- 1Department of Microbiology and Immunology, Faculty of Medicine, Canadian Centre for Vaccinology CCfV, Dalhousie University, Halifax, NS, Canada
- 2Laboratory of Immunity, Shantou University Medical College, Shantou, China
- 3Gerencia Regional de Salud de Castilla y León, Group for Biomedical Research in Sepsis (BioSepsis), Instituto de Investigación Biomédica de Salamanca (IBSAL), Salamanca, Spain
- 4Gerencia Regional de Salud de Castilla y León, Hospital Universitario Río Hortega, Valladolid, Spain
- 5Vaccine and Infectious Disease Organization-International Vaccine Centre (VIDO-InterVac), Saskatoon, SK, Canada
- 6Department of Biomedical Sciences, University of Sassari, Sassari, Italy
- 7Rwanda Biomedical Center (RBC), Kigali, Rwanda
The COVID-19 pandemic, caused by the SARS-CoV-2 coronavirus, is responsible for over 400 million cases and over 5. 5 million deaths worldwide. In response to widespread SARS-CoV-2 infection, immunization of the global population has approached 60% one dose and 54% full dose vaccination status. Emerging data indicates decreasing circulating antibody levels as well as decreases in other immune correlates in vaccinated individuals. Complicating the determination of vaccine effectiveness is the concomitant emergence of novel SARS-CoV-2 variants with substantial antigenic differences from the ancestral D614G strain. The Omicron variant (B.1.1.529) spike protein has over 30 mutations compared with the D614G spike protein, which was used to design most SARS-CoV-2 vaccines in use today. Therefore, breakthrough cases of SARS-CoV-2 infections or severe disease in fully vaccinated individuals must be interpreted with caution taking into consideration vaccine waning and the degree of vaccine variant-mismatch resulting in adaptive immune evasion by novel emerging SARS-CoV-2 variants.
Introduction
In December 2019, a pneumonia-like disease of unknown cause was discovered in Wuhan, Hubei Province, China (1). The causative virus for this outbreak was identified as a new coronavirus, which was later named severe acute respiratory syndrome coronavirus 2019 (SARS-CoV-2). The novel coronavirus 2019 (COVID-19) is responsible for over 400 million cases and over five million deaths worldwide. Messenger RNA (mRNA) based vaccines for the prevention of SARS-CoV-2, such as those from Pfizer-BioNTech or Moderna, and the urgency associated with the global pandemic have redefined the timeline for vaccine approval and rollout. Although disparities in global vaccine distribution remain central to the COVID-19 pandemic, roughly 60% of the world population has been vaccinated; that is, having received a first dose, while 54% are fully vaccinated (both doses), and over 10 billion doses have been administered in total. Although full vaccination significantly reduces the probability of being infected by SARS-CoV-2, a certain risk for viral transmission remains. However, the risk of acquiring a SARS-CoV-2 infection from a partially or fully vaccinated individual is relatively low (<1%) (2, 3). When a fully vaccinated individual contracts SARS-CoV-2, it is known as a vaccine breakthrough infection. Breakthrough cases are likely explained by a combination of immunological phenomena, including the failure to generate an immune response to viral components following vaccination, wanning vaccine immunity caused by a decline in immunological correlates of protection, or a shift in antigenicity of the circulating virus resulting in vaccine mismatch, where those vaccinated with an early vaccine iteration (such as those developed against the alpha strain) are not protected against emerging virus variants. Breakthrough cases are also influenced by a vaccine's capacity to generate sterilizing immunity, which is a form of innate immunity present in mucosal tissues (such as in the nose, throat, and upper respiratory tract) that fully prevents disease by an invading pathogen, such as SARS-CoV-2 (4). Additionally, high-risk groups such as immunosuppressed individuals and the elderly do not respond to vaccination as well as young, healthy individuals.
In the United States (US), the Centers for Disease Control and Prevention (CDC) reported that weekly case numbers and deaths remain highest for unvaccinated individuals. However, among the three major vaccine manufacturers in the US, Johnson and Johnson (J&J) has the highest rate of morbidity and mortality among fully vaccinated individuals, followed by Pfizer and Moderna. Interestingly, Moderna showed the lowest number of breakthrough cases among the three manufacturers. Those individuals 18 years of age or older who received J&J or AstraZeneca vaccines were subsequently recommended to receive a booster dose (5). Further data obtained from the CDC COVID-NET surveillance program showed that, in a three-month time span (Jan 2021–Apr 2021), over 10,000 SARS-CoV-2 breakthrough infections had been reported across 46 US states; a plausible explanation for this was the shift from the alpha variant (B.1.1.7) to more virulent forms of the virus, such as the beta (B.1.351) and delta (B.1.617.2) variants. Within these 10,000 cases, the median age was 58 years old; 63% of cases occurred in females (despite higher SARS-CoV-2 disease severity and mortality in males); 27% were asymptomatic; 10% of patients were hospitalized; and a further 2% died while in hospital (6). Among the 2% of reported deaths, the median age was 82 years. Sequence data also revealed that breakthrough cases were caused by novel variants, such as alpha (B.1.1.7, 56%), epsilon (B.1.429, 25%), B.1.427 (8%), gamma (P.1, 8%), and beta (B.1.351, 4%) (3). The constant emergence of novel variants creates the need to reassess vaccinated immune protection, waning vaccine protection, and immune status of each individual.
