
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci., 12 March 2025
Sec. Veterinary Surgery
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1543299
A 6-year-old spayed female 4.4-kg Papillon with only the left kidney presented with vomiting. Imaging unveiled ureterolithiasis and hydronephrosis, while serum chemistry displayed elevated creatinine, blood urea nitrogen, and C-reactive protein. Urinalysis revealed bacteria and bacterial phagocytes. After subcutaneous ureteral bypass (SUB) placement, kidney panels were normalized. The nephrostomy and cystostomy catheters had migrated into the renal parenchyma and bladder wall on postoperative day (POD) 212 and 369, respectively. As the migration advanced, they entered the ureter and bladder on POD 369 and 796, respectively. The SUB, excluding the nephrostomy catheter, was removed on POD 930 due to migration, obstruction, and extrusion of the SUB shunting port. On POD 937, creatinine and BUN levels remained normal. By POD 1063, the ureteroliths had disappeared. This case highlights the need for vigilant monitoring of catheter migration as a potential complication following SUB placement. Early identification and timely intervention are essential for reducing morbidity and improving patient outcomes.
Causes of ureteral obstruction include ureterolithiasis, inflammation, ureteral strictures, surgical interventions, neoplasia, and complications arising from renal transplantation (1, 2). Treatment options consist of surgical and medical management, with medical treatment demonstrating a low success rate (8–17%) (3, 4). Ureteral obstruction is considered a medical emergency, and early surgical decompression is recommended (5, 6). Traditional surgical options, such as ureterostomy, ureteral reimplantation, ureteronephrectomy, and ureteral resection with anastomosis, have been documented (4, 7). However, these methods have high complications (30–38%) and mortality rates (18–20%) (4, 8, 9). SUB is recommended for its lower mortality rate (<5%), lower complication rate (1.4–27%) and longer median survival time (762–923 days) (8, 10–12).
Long-term complications of the subcutaneous ureteral bypass (SUB), including occlusion, kinking, chronic urinary tract infection (UTI), and intermittent dysuria, have been reported (12–16). Recently, SUB migration into the gastrointestinal tract has also been reported (17–19).
This case report presents a patient in which the SUB migrated into the ureter through the renal parenchyma and the bladder. This is the first report of SUB catheter migration into both the ureter and bladder. Notably, we monitored the progression of the complication over an extended period of 1,072 days.
A 6-year-old spayed female 4.4-kg Papillon presented with a 2-day history of inappetence and lethargy after vomiting (Figure 1). The patient underwent a nephrectomy 5 years ago. Imaging revealed two ureteroliths (6–8 mm) in the left proximal ureter, proximal ureteral dilation, and renal pelvic dilation (8 mm) (Supplementary file 1). A physical examination revealed hyperthermia (40.9°C), and blood tests showed creatinine 2.5 mg/dL [reference interval (RI): 0.5–1.8 mg/dL], blood urea nitrogen (BUN) 38 mg/dL (RI: 7–27 mg/dL), and C-reactive protein 35.6 mg/dL (RI: 0–1 mg/dL), with no other remarkable findings. Urinalysis revealed cocci, rods, and phagocytic neutrophils. In-house antibiotic sensitivity testing revealed bacteria sensitive to enrofloxacin (Ashienro 50, Ashishi Life Science, Gujarat, India).
