
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 10 February 2025
Sec. Veterinary Infectious Diseases
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1529870
This article is part of the Research TopicBacteriophages, a weapon against animal bacterial pathogens and biofilmsView all 4 articles
 Bowei Zhang1†
Bowei Zhang1† Liran Song1†
Liran Song1† Yongran Wang1†
Yongran Wang1† Meimei Zhang1
Meimei Zhang1 Chong Chen1
Chong Chen1 Hui Ning1
Hui Ning1 Li Wang1
Li Wang1 Cao Qiu1
Cao Qiu1 Xinwu Wang2
Xinwu Wang2 Changjiang Sun1
Changjiang Sun1 Xin Feng1
Xin Feng1 Wenyu Han1,3
Wenyu Han1,3 Bin Wang4*
Bin Wang4* Yalu Ji1*
Yalu Ji1* Jingmin Gu1,3*
Jingmin Gu1,3*Introduction: Staphylococcus aureus (S. aureus) is one of the most important zoonotic pathogens and can be transmitted to humans through the meat diet routes, causing necrotising pneumonia.
Methods: This study investigated the therapeutic effect of bacteriophage lysin LysGH15 on necrotising pneumonia in rabbit model caused by S. aureus.
Results: In the in vitro experiments, 50 μg/mL LysGH15 not only significantly reduced the viable count (approximately 3.24 × 106 CFU/g) of chicken meat stored at 4°C for 48 h but also effectively reduced the viable count of chicken meat thawed at 4°C and 30°C, with reductions of approximately 1.42 × 106 CFU/g and 2.78 × 106 CFU/g, respectively. In the in vivo experiments, a single intranasal administration of 300 μg/rabbit increased the survival rate of rabbits to 60%. At 72 h postinfection, the number of bacteria in the lung tissues of the rabbits treated with LysGH15 was 7 × 104 CFU/g, which was significantly lower than that in the lung tissues of rabbits treated with PBS (7.76 × 106 CFU/g) or linezolid (6.38 × 105 CFU/g). In addition, LysGH15 treatment alleviated lung tissue damage in infected rabbits and significantly reduced the levels of Panton-Valentine leukocidin (PVL), alpha-toxin (Hla), and the cytokines IFN-γ, TNF-α, and IL-8 in their lung tissues, similar to those in rabbits treated with linezolid.
Discussion: These results suggest that LysGH15 has the potential to be used as a novel antimicrobial agent for the treatment of necrotising pneumonia caused by S. aureus.
Staphylococcus aureus (S. aureus) is a Gram-positive bacterium ubiquitous in the natural world and an important zoonotic and foodborne pathogen (1). Among many infectious diseases, pneumonia caused by S. aureus is one of the most serious infections and is associated with high mortality, especially in patients with fulminant necrotising pneumonia (2). Some antibiotics, such as linezolid, have been shown to have therapeutic effects on necrotising pneumonia in rabbit model. However, the problem of antibiotic resistance is becoming more serious, and the use of antibiotics in human is limited (3). Therefore, there is an urgent need to find alternative therapies to antibiotics.
Staphylococcus aureus can be transmitted to humans through various pathways, one of which is through food transmission (4). According to statistics, food poisoning incidents caused by S. aureus account for approximately 25% of total foodborne microbial food poisoning incidents (5). Most staphylococcal and enterotoxin contamination in meat products is due mainly to cross-contamination during production or raw meat storage, which allows pathogenic staphylococci to produce enough enterotoxins to cause disease (6). With increasingly strict regulations on meat additives by government departments worldwide, conventional antibacterial agents with high bactericidal effects but pose potential threats to human health can no longer be used for microbial control in chicken products (7). Identifying a safe and effective new antimicrobial agent to eliminate S. aureus in meat products has become a research hotspot.
Phage lysin is a cell wall hydrolytic enzyme synthesized by the phage gene coding in the later stage of phage infection of bacteria (8). Lysin can target peptidoglycan in bacterial cell walls, causing peptidoglycan lysis and resulting in bacterial cell wall rupture, leading to bacterial death (9). Compared with traditional antibiotic therapy, lysin has many advantages. For example, the unique mode of action of lysin avoids the resistance mechanism of antibiotics and is equally effective against antibiotic-resistant strains (10). In addition, lysin only targets specific bacterial groups, reducing collateral damage to surrounding microbial communities (11). Lysin therapy has been validated in various animal models, and no toxic side effects have been observed (8, 10).
Several studies have explored the preventive and therapeutic effects of antibodies and antibiotics on S. aureus induced necrotizing pneumonia using a rabbit model (12–16). However, there is currently no research on the therapeutic effect of phage lysin on necrotising pneumonia. Our previous studies have indicated LysGH15, a lysin encoded by the phage GH15, displays efficient lytic activity against MRSA strains and antibiotics sensitive S. aureus strains isolated from the clinics (17). In this study, we investigated the bactericidal effect of the phage lysin LysGH15 as a novel bactericidal agent in meat under different storage and thawing conditions and evaluated the therapeutic efficacy of LysGH15 on necrotising lungs caused by S. aureus in rabbit model.
All the animal studies were approved by the Animal Welfare and Research Ethics Committee at Jilin University (Permit Number: SY202309034). This study adheres to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All methods were conducted in strict accordance with the relevant guidelines and regulations. The animals were treated humanely, and every effort was made to minimize suffering.
Female New Zealand white rabbits weighing 1.5–1.8 kg was purchased from the Experimental Animal Centre of Jilin University. The rabbits were housed in cages in a temperature-controlled animal room with a 12 h light/dark cycle. Feed and fresh water were available ad libitum.
The S. aureus S6 used in this study were isolated from the lung tissues of rabbits suffering from necrotising pneumonia and has been identified as a multidrug-resistant bacterium (18). S. aureus was routinely grown in brain heart infusion (BHI) broth (Becton, Dickinson and Company, USA) at 37°C in the shaker (ISS-7100, Lab Companion, Jeio Tech Co., Ltd., Korea) with shaking at 200 rpm.
In this study, an Escherichia coli BL21 (DE3) competent cell expressing the full-length LysGH15 protein was constructed, and protein purification was performed according to a previous study (17). The purity and molecular weight of the recombinant proteins were detected via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE).
