
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol., 31 July 2023
Sec. Viral Disease Investigation
Volume 3 - 2023 | https://doi.org/10.3389/fviro.2023.1228268
 James Mburu Kangethe1,2,3*
James Mburu Kangethe1,2,3* Stephen Gichuhi4
Stephen Gichuhi4 Eddy Okoth Odari5
Eddy Okoth Odari5 Jillian Pintye6
Jillian Pintye6 Kenneth Mutai3
Kenneth Mutai3 Leila Abdullahi7
Leila Abdullahi7 Alex Maiyo8
Alex Maiyo8 Marianne W. Mureithi2,9
Marianne W. Mureithi2,9Background: Globally, 15% of reported cancers are virus-associated. Cancer-causing viruses include high-risk Human papillomavirus (HR-HPV), the causative agent of cervical cancer, and Human T-lymphotropic virus type-1 (HTLV-1), the causative agent of adult T-cell leukemia (ATL). HTLV-1 infection may enhance susceptibility to acquiring HR-HPV infections due to its retrovirus properties, resulting in increased cervical abnormalities among women living with HIV (WLHIV). In Kenya, there is a paucity of data on the burden of HR-HPV/HTLV-1 co-infection among WLHIV. We determined the prevalence of HR-HPV and HTLV-1 co-infection among WLHIV on antiretroviral therapy (ART) at Kenya’s national referral hospital, Kenyatta National Hospital (KNH).
Methodology: We conducted a cross-sectional study among WLHIV on ART attending KNH’s HIV care clinic. Study nurses collected a cervical sample with a cytobrush for HPV genotyping using Gene Xpert ® assays and HPV Genotypes 14 Real-TM Quant. Peripheral blood mononuclear cells were used for HTLV-1 DNA detection. Differences in frequency distributions of characteristics between WLHIV with and without HR-HPV and HTLV-1 co-infections were assessed using the Chi-square tests.
Results: A total of 647 WLHIV enrolled in this study with a mean age of 42.8 years (SD 8.7); 93% were on ART for >1 year and 8.8% were not virally suppressed (>1000 HIV RNA copies/mL). The HTLV-1 positivity rate among WLHIV was 3.1% overall and 7.6% among those with HR-HPV. WLHIV with HR-HPV 31 had the highest proportion of HR-HPV/HTLV-1 co-infection (31.6%). In contrast, WLHIV with HR-HPV 39 had the lowest proportion of co-infection (7.1%). Participants with HR-HPV/HTLV-1 co-infections were older compared to those without the co-infections (35.2% vs. 23.3%). A higher proportion of women with HR-HPV/HTLV-1 co-infections had their sex debut before the age of 18 years (p=0.012). Women co-infected with HR-HPV/HTLV-1 were diagnosed with HIV at ≥ 35 years compared to those without infection (70.6% vs. 41.9%, p= 0.019).
Conclusion: We found that HTLV-1 infection was more common among WLHIV on ART who also had HR-HPV and that co-infections were associated with the age of sexual debut and the age of HIV diagnosis.
Oncogenic viral infections are on the rise globally causing approximately 15% of malignancies (1). Cervical cancer ranks as the fourth most prevalent cancer in women globally with low and middle-income countries (LMICs) being worst affected. An incidence rate of > 75,000 new cervical cancer cases, resulting in approximately 50,000 deaths, are reported annually in sub-Saharan Africa (SSA) and could be on the rise due to HIV co-infections (2, 3). The World Health Organization (WHO) estimates that approximately 450,000 women will be adversely affected by cervical cancer by the year 2030, with over 80% of those affected being women in SSA (3). In Kenya, over 5000 new cervical cancer cases occur annually and result in over 3000 deaths among women living with HIV (WLHIV), despite the successful implementation of HIV care and treatment programs in the country (4).
There is a paucity of data on the prevalence and optimal detection of HR-HPV among WLHIV. The effects of ART on HR-HPV infections and cervical abnormalities have also been underexplored in resource-limited settings such as Kenya. Further, co-infections with other sexually transmitted immunosuppressive viruses, such as HTLV-1, among WLHIV with HR-HPV infections are unclear. Due to similar modes of sexual transmission by HIV, HR-HPV, and HTLV-1, the three viruses could share comparable risk factors and their co-existence could be almost similar among vulnerable populations such as WLHIV.