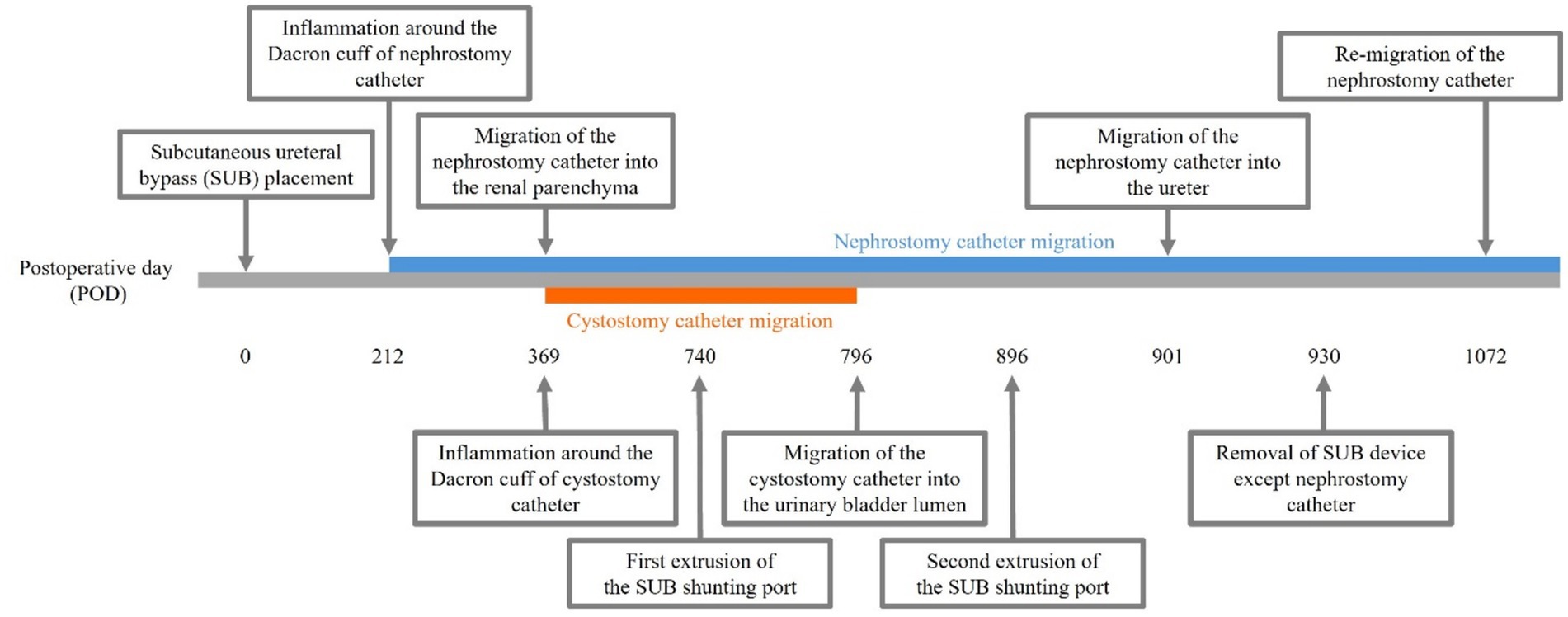
Figure 1. Case timeline. Inflammation around the Dacron cuff of the nephrostomy and cystostomy catheters was observed on postoperative days (POD) 212 and 369, respectively. By POD 901, the Dacron cuff of the nephrostomy catheter had gradually migrated through the renal parenchyma into the ureter, whereas by POD 796, the Dacron cuff of the cystostomy catheter had migrated into the bladder lumen. On POD 740, the subcutaneous ureteral bypass (SUB) shunting port protruded, prompting an attempt at primary wound closure. However, the SUB shunting port was re-exposed on POD 896, leading to the decision to remove the SUB device on POD 930. On POD 1072, the Dacron cuff had migrated into the renal pelvis again.
The patient was induced with 6 mg/kg IV propofol (Provive Inj., Pharmbio Korea, Seoul, Korea) without premedication and maintained on isoflurane (Ifran Liq., Hana Pharm, Seoul, Korea). Fluoroscope-guided placement of a SUB™ 2.0 (Norfolk Vet Products, Skokie, Illinois, United States) was conducted following a midline laparotomy. Nephrostomy and cystostomy catheters were inserted, and the Dacron cuff was secured with sterile cyanoacrylate glue and simple interrupted sutures (PDS 4–0) to the caudal pole of the kidney and the apex of the urinary bladder (Supplementary file 2). The ureteroliths remained, and the Dacron cuff was covered with perinephric fat. The catheter’s leakage and patency were verified through contrast fluoroscopy. No complications occurred during the procedure, and imaging verified successful SUB placement.
On postoperative day (POD) 1, the kidney panel (creatinine 1 mg/dL, BUN 23 mg/dL) and renal pelvic dilation improved. Following the antibiotic sensitivity tests, 2.5 mg/kg enrofloxacin (Ashienro 50, Ashishi Life Science, Gujarat, India) was prescribed orally, twice daily, for 2 months.
At 1 and 2 months postoperatively and every 1.5 months thereafter, abdominal ultrasound, serum chemistry, urinalysis, and urine cultures were conducted, followed by ultrasound-guided flushing with sterile saline and 2 mL tetra-EDTA (SUB™ Flush Kit, Norfolk Vet Products, Skokie, Illinois, United States). Prescribed targeted oral antibiotics were used, yet bacteria with comparable susceptibility profiles persisted. On POD 369, Staphylococcus pseudintermedius and Escherichia coli were identified.
