- Thyroid Unit, Division of Endocrinology and Metabolism, University of São Paulo Medical School, Hospital das Clínicas, São Paulo, Brazil
Patients with large benign goiters often present local compressive symptoms that require surgical treatment, including dysphagia, neck tightness, and airway obstruction. In contrast, patients with such goiters who remain asymptomatic may be observed after exclusion of malignancy. The use of levothyroxine (LT4) to reduce the volume of the goiter is still a controversial treatment for large goiters, and the optimal surgical procedure for multinodular goiter is still debatable. Radioiodine is a safe and effective treatment option when used alone or in combination with recombinant human TSH. This review discusses current therapeutic options to treat diffuse and multinodular non-toxic benign goiters.
Introduction
Once the diagnosis of non-toxic diffuse goiter (NDG) or non-toxic nodular goiter (NNG) is established, the following management goals should be considered:
a. Correct the underlying thyroid dysfunction, if present;
b. Verify if the goiter is growing or causing obstructive symptoms;
c. Exclude malignancy if one or more nodules are suspicious;
d. Determine whether the goiter requires therapy, and if so, weight the benefits and risks of medical and surgical interventions and reach a decision with the patient about what type of treatment should be administered.
The expression of the progressive and nodular increase of the thyroid in non-toxic benign goiters is the result of a combination of genetic and environmental factors, of which iodine deficiency is the most important (1). The treatment of benign non-toxic goiter is a challenge for clinicians and endocrinologists. Similarly to how patients with single thyroid nodules are assessed, all individuals with benign non-toxic goiter must undergo careful evaluation with serum TSH measurement and thyroid ultrasonography to guide the selection of the nodule (or nodules) to be biopsied (2). After exclusion of malignancy, treatment should be considered for individuals with compression of local structures, cosmetic concerns, and/or thyroid hyperfunction (2). The choice of treatment in patients with benign non-toxic goiter can be challenging, considering that the size of the goiter and the occurrence of symptoms often follow a non-linear association (Table 1).
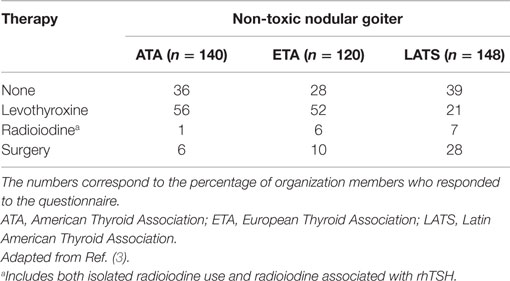
Table 1. Treatment choices reported by ATA, ETA, and LATS members based on an index case of a patient with a simple non-toxic nodular goiter, without suspicion of malignancy.
Non-Toxic Diffuse Goiter
There is no ideal therapy for NDGs (4), but for patients requiring treatment, clinical management is the most frequent choice. It is unclear whether or not early treatment of NDGs can inhibit the development of nodular goiter.
The development of NDGs occurs from a combination of genetic and environmental factors. Prolonged TSH stimulation, which frequently occurs in association with iodine deficiency, has an important role in thyroid enlargement. In these circumstances, iodine supplementation would be an adequate approach (400 μg of iodine for 8–12 months). A significant reduction in goiter size has been observed in patients with NDG supplemented with iodine (5, 6). In fact, supplementation with iodine 400 μg/day has been demonstrated to be as effective as suppressive therapy with LT4 150 μg/day (5). However, except in a few European countries, iodine is no longer used for the treatment of benign non-toxic goiter.
In contrast, a beneficial effect of LT4 has been shown in NDG (7). A volume reduction of 50% or more was detected in 31% (11/35) of the patients after 6 months (7). If suppressive treatment is considered, then administration of thyroid hormone in enough doses to inhibit or reduce TSH secretion may be used. However, it should be considered that the volume of the goiter returns to pretreatment size after LT4 withdrawal. As may be expected, many thyroid experts are unenthusiastic about treating NDG, although others advocate the use of LT4 favoring the notion that early treatment may prevent later development of nodular goiter. As with any issue in clinical medicine, the benefits of such therapy must be weighed against the potential risks of TSH suppression.
Regarding radioiodine therapy in NDG, two earlier small uncontrolled studies are available (8, 9). In the first study, 11 patients with symptoms of pressure and cosmetic complaints were selected for radioiodine ablation after refusing surgical treatment. The mean thyroid volume reduction achieved with a single dose of radioiodine within the first year was 62%, and 2 of the 11 patients (18%) developed hypothyroidism (8). In another study, 10 patients were treated with radioiodine and were followed up for 18 months with measurements of the thyroid volume by ultrasonography. The volume declined by 50% within 12–18 months. During follow-up, one patient (10%) developed persistent hypothyroidism and another (who presented positive antithyroperoxidase levels) developed transient hypothyroidism (9).
