- 1Division of Hematology and Oncology, Department of Internal Medicine, St. Marianna University School of Medicine, Kawasaki, Japan
- 2Department of Frontier Medicine, Institute of Medical Science, St. Marianna University School of Medicine, Kawasaki, Japan
- 3Department of Laboratory Molecular Genetics of Hematology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 4Department of Hematological Therapeutics, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 5Center of Stem Cell and Regenerative Medicine, Advanced Multidisciplinary Research Cluster, Institute of Research, Tokyo Medical and Dental University (TMDU), Tokyo, Japan
- 6Department of Advanced Medicine for Viral Infections, National Center for Child Health and Development, Tokyo, Japan
Systemic chronic active Epstein-Barr virus infection (sCAEBV) is an intractable disease that present activated EBV-infected T- or NK-cells and their clonal proliferation. When inflammatory symptoms persist and proceed, a lethal complication of hemophagocytic lymphohistiocytosis (HLH) develops, but its biomarker to represent the pathophysiology and an effective agent to cure have not been developed as of today. It is known that interferon-γ (IFN-γ) level in the peripheral blood increases in HLH correlatedly with the disease condition and that antagonistic anti-IFN-γ antibody is effective against HLH. We examined the plasma level of IFN-γ to investigate its role in the disease condition of sCAEBV. sCAEBV was diagnosed based on the criteria conforming to the definition of sCAEBV in the WHO classification issued in 2017. As it was previously reported, disease activity was defined as the condition positive for any one of the followings: fever, liver dysfunction, progressive skin lesions, vasculitis, and uveitis. Eighteen sCAEBV patients were examined. Their plasma IFN-γ levels were significantly higher than those of healthy donors. The levels in sCAEBV patients with disease activity were higher than those without disease activity. The mRNA expression of IFNG was detected in EBV-infected cells of all patients. We also detected a correlation between plasma IFN-γ levels and mRNA levels of EBV-infected cells in peripheral blood mononuclear cells. These results suggest that EBV-infected cells produce IFN-γ in sCAEBV. Although the difference was not significant, the patients whose plasma IFN-γ levels at diagnosis were higher than 40 pg/mL tended to result in poorer survival than those with lower levels. We concluded that plasma IFN-γ is a potential biomarker that indicates disease activity of sCAEBV. Further study shall confirm its significance.
Introduction
Systemic chronic active Epstein-Barr virus infection (sCAEBV) is an intractable, progressive disease (1, 2). The disorder is classified as a T-cell and NK-cell neoplasm in the revised edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues published in 2017 (3). sCAEBV is not only a lymphoid neoplasm but is also an inflammatory disease. In sCAEBV, the activation of EBV-infected T- or NK-cells causes continuous or recurrent systemic inflammation and progresses to a lethal complication called hemophagocytic lymphohistiocytosis (HLH). The only curative treatment strategy that can eradicate EBV-infected T- or NK-cells today is allogeneic hematopoietic stem cell transplantation (allo-HSCT) (4), but sCAEBV patients with disease activity result in poorer outcomes after allo-HSCT compared to those without. Disease activity is defined as the status with any one of the following conditions: fever, elevated alanine transaminase (ALT), vasculitis, and uveitis (5, 6). Unfortunately, medical treatment that successfully controls disease activity has not been established. It is an urgent issue to clarify the mechanism of disease activity development and to establish an effective treatment strategy for sCAEBV.
There are reports of inflammatory cytokines being responsible for the inflammation occurrence in sCAEBV (7). Among those cytokines, interferon-γ (IFN-γ) is important in the pathogenesis of HLH (8). It is now clear that this cytokine is produced and secreted in EBV-positive NK-cells and induces the differentiation and the activation of macrophages, which are responsible for HLH development in EBV-positive NK-cell neoplasms (9). In this study, we investigated the role of IFN-γ in the development of sCAEBV.
Materials and methods
Diagnosis of sCAEBV
We diagnosed patients based on the diagnostic criteria of sCAEBV (4, 6), which was developed by the Research Group of Measures Against Intractable Diseases of the Ministry of Health, Labor, and Welfare of Japan. The criteria are compatible with the definition of sCAEBV in the WHO 2017 classification.
1. Elevated EBV-DNA load in peripheral blood (PB) (>102.5 copies/μg DNA).
2. EBV infection of T- or NK-cells in the affected tissue or PB.
3. Systemic inflammatory symptoms persisting for >3 months.
4. Exclusion of other possible diseases: primary infection of EBV (infectious mononucleosis), autoimmune disease, congenital immunodeficiency, human immunodeficiency virus infection, other immunodeficiencies requiring immunosuppressive therapy, or underlying diseases with potential immunosuppression.
Definition of disease activity
Patients with any one of the defined findings of persistent inflammation were determined as to possess disease activity (4, 5). The defined findings of inflammation included: fever, liver dysfunction, progressive skin lesions, vasculitis, or uveitis.
