Mitchell, H. M., Hu, P., Chi, Y., Chen, M. H., Li, Y. Y., and Hazell, S. L. (1998). A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China). Gastroenterology 114, 256–261. doi: 10.1016/S0016-5085(98)70475-5
Moodley, Y., Linz, B., Bond, R. P., Nieuwoudt, M., Soodyall, H., Schlebusch, C. M., et al. (2012). Age of the association between Helicobacter pylori and man. PLoS Pathog. 8:e1002693. doi: 10.1371/journal.ppat.1002693
Moore, R. A., Beckthold, B., Wong, S., Kureishi, A., and Bryan, L. E. (1995). Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob. Agents Chemother. 39, 107–111. doi: 10.1128/AAC.39.1.107
Mourad-Baars, P. E., Wunderink, H. F., Mearin, M. L., Veenendaal, R. A., Wit, J. M., and Veldkamp, K. E. (2015). Low antibiotic resistance of Helicobacter pylori in The Netherlands. Helicobacter 20, 69–70. doi: 10.1111/hel.12175
Murakami, K., Sakurai, Y., Shiino, M., Funao, N., Nishimura, A., and Asaka, M. (2016). Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65, 1439–1446. doi: 10.1136/gutjnl-2015-311304
Nagy, P., Johansson, S., and Molloy-Bland, M. (2016). Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 8, 8. doi: 10.1186/s13099-016-0091-7
Naito, Y., Shimizu, T., Haruna, H., Fujii, T., Kudo, T., Shoji, H., et al. (2008). Changes in the presence of urine Helicobacter pylori antibody in Japanese children in three different age groups. Pediatr. Int. 50, 291–294. doi: 10.1111/j.1442-200X.2008.02587.x
Ngoyi, E. N. O., Ibara, B. I. A., Moyen, R., Ahoui Apendi, P. C., Ibara, J. R., Obengui, O., et al. (2015). Molecular detection of Helicobacter pylori and its antimicrobial resistance in Brazzaville, Congo. Helicobacter 20, 316–320. doi: 10.1111/hel.12204
Nguyen, T., Ramsey, D., Graham, D., Shaib, Y., Shiota, S., Velez, M., et al. (2015). The prevalence of Helicobacter pylori remains high in African American and Hispanic Veterans. Helicobacter 20, 305–315. doi: 10.1111/hel.12199
Nishizawa, T., Maekawa, T., Watanabe, N., Harada, N., Hosoda, Y., Yoshinaga, M., et al. (2015). Clarithromycin versus metronidazole as first-line Helicobacter pylori eradication: a multicenter, prospective, randomized controlled study in Japan. J. Clin. Gastroenterol. 49, 468–471. doi: 10.1097/MCG.0000000000000165
Nista, E. C., Candelli, M., Cremonini, F., Cazzato, I. A., Zocco, M. A., Franceschi, F., et al. (2004). Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 20, 1181–1188. doi: 10.1111/j.1365-2036.2004.02274.x
Oh, B., Kim, B. S., Kim, J. W., Kim, J. S., Koh, S. J., Kim, B. G., et al. (2016). The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter 21, 165–174. doi: 10.1111/hel.12270
Okamoto, T., Yoshiyama, H., Nakazawa, T., Park, I. D., Chang, M. W., Yanai, H., et al. (2002). A change in PBP1 is involved in amoxicillin resistance of clinical isolates of Helicobacter pylori. J. Antimicrob. Chemother. 50, 849–856. doi: 10.1093/jac/dkf140
Okamura, T., Suga, T., Nagaya, T., Arakura, N., Matsumoto, T., Nakayama, Y., et al. (2014). Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter 19, 214–220. doi: 10.1111/hel.12124
Padol, S., Yuan, Y., Thabane, M., Padol, I. T., and Hunt, R. H. (2006). The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am. J. Gastroenterol. 101, 1467–1475. doi: 10.1111/j.1572-0241.2006.00717.x
Pan, K. F., Zhang, L., Gerhard, M., Ma, J. L., Liu, W. D., Ulm, K., et al. (2016). A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu county, China: baseline results and factors affecting the eradication. Gut 65, 9–18. doi: 10.1136/gutjnl-2015-309197
