
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 30 January 2025
Sec. Parasitology
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1522904
To date, the primary hemoplasmas that infect cats include Mycoplasma haemofelis, ‘Candidatus Mycoplasma haemominutum’, and ‘Candidatus Mycoplasma turicensis’. In addition, other hemoplasmas Mycoplasma species have also been identified in cats. In central China, no infections or potential vectors with hemotropic mycoplasmas have been recorded in cats. To elucidate the prevalence of hemotropic mycoplasmas in both cats and parasitic ticks, this study investigated the occurrence of hemotropic mycoplasma infections in ticks and cats. A total of 78 blood samples were collected from both anemic and healthy cats, along with 284 ticks from the cats’ body surfaces and 356 ticks found in the surrounding environment. Following the morphological and molecular identification of ticks, all samples were screened for pathogens using PCR detection and sequence analysis. The results indicated the presence of 392 Haemaphysalis longicornis, 152 Rhipicephalus microplus, and 76 Rhipicephalus sanguineus sensu lato in cats and their surrounding environment. Molecular detection revealed the amplification of 156 ‘Ca. M. haemominutum’, 96 ‘Candidatus Mycoplasma haemobos’, 41 M. haemofelis, and 64 Rickettsia felis-positive amplicons from both cats and ticks. Notably, when comparing the infection rates of ‘Ca. M. haemobos’ in the environment group, no significant differences were observed in the infection rates among the three tick species from anemic or healthy cats (p > 0.05, α = 0.05). Furthermore, sequence analysis of ‘Ca. M. haemobos’ indicated two novel sequence types that were most closely related to an isolate from buffalo in China. In conclusion, in this study, in addition to ‘Ca. M. haemominutum’ and M. haemofelis, ‘Ca. M. haemobos’ was first detected in cats. ‘Ca. M. haemominutum’ appears to be associated with anemic syndrome in cats, while further research is needed to explore the relationship between ‘Ca. M. haemobos’ and clinical signs in felines. Additionally, these three hemotropic mycoplasmas were also found in three species of ticks, and transmission experiments are required to investigate the capacity of these ticks to transmit hemoplasmas Mycoplasma among animals.
Hemotropic mycoplasmas are small unculturable bacteria that attach to erythrocytes (1). Mycoplasma haemofelis (2), ‘Candidatus Mycoplasma haemominutum’ (3), and ‘Candidatus Mycoplasma turicensis’ (4) are the three main hemoplasmas that infect cats. However, an emerging hemoplasma called ‘Candidatus Mycoplasma haematoparvum like’ (5) has been detected in cats in the United States (5), Spain (6), and Japan (7). Intriguingly, in Spain, Mycoplasma wenyonii (8), which is primarily associated with cattle infection, was also found in a cat (9). Furthermore, two distinct sequence types of previously undescribed hemotropic mycoplasmas were identified in 15 European wild cats in Bosnia and Herzegovina (10). These findings suggest that cats could be infected by hemoplasmas typically infecting other hosts.
‘Candidatus Mycoplasma haemobos’ (11) is an emerging pathogen that was first detected in cattle (Bos taurus) in Japan. Later on, this pathogen was found to infect a diverse range of hosts, including water buffalo (Bubalus bubalis) (12), red deer (Cervus elaphus) (13), fallow deer (Dama dama) (13), roe deer (Capreolus capreolus) (13), goats (Capra aegagrus hircus) (14, 15), sheep (Ovis aries) (14), and dogs (Canis) (16). These natural infections are frequently accompanied by anemia (11, 14, 17), transient fever (14), lymphadenopathy (11, 17), anorexia (18), lack of appetite, and decreased milk production (18, 19). To date, natural infections of ‘Ca. M. haemobos’ have been reported worldwide, including in Africa (20, 21), Asia (14, 22–25), Europe (13, 26, 27), North America (28), and South America (12, 29). In our previous studies conducted in Henan Province, ‘Ca. M. haemobos’ was detected in goats, sheep (14), dogs (16), and cattle (15) on backyard farms. Notably, Rhipicephalus microplus and Haemaphysalis longicornis ticks infesting the body surface of these animals were also found to carry ‘Ca. M. haemobos’ (14–16). Furthermore, R. microplus ticks have been implicated as vectors and reservoirs in the transmission of ‘Ca. M. haemobos’ (30). On these backyard farms, not only dogs but also cats, which are housed for catching mice and allowed to roam freely, share the same living spaces with infected animals. Given that R. microplus and H. longicornis can parasitize goats, sheep, dogs, and cats (31–34), it remains uncertain whether cats in this region could become infected with ‘Ca. M. haemobos’ through tick infestation.
In China, Ca. M. haemominutum was first detected in cats in Guangdong province in 2009 (35), followed by its detection along with M. haemofelis and Ca. M. turicensis in cats in Shanghai in 2017 (36). To date, no infections with hemotropic mycoplasmas have been recorded in cats in central China. Given this backdrop, this study aimed to focus on the occurrence of ‘Ca. M. haemobos’ infections in cats and parasitic ticks in the Henan Province, central China. In addition, due to similar anemia symptoms exhibited in cats, other pathogens such as Ca. M. haemominutum, M. haemofelis, Ca. M. turicensis, Rickettsia felis (37), Anaplasma, Hepatozoon, Babesia, and Theileria (9) were also included in the investigation.
From April 2023 to October 2023, during the peak season for ‘Ca. M. haemobos’ infections and tick activity in southern Henan Province (112°38′ ~ 113°24′ E, 33°04′ ~ 33°37′ N), the region is characterized by a diverse topography that includes mountains, hills, and flat or gently rolling plains, situated within the subtropical continental monsoon zone. To address the research question, 19 backyard farms affected by Ca. M. haemobos’ were selected as the study site. Previous research documented infections of ‘Ca. M. haemobos’ in goats, sheep, dogs, and cattle on these farms (14–16). Subsequently, investigations were conducted on cats, parasitic ticks, and ticks found in the environment at these locations. In total, 78 EDTA-anticoagulated blood and serum samples were collected from the femoral vein of the cats. Among the sampled animals, some exhibited clinical signs such as pale oral mucous, conjunctival infection, and hematuria. In addition, all ticks found on the skin surface of each cat were collected, resulting in a total of 284 ticks. These ticks were treated individually, following the methodology established in a previous study (14). Furthermore, questing ticks (n = 356) in the environment were collected using the drag–flag method (38), using a white cotton flannel cloth (1.2 m × 1 m). The flag was systematically dragged over low vegetation (1 ~ 30 cm in height) near the edges of paths and the borders of dense vegetation.
All blood samples underwent an initial examination as follows: the partial whole blood samples were analyzed using an automated hematology analyzer (DF55Vet, Dymind, China), and the results were compared to established reference ranges (red blood cell (RBC): 6.54–12.20; hematocrit (HCT): 30.30–52.30; hemoglobin (HGB): 9.80–16.20) in accordance with the provided instructions and the reference (39). Subsequently, based on the clinical symptoms documented earlier, the cats involved in this study were categorized into two groups. The remaining blood samples from the cats were preserved at −80°C for future analysis.