The definition of liver dysfunction was an increase in ALT levels to two times higher than the upper limit of normal. Progressive skin lesions and vasculitis were diagnosed by pathological examination. Uveitis was diagnosed by the patients’ attending physicians and ophthalmologists. The disease status of patients without any of these clinical findings was determined as inactive.
Isolation and identification of sCAEBV cells
EBV-infected cells were isolated from peripheral blood mononuclear cells (PBMC) using Lymphoprep™ (Abbott Diagnostics Technologies, Ravenswood, CA, USA) and sorted into CD19-, CD4-, CD8-, or CD56-positive fractions using antibody-conjugated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). After the sorting, the levels of EBV DNA in each fraction were measured by real-time PCR using TaqMan® system (Applied Biosystems, Waltham, MA,USA) (10).
Detection of the clonality
The clonal proliferation of EBV-infected cells was detected by Southern blotting for EBV-terminal repeat (11).
Cytokine analysis
The plasma concentration of IFN-γ was measured by high sensitivity cytokine beads assay according to the manufacturer’s instructions (MILLIPLEX®MAP Kit, EMD Millipore Corporation, Burlington, MA, USA).
Cell lines
SNT8 was derived from T cell type of nasal T/NK cell lymphoma. SNK6 was derived from NK cell type of nasal T/NK cell lymphoma (12). They were cultured in Artemis Medium-2 (Nihon Techno Service, Ibaraki, Japan). Jurkat was obtained from Health Science Research Resources Bank (Osaka, Japan) and cultured in 10% FCS-RPMI.
Quantitative reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA was extracted from the target cells with ISOGEN II (Nippon Gene, Tokyo, Japan) in accordance with the manufacturer’s protocol, and cDNA was generated using PrimeScript™ RT Master Mix (Takara Bio Inc., Kusatsu, Japan). qRT-PCR analysis was performed on a LightCycler® 480 (Roche Diagnostics, Basel, Switzerland) using TaqMan® Gene Expression Assays (Applied Biosystems). The probes for IFNG and GAPDH were Hs00989291_m1 and Hs00266705_g1, respectively (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Statistical analyses