Comparable to the situation in the US, instances of COVID-19 breakthrough cases are becoming more globally recognized as a serious health threat. For example, a recent cohort study (unpublished data, referring to findings prior to peer review) in Israel found that in a sample of 1497 healthcare workers (HCWs) fully vaccinated with the Pfizer-BioNTech Comirnaty® mRNA (BNT162b2) vaccine, 39 workers tested positive for COVID-19 following RT-PCR testing. (3) Among these 39 healthcare workers, the majority of individuals were female (64%), nursing staff members (46%), and had an average age of 42 years old. The most common symptoms experienced among infected individuals included upper respiratory congestion, myalgia, and loss of smell or taste. At 6 weeks following infection, 19% of infected individuals reported experiencing ongoing loss of smell, cough, fatigue, and weakness, a phenomenon otherwise known as “long COVID-19”. Interestingly, in both the CDC and Israeli data, women appear to be at an increased risk (63 and 64%, respectively) of contracting a breakthrough COVID-19 infection. These results counter the fact that males have been widely reported as having a disproportionately higher rate of infection, disease severity, and mortality when compared to females (7). This female bias in breakthrough cases may be explained by where these cases are most likely to occur. For example, HCWs, including those donned with full personal protective equipment (PPE), are at a high risk of COVID-19 exposure. These risks may be heightened for frontline and triaging HCWs such as nurses and emergency physicians, who are the first line of medical treatment for potentially infected individuals. In a case study in Italy, one partially vaccinated and two fully vaccinated healthcare professionals (two doctors and one nurse) were infected by the same SARS-CoV-2 (B.1.1.7) positive patient (8). The patient was a 50-year-old male who reported to the emergency service with respiratory failure and pulmonary oedema, which required immediate endotracheal intubation. The patient died 2 h following admission to the ICU and during intubation all procedures were performed with full PPE, including: particulate filter respirators (P3), two pairs of gloves, face shields, and a single-use coverall (8). Noteworthy is the fact that the HCWs in this case study were not wearing goggles under their face shields, which is a recommended safety measure from the CDC. Given that the majority of frontline HCWs are females (9), possible explanations for the greater number of female breakthrough COVID-19 cases is the increased exposure, non-universal PPE standards, and sub-optimized implementation of PPE in hospitals and testing centers, such as the lack of additional eye protection in the Italian case study. In a separate study in the United States, the majority of breakthrough cases (54%) were reported in women, which further challenges the notion that men routinely exhibit disproportionately high rates of SARS-CoV-2 infection, disease severity, and mortality compared to women (7, 10). This female bias in breakthrough infections should be investigated in further detail to delineate the underlying immunological mechanisms at play, in addition to the social and behavioral factors placing women at greater risk of infection.
Immune and Virological Mechanisms of Breakthrough Cases
Potential causes for COVID-19 breakthrough cases may be explained via a myriad of mechanisms such as PPE failure; individual immune status; age; sex; variant infectivity or pathogenicity; relaxed isolation and masking measures; waning vaccine induced immune protection; and mismatch between vaccine and circulating SARS-CoV-2 variants. One retrospective cohort study found that the incidence of breakthrough cases (alpha or beta variant) among patients fully vaccinated (≥14 days after second dose) with the Comirnaty® vaccine was three times higher for immunocompromised individuals than for those with normal immune system function (11). In addition to impaired immune function, old age also places vaccinated individuals at a greater risk of a severe breakthrough infection. In a data report from the CDC from January to April 2021, the median age from 10,000 reported breakthrough cases was 52 years old, while the two percent of patients who died had a median age of 82 years old (6). An additional CDC report in July 2021 found that in a cluster of 469 COVID-19 breakthrough cases (with 346; 74% fully vaccinated) in Massachusetts, the median age was 40 years old (12). These data show that those <70 years of age are at a greater risk of death following a breakthrough case and that breakthrough cases occur across a wide range of ages. The CDC also found that the majority of individuals infected with breakthrough cases experienced mild symptoms, such as headache, cough, and sore throat, while others were asymptomatic (6, 12). Although these symptoms are indicative of moderate viral load and limited ability to infect others, studies have found that viral load levels can be as high in individuals with a breakthrough infection as those who are unvaccinated against SARS-CoV-2 (2, 12). The phenomenon of viral shedding in breakthrough cases places the elderly and immunocompromised at a higher risk of encountering a breakthrough infected individual who is unaware of their COVID-19 disease status. The ability of these vaccinated individuals to actually transmit the virus remains to be elucidated, since their mild symptoms could reflect a limited viral replication in the upper respiratory tract.
Vaccine Waning and Vaccine Effectiveness
As the large-scale, global vaccination campaign against SARS-CoV-2 continues, a void remains in our understanding of vaccine effectiveness. Clinical trial data have found the Comirnaty® mRNA vaccine to be up to 91.3% effective against COVID-19 through 6 months of follow-up and a further 96·7% effective against severe disease (13). The statistics look promising; however, given the rapid and reactionary nature of the SARS-CoV-2 vaccine response, there is little known about the durability of vaccines beyond 6 months. Early vaccine data (Table 1) show that at 3 months after the second dose of the Comirnaty® vaccine, IgG antibodies and neutralizing antibody titers decreased at a consistent rate in all individuals, while at 6 months after receipt of the second dose, neutralizing antibody titers were substantially lower among men than women, lower among persons aged 65 years or older, and lower among immunosuppressed or immunocompromised individuals (15). If not “rescued” by booster vaccine doses, specific subsets of patients such as the elderly or the immunosuppressed could face the greatest exposure to breakthrough infections due to reduced circulating antibody levels.
