
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 26 February 2025
Sec. Animal Nutrition and Metabolism
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1520678
This article is part of the Research Topic Omics Research in Canine and Feline Microbiome: Implications for Veterinary Medicine and Companion Animal Health View all 10 articles
 Lulu Wu1†
Lulu Wu1† Lixun Xue1†
Lixun Xue1† Xin Ding1
Xin Ding1 Huyan Jiang2
Huyan Jiang2 Ranran Zhang1
Ranran Zhang1 Aifang Zheng1
Aifang Zheng1 Yuan Zu1
Yuan Zu1 Shuaishuai Tan1
Shuaishuai Tan1 Xin Wang1
Xin Wang1 Zhigang Liu1,3,4,5*
Zhigang Liu1,3,4,5*Colitis is a complicated disease caused by multiple factors, seriously threatening the host health and the development of animal husbandry. Probiotics have been demonstrate to participate in the active regulation of multiple gastrointestinal disease, gut microbiota and metabolism, but research on the efficacy of Pediococcus acidilactici isolated from dogs in alleviating colitis remains scarce. Here, we aimed to investigate the ameliorative effects of Pediococcus acidilactici isolated from dogs on colitis induced by LPS and its underlying molecular mechanisms. For this purpose, we collected colon contents from 15 mice for amplicon sequencing and metabolic analysis. Results showed that Pediococcus acidilactici could relieve the colon damage and cytokine disorder caused by colitis. Microbiome analysis showed that colitis could cause a significant decrease in the gut microbial diversity and abundance, but Pediococcus acidilactici administration could restore the microbial index to the control level. Metabolomics analysis showed that 8 metabolic pathways and 5 (spermine, L-Arginine, 15-Deoxy-Delta12,14-PGJ2, prostaglandin J2, and 15(S)-HETE) metabolites may be involved in the alleviation of colitis by Pediococcus acidilactici. In summary, these findings demonstrated that the positive regulation effect of Pediococcus acidilactici on gut microbiota and metabolism may be one of its underlying mechanisms to alleviate colitis. Additionally, this study also conveyed a vital message that Pediococcus acidilactici isolated from dogs may serve as a promising candidate to ameliorate Pediococcus acidilactici.
The intestine harbors approximately 1014 microbial cells involving over 2,000 distinct species (1–3). These gut-inhabiting microbes, also known as gut microbiota, have been showed to function in host health, metabolism, intestinal homeostasis and intestinal barrier maintenance (4–9). Additionally, the gut microbiota is also essential participant and maintainer of the intestinal mucosal barrier, which play key roles in preventing pathogen invasion and maintaining intestinal homeostasis (6, 7, 10). Gut microbial community, as crucial biochemical converters, can transform the complex chemical space presented by nutrition and diet into the metabolite environment (11, 12). These metabolites including cholic acid, indole derivatives and short-chain fatty acids (SCFAs) participate in the positive regulation of the host health and intestinal homeostasis by acting on the intestine or other organ systems (13). However, gut microbial homeostats is susceptible to external factors, especially gastrointestinal related diseases (1, 14).
Inflammatory bowel disease (IBD) is a chronic intermittent disease primarily affecting the rectal and colon mucosa (15, 16). It is characterized by intestinal inflammation and damage to the epithelial barrier (17). IBD has gained significant attention in recent years due to its detrimental impact on host health. The prevalence of IBD exceeds 0.3% in developed countries, and its incidence rate is also gradually increasing in newly industrialized countries. Animals, such as dogs, cats, horses, and dairy cows, are also affected by IBD, leading to substantial economic losses and threats to animal welfare (18, 19). IBD is a complex disease influenced by factors such as diet, stress, genetics, and the environment (20). Recent studies have also linked colitis to gut microbial dysbiosis (21–23). Clinically, antibiotics, steroids, and immunosuppressants are commonly used to treat IBD. However, these therapies have drawbacks, including drug dependence, high cost, and antibiotic resistance, particularly for patients requiring long-term medication (24, 25). Therefore, the discovery of healthy and effective management options for IBD is crucial. Emerging research indicates that the regulation of gut microbiota and its metabolites holds great potential in IBD treatment (26–28). Dietary intervention, particularly the probiotics administration, is currently considered one of the most effective methods for regulating gut microbiota and metabolism (29).
Probiotics are microorganisms, such as Pediococcus acidilactici, Bifidobacterium, and Bacillus subtilis, that provide benefits to the host when consumed in sufficient amounts (30, 31). Previous studies have demonstrated the positive impact of Pediococcus acidilactici on host growth performance, digestive enzyme activity, intestinal villus height, and antioxidant capacity (32–34). Furthermore, Pediococcus acidilactici has been found to aintain gut microbial homeostasis and improve intestinal barrier function, suggesting their potential in alleviating gastrointestinal diseases (26, 27, 35). The interaction between probiotics, gut microbiota, and the host has become a significant focus in gastrointestinal disease research. Although there is substantial evidence supporting the alleviative effects of probiotics on colitis, there is a lack of studies specifically investigating the dogs source of Pediococcus acidilactici. Therefore, our objective is to evaluate whether Pediococcus acidilactici derived from dogs can alleviate colitis by modulating gut microbiota and metabolism. Meanwhile, this research will contribute to the expansion of canine probiotics applications and establish a foundation for the prevention and treatment of colitis using probiotics.
In this study, 24 specific pathogen-free (SPF) male Kunming mice (8-week-old, 42–44 g) were randomly divided into three groups following 3 days of adaptive feeding: control group (CON), Pediococcus acidilactici treatment group (RSPQ), and the colitis group (DSS). There were 8 mice in each group. The mice were maintained under standard temperature and humidity conditions and provided with a sufficient diet and drinking water. Additionally, from day 1 to day 7, the DSS and RSPQ groups received drinking water supplemented with 3% (w/v) dextran sulfate sodium salt (DSS) to induce colitis. The specific steps of Pediococcus acidilactici preparation refer to previous research (36). During days 8–14 of the experiment, the RSPQ group was supplemented with Pediococcus acidilactici (0.2 mL, 5 × 109 CFU/mL) that had been prepared in advance, while the DSS and CON groups received an equivalent volume of normal saline. At the conclusion of the experiment on day 15, all mice were euthanized, and colon tissue, colon contents, and serum samples were collected for subsequent analysis.
According to previous studies, the DNA of each samples was extracted using a commercial kit (37). We designed universal primers (338F: ACTCCTACGGGAG GCAGCAG-3 and 806R: GGACTACHVGGGTWTCTAAT) and added sequencing adapters for PCR amplification. Subsequently, the amplified products were purified, quantified, and normalized to form sequencing libraries. The DNA was quantified via utilizing UV–Vis spectrophotometer (NanoDrop 2000, United States) and DNA integrity was assessed by 0.8% agarose gel electrophoresis. Sequencing libraries were constructed using PacBio platform (Biomarker Technologies, China) according to the manufacturer’s specifications. The constructed libraries needed to be quality checked (concentration more than 2 nM), and the qualified libraries were sequenced using Illumina Novaseq 6000. The raw image data files were converted into raw sequencing sequences through base calling analysis. Meanwhile, the results were stored in the FASTQ (abbreviated as fq) file format, which contains the sequence information of the sequencing sequences (Reads) and their corresponding sequencing quality information. Moreover, the analysis of gut microbiota included the following operations: (1) Quality control of the original sequencing sequences to remove unqualified data; (2) OTUs clustering and classification based on sequence composition; (3) According to the OTUs results, taxonomic analysis of samples at various taxonomic levels was performed to explore gut microbial composition; (4) Alpha diversity indices were calculated and explore the species diversity within individual sample; (5) Beta diversity analysis was used for comparing gut microbial construction; (6) Statistical analyses were performed using GraphPad Prism (version 9.0c) and R (v3.0.3) software. Differential taxa associated with colitis were identified using Metastats analysis. Data are expressed as mean ± SEM, and statistically significant differences are denoted as p < 0.05.
In this study, we prepared tissue sections and HE staining according to previous studies (38, 39). Meanwhile, the IL-6, TNF-α, and IL-1β levels were conducted in accordance with the recommendations of the ELISA kits.
To further investigate the effects of Pediococcus acidilactici on the intestinal metabolism, we explored changes in intestinal metabolism using untargeted metabolomics. The metabolomic procedure such as sample preparation, metabolite identification, data processing and metabolic pathway analysis were determined as per previous research (9, 40).
In this research, we observed that Pediococcus acidilactici administration can restore colitis-induced weight loss in mice (Supplementary Figure S1). Moreover, the histopathological results of each group are presented in Figures 1A–C. Results indicated that the colon in the CON group was clear and no damage was observed. However, the colon of the DSS group exhibited extensive ulceration, a loss of mucosal and intestinal gland architecture, a reduced number of goblet cells, significant hyperplasia and repair of connective tissue (green arrows), and an abundance of newly formed blood vessels (gray arrows). Furthermore, a notable infiltration of lymphocytes was observed in the lamina propria of the colon tissue in the DSS group (blue arrows), alongside a limited presence of eosinophils in the intestinal glands (orange arrows). However, Pediococcus acidilactici administration can reduce the range of ulcers and restore colon damage. Serum cytokine analysis revealed that the levels of IL-6, TNF-α, and IL-1β were significantly increased in the DSS group compared with the CON group (Figures 1D–F). However, Pediococcus acidilactici administration could significantly reduce the increase in the levels of the above cytokines caused by colitis.
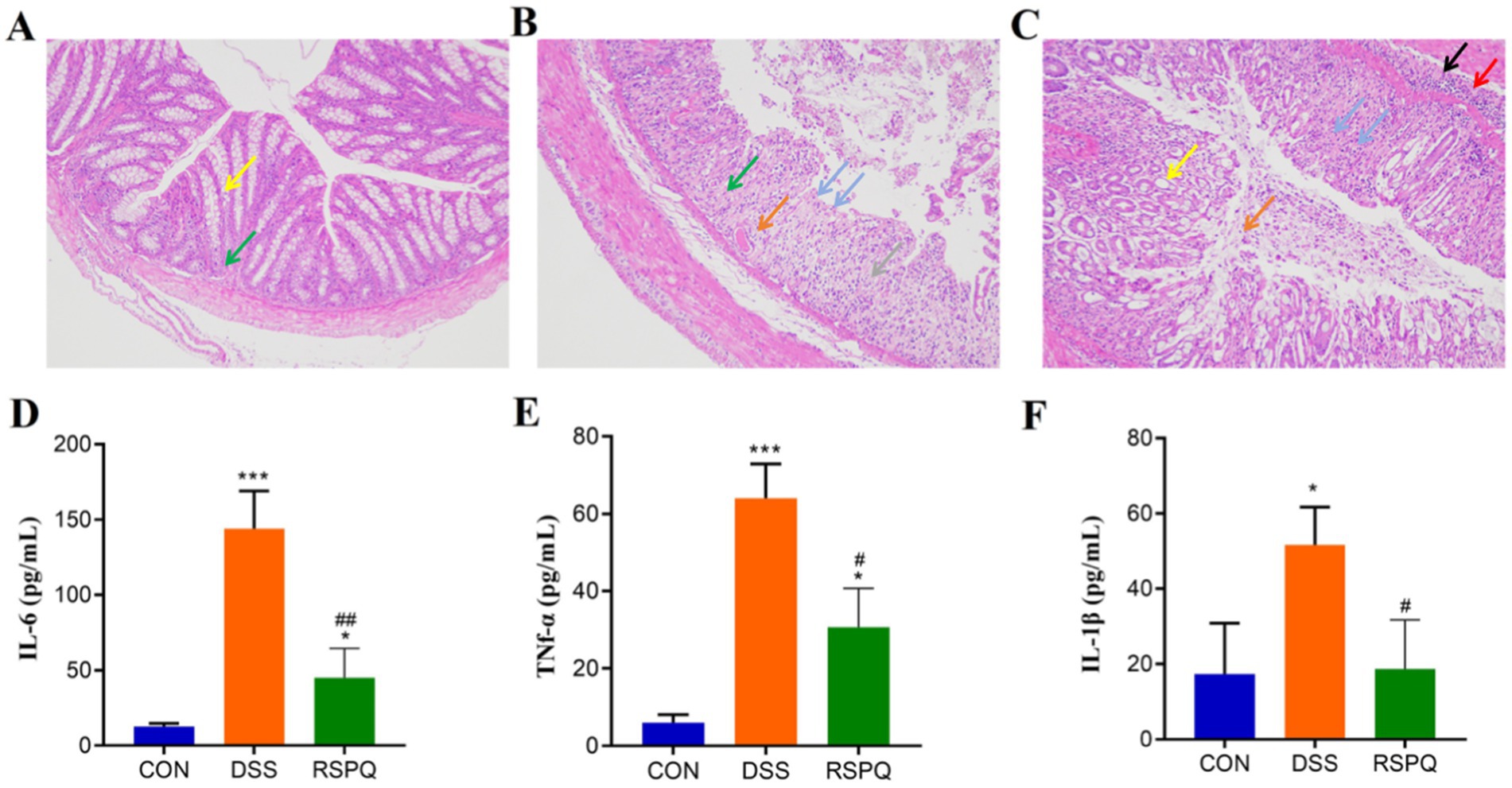
Figure 1. Pediococcus acidilactici alleviate intestinal damage and cytokine disorder caused by colitis. (A–C) Histopathological observation of colitis in the CON, DSS and RSPQ. (D–F) Serum concentrations of IL-6, TNF-α, and IL-1β. *p < 0.05 and ***p < 0.001 vs. the CON, #p < 0.05 and ##p < 0.01 vs. the DSS.