Statistical analysis was processed by Mann-Whitney test, Pearson’s correlation coefficient and Kaplan-Meier method using EZR version 1.53 (Jichi Medical University Saitama Medical Center, Saitama, Japan) (13), which is a graphical user interface for the software R version 4.02 (The R Foundation for Statical Computing, Vienna, Austria).
Results
Patients’ characteristics
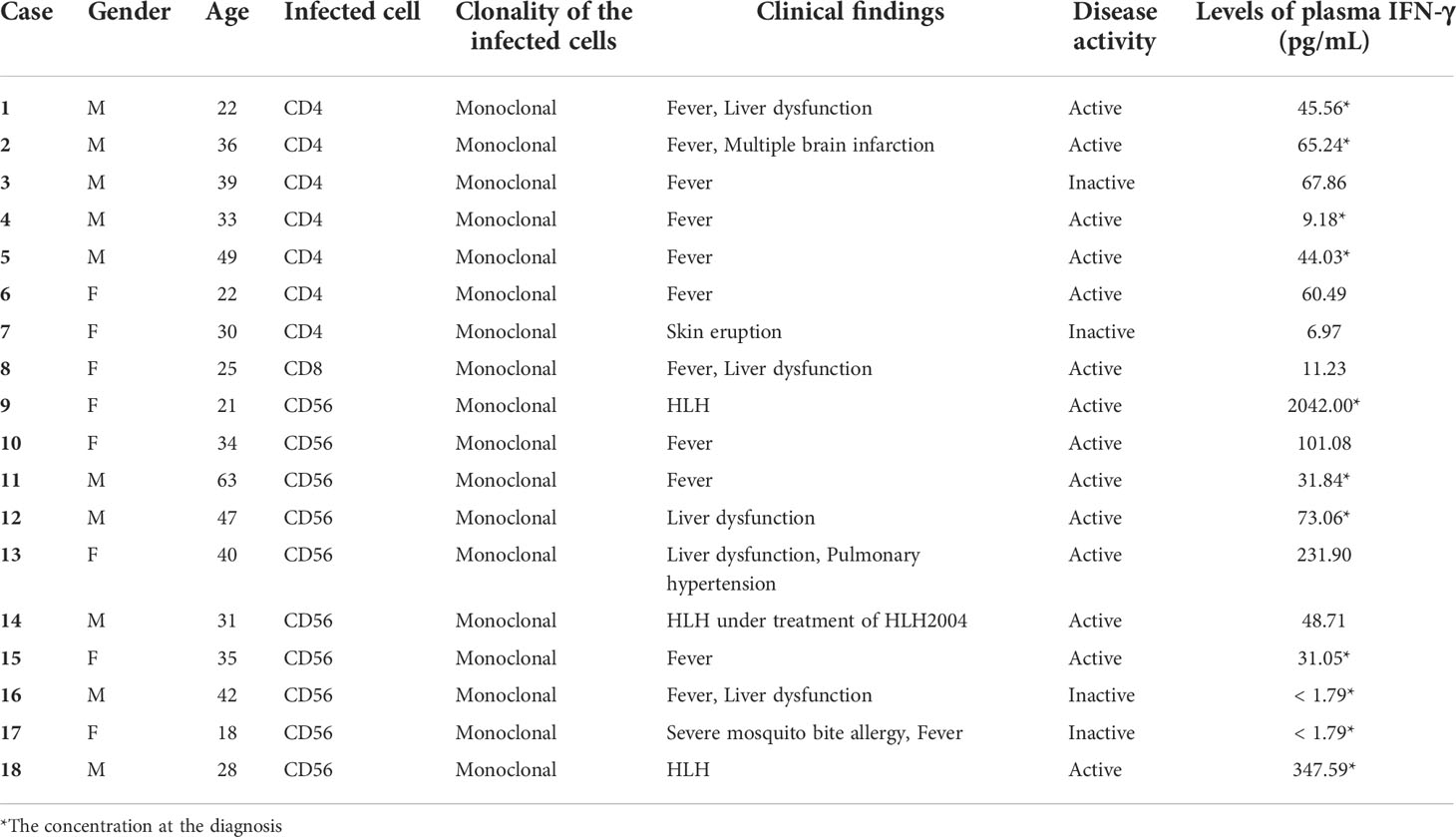
We examined 18 sCAEBV patients (age of 18-63 years, median 33 years; ten males, eight females; CD4 type n = 7, CD8 type n = 1, and CD56 type n = 10). The characteristics of the patients are summarized in Table 1. The clonal proliferation of infected cells was detected in the PBMCs of all patients. Fourteen patients had disease activity. Three patients accompanied HLH and 2 of them were untreated.
The plasma IFN-γ levels in sCAEBV patients
First, we compared the levels of plasma IFN-γ in 18 sCAEBV patients to those of 8 healthy donors. As shown in Figure 1A, the levels in sCAEBV patients were higher than those in healthy donors. We examined the levels by phenotypes of EBV-infected cells in the patients. There was no difference in the plasma IFN-γ between different phenotypes (Figure 1B). We then investigated the correlation between the plasma IFN-γ levels and the disease activity of sCAEBV. As shown in Figure 1C, the levels in sCAEBV patients with disease activity were higher than those without disease activity. There was no difference in the levels between the patients without disease activity and the healthy donors. These findings indicated that plasma IFN-γ level was associated with the disease activity of sCAEBV. We also examined the correlation between the plasma concentration of IFN-γ and Interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α), and Interleukin-1β (IL-1β), which elevated in sCAEBV patients (14, 15). As shown in Figures 1D–F, no correlation was observed.
