1
Center for Neural Science, New York University, New York, NY, USA
2
Faculdade de Ciências da Universidade do Porto, Porto, Portugal
3
Department of Psychology, University of Texas at Austin, Austin, TX, USA
4
Emotional Brain Institute Labs, Nathan Kline Institute, Orangeburg, NY, USA
Activation of GABAARs in the lateral nucleus of the amygdala (LA), a key site of plasticity underlying fear learning, impairs fear learning. The role of GABACRs in the LA and other brain areas is poorly understood. GABACRs could be an important novel target for pharmacological treatments of anxiety-related disorders since, unlike GABAARs, GABACRs do not desensitize. To detect functional GABACRs in the LA we performed whole cell patch clamp recordings in vitro. We found that GABAARs and GABABRs blockade lead to a reduction of evoked inhibition and an increase increment of excitation, but activation of GABACRs caused elevations of evoked excitation, while blocking GABACRs reduced evoked excitation. Based on this evidence we tested whether GABACRs in LA contribute to fear learning in vivo. It is established that activation of GABAARs leads to blockage of fear learning. Application of GABAC drugs had a very different effect; fear learning was enhanced by activating and attenuated by blocking GABACRs in the LA. Our results suggest that GABAC and GABAARs play opposing roles in modulation of associative plasticity in LA neurons of rats. This novel role of GABACRs furthers our understanding of GABA receptors in fear memory acquisition and storage and suggests a possible novel target for the treatment of fear and anxiety disorders.
The lateral nucleus of the amygdala (LA) is a key site of plasticity underlying fear learning (LeDoux, 2000
; Blair et al., 2001
; Maren and Quirk, 2004
), and inhibitory circuits in that structure play an important role in the regulation of fear memory and its extinction (Wilensky et al., 1999
; Akirav and Richter-Levin, 2006
; Zhang and Cranney, 2008
). In the mammalian brain, γ-aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter (Nicoll et al., 1990
). It acts through different receptor types, including the ionotropic GABAA (GABAAR) and GABAC (GABACR) receptors (both of which activate Cl− currents), and the metabotropic GABAB receptor (GABABR). Most studies that have examined the role of GABA receptors in the LA have focused on GABAARs. GABAARs and GABACRs are activated by the same ligands (GABA or GABAA agonists; Lukasiewicz et al., 1994
), but GABACRs are many times more sensitive than GABAARs to these. If GABACRs are present in the LA, they should thus contribute to amygdala functions under different conditions than GABAARs. To test this, we used different concentrations of the GABA agonist muscimol since low concentrations should activate GABACRs and high concentrations GABAARs.
The functional significance of GABACRs is generally less well understood than that of the other GABARs, and few studies have explored the contribution of GABACRs to learning, memory and plasticity. Research performed in chicks found GABAARs and GABACRs in the forebrain play opposite roles in short-term memory (STM) of avoidance learning (Gibbs and Johnston, 2005
). Also, the selective GABACR antagonist (1,2,5,6-tetrahydropyridine-4-yl) methylphosphinic acid (TPMPA), facilitates learning and memory in the Morris Water Maze task in mice (Chebib et al., 2009
).
GABACR-specific ρ1 and ρ2 subunits were detected in the amygdala of mice using in situ-hybridization [Allen Brain Atlas (Internet)]. Functional GABACR have been identified in several regions of the nervous system (Johnston et al., 1975
; Lukasiewicz et al., 1994
; Pan and Lipton, 1995
; Delaney and Sah, 1999
; Boller and Schmidt, 2001
, 2003
; Kirischuk et al., 2003
; Hartmann et al., 2004
), including the lateral part of the central nucleus of the amygdala (CE; Delaney and Sah, 1999
), but have not been reported in the LA. Demonstration of the existence of GABACRs in the LA and determining their role in amygdala circuitry could lead to a better understanding of inhibitory plasticity in this brain region. Furthermore, GABACRs could be an important target in the effort to develop pharmacological treatments for anxiety-related disorders since, unlike GABAARs, GABACRs do not desensitize (Bormann, 2000
).