Park, J. Y., Dunbar, K. B., Mitui, M., Arnold, C. A., Lam-Himlin, D. M., Valasek, M. A., et al. (2016). Helicobacter pylori clarithromycin resistance and treatment failure are common in the USA. Dig. Dis. Sci. 61, 2373–2380. doi: 10.1007/s10620-016-4091-8
Porras, C., Nodora, J., Sexton, R., Ferreccio, C., Jimenez, S., Dominguez, R. L., et al. (2013). Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control 24, 209–215. doi: 10.1007/s10552-012-0117-5
Qureshi, N. N., Gallaher, B., and Schiller, N. L. (2014). Evolution of amoxicillin resistance of Helicobacter pylori in vitro: characterization of resistance mechanisms. Microb. Drug Resist. 20, 509–516. doi: 10.1089/mdr.2014.0019
Regnath, T., Raecke, O., Enninger, A., and Ignatius, R. (2017). Increasing metronidazole and rifampicin resistance of Helicobacter pylori isolates obtained from children and adolescents between 2002 and 2015 in southwest Germany. Helicobacter. 22:e12327. doi: 10.1111/hel.12327
Rimbara, E., Noguchi, N., Kawai, T., and Sasatsu, M. (2008a). Novel mutation in 23S rRNA that confers low-level resistance to clarithromycin in Helicobacter pylori. Antimicrob. Agents Chemother. 52, 3465–3466. doi: 10.1128/AAC.00445-08
Rimbara, E., Noguchi, N., Kawai, T., and Sasatsu, M. (2008b). Mutations in penicillin-binding proteins 1, 2 and 3 are responsible for amoxicillin resistance in Helicobacter pylori. J. Antimicrob. Chemother. 61, 995–998. doi: 10.1093/jac/dkn051
Rimbara, E., Noguchi, N., Kawai, T., and Sasatsu, M. (2012). Fluoroquinolone resistance in Helicobacter pylori: role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter 17, 36–42. doi: 10.1111/j.1523-5378.2011.00912.x
Roberts, S. E., Morrison-Rees, S., Samuel, D. G., Thorne, K., Akbari, A., and Williams, J. G. (2016). Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment. Pharmacol. Ther. 43, 334–345. doi: 10.1111/apt.13474
Ruggiero, P. (2014). Use of probiotics in the fight against Helicobacter pylori. World J. Gastrointest. Pathophysiol. 5, 384–391. doi: 10.4291/wjgp.v5.i4.384
Sahara, S., Sugimoto, M., Uotani, T., Ichikawa, H., Yamade, M., Iwaizumi, M., et al. (2013). Twice-daily dosing of esomeprazole effectively inhibits acid secretion in CYP2C19 rapid metabolisers compared with twice-daily omeprazole, rabeprazole or lansoprazole. Aliment. Pharmacol. Ther. 38, 1129–1137. doi: 10.1111/apt.12492
Sarowska, J., Choroszy-Krol, I., Regulska-Ilow, B., Frej-Madrzak, M., and Jama-Kmiecik, A. (2013). The therapeutic effect of probiotic bacteria on gastrointestinal diseases. Adv. Clin. Exp. Med. 22, 759–766.
Scott, D., Weeks, D., Melchers, K., and Sachs, G. (1998). The life and death of Helicobacter pylori. Gut 43, S56–S60. doi: 10.1136/gut.43.2008.s56
Sezgin, O., Altintas, E., Ucbilek, E., and Tataroglu, C. (2006). Bismuth-based therapies for the first step eradication of Helicobacter pylori. Turk. J. Gastroenterol. 17, 90–93.
Shin, J. M., Inatomi, N., Munson, K., Strugatsky, D., Tokhtaeva, E., Vagin, O., et al. (2011). Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin e monofumarate (TAK-438). J. Pharmacol. Exp. Ther. 339, 412–420. doi: 10.1124/jpet.111.185314
Shiota, S., Reddy, R., Alsarraj, A., El-Serag, H. B., and Graham, D. Y. (2015). Antibiotic Resistance of Helicobacter pylori among male United States veterans. Clin. Gastroenterol. Hepatol. 13, 1616–1624. doi: 10.1016/j.cgh.2015.02.005
Siddiqui, T. R., Ahmed, W., Arif, A., Bibi, S., and Khan, A. (2016). Emerging trends of antimicrobial resistance in Helicobacter pylori isolates obtained from Pakistani patients: the need for consideration of amoxicillin and clarithromycin. J. Pak. Med. Assoc. 66, 710–716.
