- 1Department of Molecular Medicine, Faculty of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
- 2Department of Clinical Biochemistry and Laboratory Medicine, Faculty of Medicine, Tabriz, Iran
- 3Tuberculosis and Lung Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
- 4Rahat Breath and Sleep Research Center, Tabriz University of Medical Science, Tabriz, Iran
- 5Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 6Infectious and Tropical Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
- 7Department of Medical Biochemistry, Faculty of Medicine, Istanbul Aydin University, Istanbul, Türkiye
Breast cancer is the most prevalent neoplasm in women. ER+ (Luminal subtype), representing over 70% of breast tumors, is a genetically diverse group. Structural and Numerical-Chromosomal instability initiates tumor development and is recognized as the primary driver of genetic alteration in luminal breast tumors. Genomic instability refers to the increased tendency of cancer cells to accumulate genomic alterations during cell proliferation. The cell cycle check-point response to constant and stable genomic alterations in tumor cells drives this process. The impact of CNV patterns and aneuploidies in cell cycle and proliferation perturbation has recently been highlighted by scientists in Luminal breast tumors. The impact of chromosomal instability on cancer therapy and prognosis is not a new concept. Still, the degree of emerging genomic instability leads to prognosis alteration following cell cycle deregulation by chromosomal instability could be predicted by CNVs-based reclassification of breast tumors. In this review, we try to explain the effect of CIN in the cell cycle that ended with genomic instability and altered prognosis and the impact of CIN in decision-making for a therapy strategy for patients with luminal breast cancer.
1 Introduction
Carcinomas, malignant neoplasms of epithelial origin, are characterized by uncontrolled cell proliferation driven by the accumulation of genetic alterations (1, 2). Breast cancer, the most frequently diagnosed cancer worldwide (3, 4), is classified into four distinct subtypes based on immunohistochemical (IHC) and genetic profiling of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status. These subtypes include: Luminal A (ER+ and/or PR+, HER2-), Luminal B (ER+ and/or PR+, HER2+), HER2 positive (ER−, PR−, and HER2+), and triple-negative breast cancer (ER−, PR−, and HER2-). These subtypes exhibit distinct prognoses and responses to therapy (5–8), guiding clinical management to appropriate chemotherapy selection. To further refine IHC and fluorescence in situ hybridization (FISH), and overcome their limitation issues and cut-off challenges, the molecular signature of breast cancer subtypes is assessed by mRNA-based kit. These kits incorporate four novel proliferation markers (MKI67, PCNA, CCNA2, and KIF23), in addition to the three established biomarkers, providing a more comprehensive molecular profile for accurate subtyping and prognostication (9, 10). Notably, luminal subtypes constitute approximately 70% of breast cancers diagnosed in women, with 85% exhibiting 5-year overall survival. However, sub-classification for therapeutic purposes remains challenging due to the molecular diversity of ER+ and/or PR+ tumors (Table 1) (11–13).
Genomic instability (GI), the increased susceptibility of the genome to accumulate further alterations driving tumor clonal evolution and contributing to the heterogeneity observed in solid tumors. Genome copy number variations (CNVs), encompassing deletions, insertions, and duplications of variable-sized DNA segments, are the primary drivers of genomic instability in various cancers (14–16). The maintenance of genomic integrity, crucial for cellular function, is safeguarded by intricate mechanisms (Figure 1). Disruptions in these mechanisms can lead to genomic instability.
In breast cancer, several pathways crucial for maintaining genomic integrity are frequently altered, including some that have been successfully targeted for therapeutic intervention (15). The study of CNVs has undergone significant advancements since their initial discovery. Early research relied on techniques like array comparative genomic hybridization and SNP genotyping platforms, which enabled the identification of numerous CNVs across the human genome. With the advent of next-generation sequencing (NGS), the CNV research field has witnessed a dramatic shift. NGS offers a more comprehensive and high-throughput approach to identifying CNVs, enabling researchers to delve deeper into their genomic architecture and functional consequences (17). CNVs, a form of structural alteration characterized by variable-sized deletions, insertions, and duplications of DNA segments, represent some of the most prevalent and significant genetic alterations. Structural chromosomal instability (s-CIN), the underlying cause of CNVs in cancer, links cell cycle checkpoints, genomic instability, and mitogen-induced replication stress. Notably, CNVs in combination pathway analysis revealed that identical DNA gains encompass essential genes acting as tumorigenic drivers. The challenge lies in translating these findings into clinically relevant classifications. In breast tumors, integrative clustering based on CNVs, transcriptomic data, and clinical characteristics demonstrates the strongest correlation with survival and genomic instability development (18, 19). This approach, known as Integrative Clustering (IntClust 1-10), classifies breast cancer samples based on their genomic driver events. While luminal A and B subtypes may appear indistinguishable based on shared genomic characteristics (20), luminal B tumors exhibit greater heterogeneity in CNV patterns. This highlights the challenge of subtype classification, as tumors display a continuous spectrum of molecular formations associated with increasing levels of genetic damage (21).
This review summarizes the impact of CNVs on cell cycle behavior in the luminal subtype of breast cancer. We explore how CNVs independently or in conjunction with other factors alter the status of cell cycle checkpoints. Finally, we provide novel insights into the implications of CNVs for clinical therapeutic decision-making.
2 Literature review methodology
This review employed a comprehensive search strategy to identify relevant literature from reputable scientific databases, including Science Direct, Springer, RSC, ACS, NCBI, MDPI, Web of Science, and Google Scholar. The inclusion criteria were as follows: full-text articles published between 1994 and 2024, articles published in the English language, articles with a title, abstract, source, DOI, and year of publication.
Keywords such as “Genomic Instability in Cancer”, “Cell Cycle Deregulation in Cancer”, “Breast Cancer Subtypes”, and “Luminal Breast Cancer Genetic Variations” were used and combined with search terms using Boolean operators to refine the search. Full-text unavailable articles, non-English language, or summaries of editorials, conferences, seminars, and events were excluded.
3 Copy number variation sources and analysis
Principally, CNVs as a somatic alteration derive from several genetic mechanisms, especially gene instability, chromosomal instability (CIN), and aneuploidy, which contribute significantly toward the development and prevalence of CNVs observed in luminal breast cancers (22). The most impactful aspects of CNVs in luminal breast cancer are oncogene amplification (epithelial membrane protein 3) (23), prognostic value (phorbol-12-myristate-13-acetate-induced protein 1) (24), molecular subtyping (human epidermal growth factor receptor 2) (25), personalized treatment (Myeloid differentiation primary response 88) (26), biomarker (PGAP3, GRB7 genes) (27), and drug resistance mechanisms (MYC gene regulation) (28).
Genomic instability results from defects in several mechanisms during cell cycle progression and regulation. These mechanisms include DNA damage from both endogenous and exogenous sources, DNA damage repair, DNA replication and transcription, mitotic chromosome segregation, centrosome amplification, epigenetic modifications, and telomere maintenance. The GI can either be perpetuated or limited through the induction of mutations or aneuploidy, both of which can be enabling or catastrophic in their effects. GI is closely linked to immune evasion, cancer progression, and multidrug resistance. In hereditary cancers associated with microsatellite or chromosomal instabilities, mutations in DNA repair genes are primary drivers of GI. However, in sporadic cancers, particularly in their early stages, GI does not typically depend on mutations in DNA repair or mitotic checkpoint genes. The mutation patterns observed in sporadic cancers suggest that p53 mutations are driven by DNA damage, rather than through activation of p14ARF. Oncogene-induced DNA damage may represent a critical contributor to GI in sporadic cancers (14, 29–32).
Maintaining genomic stability necessitates the precise replication of genetic material and the equal segregation of newly duplicated chromosomes during mitosis. Disruptions in either of these processes can lead to chromosomal instability (CIN), characterized by an increased rate of chromosomal alterations (33). These chromosomal alterations serve as unique fingerprints, unveiling the intricate mechanisms by which genes become dysregulated in cancer, leading to the destabilization of the cell’s genetic integrity (34). CIN manifests as either numerical (n-CIN) or structural (s-CIN) alterations (Figure 2). n-CIN involves gains or losses of whole chromosomes, while s-CIN includes gains, losses, or rearrangements of chromosome segments. n-CIN primarily results from mitotic errors, whereas s-CIN arises from pre-mitotic events affecting chromosome integrity (33).

Figure 2. Numerical and structural chromosomal instability (CIN) (15).
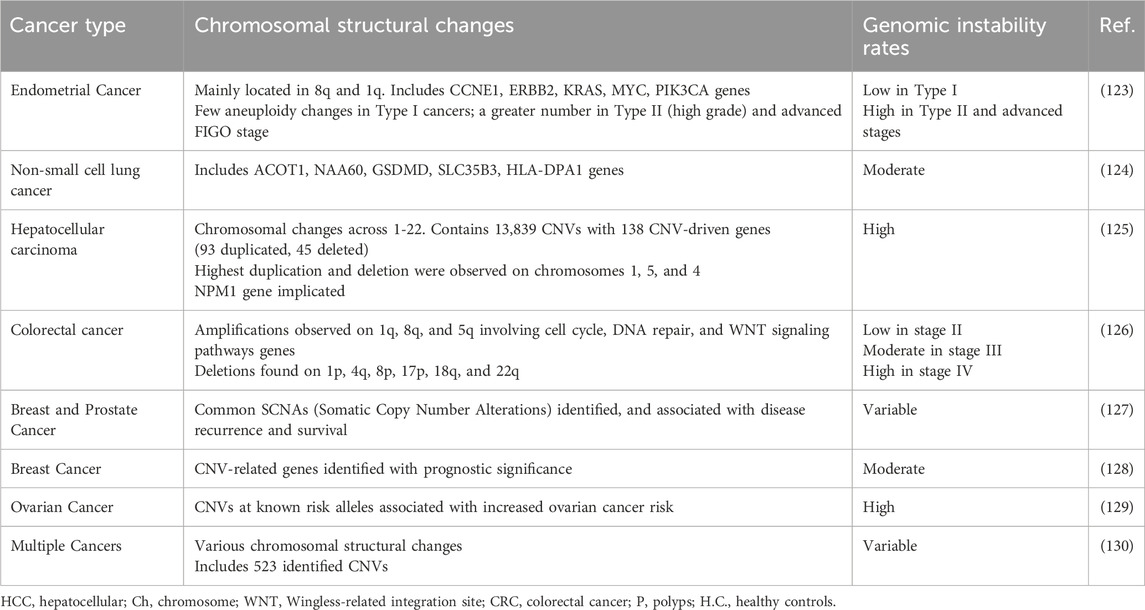
CNVs refer to variations in the copy number of specific DNA segments across different individuals’ genomes (35), approximately 200 CNVs have been identified breast cancer (36). CNVs contribute significantly to genomic diversity, varying from small alterations to complete chromosomal aneuploidies. While small CNVs are usually benign, larger ones (over 250 kb) are linked to serious consequences like developmental disorders and cancers (37). Analysis of the top 100-upregulated genes associated with various cancers reveals distinct chromosomal locations. Table 2 summarizes the findings of studies on CNVs in various types of cancer. Such validation could pave the way for the development of more effective diagnostic, prognostic, and therapeutic strategies, ultimately leading to a more promising future in the fight against cancer (34). Various techniques have been employed for CNV profiling, including SNP arrays and next-generation sequencing (NGS) technologies. Ongoing efforts are focused on developing more accurate and efficient methods for CNV detection, as well as exploring the functional implications of CNVs in biological processes and disease contexts (17). CNV holds significant promise as a valuable biomarker for tumors, with potential applications in tumor subtyping, drug response prediction, and survival time prediction (38). Targeted NGS-based CNV panels are widely used in clinical practice due to their high reproducibility and focus on a limited gene set, making them crucial for accurate clinical decision-making, particularly in cancer treatment. Insights from genome-wide CNV studies aid in designing these targeted panels. However, normal cell contamination, tumor heterogeneity, and aneuploidy hinder reliable CNV detection. Additionally, systematic errors from structural variations can bias results, making it advisable to detect these variations before conducting CNV analysis on tumor samples (38). Single-cell sequencing presents challenges for CNV analysis due to limited DNA content, making it difficult to differentiate between PCR duplication. Accurate CNV measurements become unreliable with restricted DNA quantities in single cells and circulating tumor DNA, necessitating careful adjustments for proper analysis. Additionally, integrating CNV data with other multi-omics information is essential for a comprehensive understanding of cancer development and progression (38).
