- 1Department of Companion Animal Medicine and Surgery, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia
- 2UPM-MAKNA Cancer Research Laboratory, Institute of Bioscience, Universiti Putra Malaysia, Serdang, Malaysia
- 3Department of Clinical Studies, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
- 4Department of Oncology, Lakeshore Animal Health Partners, Mississauga, ON, Canada
- 5Centre for Advancing Responsible & Ethical Artificial Intelligence, University of Guelph, Guelph, ON, Canada
- 6ANI.ML Research, ANI.ML Health Inc., Toronto, ON, Canada
Objectives: To evaluate the prognostic factors and treatment outcomes in dogs with high-grade cutaneous mast cell tumors (HGMCTs).
Methods: Medical records of dogs with a histopathologic diagnosis of HGMCTs were reviewed from a single institution. Clinical factors, treatment-related variables, and adjuvant therapies were documented to evaluate their association with clinical outcomes. Comparative and survival analyses were conducted using Kaplan–Meier survival analysis, log-rank, and Fisher’s exact tests.
Results: The overall median survival time for the 77 dogs was 317 days (range 20–3,041 days) with 6-month, 1-year, and 2-year survival rates of 69, 50, and 30%, respectively. Surgically treated dogs had significantly prolonged survival and were 6.88 times more likely to survive beyond 5.5 months. The presence of metastasis at initial staging was strongly associated with poorer outcomes, as dogs without metastasis at initial staging had 6.94 times higher odds of surviving beyond 2 years. Surgical sites with incomplete margins had a higher local recurrence rate (58%) compared to those with clean margins (26%). Despite aggressive treatment, 75% of the dogs that received concurrent surgical and adjuvant therapy experienced disease progression. Lymph node extirpation, tumor localization, number of tumors, and local recurrence were not associated with the overall outcome.
Clinical relevance: The combination of aggressive local therapy and adjuvant systemic chemotherapy provides a notable survival benefit in dogs with HGMCTs. The limited therapeutic benefit of locoregional lymph node extirpation, combined with a persistently high metastatic rate despite systemic chemotherapy, highlights the critical need for more effective regional and systemic treatment approaches for HGMCT patients.
Introduction
Mast cells are granular immune cells widely recognized for their central role in various inflammatory and immunological reactions (1, 2). These cells derive from hematopoietic stem cells and migrate to various tissues, particularly surfaces exposed to the external environment, such as the skin, respiratory tract, and gastrointestinal tract (1, 2). The mechanisms behind the neoplastic transformation of mast cells remain largely unidentified; however, underlying genetic causes and KIT mutations have been implicated (3–6).
Mast cell tumors are the most common cutaneous malignancy in dogs, exhibiting significant variation in presentation and biological behavior, ranging from benign to highly aggressive forms with markedly greater metastatic potential (7–11). Furthermore, the manifestation of paraneoplastic disorders, attributable to the release of histamine, heparin, eosinophil chemotactic factor, and proteolytic enzymes from mast cell granules, including Darier’s sign, gastrointestinal ulceration, coagulopathy, hypotension, and circulatory collapse, presents additional challenges in managing these tumors (12–14). Significant attention has been paid to the search for prognostic markers to guide treatment decisions, with factors such as histologic grade (10, 11, 15–17), mitotic count (18), clinical stage (19, 20), anatomic location (21), microvessel density (22), and c-kit gene mutations (6, 23, 24) being explored.
Histologic grade is generally considered the most reliable and consistently predictive factor for canine cutaneous mast cell tumors (MCTs) (10, 11, 16, 20). The 3-tier grading scheme (Patnaik), widely adopted since 1984, classifies cutaneous MCTs into either grade I (low-grade), II (intermediate-grade), or III (high-grade) (10), while a newly proposed 2-tier grading scheme (Kiupel) divides them into low and high grades (16). Most low-grade cutaneous mast cell tumors (LGMCTs) are effectively treated with wide surgical excision alone, though a small subset of LGMCTs may exhibit aggressive behavior, leading to metastasis and potentially death (25). In contrast, dogs with high-grade tumors have a poorer prognosis, with metastatic rates ranging from 55 to 96%, and deaths often occurring within the first year after diagnosis (10, 11, 16, 17, 20, 26). In recent years, numerous clinical studies have been conducted to evaluate the behavior and therapeutic strategies for HGMCTs, including neoadjuvant vinblastine administration (27), combination therapy with vinblastine and toceranib phosphate (28), lomustine and prednisone therapy (29), and lymph node extirpation (30). However, most were non-randomized trials with inadequately small sample sizes, likely due to the low incidence of HGMCTs (4–20%) and complexity of randomized controlled trials (10, 16, 31, 32).
The aim of this retrospective study was to expand our current understanding of the prognostic factors and outcomes in dogs with HGMCTs treated with different therapeutic protocols in the clinical setting.
Methods
Medical records from client-owned dogs presented to the Ontario Veterinary College Companion Animal Hospital, University of Guelph, were retrospectively reviewed for histopathologic diagnoses of high-grade (Kiupel), grade II (Patnaik) with histologic criteria consistent with Kiupel high-grade, or grade III cutaneous MCTs between 2007 and 2024. Only dogs with a follow-up period of at least 6 months or those that died within 6 months of an HGMCT diagnosis were included in the analysis. Dogs with multiple MCTs, at least one which was HGMCT, and those diagnosed with HGMCT post-mortem who met the inclusion criteria were also included in the analysis. Dogs with mucocutaneous or subcutaneous tumors were excluded from this study.
Data collected from the medical records included patient signalment (age, breed, sex, and body weight), diagnostic and initial staging investigations, treatment details (date of incisional or excisional biopsy, completeness of surgical excision, lymphadenectomy), as well as information on chemotherapy and radiation therapy, if administered. Post-mortem findings, when available, were also recorded. Follow-up information was retrieved from medical records or obtained through telephone communication with referring veterinarians.
