- 1Institute of Animal Husbandry and Veterinary Science, Jiangxi Academy of Agricultural Sciences, Nanchang, China
- 2Jiangxi Province Key Laboratory of Animal Green and Healthy Breeding, Nanchang, China
Introduction: Gynura procumbens (Lour.) Merr is a common traditional Chinese medicine with anti-tumor, anti-inflammatory and antioxidant activities. However, no related studies reported the potential application effect of Gynura procumbens on meat ducks. The study aims to investigate the potential effects of Gynura procumbens extract (GPE) supplementation on growth performance, carcass traits, antioxidant capacity, immunity and meat quality.
Methods: A total of 480 21-day-old female healthy ducks were randomly allocated to four treatments, each treatment containing six replicates with 20 ducks per replicate. The groups received a corn-soybean basal diet supplemented with 0 mg/kg GPE (CON), 200 mg/kg GPE (GPE200), 400 mg/kg GPE (GPE400), and 600 mg/kg GPE (GPE600), respectively. The entire experiment lasted for 7 weeks.
Results: The results showed that dietary supplementation with 600 mg/kg GPE significantly reduced the contents of serum urea nitrogen, triglyceride (TG) and total cholesterol (TC). GPE (200, 400, and 600 mg/kg) supplementation effectively reduced the contents of IL-2 and MDA. The levels of immunoglobulin M (IgM) as well as total antioxidative capacity (T-AOC) in GPE600 group dramatically elevated in comparison with the control group. Dietary GPE supplementation considerably increased the moisture content of the breast muscle. Furthermore, dietary supplementation with GPE markedly decreased the water loss rate and shear force.
Discussion: With the ban of antibiotics in poultry production, traditional Chinese medicines have been widely used in livestock and poultry production due to their high efficiency and low toxicity. Gynura procumbens extract GPE as a natural plant origin contains a series of biologically active components, including flavonoids, polyphenols, saponin, tannin and terpenoid. This study indicated that dietary supplementation with GPE can increase serum total antioxidant capacity, regulate immune function and improve meat quality to some extent in meat ducks. The recommended optimal GPE level in the diet of meat ducks is 600 mg/kg according to the results in this study.
1 Introduction
With the advent of biological and engineering technology, large numbers of new antibiotics were developed to treat diseases. In recent years, various antibiotics have been regularly used in medical treatment as well as livestock farming. The application of antibiotics in feed has greatly improved the growth performance of animals and promoted the rapid development of animal husbandry (1). Unfortunately, the extensive abuse of antibiotics resulted in significant antibiotic residues in meat and aquatic products, as well as increased the risks of drug-resistant strains mutations (2). Meanwhile, with the improvement in living standards, consumers have a stronger desire to pursue high quality natural foods. However, long-term misuse of antibiotics can undermine the original intention of ecological farming and simultaneously pose a substantial threat to public health (3–6). Therefore, there is an urgent need to search for alternative novel additives to antibiotics.
It is widely believed that nutritional regulation offers an effective strategy to replace antibiotics through the use of traditional Chinese medicine (TCM) additives. As the treasures of the Chinese nation, Chinese herbal medicines has a long history of being widely used in the treatment of various diseases among the people (7). Recently, traditional Chinese herbs have attracted increasing attention from agronomists due to their high efficacy and low toxicity. As we all know, with the gradual ban of antibiotics in livestock farming, more and more researchers have turned their attention toward the development of Chinese herbal additives. Indeed, many medicinal plants and bioactive compounds originated from natural plants have been widely used in livestock farming (8) For instance, Curcumin, a polyphenolic curcuminoid compound derived from the rhizomes of the plant Curcuma longa, has been used as feed additive for improving the intestinal health of broiler (9). Traditional Chinese medicinal herbs prescription composed of Cortex Fraxini, Pulsatilla chinensis and Eucommia ulmoides could improve ducks’ antioxidant and immune capacity (10).
As a common folk TCM, GP (Gynura procumbens (Lour.) Merr.) is an annual evergreen shrub with a fleshly stem and purple tint and is considered to possess diverse pharmacological properties, such as antioxidation, antihypertension and cardioprotective, anti-inflammatory, antihyperglycemic and anticancer activities (11). It has been extensively used in clinic as remedy for eruptive fevers, rashes, kidney diseases, hypertension, diabetes mellitus and cancer (12, 13). These beneficial pharmacological activities of GP may be attributed to the presence of its active ingredients, including flavonoids, saponin, tannin, terpenoids and glycosides (14, 15). Currently, vast attention has been focused on the broad clinical application of GPE. However, no studies are exploring the potential effects of GPE supplementation on ducks.
