- 1Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 2Collaboration Unit for Infection, Joint Research Center for Human Retrovirus Infection, Kumamoto University, Kumamoto, Japan
- 3Division of Infection and Immunity, Joint Research Center for Human Retrovirus Infection, Kumamoto University, Kumamoto, Japan
In sub-Saharan Africa (SSA) the burden of non-nucleoside reverse transcriptase inhibitor (NNRTI) HIV drug resistance (HIVDR) has been high over the years. Therefore, in 2018 the World Health Organization (WHO) recommended a regimen based on a integrase strand transfer inhibitor (INSTI), dolutegravir, as the default first-line antiretroviral therapy (ART) in countries in SSA. The scale-up of DTG-based regimens in SSA has gained significant momentum since 2018 and has continued to expand across multiple countries in recent years. However, whether or not the DTG robustness experienced in the developed world will also be achieved in SSA settings is still an important question. Evidence generated from in vitro and in vivo studies suggests that the emergence of DTG HIVDR is HIV-1 subtype dependent. These findings demonstrate that the extensive HIV-1 diversity in SSA can influence DTG effectiveness and the emergence of drug resistance. In addition, the programmatic approach to the transition to DTG adopted by many countries in the SSA region potentially exposes individuals to DTG functional monotherapy, which is associated with the emergence of DTG resistance. In this mini review, we describe the current trends of the effectiveness of DTG as reflected by viral suppression and DTG resistance. Furthermore, we explore how HIV-1 diversity and the programmatic approach in SSA could shape DTG effectiveness and DTG HIVDR in the region.
1 Introduction
Sub-Saharan Africa (SSA) contains almost two-thirds of the people living with HIV (PLHIV) worldwide (1, 2). Several global efforts have been made to combat HIV infection, such as the scale up of antiretroviral therapy (ART) (3, 4). However, despite these efforts, HIV-1 drug resistance (HIVDR) has had a significant impact on HIV control in SSA, as demonstrated by the prevalence of non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance (5, 6). In 2018 the World Health Organization (WHO) recommended the use of dolutegravir (DTG), an integrase strand transfer inhibitor (INSTI) as the preferred drug in place of NNRTIs as the first-line ART regimen in the management of HIV in resource-limited settings (7). DTG is reported to have high potency, tolerability, and effectiveness in HIV-1 viral suppression; exhibits fewer drug interactions; and possesses a high genetic barrier to resistance (8–10).
Several groups have investigated the potential of the natural variability of the integrase gene in HIV-1 strains circulating in SSA for primary INSTI resistance prior to the introduction of DTG. Most of the studies reported a lack of major INSTI resistance mutations against DTG and a low frequency of accessory INSTI resistance mutations. For example, a study in Kenya reported a number of accessory DTG drug resistance mutations in polymorphic sites at a frequency of 20% among DTG pre-treatment PLHIV, and similar studies found frequencies of 5% in Tanzania and 4.3% in Ethiopia (11–13). Similarly, only accessory INSTI resistance mutations were reported at a low frequency in studies from Mozambique (14) and South Africa (15). However, one multicenter study involving PLHIV in Kenya, Nigeria, Uganda, Zambia, and South Africa reported a 2.4% prevalence of major DTG resistance mutations among INSTI- naive PLHIV (16). In addition, major resistance mutations were also detected in three Cameroonian studies at frequencies of 0.8%, 5.4%, and 1.4% in INSTI- naive populations (17–19). The low frequency of primary INSTI resistance mutations suggests that transitioning to DTG is likely to be effective in SSA.
However, studies emerging from SSA since the rollout of DTG are reporting the rapid selection of DTG resistance mutations at rates that were not observed in the INSTI -naive population previously. A study conducted a year after the DTG rollout in Malawi reported major DTG resistance mutations in 8 out of 27 (30%) samples of PLHIV in Malawi (20). Our group’s recent national survey in Tanzania found a rapid selection of DTG drug resistance mutations within 18 months of transitioning to DTG (21). These emerging data suggest an impending danger to the success of the rollout of DTG in SSA. Evaluation of the pitfalls in the current approach is necessary to prevent the further spread of DTG drug resistance and ensure the long-term effectiveness of HIV treatment in SSA.
2 Treatment outcomes following dolutegravir based-regimens in SSA
Early evidence from SSA countries that have so far transitioned to DTG-based ART regimens indicates significant successes in early- time-point HIV viral suppression compared with NNRTI-based regimens among PLHIV (22–26). This is consistent with the fact that DTG-based ART has a better tolerability profile and can induce a rapid suppression of HIV viral load among PLHIV (8–10). Two large, randomized, landmark clinical trials, the ADVANCE and NAMSAL studies, assessed the effectiveness of DTG-based first-line ART among PLHIV in SSA and reported increased viral suppression in the DTG-based arms compared with the NNRTI-based arms (25, 26). Another study, conducted through the African Cohort Study (AFRICOS) in four countries (Kenya, Uganda, Tanzania, and Nigeria), that assessed the suppression rates following the use of tenofovir-lamivudine-dolutegravir (TLD) reported a viral suppression rate of 94.3% (22). A prospective cohort study in Lesotho (the DO-REAL study) reported that thethe country has achieved the third 95 of the 95-95-95 UNAIDS targets for the HIV cascade of care, with a viral suppression rate of >95% among PLHIV on DTG-based ART regimens (23). In a recent South African retrospective study that aimed to assess the outcomes of DTG-based ART involving two distinct cohorts of PLHIV from 2019 and 2022, it was reported that DTG was associated with improved clinical outcomes in terms of viral suppression (24). In Uganda, both clients initiating treatment and those switching from NNRTI- based to DTG-based regimens showed a high acceptability and a high viral suppression rate (94%) at 6 months following DTG use (27). These findings are promising and further validate the decision to transition to DTG-based ART in SSA.