The goals of the current study were to verify the expression of functional GABACR in the LA, and to analyze their role in circuitry using whole-cell patch clamp recordings in acute slices in vitro. To examine the relevance of the role of GABACRs on fear learning and memory, we performed in vivo auditory Pavlovian fear conditioning a learning paradigm in which an emotionally neutral auditory conditioned stimulus (CS) comes to elicit fear responses after it is paired with an aversive unconditioned stimulus (US). Because fear conditioning is rapidly acquired and long lasting and the neural circuits underlying the learning are well understood and known to critically involve the LA, fear conditioning has been a popular technique for exploring the cellular and molecular mechanisms that contribute to learning, memory and plasticity (Rodrigues et al., 2004
).
Subjects
All animal experiments were performed in accordance with our institutional guidelines after obtaining the approval of the Institutional Animal Care and Use Committee (IACUC). For the electrophysiological experiments we received 37 Sprague Dawley 21-day-old rats. For the behavioral experiments we received 28 naïve male Sprague Dawley rats, weighing 250–300 g. These rats were housed individually and placed on a 12-h light/dark cycle with ad libitum food and water. The rats were acclimatized to laboratory conditions for 3 days before undergoing surgery.
Electrophysiological Experiments
Slice preparations
The amygdala slice preparation has been described previously (Weisskopf et al., 1999
). Rats were anesthetized with subcutaneous injection of ketamine (100 mg/kg body weight) and thiazine hydrochloride (1 mg/kg). To obtain acute slices of the LA for recording, the rats were deeply anesthetized with a subcutaneous injection of ketamine (100 mg/kg body weight) and thiazine hydrochloride (1 mg/kg). After transcardial perfusion with ice-cold artificial cerebro-spinal fluid (ACSF) containing (in mM), NaCl 124, KCl 5, NaH2PO4 1.25, NaHCO3 26, MgSO4 2, CaCl2 2, glucose 10, that was continuously gassed with 5% CO2/95% O2, the brain was removed and cut into 300 μm thick sagittal slices on a vibratome in ice cold ACFS. To allow recovery, slices were incubated for 1 h in ACSF at a temperature of 36°C. For recording, the slices were transferred into a submerged type recording chamber where they were continuously superfused at 3 ml/min with ACSF at room temperature.
Electrophysiology
For whole-cell recordings, slices were transferred to a submersion-type recording chamber where they were continuously perfused with oxygenated ACSF at a rate of 4 ml/min. Whole-cell recordings were obtained from the pyramidal cells in the LA region. Patch electrodes were fabricated from borosilicate glass and had a resistance of 5.0–8.0 MΩ. The pipettes were filled with internal solution composed of (in mM): potassium gluconate, 130; sodium gluconate, 2; HEPES, 20; MgCl2, 4; Na2ATP, 4; NaGTP, 0.4; EGTA, 0.5. In order to block sodium spikes, 5 mM QX 314 (Sigma-Aldrich) was added, as was 0.5% biocytin for morphological single cell reconstruction. Neurons were visualized with an upright microscope (Nikon Eclipse E600fn) using the Nomarski-type differential interference optics through a 60× water immersion objective. Neurons with a pyramidal appearance were selected for recordings. Neurons were voltage clamped using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA). Excitatory (EPSCs) and inhibitory postsynaptic currents (IPSCs) were recorded at a holding potential of −35 mV. Synaptic responses were evoked with sharpened tungsten bipolar stimulating electrodes (2 mm diameter, World Precision Instruments, Sarasota, FL, USA) placed in the cortical and thalamic pathway, 50–100 mm from the recording electrode. Stimulation was applied, at 0.1 Hz, using a photoelectric stimulus isolation unit having a constant current output (PSIU6, Grass Instrument Co., West Warwick, RI, USA). Access resistance (8–26 MΩ) was regularly monitored during recordings, and cells were rejected if it changed by more than 15% during the experiment. The signals were filtered at 2 kHz, digitized (Digidata 1440A, Axon Instruments, Inc.), and stored on a computer using the pCLAMP10.2 software (Axon Instruments, Inc.). The peak amplitude, 10–90% rise time, and the decay time constant of IPSCs were analyzed off-line using pCLAMP10.2 software (Axon Instruments).
