1
Department of Biology, University of Central Arkansas, Conway, AR, USA
2
Department of Neurobiology and Developmental Sciences, University of Arkansas for Medical Sciences, Little Rock, AR, USA
3
Department of Biostatistics, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Cortical GABAergic (γ-aminobutyric acidergic) neurons include a recently identified subset whose projections extend over relatively long distances in adult rodents and primates. A number of these inhibitory projection neurons are located in and above the conventionally identified white matter, suggesting their persistence from, or a correspondence with, the developmental subplate. GABAergic and subplate neurons share some unique properties unlike those of the more prevalent pyramidal neurons. To better understand the GABAergic and subplate populations, we constructed a database of neural developmental events common to the three species most frequently used in experimental studies: rat, mouse, and macaque, using data from the online database www.translatingtime.net
as well as GABAergic and subplate developmental data from the empirical literature. We used a general linear model to test for similarities and differences, a valid approach because the sequence of most neurodevelopmental events is remarkably conserved across mammalian species. Similarities between the two rodent populations are striking, permitting us to identify developmental dates for GABAergic and subplate neural events in rats that were previously identified only in mice, as well as the timing in mouse development for events previously identified in rats. Primate comparative data are also compelling, although slight variability in statistical error measurement indicates differences in primate GABAergic and subplate events when compared to rodents. Although human extrapolations are challenging because fewer empirical data points are available, and because human data display more variability, we also produce estimates of dates for GABAergic and subplate neural events that have not yet been, or cannot be, determined empirically in humans.
Cortical GABAergic (γ-aminobutyric acidergic) neurons can be parceled into a number of subgroups based on variations in morphology, birthplaces, mature locations, colocalized peptides, and electrophysiological parameters (Hendry and Jones, 1991
; Ascoli et al., 2008
; Burkhalter, 2008
). Despite such diversity, conventional models of cortical function include GABAergic neurons as participators only in local connectivity, such that the designation of “interneuron” is often used interchangeably with GABAergic to describe cortical neurons that play an inhibitory role. However, cortical GABAergic categories recently were extended to include a subset of phylogenetically conserved neurons that project axons across long distances – the newly identified long-range interneurons, perhaps more precisely called cortical GABAergic projection neurons (McDonald and Burkhalter, 1993
; Gonchar et al., 1995
; Fabri and Manzoni, 1996
, 2004
; Tomioka et al., 2005
; Pinto et al., 2006
; Higo et al., 2007
; Tomioka and Rockland, 2007
).
One intriguing aspect of the latest subgroup is that the majority of the long-range GABAergic projections extend from neurons located in cortical layer I, cortical white matter, and the subgriseal region of the cortex (subjacent to cortical layer VI) (Tomioka et al., 2005
; Tomioka and Rockland, 2007
). This prompted the suggestion that the GABAergic projection neurons might be a subset of the little-studied cells that persist in the adult brain from the developmental subplate (Tomioka et al., 2005
; Tomioka and Rockland, 2007
).
Early in development, the cells of the future layer I, as well as the future subplate/white matter neurons, are merged as the preplate before neurons of the developing cortical plate split the preplate into a superficial region (later called layer 1) and a subgriseal region (later called the subplate) (Marin-Padilla, 1978
, 1988
). The exact percentage of subplate cells that survive into adulthood is somewhat difficult to identify (Chun and Shatz, 1989a
; Valverde et al., 1995
; Price et al., 1997
; Robertson et al., 2000
), but it is well-documented that some survive an early wave of cell death to remain in the mature white matter in human and non-human primate cortex (Kostovic and Rakic, 1980
; Somogyi et al., 1981
; Schiffmann et al., 1988
; Yan et al., 1996
) and in carnivores (Chun and Shatz, 1989b
). Moreover, they survive both in the white matter and as a well-defined structure subjacent to the cortex in rodents, species used for the majority of neural studies (Somogyi et al., 1984
; Lauder et al., 1986
; Huntley et al., 1988
; Luskin et al., 1988
; Reep and Goodwin, 1988
; Winer and Larue, 1989
; Cobas et al., 1991
; Woo et al., 1991
; Woo and Finlay, 1996
; Reep, 2000
).
