1
Department of Physiology, University of Ankara, Turkiye
2
Department of Psychology, The Hebrew University of Jerusalem, Israel
3
Department of Psychology and the Helen Wills Neuroscience Institute, University of California, USA
Neglect patients bisect lines far rightward of center whereas normal subjects typically bisect lines with a slight leftward bias supporting a right hemisphere bias for attention allocation. We used fMRI to assess the brain regions related to this function in normals, using two complementary tasks. In the Landmark task subjects were required to judge whether or not a presented line was bisected correctly. During the line bisection task, subjects moved a cursor and indicated when it reached the center of the line. The conjunction of BOLD activity for both tasks showed right lateralized intra-parietal sulcus and lateral peristriate cortex activity. The results provide evidence that predominantly right hemisphere lateralized processes are engaged in normal subjects during tasks that are failed in patients with unilateral neglect and highlight the importance of a right fronto-parietal network in attention allocation.
Visuospatial Attention
Different models are posited to explain the neural mechanisms of spatial attention. Mesulam (1981
, 1990)
proposed a network model of attention in which posterior parietal cortex, the cingulate cortex and the frontal cortex centered around frontal eye field (FEF) interact to support attention allocation. The cortical components within this network are also connected with subcortical structures including superior colliculus, pulvinar and striatum, which have also been implicated in attention allocation. All these regions are modulated by input from the reticular activating system. According to this model the parietal component provides an internal perceptual map of the external world whereas the frontal component coordinates the motor program for exploration, scanning, reaching and fixating.
Posner and colleagues (Posner, 1995
; Posner and Petersen, 1990
) modeled attention as consisting of posterior and anterior attention systems, as well as vigilance networks. A posterior network involving the parietal cortex, pulvinar and the superior colliculus was proposed to be involved in performing operations needed to bring attention to a location in space. An anterior network involved anterior cingulate and supplementary motor cortex and was suggested to be activated during detection of salient events and the preparation of appropriate responses. Executive control over voluntary behavior and thought processes are also proposed to be dependent on the anterior network. Several neuroimaging studies using PET or fMRI during visual attention tasks have reported activations in the attention networks supporting Mesulam’s and to some degree Posner’s models (Corbetta et al., 2000
; Gitelman et al., 1999
; Hopfinger et al., 2000
; Nobre et al., 1997
; Vandenberghe et al., 2000
). However, the lateralization of the reported activations was not consistent especially for the intra-parietal sulcus (IPS). While some studies report right lateralized activations (Gitelman et al., 1999
; Nobre et al., 1997
) most report bilateral IPS activations (Corbetta et al., 2000
; Hopfinger et al., 2000
; Vandenberghe et al., 2000
).
Corbetta et al. (2000
, 2002)
reported event related fMRI results related to visuospatial processing and suggested that two brain systems interact to mediate attention. A “dorsal” fronto-parietal network, consisting of the IPS and superior frontal cortex (corresponding to FEF), was proposed to be bilaterally activated during voluntary (“top–down”) allocation of attention and directing visuomotor behavior. Temporo-parietal junction (TPJ) and inferior frontal regions formed a separate “ventral” network proposed to be activated when salient perceptual events cause re-orientation of attention in a bottom–up manner. According to this model, whereas the dorsal attentional system is bilateral, with some regions responding more strongly when attention is shifted contralaterally, the ventral attentional system is right lateralized, for both left and right shifts of attention (Corbetta and Shulman, 2002
; Corbetta et al., 2002
). In this study, we addressed this proposed pattern of hemispheric asymmetry using a task different from the one on which Corbetta et al. based their model. The tasks we chose, line bisection and the landmark test, are ones commonly used for testing the attentional deficits of patients with unilateral neglect, a syndrome linked to damage to attentional systems, and in particular the right-lateralized ventral attentional system (Corbetta and Shulman, 2002
; Mort et al., 2003
).
