
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 07 March 2025
Sec. Animal Nutrition and Metabolism
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1562964
This article is part of the Research Topic Dietary Supplements for Optimizing Rumen Health and Nutrient Digestibility in Livestock View all 9 articles
 Hongwei Duan1,2†
Hongwei Duan1,2† Jiyou Zhang1,2†
Jiyou Zhang1,2† Na Li3
Na Li3 Liuping Chen3
Liuping Chen3 Danhong Chen3
Danhong Chen3 Hang Yang3
Hang Yang3 Qiuxia Dai3
Qiuxia Dai3 Junshi Shen1,2*
Junshi Shen1,2* Shengyong Mao1,2
Shengyong Mao1,2Introduction: Heat stress poses a significant challenge to the development of dairy industry, affecting cows’ well-being and overall productivity, leading to substantial economic losses. In this study, the impact of a specifically formulated anti-heat stress lick block supplement on milk production, milk quality, feed intake, rectal temperature, respiratory rate, and rumen fermentation in cows exposed to heat-stress was evaluated.
Methods: Twenty-four healthy Holstein lactating dairy cows were divided into two blocks based on milk yield (low and high), Parity (2–3 parity), and lactation days (114 ± 8 d). The cows in each block were randomly assigned to either a control group without lick block supplementation or a treatment group receiving lick block. The trial lasted for 6 weeks, including a 2-week adaptation phase followed by 4 weeks of feeding treatment.
Results: Heat stress levels varied from severe (THI > 88) to moderate heat stress (THI > 80) in the first 2 weeks, gradually decreasing to mild heat stress (THI > 72) in the following weeks. With the decrease in heat stress, dry matter intake (DMI) and milk production increased (Week: p < 0.05), the rectal temperature and respiratory rate of cows decreased (Week: p < 0.05). Lick block supplementation tended to increase DMI (p = 0.09), and improved milk yield (p < 0.05) without affecting (p > 0.05) milk composition, leading to increased milk yields of fat, protein, and lactose (p < 0.05). Although the overall rectal temperature of cows in the lick block group did not differ from the control group (p > 0.05), the respiratory rate of cows in the lick block group significantly decreased (p < 0.05) in the second and third weeks. Supplementation with the lick block increased (p < 0.05) rumen pH and decreased (p < 0.05) NH3-N and propionate concentrations in dairy cows, and tended to lower the acetate-to-propionate ratio (p = 0.07), total VFA concentration (p = 0.07), and butyrate concentration (p = 0.09).
Conclusion: Supplementation of anti-heat stress lick block alleviated the detrimental effects of heat stress on dairy cows within a certain range of temperature and humidity.
Holstein cows exhibit robust adaptability to cold but show poor tolerance to heat. High temperatures and humidity can disrupt the cows’ physiological equilibrium, leading to heat stress (1, 2). Previous studies have demonstrated that temperatures exceeding 25°C can easily induce heat stress in dairy cows, resulting in metabolic disorders, endocrine dysfunction, and negative impacts on the production performance, and health of dairy cows (3–5). In recent years, with the continuous growth of milk production, the heat generated by cows themselves has also been increasing. Additionally, the intensification of the global greenhouse effect has further increased the susceptibility of cows to heat stress. The thermal humidity index (THI) threshold, previously utilized to assess heat stress in cows, has been reduced from 72 to 68 (6). Gunn et al. predicted that milk yield losses across the United States will accelerate, with an average rate of 174 ± 7 kg/head/decade, due to the challenge of heat stress (7). Ranjitkar et al. also predicted that milk yield in China may decrease by 6.5 kg/head/day in 2050, with losses increasing to 7.2 kg/head/day in 2070 (8). At this rate, it must cause huge economic losses to the dairy industry and seriously hinder its healthy and sustainable development. Therefore, finding effective means to alleviate heat stress in dairy cows and reduce its effects on production performance and health is very important.
The current dairy farms primarily employ physical solutions like fans, sprinklers, and wet curtains to cool the cowshed, which were widely adopted and cost-effective practices in ranches (9). Additionally, some nutritional strategies are leveraged to further mitigate heat stress in cows including improving dietary energy levels (10), incorporating feed additives (11), and adding traditional herbal supplements (12). Several recent studies also point to the potential of various mineral elements, micronutrients, and vitamins in alleviating heat stress. For example, increasing the level of potassium (K) and sodium (Na) in the diet can enhance dry matter intake and milk yield of dairy cows exposed to heat stress, alleviating the detrimental effects of heat stress (13). Chromium (Cr) has been demonstrated to enhance cows’ production performance, immune function, glucose metabolism, and antioxidant capacity. Supplementation with an appropriate dose of Cr could alleviate the adverse effects of heat stress and enhance production performance (14). Vitamin C serves as a stress protectant for livestock (15), while vitamin E acts as a biological antioxidant and free radical scavenger, safeguarding cells and lipid-rich membranes from oxidative damage induced by heat stress (16). Furthermore, vital trace elements such as zinc (Zn), copper (Cu), manganese (Mn), and selenium (Se) have been shown to have the potential to alleviate heat stress (17). The mineral and trace elements in the diet can usually meet the production and health needs of cows. However, heat stress can lead to a decrease in feed intake and result in picky eating behavior. Therefore, it is necessary to supplement additionally to alleviate the effects of heat stress.
Lick block is a feed product made by combining salt, minerals, vitamins, and other nutrients in a specific proportion. Livestock can freely lick according to their health condition and physiological needs to supplement specific nutrients for livestock (18). At present, in countries with developed animal husbandry such as the United States, the Netherlands, and Australia, feeding lick blocks have become a necessary supplementary feeding technique for grazing and farming livestock. The Food and Agriculture Organization of the United Nations (FAO) has also promoted this low-cost and fast-acting technology to over 70 countries in Asia and Africa, achieving significant success (19). Supplementing lick block can promote saliva secretion in cows, facilitate digestion and rumination, alleviate rumen acidosis, maintain rumen acid–base balance, and be beneficial to the health of cattle and sheep (20). This experiment designed an anti-heat stress lick block targeting the needs of cows for minerals, trace elements, vitamins, and other nutrients under heat stress, and subsequently conducted an animal experiment to evaluate its anti-heat stress effect.
