- 1Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (INBIRS), Universidad de Buenos Aires (UBA)-CONICET, Buenos Aires, Argentina
- 2Respiratory Research Group, Instituto de Ciencia y Tecnología Dr. César Milstein—(Consejo Nacional de Investigaciones Científicas y Técnicas CONICET-Fundación Pablo Cassará), Ciudad de Buenos Aires, Argentina
Iota-carrageenan is a sulfated polysaccharide extracted from red seaweeds, which, formulated into a nasal spray, has already been proven safe and effective in viral upper respiratory infections. In Calu-3, a human respiratory epithelium cell line, we explored the activity of a formula of iota-carrageenan and sodium chloride against SARS-CoV-2. In this study, the assayed formula, already approved as a nasal spray for human use, effectively inhibited SARS-CoV-2 infection, providing a more substantial reference for clinical studies or developments.
Introduction
The severe acute respiratory coronavirus 2 (SARS-CoV-2) is responsible for the currently ongoing pandemic coronavirus disease (COVID-19), counting more than 243,857,028 confirmed cases and more than 4.953.246 deaths worldwide by October 26, 2021 (1, 2). There are, except for remdesivir, no approved antivirals for the treatment or prevention of SARS-CoV-2 infection. Vaccines are the only tool that has been shown to be effective in preventing COVID-19; at the time of this publication, <40% of the world population has received a complete vaccination, and in developing countries, the percentage of individuals with complete vaccination is negligible, and only 3.1% of the population has received a single dose (3).
Repurposing established medications with recognized safety profiles is a possible approach for preventing or treating the disease and shortening the time-consuming drug development stages while the vaccination is being extended.
During the first days of the infection, the virus replicates mainly in the nasal cavity and the nasopharynx; therefore, nasal sprays with antiviral activity could prevent infection or, in case it occurs, could reduce the viral load in these zones (4, 5).
Marine-derived polysaccharides, such as carrageenans, are a family of linear sulfated polysaccharides extracted from red seaweeds, widely used as thickening agents and stabilizers for food. Besides these properties, the iota-carrageenan demonstrated antiviral activity against several viruses, including respiratory viruses such as human rhinovirus, influenza A H1N1, and common cold coronavirus (6–8). Iota-carrageenan inhibits virus infection mainly based on its interaction with the surface of viral particles, preventing them from entering cells and trapping the viral particles released from the infected cells (9). It has also been shown that their inhibitory activity also relies on affecting the viral replication cycle at different steps, like entry and genome replication, and additionally activates the host's antiviral immune response (10–12).
Iota-carrageenan formulated into a nasal spray has already been proven safe and effective in common cold treatment (13). Based on these observations, the hypothesis has been raised that a nasal spray with iota-carrageenan could be effective against SARS-CoV-2. It has recently been described that iota-carrageenan has activity against the SARS-CoV-2 virus and its Spike Pseudotyped Lentivirus (SSPL) in Vero E6 cell culture (14, 15). The Vero E6 cell line (African green monkey kidney) is deficient for type 1 interferons (IFNs) and highly susceptible to many different pathogens, like measles virus, rubella virus, arboviruses, adenoviruses, influenza, and some coronavirus, including SARS-CoV-2 (16–18).
Various studies have proposed the need to study SARS-CoV-2 infection in human respiratory epithelium to get closer to the central target tissue of the disease in patients (19). Calu-3 is a cell line derived from a submucosal adenocarcinoma of the bronchi that grows in adherent culture and displays epithelial morphology. Upon stimulation with viruses or environmental toxins, the Calu-3 cell line synthesizes and releases different cytokines, including IL-6 (20), which play a central role in the inflammatory cascade associated with more severe COVID-19 (21). This cell line is considered a sensitive and efficient preclinical model to study human respiratory processes and diseases (22). Although the Calu-3 cell line comes from a submucosal adenocarcinoma of the bronchi, it is considered a suitable in vitro model of the upper airway epithelium. Calu-3 cells show a combined secretory and ciliated phenotype. Calu-3 cells have microvilli, express different cell-binding proteins (tight junctions, desmosomes, and zonulae adherent), and express MUC1 and MUC5/5AC mRNA MUC5/5AC mucins, and are covered with a uniform mucus layer, meaning that, despite its bronchial origin, the Calu-3 cell line has similar characteristics to nasal epithelial cells, being, for example, useful to study mucin gene expression and synthesis, electrolyte transport, epithelial barrier properties, regulatory mechanisms, and transport and metabolism of drugs (23–26). Clinical studies have shown that iota-carrageenan nasal sprays that are effective in vitro against viruses that infect the nasal mucosa (6) were found to be effective in preventing and reducing the symptoms and duration of the common cold caused by those viruses (13, 27–29).