Drugs
All pharmacologically active substances were bath applied for 10 min to achieve stable responses before their effects were tested. We used 0.1 μM muscimol (C5-aminomethyl acid; Sigma) as GABACR agonist. To make sure that the evoked responses were not GABAAR-mediated 20 μM bicuculline (GABAAR antagonist; Sigma) was co-applied. GABACRs were blocked with 30 μM TPMPA (Sigma). As GABABR agonist we applied 50 μM CGP52432 (Tocris).
Paired pulse depression
Neurons were stimulated by using an interstimulus interval of 100 ms. EPSCs and IPSCs were recorded.
Histochemistry
After each recording session, slices were immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C for 24 h. The slices were processed using standard histochemical techniques for visualization of biocytin with 3,3-diaminobenzidine (Sigma-Aldrich). For documentation, stained cells were photographed using a digital camera attached to a standard laboratory microscope.
Analysis
Data were analyzed by paired Student’s t-tests, because one groups, the neurons, of units that has been tested twice (control and drug).
Behavioral Studies
Surgery
Rats were anesthetized with subcutaneous injection of ketamine (100 mg/kg body weight) and thiazine hydrochloride (1 mg/kg) and treated with atropine sulfate (0.4 mg/kg). Using a stereotaxic frame, guide cannulae (22 gauge; Plastics One, Roanoke, VA, USA) fitted with internal cannulae that extended out by 1.5 mm were positioned just above the lateral and basal amygdala (LBA) using coordinates 3 mm posterior to bregma, 7.2 mm ventral to skull surface, and 5.5 mm lateral to midline). The guide cannulae were fixed to screws in the skull using cranioplastic cement (Plastics One). After the cement hardened, internal cannulae were replaced with dummy cannulae, cut 0.5 mm longer than the guides, to prevent clogging. Rats were tested the following week after recovery.
Intracranial injections
Rats were held in the experimenter’s lap while dummy cannulae were replaced with 28-gauge injector cannulae attached to 1.0 ml Hamilton syringes via polyurethane tubing. The tubing was back-filled with distilled water, and a small air bubble separated the water from the drug solution. The drug volume was 0.0003 ml that was infused bilaterally by an infusion pump at a rate of 0.05 μl/min. After drug infusion, cannulae were left in place for additional 3 min to allow diffusion of the drug away from the cannula tip, after which the dummy cannulae were replaced.
Apparatus
Fear conditioning took place in a Plexiglas rodent conditioning chamber with a metal grid floor (model E10-10; Coulbourn Instruments, Lehigh Valley, PA, USA), dimly illuminated by a single house light and enclosed within a sound-attenuating chamber (model E10-20). Testing for conditioned fear responses occurred in a brightly lit Plexiglas chamber with three house lights (ENV-001; Med Associates Inc., Georgia, VT, USA), fitted with a flat black Formica floor that had been washed with a peppermint-scented soap. Previous studies have shown that this distinct testing environment minimizes generalization from the training environment (Nader and LeDoux, 1999
; Schafe et al., 1999
). A video camera mounted at the top of the chamber recorded behavior for later scoring.
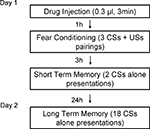
Habituation, conditioning, and testing
Figure 2
shows the behavior procedure. On day 1, rats received either muscimol (0.03 nmol/side in 0.0003 ml), TPMPA (30 nmol/side in 0.0003 ml) or ACSF vehicle (0.0003 ml) 60 min before training. All rats were habituated to the training and testing chambers for 10 min right before conditioning.
For training, rats were allowed 2–3 min to acclimate to the conditioning chamber and were then presented with three pairings of a 20-s tone CS (5 kHz, 75 dB) that co-terminated with a foot shock US (0.5 s, 0.7 mA). The intertrial interval varied randomly between 90 and 120 s. After drug infusion and conditioning, rats were returned to their home cages and to the colony.