Aneuploidy is an abnormal number of chromosomes in a cell, deviating from the typical diploid number. For instance, a human cell with 45 or 47 chromosomes instead of the usual 46 would be considered aneuploidy (39). Aneuploidy manifests in two distinct forms: stable and unstable. Stable aneuploidy exhibits consistent numerical chromosome alterations across most cells, while unstable aneuploidy is characterized by significant cell-to-cell variation in chromosome number, leading to karyotypic heterogeneity. Unstable aneuploidy notably promotes the development of diverse tumor subpopulations, contributing to both inter and intra-tumoral genomic heterogeneity. CIN is a major contributor to intra-tumoral heterogeneity, which empowers cancer cells to adapt to environmental pressures and evolve into more aggressive, treatment-resistant populations. CIN and aneuploidy profoundly affect therapeutic response by disrupting gene regulation and altering protein levels, ultimately influencing cellular responses to drug treatments. This dynamic interplay suggests that individual cancer cells with distinct chromosomal profiles may exhibit varying drug sensitivities, potentially allowing a subset of the tumor population to evade therapy and persist. A pilot study on ten Luminal B patients suggests an association between stable aneuploidy, intermediate CIN, and lympho-vascular invasion. This association between clonal heterogeneity and CIN holds potential prognostic value for breast cancer patients, particularly those with HER2+ tumors. Dual-color FISH analysis of Luminal B subtype breast cancer revealed that over 90% of patients exhibited intermediate CIN (33%-49%), while only 10% showed high CIN (52%) (40). Aneuploidy Score (AS), a measure of CIN, indicated that 75% of breast cancers had an AS above five. Luminal A tumors had lower AS compared to Luminal B. While AS was not prognostic for overall survival, progression-free survival was significantly worse in Luminal B cancers with high AS. The most frequent chromosome gains were observed in 1q, 8q, and 16p, while losses were commonly seen in 16q, 17p, and 8p (41). Notably, centromere 17 copy number, assessed by FISH and NGS-derived for CIN scores, emerged as a key prognostic factor in breast cancer (40, 42). Furthermore, Phosphatase and TENsin homolog (PTEN), a tumor suppressor gene frequently altered in breast cancer, exhibits distinct clinical characteristics compared to other cancers. Women with PTEN hamartoma tumor syndrome have an up to 85% lifetime risk of breast cancer. This alteration was identified by integrating somatic CNV and transcriptome data (43). Consequently, traditionally approved methods for CNV calling from tumor samples have demonstrated limited reliability for these proposes. Validation of these traditional methods in advance, MLPA or array-based approaches have better resolution (44).
A recent article about the reliability of DNA copy number variation linked machine learning approach CopyClust for integrative cluster classification (IntClust) for breast cancer. Particularly breast cancer subtyping is highly correlated to genomic variations of tumor, and CNVs are highly mirrored with cancer-driving genes additionally specific CNVs reflect tumor behavior and development. So IntClust classification could achieve 81% success on TCGA SNP data and 79% on WES data. Another, valuable advantage of the CopyClust algorithm is the ability to process CNV data alone when transcriptomic data is useless or not accessible. CopyClust classification has maximum clinical outcome prediction power with high accuracy (45). Table 3 comprises the traditional and updated methods in structural chromosomal variation detection in tumor samples.
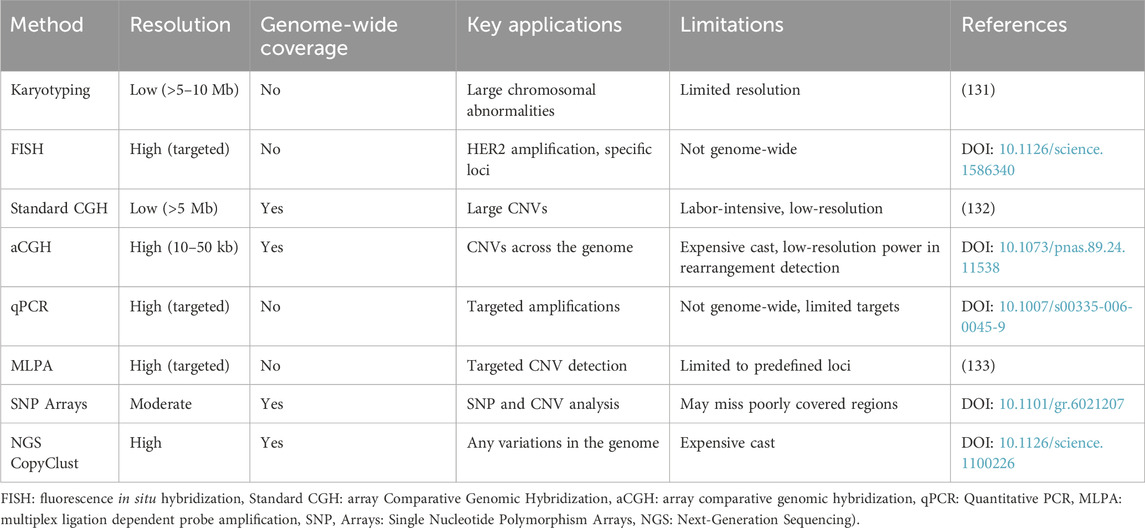
Table 3. Comparison of Traditional Methods versus updated methods in structural chromosomal variation detection in tumor samples.
3.1 Comparison of traditional methods versus updated methods in structural chromosomal variation detection in tumor samples
Traditionally approved methods for CNV calling from tumor samples did not perform well to ensure the best reliability for this purpose. Validation of these traditional methods in advance, MLPA or array-based approaches have better resolution. For multiple samples prevalent CNVs called is highly recommended (44). A recent article about the reliability of DNA copy-number-variation linked machine learning approach CopyClust for integrative cluster classification (IntClust) for breast cancer.
Particularly breast cancer subtyping is highly correlated to genomic variations of tumors, CNVs are highly mirrored with cancer-driving genes additionally specific CNVs reflect tumor behavior and development. So IntClust classification could achieve 81% success on TCGA SNP data and 79% on WES data. Another, valuable advantage of the CopyClust algorithm is the ability to process CNV data alone when transcriptomic data is useless or not accessible. CopyClust classification has maximum clinical outcome prediction power with high accuracy (45).
3.2 Genomic changes and aneuploidy in normal breast tissue
Recent studies indicate that Aneuploidy could be observed in non-cancerous normal breast tissue. The link between normal breast cells aneuploidy, certain breast cancer types, and age is notable.
A key study in the context of single-cell DNA sequencing from normal breast tissue samples of 49 healthy women. With a middle rate of 3.19% aneuploidy could be determined in the study group. These cells had changes like gain of chromosome 1q and incomplete loss of 10q, 16q, and 22q, which are characteristic chromosomal structural variations in invasive breast cancers. In particular, the prevalence of aneuploid cells elevated with age shows the possibility of natural accumulation of chromosomal instability over time (45). Non-cancerous events linked to aneuploidy in luminal cancers include: Differentiation Maintenance: Aneuploid cells control differentiation-correlated genes, like those in estrogen receptor pathways, preserving the luminal type breast cancer (DOI: 10.1038/bc2009). Cell Cycle Checkpoints: p53 activation in response to handling replication stress, may lead to more genomic stability (46). Dormancy or Senescence: The entrances of aneuploid cells to non-proliferative states, improve tumors genomic stability without invasion (47).
The possible Mechanisms of Aneuploidy Maintenance In normal tissues, without fostering tumorigenesis include Chromosomal Instability Tolerance: Autophagy and proteasomal degradation manage stress caused by aneuploidy (48), SAC1 Regulation: Fine-tuned SAC signaling stabilizes chromosomal integrity (DOI: 10.1038/nrm.2011.10) and Epigenetic Regulation: Histone modifications and DNA methylation help stabilize gene expression profiles (49).
In summary, it is widely accepted that Aneuploidy in normal breast tissue is an age-age-correlated event that is a normal consequence of early genomic instability unset. While some aneuploid cells harbor the same chromosomal alterations of luminal subtypes of breast cancers, their insignificant proliferation rate increases and non-tumorigenic mechanisms suggest self-limiting phenomena, arise from positive selection in tumor evolution. However, the influence of these cells on cancer development is not fully understood.
4 Cell cycle
The cell cycle, a tightly regulated sequence of events culminating in cell division, ensures the faithful transmission of genetic material from parent to daughter cells. This intricate machinery relies on the coordinated action of cyclins and CDKs2 throughout distinct phases: G0, G1, S, G2, and mitosis. Embedded within the cell cycle are five distinct checkpoint switches, which, according to mathematical models, may operate in a bistable manner. Activation of the G1/S restriction checkpoint and the spindle assembly checkpoint (SAC) in the G2/M transition elicits unique responses, whereas activation of the S and G2 checkpoints triggers diverse molecular outcomes. Notably, experimental validation has only confirmed the bistable switch property of the restriction checkpoint (50, 51).
4.1 G0 and G1 phases
In contrast to the quiescent G0 phase, extensive mRNA and protein synthesis, leading to cell growth and preparation for DNA replication, characterize the G1 phase. Mitogenic signaling pathways play a crucial role in G1 progression, particularly in epithelial cells (52–54). Intracellular and extracellular mitogenic signals activate the Cyclin D/CDK4/6 complex, resulting in the mono-phosphorylation of the RB3 within the E2F1/RB complex. Subsequently, the Cyclin E/CDK2 complex further phosphorylates RB, leading to the complete release of the E2F transcription factor. Active E2F1 drives the transcription of genes essential for DNA replication in the S phase. The G1/S checkpoint, also known as the restriction checkpoint, is the first critical control point in the cell cycle. This checkpoint regulates cell cycle progression by monitoring critical conditions, including growth factor availability, nutrient levels, and cell size. If these conditions are unfavorable, the cell cycle arrests in G1, returning to the G0 state. The balance between G1/S checkpoint activity and proliferation stimuli, along with the presence of the cyclin-dependent kinase inhibitor 1A (P21), determines the population of cells in G0 (55). Successful passage through this checkpoint signifies the cell’s irreversible commitment to division (51, 56).
Recent studies have highlighted the involvement of CDK2 in apoptosis regulation, DNA and RNA metabolism, and interaction with p53 in tumor development. Ectopic CDK2 expression has been linked to ductal breast carcinoma pathogenesis, with high nuclear CDK2 levels correlating with aggressive phenotypes, particularly high tumor grade, and lymph vascular invasion (57). Additionally, CDK4/6 plays a critical role in the luminal subtype of breast cancer. Analysis of 320 elderly metastatic breast cancer patients with luminal A and B subtypes revealed that CDK4/6 inhibitors facilitate treatment (58).
4.2 Deregulated G1 phase and restriction checkpoint in luminal breast cancer
CNV patterns exhibit a strong correlation with proliferation. The interplay between the G1/S checkpoint and E2F activity is modulated by mitogenic upstream signals, such as HER2 activation and Cyclin D1 expression levels. Studies on luminal breast tumors have demonstrated that CNVs impact the functional status of E2F as a transcription factor (59). CNVs regulate the phosphorylation-dependent release of E2F from the RB-E2F complex, leading to three distinct phenotypes in response to oncogene activity: G1 arrest, G1 acceleration, and G0 quiescence (60). Table 4 contains the specific genes with altered copy numbers that regulate the phosphorylation-dependent release of E2F from the RB-E2F complex.
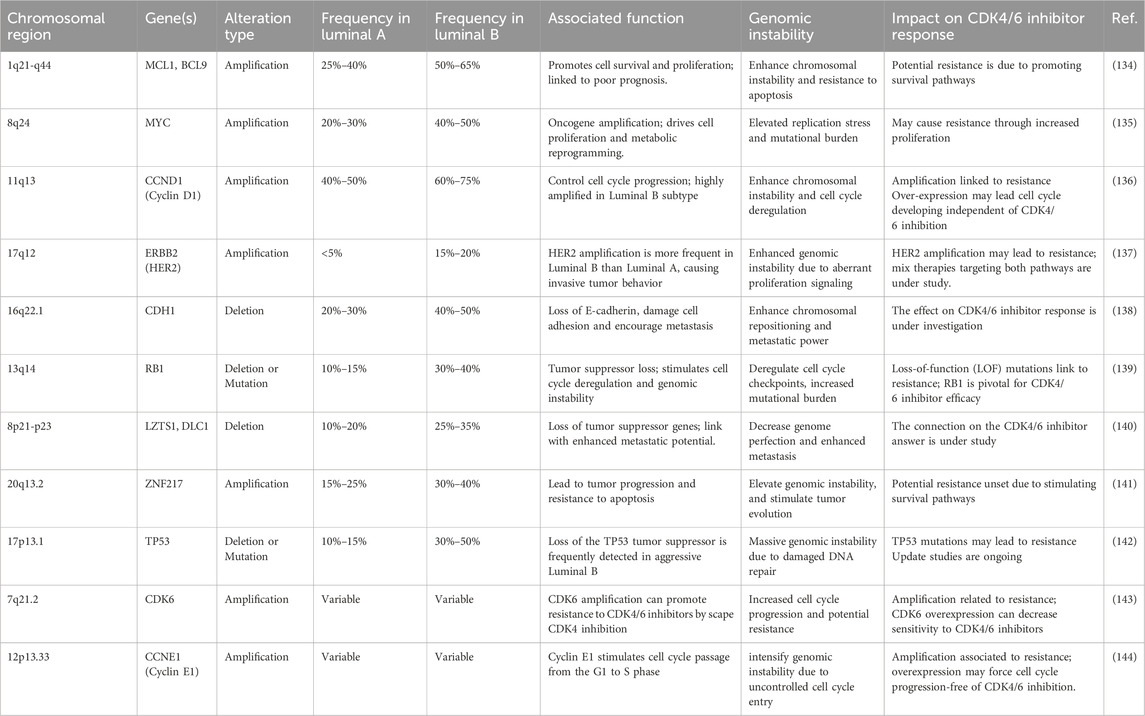
Table 4. The specific genes with altered copy numbers regulate breast cancer luminal subtypes associated with the phosphorylation-dependent release of E2F from the RB-E2F complex.
