
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol. , 10 January 2023
Sec. Virus and Host Immunity
Volume 2 - 2022 | https://doi.org/10.3389/fviro.2022.822153
 Lucia Taramasso1*
Lucia Taramasso1* Laura Labate2
Laura Labate2 Federica Briano2
Federica Briano2 Giorgia Brucci2
Giorgia Brucci2 Sara Mora3
Sara Mora3 Sabrina Blanchi1
Sabrina Blanchi1 Mauro Giacomini3,4
Mauro Giacomini3,4 Matteo Bassetti1,2
Matteo Bassetti1,2 Antonio Di Biagio1,2
Antonio Di Biagio1,2Introduction: Despite the high level of efficacy of modern antiretroviral therapy (ART) in reducing HIV viremia and the control of viral replication, some people living with HIV (PLWH) do not recover their CD4+ T cell count.
Methods: To evaluate the frequency and predictive factors of discordant immune responses, we performed a retrospective cohort study of 324 antiretroviral-naïve PLWH who initiated first-line ART between 2008 and 2018 and maintained HIV RNA < 50 copies/ml during 36 months of follow-up. PLWH were defined as immunological non-responders (INRs) when CD4+ T cell count was < 20% compared with baseline (INR20%), or < 500 cells/mm3 (INR500) or < 200 cells/mm3 (INR200) at 36 months.
Results: The prevalence of INR20%, INR500, and INR200 was 12.5%, 34.6%, and 1.5%, respectively. After adjustment for possible confounders, CD4 nadir showed a significant association with all INR definitions, with lower values predicting INR500 (aOR 0.98, 95% CI 0.98–0.99, p < 0.001) and INR200 (aOR 0.98, 95% CI 0.95–1.01, p = 0.096). Moreover, a higher baseline CD4/CD8 ratio was inversely related to the probability of being INR500 (OR 0.03, 95% CI 0.01–0.12, p < 0.001) and INR200 (OR 0.002, 95% CI 18–7–67.72, p = 0.255). By contrast, INR20% had a higher CD4 nadir and CD4/CD8 ratio than other INRs, suggesting the identification of an heterogenous population with such definition.
Discussion: The present study highlights how INR200 has become rare in the contemporary ART era, and about one-third of PLWH meet the criteria for INR500. Overcoming the threshold of 500 CD4/mm3 could be an appropriate definition of immune response, in contrast with the older definitions of INR200 and INR20%. Early diagnosis and rapid treatment initiation, before CD4 counts and the CD4/CD8 ratio begin to decline, are critical for achieving an optimal immune response.
Because of the availability of modern combined antiretroviral therapy (ART), people living with HIV (PLWH) have experienced a reduction in overall mortality and incidence of AIDS-defining conditions, improving survival and quality of life (1–3). The efficacy of ART is traditionally evaluated using two parameters: immunological recovery, namely the increase in CD4+ T-cell count (CD4), and the viral suppression, defined as an HIV RNA load of < 50 copies/ml in plasma. The extent of a CD4 increase, however, might have interpatient variations depending on age, ethnicity, test imprecision, intercurrent infection, and relative leukocyte count (4, 5), and the immunological response in the course of ART can be heterogeneous, based on individual, clinical, and genetic factors (6–8). Despite this variability, there is general agreement that CD4 count is the main marker of immunological staging (9). However, some PLWH fail to reconstitute CD4 count, even in the course of successful ART. These people are referred to as immunological non-responders (INRs). A consensus on the definition of INR has not been reached yet, owing to consistent interpatient variability of different factors, including age, HIV RNA load and CD4 count before starting ART, coinfections with HCV, HBV, and CMV, bone marrow and thymic dysfunction, genetic factors, and immune activation (10–12). INRs have been defined in the past based on CD4 count relative or absolute recovery. In studies that use relative CD4 count recovery from baseline, thresholds varied from less than 20% to 25% or 30% (13–15). Other studies consider the achievement of a predefined CD4 value, with thresholds that range from 200 to 500 cells/mm3 (16–18).
Each classification has pros and cons because of variable sensitivity and specificity. The choice of > 500 CD4+ T cells/mm3 to define full immune recovery could be justified in comparison to the general population, as it is the approximately the minimum expected CD4 count in a healthy, HIV-uninfected person. In addition, when counts are > 500 CD4+ T cells/mm3 in PLWH, their mortality rates become similar to those of the general population (19) and both mortality from AIDS- and non-AIDS-defining causes decreases as CD4 counts increase (20). On the other hand, the threshold of > 200 CD4+ T cells/mm3 might better represent the turning point between high and low risk of opportunistic diseases and AIDS-defining conditions (21). However, despite different definitions, most studies agree that INRs are at higher risk of disease progression toward AIDS-defining conditions, non-AIDS-defining conditions, and death (11, 14, 22). Because of the heterogenicity of definitions, the prevalence of INRs varies from 10% to 40% in different studies (13, 23); in the modern ART era INRs are expected to become rarer thanks to early diagnosis and immediate initiation of ART (24), and new antiretroviral agents characterized by high levels of efficacy and tolerability (25, 26).
