
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Vet. Sci., 07 February 2025
Sec. Veterinary Epidemiology and Economics
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1534114
 Xue-Tong Liu1†
Xue-Tong Liu1† Li-Dong Jiang1†
Li-Dong Jiang1† Yu-Ting Lin1†
Yu-Ting Lin1† Ran Zhao1
Ran Zhao1 Qi Wang1
Qi Wang1 Shu-Ying Zhang1,2,3*
Shu-Ying Zhang1,2,3* Emad Beshir Ata4
Emad Beshir Ata4 Xin Liu1
Xin Liu1 Yuan Wang1
Yuan Wang1 Zi-Xuan Liu1
Zi-Xuan Liu1 Cui Xu1
Cui Xu1 Ying Xiao1
Ying Xiao1 Yi-Fan Wang1
Yi-Fan Wang1 Xue Leng2
Xue Leng2 Qing-Long Gong1*
Qing-Long Gong1* Rui Du1,2,5*
Rui Du1,2,5*Background: Japanese encephalitis (JE) is an acute viral disease transmitted mainly by mosquitoes, primarily affecting Southeast Asia, and the Western Pacific. This study aimed to analyze the factors contributing to JE occurrence in pigs across China.
Methods: A systematic search was done using six databases for the published epidemiological studies on porcine JE, including the Chinese Web of Knowledge (CNKI), Wan Fang Database, ScienceDirect, Web of Science, VIP Chinese Journal Database, and PubMed.
Results: A meta-analysis of 31 studies from 2000 to 2024 found an overall prevalence of 35.2% (95% CI: 25.1–46.1). The highest prevalence occurred between 2010 and 2015 at 53.4% (95% CI: 44.2–80.6), from 2010 to 2015, increased precipitation and favorable annual temperatures led to the proliferation of mosquitoes, causing Japanese Encephalitis outbreaks among swine. While the lowest was 2.5% (95% CI: 0.2–6.6) in temperate climates. Serum samples showed the highest prevalence 38.1% (95% CI: 27.9–48.9), and ELISA testing had a higher detection rate 38.2% (95% CI: 24.5–52.9). In the farming mode subgroup, the highest prevalence was observed in the large-scale farming mode at 40.9% (95% CI: 26.4–66.3).
Conclusion: The study highlights the spread of JE across China and suggests that it may be underrecognized in some areas. Continuous monitoring and improvements in farming practices are essential for controlling the disease.
The farm animals play an essential role in maintaining the global food security (1, 2). They were subjected to different pathogens that affected their productivity (3–5), especially the swine sector is affected by different pathogens (6–9).
Japanese encephalitis (JE) also known as Epidemic encephalitis B (10), is a naturally occurring epidemic caused by the insect-born Japanese encephalitis virus (JEV); a member of the flavivirus group (11, 12) which leads to neurological disorders by affecting the central nervous system of animals (13, 14), and has been classified as a category II of animal diseases in China (15). Because of the disease zoonotic potentiality, the World Health Organization (WHO) recommends human immunization as the most effective means to control the JE (16). Though the disease can occur year-round (17), it shows distinct seasonality, peaking in summer and fall (18). Outbreaks can also be triggered by poor feeding management, unsanitary conditions, and abnormal climate changes (19). The JEV is transmitted by mosquito vectors (20), with birds and bats serving as the primary reservoir hosts. It has a broad host range, including various animal species and humans. Notably, pigs, horses, and humans exhibit observable clinical symptoms, while other infected animals generally do not show significant signs of infection (21). The pigs play a crucial role mainly as amplification hosts during human outbreaks (17, 22, 23). Pigs may exhibit prolonged viremia, lasting from weeks to months, and are susceptible to the disease at any age (24). Infection of sows during gestation period might result in abortion, stillbirth, or give birth to mummified fetuses. While, in boars, infection causes swollen testes, reduced sperm quality, diminished libido, and eventual reproductive failure (25). The main route of infection is through biting of mosquitoes vector; mainly the Culex tritaeniorhynchus, fed on diseased pigs. The virus can survive and replicate within mosquitoes, which then transmit it to other pigs and people through bites (26). Pigs play a crucial role as amplifying hosts in the JE transmission cycle, alongside water birds (27). They can develop viremia sufficient to sustain transmission and are frequently linked to epizootic spillover leading to human JE cases (27). Recent studies have revealed that pigs can shed JEV through multiple routes and maintain persistent infections, suggesting a potential for vector-free transmission among pigs (27, 28). Pigs are primary reservoirs for the JEV, which mosquitoes can transmit to humans. In Mainland China, with the improvement of living standards, the number of pigs is increasing gradually. According to government statistics, in 2014, the number of pigs in Mainland China was estimated as approximately 465,827,000, and pork is commonly consumed by the Chinese population (29). Therefore, pigs are the most important potential source for Japanese encephalitis infection in humans. Surprisingly, the virus can overcome the vector mosquito route and spread between swine herds through highly contagious oro-nasal secretions (30). The virus persists even during winter when mosquito populations are low (31), which complicate the eradication efforts. Consequently, the disease poses a serious threat to the pig farming industry, causing significant economic losses and hindering industry growth in China and globally (32).
The epidemiological situation of the disease varies between the countries but mainly found across East and Southeast Asia, including China, Japan, Korea, India, Thailand, and Vietnam (33). The causative agent can infect multiple host species including equine and swine. The JEV P3 strain was first isolated in China in 1949 and remained endemic for the next 60 years (34). Mosquito species are the primary vectors of this virus, while pigs are the main reservoirs that promote the transmission of JEV from animals to humans (26, 35). However, China has a vast hog farming industry. According to statistics, the number of pigs farrowed reached 735.1 million in 2014 (36). In 2015, 624 human cases of JE were reported in China, 19 of which were fatal (26). Furthermore, the JEV has become a major pathogen causing reproductive disorders in pigs, leading to severe economic losses (32), making it also a potential threat to human health (24).
To our knowledge, no comprehensive systematic analysis of the overall prevalence of this disease has been conducted in China. Thus, this systematic review and meta-analysis aimed to examine the prevalence of JE in Chinese swine herds and assess potential risk factors: including time of sampling, area of sample collection, testing method, and type of samples, in addition to the evaluation of raw data from the included studies, geographic factors such as longitude, latitude, elevation, rainfall, humidity, temperature, and climate conditions were examined to determine their relationship to the prevalence of the disease.
This study followed the PRISMA guidelines (Supplementary Table S1) (37, 38). Literature related to porcine JE was retrieved from six databases, including PubMed, ScienceDirect, Web of Science, CNKI, Wan Fang Data Knowledge Service Platform, and Wipro Chinese Journal Database. We reviewed all national literature on porcine JE published between January 1, 2000, and May 8, 2024, with sampling dates from 1997 to 2021.
The following formulas and MeSH terms were used in PubMed “Swine,” “Pig,” “Encephalitis, Japanese” and “China” were used in PubMed. Boolean operators “AND” were used to connect MeSH terms and “OR” to connect the entry terms.
In ScienceDirect, we searched for “Prevalence,” “Swine,” “Japanese B Encephalitis,” and “China.” In Web of Science, “Japanese B Encephalitis,” “Swine,” and “Prevalence” were used as keywords. In three Chinese databases, “liuxingxingyixingnaoyan (in Chinese)” and “zhu (in Chinese)” or “yixingnaoyan (in Chinese)” and “zhu (in Chinese)” were used to search with fuzzy search and synonym expansion in advanced searches. Detailed search formulas were provided in Supplementary Table S2. Retrieved articles were sorted and screened with Endnote X21 (version 21.2.0.17387).
Studies were included if they met the following criteria: (1) Study subjects must be pigs; (2) The objective must be to assess the prevalence of JE infection; (3) Data must include the total number of pigs tested and those testing positive; (4) The study must be conducted in China; (5) The study design must be cross-sectional; (6) The study must be published in Chinese or English. (7) The pigs must be naturally infected. Studies not meeting these criteria were excluded. Duplicate studies and review articles (non-research papers) were also excluded.
Four reviewers utilized a standardized data collection form to extract data for the meta-analysis (39). Discrepancies between reviewers or uncertainties regarding study quality were resolved by the lead author. The extracted data included: first author, sampling year, publication year, sample type, geographic area, province, latitude and longitude, elevation, mean annual temperature, humidity, max/min temperature, max daily precipitation, climate, testing method, age, sex, season of collection, feeding method, mode of swine husbandry, total swine samples, and number of positive samples for JE.