Structural chromosomal aberrations, such as CNV patterns, emerge early in tumor evolution and drive tumor progression. CNVs affect large chromosomal segments, some of which harbor known oncogenes. Depending on the proliferation potential and expression dosage, CNVs can alter the G1 phase function. Additionally, tissue-dependent expression of G1 phase inhibitors plays a role (61). Interestingly, a cancerous cell with identical oncogene expression can exhibit two distinct G1 phenotypes, depending on the E2F bistable switch status (55). Biological bistable switches are ultrasensitive biochemical systems that exhibit two distinct activation states: low and high. These switches arise in systems with multiple inhibitors and activators that create negative and positive feedback loops. A well-known example is E2F activation, which can exist in high or low levels despite steadily increasing mitogen signals. This allows the switch to be either ON or OFF (62–65).
In the E2F: OFF position, the G1 phase arrests cells based on P21 and E2F functional levels in an identical clonal population, containing variable G0 cells. Tumor masses often contain a significant population of G1-arrested and G0-phase cells, which dramatically limits the proliferation rate. This allows cells in the S phase to focus protein synthesis on DNA damage repair and replication stress defense (Figure 3) (55, 66).
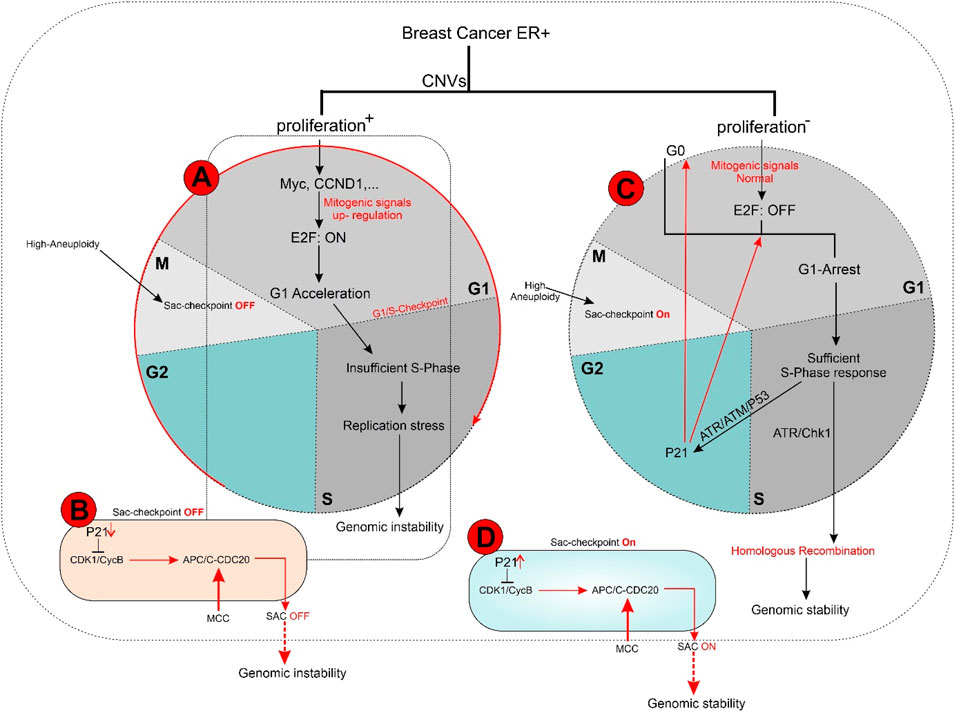
Figure 3. (A, B) In ER + breast tumors, oncogene overexpression due to identical CNVs leads to an increased proliferation rate by aberrant E2F release and accelerated G1 phase. An E2F ON status eradicates complete protein transcription in the G1 phase, which is necessary for replication stress defense in the S phase and limits response capacity to DNA damage. In turn, improper S phase checkpoint activation leads to a limited release of P21 as soon as G2 transcription activity initiation. A decreased concentration of P21 restricts its inhibitory effect on CDKs. In next G1, the lack of P21 leads to aberrant activation of CDK4 and CDK2 that in complex with specific cyclin proteins cause massive E2F activation. In the immediate M phase induction, CDK1 in CDK1/CycB complex leads to activation of APC/C-CDC20 independent of SAC inhibition. This series of molecular events initiated with identical CNVs results in a high proliferation rate and progression genomic instability due to the limited capability of DNA repair mechanisms. Additionally, SAC checkpoint’s improper function leads to sustain aneuploidy during tumor progression and is terminated with genomic instability. (C, D) In conditions where oncogene content does not reach E2F bistable capacity, the S phase displays the optimal response to DNA damage leading to P21 increased levels of transcription during G2 by activation of ATR/ATM/P53 pathway. P21 inhibitory effect on CDKs plays a critical role in the development of G0 or G1 arrest in the next cell cycle by inhibiting CDK4 and CDK2. On the other hand, upregulation of P21 in the M phase inhibits CDK1/CycB by blocking CDK1 leading to the dependency of APC/C-CDC20 function on MCC in the SAC checkpoint. Result resistance to progression aneuploidy in tumor cells. This chain of molecular events results in a low proliferation rate, low genomic instability, and insignificant aneuploidy progression.
In the E2F: ON position, G1-accelerated tumor cells exhibit a short G1 phase due to increased expression of direct and indirect mitogens (e.g., Myc and HER2) or direct E2F activators (e.g., cyclin D1 and cyclin E2) as G1 kinase complexes. Accelerated G1 leads to elevated proliferation rates, resulting in two undesirable consequences:
1. Linear tumor evolution: A single or limited number of clones can become dominant within a tumor mass due to their rapid proliferation, gaining an evolutionary advantage. This selected clone provides a suitable environment for accumulating mutations that enhance the fitness of cells to the tumor microenvironment.
2. Genomic instability: The shortened G1 phase compromises the S-phase response mechanisms, leading to genomic instability and hyper-mutation, which fuel tumor progression. This instability occurs due to elevated E2F levels, which increase cyclin E2 production while reducing the synthesis of ATR/ATM response machinery. ATM/ATR is crucial for ongoing DNA damage repair and replication stress defense, processes limited by the short G1 phase (18, 66, 67). E2F1 regulates some genes in the ATR/ATM pathway but does not fulfill all the requirements for the S-phase checkpoint machinery. Consequently, an accelerated G1 phase cannot adequately support the S-phase response to replication stress. However, as long as the G1 phase remains active, the luminal tumor can still generate sufficient factors for an appropriate S-phase response.
4.3 S phase
During the S phase, the cell duplicates its genetic material and prepares for division into two daughter cells. Activation of the CycA/CDK2 kinase in the early S phase triggers the removal of CDC6 from the ORC4, initiating DNA replication (50, 51). The S phase checkpoint is a complex system involving ATM5, ATR6, and DNA-PKcs7 kinases. These kinases act as evolutionary sensors for DNA damage, which can lead to structural malformations like DSBs8 or SSBs9 during replication.
Following DNA damage, ATR/ATM activates distinct pathways. In response to replication stress-induced single-stranded tails, the resection of DSB ends activates ATR/CHK110. Notably, ATR, not ATM, plays a crucial role in responding to replication stress arising from DNA breakage or TRCs11, which generate RNA: DNA hybrids (33, 56). While the G1/S checkpoint converges to a single response based on E2F activation status in response to diverse oncogenic stimuli, the S-phase checkpoint activation relies on a limited number of sensor molecules but generates divergent responses with varying intensities. These S checkpoint-dependent compensatory responses, which tend to spread across different cell cycle phases, ultimately lead to perturbations throughout the entire cell cycle (Figure 3) (68).
4.4 G2 phase
During the G2 phase, cellular DNA content reaches 4N, and protein synthesis resumes. Newly synthesized proteins, including those regulated by the FOXM112 transcription factor, play a crucial role in the subsequent phases of the cell cycle. G2 phase progression is driven by the CycA/CDK1 and CycB/CDK1 kinase complexes, which activate BORA13. Bora, in turn, activates Aurora-A, a key regulator of M phase entry, marking the “commitment to mitosis” (66). The G2/M transition checkpoint relies primarily on the CycA-CDK1 and CycB-CDK1 complexes, working in tandem. This checkpoint is sensitive to DNA damage, utilizing ATR/ATM sensory molecules and their partners CHK1/214. However, the “checkpoint nature” of the G2/M checkpoint is debatable due to its relatively fixed duration, even in response to ATR/ATM activation (68, 69).
4.5 Deregulation of the S and G2 phase and related checkpoints in luminal breast cancer
The S and G2 phases are crucial for ensuring the error-free replication of a cell’s genetic material. These checkpoints rely on the sensory machinery of ATR/ATM and DNA-PK molecules. While each sensor has a distinct function and activates different molecular targets, ATR/ATM activation plays a particularly influential role in the entire interphase by triggering multiple pathways. DNA damage, including DSBs and SSBs generated during DNA replication, is detected by ATR and S checkpoint sensors. A multitude of endogenous and exogenous factors can induce DNA damage in cancer. In luminal-type breast cancer, the primary source of genomic instability stems from the S phase checkpoint’s response to persistent replication stress, which can arise from various conditions, including stalled replication forks, fragile site prevalence, and oncogene overexpression (56, 70). Replication stress can arise from various conditions, including the prevalence of fragile sites -chromosomal breakpoints- and oncogene overexpression. These factors can lead to stalled replication forks. Notably, the breakpoints of CFSs15 are often located in regions of RNA: DNA hybrid zones. This change in DNA topology induces replication-transcription conflicts, which are the primary cause of single-stranded DNA fragment formation (71). The S phase checkpoint primarily relies on ATR/CHK1 rather than ATM/CHK2 to resolve replication stress (72, 73). In the context of altered DNA integrity caused by high E2F levels, the cell’s DNA damage repair capacity may exceed the threshold of ATR/CHK1, which plays a central role in this process. Consequently, the cells become unable to effectively repair DNA damage using mechanisms like HR16 (33, 56).
Studies have shown a correlation between the frequency of chromosomal breakpoints and poor prognosis in luminal B breast cancer. Additionally, a subset of luminal A tumors with oncogene overexpression that rapidly exit G1 exhibit high genomic instability. This suggests that an arrested G1 phase may facilitate proper S checkpoint activation, highlighting the importance of sufficient time for repair to ensure genomic stability (71). The convergent nature of the ATR/CHK1, ATM/CHK2, and p5317 cascades allow for a coordinated response to replication stress in the S phase, leading to G1 and G2 arrest through the co-activation of distinct compensatory and repair pathways. For instance, p53 induces P21 expression in early G2, impacting the subsequent G1 phase by extensively inhibiting CDKs (Figure 3) (55, 74).
4.6 Mitosis
The mitotic phase, the microscopically visible portion of the cell cycle, is divided into five distinct stages. While there are no clear molecular markers for the transition from G2 to M phase, the reduction of Bora protein levels may indicate the initiation of spindle assembly. The progression of M phase is tightly regulated by two checkpoints: the SAC and the mitotic exit checkpoint (68). The SAC ensures the proper attachment of spindle fibers to chromosome centromeres during anaphase. Its function relies heavily on the regulation of the APC/C18 by CycB/CDK1. Active APC/C-CDC2019 triggers the conversion of separase (75).
4.6.1 M phase dysregulation
The SAC ensures the proper attachment of spindle fibers to chromosome centromeres before anaphase, preventing the segregation of replicated chromosomes until each one is correctly connected. Deregulation of the SAC leads to aneuploidy, a frequent genomic alteration in solid tumors, including luminal breast cancer (33). Aneuploidy, also known as n-CIN20, correlates with genomic instability, chromosomal breakpoint frequency, and patient prognosis in luminal-type breast cancer (76). Aneuploid modifications are particularly pronounced in low-grade, early-stage tumors and remain remarkably stable throughout tumor development.
However, CINs and aneuploidy are distinct events, and the frequency of diploid/CIN− and aneuploid/CIN+ status differs between luminal A and luminal B subtypes. Notably, 81% of Luminal B (HER2+) tumors are aneuploid/CIN+, while only 27% of Luminal A tumors exhibit this phenotype. This variation may be attributed to differences in proliferation capacity, which is highly correlated with the onset of aneuploidy (77). Aneuploidy (n-CIS) is frequently observed in luminal B and a subset of luminal A tumors with high oncogene expression (66, 78).