Unilateral Spatial Neglect
Unilateral spatial neglect is a frequent outcome of mainly right hemisphere damage. Hemi-spatial neglect is very detrimental to the patients’ rehabilitation prognosis due to the prominent attention difficulties and lack of awareness of their deficits (Katz et al., 1999
). Unilateral neglect is more frequently associated with right hemisphere lesions and is also more severe subsequent to right rather than left hemisphere lesions (Heilman, 1998
). Cortical damage in the right inferior parietal, right temporo-parietal junction, superior temporal, frontal, and cingulate regions as well as certain subcortical structure lesions including the pulvinar and basal ganglia may cause neglect (Karnath et al., 2002
, 2004
; Mort et al., 2003
; Vallar, 2001
).
In the brunt of these cases both voluntary and involuntary attention is compromised. This could be due the fact each area is involved in both forms of attention or due to transient diaschisis in highly interconnected yet specialized cortical areas as has been reported in PET and fMRI studies (Fiorelli et al., 1991
; He et al., 2007
; Sobesky et al., 2005
).
Line Bisection-Landmark Tasks and Pseudoneglect
Several formal tests have been used to assess unilateral neglect, including search tasks, copying and drawing (Parton et al., 2004
). One of the most popular tests is the line bisection, in which patients are required to place a mark in the exact midpoint of a line drawn on a sheet of paper in front of the patient. Neglect patients bisect lines to the right of the true midline, seemingly neglecting part of or underestimating the length of the left side of the line. In contrast, neurologically intact right handed subjects tend to systematically err to the left of center, a phenomenon Bowers and Heilman first referred to as pseudoneglect (Bowers and Heilman, 1980
; Bradshaw et al., 1986
; Nalçaci et al., 1997
; Scarisbrick et al., 1987
). The misbisection to the left is not as pronounced as the rightward bias observed in neglect patients. Pseudoneglect was reported to have considerable between subject variation (Manning et al., 1990
). However, when assessed with a forced choice tachistoscopic test healthy observers showed consistent bisection errors (McCourt, 2001
). The leftward bias in controls is presumed to reflect a right hemisphere attention bias to the left field.
The Landmark task, which requires subjects to judge whether pre-marked lines are correctly bisected, is also used in unilateral spatial neglect studies, and neglect patients typically fail this test as well (Marshall and Halligan, 1995
). In contrast to line bisection, the landmark task has a reduced motor load and seems to require limited eye movement (Fink et al., 2000b
). This presumably allows one to partially dissociate perceptual from motor aspects of the bisection errors. The fact that subjects have limited motor demands has made the landmark task a test of choice in fMRI studies of spatial attention (Fink et al., 2000a
; McCourt and Olafson, 1997
). Fink et al. (2000a
,b
, 2001
, 2002)
used the Landmark task in a series of fMRI studies and reported superior posterior parietal cortex (or IPS) and temporo-parietal junction (inferior parietal lobule) activations. Activations in both of these locations tended to be more robust on the right hemisphere (Fink et al., 2000a
,b
, 2001
). Assuming that the landmark test requires voluntary, sustained, top–down attention rather then involuntary bottom–up attention, this seems to be at odds with the predictions of the recent model of Corbetta and colleagues, both in involvement of the temporo-parietal junction, part of the putative bottom–up ventral attention system, and in the right lateralization of the IPS activation. However, the descriptions of laterality patterns in specific sub-regions of the posterior parietal cortex in Fink et al.’s work do not allow a clear comparison to the model suggested by Corbetta and Shulman (2002)
.
We used both Line Bisection and Landmark paradigms in the same fMRI experiment. Both the Line Bisection and the Landmark paradigms require voluntary attention. We reasoned that the neural regions common to both tasks would define key elements of the voluntary attention network, and aimed to examine the pattern of laterality of this activity.
Subjects
Sixteen right handed subjects with no history of neurological or psychiatric disease volunteered for the study. Due to head movements and other technical problems 11 participants’ data were included in the analysis. Subjects gave informed consent according to procedures approved by the University of California Committee for the Protection of Human Subjects.