The quality of the publications was assessed using a standardized scoring method (40). Each study was evaluated on specific criteria (such as randomized sampling, assay clarity, detailed sampling methods, clear sampling timeframes, and inclusion of four or more relevant factors). Each study received a score from 0 to 5 on a standardized scale.
All calculations, including those related to the prevalence of porcine JE, were conducted using R software (version 4.0.2) using data from multiple studies. The double-arcsine transform (PFT) were selected for rate conversion based on these results and prior research findings (Table 1) (41).
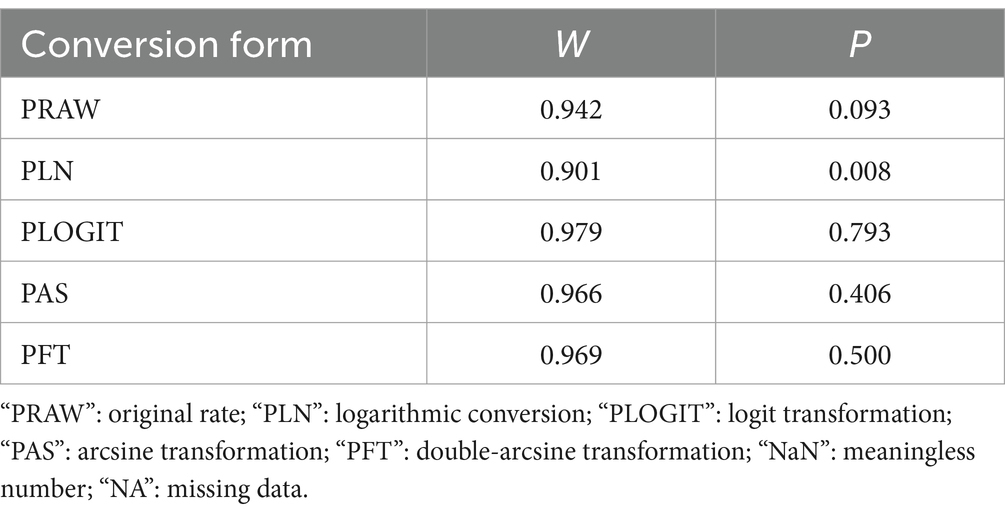
Table 1. Normal distribution test for the normal rate and the different conversion of the normal rate.
The PFT formula is:
Note: t: conversion prevalence; r = positive rate; n = sample size; se = standard deviation.
Forest plots were employed to visualize the results and assess heterogeneity between studies. Heterogeneity was calculated using Cochran’s Q-test and the I2 statistic, with 50% as the critical value for I2. The χ2 test (p < 0.05) was also applied. I2 < 50%indicates low heterogeneity, suggesting that the differences in study results were primarily due to random errors. I2 ≥ 50% indicated high heterogeneity and significant inconsistency between study results, suggesting that other factors may contribute to the observed variations. In such cases, potential factors contributing to heterogeneity require further investigation. These methods were applied to assess the statistical significance of heterogeneity in the selected studies. When heterogeneity was evident, a random-effects model was employed for meta-analysis (42). Publication bias was evaluated with funnel plots, the trim-and-fill method, and Egger’s test. Studies suggested that different subgroups may produce varying funnel plots due to changes in prevalence over time (36). Thus, each subgroup was further evaluated through funnel plots and forest plots. Sensitivity analyses were conducted to determine if any single study significantly impacted the overall estimates (43).
Heterogeneity is a critical metric in meta-analyses; thus, accurate assessing is essential to identifying key factors for preventing JE infection in pigs nationwide. To explore potential sources of heterogeneity, subgroup analyses and univariate regression were employed to identify its predictors. The factors assessed included; geographic region (Northeast vs. other regions), sampling period (post–2015 vs. pre–2010 and 2010–2015), assay method (PCR vs. ELISA, RT-RAA, LAT), season (autumn vs. spring, summer, winter), sex (boars vs. sows), age classification (nursery pigs vs. Weaned piglets and fattening pigs), sample type (serum vs. organization, brain tissue, blood), feeding system (large-scale vs. free-range), and study quality (high-quality vs. medium-quality studies). To further explore other potential sources of heterogeneity, we further assessed their geographic factors, in groups, which included longitude, latitude, elevation, rainfall, humidity, and climate.
This meta-analysis adhered to the PRISMA guidelines (Supplementary Table S1) (37, 38, 44). Correlations were analyzed for each subgroup based on testing method and region to identify heterogeneity sources. Heterogeneity in covariates was quantified using the R2 statistic. This meta-analysis lacked a review protocol and was not registered with the Cochrane Database. The R codes for this meta-analysis are available in Supplementary Table S3.
A total of 481 studies were identified from six databases. A meta-analysis was performed on 31 studies that met the inclusion and exclusion criteria (Figure 1). Among the included studies, five had quality scores between 4 and 5, 26 scored between 2 and 3, and none scored between 0 and 1.
We assumed a random-effects model because there was apparent heterogeneity in the studies (I2 = 100%, p = 0). The extent of publication bias was assessed and illustrated by a funnel plot (Figure 2). The Egger’s test (p < 0.05) revealed that, there was publication bias (p = 0.8732, Figure 3). The heterogeneity results were shown by the forest plot (Figure 4). The result of the trim and filled analysis showed that, no trimming was performed, and no data was changed, which meant there may be no significant publication bias. Therefore, our pooled estimates were relatively robust (p = 0, Figure 5; Supplementary Tables S3, S4). The publication bias should be interpreted with caution because of the inconsistency in the results.
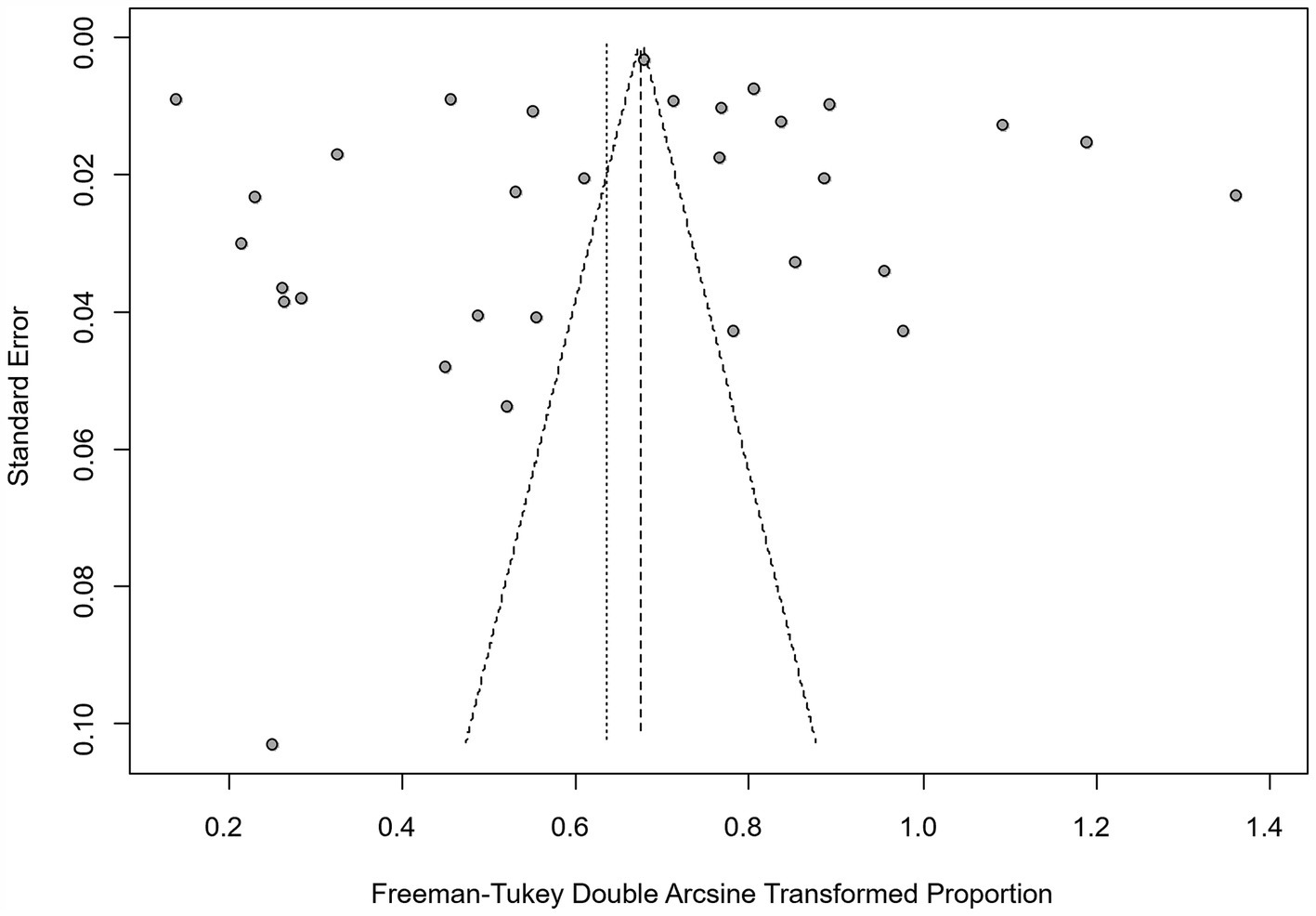
Figure 2. Funnel plot with pseudo 95% confidence interval limits for the examination of publication bias.