Nonetheless, early selection of DTG resistance is increasingly being reported in virologic failures among PLHIV on DTG-based regimens in SSA. A study conducted in Uganda among PLHIV infected with non-B HIV-1 subtypes detected several major DTG resistance mutations— E138A/K, G140A/Q, S147G, Q148R/K, and G163R —in clients experiencing virological failure on a DTG-based regimen (28). Another study conducted in Malawi detected at least one major DTG resistance mutation (R263K, E138K, or S147G) in 8 of the 27 (30%) samples from clients on TLD experiencing virological failure (20). Similarly, a national representative survey conducted by our group in Tanzania in 2020 found instances of acquired DTG HIVDR where the major DTG resistance mutations Q148K, E138K, G118R, G140A, T66A, and R263K were detected in PLHIV on DTG-based ART and experiencing high viremia (21). Preliminary findings from a study of the EMEDT cohort that evaluated the prevalence of acquired DTG resistance and failure of viral suppression have shown a very low prevalence of DTG resistance in virally suppressed populations during the transition, but a high prevalence among those not suppressed, whereby DTG resistance was detected in 15% of the population (29). These recent findings suggest that in SSA settings, emergence of DTG resistance could be a threat to the effectiveness of the ART program in the region. Table 1 summarizes the relevant recent studies that have reported on acquired major and accessory DTG resistance mutations in SSA.
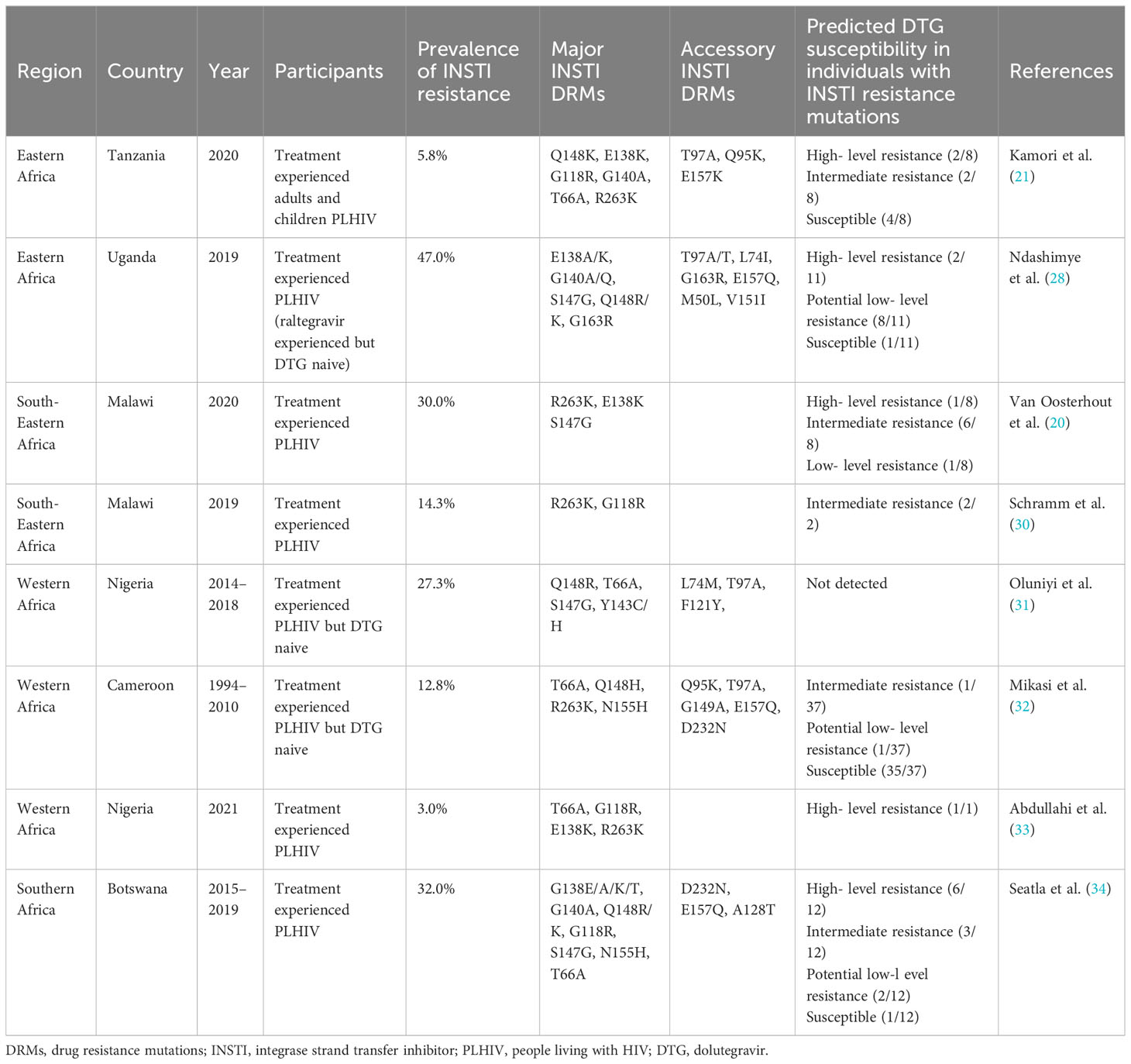
Table 1 Relevant studies reporting the acquired major and accessory INSTI resistance mutations in SSA.
3 Role of HIV-1 natural diversity on DTG resistance in SSA
3.1 Major DTG drug resistance mutations and HIV-1 diversity
A number of studies have shown the role of viral HIV-1 subtype diversity on INSTI resistance (28, 35–38). SSA is characterized by the co-circulation of multiple group M HIV-1 subtypes including A, C, D, G, H, J, K, CFR02_AG, and other inter-subtype recombinants (39, 40). In addition, HIV-1 groups O and N circulate in Central and Western Africa (41, 42). Following the WHO recommendation to introduce DTG in this relatively naive region with respect to INSTI drugs, a crucial question arises regarding the potential impact of HIV diversity on the susceptibility and selection of DTG resistance mutations. While in vitro studies suggest that DTG can be effective across HIV-1 subtypes, emerging evidence shows that natural HIV-1 variation can influence the genetic barrier to resistance and level of resistance conferred by mutations selected by DTG (43). Therefore, HIV-1 subtype diversity in SSA could play a crucial role in determining DTG effectiveness in the region.
Mutations across eight positions in the integrase region have been identified as being associated with DTG resistance, including T66K, E92Q, G118R, E138/K/A/T, G140S/A/C, Q148H/R/K, S153 F/Y, N155H, and R263K (44). However, only some, either individually or combined, are frequently reported in cases of DTG resistance in SSA (Table 1).