In the early 1980s, the first cancer-causing virus, HTLV-1 was discovered, a few years before the discovery of HIV (5). HTLV-1 is the causative agent of adult T-cell leukemia (ATL), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), HTLV-1-associated uveitis, and infective dermatitis (6). Being an RNA retrovirus, HTLV-1 has a high affinity for CD4 T-helper cells depicting an almost similar pathogenesis path to HIV (7). The synergy between HIV and HTLV-1 oncogenic retroviruses could weaken the cell-mediated immune surveillance system permitting opportunistic infections such as HR-HPV, their persistence, and the development of cervical lesions (8). Currently, there are less data on the prevalence of HR-HPV and HTLV-1 co-infections among WLHIV on ART in SSA. In the last two decades, there have been reports on the upsurge of HR-HPV/HTLV-1 co-infection in South America where an HPV prevalence of 44% was reported among HTLV-1 infected women (9, 10). In Southeast Asia, countries such as Japan have reported HTLV-1 in patients with confirmed cases of cervical cancer (11). Nevertheless, the etiology and risk factors of these co-infections could be different due to the geographic differences and biological distinctions between populations. Although Africa has been a reservoir for many infectious viral diseases, there is a paucity of data on the co-infection of HR-HPV and HTLV-1 in SSA, particularly in East Africa. In a previous study among women attending HIV and family planning clinics in Kenya’s referral hospital, an HTLV-1 prevalence of 19.5% was detected from liquid-based cytology (LBC) smears of HIV-infected women (6). The co-infection of HR-HPV and HTLV-1 has not been fully understood among immunocompromised populations such as WLHIV which are disproportionately affected by oncogenic viruses. This study aimed to determine the co-infection of HR-HPV and HTLV-1 among WLHIV on ART at KNH. Data generated from this study will inform HR-HPV/HTLV-1 disease burden among WLHIV in Kenya and will contribute to the very few data from HR-HPV/HTLV-1 co-infection in SSA.
This cross-sectional study recruited WLHIV aged 18 years and above attending the ART clinic at KNH. The study excluded the WLHIV if they were currently pregnant women, had a history of total hysterectomy, radiotherapy, or chemotherapy, and those who were currently menstruating or experiencing vaginal bleeding based on guidelines for cervical specimen collection.
KNH is the largest tertiary health facility in Kenya with a bed capacity of 1,800 and a bed occupancy rate of 90-95%. The ART clinic at KNH was established 20 years ago for the prevention and treatment of HIV and associated morbidities in men and women including adolescents and children. On average, the ART clinic offers services to nearly 10,000 clients with the highest proportion being women. Cervical cancer screening commenced 12 years ago as part of integrated service for HIV care for WLHIV at the ART.
In this study, all eligible WLHIV were offered initial partial HPV testing using Gene Xpert® HPV assays, and analysis was done at the virology molecular laboratory at KNH. The additional individual HPV genotyping was done using HPV Genotypes 14 Real-TM Quant (SACACE biotechnologies®, Italy). In addition, a blood sample was collected for the detection of HTLV-1 infections. Briefly, the study procedure involved face-to-face interviews after written informed consent was obtained. The interview covered sociodemographic, behavioral, and sexual characteristics, history of HIV diagnosis, investigation, and treatment. Thereafter, the individual participants were examined by a trained research nurse at the ART clinic including general physical and pelvic examination. In the lithotomy position, a nurse passed a sterile cusco speculum to visualize the cervix and a cytobrush was inserted into the endocervix and rotated 360 degrees two times. The cervical sample collected was sent to the laboratory for HPV genotyping. The blood sample was taken to the Kenya Medical Research Institute (KEMRI) Virology laboratory for HTLV-1 molecular detection. The research nurse also reviewed the HIV electronic medical records (EMR) of each participant and copied relevant information into the data collection form.
All participants had post-test counseling irrespective of their HPV and HTLV-1 test results. WLHIV with HTLV-1 infections were also referred to the infectious diseases unit for further management.
This paper was derived from a larger study that aimed to study the oncogenic stratification of HR-HPV and by extension associate with HTLV-1 infections. Thus, prevalence of HR-HPV was used to calculate the sample size. This was calculated using Fisher’s formula for cross-sectional studies at a 95.0% confidence interval, with a margin of error of 5%, and based on an overall prevalence of HR-HPV genotypes of 65% in women living with HIV (WLHIV) with abnormal cytology in Kenya (12). To fulfil the objectives of this study, a minimum of 350 participants were required. However, the study recruited 647 WLHIV to increase the statistical power of the study and bolster the validity of the findings.