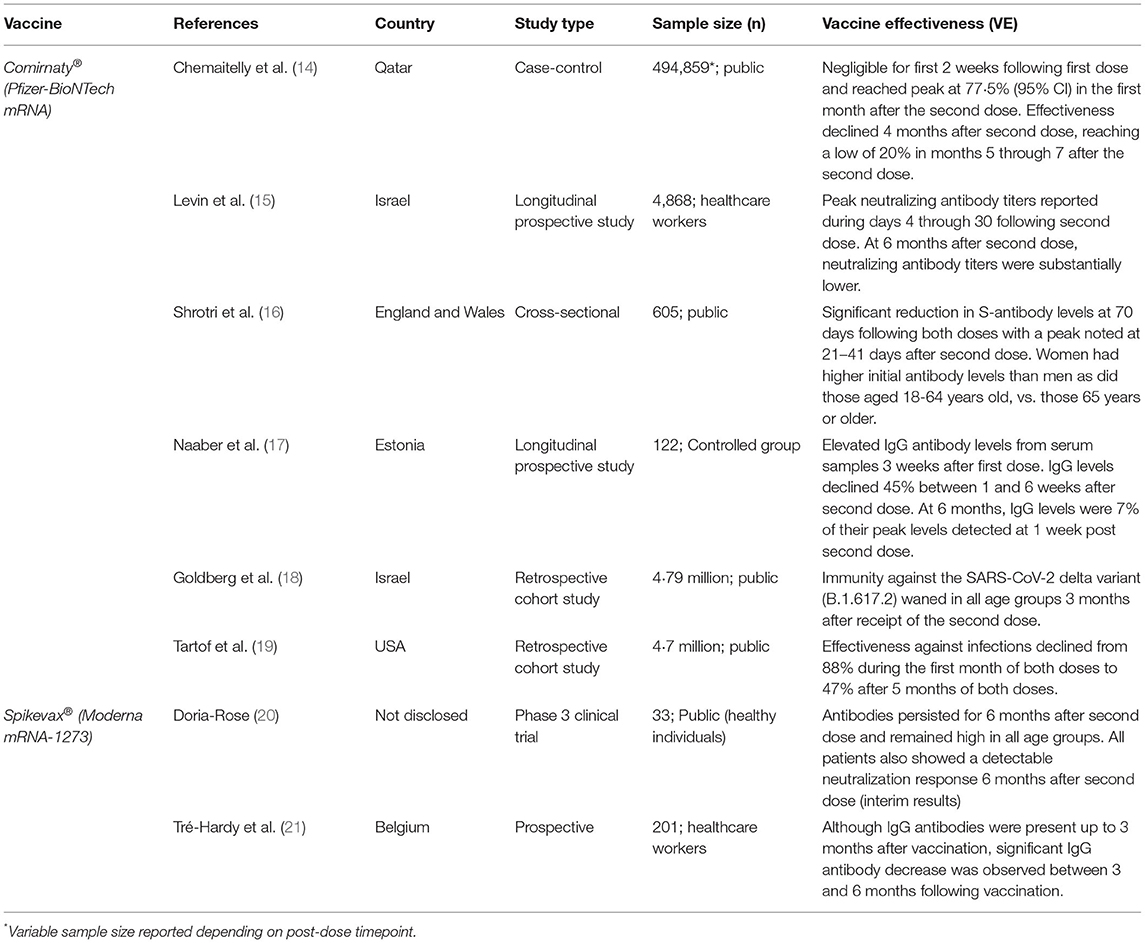
Table 1. Summary of current literature detailing the durability of available SARS-CoV-2 mRNA vaccines and their efficacy offered over time for fully vaccinated individuals.
Silent Spreaders
Here we refer to fully vaccinated individuals suffering from breakthrough infections as potential “silent spreaders” of SARS-CoV-2. Silent spreaders are unsuspecting candidates for spreading the SARS-CoV-2 virus. These individuals are those who have experienced a COVID-19 breakthrough case but due to the asymptomatic or mild nature of symptoms experienced during the disease course, have no knowledge they are infected carriers and potential spreaders of the SARS-CoV-2 virus. This unawareness is in part due to a false sense of security following vaccination, where individuals with both doses of a reputably manufactured vaccine operate assuming they can no longer contract the virus and therefore no longer need to be tested. It is also likely that those infected with a COVID-19 breakthrough case are harboring the SARS-CoV-2 delta variant (B.1.617.2). A recent study from India's deadly second SARS-CoV-2 wave in June 2021 found that among 592 fully vaccinated (Covishield and/or Covaxin) individuals with breakthrough cases, 86.7% (n = 443) were infected with the delta virus variant (22). The delta virus variant of SARS-CoV-2 is highly transmissible and is still overwhelmingly spread by unvaccinated individuals, who are themselves at high risk of serious disease. With that, vaccines are rendered most effective at combatting severe disease and due to gaps in our understanding regarding long-term protection, necessitate further clinical trials to delineate their efficacy in combatting asymptomatic disease (23). On a more intimate and domestic scale, those fully vaccinated individuals who are positive for a breakthrough case, and who display mild symptoms or none at all, are potentially endangering their family members (elderly and children), immunocompromised, partners, or colleagues. These examples emphasize the timely need for equipping the public with the knowledge required to mitigate the spread of COVID-19 from fully vaccinated individuals.
Correlates of Protection (CoP)
Perhaps the defining characteristic of long-term protection offered by a particular vaccine is its capacity to stimulate, produce, and retain key correlates of protection (CoP). CoPs are the vaccine-induced biomarkers associated with a lower risk of infection or severe disease (24). For example, among Comirnaty® -vaccinated individuals, the defining CoP was shown to be neutralizing antibody titers (3). Predictive models support neutralizing antibody titers as being highly correlated to immune protection. One model found that among seven current vaccines, the neutralization level for 50% protection against severe disease is roughly 3% of the mean convalescent titer, while 50% protection against detectable SARS-CoV-2 infection requires roughly 20% of the mean convalescent titer (25). These findings reaffirm that current SARS-CoV-2 vaccines offer greater protection against severe infection (i.e., a lower percentage of mean convalescent titer required for protection) than against mild or asymptomatic infection.
The capacity of a given vaccine to produce CoP biomarkers can vary based on factors such as the type of vaccine, the immune status of the individual receiving the dose, and whether the vaccine matches the variant (i.e., an alpha vaccine protects against the alpha variant). A 2021 study on CoP found an association between risk of disease and levels of anti-spike IgG, anti-RBD IgG, and neutralizing antibody titers, where those with higher levels of anti-spike IgG and neutralizing antibody titers exhibited a lower risk of symptomatic disease against the alpha variant (B.1.1.7) in individuals fully vaccinated with the ChAdOx1 NCoV-19 (AZD1222) vaccine. (26) A study from Gilbert et al. (2022) showed similar results, where Moderna's Spikevax® vaccine (mRNA-1273) elicited spike IgG, RBD IgG, cID50, and cID80 neutralization levels which were inversely correlated with COVID-19 risk. (27) Additional findings suggest that individuals vaccinated with both doses of Spikevax® exhibited higher antibody titer levels than those vaccinated with Comirnaty® (28).
Current literature is sparse and demonstrates the timely need for coordinated, clinical trials to further identify correlates of vaccine efficacy. One approach would be comparing banked serum samples from confirmed breakthrough individuals against fully vaccinated, non-breakthrough individuals at key follow-up timepoints. An additional approach would be a monitoring of the mucosal immune response and level of sterilizing immunity by using biofluids, such as nasopharyngeal swab isolates or salivary samples. Findings from such studies would allow more focussed efforts in vaccine development as well as a tailored approach to patient-specific management (29).