In this study, we assessed in vitro the effect of iota-carrageenan as a SARS-CoV-2 infection inhibitor in Calu-3 cells. We found that iota-carrageenan strongly inhibits virus replication in these cells in a dose-dependent manner, providing data that reinforces previous results and stimulate research on its use as a nasal spray during the pandemic.
Materials and Methods
Cells and Virus
African green monkey kidney Vero E6 cells (ATCC® CRL-1586™) and human airway epithelial Calu-3 cells (ATCC® HTB-55TM) were obtained from the American Type Culture Collection, Manassas, VA, USA. The Calu-3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Corning, NY, USA) containing 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Thermo Fisher Scientific, Waltham, MA, USA). The Vero E6 cells were cultured in complete minimal essential medium (c-MEM) (Corning, NY, USA), supplemented with 5% FBS (Thermo Fisher Scientific, Waltham, MA, USA). The cells were incubated in 95% air and 5% CO2 at 37°C.
The SARS-CoV-2 isolate used is hCoV-19/Argentina/PAIS-G0001/2020, GISAID Accession ID: EPI_ISL_499083, kindly provided by Dr. Sandra Gallegos (National University of Córdoba, Argentina). Viral master seed stock was prepared using T175 flasks of Vero E6 cells. Each flask was harvested on day two post-infection, and the supernatant was centrifuged twice at 220 x g for 15 mins to remove cellular debris. The titer of virus stock was determined by plaque assay on Vero E6 cells and expressed as plaque-forming units per ml (pfu/ml). The experiments using the virus were approved by the INBIRS Institutional Biosafety Committee and carried out in the Biosafety Level 3 with negative pressure facilities from the School of Medicine at the University of Buenos Aires.
Preparation of Sample Formulations
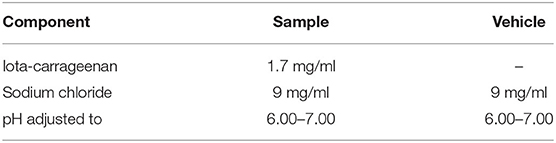
Solutions with iota-carrageenan and sodium chloride were prepared using a sterile nasal spray for therapeutic use. All the formulations and placebos were prepared at Laboratorio Pablo Cassará S.R.L. (Argentina) under aseptic conditions. The composition of active and placebo formulations is depicted in Table 1.
To determine antiviral efficacy of formulations by titer reduction assay, sample formulations were used at a final iota-carrageenan concentration of 600, 60, 6, 0.6, and 0.06 μg/ml. An equivalent concentration of placebos was used for titer reduction assay as controls. As a positive control, a dilution 1/200 of an equine hyperimmune serum (HHS) against SARS-CoV-2 was kindly provided by the Instituto Nacional de Enfermedades Infecciosas, Administración Nacional de Laboratorios e Institutos de Salud (ANLIS) “Dr. Carlos G. Malbran,” Buenos Aires, Argentina.
The present study was designed so that the concentrations to be evaluated are similar to those obtained in the nasal cavity when applying the iota-carrageenan nasal spray that was used in clinical trials in patients with the common viral cold and according to the dosage used in those clinical trials (an application of 100 microliters of a 1.2 mg/ml iota-carrageenan solution in each nostril). For this, we estimate the volume of airway surface liquid (ASL) in the nasal cavity based on a surface area of the nasal mucosa of 100 to 250 cm2 (30–33) and the height of the fluid in the nasal cavity estimated as 5–15 μm (34, 35) resulting in a range of 50 to 375 μl. If we take an average content of 200 μl of liquid from the surface of the airways in the nose plus 200 μl of the formulation after administering 100 μl of the solution in each nostril, the immediate concentration of iota-carrageenan in the nasal cavity would be 600 μg/ml, the highest concentration tested in our investigation. The expected concentrations of iota-carrageenan in the nasal cavity would be even higher if we consider a nasal formulation containing 1.7 mg/ml, like the one used in the only clinical trial published on the efficacy of a nasal spray with iota-carrageenan in the prevention of COVID-19, research led by one of the co-authors of the present work (36).
Viability Cellular Assays
Calu-3 cells were seeded by quadrupled wells using 96-well tissue culture microplates at 3 × 104 cells/well and incubated overnight at 37°C under 5% CO2. The experiment was replicated in three independent assays. Then, the cells were treated or not with iota-carrageenan 600, 60, 6, 0.6, and 0.06 μg/ml or vehicle in culture medium (DMEM supplemented with 2% FBS) for 48 h at 37°C. After incubation, cells were washed and treated with MTS/PMS (CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay, Promega, USA).