As depicted in Figure 1
, even in rodents the persisting subplate cells that remain in a distinct layer are essentially positioned in the white matter. They sit above the conventionally-identified white matter, but below a stria of intracortical connections (Reep and Goodwin, 1988
; Clancy and Cauller, 1999
; Reep, 2000
; Teague-Ross et al., 2008
), making them more comparable to the persisting white matter (interstitial) population in primates and carnivores than is often acknowledged. GABAergic neurons account for 15–25% of all the neurons in the region depicted as the persisting subplate, similar to their percentage in the cortex overall (Hendry et al., 1983
, 1987
; Chun et al., 1987
; Esclapez et al., 1987
; Meinecke and Peters, 1987
; Peduzzi, 1988
; Del Rio et al., 2000
; Tomioka et al., 2005
; Burkhalter, 2008
). The subplate may include a GABAergic population that survives from cells located at the intermediate zone/subventricular zone border early in development (Del Rio et al., 2000
), as well as some lower intermediate zone GABAergic cells that may later merge with subplate cells (Van Eden et al., 1989
).
Figure 1. (A) Schematic of a dorsal view of an adult rat brain produced from serial sections using Neurolucida (Version 8; MicroBrightField, Williston, VT, USA). Persisting subplate cells (blue) lie above the white matter (solid white). The brain outline is depicted in shadow. (B) The same brain with the infracortical stria, a fiber tract above the persisting subplate cells, shown in transparent white. The white, dashed line represents the continuity, but decreasing thickness, of the infracortical stria in posterior brain regions. (C) Overlapping coronal serial sections depicting the location of the persisting subplate cells (blue) with the conventional white matter outlined (white). Scale bar = approximately 1.0 mm. Abbreviations: ant (anterior); post (posterior).
The location of the long distance GABAergic neurons, and the possibility that some may be surviving subplate neurons, is interesting because GABAergic and subplate neurons (both developmental and those that persist across maturity, including neurons in the white matter) have a notable relationship, and share some comparable characteristics. Similar to the sometimes confusing GABAergic nomenclature recently addressed by the Pettilla committee (Ascoli et al., 2008
), surviving subplate cells have been assigned a variety of different names in mature cortex, including border neurons (Hogan and Berman, 1992
), white matter neurons (Kostovic and Rakic, 1980
), subgriseal neurons (Clancy and Cauller, 1999
), layer VII (Clancy and Cauller, 1999
; Reep, 2000
), layer VIb (Gomez-Pinilla and Cotman, 1992
), deep layer VI (McDonald and Burkhalter, 1993
), upper subplate neurons (Marin-Padilla and Marin-Padilla, 1982
), and the deep cortical band (Kristt, 1979
). In this study we will use the term “persisting subplate neurons” (Reep, 2000
) for those resilient cells that remain from the developmental subplate in and above the white matter across maturation, where they continue to participate in cortical function (Clancy et al., 1997
, 2001b
; Bayer et al., 2004
; Torres-Reveron and Friedlander, 2007
; Pinon et al., 2009
), and apparently include the GABAergic subset that sends projections for long distances (Tomioka et al., 2005
; Tomioka and Rockland, 2007
).
Subplate neurons can be activated by GABA, including intrinsic GABAergic activation from other subplate neurons (Princivalle et al., 2000
; Hanganu et al., 2001
). The subplate is equally important in GABAergic function as it is required for a developmental change that switches GABAergic input from producing a depolarizing response to its more familiar hyperpolarizing role (Lauder et al., 1986
; Kanold and Shatz, 2006
).