From February 2021 to September 2022, all eligible participants at the ART clinic were informed about the study before their routine clinic visit. Women that expressed interest in study participation were invited for further discussion with the research assistant on the study objectives and procedures. WLHIV who were eligible and willing to consent were enrolled until the desired sample size was attained.
A total of 1,080 WLHIV were approached and screened for eligibility. Out of these, 171 WLHIV were found to be ineligible due to various reasons: pregnancy (33), being on menstrual periods or experiencing vaginal bleeding (105), having a history of partial or total hysterectomy (24), undergoing chemo-radiation treatment in the past (5), being psychologically unfit (2), or requiring urgent medical attention (2). Among the 909 eligible WLHIV, 262 WLHIV did not give consent because they did not have enough time (57), and for 205 WLHIV, the reasons for non-consent were not ascertained. In total, 647 WLHIV consented and were enrolled in the study as shown in Figure 1.
The cervical smear sample in the brush was placed in a PreservCyt solution (Hologic corp.) and taken for laboratory for HPV detection of high-risk HPV types (16, 18_45, and 11 other HR-HPVs) using Xpert HPV platform (Cepheid, Sunnyvale, CA, USA). Thereafter, individual HR-HPV genotyping was conducted. First, the DNA was extracted using QIAamp DNA mini kit per the manufacturer’s protocol (Qiagen, Crawley, UK). The HPV detection was done and thereafter specific genotypes were identified and interpreted using the HPV Genotypes 14 Real-TM Quant” V67-100 FRT kit (SACACE biotechnologies®, Italy). Blood samples were separated into plasma and PBMCs prepared for HTLV-1 IgG/IgM and genotype detection respectively. The HTLV-1 ELISA was analyzed according to the manufacturer’s instructions (MP Biomedical Diagnostics, Germany) and the results were read at 450 nm and 620nm wavelength.
Briefly, the PBMCs were extracted as follows: Whole blood was diluted with phosphate buffered saline (PBS) in a falcon tube and centrifuged for 40 minutes at 400-500g without brake. Four layers were formed where the second layer was PBMC characterized by a white and cloudy blanket. These cells were gently removed using a Pasteur pipette and warm medium or PBS was used to wash off any remaining platelets. The PBMCs were preserved at an ultra-low temperature of -196°C in liquid nitrogen awaiting HTLV-1 molecular extraction. HTLV-1 PCR molecular detection was performed to assess the quality of input DNA following the manufacturer’s protocol (Bioline Ltd., London, UK).
Data were entered into the ODK tool kit and exported as a CSV file before importing into SPSS version 23.0. Descriptive statistics were presented in frequencies for categorical variables and mean or median for continuous variables. The primary outcome of this study was the co-infection of HR-HPV and HTLV-1. The differences in the frequency of demographic, clinical, and behavioral characteristics between WLHIV with and without HR-HPV and HTLV-1 co-infections were compared using Chi-square tests and fishers exact test for small numbers.
A total of 647 WLHIV provided informed consent and participated in this study. The participants ranged in age from 35 to 44 years old, with a mean age of 42.8 years (SD 8.7). Christians made up the vast majority of participants (97.1%), with 77.6% having a source of income. The average age at which participants reported initiating sexual activity was 18.3 years (SD 3.0), and 43% had given birth to three or more children. Only 29.3% of the participants reported current use of a family planning method. Nearly half of the study participants (43.4%) reported diagnosis and treatment for STDs at some point in their sexual history, and 68.5% of the cohort reported having one or more sexual partner(s) in the previous six months. The results indicate that a large proportion of the participants (91.6%) reported to have a sole partner, with nearly half (46.7%) of these partners living with HIV. Most participants (85.3%) reported their sexual partners to be circumcised. A small subset reported cigarette use (3.7%) and while 29.4% reported alcohol consumption. The social demographics, sexual, and behavioral characteristics of the participants are illustrated in Table 1.