In Italy, as well as in many other countries in the world, after 2008 new antiretroviral agents were introduced in clinical practice that completed the therapeutic armamentarium, and integrase strand transfer inhibitors (INSTIs) have begun to enter first-line therapy in PLWH (27, 28). With the aim of assessing the phenomenon of INR in an era of modern ART, we used three different definitions of immunological reconstitution, i.e., a CD4 level increase of > 20% from baseline, a CD4 level of > 200 cells/mm3 or a CD4 level of > 500 cells/mm3, and we evaluated the prevalence of INR according to these definitions in recent years in our single-center experience.
We conducted a retrospective observational study in a cohort of people diagnosed with HIV from 2008 to 2018 in our center (Policlinico IRCCS San Martino University Hospital).
The inclusion criteria were a confirmed HIV infection, being ART-naïve at the beginning of the study, being aged ≥18 years, and achieving and maintaining viral suppression (i.e., HIV RNA < 50 copies/ml) throughout the follow-up period of 36 months after ART initiation.
Exclusion criteria were at least one HIV RNA of > 50 copies/ml fter achieving viral suppression, being aged ≥ 18 years, a lack of virological and immunological data during 36 months after the study baseline, and death before 36 months from the study initiation.
Data for all PLWH included in the study were extracted from the Liguria HIV Network Database (RLH-DB). The Liguria HIV Network is a locally developed online platform with a direct connection between medical and laboratory records of PLWH through the automatic and prospective transfer of anonymous data (29, 30). Each person has an identification code, which is registered in the RLH-DB at initial engagement (i.e., at the first ambulatory visit for outpatients or first day of hospitalization for inpatients). Safety and precision are granted by the approved use of hospital anonymized codes. The use of the RLH-DB was approved by the Ligurian Ethics Committee. All people registered in the RHL-DB signed informed consent forms to be included in the study. The study has been performed in accordance with the ethics standards of the Declaration of Helsinki and with Italian national laws.
For the classification of the INR we used the following definitions: (a) ART-treated PLWH who failed to demonstrate a 20% recovery in their CD4 levels, compared with their baseline CD4 count, at 36 months after their first HIV RNA of < 50 copies/ml (who were defined as INR20%) (31–33); (b) ART-treated PLWH with a total CD4 count of < 500 cells/mm3 at 36 months after their first HIV RNA of < 50 copies/ml (who were defined as INR500) (34–38); and (c) ART-treated PLWH with a total CD4 count of < 200 cells/mm3 at 36 months after their first HIV RNA of < 50 copies/ml (who were defined as INR200) (11, 15, 17, 33, 39–42).
Plasma viral load, measured as HIV RNA copies/ml, was quantified by using the K-PCR-HIV1 (Siemens Health Care, Erlangen, Germany) kit for samples collected between 2008 and 2010; by the Nucleosens HIV (bioMerieux, Marcy-l’Etoile, France) kit between 2011 and 2018; and by Aptima HIV-1 Quant Dx Assay from 2019 onward.
CD4+ T cell counts were assessed in EDTA blood samples, which were analyzed using a BD FACSCanto flow cytometer (BD Biosciences). The following monoclonal antibodies (MABs) were used to analyze T-cell subsets: CD3FITC/CD8 PE/CD45 PercCP-Cy5.5/CD4 APC (BD Multitest).
HCV co-infection was defined as positive anti-HCV antibody test and detectable HCV RNA. HBV co-infection was defined as positive hepatitis B surface antigen (HBsAg) test. The time of HIV RNA viral decay was calculated as the difference in days between ART initiation and achievement of first HIV RNA load of < 50 copies/ml.
Clinical events were classified as AIDS defining based on CDC’s classification (43).
The primary objective of the study was to evaluate the frequency of INR20%, INR500, and INR200 at 36 months after starting ART in the period 2008–2018.
The secondary objective of the study was to evaluate the factors associated with a poor immune response in INR20%, INR500, and INR200.
Data were described using mean and standard deviation (SD) for normally distributed continuous variables, median and interquartile range (IQR) for not normally distributed continuous variables, and frequency (%) for categorical and ordinal variables. Continuous variables were compared by using the t-test or Mann–Whitney U-test, and categorical variables were compared using chi-squared or Fisher’s exact test, as appropriate. Factors associated with INR were evaluated using a binomial logistic regression model with the INR as the dependent variable; factors with a p-value ≤ 0.1 at univariate analysis were included in the multivariable model. For INR500 and INR200 analysis, only PLWH with a CD4 nadir of < 500 and <200 cells/mm3, respectively, were included in the logistic model. Since both CD4 and CD8 absolute numbers and the CD4/CD8 ratio reached significance in univariate analyses of different INR groups, a sensitivity analysis was performed to investigate the relationship between CD4/CD8 ratio, instead of absolute lymphocyte numbers, and INR. CD4 nadir was dichotomized according to the best threshold obtained from the receiver operating characteristic (ROC) curve analysis to identify the cut-off value predictive of becoming INR500 or INR200 in the study cohort. The significance level was defined as a p-value < 0.05.
During the study period, 452 PLWH were newly diagnosed in our center. Among them, 128 were excluded from the study because they were lost to follow-up (N = 98), died (N = 20), or did not meet virological criteria for study inclusion (N = 10) within 36 months of starting ART.