Inflammation was initially observed around the Dacron cuff of the nephrostomy catheter on POD 212 (Figures 2, 3). By POD 369, SUB migration into the renal parenchyma was confirmed. Additionally, on POD 369, inflammation was noted around the Dacron cuff of the cystostomy catheter (Figure 4). The bladder apex thickened, and the Dacron cuff migrated into the thickened bladder wall, eventually migrating entirely into the bladder by POD 796. Urine leakage was not suspected. By POD 901, the cuff of the nephrostomy catheter had gradually migrated through the renal parenchyma and into the ureter. On POD 901, SUB obstruction was also confirmed. No abnormalities were observed during the migration process.
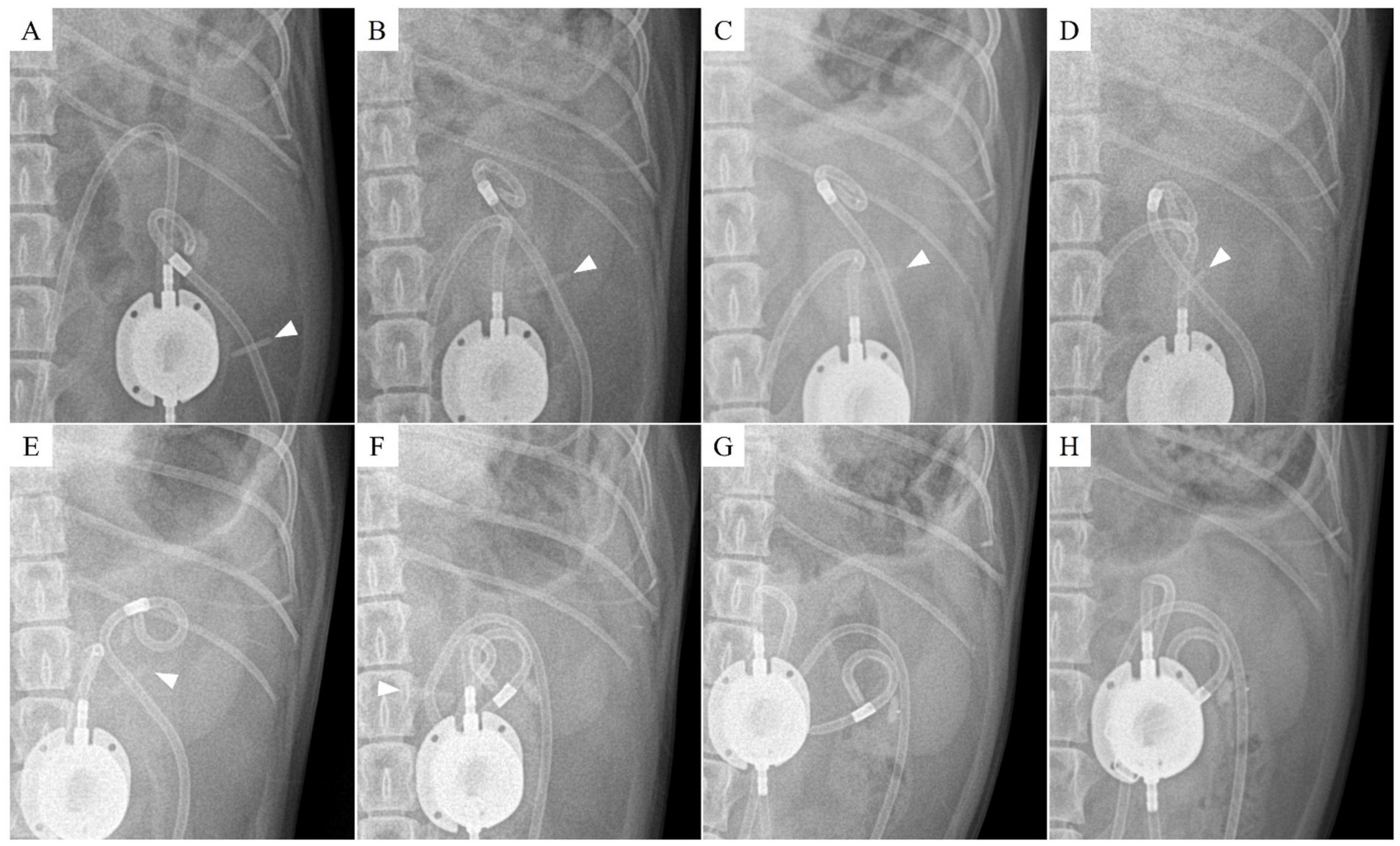
Figure 2. Radiographic findings of nephrostomy catheter migration. (A) Postoperative day (POD) 147 (before migration), (B) POD 321, (C) POD 343, (D) POD 408, (E) POD 503, (F) POD 857, (G) POD 901, and (H) POD 930. The Dacron cuff has migrated through the renal parenchyma into the ureter, while the pigtail end of the nephrostomy catheter has shifted into the proximal ureter. The arrowhead indicates the location of the Dacron cuff.