Non-Toxic Nodular Goiter
The ideal treatment for NNG is controversial (Table 1) (3), in part due to the variation in the natural history of these goiters. Some patients present an enlargement in goiter size with time along with the development of nodules, symptoms of compression, and cosmetic reasons (10). In contrast, the enlargement of the goiter may become stable or reduce spontaneously with time in around 20% of the women and 5% of the men (11).
Current alternatives for NNG treatment include
• Clinical observation for asymptomatic patients;
• Thyroid hormone suppressive therapy;
• Radioiodine therapy alone or preceded by recombinant human TSH (rhTSH); and
• Surgery.
Among these options, treatment is chosen individually for each patient in view of the risks, benefits, and availability of the various techniques, experience of the treating physician, and patient’s personal preference (Table 2).
Clinical Observation
Clinical observation, including thyroid function monitoring and ultrasonographic assessment at regular intervals, is an alternative in cases of small goiters not causing compressive symptoms and associated with normal thyroid function. If clinical observation is chosen, the possibility of malignancy should be excluded by fine-needle aspiration biopsy (FNAB) that is guided by ultrasonography.
Asymptomatic euthyroid patients with benign non-toxic goiter and without cosmetic symptoms may be simply observed with clinical and laboratory evaluation and thyroid imaging tests. In most cases, patients with asymptomatic NNG may be evaluated yearly with careful thyroid palpation and measurement of serum TSH level. Ultrasonography may be performed when the palpation of the thyroid is uncertain, whereas nodules that appear to enlarge should be evaluated with FNAB. Evaluation with neck computed tomography or magnetic resonance imaging (MRI) may be required when the goiter is subesternal.
The American Thyroid Association recommends a standard follow-up interval of 6–18 months for patients with NNG, which may be gradually prolonged if no substantial changes are observed during the first 3–5 years (12).
Suppressive Therapy with Levothyroxine
Although LT4 is broadly used in the United States, Europe, and Latin America, as shown in different surveys (3), the use of thyroid hormones to treat NNGs is controversial, and its efficacy is dependent on the degree of TSH suppression. Considering that NNG patients frequently have normal serum TSH levels, the enlargement of the thyroid in these patients is probably associated with the prolonged action of different growth factors (including TSH) on thyroid follicular cells with different synthetic and growth potentials. Advantages of this treatment modality include low cost, administration on an outpatient basis, and inhibition of the development of new nodules. In contrast, suppressive therapy has little effectiveness, as it requires permanent treatment and may have potential undesirable effects on bone (demineralization) (13) and heart (arrhythmias), especially in older individuals (14).
In a randomized placebo-controlled trial, a ≥25% goiter volume reduction was seen in 58% of the individuals with NNG and NDG during LT4 suppressive therapy over 9 months, with a return to the original volume after treatment withdrawal. Over the same period, patients randomized to the placebo group showed a 20% increase in thyroid volume (15).
In another randomized trial comparing suppressive therapy with radioiodine, patients in the radioiodine group showed a 35% reduction in goiter volume at 1 year and 44% at 2 years, whereas those who received LT4 presented 7 and 1% reduction at 1 and 2 years, respectively. A response to the therapy was found in 97% of the individuals who received radioiodine and 43% of those undergoing LT4 therapy (16). A recent meta-analysis showed lack of substantial benefits and a relative risk of only 1.9 (95% confidence interval, 0.95–3.81) of a decrease in nodular size with LT4 therapy (17). Thus, according to these randomized controlled trials, it seems that thyroid hormone suppression can interfere with the process of goitrogenesis (goiter growth and new nodule formation), with only some goiters responding and others growing despite LT4 treatment (15).
The occurrence of adverse events is more evident in older patients, a population that comprises most patients with NNG. Also, about 22% of the individuals with NNG may harbor areas with functional autonomy, which increase the concerns with LT4 therapy because of risks of bone loss and atrial fibrillation. Therefore, treatment with thyroid hormone should be avoided, especially in patients with prior serum TSH concentrations below the normal range.
Patients who start LT4 suppression must continue the therapy for a long time. The reduction in goiter size with LT4 seen in some individuals is probably due to decreased TSH secretion, especially in patients who live in areas with borderline or low iodine levels. Any decrease in NNG size that may occur with LT4 suppression is lost when the treatment is interrupted, and the nodules and goiter may grow again in size (15). In addition, keeping the serum TSH concentration in the lowest reference range or slightly below the normal range, rather than below 0.01 μU/mL, appears to be efficacious. Pending further studies, this strategy may relieve the adverse effects of LT4 therapy (18). Accordingly, an LT4 dose adjusted to obtain a non-suppressive level of serum TSH in the range of 0.5–0.8 μU/mL has been shown to significantly reduce the growth of nodules within multinodular goiters in 165 out of 356 female patients during a follow-up period of 9 years (19).