Memory B And T-Specific Immunity
Although much of this article has focussed on research efforts aimed at understanding the durability and dynamics of circulating, neutralizing antibody levels following vaccination, it is important to acknowledge recent contributions surrounding the SARS-CoV-2 adaptive immune response. A recent study found that a 3rd (booster) dose of an mRNA vaccine causes an initial increase in circulating anti-omicron neutralizing antibodies; however, these levels were 10-20 fold lower than against the original (Wuhan-Hu-1) strain (30). Despite this, a separate (pre-print) study examined the memory B cell repertoire following the 3rd mRNA vaccine dose and found an increased expansion of anti-receptor binding domain specific memory B cells (31). These memory B cells encoded antibodies exhibiting significantly increased potency compared to antibodies produced by the 2nd dose; furthermore, greater than 50% of the neutralizing antibodies produced by the memory B cells in individuals with three mRNA vaccine doses neutralized the omicron variant (31).
Recent findings suggest that the adaptive immune response also contributes to robust, long-term SARS-CoV-2 protection while also playing an important ‘second-line' defensive role following escape of omicron from neutralizing antibodies (32, 33). One study found that most vaccine- induced T cell responses were capable of recognizing all known SARS-CoV-2 variants (with average preservation >80% for omicron) following T cell repertoire analysis (33). Similarly, a study investigating T cell cross reactivity to the omicron spike protein in vaccinated (Comirnaty®, Janssen) and unvaccinated convalescent COVID-19 patients found 70–80% of the CD4+ and CD8+ T cell responses to spike were maintained across study groups (34). The same study also found similar levels of omicron cross-reactive T cells to delta and beta variants for all groups. These data submit that T cell populations are capable of cross-recognizing all SARS-CoV-2 variants, including the highly mutated omicron variant. Importantly, such findings suggest that cellular immunity is highly conserved to the omicron spike protein and that omicron spike-specific CD8+ and CD4+ T cell responses elicited by current mRNA vaccines contribute to robust protection against severe disease caused by the omicron variant, despite declining neutralizing capacity of free-circulating antibodies (34, 35).
Mucosal Immunity
For full protection against viral infections, such as SARS-CoV-2, sterilizing immunity may be required. Sterilizing immunity is achieved via the presence and secretion of neutralizing antibodies at the mucosal site of infection. For SARS-CoV-2, this refers to the secretion of IgA from mucosal tissues, including the nose, throat, and upper respiratory tract (4). Early emphasis has been placed on the maintenance of circulating levels of neutralizing antibodies in sera following intramuscular (IM) vaccination. With time, these circulating antibody levels begin to wane, therefore necessitating further vaccine “boost” doses (36). When waning levels of circulating neutralizing antibodies are coupled with emerging variants of concern, such as the omicron variant, breakthrough infections will continue to occur (37). Additionally, without sterilizing immunity, the risk of transmission of SARS-CoV-2 by vaccinated individuals cannot be overlooked.
To overcome this, vaccines are being developed to target and stimulate mucosal sites, rather than solely inducing systemic responses. To date, the only approved intranasal (IN) vaccines are live-attenuated influenza vaccines (LAIV), although 14 mucosal IN SARS-CoV-2 vaccines have progressed to the first phase of clinical trials. (36–38) A 2021 study demonstrated that similar efficacy could be achieved between a heterologous mRNA IM prime and IN Ad5 boost and two intramuscular mRNA immunizations, with the IN vaccination providing an elevated mucosal immune response to SARS-CoV-2 (37). Mucosal immunization via IN vaccination is also efficient at inducing adaptive immune responses, including secretory IgA (sIgA) antibodies and resident memory T (TRM) cells (38). Early data suggests that IN vaccines may be most effective when combined with an intramuscular mRNA vaccine, such as Comirnaty® or Spikevax® (39, 40). Additional pre-print findings (awaiting peer review) have demonstrated a correlation between anti-Spike/RBD IgA levels and breakthrough infection, where those with lower levels of anti-Spike/RBD IgA 2–4 weeks post second IM (Comirnaty® or Spikevax®) dose exhibited significantly greater rates of reinfection (p < 0.01) (40). Therefore, IN vaccination poses as a promising preventative measure against the SARS-CoV-2 virus; however, future trials are needed to determine whether IN vaccination is effective at preventing severe disease.
Vaccine Boosting
Homologous Boosting
As more long-term SARS-CoV-2 vaccine waning data becomes available, it is important to better understand the underlying implications of vaccine durability, effectiveness, and potential side effects. Although the initial vaccine boosting campaign was targeted toward older individuals (>60 years of age) and the immunocompromised, the eligibility criteria have been expanded to include those 16 years of age and older (41). Early data from a large SARS-CoV-2 vaccine booster trial in Israel shows that the rate of infection decreased by a factor of 11.3 for the booster group, vs. those who had not yet received a third dose; the same study found that the rate of severe illness was lower by a factor of 19.5 (95% CI) (42). Booster shots for SARS-CoV-2 inactivated vaccines have also shown to be beneficial for increasing levels of circulating neutralizing antibodies. For example, a 2021 cohort trial compared both levels of neutralizing antibody titers and positive antibody conversion rate among 67 individuals who had received a third booster shot, to those who had received two doses. Interestingly, they found that those who received a booster had a higher positive antibody conversion rate in the first month than those at 8 months after their second dose; the study also showed higher antibody levels at 1 month after the third dose than at 1 month after the second dose (43). In addition to greater positive antibody conversion, those on a three-dose vaccine regimen demonstrated greater neutralizing antibody levels than those on the conventional two dose regimen These results were regardless of age, sex, or vaccine procedure and demonstrate that a third dose can reverse declining neutralizing antibody levels. Although more studies are needed to support these early findings, vaccine boosting is a promising addition to the universally adopted two-dose program. It is not unlikely that, moving forward, at-risk individuals will be advised to continue receiving yearly COVID-19 booster doses, similar to influenza.
Many high- and middle-income countries including China, Canada, Israel, UAE, and the US have begun administering booster doses. The UK has procured a further 30 million Comirnaty® vaccines to be administered as boost doses to at-risk individuals. Canada's health agency has recommended and authorized Pfizer-BioNTech or Moderna booster shots for older individuals (70 and above), healthcare workers, and immunocompromised individuals (44). In the USA, health officials are encouraging all adults to seek boosters. Booster eligibility criteria is more relaxed in some densely populated areas within the US as well. For example, New York City states that any individual 18 years or older should not be turned away when seeking a booster shot, contingent on being 6 months or longer since their second shot of the Pfizer-BioNTech or Moderna mRNA vaccines (45).