Staphylococcus aureus S6 was cultured in BHI medium at 37°C and 180 rpm under oscillation and subsequently centrifuged to collect the bacteria in the logarithmic growth phase (OD600 = 0.6). The bacteria were washed three times with sterile phosphate-buffered saline (PBS) after centrifugation (8,000 × g, 5 min), and purified LysGH15 was added to the suspension of S. aureus S6 to final concentrations of 20, 50, and 100 μg/mL. The mixture was incubated at 37°C for 30 min, after which the CFU values were determined.
Raw chicken breast meat was purchased from a local retail store in Changchun (China) on October 8, 2023. Fresh chicken breast was treated according to the methods described (19). Specifically, fresh chicken breast tissue was cut into small pieces of 1 × 1 cm2 (approximately 1 g) and irradiated for 30 min on both the front and back sides at a distance of 0.3 m from the UV source for sterilization. One hundred microliters of S. aureus S6 solution (approximately 1 × 108 CFU/mL) that cultured to logarithmic growth phase (OD600 = 0.6) was evenly titrated on the surface of the meat for 15 min so that S. aureus was completely adhered to the meat. Then, 100 μL of different concentrations of LysGH15 (10 μg/mL, 50 μg/mL, or 100 μg/mL) and sterile PBS (pH = 7.0) were dripped onto the surface of the meat. To simulate thawing under normal conditions, the meat was stored at −20°C for 24 h and then thawed at 4°C. The five groups of chicken meat were collected after 0, 2, 6, 24, and 48 h. To simulate thawing under abnormal conditions, the meat was stored at −20°C for 24 h and then thawed at 30°C, and the five groups of chicken meat were collected after 0, 1, 2, 3, and 4 h. To simulate the preservation of fresh chicken meat, the meat was stored at 4°C, and the four groups of chicken meat were collected at 0, 3, 24, and 48 h. The collected chicken meat from the four groups was homogenized in 1 mL of PBS. The bacterial load was determined via S. aureus selective medium, and the results were calculated and expressed as CFU/g.
A rabbit necrotising pneumonia model was established based on a previous study (20, 21). Briefly, New Zealand white rabbits were anaesthetized with ketamine hydrochloride (35 mg/kg, i.m.) and xylazine hydrochloride (5 mg/kg, i.m.) (22) and intranasally injected with 1 mL of a single dose of S. aureus S6 (3 × 108 CFU/per rabbit) into the lungs.
To evaluate the protection rate of LysGH15 in infected rabbits, rabbits infected with S. aureus S6 were randomly divided into five groups. Three groups received an intranasal drop of 1 mL of LysGH15 (100 μg, 200 μg, or 300 μg/per rabbit) 1 h after infection; one group received an intramuscular injection of 50 mg/kg linezolid 1 h after infection; and one group received an intranasal drop of 1 mL of sterile PBS buffer 1 h after infection as a control. There were 10 rabbits in each group. The number of dead rabbits was recorded every 24 h during the 7-day observation period.
In a separate subsequent second experiment, mitigating effect of LysGH15 were studied on pathological parameters in lung tissues and blood of rabbits with induced necrotizing pneumonia, and the therapeutic dose of 300 μg/per rabbit of LysGH15 was used for evaluation. The rabbits were randomly divided into four groups: (i) rabbits treated with 1 mL of sterile PBS buffer by intranasal drip 1 h after infection; (ii) rabbits treated with a single dose of LysGH15 (1 mL, 300 μg) by intranasal drip 1 h after infection; (iii) rabbits intramuscularly injected with 50 mg/kg linezolid 1 h after infection; and (iv) rabbits without any treatment served as the control group.
Determination of the bacterial load in rabbit lung tissues. Three rabbits from each group were euthanized by carbon dioxide asphyxiation (22) at 24 and 72 h postinfection. Lung tissues (1–2 cm) were weighed and homogenized in 1 mL of PBS. Bacterial loads were determined on dilutions of the ground lung tissue.
Histopathologic analysis. Three rabbits from each group were euthanized by carbon dioxide asphyxiation at 24 and 72 h postinfection. The lungs of the rabbits were removed and immediately placed in 4% formalin. The formalin-fixed tissues were stained with hematoxylin and eosin and analyzed for changes in the histopathological characteristics of the lungs via microscopic observation.
Determination of cytokines and toxins in rabbit blood and lung tissue. The levels of cytokines in the blood and lung tissue and the Panton-Valentine leukocidal leukocytocin (PVL) and alpha toxin (Hla) levels in the lung tissue from the different groups were measured. Briefly, three rabbits from each group were euthanized by carbon dioxide asphyxiation at 24 and 72 h postinfection. The cardiac blood of each rabbit was collected, immediately placed in a 37°C incubator for 1 h and then placed at 4°C to separate the serum. In addition, 1–2 cm of lung tissue was weighed, suspended in sterile PBS and homogenized in 1 mL of PBS. The levels of the cytokines IL-8, TNF-α and IFN-γ in the blood and ground lung tissue samples and the levels of PVL and Hla in the ground lung tissue samples were determined via rabbit-specific enzyme-linked immunosorbent assay (ELISA) kits (USCN Life Science Inc., Wuhan, China) (23, 24).
SPSS version 13.0 software (SPSS, Inc., Chicago, IL, United States) was used for all the statistical analyses. All the experimental data were analyzed via one-way analysis of variance. p < 0.05 was considered a difference, and p < 0.01 was considered significant. p < 0.001 was considered extremely significant. The error bars represent the standard deviation.
As shown Figure 1A, the bactericidal activity of LysGH15 against S. aureus S6 was dose-dependent in BHI liquid culture medium, and 20, 50, and 100 μg/mL LysGH15 killed approximately 3 lg, 4 lg, and 6 lg units of S. aureus S6 within 30 min, respectively. When LyGH15 and chicken meat were stored at 4°C for 3 h, 50 μg/mL LysGH15 significantly reduced the amount of S. aureus (approximately 3.24 × 106 CFU/g) compared with that in the control group (p < 0.01) (Figure 1B). When LyGH15 and chicken meat were stored at −20°C for 24 h, 50 μg/mL LysGH15 significantly reduced the number of S. aureus (approximately 1.42 × 106 CFU/g) after thawing at 4°C for 2 h (p < 0.01) (Figure 1C). In addition, when LyGH15 and chicken meat were stored at −20°C for 24 h, 50 μg/mL LysGH15 significantly reduced the number of S. aureus (approximately 2.78 × 106 CFU/g) after thawing at 30°C for 1 h (p < 0.05) (Figure 1D).