Procedure
Functional images were collected during four conditions detailed below. In all conditions basic stimuli were black lines spanning 6.3° to 8.6° visual angle (varying randomly stepwise between 11 and 15 cm width) presented on a white background on a 13.8° × 10.3° screen (24 × 14 cm). Subjects viewed the screen from a mirror mounted on the head coil 10 cm away from their eyes. The distance between the mirror and the screen was approximately 90 cm. The lines were presented with their right or left edge 4 cm away from the right or left edge of the screen respectively. This ensured that the lines’ midline was never centered horizontally on the screen.
The experimental paradigm (Figure 1
) was created and delivered by the Presentation (version 0.55) program (Neurobehavioral system, CA, USA). Subjects used their right hand for all the responses. Conditions were as follows:
Figure 1. (A) Landmark task stimuli consisted of prebisected lines and subjects were required to judge whether they were correctly bisected or not. In the Landmark control condition subjects were presented either lines with adjacent or separate mark and the instruction was to judge whether the mark touched the line or not. In the Line Bisection task subjects were presented by a line with a cursor on the left or right edge and they moved the cursor to the center. In the line bisection control cursor appeared at the center of the lines and subjects were to move it to the edge of the line. (B) Average group reaction times for Landmark and Line Bisection tasks (for active and control conditions).
1. Line bisection task (LB): In this paradigm subjects moved a cursor on the presented lines by pressing the left button on a response box for moving the cursor to the left and the right button for moving the cursor to the right. Lines were presented initially with the cursor on the left or right edge of the line (50% probability each). Subjects were instructed to move the cursor to the line’s midline, and once there, to confirm by pressing another button at the center of the response box. Bisection error was calculated by averaging absolute deviations from the midline. Bias parameter was computed as a negative score defining a left deviation from the true midline and a positive score for right deviation.
2. Line Bisection control (LBC): Lines were presented with the cursor initially in the midline position. Subjects were required to move the cursor to the left or right edge of the line by pressing one button for left movement and another for right movement as in the Line Bisection task, and once at the edge, to confirm by pressing the third button. Left and right movements were required an equal number of times.
3. Landmark task (LM): The presented lines were prebisected by a short vertical line. Subjects were asked to judge whether the presented lines were prebisected correctly. They pushed one button for correct and another for incorrect response. Bisection marks were placed at the true midline (p = 0.4), or deviated to the right or left of the midline by 2.5%, 5% or 7.5% of the line’s length, each deviation presented with a 0.1 probability. Percentage of correct responses was calculated as subjects performance score.
4. Landmark control (LMC): All the stimuli were identical to the Landmark task except that the bisection mark was immediately adjacent to the line as in the Landmark test in 40% of trials, but was placed slightly above the line and did not actually make contact with it in the remaining 60% of the trials. Participants were instructed to press one button if the mark made contact with the line, and another button if it was separate from the line. Whether there was a gap between the mark and the line or not was orthogonal to whether the mark correctly bisected the line or not.
5. Low level baseline: In this rest condition participants looked at the white screen without any additional task. The control conditions shared with the experimental conditions the visual display of a horizontal line and a small marker, the motor demands, as well as the requirement to attend to the display. The critical difference between the experimental conditions (LB and LM) and their respective controls is in the fact that the experimental conditions exclusively required a spatial judgment (equality of length of the two parts of the line) which we reasoned requires voluntarily deploying or shifting spatial attention over both sides of space.
The experiment was conducted in a block design. Each block lasted 20 s, including 10 line presentations, each lasting 2 s. Each block was preceded by an instruction screen for 4 s, indicating which of the five conditions will follow. Each fMRI session consisted of three pseudo-randomized repetitions of five conditions and each subject performed 6 runs.