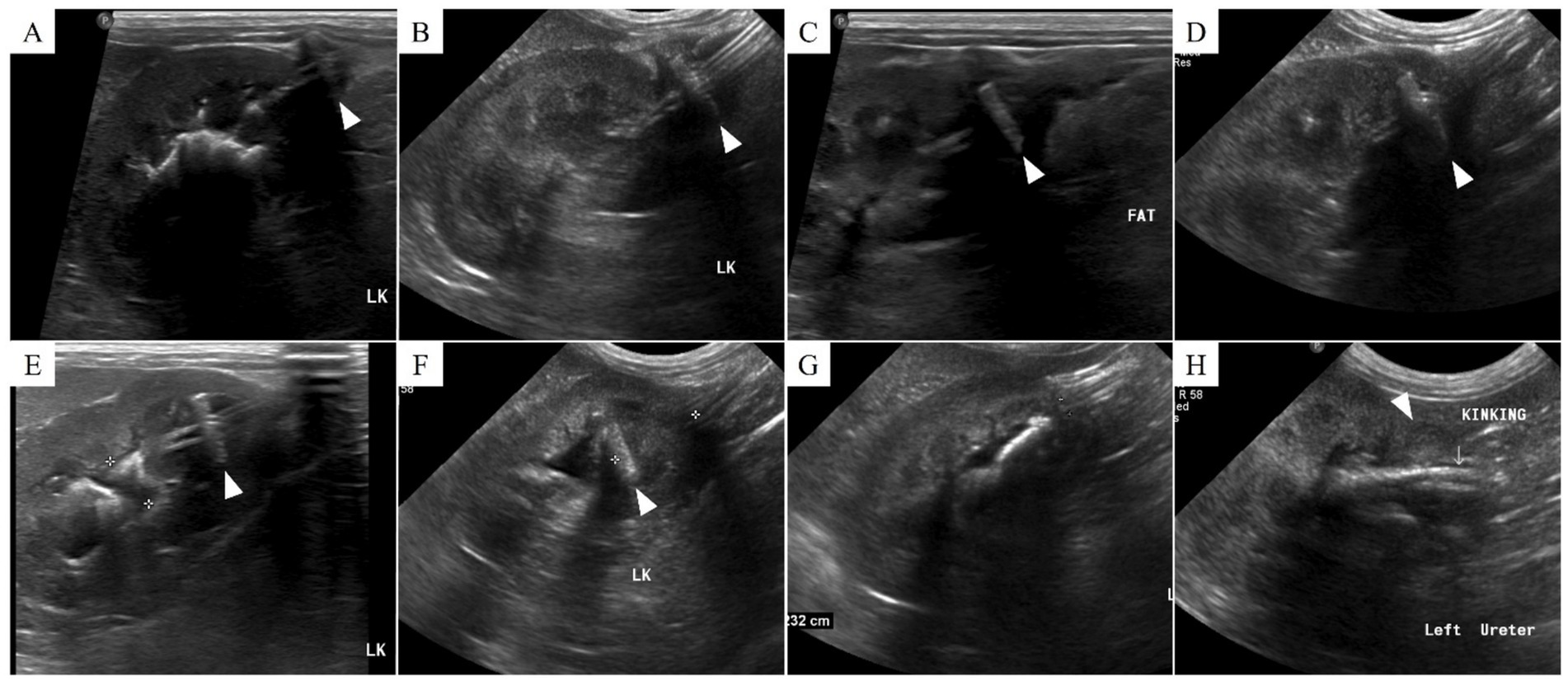
Figure 3. Ultrasonographic findings of nephrostomy catheter migration. (A) Postoperative day (POD) 148 (before migration), (B) POD 180, (C) POD 212, (D) POD 216, (E) POD 369, (F) POD 460, (G) POD 857, and (H) POD 901. On POD 212, low echogenic inflammatory lesions and fat edema are observed around the Dacron cuff. Subsequently, from (C) to (H), the Dacron cuff gradually penetrates the renal parenchyma and migrates into the ureter, resulting in kinking. The arrowhead indicates the location of the Dacron cuff.