All adult tick samples were initially identified using morphological and taxonomic identification methods (40). Hard ticks and soft ticks were differentiated at the family level based on the position of the anal groove. Subsequently, at the genus level, identification was achieved by examining the morphology of the gnathosoma. Within the same genus, ticks exhibit considerable morphological similarity, necessitating the identification of distinguishing features such as the shape, color, and patterns of the scutum; the configuration of the spiracular plates; the presence of stripes on the tarsus; and the characteristics of the pulvilli. Finally, sexual dimorphism was assessed by comparing the size of the scutum between males and females (41).
The blood samples obtained from cats were subjected to DNA extraction using the EasyPure Blood Genomic DNA Kit (TransGen Biotech, China) according to the manufacturer’s instructions. Each tick sample was homogenized in 1 mL of phosphate-buffered saline, then placed on sterile filter paper to dry, and further put into tubes prefilled with ceramic beads (MagNA Lyser Green Beads, Roche, USA). A volume of 400 μL of PBS was added to each tube, and incubation was carried out for 5 h. After the tick bodies and scuta had softened, the tick tissues were homogenized at 7,000 rpm for 90 s by the MagNA Lyser Instrument (Roche, USA), after which 200 μL of each homogenate underwent DNA extraction utilizing the Universal Genomic DNA Kit (TIANGEN, China). The extracted genomic DNA was eluted in DEPC-treated water and subsequently used as a template in PCR reactions. For molecular identification, the conserved 12S rRNA gene of tick species was selected for amplification using primers T1B and T2A (42). In alignment with previous research (43), a specific pair of primers (H-MYC-F/R) was selected to identify potential hemotropic mycoplasmas. These primers have previously demonstrated efficacy in amplifying the partial 16S rRNA gene of various species, including M. haemofelis, Ca. M. haemominutum, M. haemocanis, Ca. M. haematoparvum, Mycoplasma ovis, Candidatus Mycoplasma haemovis, M. wenyonii, and ‘Ca. M. haemobos’ (14–16, 43). Furthermore, DNA from a strain of M. wenyonii served as a positive control, while DEPC-treated water was utilized as a negative control in all PCR reactions. The PCR procedures were conducted as described in previous studies (43, 44). In addition, nested primers Rick-out/Rick-in for R. felis (45), Apla-sense and ECB primers for Anaplasma (46), HAM-1F and HPF-2R primers for Hepatozoon (47), and BTH 18S 1st F/R and BTH 18S 2nd F/R primers for Babesia and Theileria (48) were used to detect potential tick-borne pathogens associated with anemia in cats. The extracted blood and tick DNA were reconstituted in 50 μL of double-distilled water, and the quantity and quality of DNA were assessed using a spectrophotometer (UV1000, Techcomp, China). The PCR amplification reactions were performed using an EasyTaq® PCR SuperMix kit (TransGen, Beijing, China) in a total reaction of 20 μL, which included 10 μL of 2× EasyTaq® PCR SuperMix, 0.4 μM of each primer, and 20 ng template DNA. The amplification conditions were as follows: predenaturation at 94°C for 5 min, followed by 30 cycles of 30 s at 94°C, 30 s at the appropriate annealing temperature determined by the specific primers, 2 min at 72°C, and a final extension at 72°C for 10 min. The primers and annealing temperature are shown in Table 1.
Following the initial molecular screening, all mycoplasma-positive amplicons were purified and sequenced, as previously described (14). The resulting sequences were then compared to relevant sequences available in the NCBI databases utilizing a BLAST search. Subsequently, longer fragments (1,393 bp) of the 16S rRNA gene were amplified from all samples that tested positive for ‘Ca. M. haemobos’ using the MHBforw and MHBrev primers (44). These fragments were also purified and sequenced following the aforementioned protocol.
The sequences of the long 16S rRNA gene were compared and aligned using the CLUSTALW program, incorporating strains isolated from cattle, buffalo, sheep, ticks, and dogs from various regions worldwide. Subsequently, a phylogenetic analysis was performed using MEGA6 (49), employing the neighbor-joining criterion and the Kimura two-parameter model (11, 14, 22, 44, 50). The robustness of the hypothesis was tested using bootstrap analysis with 1,000 replicates.
In this study, cats were divided into two groups: Group 1 comprised 57 anemic cats that exhibited clinical signs and had hematological values below the normal reference ranges, while Group 2 consisted of 21 healthy cats with hematological values within the normal reference ranges. Statistical analysis for significant differences was performed between groups using SPSS 17 software (IBM, USA) on the chi-square test. p-value<0.05 was considered the threshold for statistical significance.
In total, 640 ticks were collected from both cats and the environment. This collection included 284 adult ticks obtained from cats, along with 336 adult ticks and 20 nymph ticks collected from the environment. Following a thorough examination using morphological and taxonomic keys, a total of 119 male adult ticks and 501 female adult ticks were identified. Subsequent molecular identification through sequencing of the 12S rRNA gene revealed that these adult ticks comprised three species from two genera within the family Ixodidae: 407 H. longicornis (199 and 208 collected from cats and the environment, respectively), 155 R. microplus (58 and 97 collected from cats and the environment, respectively), and 78 Rhipicephalus sanguineus sensu lato (R. sanguineus sl) (27 collected from cats and 51 from the environment). Furthermore, both engorged and unengorged ticks were recorded among the three tick species collected from cats and the environment. Detailed information regarding the adult tick species, their source, sexes, life stages, and numbers is presented in Table 2.
As shown in Table 3, hemotropic mycoplasma infection rates were 54.4% (31 out of 57) and 14.3% (3 out of 21) in blood samples in Group 1 and Group 2, respectively; 55.4% (102 out of 184), 26.7% (4 out of 15), and 29.3% (61 out of 208) in H. longicornis in Group 1, Group 2, and the environment, respectively; 64.0% (32 out of 50), 25.0% (2 out of 8), and 35.0% (34 out of 97) in R. microplus in Group 1, Group 2, and the environment, respectively; and 68.2% (15 out of 22), 20.0% (1 out of 5), and 17.6% (9 out of 51) in R. sanguineus sl in Group 1, Group 2, and the environment, respectively. Significant differences in mycoplasma infection rates were observed between Group 1 and Group 2 in the blood samples (p = 0.002). All hemotropic mycoplasma-positive amplicons were sequenced and aligned using the BLAST search tool in GenBank. The results indicated the prevalence rates of ‘Ca. M. haemominutum’, M. haemofelis, and ‘Ca. M. haemobos’ in the blood samples, as well as in samples from H. longicornis, R. microplus, and R. sanguineus sl. In addition, screening for other pathogens revealed the presence of R. felis in cats and three tick species, with no other pathogens detected. Moreover, two types of co-infections (‘Ca. M. haemominutum’ + R. felis and ‘Ca. M. haemobos’ + R. felis) were identified in anemic cats, H. longicornis, and R. sanguineus sl.