The Animal Care and Use Committee of Nanjing Agricultural University approved the experimental procedures used in this study (Protocol number: SYXK2017-0007).
Twenty-four healthy Holstein lactating dairy cows were divided into two blocks based on milk yield (low block: milk yield = 15.72 ± 1.46 kg; high block: milk yield = 20.65 ± 2.44 kg), Parity (2–3 parity), and lactation days (114 ± 8 d). The cows in each block were then randomly assigned to two groups: (1) CON group: not offered lick block; LB group: offered lick block. The anti-heat stress compound nutrition (HSCN) lick block used in the experiment was cooperatively designed by Nanjing Agricultural University and China Salt Jintan Co. Ltd., and the compositions are shown in Supplementary Table S1. The HSCN lick block primarily provides minerals and vitamins that contain NaCl, 894 g/kg; K, 15 g/kg; Co, 75 mg/kg; Cu, 500 mg/kg; I, 75 mg/kg; Fe, 2200 mg/kg; Mn, 2000 mg/kg; Se, 25 mg/kg; Zn, 4000 mg/kg; Cr, 15 mg/kg; VC, 3000 mg/kg; VA, 200000 IU/kg; VD, 80000 IU/kg; VE, 2000 IU/kg. All dairy cows were housed in indoor pens measuring 1.2 m × 2.2 m, featuring woodchip bedding, and had ad libitum access to fresh, clean water. Dairy cows were fed three times daily (0630; 1430; 2030) with the total mixed ratio (TMR) formulated by the NASEM standards (21), and the composition and nutrient content of the TMR are shown in Table 1. Feeding trials were conducted for 6 weeks, consisting of 2 weeks for adaptation followed by 4 weeks of feeding treatment.
The temperature and relative humidity in the barn were recorded at 0700 h, 1,400 h, and 1900 h daily throughout the experimental period using an automatic temperature and humidity recorder (TH20R; Shenzhen Huahanwei Technology Co., Ltd., Shenzhen, China). The THI was calculated according to the Specification for the Technical Assessment of Heat Stress in Dairy Cows (Ministry of Agriculture of the People’s Republic of China, 2013). THI = 0. 81*T + (0. 99*T-14.3) * RH + 46.3, where T = ambient temperature in °C and RH = relative humidity expressed as a proportion, i.e., 75% humidity is expressed as 0.75.
The respiratory rate (RR) and rectal temperature (RT) of the cows were measured at 0700 h, 1,400 h, and 1900 h twice a week. RR was measured according to the movement of the abdomen and thorax of the cows for 1 min and repeated twice. RT was measured using an animal thermometer (Dong-e–e-jiao Ahua Medical Instrument Co., Ltd., Jinan, Shandong, China).
Feed intake was measured and feed samples were collected twice a week throughout the experimental period. The samples were mixed, dried at 60°C for 48 h, and then crushed for determination of dry matter (DM), crude ash (Ash) and crude protein (CP), crude fat (CF) content in the samples according to the methods of AOAC (22). Neutral detergent fiber (NDF) and acid detergent fiber (ADF) content were analyzed according to the method described by Van Soest et al. (23).
Milk yield and composition were measured on the last 2 days of each week. Milk yield was measured using a Tunisian flowmeter (JHF-G17, Sichuan Jinhaifeng Animal Husbandry Technology Co., Ltd., Sichuan, China) during the three daily milkings. The flowmeters were calibrated before use. The milk samples were preserved with 2-bromo-2-nitropropane-1,3 diol and stored at 4°C. Milk samples collected three times daily were mixed at a ratio of 4:3:3. The milk composition was determined using a near-infrared analyser (MilkoScanTM 7RM, Foss Electric, Denmark) following the standard procedure.
At the end of the trial, rumen fluid was collected using an oral stomach tube according to the method described by Shen et al. (24). Once collected, rumen fluid pH was measured immediately using a pH meter (Ecoscan pH 5, Eutech Instruments, Singapore). After filtering with four layers of gauze, the rumen fluid was dispensed and stored at −20°C for subsequent VFA analysis. 1 mL of rumen fluid was thawed with running water and the NH3-N concentration was determined calorimetrically using ammonium chloride as standard (25). Volatile fatty acids (VFA) were determined using a gas chromatograph (Agilent 7980A, United States) using the method described by Shen et al. (26).
Data for DMI, milk yield, milk composition, RR, and RT were analyzed using the SAS Mixed procedure of SAS version 9.4 with week as repeated measures using compound symmetry covariance structure selected based on Akaike’s information criterion for optimal fit. The model included fixed effects of treatment, block, week, treatment × week interaction, treatment × block interaction, as well as random effect of dairy cow within treatment × block. Data for rumen fermentation parameters were analyzed using the SAS Mixed procedure of SAS version 9.4. The model included fixed effects of treatment, block, treatment × block interaction, and random effect of dairy cow within block × treatment. The freedom degrees were determined utilizing the Kenward-Roger option. p < 0.05 indicated significant differences, and 0.05 ≤ p ≤ 0.10 indicated a trend of variation.
In the first 2 weeks of the experiment, dairy cows experienced severe (TH I > 88) to moderate heat stress (THI > 80) (Figure 1), gradually transitioning to moderate to mild heat stress (THI > 72) in the third and fourth weeks.