The median age of HIV diagnosis was 32 years (IQR 26.0-38.0). Approximately 79% of the participants were virally suppressed (HIV-1 RNA < 50 copies/mL), 12.3% had low-level viremia (50-999 copies/mL of plasma), and 8.8% were unsuppressed (1000 copies/mL of plasma or higher). Approximately 37% of participants had an absolute CD4 cell count < 200 cells/mL. Nearly all of the participants (97.2%) were receiving ART, with 87.6% on a DTG-based regimen and 7.8% on Lopinavir/r or Atazanavir/r based regimens. However, a 2.8% of the participants were not on ART. Only a small fraction (1.1%) of participants were vaccinated against HPV. Notably, a subset of participants (20.9%) reported missing their scheduled HIV clinical appointments at least once in the past year, and 25.5% reported missing at least one daily ART intake within the past year, illustrated in Table 2.
As shown in Figure 2, the overall HTLV-1 positivity among WLHIV was 3.1%. Out of the 224 WLHIV having HR-HPV infections, the HTLV-1 positivity was 7.6% which was more frequent than among women without HR-HPV (0.9%).
WLHIV with HR-HPV 31 had the highest proportion of HR-HPV/HTLV-1 co-infection (31.6%), followed by 58 (20.7%), 59 (18.2%), 45 (17.4%), 51 (17.1%), 66 (14.3%), 35 (14.0%), 33 (13.3%), 56 (11.3%), 16 (10.9%), 52 (10.5%), 68 (7.7%), 18 (7.6%) and WLHIV with HR-HPV 39 had the lowest proportion of co-infection (7.1%), as shown in Figure 3.
Frequency of HTLV-1 positivity was higher among WLHIV with co-infection of most strains of HR-HPV (Table 3). However, a few HR-HPV genotypes (HR-HPV 18, 33, 39, and 68) did not have higher frequency of HTLV-1 positivity, though our results should be interpreted with caution due to small sample sizes.
Individuals with co-infections of HR-HPV/HTLV-1 were older compared to those without HR-HPV/HTLV-1 co-infections (35.3% vs. 23.3%). A higher proportion of women with HR-HPV/HTLV-1 co-infections had their sex debut before the age of 18 years (p=0.012). Women co-infected with HR-HPV/HTLV-1 were diagnosed with HIV at ≥ 35 years compared to those without infection (70.6% vs. 41.9%, p= 0.019), as shown in Table 4.
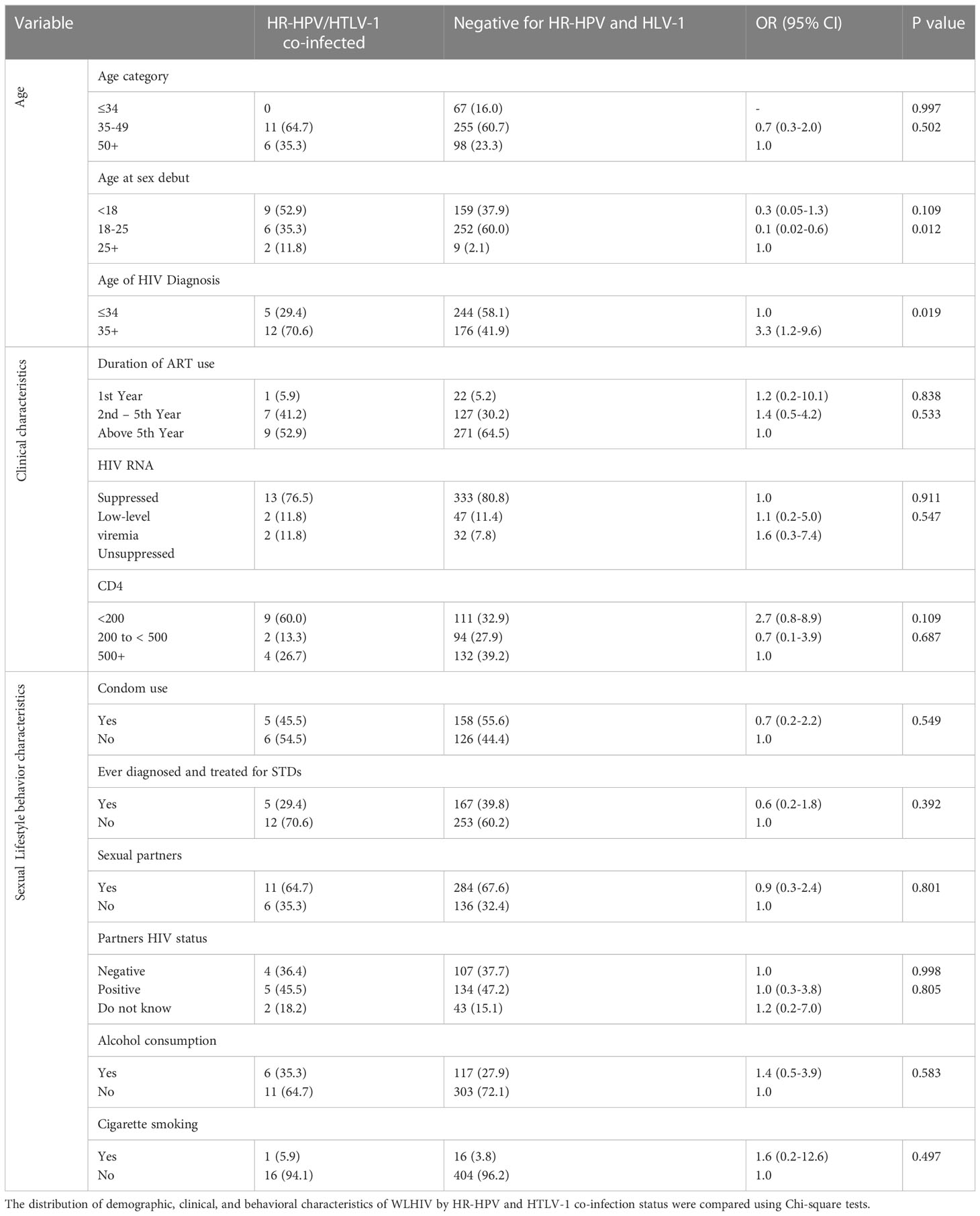
Table 4 Frequency distribution of demographic, clinical, and behavioral characteristics of WLHIV by HR-HPV and HTLV-1 co-infection status (n=17).
Despite the identification of numerous oncogenic viruses, including both AIDS and non-AIDS-defining viruses, HR-HPV and HTLV-1 co-infections among WLHIV has not been extensively studied in SSA. HIV-1, HR-HPV, and HTLV-1 are primarily transmitted through sexual contact, sharing a common route of transmission. Seminal fluid contains T-lymphocytes and macrophages, which facilitate increased infection, replication, and transmission of both HIV-1 and HTLV-1 (13–15). The integration of the HR-HPV gene into the susceptible cervical mucosa requires it to evade the cellular immune defense mechanism, particularly in WLHIV (16).