Initially, the infection rates of ‘Ca. M. haemominutum’ were analyzed. ‘Ca. M. haemominutum’ infection rates were 31.6% (18 out of 57) and 14.3% (3 out of 21) in the blood samples in Group 1 and Group 2, respectively, and no significant differences (p = 0.127) were detected. In contrast, when comparing the infection rates in H. longicornis from the environment group (14.4%, 30 out of 208), a significant difference (p < 0.001) was noted in Group 1 (37.0%, 68 out of 184), whereas no significant difference (p = 1.000) was observed in Group 2 (13.3%, 2 out of 15). Furthermore, when compared to the infection rate of R. microplus in the environment group (13.4%, 13 out of 97), the infection rates of R. microplus in both Group 1 (22.0%, 11 out of 50) and Group 2 (0%, 0 out of 8) did not exhibit significant differences (p = 0.095 and p = 0.595, respectively). In addition, when comparing the infection rates of R. sanguineus sl in the environment group (7.8%, 4 out of 51), a significant difference (p = 0.023) was found in Group 1 (31.8%, 7 out of 22), whereas no significant difference (p = 1.000) was observed in Group 2 (0%, 0 out of 5).
In the second analysis, the infection rates of ‘Ca. M. haemobos’ were compared across different groups. ‘Ca. M. haemobos’ infection rates were 15.8% (9 out of 57) and 0% (0 out of 21) in the blood samples in Group 1 and Group 2, respectively, and no statistically significant differences (p = 0.124) were observed. Furthermore, when compared to the infection rate of H. longicornis in the environment group (8.7%, 18 out of 208), no significant differences (p = 0.281 and p = 1.000, respectively) were observed in Group 1 (12.0%, 22 out of 184) and Group 2 (6.7%, 1 out of 15). Similarly, the infection rates of R. microplus in Group 1 (24.0%, 12 out of 50) and Group 2 (25.0%, 2 out of 8) showed no significant differences (p = 0.638 and p = 1.000, respectively) compared to the environment group (20.6%, 20 out of 97). In addition, when comparing the infection rate of R. sanguineus sl in the environment group (9.8%, 5 out of 51), no significant differences (p = 0.119 and p = 0.445, respectively) were found in Group 1 (22.7%, 5 out of 22) and Group 2 (20.0%, 1 out of 5). Notably, ‘Ca. M. haemobos’-positive samples, which included one H. longicornis, two R. microplus, and one R. sanguineus sl, were collected from negative cats in Group 2.
M. haemofelis infection rates were 7.0% (4 out of 57) and 0% (0 out of 21) in the blood samples in Group 1 and Group 2, respectively; 6.5% (12 out of 184), 6.7% (1 out of 15), and 6.3% (13 out of 208) in H. longicornis in Group 1, Group 2, and the environment, respectively; 16.0% (8 out of 50), 0% (0 out of 8), and 1.0% (1 out of 97) in R. microplus in Group 1, Group 2, and environment, respectively; and 9.1% (2 out of 22), 0% (0 out of 5), and 0% (0 out of 51) in R. sanguineus sl in Group 1, Group 2, and the environment, respectively. Additional details are provided in Table 3. It is important to highlight that ‘Ca. M. haemominutum’, M. haemofelis, ‘Ca. M. haemobos’, and R. felis were detected in both engorged and un-engorged ticks across all three species, as presented in Table 3.
For further analysis, the longer amplicons from all positive samples of ‘Ca. M. haemobos’ were amplified, recovered, and sequenced. A total of six different sequences were identified among these positive samples. Six strains were selected as representatives for detailed analysis: the HN1807 strain (GenBank Accession Number MH388476) and the HN1841 strain (GenBank Accession Number MH388480), which have been previously described in sheep and R. microplus (14); the HN1921 strain (GenBank Accession Number MW463059) and the HN1933 strain (GenBank Accession Number MW463060), which have been previously described in dogs (16); and two novel sequence types: HN2318 (GenBank Accession Number OR818448) and HN2340 (GenBank Accession Number OR818449). In addition, new sequences were observed in those three tick species. Specifically, the following ‘Ca. M. haemobos’ strains were included in the analysis: clones 307 (EF616467) and 311 (EF616468) (cattle, Switzerland); no. 18 (EU367965) (cattle, Japan); C115, C080, C061, and B001 (MG948630) (cattle, Cuba); I924712 (KT985638) (cattle, Malaysia); HN1804 (MH388478) (tick, China); HN1807 (MH388476) (sheep, China); HN1823 (MH388475) (goat, China); HN1921 (MW463059) (dog, China); and CMboTWN03 (KJ883516) and CMboTWN04 (KJ883517) (cat, China). Furthermore, for the purpose of phylogenetic analysis, the following isolates were included: M. haemofelis isolates Australian no. 2 (AY150977) (Australia) and UK no. 5 (AY150984) (United Kingdom); M. coccoides (AY171918) (United Kingdom, laboratory mouse); Ca. M. turicensis 94–100 (DQ825454) (Tanzania, lion) and B3 (DQ464423) (Australia, cat); M. wenyonii Massachusetts (CP003703) (USA, cattle) and CGXD (EF221880) (China); and M. pneumoniae NBRC 14401 (NR 113659) (Japan, human). The two new representative isolates were compared to other ‘Ca. M. haemobos’ strains available in GenBank, revealing that the 16S rRNA sequences of the two new isolates exhibited 99.34–99.57% identity with those of other isolates. A phylogenetic tree was inferred based on the 16S rRNA sequence (Figure 1), indicating that the two new representative isolates clustered within the species of ‘Ca. M. haemobos’. Moreover, the two new isolates identified in the present study were found to be most closely related to an isolate (China-1) (EF424082) from buffalo in China, while being most distantly related to the isolate from Switzerland (clone 307).