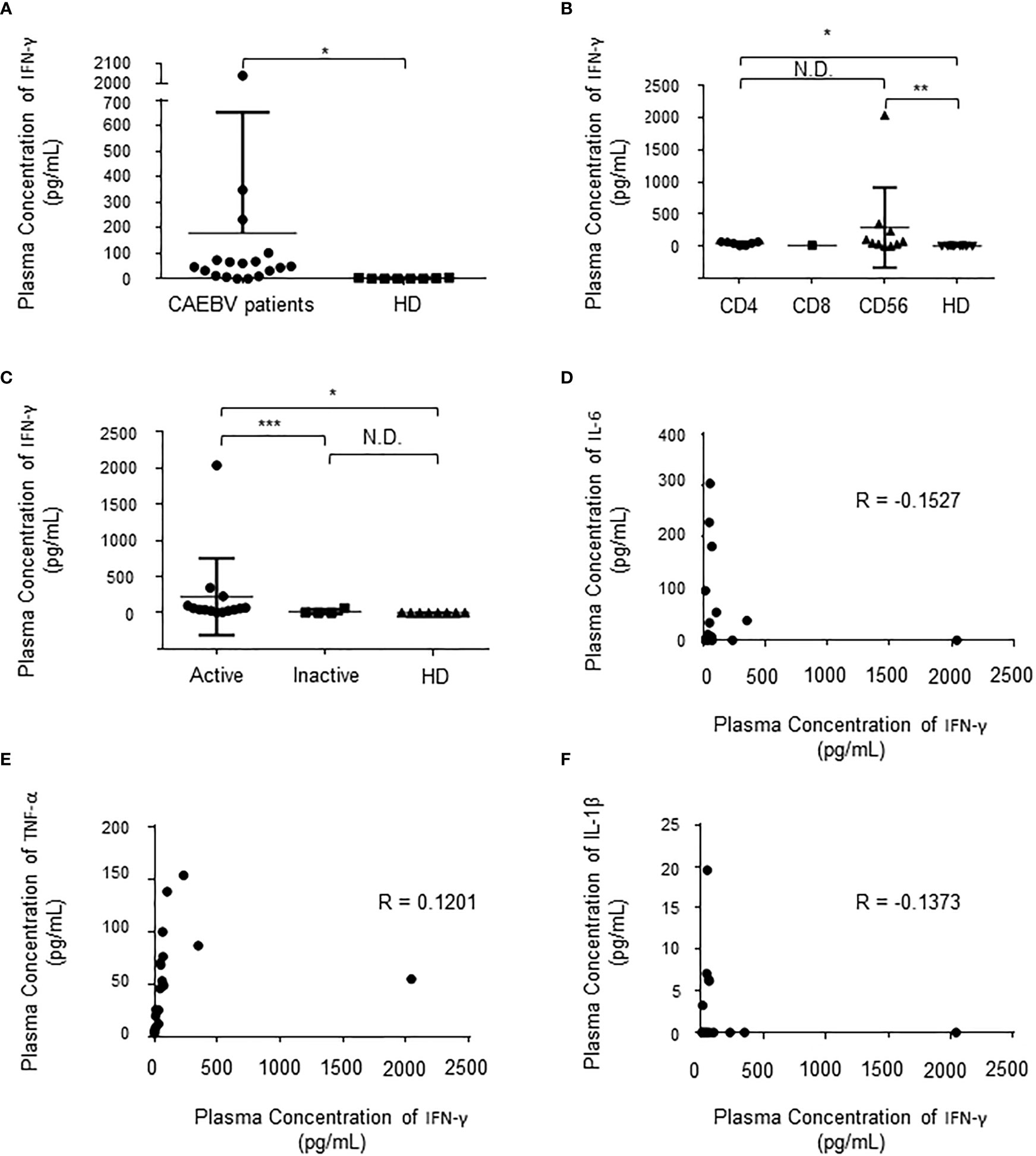
Figure 1 The plasma concentration of IFN-γ in sCAEBV patients. (A). The concentration in sCAEBV patients and healthy donors. Significant difference is indicated as * for p < 0.001 between the patients and the healthy donors. HD: healthy donors. (B). The concentrations by EBV-infected cell types. Significant differences are indicated as * for p < 0.001 and ** for p < 0.01. N.D: no difference. HD: healthy donors. (C). The concentrations by disease activity. Significant differences are indicated as * for p < 0.001 and *** for p < 0.05. N.D: no difference. HD: healthy donors. (D–F). The correlation between the plasma concentration of IFN-γ and the plasma concentration of IL-6 (D), TNF-α (E), and IL-1β (F). R: correlation coefficient.
IFN-γ-producing cells in sCAEBV
Next, we tried to identify the phenotype of IFN-γ-producing cells in 3 patients. We separated lymphocytes from patients’ PB into fractions. As shown in Figure 2A, the expression of IFNG mRNA was detected in the fractions of EBV-infected cell of all patients. The expression in patients was higher in comparison to that of healthy donors. Interestingly, the mRNA was also elevated in non-infected cells in the patients. Then, we investigated the relation between IFNG mRNA expression in the EBV-infected cells and the plasma concentration of IFN-γ. There was a statistical correlation between the plasma IFN-γ levels and the mRNA levels of EBV-infected cells (Figure 2B). A patient whose plasma IFN-γ was extremely high harbored HLH with markedly high expression of IFNG mRNA in EBV-infected cells. EBV-DNA load in the peripheral blood reflects the number of EBV-infected cells (16). We examined the correlation between EBV-DNA load and the concentration of IFN-γ in the plasma in 11 cases of which the values of EBV-DNA load were measures when IFN-γ was measured at diagnosis (Table 1). As shown in Figure 2C, there was no correlation between the two values. We reached to a conclusion that IFN-γ was produced in EBV-infected cells in sCAEBV patients.