Testing took place for STM and long-term memory (LTM). The STM test consisted of two CSs presentations 3 h after conditioning and the LTM test 18 CS presentations 24 h after conditioning. Rats were videotaped during testing for later scoring. After a 3-min acclimation period to the test chamber, rats were presented with 20 s tones (5 kHz, 75 dB). After tone testing, rats were returned to their home cages and to the colony. Fear memory was evaluated from the videotape by measuring the number of seconds during each tone presentation where rats engaged in freezing behavior, defined as a lack of all movement with the exception of respiration.
Data were analyzed with the unpaired Student’s t-test, because two separate independent and identically distributed samples were obtained, where one from each of the two populations were compared. Measures were compared by ANOVA with post hoc testing where appropriate (p < 0.05).
Histology
To verify injector tip location, rats were anesthetized with an overdose of Nembutal (100 mg/kg, i.p.) and perfused transcardially with 0.9% NaCl followed by 10% buffered Formalin. Brains were postfixed in 10% buffered Formalin and subsequently blocked, sectioned on a cryostat at 50 μm. Sections were cover slipped with Permount and examined under light microscopy for injector tip penetration into the amygdala.
We recorded from 47 pyramidal cells across the LA. Input resistances of recorded neurons in patch clamp experiments ranged from 104.7 to 198.0 MΩ (mean 160.2 MΩ, SD 64.5), resting membrane potentials varied between −55.7 and −68.3 mV (mean −61.3 mV, SD 5.0).
Effects of Muscimol on Postsynaptic Currents
In the first experiments we used whole-cell patch clamp recordings in acute slices in vitro to test whether GABACRs are present and participate in synaptic transmission within the LA. To elicit postsynaptic currents, electric stimulation was applied to the cortical or thalamic pathway to mimic, in vitro, testing the effects of stimulation of sensory inputs known to occur during fear conditioning in vivo (Romanski et al., 1993
; Repa et al., 2001
). The effects of the 1 μM muscimol, the GABA agonist, on the electrically evoked responses was assessed since this concentration has been reported to selectively activate GABACRs as opposed to GABAARs (Pasternack et al., 1999
; Boller and Schmidt, 2001
; Schmidt et al., 2001
). To determine whether this 1 μM muscimol primarily activated GABACRs we also examined the effects of blockade of GABAARs and GABABRs on the electrically evoked responses by adding the GABAAR antagonist bicuculline and the GABABR antagonist CGP 52432 separately to the bath during recordings.
EPSCs and IPSCs, evoked through external and internal capsule stimulation, were recorded from 23 pyramidal cells. Application of 1 μM muscimol, the concentration selective for activation of GABACRs, led to a decrease of IPSCs by 84 ± 13.7% (p < 0.05) and an increase in EPSCs of 90 ± 21.5% (p < 0.05) in all cells (Figures 1
A,B). CGP 52432 the GABABR antagonist decreased inhibition by 82 ± 9.9% (p < 0.05) and increased excitation 75 ± 7.1% (p < 0.05) in all pyramidal cells. Co-application of bicuculline to 1 μM muscimol and CGP blocked all the IPSCs (100%, p < 0.05) and the excitatory current increased by additional 330 ± 82% (p < 0.05; Figure 1
A). These results suggest that muscimol, in a concentration that selectively activates GABACRs, reduced inhibition in the LA and increased excitation. This is the opposite of the effects of activating GABAARs and GABABRs, which, upon ligand activation, hyperpolarize pyramidal neurons and reduce excitation. Consistent with this, we found that GABAARs and GABABRs blockade led to reduction of evoked inhibition and increment of excitation.
Figure 1. In vitro patch clamp recordings, showed functional GABACRs in the rat LA. (A) Recorded traces of a pyramidal cell in the LA during control, application of 1 μM muscimol (GABACR agonist), addition of 50 μM CGP 52432 (GABABR antagonist), and co-application of 10 μM bicuculline (GABAAR antagonist). (B) Average effect of 1 μM muscimol on synaptic currents of pyramidal cells in the LA compared to Control (n = 23). (C) Traces of synaptic currents of a pyramidal cell in the LA under control conditions, application of 30 μM TPMPA (GABACR agonist), 50 μM CGP 52432 (GABABR antagonist), and addition of bicuculline to CGP 52432. (D) Effect of 30 μM TPMPA on all recorded pyramidal cells in the LA compared to control (n = 14).