The present study reported an overall prevalence of 3.1% for HTLV-1 among WLHIV. Among WLHIV with HR-HPV infections, the prevalence of HTLV-1 was higher at 7.6%. This observed prevalence of HTLV-1 co-infection was lower than the previously reported rate of 19.5% among WLHIV by Maranga et al. (6) Nevertheless, the observed discrepancy in the prevalence of HTLV-1 co-infection among WLHIV with HR-HPV could be attributed to the differences in the biological samples utilized for HTLV-1 detection. In our study, PBMCs were used for molecular isolation of HTLV-1, while Maranga et al. employed liquid-based cytology (LBC) smears for HTLV-1 detection. In a separate study that aimed to assess if HTLV-1 increased susceptibility to cervical HPV infections among Brazilian women, it was noted that the prevalence of HPV was significantly higher among HTLV-1-infected women than in the control group, with rates of 44% versus 22.5%, respectively (10). In the present study, a statistically significant positive association (p ≤ 0.05) was observed between HTLV-1 infection and several HR-HPV types (16, 31, 35, 45, 51, 52, 56, 58, 59, and 66) in WLHIV. This strong association between HTLV-1 and HR-HPV infections among WLHIV may be due to the combined effects induced by HIV and HTLV-1. Such induction could lead to T-cell dysfunction, thereby increasing susceptibility to cervical HR-HPV infections. Similar to HIV, HTLV-1 infection causes an impulsive propagation of activated lymphocytes, resulting in elevated expression of proinflammatory cytokines and poor response to recall antigens, ultimately promoting immunosuppression (17, 18). Moreover, the observed higher prevalence of HR-HPV infections among WLHIV with HTLV-1 positivity may be attributable to the three viruses sharing sexual contact as a mode of transmission.
WLHIV with HR-HPV 31 had the highest proportion of HR-HPV/HTLV-1 co-infection (31.6%), followed by 58 (20.7%), where HR-HPV 39 had the lowest HR-HPV/HTLV-1 co-infection (7.1%). The higher prevalence of type 31 co-infection with HTLV-1 in this study may be due to regional differences in HPV prevalence or differences in the population studied, differences in the sample size, laboratory methods used for HR-HPV/HTLV-1 testing. It is also possible that the prevalence of specific HR-HPV genotypes varies over time and may be influenced by changes in sexual behavior or HPV vaccination rates in different population. Additionally, the immune status of the WLHIV participants may have played a role in the different distribution of HR-HPV genotypes observed in this study.