The SAC checkpoint relies on distinct sensors and activators to ensure accurate chromosome segregation. Its M phase restriction is highly dependent on the phosphorylation of APC/C-CDC20 by the MCC complex under normal growth conditions. The SAC checkpoint’s bistable character arises from a double-negative feedback loop involving CycB/CDK1 and the MCC complex. In the OFF state, following complete chromosome alignment on kinetochores, the MCC complex is degraded, leading to the formation of APC/C-Cdc20, which is activated by CycB/CDK1. This activation triggers the degradation of securin and Cyclin B, enabling successful chromosome separation. Conversely, when the SAC is in the ON state, unattached kinetochores provide a signal to block APC/C using the MCC complex (56, 75, 79–81). Recent studies have provided a deeper understanding of the SAC machinery and highlighted the regulatory role of CycB/CDK1. While CDK1 levels remain relatively stable throughout the cell cycle, Cyclin B expression increases during the metaphase-anaphase transition due to the activity of FOXM1-MuvB transcription factors. CycB/CDK1 initiates the activation of APC/C-CDC20, followed by its rapid degradation by active APC/C-CDC20. Interestingly, CycB/CDK1 also regulates its transcription factor, FOXM1-MuvB (68, 82). Previous studies have demonstrated the significant inhibitory effect of P21 on CDK4, CDK2, and CDK1 in the G1, G2, and M phases (83). P21 is regulated by P53, the effector molecule downstream of the ATR/ATM response to DNA damage-induced stress, including (CIN). Therefore, the ATR/ATM/P53 pathway can influence cell cycle transitions through P21’s function. Recent studies indicate that P21’s inhibitory effect on multiple cell cycle kinases, particularly those crucial for G1 and M phase checkpoints, exhibits a bistable switch property (66, 83). The balance between the SAC: OFF and SAC: ON states depends on oncogene activity and/or the frequency of n-CINs in tumors, influencing SAC checkpoint dynamics and tolerance versus stable aneuploidy. This balance could be targeted by anti-SAC agents (75, 84). These findings suggest that ER+ breast tumors exhibit a range of molecular diversity. The presence of identical CNVs and consistent cell cycle performance suggests potential therapeutic opportunities. This is based on the default aneuploidy that arises early in luminal tumor development and remains remarkably stable throughout tumor evolution.
5 Genetic alterations responsible for cell cycle checkpoint dysregulation in luminal-type breast cancers
Cancer classification based on driver genetic alterations reveals two major classes: “mutation driver” and “copy number variant driver.” Breast cancer is a prime example of the CNV-enriched class (85). In breast cancer, the primary driver of genomic alterations is consistent structural chromosomal and numerical aberrations. These CNVs frequently coincide with genes identified as neoplasm drivers, highlighting their functional significance. Notably, over 85% of genetic alterations shared by luminal subtypes are stable CNVs, occurring early in tumor progression and remaining remarkably stable throughout tumor evolution (86, 87). Their stability implies they are selected during the tumor’s evolution due to their role in providing growth advantages.
In the luminal A subtype, certain genetic changes tend to occur consistently. These include: Stable amplification (increased copies of genes): 1q region: for example, PIK3C2B and MCL1 genes, 8q region: such as the MYC gene, 16p region: including the FUS and DOK2 genes. Stable deletions (loss of genetic material): 16q region: for example, the loss of CDH1, which is associated with the loss of E-cadherin (a protein important for cell adhesion), 11q region: including the loss of ATM or CENPF genes.
Some specific stable CNVs that transpire in the luminal B subtype are amplification and deletion alteration. The main amplification of stable copy number variations is 17q (e.g., ERBB2, GRB7), 11q13 (e.g., CCND1, involved in cell cycle regulation), and 8q24 (e.g., MYC). Moreover, important deletions of stable copy number variations in luminal B are 13q (e.g., RB1 loss, contributing to increased proliferation) and 11q23 (ATM loss, impacting DNA repair) (27, 88).
In ER+ luminal tumors, overexpression of these genes correlates with altered prognosis and genomic instability. Annotation analysis further reveals a strong association between CNV patterns and cell proliferation rates in early-stage tumors (21, 89, 90). This link is further supported by the overlap between cell cycle gene sets and proliferation signatures in tumor samples (91). Notably, the expression of cell cycle effectors and regulators increases in concert with tumor cell proliferation rates. These genes, periodically expressed during the cell cycle, play critical roles in DNA replication, chromosome segregation, and spindle assembly (59, 92).
Recent evidence suggests that CCAs21, unlike NCCAs22, are the primary drivers of genomic instability and karyotype changes in early-stage tumors (93). Single-cell sequencing studies reveal that mammary epithelial cells in pre-cancerous lesions undergo numerous structural chromosomal aberrations, most of which are transient. Only a limited number of these alterations confer a selective advantage and are subjected to clonal selection (94, 95). The selection of initial clones with stable CNVs during tumor progression, even in the absence of proliferation gene modifications, can be explained by the immortalization model (86, 96).
The initial genomic variations in breast cancer, primarily consisting of stable CNVs, occur early in tumor progression and remain prevalent. In luminal subtype tumors, clonal evolution follows a punctuated pattern, with sub-branches emerging during tumor development, mainly through mutations and rarely through additional CNVs. In luminal B and a small subset of luminal A tumors, these new clones harbor oncogenes, altering the pattern of tumor evolution. This linear evolution leads to the accumulation of tumorigenic mutations in a single or limited number of sub-clones with proliferation support. In non-proliferative luminal A tumors, neutral evolution may occur, resulting in numerous sub-clones with no proliferation advantage (97, 98). However, when the initial clone lacks sufficient growth stimuli and selection occurs via a hallmark other than proliferation, such as immortalization, proliferation deficiency does not guarantee that mutated cancer cells in the clone will reach a significant population (96). Consequently, these tumors, with a low tumor mutation burden, consist of a large, slowly growing single clone that forms the bulk of the tumor mass, accompanied by numerous sub-clones with minimal abundance (98).
CNVs exert their phenotypic effects on tumors by encompassing long chromosome segments harboring multiple genes susceptible to amplifications and deletions. One crucial phenotypic property influenced by CNVs is proliferation, which directly impacts prognosis and therapeutic strategies, particularly in luminal breast tumors (99). However, neither Ki67 detection (a proliferation marker) nor histological findings provide reliable indicators of tumor proliferation propensity. The chromosomal instability index, exhibiting a strong correlation with CNV profiles in luminal breast tumors, offers a more accurate measure for predicting tumor progression compared to other markers (100).
(86, 93–96) Array-CGH23 results offer a promising approach for separating luminal subtypes based on their distinct chromosomal structural and numerical instability profiles. High-resolution CNV assays have revealed that specific CNVs are independently associated with low proliferation rates, low genomic instability, and favorable prognosis (101). These CNVs include gains of 1q and 16p and a loss of 16q, typically observed in luminal A tumors according to IHC24 criteria. Conversely, another group characterized by 8q loss and gains of 11q, 20q, and 17q is associated with high proliferation, intermediate-to-high genomic instability, and poor patient prognosis (86).
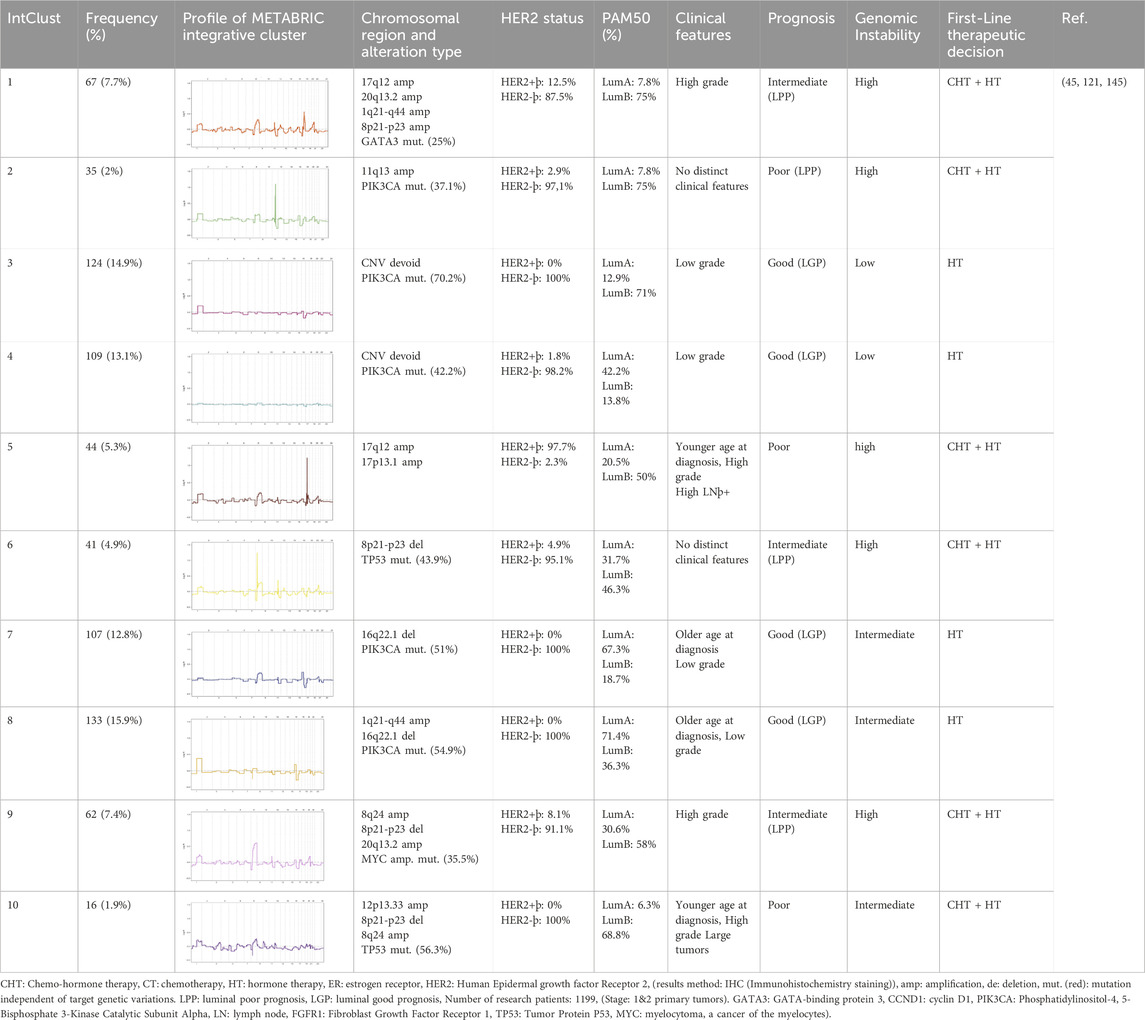
The Integrative Clustering (IntClust) approach, based on transcriptome data and prognosis, categorizes breast cancer tumors into 10 distinct groups (Table 5). Each IntClust exhibits a specific CNV pattern, intermediate/high genomic instability, and intermediate/poor 10-year prognosis. IntClust-1 and IntClust-5 largely correspond to conventional luminal B tumors and are closely related to the HER2 subtype due to 17q gain, which encompasses the ERBB2 (HER2) gene. IntClust-6 and IntClust-9 exhibit intermediate prognosis with variable IHC results. IntClust-6 is characterized by FGFR1 (Fibroblast Growth Factor Receptor 1) oncogene amplification on 8p, while IntClust-9 is defined by gains of 8q and 20q, leading to Myc and BHLH Transcription Factor overexpression, respectively. IntClust-2, associated with CCND1 (Cyclin D1) gene amplification on chromosome 11q, displays high genomic instability and a poor 10-year survival rate. IntClusts 3, 4, 7, and 8 primarily correspond to luminal A tumors with low to intermediate genomic instability and favorable 10-year survival. IntClust 3 and 4 exhibit minimal CNVs, while IntClust 7 and 8 subtypes display gains of 1q and 16p and a loss of 16q. Notably, 8q amplification, encompassing the Myc gene, is prevalent in IntClust7 but does not affect genomic instability, likely due to expression dosage insufficiency (7, 20, 59, 102, 103). Recent studies have confirmed that each CNV has a unique phenotypic impact. Chromosome 8q amplification, encompassing the Myc oncogene, is prevalent in ER+ breast tumors but has minimal effects on proliferation in many cases. This may be attributed to interactions between distant copy number variants and gene dosage dependencies (18, 61, 99). Table 5 provides an example of an integrated classification of breast cancer.