In fact, it is difficult to comprehensively characterize one population without including the other. Both GABAergic and subplate populations have been implicated in similar disorders associated with development, including epilepsy and schizophrenia (Akbarian et al., 1995
, 1996
; Kirkpatrick et al., 1999
; Lein et al., 1999
; Kanold, 2004
; Woo et al., 2004
; Levitt, 2005
; Lewis et al., 2005
; Eastwood and Harrison, 2006
; Freund and Katona, 2007
; Leviton and Gressens, 2007
; Lazar et al., 2008
; Metin et al., 2008
). Both populations may be particularly vulnerable to developmental insults, including those associated with premature birth (Nie and Wong-Riley, 1996
; Ulfig, 2002
; McQuillen and Ferriero, 2005
), and in the white matter damage that often follows intrauterine infection (Dammann et al., 2002
; Jensen et al., 2003
; Kostovic and Judas, 2006
; Robinson et al., 2006
; Leviton and Gressens, 2007
). Both populations play roles that change across development and maturity (Owens and Kriegstein, 2002
; Ben-Ari et al., 2004
; Kanold and Shatz, 2006
; Friedlander, 2008
), and it has been proposed that both populations may establish mechanisms during early development that lie dormant until triggered at later ages (Kanold et al., 2003
; Butt et al., 2007
).
Both GABAergic and subplate populations include numerous and diverse morphological subsets that are different from the more prevalent cortical pyramidal neurons (although each population may include cells with pyramidal morphology), and both populations contain a subset whose projections may travel long distances, sometimes crossing areal boundaries, as well as a subset that focuses projections on cortical layer I (Cauller et al., 1998
; Clancy and Cauller, 1999
; Tomioka et al., 2005
; Silberberg and Markram, 2007
; Tomioka and Rockland, 2007
). Both populations are similarly heterogeneous in their electrophysiological properties (Friauf et al., 1990
; Luhmann et al., 2000
; Hanganu et al., 2001
; Voigt et al., 2001
; Miyoshi et al., 2004
, 2007
; Torres-Reveron and Friedlander, 2007
), and in the numerous signaling chemicals they sequester (Chun and Shatz, 1989a
,b
; Bredt and Snyder, 1992
; Gao et al., 1999
; Tao et al., 1999
; Robertson et al., 2000
; Clancy et al., 2001b
; Heuer et al., 2003
; Bayer et al., 2004
; Garbossa et al., 2004
; Tomioka et al., 2005
; Tomioka and Rockland, 2007
; Burkhalter, 2008
). Moreover, subsets of both populations may share a common non-cortical birthplace in the ganglionic eminences (Tamamaki et al., 1997
; Lavdas et al., 1999
; Anderson et al., 2001
; McQuillen and Ferriero, 2005
), raising the possibility that some may descend from similar sets of precursors. Supporting this notion, both populations use somewhat similar molecular modes of migration, different from the mechanism used by pyramidal cells (Gilmore and Herrup, 2001
).
Although the contribution of the GABAergic interneurons to cortical function is undisputed, and the critical role of the subplate in cortical development is well-accepted (McConnell et al., 1989
; Ghosh et al., 1990
; De Carlos and O’Leary, 1992
), conventional models of mature cognitive function do not yet incorporate contributions of either the projection GABAergic or the persisting subplate neurons. When numbers are reduced compared to other neural populations, there may be a tendency to simply dismiss those that persist as “sparse,” “remnants,” or “relics”. Unfortunately, such terminology implies a fairly unessential function, and it seems important to avoid such categorization until additional information on their function is available. Persisting subplate cells in adult mammalian species have thus far eluded characterization as an easily observable and/or organized structure. Several studies, however, have suggested revising the whole idea of a “remnant” population, and provided evidence for the organization and participation of the persisting subplate cells in mature cortical function (Clancy et al., 2001b
; Colombo and Bentham, 2006
; Torres-Reveron and Friedlander, 2007
; Chang et al., 2008
; Friedlander, 2008
; Friedlander and Torres-Reveron, 2009
; Suarez-Sola et al., 2009
).