Tumor characteristics evaluated included anatomic location (head/neck, trunk, limb, perineum/inguinal/prepuce/tail, or multifocal if tumors were located at more than one of these sites) and whether there was a history of previous MCT. Surgical excision was considered complete if no microscopic residual tumor was present at the resection margin, and the presence of residual tumor at the resection margin was considered an incomplete excision. Local recurrence was defined as regrowth of a tumor at the surgical site or as indicated in the medical records, and tumors that developed at distant sites following treatment initiation were classified as de novo lesions. The location of metastatic disease was recorded based on cytologic or histologic findings during restaging procedures or post-mortem examination.
Median survival time was calculated from the date of treatment initiation (either surgery or neoadjuvant chemotherapy) to the date of death or censoring. Dogs were censored if they were lost to follow-up, dead due to MCT-unrelated causes, or alive at the time of statistical analysis. Continuous data were analyzed for normality using the Shapiro–Wilk test. Normally distributed data were expressed as mean ± SD, and non-normally distributed data were expressed as median (range). Survival plots were generated using the Kaplan–Meier product-limit method. Variables evaluated for association with MST included sex, anatomic location, presence of metastasis at initial staging, treatment protocol (surgical excision, lymphadenectomy, and administration of chemotherapy with or without radiotherapy [RT]), and completeness of surgical excision. Associations between various categorical variables and outcomes (surgical margin, local recurrence, and metastasis) were evaluated using Fisher’s exact test. All statistical analyses were performed using SPSS Statistics version 29.0 (IBM Corp., Armonk, NY, USA), and p-values of <0.05 were considered significant.
Results
Seventy-seven client-owned dogs were included in this study. The mean age at diagnosis was 8.3 ± 0.3 years (range 2.0–13.8 years), and the median weight was 28.5 kg (range 3.4–83.0 kg). The study population consisted of 39 spayed females, 1 intact female, 34 castrated males, and 3 intact males. Twenty-six breeds were identified; Labrador Retrievers (n = 17, 22%), mixed breeds (n = 17, 22%), and Golden Retrievers (n = 7, 9%) were over-represented. With six retriever mixes included in the mixed breed category, retrievers accounted for up to 39% (n = 30) of the study population. Bulldog-related breeds – such as Boston Terrier (n = 3), American Bulldog (n = 2), French Bulldog (n = 2), English Bulldog (n = 1), and Alapaha Blue Blood Bulldog (n = 1), comprised 12%.
The primary HGMCT location was recorded as trunk (n = 28, 36%), limb (n = 20, 26%), head/neck (n = 19, 25%), perineum/inguinal/tail/prepuce (n = 7, 9%), and multifocal (n = 3, 4%). Staging investigations were not standardized but included thoracic radiographs (n = 59, 77%), abdominal ultrasound (n = 67, 87%), cytologic and/or histologic evaluation of regional lymph nodes (n = 32, 41%), cytology of the liver (n = 46, 60%), spleen (n = 50, 65%), and bone marrow (n = 2, 0.03%), and imaging via computed tomography (n = 2, 0.03%) and magnetic resonance (n = 1, 0.01%). Metastasis was identified in 25 dogs (37%), including 22 with regional lymph node involvement, two with metastasis to both the lymph nodes and spleen, and one with dissemination to the lymph nodes, spleen and liver. Additionally, 23 dogs (30%) with HGMCT also had LGMCT or subcutaneous MCT. Dogs underwent various non-standardized restaging procedures at clinician or owner discretion, including thoracic radiographs, abdominal ultrasound, and cytologic examination of the liver, spleen, regional lymph nodes, and new tumors every 3 or 6 months. Post-mortem examinations (PM) were conducted on eight dogs. At the end of the study, metastasis was documented in 58% of the study population (n = 45). The most commonly affected sites were the lymph nodes (n = 27, with 6 confirmed through PM), spleen (n = 14, with 4 confirmed through PM), and liver (n = 10, with 5 confirmed through PM). Less frequently affected sites, all identified through PM, included the kidneys (n = 3), lungs (n = 2), heart (n = 2), and bone marrow (n = 2). Single occurrences were observed in the adrenal gland, pleura, pancreas, gastrointestinal tract, omentum, and mesentery.
The overall median survival time (MST) was 317 days (range 20–3,041 days), with 6-month, 1-year, and 2-year survival rates of 69, 50, and 30%, respectively. The primary tumor location of the HGMCTs, and presence of additional LGMCT or subcutaneous MCT did not have a significant impact on metastasis or MST. However, dogs presenting with metastasis at initial staging had a significantly shorter MST (p < 0.001, MST = 182 days, Figure 1). Notably, dogs without metastasis at initial staging had 6.94 times higher odds of surviving beyond 2 years.