Immunostaining of 145 paraffin-embedded luminal breast cancer samples further supports the association between cell cycle activity and patient prognosis. Antibodies against MCM2 (Mini-chromosome Maintenance Complex Component 2), GMNN (Geminin DNA Replication Inhibitor, also known as “geminin”), AURKA (Aurora Kinase A), and Plk1 (Polo-like kinase 1) – all markers of cell cycle progression–were used for staining. Notably, 21% of non-stained ER+ samples were confirmed to be quiescent (G0 phase), 30% exhibited MCM2 staining alone indicating a prolonged G1 phase and 49% were stained by all antibodies, suggesting active progression through S, G2, and M phases. These findings demonstrate that approximately half of luminal breast cancer cells exhibit altered cell cycle activity, while the remaining half of mammary epithelial cells retain intrinsic sensitivity to mitogenic signaling. Consequently, tumors with an activated cell cycle may benefit from the use of phase-specific chemotherapeutic agents (Figure 3) (104, 105).
6 Discussion
Tumors with high CDK2 transcripts were more likely to have higher expressions of genes involved in the cell cycle, homologous recombination, and p53 signaling. Genes/pathways involved in BC cell survival and proliferation were associated with worse outcomes, as opposed to most immune-related genes/signatures, especially in the CDK4/6i arm. CD24 was the only gene significantly associated with worse PFS in both arms. Tertiary lymphoid structures and higher tumor-infiltrating lymphocytes also showed favorable survival trends in the CDK4/6i arm (106).
The cell cycle, characterized by its invariant phase durations and independence between phases, normally proceeds through a tightly regulated sequence of molecular interactions, culminating in the error-free duplication of a mother cell into two daughter cells. The timing and impact of each molecular reaction are intricately linked to the cell’s genomic structure and epigenetic factors. Checkpoints within each phase play a critical role in ensuring the fidelity of cell cycle progression (99, 107, 108).
Luminal-type breast tumors are classified into Luminal A (ER+ and/or PR+ and HER2-) and Luminal B (ER+ and/or PR+ and HER2+) subtypes based on immunohistochemical staining. Tumor cells exhibit varying degrees of genomic alterations, which can perturb cell cycle progression. Disruption of checkpoint regulation in response to replication stress and DNA damage can lead to genomic instability (33, 56). This instability increases the propensity for cells to acquire new mutations and alter tumor evolution patterns, potentially affecting prognosis and treatment success.
Proper checkpoint activation triggers cell cycle arrest and activates DNA repair mechanisms to maintain genomic integrity and ensure the inheritance of a complete genome by daughter cells. If cellular compensatory mechanisms fail to repair the damage, prolonged arrest can initiate apoptosis (109). In cancer, the normally uncoupled nature of cell cycle phases becomes disrupted (110). This disruption can be driven by enhanced cross-talk signaling or the activity of transcription factors like E2F. Identifying these molecular players is crucial for selecting optimal therapies for each cancer type (111).
In the treatment of luminal breast cancer, the efficacy of CDK inhibitors in Luminal A and Topoisomerase II (Top II) inhibitors in Luminal B subtypes remains under investigation in clinical trials. Accurately predicting cell cycle alterations is crucial for selecting patient groups who will benefit from these therapies. This goal is achievable through the detection of copy number variations in early-stage tumors, leveraging the distinct genetic profiles of Luminal A and B subtypes (110). This approach holds promise for personalized treatment strategies, tailoring therapy to the specific genomic landscape of each patient’s tumor.
The recent FDA approval of CDK inhibitors, such as Palbociclib, has revolutionized the treatment landscape for HR+ metastatic breast cancer. These treatments have proven to be highly effective in improving patient outcomes when used in combination with tamoxifen, aromatase inhibitors, or Fulvestrant. This therapeutic regimen has also shown promise in hormone-receptor-positive, HER2-negative early-stage breast cancer, highlighting its potential for broader application (112).
Tailoring personalized medicine for breast cancer types Luminal A and Luminal B use their unique traits, particularly how they control the G1/S cell cycle step. Luminal A cancers are ER-positive (ER+), PR-positive (PR+), and HER2-negative (HER2-), with slow growth rates. Luminal B cancers, which are also ER+, tend to have lower PR levels, grow faster and may show HER2.
The G1/S checkpoint plays a key role in cell cycle control. Estrogen receptor signals boost cyclin D levels, which activates the CDK4/6-cyclin D complex. The activated complex adds phosphate to the Rb protein. Phosphorylation frees E2F transcription factors, allowing the cell cycle to move forward.
In Luminal A tumors, cell cycle regulatory systems like active p16INK4a and balanced CDK4/6 activity keep the G1/S step in arrest, leading to a slower growth phenomenon. Luminal B tumors, in contrast, often have issues displaying excessive CDK4/6 levels or cyclin D overexpression, loss of Rb, or p53 gene mutations, which cause fast uncontrolled proliferation.
Focusing on the CDK4/6-cyclin D is a possible efficient strategy. Palbociclib, a CDK4/6 inhibitor, stops the cell cycle in G1 by blocking Rb phosphorylated activation, sequestering E2F, and stopping growth. Studies like PALOMA-2 showed great gains in progression-free survival when Palbociclib was applied with letrozole for ER + breast cancers (113). For Luminal A, CDK4/6 inhibitors enhance hormone therapy efficiency, reducing recurrence/relapse events while displaying low toxicity. In Luminal B, these medications reduce the proliferation rate and synergize with other treatments like anti-HER2 drugs. This personalized guide, supported by molecular profiling data, optimizes treatment efficacy and significantly advances breast cancer medication management (102) DOI: 10.1016/j.molonc.2010.12.005).
The prognostic value of intrinsic subtypes in metastatic breast cancer suggests that tailoring chemotherapy regimens based on these subtypes may lead to improved outcomes (114, 115). While early-stage genetic profiles are generally considered invariant during metastasis, recent evidence suggests that tumor cells can acquire an E2F: OFF state through CDK4/6 inhibition (56). Notably, primary luminal A tumors with overexpression of mitogenic genes and CCND1, but not CCNE2, exhibit particular sensitivity to Palbociclib (56). This sensitivity is attributed to an indirect increase in P21 levels induced by Palbociclib, leading to cell cycle arrest in the G1 phase and accumulation of the G0 population within the tumor mass (116).
Recent studies suggest that the combination of limited oncogene activity and an intact spindle assembly checkpoint (SAC) function leads to genomic stability and a favorable prognosis. Conversely, perturbations in the cell cycle caused by overexpressed oncogenes and stable aneuploidy, coupled with a defective SAC function, induce genomic instability and a poor prognosis. The prevalence of stable aneuploidy and intact SAC machinery guides the therapeutic efficacy of paclitaxel and anthracyclines, a group of chemotherapeutic agents (110).
The prevalence of aneuploidy in Luminal B and a subset of Luminal A tumors suggests that these subtypes should be further categorized into two subgroups: diploid/CIN− and aneuploid/CIN+, based on copy number variation patterns (77). While recent studies have indicated that SAC function may not be a significant factor in tumor progression, its unique machinery makes it a potential target for anti-cancer therapies. Microtubule-targeting agents (MTAs) like paclitaxel, currently used in metastatic breast cancer, have limited efficacy in Luminal B therapy due to the high aneuploidy and proliferation rate of these tumors (75).
Prolonged SAC inactivation forces cells to choose between two fates: initiating an SAC-originated apoptotic signal or progressing through the cell cycle with significant chromosome aneuploidy, exceeding the cellular tolerance for CINs (84). However, the impact of CNV patterns in cancer clinic utilization and the relationship between CNVs and the degree of genomic instability is not as straightforward in context. Recent studies indicate that in luminal breast tumors, the status of G1/S could control the proliferation rate of tumors due to the predictable E2F function in the presence of oncogenes overexpression, leading to a low genomic instability and a good prognosis. An identical CNV type leads to the E2F status change that acts as a critical transcription factor in G1 transition and S phase initiation. On the other hand, the status of the M phase checkpoint is highly dependent on CDK1/CycB function in APC/C-CDC20 activation. Cyclin B transcription is regulated by multiple transcription factors that only FoxM1 induced by CDK1/CycB among them, and others activated by various pathways. FoxM1 transcription factor that is activated by CDK1/CycB is one of the multiple transcription factors that lead to Cyclin B transcription. These findings suggest a linkage between the maintenance of aneuploidy/CIN and non-tumorigenic events in primary luminal breast tumors. These are cases where genomic instability results are biased from those predicted by CNV results (83, 117, 118).
However, the clinical implications of CNV patterns and their relationship to genomic instability remain complex. Recent studies in luminal breast tumors suggest that G1/S status may control tumor proliferation due to the predictable E2F function in the presence of oncogene overexpression, leading to low genomic instability and a favorable prognosis. Notably, an identical CNV type can induce changes in E2F status, which acts as a critical transcription factor in G1 transition and S phase initiation.
On the other hand, the M phase checkpoint status is tightly linked to CDK1/CycB function, which is essential for APC/C-CDC20 activation. A complex network of transcription factors, including FoxM1, which is specifically induced by CDK1/CycB, regulates cyclin B transcription. These findings suggest a potential link between the maintenance of aneuploidy/CIN and non-tumorigenic events in primary luminal breast tumors, where genomic instability results may deviate from those predicted by CNV analysis (83, 118).
6.1 Challenges
The copy of cell cycle genes can be changed directly by CNVs, which leads to their under-expression or overexpression. However, the exact mechanism that CNVs are involved in cell cycle control is unknown. To create specific treatments, it is important to distinguish between those CNVs that drive cell cycle progression and those that do not. Driver CNVs can be identified through functional studies and inter-tumor analyses. Luminal tumors display major differences in CNV profiles of different sub-clones. Single-cell sequencing is necessary to assess how CNVs affect cellular cycle dynamics and identify their clones’ evolution patterns. CNVs are frequently observed together with other genetic alterations, and to address treatment strategies for cell cycle malfunction, it is important to comprehend their interaction. Different technologies known as multi-omics are used to generate data that show an overview of the molecular signature and influence on the cell cycle’s regulation. Even though the CNVs bring hope to picking patients responsive to cell cycle deregulation-targeted treatments, the implementation of these discoveries in a medical environment is still difficult. Knowledge of the links between CNVs and cell cycle checkpoints can give a foundation for individualized medication approaches. Enabling patient-specific therapeutic interventions with specific CNV patterns and state of checkpoints would enhance treatment efficiency and patient outcomes. It is necessary to make sure that strong models predicting future events simultaneously help in realizing biomarkers linked to CNV-induced cell-cycle changes on an individualized basis. To successfully face these challenges, it is necessary to use collaborative methods of working and using genomics, cell biology, and clinical oncology knowledge. Understanding how CNVs interact with aberrations in cell cycle regulation is pivotal to the development of better luminal-type breast cancer treatment strategies, which are personalized as well.
7 Conclusion
The effect of CNVs on cell cycle dysregulation in luminal-type breast cancer has been thoroughly discussed in this study. We have discussed how CNVs can cause genomic instability and tumor growth by altering cell cycle checkpoints when they occur alone or in conjunction with other variables. Results point to a critical role for CNVs in the initiation and spread of luminal-type breast cancer. By identifying how CNVs disrupt cell cycle checkpoints, the findings offer a foundational framework for developing targeted therapeutic strategies. These findings have given the basic framework to develop targeted therapeutic strategies by pointing out how CNVs disrupt cell cycle checkpoints. This may integrate CNV profiling into clinical practice and allow patient stratification based on genomic alterations to open the door to precision oncology interventions. Because of the genes targeted by CNVs, there is indeed a prospect to build very personal therapeutic regimens. Examples are CNV correction, leveraging CRISPR-Cas9 technology against the aberrant copies induced, or the application of inhibitors that may be developed for dysregulated pathways within the cell cycle. New findings point toward an exceptional ability of biomarker-driven therapies in general, a successful experience well noted in the HER2 and CDK4/6 pathway treatments. Future studies should be focused on high-throughput genomic analyses and integrative multi-omics approaches to identify actionable targets affected by CNVs. Thus, the correlation of CNVs with therapeutic responses will advance the development of novel treatment modalities in concert with individual patient profiles and improve outcomes in this aggressive subtype of breast cancer.
Author contributions
AM: Writing–original draft, Writing–review and editing. DJ-G: Writing–original draft, Writing–review and editing. KA: Writing–original draft, Writing–review and editing. PP: Writing–original draft, Writing–review and editing. ZA: Writing–original draft, Writing–review and editing. BN: Writing–original draft, Writing–review and editing. NZ: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge the Tabriz University of Medical Science for its scientific support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Spindle Assembly Checkpoint.
2Cyclin-Dependent Kinases.
3Retinoblastoma Protein.
4Origin Recognition Complex.
5Ataxia Telangiectasia Mutated.
6Ataxia Telangiectasia and Rad3-Related Protein.
7DNA-Dependent Protein Kinase Catalytic Subunit.
8Double-Strand Breaks.
9Single-Strand Breaks.
10Checkpoint Kinase 1.
11Transcription-Replication Conflicts.
12Forkhead Box M1.
13BORA Aurora Kinase A Activator.
14Checkpoint Kinase 1&2.