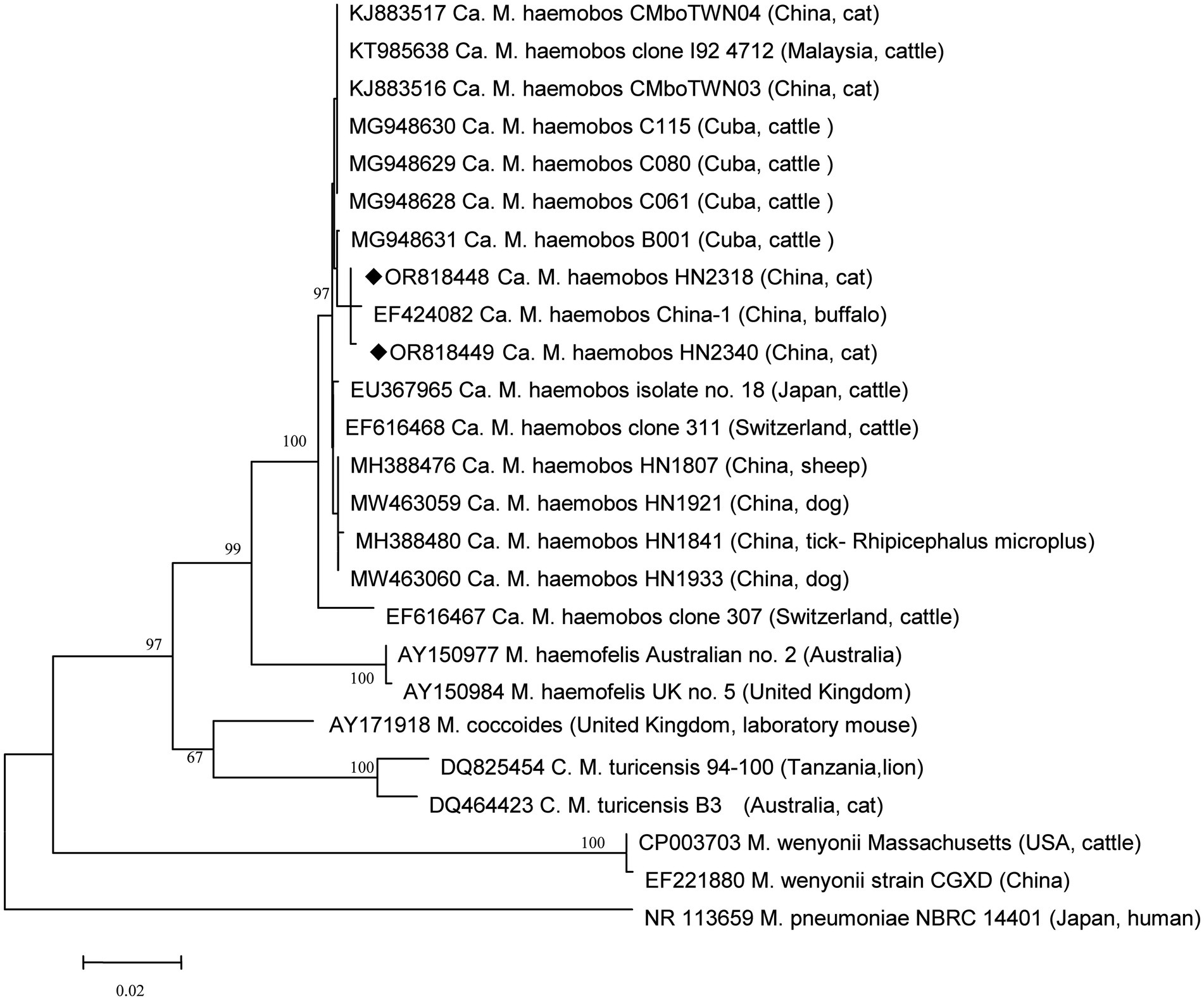
Figure 1. Phylogenetic analysis of ‘Ca. M. haemobos’ from cats in Henan Province, central China, and reference strains using the 16S rRNA sequences. New isolates in this study are highlighted with a symbol (◆).
To date, no studies focusing on ‘Ca. M. haemobos’ in cats have been reported in central China. Given the limited evidence regarding the association between ‘Ca. M. haemobos’ and infections in cats, this study aimed to clarify this issue. Although no significant difference in infection rates was observed between anemic cats and healthy cats, the potential association between ‘Ca. M. haemobos’ and anemic syndrome in cats cannot be excluded. The presence of other pathogens in the anemic cats and the limited sample size in this study may constrain our analysis and conclusions. Further research involving experimental infections in cats is warranted to elucidate the relationship between ‘Candidatus Mycoplasma haemobos’ and feline health. In Henan Province in central China, ‘Ca. M. haemobos’ has been identified in blood samples collected from sick cattle and goats with a rate of 63.9% (23 out of 36) and 58.2% (32 out of 55) (15), blood samples from sick sheep with a rate of 40.0% (10 out of 25) (51), and blood samples from healthy dogs and anemic dogs with the rates of 28.6% (4 out of 14) and 63.4% (26 out of 41), respectively. This study represents the first report of ‘Ca. M. haemobos’ in cats within the country, and the prevalence rate in cats is not much different than in other species. Phylogenetic analysis revealed that the two new isolates identified in this study are not most closely related to the isolates found in goats, sheep, dogs, and ticks in the region described in previous research (14–16), but rather to an isolate from buffalo in China (52). Two possible explanations for this finding included the frequent importation and trading of livestock in China, which may facilitate the spread of ‘Ca. M. haemobos’ to new areas, or the potential evolution of the pathogen as it adapts to new hosts or vectors, such as cats and ticks. Previous studies have indicated that R. microplus and H. longicornis can harbor ‘Ca. M. haemobos’ and are associated with its transmission in goats, cattle, and dogs. Given that the cats in this study share their habitat with these animals and that these ticks can infest cats, the role of ticks in the transmission of ‘Ca. M. haemobos’ to cats remains unclear. Therefore, an investigation was conducted on all ticks parasitizing cats and those present in the environment to address this question.
In Hungary, 21 cattle were diagnosed as positive for ‘Ca. M. haemobos’, and four species of ticks were collected from these animals: Dermacentor reticulatus, Haemaphysalis inermis, Ixodes ricinus, and Dermacentor marginatus. However, all ticks were negative for ‘Ca. M. haemobos’ (53). This study identified three species of ticks. In addition to R. microplus and H. longicornis ticks, which have been documented on goats, cattle, and dogs (14–16), R. sanguineus sl ticks were newly identified as carriers of ‘Ca. M. haemobos’. Notably, ‘Ca. M. haemobos’-positive ticks were detected on both positive and negative cats, including ticks that were not engorged. This finding is consistent with previous research, indicating that the eggs of R. microplus can acquire ‘Ca. M. haemobos’ from female ticks and retain the pathogen during development stages (30). Consequently, it is plausible that the tick collected from a negative cat was indeed positive for the pathogen. Although the presence of ‘Ca. M. haemobos’ in H. longicornis ticks has been reported in earlier studies (15, 16), the potential for transovarial transmission of ‘Ca. M. haemobos’ by female H. longicornis ticks remains to be elucidated. Given the high positive rates of ‘Ca. M. haemobos’ in H. longicornis ticks associated with dogs (24 out of 150) (16), cattle (20 out of 45), goats (6 out of 16) (15), and cats in this study (41 out of 407), it is crucial to investigate whether H. longicornis ticks can act as vectors during their developmental stages and to conduct experimental transmission studies of ‘Ca. M. haemobos’ to potential hosts such as goats, cattle, dogs, and cats in future research. To date, R. sanguineus sl ticks have been identified on cattle, goats, and dogs (54), as well as on cats in four provinces (Hebei, Anhui, Zhejiang, and Guangxi) in China. However, their presence on cats in Henan Province had not been previously reported (55). This study confirms the occurrence of R. sanguineus sl on cats in this region for the first time. While numerous pathogens have been detected in R. sanguineus sl (34), the presence of ‘Ca. M. haemobos’ in this tick had not been documented until this study. Among the positive ticks, one was collected from a negative cat. Considering that R. sanguineus sl is a three-host tick (56), this positive tick may have acquired ‘Ca. M. haemobos’ from a previous host during blood feeding or may have carried the pathogen from an earlier development stage, such as from eggs. In either scenario, ‘Ca. M. haemobos’ could persist in R. sanguineus sl ticks for a certain duration. Furthermore, other livestock species, such as rabbits, pigs, and horses, also serve as hosts for the aforementioned ticks (57, 58). The potential for these animals to become infected with ‘Ca. M. haemobos’ while infested with ticks remains unknown, and future investigations should be carried out to determine the prevalence of ‘Ca. M. haemobos’ across various livestock species. Furthermore, no statistically significant differences (p > 0.05) were observed in the infection rates of ‘Ca. M. haemobos’ among three tick species in cats from both Group 1 and Group 2 when compared to the infection rates of ‘Ca. M. haemobos’ in the environment group. In addition, the majority of positive ticks collected from the environment were not engorged. These findings suggested that the presence of ‘Ca. M. haemobos’ in ticks collected from cats may not be attributable to the parasite acquired through a blood meal. It is possible that ‘Ca. M. haemobos’ can persist in the three tick species for a certain duration before or after infesting the host animals.