Hetero Boosting
Current countries administering booster doses have opted to administer both the Comirnaty® and Spikevax® mRNA vaccines, regardless of which vaccine type was administered for the prime (dose 1) and boost (dose 2) vaccine of a given individual. Reaching a consensus as to whether mixing and matching with different vaccine brands and types has been fraught with uncertainty. Due to the halting of vaccination of AstraZeneca's ChAdOx1 nCov-19 (ChAd) vaccine in many countries, individuals were left partially vaccinated with the option of receiving a second dose with an mRNA vaccine, such as Comirnaty® or Spikevax®. Data from mixed-dose individuals suggested that receiving a heterologous ChAd-Comirnaty® dosing resulted in a greater IgG and IgA response to the SARS-CoV-2 spike protein with increased neutralizing antibody titer levels than that seen in individuals who received homologous ChAd-ChAd dosing (46, 47). In addition, the neutralizing antibody titer levels were roughly threefold higher in the serum of the ChAd-Comirnaty® than the serum of the homologous Comirnaty® group. A separate study from Nordström et al. (2021) found that a heterologous prime-boost vaccine schedule consisting of ChAd-Comirnaty® was 67% effective while ChAd-Spikevax® prime-boost offered 79% vaccine efficacy, compared to a homologous prime-boost regiment of ChAd-ChAd, offering only 50% efficacy (48). These findings suggest that further prospective clinical trials are needed to compare long-term vaccine efficacy following a third booster dose of either homologous or heterologous vaccine dosing. The data also suggests that the Spikevax® vaccine may be equipped to offer similar or exceeding protection to that of the Comirnaty® vaccine, potentially expediting the global rollout for individuals who require a third dose in a timely manner. Additionally, Moderna's booster dose (3rd dose) is to be administered at half the volume (0.25 mL, 50 mcg) of the first two doses (0.5 mL, 100 mcg). The decision to administer the Spikevax® booster at half the volume of the first two doses was to reduce adverse side effects consistent with a full dose, such as fatigue, body aches, and fever, while also helping address global vaccine shortages. A half dose (50 mcg) of Spikevax® was found to increase neutralizing antibody levels 37-fold higher than pre-boost levels and a full dose booster (100 mcg) was found to increase these levels further (83-fold higher than pre-boost levels) (49). It is important to highlight individuals at risk who would most benefit from a booster dose, such as people living in care homes, the elderly, front line healthcare workers, and people with underlying health conditions or impaired immune function (50). With greater priority assigned to identifying individuals who require a third dose, the already limited global COVID-19 vaccine supply can be more equitably distributed to poorer countries who have not yet received a first dose.
Breakthrough and Variants of Concern
As an increasing portion of the global population is vaccinated against SARS-CoV-2, a growing concern is the emergence of additional, potentially more virulent, variants of concern (VoCs). In late November 2021, a novel SARS-CoV-2 variant termed the omicron (o) variant (B.1.1.529) was discovered in Botswana and a few days later in an individual in Hong Kong who had traveled from South Africa (51). The omicron variant has since been listed as a variant of concern largely due to its rapid transmission and highly mutated spike protein (Figure 1) with 50 genetic mutations and more than 30 spike protein mutations (52). Confirmed cases of the omicron variant have been reported in over 80 countries, including Canada, Hong Kong, Australia, Botswana, France, Germany, Portugal, Italy, and the Netherlands. The emergence of the fifth VoC in April 2021, the delta SARS-CoV-2 variant, was associated with global surges in cases, higher viral loads, longer duration of infectiousness, and high rates of reinfection (53). Therefore, it remains of great concern to rapidly elucidate the underlying immunological mechanisms that dictate disease course for individuals infected with emerging novel variants.
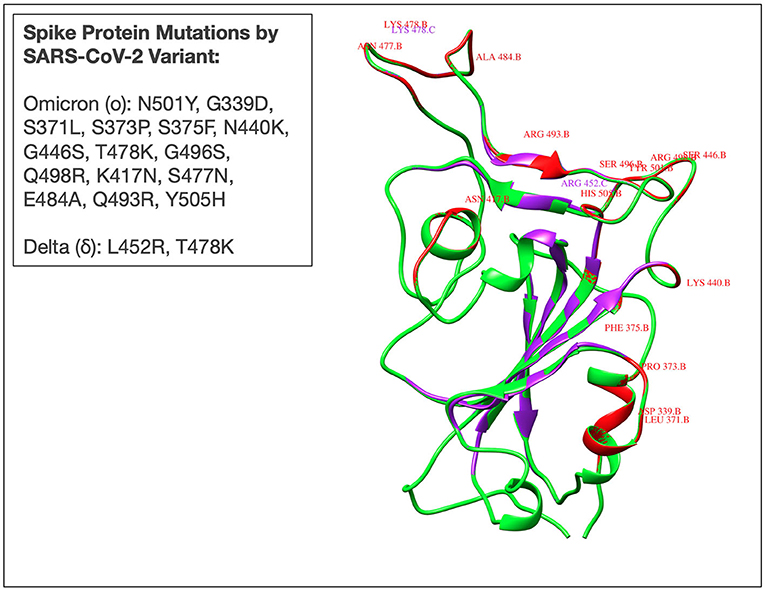
Figure 1. Superimposed three-dimensional (3D) structure of the receptor binding domain (RBD) of spike protein derived from Omicron (B.1.1.529), Delta (B.1.617), and Wuhan (ancestral- D614G) SARS-CoV-2 variants. Red and purple colours reflect the omicron and delta RBD, respectively. The Wuhan RBD is represented by a green colour. Mutations in delta (L452R, T478K), and omicron (N501Y, G339D, S371L, S373P, S375F, N440K, G446S, T478K, G496S, Q498R, K417N, S477N, E484A, Q493R, Y505H) are also represented by corresponding colours. Mutant 3D models for delta and omicron variants were generated using UCSF Chimera software from the resource of Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01,081). The PDB ID: 7T9J was used as a template for the mutant model's prediction.