A potential benefit of thyroid hormone therapy is a reduction in the risk of thyroid oncogenesis (20). However, this hypothesis is still speculative, and more studies are still needed.
In general, since the volume of the thyroid reduces in only 30% of the patients with NNGs treated with LT4 suppression, recommendations for this type of treatment have decreased (10).
Surgery
The best surgical procedure to treat patients with NNG is still controversial (21). A recent meta-analysis that included 1305 participants evaluated the impact of total/near-total thyroidectomy versus subtotal thyroidectomy in adults with non-toxic multinodular goiter. The key results of this study were (a) the recurrence of goiter was lower in patients who underwent thyroidectomy compared with those who underwent subtotal thyroidectomy, with rates of goiter recurrence of 84 in 1000 patients with subtotal thyroidectomy and 5 in 1000 patients with total thyroidectomy; (b) no clear benefits or harms were observed with subtotal or total thyroidectomy in patients undergoing surgical intervention due to recurrence in goiter size, complications such as permanent recurrent laryngeal nerve palsy, or occurrence of thyroid carcinoma; and (c) cancer detection was lower (6.1%) in patients undergoing subtotal thyroidectomy compared with 7.3% of those undergoing total thyroidectomy, but this difference was not significant (21).
In most patients with large obstructive goiters, cosmetic complaints, or retrosternal NNGs for whom surgery is recommended, total thyroidectomy is preferable over subtotal thyroidectomy (22, 23). In the long term, patients undergoing subtotal thyroidectomy have higher recurrence rates than those undergoing total thyroidectomy, and 2.5–42% are required to undergo a new intervention. In addition, 3.5% of the NNG patients undergoing subtotal thyroidectomy require another surgical intervention to remove remaining thyroid tissue because of incidental thyroid carcinoma (24). Rates of permanent complications, such as hypoparathyroidism and vocal palsy, are similar with both total and subtotal surgeries; however, total thyroidectomy is preferred due to an increased risk of these complications associated with a new intervention (25). In patients with unilateral NNG, some authors recommend unilateral thyroidectomy based on a low rate of recurrence (2%) and high rate of maintenance of euthyroidism (73%) (26).
Post-surgical recurrence rates are directly proportional to the volume of the remaining thyroid tissue. Hegedüs et al. (27) studied 202 consecutive patients who underwent surgical resection due to benign non-toxic goiter. They found a recurrence rate of 35% detected by ultrasonography during a median follow-up of 10 years (27). In a retrospective study, the assessment of 112 individuals 30 years after surgery showed rates of disease recurrence of 40–45% during a 30-year follow-up period (28). In another study, the recurrence rate of the goiters was 18% when assessed by ultrasonography 9 years after surgery (29).
This high probability of goiter recurrence after subtotal thyroidectomy resulted in a preventive use of LT4. However, only one (30) out of four randomized trials (27, 30–32) has demonstrated LT4 to be effective in this setting.
A cervical incision is often used to approach intrathoracic goiters; however, 10–30% of the patients require sternotomy or thoracotomy (33).
After total thyroidectomy, patients must start LT4 replacement at a dose of 1.4–2.2 μg/kg/day (34). Adjustments in LT4 dose must be based on the patient’s age (35), according to the usual recommendations for patients with hypothyroidism. However, in patients undergoing partial thyroidectomy, treatment with LT4 should be implemented after the establishment of hypothyroidism and not preventively against goiter recurrence, because this benefit has not been confirmed in randomized studies (36).
The benefits and risks of a surgical procedure must be carefully considered, since the incidence of NNG increases with age, affecting particularly elderly individuals who often have other comorbidities. As discussed below, rhTSH-stimulated radioiodine may be a treatment alternative for these patients.
Therapy with Radioiodine
Therapy with radioiodine may be recommended in cases of NNG affecting patients who refuse or have contraindications for surgery. Over the past years, this type of treatment has increased in patients with nodular goiter and is associated with a substantial decrease in glandular volume, reaching 30–40% in the first year, and 50–60% in the fourth year. Obstructive symptoms improve in most individuals (37), with reports of a single dose administered orally restoring euthyroidism over a period of 2–4 months (2).
Radioiodine for the treatment of goiter was introduced approximately three decades ago. In an initial report, 25 individuals with a mean glandular volume of 73 cm3, who received 100 μCi of radioiodine per gram of thyroid tissue corrected to 100% uptake in 24 h, showed an approximate reduction in goiter volume of 41% after 1 year of follow-up (38). The larger the volume of the gland and the lower the radioiodine uptake (RAIU), the higher should be the radioiodine activity to be administered. Subsequent studies have unanimously corroborated this observation (39, 40). Patients with very large goiters (>100 cm3) have a smaller decrease in glandular volume (about 35%) even with administration of similar radioiodine doses (37).