The remaining 324 PLWH were included in the study. Among them, 91 out of 324 (28%) were female and 240 out of 324 (74%) were Italian. The median age of study participants was 41 years (IQR 18–80 years). The risk factors for HIV acquisition were as follows: unprotected intercourse, 278 PLWH (heterosexual, 183 [56%]; men who had sex with men [MSM], 95 [29%]); intravenous drug use (IVDU), 29 (9%) PLWH; and other/unknown, 17 (5%) PLWH.
The mean CD4 nadir of study participants before they received ART was 276 (± 222) cells/mm3, and the mean CD4 count after 36 months of ART was 664 (± 326) cells/mm3.
According to the CDC classification, 121 (37%), 131 (40%), and 71 (22%) PLWH were on stage A, B, and C, respectively, at time of diagnosis (Table 1).
Overall, 41 out of 324 (12.6%) study participants met the criteria for INR20%; 32 were male (78%) and the mean age was 42 years (±14.08 years). INR20% were less frequently in CDC stage C (7.3% vs. 24%, p = 0.02), had a lower CD4 nadir (254 cells/mm3 in INR20% vs. 420 cells/mm3 in full responders, p < 0.001), and a lower HIV RNA zenith (4.44 log10 in INR20% vs. 4.96 log10 in full responders, p = 0.01). The mean CD8+T cell counts were similar in INR20% and full responders at baseline (p = 0.41), but were lower in INR20% at 36 months (731 vs. 916 cells/mm3, p = 0.006).
Age (p = 0.07), sex (p = 0.35), risk factor for HIV infection (p = 0.23), country of origin (p = 0.31), and prevalence of HCV RNA, HBsAg, and CMV-IgG positivity were similar between INR20% and full responders (p =0.91, p = 0.38, and p = 0.50, respectively). In addition, the dynamics of HIV RNA decay during follow-up was similar in the two groups (p = 0.38), and no difference was found in time of exposure to different ART regimens in INR20% and full responders. The mortality rate was similar in the two groups (p = 0.39; Table 2).
A total of 112 out 324 (34.6%) PLWH met the criteria for INR500 at 36 months; 86 were male (77%) and the median age was 42 years (±12.06 years). INR500 were more often non-Italian (40% vs. 22%, p = 0.01) and in CDC stage C at the time of HIV diagnosis (37% vs.14%, p < 0.001) compared with full responders. CD4 nadir (109 vs. 364 cells/mm3, p < 0.001) and 36-month CD8+ T cell counts (780 vs. 949 cells/mm3, p <0.001) were lower in INR500, and, at baseline, CD8+ T cell counts were higher in INR500 (1030 vs.768 cells/mm3, p = 0.038). According to the ROC curve, a CD4 nadir ≤ 200 cells/mm3 showed 83.93% specificity and 71.43% sensitivity in predicting becoming INR500 [area under the curve (AUC) 0.839;143 people had CD4 nadir ≤ 200 cells/mm3 in the study cohort] in this cohort. The CD4/CD8 ratio was lower in INR500 at both baseline and 36 months’ evaluation (0.14 vs.0.43, p < 0.001 and 0.48 vs. 0.92, p < 0.001, respectively).
No difference was found in time to achievement of HIV RNA of < 50 copies/ml (p = 0.71) or in the zenith of HIV RNA (p = 0.31). No differences were found in time of exposure to different ART classes or in the other patient characteristics that were evaluated. The mortality rate was similar in the two groups (p = 0.22; Table 3).
In the study population, 5 out of 324 (1.5%) PLWH did not recover at least 200 CD4/mm3 at 36 months after ART initiation and were thus classified as INR200. Among them, four were male (80%) and the median age was 51 years (±12.06 years). They had a lower CD4 nadir (33 vs. 279 cells/mm3, p < 0.001) and a lower CD4/CD8 ratio at both baseline and 36 months’ follow-up than full responders (0.14 vs. 0.42, p = 0.012, and 0.17 vs. 0.78, p < 0.001, respectively). According to the ROC curve, a CD4 nadir ≤ 65 cells/mm3 showed 56.5% specificity and 100% sensitivity in predicting becoming INR200 (AUC 0.765) in this cohort (65 people had a CD4 nadir ≤ 65 cells/mm3 in the study cohort). The CDC stage at diagnosis was not significantly different in INR200 than in full responders (p = 0.559). The crude mortality was higher in this group because of one death out of five INR200 (Table 4). No other significant differences were found in the other analyzed characteristics and drug exposure in the study population.
Univariate analysis of factors associated with INR20%, INR500, and INR200 are shown in Supplementary Tables 1, 2, and 3. After adjustment for possible confounders, CD4 nadir remained the only factor that maintained a significant association with all INR definitions, with higher values predicting INR20% [adjusted odds ratio (aOR) 1.003, 95% CI 1.001–1.004, p < 0.001] and lower values predicting INR500 (aOR 0.984, 95% CI 0.980–0.988, p < 0.001) and INR200 (aOR 0.98, 95% CI 0.95–1.001, p = 0.098). Both INR20% and INR500 were associated with lower CD8+ T cell counts after 36 months of suppressive ART (aOR 0.99, 95% CI 0.99–1.00, p = 0.010, and aOR 0.99, 95% CI 0.99–1.00, p < 0.001, respectively). The complete multivariable analysis is shown in Table 5.