Effects of GABACR Antagonists on Postsynaptic Currents
In the next set of experiments the effects of a blockade of GABACRs on the electrically evoked responses in pyramidal cells was examined (n = 14). The selective GABACR antagonist TPMPA was bath-applied and led to enhancement of inhibition: IPSC amplitudes were increased (41 ± 18.2%, p < 0.05), and EPSC amplitudes were reduced by 60 ± 26.7% (p < 0.05; Figures 1
C,D). GABACRs are resistant to GABAAR antagonist bicuculline and GABABRs antagonist CGP 52432. We added bicuculline and CGP 52432 to TPMPA to verify that the recorded impact of TPMPA applications were a GABACR related effect. The IPSCs were completely blocked (100 ± 0%) in all 14 pyramidal cells, leading to increased EPSC amplitudes (350 ± 75%, p < 0.05; Figure 1
C), so all inhibition was extinguished. Blocking GABAARs and GABABRs had the opposite effect of blocking GABACRs.
Next we verified that TPMPA application blocks the effect of muscimol. We applied just 1 μM muscimol to the bath for 15 min, and after this we added TPMPA (n = 10). One micromolar muscimol led to a decrease of IPSCs by 86 ± 11.5% (p < 0.05) and an increase in EPSCs of 89 ± 11.9% (p < 0.05) in all cells. After the addition if TPMPA the effect of muscimol was completely blocked and IPSC amplitudes were increased (71 ± 10.6%, p < 0.05), and EPSC amplitudes were reduced by 84 ± 23.9% (p < 0.05).
In summary, our physiological studies show that blockade of GABACRs with TPMPA increases inhibition and their activation with 1 μM muscimol leads to the opposite. If the GABACRs were located on the postsynaptic side, we would have expected for muscimol to increase inhibition and TPMPA enlarge excitation. The fact that muscimol lead to a decrease in inhibition and TPMPA to an increase in excitation. This suggests the possibility that the GABACRs are located on the presynaptic side. We next tested this hypothesis directly.
Paired Pulse Depression and Facilitation was Affected by Muscimol and TPMPA
Paired pulse depression (PPD) and facilitation (PPF) are tests of presynaptic effects. To examine whether GABACRs act on presynaptic sites, we used PPD and PPF of IPSCs and EPSCs by using an interstimulus interval of 100 ms. PPD of IPSCs is associated with decreased GABA release and a PPD of EPSCs with increased GABA release. In all 12 neurons recorded, TPMPA increased the PPD of EPSCs by 35.6 ± 7.3% (p < 0.05). Muscimol significantly increased the PPD ratio and of IPSCs by 42.5 ± 5.2% (p < 0.05) and increased the PPF ratio of EPSCs by 39.2 ± 3.9% (p < 0.05) (Figure 2
). These data support our hypothesis that GABACRs could be located on the presynaptic sites of GABAergic interneurons in the LA. Therefore, the function of GABACRs seems to be opposite from GABAARs and GABABRs in vitro. Based on these results we next asked the question, if this function of GABACRs would affect learning and memory abilities in the living animal.
Figure 2. Paired pulse depression was affected by muscimol and TPMPA Testing for paired pulse depression (PPD) in 12 Pyramidal cells by using an interstimulus interval of 100 ms. TPMPA increased the PPD of EPSCs by 35.6 ± 7.3% (p < 0.05). Muscimol significantly increased the PPD ratio and of IPSCs by 42.5 ± 5.2% (p < 0.05) and increased the PPF ratio of EPSCs by 39.2 ± 3.9% (p < 0.05).