In some European countries such as Denmark and the Netherlands (to some degree), radioiodine has currently replaced surgery as the treatment of choice for NNG (10). Depending on each country’s regulations, radioiodine administered for treatment purposes may be delivered in fractions on an outpatient basis, reducing the costs of hospitalization (41).
Some patients develop temporary mild thyrotoxicosis within the initial 2 weeks of treatment, and about 45% of them develop hypothyroidism, requiring thyroid hormone replacement for life (16). The occurrence of hyperthyroidism due to Graves’ disease associated with increased serum concentrations of TSH receptor antibodies has also been described in patients with increased baseline levels of thyroid peroxidase antibodies after radioiodine treatment for NNG (42).
Based on measurements of whole-body radiation exposure, the theoretical lifetime risk of development of cancer outside the thyroid gland has been calculated as 1.6%. This is due to the use of high doses of radioiodine in patients with very large goiters (the mean goiter volume used in the calculation was ~220 g). When administered to individuals aged 65 years or older, the estimated risk is ~0.5% (43).
Recombinant Human TSH-Stimulated Radioiodine Therapy
Low isotope accumulation in inactive and partially suppressed areas around the nodule is a limitation of radioiodine treatment in patients with NNGs. This problem may be solved by increasing the RAIU in such nodular goiters (44). For this purpose, recent studies using rhTSH in preparation for radioiodine therapy have shown good results (45).
Several studies have assessed the adjuvant role of rhTSH in the radioiodine treatment of NNG. Administration of rhTSH is associated with a twofold to fourfold increase in RAIU by the thyroid (46). It should be noted that the baseline serum TSH may be a confounding factor since the increase in the 24-h thyroid RAIU correlates inversely with this variable. Since patients with NNG often present low serum TSH, the radioiodine is only taken up by some “hot” areas encircled by suppressed thyroid tissue that is inactive on scintigraphy. This phenomenon can be explained by a suppression of the paranodular parenchyma as a consequence of the low TSH level. Upon stimulation with rhTSH, these dormant areas, which concentrate radioiodine weakly, reactivate and eventually amplifies the effect of the radioiodine in the gland, promoting a further reduction in the volume of the goiter. In fact, rhTSH has been shown to distribute the radioiodine more homogeneously in the goiter, allowing a decrease in the dose of radioiodine to be administered (44). Most studies analyzing rhTSH in this setting have used fixed doses of radioiodine ranging from ~14 to ~42 mCi (37) and treatment with one or two rhTSH doses ranging from 0.1 to 0.3 mg administered 24 h prior to the radioiodine. However, the dose with ideal efficacy and safety is yet to be defined (47).
Combined therapy with rhTSH and radioiodine is well tolerated, and potential side effects that occur are similar to those observed in individuals receiving radioiodine alone. However, patients may experience a short increase in thyroid hormone levels within 48 h from the administration of radioiodine, leading to transient mild thyrotoxicosis, which may be followed by hypothyroidism during the first 30 days after the therapy (48, 49). Other acute adverse events that have been reported include painful transient thyroiditis, thyroid swelling, compression of the trachea, and, often, heart-related symptoms. Administration of glucocorticoids and β-blockers may help minimize these symptoms (50).
Recent studies (37) have shown that these adverse events may be dependent on the dose and are negligible with lower doses of rhTSH. Bonnema et al. (44) have shown that the ideal rhTSH dose to improve treatment with radioiodine may be about 0.03–0.1 mg. This dose range increases the thyroid RAIU substantially, with a minimal risk of thyroid swelling and transient thyrotoxicosis.
Studies assessing the long-term adverse effects of the combined use of rhTSH and radioiodine therapy showed an increased rate of permanent hypothyroidism. Three randomized controlled studies have reported 1-year rates of permanent hypothyroidism between 21 and 65% in rhTSH-pretreated patients compared with rates between 7 and 21% in patients in whom rhTSH was not administered (51–53). Due to its effect on the thyroid, rhTSH can potentially trigger an autoimmune response. Following treatment with radioiodine stimulated with rhTSH, 8 of 15 subjects with NNG developed antiperoxidase antibodies. However, no differences in autoimmunity were observed at 12 months between the subjects who underwent therapy with radioiodine alone versus those pretreated with rhTSH before radioiodine (54).
No studies have specifically assessed the risk of malignancy after NNG treatment with radioiodine with rhTSH prestimulation. However, by increasing the uptake of radioiodine by the thyroid, rhTSH results in less radioactivity delivered to the thyroid and to the rest of the body, which potentially reduces the theoretical risk of radioiodine-induced malignancy (37).