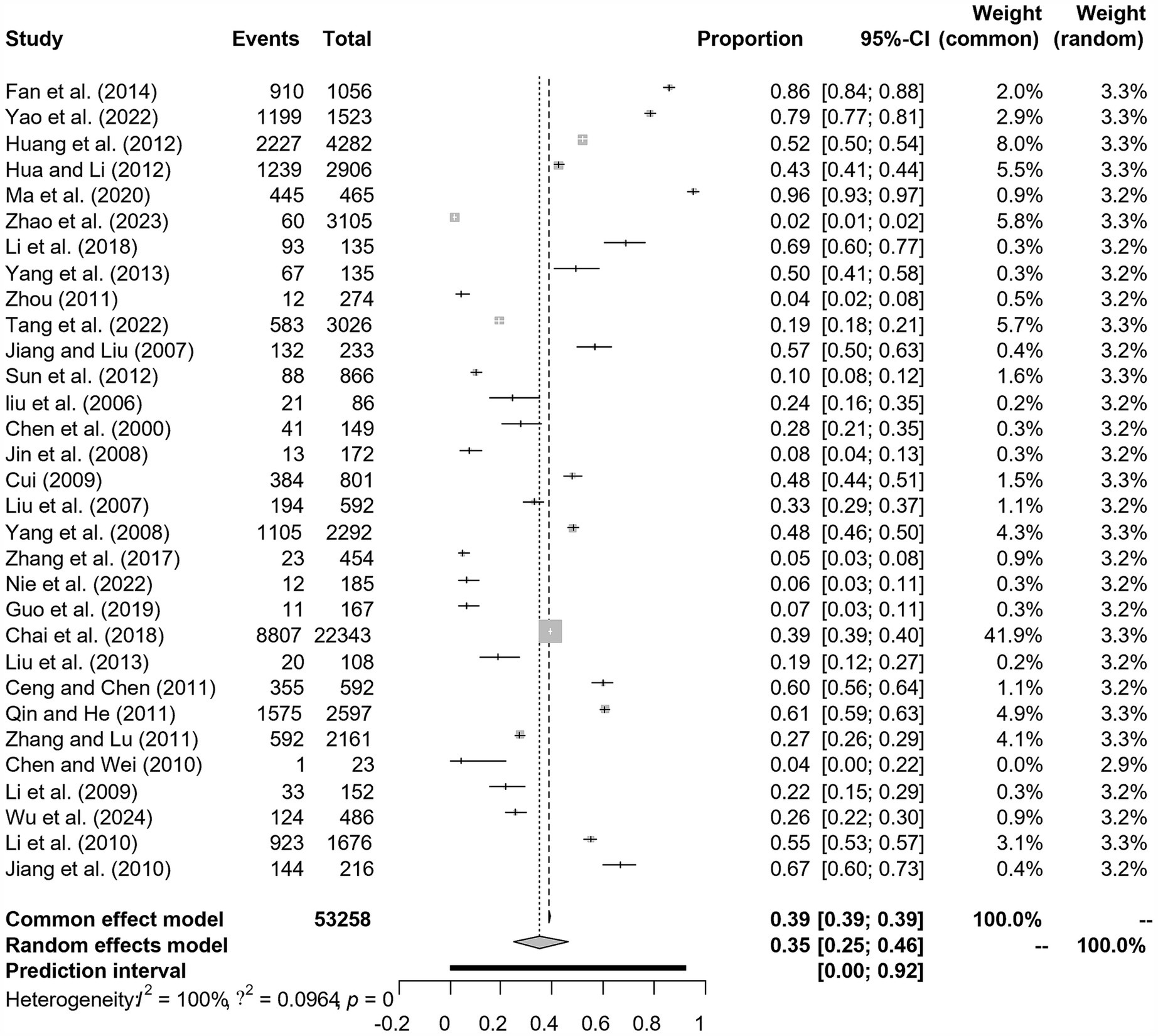
Figure 4. Forest plot of prevalence of epidemic encephalitis B in pig amongst studies conducted in China.
Sensitivity analyses showed that, excluding any single study did not change the overall results, which remained consistent with prior analyses (Figure 6). Therefore, the findings of this review and meta-analysis were robust and reliable.
In China, all provinces showed a high prevalence of JE, except for Qinghai, Tibet, and Xinjiang, which were unaffected regions (45). Our meta-analysis covered seven geographic subregions: East China, South China, North China, Central China, Southwest China, Northwest China, and Northeast China. The overall prevalence of JE in the national swine population was 35.2% (95% CI: 25.1–46.1; Table 2). South China had the highest prevalence among regions at 43.8% (95% CI: 21.6–67.4; Table 2) (Supplementary Figure S1). Jiangxi Province had the highest prevalence at 86.0% (95% CI: 24.8–100.0; Table 3) (Supplementary Figure S10), followed by Chongqing Municipality at 77.4% (95% CI: 71.1–83.2; Table 3) (Supplementary Figure S10).
In this study, subgroup analyses were conducted based on sampling time, region, season, testing method, age, province, sex, breeding mode, sample type, and quality score. Sampling time, region, testing method, and sample type were identified as significant risk factors for JEV infection in pigs (p < 0.05, Table 2). The prevalence of JE was 63.4% (95% CI: 44.2–80.6; Table 2) (Supplementary Figure S2) with studies conducted between 2010 and 2015 were higher than other periods. The infection rate in South China was 43.8% (95% CI: 21.6–67.4; Table 2) (Supplementary Figure S1), which was higher than the other regions. While the lowest rate in the northeast was recorded in 7.4%, (95%CI: 0.3–21.9; Table 2). In the climate subgroup, the prevalence in temperate monsoon climates was 12.7% (95% CI: 5.7–21.9; Table 2) (Supplementary Figure S19) compared to 5.1% (95% CI: 3.2–7.3; Table 2) (Supplementary Figure S19) in highland alpine regions. Within the testing method subgroup, the prevalence using ELISA was 38.2% (95% CI: 24.5–52.9; Table 2) (Supplementary Figure S4), while RT-RAA had the lowest prevalence rate in 6.5% (95%CI: 3.3–10.5; Table 2) (Supplementary Figure S4). The prevalence among samples tested as serum was 38.1% (95% CI: 27.9–48.9; Table 2) (Supplementary Figure S3). The prevalence of porcine JE in the assay method subgroups ranged from 38.2% (95% CI: 24.5–52.9; Table 2) to 6.5% (95% CI: 3.3–10.5). Among all sample types, serological testing samples had the highest prevalence of 38.1% (95% CI: 27.9–48.9; Table 2), whereas tissue samples had the lowest prevalence (4.4, 95% CI: 0.0–17.7; Table 2). In the seasonal subgroups, winter had the highest prevalence of 51.3% (95% CI: 13.6–88.2; Table 2) and autumn had the lowest prevalence of 23.8% (95% CI: 5.4–49.3; Table 2) (Supplementary Figure S6). Among the age subgroups, fattening pigs were more affected, with a prevalence of 49.7% (95% CI: 29.8–69.7; Table 2), meanwhile nursery pigs had the lowest prevalence of 31.2% (95% CI: 14.6–50.8) (Supplementary Figure S8). Among the sex subgroups, the prevalence was higher in saws (50, 95%CI: 26.8–73.3; Table 2) than in boars (40.6, 95%CI: 19.4–63.7; Table 2) (Supplementary Figure S7). Among the different farming modes, the positive detection rate was significantly higher in mass culture (41.0, 95% CI: 26.4–56.3; Table 2) than in free-range mode (35.8, 95% CI: 14.9–59.7; Table 2) (Supplementary Figure S5). In the quality score subgroup, the prevalence of score 3–4 (35.1, 95% CI: 24.2–46.9; Table 2) was higher than 0–2 (29.3, 95% CI: 11.4–51.2) (Supplementary Figure S9).