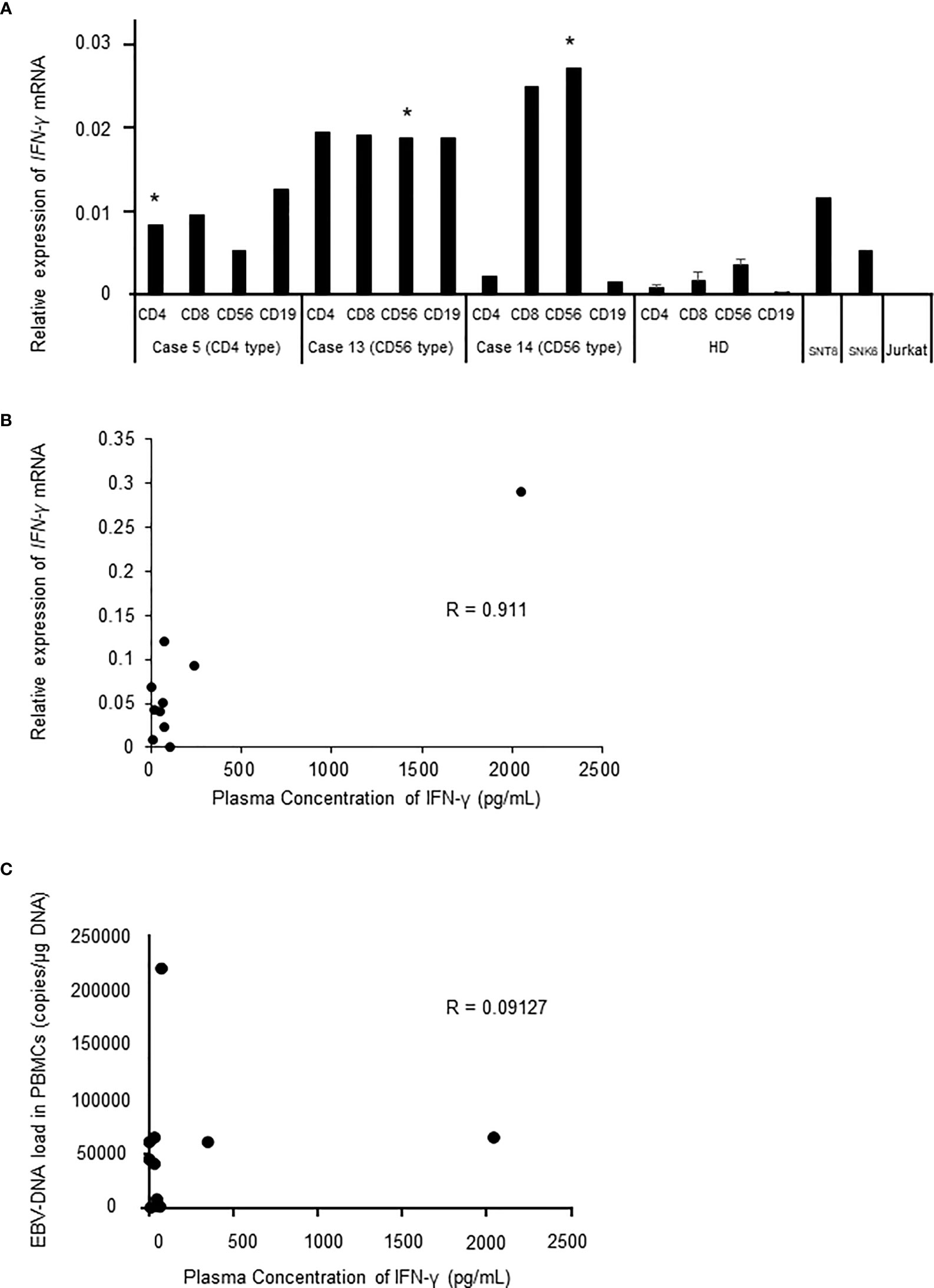
Figure 2 The mRNA expression of IFNG in sCAEBV patients (A). IFNG expression of sCAEBV patients by lymphocyte fraction. EBV-infected cell fractions are indicated as *. SNT8 cells and SNK6 cells are positive controls. Jurkat cells are negative control. HD: healthy donors. (B). The correlation between mRNA expression and the plasma levels of IFN-γ in sCAEBV patients. R: correlation coefficient. (C). The correlation between EBV-DNA load in the peripheral blood mononuclear cells (PBMCs) and the plasma levels of IFN-γ in sCAEBV patients.
Do plasma IFN-γ levels affect the survival of sCAEBV?