To avoid unintentional stimulation of the thyroid, a “modified-release rhTSH” (MRrhTSH) has been recently introduced. This compound has a slightly different serum profile than that of rhTSH, with a more delayed peak and, therefore, a possible lower risk of goiter enlargement. A phase II study of MRrhTSH use in patients with NNG undergoing radioiodine therapy was recently published. After 6 months, patients pre-stimulated with MRrhTSH 0.01 mg or placebo before radioiodine therapy had a 23% reduction in the goiter volume, whereas the reduction was 33% in individuals pre-stimulated with MRrhTSH 0.03 mg (55).
Both rhTSH and MRrhTSH are not approved by the US Food and Drug Administration or European Medicines Agency to treat NNG in association with radioiodine; so, their use for this purpose is currently off-label.
An alternative approach to increase the thyroid RAIU is to administer methimazole to stimulate the secretion of endogenous TSH. Albino et al. (56) pretreated nine patients with nodular goiter and subclinical hyperthyroidism with methimazole, aiming at increasing the serum level of TSH above 6 μU/mL. When this level was achieved, radioiodine 30 mCi was administered. The 24-h thyroid RAIU increased from 21 to 78%, and the mean goiter reduction at 1 year was 46%. In eight individuals (89%) with subclinical hyperthyroidism, the thyroid function normalized after 1 year. Five patients (56%) developed overt hypothyroidism, and no adverse clinical events were observed. However, whether a marginal hypothyroid state obtained with methimazole is as effective as rhTSH to increase the thyroid RAIU and to augment the reduction in goiter size after radioiodine therapy remains to be clarified with controlled trials.
In summary, individuals with NDG should receive clinical rather than surgical treatment. NNG is a highly prevalent disease, even in regions without iodine deficiency. Many individuals are asymptomatic, and when symptoms are present, the most common clinical manifestations result from local compressive effects. Individuals with NDG should be thoroughly evaluated to exclude malignancy. They should then receive individualized therapy after assessment of risks and benefits of each treatment option and discussion with their physicians. The first therapeutic option is total thyroidectomy, followed by treatment with radioiodine alone or after rhTSH stimulation to increase the radioiodine efficacy.
It is unclear if suppressive therapy with thyroid hormone is effective in patients with NNG. The choice of this therapy has decreased because of concerns regarding eventual long-term side events due to subclinical hyperthyroidism, and due to the fact after therapy interruption, most goiters grow again in size. All these therapies have potential advantages and disadvantages and are associated with short-term and long-term side events.
Surgery or Radioiodine Therapy?
Table 3 presents various circumstances associated with the development of multinodular goiter, as well as the treatments (surgery or radioiodine) recommended in each case.
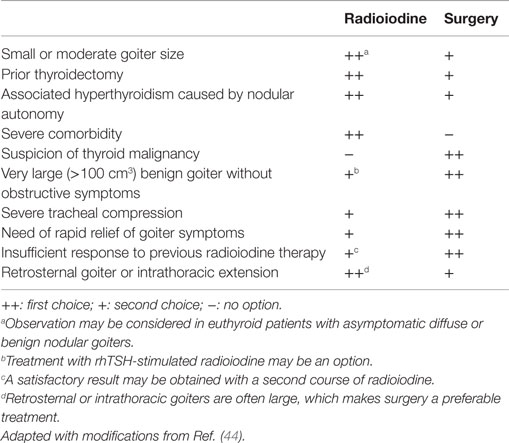
Table 3. Circumstances of preferred thyroidectomy or radioiodine therapy in patients with multinodular goiter.
Final Remarks
The therapeutic goals are to treat eventual thyroid dysfunction and reduce the size of the gland or prevent an additional increase in size. Without robust data from randomized studies, treatment decisions must be individualized according to the characteristics of the patients. Several individuals with large benign goiters may present symptoms of pressure, including dysphagia, neck tightness, or sensation of airway obstruction. Such patients frequently require surgical treatment to improve their symptoms. Asymptomatic patients in whom malignancy is excluded may be only observed. Controversy remains regarding the effectiveness of LT4 suppression implemented to reduce the size of the goiter. Radioactive iodine alone or with rhTSH is safe and effective and may be a reasonable therapeutic option.
Finally, which is the ideal treatment for benign NDG and NNG goiters? Once the diagnosis and indication for treatment of non-toxic goiter has been made, the treating physician and patient should discuss each of the treatment options, including the benefits, side effects, expected speed of recovery, drawbacks, and then decide on the best treatment modality for that particular patient, taking into account the patient’s age and comorbidities.
Ethical Approval
This review did not include studies conducted by the author with the participation of humans or animals.
Informed Consent
For this type of article, a formal consent is not required.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author would like to thank Milena Braga-Basaria for editorial suggestions and revision of the manuscript.