Although not statistically significant, WLHIV with a CD4+ cell count of less than 200 cells/microliter had the highest co-infection rate of HR-HPV/HTLV-1 (60%) compared to other CD4+ cell count categories. However, the CD4+ cell count alone may not be a reliable prognostic indicator when retroviruses are involved, as it may mask immunosuppression and must be interpreted with caution, as reported by Nasir et al. in their study among ART-naive HIV-infected participants (19). Consistent with a previous study conducted in Jamaica, a Brazilian study found an association between HTLV-1 infections and cervical HPV infections and persistence (20). The observed lower prevalence of HR-HPV/HTLV-1 co-infection in our study may be attributed to the high rate of ART enrolment, with 76% of participants being on ART for more than five years and 79% having suppressed HIV-1 RNA viral load. The high rate of HIV viral suppression suggests effective control of HIV replication and reduced attack on the immune system by the virus. However, given that SSA bears more than two-thirds of the global HIV burden, it is crucial to comprehensively assess HR-HPV/HTLV-1 co-infections in this context and establish the potential role of ART in preventing transmission and managing HTLV-1 infections, especially considering the absence of an effective cure or vaccine for HTLV-1 infections. An evaluation of efficient pre-exposure prophylaxis (PrEP) to prevent HTLV-1 transmission and the potential for a long-acting injectable that could simultaneously act against both HIV-1 and HTLV-1 should be considered. In addition, vaccination against HPV could play a significant role in reducing HR-HPV/HTLV-1 co-infections in WLHIV. While long-term solutions are being sought, condom use among WLHIV could offer interim measures for preventing transmission of these retroviral infections. Notably, a majority of WLHIV (55.6%) who used condoms did not have HR-HPV/HTLV-1 co-infections, while those who reported having unprotected sex had higher HR-HPV/HTLV-1 co-infections (54.5%). This finding is consistent with a Jamaican study on individuals with sexually transmitted diseases (21).
Although not statistically significant, the consumption of alcohol and smoking of cigarettes were more common among respondents with HR-HPV/HTLV-1 co-infections compared to those without. Smoking is known to compromise the Langerhans cells of the cervix, thereby reducing the effectiveness of local immune cells and increasing the risk of viral sexually transmitted infections (STIs) such as HR-HPV and HTLV-1 (22, 23). In a cohort study of women with normal cytology in Hawaii, Goodman et al. also found that alcohol consumption was associated with increased acquisition of cervical HPV (24, 25). These lifestyle characteristics, combined with multiple sexual partners as reported in our study, could have contributed to the higher prevalence of HR-HPV/HTLV-1 co-infections among WLHIV due to increased promiscuity.
Effective public health interventions are essential in addressing HR-HPV/HTLV-1 co-infections, including increased condom use, universal screening of blood, organs, and tissues to prevent HTLV-1 transmission, and optimal HPV vaccination among eligible WLHIV. Despite high rates of ART usage and HIV 1 RNA viral suppression, HR-HPV/HTLV-1 co-infections are increasing among WLHIV in Kenya, suggesting a need for further research into the persistent nature of HR-HPV in the presence of HTLV-1 and the development of cervical abnormalities in the ART era. Our study results are not generalizable since only WLHIV were studied and it was facility based. Our study had some limitations. The study encountered a notable phenomenon where more than 200 eligible women declined to participate, particularly among the younger age group of 18 to 22 years. However, the underlying reasons behind their decision were not investigated or determined in this study. Further, being a facility based study among WLHIV, it may not be generalized.
In conclusion, this study found a lower prevalence of HTLV-1 co-infection among women living with HIV (WLHIV) compared to previous reports. However, a higher prevalence of HTLV-1 was observed among WLHIV co-infected with HR-HPV. The strong association between HTLV-1 and HR-HPV infections among WLHIV may be attributed to the combined effects of HIV and HTLV-1 on T-cell dysfunction and immunosuppression. Efforts should focus on implementing interventions such as condom use, HPV vaccination, and universal screening to address the increasing rates of HR-HPV/HTLV-1 co-infections among WLHIV.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Nairobi and Kenyatta National Hospital Ethics Review Committee. The patients/participants provided their written informed consent to participate in this study.