With the recent indication that the long distance projection GABAergic neurons are associated with the persisting subplate population, including neurons that remain in isolated positions in both cortex and white matter, a role for the persisting subplate population is strengthened. Indeed, the mathematical principles underlying small-world networks suggest that sparse connectivity is a plausible design underlying important cognitive function. Long-range inhibition, even from relatively sparse connections, can be a potent network component (Sur and Rubenstein, 2005
). In small-world networks (Watts and Strogatz, 1998
) (inspired by the same mathematics behind “Six Degrees of Kevin Bacon”), clusters of cells link to their nearest neighbors, while some connect to distant clusters. This pattern can serve as the basis for a surprisingly strong communication network, especially when it is amplified by local input, as is likely the case for both the long-range GABAergic and the persisting subplate populations.
Most characteristics of GABAergic and subplate cells are conserved across species (Levitt, 2005
; Wang and Kriegstein, 2009
), and even some GABAergic features previously considered to be exclusive to primates, or wholly exclusive to humans, were later identified in other species (Meyer et al., 1998
; Yuste, 2005
; Petanjek et al., 2009
). However, species differences have been reported in both populations, including in birthplace, migration, and final locations in mature cortices, suggesting that both populations may drive and/or be driven by evolutionary processes (Peduzzi, 1988
; Letinic et al., 2002
; Rakic, 2003
, 2006
; Watakabe et al., 2006
; Petanjek et al., 2008
; Suarez-Sola et al., 2009
). In addition to the evolutionary aspects, the question of species differences has pragmatic impact because rodent models are necessary in studies for both normal (Rakic, 2006
) and abnormal neural development (Goffinet and Rakic, 2000
; Levitt, 2005
; Robinson et al., 2005
), and non-human primate and rodent studies are used routinely to fill in gaps in knowledge of human development e.g. (de Graaf-Peters and Hadders-Algra, 2006
).
In a series of previous studies, mathematical models have been used to successfully identify both similarities and relative differences in the timing of neural “events” when comparing primate and non-primate development (http://www.translatingtime.net
) (Clancy et al., 2001a
, 2007a
,b
; Nagarajan and Clancy, 2008
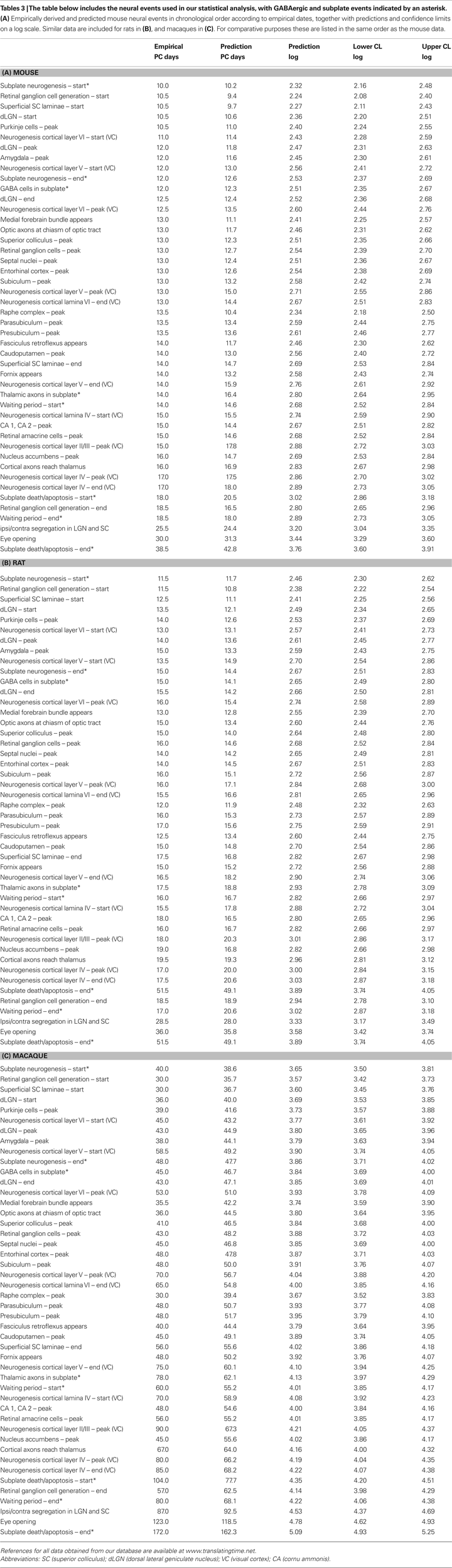
). For the purpose of this review, “neural events” are defined as milestones pertaining to neural development such as the post conception (PC) date that neurons destined for the various cortical layers are generated. (Complete lists are included in Table 3
at the end of this review.) Mathematical approaches are valid because despite species differences, including differences in the duration of development, the size of most brain regions scales similarly across species (Finlay and Darlington, 1995
; Finlay et al., 1998