References
1. Krohn K, Fuhrer D, Bayer Y, Eszlinger M, Brauer V, Neumann S, et al. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev (2005) 26:504–24. doi: 10.1210/er.2004-0005
2. Knobel M. Etiopathology, clinical features, and treatment of diffuse and multinodular nontoxic goiters. J Endocrinol Invest (2016) 39:357–73. doi:10.1007/s40618-015-0391-7
3. Diehl LA, Garcia V, Bonnema SJ, Hegedüs L, Albino CC, Graf H, et al. Management of the nontoxic multinodular goiter in Latin America: comparison with North America and Europe, an electronic survey. J Clin Endocrinol Metab (2005) 90:117–23. doi:10.1210/jc.2004-1722
4. Samuels MH. Evaluation and treatment of sporadic nontoxic goiter – some answers and more questions [editorial]. J Clin Endocrinol Metab (2001) 86:994–7. doi:10.1210/jcem.86.3.7384
5. Hintze G, Kobberling J. Treatment of iodine deficiency goiter with iodine, levothyroxine or a combination of both. Thyroidology (1992) 4:37–40.
6. Peters H, Hackel D, Schleusener H. Treatment of euthyroid struma. Comparable volume reduction with 400 micrograms iodine, 100 micrograms levothyroxine combined with 100 micrograms iodine or individually dosed levothyroxine. Med Klin (Munich) (1997) 92:63–7. doi:10.1007/BF03042286
7. Güllü S, Gürses MA, Başkal N, Uysal AR, Kamel AN, Erdoğan G. Suppressive therapy with levothyroxine for euthyroid diffuse and nodular goiter. Endocr J (1999) 46:221–6. doi:10.1507/endocrj.46.221
8. Hegedüs L, Bennedbaek FN. Radioiodine for non-toxic diffuse goitre. Lancet (1997) 350:409–10. doi:10.1016/S0140-6736(05)64132-3
9. Nygaard B, Farber J, Veje A, Hansen JE. Thyroid volume and function after 131I treatment of diffuse non-toxic goitre. Clin Endocrinol (Oxf) (1997) 46:493–6. doi:10.1046/j.1365-2265.1997.1760987.x
10. Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev (2003) 24:102–32. doi:10.1210/er.2002-0016
11. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) (1995) 43:55–68. doi:10.1111/j.1365-2265.1995.tb01894.x
12. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19:1167–214. doi:10.1089/thy.2009.0110
13. Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab (1996) 81:4278–89. doi:10.1210/jcem.81.12.8954028
14. Rieu M, Bekka S, Sambor B, Berrod JL, Fombeur JP. Prevalence of subclinical hyperthyroidism and relationship between thyroid hormonal status and thyroid ultrasonographic parameters in patients with non-toxic nodular goitre. Clin Endocrinol (Oxf) (1993) 39(1):67–71. doi:10.1111/j.1365-2265.1993.tb01752.x
15. Berghout A, Wiersinga WM, Drexhage HA, Smits NJ, Touber JL. Comparison of placebo with L-thyroxine alone or with carbimazole for treatment of sporadic non-toxic goitre. Lancet (1990) 336:193–7. doi:10.1016/0140-6736(90)91730-X
16. Wesche MF, Tiel-V Buul MM, Lips P, Smits NJ, Wiersinga WM. A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab (2001) 86:998–1005. doi:10.1210/jcem.86.3.7244
17. Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta-analysis. J Clin Endocrinol Metab (2002) 87:4154–9. doi:10.1210/jc.2001-011762
18. Grussendorf M, Reiners C, Paschke R, Wegscheider K, LISA Investigators. Reduction of thyroid nodule volume by levothyroxine and iodine alone and in combination: a randomized, placebo-controlled trial. J Clin Endocrinol Metab (2011) 96:2786–95. doi:10.1210/jc.2011-0356
19. Puzziello A, Carrano M, Angrisani E, Marotta V, Faggiano A, Zeppa P, et al. Evolution of benign thyroid nodules under levothyroxine non-suppressive therapy. J Endocrinol Invest (2014) 37:1181–6. doi:10.1007/s40618-014-0128-z
20. Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27914 patients. Endocr Relat Cancer (2010) 17:231–9. doi:10.1677/ERC-09-0251
21. Cirocchi R, Trastulli S, Randolph J, Guarino S, Di Rocco G, Arezzo A, et al. Total or near-total thyroidectomy versus subtotal thyroidectomy for multinodular non-toxic goitre in adults. Cochrane Database Syst Rev (2015) 7(8):CD010370. doi:10.1002/14651858.CD010370.pub2
22. Agarwal G, Aggarwal V. Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J Surg (2008) 32:1313–24. doi:10.1007/s00268-008-9579-8
23. Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg (2008) 32:1301–12. doi:10.1007/s00268-008-9477-0
24. Thomusch O, Machens A, Sekulla C, Ukkat J, Lippert H, Gastinger I, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg (2000) 24:1335–41. doi:10.1007/s002680010221
25. Yoldas T, Makay O, Icoz G, Kose T, Gezer G, Kismali E, et al. Should subtotal thyroidectomy be abandoned in multinodular goiter patients from endemic regions requiring surgery? Int Surg (2015) 100:9–14. doi:10.9738/INTSURG-D-13-00275.1
26. Bauer PS, Murray S, Clark N, Pontes DS, Sippel RS, Chen H. Unilateral thyroidectomy for the treatment of benign multinodular goiter. J Surg Res (2013) 184:514–8. doi:10.1016/j.jss.2013.04.045
27. Hegedüs L, Nygaard B, Hansen JM. Is routine thyroxine treatment to hinder postoperative recurrence of nontoxic goiter justified? J Clin Endocrinol Metab (1999) 84:756–60. doi:10.1210/jcem.84.2.5478
28. Rojdmark J, Jarhult J. High long term recurrence rate after subtotal thyroidectomy for nodular goitre. Eur J Surg (1995) 161:725–7.