15Common Fragile Sites.
16Homologous Recombination.
17Tumor Protein p53.
18Anaphase-Promoting Complex/Cyclosome.
19Cyclosome-Cell Division Cycle 20.
20Numerical Chromosome Instability.
21Clonal Chromosomal Aberrations.
22Non-Clonal Aberrations.
23Array Comparative Genomic Hybridization.
24Immunohistochemistry.
References
1. Takeshima, H, and Tjnpo, U. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. (2019) 3(1):1–8.
2. Pal, B, Chen, Y, Vaillant, F, Capaldo, BD, Joyce, R, Song, X, et al. A single-cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. The EMBO J (2021) 40(11):e107333. doi:10.15252/embj.2020107333
3. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics. CA: a Cancer J clinicians (2022) 72(1):7–33. doi:10.3322/caac.21708
4. Hashemi, F, Mohajeri, N, Radnia, F, and Zarghami, N. Design of an efficient fluorescent nanoplatform carrier for hydrophobic drugs along with green carbon dot: possible application in cancer image-guided drug therapy. Photodiagnosis Photodynamic Ther (2022) 37:102738. doi:10.1016/j.pdpdt.2022.102738
5. Blows, FM, Driver, KE, Schmidt, MK, Broeks, A, Van Leeuwen, FE, Wesseling, J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med (2010) 7(5):e1000279. doi:10.1371/journal.pmed.1000279
6. Perou, CM, Sørlie, T, Eisen, MB, Van De Rijn, M, Jeffrey, SS, Rees, CA, et al. Molecular portraits of human breast tumours. nature (2000) 406(6797):747–52. doi:10.1038/35021093
7. Dawson, SJ, Rueda, OM, Aparicio, S, and Caldas, C. A new genome-driven integrated classification of breast cancer and its implications. The EMBO J (2013) 32(5):617–28. doi:10.1038/emboj.2013.19
8. Turk, Z, Armani, A, Jafari-Gharabaghlou, D, Madakbas, S, Bonabi, E, and Zarghami, N. A new insight into the early detection of HER2 protein in breast cancer patients with a focus on electrochemical biosensors approaches: a review. Int J Biol Macromolecules (2024) 272:132710. doi:10.1016/j.ijbiomac.2024.132710
9. Di Palma, S, Koliou, P, Simonovic, A, Costa, D, Faulkes, C, Kobutungi, B, et al. Breast cancer molecular subtyping in practice: a real-world study of the APIS breast cancer subtyping assay in a consecutive series of breast core biopsies. Int J Mol Sci (2024) 25(5):2616. doi:10.3390/ijms25052616
10. Sarhadi, S, Armani, A, Jafari-Gharabaghlou, D, Sadeghi, S, and Zarghami, N. Cross-platform gene expression profiling of breast cancer: exploring the relationship between breast cancer grades and gene expression pattern. Heliyon (2024) 10(8):e29736. doi:10.1016/j.heliyon.2024.e29736
11. Özmen, V, Özmen, T, and Doğru, V. Breast cancer in Turkey; an analysis of 20.000 patients with breast cancer. Eur J Breast Health (2019) 15(3):141–6. doi:10.5152/ejbh.2019.4890
12. Ciriello, G, Sinha, R, Hoadley, KA, Jacobsen, AS, Reva, B, Perou, CM, et al. The molecular diversity of Luminal A breast tumors. Breast Cancer Res Treat (2013) 141(3):409–20. doi:10.1007/s10549-013-2699-3
13. Mohammadinejad, S, Jafari-Gharabaghlou, D, and Zarghami, N. Development of PEGylated PLGA nanoparticles co-loaded with bioactive compounds: potential anticancer effect on breast cancer cell lines. Asian Pac J Cancer Prev (2022) 23(12):4063–72. doi:10.31557/apjcp.2022.23.12.4063
14. Negrini, S, Gorgoulis, VG, and Halazonetis, TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cel Biol (2010) 11(3):220–8. doi:10.1038/nrm2858
15. Duijf, PH, Nanayakkara, D, Nones, K, Srihari, S, Kalimutho, M, and Khanna, KK. Mechanisms of genomic instability in breast cancer. Trends Molecular Medicine (2019) 25(7):595–611. doi:10.1016/j.molmed.2019.04.004
16. R Colnaghi, G Carpenter, M Volker, and M O’Driscoll, editors. The consequences of structural genomic alterations in humans: genomic disorders, genomic instability and cancer. Seminars in cell and developmental biology. Elsevier (2011).
17. Zhang, F, Gu, W, Hurles, ME, and Lupski, JR. Copy number variation in human health, disease, and evolution. Annu Rev genomics Hum Genet (2009) 10:451–81. doi:10.1146/annurev.genom.9.081307.164217
18. Gatza, ML, Silva, GO, Parker, JS, Fan, C, and Perou, CM. An integrated genomics approach identifies drivers of proliferation in luminal-subtype human breast cancer. Nat Genet (2014) 46(10):1051–9. doi:10.1038/ng.3073
19. Bergamaschi, A, Kim, YH, Wang, P, Sørlie, T, Hernandez-Boussard, T, Lonning, PE, et al. Distinct patterns of DNA copy number alteration are associated with different clinicopathological features and gene-expression subtypes of breast cancer. Genes, Chromosomes and Cancer (2006) 45(11):1033–40. doi:10.1002/gcc.20366
20. Ali, HR, Rueda, OM, Chin, S-F, Curtis, C, Dunning, MJ, Aparicio, SA, et al. Genome-driven integrated classification of breast cancer validated in over 7,500 samples. Genome Biol (2014) 15(8):431–14. doi:10.1186/s13059-014-0431-1
21. Tishchenko, I, Milioli, HH, Riveros, C, and Moscato, P. Extensive transcriptomic and genomic analysis provides new insights about luminal breast cancers. PloS one (2016) 11(6):e0158259. doi:10.1371/journal.pone.0158259
22. Shahrouzi, P, Forouz, F, Mathelier, A, Kristensen, VN, and Duijf, PHG. Copy number alterations: a catastrophic orchestration of the breast cancer genome. Trends Mol Med (2024) 30(8):750–64. doi:10.1016/j.molmed.2024.04.017
23. Wang, Y-W, Tuan, Y-L, Wang, J-Y, Chang, H-Y, Chu, C-A, Chen, Y-L, et al. Potential of epithelial membrane protein 3 as a novel therapeutic target for human breast cancer. Oncol Rep (2024) 53(1):16–3. doi:10.3892/or.2024.8849
24. Zhang, Y, Xu, H, Han, X, Yu, Q, Zheng, L, and Xiao, H. PMAIP1-mediated glucose metabolism and its impact on the tumor microenvironment in breast cancer: integration of multi-omics analysis and experimental validation. Translational Oncol (2025) 52:102267. doi:10.1016/j.tranon.2024.102267
25. Koon Sun Pat, M, Manraj, M, and Manraj, S. Breast cancer survival analysis in the Republic of Mauritius by age, stage at diagnosis and molecular subtype: a retrospective cohort study. Int J Cancer (2025) 156(2):331–8. doi:10.1002/ijc.35172
26. Yu, X, Zhang, Q, Li, B, Sun, S, Li, J, and Li, W. Unveiling the prognostic power and immune landscape of MyD88 in breast cancer: an integrative bioinformatics and IHC approach. J Cancer (2025) 16(1):201–13. doi:10.7150/jca.103403
27. Pan, X, Hu, X, Zhang, Y-H, Chen, L, Zhu, L, Wan, S, et al. Identification of the copy number variant biomarkers for breast cancer subtypes. Mol Genet Genomics (2019) 294:95–110. doi:10.1007/s00438-018-1488-4
28. Mekonnen, N, Yang, H, Rajasekaran, N, Song, K, Choi, Y-L, and Shin, YK. Indirect targeting of MYC and direct targeting in combination with chemotherapies are more effective than direct mono-targeting in triple negative breast cancer. Translational Oncol (2025) 51:102204. doi:10.1016/j.tranon.2024.102204
29. Dall'Olio, FG, Zrafi, W, Mamann, A, Job, B, Michiels, S, Tomasini, P, et al. Genomic instability in advanced non-small cell lung cancer (NSCLC) treated with maintenance durvalumab in the UNICANCER SAFIR02-Lung/IFCT1301 trial. American Society of Clinical Oncology (2024).
30. Li, W, Wu, H, and Xu, J. Construction of a genomic instability-derived predictive prognostic signature for non-small cell lung cancer patients. Cancer Genet (2023) 278-279:24–37. doi:10.1016/j.cancergen.2023.07.008
31. Liu, Y, Bi, X, Leng, Y, Chen, D, Wang, J, Ma, Y, et al. A deep-learning-based genomic status estimating framework for homologous recombination deficiency detection from low-pass whole genome sequencing. Heliyon (2024) 10:e26121. doi:10.1016/j.heliyon.2024.e26121
32. Sahabi, S, Jafari-Gharabaghlou, D, and Zarghami, N. A new insight into cell biological and biochemical changes through aging. Acta Histochem (2022) 124(1):151841. doi:10.1016/j.acthis.2021.151841
33. Wilhelm, T, Said, M, and Naim, V. DNA replication stress and chromosomal instability: dangerous liaisons. Genes (2020) 11(6):642. doi:10.3390/genes11060642
34. Sayad, S, Hiatt, M, and Mustafa, H. Connecting cancers to chromosomal locations through copy number variations. bioRxiv. (2023). doi:10.1101/2023.10.03.560705
35. Pool, EJ, Walters, CR, and Pool, EJ. The effects of silver nanoparticles on RAW 264.7. Macrophages and human whole blood cell cultures. Front Biosci (2019) 24(2):347–65. doi:10.2741/4722
36. Luo, X, Qin, F, Cai, G, and Xiao, F. Integrating genomic correlation structure improves copy number variations detection. Bioinformatics (2021) 37(3):312–7. doi:10.1093/bioinformatics/btaa737
37. Macé, A, Kutalik, Z, and Valsesia, A. Copy number variation. In: E Evangelou, editor. Genetic epidemiology: methods and protocols. New York, NY: Springer New York (2018). p. 231–58.
39. Leclerc, S, and Kitagawa, K. The role of human centromeric RNA in chromosome stability. Front Mol Biosciences (2021) 8:642732. doi:10.3389/fmolb.2021.642732
40. Camargo-Herrera, V, Castellanos, G, Rangel, N, Jiménez-Tobón, GA, Martínez-Agüero, M, and Rondón-Lagos, M. Patterns of chromosomal instability and clonal heterogeneity in luminal B breast cancer: a pilot study. Int J Mol Sci (2024) 25(8):4478. doi:10.3390/ijms25084478
41. Voutsadakis, IA. The landscape of chromosome instability in breast cancers and associations with the tumor mutation burden: an analysis of data from TCGA. Cancer Invest (2021) 39(1):25–38. doi:10.1080/07357907.2020.1863418
42. Lee, K, Kim, HJ, Jang, MH, Lee, S, Ahn, S, and Park, SY. Centromere 17 copy number gain reflects chromosomal instability in breast cancer. Scientific Rep (2019) 9(1):17968. doi:10.1038/s41598-019-54471-w
43. Brewer, T, Yehia, L, Bazeley, P, and Eng, C. Integrating somatic CNV and gene expression in breast cancers from women with PTEN hamartoma tumor syndrome. NPJ Genomic Med (2023) 8(1):14. doi:10.1038/s41525-023-00361-0
44. Gabrielaite, M, Torp, MH, Rasmussen, MS, Andreu-Sánchez, S, Vieira, FG, Pedersen, CB, et al. A comparison of tools for copy-number variation detection in germline whole exome and whole genome sequencing data. Cancers (Basel) (2021) 13(24):6283. doi:10.3390/cancers13246283
45. Young, CC, Eason, K, Manzano Garcia, R, Moulange, R, Mukherjee, S, Chin, SF, et al. Development and validation of a reliable DNA copy-number-based machine learning algorithm (CopyClust) for breast cancer integrative cluster classification. Sci Rep (2024) 14(1):11861. doi:10.1038/s41598-024-62724-6
46. Savarese, F, and Grosschedl, R. Blurring cis and trans in gene regulation. Cell (2006) 126(2):248–50. doi:10.1016/j.cell.2006.07.008
47. Kanu, N, Grönroos, E, Martinez, P, Burrell, RA, Yi Goh, X, Bartkova, J, et al. SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair. Oncogene (2015) 34(46):5699–708. doi:10.1038/onc.2015.24
48. Cavalli, FMG, Remke, M, Rampasek, L, Peacock, J, Shih, DJH, Luu, B, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell (2017) 31(6):737–54.e6. doi:10.1016/j.ccell.2017.05.005
49. Danforth, DN. Genomic changes in normal breast tissue in women at normal risk or at high risk for breast cancer. Breast Cancer Basic Clin Res (2016) 10:BCBCR.S39384–46. doi:10.4137/bcbcr.s39384