, 2001
). Central to this meta analysis, the timing of events that occur in most neural regions is remarkably conserved (Finlay and Darlington, 1995
; Finlay et al., 1998
, 2001
).
Moreover, even neural regions that do display species-related duration differences can be modeled with appropriate mathematical adjustments (Clancy et al., 2000
, 2001a
). As a practical illustration, in a careful study of neurogenesis of the primate cortical plate (Smart et al., 2002
), the authors urged caution when comparing histogenesis results between monkey and mouse, pointing out what seemed to be a disparity in that mouse PC days 11–12 compare to monkey PC days 46–65. In fact, our statistical modeling approach indicates the two time periods are remarkably comparable, predicting PC 11–12 in mice to correspond to PC 44–50 in macaques (these and other comparisons can be accessed at http://www.translatingtime.net
).
The most pragmatic application of statistical modeling is that neural events empirically derived in one species can be compared and successfully applied to another. Therefore, given the potentially important contributions of the long distance GABAergic and persisting subplate populations, we reasoned any additional information about these two populations, including comparative cross-species data, is likely to be useful. At this time, no empirical developmental data are yet available specifically for the cortical GABAergic projection neurons. However developmental data are available for both general populations that include them, the GABAergic and subplate populations. We assembled a database of GABAergic and subplate developmental events (e.g. the PC day subplate neurogenesis begins, the day GABAergic cells are first found in the subplate). We then applied cross species statistical modeling, and tested if species similarities and differences might be indicated by statistical analysis of the developmental sequences.
For this review we generated three sets of results: (i) We compare and translate GABAergic and subplate developmental neural events between rats and mice, since a majority of the events have been documented in the literature in these two species. (ii) In order to understand the impact of translation from rodents to primates, we present the GABAergic and subplate predictions by pooling the events from mice, rats, and macaques, as sufficient data are available in macaques to allow such conversions. More importantly for this review, we discuss the prediction of common events across these three species with and without subplate and GABAergic events. (iii) Finally, we discuss the results obtained by pooling the data for mice, rats, macaques and humans. As would be expected, the number of empirically derived events in humans is considerably lower as compared to other three species. However, the results we present indicate that it might be possible to arrive at meaningful approximations to unknown human events by “translating” empirically derived events across other species.