In addition, geographic factors were analyzed to further investigate the risk factors for the prevalence of porcine JE, such as latitude, longitude, rainfall, altitude, climate, and temperature variation. In the northern latitude subgroup, the highest prevalence was found at 20–30 degrees north latitude (44.8, 95% CI: 32.4–57.4; Table 4), whereas the lowest prevalence was found at 40–50 degrees north latitude (10.9, 95% CI: 10.1–11.7; Table 4). In the east longitude subgroup, the prevalence was higher in the 90–110 degree longitude range compared to the other two groups (49.5, 95% CI: 48.7–50.4; Table 4) (Supplementary Figure S12). In the altitude subgroup, the prevalence of positive detections was higher in the altitude range 0–1,000 (47.1, 95% CI: 25.8–68.9; Table 4) than in the range 4,000–15,000 (21.7, 95% CI: 6.2–43.1; Table 4) (Supplementary Figure S13). The highest positive detection rate was observed at rainfall levels of 150–200 (63.7, 95% CI: 17.1–98.1; Table 4) compared to 0–50 (24.9, 95% CI: 10.4–43.1; Table 4) (Supplementary Figure S14), and the highest prevalence rate was observed in the humidity subgroups of 75–85% at 46.6% (95% CI: 45.9–47.3; Table 4), while the lowest prevalence was observed at 40–65% (12.4, 95% CI: 4.5–23.2; Table 4) (Supplementary Figure S15). In the temperature subgroup, the highest prevalence of 53.7% (95% CI: 34.7–72.2; Table 4) was observed when the temperature reached 20–25°C, while the lowest prevalence of 14.8% (95% CI: 5.7–27.2; Table 4) (Supplementary Figures S16–118) was observed when the temperature was ranged from 0 to 10°C. The prevalence was highest when the temperature reached 20–25°C, while the lowest prevalence was observed when the temperature was 0–10°C (95% CI: 5.7–27.2; Table 4) (Supplementary Figures S16–118).
Heterogeneity across subgroups was explained by the assay method (covariate) (range 0–79.25%; R2-method) and geographic region (covariate) (range 60.97–97.04%; R2-country).
Porcine JE is a zoonotic infectious disease, that affects both humans and animals. Geographically, it is endemic in regions in the Far East, South, and Southeast Asian countries (46, 47) including South Korea, Thailand, Java (Indonesia), and the Primrosy region of Siberia (Russia), and in Kerala, and Haryana, India (48, 49). Recently, cases of JE have also been reported in mainland Australia, Guam, and USA (50). Surprisingly, the morbidity and mortality rates due to infection with JE have increased in China, except in Northern, Northeast China, Qinghai, Xinjiang, and Tibet. Meanwhile, the prevalence of JEV is rising globally in endemic areas, posing a serious threat to public health and the livestock industry (51). Pigs are intermediate hosts for the JEV, whereas humans are the final hosts, and the infected carrier pigs are the primary source of transmission. Clinically, the disease leads to abortion, stillbirth, mummified fetuses in sows, and testicular inflammation in boars (25). This is clearly reflecting the expanding range of the disease’s endemicity, and posing a growing public health concern (32).
To our knowledge, this is the first meta-analysis on the prevalence of porcine JE in China. The findings of this study could inform actionable control measures to improve animal husbandry practices. The analysis of the obtained results revealed, significant variations in the prevalence of JE in pigs across regions, sampling periods, and breeding practices (p < 0.05). The national swine population showed an overall prevalence rate of 35.2% for JE (Table 2). At the regional level, the South China showed a high significant (p < 0.05) prevalence (43.8, 95% CI: 21.6–67.4; Table 2) compared to the other regions (Table 2). Also, Jiangxi Province had the highest prevalence, followed by Chongqing Municipality (Table 2). Both provinces are located within the subtropical monsoon climate zone, and characterized by hot summers, mild winters, four distinct seasons, and a well-developed monsoon pattern, all of which are likely to influence the spreading of the disease. Numerous analyses have indicated that the incidence of JEV infection has a seasonal pattern and closely related to geographical distribution, and climate (19). Study in southwest China found significant associations between JE incidence and agricultural and climatic variables, including monthly precipitation and monthly mean minimum and maximum temperatures (52). This climate provides favorable conditions for its spread. The region’s average annual temperature ranges from 20°C to 25°C. This warm climate promotes the reproduction and transmission of vector organisms, such as mosquitoes. Consequently, swine populations in the subtropical region face a higher risk of infection, leading to elevated prevalence rates. Furthermore, the higher elevations, cold and arid climate, and low annual precipitation in the western region are unfavorable conditions for mosquito survival and reproduction, leading to weaker transmission of JE. Meanwhile, the low elevation, abundant plains, high precipitation, and vegetation of South China create optimal conditions for mosquito proliferation, thereby facilitating the local spreading of JEV (53–55).
The prevalence of JE between 2010 and 2015 was 63.4%, that was higher than in other periods. A total of 858 pig serum samples from both large-scale and rural free-range farms in Longyan City, Fujian Province, were tested for JEV antibody levels between 2011 and 2014. The elevated JE prevalence from 2010 to 2015 was influenced by several factors. A substantial research has consistently demonstrated a significant positive correlation between increasing temperatures and both the proliferation of mosquito populations and elevated incidence of mosquito-borne diseases (56). Average annual precipitation of 100–150 millimeters and temperatures between 15 and 20°C fostered mosquito proliferation, correlated positively with JE incidence and leading to a rise in in infected cases. Distinct climatic subtypes within temperate regions showed varying JE prevalence patterns. In Gansu Province, China, the cases appeared in a temperate arid climate, indicating a possible spread to new areas (57). In temperate zones, the disease transmission is typically epidemic and seasonal, with most cases occurring during summer months (58). This contrasts with subtropical and tropical regions where transmission can occur year-round, peaking during the rainy season (58). The seasonal nature of JE in temperate areas limits the overall prevalence compared to regions with continuous transmission (59). Serological testing revealed that, the prevalence of JEV in immunized pigs from large-scale and free-range farms were 72.17 and 57.72%, respectively. In comparison, the seropositivity rate in immunized pigs was 69.71%, slightly higher than the 68.89% in unimmunized pigs (60). Significant differences were observed between the two cases, and due to the divergent objectives of the studies, investigations involving immunized pigs were excluded from our analysis, while only studies utilizing non-immunized pigs were included. The JE remains a serious concern in Fujian Province and requires continued attention. One of the included articles showed that, 78 porcine JEV nucleic acids were detected in 263 samples collected from 14 different swine farms in the south from 2011 to 2018, with a positivity rate of 29.7% (61). The emergence of this cause may be due to the location in the tropics and subtropics, where the warm and humid climate, the high density of mosquitoes, and the large number of domestic pigs provide the natural conditions for the spread and reproduction of JEV (22).