Selection of R263K was first observed in vivo in the SAILING clinical trial among individuals on a DTG-based regimen experiencing virologic failure (6). Since then, this mutation has been frequently selected among individuals on DTG (45). Viruses harboring R263K show moderate resistance to DTG but have significantly impaired integrase enzymatic function and viral replication (46). The deleterious effect of the mutation tends to differ between subtypes, and has been shown to be higher in subtype C than in subtype B (47, 48). Nevertheless, in vivo selection of R263K has been observed in subtypes C, D, CRF02_AG, and B (21, 49–53). Furthermore, some studies suggest that R263K mutations may protect against further selection of resistance mutations within the integrase region (46, 54, 55) and in the reverse transcriptase region (48). However, studies from Malawi and Nigeria have demonstrated that this may not be the case. In the Nigeria study, one individual harbored a combination of T66A, G118R, and E138K, in addition to an R263K mutation (33). In the Malawi study, the R263K mutation co-occurred with two other major INSTI resistance mutations (E138K and S147G) and the accessory mutation E157Q in one individual. Overall, out of eight participants with major INSTI resistance mutations (all infected with subtype C), seven had selected the R263K mutation. Three of these participants also harbored the accessory mutation E157Q, while the remaining participants had no additional mutations in integrase, except for M50I in one individual (20). Importantly, all individuals in the Malawi study exhibited significantly high levels of viremia. In another study from Tanzania by our group, we identified four viremic individuals who were on TLD and harbored major INSTI resistance mutations; in one individual infected with subtype C, R263K was detected as a single INSTI resistance-associated mutation (21). Recently, R263K was reported in one drug-naive individual infected with subtype C in Ethiopia (52). However, exposure to DTG in this case was not ruled out. These emerging data suggest that although the R263K mutation was thought to be deleterious to the subtype C virus, it may still play an important role in DTG resistance in the SSA region.
G118R is another important resistance mutation selected by DTG and rarely by other INSTIs (56). It is associated with a 5–10 -fold reduced susceptibility to DTG. The mutation is associated with a significant reduction in both strand transfer and 3′ processing activities. Notably, strand transfer activity is relatively spared in subtype C compared to subtype B. The selection of a secondary mutation such as H51Y and/or E138K in addition to G118R was shown to affect the integrase enzymatic activity and DTG resistance profile in an HIV- 1 subtype-dependent manner (37). In one study, G118R substitution was selected alone and in combination with H51Y in tissue culture selection experiments in CRF02_AG and subtype C viruses but never in subtype B viruses (37). This further demonstrates the influence of polymorphisms on the integrase backbone between HIV subtypes in the selection of DTG resistance mutations. The study by our group identified two out of four individuals with major INSTI resistance mutations, harboring the G118R substitution as a single mutation in one case (infected with subtype A1) and in combination with T66I and E138K in another case (subtype A1C) (21). Interestingly, the latter combination of mutations was also observed in a South African individual failing DTG-based triple therapy (57). Rapid selection of G118R has also been reported in another recent study in SSA (30). These findings suggest that G118R could be an important DTG resistance pathway across non-B subtypes circulating in the SSA region.
The combination of E138K, G140A/S, and Q148K is another important pathway to DTG resistance usually observed in INSTI- exposed individuals. This combination results in a high level of resistance to DTG. Interestingly, in our study one individual (infected with subtype A1D) with no known prior exposure to INSTIs had selected this combination of mutations (21). This evidence supports the idea that the viral diversity in SSA may influence the patterns and possibly the frequency of DTG resistance in SSA. Phenotypic resistance information in the context of combinations of mutations in non-B backbones is needed to better understand DTG resistance in the SSA context.
3.2 Naturally arising INSTI resistance-associated mutations and HIV-1 diversity in SSA
Given that the genetic backbone can significantly influence the genetic barrier to a specific mutation, natural integrase variations have implications for the susceptibility or selection of resistance mutations to DTG. Integrase is a relatively conserved protein (58); however, significant variations among HIV subtypes exist. Five polymorphic integrase positions (M50, L74, T97, V151, and E157) have been associated with low-level resistance to first- and/or second-generation INSTIs or an increase in the level of resistance when they occur alongside other INSTI drug resistance mutations (59, 60). Specifically, mutations M50, L74, and T97, in combination with other mutations, have been associated with reduced susceptibility to DTG (59, 61). The frequency of naturally occurring variations in these polymorphic positions depends on the subtypes (Figure 1). Interestingly, there is wide sub-regional variation in the intra- and inter-subtype prevalence of these polymorphic mutations in SSA (Figure 1).