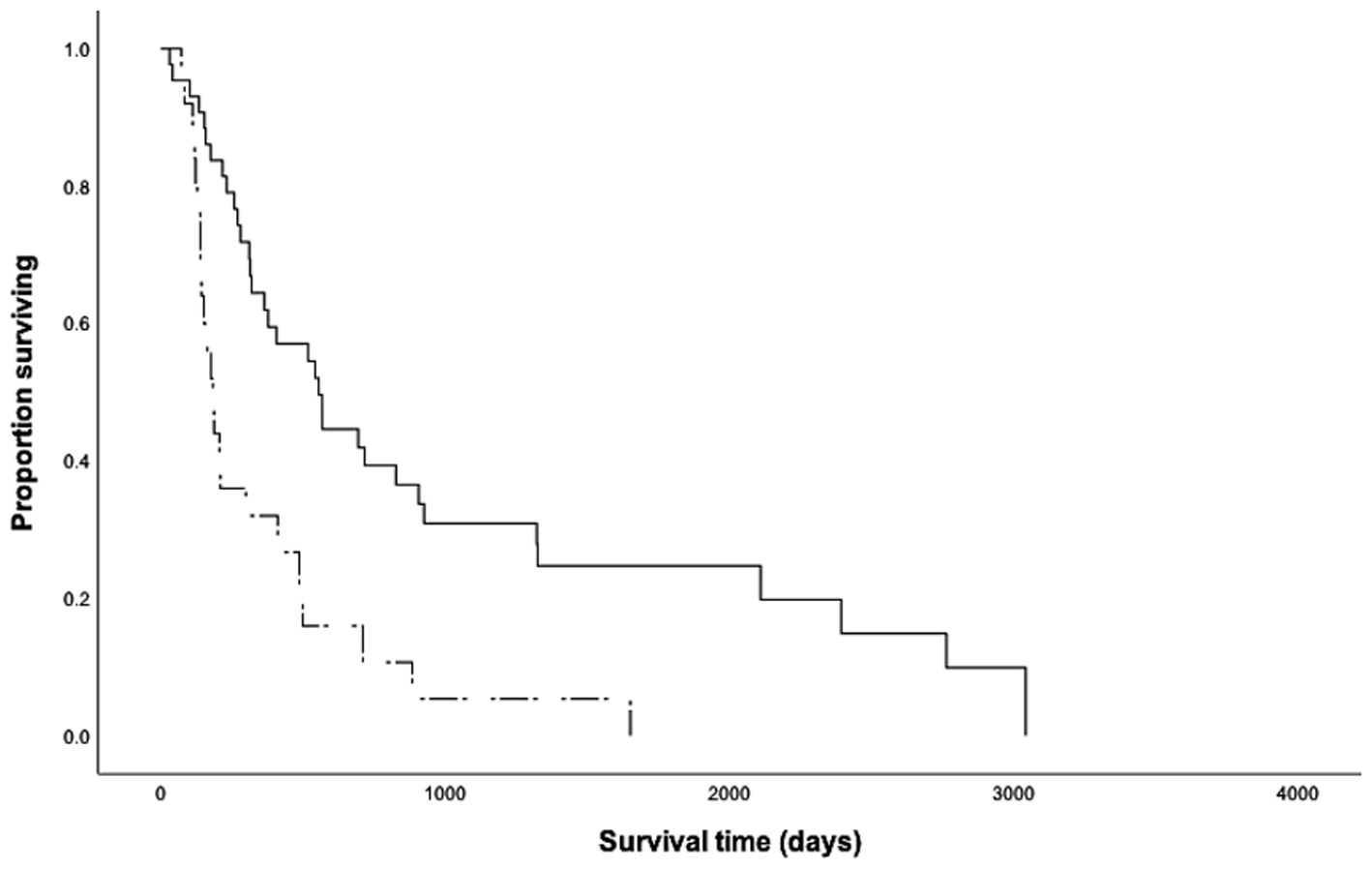
Figure 1. Kaplan Meier survival curves for 68 dogs with high-grade cutaneous mast cell tumors, with (n = 25, dashed line) and without (n = 43, solid line) metastasis at initial staging. The median survival time of dogs without metastasis (554 days) was significantly longer than that of dogs with metastasis at initial staging (182 days, p < 0.001).
Of the cohort, 65 dogs received chemotherapy following tumor resection, six dogs underwent surgical treatment only, five dogs were treated with chemotherapy with or without RT, and one dog did not receive any treatment based on clinician or owner discretion. First-line and salvage chemotherapies included vinblastine-based protocols, CCNU-based protocols, tyrosine kinase inhibitors (toceranib, imatinib or masitinib), vincristine with cyclophosphamide, intralesional triamcinolone injection, hydroxyurea, and prednisone. The MST of dogs that underwent surgical treatment (n = 71, MST = 385 days) was longer than that of dogs that received non-surgical treatment only (n = 6, MST = 137 days, p = 0.016, Figure 2). Dogs that underwent surgical treatment had 6.88-fold higher odds of surviving beyond 5.5 months. Despite aggressive treatment, 75% (n = 49) of