Eye Movement Recording and Analysis
Tasks were performed without eye fixation, as in the clinical tests after which the tasks are designed. Eye position was monitored in the scanner at 60 Hz with an infrared video graphic camera equipped with a telephoto lens (model 504LRO; Applied Science Laboratories, Bedford, MA, USA) that focused on the right eye viewed from a small dielectric flat surface mirror mounted inside the RF coil. Nine-point calibrations were performed at the beginning of the session and between runs when necessary. Eight subjects’ eye data were available out of 11 because of technical difficulties. The eye movement data were analyzed using ILAB software (Gitelman, 2002
). For each of the four conditions (not for resting condition), we calculated the length of scan path of the subject’s gaze on the screen.
fMRI Procedure
Imaging was performed using a 4.0-Tesla Varian Inova scanner equipped with a fast gradient system for echoplanar imaging and a standard RF head coil. A gradient-echo, echo-planar sequence (TR = 2000 ms, TE = 28 ms, matrix size = 64 × 64, FOV = 22.4 cm) was used to acquire data sensitive to the blood oxygen level dependent (BOLD) signal. Each functional volume contained eighteen 6 mm thick axial slices with 1 mm gap, which covered the subject’s whole brain from the vertex to the base of cerebellum. Using a midsagital scout image, the slices were oriented parallel to the anterior–posterior commissure (AC-PC). Each subject was scanned in 6 runs of 180 volumes (3 repetitions × 5 conditions × 12 TRs per condition, including the instructions).
fMRI Data Analysis
Data processing and analysis were performed using MATLAB (The Mathworks, Inc., Natick, MA, USA) and SPM5 (Statistical Parametric Mapping software; Wellcome Department of Cognitive Neurology, London, UK)
1
. SPM was used for realignment, spatial normalization, smoothing and creating statistical maps of significant relative regional BOLD response changes (Friston et al., 1995b
,c
).
The first 10 images of each time series, during which a steady state tissue magnetization was achieved, were discarded. The remaining 180 volumes were realigned to correct for head movements between scans. The functional images were then coregistered to the high resolution 3D anatomical image. Structural and functional images were spatially normalized into a standardized anatomical framework using the default T1 template provided in SPM5, based on the averaged-brain of the Montreal Neurological Institute and approximating the normalized probabilistic spatial reference frame of Talairach and Tournoux (1988)
. Smoothing was performed with a Gaussian kernel of 10 mm full-width half-maximal.
Low-frequency cosine waves modeled and removed subject-specific low frequency drifts in signal, and global means were normalized by proportional scaling. Data were analyzed using the general linear model as implemented in SPM5, modeling the conditions using boxcar functions convolved with a “canonical” hemodynamic response function (Friston et al., 1995a
).
Statistical parametric maps for specific effects were calculated by applying appropriate contrasts to the parameter estimates. We obtained one map per subject per contrast, and put them into one-sample t-tests random-effect analyses, allowing inferences to the general population. The presented results are significant at a level of at least p < 0.05 corrected for multiple comparisons. Voxels presenting a p < 0.001 and belonging to clusters of at least 41 voxels were considered as activated. These parameters were chosen on the basis of Monte Carlo simulations processed with the AlphaSim program
2
. Common activities for Landmark and Line Bisection tasks were assessed. To this end LM-LMC contrast was inclusively masked by LB-LBC (threshold for masking image was p < 0.05 uncorrected). The differential activations between the Landmark and the Line Bisection was also obtained with [(LM-LMC) − (LB-LBC)] and [(LB-LBC) − (LM-LMC)] contrasts. Masking was applied to the interaction contrasts to exclude the effect of deactivations. [LM-LMC] − [LB-LBC] was exclusively masked with LBC-LB and [LB-LBC] − [LM-LMC] was exclusively masked with LMC-LM.
A region of interest (ROI) analysis was performed based on the activated clusters resulting from the Landmark and Line bisection conjunction analysis. To reveal the significance of the lateralization in the results symmetrical left hemisphere ROI was created by mirroring the right hemisphere cluster resulting from the conjunction analysis. That is, following normalization, the sign of the x coordinates of the active cluster found over the right hemisphere was flipped to determine the mirror region in the left hemisphere. Average BOLD responses were extracted from and averaged within these ROIs from the individual subject images. Wilcoxon Signed Rank Test was used to compare the hemodynamic responses of the left and right ROIs.