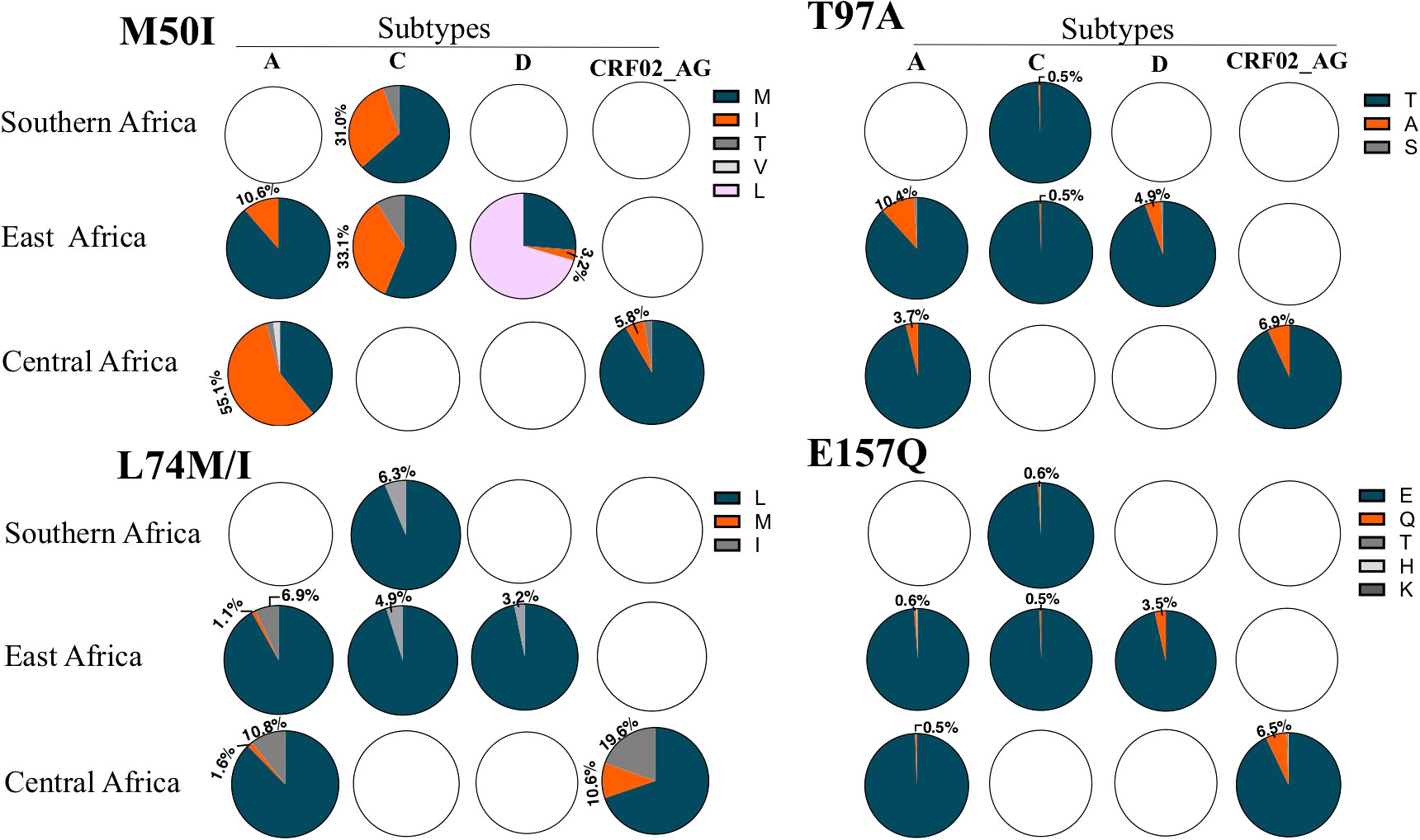
Figure 1 The frequency of polymorphisms in four integrase positions associated with DTG resistance. The figure depicts the frequency of polymorphisms analyzed from sequences deposited in the Stanford University HIV database. Only HIV subtypes with over 100 sequences per subtype per sub region are shown. The Western Africa region had fewer than 100 sequences per subtype and therefore was not included. Only the percentages of relevant substitutions are indicated. Blank pie charts indicate that <100 sequences were available for the particular HIV-1 subtype.
The M50I polymorphism can increase DTG resistance conferred by the R263K substitution in HIV subtype B (59). M50I has also been selected by DTG in in vitro passage experiments (59). The polymorphism is highly prevalent in non-B subtypes circulating in SSA. In HIV subtype A, M50I present in >50% of the sequences obtained from central Africa while occurring in about 10% of Subtype A sequences from Eastern Africa. Nearly one- third of subtype C sequences from Eastern and Southern Africa harbor this polymorphism. Much lower frequencies are observed in HIV subtypes D and CRF02-AG (Figure 1). Although selected by DTG, the M50I substitution does not seem to compensate for the viral fitness cost of R263K, at least in HIV subtype B (59). This could indicate that the selection is driven by the advantage in the resistance profile against DTG. Amid the rollout of the DTG, whether or not the high background prevalence of the M50I polymorphism in HIV subtypes circulating in SSA regions could influence the R263K resistance pathway against DTG needs to be investigated.
L74 residue is located in the catalytic core domain and is a part of the hydrophobic cluster near the active site of integrase (62). The role of L74 substitutions in DTG resistance remains inconclusive. The L74M substitution can be selected by DTG and increase resistance conferred by G118R or E138K mutations (26). On the other hand, L74F in combination with V75I has been reported to increase resistance levels of N155H or G140S together with levels of Q148H against DTG by several orders of magnitude (63). In contrast, L74I is weakly associated with selection by INSTI. Analysis of sequences obtained in the SSA region indicate diversity in the prevalence of L74 polymorphisms. Subtype A and CRF02_ AG from the Central African region tended to have a high prevalence of L74I and L74M polymorphisms, with CRF02_AG showing a combined prevalence of >20%. In contrast, a lower prevalence of L74M/I polymorphisms was observed in subtypes A, C, and D from the Eastern and Southern African region (Figure 1).
T97A is a relative common polymorphism enriched in non-B subtypes. In particular, sequences from Eastern African subtype A show a frequency of 10.4% compared to 3.7% in those obtained from Central Africa. A relatively high prevalence of 6.9% is also seen in CRF02_AG from Central Africa, while subtype C from Eastern and Southern Africa shows a prevalence of less than 1% (Figure 1). The polymorphism T97A has been shown to have no impact on the outcome of DTG treatment in INSTI- naive individuals (64). However, selection of this mutation has been associated with increased resistance to DTG in INSTI- experienced individuals with major INSTI resistance mutations at positions 140 and 148 (61, 65). On the other hand, co-occurrence of the T97A mutation with DTG- selected N155H and R263K resistance mutations improves neither the level of resistance nor replicative capacity (55). This suggest that T97A mutation may be important in conferring resistance to DTG only in certain resistance pathways. Although first generation INSTIs tend to select the 140 and 148 mutations, these mutations have also been selected by DTG in vivo, suggesting the potential relevance of T97A substitution in DTG resistance (13).
The E157Q substitution is also relatively common in INSTI- naive sequences from SSA. While a prevalence of less than 1% has been observed in subtypes A and C, in subtypes D (Eastern Africa) and CRF02_AG (Central Africa) a prevalence of 3.5% and 6.5%, respectively, has been reported. To date there is limited evidence of selection of this mutation by dolutegravir; however, its occurrence together with R263K has been associated with the compensation of viral fitness. The combination of these mutations has been reported in vivo in individuals infected with subtype C (20), suggesting that E157Q polymorphisms may have some role in conferring DTG resistance in the SSA context.