In this research, we explored the differences of the gut microbial abundance and diversity by comparing ACE, Chao1, PD_whole_tree and Shannon. There were statistically significant differences in the gut microbial ACE (587.55 ± 40.35 versus 433.04 ± 27.88, p < 0.05), Chao1 (583.76 ± 40.46 versus 428.63 ± 28.09, p < 0.05), PD_whole_tree (58.05 ± 6.46 versus 40.19 ± 2.27, p < 0.05) and Shannon (6.52 ± 0.06 versus 5.63 ± 0.31, p < 0.05) indices between DSS and CON groups, whereas the above-mentioned indices were not significantly different between the CON and RSPQ groups (Figures 2A–D). Intergroup analysis intuitively revealed that colitis could significantly decrease gut microbial abundance and diversity, thereby causing gut microbial dysbiosis. However, Pediococcus acidilactici administration could restore the gut microbial diversity and abundance to the control level and maintain gut microbial homeostasis. Moreover, PCoA plots generated from the weighted and unweighted UniFrac distances were applied to assess the beta diversity. Results revealed that the individuals in these groups were clustered together, suggesting no significant differences in the gut microbial construction (Figures 2E,F).
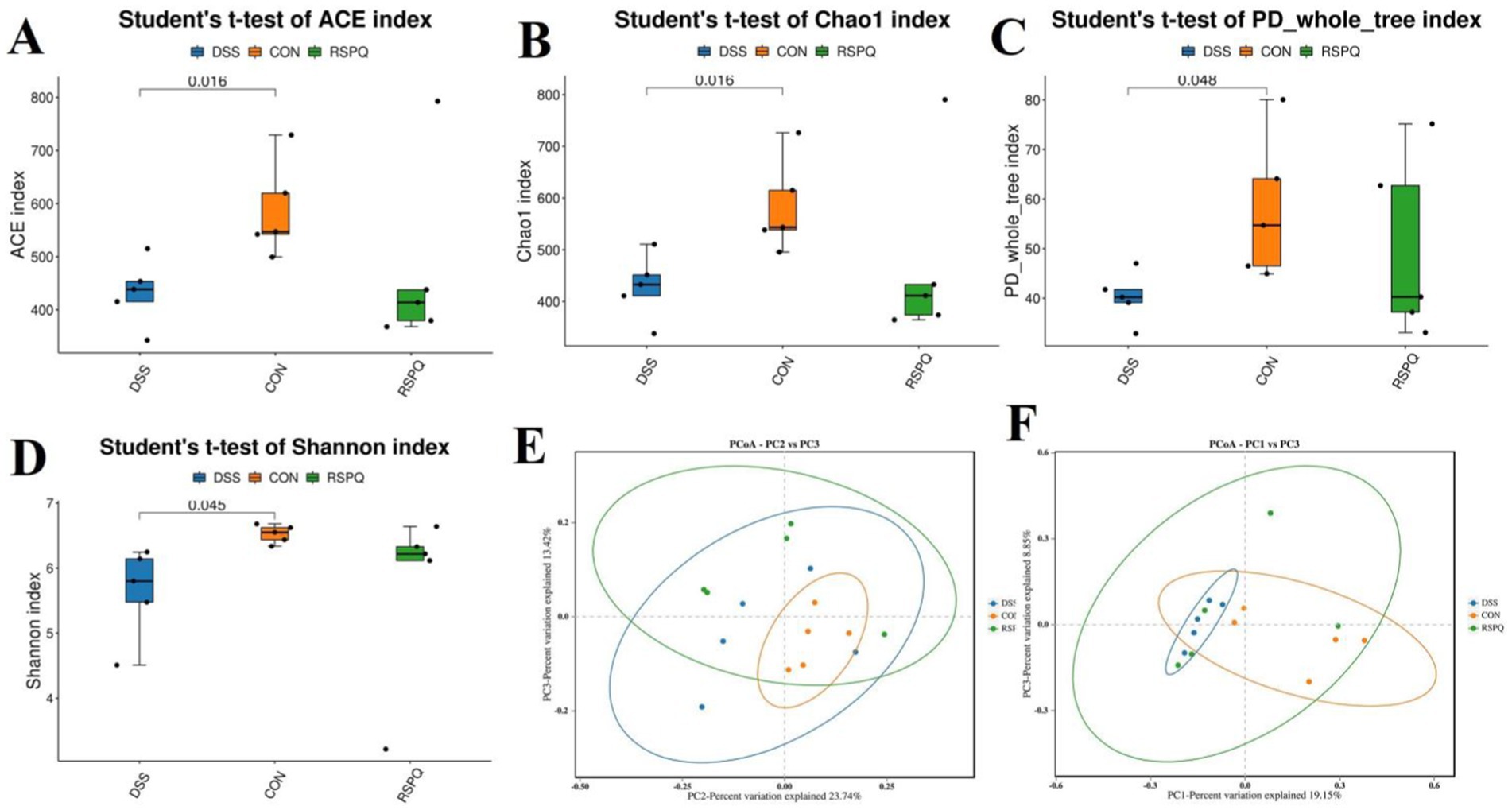
Figure 2. Pediococcus acidilactici administration reversed shifts in gut microbial diversity and structure associated with colitis in mice. (A) Chao1 index; (B) ACE index; (C) PD_whole_tree index; (D) Shannon index. PCoA plots based on the weighted (E) and unweighted (F) uniFrac distance. Each point on the graph represents a sample, with distinct colors denoting different groups. The distance between points illustrates the degree of variation among the samples.
There were 30 phyla and 571 genera identified from acquired samples, varying from 18 to 26 phyla and 131–299 genera per sample. Specifically, the gut microbiota in the CON, DSS and RSPQ groups were predominated by Firmicutes (CON = 40.93%, DSS = 41.71%, RSPQ = 48.23%), Bacteroidota (CON = 54.74%, DSS = 35.32%, RSPQ = 26.50%) and Proteobacteria (CON = 1.12%, DSS = 18.79%, RSPQ = 18.25%) (Figure 3A). Moreover, other phyla such as Campylobacterota (CON = 0.24%, DSS = 0.50%, RSPQ = 0.64%), Verrucomicrobiota (CON = 0.036%, DSS = 0.024%, RSPQ = 1.79%), Actinobacteriota (CON = 0.48%, DSS = 0.35%, RSPQ = 0.42%), Acidobacteriota (CON = 0.37%, DSS = 0.16%, RSPQ = 0.30%) and Cyanobacteria (CON = 0.053%, DSS = 0.10%, RSPQ = 0.68%) in the CON, DSS and RSPQ groups were represented with a lower abundance. Among recognized genera, unclassified_Muribaculaceae (19.87%), unclassified_Lachnospiraceae (15.04%) and Lachnospiraceae_NK4A136_group (13.00%) were the most prevalent bacteria in the CON group, accounting for approximately 47.92 of overall composition (Figure 3B). Additionally, the dominant bacterial genera observed in the DSS group were Bacteroides (22.55%), Escherichia_Shigella (14.79%) and unclassified_Lachnospiraceae (11.38%), whereas Bacteroides (16.31%) was the most predominant bacterial genus in the RSPQ groups, followed by Lachnospiraceae_NK4A136_group (15.13%) and Escherichia_Shigella (12.85%). Furthermore, clustering heatmap also showed the composition and abundance distribution of gut microbiota in the CON, DSS and RSPQ groups and demonstrated the significant effects of colitis on gut microbiota (Figure 3C).