Silva, F. M., Navarro-Rodriguez, T., Barbuti, R. C., Mattar, R., Hashimoto, C. L., and Eisig, J. N. (2010). Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter 15, 46–52. doi: 10.1111/j.1523-5378.2009.00734.x
Sivapalasingam, S., Rajasingham, A., Macy, J. T., Friedman, C. R., Hoekstra, R. M., Ayers, T., et al. (2014). Recurrence of Helicobacter pylori infection in Bolivian children and adults after a population-based “screen and treat” strategy. Helicobacter 19, 343–348. doi: 10.1111/hel.12137
Smiley, R., Bailey, J., Sethuraman, M., Posecion, N., and Showkat Ali, M. (2013). Comparative proteomics analysis of sarcosine insoluble outer membrane proteins from clarithromycin resistant and sensitive strains of Helicobacter pylori. J. Microbiol. 51, 612–618. doi: 10.1007/s12275-013-3029-5
Song, Z., Zhang, J., He, L., Chen, M., Hou, X., Li, Z., et al. (2014). Prospective multi-region study on primary antibiotic resistance of Helicobacter pylori strains isolated from Chinese patients. Dig. Liver Dis. 46, 1077–1081. doi: 10.1016/j.dld.2014.08.038
Song, Z., Zhou, L., Zhang, J., He, L., Bai, P., and Xue, Y. (2016). Hybrid therapy as first-line regimen for Helicobacter pylori eradication in populations with high antibiotic resistance rates. Helicobacter 21, 382–388. doi: 10.1111/hel.12294
Stone, G. G., Shortridge, D., Flamm, R. K., Versalovic, J., Beyer, J., Idler, K., et al. (1996). Identification of a 23S rRNA gene mutation in clarithromycin-resistant Helicobacter pylori. Helicobacter 1, 227–228. doi: 10.1111/j.1523-5378.1996.tb00043.x
Su, P., Li, Y., Li, H., Zhang, J., Lin, L., Wang, Q., et al. (2013). Antibiotic resistance of Helicobacter pylori isolated in the southeast coastal region of China. Helicobacter 18, 274–279. doi: 10.1111/hel.12046
Su, Z., Xu, H., Zhang, C., Shao, S., Li, L., Wang, H., et al. (2006). Mutations in Helicobacter pylori porD and oorD genes may contribute to furazolidone resistance. Croat. Med. J. 47, 410–415.
Sugano, K., Tack, J., Kuipers, E. J., Graham, D. Y., El-Omar, E. M., Miura, S., et al. (2015). Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367. doi: 10.1136/gutjnl-2015-309252
Sugimoto, M., Sahara, S., Ichikawa, H., Kagami, T., Ban, H., Otsuka, T., et al. (2017). Four-times-daily dosing of rabeprazole with sitafloxacin, high-dose amoxicillin, or both for metronidazole-resistant infection with Helicobacter pylori in Japan. Helicobacter 22:e12319. doi: 10.1111/hel.12319
Sugimoto, M., Sahara, S., Ichikawa, H., Kagami, T., Uotani, T., and Furuta, T. (2015). High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment. Pharmacol. Ther. 42, 477–483. doi: 10.1111/apt.13280
Sun, Q., Liang, X., Zheng, Q., Liu, W., Xiao, S., Gu, W., et al. (2010). High efficacy of 14-day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 15, 233–238. doi: 10.1111/j.1523-5378.2010.00758.x
Szajewska, H., Horvath, A., and Kolodziej, M. (2015). Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 41, 1237–1245. doi: 10.1111/apt.13214
Szajewska, H., Horvath, A., and Piwowarczyk, A. (2010). Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment. Pharmacol. Ther. 32, 1069–1079. doi: 10.1111/j.1365-2036.2010.04457.x
Tadesse, E., Daka, D., Yemane, D., and Shimelis, T. (2014). Seroprevalence of Helicobacter pylori infection and its related risk factors in symptomatic patients in southern Ethiopia. BMC Res. Notes 7:834. doi: 10.1186/1756-0500-7-834
Take, S., Mizuno, M., Ishiki, K., Imada, T., Okuno, T., Yoshida, T., et al. (2012). Reinfection rate of Helicobacter pylori after eradication treatment: a long-term prospective study in Japan. J. Gastroenterol. 47, 641–646. doi: 10.1007/s00535-012-0536-9
Tankovic, J., Lascols, C., Sculo, Q., Petit, J. C., and Soussy, C. J. (2003). Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 47, 3942–3944. doi: 10.1128/AAC.47.12.3942-3944.2003
Thung, I., Aramin, H., Vavinskaya, V., Gupta, S., Park, J. Y., Crowe, S. E., et al. (2016). Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 43, 514–533. doi: 10.1111/apt.13497
Tong, J. L., Ran, Z. H., Shen, J., Zhang, C. X., and Xiao, S. D. (2007). Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment. Pharmacol. Ther. 25, 155–168. doi: 10.1111/j.1365-2036.2006.03179.x
Tseng, Y. S., Wu, D. C., Chang, C. Y., Kuo, C. H., Yang, Y. C., Jan, C. M., et al. (2009). Amoxicillin resistance with beta-lactamase production in Helicobacter pylori. Eur. J. Clin. Invest. 39, 807–812. doi: 10.1111/j.1365-2362.2009.02166.x
Uchida, T., Miftahussurur, M., Pittayanon, R., Vilaichone, R. K., Wisedopas, N., Ratanachu-Ek, T., et al. (2015). Helicobacter pylori infection in Thailand: a nationwide study of the CagA phenotype. PLoS ONE 10:e0136775. doi: 10.1371/journal.pone.0136775
Uygun, A., Ozel, A. M., Yildiz, O., Aslan, M., Yesilova, Z., Erdil, A., et al. (2008). Comparison of three different second-line quadruple therapies including bismuth subcitrate in Turkish patients with non-ulcer dyspepsia who failed to eradicate Helicobacter pylori with a 14-day standard first-line therapy. J. Gastroenterol. Hepatol. 23, 42–45.