4 Role of SSA ART programs in the emergence of DTG resistance
The accelerated rollout of a fixed- dose combination of TLD in PEPFAR-funded ART programs throughout SSA has been commendably rapid and highly effective (29). The push is also aided by the donor’s desire to phase out the acquisition of the NNRTI-based regimen, not only because of the clinical benefits, but also because of the lower cost of DTG-based fixed combination treatment (66). The transition involves the initiation of all treatment -naive individuals as well as switching all eligible individuals on a NNRTI-based first- line regimen to a DTG-based fixed combination regimen (67). The adopted strategy in many countries does not require confirmation of virological suppression prior to switching to DTG. This is in line with the initial studies that suggested the lack of need for viral load estimation and resistance genotyping during switching to a DTG-based fixed combination regimen (25, 26). Therefore, there may be a substantial number of clients experiencing virological failure that are being switched to TLD with undetected resistance to NRTIs. In this case such clients can be subjected to DTG functional monotherapy, which is associated with the selection of DTG resistance. Indeed, the emerging data since the rollout indicate that DTG resistance is more frequently selected in treatment- experienced than in ART- naive individuals (20, 21). In fact, all of the observed cases of DTG resistance reported in our study from Tanzania (21), the Malawi study (20), the Uganda study (28), and the Nigerian study (33) were from treatment- experienced individuals that transitioned to TLD. The drug resistance profile in these individuals showed extensive NRTI resistance mutations that render the tenofovir and lamivudine backbone in the DTG fixed combination treatment less susceptible (20, 21, 28, 33).
DTG functional monotherapy together with infrastructural and other programmatic challenges, including suboptimal ART adherence, lack of adherence to viral load testing guidelines, poor retention in care, and limited viral load coverage, may significantly impair the effectiveness of DTG-based fixed combination treatments and promote the selection of DTG resistance in SSA (68–72). Therefore, in order to curb DTG resistance in SSA, it is essential to ensure that national ART programs in SSA carry out intensified monitoring of individuals on DTG-based fixed combination, conduct routine resistance surveillance, and consider the revision of existing ART guidelines to estimate viral loads prior to switching to DTG.
5 Conclusion and future perspectives
Recent data since the rollout of DTG provide good indications that DTG can steer the SSA region toward achieving the third 95 of the 95-95-95 UNAIDS targets for the HIV cascade of care. However, it is also becoming clear that SSA settings expose DTG to factors that threaten its effectiveness and promote the rapid selection of resistance mutations not previously encountered in other regions. The diversity of HIV-1 integrase in SSA could allow for the selection of DTG resistance mutations or combinations of mutations that are rarely observed in HIV subtype B. Therefore, measures to control the development of drug resistance are equally important in this era of DTG. Studies to understand DTG resistance in the context of SSA should be conducted to inform the ART programs in the region.
Author contributions
DK and GB: Conceptualization, Formal Analysis, Resources, Writing - original draft, Writing – review & editing
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors wish to acknowledge Dr. Macdonald Mahiti for his critical review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author DK declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kharsany AB, Karim QA. HIV infection and AIDS in sub-saharan africa: current status, challenges and opportunities. Open AIDS J (2016) 8(10):34–48. doi: 10.2174/1874613601610010034
3. Marston M, Michael D, Wringe A, Isingo R, Clark BD, Jonas A, et al. The impact of antiretroviral therapy on adult mortality in rural Tanzania. Trop Med Int Health (2012) 17(8):e58–65. doi: 10.1111/j.1365-3156.2011.02924.x
4. Johnson LF. Access to antiretroviral treatment in South Africa, 2004-2011. Afr J HIV Med (2012) 13:22–7. doi: 10.4102/sajhivmed.v13i1.156
5. de Waal R, Lessells R, Hauser A, Kouyos R, Davies MA, Egger M, et al. HIV drug resistance in sub-Saharan Africa: public health questions and the potential role of real-world data and mathematical modelling. J Virus Erad (2018) 4(Suppl 2)55–8. doi: 10.1016/S2055-6640(20)30347-2
6. Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet (2012) 380:1250–8. doi: 10.1016/S0140-6736(12)61038-1
7. WHO. Guidelines on the public health response to pretreatment HIV drug resistance. (2017). Available at: https://www.who.int/publications/i/item/9789241550055.
8. Llibre JM, Pulido F, García F, García Deltoro M, Blanco JL, R D. Genetic barrier to resistance for dolutegravir. AIDS Rev (2015) 17(1):56–64.
9. Brenner BG MAW. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res (2017) 15(239):1–9. doi: 10.1016/j.virusres.2016.07.006