Currently, in the process of duck farming, Low immunity among duck flocks remains a pressing challenge for farmers and is widely recognized as a key factor contributing to high duck mortality rates. Additionally, ducks are highly susceptible to stress responses triggered by high-density farming, which results in oxidative damage and further causes the reduction of growth performance (16). Therefore, maintaining the balance of the oxidant/antioxidant and enhancing immunity is an effective approach to promote the health growth of ducks and plays a fundamental role in improving the quality of duck meat (17). Numerous clinical studies highlight that GPE is abundant in polyphenols and flavonoids, which plays an important role in boosting immunity and strengthening antioxidant defenses (18, 19). Despite GPE’s widespread clinical applications, no related studies to date have explored its potential effects on ducks. Our preliminary study has indicated that dietary supplementation with GPE could boost duck’s immunity. Therefore, the current study was designed to explore the potential effect of dietary supplementation with GP on growth performance, immunity and antioxidative capacity of ducks and further elucidate the important role of GPE in improving meat quality.
2 Materials and methods
2.1 Animals and experimental design
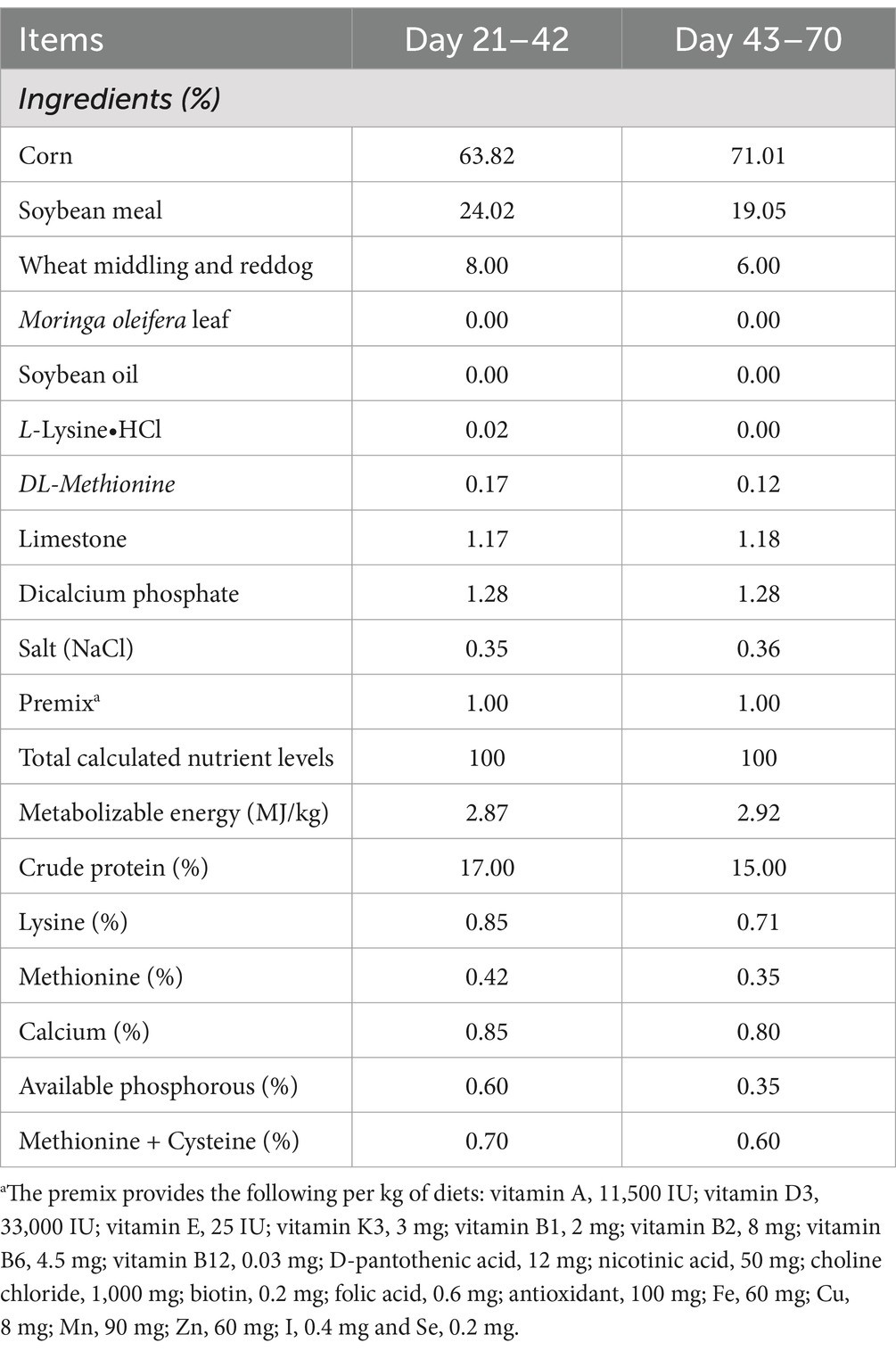
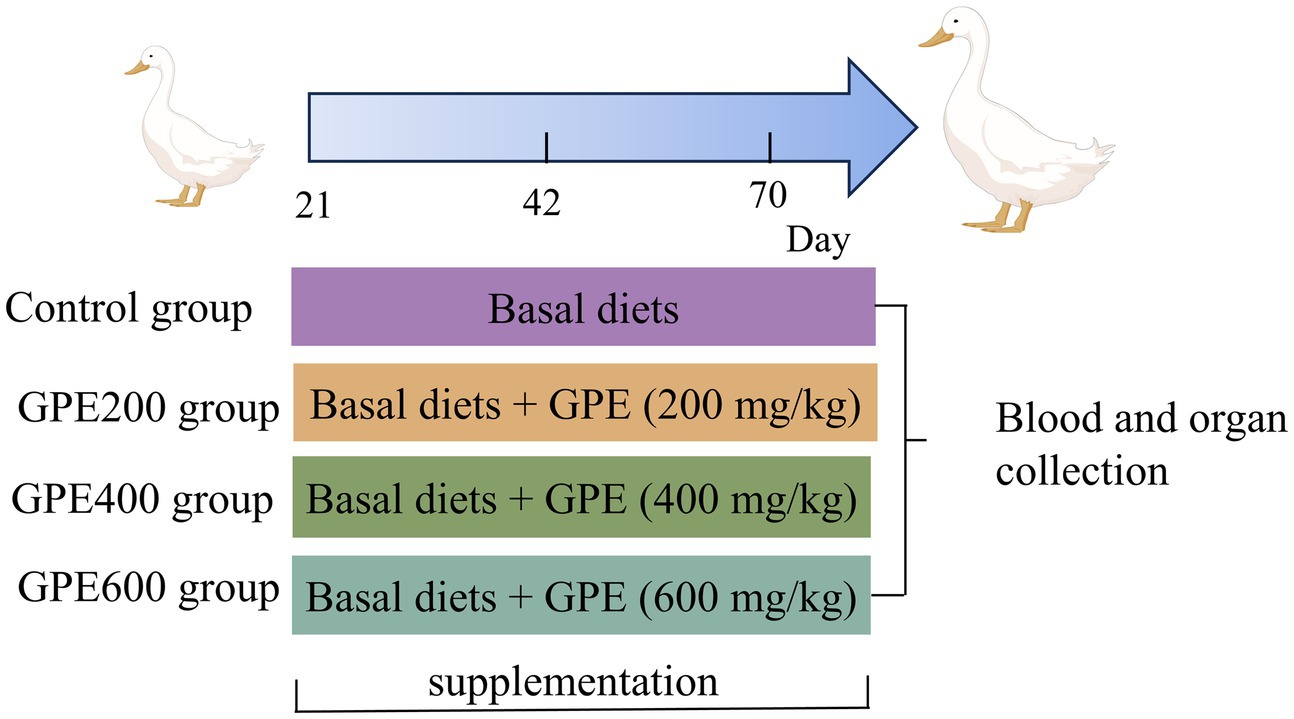
The experiment was carried out with permission from the Experimental Animal Ethics Committee in Jiangxi Academy of Agricultural Sciences. A total of 480 21-day-old female healthy ducks with similar body weight were bought from a local commercial hatchery (Jian, China). Subsequently, they were randomly assigned into four treatments with six replicates each comprising 20 ducks. The four dietary treatments consisted of the control and three levels of GPE (200 mg/kg, 400 mg/kg, and 600 mg/kg). The basal diet was formulated to meet the nutrient requirements of meat ducks (NY/T 2122–2012, China). The ingredient compositions and nutrient contents of the basal diets for ducks are listed in Table 1. These ducks were rearing on net bed with splash pool and received water ad libitum through the entire experiment period. The whole experiment was sustained for 7 weeks, the schematic diagram showing the study design is displayed in Figure 1.
2.2 Growth performance
After 12 h of fasting, the body weight (BW) from each replicate cage was measured on days 21, 42, and 70. Mortality was monitored daily, with the weight of dead ducks recorded and feed intake per cage was recorded every day through the experimental period. Subsequently, the average body weight, average daily feed intake, average daily gain, and the ratio of feed to weight gain (F/G) were calculated with correction for mortalities.
2.3 Blood sample collection
Two ducks from each replicate were randomly selected at 42 and 70 days of age, respectively. Blood was obtained from wing vein and centrifugated at 3,000 g, for 10 min at 4°C to prepare serum for subsequent measurement.
2.4 Measurement of serum biochemical indices, antioxidant activity and immunity
For biochemical assays, the freshly collected serum samples were used to measure the activities of total antioxidant capacity (T-AOC, Cat no, A015-2-1), glutathione peroxidase (GSH-Px, Cat no, A005-1-2), superoxide dismutase (SOD, Cat no, A001-3-2), malondialdehyde (MDA, Cat no, A003-1-2), blood urea nitrogen (BUN, Cat no, C013-2-1), alkaline phosphatase (ALP, Cat no, A059-2-2), aspartate aminotransferase (AST, Cat no, C010-2-1), alanine aminotransferase (ALT, Cat no, C009-2-1), triglyceride (TG, Cat no, A110-2-1) and albumin (ALB, Cat no, A028-2-1) using corresponding commercial assay kits (Jiancheng Bioengineering, Nanjing, China). The contents of immunoglobulin A (IgA, Cat no, ml036901), immunoglobulin G (IgG, Cat no, ml036900) and immunoglobulin M (IgM, Cat no, ml061224) were quantified with ELISA assay kits (Shanghai Enzyme-linked Biotechnology, China) according to the manufacturer’s instructions. Additionally, sample quality control and testing parameter setting were strictly adhered to the product specification.
2.5 Carcass traits and organ indices
At the end of the trial (70 days of age), two ducks from each replicate were randomly selected, weighed individually and sacrificed after 12 h of feed deprivation. Subsequently, ducks were sacrificed by exsanguination from the jugular vein. The weight of the defeather carcass was determined as the carcass weight. The carcass was weighed after removing the trachea, esophagus, spleen, pancreas, gallbladder, reproductive organ, intestinal tract, gizzard content and gizzard-membrane which was recorded as semi-eviscerated weight. The eviscerated weight was measured as eviscerated weight after removing the heart, liver, lung, gizzard, glandular stomach, and abdominal fat. Finally, the rate of dressing, semi-eviscerated, eviscerated, breast muscle, leg muscle, and abdominal fat were calculated. The weight of dissected heart, liver, spleen, lung, gizzard and glandular stomach was recorded, respectively, and organ index was calculated.
2.6 Evaluation of liver histopathological changes
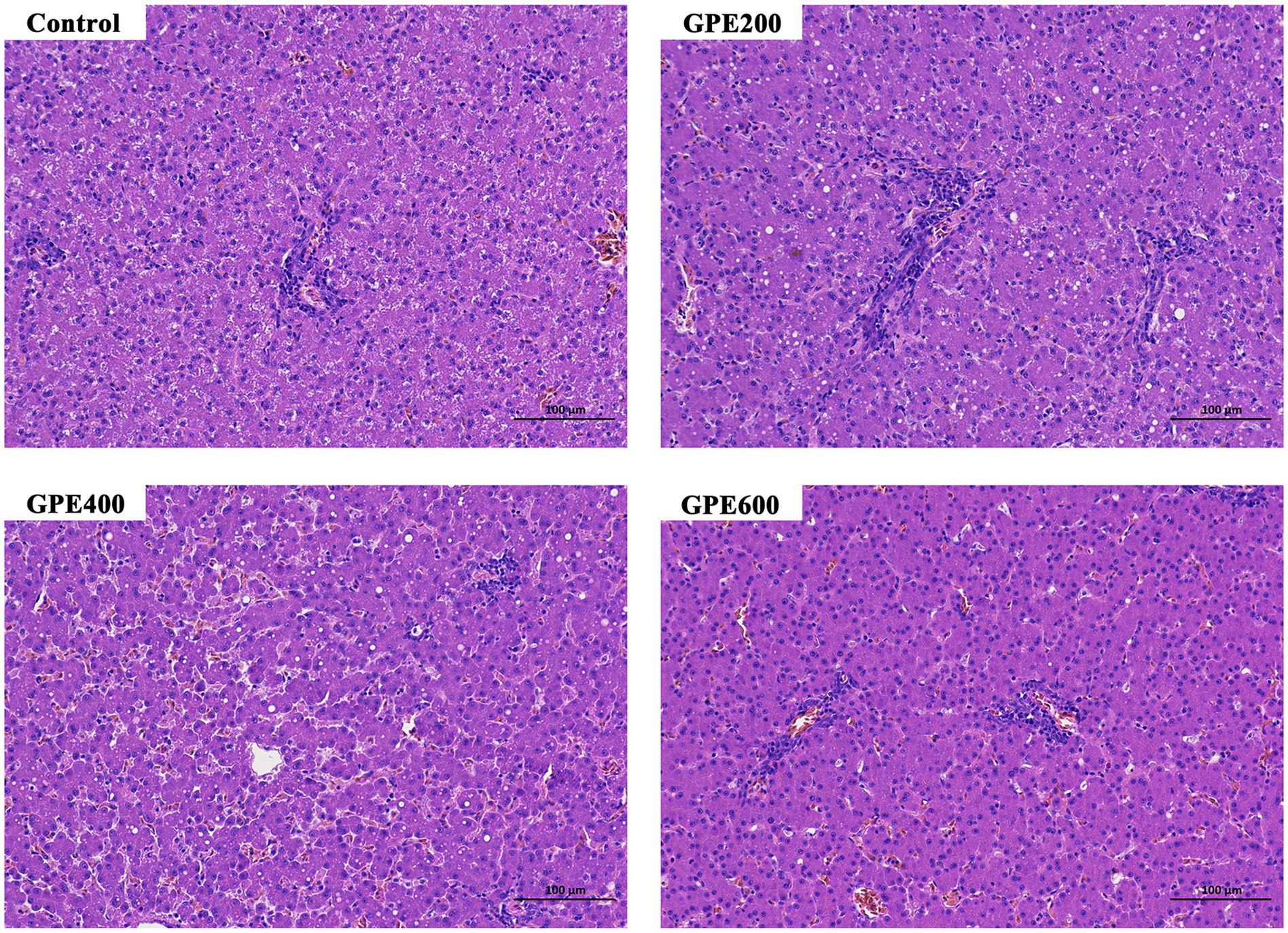
For histopathological analysis, liver tissues of ducks were collected in ice-cold phosphate-buffered saline and subsequently transferred into 4% fresh paraformaldehyde for fixation at room temperature for at least 24 h. Subsequently, the specimens were further processed for dehydration. After dehydration with ethanol and xylene, the tissues were embedded in paraffin, sectioned at a thickness of 5 μm and subjected to hematoxylin and eosin (H&E). Afterward, the above samples were photographed under a light microscope (BX53, Olympus).