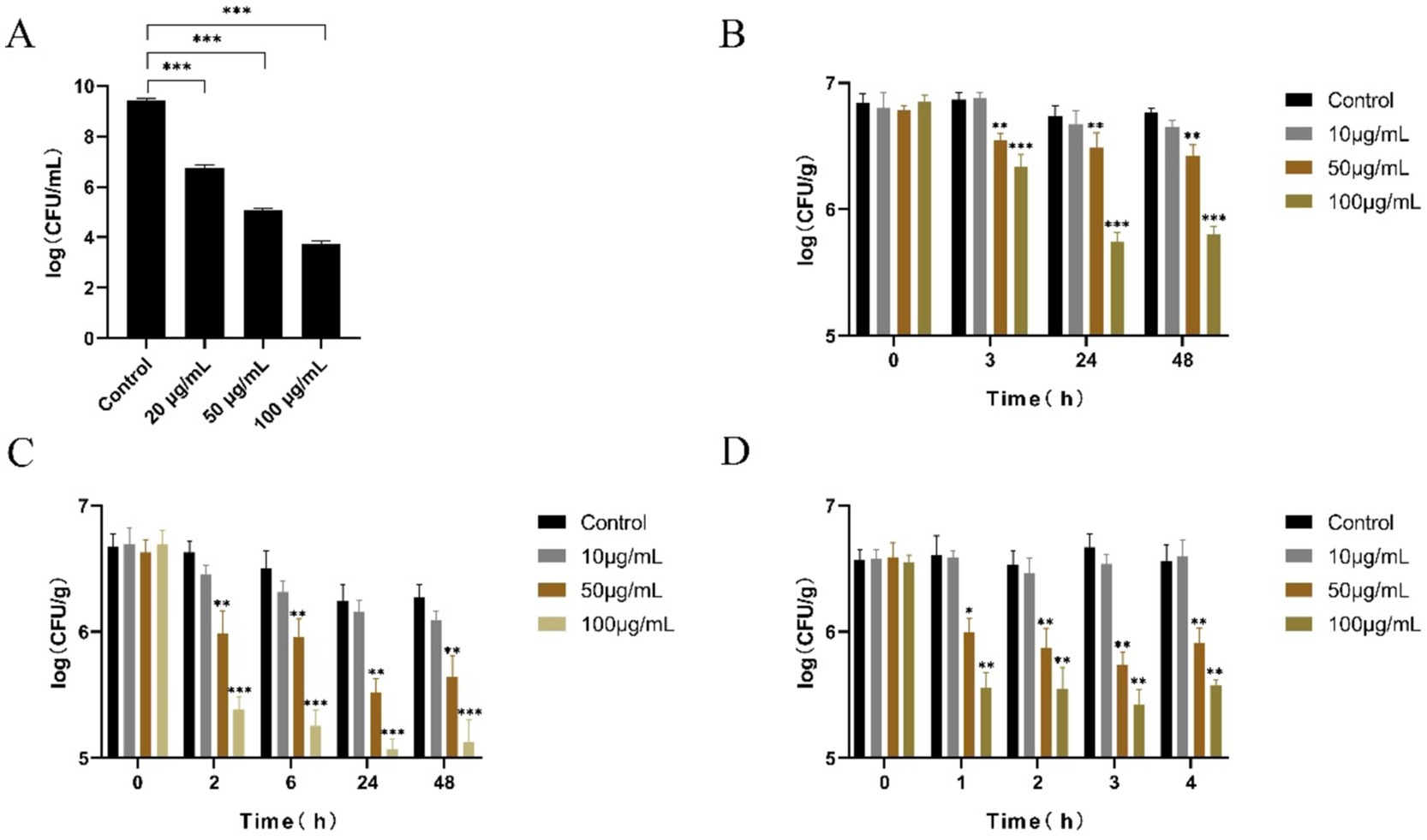
Figure 1. The bactericidal activity of LyGH15 against Staphylococcus aureus in BHI medium and chicken meat under different storage conditions. (A) Determination of the bactericidal activity of different concentrations of LysGH15 against S. aureus in BHI medium. (B) LyGH15 and chicken meat were stored at 4°C, and the bacterial load in the chicken meat was measured at different time points. (C) LyGH15 and chicken meat were stored at −20°C for 24 h and then thawed at 4°C, after which the bacterial load in the chicken meat was measured at different time points after thawing. (D) LyGH15 and chicken meat were stored at −20°C for 24 h and then thawed at 30°C, after which the bacterial load in the chicken meat was measured at different time points after thawing. The data represent the means ± standard deviations (SDs) of triplicate experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
As shown Figure 2A, in the control group, a single dose of 3 × 108 CFU/per rabbit of S. aureus resulted in a 60% (6/10) mortality rate within 2 days and a 100% (10/10) mortality rate within 5 days. Compared with the control group, the LysGH15-treated group presented prolonged survival time and an increased final survival rate (Figure 2A). On day 5 postinfection, a single dose of 100 μg/per rabbit of LysGH15 increased the survival rate of the rabbits to 20% (2/10). A single dose of 200 μg or 300 μg/per rabbit of LysGH15 increased the survival rate of the rabbits to 60% (6/10) and 80% (8/10), respectively, and the survival rate was ultimately maintained at 60% (6/10). In addition, the linezolid (50 mg/kg) treatment increased the survival rate of rabbits to 40% (4/10) on day 5 postinfection, and the survival rate was ultimately maintained at 20% (2/10).