Due to the recency of the omicron variant emergence, little is known about its virulence and whether currently vaccinated individuals are protected. Available data does, however, lend itself to interpretation. Due to the large number of mutations (>30) observed in omicron (many of which overlap with those observed in alpha, beta, gamma, and delta VoCs), it is not unreasonable to predict that the omicron variant may be associated with higher viral binding affinity, greater antibody escape, and increased transmissibility (53, 54). Recent modeling using S-gene target failure (SDFT) suggests that the rate of infection of the omicron variant will be 100-fold higher compared to that of the delta variant of SARS-CoV-2 (55). There is limited data detailing whether the immune protection for fully vaccinated individuals is effective at preventing omicron infection; however, vaccine manufacturers remain optimistic that immunization will offer protection against severe infection caused by the omicron variant (56). Due to the large number of mutations present in the omicron variant, the delineation of the efficacy of current SARS-CoV-2 vaccines in preventing mild infections caused by the omicron variant remains of high importance.
A recent study (pre-print) from South Africa found that individuals fully vaccinated with the Comirnaty® mRNA vaccine may still be vulnerable to breakthrough infections with omicron. The study compared neutralization levels for the ancestral SARS-CoV-2 variant (D614G) and the novel omicron variant (B.1.1.529) in cells from individuals vaccinated with the Comirnaty® vaccine (57). Their results showed that the geometric mean titer (GMT) FRNT50 (inverse of the plasma dilution required for 50% reduction in infection foci number) was 1,321 for D614G, but dropped to 32 for the omicron variant, a 41-fold decrease. Additional studies are required to fully understand the level of protection offered by current vaccines against the omicron variant, such as the breadth of vaccine-induced cellular immune protection against the variant. Current emphasis has been placed on determining whether a third vaccine (booster) dose will restore protection and prevent or reduce breakthrough infections, especially in at-risk groups, such as the elderly, who typically mount less robust and coordinated cellular immune responses. For these individuals, a bolstered level of circulating antibodies from a booster shot may be required for adequate protection from the omicron variant and additional emerging VoCs.
Another recent study demonstrated that individuals fully vaccinated with the Comirnaty® mRNA vaccine with additional booster shots remain at risk of developing an omicron infection (58). Among the seven participants in the study, six were fully vaccinated with the Comirnaty® vaccine, and five of these individuals received a third (booster dose) of Comirnaty®, while the sixth individual received an additional full dose (100 mcg) of the Spikevax® vaccine. Five of these individuals tested positive for the omicron variant and had mean viral loads of 4.16 x 10E7 RNA copies per mL of swab eluate, with a mean age of 27.7 years. These findings suggest that omicron can produce a breakthrough infection in individuals who have received a booster dose, who are of young age, and who are otherwise healthy. Additionally, this data indicates that greater than three vaccine doses may be required for protection and a wider age range is at risk of developing an omicron breakthrough infection. Additional (pre-print) findings from the UK suggest that two doses with Comirnaty® or ChAdOx1 offer insufficient levels of protection against infection and mild disease caused by the omicron variant (59). Furthermore, a 2022 study found that the omicron variant has roughly an 88% likelihood of escaping neutralizing antibodies produced by current vaccines, some 14 times as high as that of the delta variant (60). Although there have been limited reports of severe disease caused by the omicron variant, its rapid spread has caused surges in hospitalizations worldwide, where the United States is reporting >2,000 omicron related deaths per day (61). As increasingly virulent, heavily mutated variants of SARS-CoV-2 emerge, further investigation and development surrounding second-generation, multivalent vaccines is required. The design of such vaccines should be focussed on addressing the deficiencies of existing vaccines, including: (i) reducing reliance on booster doses for long-term protection; (ii) increasing robust memory responses; and (iii) are preventative in nature (62).
Conclusion
Perhaps the most robust SARS-CoV-2 transmission mitigation measure remains striving for a universally vaccinated population. Curbing the spread relies on both the individual and the scientific community, the latter being ultimately responsible for equipping the public with the knowledge required to make informed decisions about vaccination. Those resisting vaccination are directly endangering others while also jeopardizing the efforts of many global health leaders. This results in the emergence of potentially harmful, highly mutated variants, such as the delta and omicron variants, which will continue to persist in the population further saturating health care systems and intensive care units.
Additional efforts should be focussed on assessing the efficacy and durability of current SARS-CoV-2 vaccines. Highly mutated viral variants are demonstrating an increased ability to evade the vaccine-induced immune response especially due to shifts in antigenicity, which creates instances of vaccine mismatch where individuals fully vaccinated with early vaccine iterations fail to mount an immune response to mutated viral components. The omicron (B.1.1.529) variant is an example of this phenomena, where many individuals remain at risk of a breakthrough infection despite being fully vaccinated (with a booster) due to the many genetic and protein-level mutations. The risk of breakthrough infection underscores the need for further clinical trials designed to evaluate not only the durability of protection offered by a given vaccine, but also the efficacy of multivalent vaccines and heterologous prime-boost vaccination regimens. As variants of concern emerge, vaccine manufacturers will need to shift vaccine development toward novel and circulating virus stains.
Central to mitigating SARS-CoV-2 transmission is the continued testing of fully vaccinated individuals, which may be achieved by increased mobile testing sites, dedicated regional testing centers, and greater access to self-administered testing kits, such as those from Maple™, Bio-Rad™, or Abbott™. Particular attention should be paid to individuals who have traveled (domestic or international), experienced symptoms (mild or otherwise), or have come in contact with a known infected individual. The CDC also recommends that, in indoor areas with high-transmission potential, individuals wear masks regardless of vaccination status (63). Tracing databases and case logging applications should be modified to incorporate confirmed COVID-19 breakthrough cases. Additionally, increased serosurveillance and monitoring of major vaccine manufacturers and their respective rates of breakthrough cases should also be tracked, given the discrepancies reported by the CDC. At the level of the individual, precautionary measures such as isolation, personal hand hygiene, limited social gatherings, and masking policies should be followed according to local or federal guidelines. Breakthrough cases present a new global challenge for managing the spread of COVID-19; however, with adequate resources, appropriate scientific dissemination, and a dedicated multi-disciplinary workforce, COVID-19 breakthrough cases can be closely managed and tightly regulated.
The authors of this paper acknowledge that special efforts are needed to elucidate the degree to which those who have breakthrough infections are also able to effectively transmit the virus to other individuals. Further epidemiological studies specifically designed to address this issue should be focussed on specific groups such as the immunocompromised individuals, those suffering from chronic diseases, and the elderly, as well as to the influence of each given type/modality of vaccine. Results from such studies will help shape the design of future public health policies aimed at disseminating risk-related information pertinent to the COVID-19 pandemic.