50. Morgan, DO. The cell cycle: principles of control. Yale Journal of Biology and Medicine (YJBM) (2007).
51. Fischer, M, Dang, CV, and DeCaprio, JA. Control of cell division. Hematology. Elsevier (2018). p. 176–85.
52. Shehata, M, Waterhouse, PD, Casey, AE, Fang, H, Hazelwood, L, and Khokha, R. Proliferative heterogeneity of murine epithelial cells in the adult mammary gland. Commun Biol (2018) 1(1):111–0. doi:10.1038/s42003-018-0114-7
53. Kwon, JS, Everetts, NJ, Wang, X, Wang, W, Della Croce, K, Xing, J, et al. Controlling depth of cellular quiescence by an Rb-E2F network switch. Cell Rep (2017) 20(13):3223–35. doi:10.1016/j.celrep.2017.09.007
54. Amirsaadat, S, Jafari-Gharabaghlou, D, Dadashpour, M, and Zarghami, N. Potential anti-proliferative effect of nano-formulated curcumin through modulating micro RNA-132, Cyclin D1, and hTERT genes expression in breast cancer cell lines. J Cluster Sci (2023) 34(5):2537–46. doi:10.1007/s10876-023-02404-z
55. Barr, AR, Cooper, S, Heldt, FS, Butera, F, Stoy, H, Mansfeld, J, et al. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat Commun (2017) 8(1):14728–17. doi:10.1038/ncomms14728
56. Matthews, HK, Bertoli, C, and de Bruin, RA. Cell cycle control in cancer. Nat Rev Mol Cell Biol (2022) 23(1):74–88. doi:10.1038/s41580-021-00404-3
57. Lashen, A, Alqahtani, S, Shoqafi, A, Algethami, M, Jeyapalan, JN, Mongan, NP, et al. Clinicopathological significance of cyclin-dependent kinase 2 (CDK2) in ductal carcinoma in situ and early-stage invasive breast cancers. Int J Mol Sci (2024) 25(9):5053. doi:10.3390/ijms25095053
58. O’Connor, TN, Schultz, E, Wang, J, O’Connor, T, Levine, E, Knudsen, ES, et al. Real-world experience among elderly metastatic breast cancer patients treated with CDK4/6 inhibitor-based therapy. Cancers. (2024) 16(9):1749. doi:10.3390/cancers16091749
59. Whitfield, ML, George, LK, Grant, GD, and Perou, CM. Common markers of proliferation. Nat Rev Cancer (2006) 6(2):99–106. doi:10.1038/nrc1802
60. Kent, LN, and Leone, G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer (2019) 19(6):326–38. doi:10.1038/s41568-019-0143-7
61. Baslan, T, Kendall, J, Volyanskyy, K, McNamara, K, Cox, H, D'Italia, S, et al. Novel insights into breast cancer copy number genetic heterogeneity revealed by single-cell genome sequencing. elife (2020) 9:e51480. doi:10.7554/elife.51480
62. Tyson, JJ, Chen, KC, and Novak, B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cel Biol (2003) 15(2):221–31. doi:10.1016/s0955-0674(03)00017-6
63. Stallaert, W, Kedziora, KM, Chao, HX, and Purvis, JE. Bistable switches as integrators and actuators during cell cycle progression. FEBS Lett (2019) 593(20):2805–16. doi:10.1002/1873-3468.13628
64. Rombouts, J, and Gelens, L. Dynamic bistable switches enhance robustness and accuracy of cell cycle transitions. PLoS Comput Biol (2021) 17(1):e1008231. doi:10.1371/journal.pcbi.1008231
65. Yao, G, Lee, TJ, Mori, S, Nevins, JR, and You, L. A bistable Rb–E2F switch underlies the restriction point. Nat Cel Biol (2008) 10(4):476–82. doi:10.1038/ncb1711
66. Otto, T, and Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer (2017) 17(2):93–115. doi:10.1038/nrc.2016.138
67. Gemble, S, Bernhard, S, Srivastava, N, Wardenaar, R, Nano, M, Macé, A-S, et al. Mechanisms of genetic instability in a single S-phase following whole genome doubling. (2021).
68. D Martínez-Alonso, and M Malumbres, editors. Mammalian cell cycle cyclins. Seminars in cell and developmental biology. Elsevier (2020).
69. Willems, E, Dedobbeleer, M, Digregorio, M, Lombard, A, Lumapat, PN, and Rogister, B. The functional diversity of Aurora kinases: a comprehensive review. Cell division (2018) 13(1):7–17. doi:10.1186/s13008-018-0040-6
70. Hartwell, LH, and Kastan, MB. Cell cycle control and cancer. Science (1994) 266(5192):1821–8. doi:10.1126/science.7997877
71. Wilson, TE, Arlt, MF, Park, SH, Rajendran, S, Paulsen, M, Ljungman, M, et al. Large transcription units unify copy number variants and common fragile sites arising under replication stress. Genome Res (2015) 25(2):189–200. doi:10.1101/gr.177121.114
72. Williams, RM, and Zhang, X. Roles of ATM and ATR in DNA double strand breaks and replication stress. Prog Biophys Mol Biol (2021) 161:27–38. doi:10.1016/j.pbiomolbio.2020.11.005
73. Ozeri-Galai, E, Schwartz, M, Rahat, A, and Kerem, B. Interplay between ATM and ATR in the regulation of common fragile site stability. Oncogene (2008) 27(15):2109–17. doi:10.1038/sj.onc.1210849
74. Vijayraghavan, S, Tsai, F-L, and Schwacha, A. A checkpoint-related function of the MCM replicative helicase is required to avert accumulation of RNA: DNA hybrids during S-phase and ensuing DSBs during G2/M. PLoS Genet (2016) 12(8):e1006277. doi:10.1371/journal.pgen.1006277
75. Marques, S, Fonseca, J, Silva, P, and Bousbaa, H. Targeting the spindle assembly checkpoint for breast cancer treatment. Curr Cancer Drug Targets (2015) 15(4):272–81. doi:10.2174/1568009615666150302130010
76. Martínez-A, C, and van Wely, KH. Are aneuploidy and chromosome breakage caused by a CINgle mechanism? Cell Cycle (2010) 9(12):2275–80. doi:10.4161/cc.9.12.11865
77. Yanagawa, M, Ikemot, K, Kawauchi, S, Furuya, T, Yamamoto, S, Oka, M, et al. Luminal A and luminal B (HER2 negative) subtypes of breast cancer consist of a mixture of tumors with different genotype. BMC Res Notes (2012) 5(1):376–8. doi:10.1186/1756-0500-5-376
78. Pfister, K, Pipka, JL, Chiang, C, Liu, Y, Clark, RA, Keller, R, et al. Identification of drivers of aneuploidy in breast tumors. Cell Rep (2018) 23(9):2758–69. doi:10.1016/j.celrep.2018.04.102
79. Wenzel, ES, and Singh, AT. Cell-cycle checkpoints and aneuploidy on the path to cancer. In vivo (Athens, Greece) (2018) 32(1):1–5. doi:10.21873/invivo.11197
80. Faria, J, Barbosa, J, Moura, IM, Reis, RM, and Bousbaa, H. The spindle assembly checkpoint and aneuploidy. Aneuploidy: etiology. The New England Journal of Medicine (2012). 59–76. Disorders and Risk Factors.
81. Barbosa, J, Nascimento, AV, Faria, J, Silva, P, and Bousbaa, H. The spindle assembly checkpoint: perspectives in tumorigenesis and cancer therapy. Front Biol (2011) 6(2):147–55. doi:10.1007/s11515-011-1122-x
82. Lara-Gonzalez, P, Moyle, MW, Budrewicz, J, Mendoza-Lopez, J, Oegema, K, and Desai, A. The G2-to-M transition is ensured by a dual mechanism that protects cyclin B from degradation by Cdc20-activated APC/C. Developmental Cel (2019) 51(3):313–25. e10. doi:10.1016/j.devcel.2019.09.005
83. Fischer, M, and Müller, GA. Cell cycle transcription control: DREAM/MuvB and RB-E2F complexes. Crit Rev Biochem Mol Biol (2017) 52(6):638–62. doi:10.1080/10409238.2017.1360836
84. Cohen-Sharir, Y, McFarland, JM, Abdusamad, M, Marquis, C, Bernhard, SV, Kazachkova, M, et al. Aneuploidy renders cancer cells vulnerable to mitotic checkpoint inhibition. Nature (2021) 590(7846):486–91. doi:10.1038/s41586-020-03114-6
85. Ciriello, G, Miller, ML, Aksoy, BA, Senbabaoglu, Y, Schultz, N, and Sander, C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet (2013) 45(10):1127–33. doi:10.1038/ng.2762
86. Rennstam, K, Ahlstedt-Soini, M, Baldetorp, B, Bendahl, P-O, Borg, Å, Karhu, R, et al. Patterns of chromosomal imbalances defines subgroups of breast cancer with distinct clinical features and prognosis. A study of 305 tumors by comparative genomic hybridization. Cancer Res (2003) 63(24):8861–8.
87. Arsuaga, J, Borrman, T, Cavalcante, R, Gonzalez, G, and Park, C. Identification of copy number aberrations in breast cancer subtypes using persistence topology. Microarrays (2015) 4(3):339–69. doi:10.3390/microarrays4030339
88. Cava, C, Pisati, M, Frasca, M, and Castiglioni, I. Identification of breast cancer subtype-specific biomarkers by integrating copy number alterations and gene expression profiles. Medicina (2021) 57(3):261. doi:10.3390/medicina57030261
89. Fumagalli, C, Ranghiero, A, Gandini, S, Corso, F, Taormina, S, De Camilli, E, et al. Inter-tumor genomic heterogeneity of breast cancers: comprehensive genomic profile of primary early breast cancers and relapses. Breast Cancer Res (2020) 22(1):107–11. doi:10.1186/s13058-020-01345-z
90. Aure, MR, Vitelli, V, Jernström, S, Kumar, S, Krohn, M, Due, EU, et al. Integrative clustering reveals a novel split in the luminal A subtype of breast cancer with impact on outcome. Breast Cancer Res (2017) 19(1):44–18. doi:10.1186/s13058-017-0812-y
91. Perou, CM, Jeffrey, SS, Van De Rijn, M, Rees, CA, Eisen, MB, Ross, DT, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci (1999) 96(16):9212–7. doi:10.1073/pnas.96.16.9212
92. Rhodes, DR, Yu, J, Shanker, K, Deshpande, N, Varambally, R, Ghosh, D, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci (2004) 101(25):9309–14. doi:10.1073/pnas.0401994101
93. Heng, HH, Stevens, JB, Liu, G, Bremer, SW, Ye, KJ, Reddy, PV, et al. Stochastic cancer progression driven by non-clonal chromosome aberrations. J Cell Physiol (2006) 208(2):461–72. doi:10.1002/jcp.20685
94. Wang, Y, Waters, J, Leung, ML, Unruh, A, Roh, W, Shi, X, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature (2014) 512(7513):155–60. doi:10.1038/nature13600
95. Navin, N, Kendall, J, Troge, J, Andrews, P, Rodgers, L, McIndoo, J, et al. Tumour evolution inferred by single-cell sequencing. Nature (2011) 472(7341):90–4. doi:10.1038/nature09807
96. Horne, SD, Wexler, M, Stevens, JB, and Heng, HH. Insights on processes of evolutionary tumor growth. In: Atlas of genetics and cytogenetics in oncology and haematology (2016).