Infection Assays
In three independent experiments, Calu-3 cells were seeded in 96-well tissue culture microplates at 3x104 cells/well. After 48 h of incubation at 37°C, treated or not with iota-carrageenan 600, 60, 6, 0.6, and 0.06 μg/ml or vehicle and 2 h later infected with SARS-CoV-2 (multiplicity of infection (MOI) = 0.01 and 0.1) in serum-free DMEM (Thermo Fisher) for 1 h at 37°C. Then, cells were washed and placed in culture medium (DMEM supplemented with 2% FBS) containing iota-carrageenan or vehicle for 48 h. After that, supernatants were harvested and stored at −80°C.
In another set of experiments, Calu-3 cells were treated with iota-carrageenan (600 to 0.6 μg/ml) 2 h before the infection only (Pretreatment), while being infected (Simultaneous), or after the cells were washed (Post-treatment). Cells exposed to iota-carrageenan throughout the experiment were also included (2 h of pretreatment, 1 h during infection, and 48 h after infection) (Continuous). Supernatants were harvested 48 h after infection, and virus production was measured.
To determine the iota-carrageenan neutralization activity, Calu-3 cells were pre-treated with iota-carrageenan or vehicle (600 μg/ml to 0.06 μg/ml) for 2 h. After 2 h of pretreatment, cells were infected (MOI: 0.01) with SARS-CoV-2 and incubated for 48 h in the presence of iota-carrageenan. Supernatants were harvested, and then the viral titer was quantified in Vero E6 cells. Cytopathic effect was quantified by crystal violet staining and measured as absorbance at 585 nm by triplicate. The percentage of inhibition was calculated with respect to control.
Viral Titration
Vero E6 cells were seeded into 96-well microplates and grown overnight at 37°C under 5% CO2. Ten-fold dilutions of virus samples from Calu-3 cells were added to monolayers of 80% confluent Vero E6 cells at 37°C for 1 h in serum-free DMEM. After incubation, the inoculum was removed, and monolayers were overlaid with DMEM supplemented with 2% of FBS. The cells were incubated at 37°C for 72 h and fixed using 4% formaldehyde. Finally, cells were stained with 0.1% crystal violet in 20% ethanol and counted. Virus endpoint titer was determined using the Reed-Muench formula and expressed as 50% tissue culture infectious dose (TCID50) per ml.
SARS-CoV-2 Viral Load Quantification by RT-qPCR
The supernatants were harvested 48 h post-infection, and RNA was extracted using a Chemagic 360-D automated extraction equipment (Perkin-Elmer). SARS-CoV-2 RNA was quantified using DisCoVery SARS-CoV-2 RT-PCR Detection Kit Rox (Transgen Biotech), which allows multiplex detection of viral genes N, ORF1ab, and human gene (RNase P gene).
Statistical Analysis
In the cellular viability and infection assays, the results were analyzed statistically by One-way ANOVA followed by Tukey's or Dunnett's (when compared to vehicle), multiple comparisons tests, using GraphPad Prism version 9.1.0, GraphPad Software, San Diego, California USA, www.graphpad.com.
Results
The antiviral effects of iota-carrageenan on SARS-CoV-2 were tested in a dose-dependent manner. In the first set of experiments, Calu-3 cells were pre-treated with different iota-carrageenan concentrations (600–0.06 μg/ml), and cell viability was quantified (Figure 1A). No difference in cell viability was observed in iota-carrageenan-treated cells compared to vehicle-treated control cells.