29. Berghout A, Wiersinga WM, Drexhage HA, van Trotsenburg P, Smits NJ, van der Gaag RD, et al. The long-term outcome of thyroidectomy for sporadic non-toxic goitre. Clin Endocrinol (Oxf) (1989) 31:193–9. doi:10.1111/j.1365-2265.1989.tb01242.x
30. Miccoli P, Antonelli A, Iacconi P, Alberti B, Gambuzza C, Baschieri L. Prospective, randomized, double-blind study about effectiveness of levothyroxine suppressive therapy in prevention of recurrence after operation: result at the third year of follow-up. Surgery (1993) 114:1097–101.
31. Bistrup C, Nielsen JD, Gregersen G, Franch P. Preventive effect of levothyroxine in patients operated for non-toxic goitre: a randomized trial of one hundred patients with nine years follow-up. Clin Endocrinol (Oxf) (1994) 40:323–7. doi:10.1111/j.1365-2265.1994.tb03926.x
32. Geerdsen JP, Frølund L. Thyroid function after surgical treatment of nontoxic goitre. A randomized study of postoperative thyroxine administration. Acta Med Scand (1986) 220:341–5. doi:10.1111/j.0954-6820.1986.tb02775.x
33. Mercante G, Gabrielli E, Pedroni C, Formisano D, Bertolini L, Nicoli F, et al. CT cross-sectional imaging classification system for substernal goiter based on risk factors for an extracervical surgical approach. Head Neck (2010) 33:792–9. doi:10.1002/hed.21539
34. Baehr KM, Lyden E, Treude K, Erickson J, Goldner W. Levothyroxine dose following thyroidectomy is affected by more than just body weight. Laryngoscope (2012) 122:834–8. doi:10.1002/lary.23186
35. Pieracci FM, Fahey TJ 3rd. Substernal thyroidectomy is associated with increased morbidity and mortality as compared with conventional cervical thyroidectomy. J Am Coll Surg (2007) 205:1–7. doi:10.1016/j.jamcollsurg.2007.03.010
36. Ross DS. Thyroid hormone suppressive therapy of sporadic nontoxic goiter. Thyroid (1992) 2:263–9. doi:10.1089/thy.1992.2.263
37. Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev (2012) 33:920–80. doi:10.1210/er.2012-1030
38. Hegedüs L, Hansen BM, Knudsen N, Hansen JM. Reduction of size of thyroid with radioactive iodine in multinodular non-toxic goitre. BMJ (1988) 297:661–2. doi:10.1136/bmj.297.6649.661
39. Huysmans DA, Hermus AR, Corstens FH, Barentsz JO, Kloppenborg PW. Large, compressive goiters treated with radioiodine. Ann Intern Med (1994) 121:757–62. doi:10.7326/0003-4819-121-10-199411150-00005
40. Bonnema SJ, Bertelsen H, Mortensen J, Andersen PB, Knudsen DU, Bastholt L, et al. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocrinol Metab (1999) 84:3636–41. doi:10.1210/jcem.84.10.6052
41. Howarth DM, Epstein MT, Thomas PA, Allen LW, Akerman R, Lan L. Outpatient management of patients with large multinodular goitres treated with fractionated radioiodine. Eur J Nucl Med (1997) 24:1465–9. doi:10.1007/s002590050175
42. Nygaard B, Knudsen JH, Hegedüs L, Scient AV, Hansen JE. Thyrotropin receptor antibodies and Graves’ disease, a side-effect of 131I treatment in patients with nontoxic goiter. J Clin Endocrinol Metab (1997) 82:2926–30. doi:10.1210/jc.82.9.2926
43. Huysmans DA, Buijs WC, van de Ven MT, van den Broek WJ, Kloppenborg PW, Hermus AR, et al. Dosimetry and risk estimates of radioiodine therapy for large, multinodular goiters. J Nucl Med (1996) 37:2072–9.