In the sensitivity analysis including the CD4/CD8 ratio instead of the absolute numbers of CD4 and CD8+ T cells (Table 6), a higher baseline CD4/CD8 ratio was confirmed to be inversely related to the probability of poor immune recovery for INR500 (OR 0.03, 95% CI 0.01–0.12, p < 0.001) and INR200 (OR 0.002, 95% CI 18–7–67.72, p = 0.255), and it was directly related to the odds of being INR20% (OR 6.14, 95%CI 2.36–15.97, p<0.001).
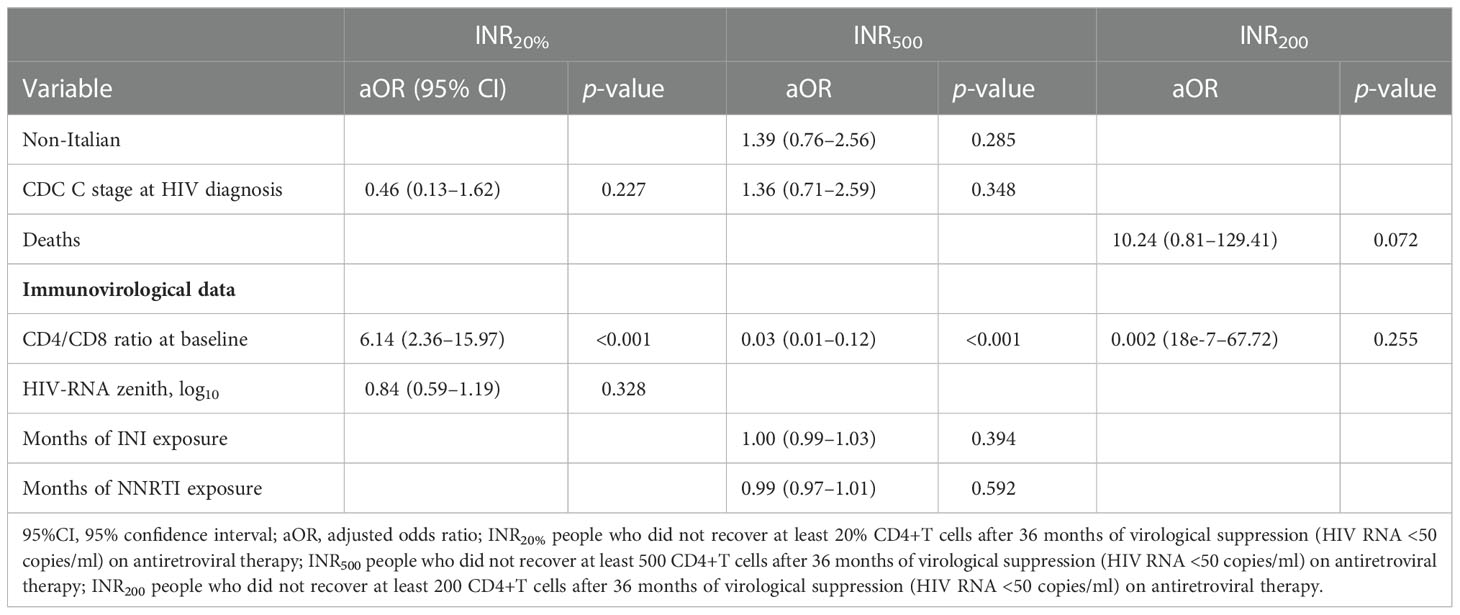
Table 6 Multivariable analysis of factors associated to INR20%, INR500 and INR200, including CD4/CD8 ratio at baseline.
At the 36-month evaluation, the relationship between CD4/CD8 ratio and INR was confirmed, with OR 0.05 (95% CI 0.02–0.13, p < 0.001) and 1.9–14 (95% CI 4.5–25–7.88–4, p = 0.011) of being INR500 and INR200, respectively, and OR 1.30 (95% CI 0.72–2.33) of being INR20% for each unit of increase of CD4/CD8 ratio (Table 7).