Vafaeimanesh, J., Rajabzadeh, R., Ahmadi, A., Moshtaghi, M., Banikarim, S., Hajiebrahimi, S., et al. (2013). Effect of Helicobacter pylori eradication on glycaemia control in patients with type 2 diabetes mellitus and comparison of two therapeutic regimens. Arab J. Gastroenterol. 14, 55–58. doi: 10.1016/j.ajg.2013.03.002
Wang, B., Lv, Z. F., Wang, Y. H., Wang, H., Liu, X. Q., Xie, Y., et al. (2014). Standard triple therapy for Helicobacter pylori infection in China: a meta-analysis. World J. Gastroenterol. 20, 14973–14985. doi: 10.3748/wjg.v20.i40.14973
Wang, J., Xu, L., Shi, R., Huang, X., Li, S. W., Huang, Z., et al. (2011). Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion 83, 253–260. doi: 10.1159/000280318
Wang, Y. H., Lv, Z. F., Zhong, Y., Liu, D. S., Chen, S. P., and Xie, Y. (2017). The internalization of Helicobacter pylori plays a role in the failure of H. pylori eradication. Helicobacter. 22:e12324. doi: 10.1111/hel.12324
Wang, Z. H., Gao, Q. Y., and Fang, J. Y. (2013). Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J. Clin. Gastroenterol. 47, 25–32. doi: 10.1097/MCG.0b013e318266f6cf
Wotherspoon, A. C., Ortiz-Hidalgo, C., Falzon, M. R., and Isaacson, P. G. (1991). Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 338, 1175–1176. doi: 10.1016/0140-6736(91)92035-Z
Wroblewski, L. E., Peek, R. M. Jr., and Wilson, K. T. (2010). Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin. Microbiol. Rev. 23, 713–739. doi: 10.1128/CMR.00011-10
Xie, Y., Zhu, Y., Zhou, H., Lu, Z. F., Yang, Z., Shu, X., et al. (2014). Furazolidone-based triple and quadruple eradication therapy for Helicobacter pylori infection. World J. Gastroenterol. 20, 11415–11421. doi: 10.3748/wjg.v20.i32.11415
Yamade, M., Sugimoto, M., Uotani, T., Nishino, M., Kodaira, C., and Furuta, T. (2011). Resistance of Helicobacter pylori to quinolones and clarithromycin assessed by genetic testing in Japan. J. Gastroenterol. Hepatol. 26, 1457–1461. doi: 10.1111/j.1440-1746.2011.06815.x
Yan, T. L., Hu, Q. D., Zhang, Q., Li, Y. M., and Liang, T. B. (2013). National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment. Pharmacol. Ther. 37, 963–968. doi: 10.1111/apt.12293
Yang, J. C., Lin, C. J., Wang, H. L., Chen, J. D., Kao, J. Y., Shun, C. T., et al. (2015). High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 13, 895–905. doi: 10.1016/j.cgh.2014.10.036
Zhang, D. H., Zhou, L. Y., Lin, S. R., Ding, S. G., Huang, Y. H., Gu, F., et al. (2009). Recent changes in the prevalence of Helicobacter pylori infection among children and adults in high- or low-incidence regions of gastric cancer in China. Chin. Med. J. 122, 1759–1763.