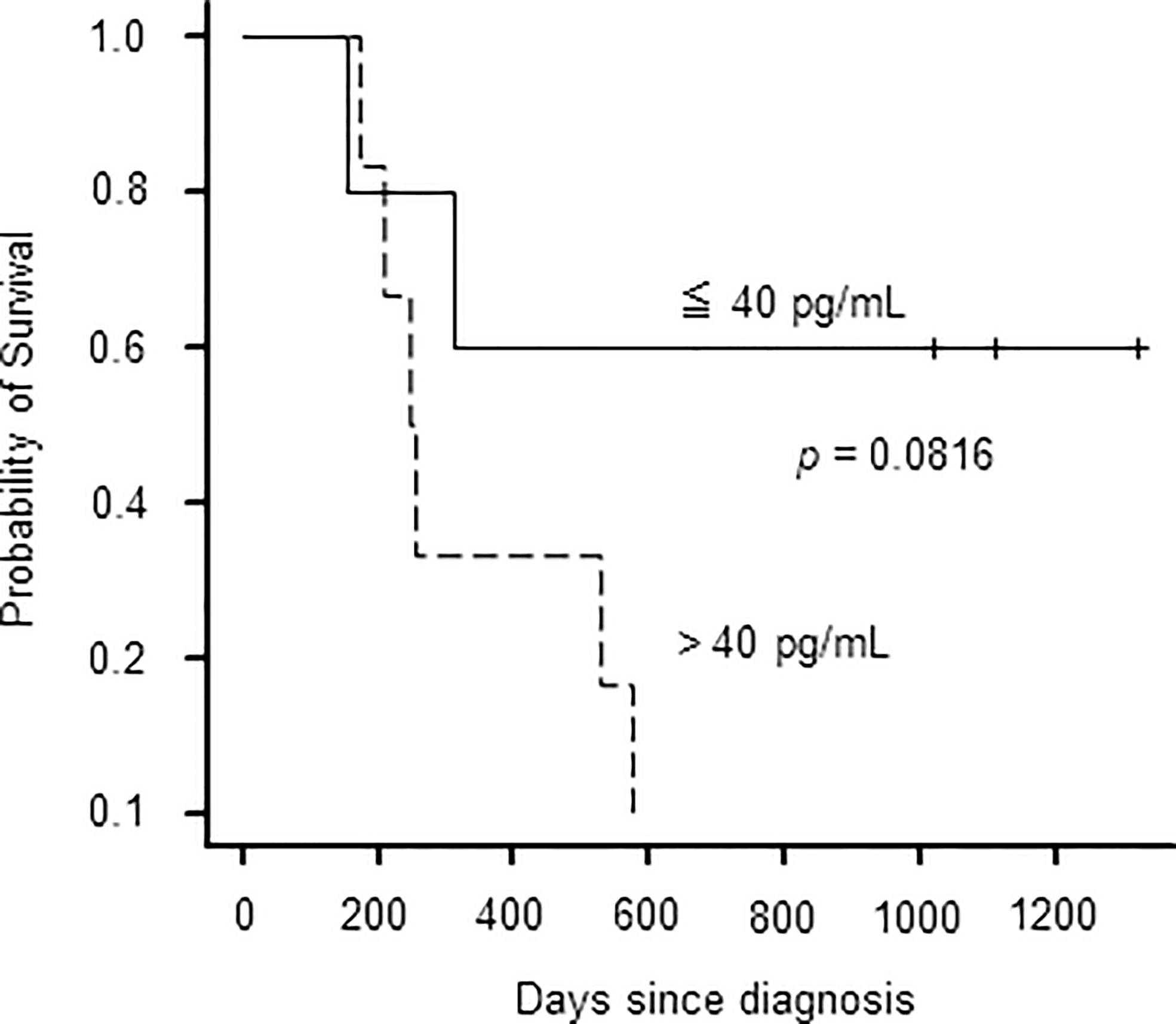
Previous reports state that disease activities at allo-HSCT are associated with poor outcomes of sCAEBV (5). We investigated the correlation between plasma IFN-γ levels and the survival of the patients because plasma IFN-γ levels correlated with disease activities. We examined 11 patients whose plasma IFN-γ levels were measured at diagnosis. Patients with plasma IFN-γ levels higher than 40 pg/mL at diagnosis tended to have poorer survival than those with low IFN-γ levels although the difference was not significant (Figure 3).
The plasma IFN-γ levels during the clinical course
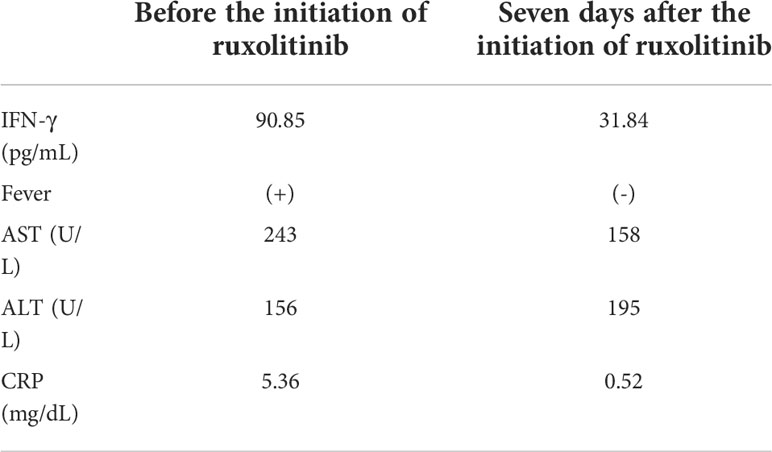
Finally, we examined if plasma IFN-γ level decreased to coincide with the resolution of disease activity of sCAEBV. Case 18 received an allo-HSCT, but did not achieve complete response. In EBV-infected T- or NK-cells of sCAEBV, STAT3, a substrate of JAK1/2, is constitutively activated and mediates the expression of mRNA of IFN-γ (7). Based on a report stating the efficacy of ruxolitinib on inflammatory symptoms of sCAEBV (17), we treated Case 18 with ruxolitinib. We started its administration 5 mg/day and increased to 10 mg/day. Fever resolved on the next day of the ruxolitinib initiation. The clinical findings on day 7 are shown in Table 2. The patient became afebrile and his liver function improved. Interestingly, the concentration of IFN-g in the plasma decreased after this treatment.