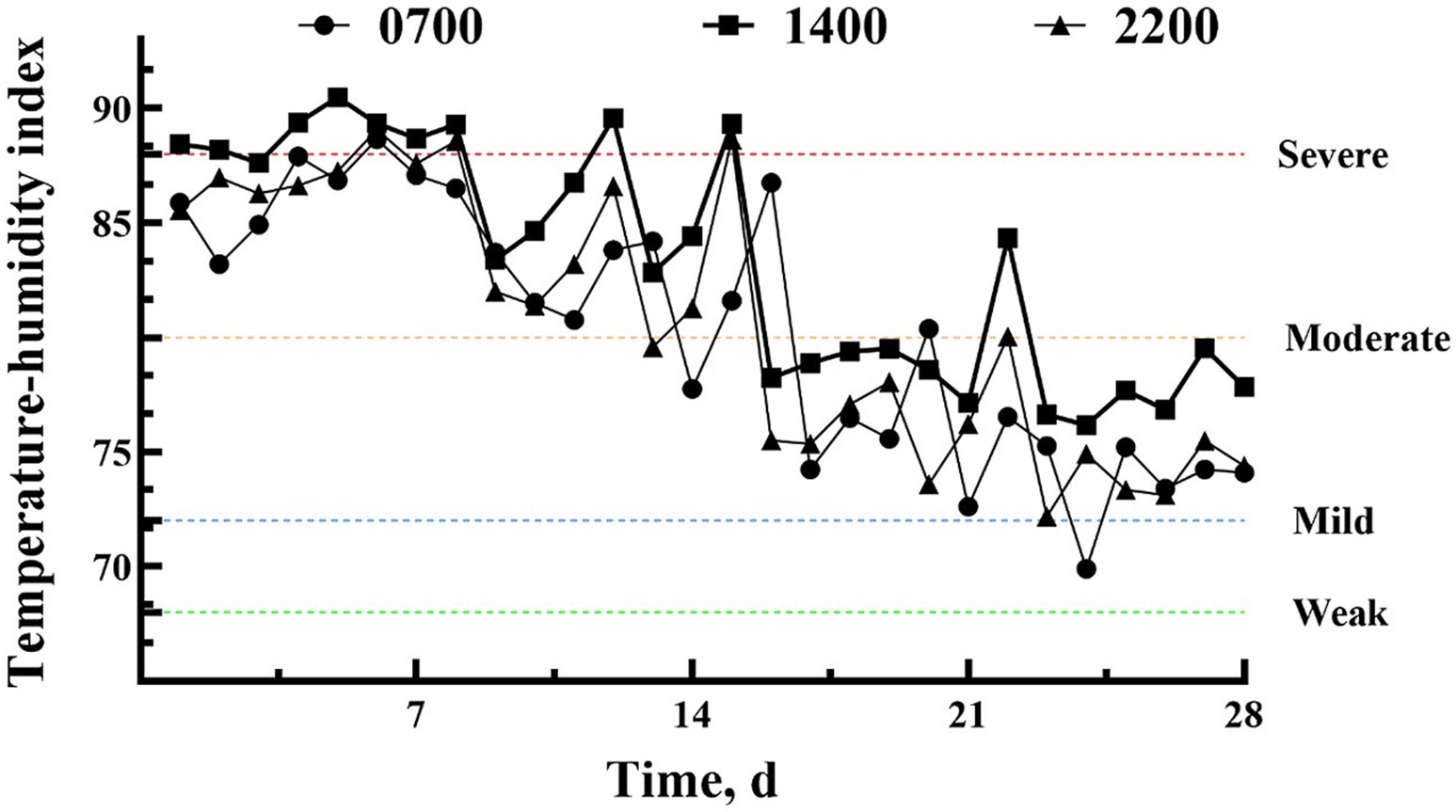
Figure 1. The temperature and humidity index in the cowshed at 0700 h, 1,400 h, and 1900 h during the experiment.
There was no interaction between the weeks and treatment on the effects of DMI and milk yield (Figure 2). Both DMI and milk yield show an increase (week: p < 0.01) as the degree of heat stress decreased from the first to the fourth week (Figures 1, 2). There was no difference in the initial milk yield difference between two groups. Offer the HSCN lick block to dairy cows increased (p < 0.01) the milk yield from the first week to the fourth week (Figure 2A), with an increased tendency (p = 0.09) of DMI (Figure 2B).
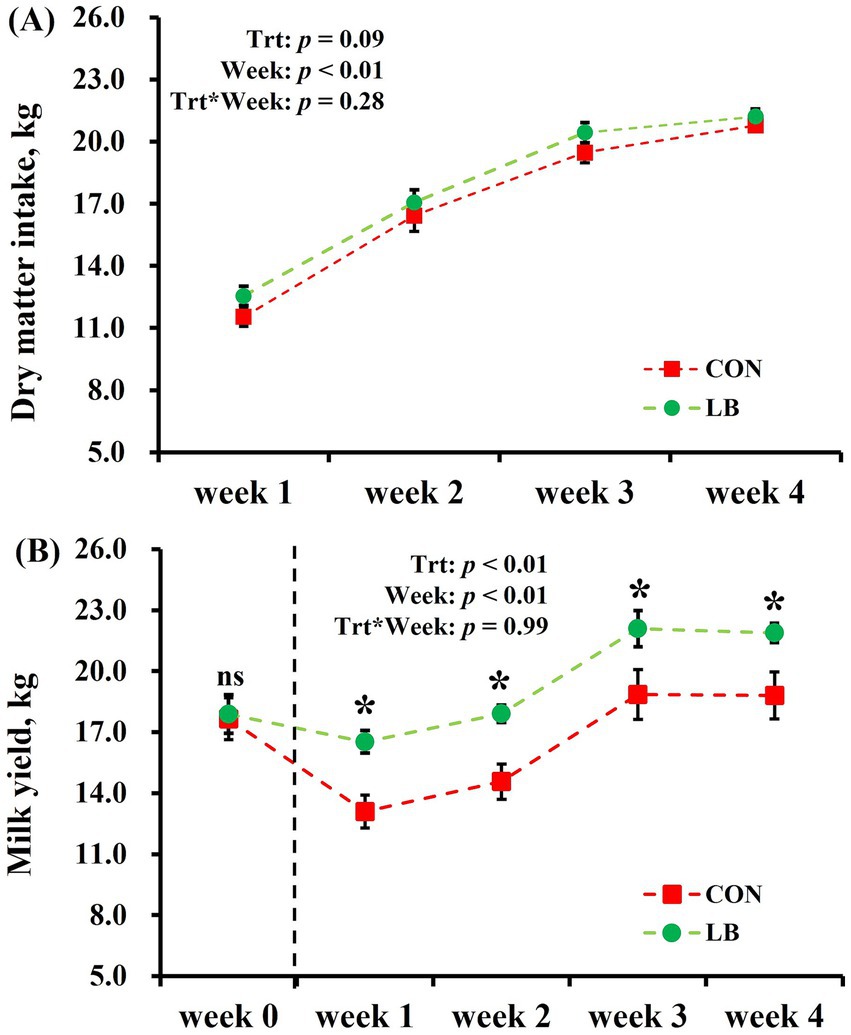
Figure 2. Dry matter intake (A) and Milk yield (B) of dairy cows offered and not offered access to HSCN lick block. *Indicates a statistically significant difference (p < 0.05).