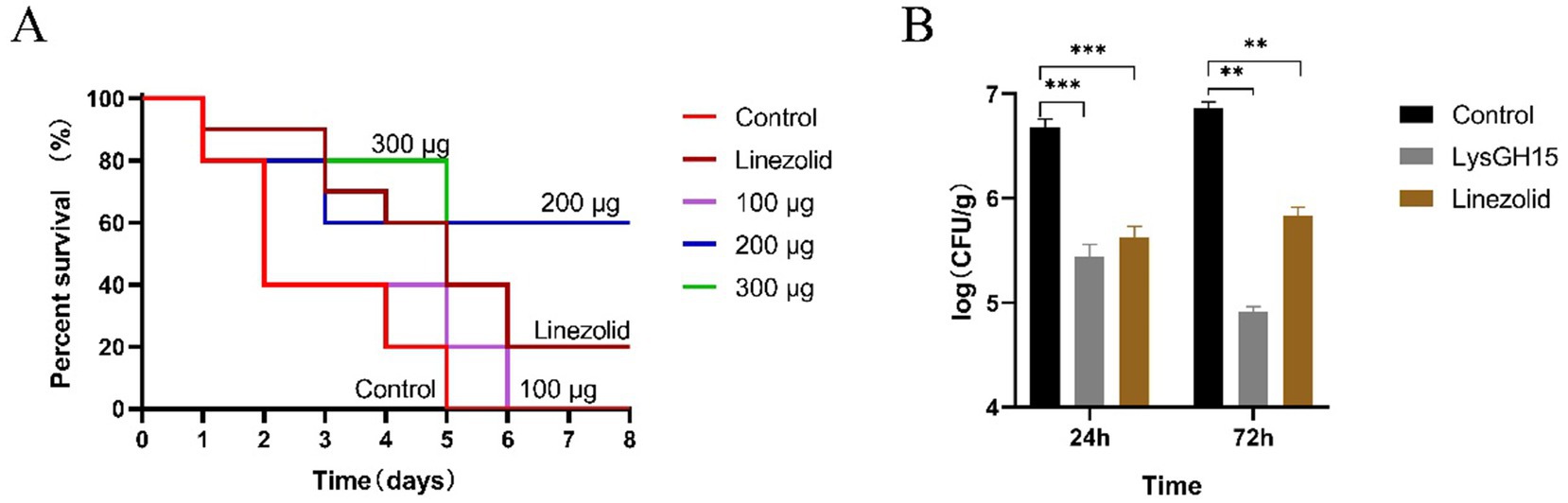
Figure 2. LysGH15 improved the survival outcome of rabbits infected with a lethal dose of S. aureus S6. (A) Survival rates. The rabbits were intranasally challenged with 3 × 108 CFU of S. aureus S6, and 1 h later, the challenged rabbits were intranasally treated with different doses of LysGH15. The control rabbits were treated with linezolid or PBS under the same conditions. Each group contained 10 rabbits. (B) Colony counts from the rabbit lung samples. Each group contained three rabbits. The lungs of the rabbits were harvested and homogenized at 24 h and 72 h after infection with S. aureus S6. Each bar represents the average count of three rabbits. The values represent the means and standard deviations. **p < 0.01, ***p < 0.001.
At 24 and 72 h postinfection, the bacterial counts in the lung tissues of the control rabbits reached approximately 4.36 × 106 CFU/g and 7.76 × 106 CFU/g, respectively (Figure 2B). In contrast, a single dose of 300 μg of LysGH15 significantly reduced the bacterial load in rabbit lung tissues, with rabbit lung tissue bacterial counts reaching approximately 2.92 × 105 CFU/g and 7.11 × 104 CFU/g at 24 h and 72 h postinfection, respectively. In the linezolid treatment group, the rabbit lung tissue bacterial counts reached approximately 4.44 × 105 CFU/g and 6.38 × 105 CFU/g at 24 and 72 h postinfection, respectively (Figure 2B).
LysGH15 significantly improved the pathological damage caused by S. aureus to rabbit lungs. On the surface, the lung tissue from the control rabbits was pink, and the surface was rosy and smooth (Figure 3A). However, the lungs of the rabbits infected with S. aureus were dark red or even purple-black (Figures 3B,E). In contrast, the lungs of the rabbits in the LysGH15 treatment group and antibiotic treatment group had similar appearances, with a smooth surface and pink color 24 h after treatment (Figures 3C,D) and a dark red color in the local area 48 h after treatment (Figures 3F,G). According to the pathological analysis, the alveolar structure of the control lung tissue was clear, and there was no inflammatory cell infiltration in the alveolar spaces (Figure 3H). In contrast, after infection with S. aureus, the rabbit lung membranes were thickened, the alveolar intervals were thickened, capillary dilatation and congestion of the alveolar walls occurred, and the alveolar epithelium was necrotic and exfoliated, accompanied by a large amount of inflammatory cell infiltration (Figures 3I,L). In contrast, inflammation and pathological changes were significantly alleviated in the lung tissue of the rabbits that were treated with lysin LysGH15, and similar results were observed in the linezolid treatment group (Figures 3J,K,M,N).
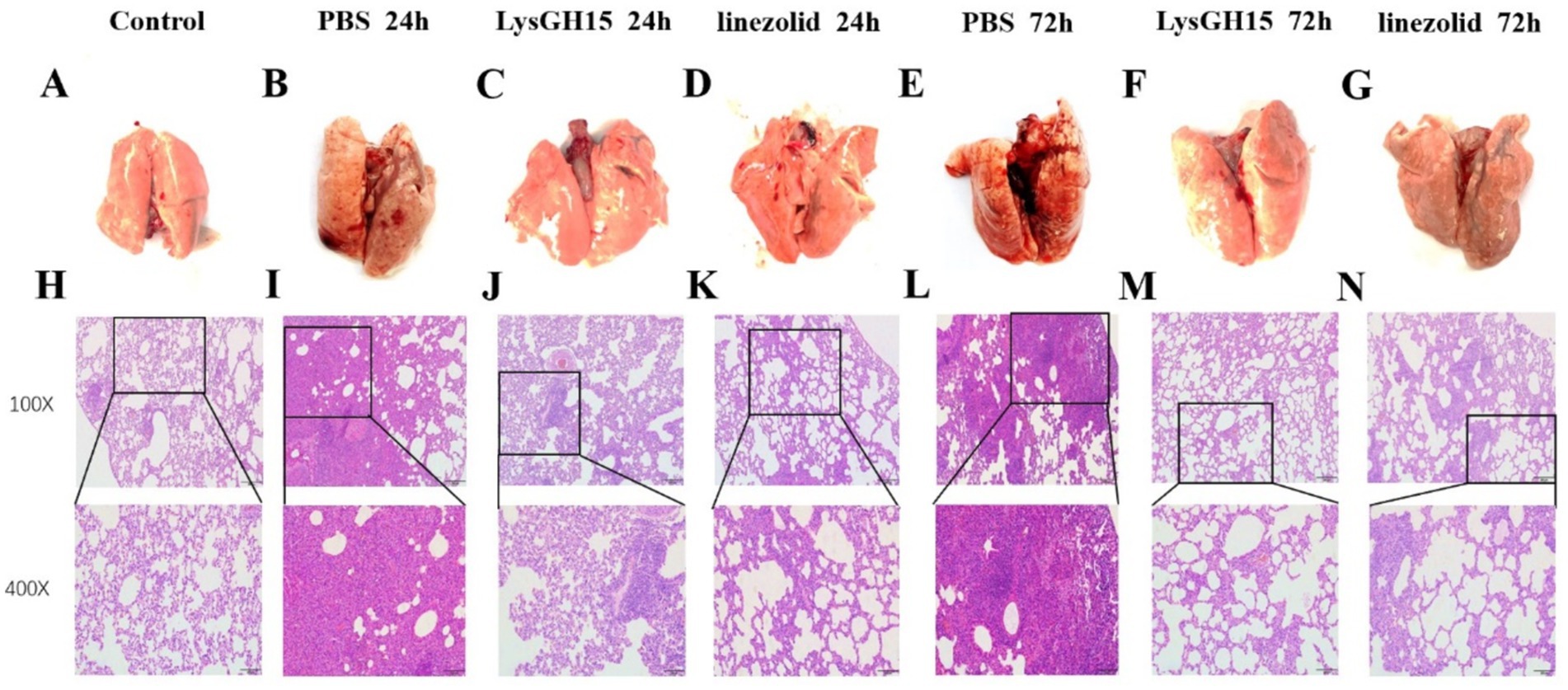
Figure 3. Gross pathology and histopathology of the lung tissue. At 24 h and 72 h postinfection, the lungs were removed from the rabbits treated with 300 μgLysGH15, linezolid or PBS. The lungs of healthy rabbits were used as controls (A-G). The tissue samples were stained with hematoxylin and eosin (H-N).