97. Poudel, P, Nyamundanda, G, Patil, Y, Cheang, MCU, and Sadanandam, A. Heterocellular gene signatures reveal luminal-A breast cancer heterogeneity and differential therapeutic responses. NPJ breast cancer (2019) 5(1):21–10. doi:10.1038/s41523-019-0116-8
98. Vendramin, R, Litchfield, K, and Swanton, C. Cancer evolution: Darwin and beyond. The EMBO J (2021) 40(18):e108389. doi:10.15252/embj.2021108389
99. Dexter, TJ, Sims, D, Mitsopoulos, C, Mackay, A, Grigoriadis, A, Ahmad, AS, et al. Genomic distance entrained clustering and regression modelling highlights interacting genomic regions contributing to proliferation in breast cancer. BMC Syst Biol (2010) 4(1):127–14. doi:10.1186/1752-0509-4-127
100. Vincent-Salomon, A, Benhamo, V, Gravier, E, Rigaill, G, Gruel, N, Robin, S, et al. Genomic instability: a stronger prognostic marker than proliferation for early stage luminal breast carcinomas. PLoS One (2013) 8(10):e76496. doi:10.1371/journal.pone.0076496
101. Bonnet, F, Guedj, M, Jones, N, Sfar, S, Brouste, V, Elarouci, N, et al. An array CGH based genomic instability index (G2I) is predictive of clinical outcome in breast cancer and reveals a subset of tumors without lymph node involvement but with poor prognosis. BMC Med genomics (2012) 5(1):54–18. doi:10.1186/1755-8794-5-54
102. Curtis, C, Shah, SP, Chin, S-F, Turashvili, G, Rueda, OM, Dunning, MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature (2012) 486(7403):346–52. doi:10.1038/nature10983
103. Imani, M, Mohajeri, N, Rastegar, M, and Zarghami, N. Synthesis and characterization of N-rich fluorescent bio-dots as a reporter in the design of dual-labeled FRET probe for TaqMan PCR: a feasibility study. Biotechnol Appl Biochem (2023) 70(2):645–58. doi:10.1002/bab.2387
104. Loddo, M, Kingsbury, S, Rashid, M, Proctor, I, Holt, C, Young, J, et al. Cell-cycle-phase progression analysis identifies unique phenotypes of major prognostic and predictive significance in breast cancer. Br J Cancer (2009) 100(6):959–70. doi:10.1038/sj.bjc.6604924
105. Shetty, A, Loddo, M, Fanshawe, T, Prevost, A, Sainsbury, R, Williams, G, et al. DNA replication licensing and cell cycle kinetics of normal and neoplastic breast. Br J Cancer (2005) 93(11):1295–300. doi:10.1038/sj.bjc.6602829
106. Schettini, F, Palleschi, M, Mannozzi, F, Brasó-Maristany, F, Cecconetto, L, Galván, P, et al. CDK4/6-Inhibitors versus chemotherapy in advanced HR+/HER2-Negative breast cancer: results and correlative biomarker analyses of the KENDO randomized phase II trial. The Oncologist (2024):oyad337.
107. Chao, HX, Fakhreddin, RI, Shimerov, HK, Kedziora, KM, Kumar, RJ, Perez, J, et al. Evidence that the human cell cycle is a series of uncoupled, memoryless phases. Mol Syst Biol (2019) 15(3):e8604. doi:10.15252/msb.20188604
108. Bueno, MJ, and Malumbres, M. MicroRNAs and the cell cycle. Biochim Biophys Acta (BBA)-Molecular Basis Dis (2011) 1812(5):592–601. doi:10.1016/j.bbadis.2011.02.002
109. Chen, J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harbor Perspect Med (2016) 6(3):a026104. doi:10.1101/cshperspect.a026104
110. Bower, JJ, Vance, LD, Psioda, M, Smith-Roe, SL, Simpson, DA, Ibrahim, JG, et al. Patterns of cell cycle checkpoint deregulation associated with intrinsic molecular subtypes of human breast cancer cells. NPJ Breast Cancer (2017) 3(1):9–12. doi:10.1038/s41523-017-0009-7
111. Heldt, FS, Barr, AR, Cooper, S, Bakal, C, and Novák, B. A comprehensive model for the proliferation–quiescence decision in response to endogenous DNA damage in human cells. Proc Natl Acad Sci (2018) 115(10):2532–7. doi:10.1073/pnas.1715345115
112. Mayer, EL, Dueck, AC, Martin, M, Rubovszky, G, Burstein, HJ, Bellet-Ezquerra, M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. The Lancet Oncol (2021) 22(2):212–22. doi:10.1016/s1470-2045(20)30642-2
113. Finn, RS, Martin, M, Rugo, HS, Jones, S, Im, SA, Gelmon, K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med (2016) 375(20):1925–36. doi:10.1056/nejmoa1607303
114. Zardavas, D, Baselga, J, and Piccart, M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol (2013) 10(4):191–210. doi:10.1038/nrclinonc.2013.29
115. Prat, A, Ellis, MJ, and Perou, CM. Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol (2012) 9(1):48–57. doi:10.1038/nrclinonc.2011.178
116. Serra, F, Lapidari, P, Quaquarini, E, Tagliaferri, B, Sottotetti, F, and Palumbo, R. Palbociclib in metastatic breast cancer: current evidence and real-life data. Drugs in context. (2019) 8:1–16. doi:10.7573/dic.212579
117. Memari, F, Tavakolpour, V, Mohajeri, N, Poopak, B, Fallah, P, Alizadeh, E, et al. Distinct power of bone marrow microRNA signatures and tumor suppressor genes for early detection of acute leukemia. Clin Transl Oncol (2022) 24(7):1372–80. doi:10.1007/s12094-022-02781-3
118. Müller, GA, Wintsche, A, Stangner, K, Prohaska, SJ, Stadler, PF, and Engeland, K. The CHR site: definition and genome-wide identification of a cell cycle transcriptional element. Nucleic Acids Res (2014) 42(16):10331–50. doi:10.1093/nar/gku696
119. Rasool, R, Ullah, I, Mubeen, B, Alshehri, S, Imam, SS, Ghoneim, MM, et al. Theranostic interpolation of genomic instability in breast cancer. Int J Mol Sci (2022) 23(3):1861. doi:10.3390/ijms23031861
120. Rueda, OM, Sammut, S-J, Seoane, JA, Chin, S-F, Caswell-Jin, JL, Callari, M, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature (2019) 567(7748):399–404. doi:10.1038/s41586-019-1007-8
121. Alba, E, Rueda, OM, Lluch, A, Albanell, J, Chin, S-F, Chacon, JI, et al. Integrative cluster classification to predict pathological complete response to neoadjuvant chemotherapy in early breast cancer. American Society of Clinical Oncology (2018).
122. van den Bosch, T, Rueda, OM, Caldas, C, Vermeulen, L, and Miedema, DM. Copy number heterogeneity identifies ER+ breast cancer patients that do not benefit from adjuvant endocrine therapy. Br J Cancer (2022) 127:1332–9. doi:10.1038/s41416-022-01906-3
123. Dugo, E, Piva, F, Giulietti, M, Giannella, L, Ciavattini, A, and Gough, L. Copy number variations in endometrial cancer: from biological significance to clinical utility. Int J Gynecol Cancer (2024) 34(7):1089–97. doi:10.1136/ijgc-2024-005295
124. Heo, Y, Heo, J, Han, SS, Kim, WJ, Cheong, HS, and Hong, Y. Difference of copy number variation in blood of patients with lung cancer. Int J Biol Markers (2021) 36(1):3–9. doi:10.1177/1724600820980739
125. Zhou, C, Zhang, W, Chen, W, Yin, Y, Atyah, M, Liu, S, et al. Integrated analysis of copy number variations and gene expression profiling in hepatocellular carcinoma. Sci Rep (2017) 7(1):10570. doi:10.1038/s41598-017-11029-y
126. Li, J, Dittmar, RL, Xia, S, Zhang, H, Du, M, Huang, CC, et al. Cell-free DNA copy number variations in plasma from colorectal cancer patients. Mol Oncol (2017) 11(8):1099–111. doi:10.1002/1878-0261.12077
127. Chen, Y, Sadasivan, SM, She, R, Datta, I, Taneja, K, Chitale, D, et al. Breast and prostate cancers harbor common somatic copy number alterations that consistently differ by race and are associated with survival. BMC Med Genomics (2020) 13(1):116. doi:10.1186/s12920-020-00765-2
128. Hu, W, Li, M, Zhang, Q, Liu, C, Wang, X, Li, J, et al. Establishment of a novel CNV-related prognostic signature predicting prognosis in patients with breast cancer. J Ovarian Res (2021) 14(1):103. doi:10.1186/s13048-021-00823-y
129. Borregales, LD, DeMeo, G, Gu, X, Cheng, E, Dudley, V, Schaeffer, EM, et al. Response to takahashi. JNCI: J Natl Cancer Inst (2022) 114(11):1557–8. doi:10.1093/jnci/djac146
130. Yan, K, Niu, L, Wu, B, He, C, Deng, L, Chen, C, et al. Copy number variants landscape of multiple cancers and clinical applications based on NGS gene panel. Ann Med (2023) 55(2):2280708. doi:10.1080/07853890.2023.2280708
131. Conrad, B, and Antonarakis, SE. Gene duplication: a drive for phenotypic diversity and cause of human disease. Annu Rev Genomics Hum Genet (2007) 8:17–35. doi:10.1146/annurev.genom.8.021307.110233
132. Kallioniemi, A, Kallioniemi, OP, Sudar, D, Rutovitz, D, Gray, JW, Waldman, F, et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science (1992) 258(5083):818–21. doi:10.1126/science.1359641
133. Eijk-Van Os, PG, and Schouten, JP. Multiplex Ligation-dependent Probe Amplification (MLPA®) for the detection of copy number variation in genomic sequences. Methods Mol Biol (2011) 688:97–126. doi:10.1007/978-1-60761-947-5_8
134. Voutsadakis, IA. Breast cancer sub-types display heterogeneity in gene amplification and mRNA expression of the anti-apoptotic members of BCL2 family. Gene (2023) 857:147179. doi:10.1016/j.gene.2023.147179
135. Chen, Y, and Olopade, OI. MYC in breast tumor progression. Expert Rev Anticancer Ther (2008) 8(10):1689–98. doi:10.1586/14737140.8.10.1689
136. Reis-Filho, JS, Savage, K, Lambros, MB, James, M, Steele, D, Jones, RL, et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol (2006) 19(7):999–1009. doi:10.1038/modpathol.3800621
137. Lamy, PJ, Fina, F, Bascoul-Mollevi, C, Laberenne, AC, Martin, PM, Ouafik, L, et al. Quantification and clinical relevance of gene amplification at chromosome 17q12-q21 in human epidermal growth factor receptor 2-amplified breast cancers. Breast Cancer Res (2011) 13(1):R15. doi:10.1186/bcr2824
138. Rakha, EA, Armour, JA, Pinder, SE, Paish, CE, and Ellis, IO. High-resolution analysis of 16q22.1 in breast carcinoma using DNA amplifiable probes (multiplex amplifiable probe hybridization technique) and immunohistochemistry. Int J Cancer (2005) 114(5):720–9. doi:10.1002/ijc.20738
139. Shahrouzi, P, Azimzade, Y, Brankiewicz-Kopcinska, W, Bhatia, S, Kunke, D, Richard, D, et al. Loss of chromosome cytoband 13q14.2 orchestrates breast cancer pathogenesis and drug response. Breast Cancer Res (2024) 26(1):170. doi:10.1186/s13058-024-01924-4
140. Kang, J. Genomic alterations on 8p21-p23 are the most frequent genetic events in stage I squamous cell carcinoma of the lung. Exp Ther Med (2015) 9(2):345–50. doi:10.3892/etm.2014.2123
141. Krig, SR, Miller, JK, Frietze, S, Beckett, LA, Neve, RM, Farnham, PJ, et al. ZNF217, a candidate breast cancer oncogene amplified at 20q13, regulates expression of the ErbB3 receptor tyrosine kinase in breast cancer cells. Oncogene (2010) 29(40):5500–10. doi:10.1038/onc.2010.289
142. Pouladi, N, Abdolahi, S, Farajzadeh, D, and Feizi, MAHP. Association of the 17p13.1 region gene variants rs1042522 and rs2287499 with risk of breast cancer in Iranian-Azeri population. Meta Gene (2019) 19:117–22. doi:10.1016/j.mgene.2018.11.009
143. Wang, H, Ba, J, Kang, Y, Gong, Z, Liang, T, Zhang, Y, et al. Recent progress in CDK4/6 inhibitors and PROTACs. Molecules (2023) 28(24):8060. doi:10.3390/molecules28248060
144. Bruner, HC, and Derksen, PWB. Loss of E-cadherin-dependent cell-cell adhesion and the development and progression of cancer. Cold Spring Harb Perspect Biol (2018) 10(3):a029330. doi:10.1101/cshperspect.a029330
Keywords: cell cycle, luminal breast cancer, chromosomal instability, copy number variation, therapeutic strategy
Citation: Mahdi Khamaneh A, Jafari-Gharabaghlou D, Ansarin K, Pazooki P, Akbarpour Z, Naghili B and Zarghami N (2025) A new insight into the impact of copy number variations on cell cycle deregulation of luminal-type breast cancer. Oncol. Rev. 19:1516409. doi: 10.3389/or.2025.1516409
Received: 24 October 2024; Accepted: 16 January 2025;
Published: 12 February 2025.
Edited by:
Dirk Geerts, Amsterdam University Medical Center, NetherlandsReviewed by:
Lakshmi Muthuswamy, Blueprint Medicines, United StatesLing Ding, University of Pittsburgh Medical Center, United States
Copyright © 2025 Mahdi Khamaneh, Jafari-Gharabaghlou, Ansarin, Pazooki, Akbarpour, Naghili and Zarghami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nosratollah Zarghami, emFyZ2hhbWluQGdtYWlsLmNvbQ==; Behrooz Naghili, bmFnaGlseWJAZ21haWwuY29t
 Amir Mahdi Khamaneh1
Amir Mahdi Khamaneh1 Davoud Jafari-Gharabaghlou
Davoud Jafari-Gharabaghlou Pouya Pazooki
Pouya Pazooki Nosratollah Zarghami
Nosratollah Zarghami