Behavioral and Eye Movement Data
The Landmark task performance (percent correct) of subjects was high (mean = 78%, S.E. ± 1.7%). Line bisection scores were as follows: Bisection error, mean = 11.9%, S.E. ± 1.1%; Bias, mean = −0.1, S.E. ± 0.7.
A two-way ANOVA was performed to investigate the effects of task (Landmark or Line Bisection) and condition (active or control) upon reaction times (RT) (see Figure 1
lower panel). The subjects were faster during the Landmark compared to the Line Bisection task [main effect of Task: F (1, 10) = 1276, p < 0.001]. The responses were also faster during control conditions compared to the active task conditions [main effect of condition: F (1, 10) = 182, p < 0.001]. These two effects significantly interacted however, as the active versus control RT difference was larger in the Landmark than in the Line Bisection task [F (1, 10) = 30, p < 0.001].
A similar two-way ANOVA was performed to investigate the eye movement data. The effects of task (Landmark or Line Bisection) and condition (active or control) upon the average scan path was assessed. The average scan path (in pixels) was longer during Line Bisection compared to the Landmark [main effect of Task: F (1, 7) = 9.9, p < 0.05]. The main effect of condition was not significant (p > 0.05). Task and condition effects significantly interacted [F (1, 7) = 15.5, p < 0.01]. While there was no eye movement difference (p > 0.05) between Landmark task (3175 ± 478; mean ± S.E.) and Line Bisection tasks (3249 ± 553), the average scan path was longer (t1,7 = 4.8, p < 0.01) in the Line Bisection control (4005 ± 516) than in the Landmark control (1846 ± 424).
fMRI Data
Landmark task
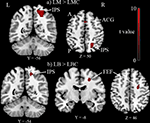
The Landmark task (LM-LMC contrast) activated right IPS, anterior cingulate girus, and right lateral peristriate cortex (p < 0.05 corrected for multiple comparisons, see Table 1 and Figure 2
A).
Figure 2. Group results (n = 11) thresholded at p < 0.05 corrected for multiple comparisons. (A) Landmark task (LM) versus Landmark control (LMC) activated intra-parietal sulcus (IPS), anterior cingulate girus (ACG) and lateral peristriate cortex (not shown in this picture). (B) Line bisection task (LB) compared to its control (LBC) activated again IPS, FEF also lateral peristriate cortex not shown in this picture. A, anterior; P, posterior; L, left; R, right.
Line bisection task
The Line bisection task (LB-LBC contrast) activated right IPS, right FEF and right lateral peristriate cortex (p < 0.05 corrected for multiple comparisons, see Table 1 and Figure 2
B).
Common activities for Landmark and Line Bisection
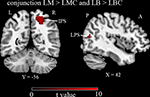
The activity related to the two tasks (compared to their respective controls) overlapped in right hemisphere IPS and LPS (see Table 2 and Figure 3
). Since no left IPS activity was found, ROI analysis was performed based on right IPS and its left hemisphere mirror site (see “Materials and Methods”). Right IPS activity (percentage signal change) was greater than left for both LM-LMC (z1,10 = 2.9, p < 0.01) and LB-LBC (z1,10 = 2.3, p < 0.05) contrasts. While this left–right comparison may seem biased by the fact that the left ROI was created based on the right IPS ROI which was defined functionally, it is only meant to reaffirm the difference between the two hemispheres in this region. Note that simply showing that the right IPS shows activity whereas the left does not is insufficient statistically to establish this difference. In an attempt to define the left ROI functionally we reduced the threshold to 0.05, uncorrected. This revealed a cluster of 2 voxels at [−22, −60, 50] which were already included in the mirror ROI described above. Thus, although the selection of the left IPS ROI was based on the right IPS ROI, the left IPS ROI did reflect the maximal activity for this contrast over the left hemisphere.
Figure 3. Group results thresholded at p < 0.05 corrected for multiple comparisons. Intra-parietal sulcus (IPS) and lateral peristriate cortex (LPS) activated (please see Table 2
for coordinates) commonly for both Landmark and Line Bisection tasks (LM-LMC inclusively masked with LB-LBC). A, anterior; P, posterior; L, left; R, right.