Zhang, M. M., Qian, W., Qin, Y. Y., He, J., and Zhou, Y. H. (2015). Probiotics in Helicobacter pylori eradication therapy: a systematic review and meta-analysis. World J. Gastroenterol. 21, 4345–4357. doi: 10.3748/wjg.v21.i14.4345
Zhang, W., Chen, Q., Liang, X., Liu, W., Xiao, S., Graham, D. Y., et al. (2015). Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 64, 1715–1720. doi: 10.1136/gutjnl-2015-309900
Zhang, Y. X., Zhou, L. Y., Song, Z. Q., Zhang, J. Z., He, L. H., and Ding, Y. (2015). Primary antibiotic resistance of Helicobacter pylori strains isolated from patients with dyspeptic symptoms in Beijing: a prospective serial study. World J. Gastroenterol. 21, 2786–2792. doi: 10.3748/wjg.v21.i9.2786
Zhang, Z., Liu, Z. Q., Zheng, P. Y., Tang, F. A., and Yang, P. C. (2010). Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J. Gastroenterol. 16, 1279–1284. doi: 10.3748/wjg.v16.i10.1279
Zheng, Q., Chen, W. J., Lu, H., Sun, Q. J., and Xiao, S. D. (2010). Comparison of the efficacy of triple versus quadruple therapy on the eradication of Helicobacter pylori and antibiotic resistance. J. Dig. Dis. 11, 313–318. doi: 10.1111/j.1751-2980.2010.00457.x
Zhou, L. Y., Song, Z. Q., Xue, Y., Li, X., Li, Y. Q., and Qian, J. M. (2017). Recurrence of Helicobacter pylori infection and the affecting factors: a follow-up study. J. Dig. Dis. 18, 47–55. doi: 10.1111/1751-2980.12440
Zhou, L., Sung, J. J., Lin, S., Jin, Z., Ding, S., Huang, X., et al. (2003). A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin. Med. J. 116, 11–14.
Zhou, L., Zhang, J., Chen, M., Hou, X., Li, Z., Song, Z., et al. (2014). A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am. J. Gastroenterol. 109, 535–541. doi: 10.1038/ajg.2014.26
Zhou, L., Zhang, J., Song, Z., He, L., Li, Y., Qian, J., et al. (2016). Tailored versus triple plus bismuth or concomitant therapy as initial Helicobacter pylori treatment: a randomized trial. Helicobacter 21, 91–99. doi: 10.1111/hel.12242
Zhu, Z. H., Huang, D. Q., Xie, Y., Liu, L. L., and Lu, N. H. (2013). Characterization of 23S rRNA gene mutation in primary and secondary clarithromycin-resistant Helicobacter pylori strains from East China. Turk. J. Gastroenterol. 24, 5–9.
Zollner-Schwetz, I., Leitner, E., Plieschnegger, W., Semlitsch, G., Stepan, V., Reiter, L., et al. (2016). Primary resistance of Helicobacter pylori is still low in southern Austria. Int. J. Med. Microbiol. 306, 206–211. doi: 10.1016/j.ijmm.2016.04.003
Zou, J., Dong, J., and Yu, X. (2009). Meta-analysis: lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter 14, 97–107. doi: 10.1111/j.1523-5378.2009.00716.x
Zullo, A., Lorenzetti, R., and Hassan, C. (2007). A quintuple therapy for H. pylori eradication. Am. J. Gastroenterol. 102, 2601; author reply 2601–2602. doi: 10.1111/j.1572-0241.2007.01514_1.x
Keywords: Helicobacter pylori, infection, reinfection, resistance, treatment
Citation: Hu Y, Zhu Y and Lu N-H (2017) Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell. Infect. Microbiol. 7:168. doi: 10.3389/fcimb.2017.00168
Received: 14 November 2016; Accepted: 18 April 2017;
Published: 05 May 2017.
Edited by:
Lorenza Putignani, Bambino Gesú Ospedale Pediatrico (IRCCS), ItalyReviewed by:
Teresa Olczak, University of Wroclaw, PolandSusan M. Bueno, Pontifical Catholic University of Chile, Chile
Copyright © 2017 Hu, Zhu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Zhu, zhuyin27@sina.com.cn
Nong-Hua Lu, lunonghua@ncu.edu.cn
 Yi Hu
Yi Hu Yin Zhu
Yin Zhu Nong-Hua Lu
Nong-Hua Lu