10. Kanters S, Vitoria M, Zoratti M, Doherty M, Penazzato M, Rangaraj A, et al. Comparative efficacy, tolerability and safety of dolutegravir and efavirenz 400mg among antiretroviral therapies for first-line HIV treatment: A systematic literature review and network meta-analysis. EClinicalMedicine (2020) 16(28):100573. doi: 10.1016/j.eclinm.2020.100573
11. Mabeya S, Nyamache A, Ngugi C, Nyerere ARL. Characterization of HIV-1 integrase gene and resistance associated mutations prior to roll out of integrase inhibitors by Kenyan national HIV-treatment program in Kenya. Ethiop J Health Sci (2020) 30(1):37–44. doi: 10.4314/ejhs.v30i1.6
12. Arimide DA, Szojka ZI, Zealiyas K, Gebreegziabxier A, Adugna F, Sasinovich S, et al. Pre-treatment integrase inhibitor resistance and natural polymorphisms among HIV-1 subtype C infected patients in Ethiopia. Viruses (2022) 14(4):729. doi: 10.3390/v14040729
13. Masoud S, Kamori D, Barabona G, Mahiti M, Sunguya B, Lyamuya E, et al. Circulating HIV-1 integrase genotypes in Tanzania: implication on the introduction of integrase inhibitors-based antiretroviral therapy regimen. AIDS Res Hum Retroviruses (2020) 36(6):539–43. doi: 10.1089/aid.2020.0021
14. Oliveira MF, Ramalho DB, Abreu CM, Vubil A, Mabunda N, Ismael N, et al. Genetic diversity and naturally polymorphisms in HIV type 1 integrase isolates from Maputo, Mozambique: implications for integrase inhibitors. AIDS Res Hum Retroviruses (2012) 28(12):1788–92. doi: 10.1089/aid.2012.0058
15. Bessong PO, Nwobegahay. Genetic analysis of HIV-1 integrase sequences from treatment naive individuals in northeastern South Africa. Int J Mol Sci (2013) 14:5013–24. doi: 10.3390/ijms14035013
16. Inzaule SC, Hamers RL, Noguera-Julian M, Casadellà M, Parera M, Rinke de Wit TF, et al. Primary resistance to integrase strand transfer inhibitors in patients infected with diverse HIV-1 subtypes in sub-Saharan Africa. J Antimicrob Chemother (2018) 73(5):1167–72. doi: 10.1093/jac/dky005
17. Semengue ENJ, Armenia D, Inzaule S, Santoro MM, Dambaya B, Takou D, et al. Baseline integrase drug resistance mutations and conserved regions across HIV-1 clades in Cameroon: implications for transition to dolutegravir in resource-limited settings. J Antimicrob Chemother (2021) 76(5):1277–85. doi: 10.1093/jac/dkab004
18. Mikasi SG, Gichana JO, van der Walt C, Brado D, Obasa AE, Njenda D, et al. HIV-1 integrase diversity and resistance-associated mutations and polymorphisms among integrase strand transfer inhibitor-naive HIV-1 patients from Cameroon. AIDS Res Hum Retroviruses (2020) 36(5):450–5. doi: 10.1089/aid.2019.0264
19. Mikasi SG, Isaacs D, Ikomey GM, Shimba H, Cloete R, Jacobs GB. Short communication: HIV-1 drug resistance mutation analyses of Cameroon-derived integrase sequences. AIDS Res Hum Retroviruses (2021) 37(1):54–6. doi: 10.1089/aid.2020.0022
20. van Oosterhout JJ, Chipungu C, Nkhoma L, Kanise H, Hosseinipour MC, Sagno JB, et al. Dolutegravir resistance in Malawi’s national HIV treatment program. Open Forum Infect Dis (2022) 9(5):ofac148. doi: 10.1093/ofid/ofac148
21. Kamori D, Barabona G, Rugemalila J, Maokola W, Masoud SS, Mizinduko M, et al. Emerging integrase strand transfer inhibitor drug resistance mutations among children and adults on ART in Tanzania: findings from a national representative HIV drug resistance survey. J Antimicrob Chemother (2023) 78(3):779–87. doi: 10.1093/jac/dkad010
22. Esber A, Dear N, Shah N, Kibuuka H, Maswai J, Owuoth J, et al. Brief report: virologic impact of the dolutegravir transition: prospective results from the multinational african cohort study. J Acquir Immune Defic Syndr (2022) 91(3):285–9. doi: 10.1097/QAI.0000000000003065
23. Brown JA, Nsakala BL, Mokhele K, Rakuoane I, Muhairwe J, Urda L, et al. Viral suppression after transition from nonnucleoside reverse transcriptase inhibitor- to dolutegravir-based antiretroviral therapy: A prospective cohort study in Lesotho (the DO-REAL study) reported that the the country has achieved the third 95 of the 95-95-95 UNAIDS targets for the HIV cascade of care, with a viral suppression rate of >95% among PLHIV on DTG-based ART regimens . HIV Med (2022) 23(3):287–93. doi: 10.1111/hiv.13189
24. Dorward J, Sookrajh Y, Khubone T, van der Molen J, Govender R, Phakathi S, et al. Implementation and outcomes of dolutegravir-based first-line antiretroviral therapy for people with HIV in South Africa: a retrospective cohort study. Lancet HIV (2023) 10(5):e284–e94. doi: 10.1016/S2352-3018(23)00047-4
25. Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med (2019) 381(9):803–15. doi: 10.1056/NEJMoa1902824
26. NAMSAL ANRS 12313 Study Group, Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, Eymard-Duvernay S, Leroy S, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med (2019) 381(9):816–26. doi: 10.1056/NEJMoa1904340
27. Nabitaka VM, Nawaggi P, Campbell J, Conroy J, Harwell J, Magambo K, et al. High acceptability and viral suppression of patients on Dolutegravir-based first-line regimens in pilot sites in Uganda: A mixed-methods prospective cohort study. PloS One (2020) 15(5):e0232419. doi: 10.1371/journal.pone.0232419
28. Ndashimye E, Avino M, Olabode AS, Poon AFY, Gibson RM, Li Y, et al. Accumulation of integrase strand transfer inhibitor resistance mutations confers high-level resistance to dolutegravir in non-B subtype HIV-1 strains from patients failing raltegravir in Uganda. J Antimicrob Chemother (2020) 75(12):3525–33. doi: 10.1093/jac/dkaa355