2.7 Meat quality
After slaughter, the pH values of breast muscle and leg muscle were measured with a portable pH meter (pH-Star, Matthaus GmbH & Co. KG, Eckelsheim, Germany) in the pectoralis major muscle and leg muscle at about 1 cm depth as Huang previously described (20). Color changes were evaluated at each sampling by measuring L*, a*, and b* parameters using a reflectance spectrophotometer (X-Rite SP64, United States), L*, a*, and b* values represented the degree of lightness, greenness or redness, and blueness or yellowness, respectively. Subsequently, the shear force was measured. The procedure was as follows: the raw breast muscles were cut into three strips (1 × 1 × 3 cm) perpendicular to the muscle fiber direction to evaluate the tenderness using a digital-display muscle tenderness meter (C-LM3B, Tenova, Harbin, China). The water loss rate was measured according to the modified method of Li et al. (21). In brief, collected meat samples (0.125 cm3) were weighed and carefully wrapped in absorbent and placed into the machine for testing. The program was set to 300 N for 5 min. After 5 min, the samples were weighed again. Finally, the water loss rate was calculated as a percentage:
2.8 Determination of IMP content in muscle
The inosine Monophosphate (IMP) concentration was determined with high-performance liquid chromatography (HPLC, Agilent 1290, Agilent Technologies, United States). The IMP content in the samples was measured using an acidic extraction method on approximately 1 g of dry weight samples. In brief, 25 mL of phosphoric acid was first added to the sample. Subsequently, it was gently mixed and incubated on ice for 30 min with intermittent gentle shaking. After extraction for 30 min in ice-water bath, the supernatant was collected after centrifugation (3,000 rpm/min, 5 min) and then transferred to a clean 50 mL centrifuge tube. Immediately afterward, the supernatant was adjusted to pH 6.5 with 5 M sodium hydroxide solution and topped to 50 mL with the additional supernatant. Afterward, the obtained solution was filtered through 0.45 μm organic filter membrane before being used for the HPLC analysis. Finally, the content of IMP in the filtrate was analyzed by HPLC. The HPLC conditions were as described above: column: Phenomenex Luna C18 100 Å column (250 mm × 4.6 mm, 5 μm); mobile phase: 0.05 M triethylammonium dihydrogen phosphate/acetonitrile (95/5); flow rate: 1 mL/min; detection wavelength: 254 nm; column temperature: 25°C.
2.9 Chemical composition of the duck breast muscle
Protein, fat and moisture contents in duck breast muscle (stored at 4°C) were determined following the guidelines from the Associate of Analytical Chemists.
2.10 Analysis of antioxidative enzyme activities in duck breast muscle
Duck breast muscle (100 mg, stored in air 4°C for 24 h) was accurately weighed and added with ice-cold phosphate buffer solution (4°C, 0.01 mol/L, pH = 7.2–7.4) to prepare 10% w/v homogenate. Firstly, the protein content of 10% homogenate was measured by bicinchoninic acid (BCA) protein assay reagent. Subsequently, the activities of SOD (Cat no, A001-3-2), GSH-Px (Cat no, A005-1-2), T-AOC (Cat no, A015-2-1), total cholesterol (TC, Cat no, A111-2-1) and the concentration of MDA (Cat no, A003-1-2) were determined using the commercial kits (Nanjing Jiancheng Biology Engineering Institute, Nanjing, China) following the instructions of the manufacturer, respectively.
2.11 Statistical analysis
All results are presented as the means ± (SEM) standard errors of the means. The experimental data were statistically analyzed using the SPSS version 19.0 software (SPSS Inc., Chicago, IL, United States). For the comparison between two experiment groups, statistical significance was assessed using Student’s t-test. For multiple groups, the statistical significance of the differences was carried out by one-way analysis followed by Turkey test. Differences between groups were considered statistically significant for p < 0.05.
3 Results
3.1 Growth performance
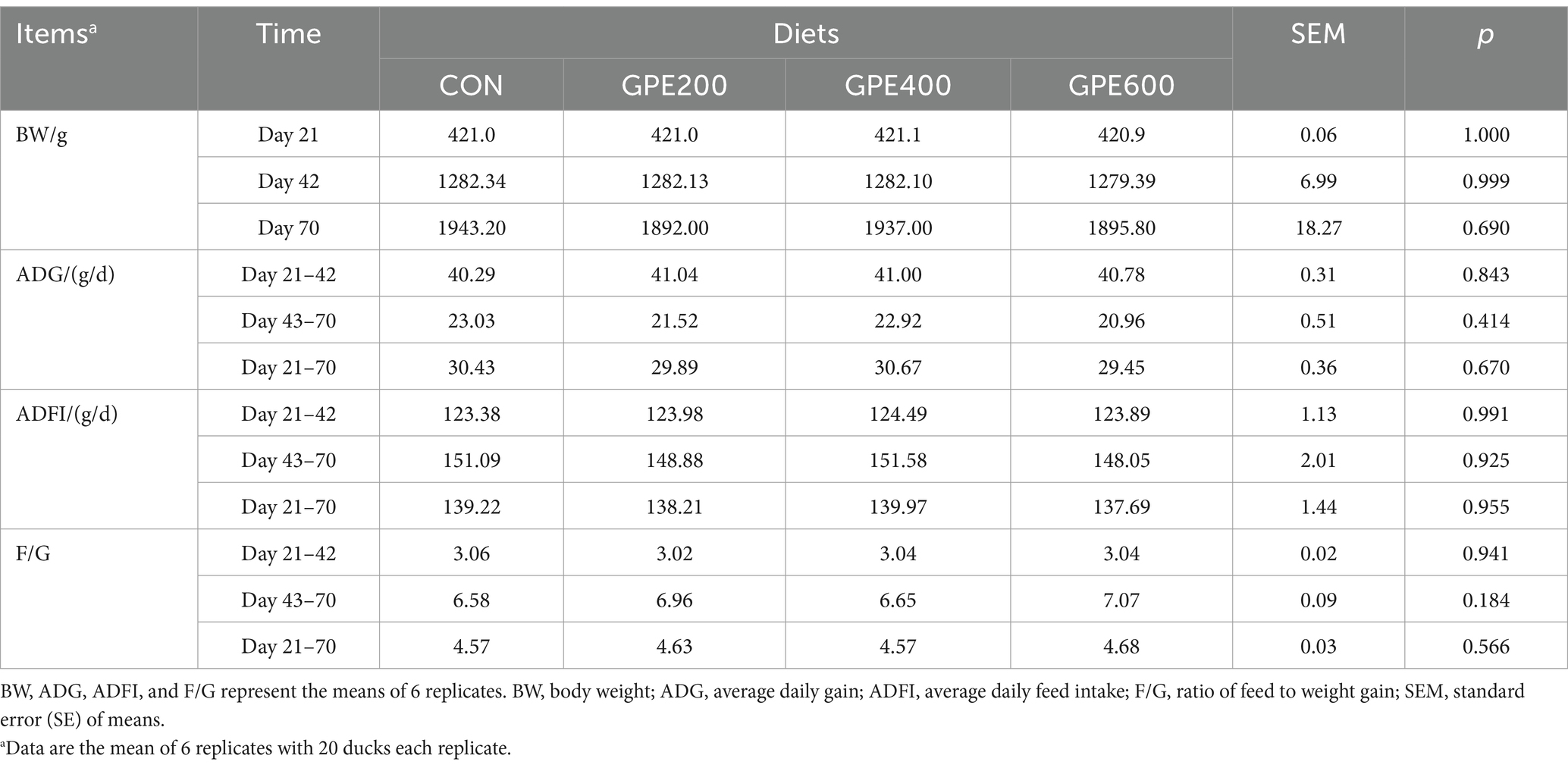
As showcased in Table 2, in comparison to the control group, there was no significant difference in body weight, average daily feed intake (ADFI) and average daily gain (ADG) with increasing levels of GPE throughout the experimental period. Additionally, there was no statistically considerable variation in F/G between the control and the treatment groups.