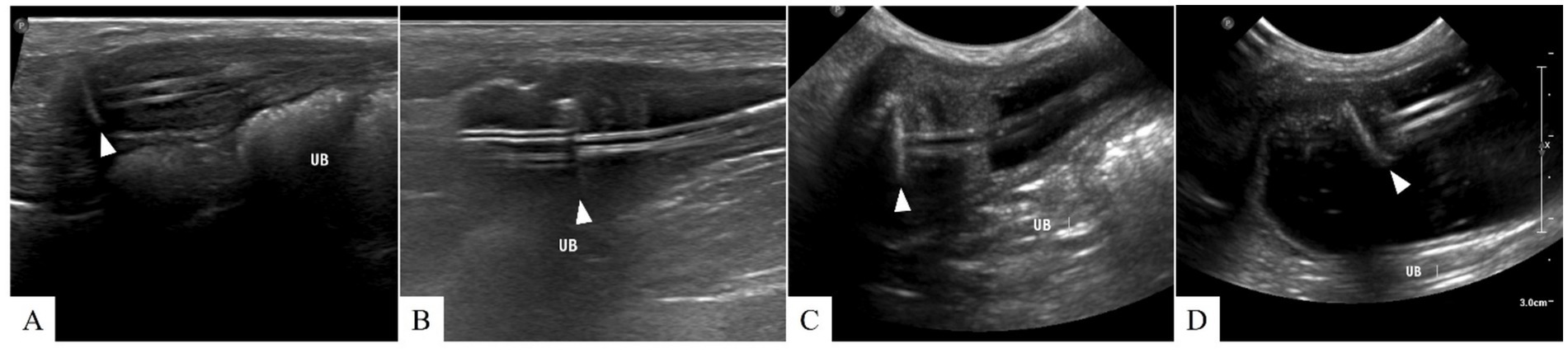
Figure 4. Ultrasonographic findings of cystostomy catheter migration. (A) Postoperative day (POD) 148 (before migration), (B) POD 369, (C) POD 654, and (D) POD 796. On POD 369, low echogenic inflammatory lesions and fat edema are observed around the Dacron cuff. From (B) to (D), the cystostomy catheter migrates into the bladder lumen, resulting in severe bladder wall inflammation. No signs of urine leakage are suspected after migration. The arrowhead indicates the location of the Dacron cuff.
On POD 740, the SUB shunting port protruded, and primary wound closure was attempted. Primary and secondary closure attempts were unsuccessful when the SUB shunting port was re-exposed on POD 896.
On POD 930, due to migration, SUB obstruction, and extrusion of the SUB shunting port, it was decided to remove the SUB. The kidney panel was within the normal range. The patient was anesthetized using the same procedure as before. The midline laparotomy uncovered omental adhesions at the cystostomy catheter insertion site, significantly thickening the bladder wall. Preoperative intravenous pyelography (IVP) verified ureteral patency, while contrast fluoroscopy via the SUB shunting port revealed no nephrostomy catheter opacification during operation, indicating that the ureter was patent. The SUB was firmly affixed to tissue, and the nephrostomy catheter had penetrated the kidney into the ureter, prompting concerns about renal injury upon removal. During the surgery, only the SUB shunting port and cystostomy catheter were excised, while the nephrostomy catheter was ligated to prevent urine leakage.
On POD 937, the kidney panel at discharge remained within the normal range (creatinine 0.81 mg/dL, BUN 14.12 mg/dL). The ultrasound revealed two ureteroliths upon SUB removal, but 133 days later (POD 1063 from the initial surgery), they had disappeared. On POD 1072, the Dacron cuff had migrated into the renal pelvis again.
Ureteral obstruction is managed medically or surgically, with its severity and duration impacting the glomerular filtration rate (GFR) (2, 20). Traditional surgical options for ureteral obstruction include ureterostomy, ureteral reimplantation, ureteronephrectomy, and ureteral resection with anastomosis (3–7). However, these methods have high complications (30–38%) and mortality rates (18–20%) (4, 8, 9). SUB is recommended for its lower mortality rate (<5%), lower complication rate (1.4–27%), and longer median survival time (762–923 days) (8, 10–12).