In addition to ‘Ca. M. haemobos’, ‘Ca. M. haemominutum’, M. haemofelis, and R. felis were also detected in cats, H. longicornis, R. microplus, and R. sanguineus sl. Specifically, infections with ‘Ca. M. haemominutum’ and M. haemofelis in cats have been reported with prevalence rates of 3.4 and 0.9% in 668 client-owned cats in Beijing and Shanghai, China, respectively (36). Of those 668 cats, 131 were anemic with a hemotropic mycoplasma infection rate of 9.2%. Furthermore, the prevalence rates in Shanghai and Beijing were all lower than those in Henan in this study. Several factors may explain this difference: 668 cats in Shanghai and Beijing were housed in the city and had less exposure to the wild; blood samples were collected not only in the summer season and nearly half of the cats were using ectoparasiticides. In addition, the study did not record whether the positive cats had been infested by ticks. In Iran, 361 blood samples were collected from healthy cats for hemotropic mycoplasma screening; the results showed that the rates of ‘Ca. M. haemominutum’ and M. haemofelis were 10.5 and 2.2%, respectively (59). Similarly in Brazil, ‘Ca. M. haemominutum’ and M. haemofelis were 8.9 and 4.4%, respectively, in 45 healthy stray cats (60). These positive rates are similar to those in healthy cats in our study. In Romania, ‘Ca. M. haemominutum’ and M. haemofelis were 15.7 and 5.9%, respectively, in 51 unhealthy cats (61). These positive rates are lower than those in anemic cats in our study. Based on the above studies, it is indicated that the infection rate of Mycoplasma haemofelis in cats may be related to factors such as countries, regions, the health status of cats, feeding methods, and the season of sample collection.
In northern Switzerland, feline hemotropic mycoplasmas were identified in several Ixodes sp. (2.8%, 2 out of 71) and Rhipicephalus sp. ticks (4.3%, 1 out of 23) collected from animals (62). In Italy, Ixodes ricinus and Ixodes trianguliceps ticks (0.6%, 3 out of 50) were found to be positive for ‘Ca. M. haemominutum’; meanwhile, Ixodes trianguliceps (0.2%, 1 out of 540) was also found to be positive for M. haemofelis (63). In Japan, among eight pools of unfed Ixodes ovatus ticks collected from vegetation, three pools were positive for ‘Ca. M. haemominutum’ (64), and, similarly, Ixodes tanuki ticks (3.3%, 1 out of 30) collected from Tsushima leopard cats were also found to carry this pathogen (65). These studies suggest that ticks have the potential to serve as carriers for feline hemotropic mycoplasmas. These positive rates are lower than those in the three tick species in our study. However, in Italy, one study showed that no hemotropic mycoplasmas have been detected in 17 R. sanguineus sl tick samples collected from cats (66). To the best of our knowledge, neither ‘Ca. M. haemominutum’ nor M. haemofelis has been documented in H. longicornis or R. microplus ticks in China. Our research demonstrates significant differences in the infection rates of ‘Ca. M. haemominutum’ between Groups 1 and 2 for H. longicornis and R. sanguineus sl. These findings suggest that these tick species may serve as potential vectors for this mycoplasma in cats, and that ‘Ca. M. haemominutum’ should be associated with anemia in cats in Group 1. Further studies should evaluate the competence of these ticks in transmitting ‘Ca. M. haematobium’ to cats. R. felis has been detected in Ixodes granulatus ticks from rodents (67) and R. sanguineus sl ticks from dogs (68) in Taiwan. In addition, Rickettsia spp. have been identified in H. longicornis and R. microplus ticks from non-cat hosts, as well as through flagging over vegetation in Jiangxi province, located in southeastern China (69). In Jiangsu province, R. felis was first identified in R. sanguineus sl ticks (45). To date, studies on R. felis infections in cats in China remain limited. Our findings suggest that the prevalence of R. felis should not be overlooked in backyard farms in China, particularly as the presence of these ticks on infected cats may increase the public health risk for individuals in agricultural settings.
This study represents the first investigation of hemoplasmas Mycoplasma in cats and ticks in central China. In addition to ‘Ca. M. haemominutum’ and M. haemofelis, ‘Ca. M. haemobos’ was first detected in cats. ‘Ca. M. haemominutum’ appears to be associated with anemic syndrome in cats, while further research is warranted to explore the relationship between ‘Ca. M. haemobos’ and clinical signs in felines. Specifically, two types of ‘Ca. M. haemobos’ sequences have been detected both in ticks and cats. Meanwhile, these three hemotropic mycoplasmas were also found in the parasitic ticks and questing ticks, including H. longicornis, R. microplus, and R. sanguineus sl. Among these findings, ‘Ca. M. haemominutum’ and M. haemofelis in H. longicornis and R. microplus, and ‘Ca. M. haemobos’ in R. sanguineus sl. were first documented in China. In the future, transmission experiments are needed to investigate the capacity for transmitting hemoplasmas Mycoplasma among animals by these ticks.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The animal studies were approved by the Animal Welfare and Ethics Committee of Nanyang Normal University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
HS: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. GL: Data curation, Investigation, Methodology, Resources, Writing – review & editing. DL: Data curation, Software, Visualization, Writing – review & editing. HZ: Resources, Validation, Writing – review & editing. SJ: Investigation, Validation, Writing – review & editing. YH: Funding acquisition, Validation, Writing – review & editing. LW: Supervision, Writing – review & editing. LY: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The Natural Science Foundation of Henan province (Grant no. 242300421334), Scientific and Technological Project of Henan Province (Grant no. 222102110260), Key Research Projects of Higher Education Institutions in Henan Province (CN) (Grant no. 22A180026 and 22B230001) and the NanYang Science and Technology Research Project (KJGG136) supported the sample collection, analysis and interpretation of data in this study.
We thank AJE (www.AJE.com) for their linguistic assistance during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1522904/full#supplementary-material
Supplementary Figure 1 | The results of PCR analysis of the partial 16S rRNA gene of hemotropic mycoplasmas conducted on some blood and tick samples. The DNA marker is represented by the letter M, while negative and positive controls are indicated by “-” and “+”. Blood and tick samples numbered 1 through 22 were analyzed.
Supplementary Figure 2 | The results of PCR analysis of the long 16S rRNA gene of ‘Ca. M. haemobos’ conducted on some blood and tick samples that tested positive for ‘Ca. M. haemobos’ by sequencing. The DNA marker is represented by the letter M, while negative control is indicated by “-”. Positive blood and tick samples numbered 1 through 23 were analyzed.
Supplementary Table 1 | Isolates, organism, host and GenBank number of hemotropic mycoplasmas samples.