3.2 Serum biochemical parameters
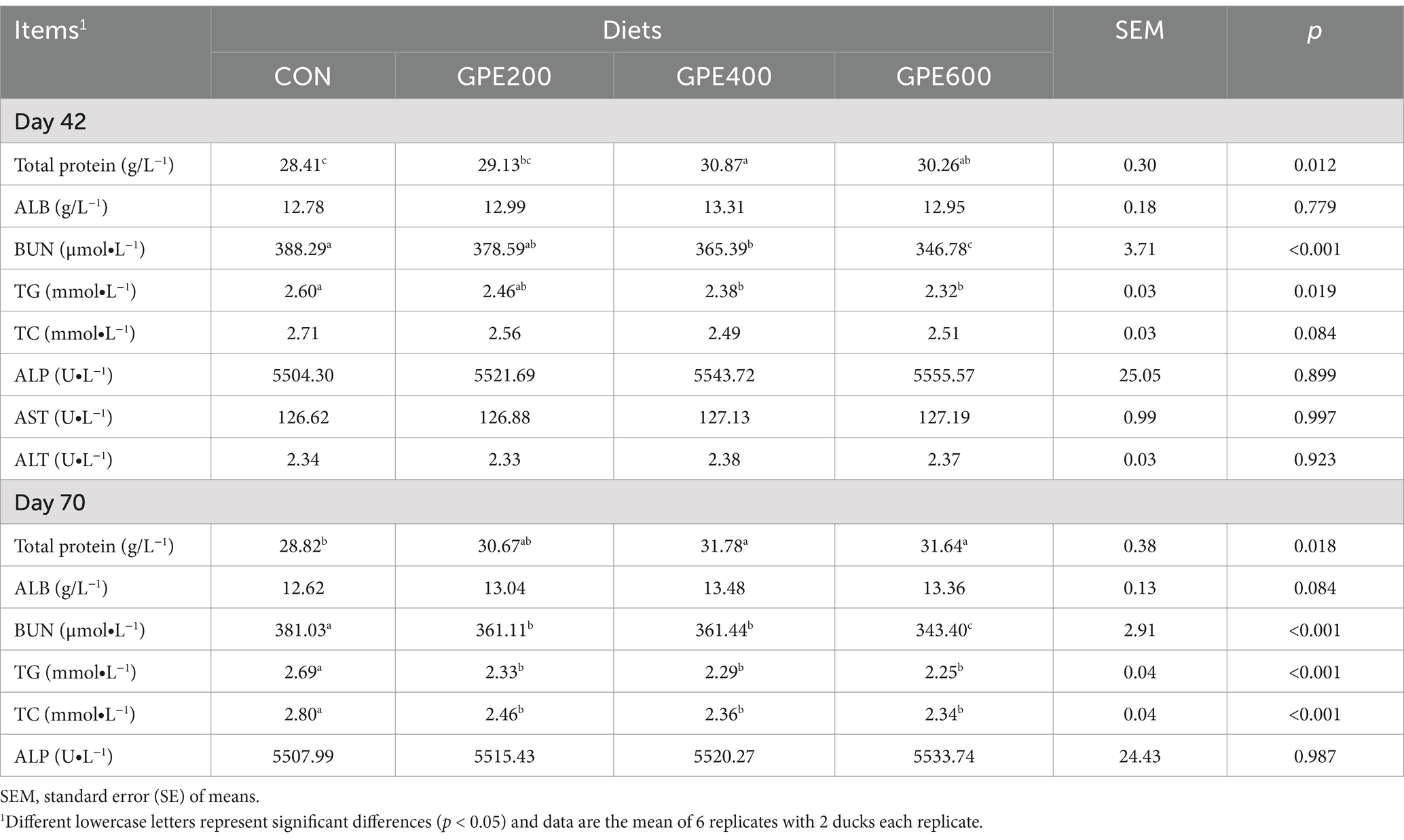
To probe whether GPE exerted potential protective effect on antioxidation, lipid metabolism and liver function, related biochemical indexes were measured by biochemical kits. The results are presented in Table 3. Relative to the control group, dietary GPE supplementation prominently (all for p < 0.05) decreased the activities of BUN and TG at 42 and 70 days of age. Moreover, dietary GPE inclusion significantly elevated the total protein level. Notably, at 42 and 70 days of age, as the concentration of GPE increased, BUN and TG activities progressively decreased, reaching their lowest at a dietary concentration of 600 mg/kg GPE. However, supplementation with GPE had no discernible effect on the contents of ALP, AST, ALT and ALB (all for p > 0.05).
3.3 Serum immunity and inflammatory parameters
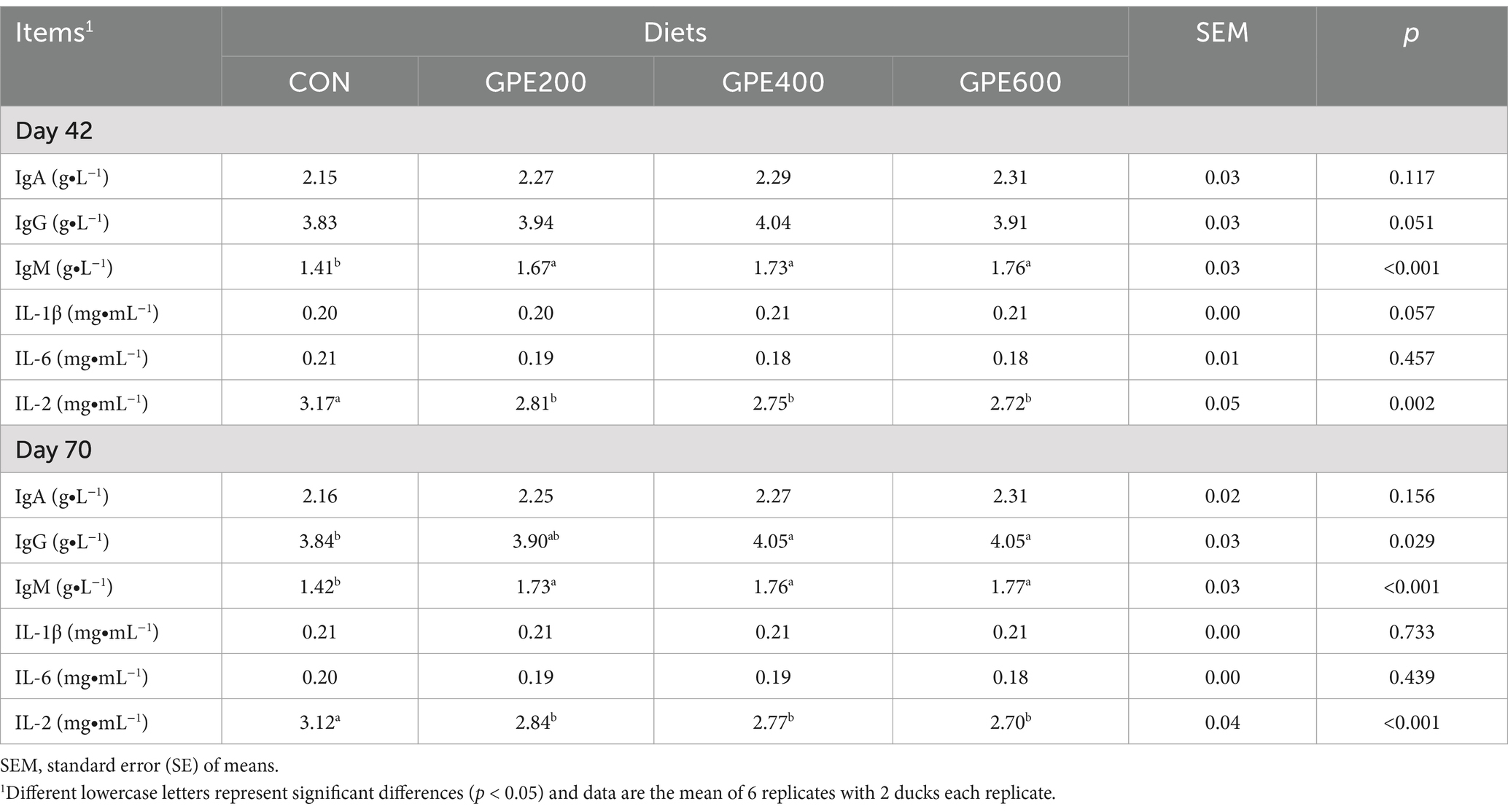
To further interrogate the effect of GPE on cellular immunity, the ELISA analysis was used to detect the concentrations of serum IL-6, IL-1β, IgA, IgM, and IgG. As depicted in Table 4, with respect to the control group, the IgM level was markedly (p < 0.01) enhanced at 42 and 70 days of age after dietary supplementation with GPE. Additionally, at 42 days of age, supplementation with GPE had no obvious effect on IgG activity. However, supplementation with GPE apparently elevated the IgG and IgM activities at 70 days of age. Furthermore, the concentration of IL-2 was notably lowered in GPE-treated groups at 42 and 70 days of age compared with the control group. Of note, there are no significant differences observed in IL-1β, IL-6, and IgA. These results demonstrated that dietary supplementation with GPE could improve immune function by enhancing the activities of immune factors and modulating the levels of inflammatory cytokines.