Aggressive surgical intervention was deemed necessary due to imaging findings indicative of hydronephrosis, renal damage, and retroperitoneal inflammation, corroborated by serum chemistry confirming azotemia despite the absence of anuria. Given the presence of a single kidney, preserving the glomerular filtration rate was imperative. Moreover, the accompanying infection raised concerns about the progression to pyelonephritis, septicemia, and mortality (21). Retropulsion of proximal ureteroliths to the renal pelvis for pyelolithotomy was also considered (22). However, the renal pelvis presented insufficient dilation (8 mm), thereby complicating the surgical procedure (23). Additionally, stricture concerns prompted the SUB placement.
SUB complications include intermittent dysuria (12.5–38.5%), obstruction (5.3–33.3%), UTI (8–30.8%), mineralization (25%), kinking (4–12.5%), leakage (3.5%), transmural migration into the small intestine (1%) (18, 19), and intestinal perforation of the nephrostomy catheter and Dacron cuff without catheter migration (2 cats), extrusion of the SUB shunting port (1 cat) (17), and enterovesicular fistula at the cystostomy catheter site (1 cat) (12–15, 24). However, there are no reports concerning catheter migration through the renal parenchyma and bladder wall into the ureteral and bladder lumina.
SUB migration may result from a foreign body reaction, potentially involving the Dacron cuff and cyanoacrylate glue (18, 19, 25). This is similar to reports of biomaterials causing transmural migration into the gastrointestinal tract, bladder, lungs, and trachea due to foreign body reaction (26–28). Late Dacron patch inflammatory complications in humans have been reported up to 7 years post-implantation, suggesting secondary foreign body reactions from the Dacron cuff in the abdominal cavity could cause transmural migration (25). In this patient, inflammation likely first developed around the Dacron cuff, possibly triggering a foreign body reaction that contributed to catheter migration.
Prior to SUB placement surgery, this patient had already been diagnosed with pyelonephritis caused by both rods and cocci. Postoperatively, bacterial biofilm formation on the surface of the SUB device may have contributed to persistent infection, making it difficult to control. Consequently, chronic UTI may have potentially facilitated the SUB migration (17–19, 26, 27).
In this patient, despite two ureteroliths remaining, IVP confirmed ureteral patency, facilitating SUB removal. When infected, biofilm on the SUB may require removal of the SUB and high-dose antibiotic therapy (29–31). Even after SUB replacement, persistent infections in surrounding tissues might result in secondary infection (32). Furthermore, its removal or replacement should be decided based on ureteral patency (18). Focal inflammation and ureteral muscle spasm can exacerbate ureteral obstruction (33), and half of SUB reobstruction cases regained ureteral patency (13, 20).
There were no signs of suspected urine leakage, likely due to severe omental adhesions and gradual migration. However, in cases of SUB migration, it is crucial to confirm the absence of any leakage, whether from the intestine, bladder, or other sources, as cases of intestinal leakage with septic peritonitis have been reported (25).
A limitation of this case is that the nephrostomy catheter was not removed during the second surgery. Despite our recommendation for revision surgery to remove the remaining nephrostomy catheter, the procedure was not performed. The owner declined it, as the patient was clinically stable. However, in cases of migration, it is generally preferable to remove the entire SUB from the outset.
In conclusion, the long-term management of SUB devices should include monitoring for potential complications, such as the gradual migration of the nephrostomy and cystostomy catheters into the renal pelvis and bladder lumen, as observed in this patient. Prompt identification of this complication is crucial for reducing morbidity and facilitating timely, appropriate intervention.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for this study in accordance with the local legislation and institutional requirements because the case report is a description of a clinical case. This case report involves a client-owned animal, and written informed consent was obtained from the owner for both the animal’s participation and the publication of this report.