1. Neimark, H, Johansson, KE, Rikihisa, Y, and Tully, JG. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of 'Candidatus Mycoplasma haemofelis', 'Candidatus Mycoplasma haemomuris', 'Candidatus Mycoplasma haemosuis' and 'Candidatus Mycoplasma wenyonii'. Int J Syst Evol Microbiol. (2001) 51:891–9. doi: 10.1099/00207713-51-3-891
2. VLN. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int J Syst Bacteriol. (1984) 34:355–7. doi: 10.1099/00207713-34-3-355
3. Foley, JE, and Pedersen, NC. 'Candidatus Mycoplasma haemominutum', a low-virulence epierythrocytic parasite of cats. Int J Syst Evol Microbiol. (2001) 51:815–7. doi: 10.1099/00207713-51-3-815
4. Willi, B, Boretti, FS, Cattori, V, Tasker, S, Meli, ML, Reusch, C, et al. Identification, molecular characterization, and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anemia in Switzerland. J Clin Microbiol. (2005) 43:2581–5. doi: 10.1128/jcm.43.6.2581-2585.2005
5. Sykes, JE, Drazenovich, NL, Ball, LM, and Leutenegger, CM. Use of conventional and real-time polymerase chain reaction to determine the epidemiology of hemoplasma infections in anemic and nonanemic cats. J Vet Intern Med. (2007) 21:685–93. doi: 10.1892/0891-6640(2007)21[685:uocarp]2.0.co;2
6. Martínez-Díaz, VL, Silvestre-Ferreira, AC, Vilhena, H, Pastor, J, Francino, O, and Altet, L. Prevalence and co-infection of haemotropic mycoplasmas in Portuguese cats by real-time polymerase chain reaction. J Feline Med Surg. (2013) 15:879–85. doi: 10.1177/1098612x13480985
7. Walker Vergara, R, Morera Galleguillos, F, Gómez Jaramillo, M, Pereira Almosny, NR, Arauna Martínez, P, Grob Behne, P, et al. Prevalence, risk factor analysis, and hematological findings of hemoplasma infection in domestic cats from Valdivia, southern Chile. Comp Immunol Microbiol Infect Dis. (2016) 46:20–6. doi: 10.1016/j.cimid.2016.03.004
8. Neimark, H, Johansson, KE, Rikihisa, Y, and Tully, JG. Revision of haemotrophic Mycoplasma species names. Int J Syst Evol Microbiol. (2002) 52:683. doi: 10.1099/00207713-52-2-683
9. Álvarez-Fernández, A, Maggi, R, Martín-Valls, GE, Baxarias, M, Breitschwerdt, EB, and Solano-Gallego, L. Prospective serological and molecular cross-sectional study focusing on Bartonella and other blood-borne organisms in cats from Catalonia (Spain). Parasit Vectors. (2022) 15:6. doi: 10.1186/s13071-021-05105-6
10. Hodžić, A, Alić, A, and Duscher, GG. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: A molecular study. Ticks Tick Borne Dis. (2018) 9:589–93. doi: 10.1016/j.ttbdis.2018.01.017
11. Tagawa, M, Matsumoto, K, and Inokuma, H. Molecular detection of Mycoplasma wenyonii and 'Candidatus Mycoplasma haemobos' in cattle in Hokkaido. Japan Vet Microbiol. (2008) 132:177–80. doi: 10.1016/j.vetmic.2008.05.006
12. Santos, NJR, Brito, DRB, Abate, HL, Paixao, SF, Soares, EDS, Vieira, T, et al. Hemotropic mycoplasmas infection in water buffaloes (Bubalus bubalis) from northeastern Brazil. Comp Immunol Microbiol Infect Dis. (2018) 56:27–9. doi: 10.1016/j.cimid.2017.12.003
13. Hornok, S, Sugar, L, Fernandez de Mera, IG, de la Fuente, J, Horvath, G, Kovacs, T, et al. Tick-and fly-borne bacteria in ungulates: the prevalence of Anaplasma phagocytophilum, haemoplasmas and rickettsiae in water buffalo and deer species in Central Europe, Hungary. BMC Vet Res. (2018) 14:98. doi: 10.1186/s12917-018-1403-6
14. Shi, H, Hu, Y, Leng, C, Shi, H, Jiao, Z, Chen, X, et al. Molecular investigation of "Candidatus Mycoplasma haemobos" in goats and sheep in Central China. Transbound Emerg Dis. (2019) 66:22–7. doi: 10.1111/tbed.13021
15. Shi, H, Hui, R, Zhou, M, Wang, L, Li, G, Bai, Y, et al. Abortion outbreak in pregnant goats and cows with coinfection of ‘Candidatus Mycoplasma haemobos’ and HoBi-like pestivirus. Vet Microbiol. (2023) 279:109690. doi: 10.1016/j.vetmic.2023.109690
16. Shi, H, Li, B, Li, J, Chen, S, Wang, L, Bai, Z, et al. Molecular detection of haemophilic pathogens reveals evidence of Candidatus Mycoplasma haemobos in dogs and parasitic ticks in Central China. BMC Vet Res. (2022) 18:254. doi: 10.1186/s12917-022-03361-x
17. Hoelzle, K, Winkler, M, Kramer, MM, Wittenbrink, MM, Dieckmann, SM, and Hoelzle, LE. Detection of Candidatus Mycoplasma haemobos in cattle with anaemia. Vet J. (2011) 187:408–10. doi: 10.1016/j.tvjl.2010.01.016
18. Baggenstos, R, Wenzinger, B, Meli, ML, Hofmann-Lehmann, R, and Knubben-Schweizer, G. Haemoplasma infection in a dairy cow. Tierarztl Prax Ausg G Grosstiere Nutztiere. (2012) 40:397–400. doi: 10.1055/s-0038-1623137
19. Tagawa, M, Yamakawa, K, Aoki, T, Matsumoto, K, Ishii, M, and Inokuma, H. Effect of chronic hemoplasma infection on cattle productivity. J Vet Med Sci. (2013) 75:1271–5. doi: 10.1292/jvms.13-0119
20. Happi, AN, Osifade, O, Oluniyi, PE, and Ogunro, BN. Comparison of light microscopy and polymerase chain reaction for the detection of Haemoparasites in cattle in Nigeria. Acta Parasitol. (2020) 65:44–56. doi: 10.2478/s11686-019-00123-y