44. Bonnema SJ, Fast S, Hegedüs L. The role of radioiodine therapy in benign nodular goiter. Best Pract Res Clin Endocrinol Metabol (2014) 28:619–31. doi:10.1016/j.beem.2014.02.001
45. Medeiros-Neto G, Marui S, Knobel M. An outline concerning the potential use of recombinant human thyrotropin for improving radioiodine therapy of multinodular goiter. Endocrine (2008) 33:109–17. doi:10.1007/s12020-008-9077-7
46. Nielsen VE, Bonnema SJ, Boel-Jørgensen H, Veje A, Hegedüs L. Recombinant human thyrotropin markedly changes the 131I kinetics during 131I therapy of patients with nodular goiter: an evaluation by a randomized double-blinded trial. J Clin Endocrinol Metab (2005) 90:79–83. doi:10.1210/jc.2004-1550
47. Graf H. Recombinant human TSH and radioactive iodine therapy in the management of benign multinodular goiter. Eur J Endocrinol (2015) 172:R47–52. doi:10.1530/EJE-14-0608
48. Paz-Filho GJ, Mesa-Junior CO, Olandoski M, Woellner LC, Goedert CA, Boguszewski CL, et al. Effect of 30 mCi radioiodine on multinodular goiter previously treated with recombinant human thyroid-stimulating hormone. Braz J Med Biol Res (2007) 40:1661–70. doi:10.1590/S0100-879X2006005000186
49. Romao R, Rubio IGS, Tomimori EK, Camargo RY, Knobel M, Medeiros-Neto G. High prevalence of side effects after rhTSH stimulated radioiodine treatment with 30 mCi in patients with multinodular goiter and subclínical/clínical hyperthyroidism. Thyroid (2009) 19:945–51. doi:10.1089/thy.2008.0394
50. Fast S, Nielsen VE, Bonnema SJ, Hegedüs L. Time to reconsider nonsurgical therapy of benign non-toxic multinodular goitre: focus on recombinant human TSH augmented radioiodine therapy. Eur J Endocrinol (2009) 160:517–28. doi:10.1530/EJE-08-0779
51. Nielsen VE, Bonnema SJ, Boel-Jørgensen H, Grupe P, Hegedüs L. Stimulation with 0.3-mg recombinant human thyrotropin prior to iodine 131 therapy to improve the size reduction of benign nontoxic nodular goiter: a prospective randomized double-blind trial. Arch Intern Med (2006) 166:1476–82. doi:10.1001/archinte.166.14.1476
52. Bonnema SJ, Nielsen VE, Boel-Jørgensen H, Grupe P, Andersen PB, Bastholt L, et al. Improvement of goiter volume reduction after 0.3 mg recombinant human thyrotropin-stimulated radioiodine therapy in patients with a very large goiter: a double-blinded, randomized trial. J Clin Endocrinol Metab (2007) 92:3424–8. doi:10.1210/jc.2007-0501
53. Fast S, Nielsen VE, Grupe P, Boel-Jørgensen H, Bastholt L, Andersen PB, et al. Prestimulation with recombinant human thyrotropin (rhTSH) improves the long-term outcome of radioiodine therapy for multinodular nontoxic goiter. J Clin Endocrinol Metab (2012) 97:2653–60. doi:10.1210/jc.2011-3335
54. Rubio IG, Perone BH, Silva MN, Knobel M, Medeiros-Neto G. Human recombinant TSH preceding a therapeutic dose of radioiodine for multinodular goiters has no significant effect in the surge of TSH-receptor and TPO antibodies. Thyroid (2005) 15:134–9. doi:10.1089/thy.2005.15.134
55. Graf H, Fast S, Pacini F, Pinchera A, Leung A, Vaisman M, et al. Modified-release recombinant human TSH (MRrhTSH) augments the effect of 131I therapy in benign multinodular goiter. Results from a multicenter international, randomized, placebo-controlled study. J Clin Endocrinol Metab (2011) 96:1368–76. doi:10.1210/jc.2010-1193
Keywords: benign goiter, non-toxic, diffuse, multinodular, treatment
Citation: Knobel M (2016) Which Is the Ideal Treatment for Benign Diffuse and Multinodular Non-Toxic Goiters? Front. Endocrinol. 7:48. doi: 10.3389/fendo.2016.00048
Received: 04 April 2016; Accepted: 11 May 2016;
Published: 23 May 2016
Edited by:
Alessandro Antonelli, University of Pisa, ItalyReviewed by:
Salvatore Ulisse, Sapienza University of Rome, ItalyAkira Hishinuma, Dokkyo Medical University, Japan
Copyright: © 2016 Knobel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meyer Knobel, bWV5ZXIua25vYmVsJiN4MDAwNDA7aGMuZm0udXNwLmJy
 Meyer Knobel
Meyer Knobel