In addition, several key inflammatory cytokines and toxins in the blood and lung tissues of rabbits in each group were measured. As shown in Figure 4, the levels of the cytokines IFN-γ, TNF-α, and IL-8 in the blood and lung tissues were significantly greater in the infected rabbits than in the control rabbits at 24 and 72 h postinfection. Compared with the PBS treatment, LysGH15 treatment also significantly reduced the cytokine IFN-γ, TNF-α and IL-8 in the blood (Figures 4A–C) and lung tissues (Figures 4D–F) of rabbits. In addition, LysGH15 treatment significantly reduced the levels of toxins PVL and Hla in the lung tissues of rabbits (Figures 5A,B). These decreases were similar to the linezolid treatment group (Figures 4, 5).
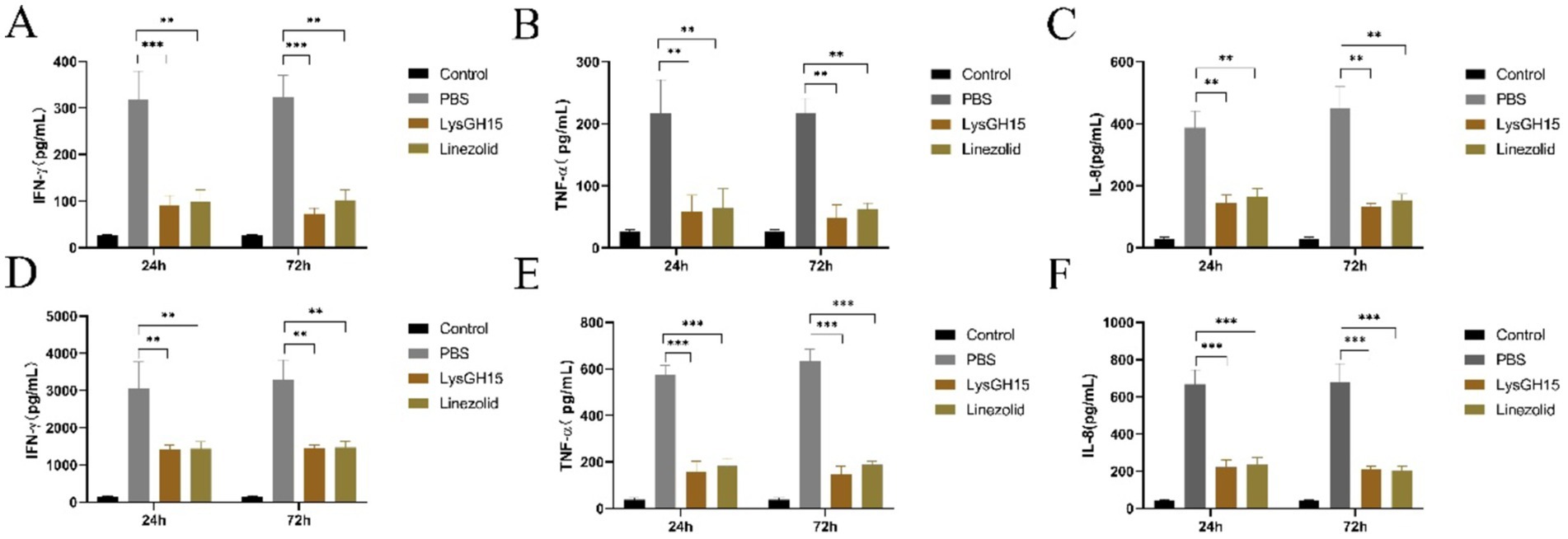
Figure 4. Cytokine levels in the blood and lung tissues. (A–C) Determination of the levels of the cytokines IFN-γ, TNF-α, and IL-8 in the blood. (D–F) Determination of the levels of the cytokines IFN-γ, TNF-α, and IL-8 in the lung tissue homogenate. Each group contained 3 rabbits. **p < 0.01, ***p < 0.001.