21. Byamukama, B, Tumwebaze, MA, Tayebwa, DS, Byaruhanga, J, Angwe, MK, Li, J, et al. First molecular detection and characterization of Hemotropic Mycoplasma species in cattle and goats from Uganda. Animals. (2020) 10:1624. doi: 10.3390/ani10091624
22. Sasaoka, F, Suzuki, J, Watanabe, Y, Fujihara, M, Nagai, K, Hirata, T, et al. Two genotypes among 'Candidatus Mycoplasma haemobos' strains based on the 16S-23S rRNA intergenic spacer sequences. J Vet Med Sci. (2013) 75:361–4. doi: 10.1292/jvms.12-0349
23. Fujihara, Y, Sasaoka, F, Suzuki, J, Watanabe, Y, Fujihara, M, Ooshita, K, et al. Prevalence of hemoplasma infection among cattle in the western part of Japan. J Vet Med Sci. (2011) 73:1653–5. doi: 10.1292/jvms.11-0269
24. Galon, EMS, Yba Nez, RHD, Adjou Moumouni, PF, Tumwebaze, MA, Fabon, RJA, Callanta, MRR, et al. Molecular survey of tick-borne pathogens infecting backyard cattle and water buffaloes in Quezon province. Philippines J Vet Med Sci. (2020) 82:886–90. doi: 10.1292/jvms.19-0636
25. Altay, K, Sahin OFErol, U, and Aytmirzakizi, A. First molecular detection and phylogenetic analysis of Mycoplasma wenyonii and Candidatus Mycoplasma haemobos in cattle in different parts of Kyrgyzstan. Biologia. (2023) 78:633–40. doi: 10.1007/s11756-022-01292-4
26. Ayling, RD, Bisgaard-Frantzen, S, Adler, A, Blowey, RW, Barlow, AM, Millar, MF, et al. Detection of 'Candidatus Mycoplasma haemobos', Mycoplasma wenyonii and Anaplasma phagocytophilum from cattle in England. Vet Rec. (2012) 170:543. doi: 10.1136/vr.100636
27. Erol, U, and Sahin OFAltay, K. Molecular prevalence of bovine hemoplasmosis in Turkey with first detection of Mycoplasma wenyonii and Candidatus Mycoplasma haemobos in cattle and water buffalo. Vet Res Commun. (2023) 47:207–15. doi: 10.1007/s11259-022-09943-2
28. de Souza, FL, Bolin, S, Abuelo, A, Norby, B, and Ruegg, PL. Apparent prevalence of hemotropic mycoplasma in dairy calves and replacement heifers on Michigan farms. J Dairy Sci. (2024) 107:4987–5000. doi: 10.3168/jds.2023-24395
29. Martinez-Ocampo, F, Rodriguez-Camarillo, SD, Amaro-Estrada, I, and Quiroz-Castaneda, RE. Draft genome sequence of "Candidatus Mycoplasma haemobos, " a Hemotropic Mycoplasma identified in cattle in Mexico. Genome Announc. (2016) 4:e00656–16. doi: 10.1128/genomeA.00656-16
30. Shi, H, Duan, L, Liu, F, Hu, Y, Shi, Z, Chen, X, et al. Rhipicephalus (Boophilus) microplus ticks as reservoir and vector of 'Candidatus Mycoplasma haemobos' in China. Vet Parasitol. (2019) 274:108929. doi: 10.1016/j.vetpar.2019.108929
31. de Miranda, RL, de Castro, JR, Olegário, MM, Beletti, ME, Mundim, AV, O'Dwyer, LH, et al. Oocysts of Hepatozoon canis in Rhipicephalus (Boophilus) microplus collected from a naturally infected dog. Vet Parasitol. (2011) 177:392–6. doi: 10.1016/j.vetpar.2011.01.044
32. Szabó, MP, de Souza, LG, Olegário, MM, Ferreira, FA, and de Albuquerque Pajuaba Neto, A. Ticks (Acari: Ixodidae) on dogs from Uberlândia, Minas Gerais, Brazil. Transbound Emerg Dis. (2010) 57:72–4. doi: 10.1111/j.1865-1682.2010.01111.x
33. Lu, X, Lin, XD, Wang, JB, Qin, XC, Tian, JH, Guo, WP, et al. Molecular survey of hard ticks in endemic areas of tick-borne diseases in China. Ticks Tick Borne Dis. (2013) 4:288–96. doi: 10.1016/j.ttbdis.2013.01.003
34. Colella, V, Nguyen, VL, Tan, DY, Lu, N, Fang, F, Zhijuan, Y, et al. Zoonotic Vectorborne pathogens and Ectoparasites of dogs and cats in eastern and Southeast Asia. Emerg Infect Dis. (2020) 26:1221–33. doi: 10.3201/eid2606.191832
35. Zhuang, QJ, Zhang, HJ, Lin, RQ, Yuan, ZG, and Zhu, XQ. The occurrence of Mycoplasma haemofelis and Candidatus Mycoplasma Haemominutum in cats in China confirmed by sequence-based analysis of ribosomal DNA. J Animal Vet Adv. (2010) 9:635–8. doi: 10.3923/javaa.2010.635.638
36. Zhang, Y, Zhang, Z, Lou, Y, and Yu, Y. Prevalence of hemoplasmas and Bartonella species in client-owned cats in Beijing and Shanghai. China J Vet Med Sci. (2021) 83:793–7. doi: 10.1292/jvms.20-0681
37. Bouyer, DH, Stenos, J, Crocquet-Valdes, P, Moron, CG, Popov, VL, Zavala-Velazquez, JE, et al. Rickettsia felis: molecular characterization of a new member of the spotted fever group. Int J Syst Evol Microbiol. (2001) 51:339–47. doi: 10.1099/00207713-51-2-339
38. Newman, BC, Sutton, WB, Wang, Y, Schweitzer, CJ, Moncayo, AC, and Miller, BT. A standardized method for the construction of a tick drag/flag sampling approach and evaluation of sampling efficacy. Exp Appl Acarol. (2019) 79:433–46. doi: 10.1007/s10493-019-00429-6
39. Yang, T, Zhou, Y, Lu, Z, and Xia, Z. Case report of secondary Evans syndrome in a cat infected with hemotropic mycoplasma. Chinese J Vet Med. (2024) 60:137–40.
41. Nicholson, WL, Sonenshine, DE, Noden, BH, and Brown, RN. Chapter 27- ticks (Ixodida) In: GR Mullen, editor. Medical and veterinary entomology (third edition). Durden LA: Academic Press (2019). 603–72.