the dogs that received concurrent surgical and non-surgical therapy (n = 65) experienced disease progression, including de novo lesions (n = 32, 49%), local recurrence (n = 17, 26%) and metastases to the lymph nodes (n = 24, 37%), spleen (n = 12, 18%) and liver (n = 8, 12%). Although not statistically significant, surgical patients who received combined chemotherapy and RT (n = 17, MST = 716 days) showed better survival outcomes than those who received chemotherapy alone (n = 48, MST = 317 days, Table 1). The dog, which did not undergo any treatment, survived for 409 days before humane euthanasia was performed due to disease progression.
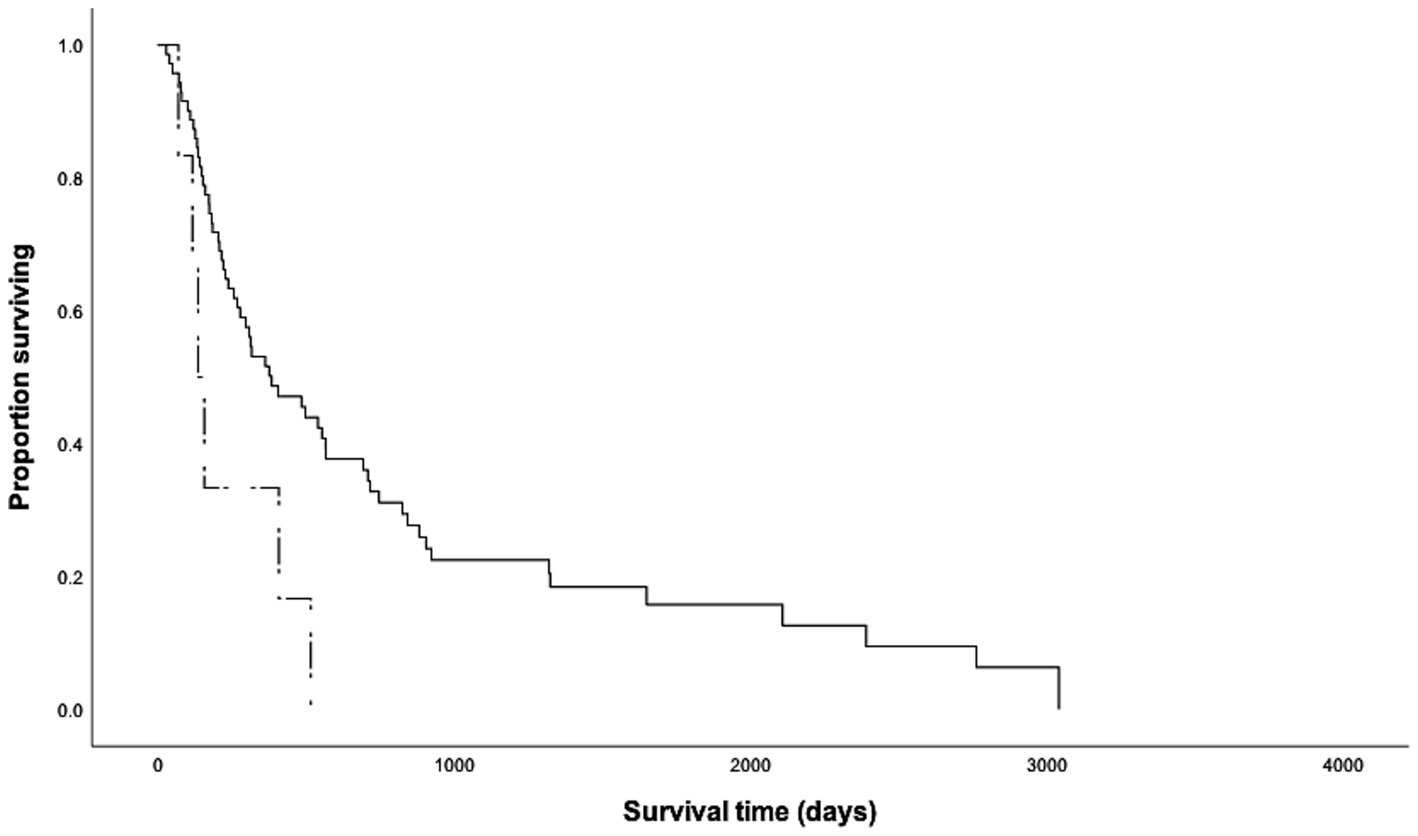
Figure 2. Kaplan Meier survival curves for 77 dogs with high-grade cutaneous mast cell tumors that underwent (n = 71, solid line) and did not undergo (n = 6, dashed line) surgical excision of the tumors. The median survival time of dogs that underwent surgery (385 days) was significantly longer than that of dogs that did not undergo surgical excision (137 days, p = 0.016).
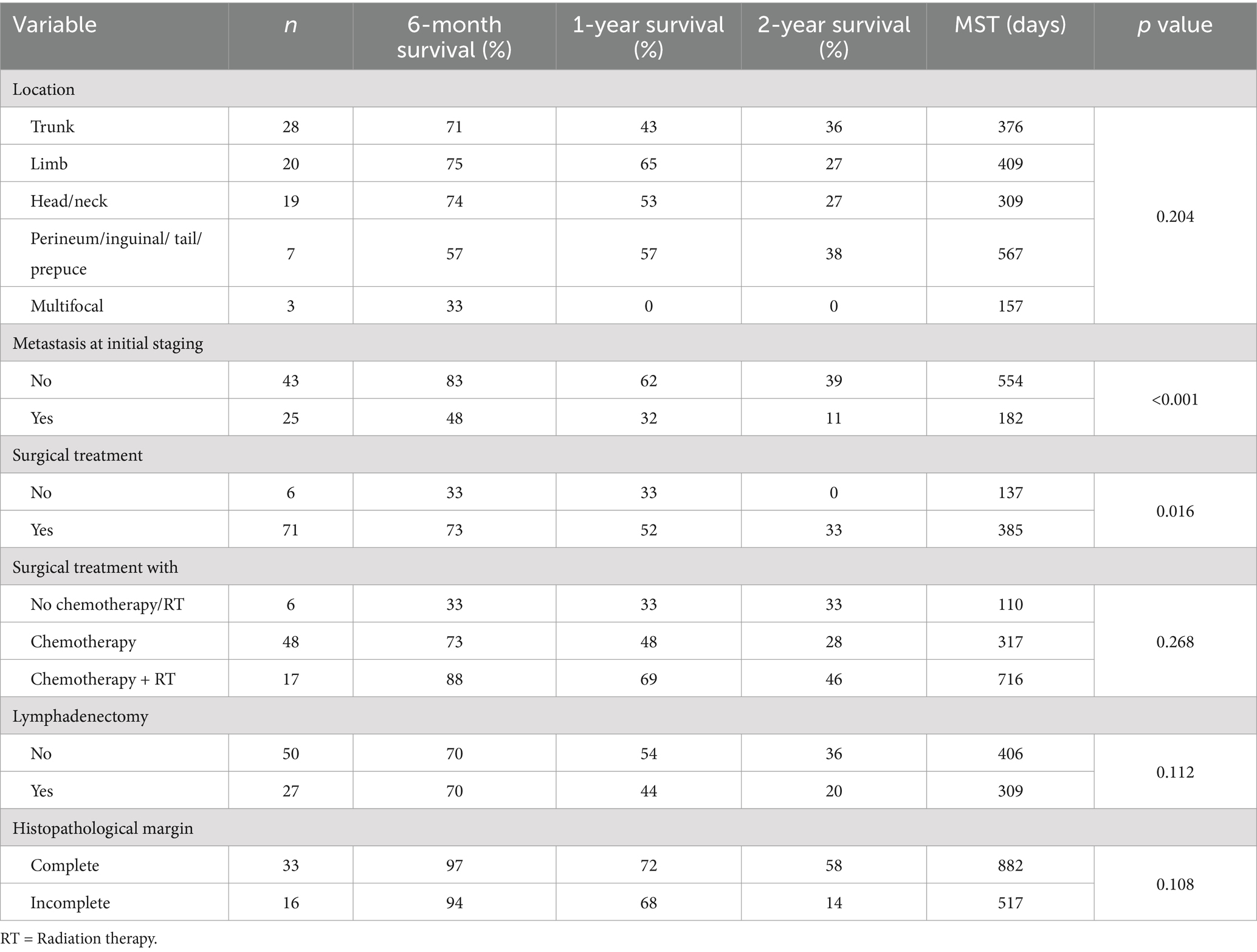
Table 1. Univariate analysis evaluating the influence of selected clinical factors on survival in dogs with high-grade cutaneous mast cell tumors.