No interaction (p > 0.10) of week × treatment was detected on any of the analyzed milk fat, milk protein, milk lactose, total solids, and milk urea nitrogen of dairy cows (Table 2). Supplementation lick block increased (p < 0.05) the milk yield of dairy cows including 3.5% FCM and ECM without (p > 0.05) affecting milk composition, which led to an increase (p < 0.01) in the total synthesis of milk fat, milk protein, lactose, and total solids.

Table 2. Milk yield and composition of dairy cows offered and not offered access to HSCN lick block.
No interaction (p > 0.10) of week × treatment was detected on any of the analyzed SCC and SCS of dairy cows (Figure 3). The SCC and SCS of milk did not differ between the first to fourth weeks of the experimental period.
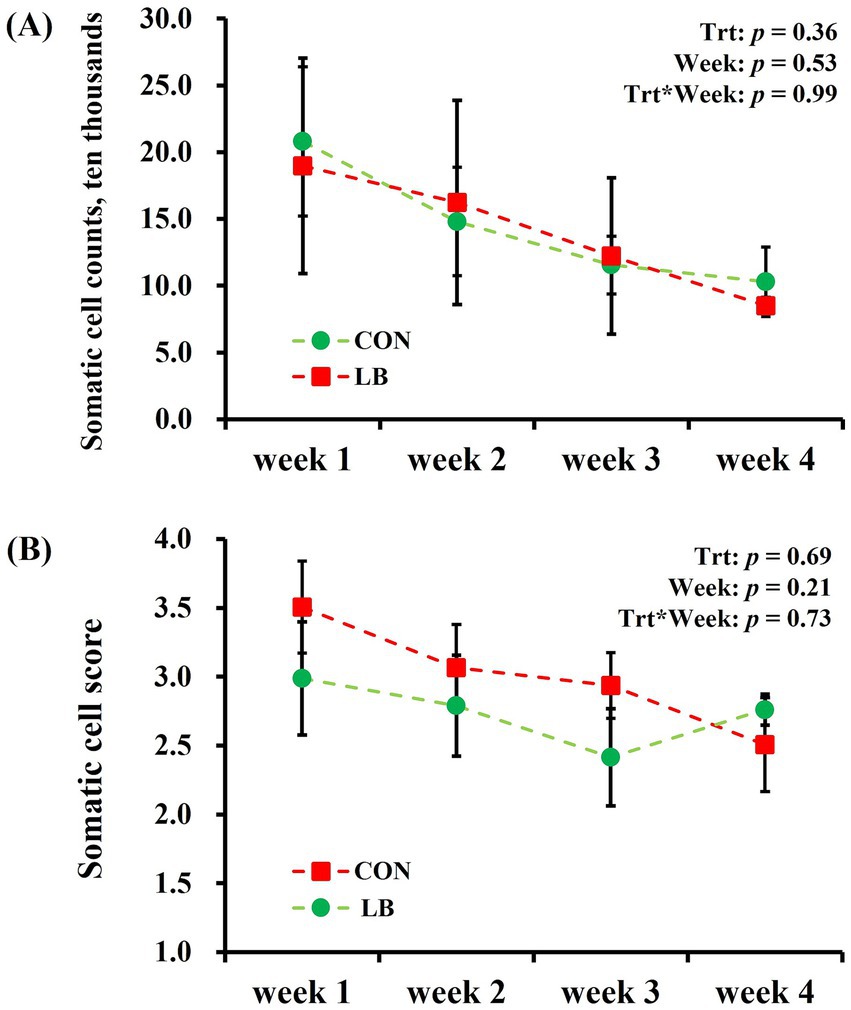
Figure 3. Somatic cell counts (A) and Somatic cell score (B) of dairy cows offered and not offered access to HSCN lick block.