Our database was gleaned from the published literature, including the timing of 20 GABAergic and subplate neural events established in two or more mammalian species. We then incorporated these dates into our previously established database of 101 neural events freely available at http://www.translatingtime.net
: (i) For translation of GABAergic and subplate developmental neural events between rats and mice, we considered a total of 135 events, comprised of dates from the empirical literature for mice, rats, and both mice and rats. (ii) For translation from rodents to primates, we considered the common events for mice, rats, and macaques, with GABAergic and subplate events (46 events) and without (38 events). We restricted the analysis to the common events in order to facilitate comparison of the regression results across these three species. (iii) Finally, we discuss the results obtained by pooling data on mice, rats, macaques and humans. This portion of the analysis consisted of events empirically derived in at least one of the species (119 events total). Tables that list the specific neural events, including empirically derived dates as well as the predictions generated by our analyses, are included at the end of this review (Table 3
). In our analyses, data are standardized whenever possible such that the 24-h period following conception is designated PC 1, and the 24-h period immediately following birth is considered postnatal (PN) day 0. The “start” date is the day on which 5% of the neurons of a given structure are generated, and “end” is assigned similarly. If no clear “peak” is evident in the empirical neurogenesis data, a midpoint is used.
Because the timing of most developmental events in mammalian brains follows a similar pattern across species (Finlay and Darlington, 1995
), standard regression techniques can be used to compare cross-species neural development (Clancy et al., 2001a
, 2007a
,b
). The present study uses a general linear model to translate the events across the species. The response values (i.e. empirically derived event timings) were log-transformed and each of the predictors representing the species and the events were represented by dummy variables (Darlington, 1990
). The model parameters were subsequently estimated using least squares regression. Confidence limits were determined in the log-scale for each of the estimated event timings (Statistical software “R” 2.8.1).
Although we found empirical data for many developmental GABAergic and subplate data points in both rats and mice, there were a numbers of events for which we found empirical data points in only one of these important experimental species. Predictions for GABAergic and subplate events in the rodent species using the general linear model from the documented events are depicted in Table 1
. The predicted values along with their confidence intervals are shown in the log-scale. The predicted values are also transformed back in the original scale (PC days) for clarity. Because so many developmental data points are available in these two species, cross-rodent conversions between these closely related species are especially compelling (Nagarajan and Clancy, 2008
).
Comparisons of Common Events in Rats, Mice, and Macaques with and without Subplate and GABAergic Events
There are reported species differences, most notably in rodent/primate comparisons, in the relative timing (heterochrony) and location of neurogenesis for both the GABAergic and subplate cell populations (Letinic et al., 2002
; Smart et al., 2002
; Rakic, 2003
, 2006
). Because a mathematical modeling approach has previously permitted identification of two neural systems whose temporal milestones “shift” in primates – the limbic system (shifted to occur earlier in primates) and cortical neurogenesis events (shifted to occur later when compared to non-primates) (Clancy et al., 2000
), we anticipated our model would be able to establish if similar discrepancies occur in GABAergic and subplate neural development events when comparing the timing of rodent/primate events. In order to facilitate a direct comparison between rodents and macaques, we selected the common events across these species including the GABA/subplate events (46 events) and excluding them (38 events).
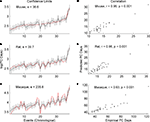
As depicted in the graphs in Figure 2
, the sums of absolute error (e) between the empirical values and those predicted by the general linear model are similar for mice and rats (mice e = 36.6; rats e = 39.7), but much higher for macaques (e = 235.8). However, the correlation coefficient between the empirical and predicted values is significant for all three species (mice r = 0.95; rats r = 0.96; macaques r = 0.93; p < 0.001) indicating that a general linear model is a useful tool in cross species translations. Yet the results of the sum of absolute error do indicate that translation of the events from rodent to primates can be more challenging. Some of the discrepancy in the sum of squared error may be attributed to additional variability in the macaque events as opposed to those of rats and mice.
Figure 2. The predicted (black) and empirical values (red) of the post-conceptional days in the log-scale, i. e. log(PC days), of the 38 common events excluding GABAergic and subplate events across mice, rats and macaques are shown in (A), (B) and (C) respectively. The rat and macaque events are in the same chronological order as that of mouse events. The confidence limits (log-scale) about the predicted values and the sum of absolute error (e) between the empirical and the predicted values are also shown in (A), (B) and (C). The correlation coefficient (r) between the predicted and the empirical values for mice, rats and macaques are shown in (D), (E) and (F) respectively. The names of the events are included in Table 2
at the end of this review.