Lymphadenectomy was performed in 27 dogs (35%), with histopathologic analysis available for 25 of them. Lymph node metastasis was detected in 63% (n = 17) of the excised nodes. Lymph node extirpation did not result in a significant survival advantage (Table 1), nor did it lead to a notable reduction in metastatic spread. Moreover, lymphadenectomy did not provide a clear survival benefit for dogs with metastatic lymph nodes (p = 0.694). The MST for dogs with metastatic lymph node that did not undergo lymphadenectomy was 161 days, compared to 182 days for those that underwent the procedure.
To assess the impact of surgical margins on local recurrence, only surgical sites with a minimum follow-up period of 6 months postoperatively were analyzed, unless recurrence occurred within 6 months. A total of 54 HGMCTs, including recurrent HGMCTs, surgically excised from 49 dogs, met this criterion (64% of included dogs). Incomplete surgical margins were significantly associated with local recurrence (odds ratio = 3.97, p = 0.037, Table 2), but not with the development of postoperative metastasis or survival time. Of the 19 masses with incomplete surgical margins, 11 (58%) developed local regrowth, while nine (26%) of the 35 masses with complete margins recurred. The median time to local recurrence was 140 days. Seven of the 19 surgical sites with incomplete margins were treated with RT, and 43% (n = 3) of these sites experienced MCT regrowth. Data from this study failed to demonstrate a significant local recurrence benefit from concurrent chemotherapy and RT (Table 2).
At the end of the study, 62 dogs were dead or euthanized for the following: MCT-related causes (n = 52), undetermined causes (n = 6), and causes unrelated to MCT (n = 4). Fifteen dogs were censored from the analysis, including 12 that were still alive and three that were lost to follow-up. A subset of the study population had one or more of the following comorbidities: cardiovascular disorders (n = 9), neurologic diseases (n = 5), musculoskeletal disorders (n = 3), melanoma (n = 2), hemangiosarcoma (n = 1), squamous cell carcinoma (n = 1), renal adenocarcinoma (n = 1), anaplastic carcinoma (n = 1), soft tissue sarcoma (n = 1), primary lung histiocytic sarcoma (n = 1), duodenal mass (n = 1), and chronic renal insufficiency (n = 1). No association was found between sex, number of MCTs at presentation, local recurrence, and survival time.
Discussion
This study reports the outcomes of 77 dogs with HGMCTs treated with various regimens based on clinician and owner discretion. Various studies on canine HGMCTs treated with surgery alone have shown varying survival times, ranging from 98 days to 278 days (16, 19, 33, 34). The MST for all dogs in this study was 317 days, with survival rates at 6-month, 1-year, and 2-year of 69, 50, and 30%, respectively. Our results confirm that dogs with HGMCTs can experience a fair outcome if treated, particularly with surgical intervention. There was a trend toward improved survival time in dogs with complete surgical margins (MST = 882 days) and those receiving concurrent surgery, chemotherapy, and RT (MST = 716 days), emphasizing the importance of local control in dogs with HGMCTs. Hume et al. (15) also reported a survival benefit associated with local tumor control in dogs with grade III MCTs. It should be noted that the number of dogs that did not undergo surgical treatment in our study was small (n = 6), and four of these dogs were presented with multiple masses and/or metastasis at initial staging. Combined with the absence of stratification by clinical stage and tumor diameter, these factors likely impacted the strength of our statistical analysis for this subset of the population.
Dogs with LGMCTs can have a good long-term prognosis following complete excision of the primary tumor, making wide surgical excision with adequate margins crucial for a successful outcome (35). Our findings showed that inadequate surgical margins in HGMCTs increased the risk of local tumor regrowth, which occurred in 58% of dogs. Even with complete excision, a significant recurrence risk remained, with 26% of tumors recurring after full resection. This finding is consistent with a previous study, which reported a significantly higher local recurrence rate in HGMCTs (36%) compared to LGMCTs (4%), despite complete resection (35). However, the accuracy of surgical margin assessment in this study may have been compromised by the method used to quantify histologic tumor-free margins. Tangential sectioning, a technique known for its higher sensitivity in identifying incomplete margins (36), was not consistently applied and could have resulted in false-negative classifications. In veterinary oncology, the definition of a complete histologic excision remains undefined, with varying tumor-free margin widths applied inconsistently and often lacking supporting evidence. To address this gap, we adopted the R classification system, widely utilized in human oncology, where a histologic tumor-free margin greater than 0 mm is considered a complete excision and is highly prognostic for most malignant tumors in humans (37).
Theoretically, one would expect local regrowth in all patients with incomplete surgical excision, which was not observed in this study. Several factors have been postulated to explain this, including immune infiltration and eradication of tumor cells post-operatively, the inhibitory effects of anti-invasion factors from connective tissues, the inability of the residual cells to secrete autocrine growth factors to support their survival, and an inadequate follow-up period causing erroneous patient categorization during analyses (38). Interestingly, the local recurrence rate remained high even for completely resected tumors. These recurrent lesions could originate from the surrounding satellite tumor cell populations that were not removed during surgery or from de novo tumors near the surgical scars, rather than true local recurrences. In contrast to other literature, we could not find evidence of a negative association between local recurrence and metastasis, as well as survival time (15, 39).