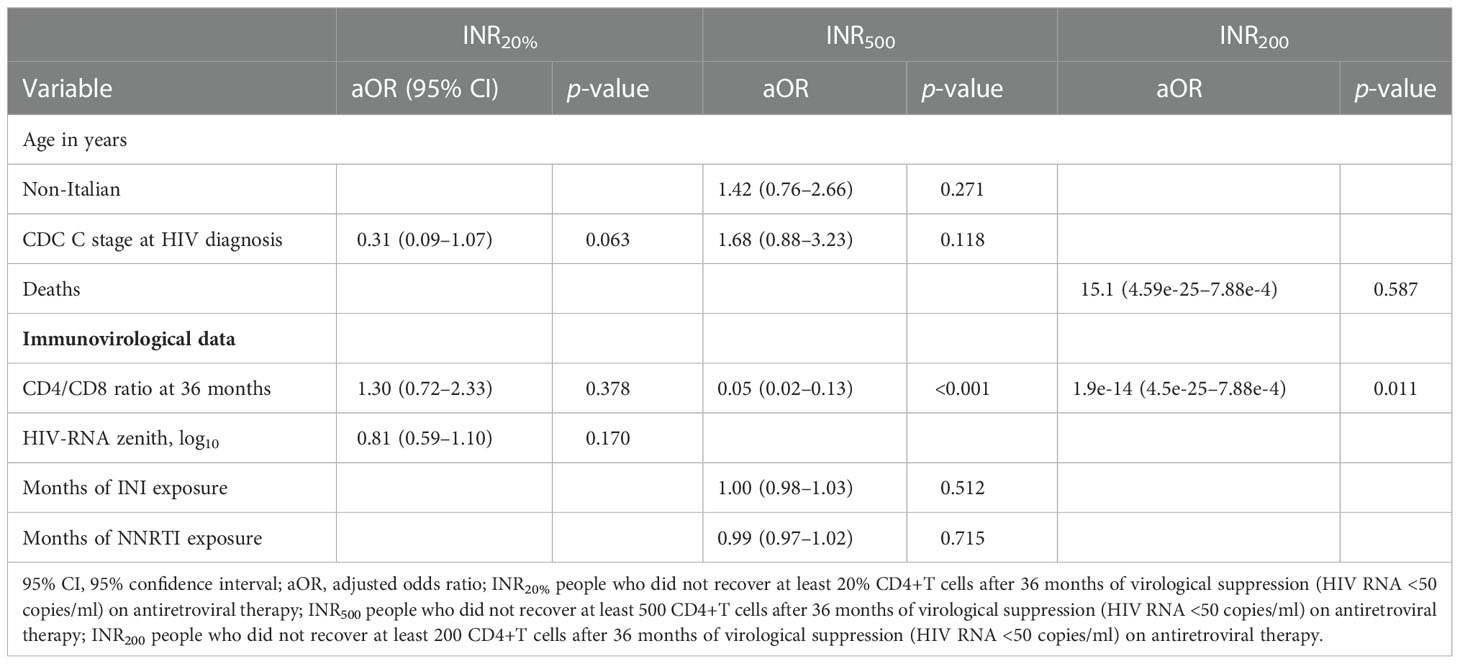
Table 7 Multivariable analysis of factors associated to INR20%, INR500 and INR200, including CD4/CD8 ratio at 36 months evaluation.
In the present study we found a prevalence of INR ranging between 1.5% and 34.6%, exploring different definition of immunological non-response.
The most restrictive definition that we used, namely that of INR200, revealed an INR200 rate of only 1.5%, which was lower than that reported in older studies that used the same threshold, which ranged between 15% and 26% (11, 42, 44), but which is consistent with the results of others (45). However, comparison among studies might be influenced by different study periods and antiretrovirals used in different settings and by heterogeneous duration of follow-up. The higher frequency of INR200 found in previous years might reflect the use of older drugs that are currently considered sub-optimal or less tolerable, possibly compromising overall ART efficacy and, consequently, also immunological recovery (11, 42, 44). On the contrary, newer drugs such as INSTIs (46, 47), and also PIs, when compared with older ART approaches (48), might favor a more effective immune recovery. However, in our study, we did not find a consistent correlation between INR rate and months of exposure to different classes of antiretrovirals. It is notable that the study was conducted in an era of modern ART; if different ART classes were used in first-line therapy, the usefulness of comparing newer and older drugs is limited by the absence of an historical control group. In addition, the observation about the role of a low CD4 nadir underlying the INR200 phenomenon underscores the very important role of early diagnosis in preventing the INR phenomenon; increased awareness of the risk of infection and improved timing of diagnosis could explain, at least in part, the reduction in the current number of INR200.
On the other hand, when assessing frequency of INR500, we still found about 35% of PLWH with a discordant viro-immunological response, in line with the frequency found in previous studies (34, 45, 46), suggesting that, even if modern therapies have contributed to make INR200 rarer, the complete viro-immunological response is still not guaranteed nor consistently improved compared with the past, when using the CD4 threshold of 500 cells/mm3. Even if the highest risk of clinical events and death has been associated with INR200 (11, 42, 44), PLWH with a CD4 count in the range 200–499 cells/mm3 still have higher mortality rates than those with a CD4 count > 500 cells/mm3 (49). Therefore, the goal of ART should be to get patients over this threshold and reaching an immunological condition as close as possible to that of the general population. Moreover, INR500 have been shown to have higher percentages of activated CD4+ T cells, regulatory T cells (Treg), effector Treg and terminal effector Treg suggesting, in this group of PLWH, a residual immune activation persisting after many years of ART (18, 35). In addition, immune responses against vaccines are still different among PWH with CD4+ T cell counts of < 200, 200–500, or > 500 cells/mm3, and in those with counts of > 500 cells/mm3 responses comparable to those in the HIV-uninfected population have been described (50). The persistence of a certain grade of inflammation might also be indirectly inferred from the CD4/CD8 ratio, a surrogate marker of T-cell compartment balance, reflecting both CD4 T-cell recovery and CD8 T-cell activation, expansion, and senescence (51, 8). Few studies have investigated the relationship between CD4/CD8 ratio and immune recovery, supporting an association between lower baseline levels and immunodiscordant response to ART (46, 52). In our study, we found not only that a higher CD4/CD8 ratio was protective toward becoming either INR200 or INR500 at time of ART initiation, but also that that CD4/CD8 ratio was more likely to be lower after 36 months of ART in INR200 and INR500 than in full responders, supporting the hypothesis of a possible persistent immune activation impairing reconstitution of the immune system and CD4 gains (53). Moreover, CD4 nadir was confirmed to be the stronger predictor of immunological non-response in INR200 and INR500, in accordance with data from the literature (18, 34, 44, 45, 54), and all other epidemiological and clinical variables considered did not consistently correlate with INR. Instead, these data were not confirmed when using the definition of INR20%. In fact, in our study, the odds of being INR20% was higher in PLWH with a higher CD4 nadir and higher CD4/CD8 ratio. Being INR20% has been described in the past as a predictor of clinical progression (14); however, the definition of INR20% is highly influenced by baseline CD4– T cell count and, in an era of modern ART, where therapy is initiated regardless of total CD4 count at HIV diagnosis, this definition may be misleading, as it may imply the inclusion of early-treated patients with unimpaired baseline CD4 counts. Consequently, these PLWH do not experience an increase in CD4 above the predefined threshold of 20% from baseline, but this should not be interpreted as a poor response. On the contrary, for PLWH with extremely low CD4 counts, a small CD4 gain could even exceed the threshold of 20% while remaining with a very low absolute number of lymphocytes and CD4/CD8 ratio. Therefore, even if the definition can still be applied to certain clinical situations in PLWH stratified based on baseline CD4 counts, or in certain research contexts, INR20% might not be a suitable definition for identifying in general PLWH with poor responses in clinical practice.