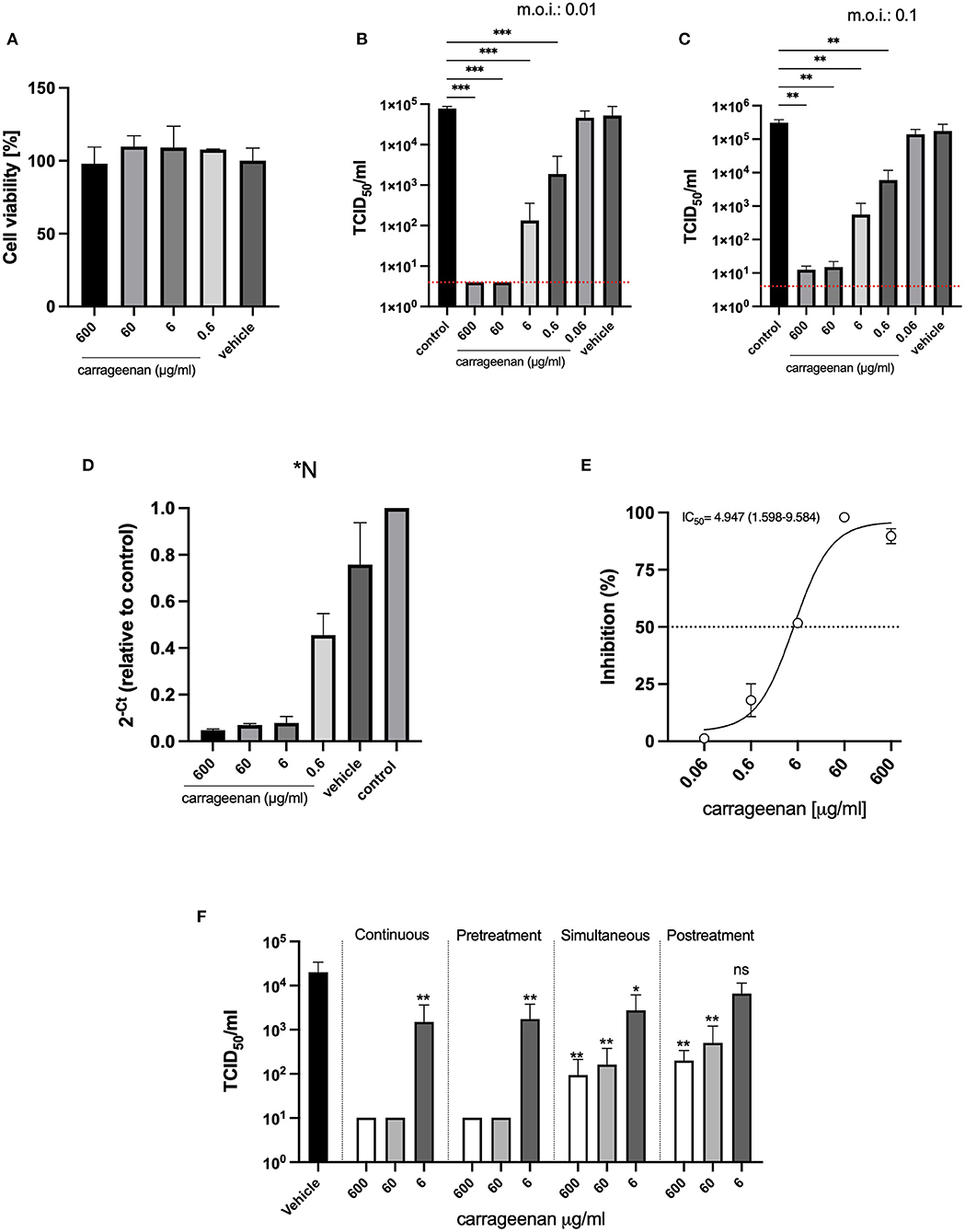
Figure 1. (A) Cellular viability assays. Calu-3 cells were treated with iota-carrageenan or vehicle (600–0 μg/mL) for 48 h at 37°C. After incubation, cellular viability was analyzed, and no statistically significant difference was found between the groups compared to the vehicle control group (Group 600 μg/ml, p = 0.9987, Group 60 μg/ml, p = 0.7371, Group 6 μg/ml, p = 0.7875; and Group 0.6 μg/ml, p = 0.8652). Data are expressed as mean ± SD derived from three independent experiments. (B,C) Infection assays. Calu-3 cells were pretreated with iota-carrageenan or vehicle (600 μg/mL to 0 μg/mL) for 2 h. After the pretreatment, cells were infected in two different conditions MOI: 0.01 (B) and MOI: 0.1 (C) with SARS-CoV-2 and incubated for 48 h in the presence of iota-carrageenan. Supernatants were harvested and virus yield was determined by titration. Data are expressed as mean ± SD derived from three independent experiments. The p-values in (B,C) figures are p ≤ 0.0006 (***) and p ≤ 0.0068 (**), respectively. In graphs (B,C) the red dotted lines indicate the level of inhibition achieved with the positive control, equine hyperimmune serum against SARS-CoV-2. (D) Viral RNA quantification (RT-qPCR). Conditioned medium was harvested 48 h post-infection. RNA was extracted from samples, and SARS-CoV-2 RNA was quantified by multiplex detection of viral genes N, ORF1ab, and human gene (RNase P gene). (E) Inhibitory effectiveness of iota-carrageenan (IC50). Calu-3 cells were pretreated with iota-carrageenan or vehicle (600 μg/ml to 0.06 μg/ml) for 2 h. After the pretreatment, cells were infected (MOI: 0.01) with SARS-CoV-2 and incubated for 48 h in the presence of iota-carrageenan. Supernatants were harvested, and then the viral titer was quantified in Vero E6 cells. Cytopathic effect was quantified by crystal violet staining and measured as absorbance at 585 nm by triplicate. The percentage of inhibition was calculated with respect to control. IC50 is indicated in the graph. Data are expressed as mean ± SD derived from three independent experiments. (F) Addition of iota-carrageenan at different times of infection. Calu-3 cells were treated with iota-carrageenan (600 μg/ml to 0 μg/ml) 2 h before the infection (Pretreatment), while being infected (Simultaneous), or after the already infected cells were washed (Posttreatment). Cells exposed to iota-carrageenan throughout the experiment were also included (Continuous). Supernatants were harvested 48 h after infection, and virus production was measured. Data are expressed as mean ± SD derived from three independent experiments. Differences between each group and the vehicle-treated group are depicted, and the dotted blue line indicates the limit of detection. The p-values for the “Continuous” treatment are p = 0.0076 (**) for the 6 μg/ml group, the 600 μg/ml and 60 μg/ml groups remained below the limit of detection, in the “Pretreatment” experiment, p = 0.0084 (**) for the 6 μg/ml group, and the 600 μg/ml and 60 μg/ml groups remained below the limit of detection, for the “Simultaneous” treatment p = 0.0127 (*), p = 0.0044 (**), and p = 0.0043 (**) for the 6, 60, and 600 μg/ml groups respectively, in the “Posttreatment” assay, p = 0.0613 (ns), p = 0.0051 (**), and p = 0.0045 (**) for the 6, 60, and 600 μg/ml groups respectively.