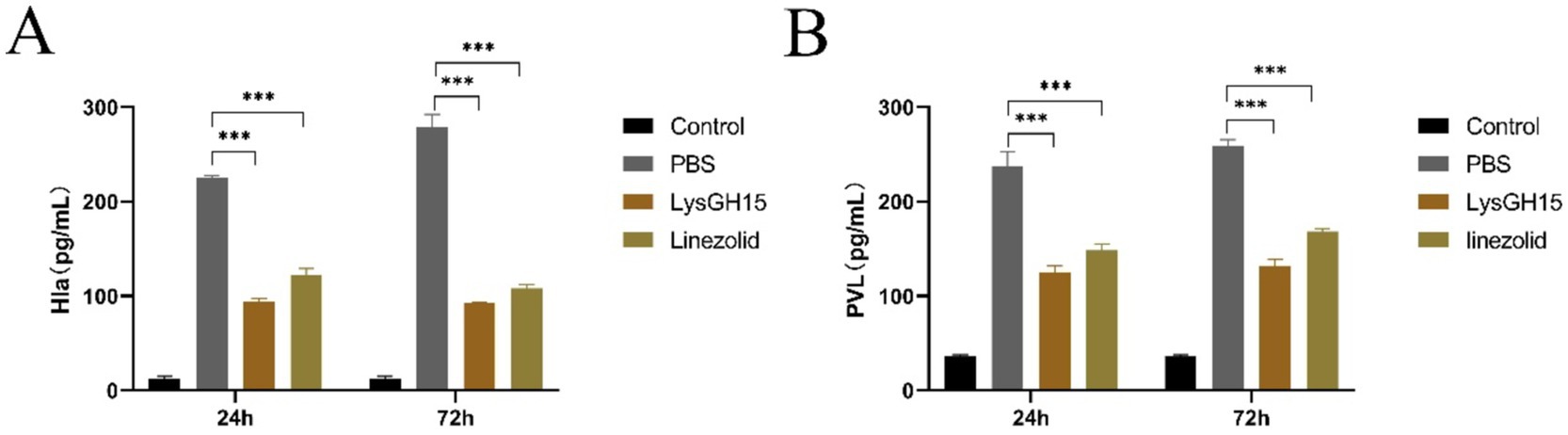
Figure 5. Toxin levels in the lung tissues. Determination of the PVL (A) and Hla (B) toxin concentration in the lung tissue homogenate. The lungs of the healthy rabbits were used as controls. Each group contained 3 rabbits. ***p < 0.001.
As an important zoonotic pathogen, the emergence and spread of multidrug-resistant S. aureus in different environments continue to pose significant challenges to its prevention and treatment in humans (25, 26). Although antibiotics linezolid and Tedizolid have improved necrotising pneumonia caused by S. aureus in rabbit model, the improvement effect needs to be further improved (20). Previous studies have shown that bacteriophages have a better protective effect against S. aureus necrotic pneumonia in rabbits than the antibiotic linezolid (18). In comparison, the lysins encoded by phage has greater bactericidal efficiency than phage, which is attributed to the fact that lysins can directly act on the peptidoglycan scaffold of the cell wall from the outside, hydrolyzing peptidoglycan to kill bacteria (27). Due to the lack of outer membrane protection in the cell wall of Gram-positive bacteria, lysins are particularly effective in Gram-positive bacteria, leading to osmotic shock and cell rupture, thereby achieving the goal of rapidly killing pathogens (28). Therefore, the use of phage lysins is more promising, by enabling the development of broad host range potent antimicrobials (29, 30).
Although there have been numerous studies on phage lysins in animal models of bacterial infection, there is currently no research on the treatment of lysins in rabbit models of necrotizing pneumonia. In this study, we evaluated the therapeutic effect of S. aureus phage lysin on necrotic pneumonia in rabbits. Because S. aureus can rapidly cause necrotising pneumonia in rabbits (21), combined with previous studies on phage therapy (18), we chose to treat infected rabbits with a single dose of LysGH15 administrated 1 h after infection. Encouragingly, the protective effect of single-dose LysGH15 treatment on infected rabbits was superior to that of antibiotics. Lysin can rapidly act and lyse bacteria (31), in contrast, most antibiotics, including linezolid, inhibit basic bacterial metabolic steps, leading to slow deterioration of cellular conditions and ultimately cell death (31). Therefore, antibiotics take a long time to kill bacteria. This is a potential explanation for the superiority of lysins over linezolid in reducing bacterial load and improving survival outcomes.
Staphylococcal pneumonia is largely driven by Hla and PVL (20, 32). In this study, treatment with 300 μg/rabbit lysin LyGH15 significantly reduced the levels of the two toxins in infected rabbit lung tissue at 24 and 72 h post-treatment. However, after LyGH15 treatment, 40% of the infected rabbits still died one after another, which may be due to the lethal damage caused by the toxins PVL and Hla to the rabbits at the beginning of the treatment window. Research has shown that attenuated forms of Hla or PVL alone can prevent fatal pneumonia in only partially infected rabbits (32). Therefore, combining lysin with vaccines targeting Hla and PVL toxins may provide better protection against necrotising pneumonia caused by S. aureus.
Due to the fact that S. aureus can be transmitted to humans through meat products, this study also explored the sterilization effect of LyGH15 on S. aureus in frozen chicken meat during thawing under recommended and temperature abuse conditions. After processing raw chicken meat, storing it at a refrigerated temperature is recommended (19). In this study, the lysin LyGH15 significantly reduced the number of S. aureus in chicken meat during refrigeration. In addition, frozen storage is a method of maintaining meat quality and controlling the growth of bacterial pathogens. S. aureus can survive under such harsh conditions (33), which is consistent with our findings (Figures 1C,D). Usually, frozen meat is thawed at 4°C. If frozen meat is not properly handled, such as when left on a countertop, rising temperatures will rapidly breed pathogenic bacteria (19, 34). However, our research results indicate that, in chicken meat contaminated with S. aureus, whether thawed at 4°C or a high temperature of 30°C, LyGH15 can effectively reduce the number of S. aureus in chicken meat.
The activity of phage and lysins against pathogenic bacteria has been confirmed in food raw materials and specific foods, which clearly indicates that they can be used to combat foodborne pathogens in the food sector (35). In addition, phage have become commercially available products in some cases, which can eliminate pathogenic bacteria in livestock and various food matrices (36). However, the application of lysins in food protection has not been fully explored. The important difference between lysins and phage is that lysins have a lower likelihood of developing drug resistance, while phage is unique in their autodosing and evolution capacity (37). Therefore, with the continuous increase and deepening of lysins in clinical research, engineered phage lysins are expected to become an effective tool for combating foodborne pathogens.
In this study, the results of bactericidal activity experiments on chicken meat revealed that LysGH15 could effectively control the contamination of S. aureus in meat products under chilled and thawed conditions. In vivo experiments revealed that LysGH15 treatment effectively reduced the number of bacteria in infected rabbit lungs, inhibited the production of bacterial toxins, reduced the production of cytokines, significantly improved the pathological manifestations of lung tissues, and ultimately increased the survival rate. These results suggest that LysGH15 has the potential to be used as a novel antimicrobial agent for controlling the growth of S. aureus in chilled and thawed chicken meat and for the treatment of necrotising pneumonia caused by S. aureus.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, HM015284.
All the animal studies were approved by the Animal Welfare and Research Ethics Committee at Jilin University (Permit Number: SY202309034). The study was conducted in accordance with the local legislation and institutional requirements.