The present study has several limitations. The retrospective design and relatively small sample size of the cohort limit the strength and generalizability of the findings. Moreover, data on biomarkers of immune activation, on viral reservoir, as well as data on herpetic viral co-infections, genetics, and behavioral or dietary factors were not available, limiting the possibility of investigation of further variables influencing immunological responses. In addition, the exclusion of people lost to follow-up or dead before the predefined time point of 36 months might have contributed to underestimation of INRs. Despite these limits, the study highlights how INR200 have become very rare in the contemporary ART era, and still about one-third of PLWH meet the criteria for INR500. Overcoming the threshold of 500 CD4/mm3 could be more appropriate to define full responders, in contrast with the older definitions of INR200 and INR20%. Although our results do not show a benefit from choosing different ART strategies to improve immune recovery, early diagnosis, and rapid treatment initiation before CD4 counts and CD4/CD8 ratio begin to decline are critical to achieving an optimal immune response.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ligurian Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
LT, LL, FB, GB, SB, AB, and MB performed the research. SM, SB, and MG managed the database and checked the accuracy of the data. LT, LL, and AB designed the research study. LT analyzed the data. LT, LL, FB, and GB wrote the paper. MB and AB reviewed the final version of the paper and the scientific contents of the study. All authors have read and approved the final version of the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2022.822153/full#supplementary-material
1. Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med (2006) 355:2283–96. doi: 10.1056/NEJMoa062360
2. Lohse N, Hansen A-BE, Pedersen G, Kronborg G, Gerstoft J, Sørensen HT, et al. Survival of persons with and without HIV infection in Denmark 1995-2005. Ann Intern Med (2007) 146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003
3. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV (2017) 4:e349–56. doi: 10.1016/S2352-3018(17)30066-8
4. Gordon CL, Cheng AC, Cameron PU, Bailey M, Crowe SM, Mills J. Quantitative assessment of intra-patient variation in CD4+ T cell counts in stable, virologically-suppressed, HIV-infected subjects. PloS One (2015) 10:e0125248. doi: 10.1371/journal.pone.0125248
5. Milanés-Guisado Y, Gutiérrez-Valencia A, Trujillo-Rodríguez M, Espinosa N, Viciana P, López-Cortés LF. Absolute CD4+ T cell count overstate immune recovery assessed by CD4+/CD8+ ratio in HIV-infected patients on treatment. PloS One (2018) 13:e0205777. doi: 10.1371/journal.pone.0205777
6. van Lelyveld SFL, Gras L, Kesselring A, Zhang S, De Wolf F, Wensing AMJ, et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS (2012) 26:465–74. doi: 10.1097/QAD.0b013e32834f32f8
7. Takuva S, Maskew M, Brennan AT, Long L, Sanne I, Fox MP. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a south African cohort. J Int AIDS Soc (2014) 17:18651. doi: 10.7448/IAS.17.1.18651
8. Pacheco YM, Jarrin I, Rosado I, Campins AA, Berenguer J, Iribarren JA, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res (2015) 117:69–74. doi: 10.1016/j.antiviral.2015.03.002
9. Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the international antiviral society-USA panel. JAMA (2020) 324:1651–69. doi: 10.1001/jama.2020.17025
10. Di Biagio A, Rusconi S, Marzocchetti A, Signori A, Schiavetti I, Bruzzone B, et al. The role of baseline HIV-1 RNA, drug resistance, and regimen type as determinants of response to first-line antiretroviral therapy. J Med Virol (2014) 86:1648–55. doi: 10.1002/jmv.24017
11. Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis (2014) 58:1312–21. doi: 10.1093/cid/ciu038
12. Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J Leukoc Biol (2020) 107:597–612. doi: 10.1002/JLB.4MR1019-189R
13. Gazzola L, Tincati C, Bellistrì GM, d’Arminio MA, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis (2009) 48:328–37. doi: 10.1086/595851
14. Lapadula G, Cozzi-Lepri A, Marchetti G, Antinori A, Chiodera A, Nicastri E, et al. Risk of clinical progression among patients with immunological nonresponse despite virological suppression after combination antiretroviral treatment. AIDS (2013) 27:769–79. doi: 10.1097/QAD.0b013e32835cb747
15. Rusconi S, Vitiello P, Adorni F, Colella E, Focà E, Capetti A, et al. Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: An Italian randomized clinical trial. PloS One (2013) 8:e80157. doi: 10.1371/journal.pone.0080157