Next, Calu-3 cells were pre-treated with iota-carrageenan (600–0.06 μg/ml) for 2 h and then infected with SARS-CoV-2 (MOI: 0.01); after that, cells were washed to remove the viral inoculum, and fresh medium was added. Forty-eight hours later, supernatants were harvested. The SARS-CoV-2 production was evaluated by adding the supernatants to Vero E6 cells for 1 h. After incubation, the inoculum was removed, and monolayers were incubated at 37 °C for 72 h. Then cells were fixed and stained with crystal violet. Virus endpoint titer was determined by the Reed-Muench formula and expressed as TCID50/ml (37) (Figures 1B,C). No antiviral activity was only observed at the lowest concentration of iota-carrageenan (0.06 μg/ml), and complete inhibition was done using an equine hyperimmune serum (HHS) against SARS-CoV-2 (Figures 1B,C red dotted line). Lastly, there was no reduction in virus production with vehicle formulation, suggesting that the iota-carrageenan, not the sample excipient components, inhibited the SARS-CoV-2 replication (Figure 1B). Finally, Figure 1C shows that inhibition of viral production is also observed when pretreatment with carrageenan is applied to the cells, followed by an infection at a higher MOI (0.1). These results were also confirmed by RT-PCR (Figure 1D). Furthermore, the results were expressed as the inhibitory concentration 50% (IC50), defined as the compound concentration required to reduce virus yield by 50% (Figure 1E). The calculated IC50 was 4.947 μg/ml (1.598–9.584), suggesting low concentrations of iota-carrageenan are enough to inhibit Calu-3 cells SARS-CoV-2 infection.
The previous experimental design evaluated mainly the prophylactic use of the iota-carrageenan solution as cells were pre-incubated before viral addition. To assess if the treatment could be useful not only for prevention but also after infection, we performed other sets of experiments; we treated with three concentrations of iota-carrageenan at different times: (a) Exposed to iota-carrageenan throughout the experiment (Figure 1F, Continuous). (b) Calu-3 cells were treated with iota carrageenan only 2 h before the infection, then cells were washed and infected with SARS CoV-2 (MOI: 0,01) for 1 h, washed again, and cultured for 48 h (Figure 1F, Pretreatment). (c) Calu-3 cells were treated with iota-carrageenan at the same time that the virus was added, incubated for 1 h, and then cells were washed and cultured for 48 h (Figure 1F, Simultaneous). (d) Calu-3 cells were treated with iota-carrageenan after infection during 48 h (Figure 1F, Posttreatment). In a, b, c, and d, after 48 h of infection, the supernatants were harvested, and viral production was quantified in Vero E6 cells by TCDI50/ml (Figure 1F). Iota-carrageenan treatment inhibited viral production in all experimental designs. However, the maximal inhibitory effect was obtained only when Calu-3 cells were pre-treated with iota-carrageenan, where 600 and 60 μg/ml concentrations completely abrogated the infection. Simultaneous and post-treatment of Calu-3 cells with 600 and 60 μg/ml of iota-carrageenan also partially inhibited SARS-CoV-2 infection (Figure 1F).
Discussion
In this study, we found that iota-carrageenan drastically inhibits SARS-CoV-2 production in a dose-dependent manner. The results show a more significant inhibitory action with the prophylactic application. As described previously for iota-carrageenan (12), our results suggest that the inhibitory effect could mainly be explained by interference with the viral entry because pretreatment assays showed the most marked inhibition of SARS-CoV-2 infection. On the other hand, although the application after the exposure to the virus was less effective, an inhibitory effect is also observed with the application of iota-carrageenan in concentrations that are reached with the in vivo application of nasal sprays containing iota-carrageenan.