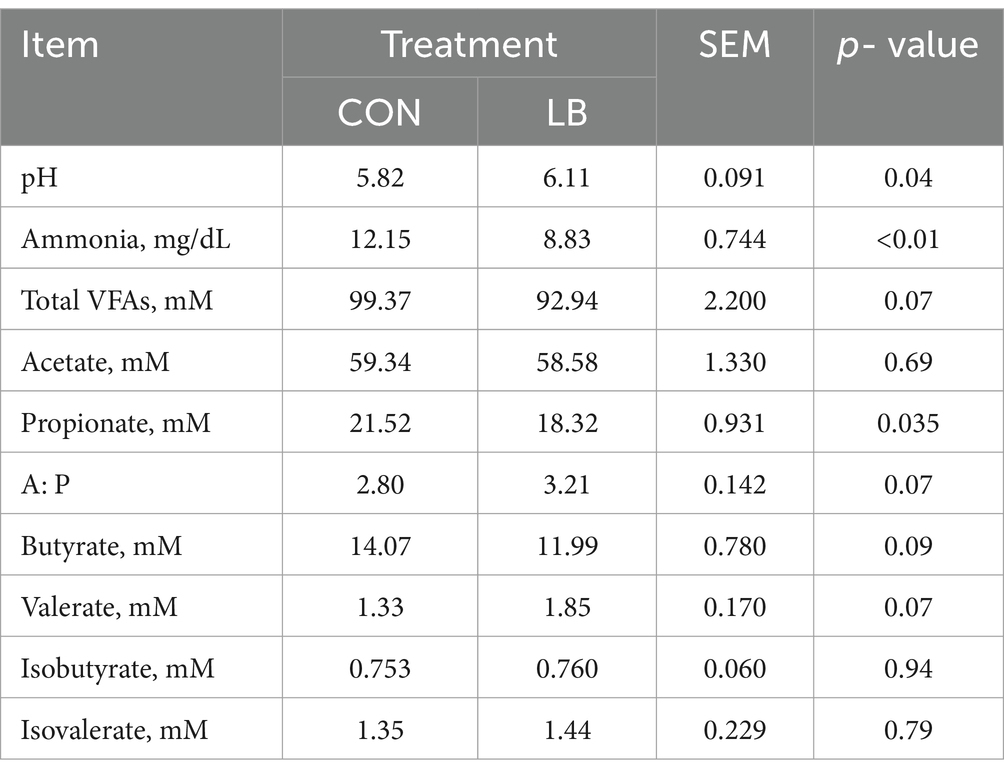
Table 3. The rumen fermentation characteristics in dairy cows offered and not offered access to HSCN lick block.
No interaction (p > 0.10) of week × treatment was detected on the RR, but there was an interaction (p < 0.05) of treatment × time on the RT (Figure 4). Independent analyses showed that the lick block group had lower (p < 0.05) RR than the control group in the second and third weeks. RT decreased (Week: p < 0.05) with the decrease in the degree of heat stress from the first to the fourth week, and no difference (p > 0.05) was observed in the RT between the two groups.
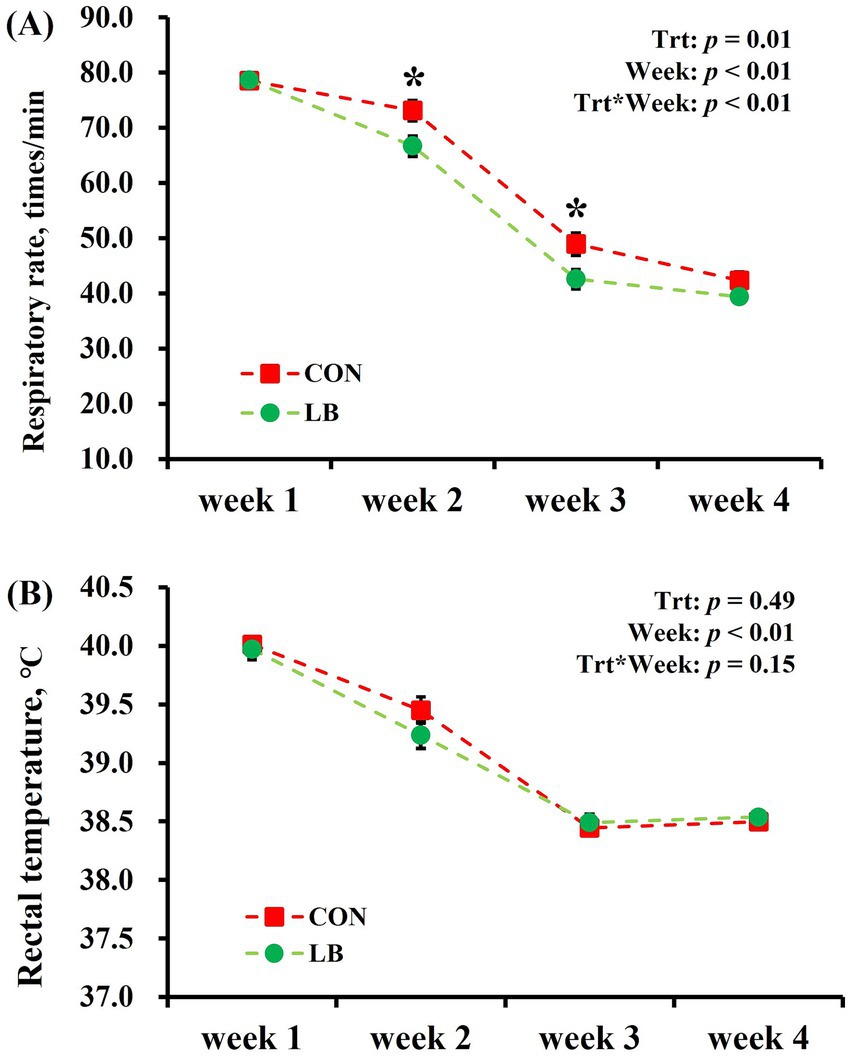
Figure 4. The respiratory rate (A) and rectal temperature (B) of dairy cows offered and not offered access to HSCN lick block. *Indicates a statistically significant difference (p < 0.05).
Dairy cows offered access to an HSCN lick block had a greater rumen pH and lower NH3-N and propionate concentration (p < 0.05). Meanwhile, the acetate-to-propionate ratio (p = 0.07), total VFA concentration (p = 0.07), and butyrate concentration (p = 0.09) had a declining trend in the LB group (Table 3).