The study was conceptualized and designed by JK, SG, EO, JP, KM, and MM. Data collection was conducted in the field by JK and AM. Data analysis was performed by JK and KM, JP, SG, EO, and MM. All authors contributed to the manuscript’s development, reviewed and edited it, and gave their final approval.
This research study and JK a graduate research fellow was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No. G-19-57145), Sida (Grant No:54100113), Uppsala Monitoring Center, Norwegian Agency for Development Cooperation (Norad), and by the Wellcome Trust [reference no. 107768/Z/15/Z] and the UK Foreign, Commonwealth & Development Office, with support from the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme. The statements made and views expressed are solely the responsibility of the Fellow. Additionally, the study was supported by the Union for African Population Studies (UAPS)- AFRES DATA Program and the HIV research Trust.
The authors express their gratitude to the Medical Research, Training and Innovation Department at Kenyatta National Hospital for technical support during the study. Technical guidance and support during the data collection phase from Jerusha Malubi and Lydia Ivita are also acknowledged. The study participants are appreciated for their substantial support. This research study and JK a graduate research fellow was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No. G-19-57145), Sida (Grant No:54100113), Uppsala Monitoring Center, Norwegian Agency for Development Cooperation (Norad), and by the Wellcome Trust [reference no. 107768/Z/15/Z] and the UK Foreign, Commonwealth & Development Office, with support from the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) programme. The statements made and views expressed are solely the responsibility of the Fellow. Additionally, the study was supported by the Union for African Population Studies (UAPS)- AFRES DATA Program and the HIV research Trust.
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in conducting this research study and writing this article.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are solely accountable for the statements made and opinions expressed, and they do not represent an official stance of the institution or funder.
1. Tempera I, Lieberman PM. Oncogenic viruses as entropic drivers of cancer evolution. Front Virol (2021) 1:28. doi: 10.3389/fviro.2021.753366
2. Huang J, Deng Y, Boakye D, Tin MS, Lok V, Zhang L, et al. Global distribution, risk factors, and recent trends for cervical cancer: A worldwide country-level analysis. Gynecol Oncol (2022) 164(1):85–92. doi: 10.1016/j.ygyno.2021.11.005
3. Mboumba Bouassa RS, Prazuck T, Lethu T, Jenabian MA, Meye JF, Bélec L. Cervical cancer in sub-Saharan Africa: a preventable noncommunicable disease. In: Expert Review of Anti-Infective Therapy, vol. Vol. 15. United Kingdom (UK): Taylor & Francis (2017). p. 613–27. doi: 10.1080/14787210.2017.1322902
4. Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Heal (2021) 9(2):e161–9. doi: 10.1016/S2214-109X(20)30459-9
5. Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene (2005) 24(39):5926–30. doi: 10.1038/sj.onc.1208980
6. He X, Maranga IO, Oliver AW, Gichangi P, Hampson L, Hampson IN. Analysis of the prevalence of HTLV-1 proviral DNA in cervical smears and carcinomas from HIV positive and negative Kenyan women. Viruses (2016) 8(9):245. doi: 10.3390/v8090245
7. Futsch N, Mahieux R, Dutartre H. HTLV-1, the other pathogenic yet neglected human retrovirus: from transmission to therapeutic treatment. Viruses (2018) 10(1):1. doi: 10.3390/v10010001
8. Blas MM, Alva IE, Garcia PJ, Carcamo C, Montano SM, Mun R. Association between Human Papillomavirus and Human T-Lymphotropic Virus in Indigenous Women from the Peruvian Amazon, Vol. 7. (United States of America (USA): PLOS One) (2012). pp. 4–9.