29. WHO. Update on the transition to dolutegravir-based antiretroviral therapy. In: Report of a WHO meeting (2022).
30. Schramm B, Temfack E, Descamps D, Nicholas S, Peytavin G, Bitilinyu-Bangoh JE, et al. Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study. Lancet HIV (2022) 9(8):e544–e53. doi: 10.1016/S2352-3018(22)00136-9
31. Oluniyi PE, Ajogbasile FV, Zhou S, Fred-Akintunwa I, Polyak CS, Ake JA, et al. HIV-1 drug resistance and genetic diversity in a cohort of people with HIV-1 in Nigeria. AIDS (2022) 36(1):137–46. doi: 10.1097/QAD.0000000000003098
32. Mikasi SG, Isaacs D, Chitongo R, Ikomey GM, Jacobs GB, Cloete R. Interaction analysis of statistically enriched mutations identified in Cameroon recombinant subtype CRF02_AG that can influence the development of Dolutegravir drug resistance mutations. BMC Infect Dis (2021) 21(1):379. doi: 10.1186/s12879-021-06059-x
33. Abdullahi A, Kida IM, Maina UA, Ibrahim AH, Mshelia J, Wisso H, et al. Limited emergence of resistance to integrase strand transfer inhibitors (INSTIs) in ART-experienced participants failing dolutegravir-based antiretroviral therapy: a cross-sectional analysis of a Northeast Nigerian cohort. J Antimicrob Chemother (2023) 27:dkad195. doi: 10.1093/jac/dkad195
34. Seatla KK, Maruapula D, Choga WT, Ntsipe T, Mathiba N, Mogwele M, et al. HIV-1 subtype C drug resistance mutations in heavily treated patients failing integrase strand transfer inhibitor-based regimens in Botswana. Viruses (2021) 13(4):594. doi: 10.3390/v13040594
35. Bar-Magen T, Donahue DA, McDonough EI, Kuhl BD, Faltenbacher VH, Xu H, et al. HIV-1 subtype B and C integrase enzymes exhibit differential patterns of resistance to integrase inhibitors in biochemical assays. AIDS (2010) 24(14):2171–9. doi: 10.1097/QAD.0b013e32833cf265
36. Han YS, Mesplède T, Wainberg MA. Differences among HIV-1 subtypes in drug resistance against integrase inhibitors. Infect Genet Evol (2016) 46:286–91. doi: 10.1016/j.meegid.2016.06.047
37. Quashie PK, Oliviera M, Veres T, Osman N, Han YS, Hassounah S, et al. Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase. J Virol (2015) 89(6):3163–75. doi: 10.1128/JVI.03353-14
38. Seatla KK, Avalos A, Moyo S, Mine M, Diphoko T, Mosepele M, et al. Four-class drug-resistant HIV-1 subtype C in a treatment experienced individual on dolutegravir-based antiretroviral therapy in Botswana. AIDS (2018) 32(13):1899–902. doi: 10.1097/QAD.0000000000001920
39. Elangovan R, Jenks M, Yun J, Dickson-Tetteh L, Kirtley S, Hemelaar J, et al. Global and regional estimates for subtype-specific therapeutic and prophylactic HIV-1 vaccines: A modeling study. Front Microbiol (2021) 12:690647. doi: 10.3389/fmicb.2021.690647
40. Giovanetti M, Ciccozzi M, Parolin C, Borsetti A. Molecular epidemiology of HIV-1 in african countries: A comprehensive overview. Pathogens (2020) 9(12):1072. doi: 10.3390/pathogens9121072
41. Appah A, Beelen CJ, Kirkby D, Dong W, Shahid A, Foley B, et al. Molecular epidemiology of HIV-1 in Ghana: subtype distribution, drug resistance and coreceptor usage. Viruses (2022) 15(1):128. doi: 10.3390/v15010128
42. Gartner MJ, Roche M, Churchill MJ, Gorry PR, Flynn JK. Understanding the mechanisms driving the spread of subtype C HIV-1. EBioMedicine (2020) 53:102682. doi: 10.1016/j.ebiom.2020.102682
43. Depatureaux A, Mesplède T, Quashie P, Oliveira M, Moisi D, Plantier JC, et al. HIV-1 group O resistance against integrase inhibitors. J Acquir Immune Defic Syndr (2015) 70(1):9–15. doi: 10.1097/QAI.0000000000000698
44. Wensing AM, Calvez V, Ceccherini-Silberstein F, Charpentier C, Günthard HF, Paredes R, et al. update of the drug resistance mutations in HIV-1. Top Antivir Med (2022) 30(4):559–74.
45. Underwood M, Horton J, Nangle K, Hopking J, Smith K, Aboud M, et al. Integrase inhibitor resistance mechanisms and structural characteristics in antiretroviral therapy-experienced, integrase inhibitor-naive adults with HIV-1 infection treated with dolutegravir plus two nucleoside reverse transcriptase inhibitors in the DAWNING study. Erratum: Antimicrob Agents Chemother (2023) 67(3):e0032622. doi: 10.1128/aac.00326-22
46. Pham HT, Labrie L, Wijting IEA, Hassounah S, Lok KY, Portna I, et al. The S230R integrase substitution associated with virus load rebound during dolutegravir monotherapy confers low-level resistance to integrase strand-transfer inhibitors. J Infect Dis (2018) 218(5):698–706. doi: 10.1093/infdis/jiy175
47. Mesplède T, Quashie PK, Hassounah S, Osman N, Han Y, Liang J, et al. The R263K substitution in HIV-1 subtype C is more deleterious for integrase enzymatic function and viral replication than in subtype B. AIDS (2015) 29(12):1459–66. doi: 10.1097/QAD.0000000000000752
48. Singhroy DN, Wainberg MA, Mesplède T. Combination of the R263K and M184I/V resistance substitutions against dolutegravir and lamivudine decreases HIV replicative capacity. Antimicrob Agents Chemother (2015) 59(5):2882–5. doi: 10.1128/AAC.05181-14
49. Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study8. Lancet (2014) 383(9911):30. doi: 10.1016/S0140-6736(13)61221-0
50. Quashie PK, Mesplède T, Han YS, Oliveira M, Singhroy DN, Fujiwara T, et al. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol (2012) 86(5):2696–70. doi: 10.1128/JVI.06591-11
51. Ahmed N, Flavell S, Ferns B, Frampton D, Edwards SG, Miller RF, et al. Development of the R263K mutation to dolutegravir in an HIV-1 subtype D virus harboring 3 class-drug resistance. Open Forum Infect Dis (2018) 6(1):ofy329. doi: 10.1093/ofid/ofy329
52. Kiros M, Tefera DA, Andualem H, Geteneh A, Tesfaye A, Woldemichael TS, et al. Low level of HIV-1C integrase strand transfer inhibitor resistance mutations among recently diagnosed ART-naive Ethiopians. Sci Rep (2023) 13(1):6546. doi: 10.1038/s41598-023-33850-4
53. Diaz RS, Hunter JR, Camargo M, Dias D, Galinskas J, Nassar I, et al. Dolutegravir-associated resistance mutations after first-line treatment failure in Brazil. BMC Infect Dis (2023) 23(1):347. doi: 10.1186/s12879-023-08288-8