Heat stress causes a sharp drop in feed intake, resulting in poor nutrient intake, decreased productivity, and poor offspring growth. It can also trigger inflammatory responses in the body that affect the health of dairy cows (1). When the THI exceeds 68, cows exhibit signs of heat stress such as elevated body temperature, increased respiratory rate, and water intake, while decreasing feed intake, and milk yield (27). This study incorporates specific ratios of potassium, selenium, chromium, zinc, vitamin A, and vitamin E, which have the potential to reduce heat stress, to formulate an anti-heat stress lick block. These blocks were provided for free access to cows potentially experiencing heat stress, with the aim of assessing the effectiveness of the anti-heat stress intervention.
During the duration of the experiment, the THI remained consistently between 69 to 90, signifying continuous heat stress exposure for dairy cows. Previous studies have shown that cows exposed to heat stress have significantly lower feed intake and milk yield. The higher the level of heat stress, the greater the decline in these metrics (28, 29). In the current study, dairy cows exposed to heat stress in the LB group had a higher feed intake and milk yield compared to the CON group, likely due to the lick block offered. This suggests that the heat stress experienced by the cows was mitigated. Additionally, a previous study indicated that heat stress negatively affects milk quality by lowering both milk fat and milk protein levels (30). In contrast, this study found that offering lick blocks to dairy cows enhanced the synthesis of milk fat and protein. This improvement was most likely related to the lower level of heat stress experienced by the cows. With a lower degree of heat stress, cows were more efficient in synthesizing milk fat and milk protein; meanwhile, the higher feed intake provided more substrates for the synthesis of milk fat and protein.
Somatic cell count serves as a crucial indicator for assessing the health status of dairy cows. Transforming somatic cell numbers to obtain a somatic cell score, which adheres more closely to a normal distribution and facilitates statistical analysis, is a common practice (31). A previous study demonstrated a significant negative correlation between somatic cell score and milk yield, indicating that higher somatic cell scores are associated with decreased milk production (32). In the current study, a lower somatic cell score corresponded to an increase in milk yield as heat stress lessened, corroborating the findings of Bellagi et al. (33). It is noteworthy that the LB group had a lower somatic cell score compared to the CON group for the first 3 weeks. Although this difference was only numerical, it suggested to some extent that heat stress in cows was alleviated.
A previous study revealed a notable increase in cows’ respiratory rate at high THI levels (34). Concurrently, the challenge of effectively dissipating the cows’ excess metabolic heat in such conditions leads to elevated body temperatures among dairy cows (35). Throughout the experimental duration, the cows experienced persistently high THI levels. Remarkably, lactating dairy cows offered access to HSCN block had a lower respiratory rate, potentially indicating that the provision of the lick block mitigated heat stress in the cows. However, contrary to expectations, there was no substantial variation in body temperature between two groups. This unexpected outcome may be attributed to the high barn temperatures during the experiment, which impeded effective heat dissipation.
Rumen homeostasis is crucial for ensuring the efficient digestion of nutrients and the overall health of ruminants. Rumen pH as an important indicator of rumen homeostasis influenced by multiple factors such as diet type, animal feeding and drinking, and animal health. Previously, a study highlighted the impact of heat stress on altering animals’ feed intake behavior, showing a decrease in forage intake and an increase in concentrate intake (36). Excessive concentrate intake can trigger a short-term surge in VFA production in the rumen, causing a rapid decline in rumen pH and escalating the risk of rumen acidosis. Additionally, the increased respiratory rate and decreased salivary secretion of dairy cows caused by heat stress will also decrease the concentration of carbonates available for exchange in blood and saliva, ultimately resulting in lower rumen pH (2). Supplementary lick blocks have previously been found to increase salivary secretion, which contains many buffering substances that help maintain rumen homeostasis (20). Meanwhile, the decline in respiratory rate allowed for more carbonates to be used for exchange in the blood entering the rumen, which may explain the elevated rumen pH.
The ratio of concentrate to roughage in the diet affects the content of acetate, propionate, and butyrate produced during rumen fermentation. When feeding diets with higher fiber levels, the total VFA in the rumen will decrease, while the pH and acetate levels will increase. In contrast, when the ratio of concentrate (starch) is increased, the proportion of propionate increases dramatically (37, 38). In the present study, although the proportion of concentrate and roughage consumed by the cows was not measured, dairy cows in LB group had a lower concentrations of rumen TVFA, propionate, and butyrate and a great concentration of acetate. This potentially indicates that dairy cows in the LB group exhibited less picky eating behavior and consumed more roughage, which was similarly reported in a study by Nonaka et al. (39). The supplementation of lick block alleviated the heat stress and improved the body and rumen health of the dairy cows, and the tendency to decrease the total acid concentration also may be related to the enhancement of rumen absorption function. Additionally, in the present study, the reduced ammonia-nitrogen concentration in the lick block treatment may be related to the improved rumen fermentation, which resulted in more ammonia being used to synthesize microbial proteins.
Supplementation with anti-heat stress lick block improved rumen fermentation and reduced the respiratory rate of dairy cows, resulting in increased feed intake, milk yield, and both milk fat and milk protein yields. Therefore, the supplementation of anti-heat stress lick block has a beneficial effect on alleviating heat stress in dairy cows and enhancing productivity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by The Animal Care and Use Committee of Nanjing Agricultural University. The study was conducted in accordance with the local legislation and institutional requirements.
HD: Data curation, Validation, Visualization, Writing – original draft. JZ: Project administration, Supervision, Validation, Visualization, Writing – original draft. NL: Funding acquisition, Project administration, Supervision, Writing – review & editing. LC: Funding acquisition, Investigation, Project administration, Writing – review & editing. DC: Data curation, Funding acquisition, Project administration, Writing – review & editing. HY: Data curation, Funding acquisition, Investigation, Project administration, Writing – review & editing. QD: Data curation, Funding acquisition, Investigation, Project administration, Writing – review & editing. JS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing. SM: Formal analysis, Funding acquisition, Investigation, Resources, Software, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Key Research and Development Program of China no. (2022YFD1301001).