42. Beati, L, and Keirans, JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. (2001) 87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2
43. Jensen, WA, Lappin, MR, Kamkar, S, and Reagan, WJ. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis in naturally infected cats. Am J Vet Res. (2001) 62:604–8. doi: 10.2460/ajvr.2001.62.604
44. Meli, ML, Willi, B, Dreher, UM, Cattori, V, Knubben-Schweizer, G, Nuss, K, et al. Identification, molecular characterization, and occurrence of two bovine hemoplasma species in Swiss cattle and development of real-time Taq man quantitative PCR assays for diagnosis of bovine hemoplasma infections. J Clin Microbiol. (2010) 48:3563–8. doi: 10.1128/JCM.02224-09
45. Zhang, J, Lu, G, Kelly, P, Zhang, Z, Wei, L, Yu, D, et al. First report of Rickettsia felis in China. BMC Infect Dis. (2014) 14:682. doi: 10.1186/s12879-014-0682-1
46. Santos, F, Coppede, JS, Pereira, AL, Oliveira, LP, Roberto, PG, Benedetti, RB, et al. Molecular evaluation of the incidence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs from Ribeirão Preto, Brazil. Vet J. (2009) 179:145–8. doi: 10.1016/j.tvjl.2007.08.017
47. Hodžić, A, Alić, A, Beck, R, Beck, A, Huber, D, Otranto, D, et al. Hepatozoon martis n. sp. (Adeleorina: Hepatozoidae): morphological and pathological features of a Hepatozoon species infecting martens (family Mustelidae). Ticks Tick Borne Dis. (2018) 9:912–20. doi: 10.1016/j.ttbdis.2018.03.023
48. Masatani, T, Hayashi, K, Andoh, M, Tateno, M, Endo, Y, Asada, M, et al. Detection and molecular characterization of Babesia, Theileria, and Hepatozoon species in hard ticks collected from Kagoshima, the southern region in Japan. Ticks Tick Borne Dis. (2017) 8:581–7. doi: 10.1016/j.ttbdis.2017.03.007
49. Tamura, K, Stecher, G, Peterson, D, Filipski, A, and Kumar, S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. (2013) 30:2725–9. doi: 10.1093/molbev/mst197
50. Roblejo-Arias, L, Díaz-Sánchez, AA, Corona-González, B, Meli, ML, Fonseca-Rodríguez, O, Rodríguez-Mirabal, E, et al. First molecular evidence of Mycoplasma haemocanis and 'Candidatus Mycoplasma haematoparvum' infections and its association with epidemiological factors in dogs from Cuba. Acta Trop. (2022) 228:106320. doi: 10.1016/j.actatropica.2022.106320
51. Shi, H, Kan, Y, Yao, L, Leng, C, Tang, Q, Ji, J, et al. Identification of natural infections in sheep/goats with HoBi-like Pestiviruses in China. Transbound Emerg Dis. (2016) 63:480–4. doi: 10.1111/tbed.12551
52. Su, QL, Song, HQ, Lin, RQ, Yuan, ZG, Yang, JF, Zhao, GH, et al. The detection of "Candidatus Mycoplasma haemobos" in cattle and buffalo in China. Trop Anim Health Prod. (2010) 42:1805–8. doi: 10.1007/s11250-010-9640-0
53. Hornok, S, Micsutka, A, Fernandez de Mera, IG, Meli, ML, Gonczi, E, Tanczos, B, et al. Fatal bovine anaplasmosis in a herd with new genotypes of Anaplasma marginale, Anaplasma ovis and concurrent haemoplasmosis. Res Vet Sci. (2012) 92:30–5. doi: 10.1016/j.rvsc.2010.10.011
54. Intirach, J, Lv, X, Han, Q, Lv, ZY, and Chen, T. Morphological and molecular identification of hard ticks in Hainan Island, China. Genes. (2023) 14:1592. doi: 10.3390/genes14081592
55. Liu, Q. Research Progress of ticks and tick-borne disease. J Anhui Agric Sci. (2013) 41:1107–1109. doi: 10.13989/j.cnki.0517-6611.2013.03.005
56. Dantas-Torres, F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. (2008) 152:173–85. doi: 10.1016/j.vetpar.2007.12.030
57. BIng, H, and Xinhua, H. The epidemiology, preventive and treat measures of Boophilus microplus in Xinyang area. Shandong J Animal Sci Vet Med. (2011) 32:27–8.
58. Ruili, J. Invesigation of hard ticks distribution in Fuzhou and researches on the biological characteristics of Rhipicephalus (Boophilus) microplus. Fujian normal university. (2011).
59. Hoseinpoor, E, Goudarztalejerdi, A, and Sazmand, A. Molecular prevalence and phylogenetic analysis of hemotropic Mycoplasma species in cats in different regions of Iran. BMC Microbiol. (2024) 24:198. doi: 10.1186/s12866-024-03356-8
60. Yamakawa, AC, Haisi, A, Kmetiuk, LB, Pellizzaro, M, Mendes, JCR, Canavessi, AMO, et al. Molecular detection of feline hemoplasmas and retroviruses in free-roaming and shelter cats within a university campus. JFMS Open Rep. (2023) 9:20551169221148672. doi: 10.1177/20551169221148672
61. Imre, M, Văduva, C, Dărăbuș, G, Morariu, S, Herman, V, Plutzer, J, et al. Molecular detection of hemotropic mycoplasmas (hemoplasmas) in domestic cats (Felis catus) in Romania. BMC Vet Res. (2020) 16:399. doi: 10.1186/s12917-020-02626-7
62. Willi, B, Boretti, FS, Meli, ML, Bernasconi, MV, Casati, S, Hegglin, D, et al. Real-time PCR investigation of potential vectors, reservoirs, and shedding patterns of feline hemotropic mycoplasmas. Appl Environ Microbiol. (2007) 73:3798–802. doi: 10.1128/aem.02977-06
63. Duplan, F, Davies, S, Filler, S, Abdullah, S, Keyte, S, Newbury, H, et al. Anaplasma phagocytophilum, Bartonella spp., haemoplasma species and Hepatozoon spp. in ticks infesting cats: a large-scale survey. Parasit Vectors. (2018) 11:201. doi: 10.1186/s13071-018-2789-5
64. Taroura, S, Shimada, Y, Sakata, Y, Miyama, T, Hiraoka, H, Watanabe, M, et al. Detection of DNA of 'Candidatus Mycoplasma haemominutum' and Spiroplasma sp. in unfed ticks collected from vegetation in Japan. J Vet Med Sci. (2005) 67:1277–9. doi: 10.1292/jvms.67.1277
65. Tateno, M, Sunahara, A, Nakanishi, N, Izawa, M, Matsuo, T, Setoguchi, A, et al. Molecular survey of arthropod-borne pathogens in ticks obtained from Japanese wildcats. Ticks Tick Borne Dis. (2015) 6:281–9. doi: 10.1016/j.ttbdis.2015.01.009
66. Pennisi, MG, Persichetti, MF, Serrano, L, Altet, L, Reale, S, Gulotta, L, et al. Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy). Parasit Vectors. (2015) 8:512. doi: 10.1186/s13071-015-1128-3
67. Shih, CM, Yang, PW, and Chao, LL. Molecular detection and genetic identification of Rickettsia infection in Ixodes granulatus ticks, an incriminated vector for geographical transmission in Taiwan. Microorganisms. (2021) 9:1309. doi: 10.3390/microorganisms9061309
68. Shih, CM, and Chao, LL. First detection and genetic identification of Rickettsia infection in Rhipicephalus sanguineus (Acari: Ixodidae) ticks collected from southern Taiwan. Exp Appl Acarol. (2021) 85:291–304. doi: 10.1007/s10493-021-00669-5
Keywords: cat, Haemaphysalis longicornis, Rhipicephalus microplus, Candidatus Mycoplasma haemobos, Rhipicephalus sanguineus sensu lato
Citation: Shi H, Li G, Li D, Zhai H, Ji S, Hu Y, Wang L and Yao L (2025) Molecular investigation reveals three hemotropic mycoplasmas in cats and three tick species in China. Front. Vet. Sci. 12:1522904. doi: 10.3389/fvets.2025.1522904
Received: 19 November 2024; Accepted: 06 January 2025;
Published: 30 January 2025.
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
Benjamin Cull, University of Minnesota Twin Cities, United StatesCopyright © 2025 Shi, Li, Li, Zhai, Ji, Hu, Wang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongfei Shi, a2NuMUAxNjMuY29t; Lunguang Yao, bHVuZ3Vhbmd5YW9AMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.