We then ran the same analysis including GABAergic and subplate events because were especially interested if the development of these two populations might exhibit any differences when compared to the other neural events, based on the reports that these two populations might have particular species-specific differences (Kostovic and Rakic, 1990
; Smart et al., 2002
).
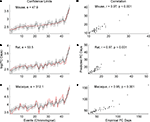
In each of the three species, the correlation coefficient values (r) do not change significantly with the inclusion of GABAergic and subplate events (Figure 3
) when compared to the values generated without including these events (Figure 2
). The r for mice increases from 0.95 to 0.97; for rats it increases from 0.96 to 0.97; for macaques it increases from 0.93 to 0.95 (p = 0.001 for all). When GABAergic and subplate events are considered, once again there is considerable similarity in the sum of absolute error between the empirical and predicted values in the rodents; mice (e = 47.9; p = 0.001) and rats (e = 53.5; p = 001), and again error for macaques is higher (e = 312.1; p = 001). However, the magnitude of the error (e) was higher across all three species when the data includes GABAergic and subplate events (Figure 3
) as opposed to when they are excluded (Figure 2
). For mice it increases from 36.6 to 47.9; for rats from 39.7 to 53.5; and for macaques it increases from 235.8 to 312.1 (p = 0.001 for all). This would appear to indicate something is indeed different in these GABAergic and subplate events when comparing rodents to primates, and it is more distinctive in primates.
Figure 3. The predicted (black) and empirical values (red) of the post-conceptional days in the log-scale, i. e. log(PC days), of the 46 common events including GABAergic and subplate events across mice, rats and macaques are shown in (A), (B) and (C) respectively. The rat and macaque events are in the same chronological order as the mouse events. The confidence limits (log-scale) for the predicted values and the sum of absolute error (e) between the empirical and the predicted values are also shown in (A), (B) and (C). The correlation coefficient (r) between the predicted and the empirical values for mice, rats and macaques are shown in (D), (E) and (F) respectively. The specific events are included in Table 3
at the end of this review.
These analyses are necessarily preliminary as they are based on a somewhat limited dataset, but they do support the neuroanatomical data noting slight species differences in the GABAergic and subplate populations (Peduzzi, 1988
; Letinic et al., 2002
; Rakic, 2003
). Clearly a more detailed study with increased numbers of empirical events as they become available will be necessary in order to gain sufficient insight into the impact of GABAergic and subplate events on evolutionary modifications between rodent and primate species.
The difficulty of establishing precise empirical dates for neural events in human development is extremely challenging, particularly due to individual variation and observational error. The limitations are unavoidable: samples sizes are necessarily small, dates of conception are estimated, sampling intervals are essentially opportune, the most convincing techniques to establish dates of neurogenesis require invasive techniques, to name but a few. However, it is possible to arrive at meaningful approximation to unknown human events using statistical techniques to translating empirically derived dates from other mammalian species (Clancy et al., 2000
).
Predictions for human GABAergic and subplate events translated using empirically derived statistical comparisons from our database (119 events) for at least one of the species (rat, mouse and macaque) are included in Table 2
. For the predictions listed in Table 2
, we used the statistical model to predict human events for data points that are not yet known, as well as for some events that are reported in the empirical literature. In most cases where empirical data are available, the model predictions were for dates earlier than those reported in the literature. This may be an indication of variability, but we have reason to propose that our data are accurate based on a principle, well known to statisticians, called the “bootstrap effect” (Cronbach and Meehl, 1955
). Since the estimates generated by any mathematical model are based on all the empirical data used to build the model, errors can be “averaged out” such that the model’s estimates may even be more accurate than empirical data. This seems especially compelling for human data, where techniques are extraordinarily challenging and sampling/observation intervals are often wide, such that an event might not be documented until well after it has occurred.