Behavioral Effects of GABACR Activation
We tested whether GABACRs participate in fear learning and memory using auditory Pavlovian fear conditioning. Because our electrophysiological results showed that direct activation of GABACRs reduced evoked inhibition and enhanced excitation in LA pyramidal neurons, we hypothesized that stimulation of GABACRs in the LA prior to fear conditioning should enhance the acquisition of fear memory formation. To test this hypothesis, rats were chronically implanted with bilateral cannulae in the LA and muscimol or vehicle (ACSF) were injected in the LA in separate groups of animals prior to fear conditioning. Based on a previous study (Wilensky et al., 1999
), where 4.4 nm muscimol was used to activate GABAARs, we used a very weak concentration (0.03 nM) of muscimol 1 h before FC (Figure 3
). As noted GABACRs are at least 10-fold more sensitive to muscimol than GABAARs (Bormann, 2000
), and our concentration was more than 100 times less than that used in previous studies to block fear learning. We tested STM 3 h after conditioning and LTM 24 h later. We analyzed pre-tone freezing, the average was 5 ± 0.3%, and there was no significant difference between groups (p > 0.05).
Figure 3. Design of in vivo behavior experiments. Outline of general behavioral procedures and timing of pre-training injections relative to training.
Figure 4
A shows the mean ± SE percent freezing during the test tone presentations for rats injected before conditioning with 0.03 nM muscimol (n = 6) and saline (n = 7). The results show a significant difference between the saline and muscimol groups for FC (p < 0.05), STM (p < 0.05), and LTM (p < 0.05) (Figures 4
A–C). Administration of 0.03 nM muscimol enhanced fear acquisition and consolidation.
Figure 4. Intracranial injections of 0.03 nM muscimol into the LA enhanced fear learning and memory. (A) Image shows the mean ± SE percent freezing during the test tone presentations for rats injected before conditioning with 0.03 nM muscimol (n = 6) and saline (n = 7). (B–D) Diagrams show a significant difference between the control (vehicle injection) and muscimol groups for FC, fear conditioning; STM, short-term memory test; and LTM, long-term memory test (p < 0.05).
Behavioral Effects of GABACR Blockade
Because the electrophysiological data indicate that blocking GABACRs with TPMPA enhances evoked inhibition, we hypothesized that blocking GABACRs with TPMPA would impair fear learning and memory in rats. As before, bilateral cannulae injections of 30 nM TPMPA (n = 7) into the LA were performed 1 h before FC whereas the control group received intra-LA administration of ACSF (n = 6) and both STM and LTM were assessed. In Figure 5
A we show mean ± SE percent freezing levels for TPMPA and control treated animals, demonstrating that intra-LA microinjections of TPMPA significantly impaired fear learning and memory at STM (p < 0.05) and LTM (p < 0.05) time points (Figures 5
B–D).
Figure 5. Intracranial injections of 30 nM TPMPA into the LA impaired fear learning and memory. (A) Whole experiment with mean ± SE percent freezing levels for injections of 30 nM TPMPA (n = 7) and control (n = 6). (B–D) Diagrams show TPMPA impaired fear learning for FC, fear conditioning; STM, short-term memory test; and LTM, long-term memory test (p < 0.05).
Histology
Histological reconstruction of cannulae placements revealed that all injector tips were located in the LA in 26 out of 47 animals and were thus included in the analysis.
Our results indicate that GABACRs are involved in intra-LA neuronal communication by modulating a considerable fraction of postsynaptic currents. As justified below, we interpret this to suggest that GABACRs could be located on the presynaptic side on the axons of the interneurons and act as autoinhibitors to reduce synaptic GABA release. Infusion of GABACR agonists and antagonists in LA in conjunction with auditory Pavlovian fear conditioning showed that GABAARs and GABACRs play opposing roles in fear acquisition and consolidation: GABAARs impair and GABACRs enhance fear learning and memory. Our results demonstrate a novel role of GABACRs, which advances our understanding of the function of GABACRs in the brain, our knowledge of the circuitry of the LA, and the mechanisms by which fear memories are formed and stored.