3.4 Serum antioxidant status
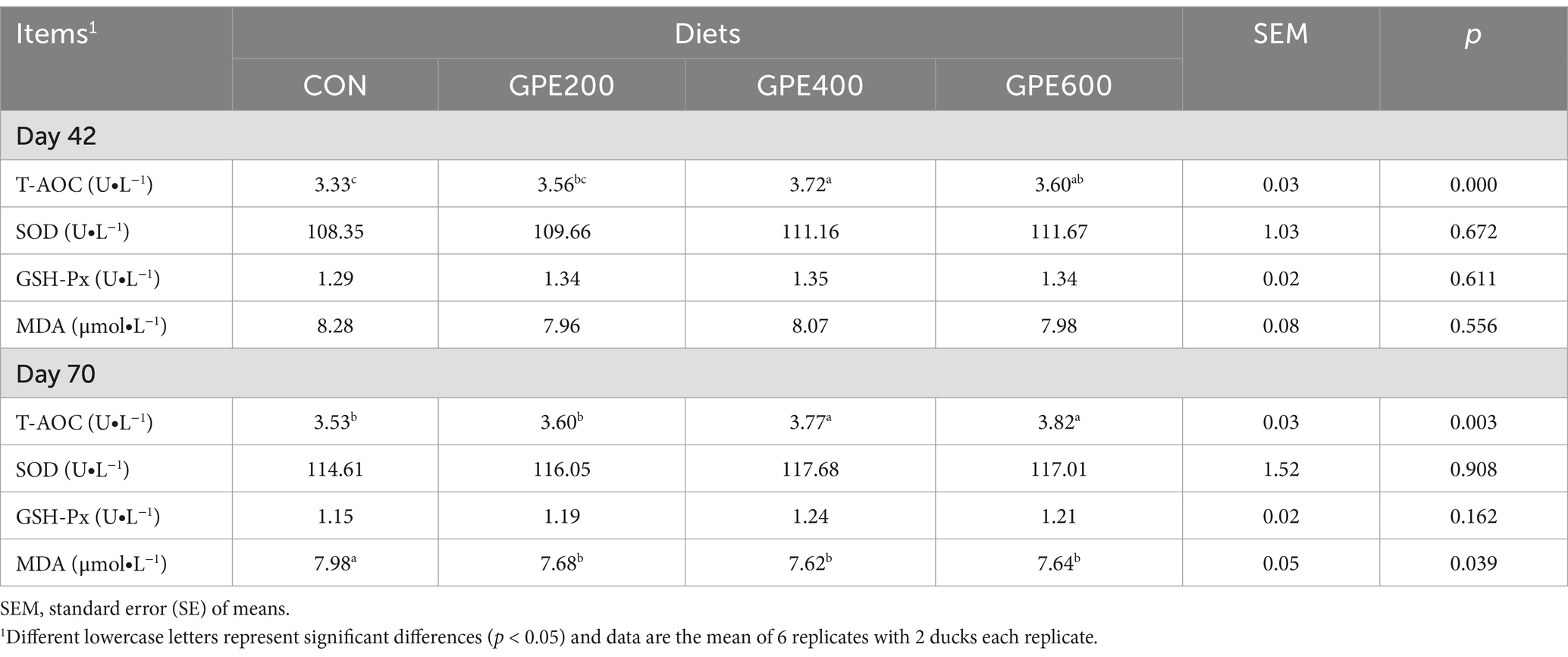
Antioxidant activity refers to the ability to eliminate free radicals in the body. To evaluate the antioxidant potential of GPE, the levels of oxidative stress-related indicators (T-AOC, SOD, MDA, and GSH-Px) were detected by corresponding assay kits. As displayed in Table 5, the level of T-AOC was distinctly elevated at 42 and 70 days of age following GPE supplementation, showing a dose-dependent manner with higher concentrations of GPE. Furthermore, in contrast to the control group, at 70 days of age, dietary supplementation with GPE conspicuously reduced MDA level, with 600 mg/kg GPE treatment showing the lowest levels. However, no prominent changes are observed in SOD and GSH-Px throughout the duration of the experiment after dietary supplementation with GPE.
3.5 Carcass traits
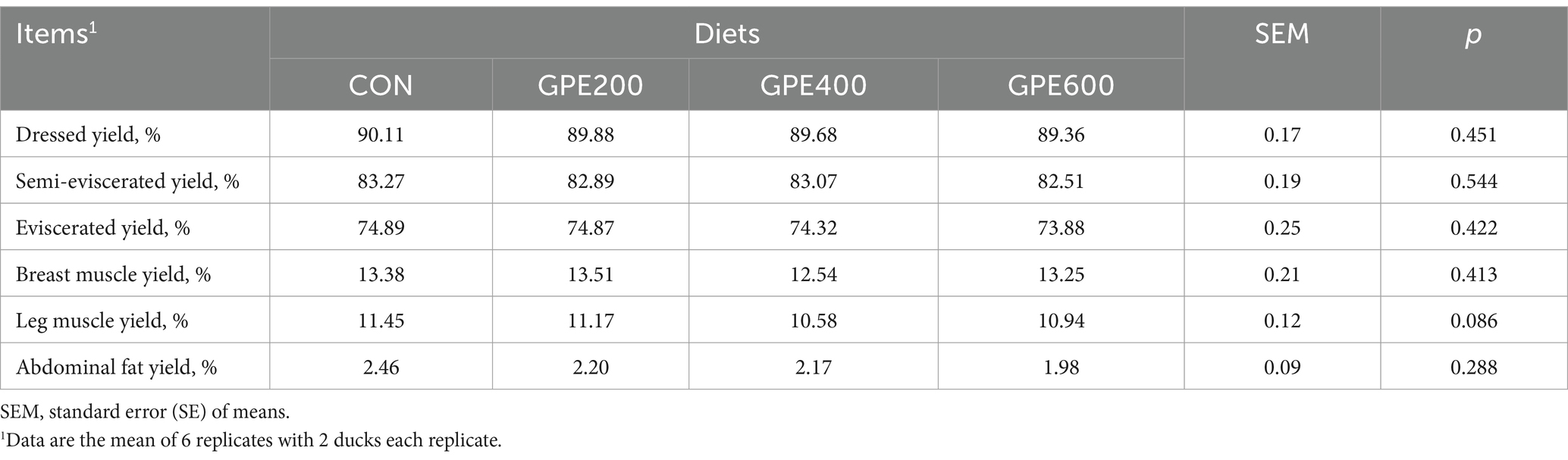
The effect of GPE supplementation on carcass traits of ducks were displayed in Table 6. Compared to the control group, all treatments did not show any significant effects on semi-eviscerated yield, dressed yield, eviscerated yield, breast muscle yield, leg muscle yield and abdominal fat yield. Although there were no differences in these indicators, it was worth noting that dietary supplementation with GPE decreased abdominal fat, especially 600 mg/kg.
3.6 Organ indexes
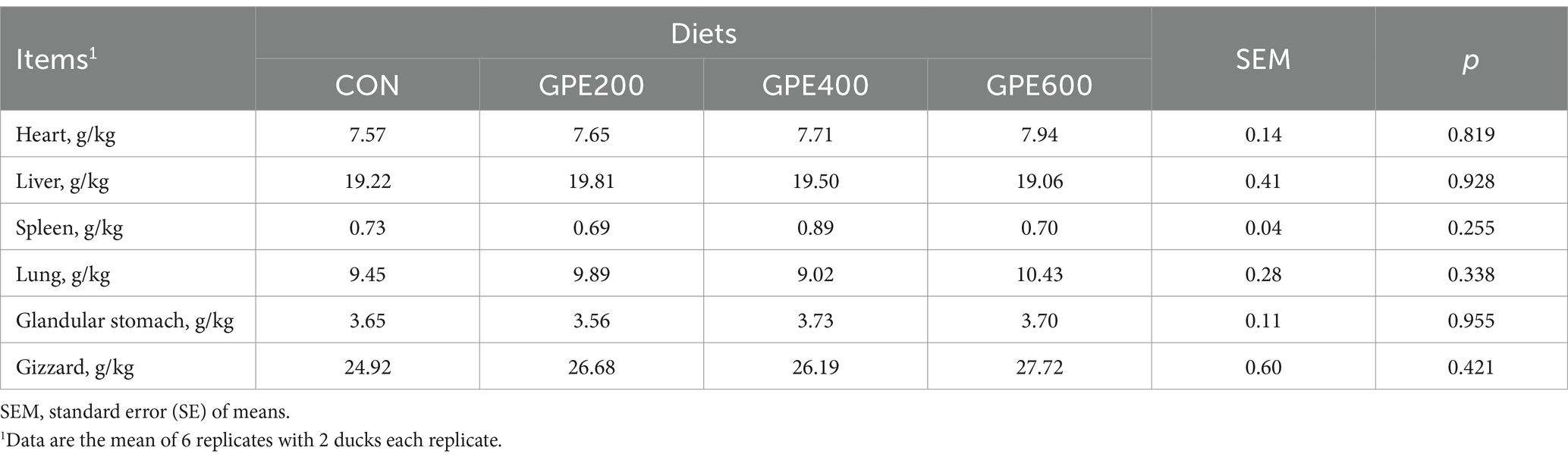
The effects of GPE on relative weights of liver, kidney, spleen, lung, gizzard and glandular stomach are illustrated in Table 7. Moreover, the kidney, spleen, lung, gizzard and glandular stomach weight were no obvious differences between the control group and the different dosages of GPE-treated groups. These results revealed that GPE had no obvious toxic and side effects to major organs in vivo.
3.7 Hepatic morphology
To further explore whether GPE exerted potential toxic effect on the liver, the histological architecture of the liver was observed by microscope following H&E staining. As showcased in Figure 2, in the control group, the structure of liver tissues was intact with regular arrangement of hepatocyte cells. However, in parallel to the control group, dietary inclusion of GPE did not result in any noticeable morphological changes in hepatocytes. This finding suggested that GPE supplementation had no potential cytotoxic effects on the liver, highlighting its suitability and safely as a feed additive.
3.8 Meat quality
To estimate the effect of GPE supplementation on muscle characteristics and meat quality traits, the related parameters of meat quality (pH, color, water loss rate, and shear force) were measured. The effect of dietary GPE on the meat quality of ducks were presented in Table 8. Dietary supplementation with GPE showed no effect on the pH of breast or leg muscles. Additionally, no significant differences in meat color values were detected, including a*, b*, and L*. Notably, in parallel to the control group, dietary supplementation with GPE resulted in a substantial depression of water loss rate and shear force. These results indicated that diets with GPE supplementation could effectively improve shear force and reduce water loss rate, leading to an overall improvement in meat quality.
3.9 The IMP content of breast muscle
As shown in Table 8, the IMP content in the breast muscle meat of the GPE-treated groups was not significantly different from that of the control group at 70 days, indicating that GPE supplementation has no impact on modulating IMP level.
3.10 Chemical composition of the duck breast muscle
As displayed in Table 9, there was no noticeable effect of GPE on protein and fat (p > 0.05) content in the duck breast muscle. However, the inclusion of GPE (600 mg/kg) apparently improved the content of moisture in duck breast muscle. These findings suggested that GPE supplementation had no significant effect on the content of protein and fat but increased moisture, ultimately improving meat quality to some extent.