16. Sennepin A, Baychelier F, Guihot A, Nel I, Ho Tsong Fang R, Calin R, et al. NKp44L expression on CD4+ T cells is associated with impaired immunological recovery in HIV-infected patients under highly active antiretroviral therapy. AIDS (2013) 27:1857–66. doi: 10.1097/qad.0b013e328361a3fe
17. Gaardbo JC, Hartling HJ, Ronit A, Springborg K, Gjerdrum LMR, Ralfkiær E, et al. Regulatory T cells in HIV-infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J Acquir Immune Defic Syndr (2014) 66:349–57. doi: 10.1097/QAI.0000000000000173
18. Saison J, Ferry T, Demaret J, Maucort-Boulch D, Venet F, Perpoint T, et al. Relationship between discordant response to HAART, tregs, immune activation and low-level viraemia. J Int AIDS Soc (2014) 17:19672. doi: 10.7448/IAS.17.4.19672
19. Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord, Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A, et al. All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: Evidence from a large European observational cohort collaboration. Int J Epidemiol (2012) 41:433–45. doi: 10.1093/ije/dyr164
20. Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet (2014) 384:241–8. doi: 10.1016/S0140-6736(14)60604-8
21. Centers for Disease Control and Prevention (CDC). Terms, definitions, and calculations used in CDC HIV surveillance publications (2016). Available at: https://www.cdc.gov/hiv/pdf/statistics/systems/nhbs/cdc-hiv-terms-surveillance-publications-2014.pdf (Accessed October 11, 2022).
22. Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis (2006) 6:280–7. doi: 10.1016/S1473-3099(06)70463-7
23. Massanella M, Negredo E, Clotet B, Blanco J. Immunodiscordant responses to HAART–mechanisms and consequences. Expert Rev Clin Immunol (2013) 9:1135–49. doi: 10.1586/1744666X.2013.842897
24. INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med (2015) 373:795–807. doi: 10.1056/NEJMoa1506816
25. DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents, A Working Group of the Office of AIDS Research, Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. (Last accessed 28 November 2022).
26. EACS European Aids Clinical Society Guidelines. (2021). Available at: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. (Last accessed 20 October 2021).
27. Hammer SM, Eron JJ, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the international AIDS society-USA panel. JAMA (2008) 300:555–70. doi: 10.1001/jama.300.5.555
28. Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JVR, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: A multicentre, double-blind randomised controlled trial. Lancet (2009) 374:796–806. doi: 10.1016/S0140-6736(09)60918-1
29. Fraccaro P, Pupella V, Gazzarata R, Dentone C, Cenderello G, De Leo P, et al. The ligurian human immunodeficiency virus clinical network: A web tool to manage patients with human immunodeficiency virus in primary care and multicenter clinical trials. Med 2 0 (2013) 2:e5. doi: 10.2196/med20.2712
30. Gazzarata R, Giannini B, Giacomini M. A SOA-based platform to support clinical data sharing. J Healthc Eng (2017) 2017. doi: 10.1155/2017/2190679
31. Marziali M, De Santis W, Carello R, Leti W, Esposito A, Isgrò A, et al. T-Cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS (2006) 20:2033–41. doi: 10.1097/01.aids.0000247588.69438.fd
32. Isgrò A, Leti W, De Santis W, Marziali M, Esposito A, Fimiani C, et al. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis (2008) 46:1902–10. doi: 10.1086/588480
33. Li T, Wu N, Dai Y, Qiu Z, Han Y, Xie J, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis (2011) 53:944–51. doi: 10.1093/cid/cir552
34. Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis (2005) 41:361–72. doi: 10.1086/431484
35. Saison J, Ferry T, Demaret J, Maucort Boulch D, Venet F, Perpoint T, et al. Association between discordant immunological response to highly active anti-retroviral therapy, regulatory T cell percentage, immune cell activation and very low-level viraemia in HIV-infected patients. Clin Exp Immunol (2014) 176:401–9. doi: 10.1111/cei.12278
36. Jarrin I, Pantazis N, Dalmau J, Phillips AN, Olson A, Mussini C, et al. Does rapid HIV disease progression prior to combination antiretroviral therapy hinder optimal CD4+ T-cell recovery once HIV-1 suppression is achieved? AIDS (2015) 29:2323–33. doi: 10.1097/QAD.0000000000000805
37. Girard A, Vergnon-Miszczycha D, Depincé-Berger A-E, Roblin X, Lutch F, Lambert C, et al. Brief report: A high rate of β7+ gut-homing lymphocytes in HIV-infected immunological nonresponders is associated with poor CD4 T-cell recovery during suppressive HAART. J Acquir Immune Defic Syndr (2016) 72:259–65. doi: 10.1097/QAI.0000000000000943