In Vitro Patch Clamp Recordings
Previous studies noted the existence of GABACRs in the lateral part of the CE (Delaney and Sah, 1999
). If GABACRs are also present in the LA, their activation would be expected to influence the amplitudes of postsynaptic currents that are evoked by electric stimulation. To test this we used coronal slices that included the LA since inhibitory responses of neurons are known to be stronger than in horizontal slices (Samson et al., 2003
). Pyramidal cell afferents from the cortex and thalamus were stimulated electrically. This simultaneously elicits monosynaptic EPSCs, through direct activation of excitatory thalamic and cortical afferents onto LA pyramidal cells, and heterosynaptic through a direct stimulation, and IPSCs, through indirect afferent activation of GABAergic interneurons. An increase of the excitatory amplitudes of the postsynaptic currents in the presence of muscimol at low concentrations (see above) was regarded as an indication of the expression of GABACRs by the presynaptic interneurons to the pyramidal cells. In addition, these results demonstrate that GABACRs activation reduces feed forward or feedback inhibition and enhances excitatory transmission. Such an effect of muscimol was observed in all of the recorded pyramidal cells.
To answer the question of whether endogenous activation of GABACRs contributes to information processing within the LA, the specific GABACR blocker TPMPA (Ragozzino et al., 1996
; Bormann, 2000
) was applied and the effects on evoked postsynaptic responses of pyramidal cells were investigated. If the GABACRs are located on the presynaptic interneurons their blockade should increase inhibition of the pyramidal cells due to higher GABA release. This effect was registered in all 14 pyramidal cells we recorded from.
Next we examined PPD and PPF of IPSCs and EPSCs in the presence of TPMPA, and separately muscimol (Figure 2
). TPMPA blocked the GABACRs on the presynaptic side so that during the second pulse GABA was still being released. The second pulse showed a stronger reduction of excitation, which was recorded as smaller EPSCs (PPD). On the other hand, muscimol application activated the presynaptic GABACRs. During the second pulse the IPSC amplitudes were decreased (PPD) and EPSCs increased (PPF). This was a result of suppressed GABA release.
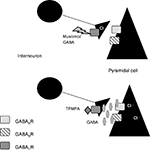
These results lead us to the conclusion that GABACRs could be located on the presynaptic side. We propose in our model that GABACRs would act as autoinhibitors on interneurons (Figure 6
). A similar function for GABACRs was described in the retina, where GABACRs are expressed predominantly at the bipolar cell terminals wherefrom they mediate feedback inhibition from amacrine cells (Lukasiewicz and Werblin, 1994
; Vaquero and de la Villa, 1999
; Euler and Masland, 2000
; Shields et al., 2000
). In general inhibition mediated by GABACRs is slower compared to the kinetics of GABAARs, which induce much faster responses to GABA, suppress glutamate release more rapidly and transiently (Pan and Lipton, 1995
). The presence of the three receptor types on the synapse leads to a much larger dynamic range in the overall response to GABA than each subtype alone.
Figure 6. Model for our hypothesis: GABACRs are located on the presynaptic side. Based on our results with GABACRs ago- and antagonists we assume that the receptors are located on the presynaptic side and act as autoinhibitors on the interneurons in the LA.
In our model muscimol or GABA would activate the GABACRs on the axons of the interneurons, which would lead to less or no GABA release that would inhibit the Pyramidal cells. Application of TPMPA would block the presynaptic GABACRs, the axon would release GABA which activates the GABAARs and GABABRs that inhibit the Pyramidal cells.
In Vivo Auditory Pavlovian Fear Conditioning
LA is a key site of plasticity underlying fear learning (LeDoux, 2000
; Blair et al., 2001
; Maren and Quirk, 2004
). It is known that inhibitory circuits in LA and related amygdala areas play an important role in fear memory and its extinction (Wilensky et al., 1999
; Akirav and Richter-Levin, 2006
; Zhang and Cranney, 2008
). Most studies have focused on GABAARs, and to a lesser degree and GABABRs. GABAARs have a very large chloride channel (Farrant and Nusser, 2005
), and drugs targeting this receptor produce strong cellular hyper polarization and have a profound impact on processing in neural circuits.