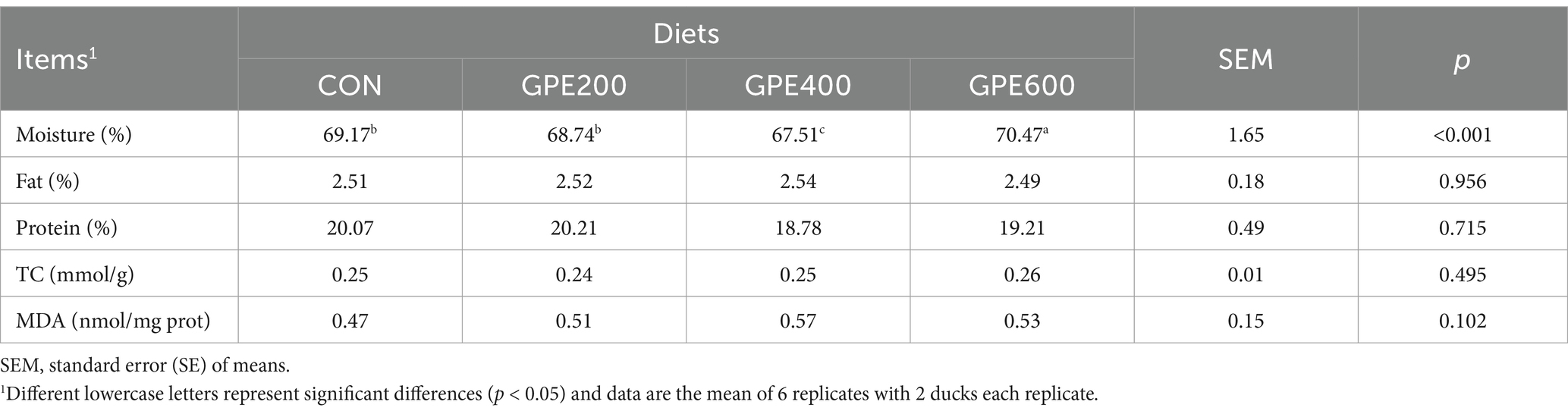
Table 9. Effect of GPE supplementation on protein, fat, moisture content and antioxidant capacity of duck breast muscle.
3.11 Antioxidant capacity of the duck breast muscle
As depicted in Table 9, in contrast to the control group, the levels of MDA and TC did not show any significant variations after GPE supplementation. These results demonstrated that GPE inclusion had no significant impact on improving antioxidant capacity of breast muscle or modulating its TC level.
4 Discussion
Over the last several decades, therapeutic practices in global animal production have heavily relied on synthetic antibiotics, with approximately 70% usage (22, 23). This widespread reliance is primarily due to their remarkable efficacy in boosting antioxidative defenses, mitigating inflammatory responses, and improving both overall health status and production performance (24). However, with increasing concern about the negative impact of antibiotic use aspects on public health, many countries have gradually been banning the use of antibiotics in animal husbandry production (25–27). Despite strict restrictions on the use of antibiotics, maintaining poultry health remains a formidable challenge in livestock and poultry production. In efforts to address this issue, researchers have gradually turned their attention to natural plants which could enhance immunity and antioxidant capacity of animals and birds (28). Therefore, it is imperative to search for efficient, eco-friendly, and safe feed additives that can enhance animals’ production potential, improve body health, and serve as a viable alternative to antibiotics in feed.
Through a long history of clinical practice, TCM has developed a unique theoretical framework and abundant clinical experience aimed at enhancing the health of both humans and animals (29). In recent, with the ban on antibiotics, TCM has been widely used in livestock farming due to its high efficacy and low toxicity (30). Not only these, new natural compounds derived from TCM have been also increasingly used as feed additives (31, 32). Furthermore, many lines of evidence have indicated that dietary supplementation with TCM has a positive effect on improving animal health, providing a promising and effective alternative to antibiotics in livestock production (33). A large body of animal experiment results also confirmed that dietary supplementation with TCM has more advantages in promoting livestock growth and improving disease resistance in comparison to conventional feed additives (34). Therefore, more and more researchers are also aware of the vital role of TCM in livestock farming (35). It can thus be stated that TCM has the potential to be a new feed additive to improve livestock productivity and health status.
GP is a traditional folk herbal medicine widely used in China, which is clinically used for the treatment of eruptive fevers and inflammatory-related diseases. It contained a series of biologically active components, including flavonoids, polyphenols, saponin, tannin and terpenoid (18, 36). Research has indicated that GP leaf extract could enhance hepatic antioxidation capacity and decrease pro-inflammatory factor levels in mice, effectively mitigating cadmium-induced liver toxicity (37). Furthermore, other investigators have found that dietary supplementation of GP or GP-containing herbal prescriptions could reduce the egg yolk cholesterol, suppress harmful excreta microflora and improve layers performance, which was ascribed to the bioactive compounds in GP (38). From this, GPE has the potential to be a new feed additive to improve livestock farming applications. Therefore, to delve deeper into whether the potential effects of GPE on ducks are associated with immune enhancement and improved antioxidant functions, we measured immune and anti-oxidation-related parameters.
Serum biochemical parameters provide valuable information for evaluating the health status of ducks and reflect many metabolic alternations of organs and tissues. ALT, AST and ALP are the commonly used indicators for assessing liver function. Once liver was injured, hepatocytes were destroyed contributing to the sharply increased serum aminotransferases (39). Urea nitrogen is the primary metabolite of protein and amino acid, which reflects the utilization efficiency of nitrogen (40). Serum total protein and ALB could reflect the metabolic status of protein in the body to a certain extent (41). TC is an important index that could indirectly reflect the ability of hepatic lipid synthesis (42). Additionally, TG is the recognized indicator, which is used to further evaluate the function of hepatic lipid metabolism (43).
In this work, there is no statistically significant difference in ALT, AST, and ALP levels between the control group and treatment groups, indicating dietary supplementation of GPE did not exert any toxic effect on the liver. Notably, dietary supplementation of GPE dramatically reduced the levels of total protein and BUN, suggesting the utilization efficiency of nitrogen was dramatically increased after GPE supplementation. Furthermore, dietary supplementation of GPE improved hepatic lipid metabolism, as evidenced by the reduced levels of TC and TG. Concordant with our results, Murugaiyah et al. reported that serum TC level was decreased in chemical and high fat diet (HFD)-induced hyperlipidemic rats treated with GPE (44). Hence, these results illustrated that dietary supplementation with GPE could modulate hepatic lipid metabolism and improve nitrogen utilization efficiency in ducks.
Accumulated lines of evidence have indicated that immunity is intimately correlated with livestock health (45). The immune system guards the body against foreign substances and protects it from invasion by pathogenic organisms. As the important regulators of immune function, Ig is a class of specific active proteins that can be converted into antibodies by antigen induction, such as IgM, IgG, IgA and so on (46). The immunoglobulin activities (IgA, IgM, and IgG) in the serum are important indicators to estimate the non-specific immunity status of the animal. Immunoglobulin A (IgA), an important serum immunoglobulin, plays a crucial role in the immune defense of mucosal surfaces (47). Additionally, when the body is exposed to an external stimulus, the immune system is primed, triggering the release of several inflammatory factors, such as IL-1β and IL-6 (48). In the present experiment, dietary inclusion of GPE could dramatically elevate the contents of IgM and IgG at day 70 of age in compared to the control group. The IL-2 level was considerably inhibited by dietary GP supplementation. Curiously, treatment with GPE has no evident difference in IL-6 and IL-1β activities. Consistent with our observations, Wu et al. found that dietary supplementation with flavonoids from bamboo leaf could elevate the serum IgM activity in comparison to the control group, which might be attributed to the potent immunomodulatory functions of flavonoids (49). Additionally, Huang et al. also noted that supplementation with flavonoid-rich Fenugreek extract had a positive effect on modulating immunity in broiler (50). The enhanced immunity may result from GPE’S ability to stimulate immune cells to release immune factors, thereby attenuating inflammation (51).
In addition to modulating immunity function, numerous studies have revealed that oxidative stress was also a crucial contributor to inferior growth performance of ducks (52). To further elucidate whether the potential effect of GPE was intimately associated with its antioxidant function, the oxidative stress-related indicators were determined. MDA, as the final product of lipid peroxidation, is widely considered a reliable marker reflecting the degree of oxidative damage (53). GSH-Px are the key antioxidant enzymes that can scavenge ROS generated from oxidant stress (54). SOD is a pivotal endogenous antioxidant enzyme that acts as a component of first-line defense against oxidative damage. It catalyzes the dismutation of superoxide anion, forming hydrogen peroxide and molecular oxygen (55). T-AOC was the main parameter to measure the total antioxidant level of the enzymatic and nonenzymatic systems (56). In the present experiment, our results indicated that dietary supplementation with GPE could dramatically elevate the activity of T-AOC and reduce MDA content in serum, which might largely be attributed to its primary bioactive ingredient, polyphenolic. Similar to our finding, Ao et al. demonstrated that grape seeds were also richer in polyphenolic compounds and confirmed that supplementation with grape seeds dramatically enhanced antioxidative activity by improving T-AOC in broilers (57). The excellent antioxidant capacity might be closely associated with polyphenol, flavonoids in GPE, all of which exhibit potent antioxidant and free radical scavenging activities (36).
Carcass characteristics have been recognized as crucial response parameters to assess dietary energy and amino acid status in livestock dietary, such as yields of breast muscle, thigh muscle, and abdominal fat (58). Our findings suggested that no significant variance in dressing yield was observed between the control group and the three treatment groups. Additionally, there was no statistically significant difference in dressing percentage, semi-eviscerated yield, eviscerated yield, breast muscle yield and abdominal fat yield between the various treated groups. In agreement with our results, Ma et al. reported that there were no pronounced effects on dressing percentage, eviscerated yield, breast and thigh percentage after supplementation with flavonoids derived from sea buckthorn fruits (59). Altogether, these findings indicated that dietary supplementation with GPE did not show any substantial effect on the carcass characteristics.