BL: Conceptualization, Data curation, Investigation, Writing – original draft. JS: Investigation, Methodology, Writing – review & editing. S-WJ: Conceptualization, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1543299/full#supplementary-material
1. Clarke, DL. Feline ureteral obstructions part 1: medical management. J Small Anim Pract. (2018) 59:324–33. doi: 10.1111/jsap.12844
3. Berent, AC. Ureteral obstructions in dogs and cats: a review of traditional and new interventional diagnostic and therapeutic options. J Vet Emerg Crit Care. (2011) 21:86–103. doi: 10.1111/j.1476-4431.2011.00628.x
4. Kyles, AE, Hardie, EM, Wooden, BG, Adin, CA, Stone, EA, Gregory, CR, et al. Management and outcome of cats with ureteral calculi: 153 cases (1984–2002). J Am Vet Med Assoc. (2005) 226:937–44. doi: 10.2460/javma.2005.226.937
5. Milligan, M, and Berent, AC. Medical and interventional management of upper urinary tract uroliths. Vet Clin North Am Small Anim Pract. (2019) 49:157–74. doi: 10.1016/j.cvsm.2018.11.004
6. Lulich, JP, Berent, AC, Adams, LG, Westropp, JL, Bartges, JW, and Osborne, CA. ACVIM small animal consensus recommendations on the treatment and prevention of uroliths in dogs and cats. J Vet Intern Med. (2016) 30:1564–74. doi: 10.1111/jvim.14559
7. Clarke, DL. Feline ureteral obstructions Part 2: surgical management. J Small Anim Pract. (2018) 59:385–97. doi: 10.1111/jsap.12861
8. Berent, AC, Weisse, CW, Todd, K, and Bagley, DH. Technical and clinical outcomes of ureteral stenting in cats with benign ureteral obstruction: 69 cases (2006–2010). J Am Vet Med Assoc. (2014) 244:559–76. doi: 10.2460/javma.244.5.559
9. Roberts, SF, Aronson, LR, and Brown, DC. Postoperative mortality in cats after ureterolithotomy. Vet Surg. (2011) 40:438–43. doi: 10.1111/j.1532-950X.2011.00836.x
10. Deroy, C, Rossetti, D, Ragetly, G, Hernandez, J, and Poncet, C. Comparison between double-pigtail ureteral stents and ureteral bypass devices for treatment of ureterolithiasis in cats. J Am Vet Med Assoc. (2017) 251:429–37. doi: 10.2460/javma.251.4.429
11. Kulendra, NJ, Syme, H, Benigni, L, and Halfacree, Z. Feline double pigtail ureteric stents for management of ureteric obstruction: short- and long-term follow-up of 26 cats. J Feline Med Surg. (2014) 16:985–91. doi: 10.1177/1098612X14531763
12. Berent, AC, Weisse, CW, Bagley, DH, and Lamb, K. Use of a subcutaneous ureteral bypass device for treatment of benign ureteral obstruction in cats: 174 ureters in 134 cats (2009–2015). J Am Vet Med Assoc. (2018) 253:1309–27. doi: 10.2460/javma.253.10.1309
13. Livet, V, Pillard, P, Goy-Thollot, I, Maleca, D, Cabon, Q, Remy, D, et al. Placement of subcutaneous ureteral bypasses without fluoroscopic guidance in cats with ureteral obstruction: 19 cases (2014–2016). J Feline Med Surg. (2017) 19:1030–9. doi: 10.1177/1098612X16670572
14. Vrijsen, E, Devriendt, N, Mortier, F, Stock, E, Van Goethem, B, and de Rooster, H. Complications and survival after subcutaneous ureteral bypass device placement in 24 cats: a retrospective study (2016–2019). J Feline Med Surg. (2021) 23:759–69. doi: 10.1177/1098612X20975374
15. Kulendra, NJ, Borgeat, K, Syme, H, Dirrig, H, and Halfacree, Z. Survival and complications in cats treated with subcutaneous ureteral bypass. J Small Anim Pract. (2021) 62:4–11. doi: 10.1111/jsap.13226
16. Fouhety, A, and Boursier, J-F. Infection and extrusion of a subcutaneous access port in a cat: a long-term postoperative complication of a subcutaneous ureteral bypass device. J Feline Med Surg Open Rep. (2020) 6:205511692091176. doi: 10.1177/2055116920911765
17. Lee, SM, and Tuan, J. Surgical repositioning with omentalisation of an exposed subcutaneous ureteral bypass shunting port in a cat. J Feline Med Surg Open Rep. (2024) 10:20551169241257884. doi: 10.1177/20551169241257884
18. Véran, E, Vachon, C, Byron, J, Howard, J, Berent, A, Weisse, C, et al. Multicenter retrospective evaluation of transmural migration of subcutaneous ureteral bypass devices within the digestive tract in cats. J Vet Intern Med. (2022) 36:1677–85. doi: 10.1111/jvim.16511
19. Boullenger, J, Lafuma, F, Baudin Trehiou, C, Blond, L, Gibert, S, and Kulendra, N. Transmural migration of a subcutaneous ureteral bypass into the intestine in three cats. J Small Anim Pract. (2022) 63:792–6. doi: 10.1111/jsap.13502
20. Pilot, M, Broome, C, Hammond, G, Ward, PM, and McLauchlan, G. Bladder catheter dislodgement as a complication following placement of a subcutaneous ureteral bypass device. Vet Rec Case Rep. (2017) 5:e000479. doi: 10.1136/vetreccr-2017-000479
21. McLoughlin, MA. Surgical emergencies of the urinary tract. Vet Clin North Am Small Anim Pract. (2000) 30:581–601. doi: 10.1016/S0195-5616(00)50040-0