54. Liang J, Mesplède T, Oliveira M, Anstett K, Wainberg MA. The combination of the R263K and T66I resistance substitutions in HIV-1 integrase is incompatible with high-level viral replication and the development of high-level drug resistance. J Virol (2015) 89(22):11269–74. doi: 10.1128/JVI.01881-15
55. Anstett K, Mesplede T, Oliveira M, Cutillas V, Wainberg MA. Dolutegravir resistance mutation R263K cannot coexist in combination with many classical integrase inhibitor resistance substitutions. J Virol (2015) 89(8):4681–4. doi: 10.1128/JVI.03485-14
56. Quashie PK, Mesplède T, Han YS, Veres T, Osman N, Hassounah S, et al. Biochemical analysis of the role of G118R-linked dolutegravir drug resistance substitutions in HIV-1 integrase. Antimicrob Agents Chemother (2013) 57(12):6223–35. doi: 10.1128/AAC.01835-13
57. Mahomed K, Wallis CL, Dunn L, Maharaj S, Maartens G, Meintjes G. Case report: Emergence of dolutegravir resistance in a patient on second-line antiretroviral therapy. South Afr J HIV Med (2020) 21(1):1062. doi: 10.4102/sajhivmed.v21i1.1062
58. Rhee SY, Liu TF, Kiuchi M, Zioni R, Gifford RJ, Holmes SP, et al. Natural variation of HIV-1 group M integrase: implications for a new class of antiretroviral inhibitors. Retrovirology (2008) 5(74). doi: 10.1186/1742-4690-5-74
59. Wares M, Mesplède T, Quashie PK, Osman N, Han Y, Wainberg MA. The M50I polymorphic substitution in association with the R263K mutation in HIV-1 subtype B integrase increases drug resistance but does not restore viral replicative fitness. Retrovirology (2014) 11(7). doi: 10.1186/1742-4690-11-7
60. Tzou PL, Rhee SY, Descamps D, Clutter DS, Hare B, Mor O, et al. ntegrase strand transfer inhibitor (INSTI)-resistance mutations for the surveillance of transmitted HIV-1 drug resistance. J Antimicrob Chemother (2020) 75(1):170–82. doi: 10.1093/jac/dkz417
61. George JM, Kuriakose SS, Dee N, Stoll P, Lalani T, Dewar R, et al. Rapid development of high-level resistance to dolutegravir with emergence of T97A mutation in 2 treatment-experienced individuals with baseline partial sensitivity to dolutegravir. Open Forum Infect Dis (2018) 5(10):ofy221. doi: 10.1093/ofid/ofy221
62. Rogers L OA, Jacobs GB, Sarafianos SG, Sönnerborg A, Neogi U, Singh K. Structural implications of genotypic variations in HIV-1 integrase from diverse subtypes. Front Microbiol (2018) 9:1754. doi: 10.3389/fmicb.2018.01754
63. Hachiya A, Kirby KA, Ido Y, Shigemi U, Matsuda M, Okazaki R, et al. Impact of HIV-1 integrase L74F and V75I mutations in a clinical isolate on resistance to second-generation integrase strand transfer inhibitors. Antimicrob Agents Chemother (2017) 61(8):e00315–17. doi: 10.1128/AAC.00315-17
64. Abram ME, Ram RR, Margot NA, Barnes TL, White KL, Callebaut C, et al. Lack of impact of pre-existing T97A HIV-1 integrase mutation on integrase strand transfer inhibitor resistance and treatment outcome. PloS One (2017) 12(2):e0172206. doi: 10.1371/journal.pone.0172206
65. Cheung PK, Shahid A, Dong W, Lepik KJ, Montaner JSG, Brockman MA, et al. Impact of combinations of clinically observed HIV integrase mutations on phenotypic resistance to integrase strand transfer inhibitors (INSTIs): a molecular study. J Antimicrob Chemother (2022) 77(4):979–88. doi: 10.1093/jac/dkab498
66. Raizes E, Hader S, Birx D. The US president’s emergency plan for AIDS relief (PEPFAR) and HIV drug resistance: mitigating risk, monitoring impact. J Infect Dis (2017) 216(5):805–S7. doi: 10.1093/infdis/jix432
67. WHO. Update of recommendations on first- and second-line antiretroviral regimens. Geneva, Switzerland: WHO/CDS/HIV/1915 (2019).
68. Glass TR, Motaboli L, Nsakala B, Lerotholi M, Vanobberghen F, Amstutz A, et al. The viral load monitoring cascade in a resource-limited setting: A prospective multicentre cohort study after introduction of routine viral load monitoring in rural Lesotho. PloS One (2019) 14(8):e0220337. doi: 10.1371/journal.pone.0220337
69. Etoori D, Ciglenecki I, Ndlangamandla M, Edwards CG, Jobanputra K, Pasipamire M, et al. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second-line switches in Swaziland. J Int AIDS Soc (2018) 21(10):e25194. doi: 10.1002/jia2.25194
70. Pham MD, Nguyen HV, Dea A. Viral load monitoring for people living with HIV in the era of test and treat: progress made and challenges ahead – a systematic review. BMC Public Health (2022) 22(1203):1203. doi: 10.1186/s12889-022-13504-2
71. Lubega P, Nalugya SJ, ANea K. Adherence to viral load testing guidelines, barriers, and associated factors among persons living with HIV on ART in Southwestern Uganda: a mixed-methods study. BMC Public Health (2022) 22:1268. doi: 10.1186/s12889-022-13674-z
72. Asio J, Watera C, Namuwenge N, Kirungi W, Musinguzi J, Mugagga K, et al. Uganda HIV Drug Resistance Technical Working Group. Population-based monitoring of HIV drug resistance early warning indicators in Uganda: A nationally representative survey following revised WHO recommendations. PloS One (2020) 15(4):e0230451. doi: 10.1371/journal.pone.0230451
Keywords: HIV drug resistance, dolutegravir, antiretroviral treatment, HIV-1 subtypes, viral suppression, sub-Saharan Africa, people living with HIV, mutations
Citation: Kamori D and Barabona G (2023) Dolutegravir resistance in sub-Saharan Africa: should resource-limited settings be concerned for future treatment? Front. Virol. 3:1253661. doi: 10.3389/fviro.2023.1253661
Received: 05 July 2023; Accepted: 04 September 2023;
Published: 25 September 2023.
Edited by:
Marcel Tongo, Center for Research on Emerging and Re-Emerging Diseases (CREMER), CameroonReviewed by:
Emmanouil Magiorkinis, Athens Chest Hospital Sotiria, GreeceCopyright © 2023 Kamori and Barabona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doreen Kamori, ZG9yZWVuZG9uYWxkQHlhaG9vLmNvbQ==; ZG9yZWVua2Ftb3JpQGdtYWlsLmNvbQ==
 Doreen Kamori
Doreen Kamori Godfrey Barabona
Godfrey Barabona