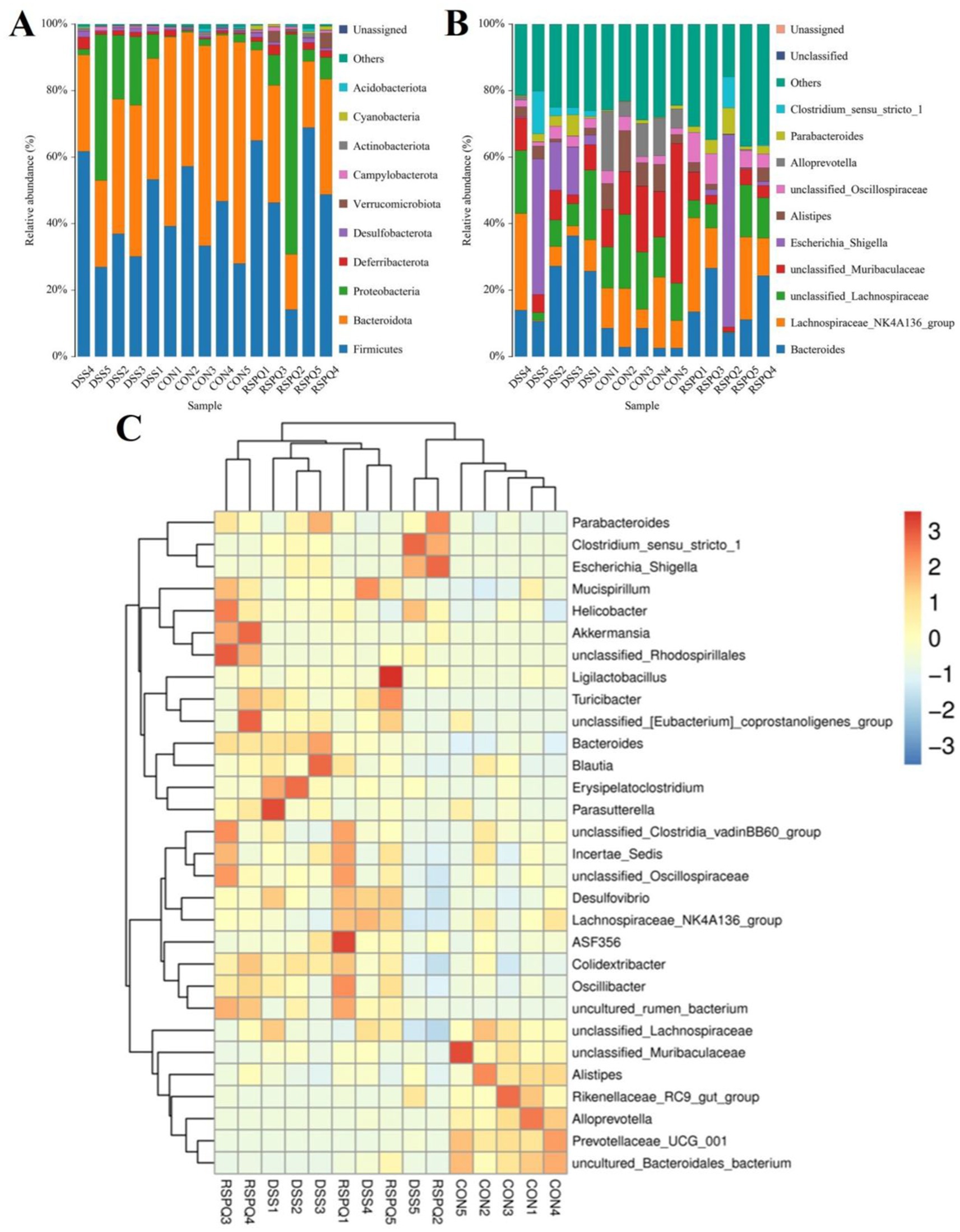
Figure 3. Relative abundance distribution of different samples at the phylum (A) and genus (B) levels. Only the top 10 most abundant bacterial phyla and genera are displayed in each sample. (C) Cluster heatmap of different samples at the genus level. The gradient of blue to red represents the alteration of abundance from low to high.
Metastats analysis was used for distinguishing the differential taxa at different classification levels to further explore the effects of Pediococcus acidilactici administration on gut microbiota in mice with colitis. At the phylum level, the gut microbiota in the DSS group exhibited significant increase in the relative proportions of Desulfobacterota and Proteobacteria, whereas Bacteroidota, Patescibacteria and Bdellovibrionota decreased dramatically as compared to CON group (Figure 4A). Moreover, 49 bacterial genera were found to be significantly different between CON and DSS groups. Among them, the relative abundances of 15 bacterial genera (unclassified_Erysipelatoclostridiaceae, Bacteroides, Akkermansia, Turicibacter, Erysipelatoclostridium, unclassified_env.OPS_17, Streptococcus, unclassified_Desulfovibrionaceae, Bilophila, Romboutsia, Enterorhabdus, Escherichia_Shigella, Enterococcus, uncultured_Clostridiales_bacterium, and uncultured_rumen_bacterium) significantly increased, whereas the relative richness of 34 bacterial genera (uncultured_Muribaculaceae_bacterium, Prevotellaceae_UCG_001, unclassified_Clostridia, uncultured_Bacteroidales_bacterium, unclassified_Erysipelotrichaceae, Alloprevotella, ZOR0006, unclassified_RF39, unclassified_SBR1031, A2, Alistipes, Muribaculum, Candidatus_Arthromitus, Odoribacter, Roseburia, Marvinbryantia, [Eubacterium]_nodatum_group, Prevotellaceae_NK3B31_group, unclassified_Rokubacteriales, unclassified_Gaiellales, Paenibacillus, unclassified_Xanthobacteraceae, Sphingomonas, Mesoplasma, unclassified_Muribaculaceae, uncultured_soil_bacterium, unclassified_Comamonadaceae, Candidatus_Saccharimonas, unclassified_BIrii41, unclassified_Acetobacteraceae, unclassified_Isosphaeraceae, Rikenellaceae_RC9_gut_group, Anaerotruncus, and unclassified_TRA3_20) memorably decreased during colitis (Figure 4B). At the phylum level, the abundances of Patescibacteria and Fusobacteriota was observably more preponderant in RSPQ than in the DSS, whereas the abundances of Proteobacteria was lower (Figure 5A). Moreover, we observed that the relative abundances of six genera (unclassified_Erysipelatoclostridiaceae, Rikenella, unclassified_env.OPS_17, Erysipelatoclostridium, Acetatifactor and Aquisphaera) obviously decreased significantly increased, whereas the relative abundances of 12 genera (Iunclassified_Clostridia, unclassified_Bacilli, Robiginitalea, unclassified_TRA3_20, uncultured_Mollicutes_bacterium, unclassified_Anaerolineaceae, uncultured_rumen_bacterium, Fusobacterium, [Eubacterium]_nodatum_group, Pediococcus, Bdellovibrio and Akkermansia) significantly increased in RSPQ as compared to DSS (Figure 5B).
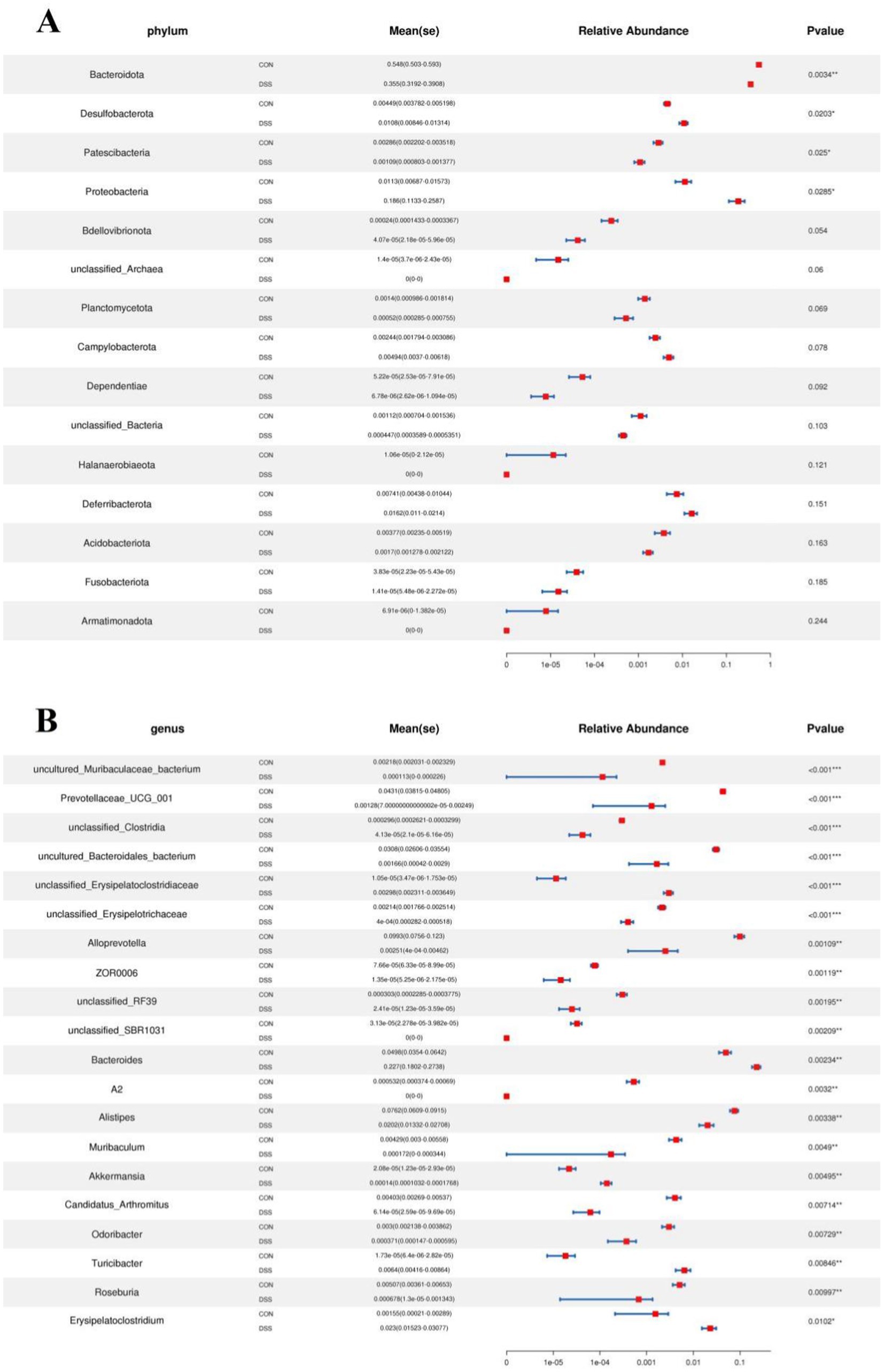
Figure 4. Taxa that were significantly different between the CON and DSS groups at the phylum (A) and genus (B) levels. All of the data represent means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
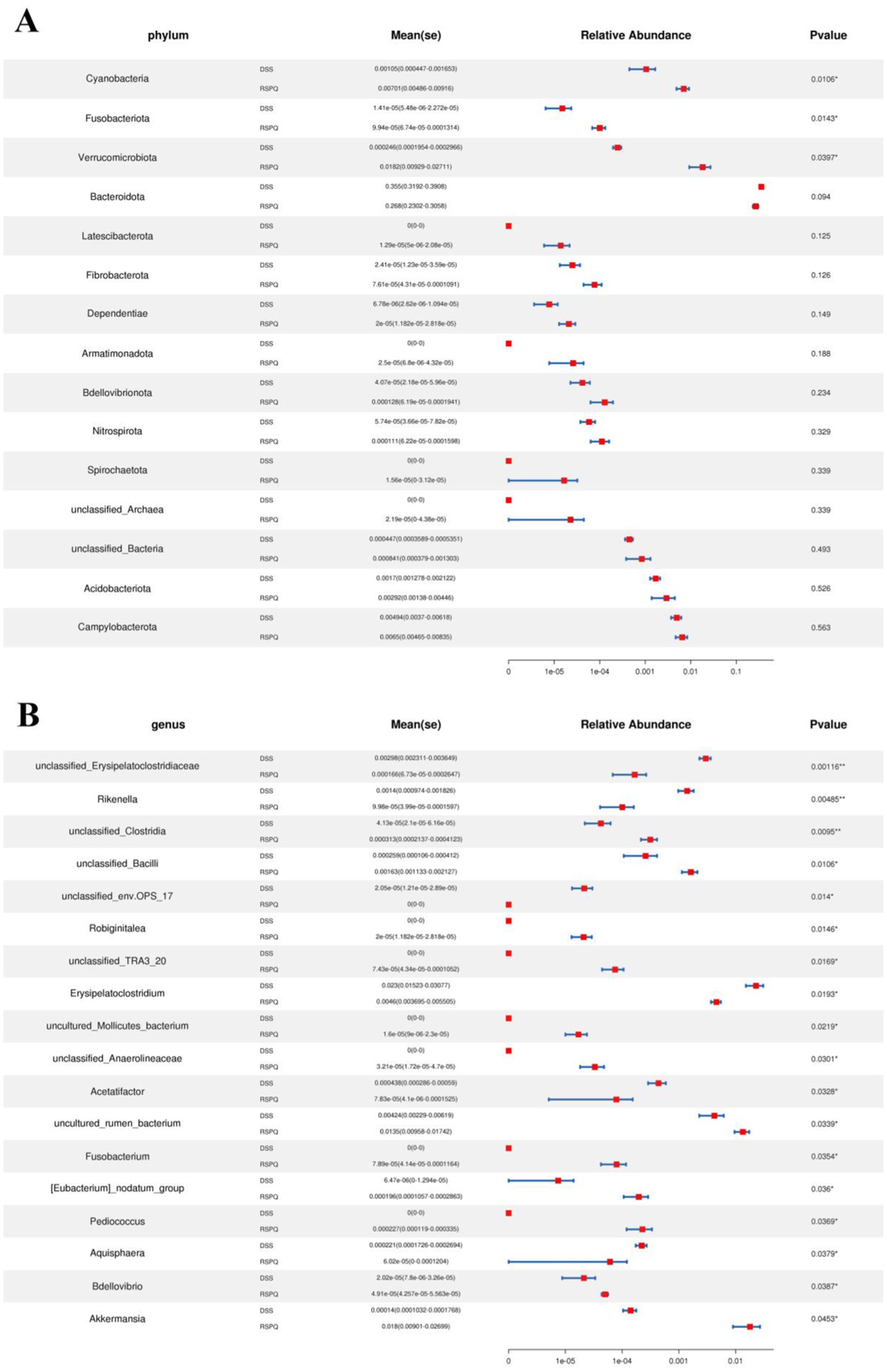
Figure 5. Taxa that were significantly different between the RSPQ and DSS groups at the phylum (A) and genus (B) levels. All of the data represent means ± SD. *p < 0.05, **p < 0.01.