NL, LC, DC, HY, and QD were employed by China Salt Jintan Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1562964/full#supplementary-material
1. Becker, CA, Collier, RJ, and Stone, AE. Invited review: physiological and behavioral effects of heat stress in dairy cows. J Dairy Sci. (2020) 103:6751–70. doi: 10.3168/jds.2019-17929
2. Das, R, Sailo, L, Verma, N, Bharti, P, Saikia, J, Imtiwati, N, et al. Impact of heat stress on health and performance of dairy animals: a review. Vet World. (2016) 9:260–8. doi: 10.14202/vetworld.2016.260-268
3. Kim, SH, Ramos, SC, Valencia, RA, Cho, YI, and Lee, SS. Heat stress: effects on rumen microbes and host physiology, and strategies to alleviate the negative impacts on lactating dairy cows. Front Microbiol. (2022) 13:804562. doi: 10.3389/fmicb.2022.804562
4. Wilson, SJ, Marion, RS, Spain, JN, Spiers, DE, Keisler, DH, and Lucy, MC. Effects of controlled heat stress on ovarian function of dairy cattle. 1. Lactating Cows1. J Dairy Sci. (1998) 81:2124–31. doi: 10.3168/jds.S0022-0302(98)75788-1
5. Bernabucci, U, Basiricò, L, Morera, P, Dipasquale, D, Vitali, A, Piccioli, CF, et al. Effect of summer season on milk protein fractions in Holstein cows. J Dairy Sci. (2015) 98:1815–27. doi: 10.3168/jds.2014-8788
6. De Rensis, F, Garcia-Ispierto, I, and López-Gatius, F. Seasonal heat stress: clinical implications and hormone treatments for the fertility of dairy cows. Theriogenology. (2015) 84:659–66. doi: 10.1016/j.theriogenology.2015.04.021
7. Gunn, KM, Holly, MA, Veith, TL, Buda, AR, Prasad, R, Rotz, CA, et al. Projected heat stress challenges and abatement opportunities for U.S. milk production. PLoS One. (2019) 14:e0214665. doi: 10.1371/journal.pone.0214665
8. Ranjitkar, S, Bu, D, Van, WM, Ma, Y, Ma, L, Zhao, L, et al. Will heat stress take its toll on milk production in China? Clim Chang. (2020) 161:637–52. doi: 10.1007/s10584-020-02688-4
9. Collier, RJ, Dahl, GE, and VanBaale, MJ. Major advances associated with environmental effects on dairy cattle. J Dairy Sci. (2006) 89:1244–53. doi: 10.3168/jds.S0022-0302(06)72193-2
10. Wang, JP, Bu, DP, Wang, JQ, Huo, XK, Guo, TJ, Wei, HY, et al. Effect of saturated fatty acid supplementation on production and metabolism indices in heat-stressed mid-lactation dairy cows. J Dairy Sci. (2010) 93:4121–7. doi: 10.3168/jds.2009-2635
11. Zimbelman, RB, Collier, RJ, and Bilby, TR. Effect of feeding rumen-protected niacin on core body temperature and milk production in lactating Holstein cows during summer heat stress. J Dairy Sci. (2008) 91:E142. doi: 10.1016/j.anifeedsci.2013.01.005
12. Shan, CH, Guo, JJ, Sun, XS, Li, N, Yang, XY, Gao, YH, et al. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J Anim Sci. (2018) 96:4444–57. doi: 10.1093/jas/sky270
13. Sanchez, WK, McGuire, MA, and Beede, DK. Macromineral nutrition by heat stress interactions in dairy cattle: review and original research. J Dairy Sci. (1994) 77:2051–79. doi: 10.3168/jds.S0022-0302(94)77150-2
14. Kafilzadeh, F, Shabankareh, HK, and Targhibi, MR. Effect of chromium supplementation on productive and reproductive performances and some metabolic parameters in late gestation and early lactation of dairy cows. Biol Trace Elem Res. (2012) 149:42–9. doi: 10.1007/s12011-012-9390-0
15. Yoshimura, EH, Santos, NW, Machado, E, Agustinho, BC, Pereira, LM, De, ASC, et al. Effects of dairy cow diets supplied with flaxseed oil and propolis extract, with or without vitamin E, on the ruminal microbiota, biohydrogenation, and digestion. Anim Feed Sci Technol. (2018) 241:163–72. doi: 10.1016/j.anifeedsci.2018.04.024
16. Gao, J, Lin, H, Wang, XJ, Song, ZG, and Jiao, HC. Vitamin E supplementation alleviates the oxidative stress induced by dexamethasone treatment and improves meat quality in broiler chickens. Poult Sci. (2010) 89:318–27. doi: 10.3382/ps.2009-00216
17. Li, M, Li, DG, Tong, X, Nan, XM, Ding, DY, Xu, B, et al. Nutritional strategies for alleviating the detrimental effects of heat stress in dairy cows: a review. Int J Biometeorol. (2019) 63:1283–302. doi: 10.1007/s00484-019-01744-8
18. Tekeba, E, Wurzinger, M, and Zollitsch, W. Effects of urea-molasses multi-nutrient blocks as a dietary supplement for dairy cows in two milk production systems in North-Western Ethiopia. Livest Res Rural Dev. (2012) 2024:24.
19. Vu, DD, Cuong, LX, Dung, CA, and Hai, PH. Use of urea-molasses-multinutrient block and urea-treated rice straw for improving dairy cattle productivity in Vietnam. Prev Vet Med. (1999) 38:187–93. doi: 10.1016/s0167-5877(98)00124-x
20. Srinivas, B, and Gupta, BN. Rumen fermentation, bacterial and total volatile fatty acid (TVFA) production rates in cattle fed on urea-molasses-mineral block licks supplement. Anim Feed Sci Technol. (1997) 65:275–86. doi: 10.1016/S0377-8401(96)01062-0
21. NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient requirements of dairy cattle. 8th ed. Washington, DC: National Academies Press (2021).