Various methods have been used in epidemiological studies of JEV, including virus isolation, RT-PCR, RT-qPCR, and microdroplet digital PCR (ddPCR) (13). Virus isolation is a time-consuming, labor-intensive process that often taking over a week to complete, this limits its use in large-scale epidemiologic investigations. The serum neutralization test (SNT) is the standard method for serological detection of JEV, but cross-reactivity between the different flaviviruses within the same genus was recorded using this tool which reflects the inaccurate results (62). On the other side, the previously mentioned molecular techniques usually take 2–3 h for completion (63–65). False positivity varies depending on the used tools and could affect the accurate estimation of the disease prevalence. Accordingly, four major detection methods for JE were usually applied including; ELISA, PCR, RT-RAA, and LAT. ELISA is a fundamental technique in immunology and molecular biology, utilizing antigen–antibody binding with enzymatic and colorimetric assays for quantitative analysis of target molecules. It detects and quantifies specific proteins, peptides, antibodies, or antigens in biological samples, making it essential in research and diagnostics (66). This technique is extensively used to detect antibodies and antigens for diagnosing and JEV monitoring but is prone to cross-reactivity with other flaviviruses like yellow fever virus, which can lead to false results and affect prevalence estimates. To address this issue more effectively, it is suggested to develop more specific detection methods for antigen, including secondary screening alongside PCR assays or alternative immunological detection techniques in future studies to mitigate the impact of cross-reactivity. PCR utilizes the semi-conservative replication of DNA for in vitro enzymatic synthesis and amplification of specific nucleic acid sequences. The specificity of this technique is achieved through the utilization of oligonucleotide primers complementary to the flanking regions of the target sequence (67). RT-PCR involves the conversion of mRNA into cDNA utilizing reverse transcriptase, which subsequently serves as the template for amplifying the target fragment. The RNA template employed in this procedure may comprise total RNA, mRNA, or in vitro transcribed RNA (68). LAT is an indirect agglutination assay using latex particles as carriers. Soluble antigens are adsorbed on these particles, allowing specific antibodies to bind and promote agglutination (69). It was found that, ELISA was significantly (p = 0.0056, Table 5) the commonly used tool. It offers several advantages, including rapidity, high efficiency, low cost, specificity, high sensitivity, simplicity, and no need for high aseptic procedures. Also, it enables the simultaneous testing of multiple serum samples (70). Given the large pig population, rapid turnover, and high infection rates of JEV in the country (71), the specificity, reproducibility, and operational simplicity of ELISA render it an optimal method for the detection of porcine JE antibodies due to infection adding to the evaluation of antibody titers following immunization (70). It is noteworthy that, some studies did not explain whether the pigs had been immunized with swine JE vaccine or not. So, false-positive results contribute to heterogeneity in the results (72).
It was recorded that; Pigs are one of the main hosts of JEV (73, 74). The prolonged viremia in the blood of pigs infected with the JEV, characterized by high viral loads and infectiousness, which could be the main source of human infection (35). Once the virus enters the host, it rapidly invades the bloodstream and replicates in internal organs such as (heart, liver, spleen, kidneys), causing brief viremia that lasts 3–7 days. The virus can cross the blood–brain barrier, invade the central nervous system, and replicate in brain tissue, causing lesions and neurological symptoms (75, 76). In the present study, our analysis of various sample types showed that serum had a higher detected prevalence compared to other tissues. Analysis of JEV serum data from Chinese swine herds showed that the prevalence and distribution of JEV in pigs also exhibited seasonal and geographic variation; JEV infections appeared 1–2 months earlier in southern China than in northern parts (26). These characteristics not only allow pigs to play an important role in the JEV transmission chain, but also provide a warning to the public health community that pigs are potential reservoirs of viruses that may directly or indirectly infect humans, especially if they have high viral loads in their blood with the ability to cross the blood–brain barrier, enter the central nervous system and replicate in brain tissue (77), causing neurological lesions that lead to clinical manifestations such as neurological symptoms, meningitis, encephalitis, and other serious diseases (11, 78).
Immunization greatly affects disease incidence in pig populations. Significant emphasis was placed on rigorous screening of unvaccinated pig herds, excluding articles that did not specify immunized populations and antibody protection rates. All included studies came from large-scale farms and free-range herds with unvaccinated pigs. According to the World Health Organization, the vaccine currently used for JE is the SA14-14-2 strain (79), and studies have shown vaccine efficacy to be between 80 and 99% after a single dose and 98% or higher after two doses (80). Therefore, for studies that did not explicitly state whether the subjects had been vaccinated, when the seropositive rate of pigs exceeded 90%, we considered the herd to be immune. For studies that did not explicitly state whether subjects had been immunized, we assumed that the seropositivity rate among pigs exceeded 90%, as vaccinated pigs generate antibodies, resulting in a higher antibody positivity rate. Through rigorous screening, we minimized immune factor confounding to accurately analyze the JE prevalence.
Surprisingly, the infection rate was higher in winter than in other seasons, though the difference was not statistically significant (Table 2). The incidence and prevalence of the disease show clear seasonality, typically peaking from July to September, then sharply declining after October. The disease is usually sporadic but can also become endemic (14). In our study, the phenomenon of higher prevalence in winter may be related to the regions included in the study. Especially in Hainan, Guangdong, and Yunnan provinces, which have warmer climates with insignificant seasonal variations, mosquitoes are active throughout the year. Therefore, even in winter, the mosquito population remains high, leading to higher infection rates in that season, which in turn may have influenced the bias of the study results. This disease peaks in prevalence during China’s rainy summer and autumn. Epidemic peaks occur from June to July in southern regions, from July to August in northern regions, and from August to September in northeastern regions. For instance, irrigated rice fields provide ideal breeding grounds for Culex tritaeniorhynchus, the primary vector for JEV transmission (81). Variations in environmental conditions and temperatures affect mosquito activity, leading to distinct disease transmission patterns across different areas (82). The increased precipitation during the summer and fall seasons creates more favorable breeding conditions for mosquitoes, resulting in a substantial increase in both of their population density and activity levels (57). As a consequence, this exacerbates the transmission of JEV. In areas with intensive rice farming and pig production, JE transmission is likely to increase due to the creation of suitable environments for vector mosquitoes and amplifying hosts (19). Studies indicate that tropical regions lack seasonality, allowing the disease to occur year-round (83). Interestingly, the same observation of high incidence rate was recorded in winter compared to the other seasons but with a different insect-born pathogen (2, 11).
The epidemiology of porcine JE is mainly driven by mosquito as the primary virus vector (84). It has a well-defined transmission route, mainly through mosquito bites, so mosquito control is a key measure to prevent disease transmission. In areas where the climate is more stable and mosquitoes are active throughout the year, especially in tropical and subtropical areas, prevention and control strategies for epidemics should focus on strengthening herd management and immunization (15). However, swine JE lacks specific antiviral treatments, so management relies on supportive care and immune enhancement. Prevention involves immunization, vector control, and managing pig populations (85). Live JEV vaccines are recommended in endemic or high-risk regions. Since JE transmission is linked to blood-feeding arthropods like mosquitoes, controlling these vectors by the different tools is crucial for prevention (86).
Our meta-analysis included five studies with quality scores of 4 or 5, 26 studies with scores of 2 or 3, and none with scores of 0 or 1. Our review for the moderate-quality studies revealed that several detailed descriptions of seasons, random sampling methods, and sampling procedures were lacked. Neglecting of seasonal factors may lead to seasonal bias in epidemiologic results, especially for those diseases that are strongly influenced by climatic and environmental changes, and the lack of seasonal descriptions will limit the accuracy and extrapolation of results. Lack of random sampling or poor description may then lead to sample selection bias, making the results of the study unable to truly reflect the characteristics of the target group, thus affecting the reliability and scientific value of the results. In addition, unclear details of the sampling method may lead to reduced comparability across studies, thus affecting the accuracy of meta-analyses. Therefore, it is recommended that, future researchers in the future should cover these shortages to improve the reliability of their findings. This study used regression analysis to investigate factors affecting JE spreading, identifying a significant correlation between sample size and JE prevalence. However, the analyses did not account for all potential confounding variables. Future research should include more covariates to improve generalizability and establish stronger causal relationships.
This meta-analysis has several strengths, including a broad temporal range, extensive geographic coverage, and well-defined analytical methods, but also some limitations were present. Firstly, the selected articles were limited to Chinese or English, potentially excluding relevant studies in other languages. Secondly, the articles were sourced from six databases only, which may have excluded relevant studies from other sources. Lastly, the study concentrates on specific Chinese provinces, underrepresenting regions like Qinghai, Tibet, and Xinjiang. This limited representation may impact findings and compromise external validity and robustness. Future studies should adopt a more comprehensive sampling approach, especially in underrepresented western provinces, to better assess national prevalence.