The PCA analysis indicated that colitis caused distinct changes in intestinal metabolism, while Pediococcus acidilactici administration ameliorated intestinal metabolism in mice with colitis (Figures 6, 7). To further reveal the positive regulation of Pediococcus acidilactici on intestinal metabolism, OPLS-DA score plots was applied for pattern discriminant analysis. Results indicated that there was a clear separation among CON, DSS, and RSPQ groups and no fitting occur. There are 957 (455 in positive mode, 502 in negative-ion mode) differential metabolites were detected between CON and DSS groups (Figures 8A,B). Among significantly different metabolites, 740 metabolites were down-regulated, whereas 217 metabolites were up-regulated in DSS group. Additionally, we observed that there were 1,018 metabolites exhibiting significant differences between the CON and RSPQ groups (Figures 9A,B). Among these, 208 (89 in positive mode, 119 in negative-ion mode) metabolites were significantly increased, while 810 (403 in positive mode, 407 in negative-ion mode) metabolites were significantly decreased in the RSPQ group compared to the CON group. For the comparison of the DSS and RSPQ groups, 243 metabolites (121 in positive mode, 122 in negative-ion mode) were totally identified, while the richness of 28 metabolites increased dramatically, whereas 215 metabolites showed the opposite trend.
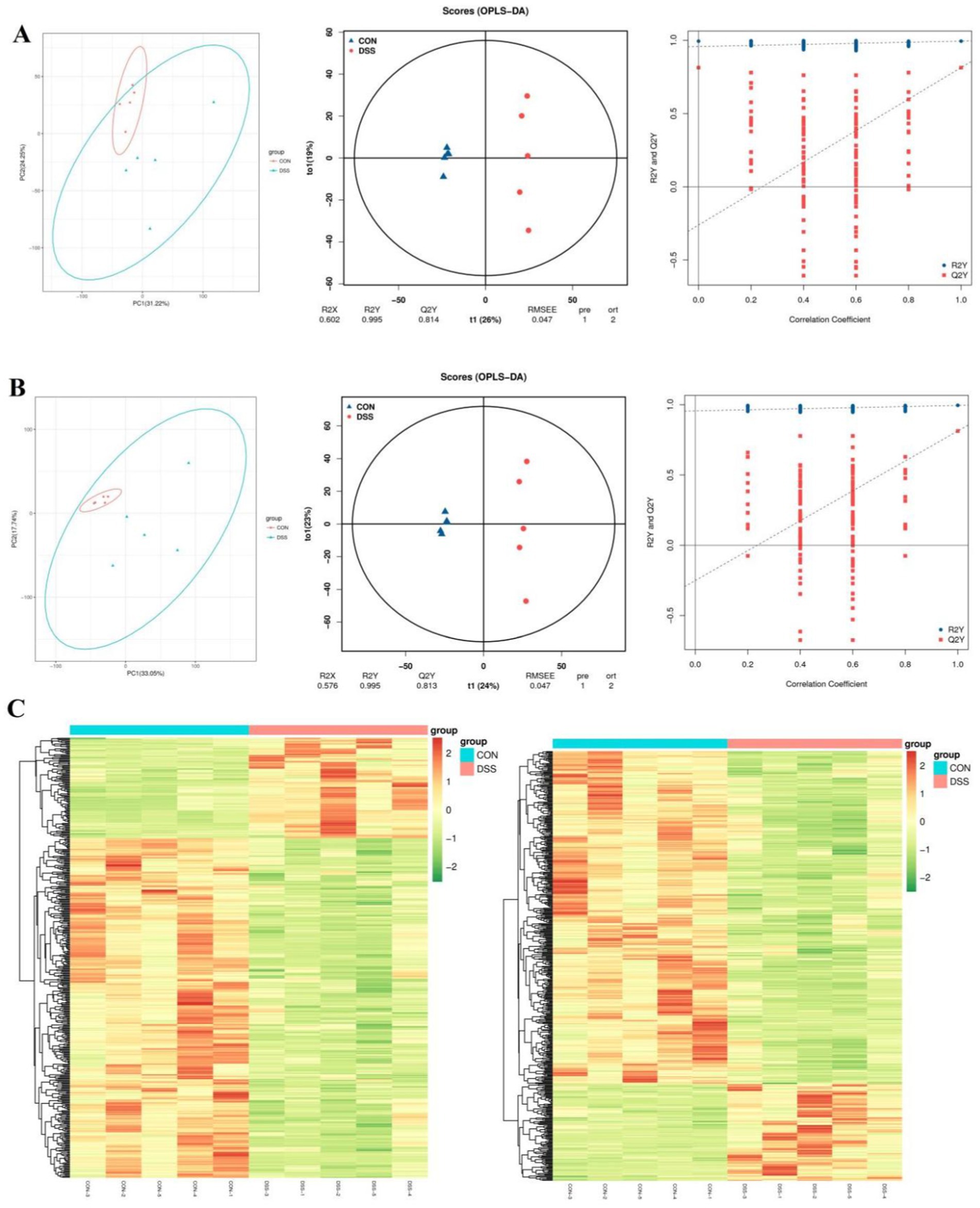
Figure 6. Multivariate statistical analysis of intestinal metabolism in CON and DSS groups. (A,B) PCA score plots, OPLS-DA score plots and permutation tests of the OPLS-DA in positive-ion and negative-ion modes. (C) Cluster heat map of differential metabolites in CON and DSS groups in positive-ion and negative-ion modes.
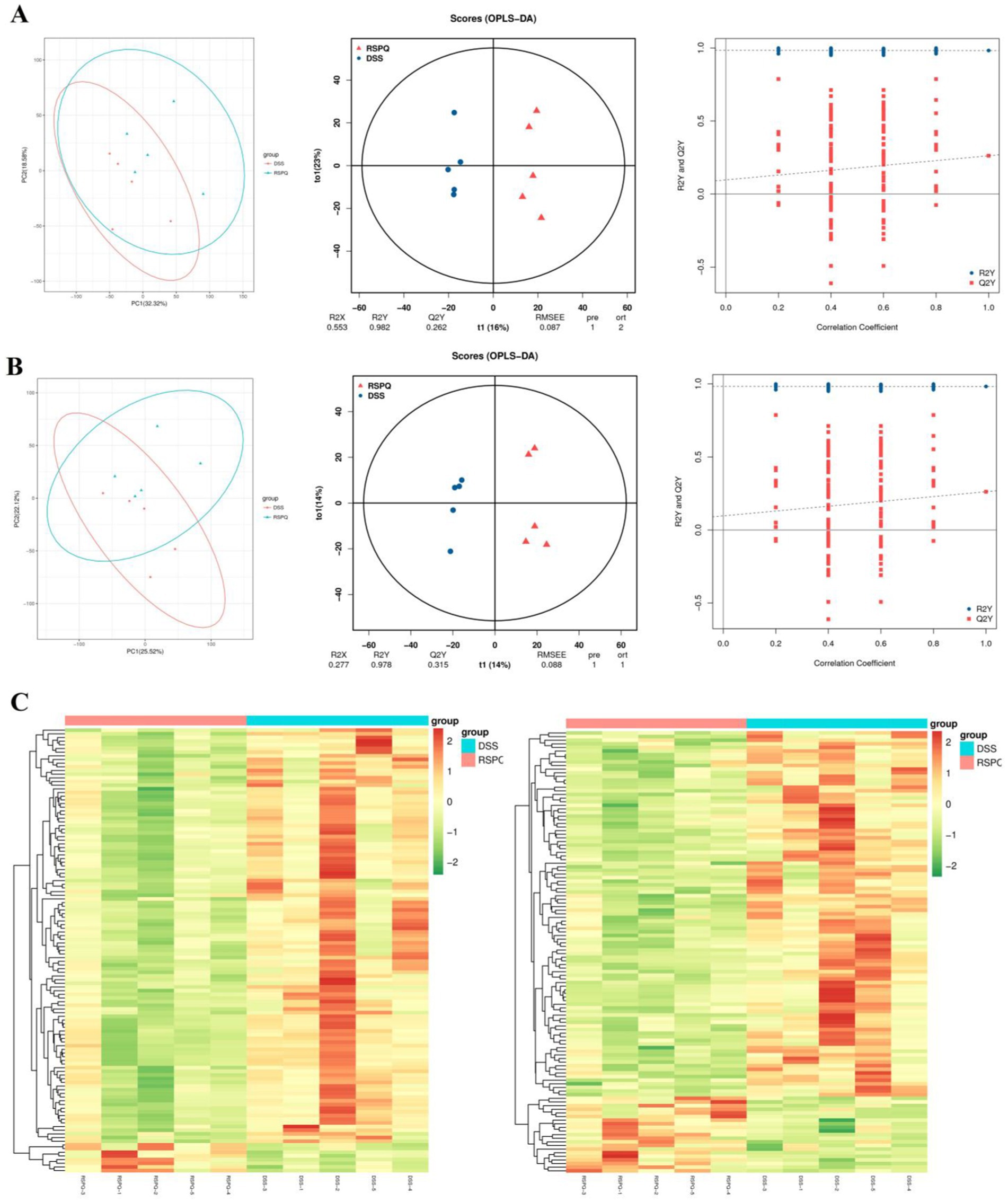
Figure 7. Multivariate statistical analysis of intestinal metabolism associated with Pediococcus acidilactici and colitis. (A,B) PCA score plots, OPLS-DA score plots and permutation tests of the OPLS-DA in positive-ion and negative-ion modes. (C) Cluster heat map of differential metabolites in CON and DSS groups in positive-ion and negative-ion modes.