We have some direct evidence of the value of the bootstrap effect from a previous study (Clancy et al., 2000
) that consistently predicted a much earlier date for human eye opening than any date then found in the empirical literature. When four-dimensional sonograms were perfected, it became clear that the statistical model had been accurate (Clancy et al., 2007a
).
The pragamatic value of the cross species translations in direct savings of time and resources when intervals for studies might be narrowed is a compelling reason to add this type of analysis to the growing array of modern neuroanatomical tools. In some case, it might eliminate the necessity of repeating a study already accomplished in one species (as our data suggest is possible when translating from mice to rats, or rats to mice), or at the least contribute to a narrowing of time intervals (as our data suggest is possible for humans). And although correlations between data available in the empirical literature and data produced by the model were already significant, we expect future predictions will become more accurate as additional data points are added to our database.
On the other hand, we certainly do not suggest that there are no differences in the brain development of diverse mammalian species. Mathematical modeling can successfully adjust for some rodent/primate differences (Clancy et al., 2001a
), such that comparisons and predictions are not overly distorted by differences in brain sizes, or what might seem to be a relatively prolonged time window for neurogenesis in primates when compared to rats. Yet there are questions that analysis of our database does not yet permit us to address such as the possibility of an effect on differences in cell cycle mechanisms (Dehay and Kennedy, 2007
), or the effect of variability in the location of GABAergic proliferation when comparing rodents to humans (Letinic et al., 2002
; Molnar and Cheung, 2006
; Molyneaux et al., 2007
). Such differences may be exemplified by a recent statistical analysis of the pulvinar (Chalfin et al., 2007
), which in humans also has a dual source of neurogenesis (Letinic and Rakic, 2001
). However, it is clear that the timing of neurogenesis alone is an important factor in development, given the evidence that timing might predict such properties as laminar position, electrophysiology responses, and neuronal morphology, including projection patterns (Caviness, 1982
; Rakic, 1988
; Takahashi et al., 1999
; Lai et al., 2008
). As datapoints are added to our database, we hope to test if a neuron’s role is more closely related to the location of its birthplace, or the timing of its birth date.
One additional limitation we should point out arises because early PN days in rodent brain development correspond to in utero timing in humans. The result is that the effects of a perinatal wave of synaptogenesis (Zecevic and Rakic, 1991
), the onslaught of experience surrounding birth, and the mother/offspring interaction are not included, as we have not yet identified data points associated with these events that fit into statistical models.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank James Hyde for assistance with figure production, Amanda James and Kristi Erbach for excellent technical assistance, and Richard Darlington for suggestions on the statistical analyses. This project was supported by NIH Grant Number P20 RR-16460 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources and NSF Grant Number 0849627 to BC, and NSF Grant Number 0849684 to RN.
Ascoli, G. A., Alonso-Nanclares, L., Anderson, S. A., Barrionuevo, G., Benavides-Piccione, R., Burkhalter, A., Buzsaki, G., Cauli, B., Defelipe, J., Fairen, A., Feldmeyer, D., Fishell, G., Fregnac, Y., Freund, T. F., Gardner, D., Gardner, E. P., Goldberg, J. H., Helmstaedter, M., Hestrin, S., Karube, F., Kisvarday, Z. F., Lambolez, B., Lewis, D. A., Marin, O., Markram, H., Munoz, A., Packer, A., Petersen, C. C., Rockland, K. S., Rossier, J., Rudy, B., Somogyi, P., Staiger, J. F., Tamas, G., Thomson, A. M., Toledo-Rodriguez, M., Wang, Y., West, D. C., and Yuste, R. (2008). Petilla terminology: nomenclature of features of, GABAergic interneurons of the cerebral cortex. Nat. Rev. 9, 557–568.
Maric, D., Liu, Q. Y., Maric, I., Chaudry, S., Chang, Y. H., Smith, S. V., Sieghart, W., Fritschy, J. M., and Barker, J. L. (2001). GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl− channels. J. Neurosci. 21, 2343–2360.