Different articles have been published that raise the hypothetical usefulness of nasal sprays with carrageenan for both prevention and treatment of COVID-19 (38–40), although some clinical trials are underway (Carrageenan Nasal Spray for COVID-19 Prophylaxis [ICE-COVID], Swansea University, ClinicalTrials.gov Identifier: NCT04590365; Prophylactic Treatment With Carragelose Nasal Spray to Prevent SARS-CoV-2, COVID-19, Infections in Health Care Workers, Marinomed Biotech AG, ClinicalTrials.gov Identifier: NCT04681001). So far, a unique trial has been completed, and its results published in a peer-reviewed journal indexed in PubMed (36). This pilot, pragmatic, multicenter, randomized, double-blind, and placebo-controlled study evaluates the efficacy of a nasal spray containing iota-carrageenan in the prophylaxis of COVID-19 in hospital personnel dedicated to the care of patients with COVID-19 (ClinicalTrials.gov Identifier: NCT04521322). The study assigned clinically healthy physicians, nurses, kinesiologists, and other healthcare providers treating hospitalized patients for COVID-19 in a 1:1 ratio to receive four daily doses of iota-carrageenan spray or placebo for 21 days. The primary endpoint was the occurrence of clinical COVID-19, confirmed by the reverse transcriptase-polymerase chain reaction test, over the 21 days. The results show that a total of 394 people were randomly assigned to receive iota-carrageenan or placebo. Both treatment groups had similar baseline characteristics. The incidence of COVID-19 was significantly different between subjects who received the nasal spray with iota-carrageenan (2 of 196 [1.0%]) and those who received a placebo (10 of 198 [5.0%]). The iota-carrageenan spray was associated with a 79.8% reduction in the relative risk of becoming ill (95% CI: 5.3–95.4; P = 0.03) and an absolute risk reduction of 4% (CI 95%: 0.6–7.4).
If these results were repeated in other clinical trials in different populations, a new tool would be available for managing the pandemic, especially considering that carrageenan is contained in nasal sprays registered as medical devices and sold as over-the-counter (OTC) products in Europe, Asia, and Australia. Another nasal spray with iota-carrageenan has been recently approved in the USA (FDA Medical Devices Databases Establishment Registration & Device Listing D 441354), and in Argentina and other Latin American countries, the nasal spray with carrageenan used in the published clinical trial has been approved by regulatory authorities and available for use since 2013 (ANMAT disposition 5158–2013).
As we already mentioned, although the application after the exposure to the virus was less effective in our experiments, an inhibitory effect is also observed with the application of iota-carrageenan in concentrations that are reached with the clinical application of nasal sprays. Clinical trials should be carried out to determine if the early use of an iota-Carrageenan nasal spray in individuals with recent-onset COVID-19 can decrease the severity or shorten the symptomatic period, as described in colds caused by other viruses.
Conclusion
In summary, our results confirm that a formulation of iota-carrageenan and sodium chloride available as a nasal spray applied in concentrations within the range of those achieved with the application used in clinical use effectively inhibited SARS-CoV-2 infection in vitro in human respiratory epithelial cell line culture, strengthening the hypothesis that a nasal spray with iota-carrageenan may be helpful in the prevention or treatment of COVID-19 and reinforces the interest in the development of clinical trials on this topic.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
AC, AD, and JF conceptualized the study. AC, AV, AP, CP, and AD developed the methodology. JF wrote and prepared the original draft. AC, AD, and CP wrote, reviewed, and edited the manuscript. AC and AD supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was partially supported by the Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (INBIRS) and the Instituto de Ciencia y Tecnología Dr. César Milstein—(Consejo Nacional de Investigaciones Científicas y Técnicas CONICET- Fundación Pablo Cassará).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
2. WHO. WHO Coronavirus (COVID-19) Dashboard|With Vaccination Data. (2021). Available online at: https://covid19.who.int/ (accessed October 20, 2021).
3. Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. (2021) 5:947–53. doi: 10.1038/s41562-021-01122-8
4. Liu Y, Qu H-Q, Qu J, Tian L, Hakonarson H. Expression pattern of the SARS-CoV-2 Entry Genes ACE2 and TMPRSS2 in the respiratory tract. Viruses. (2020) 12:1174. doi: 10.3390/v12101174
5. Ahn JH, Kim J, Hong SP, Choi SY, Yang MJ, Ju YS, et al. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest. (2021) 131:517. doi: 10.1172/JCI148517
6. Grassauer A, Weinmuellner R, Meier C, Pretsch A, Prieschl-Grassauer E, Unger H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J. (2008) 5:107. doi: 10.1186/1743-422X-5-107
7. Leibbrandt A, Meier C, König-Schuster M, Weinmüllner R, Kalthoff D, Pflugfelder B, et al. Iota-Carrageenan Is a Potent Inhibitor of Influenza a Virus Infection. PLoS ONE. (2010) 5:e14320. doi: 10.1371/journal.pone.0014320
8. Morokutti-Kurz M, König-Schuster M, Koller C, Graf C, Graf P, Kirchoff N, et al. The intranasal application of zanamivir and carrageenan is synergistically active against influenza a virus in the murine model. PLoS ONE. (2015) 10:e0128794. doi: 10.1371/journal.pone.0128794
9. Eccles R. Iota-carrageenan as an antiviral treatment for the common cold. Open Virology J. (2020) 14:9–15. doi: 10.2174/1874357902014010009
10. Álvarez-Viñas M, Souto S, Flórez-Fernández N, Torres MD, Bandín I, Domínguez H. Antiviral activity of carrageenans and processing implications. Mar Drugs. (2021) 19:437. doi: 10.3390/md19080437
11. Chen X, Han W, Wang G, Zhao X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int J Biol Macromol. (2020) 164:331–43. doi: 10.1016/j.ijbiomac.2020.07.106
12. Hans N, Malik A, Naik S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: mini review. Biores Tech Rep. (2020) 13:100623. doi: 10.1016/j.biteb.2020.100623
13. Koenighofer M, Lion T, Bodenteich A, Prieschl-Grassauer E, Grassauer A, Unger H, et al. Carrageenan nasal spray in virus confirmed common cold: individual patient data analysis of two randomized controlled trials. Multidiscip Resp Med. (2014) 9:57. doi: 10.4081/mrm.2014.392
14. Song S, Peng H, Wang Q, Liu Z, Dong X, Wen C, et al. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. (2020) 11:7415–20. doi: 10.1039/D0FO02017F
15. Morokutti-Kurz M, Fröba M, Graf P, Große M, Grassauer A, Auth J, et al. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS One. (2021) 16:e0237480. doi: 10.1371/journal.pone.0237480
16. Osada N, Kohara A, Yamaji T, Hirayama N, Kasai F, Sekizuka T, et al. The genome landscape of the African green monkey kidney-derived vero cell line. DNA Res. (2014) 21:673–83. doi: 10.1093/dnares/dsu029
17. Barrett PN, Terpening SJ, Snow D, Cobb RR, Kistner O. Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases. Expert Rev Vaccines. (2017) 16:883–94. doi: 10.1080/14760584.2017.1357471
18. Banerjee A, Nasir JA, Budylowski P, Yip L, Aftanas P, Christie N, et al. Isolation, sequence, infectivity, and replication kinetics of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis. (2020) 26:2054–63. doi: 10.3201/eid2609.201495
19. Holwerda M., V'kovski P., Wider M., Thiel V., Dijkman R. (2020). Identification of an antiviral compound from the pandemic response box that efficiently inhibits SARS-CoV-2 infection In vitro. Microorg. 8:1872. doi: 10.3390/microorganisms8121872
20. Zhu Y, Miller TL, Singhaus CJ, Shaffer TH, Chidekel A. Effects of oxygen concentration and exposure time on cultured human airway epithelial cells. Pediatr Crit Care Me. (2008) 9:224–9. doi: 10.1097/PCC.0b013e318166fbb5
21. Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MD. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth F R. (2020) 53:13–24. doi: 10.1016/j.cytogfr.2020.05.009
22. Zhu Y, Chidekel A, Shaffer TH. Cultured Human Airway Epithelial Cells (Calu-3): A Model of human respiratory function, structure, and inflammatory responses. Critical Care Res Pract. (2010) 2010:394578. doi: 10.1155/2010/394578
23. Witschi C, Mrsny RJ. In vitro evaluation of microparticles and polymer gels for use as nasal platforms for protein delivery. Pharmaceut Res. (1999) 16:382–90. doi: 10.1023/A:1018869601502
24. Wang S, Chow MSS, Zuo Z. An approach for rapid development of nasal delivery of analgesics—identification of relevant features, in vitro screening and in vivo verification. Int J Pharmaceut. (2011) 420:43–50. doi: 10.1016/j.ijpharm.2011.08.019