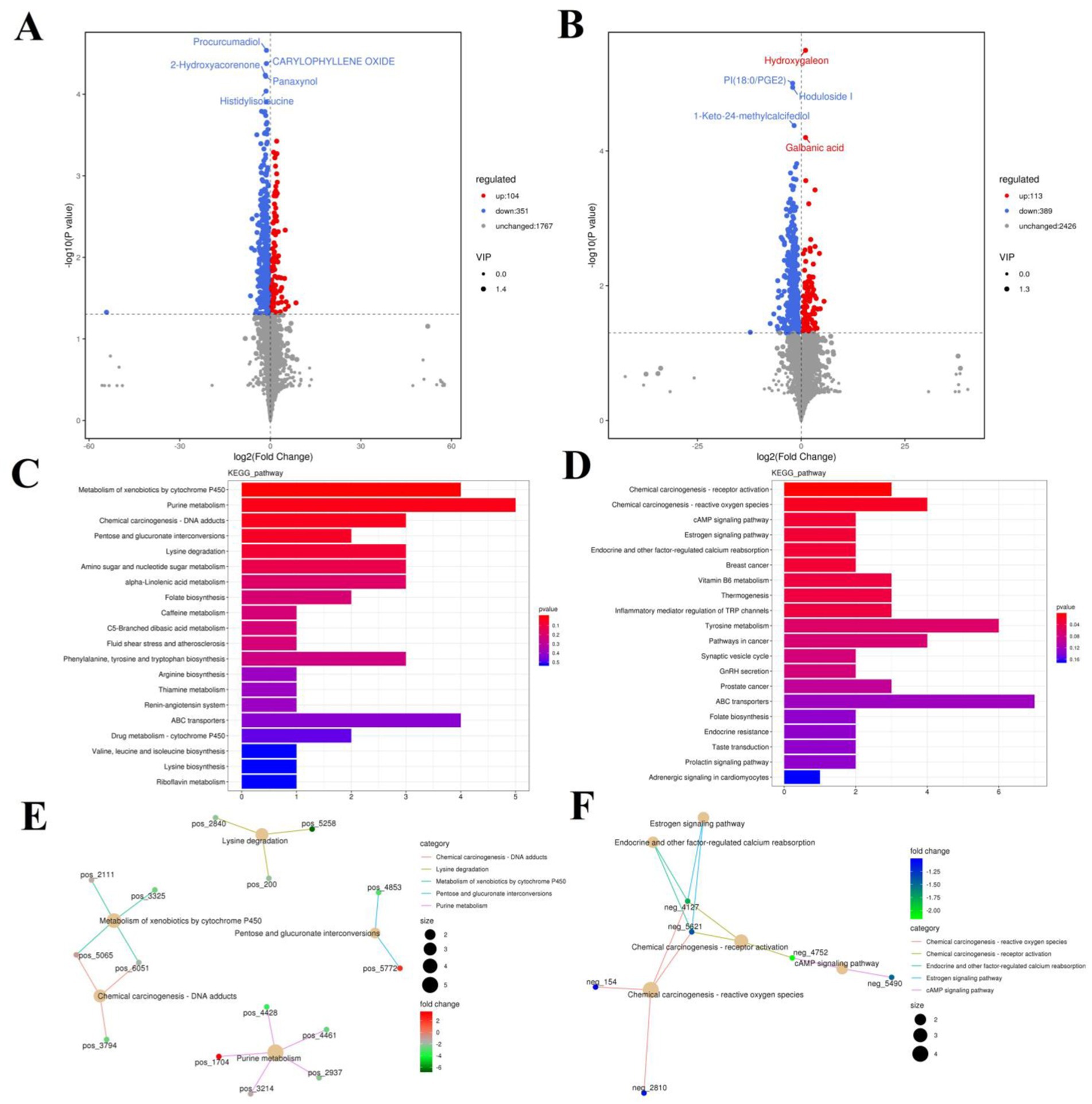
Figure 8. Effects of colitis on intestinal metabolism. (A,B) Volcano plot of differential metabolites between CON and DSS groups in positive-ion and negative-ion modes. The red dots represent the increased metabolites. The blue dots represent the decreased metabolites. (C,D) Intestinal metabolic pathways associated with colitis in positive-ion and negative-ion modes. (E,F) The representative network diagram of metabolites and related metabolic pathways between CON and DSS groups in positive-ion and negative-ion modes.
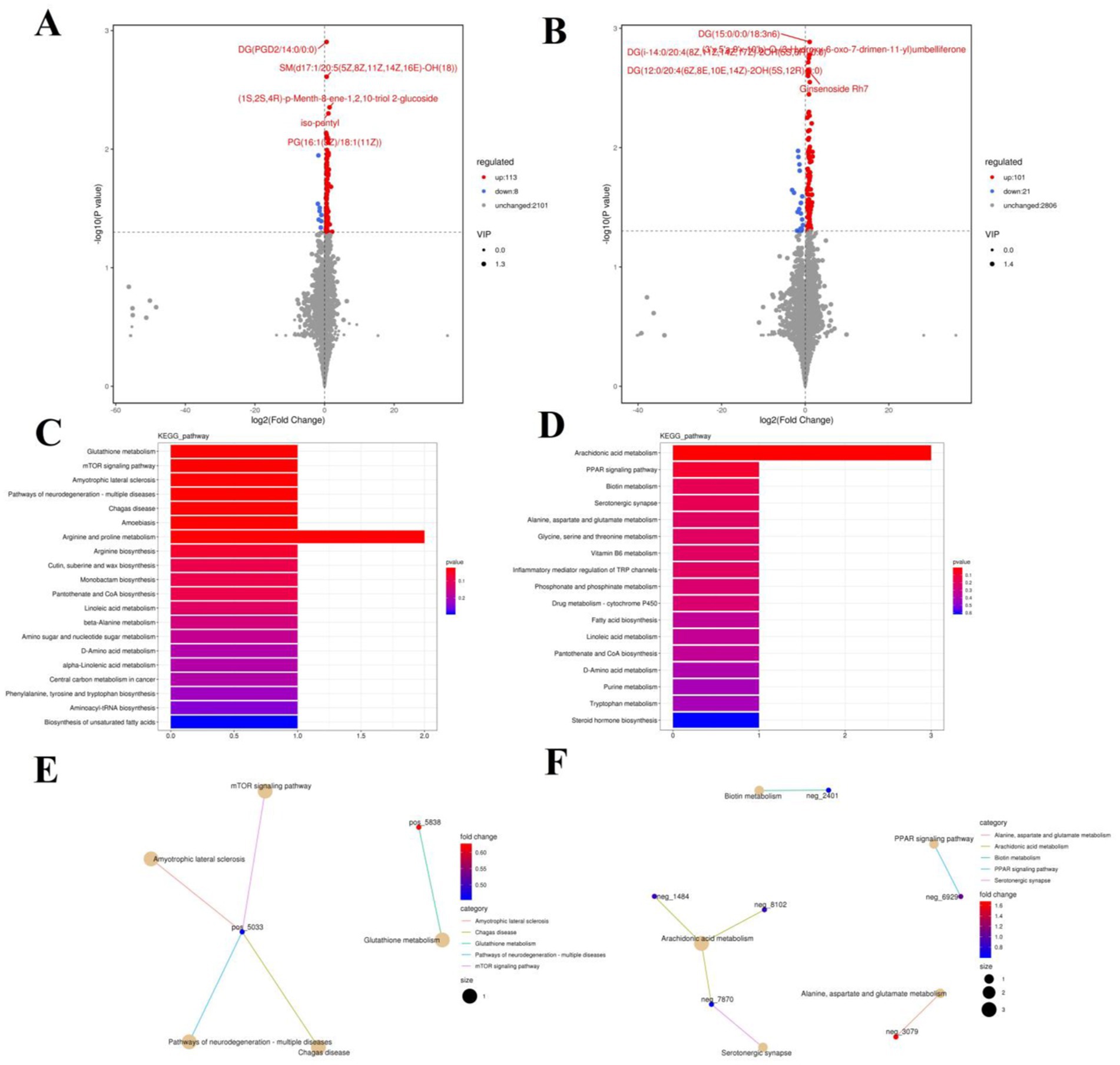
Figure 9. Effects of colitis on intestinal metabolism. (A,B) Volcano plot of differential metabolites between DSS and RSPQ groups in positive-ion and negative-ion modes. The red dots represent the increased metabolites. The blue dots represent the decreased metabolites. (C,D) Intestinal metabolic pathways associated with colitis in positive-ion and negative-ion modes. (E,F) The representative network diagram of metabolites and related metabolic pathways between DSS and RSPQ groups in positive-ion and negative-ion modes.
The representative enriched pathways associated with colitis and Pediococcus acidilactici administration were present in Figures 8C,D and Figure 9C,D. Among enriched pathways between CON and DSS groups, 13 pathways with a significant difference were the metabolism of xenobiotics by cytochrome P450, purine metabolism, chemical carcinogenesis-DNA adducts, pentose and glucuronate interconversions, chemical carcinogenesis-receptor activation, chemical carcinogenesis-reactive oxygen species, cAMP signaling pathway, estrogen signaling pathway, endocrine and other factor-regulated calcium reabsorption, breast cancer, vitamin B6 metabolism, thermogenesis, inflammatory mediator regulation of TRP channels, which involved in 24 potential biomarkers including L-Noradrenaline, dimethylarsinous acid, oleoylethanolamide, 4-Pyridoxic acid, 2-Oxo-3-hydroxy-4-phosphobutanoate, 2-(Hydroxymethyl)-4-oxobutanoate, estradiol, 5-HETE, histamine, 15(S)-HETE, 5-Amino-4-imidazolecarboxyamide, dGMP, xanthosine, dIMP, Sudan I, digalacturonate, and D-Fructuronate, etc. (Table 1). Moreover, there were 8 pathways that were significantly different between the DSS and RSPQ, namely glutathione metabolism, mTOR signaling pathway, amyotrophic lateral sclerosis, pathways of neurodegeneration-multiple diseases, chagas disease, amoebiasis, arginine and proline metabolism, and arachidonic acid metabolism (Table 2). These pathways are linked to five potential biomarkers: spermine, L-Arginine, 15-Deoxy-Delta12,14-PGJ2, prostaglandin J2, and 15(S)-HETE. The metabolic diagrams related to colitis or Pediococcus acidilactici administration in the intestine were shown in Figures 8E,F and Figures 9E,F.