The organ indexes are important indicators, which could partially reflect the health status of the body. When the body is challenged by an external stimulus, the internal organs of the body will undergo corresponding changes. Among them, the spleen is an important immune organ, which protects the host from microbial infections and mechanical injuries. The liver is a central organ for lipid metabolism and plays a vital role in detoxification. The lung is a particularly sensitive organ, which defenses the body from external stimulus. Our present results showed that dietary supplementation of GPE had no implications on the various organs, such as heart, liver, kidney, spleen, gizzard and glandular stomach. These data demonstrated that dietary supplementation with GPE did not exert toxic side effects on these organs, confirming that dietary supplementation with GPE is a safe strategy for improving animal health.
As we all know, in addition to their impact on the growth and health of livestock, abnormal oxidative stress and immunity influence have also a negative effect on the quality of livestock meat (60). Meat quality is an essential element that influences consumer’s purchasing decisions. As the consumer’s demand for meat quality is increasing, improving the quality of meat becomes a highly effective approach to boost the consumer’s purchase desire (61). Recently, more and more traditional Chinese medicine, such as Astragalus mongholicus Bunge and Largehead Atractylodes Rh, have been confirmed to exhibit a favorable effect on the improvement of meat quality (33, 62). Therefore, to investigate whether the potential effect of GPE was closely linked to improving meat quality, the meat quality relevant parameters were measured. In this work, dietary supplementation with GPE apparently improved the moisture content of breast muscle, while it had no notable variation in protein, fat and IMP contents of the breast muscle. In keeping with our observation, Ahmad et al. found that β-GOS and methionine co-supplementation did not show any promising effects on meat quality in broilers (63). Furthermore, dietary supplementation of GPE could apparently reduce the shear force and the water loss rate. In addition, no marked difference in breast muscle pH, leg muscle pH and breast muscle color were observed in any of the experimental groups. As we all know, pH value is a vital reference for judging the change of acidity in the process of muscle tissue fermentation and speed of fermentation after slaughtering, which has a substantial impact on meat quality. Therefore, maintaining a stable pH is essential for the proper maturation of muscles. Recent studies have demonstrated that a rapid pH reduction causes denaturation of myofibrillar proteins, leading to poor water-holding capacity and deteriorated drip loss (64). In this investigation, the reduced water loss in the breast muscle may be attributed to a stable pH value. The above results suggested that dietary supplementation with GPE prominently enhanced the tenderness and water-holding capacity of duck meat, leading to a notable improvement in its overall quality. Consistent with our results, Jin et al. reported that dietary supplementation with curcumin influenced the color of leg muscle while enhanced a prominent trend toward improving the water-holding capacity in breast muscle of meat ducks (65). The integrity of the cell membrane is a crucial factor that influences the water-holding capacity and tenderness of meat. Enhancement in meat quality may be caused by the fact the stability and integrity of the cell membrane were changed due to the improved antioxidant capacity of meat (66).
Taken together, our study first demonstrated that there was no significant effect on growth performance after dietary supplementation with GPE. Noteworthy, dietary supplementation with GPE could enhance immunity and regulate oxidative stress. Moreover, supplementation of GPE could improve meat quality, which was primarily manifested by improvements in the shear force and water loss rate. It might offer a strong rationale for developing and utilizing GPE and GP-containing traditional Chinese herbal prescriptions as a novel feed additive. Furthermore, our discovery would have far-reaching implications for expanding the potential utility of GPE.
Apart from these strengths, there are several additional limitations to our study. Firstly, despite GPE exhibiting favorable effect on ducks, it is not clearly established which bioactive ingredients are associated with its excellent modulation effect. Secondly, the study is currently restricted to ducks only and has not been extended to other conventional animals. Thirdly, our study only focused on the modulation effect of GPE in ducks, which neglected the dynamic process of GPE in vivo. To address these limitations, the detailed bioactive ingredients still need a deeper investigation. In addition, the specific regulatory mechanism of GPE as immunomodulators and antioxidants deserves to be further investigated. Furthermore, more different kinds of animals were required to investigate its potential effect before widespread application. By explicitly noting these constricts, we aim to elucidate which active components exhibited the best modulation effect on ducks. Besides, we also encourage ongoing research to investigate the dynamic process of GPE in vivo for a clearer understanding of the underlying molecular mechanisms.
5 Conclusion
In conclusion, this study showed that dietary supplementation with GPE could increase serum total antioxidant capacity, regulate immunity function and improve meat quality to some extent in meat ducks. These results can provide further evidence using GPE as a feed additive in meat duck production. The recommended optimal GPE level in the diet of meat ducks is 600 mg/kg according to the results in this study.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by the Experimental Animal Ethics Committee in Jiangxi Academy of Agricultural Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GA: Conceptualization, Writing – original draft. PX: Formal analysis, Writing – original draft. JC: Data curation, Methodology, Writing – review & editing. WSo: Data curation, Writing – review & editing. QS: Validation, Writing – review & editing. CX: Data curation, Writing – review & editing, Investigation. WSu: Data curation, Writing – review & editing, Visualization. ZZ: Formal analysis, Visualization, Writing – review & editing. QW: Funding acquisition, Project administration, Writing – review & editing. XC: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by grants from Jiangxi Province Key Research and Development Program (20224BBF62003), the Earmarked Fund for Modern Agro-industry Technology Research System of China (CARS-42-43), Jiangxi Province Modern Agricultural Poultry Industry Technical System of China (JXARS-09) and Gan-Po Talented Youth Support Program • the High-level and High-skill Leading Talent Training Project of Jiangxi Province (2023).
Acknowledgments
The authors are grateful to Professor Dong Wu and his team of the National Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Chinese Medicine for generously providing the GPE.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADFI, Average daily feed intake; ADG, Average daily gain; ALB, Albumin; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; ALP, Alkaline phosphatase; BCA, Bicinchoninic acid; BUN, Blood urea nitrogen; BW, Body weight; ELISA, Enzyme-linked immunosorbent assay; F/G, Feed to weight gain ratio; GSH-Px, Glutathione peroxidase; GPE, Gynura procumbens extract; HFD, High fat diet; IMP, Inosine monophosphate; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; IL-6, Interleukin-6; IL-1β, Interleukin-1β; MDA, Malondialdehyde; SEM, Standard error of means; SOD, Superoxide dismutase; H&E, Hematoxylin and eosin; T-AOC, Total antioxidant capacity; TC, Total cholesterol; TCM, Traditional Chinese Medicine; TG, Triglyceride.
References
1. Pokharel, S, Shrestha, P, and Adhikari, B. Antimicrobial use in food animals and human health: time to implement ‘one Health’approach. Antimicrob Resist Infect Control. (2020) 9:1–5. doi: 10.1186/s13756-020-00847-x
2. Muaz, K, Riaz, M, Akhtar, S, Park, S, and Ismail, A. Antibiotic residues in chicken meat: global prevalence, threats, and decontamination strategies: a review. J Food Protect. (2018) 81:619–27. doi: 10.4315/0362-028X.JFP-17-086
3. Shao, Y, Wang, Y, Yuan, Y, and Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci Total Environ. (2021) 798:149205. doi: 10.1016/j.scitotenv.2021.149205
4. Gul, ST, and Alsayeqh, AF. Probiotics improve physiological parameters and meat production in broiler chicks. Int J Vet Sci. (2023) 12:182–91. doi: 10.47278/journal.ijvs/2022.191
5. Coniglio, M, Luna, M, Provensal, P, Watson, S, Ortiz, M, Ludueña, H, et al. Use of the probiotic Saccharomyces cerevisiae var. boulardii RC009 in the rearing stage of calves. Int J Agricult Biosci. (2023) 12:188–92. doi: 10.47278/journal.ijab/2023.063
6. Rashid, S, Tahir, S, Akhtar, T, Altaf, S, Ashraf, R, and Qamar, W. Bacillus-based probiotics: an antibiotic alternative for the treatment of salmonellosis in poultry. Pak. Vet J. (2023):43. doi: 10.29261/pakvetj/2023.017