BZ: Data curation, Formal analysis, Investigation, Writing – original draft. LS: Data curation, Visualization, Writing – original draft. YW: Formal analysis, Methodology, Writing – original draft. MZ: Investigation, Visualization, Writing – original draft. CC: Formal analysis, Resources, Writing – original draft. HN: Software, Writing – original draft. LW: Formal analysis, Writing – original draft. CQ: Visualization, Writing – original draft. XW: Funding acquisition, Investigation, Writing – original draft. CS: Formal analysis, Writing – review & editing. XF: Investigation, Visualization, Writing – review & editing. WH: Conceptualization, Project administration, Writing – review & editing. BW: Writing – review & editing. YJ: Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. JG: Funding acquisition, Project administration, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant nos. 32072824 and 32222083), the Fundamental Research Funds for the Central Universities, the Postdoctoral Fellowship Program of CPSF (grant no. GZC20230952), and the Science and Technology Development Project of Jilin Province (grant nos. YDZJ202301ZYTS358 and 20240602050RC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rao, RT, Madhavan, V, Kumar, P, Muniraj, G, Sivakumar, N, and Kannan, J. Epidemiology and zoonotic potential of livestock-associated Staphylococcus aureus isolated at Tamil Nadu, India. BMC Microbiol. (2023) 23:326. doi: 10.1186/s12866-023-03024-3
2. Diep, BA, Le, VT, Visram, ZC, Rouha, H, Stulik, L, Dip, EC, et al. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of Leukocidins in addition to alpha-Hemolysin. Antimicrob Agents Chemother. (2016) 60:6333–40. doi: 10.1128/AAC.01213-16
3. Alonso, LL, Podio, NS, Marino, DJG, Almada, NS, Gange, JM, Bernigaud, I, et al. Evaluating antibiotic occurrence, degradation, and environmental risks in poultry litter within Argentina's agricultural hub. Sci Total Environ. (2024) 920:170993. doi: 10.1016/j.scitotenv.2024.170993
4. Karadal, F, Onmaz, NE, Hizlisoy, H, Yildirim, Y, Al, S, Gonulalan, Z, et al. Toxigenic Staphylococcus aureus in some animal-originated food products marketed in Turkey: presence and public health concerns. Vet Ital. (2024) 60. doi: 10.12834/VetIt.3022.21271.2
5. Albashir, D, Lu, H, Gouda, M, Acharya, DR, Danhassan, UA, Bakur, A, et al. A novel polydiacetylene-functionalized fibrinogen paper-based biosensor for on-spot and rapid detection of Staphylococcus aureus. Food Chem. (2024) 458:140291. doi: 10.1016/j.foodchem.2024.140291
6. Nadiya, S, Kolla, HB, and Reddy, PN. Optimization and evaluation of a multiplex PCR assay for detection of Staphylococcus aureus and its major virulence genes for assessing food safety. Braz J Microbiol. (2023) 54:311–21. doi: 10.1007/s42770-023-00906-6
7. Akermi, S, Chaari, M, Elhadef, K, Fourati, M, Chakchouk Mtibaa, A, Agriopoulou, S, et al. Disclosing the functional potency of three oxygenated monoterpenes in combating microbial pathogenesis: from targeting virulence factors to chicken meat preservation. Food Secur. (2024) 13:965. doi: 10.3390/foods13060965
8. Rahman, MU, Wang, W, Sun, Q, Shah, JA, Li, C, Sun, Y, et al. Endolysin, a promising solution against antimicrobial resistance. Antibiotics. (2021) 10:1277. doi: 10.3390/antibiotics10111277
9. Matsui, H, Uchiyama, J, Ogata, M, Nasukawa, T, Takemura-Uchiyama, I, Kato, SI, et al. Use of recombinant Endolysin to improve accuracy of group B Streptococcus tests. Microbiol Spectr. (2021) 9:e0007721. doi: 10.1128/Spectrum.00077-21
10. Shah, S, Das, R, Chavan, B, Bajpai, U, Hanif, S, and Ahmed, S. Beyond antibiotics: phage-encoded lysins against gram-negative pathogens. Front Microbiol. (2023) 14:1170418. doi: 10.3389/fmicb.2023.1170418
11. Mondal, SI, Draper, LA, Ross, RP, and Hill, C. Bacteriophage endolysins as a potential weapon to combat Clostridioides difficile infection. Gut Microbes. (2020) 12:1813533. doi: 10.1080/19490976.2020.1813533
12. Euler, CW, Raz, A, Hernandez, A, Serrano, A, Xu, S, Andersson, M, et al. PlyKp104, a novel phage Lysin for the treatment of Klebsiella pneumoniae, Pseudomonas aeruginosa, and other gram-negative ESKAPE pathogens. Antimicrob Agents Chemother. (2023) 67:e0151922. doi: 10.1128/aac.01519-22
13. Sosa, BR, Niu, Y, Turajane, K, Staats, K, Suhardi, V, Carli, A, et al. John Charnley award: the antimicrobial potential of bacteriophage-derived lysin in a murine debridement, antibiotics, and implant retention model of prosthetic joint infection. Bone Joint J. (2020) 102:3–10. doi: 10.1302/0301-620X.102B7.BJJ-2019-1590.R1
14. Wang, Z, Liu, X, Shi, Z, Zhao, R, Ji, Y, Tang, F, et al. A novel lysin Ply1228 provides efficient protection against Streptococcus suis type 2 infection in a murine bacteremia model. Vet Microbiol. (2022) 268:109425. doi: 10.1016/j.vetmic.2022.109425
15. Wang, Z, Ma, J, Wang, J, Yang, D, Kong, L, Fu, Q, et al. Application of the phage Lysin Ply5218 in the treatment of Streptococcus suis infection in piglets. Viruses. (2019) 11:715. doi: 10.3390/v11080715
16. Xu, J, Yang, H, Bi, Y, Li, W, Wei, H, and Li, Y. Activity of the chimeric Lysin ClyR against common gram-positive Oral microbes and its Anticaries efficacy in rat models. Viruses. (2018) 10:380. doi: 10.3390/v10070380
17. Gu, J, Xu, W, Lei, L, Huang, J, Feng, X, Sun, C, et al. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. (2011) 49:111–7. doi: 10.1128/JCM.01144-10