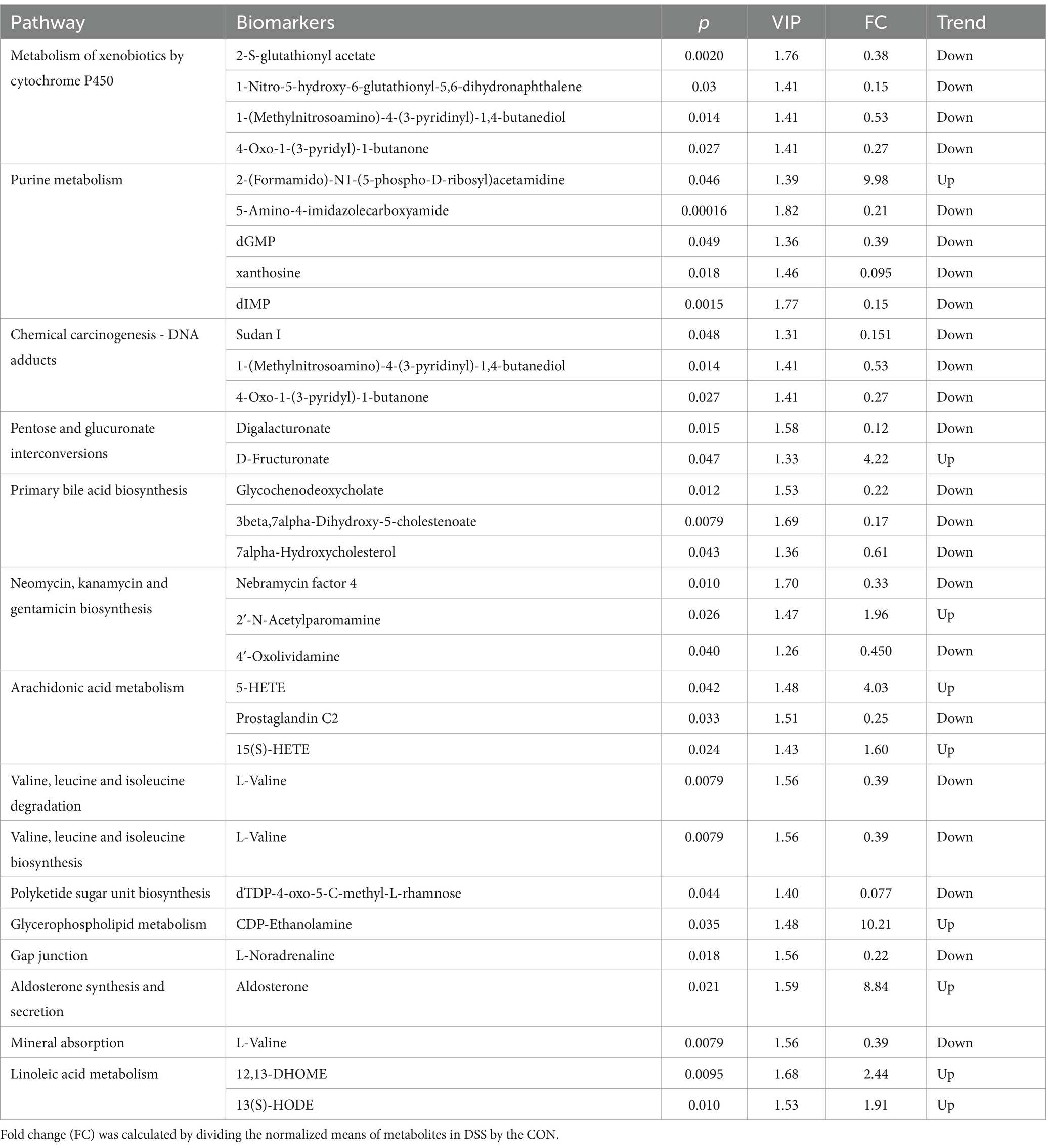
Table 1. Identification of potential biomarkers associated with differential metabolic pathways between the CON and DSS groups.
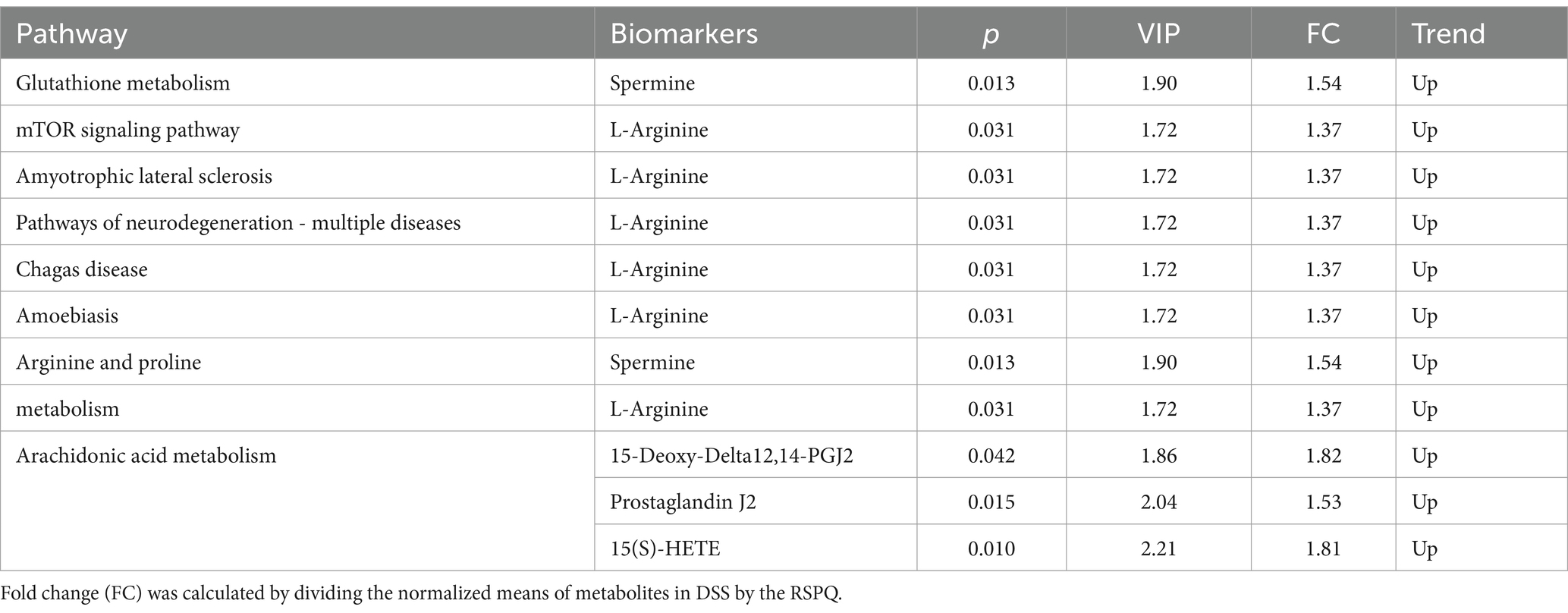
Table 2. Identification of potential biomarkers associated with differential metabolic pathways between the RSPQ and DSS groups.
The adverse effects of colitis on both human and animal health have attracted increasing attention (41). Although the specific pathogenesis of colitis has not yet been fully elucidated, it has been demonstrated to be closely associated with multiple factors such as environment, bacterial infection, oxidative stress, and genetics (42, 43). Recently, some studies involving gut microbiota have reported its important role in the development of colitis (44). Antibiotics remain the most commonly used treatment for colitis. However, antibiotics are no longer recommended for long-term use, due to the negative effects on hots health such as bacterial resistance, drug residues, and gut microbial imbalance. Probiotics have received widespread attention due to their important roles in gut microbiota, immunity, and metabolism (45, 46). Thus, in vitro supplementation of probiotics is also considered an effective strategy to alleviate colitis. In this study, we systematically explored the alleviating effect of Pediococcus acidilactici isolated from dogs on colitis using microbiome and metabolomics technologies.
To investigate the possible mechanism, we hypothesized that Pediococcus acidilactici administration could mediate the gut microbiota and their metabolites to further attenuate the colitis. Gut microbial composition and diversity were previously demonstrated to be associated with multiple intestinal diseases (47). In this study, colitis caused intestinal injury and decreased gut microbial alpha diversity, which is consistent with the findings of previous studies (47, 48). Early investigations suggested that intestinal injury inevitably could affecte intestinal environment and microbial survival, which in turn force existing microorganisms to adapt to new intestinal environment and alter the gut microbial composition and diversity, perturbing gut microbial homeostasis (49). The stable gut microbiota is an important biological barrier against the colonization and overgrowth of pathogens and conditioned pathogens (50). Conversely, gut microbial dysbiosis can affect intestinal mucosal barrier and immunologic function, thus increasing susceptibility to pathogens (50, 51). In this study, we observed that Pediococcus acidilactici administration could alleviate colon damage and reverse the reduction of microbial index caused by colitis.
We further dissected the relationship between colitis and gut microbiota and observed that colitis could cause changes in some specific bacteria that may play crucial roles in intestinal homeostasis and function. In this research, colitis cause a decline in the relative abundances of Bacteroidetes, Prevotellaceae, Alloprevotella, Rikenellaceae, Alistipes, and Roseburia and an increase in the proportion of Proteobacteria, Turicibacter, Bilophila, Escherichia_Shigella, and Enterococcus. Previous research indicated that the gut-residing Bacteroidetes is responsible for degrading carbohydrates and proteins (52). Meanwhile, it has been demonstrated to it can facilitate the development of the gastrointestinal immune system (53). As a pro-inflammatory bacterium, the levels of Turicibacter is significantly increased during enteritis (54). Earlier investigations showed that the increased Bilophila may contribute to the development of appendicitis and colitis (55). Escherichia_Shigella has been reported to be associated with diarrhea and intestinal infections (56). Enterococcus are common pathogens that can cause sepsis, pericarditis, and meningitis (57). Additionally, Enterococcus have been demonstrated to have both intrinsic and acquired drug resistance, which severely affects the treatment of their infections (58). Prevotellaceae has been shown to possess the ability to degrade polysaccharide and high-carbohydrate substrates (59). Thus, the decreased abundance of Prevotellaceae in the intestine may negatively impact host nutrient uptake. Alloprevotella has been demonstrated to be closely related to the decreased lifetime cardiovascular disease risk due to its ability to produce acetate and succinate (4, 5). As a common beneficial intestinal bacteria, Rikenellaceae could degrade plant-derived polysaccharides and alleviate the colitis by mediating T-regulatory cell differentiation (60). Alistipes and Roseburia are potential producers of SCFAs. Previous investigations involving SCFAs have indicated their vital roles in immune system, cell proliferation, energy intake and intestinal metabolism (61, 62). Additionally, SCFAs also participate in the positive regulation of gut microbiota and intestinal barrier, which are essential for host health (63). However, Pediococcus acidilactici administration can improve the gut microbial composition of patients with colitis. Notably, Pediococcus acidilactici administration can increase the relative abundances of Akkermansia. Akkermansia has long been recognized as a beneficial intestinal bacterium, and its abundance gradually decreases with the development of enteritis (64). Numerous studies indicated that Akkermansia can alleviate intestinal inflammation and prevent intestinal cancer by regulating the immune response in the spleen, intestines, and mesenteric lymph nodes (65, 66). Moreover, Akkermansia has been demonstrated to be negatively related to obesity and diabetes (67, 68). These results showed the vital roles of Pediococcus acidilactici administration in regulating gut microbiota, which contributed to maintaining intestinal colonization resistance and homeostasis.
Previous studies indicated that the gut microbiota can systematically regulate host health by producing metabolites. Therefore, we also explored the changes in intestinal metabolism. In this study, we observed that eight metabolic pathways (glutathione metabolism, arginine and proline metabolism, arachidonic acid metabolism, etc.) and 5 (spermine, L-Arginine, 15-Deoxy-Delta12,14-PGJ2, prostaglandin J2, and 15(S)-HETE) metabolites were involved in the alleviating effect of Pediococcus acidilactici administration. These significantly changed metabolites and metabolic pathways may play a key role in Pediococcus acidilactici alleviating colitis. Previous studies have demonstrated that arachidonic acid can alleviate oxidative stress by increasing the levels of superoxide dismutase (SOD) and catalase (CAT) (69). Furthermore, arachidonic acid has been shown to inhibit oxidative stress by reducing mitochondrial membrane potential and blocking reverse electron transport (RET) through uncoupling, which in turn inhibits RET-dependent reactive oxygen species (ROS) generation (70). As an important intracellular metabolic regulator and antioxidant, glutathione can participate in the tricarboxylic acid cycle and sugar metabolism in the body (71). It can also activate multiple enzymes, thereby facilitating the metabolism of carbohydrates, fats and proteins. Additionally, glutathione play a crucial role in maintaining the function of the immune system and the stability of the red blood cell membrane structure (72). Recent research indicates that glutathione can rectify the imbalance of acetylcholine and cholinesterase, exert an anti-allergic effect, and enhance the skin’s antioxidant capacity (73). Previous research has indicated that oxidative stress is a significant factor contributing to the development of colitis. Thus, Pediococcus acidilactici may alleviate the development of colitis by mediating arachidonic acid metabolism and glutathione metabolism.