The differential activations between the Landmark and the Line Bisection was assessed with [(LM-LMC) − (LB-LBC)] and [(LB-LBC) − (LM-LMC)] contrasts. No significant activation passed the corrected threshold (p < 0.001 and ≥41 voxels) for these contrasts. Thus, we did not find evidence for any difference in the activation caused by the two tasks.
We used both the Landmark task and the line bisection task to disentangle the question of right hemisphere dominance within the attention networks. The line bisection task is frequently used as a bedside measure of hemi-spatial neglect. Both these tasks require voluntary attention and both activated the dorsal attention system as was predicted. However, the activations were not symmetrical and showed right hemisphere lateralization.
Task Specific Activations for Landmark and Line Bisection
The present study found right IPS, anterior cingulate girus (ACG) and right LPS activations for the Landmark task (LM-LMC contrast). IPS, ACG and LPS activations (as well as frontal and cerebellar activations) were first reported for the Landmark task by Fink and his coworkers (Fink et al., 2000a
, 2001
, 2002
; Weiss et al., 2003
). They reported extensive inferior parietal lobe (IPL) and prefrontal activations during the Landmark task contrary to our findings. This could be due in part to the presentation style of the stimuli and to the choice of a control condition. In most of the above cited work Fink and colleagues presented the stimuli in the four screen quadrants (Fink et al., 2000a
, 2002
; Weiss et al., 2003
) while we did that in the center of the screen. In addition the line stimuli spanned 16° to 24° visual angle (greater than our stimuli spanning 6.3° to 8.6° visual angle) in the majority of the previous work (Fink et al., 2000a
, 2001
, 2002
). The choice of control condition was simpler in some previous work – to judge if there is a mark on the line or not – (Fink et al., 2000a
, 2001
). Moreover, in one experiment only a low level baseline was used and highly significant prefrontal and IPL activations were reported (Fink et al., 2002
). This suggests that some of the activations reported may not be specific to the Landmark task.
The Line bisection task (LB-LBC contrast) activated IPS, FEF and LPS. This is consistent with the findings of Weiss and his coworkers who required subjects to use a laser pointer to bisect the lines presented on the screen during PET measurements (Weiss et al., 2000
, 2003
). Although their results well match with our line bisection task activations, they reported IPL activity consistently in addition to the activation locations revealed in our results. Again the presentation style of the stimuli could be the reason for the differences in their results. Weiss and coworkers presented the lines in the four screen quadrants though their lines spanned 8.5° to 9° visual angle –more similar to ours. Lastly the control condition requiring just pointing to a dot on the screen (Weiss et al., 2000
) might not involve some of the spatial processes compared to our line bisection control which requires moving a cursor on a whole line. While the FEF activity in Line bisection (and not in the Landmark task) could be ascribed to increased eye movements required in this task, in fact there were nominally more eye movements in the Line Bisection control condition than in the Line Bisection condition itself (see Section “Behavioral and Eye Movement Data”).
In both the Landmark and Line Bisection task conditions subjects should see the line as a whole and then judge the midline. The perceptual judgment of total line length and the estimation of the midline may require a global distribution of the spatial attention to the visual field which activates IPS (Çiçek et al., 2007
; Molenberghs et al., 2008
). The space that must be covered during Landmark and Line Bisection task is more extensive than in the control conditions. In the Line Bisection control condition subjects only move the cursor to the edge of the line and in the Landmark control a very simple visual discrimination is needed. Thus control conditions seem to engage more focal spatial attention.