18. Ji, Y, Cheng, M, Zhai, S, Xi, H, Cai, R, Wang, Z, et al. Preventive effect of the phage VB-SavM-JYL01 on rabbit necrotizing pneumonia caused by Staphylococcus aureus. Vet Microbiol. (2019) 229:72–80. doi: 10.1016/j.vetmic.2018.12.021
19. Abhisingha, M, Dumnil, J, and Pitaksutheepong, C. Effect of lysin EN4 in combination with sodium bicarbonate on reduction of Salmonella in chilled and thawed chicken meat. Int J Food Microbiol. (2023) 387:110058. doi: 10.1016/j.ijfoodmicro.2022.110058
20. Diep, BA, Afasizheva, A, Le, HN, Kajikawa, O, Matute-Bello, G, Tkaczyk, C, et al. Effects of linezolid on suppressing in vivo production of staphylococcal toxins and improving survival outcomes in a rabbit model of methicillin-resistant Staphylococcus aureus necrotizing pneumonia. J Infect Dis. (2013) 208:75–82. doi: 10.1093/infdis/jit129
21. Diep, BA, Chan, L, Tattevin, P, Kajikawa, O, Martin, TR, Basuino, L, et al. Polymorphonuclear leukocytes mediate Panton-valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci USA. (2010) 107:5587–92. doi: 10.1073/pnas.0912403107
22. Hagiwara, M, Shibuta, S, Takada, K, Kambayashi, R, Nakajo, M, Aimoto, M, et al. The anaesthetized rabbit with acute atrioventricular block provides a new model for detecting drug-induced torsade de pointes. Br J Pharmacol. (2017) 174:2591–605. doi: 10.1111/bph.13870
23. Jacquier, V, Estelle, J, Schmaltz-Panneau, B, Lecardonnel, J, Moroldo, M, Lemonnier, G, et al. Genome-wide immunity studies in the rabbit: transcriptome variations in peripheral blood mononuclear cells after in vitro stimulation by LPS or PMA-Ionomycin. BMC Genomics. (2015) 16:26. doi: 10.1186/s12864-015-1218-9
24. Pauchard, LA, Blot, M, Bruyere, R, Barbar, SD, Croisier, D, Piroth, L, et al. Linezolid and atorvastatin impact on pneumonia caused by Staphyloccocus aureus in rabbits with or without mechanical ventilation. PLoS One. (2017) 12:e0187187. doi: 10.1371/journal.pone.0187187
25. Douglas, EJA, Wulandari, SW, Lovell, SD, and Laabei, M. Novel antimicrobial strategies to treat multi-drug resistant Staphylococcus aureus infections. Microb Biotechnol. (2023) 16:1456–74. doi: 10.1111/1751-7915.14268
26. Miller, WR, and Arias, CA. ESKAPE pathogens: antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat Rev Microbiol. (2024) 22:598–616. doi: 10.1038/s41579-024-01054-w
27. Chang, RYK, Nang, SC, Chan, HK, and Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv Drug Deliv Rev. (2022) 187:114378. doi: 10.1016/j.addr.2022.114378
28. Fischetti, VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. (2008) 11:393–400. doi: 10.1016/j.mib.2008.09.012
29. Angelopoulou, A, Warda, AK, Hill, C, and Ross, RP. Non-antibiotic microbial solutions for bovine mastitis – live biotherapeutics, bacteriophage, and phage lysins. Crit Rev Microbiol. (2019) 45:564–80. doi: 10.1080/1040841X.2019.1648381
30. Gondil, VS, Harjai, K, and Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int J Antimicrob Agents. (2020) 55:105844. doi: 10.1016/j.ijantimicag.2019.11.001
31. Gerstmans, H, Rodriguez-Rubio, L, Lavigne, R, and Briers, Y. From endolysins to Artilysin(R)s: novel enzyme-based approaches to kill drug-resistant bacteria. Biochem Soc Trans. (2016) 44:123–8. doi: 10.1042/BST20150192
32. Tran, VG, Venkatasubramaniam, A, Adhikari, RP, Krishnan, S, Wang, X, Le, VTM, et al. Efficacy of active immunization with attenuated alpha-Hemolysin and Panton-valentine Leukocidin in a rabbit model of Staphylococcus aureus necrotizing pneumonia. J Infect Dis. (2020) 221:267–75. doi: 10.1093/infdis/jiz437
33. Yu, K, Yang, L, Zhang, S, and Zhang, N. Strong, tough, high-release, and antibacterial nanocellulose hydrogel for refrigerated chicken preservation. Int J Biol Macromol. (2024) 264:130727. doi: 10.1016/j.ijbiomac.2024.130727
34. Al-Holy, MA, Olaimat, AN, Al-Nabulsi, AA, Al-Qadiri, H, Abughoush, MH, Osaili, TM, et al. Survival and growth behavior of common foodborne pathogens in falafel paste under different storage temperatures. Int J Food Microbiol. (2024) 413:110609. doi: 10.1016/j.ijfoodmicro.2024.110609
35. Xu, Y. Phage and phage lysins: new era of bio-preservatives and food safety agents. J Food Sci. (2021) 86:3349–73. doi: 10.1111/1750-3841.15843
36. Kocot, AM, Briers, Y, and Plotka, M. Phages and engineered lysins as an effective tool to combat gram-negative foodborne pathogens. Compr Rev Food Sci Food Saf. (2023) 22:2235–66. doi: 10.1111/1541-4337.13145
Keywords: Staphylococcus aureus, lysin, LysGH15, necrotising pneumonia, rabbit
Citation: Zhang B, Song L, Wang Y, Zhang M, Chen C, Ning H, Wang L, Qiu C, Wang X, Sun C, Feng X, Han W, Wang B, Ji Y and Gu J (2025) Therapeutic efficacy of LysGH15 against necrotising pneumonia caused by Staphylococcus aureus in a rabbit model. Front. Vet. Sci. 12:1529870. doi: 10.3389/fvets.2025.1529870
Received: 18 November 2024; Accepted: 20 January 2025;
Published: 10 February 2025.
Edited by:
Chao-ting Xiao, Hunan University, ChinaReviewed by:
Samar El-Masry, Ain Shams University, EgyptCopyright © 2025 Zhang, Song, Wang, Zhang, Chen, Ning, Wang, Qiu, Wang, Sun, Feng, Han, Wang, Ji and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingmin Gu, amluZ21pbjA2MjlAMTYzLmNvbQ==; Yalu Ji, aml5YWx1MTIwQDE2My5jb20=; Bin Wang, d2FuZ2JpbjA2MDRAamx1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.