Our physiological results in coronal slices suggest that GABACRs could be present in the LA on the presynaptic terminals of inhibitory inputs onto pyramidal cells, and act as autoinhibitors on the interneurons. If our interpretation is accurate, their activation should also influence fear learning and memory.
To test this we examined the effects of intra-LA infusion of a GABACR agonist and antagonist on auditory Pavlovian fear conditioning. Previously 4.4 nM muscimol was used as a GABAAR agonist (Wilensky et al., 1999
). It is known that GABACR are at least 10-fold more sensitive to GABA and muscimol than GABAARs (Bormann, 2000
). We used 0.03 nM muscimol as a GABACR agonist, and found a significant difference to the control (Figure 4
) for FC, STM, and LTM. We found that activation of GABACRs with low concentrations of muscimol enhanced fear learning and memory and that blocking GABACRs with TPMPA produced the opposite effect (Figure 5
). These results showed two things: (1) GABACRs modulates fear acquisition and consolidation; and (2) the role of GABACRs is opposite from GABAARs: their activation enhances fear learning and memory.
Implications for Anxiety Disorders
GABAergic agonists (e.g., benzodiazepines), are commonly used drugs to treat anxiety-related disorders, especially by targeting GABAARs. Yet, beyond their potent anxiolytic properties, these drugs also lead to side effects that include sedation, motor and memory impairments. These side effects are, in part, due to the fact that GABAARs are ubiquitously distributed throughout the mammalian brain. Another problem is that these drugs often lead to dependence. A third problem is that GABAARs desensitize, possibly explaining why patients with generalized anxiety disorder or panic disorder show lower benzodiazepine binding in some forebrain areas (Kaschka et al., 1995
; Tiihonen et al., 1997
; Malizia et al., 1998
). Due to the non-specific effects of benzodiazepines, their potential for dependence, and their tendency to desensitize, GABAARs are not optimal as a target for long-term treatment of patients with anxiety disorders. As a result, there has been a continued search for new, more specific, anxiolytic agents, either by indirect modulation of GABAARs via targeting norepinephrine (NE), serotonin, and dopamine, or by research aimed at altering specific GABAARs subunits. Given that amygdala processing is altered in anxiety disorders (LeDoux, 2007
; Monk, 2008
), our results suggest that GABACRs in the amygdala might be useful alternative target for the development of anti-anxiety drugs.
The present results expand our understanding of the role of GABA receptors in fear learning, and suggest possible ways to improve the treatment of anxiety-related disorders. However further studies are needed to fully understand the role of GABACRs in the amygdala, where neuromodulators like NE play crucial roles in synaptic plasticity and learning and memory (Cahill et al., 1994
; McGaugh, 2000
; Debiec and Ledoux, 2004
). The GABAARs antagonist, picrotoxin, and high dosages of muscimol, which target GABAARs, are known to modulate the NE levels in the amygdala (Hatfield et al., 1999
). It would be very important to know if and how GABACRs agonists and antagonists influence NE and other neuromodulator concentrations in the LA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work has been supported by the Portuguese Fundacao para a Ciencia e Tecnologia and the GABBA PhD program in Porto, Portugal, as well as postdoctoral fellowship from AHFMR, NSERC, and CIHR to Marie-H. Monfils, and by the grants P50 MH58911, R01 MH046516 to Joseph E. LeDoux.
Chebib, M., Hinton, T., Schmid, K. L., Brinkworth, D., Qian, H., Matos, S., Kim, H. L., Abdel-Halim, H., Kumar, R. J., Johnston, G. A., and Hanrahan, J. R. (2009). Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J. Pharmacol. Exp. Ther. 328, 448–457.
Tiihonen, J., Kuikka, J., Rasanen, P., Lepola, U., Koponen, H., Liuska, A., Lehmusvaara, A., Vainio, P., Kononen, M., Bergstrom, K., Yu, M., Kinnunen, I., Akerman, K., and Karhu, J. (1997). Cerebral benzodiazepine receptor binding and distribution in generalized anxiety disorder: a fractal analysis. Mol. Psychiatry 2, 463–471.