22. AOAC International. Official methods of analysis of AOAC. Rockville, MD: AOAC International (2005).
23. Soest Van, PJ. Development of a comprehensive system of feed analyses and its application to forages. J Anim Sci. (1967) 26:119–28. doi: 10.2527/jas1967.261119x
24. Shen, JS, Chai, Z, Song, LJ, Liu, JX, and Wu, YM. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows1. J Dairy Sci. (2012) 95:5978–84. doi: 10.3168/jds.2012-5499
25. Chaney, AL, and Marbach, EP. Modified reagents for determination of urea and Ammonia. Clin Chem. (1962) 7:892–132. doi: 10.1016/0009-8981(62)90080-3
26. Shen, J, Liu, Z, Yu, Z, and Zhu, W. Monensin and Nisin affect rumen fermentation and microbiota differently in vitro. Front Microbiol. (2017) 8:8. doi: 10.3389/fmicb.2017.01111
27. Kadzere, CT, Murphy, MR, Silanikove, N, and Maltz, E. Heat stress in lactating dairy cows: a review. Livest Prod Sci. (2002) 77:59–91. doi: 10.1016/S0301-6226(01)00330-X
28. Knyves, T, Zlatkovi, N, Memii, N, Luka, D, and Mievi, B. Relationship of temperature-humidity index with milk production and feed intake of Holstein-frisian cows in different year seasons. Thai Vet Med. (2017) 47:15–23. doi: 10.56808/2985-1130.2807
29. Lim, DH, Kim, TI, Park, SM, Ki, KS, and Kim, Y. Evaluation of heat stress responses in Holstein and Jersey cows by analyzing physiological characteristics and milk production in Korea. J Anim Sci Technol. (2021) 63:872–83. doi: 10.5187/jast.2021.e62
30. Majki, M, Cincovi, MR, Beli, B, Plava, N, and Radinovi, M. Relationship between milk production and metabolic adaptation in dairy cows during heat stress. Acta Agric Serb. (2017) 22:123–31. doi: 10.5937/AASer1744123M
31. Beaudeau, F, Fourichon, C, Seegers, H, and Bareille, N. Risk of clinical mastitis in dairy herds with a high proportion of low individual milk somatic-cell counts - ScienceDirect. Prev Vet Med. (2002) 53:43–54. doi: 10.1016/s0167-5877(01)00275-6
32. Litwińczuk, Z, Król, J, Brodziak, A, and Barłowska, J. Changes of protein content and its fractions in bovine milk from different breeds subject to somatic cell count. J Dairy Sci. (2011) 94:684–91. doi: 10.3168/jds.2010-3217
33. Bellagi, R, Martin, B, Chassaing, C, Najar, T, and Pomiès, D. Evaluation of heat stress on Tarentaise and Holstein cow performance in the Mediterranean climate. Int J Biometeorol. (2017) 61:1371–9. doi: 10.1007/s00484-017-1314-4
34. Dalcin, VC, Fischer, V, Daltro, DDS, Alfonzo, EPM, Stumpf, MT, Kolling, GJ, et al. Physiological parameters for thermal stress in dairy cattle. R Bras Zootec. (2016) 45:458–65. doi: 10.1590/S1806-92902016000800006
35. Kovács, L, Kézér, FL, Ferenc, R, Viktor, J, and Szenci, O. Heart rate, cardiac vagal tone, respiratory rate, and rectal temperature in dairy calves exposed to heat stress in a continental region. Int J Biometeorol. (2018) 62:1791–7. doi: 10.1007/s00484-018-1581-8
36. Sammad, A, Wang, YJ, Umer, S, Lirong, H, Khan, I, Khan, A, et al. Nutritional physiology and biochemistry of dairy cattle under the influence of heat stress: consequences and opportunities. Animals. (2020) 10:793. doi: 10.3390/ani10050793
37. Leng, RA, and Brett, DJ. Simultaneous measurements of the rates of production of acetic, propionic and butyric acids in the rumen of sheep on different diets and the correlation between production rates and concentrations of these acids in the rumen. Br J Nutr. (1966) 20:541–52. doi: 10.1079/BJN19660053
38. Satter, LD, Suttie, JW, and Raumgardt, BR. Dietary induced changes in volatile fatty acid formation from α-cellulose-C14 and hemicellulose-C14. J Dairy Sci. (1964) 47:1365–70. doi: 10.3168/jds.S0022-0302(64)88919-0
39. Nonaka, I, Takusari, N, Tajima, K, Suzuki, T, Higuchi, K, and Kurihara, M. Effects of high environmental temperatures on physiological and nutritional status of prepubertal Holstein heifers. Livest Sci. (2008) 113:14–23. doi: 10.1016/j.livsci.2007.02.010
40. Sklan, D, Ashkenazi, R, Brun, A, Devorin, A, and Tabor, K. Fatty acids, calcium soaps of fatty acids, and cottonseeds fed to high yielding cows. J Dairy Sci. (1992) 75:2463–72. doi: 10.3168/jds.S0022-0302(92)78008-4
Keywords: heat stress, dairy cow, lick block, lactation performance, heat stress index, rumen fermentation
Citation: Duan H, Zhang J, Li N, Chen L, Chen D, Yang H, Dai Q, Shen J and Mao S (2025) Anti-heat stress lick block supplementation alleviated the detrimental effects of heat stress on dairy cows. Front. Vet. Sci. 12:1562964. doi: 10.3389/fvets.2025.1562964
Received: 18 January 2025; Accepted: 24 February 2025;
Published: 07 March 2025.
Edited by:
Yanfeng Xue, Anhui Agricultural University, ChinaReviewed by:
Bahram Chachar, Lasbela University of Agriculture, Water and Marine Sciences, PakistanCopyright © 2025 Duan, Zhang, Li, Chen, Chen, Yang, Dai, Shen and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junshi Shen, shenjunshi@njau.edu.cn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.