Discussion
In this study, we focused on IFN-γ in the plasma of sCAEBV patients, and we investigated the correlation of the IFN-γ concentration and the clinical features of patients. The concentration was higher in the plasma of sCAEBV patients compared to that of healthy donors, and we could assume that high concentration level is associated with the formation of pathophysiology of sCAEBV.
We had reported that IFN-γ is produced in EBV-infected T- and NK-cells of sCAEBV patients (7). We had also reported that constitutively activated STAT3 in EBV-infected cells contributed to the production of IFN-γ (7). In this study, we detected an elevated expression of IFNG mRNA in EBV-infected cell fraction and a correlation between the plasma IFN-γ levels and the mRNA levels of EBV-infected cells. These results suggest that EBV-infected cells produce IFN-γ in sCAEBV. We also detected IFNG mRNA expression in the fractions of the non-infected cells of the patients PBMCs. Not only T or NK cells but also B cells, monocytes, and macrophages can produce IFN-γ (18). Thus, these EBV-negative cells may be activated and may play some roles in the production of IFN-γ in sCAEBV. Various types of immune cells not only exist in PB but also infiltrate the lesions of sCAEBV including EBV-negative cells. We need to examine the infiltrating cells of pathophysiological tissues as well as examining PB.
IFN-γ is a cause of inflammatory symptoms and macrophage activation leading to HLH, a lethal complication. In the present study, we found that the concentration of IFN-γ is significantly higher in sCAEBV patients with disease activity compared to sCAEBV patients without disease activity. Two patients with the highest plasma IFN-γ levels had untreated HLH. In Case 18, as the activity of sCAEBV improved after starting ruxolitinib administration, the concentration of IFN-γ in the plasma decreased. These findings led us to assume that IFN-γ is a potent biomarker of disease activity and a cause of HLH. There was no significant correlation with IFN-γ concentration and the survival time probably because this study’s sample size was too small. Further study is necessary to prove the effects of IFN-γ on the outcome of sCAEBV.
Since IFN-γ is a cause of inflammation and HLH development in sCAEBV, it is indispensable to suppress IFN-γ to manage sCAEBV. Ruxolitinib, a JAK1/JAK2 inhibitor, is reported to reduce the expression of IFNG mRNA in EBV-infected T- and NK-cells of sCAEBV patients. Ruxolitinib had effects on the inflammatory symptoms of Case 18. We highly expect the drug to be a potent therapeutic remedy (7). Emapalumab, an IFN-γ-blocking antibody, has shown its usefulness in a clinical trial of pediatric primary HLH (19). This result suggests its potential efficacy in controlling the disease condition of sCAEBV.
Our study has a limitation for not targeting enough number of patients because sCAEBV is a rare disease. To explore the validity of IFN-γ as a biomarker and its correlation with prognosis, we must accumulate more cases and more biological samples. There is a movement to establish a registry system of sCAEBV in Japan. We hope this system will be effectively used to for further investigation targeting a larger number of registered cases of sCAEBV.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Tokyo Medical and Dental University (TMDU) (Approval #G2000-176) and St. Marianna University School of Medicine (Approval #4753). Ruxolitinib treatment for sCAEBV was approved by the institutional review board of TMDU. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YU and AA designed the research, performed the experiments, analyzed the data, and wrote the draft. AO, MY, MN, NW, and KI-I performed the experiments and analyzed the data. TH and NS analyzed the data. AA collected samples. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the grants, “Practical Research Project for Rare/Intractable Diseases (18ek0109334h0001, 19ek0109334h0002, 20ek0109334h0003, 21ek0109334h0004)” from the Japan Agency for Medical Research and Development (AMED) and “Grant-in-Aid for Early-Career Scientists (21K16254)” from Japan Society for the Promotion of Science Research.
Acknowledgments
We give special thanks to Ayako Komoto for her excellent editorial support to the authors during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arai A. Advances in the study of chronic active Epstein-Barr virus infection: Clinical features under the 2016 WHO classification and mechanisms of development. Front Pediatr (2019) 7:14. doi: 10.3389/fped.2019.00014
2. Kimura H, Cohen JI. Chronic active Epstein-Barr virus disease. Front Immunol (2017) 8:1867. doi: 10.3389/fimmu.2017.01867
3. L Q-M. WHO classification of tumours of haematopoietic and lymphoid tissue. Lyon Int Agency Res Cancer (2017) 2:2 355–63.