Nodal metastasis was observed in 37% of the patients at initial diagnosis and was associated with a poorer survival outcome, which is comparable to earlier reports (15, 19, 40). Data from this current study surprisingly failed to demonstrate a significant survival advantage of lymphadenectomy in dogs with nodal metastasis. This may be due to the small number of patients with nodal metastasis present at the time of lymphadenectomy and may have affected our statistical analyses. Several studies have shown a favorable therapeutic effect of metastatic lymph node extirpation in dogs with MCTs, and recently, therapeutic lymphadenectomy has gained increased attention in veterinary surgical oncology (15, 30, 41, 42). This study’s lack of an associated survival benefit could be attributed to the non-selective nodal dissection technique, potentially missing metastatic lymph nodes. It has been shown that lymphatic draining pattern of tumors may be aberrant and does not correspond to regional lymph nodes in up to 63% of canine patients due to tumor-induced lymphagiogenesis (43–45). Therefore, non-selective nodal dissection may result in undertreatment and undermine the therapeutic efficacy of lymphadenectomy. Additionally, dogs in this study underwent different staging procedures, meaning that some patients might have been under-staged at the time of initial diagnosis.
It is well established that HGMCTs are highly metastatic. Consequently, the administration of adjuvant chemotherapy has become the standard of care, even when visible metastasis is not observed, to reduce the probability of systemic dissemination of tumor cells (20). Adjuvant systemic therapy did not indefinitely halt disease progression; 79% (n = 31/39) of the dogs initially free from metastasis later experienced progression, with 38% (n = 15) developing metastatic disease despite chemotherapy. Additionally, 65% of dogs succumbed to their disease despite combination treatment with surgery and chemotherapy, with or without RT. This underscores the need for more effective treatment regimens or strategies for this subset of dogs. Nonetheless, the contribution of adjuvant systemic therapy, with or without RT, to the prolonged survival time should not be disregarded. The MST, 1-year, and 2-year survival rates in this study are longer than those reported in previous studies on dogs that underwent surgical treatment only (16, 19, 33, 34).
The predilection of retrievers and bulldog-related breeds to MCTs observed in this study is consistent with previous reports (46, 47). Mast cell tumors in inguinal and perineal areas have historically been associated with an unfavorable outcome, and recent literature has shown an increased risk of HGMCT development in these locations (32). However, our data indicates that the majority of the HGMCTs were located on the trunk (36%), limb (26%), and head/neck (25%), and we were unable to demonstrate a significant association between these locations and prognosis. Sfiligoi et al. (48) also suggested that dogs with MCTs at the perineal and inguinal areas may not have a worse prognosis. This finding warrants further investigation to determine the prognostic impact of tumor localization, which could help clinicians make more informed treatment decisions.
The retrospective nature of this study presents limitations that should be noted. A primary limitation is the lack of stratification by clinical stage and tumor diameter, potentially introducing bias into the findings. Moore et al. (18) demonstrated that dogs with stage I HGMCT and tumor diameters less than 25 mm can achieve favorable outcomes, underscoring the necessity of such stratification in future analyses. Furthermore, different pathologists and clinicians examined the histopathological slides and patients in this study population, respectively, leading to potential inter-pathologist and clinician variability. Other limitations include the absence of a control group for comparing outcomes between treated and untreated dogs and the small sample size in certain treatment groups. The progression-free interval was not examined as the date of disease progression was not uniformly available in the medical records and some dogs were classified as relapse based on the attending clinician’s clinical judgement without further diagnostic confirmation.
Results from this study suggest that local tumor control and adjuvant medical treatment provide a survival advantage for dogs with HGMCTs compared with findings from other studies that evaluated the outcomes of dogs with HGMCT that received surgical treatment only. Early diagnosis and intervention of HGMCTs are essential as metastasis at initial diagnosis negatively impacts prognosis. Lymphadenectomy did not improve outcomes in this study; however, further investigations into the benefits of sentinel lymph node mapping and biopsy in dogs with HGMCT is recommended. The high rate of local recurrence in completely resected HGMCTs underscores the need for more reliable outcome data to assist surgeons in making informed decisions on resection techniques and improving clinical results. Finally, the failure of adjuvant chemotherapy to impede disease progression remains a significant problem, highlighting the urgent need for better treatment strategies for HGMCTs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because of the retrospective nature of the study. Written informed consent was not obtained from the owners for the participation of their animals in this study because of the retrospective nature of the study.
Author contributions
SO: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CM: Data curation, Methodology, Writing – review & editing. CP: Validation, Writing – review & editing. DR: Validation, Writing – review & editing. MO: Conceptualization, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Partially funded by Animal Health Partners Research Chair in Veterinary Medical Innovation.
Conflict of interest
CP was employed by ANI.ML Research, ANI.ML Health Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Galli, SJ, Nakae, S, and Tsai, M. Mast cells in the development of adaptive immune responses. Nat Immunol. (2005) 6:135–42. doi: 10.1038/ni1158