Taken together, this study investigated the alleviating effect of Pediococcus acidilactici on colitis. Results indicated that Pediococcus acidilactici could alleviate colonic injury and reverse the decline in gut microbial diversity and abundance associated with colitis. Additionally, we also observed a positive effect of Pediococcus acidilactici on intestinal metabolism in patients with colitis. Arachidonic acid metabolism and glutathione metabolism may be potential pathways for Pediococcus acidilactici to exert its effects. This study is an important exploration of whether Pediococcus acidilactici isolated from dogs can alleviate colitis. Meanwhile, it also contributes to expanding the probiotic application and provide a theoretical basis for alleviating colitis from the microbial and metabolic perspective. However, it is important to acknowledge the limitations of this study, particularly the small sample size.
The original sequence data was submitted to the Sequence Read Archive (SRA) (NCBI, USA) with the accession no. PRJNA1179910.
The animal study was approved by the Animal Welfare and Ethics Committee of Anqing Normal University. The study was conducted in accordance with the local legislation and institutional requirements.
LW: Writing – review & editing. LX: Writing – review & editing. XD: Writing – review & editing. HJ: Writing – review & editing. RZ: Writing – review & editing. AZ: Writing – review & editing. YZ: Writing – review & editing. ST: Writing – review & editing. XW: Writing – review & editing. ZL: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Engineering Technology Research Center for Aquatic Organism Conservation and Water Ecosystem Restoration in University of Anhui Province topics open to the outside world (No. AO202301), Anhui Provincial Higher Education Key Scientific Research Project (2023AH050504 and 2024AH051130), and Key Lab. of Biodiversity Conservation and Characteristic Resource Utilization in Southwest Anhui topics open to the outside world (Wxn202402).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1520678/full#supplementary-material
1. Li, A, Liu, B, Li, F, He, Y, Wang, L, Fakhar-E-Alam, KM, et al. Integrated bacterial and fungal diversity analysis reveals the gut microbial alterations in diarrheic giraffes. Front Microbiol. (2021) 12:712092. doi: 10.3389/fmicb.2021.712092
2. Li, A, Wang, Y, He, Y, Liu, B, Iqbal, M, Mehmood, K, et al. Environmental fluoride exposure disrupts the intestinal structure and gut microbial composition in ducks. Chemosphere. (2021) 277:130222. doi: 10.1016/j.chemosphere.2021.130222
3. Liao, J, Liu, Y, Yi, J, Li, Y, Li, Q, Li, Y, et al. Gut microbiota disturbance exaggerates battery wastewater-induced hepatotoxicity through a gut-liver axis. Sci Total Environ. (2022) 809:152188. doi: 10.1016/j.scitotenv.2021.152188
4. Han, B, Shi, L, Bao, MY, Yu, FL, Zhang, Y, Lu, XY, et al. Dietary ellagic acid therapy for cns autoimmunity: targeting on alloprevotella rava and propionate metabolism. Microbiome. (2024) 12:114. doi: 10.1186/s40168-024-01819-8
5. Han, Y, Zhang, Z, Yang, H, Zhang, N, Lin, C, Raza, A, et al. Protective effects of shlo on lps-induced lung injury via tlr4/myd88-erk signaling pathway and intestinal flora regulation. Pak Vet J. (2024) 44:776–84. doi: 10.29261/pakvetj/2024.255
6. Li, A, Liu, F, Si, W, Wang, Y, Wang, D, Yuan, Z, et al. Pesticide butachlor exposure perturbs gut microbial homeostasis. Ecotoxicol Environ Saf. (2024) 281:116646. doi: 10.1016/j.ecoenv.2024.116646
7. Li, J, Jia, J, Teng, Y, Xie, C, Li, C, Zhu, B, et al. Gastrodin alleviates dss-induced colitis in mice through strengthening intestinal barrier and modulating gut microbiota. Food Secur. (2024) 13:2460. doi: 10.3390/foods13152460
8. Wang, D, Zeng, J, Ma, H, Fouad, D, and Su, Z. Comparative analysis of the gut microbiota between two horse species. Pak Vet J. (2024) 44:449–57. doi: 10.29261/pakvetj/2024.151
9. Wang, L, Nabi, F, Zhang, X, Zhou, G, Shah, QA, Li, S, et al. Effects of Lactobacillus plantarum on broiler health: integrated microbial and metabolomics analysis. Probiotics Antimicrob Proteins. (2024):1–19. doi: 10.1007/s12602-024-10336-x
10. Wang, R, Yang, X, Liu, J, Zhong, F, Zhang, C, Chen, Y, et al. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun. (2022) 13:2522. doi: 10.1038/s41467-022-30240-8
11. Li, A, Wang, Y, Kulyar, MF, Iqbal, M, Lai, R, Zhu, H, et al. Environmental microplastics exposure decreases antioxidant ability, perturbs gut microbial homeostasis and metabolism in chicken. Sci Total Environ. (2023) 856:159089. doi: 10.1016/j.scitotenv.2022.159089
12. Luo, M, Han, Y, Chen, Y, Du, H, Chen, B, Gao, Z, et al. Unveiling the role of gut microbiota in curcumin metabolism using antibiotic-treated mice. Food Chem. (2024) 460:140706. doi: 10.1016/j.foodchem.2024.140706
13. Liang, X, Wang, R, Luo, H, Liao, Y, Chen, X, Xiao, X, et al. The interplay between the gut microbiota and metabolism during the third trimester of pregnancy. Front Microbiol. (2022) 13:1059227. doi: 10.3389/fmicb.2022.1059227
14. Meng, A, Zhang, X, Pubu, P, Ali, M, Wang, J, Xu, C, et al. Protective effect of lentinan against LPS-induced injury in mice via influencing antioxidant enzyme activity, inflammatory pathways, and gut microbiota. Pak Vet J. (2024) 44:647–56. doi: 10.29261/pakvetj/2024.225
15. Sasahara, Y, Uchida, T, Suzuki, T, and Abukawa, D. Primary immunodeficiencies associated with early-onset inflammatory bowel disease in southeast and east asia. Front Immunol. (2021) 12:786538. doi: 10.3389/fimmu.2021.786538
16. Wang, L, Shao, L, Chen, MY, Wang, L, Yang, P, Tan, FB, et al. Panax notoginseng alleviates colitis via the regulation of gut microbiota. Am J Chin Med. (2023) 51:107–27. doi: 10.1142/S0192415X23500076
17. Yu, H, Zhang, S, Li, R, Ma, C, Zhang, Q, Xia, F, et al. Berberine alleviates inflammation and suppresses pla2-cox-2-pge2-ep2 pathway through targeting gut microbiota in dss-induced ulcerative colitis. Biochem Biophys Res Commun. (2024) 695:149411. doi: 10.1016/j.bbrc.2023.149411
18. Cardenas, VR, Lozzi, RD, Roman, HO, Arco, M, Moreno, FR, and Pastorino, M. Cat scratch lesions as a manifestation of chronic colitis due to spirochetosis. Rev Esp Enferm Dig. (2022) 114:693–4. doi: 10.17235/reed.2022.9078/2022
19. King, AP, Donovan, TA, Cohen, E, Marin, J, and Le Roux, AB. Short colon syndrome in cats. J Vet Intern Med. (2024) 38:2138–50. doi: 10.1111/jvim.17103
20. Lyu, B, Wang, Y, Fu, H, Li, J, Yang, X, Shen, Y, et al. Intake of high-purity insoluble dietary fiber from okara for the amelioration of colonic environment disturbance caused by acute ulcerative colitis. Food Funct. (2022) 13:213–26. doi: 10.1039/d1fo02264d
21. Jiang, P, Zhang, Y, Li, X, and Chen, J. Geniposidic acid attenuates dss-induced colitis through inhibiting inflammation and regulating gut microbiota. Phytother Res. (2023) 37:3453–66. doi: 10.1002/ptr.7819
22. Xie, Q, Zhang, Y, Zhang, Z, Gong, S, Mo, Q, and Li, J. Characteristics and dynamic changes of gut microbiota in cats with colitis. Pak Vet J. (2024) 44:414–22. doi: 10.29261/pakvetj/2024.175
23. Zhou, G, Zhang, N, Meng, K, and Pan, F. Interaction between gut microbiota and immune checkpoint inhibitor-related colitis. Front Immunol. (2022) 13:1001623. doi: 10.3389/fimmu.2022.1001623
24. Li, S, Jin, Y, Fu, W, Cox, AD, Lee, D, and Reddivari, L. Intermittent antibiotic treatment accelerated the development of colitis in il-10 knockout mice. Biomed Pharmacother. (2022) 146:112486. doi: 10.1016/j.biopha.2021.112486
25. Ozkul, C, Ruiz, VE, Battaglia, T, Xu, J, Roubaud-Baudron, C, Cadwell, K, et al. A single early-in-life antibiotic course increases susceptibility to dss-induced colitis. Genome Med. (2020) 12:65. doi: 10.1186/s13073-020-00764-z
26. Fu, R, Wang, L, Meng, Y, Xue, W, Liang, J, Peng, Z, et al. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front Nutr. (2022) 9:1062961. doi: 10.3389/fnut.2022.1062961
27. Fu, W, Chen, C, Xie, Q, Gu, S, Tao, S, and Xue, W. Pediococcus acidilactici strain alleviates gluten-induced food allergy and regulates gut microbiota in mice. Front Cell Infect Microbiol. (2022) 12:845142. doi: 10.3389/fcimb.2022.845142
28. Meng, Z, Sun, W, Liu, W, Wang, Y, Jia, M, Tian, S, et al. A common fungicide tebuconazole promotes colitis in mice via regulating gut microbiota. Environ Pollut. (2022) 292:118477. doi: 10.1016/j.envpol.2021.118477
29. Jadhav, A, Jagtap, S, Vyavahare, S, Sharbidre, A, and Kunchiraman, B. Reviewing the potential of probiotics, prebiotics and synbiotics: advancements in treatment of ulcerative colitis. Front Cell Infect Microbiol. (2023) 13:1268041. doi: 10.3389/fcimb.2023.1268041
30. Chandhni, PR, Pradhan, D, Sowmya, K, Gupta, S, Kadyan, S, Choudhary, R, et al. Ameliorative effect of surface proteins of probiotic lactobacilli in colitis mouse models. Front Microbiol. (2021) 12:679773. doi: 10.3389/fmicb.2021.679773
31. Cordeiro, BF, Alves, JL, Belo, GA, Oliveira, ER, Braga, MP, Da, SS, et al. Therapeutic effects of probiotic minas frescal cheese on the attenuation of ulcerative colitis in a murine model. Front Microbiol. (2021) 12:623920. doi: 10.3389/fmicb.2021.623920
32. Cho, H, Lee, GY, Ali, MS, and Park, S. Effects of dietary intake of heat-inactivated Limosilactobacillus reuteri PSC102 on the growth performance, immune response, and gut microbiota in weaned piglets. Pak Vet J. (2024) 44:819–25. doi: 10.29261/pakvetj/2024.224
33. Luo, C, Wang, L, Chen, Y, and Yuan, J. Supplemental enzyme and probiotics on the growth performance and nutrient digestibility of broilers fed with a newly harvested corn diet. Animals (Basel). (2022) 12:2381. doi: 10.3390/ani12182381