25. Zhang L, Du S-Y, Lu Y, Liu C, Tian Z-H, Yang C, et al. Puerarin transport across a Calu-3 cell monolayer – an in vitro model of nasal mucosa permeability and the influence of paeoniflorin and menthol. Drug Des Dev Ther. (2016) 10:2227–37. doi: 10.2147/DDDT.S110247
26. Wu C, Li B, Zhang Y, Chen T, Chen C, Jiang W, et al. Intranasal delivery of paeoniflorin nanocrystals for brain targeting. Asian J Pharm Sci. (2020) 15:326–35. doi: 10.1016/j.ajps.2019.11.002
27. Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Prieschl-Grassauer E. Efficacy and safety of an antiviral Iota-Carrageenan nasal spray: a randomized, double-blind, placebo-controlled exploratory study in volunteers with early symptoms of the common cold. Respir Res. (2010) 11:108. doi: 10.1186/1465-9921-11-108
28. Fazekas T, Eickhoff P, Pruckner N, Vollnhofer G, Fischmeister G, Diakos C, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. Bmc Complem Altern M. (2012) 12:147–147. doi: 10.1186/1472-6882-12-147
29. Ludwig M, Enzenhofer E, Schneider S, Rauch M, Bodenteich A, Neumann K, et al. Efficacy of a Carrageenan nasal spray in patients with common cold: a randomized controlled trial. Respir Res. (2013) 14:124. doi: 10.1186/1465-9921-14-124
30. Garcia GJM, Schroeter JD, Segal RA, Stanek J, Foureman GL, Kimbell JS. Dosimetry of nasal uptake of water-soluble and reactive gases: A first study of interhuman variability. Inhal Toxicol. (2009) 21:607–18. doi: 10.1080/08958370802320186
31. Pires A, Fortuna A, Alves G, Falcão A. Intranasal Drug Delivery: How, Why and What for? J Pharm Pharm Sci. (2009) 12:288–311. doi: 10.18433/J3NC79
32. Bitter C, Suter-Zimmermann K, Surber C. Nasal drug delivery in humans. Curr Probl Dermatol. (2011) 40:20–35. doi: 10.1159/000321044
33. Gizurarson S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr Drug Deliv. (2012) 9:566–82. doi: 10.2174/156720112803529828
34. Wagenmann M, Naclerio RM. Anatomic and physiologic considerations in sinusitis. J Allergy Clin Immun. (1992) 90:419–23. doi: 10.1016/0091-6749(92)90161-T
35. Helassa N, Garnett JP, Farrant M, Khan F, Pickup JC, Hahn KM, et al. A novel fluorescent sensor protein for detecting changes in airway surface liquid glucose concentration. Biochem J. (2014) 464:213–20. doi: 10.1042/BJ20141041
36. Figueroa JM, Lombardo ME, Dogliotti A, Flynn LP, Giugliano R, Simonelli G, et al. Efficacy of a nasal spray containing iota-carrageenan in the postexposure prophylaxis of COVID-19 in hospital personnel dedicated to patients care with COVID-19 Disease. Int J Gen Medicine Volume. (2021) 14:6277–86. doi: 10.2147/IJGM.S328486
37. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. (1938) 27:493–7. doi: 10.1093/oxfordjournals.aje.a118408
38. Frediansyah SSA. The antiviral activity of iota-, kappa-, and lambda-carrageenan against COVID-19: a critical review. Clin Epidemiol Glob Heal. (2021) 12:100826. doi: 10.1016/j.cegh.2021.100826
39. Hoseini-Tavassol Z, Ejtahed H-S, Soroush A-R, Sajjadpour Z, Hasani-Ranjbar S, Larijani B. Natural derived nasal spray; a proposed approach for COVID-19 disease control. Infect Disord—Drug Targets. (2021) 21. doi: 10.2174/1871526521666210218201113
Keywords: SARS-CoV-2, COVID-19, iota-carrageenan, respiratory epithelium, antivirals
Citation: Varese A, Paletta A, Ceballos A, Palacios CA, Figueroa JM and Dugour AV (2021) Iota-Carrageenan Prevents the Replication of SARS-CoV-2 in a Human Respiratory Epithelium Cell Line in vitro. Front. Virol. 1:746824. doi: 10.3389/fviro.2021.746824
Received: 24 July 2021; Accepted: 31 October 2021;
Published: 15 December 2021.
Edited by:
Elsa Beatriz Damonte, Universidad de Buenos Aires, ArgentinaReviewed by:
Nuria Izquierdo-Useros, IrsiCaixa, SpainHyung-Joo Kwon, Hallym University, South Korea
Copyright © 2021 Varese, Paletta, Ceballos, Palacios, Figueroa and Dugour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Vanesa Dugour, YWR1Z291ckBmdW5kYWNpb25jYXNzYXJhLm9yZy5hcg==
†These authors have contributed equally to this work
 Augusto Varese
Augusto Varese Ana Paletta1†
Ana Paletta1† Ana Ceballos
Ana Ceballos Carlos Adolfo Palacios
Carlos Adolfo Palacios Juan Manuel Figueroa
Juan Manuel Figueroa Andrea Vanesa Dugour
Andrea Vanesa Dugour