The current meta-analysis showed that the prevalence of JE infection in swine is widely distributed across China. Additionally, the disease is more prevalent in regions with consistently hot and humid climates. Thus, we recommend continuous surveillance of swine populations and implementing isolation measures to reduce mosquito contact with herds. Furthermore, awareness of JE should be raised in regions where the disease receives less attention, and epidemiological investigations should be promptly conducted to ensure timely control of its spread. The high prevalence of this disease swine can cause significant economic losses for farmers and herdsmen adding to increasing the risk of infection. Therefore, attention to animal welfare and application of all precaution measures to limit the spread of JE is crucial for intensive pig farming. This study lays a foundation for future research on strategies to control JE.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
X-TL: Writing – original draft, Writing – review & editing, Software, Visualization. L-DJ: Data curation, Writing – review & editing, Formal analysis. Y-TL: Data curation, Writing – review & editing, Formal analysis. RZ: Formal analysis, Writing – review & editing. QW: Methodology, Writing – review & editing, Visualization. S-YZ: Conceptualization, Funding acquisition, Writing – review & editing. EA: Writing – review & editing, Investigation. XiL: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. Z-XL: Investigation, Writing – review & editing. CX: Investigation, Writing – review & editing. YX: Investigation, Writing – review & editing. Y-FW: Investigation, Writing – review & editing. XuL: Writing – review & editing, Investigation, Supervision. Q-LG: Conceptualization, Writing – review & editing, Methodology, Software. RD: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by Jilin Province Science and Technology Development Project (20240304190SF).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1534114/full#supplementary-material
1. Niu, TM, Yu, LJ, Zhao, JH, Zhang, RR, Ata, EB, Wang, N, et al. Characterization and pathogenicity of the porcine epidemic diarrhea virus isolated in China. Microb Pathog. (2023) 174:105924. doi: 10.1016/j.micpath.2022.105924
2. Ata, EB, Abdel-Aziz, TH, Abdel-Ghany, HSM, Elsawy, BSM, Abdullah, H, Abouelsoued, D, et al. Molecular and serological diagnosis of the circulating Trypanosoma evansi in Egyptian livestock with risk factors assessment. Microb Pathog. (2024) 197:107073. doi: 10.1016/j.micpath.2024.107073
3. Kasem, S, Yu, MHH, Alkhalefa, N, Ata, EB, Nayel, M, Abdo, W, et al. Impact of equine herpesvirus-1 ORF15 (Eul45) on viral replication and neurovirulence. Vet Microbiol. (2024) 298:110234. doi: 10.1016/j.vetmic.2024.110234
4. Ibrahim, HS, Alsenosy, AA, El-Ktany, EM, Ata, EB, and Abas, OM. Anthelmintic efficacy and pharmacodynamic effects of levamisole-oxyclozanide combination as (Levanide®) in fattening calves. Egypt J Vet Sci. (2023) 54:1245–54. doi: 10.21608/ejvs.2023.219811.1532
5. Shalaby, H, Kandil, O, Hendawy, S, Elsawy, BS, Ashry, HM, El-Namaky, A, et al. Dynamics of Haemonchus contortus coproantigen appearance in feces of experimentally infected sheep. Egypt J Vet Sci. (2024) 55:1307–14. doi: 10.21608/ejvs.2024.251684.1693
6. Ata, EB, Li, ZJ, Shi, CW, Yang, GL, Yang, WT, and Wang, CF. African swine fever virus: a raised global upsurge and a continuous threaten to pig husbandry. Microbe Pathog. (2022) 167:105561. doi: 10.1016/j.micpath.2022.105561
7. Hu, TY, Lian, YB, Qian, JH, Yang, YL, Ata, EB, Zhang, RR, et al. Immunogenicity of engineered probiotics expressing conserved antigens of influenza virus and FLIC flagellin against H9N2 AI infection in mice. Res Vet Sci. (2022) 153:115–26. doi: 10.1016/j.rvsc.2022.10.024
8. Sha, W, Beshir Ata, E, Yan, M, Zhang, Z, and Fan, H. Swine colibacillosis: analysis of the gut bacterial microbiome. Microorganisms. (2024) 12:1233. doi: 10.3390/microorganisms12061233
9. Yang, W-T, Yang, W, Jin, Y-B, Ata, EB, Zhang, R-R, Huang, HB, et al. Synthesized swine influenza NS1 antigen provides a protective immunity in a mice model. J Vet Sci. (2020) 21:e66. doi: 10.4142/jvs.2020.21.e66
10. Hao, Y, Sheng, K, and Ruan, WK. Expression of non-structural proteins in Japanese encephalitis virus and their interaction with host hnRNP K in Chinese. College of Animal Science and Technology, Beijing University of Agriculture (2024), 39, 37–42. doi: 10.13473/j.cnki.issn.1002-3186.2024.0108
11. Ashraf, U, Ding, Z, Deng, S, Ye, J, Cao, S, and Chen, Z. Pathogenicity and virulence of Japanese encephalitis virus: neuroinflammation and neuronal cell damage. Virulence. (2021) 12:968–80. doi: 10.1080/21505594.2021.1899674
12. Wang, LP, Yuan, Y, Liu, YL, Lu, QB, Shi, LS, Ren, X, et al. Etiological and epidemiological features of acute meningitis or encephalitis in China: a nationwide active surveillance study. Lancet Reg Health West Pac. (2022) 20:100361. doi: 10.1016/j.lanwpc.2021.100361
13. Nie, M, Zhou, Y, Li, F, Deng, H, Zhao, M, Huang, Y, et al. Epidemiological investigation of swine Japanese encephalitis virus based on RT-RAA detection method. Sci Rep. (2022) 12:9392. doi: 10.1038/s41598-022-13604-4
14. Li, F, Li, H, Yang, L, Wang, L, Gu, L, Zhong, G, et al. The spatial-temporal pattern of Japanese encephalitis and its influencing factors in Guangxi, China. Infect Genet Evol. (2023) 111:105433. doi: 10.1016/j.meegid.2023.105433
15. Wang, Q, Yang, S, Yang, K, Li, X, Dai, Y, Zheng, Y, et al. CD4 is an important host factor for Japanese encephalitis virus entry and replication in PK-15 cells. Vet Microbiol. (2023) 287:109913. doi: 10.1016/j.vetmic.2023.109913
16. Paul, KK, Sazzad, HMS, Rahman, M, Sultana, S, Hossain, MJ, Ledermann, JP, et al. Hospital-based surveillance for Japanese encephalitis in Bangladesh, 2007–2016: implications for introduction of immunization. Int J Infect Dis. (2020) 99:69–74. doi: 10.1016/j.ijid.2020.07.026
17. Impoinvil, DE, Baylis, M, and Solomon, T. Japanese encephalitis: on the one health agenda. Curr Top Microbiol Immunol. (2013) 365:205–47. doi: 10.1007/82_2012_243
18. De, Y, Zou, WZ, and Liu, H. Overview of porcine epidemic encephalitis B and its prevention and treatment in pigs. Chinese Livestock Poultry Breed Chinese. (2022) 18:138–40.
19. Erlanger, TE, Weiss, S, Keiser, J, Utzinger, J, and Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. (2009) 15:1–7. doi: 10.3201/eid1501.080311
20. Ricklin, ME, García-Nicolás, O, Brechbühl, D, Python, S, Zumkehr, B, Nougairede, A, et al. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat Commun. (2016) 7:10832. doi: 10.1038/ncomms10832
21. Liu, QL, Jian, WX, Shi, CQ, Xia, ZH, Yang, GY, Hong, J, et al. Monitoring immune antibodies against Japanese encephalitis in pigs from a large-scale farm in Yuping County, Guizhou Province from 2020 to 2022 (in Chinese). Animals Breed Feed. (2024) 23:75–8. doi: 10.13300/j.cnki.cn42-1648/s.2024.11.016
22. Van den Hurk, AF, Ritchie, SA, and Mackenzie, JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. (2009) 54:17–35. doi: 10.1146/annurev.ento.54.110807.090510
23. Simpson, DI, Smith, CE, Marshall, TF, Platt, GS, Way, HJ, Bowen, ETW, et al. Arbovirus infections in Sarawak: the role of the domestic pig. Trans R Soc Trop Med Hyg. (1976) 70:66–72. doi: 10.1016/0035-9203(76)90010-9