4. Yonese I, Sakashita C, Imadome KI, Kobayashi T, Yamamoto M, Sawada A, et al. Nationwide survey of systemic chronic active EBV infection in Japan in accordance with the new WHO classification. Blood Adv (2020) 4(13):2918–26. doi: 10.1182/bloodadvances.2020001451
5. Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood (2012) 119(3):673–86. doi: 10.1182/blood-2011-10-381921
6. Yamamoto M, Sato M, Onishi Y, Sasahara Y, Sano H, Masuko M, et al. Registry data analysis of hematopoietic stem cell transplantation on systemic chronic active Epstein-Barr virus infection patients in Japan. Am J Hematol (2022) 97(6):780–90. doi: 10.1002/ajh.26544
7. Onozawa E, Shibayama H, Takada H, Imadome K, Aoki S, Yoshimori M, et al. STAT3 is constitutively activated in chronic active Epstein-Barr virus infection and can be a therapeutic target. Oncotarget (2018) 9:31077–89. doi: 10.18632/oncotarget.25780
8. Henter JI, Elinder G, Soder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood (1991) 78(11):2918–22. doi: 10.1182/blood.V78.11.2918.2918
9. Yoshimori M, Nishio M, Ohashi A, Tateishi M, Mimura A, Wada N, et al. Interferon-gamma produced by EBV-positive neoplastic NK-cells induces differentiation into macrophages and procoagulant activity of monocytes, which leads to HLH. Cancers (Basel) (2021) 13(20):5097. doi: 10.3390/cancers13205097
10. Kimura H, Morita M, Yabuta Y, Kuzushima K, Kato K, Kojima S, et al. Quantitative analysis of Epstein-Barr virus load by using a real-time PCR assay. J Clin Microbiol (1999) 37(1):132–6. doi: 10.1128/jcm.37.1.132-136.1999
11. Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell (1986) 47(6):883–9. doi: 10.1016/0092-8674(86)90803-2
12. Zhang Y, Nagata H, Ikeuchi T, Mukai H, Oyoshi MK, Demachi A, et al. Common cytological and cytogenetic features of Epstein-Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br J Haematol (2003) 121(5):805–14. doi: 10.1046/j.1365-2141.2003.04359.x
13. Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant (2013) 48(3):452–8. doi: 10.1038/bmt.2012.244
14. Arai A, Nogami A, Imadome KI, Kurata M, Murakami N, Fujiwara S, et al. Sequential monitoring of serum IL-6, TNF-α, and IFN-γ levels in a CAEBV patient treated by plasma exchange and immunochemotherapy. Int J Hematol (2012) 96(5):669–73. doi: 10.1007/s12185-012-1170-2
15. Ohashi A, Uemura Y, Yoshimori M, Wada N, Imadome KI, Yudo K, et al. The plasma level of interleukin-1β can be a biomarker of angiopathy in systemic chronic active Epstein-Barr virus infection. Front Microbiol (2022) 13:874998. doi: 10.3389/fmicb.2022.874998
16. Ito Y, Suzuki M, Kawada J, Kimura H. Diagnostic values for the viral load in peripheral blood mononuclear cells of patients with chronic active Epstein-Barr virus disease. J Infect Chemother (2016) 22(4):268–71. doi: 10.1016/j.jiac.2015.11.002
17. Song Y, Wang J, Wang Y, Wang Z. Ruxolitinib in patients with chronic active Epstein-Barr virus infection: A retrospective, single-center study. Front Pharmacol (2021) 12:710400. doi: 10.3389/fphar.2021.710400
18. De Benedetti F, Prencipe G, Bracaglia C, Marasco E, Grom AA. Targeting interferon-γ in hyperinflammation: opportunities and challenges. Nat Rev Rheumatol (2021) 17(11):678–91. doi: 10.1038/s41584-021-00694-z
Keywords: systemic chronic active Epstein-Barr virus infection (sCAEBV), Epstein- Barr virus, T cell, NK cell, interferon-γ, hemophagocytic lymphohistiocytosis (HLH), disease activity
Citation: Uemura Y, Ohashi A, Yoshimori M, Nishio M, Hirakawa T, Shimizu N, Wada N, Imadome K-I and Arai A (2022) Plasma interferon-γ concentration: a potential biomarker of disease activity of systemic chronic active Epstein-Barr virus infection. Front. Virol. 2:999929. doi: 10.3389/fviro.2022.999929
Received: 09 August 2022; Accepted: 09 September 2022;
Published: 29 September 2022.
Edited by:
Andrea Lombardi, University of Milan, ItalyReviewed by:
Hisashi Iizasa, Shimane University, JapanMasashi Fukayama, Asahi General Hospital, Japan
Copyright © 2022 Uemura, Ohashi, Yoshimori, Nishio, Hirakawa, Shimizu, Wada, Imadome and Arai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayako Arai, YXJhLmhlbWFAbWFyaWFubmEtdS5hYy5qcA==
 Yu Uemura1
Yu Uemura1 Ayaka Ohashi
Ayaka Ohashi Tsuneaki Hirakawa
Tsuneaki Hirakawa Ayako Arai
Ayako Arai