2. Elieh Ali Komi, D, Wöhrl, S, and Bielory, L. Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol. (2016) 58:342–65. doi: 10.1007/s12016-019-08769-2
3. London, CA, Kisseberth, WC, Galli, SJ, Geissler, EN, and Helfand, SC. Expression of stem cell factor receptor (c-kit) by the malignant mast cells from spontaneous canine mast cell tumours. J Comp Pathol. (1996) 115:399–414. doi: 10.1016/S0021-9975(96)80074-0
4. London, CA, Galli, SJ, Yuuki, T, Hu, ZQ, Helfand, SC, and Geissler, EN. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp Hematol. (1999) 27:689–97. doi: 10.1016/S0301-472X(98)00075-7
5. Letard, S, Yang, Y, Hanssens, K, Palmérini, F, Leventhal, PS, Guéry, S, et al. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res. (2008) 6:1137–45. doi: 10.1158/1541-7786.MCR-08-0067
6. Downing, S, Chien, MB, Kass, PH, Moore, PF, and London, CA. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c- kit in mast cell tumors of dogs. Am J Vet Res. (2002) 63:1718–23. doi: 10.2460/AJVR.2002.63.1718
7. Bostock, DE. Neoplasms of the skin and subcutaneous tissues in dogs and cats. Br Vet J. (1986) 142:1–19. doi: 10.1016/0007-1935(86)90002-3
8. Villamil, JA, Henry, CJ, Bryan, JN, Ellersieck, M, Schultz, L, Tyler, JW, et al. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. J Am Vet Med Assoc. (2011) 239:960–5. doi: 10.2460/JAVMA.239.7.960
9. Rothwell, TL, Howlett, CR, Middleton, DJ, Griffiths, DA, and Duff, BC. Skin neoplasms of dogs in Sydney. Aust Vet J. (1987) 64:161–4. doi: 10.1111/J.1751-0813.1987.TB09673.X
10. Patnaik, AK, Ehler, WJ, and MacEwen, EG. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet Pathol. (1984) 21:469–74. doi: 10.1177/030098588402100503
11. Murphy, S, Sparkes, AH, Brearley, MJ, Smith, KC, and Blunden, AS. Relationships between the histological grade of cutaneous mast cell tumours in dogs, their survival and the efficacy of surgical resection. Vet Rec. (2004) 154:743–6. doi: 10.1136/VR.154.24.743
12. Howard, EB, Sawa, TR, Nielsen, SW, and Kenyon, AJ. Mastocytoma and gastroduodenal ulceration: gastric and duodenal ulcers in dogs with mastocytoma. Vet Pathol. (1969) 6:146–58. doi: 10.1177/030098586900600205
13. Ishiguro, T, Kadosawa, T, Takagi, S, Kim, G, Ohsaki, T, Bosnakovski, D, et al. Relationship of disease progression and plasma histamine concentrations in 11 dogs with mast cell tumors. J Vet Intern Med. (2003) 17:194–8. doi: 10.1111/J.1939-1676.2003.TB02433.X
14. O’Keefe, DA, Couto, CG, Burke-Schwartz, C, and Jacobs, RM. Systemic mastocytosis in 16 dogs. J Vet Intern Med. (1987) 1:75–80. doi: 10.1111/J.1939-1676.1987.TB01990.X
15. Hume, CT, Kiupel, M, Rigatti, L, Shofer, FS, Skorupski, KA, and Sorenmo, KU. Outcomes of dogs with grade 3 mast cell tumors: 43 cases (1997-2007). J Am Anim Hosp Assoc. (2011) 47:37–44. doi: 10.5326/JAAHA-MS-5557
16. Kiupel, M, Webster, JD, Bailey, KL, Best, S, DeLay, J, Detrisac, CJ, et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet Pathol. (2011) 48:147–55. doi: 10.1177/0300985810386469
17. Takeuchi, Y, Fujino, Y, Watanabe, M, Takahashi, M, Nakagawa, T, Takeuchi, A, et al. Validation of the prognostic value of histopathological grading or c-kit mutation in canine cutaneous mast cell tumours: a retrospective cohort study. Vet J. (2013) 196:492–8. doi: 10.1016/J.TVJL.2012.11.018
18. Moore, AS, Frimberger, AE, Taylor, D, and Sullivan, N. Retrospective outcome evaluation for dogs with surgically excised, solitary Kiupel high-grade, cutaneous mast cell tumours. Vet Comp Oncol. (2020) 18:402–8. doi: 10.1111/vco.12565
19. Murphy, S, Sparkes, AH, Blunden, AS, Brearley, MJ, and Smith, KC. Effects of stage and number of tumours on prognosis of dogs with cutaneous mast cell tumours. Vet Rec. (2006) 158:287–91. doi: 10.1136/VR.158.9.287
20. London, CA, and Thamm, DH. Mast cell tumours. Withrow and MacEwen’s small animal clinical oncology. St. Louis: Saunders Elsevier (2013). p. 335–355
21. Hillman, LA, Garrett, LD, de Lorimier, LP, Charney, SC, Borst, LB, and Fan, TM. Biological behavior of oral and perioral mast cell tumors in dogs: 44 cases (1996-2006). J Am Vet Med Assoc. (2010) 237:936–42. doi: 10.2460/JAVMA.237.8.936
22. Preziosi, R, Sarli, G, and Paltrinieri, M. Prognostic value of intratumoral vessel density in cutaneous mast cell tumours of the dog. J Comp Pathol. (2004) 130:143–51. doi: 10.1016/J.JCPA.2003.10.003
23. Kiupel, M, Webster, JD, Kaneene, JB, Miller, R, and Yuzbasiyan-Gurkan, V. The use of KIT and tryptase expression patterns as prognostic tools for canine cutaneous mast cell tumors. Vet Pathol. (2004) 41:371–7. doi: 10.1354/vp.41-4-371
24. Mullins, MN, Dernell, WS, Withrow, SJ, Ehrhart, EJ, Thamm, DH, and Lana, SE. Evaluation of prognostic factors associated with outcome in dogs with multiple cutaneous mast cell tumors treated with surgery with and without adjuvant treatment: 54 cases (1998–2004). J Am Vet Med Assoc. (2006) 228:91–5. doi: 10.2460/JAVMA.228.1.91
25. Bae, S, Milovancev, M, Bartels, C, Irvin, VL, Tuohy, JL, Townsend, KL, et al. Histologically low-grade, yet biologically high-grade, canine cutaneous mast cell tumours: a systematic review and meta-analysis of individual participant data. Vet Comp Oncol. (2020) 18:580–9. doi: 10.1111/vco.12581
26. Weisse, C, Shofer, FS, and Sorenmo, K. Recurrence rates and sites for grade II canine cutaneous mast cell tumors following complete surgical excision. J Am Anim Hosp Assoc. (2002) 38:71–3. doi: 10.5326/0380071
27. Anderson, K, Pellin, M, Snyder, E, and Clarke, D. Tumor grade and mitotic count are prognostic for dogs with cutaneous mast cell tumors treated with surgery and adjuvant or neoadjuvant vinblastine chemotherapy. Vet Sci. (2024) 11:363. doi: 10.3390/VETSCI11080363
28. Todd, JE, Nguyen, SM, White, J, Langova, V, Thomas, PM, and Tzannes, S. Combination vinblastine and palladia for high-grade and metastatic mast cell tumors in dogs. Can Vet J. (2021) 62:1335–40.
29. Hay, JK, and Larson, VS. Lomustine (CCNU) and prednisone chemotherapy for high-grade completely excised canine mast cell tumors. Can Vet J. (2019) 60:1326–30.