24. Wang, H, Li, Y, Liang, X, and Liang, G. Japanese encephalitis in Mainland China. Jpn J Infect Dis. (2009) 62:331–6.
25. Mansfield, KL, Hernández-Triana, LM, Banyard, AC, Fooks, AR, and Johnson, N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol. (2017) 201:85–92. doi: 10.1016/j.vetmic.2017.01.014
26. Chai, C, Wang, Q, Cao, S, Zhao, Q, Wen, Y, Huang, X, et al. Serological and molecular epidemiology of Japanese encephalitis virus infections in swine herds in China, 2006–2012. J Vet Sci. (2018) 19:151–5. doi: 10.4142/jvs.2018.19.1.151
27. Park, SL, Huang, YS, and Vanlandingham, DL. Re-examining the importance of pigs in the transmission of Japanese encephalitis virus. Pathogens. (2022) 11:575. doi: 10.3390/pathogens11050575
28. Lyons, AC, Huang, YS, Park, SL, Ayers, VB, Hettenbach, SM, Higgs, S, et al. Shedding of Japanese encephalitis virus in oral fluid of infected swine. Vector Borne Zoonotic Dis. (2018) 18:469–74. doi: 10.1089/vbz.2018.2283
29. Zhang, XX, Ren, WX, Tan, QD, Hou, GY, Fei, YC, Zhao, LJ, et al. Meta-analysis of toxoplasma gondii in pigs intended for human consumption in Mainland China. Acta Trop. (2019) 198:105081. doi: 10.1016/j.actatropica.2019.105081
30. Banerjee, S, Sen Gupta, PS, and Bandyopadhyay, AK. Insight into SNPs and epitopes of E protein of newly emerged genotype-I isolates of JEV from Midnapur, West Bengal, India. BMC Immunol. (2017) 18:13. doi: 10.1186/s12865-017-0197-9
31. Ning-Qing, C. Control of arboviral encephalitis in China (Author's Transl). Med Trop (Mars). (1980) 40:555–9.
32. Yuan, L, Wu, R, Liu, H, Wen, X, Huang, X, Wen, Y, et al. Tissue tropism and molecular characterization of a Japanese encephalitis virus strain isolated from pigs in Southwest China. Virus Res. (2016) 215:55–64. doi: 10.1016/j.virusres.2016.02.001
33. WHO. Japanese encephalitis vaccines: who position paper, February 2015–recommendations. Vaccine. (2016) 34:302–3. doi: 10.1016/j.vaccine.2015.07.057
34. Li, YX, Li, MH, Fu, SH, Chen, WX, Liu, QY, Zhang, HL, et al. Japanese encephalitis, Tibet, China. Emerg Infect Dis. (2011) 17:934–6. doi: 10.3201/eid1705.101417
35. Weaver, SC, and Barrett, AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. (2004) 2:789–801. doi: 10.1038/nrmicro1006
36. Ni, HB, Gong, QL, Zhao, Q, Li, XY, and Zhang, XX. Prevalence of Haemophiles parasuis "Glaesserella Parasuis" in pigs in China: a systematic review and meta-analysis. Prev Vet Med. (2020) 182:105083. doi: 10.1016/j.prevetmed.2020.105083
37. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
38. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
39. Wang, W, Gong, QL, Zeng, A, Li, MH, Zhao, Q, and Ni, HB. Prevalence of cryptosporidium in pigs in China: a systematic review and meta-analysis. Trans bound Emerg Dis. (2021) 68:1400–13. doi: 10.1111/tbed.13806
40. Ran, X, Cheng, J, Wang, M, Chen, X, Wang, H, Ge, Y, et al. Brucellosis seroprevalence in dairy cattle in China during 2008-2018: a systematic review and meta-analysis. Acta Trop. (2019) 189:117–23. doi: 10.1016/j.actatropica.2018.10.002
41. Barendregt, JJ, Doi, SA, Lee, YY, Norman, RE, and Vos, T. Meta-analysis of prevalence. J Epidemiol Community Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
42. Assefa, A, and Bihon, A. Bovine cysticercosis in Ethiopia: a systematic review and meta-analysis of prevalence from abattoir-based surveys. Prev Vet Med. (2019) 169:104707. doi: 10.1016/j.prevetmed.2019.104707
43. Gong, QL, Li, D, Diao, NC, Liu, Y, Li, BY, Tian, T, et al. Mink Aleutian disease seroprevalence in China during 1981–2017: a systematic review and meta-analysis. Microb Pathog. (2020) 139:103908. doi: 10.1016/j.micpath.2019.103908
44. Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 349:g7647. doi: 10.1136/bmj.g7647
45. Japanese encephalitis surveillance and immunization--Asia and the Western Pacific, 2012. MMWR Morb Mortal Wkly Rep. (2013) 62:658–62.
46. Dhanda, V, Thenmozhi, V, Kumar, NP, Hiriyan, J, Arunachalam, N, Balasubramanian, A, et al. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala in 1996. Indian J Med Res. (1997) 106:4–6.
47. Schuh, AJ, Li, L, Tesh, RB, Innis, BL, and Barrett, AD. Genetic characterization of early isolates of Japanese encephalitis virus: genotype II has been circulating since at least 1951. J Gen Virol. (2010) 91:95–102. doi: 10.1099/vir.0.013631-0
48. Williams, DT, Wang, LF, Daniels, PW, and Mackenzie, JS. Molecular characterization of the first Australian isolate of Japanese encephalitis virus, the Fu strain. J Gen Virol. (2000) 81:2471–80. doi: 10.1099/0022-1317-81-10-2471
49. Nabeshima, T, Loan, HTK, Inoue, S, Sumiyoshi, M, Haruta, Y, Nga, PT, et al. Evidence of frequent introductions of Japanese encephalitis virus from South-East Asia and continental East Asia to Japan. J Gen Virol. (2009) 90:827–32. doi: 10.1099/vir.0.007617-0
50. Sikazwe, C, Neave, MJ, Michie, A, Mileto, P, Wang, J, Cooper, N, et al. Molecular detection and characterization of the first Japanese encephalitis virus belonging to genotype IV acquired in Australia. PLoS Negl Trop Dis. (2022) 16:e0010754. doi: 10.1371/journal.pntd.0010754
51. Sewgobind, S, Johnson, N, and Mansfield, KL. Jmm profile: Japanese encephalitis virus: an emerging threat. J Med Microbiol. (2022) 71. doi: 10.1099/jmm.0.001620
52. Zhao, X, Cao, MQ, Feng, HH, Fan, H, Chen, F, Feng, Z, et al. Japanese encephalitis risk and contextual risk factors in Southwest China: a Bayesian hierarchical spatial and spatiotemporal analysis. Int J Environ Res Public Health. (2014) 11:4201–17. doi: 10.3390/ijerph110404201
53. Li, X, Gao, X, Ren, Z, Cao, Y, Wang, J, and Liang, G. A spatial and temporal analysis of Japanese encephalitis in Mainland China, 1963–1975: a period without Japanese encephalitis vaccination. PLoS One. (2014) 9:e99183. doi: 10.1371/journal.pone.0099183
54. Zheng, Y, Li, M, Wang, H, and Liang, G. Japanese encephalitis and Japanese encephalitis virus in Mainland China. Rev Med Virol. (2012) 22:301–22. doi: 10.1002/rmv.1710
55. Liang, G, Li, X, Gao, X, Fu, S, Wang, H, Li, M, et al. Arboviruses and their related infections in China: a comprehensive field and laboratory investigation over the last 3 decades. Rev Med Virol. (2018) 28:1–21. doi: 10.1002/rmv.1959
56. Wang, J, Ogden, NH, and Zhu, H. The impact of weather conditions on Culex pipiens and Culex restuans (Diptera: Culicidae) abundance: a case study in Peel region. J Med Entomol. (2011) 48:468–75. doi: 10.1603/me10117
57. Wang, L, Hu, W, Soares Magalhaes, RJ, Bi, P, Ding, F, Sun, H, et al. The role of environmental factors in the spatial distribution of Japanese encephalitis in Mainland China. Environ Int. (2014) 73:1–9. doi: 10.1016/j.envint.2014.07.004
58. Aditi, S, Shailendra, K, Saxena,, Srivastava, AK, and Asha, M. Japanese encephalitis: a persistent threat. Proc Natl Acad Sci India Sect B Biol Sci. (2012) 82:55–68.