38. Norris PJ, Zhang J, Worlock A, Nair SV, Anastos K, Minkoff HL, et al. Systemic cytokine levels do not predict CD4(+) T-cell recovery after suppressive combination antiretroviral therapy in chronic human immunodeficiency virus infection. Open Forum Infect Dis (2016) 3:ofw025. doi: 10.1093/ofid/ofw025
39. Marchetti G, Gazzola L, Trabattoni D, Bai F, Ancona G, Ferraris L, et al. Skewed T-cell maturation and function in HIV-infected patients failing CD4+ recovery upon long-term virologically suppressive HAART. AIDS (2010) 24:1455–60. doi: 10.1097/QAD.0b013e328339cf40
40. Soria A, Guerini FR, Bandera A, Bolognesi E, Uglietti A, Fusco C, et al. KIR-HLA genotypes in HIV-infected patients lacking immunological recovery despite effective antiretroviral therapy. PloS One (2011) 6:e27349. doi: 10.1371/journal.pone.0027349
41. Zoufaly A, ander Heiden M, Kollan C, Bogner JR, Fätkenheuer G, Wasmuth JC, et al. Clinical outcome of HIV-infected patients with discordant virological and immunological response to antiretroviral therapy. J Infect Dis (2011) 203:364–71. doi: 10.1093/jinfdis/jiq055
42. Lapadula G, Chatenoud L, Gori A, Castelli F, Di Giambenedetto S, Fabbiani M, et al. Risk of severe non AIDS events is increased among patients unable to increase their CD4+ T-cell counts >200+/μl despite effective HAART. PloS One (2015) 10:e0124741. doi: 10.1371/journal.pone.0124741
43. Centers for Disease Control and Prevention (CDC). Duration of isolation and precautions for adults with COVID-19 (2020). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html.
44. Engsig FN, Gerstoft J, Kronborg G, Larsen CS, Pedersen G, Røge B, et al. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: A cohort study. BMC Infect Dis (2010) 10:318. doi: 10.1186/1471-2334-10-318
45. Kaufmann GR, Bloch M, Finlayson R, Zaunders J, Smith D, Cooper DA. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS (2002) 16:359–67. doi: 10.1097/00002030-200202150-00007
46. Roul H, Mary-Krause M, Ghosn J, Delaugerre C, Pialoux G, Cuzin L, et al. CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS (2018) 32:2605–14. doi: 10.1097/QAD.0000000000002010
47. Fabbiani M, Borghetti A, Squillace N, Colafigli M, Taramasso L, Lombardi A, et al. Integrase inhibitors use and cytomegalovirus infection predict immune recovery in people living with HIV starting first-line therapy. J Acquir Immune Defic Syndr (2021) 86:119–27. doi: 10.1097/QAI.0000000000002525
48. Dronda F, Moreno S, Moreno A, Casado JL, Pérez-Elías MJ, Antela A. Long-term outcomes among antiretroviral-naive human immunodeficiency virus-infected patients with small increases in CD4+ cell counts after successful virologic suppression. Clin Infect Dis (2002) 35:1005–9. doi: 10.1086/342695
49. Maman D, Pujades-Rodriguez M, Nicholas S, McGuire M, Szumilin E, Ecochard R, et al. Response to antiretroviral therapy: improved survival associated with CD4 above 500 cells/μl. AIDS (2012) 26:1393–8. doi: 10.1097/QAD.0b013e328352d054
50. Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in people living with human immunodeficiency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin Infect Dis (2022) 75:e552–63. doi: 10.1093/cid/ciac238
51. Lu W, Mehraj V, Vyboh K, Cao W, Li T, Routy J-P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc (2015) 18:20052. doi: 10.7448/IAS.18.1.20052
52. Rosado-Sánchez I, Herrero-Fernández I, Álvarez-Ríos AI, Genebat M, Abad-Carrillo MA, Ruiz-Mateos E, et al. A lower baseline CD4/CD8 T-cell ratio is independently associated with immunodiscordant response to antiretroviral therapy in HIV-infected subjects. Antimicrob Agents Chemother (2017) 61:e00605–17. doi: 10.1128/AAC.00605-17
53. Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T Cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis (2003) 187:1534–43. doi: 10.1086/374786
Keywords: immunological non responders, HIV, immune recovery, discordant immune response, antiretrovirals, CD4+T-cell count
Citation: Taramasso L, Labate L, Briano F, Brucci G, Mora S, Blanchi S, Giacomini M, Bassetti M and Di Biagio A (2023) CD4+ T lymphocyte recovery in the modern antiretroviral therapy era: Toward a new threshold for defining immunological non-responders. Front. Virol. 2:822153. doi: 10.3389/fviro.2022.822153
Received: 25 November 2021; Accepted: 12 December 2022;
Published: 10 January 2023.
Edited by:
Lara Manganaro, National Institute of Molecular Genetics (INGM), ItalyReviewed by:
Maura Statzu, Emory University, United StatesCopyright © 2023 Taramasso, Labate, Briano, Brucci, Mora, Blanchi, Giacomini, Bassetti and Di Biagio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Taramasso, dGFyYW1hc3NvLmx1Y2lhQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.