30. Chalfon, C, Sabattini, S, Finotello, R, Faroni, E, Guerra, D, Pisoni, L, et al. Lymphadenectomy improves outcome in dogs with resected Kiupel high-grade cutaneous mast cell tumours and overtly metastatic regional lymph nodes. J Small Anim Pract. (2022) 63:661–9. doi: 10.1111/JSAP.13525
31. Pierini, A, Lubas, G, Gori, E, Binanti, D, Millanta, F, and Marchetti, V. Epidemiology of breed-related mast cell tumour occurrence and prognostic significance of clinical features in a defined population of dogs in west-Central Italy. Vet Sci. (2019) 6:53. doi: 10.3390/vetsci6020053
32. Śmiech, A, Ślaska, B, Łopuszyński, W, Jasik, A, Bochyńska, D, and Dąbrowski, R. Epidemiological assessment of the risk of canine mast cell tumours based on the Kiupel two-grade malignancy classification. Acta Vet Scand. (2018) 60:70. doi: 10.1186/s13028-018-0424-2
33. Sabattini, SI, Scarpa, F, Berlato, D, and Bettini, GI. Histologic grading of canine mast cell tumor: is 2 better than 3? Vet Pathol. (2015) 52:70–3. doi: 10.1177/0300985814521638
34. Bostock, DE. The prognosis following surgical removal of mastocytomas in dogs. J Small Anim Pract. (1973) 14:27–40. doi: 10.1111/J.1748-5827.1973.TB06891.X
35. Donnelly, L, Mullin, C, Balko, J, Goldschmidt, M, Krick, E, Hume, C, et al. Evaluation of histological grade and histologically tumour-free margins as predictors of local recurrence in completely excised canine mast cell tumours. Vet Comp Oncol. (2015) 13:70–6. doi: 10.1111/VCO.12021
36. Dores, CB, Milovancev, M, and Russell, DS. Comparison of histologic margin status in low-grade cutaneous and subcutaneous canine mast cell tumours examined by radial and tangential sections. Vet Comp Oncol. (2018) 16:125–30. doi: 10.1111/VCO.12321
37. Liptak, JM. Histologic margins and the residual tumour classification scheme: is it time to use a validated scheme in human oncology to standardise margin assessment in veterinary oncology? Vet Comp Oncol. (2020) 18:25–35. doi: 10.1111/VCO.12555
38. Misdorp, W. Incomplete surgery, local immunostimulation, and recurrence of some tumour types in dogs and cats. Vet Q. (1987) 9:279–86. doi: 10.1080/01652176.1987.9694113
39. Hahn, KA, King, GK, and Carreras, JK. Efficacy of radiation therapy for incompletely resected grade-III mast cell tumors in dogs: 31 cases (1987–1998). J Am Vet Med Assoc. (2004) 224:79–82. doi: 10.2460/JAVMA.2004.224.79
40. Krick, EL, Billings, AP, Shofer, FS, Watanabe, S, and Sorenmo, KU. Cytological lymph node evaluation in dogs with mast cell tumours: association with grade and survival. Vet Comp Oncol. (2009) 7:130–8. doi: 10.1111/J.1476-5829.2009.00185.X
41. Marconato, L, Polton, G, Stefanello, D, Morello, E, Ferrari, R, Henriques, J, et al. Therapeutic impact of regional lymphadenectomy in canine stage II cutaneous mast cell tumours. Vet Comp Oncol. (2018) 16:580–9. doi: 10.1111/vco.12425
42. Baginski, H, Davis, G, and Bastian, RP. The prognostic value of lymph node metastasis with grade 2 MCTs in dogs: 55 cases (2001–2010). J Am Anim Hosp Assoc. (2014) 50:89–95. doi: 10.5326/JAAHA-MS-5997
43. Ran, S, Volk, L, Hall, K, and Flister, MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. (2010) 17:229–51. doi: 10.1016/J.PATHOPHYS.2009.11.003
44. Worley, DR. Incorporation of sentinel lymph node mapping in dogs with mast cell tumours: 20 consecutive procedures. Vet Comp Oncol. (2014) 12:215–26. doi: 10.1111/J.1476-5829.2012.00354.X
45. Ferrari, R, Chiti, LE, Manfredi, M, Ravasio, G, De Zani, D, Zani, DD, et al. Biopsy of sentinel lymph nodes after injection of methylene blue and lymphoscintigraphic guidance in 30 dogs with mast cell tumors. Vet Surg. (2020) 49:1099–108. doi: 10.1111/VSU.13483
46. Mochizuki, H, Motsinger-Reif, A, Bettini, C, Moroff, S, and Breen, M. Association of breed and histopathological grade in canine mast cell tumours. Vet Comp Oncol. (2017) 15:829–39. doi: 10.1111/VCO.12225
47. Warland, J, and Dobson, J. Breed predispositions in canine mast cell tumour: a single Centre experience in the United Kingdom. Vet J. (2013) 197:496–8. doi: 10.1016/J.TVJL.2013.02.017
Keywords: canine, high-grade cutaneous mast cell tumor, surgical excision, lymphadenectomy, adjuvant therapy
Citation: Ong SM, McKenna C, Pinard C, Richardson D and Oblak ML (2025) Clinical outcomes of dogs with high-grade cutaneous mast cell tumors. Front. Vet. Sci. 11:1519636. doi: 10.3389/fvets.2024.1519636
Edited by:
Antonio Giuliano, City University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Petros Frezoulis, Southfields Veterinary Specialists, United KingdomAngelo Capasso, Dick White Referrals, United Kingdom
Copyright © 2025 Ong, McKenna, Pinard, Richardson and Oblak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siew Mei Ong, c2lld21laUB1cG0uZWR1Lm15; Michelle L. Oblak bW9ibGFrQHVvZ3VlbHBoLmNh
 Siew Mei Ong
Siew Mei Ong Charly McKenna
Charly McKenna Christopher Pinard3,4,5,6
Christopher Pinard3,4,5,6 Michelle L. Oblak
Michelle L. Oblak