59. Li, RF, Zhao, XH, Tian, Y, Shi, YJ, Gu, XY, and Wang, S. Different responses of Japanese encephalitis to weather variables among eight climate subtypes in Gansu, China, 2005-2019. BMC Infect Dis. (2023) 23:114. doi: 10.1186/s12879-023-08074-6
60. Li, XH, Wei, CH, Dai, AL, Chen, SY, and Yang, XY. Epidemiological investigation of epidemic encephalitis B in pigs in Longyan city, China in Chinese. Heilongjiang Animal Sci Vet Med. (2017) 2:119–22. doi: 10.13881/j.cnki.hljxmsy.2017.0316
61. Sun, Y, Ding, H, Zhao, F, Yan, Q, Li, Y, Niu, X, et al. Genomic characteristics and E protein bioinformatics analysis of JEV isolates from South China from 2011 to 2018. Vaccines (Basel). 10:1303. doi: 10.3390/vaccines10081303
62. Beck, C, Lowenski, S, Durand, B, Bahuon, C, Zientara, S, and Lecollinet, S. Improved reliability of serological tools for the diagnosis of West Nile fever in horses within Europe. PLoS Negl Trop Dis. (2017) 11:e0005936. doi: 10.1371/journal.pntd.0005936
63. Shao, N, Li, F, Nie, K, Fu, SH, Zhang, WJ, et al. TaqMan real-time RT-PCR assay for detecting and differentiating Japanese encephalitis virus. Biomed Environ Sci. (2018) 31:208–14. doi: 10.3967/bes2018.026
64. Santhosh, SR, Parida, MM, Dash, PK, Pateriya, A, Pattnaik, B, Pradhan, HK, et al. Development and evaluation of SYBR green I-based one-step real-time RT-PCR assay for detection and quantitation of Japanese encephalitis virus. J Virol Methods. (2007) 143:73–80. doi: 10.1016/j.jviromet.2007.02.011
65. Wu, X, Lin, H, Chen, S, Xiao, L, Yang, M, An, W, et al. Development and application of a reverse transcriptase droplet digital PCR (RT-ddPCR) for sensitive and rapid detection of Japanese encephalitis virus. J Virol Methods. (2017) 248:166–71. doi: 10.1016/j.jviromet.2017.06.015
66. Cui, Y. Development of Nanobody and construction of immunoassay for staphylococcal enterotoxin (in Chinese). Northwest A&F Univ. (2024). doi: 10.27409/d.cnki.gxbnu.2024.002195
67. Sun, SY, Wu, JJ, and Yang, Z. Progress in serological detection of hepatitis B. Modern Med Health Res Electronic J. (2023) 7:134–7.
68. Niu, YH, Yang, K, Wang, FM, Shen, HQ, and Zhao, BH. Research advances and application of the polymerase chain reaction (PCR) in shrimp virus inspection. Hebei Fisheries. (2009) 1:14–24.
69. Liu, ZL, Liu, ZY, Li, ZJ, and Guo, L. Research progress of bovine rotavirus. Graziery Vet Sci. (2019) 20:1–3.
70. Mei, L, Wu, P, Ye, J, Gao, G, Shao, L, Huang, S, et al. Development and application of an antigen capture ELISA assay for diagnosis of Japanese encephalitis virus in swine, human and mosquito. Virol J. (2012) 9:4. doi: 10.1186/1743-422X-9-4
71. Li, Y, Hou, L, Ye, J, Liu, X, Dan, H, Jin, M, et al. Development of a convenient immunochromatographic strip for the diagnosis of infection with Japanese encephalitis virus in swine. J Virol Methods. (2010) 168:51–6. doi: 10.1016/j.jviromet.2010.04.015
72. Liu, Y, Gong, QL, Nie, LB, Wang, Q, Ge, GY, Li, DL, et al. Prevalence of porcine circovirus 2 throughout China in 2015–2019: a systematic review and meta-analysis. Microb Pathog. (2020) 149:104490. doi: 10.1016/j.micpath.2020.104490
73. Zheng, B, Wang, X, Liu, Y, Li, Y, Long, S, Gu, C, et al. Japanese encephalitis virus infection induces inflammation of swine testis through RIG-I-NF-kB signaling pathway. Vet Microbiol. (2019) 238:108430. doi: 10.1016/j.vetmic.2019.108430
74. Liu, H, Liu, ZJ, Jing, J, Ren, JQ, Liu, YY, Guo, HH, et al. Reverse transcription loop-mediated isothermal amplification for rapid detection of Japanese encephalitis virus in swine and mosquitoes. Vector Borne Zoonotic Dis. (2012) 12:1042–52. doi: 10.1089/vbz.2012.0991
75. Zeng, Q, Liu, J, Li, Z, Zhang, Y, Zu, S, Ding, X, et al. Japanese encephalitis virus NS4B inhibits interferon beta production by targeting TLR3 and TRIF. Vet Microbiol. (2023) 284:109849. doi: 10.1016/j.vetmic.2023.109849
76. Pierson, TC, and Diamond, MS. The continued threat of emerging flaviviruses. Nat Microbiol. (2020) 5:796–812. doi: 10.1038/s41564-020-0714-0
77. Li, X, Li, J, Wu, G, Wang, M, and Jing, Z. Detection of Japanese encephalitis by metagenomic next-generation sequencing of cerebrospinal fluid: a case report and literature review. Front Cell Neurosci. (2022) 16:856512. doi: 10.3389/fncel.2022.856512
78. Sharma, KB, Vrati, S, and Kalia, M. Pathobiology of Japanese encephalitis virus infection. Mol Asp Med. (2021) 81:100994. doi: 10.1016/j.mam.2021.100994
79. National Health Commission of the people′s republic of China. Immunization schedules and instructions for vaccines of the national immunization program (2021 version). Chinese J Viral Dis. (2021) 4:241–5. doi: 10.16505/j.2095-0136.2021.0021
80. Global Advisory Committee on vaccine safety, 9-10 June 2005. Wkly Epidemiol Rec. (2005) 80:242–7.
81. Tu, T, Xu, KQ, Xu, L, Gao, Y, Zhou, Y, He, YM, et al. Association between meteorological factors and the prevalence dynamics of Japanese encephalitis. PLoS One. (2021) 16:e0247980. doi: 10.1371/journal.pone.0247980
82. Li, LH, Li, Y, and Bi, YH. Mosquito and pig with Japanese encephalitis (in Chinese). Swine Ind Sci. (2008) 6:34–6.
83. Sakamoto, R, Tanimoto, T, Takahashi, K, Hamaki, T, Kusumi, E, and Crump, A. Flourishing Japanese encephalitis, associated with global warming and urbanisation in Asia, demands widespread integrated vaccination programmes. Ann Glob Health. (2019) 85:111. doi: 10.5334/aogh.2580
84. Walsh, MG, Pattanaik, A, Vyas, N, Saxena, D, Webb, C, Sawleshwarkar, S, et al. High-risk landscapes of Japanese encephalitis virus outbreaks in India converge on wetlands, rain-fed agriculture, wild Ardeidae, and domestic pigs and chickens. Int J Epidemiol. (2022) 51:1408–18. doi: 10.1093/ije/dyac050
85. Ladreyt, H, Durand, B, Dussart, P, and Chevalier, V. How central is the domestic pig in the epidemiological cycle of Japanese encephalitis virus? A review of scientific evidence and implications for disease control. Viruses. (2019) 11:949. doi: 10.3390/v11100949
Keywords: Japanese encephalitis, prevalence, pigs, zoonosis, meta-analysis
Citation: Liu X-T, Jiang L-D, Lin Y-T, Zhao R, Wang Q, Zhang S-Y, Ata EB, Liu X, Wang Y, Liu Z-X, Xu C, Xiao Y, Wang Y-F, Leng X, Gong Q-L and Du R (2025) Prevalence of Japanese encephalitis in pigs in Mainland China during 2000–2024: a systemic review and meta-analysis. Front. Vet. Sci. 12:1534114. doi: 10.3389/fvets.2025.1534114
Received: 25 November 2024; Accepted: 27 January 2025;
Published: 07 February 2025.
Edited by:
Abdul Wahaab, The Pennsylvania State University (PSU), United StatesReviewed by:
Sawar Khan, Central South University, ChinaCopyright © 2025 Liu, Jiang, Lin, Zhao, Wang, Zhang, Ata, Liu, Wang, Liu, Xu, Xiao, Wang, Leng, Gong and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Ying Zhang, emhhbmdzaHV5aW5nMTIwMUAxNjMuY29t; Qing-Long Gong, Z29uZ3Fpbmdsb25nMTAwMUAxNjMuY29t; Rui Du, ZHVydWkxOTcxMDFAc2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.