Conjunction of Landmark and Line Bisection
The common activations for the Landmark and Line Bisection paradigms were found in the right IPS and right LPS. These findings are also in line with previous functional brain imaging results using line bisection or Landmark paradigms in separate studies (Fink et al., 2001
, 2002
; Weiss et al., 2000
, 2003
). IPS, FEF and peristriate regions were activated previously during studies using well controlled spatial attention paradigms with left and rightward cueing (Corbetta and Shulman, 2002
; Corbetta et al., 2000
; Gitelman et al., 1999
; Hopfinger et al., 2000
). The common activation regions for these tasks and our tasks overlap with the proposed dorsal voluntary attention network around IPS, FEF and lateral occipital cortex (Corbetta and Shulman, 2002
; Corbetta et al., 2000
). Although conjunction analysis did not reveal FEF activation this area was active during the Line Bisection task (compared to control).
Right Lateralization
In the present study, task specific activities for the Landmark and Line bisection tasks as well as conjunction activities were more extensive in the right hemisphere than the left hemisphere. ROI analysis performed based on IPS cluster showed significantly more activation on the right hemisphere for these tasks.
Right hemisphere dominance in line bisection is supported by a combination of electrical and hemodynamic neuroimaging data, as well as lesion data. Patients with right hemisphere lesions show lower performance on line bisection tasks compared to the left hemisphere lesioned groups (Mennemeier et al., 1997
). Vallar et al. (1999)
reported parietal occipital and frontal fMRI activations during a modified form of line bisection task during which 89% of the activated voxels were on the right hemisphere. Greater activations on the right hemisphere relative to the left hemisphere during line bisection tasks where shown in other imaging studies using fMRI and SPECT (Fink et al., 2000a
,b
, 2001
, 2002
; Marshall et al., 1997
) and also using transcranial doppler sonography (Flöel et al., 2001
, 2002
). High-density event-related potential recording during line bisection performance showed early right TPJ and later right parietal (in the vicinity of IPS) localized potentials (Foxe et al., 2003
).
Our asymmetrical activations of the dorsal attention network are inconsistent however with the symmetrical activation pattern reported by Corbetta for this network (Corbetta et al., 2000
). Corbetta and his coworkers used the Posner paradigm and dissociated cue related activity during which subjects endogenously attended to the left or the right hemi-space from target related activity which required subjects to respond to a stimulus presented from left or right side of the screen. The post-cue period, representing voluntary attention processes, activated the dorsal attention network bilaterally whereas target period engaging involuntary attention processes activated the ventral attention network with right hemisphere dominance. In the present study, the Landmark and Line bisection activated areas overlapping the dorsal attention network more than the ventral parts, possibly because the role of sustained attention is larger than reorienting responses in this task. Nevertheless, the activation of the dorsal attention network was clearly right lateralized. A possible explanation for the more right lateralized activity may lie in the fact that our task required spatial judgments in addition to sustained attention (Kukolja et al., 2006
). Object based spatial processing requirements (rather than egocentric) in our tasks may be another reason for right lateralization (Galati et al., 2000
). Anderson (1996)
proposed a mathematical model of line bisection behavior using object based frame of reference and showed simulation results reminiscent of human subjects’ performance. The model proposed that “subjects bisect lines at the point where they perceive the salience of the two line segments created by their bisection mark to be equal”. In line with our brain activity results Anderson’s model assumed that right hemisphere established most of the magnitude of a point’s salience in horizontal axis (Anderson, 1996
).
Using the Landmark and Line bisection tasks together and looking at the conjunction results might give a clearer view about the brain areas related to the deployment of visuospatial attention. Our data supports the tasks engagement of dorsal fronto-parietal network revolving around IPS and LPS in both tasks and FEF for the line bisection task. The tasks evoked a pronounced right lateralization in agreement with ERP studies and clinical observations. This suggests that not only the ventral attention system, but also the dorsal, may have a predilection for the right hemisphere with some tasks. The overall pattern of results supports a dominant role of the right hemisphere network in voluntary spatial attention. This asymmetry may underlie the dominant role of right hemisphere lesions in hemi-spatial neglect.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Marc D’Esposito for his valuable contributions on the presented work’s experimental design and to the all Knight Lab members. This work was supported by The Scientific and Technological Research Council of Turkey grant SBAG-AYD-429 to MÇ and NINDS grant NS21135 to RTK.