34. Yang, Y, Yan, G, Meng, X, Wang, X, Zhao, Z, Zhou, S, et al. Effects of Lactobacillus plantarum and Pediococcus acidilactici co-fermented feed on growth performance and gut microbiota of nursery pigs. Front Vet Sci. (2022) 9:1076906. doi: 10.3389/fvets.2022.1076906
35. Xu, W, Liu, Q, Fan, S, Wang, X, Lu, S, Liu, J, et al. Effect of ginsenoside fermented by Pediococcus acidilactici XM-06 on preventing diarrhea in mice via regulating intestinal barrier function and gut microbiota. J Funct Foods. (2024) 123:106594. doi: 10.1016/j.jff.2024.106594
36. Liu, J, Wang, Y, Li, A, Iqbal, M, Zhang, L, Pan, H, et al. Probiotic potential and safety assessment of Lactobacillus isolated from yaks. Microb Pathog. (2020) 145:104213. doi: 10.1016/j.micpath.2020.104213
37. Hu, J, Nie, Y, Chen, J, Zhang, Y, Wang, Z, Fan, Q, et al. Gradual changes of gut microbiota in weaned miniature piglets. Front Microbiol. (2016) 7:1727. doi: 10.3389/fmicb.2016.01727
38. Chen, Y, Tian, P, Li, Y, Tang, Z, and Zhang, H. Thiram exposure: disruption of the blood-testis barrier and altered apoptosis-autophagy dynamics in testicular cells via the bcl-2/bax and mtor/atg5/p62 pathways in mice. Pest Biochem Physiol. (2024) 203:106010. doi: 10.1016/j.pestbp.2024.106010
39. Wu, S, Liu, K, Huang, X, Sun, Q, Wu, X, Mehmood, K, et al. Molecular mechanism of mir-203a targeting runx2 to regulate thiram induced-chondrocyte development. Pest Biochem Physiol. (2024) 200:105817. doi: 10.1016/j.pestbp.2024.105817
40. Kasimir, M, Behrens, M, Schulz, M, Kuchenbuch, H, Focke, C, and Humpf, HU. Intestinal metabolism of alpha-and beta-glucosylated modified mycotoxins t-2 and ht-2 toxin in the pig cecum model. J Agric Food Chem. (2020) 68:5455–61. doi: 10.1021/acs.jafc.0c00576
41. Sidaway, P. Faecal transplantation reverses colitis. Nat Rev Clin Oncol. (2019) 16:66. doi: 10.1038/s41571-018-0139-3
42. Hwang, J, Jin, J, Jeon, S, Moon, SH, Park, MY, Yum, DY, et al. Sod1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. (2020) 37:101760. doi: 10.1016/j.redox.2020.101760
43. Moradipoor, F, Jivad, N, Asgharzadeh, S, Zare, E, and Amini-Khoei, H. Neuroimmune response and oxidative stress in the prefrontal cortex mediate seizure susceptibility in experimental colitis in male mice. J Biochem Mol Toxicol. (2024) 38:e23755. doi: 10.1002/jbt.23755
44. Zhang, S, Sun, Y, Nie, Q, Hu, J, Li, Y, Shi, Z, et al. Effects of four food hydrocolloids on colitis and their regulatory effect on gut microbiota. Carbohydr Polym. (2024) 323:121368. doi: 10.1016/j.carbpol.2023.121368
45. Gao, R, Zhang, X, Huang, L, Shen, R, and Qin, H. Gut microbiota alteration after long-term consumption of probiotics in the elderly. Probiotics Antimicrob Proteins. (2019) 11:655–66. doi: 10.1007/s12602-018-9403-1
46. Wang, X, Zhang, P, and Zhang, X. Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules. (2021) 26:6076. doi: 10.3390/molecules26196076
47. Guo, S, Geng, W, Chen, S, Wang, L, Rong, X, Wang, S, et al. Ginger alleviates dss-induced ulcerative colitis severity by improving the diversity and function of gut microbiota. Front Pharmacol. (2021) 12:632569. doi: 10.3389/fphar.2021.632569
48. Li, F, Han, Y, Cai, X, Gu, M, Sun, J, Qi, C, et al. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. (2020) 11:1063–73. doi: 10.1039/c9fo01519a
49. Li, C, Wang, M, Chen, X, and Chen, W. Taraxasterol ameliorates dextran sodium sulfate-induced murine colitis via improving intestinal barrier and modulating gut microbiota dysbiosis. Acta Biochim Biophys Sin. (2022) 54:340–9. doi: 10.3724/abbs.2022019
50. Chen, Y, Mai, Q, Chen, Z, Lin, T, Cai, Y, Han, J, et al. Dietary palmitoleic acid reprograms gut microbiota and improves biological therapy against colitis. Gut Microbes. (2023) 15:2211501. doi: 10.1080/19490976.2023.2211501
51. Shen, B, Wang, J, Guo, Y, Gu, T, Shen, Z, Zhou, C, et al. Dextran sulfate sodium salt-induced colitis aggravates gut microbiota dysbiosis and liver injury in mice with non-alcoholic steatohepatitis. Front Microbiol. (2021) 12:756299. doi: 10.3389/fmicb.2021.756299
52. Pan, X, Raaijmakers, JM, and Carrion, VJ. Importance of bacteroidetes in host-microbe interactions and ecosystem functioning. Trends Microbiol. (2023) 31:959–71. doi: 10.1016/j.tim.2023.03.018
53. Sequeira, RP, McDonald, J, Marchesi, JR, and Clarke, TB. Commensal bacteroidetes protect against klebsiella pneumoniae colonization and transmission through il-36 signalling. Nat Microbiol. (2020) 5:304–13. doi: 10.1038/s41564-019-0640-1
54. Gerges, P, Bangarusamy, DK, Bitar, T, Alameddine, A, Nemer, G, and Hleihel, W. Turicibacter and catenibacterium as potential biomarkers in autism spectrum disorders. Sci Rep. (2024) 14:23184. doi: 10.1038/s41598-024-73700-5
55. Feng, Z, Long, W, Hao, B, Ding, D, Ma, X, Zhao, L, et al. A human stool-derived bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. (2017) 9:59. doi: 10.1186/s13099-017-0208-7
56. Bin, P, Tang, Z, Liu, S, Chen, S, Xia, Y, Liu, J, et al. Intestinal microbiota mediates enterotoxigenic escherichia coli-induced diarrhea in piglets. BMC Vet Res. (2018) 14:385. doi: 10.1186/s12917-018-1704-9
57. Karasawa, Y, Kato, J, Kawamura, S, Kojima, K, Ohki, T, Seki, M, et al. Risk factors for acute cholangitis caused by enterococcus faecalis and enterococcus faecium. Gut Liver. (2021) 15:616–24. doi: 10.5009/gnl20214
58. Toc, DA, Pandrea, SL, Botan, A, Mihaila, RM, Costache, CA, Colosi, IA, et al. Enterococcus raffinosus, enterococcus durans and enterococcus avium isolated from a tertiary care hospital in Romania-retrospective study and brief review. Biology Basel. (2022) 11:598. doi: 10.3390/biology11040598
59. Cuevas-Sierra, A, Riezu-Boj, JI, Guruceaga, E, Milagro, FI, and Martinez, JA. Sex-specific associations between gut prevotellaceae and host genetics on adiposity. Microorganisms. (2020) 8:938. doi: 10.3390/microorganisms8060938
60. Seo, SH, Unno, T, Park, SE, Kim, EJ, Lee, YM, Na, CS, et al. Korean traditional medicine (jakyakgamcho-tang) ameliorates colitis by regulating gut microbiota. Meta. (2019) 9:226. doi: 10.3390/metabo9100226
61. Liu, XF, Shao, JH, Liao, YT, Wang, LN, Jia, Y, Dong, PJ, et al. Regulation of short-chain fatty acids in the immune system. Front Immunol. (2023) 14:1186892. doi: 10.3389/fimmu.2023.1186892
62. Schwarz, A, Bruhs, A, and Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. (2017) 137:855–64. doi: 10.1016/j.jid.2016.11.014
63. Liu, M, Peng, R, Tian, C, Shi, J, Ma, J, Shi, R, et al. Effects of the gut microbiota and its metabolite short-chain fatty acids on endometriosis. Front Cell Infect Microbiol. (2024) 14:1373004. doi: 10.3389/fcimb.2024.1373004
64. He, KY, Lei, XY, Wu, DH, Zhang, L, Li, JQ, Li, QT, et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes. (2023) 15:2293312. doi: 10.1080/19490976.2023.2293312
65. Ansaldo, E, Slayden, LC, Ching, KL, Koch, MA, Wolf, NK, Plichta, DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. (2019) 364:1179–84. doi: 10.1126/science.aaw7479
66. Ghotaslou, R, Nabizadeh, E, Memar, MY, Law, W, Ozma, MA, Abdi, M, et al. The metabolic, protective, and immune functions of akkermansia muciniphila. Microbiol Res. (2023) 266:127245. doi: 10.1016/j.micres.2022.127245
67. Huwart, S, de Wouters, DA, Rastelli, M, Van Hul, M, de Vos, WM, Luquet, S, et al. Food reward alterations during obesity are associated with inflammation in the striatum in mice: beneficial effects of akkermansia muciniphila. Cells. (2022) 11:2534. doi: 10.3390/cells11162534
68. Zhou, Q, Zhang, Y, Wang, X, Yang, R, Zhu, X, Zhang, Y, et al. Gut bacteria akkermansia is associated with reduced risk of obesity: evidence from the American gut project. Nutr Metab. (2020) 17:90. doi: 10.1186/s12986-020-00516-1
69. Wang, ZJ, Liang, CL, Li, GM, Yu, CY, and Yin, M. Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem Biol Interact. (2006) 163:207–17. doi: 10.1016/j.cbi.2006.08.005
70. Schonfeld, P, and Wojtczak, L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim Biophys Acta. (2007) 1767:1032–40. doi: 10.1016/j.bbabio.2007.04.005
71. Lopez-Huertas, E, and Palma, JM. Changes in glutathione, ascorbate, and antioxidant enzymes during olive fruit ripening. J Agric Food Chem. (2020) 68:12221–8. doi: 10.1021/acs.jafc.0c04789
72. Lu, C, Jiang, Y, Yue, Y, Sui, Y, Hao, M, Kang, X, et al. Glutathione and neodiosmin feedback sustain plant immunity. J Exp Bot. (2023) 74:976–90. doi: 10.1093/jxb/erac442
73. Gunderson, MP, Nguyen, BT, Cervantes, RJ, Holden, LL, French, J, Smith, BD, et al. Response of phase i and ii detoxification enzymes, glutathione, metallothionein and acetylcholine esterase to mercury and dimethoate in signal crayfish (pacifastacus leniusculus). Chemosphere. (2018) 208:749–56. doi: 10.1016/j.chemosphere.2018.05.183
Keywords: Pediococcus acidilactici, colitis, gut microbiota, metabolite, arachidonic acid metabolism
Citation: Wu L, Xue L, Ding X, Jiang H, Zhang R, Zheng A, Zu Y, Tan S, Wang X and Liu Z (2025) Integrated microbiome and metabolomics analysis reveals the alleviating effect of Pediococcus acidilactici on colitis. Front. Vet. Sci. 12:1520678. doi: 10.3389/fvets.2025.1520678
Received: 12 November 2024; Accepted: 22 January 2025;
Published: 26 February 2025.
Edited by:
Xihong Zhou, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Houqiang Luo, Wenzhou Vocational College of Science and Technology, ChinaCopyright © 2025 Wu, Xue, Ding, Jiang, Zhang, Zheng, Zu, Tan, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Liu, bGl1MTIwMjRAc2luYS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.