10. Lôpo SS, Oliveira PM, Santana IU, Pena GB, Torrales MB, Mascarenhas RE, et al. Evidence of a higher prevalence of HPV infection in HTLV-1-infected women: A cross-sectional study | Evidência de maior prevalência de infecção pelo HPV em mulheres infectadas pelo HTLV-1: Um estudo de corte transversal. Rev Soc Bras Med Trop (2012) 45(3):305–8. doi: 10.1590/S0037-86822012000300005
11. Miyazaki K, Yamaguchi K, Tohya T, Ohba T, Takatsuki K, Okamura H. Human T-cell leukemia virus type I infection as an oncogenic and prognostic risk factor in cervical and vaginal carcinoma. Obstet Gynecol (1991) 77(1):107–10. doi: 10.1016/0020-7292%2891%2990515-7
12. Menon S, Luchters S, Rossi R, Callens S, Kishor M, Bogers J, et al. Human papillomavirus correlates of high-grade cervical dysplasia in HIV-infected women in Mombasa, Kenya: a cross-sectional analysis. Virol J (2018) 15(1):54. doi: 10.1186/s12985-018-0961-3
13. Bradshaw D, Taylor GP. HTLV-1 Transmission and HIV pre-exposure prophylaxis: a scoping review. Front Med Front Media S A (2022) 9:1034. doi: 10.3389/fmed.2022.881547
14. Mamo L, Epstein S. The new sexual politics of cancer: Oncoviruses, disease prevention, and sexual health promotion oa. Biosocieties (2017) 12(3):367–91. doi: 10.1057/biosoc.2016.10
15. Moriuchi M, Moriuchi H. Seminal fluid enhances replication of human T-cell leukemia virus Type 1: implications for sexual transmission. J Virol (2004) 78(22):12709–11. doi: 10.1128/JVI.78.22.12709-12711.2004
16. Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination - Review of current perspectives. J Oncol (2019) 2019:3257939. doi: 10.1155/2019/3257939
17. Aghajanian S, Teymoori-Rad M, Molaverdi G, Mozhgani SH. Immunopathogenesis and cellular interactions in human T-cell leukemia virus type 1 associated myelopathy/tropical Spastic paraparesis. Front Microbiol Front Media S A (2020) 11:3141. doi: 10.3389/fmicb.2020.614940
18. Ghezeldasht SA, Shirdel A, Assarehzadegan MA, Hassannia T, Rahimi H, Miri R, et al. Human T lymphotropic virus type I (HTLV-I) oncogenesis: molecular aspects of virus and host interactions in pathogenesis of Adult T cell Leukemia/Lymphoma (ATL) (2013). Available at: www.mums.ac.ir/basic_medical/en/index.
19. Nasir IA, Ahmad AE, Emeribe AU, Shehu MS, Medugu JT, Babayo A. Molecular detection and clinical implications of HTLV-1 infections among antiretroviral therapy-Naïve HIV-1-infected individuals in Abuja, Nigeria. Virol (Auckl) (2015) 6(6):17. doi: 10.4137/VRT.s35331
20. Strickler HD, Rattray C, Escoffery C, Manns A, Schiffman MH, Brown C, et al. Human T-Cell lymphotropic virus type I and severe neoplasia of the cervix in jamaica. Int J Cancer (1995) 61(1):23–6. doi: 10.1002/ijc.2910610105
21. Murphy EL, Figueroa JP, Gibbs WN, Brathwaite A, Holding-Cobham M, Waters D, et al. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann Intern Med (1989) 111(7):555–60. doi: 10.7326/0003-4819-111-7-555
22. Vaccarella S, Herrero R, Snijders PJF, Dai M, Thomas JO, Hieu NT, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol (2008) 37(3):536–46. doi: 10.1093/ije/dyn033
23. Appleby P, Beral V, Berrington De González A, Colin D, Franceschi S, Goodill A, et al. Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer (2006) 118(6):1481–95. doi: 10.1002/ijc.21493
24. Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res (2008) 68(21):8813–24. doi: 10.1158/0008-5472.CAN-08-1380
Keywords: women living with HIV, high-risk human papillomavirus, Kenya, human T lymphotropic virus 1, ART
Citation: Kangethe JM, Gichuhi S, Odari EO, Pintye J, Mutai K, Abdullahi L, Maiyo A and Mureithi MW (2023) Co-infection of high-risk Human papillomavirus and Human T-lymphotropic virus-1 among women living with HIV on antiretroviral therapy at a tertiary hospital in Kenya. Front. Virol. 3:1228268. doi: 10.3389/fviro.2023.1228268
Received: 24 May 2023; Accepted: 11 July 2023;
Published: 31 July 2023.
Edited by:
Stefano Aquaro, University of Calabria, ItalyReviewed by:
Luiz Fernando Almeida Machado, Federal University of Pará, BrazilCopyright © 2023 Kangethe, Gichuhi, Odari, Pintye, Mutai, Abdullahi, Maiyo and Mureithi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Mburu Kangethe, amFtZXMubWJ1cnVAdW9uYmkuYWMua2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.