Author Contributions
BH: writing (original draft), investigation, editing, conceptualization, and data curation. MR, JB-M, AAK, and PN: editing, review, and conceptualization. CR: editing, review, conceptualization, and writing. SR, AT, AM-A-R: conceptualization and review. AK: figure creation/visualization. DK: conceptualization, editing, review, supervision, methodology, and investigation writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from Canadian Institutes of Health Research, Genome Canada/Atlantic Genome, Research Nova Scotia, Dalhousie Medical Research Foundation, and the Li-Ka Shing Foundation.
Conflict of Interest
DK is a Canada Research Chair in Translational Vaccinology and Inflammation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Nikki Kelvin (The Journal of Infection in Developing Countries) provided editing assistance throughout the preparation of this manuscript.
References
1. He F, Deng Y, Li W. Coronavirus disease 2019: What we know? J Med Virol. (2020) 92:719–25. doi: 10.1002/jmv.25766
2. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 vaccination. JAMA. (2021) 326:2018–20. doi: 10.1001/jama.2021.19063
3. Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. (2021) 385:1474–84. doi: 10.1056/NEJMoa2109072
4. Will the COVID-19 Vaccines Provide Sterilizing Immunity? Verywell Health. (2022). Available online at: https://www.verywellhealth.com/covid-19-vaccines-and-~sterilizing-immunity-5092148
5. Aufrichtig A, Walker AS. Who had covid-19 vaccine breakthrough cases? The New York Times (2021 October 28). Available online at: https://www.nytimes.com/interactive/2021/10/28/us/covid-breakthrough-cases.html
6. Birhane M, Bressler S, Chang G, Clark T, Dorough L, Fischer M, et al. COVID-19 vaccine breakthrough infections reported to CDC — United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:792–3. doi: 10.15585/mmwr.mm7021e3
7. Bwire GM. Coronavirus: Why men are more vulnerable to covid-19 than women? SN Compr Clin Med. (2020) 2:874-6. doi: 10.1007/s42399-020-00341-w
8. Loconsole D, Sallustio A, Accogli M, Leaci A, Sanguedolce A, Parisi A, et al. Investigation of an outbreak of symptomatic SARS-CoV-2 VOC 202012/01-lineage B.1.1.7 infection in healthcare workers, Italy. Clin Microbiol Infect. (2021) 27:1174.e1-e4. doi: 10.1016/j.cmi.2021.05.007
9. Boniol M, McIsaac M, Xu L, Wuliji T, Diallo K, Campbell J. Gender equity in the health workforce: analysis of 104 countries. Report No.: WHO/HIS/HWF/Gender/WP1/2019.1. World Health Organization (2019). Available online at: https://apps.who.int/iris/handle/10665/311314
10. Servellita V, Morris MK, Sotomayor-Gonzalez A, Gliwa AS, Torres E, Brazer N, et al. Predominance of antibody-resistant SARS-CoV-2 variants in vaccine breakthrough cases from the San Francisco Bay Area, California. Nat Microbiol. (2022) 7:277–88. doi: 10.1038/s41564-021-01041-4
11. Di Fusco M, Moran MM, Cane A, Curcio D, Khan F, Malhotra D, et al. Evaluation of COVID-19 vaccine breakthrough infections among immunocompromised patients fully vaccinated with BNT162b2. J Med Econ. (2021) 24:1248–60. doi: 10.1080/13696998.2021.2002063
12. Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV- 2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1059–62. Available online at: https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7031e2-H.pdf
13. Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. (2021) 385:1761–73. doi: 10.1056/NEJMoa2110345
14. Cele S, Jackson L, Khan K, Khoury D, Moyo-Gwete T, Tegally H, et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. [Preprint]. doi: 10.1101/2021.12.08.21267417
15. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. (2021) 385:e84. doi: 10.1056/NEJMoa2114583
16. Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, Keeton R, et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet. (2022). Available online at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)00090-3/fulltext
17. Andrews N, Stowe J, Kirsebom F, Toffa S, Gallagher E, DPhil CG, et al. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. medRxiv. (2021). Available online at: https://www.medrxiv.org/content/10.1101/2021.12.14.21267615v1.full.pdf
18. Chen J, Wang R, Gilby NB, Wei G-W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J Chem Inf Model. (2022) 62:412–22.
19. U.S. Covid Death Toll Surpasses 900,000 as Omicron's Spread Slows - The New York Times [Internet]. [cited 2022 Feb 7]. Available from: https://www.nytimes.com/2022/02/04/us/us-covid-deaths.html
20. Haque A, Pant AB. Mitigating Covid-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J Autoimmun. (2022) 127:102792.
21. CDC. COVID-19 Vaccination. Centers for Disease Control and Prevention (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html
22. Gupta N, Kaur H, Yadav PD, Mukhopadhyay L, Sahay RR, Kumar A, et al. Clinical characterization and genomic analysis of samples from COVID-19 breakthrough infections during the second wave among the various states of india. Viruses. (2021) 13:1782. doi: 10.3390/v13091782
23. Krause PR, Fleming TR, Peto R, Longini IM, Figueroa JP, Sterne JAC, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. (2021) 398:1377– 80. doi: 10.1016/S0140-6736(21)02046-8
24. Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. (2021) 27:205–11. doi: 10.1038/s41591-021-01230-y
25. Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. (2021) 385:e83. doi: 10.1056/NEJMoa2114114
26. Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike- antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. (2021) 398:385–7. doi: 10.1016/S0140-6736(21)01642-1
27. Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. (2021) 10:100208. doi: 10.1016/j.lanepe.2021.100208
28. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. (2021) 385:e85. doi: 10.1056/NEJMoa2114228
29. Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. (2021) 398:1407–16. Available online at: https://www.thelancet.com/action/showPdf?pii=S0140-6736%2821%2902183-8
30. Doria-Rose N, Suthar MS, Makowski M, O'Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. (2021) 384:2259–61. doi: 10.1056/NEJMc2103916
31. Tré-Hardy M, Cupaiolo R, Wilmet A, Antoine-Moussiaux T, Della Vecchia A, Horeanga A, et al. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: A third dose is expected. J Infect. (2021) 83:559-64. Available online at: https://www.sciencedirect.com/science/article/pii/S0163445321004333
32. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. (2021) 27:1205–11. doi: 10.1038/s41591-021-01377-8
33. Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. (2021) 27:2032-40. doi: 10.1038/s41591-021-01540-1
34. Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022 Jan 7;375(6576):43–50.
35. Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination With BNT162b2 and mRNA-1273. JAMA. (2021) 326:1533–5. doi: 10.1001/jama.2021.15125
36. Krammer F. Correlates of protection from SARS-CoV-2 infection. Lancet. (2021) 397:1421–3. doi: 10.1016/S0140-6736(21)00782-0
37. Schmidt F, Muecksch F, Weisblum Y, Da Silva J, Bednarski E, Cho A, et al. Plasma neutralization of the SARS-CoV-2 omicron variant. N Engl J Med. (2022) 386:599–601. doi: 10.1056/NEJMc2119641
38. Muecksch F, Wang Z, Cho A, Gaebler C, Tanfous TB, DaSilva J, et al. Increased potency and breadth of SARS-CoV-2 neutralizing antibodies after a third mRNA vaccine dose. bioRxiv. (2022). Available online at: https://www.biorxiv.org/content/10.1101/2022.02.14.480394v1
39. Mandavilli A. Got a covid booster? you probably won't need another for a long time. The New York Times. (2022 February 21). Available online at: https://www.nytimes.com/2022/02/21/health/covid-vaccine-antibodies-t-cells.html
40. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. (2022) 185:847–59. Available online at: https://www.sciencedirect.com/science/article/pii/S0092867422000733
41. Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize omicron. Nature. (2022) 603:488-92. doi: 10.1038/s41586-022-04460-3
42. Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 omicron. Nature. (2022) 603:493-9. doi: 10.1038/s41586-022-04465-y
43. Focosi D, Maggi F, Casadevall A. Mucosal vaccines, sterilizing immunity, and the future of SARS-CoV-2 virulence. Viruses. (2022) 14:187. doi: 10.3390/v14020187
44. Lapuente D, Fuchs J, Willar J, Vieira Antão A, Eberlein V, Uhlig N, et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. (2021) 12:6871. doi: 10.1038/s41467-021-27063-4
45. Alu A, Chen L, Lei H, Wei Y, Tian X, Wei X. Intranasal COVID-19 vaccines: From bench to bed. eBioMedicine. (2022) 76:103841. doi: 10.1016/j.ebiom.2022.103841
46. Zhou R, Wang P, Wong Y-C, Xu H, Lau S-Y, Liu L, et al. Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models. eBioMedicine. (2022) 75:103762. doi: 10.1016/j.ebiom.2021.103762
47. Sheikh-Mohamed S, Isho B, Chao GYC, Zuo M, Cohen C, Lustig Y, et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. medRxiv. (2022). Available online at: https://www.medrxiv.org/content/10.1101/2021.08.01.21261297v4
48. CDC. COVID-19 Booster Shot. Centers for Disease Control and Prevention (2021). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
49. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. (2021) 385:1393–400. doi: 10.1056/NEJMoa2115926
50. Yue L, Xie T, Yang T, Zhou J, Chen H, Zhu H, et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS-CoV-2 vaccine. J Med Virol. (2022) 94:35–8. doi: 10.1002/jmv.27334
52. Fadulu L. New York City tells health providers to give booster shots to all adults who want them. The New York Times. (2021 November 15). Available online at: https://www.nytimes.com/2021/11/15/nyregion/nyc-booster-shots-adults.html
53. Deming ME, Lyke KE. A ‘mix and match' approach to SARS-CoV-2 vaccination. Nat Med. (2021) 27:1510–1.
54. Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Morillas Ramos G, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. (2021) 27:1525–9.
55. Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. Lancet. (2021). Available online at: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(21)00235-0/fulltext
56. Moderna Announces preliminary booster data updates strategy to address omicron variant. (2022). Available online at: https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-~Preliminary-Booster-Data-and-Updates-Strategy-to-Address-Omicron-Variant/default.aspx
57. Wise J. Covid-19: Booster doses to be offered to 30 million people in UK. BMJ. (2021) 374:n2261.
58. GISAID - hCov19 Variants. (2021). Available online at: https://www.gisaid.org/hcov19-variants/
59. Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. (2021) 375:n2943.
60. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. (2021). Available online at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02758-6/fulltext
61. Greaney AJ, Starr TN, Gilchuk P, Zost SJ, Binshtein E, Loes AN, et al. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. (2021) 29:44-57.e9.
62. Rao S, Singh M. The newly detected B.1.1.529 (omicron) variant of SARS-CoV-2 with multiple mutations: implications for transmission, diagnostics, therapeutics, and immune evasion. DHR Proc. (2021) 1:7–10.
Keywords: SARS-CoV-2, breakthrough cases, immune evasion, vaccine mismatch, variants of concern (VoCs)
Citation: Hewins B, Rahman M, Bermejo-Martin JF, Kelvin AA, Richardson CD, Rubino S, Kumar A, Ndishimye P, Toloue Ostadgavahi A, Mahmud-Al-Rafat A and Kelvin DJ (2022) Alpha, Beta, Delta, Omicron, and SARS-CoV-2 Breakthrough Cases: Defining Immunological Mechanisms for Vaccine Waning and Vaccine-Variant Mismatch. Front. Virol. 2:849936. doi: 10.3389/fviro.2022.849936
Received: 06 January 2022; Accepted: 21 February 2022;
Published: 06 May 2022.
Edited by:
Seshadri Vasan, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaReviewed by:
Hyesun Jang, J. Craig Venter Institute (La Jolla), United StatesEliseo Albert, Hospital Clínico Universitario de Valencia, Spain
Copyright © 2022 Hewins, Rahman, Bermejo-Martin, Kelvin, Richardson, Rubino, Kumar, Ndishimye, Toloue Ostadgavahi, Mahmud-Al-Rafat and Kelvin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David J. Kelvin, RGF2aWQuS2VsdmluQGRhbC5jYQ==
 Benjamin Hewins
Benjamin Hewins Motiur Rahman1
Motiur Rahman1 Jesus F. Bermejo-Martin
Jesus F. Bermejo-Martin Salvatore Rubino
Salvatore Rubino Anuj Kumar
Anuj Kumar Ali Toloue Ostadgavahi
Ali Toloue Ostadgavahi Abdullah Mahmud-Al-Rafat
Abdullah Mahmud-Al-Rafat