22. McLoughlin, MA. Textbook of small animal surgery. 3rd Philadelphia, PA: WB Saunders (2003). 1619–1672.
23. Fossum, TW, Hedlund, CS, Hulse, DA, Johnson, AL, Seim, HB, Willard, MD, et al. Small animal surgery. 5th ed. St. Louis: Elsevier (2018).
24. Kopecny, L, Palm, CA, Drobatz, KJ, Balsa, IM, and Culp, WTN. Risk factors for positive urine cultures in cats with subcutaneous ureteral bypass and ureteral stents (2010-2016). J Vet Intern Med. (2019) 33:178–83. doi: 10.1111/jvim.15343
25. Johnston, SK, Bennett, T, and Miller, AJ. Intestinal perforation involving the Dacron cuff of nephrostomy tubes following subcutaneous ureteral bypass system implantation for ureteral obstructions in two cats. J Feline Med Surg Open Rep. (2021) 7:20551169211013295. doi: 10.1177/20551169211013295
26. Picchio, M, Muggianu, A, Mancini, F, Tintisona, O, and Spaziani, E. Complete mesh migration into the small bowel after incisional hernia repair: a case report and literature review. Acta Chir Belg. (2017) 117:118–21. doi: 10.1080/00015458.2016.1229399
27. Zengin, K, Sen, B, Ozben, V, and Taskin, M. Detachment of the connecting tube from the port and migration into jejunal wall. Obes Surg. (2006) 16:206–7. doi: 10.1381/096089206775565131
28. Zantvoord, Y, van der Weiden, RMF, and van Hooff, MHA. Transmural migration of retained surgical sponges. Obstet Gynecol Surv. (2008) 63:465–71. doi: 10.1097/OGX.0b013e318173538e
29. Josse, J, Valour, F, Maali, Y, Diot, A, Batailler, C, Ferry, T, et al. Interaction between staphylococcal biofilm and bone: how does the presence of biofilm promote prosthesis loosening? Front Microbiol. (2019) 10:1602. doi: 10.3389/fmicb.2019.01602
30. Wagner, C, and Hänsch, GM. Mechanisms of bacterial colonization of implants and host response. Adv Exp Med Biol. (2017) 971:15–27. doi: 10.1007/5584_2016_173
31. Rosman, CWK, van Dijl, JM, and Sjollema, J. Interactions between the foreign body reaction and Staphylococcus aureus biomaterial-associated infection. Winning strategies in the derby on biomaterial implant surfaces. Crit Rev Microbiol. (2022) 48:624–40. doi: 10.1080/1040841X.2021.2011132
32. Conlon, BP. Staphylococcus aureus chronic and relapsing infections: evidence of a role for persister cells. BioEssays. (2014) 36:991–6. doi: 10.1002/bies.201400080
Keywords: ureteral obstruction, ureterolithiasis, hydronephrosis, foreign body reaction, Dacron cuff
Citation: Lee B, Seo J and Jeong S-W (2025) Case Report: Long-term complications of subcutaneous ureteral bypass migration in an adult female Papillon. Front. Vet. Sci. 12:1543299. doi: 10.3389/fvets.2025.1543299
Received: 11 December 2024; Accepted: 24 February 2025;
Published: 12 March 2025.
Edited by:
Floryne Ottilie Buishand, Royal Veterinary College (RVC), United KingdomReviewed by:
Ivan Santos, Rondônia Federal University, BrazilCopyright © 2025 Lee, Seo and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Boram Lee, cmVkcXVlZW4wMzA5QG5hdmVyLmNvbQ==
Jeonghyun Seo, https://orcid.org/0009-0004-7945-3789
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.