7. Sun, J, Ren, J, Hu, X, Hou, Y, and Yang, Y. Therapeutic effects of Chinese herbal medicines and their extracts on diabetes. Biomed Pharmacother. (2021) 142:111977. doi: 10.1016/j.biopha.2021.111977
8. Hegazy, SA, Abd, ES, Khorshed, M, and Salem, F. Productive and immunological performance of small ruminants offered some medicinal plants as feed additives. Int J Vet Sci. (2023) 12:120–5. doi: 10.47278/journal.ijvs/2022.163
9. Krishnaveni, P, Thangapandiyan, M, Raja, P, and Rao, GJPVJ. Pathological and molecular studies on antitumor effect of curcumin and curcumin solid lipid nanoparticles. Pak Vet J. (2023) 43:315–20. doi: 10.29261/pakvetj/2023.022
10. Bai, L, Song, X, Fu, Y, Chen, S, Tian, Y, Jia, R, et al. Effects of a mixed extract of cortex Fraxini, Pulsatilla chinensis, and Eucommia ulmoides on immunity and antioxidant activity in hemp ducks. Livest Sci. (2019) 221:63–9. doi: 10.1016/j.livsci.2019.01.009
11. Tan, H-L, Chan, K-G, Pusparajah, P, Lee, L-H, and Goh, B-H. Gynura procumbens: an overview of the biological activities. Front Pharmacol. (2016) 7:52. doi: 10.3389/fphar.2016.00052
12. Hassan, Z, Yam, MF, Ahmad, M, and Yusof, AP. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules. (2010) 15:9008–23. doi: 10.3390/molecules15129008
13. Hoe, S-Z, Kamaruddin, MY, and Lam, S-K. Inhibition of angiotensin-converting enzyme activity by a partially purified fraction of Gynura procumbens in spontaneously hypertensive rats. Med Prin Pract. (2007) 16:203–8. doi: 10.1159/000100391
14. Haque, E, Kamal, MS, Tahsin, MR, Ahmed, R, Choudhury, JA, Chowdhury, AA, et al. Current knowledge regarding pharmacological profile and chemical constituents of Gynura procumbens. Curr Top Med Chem. (2021) 21:2671–86. doi: 10.2174/1568026621666211004094902
15. Ijaz, MU, Ishtiaq, A, Ehsan, N, Imran, M, and Zhu, G-p. Hepatoprotective potential of Genkwanin against aflatoxin B1-induced biochemical, inflammatory and histopathological toxicity in rats. Pak. Vet J. (2022) 42:493–8. doi: 10.29261/pakvetj/2022.048
16. Nasr, MAF, Alkhedaide, AQ, Radwan, MME, Hafez, ASE, Hussein, MA, and El Bayomi, RM. Growth, carcass parameters, biochemical and oxidative stress indices, and meat traits of duck breeds under different stocking densities. Poult Sci. (2022) 101:101992. doi: 10.1016/j.psj.2022.101992
17. Celi, P, and Gabai, G. Oxidant/antioxidant balance in animal nutrition and health: the role of protein oxidation. Front Vet Sci. (2015) 2:48. doi: 10.3389/fvets.2015.00048
18. Kaewseejan, N, Sutthikhum, V, and Siriamornpun, S. Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J Funct Foods. (2015) 12:120–8. doi: 10.1016/j.jff.2014.11.001
19. Mumu, SK, and Mustafa, A. Modulation of acute stress and immune response in tilapia, Oreochromis niloticus, using longevity spinach, Gynura procumbens extract, as nutraceuticals. J Immunoass Immunoch. (2022) 43:678–94. doi: 10.1080/15321819.2022.2080558
20. Huang, J, Yang, J, Huang, F, Huang, M, Chen, K, Xu, X, et al. Effect of fast pH decline during the early postmortem period on calpain activity and cytoskeletal protein degradation of broiler M. pectoralis major. Poult Sci. (2016) 95:2455–63. doi: 10.3382/ps/pew206
21. Li, X, Yang, B, Dong, Z, Geng, D, Wang, C, Guo, Q, et al. Growth performance, carcass traits, meat quality, and blood variables of small-sized meat ducks with different feed efficiency phenotypes. Poult Sci. (2023) 102:102818. doi: 10.1016/j.psj.2023.102818
22. Cuong, NV, Padungtod, P, Thwaites, G, and Carrique-Mas, JJ. Antimicrobial usage in animal production: a review of the literature with a focus on Low-and middle-income countries. Antibiotics (Basel). (2018) 7:75. doi: 10.3390/antibiotics7030075
23. Li, X, Zhu, X, and Xue, Y. Drug resistance and genetic relatedness of Escherichia coli from mink in Northeast China. Pak Vet J. (2023) 43:824–7. doi: 10.29261/pakvetj/2023.062
24. Kabploy, K, Bunyapraphatsara, N, Morales, NP, and Paraksa, N. Effect of antibiotic growth promoters on anti-oxidative and anti-inflammatory activities in broiler chickens. Thai J Vet Med. (2016) 46:89–95. doi: 10.56808/2985-1130.2722
25. Low, CX, Tan, LT-H, Ab Mutalib, N-S, Pusparajah, P, Goh, B-H, Chan, K-G, et al. Unveiling the impact of antibiotics and alternative methods for animal husbandry: a review. Antibiotics. (2021) 10:578. doi: 10.3390/antibiotics10050578
26. Saad, N, El-Abasy, MA, El-Khayat, F, Ali, NG, and Ismail, MM. Efficacy of chitosan nanoparticles as a natural antibacterial agent against pathogenic bacteria causing omphalitis in poultry. Pak Vet J. (2023) 43:573–8. doi: 10.29261/pakvetj/2023.065
27. Coniglio, M, Luna, M, Provensal, P, Watson, S, Ortiz, M, Ludueña, H, et al. The impact of Saccharomyces cerevisiae var. Boulardii RC009 on productive parameters in weaned calves and cull cows. Agrobiol Rec. (2023) 13:1–6. doi: 10.47278/journal.abr/2023.021
28. Ismael, E, Ismail, EM, Khalefa, HS, Elleithy, EM, Elmosalamy, SH, Marouf, S, et al. Evaluation of Saccharomyces cerevisiae yeast fermentate and xylanase in reduced energy diet fed to broiler chicken. Int J Vet Sci. (2022) 11:141–50. doi: 10.47278/journal.ijvs/2021.096
29. Tong, Z, He, W, Fan, X, and Guo, A. Biological function of plant tannin and its application in animal health. Front Vet Sci. (2021) 8:803657. doi: 10.3389/fvets.2021.803657
30. Akbarian, A, Golian, A, Gilani, A, Kermanshahi, H, Zhaleh, S, Akhavan, A, et al. Effect of feeding citrus peel extracts on growth performance, serum components, and intestinal morphology of broilers exposed to high ambient temperature during the finisher phase. Livest Sci. (2013) 157:490–7. doi: 10.1016/j.livsci.2013.08.010
31. Zhu, C, Huang, K, Bai, Y, Feng, X, Gong, L, Wei, C, et al. Dietary supplementation with berberine improves growth performance and modulates the composition and function of cecal microbiota in yellow-feathered broilers. Poult Sci. (2021) 100:1034–48. doi: 10.1016/j.psj.2020.10.071
32. Hafez, MH, El-Kazaz, SE, Alharthi, B, Ghamry, HI, Alshehri, MA, Sayed, S, et al. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals. (2022) 12:958. doi: 10.3390/ani12080958
33. Abdallah, A, Zhang, P, Zhong, Q, and Sun, Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr Drug Metab. (2019) 20:54–64. doi: 10.2174/1389200219666180523102920
34. Ran, M, Cha, C, Xu, Y, Zhang, H, Yang, Z, Li, Z, et al. Traditional Chinese herbal medicine complex supplementation improves reproductive performance, serum biochemical parameters, and anti-oxidative capacity in periparturient dairy cows. Anim Biotechnol. (2022) 33:647–56. doi: 10.1080/10495398.2020.1819823
35. Li, Y, Mei, H, Liu, Y, Li, Z, Qamar, H, Yu, M, et al. Dietary supplementation with Rutin alters meat quality, fatty acid profile, antioxidant capacity, and expression levels of genes associated with lipid metabolism in breast muscle of Qingyuan partridge chickens. Food Secur. (2023) 12:2302. doi: 10.3390/foods12122302
36. Nasiruddin, M, and Sinha, SN. Phytochemical screening and antioxidant, antibacterial efficacy of Gynura procumbens (Lour.) Merr. Asian J med. Biol Res. (2020) 6:187–95. doi: 10.3329/ajmbr.v6i2.48049
37. Wibowo, AT, Zubaidah, U, Savitri, AD, Faukib, MS, Salsabila, NS, and Manuhara, YSW. Biological properties of Gynura procumbens leaves extract to MDA levels and antioxidant activities in liver of mice. Res J Pharmy Technol. (2022) 15:5829–34. doi: 10.52711/0974-360X.2022.00984
38. Lokhande, A, Ingale, S, Lee, S, Sen, S, Khong, C, Chae, B, et al. Effects of dietary supplementation with Gynura procumbens (Merr.) on egg yolk cholesterol, excreta microflora and laying hen performance. Brit Poult Sci. (2014) 55:524–31. doi: 10.1080/00071668.2014.938020
39. Neag, MA, Catinean, A, Muntean, DM, Pop, MR, Bocsan, CI, Botan, EC, et al. Probiotic Bacillus spores protect against acetaminophen induced acute liver injury in rats. Nutrients. (2020) 12:12. doi: 10.3390/nu12030632
40. Liang, YQ, Zheng, XC, Wang, J, Yang, HM, and Wang, ZY. Different amino acid supplementation patterns in low-protein diets on growth performance and nitrogen metabolism of goslings from 1 to 28 days of age. Poult Sci. (2023) 102:102395. doi: 10.1016/j.psj.2022.102395
41. Almasaudi, AS, Dolan, RD, Edwards, CA, and Mcmillan, DC. Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal Cancer. Cancers. (2020) 12:1986. doi: 10.3390/cancers12071986
42. Fan, Y, Yan, L-T, Yao, Z, and Xiong, G-Y. Biochanin a regulates cholesterol metabolism further delays the progression of nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes. (2021) 14:3161–72. doi: 10.2147/DMSO.S315471
43. Xue, J, Wang, Y, Li, B, Yu, S, Wang, A, Wang, W, et al. Triglycerides to high-density lipoprotein cholesterol ratio is superior to triglycerides and other lipid ratios as an indicator of increased urinary albumin-to-creatinine ratio in the general population of China: a cross-sectional study. Lipids Health Dis. (2021) 20:13–2. doi: 10.1186/s12944-021-01442-8
44. Murugaiyah, V, Saeed, MAA, Kuong, Y-M, Murugesu, K, Parasuraman, S, Asmawi, MZ, et al. Lipid-lowering effect of hydroalcoholic extracts of Gynura procumbens in chemical-and high-fat diet-induced hyperlipidemic rats. Pharmacogn Mag. (2018) 14:184. doi: 10.4103/pm.pm_451_17
45. Wickramasuriya, SS, Park, I, Lee, K, Lee, Y, Kim, WH, Nam, H, et al. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccine. (2022) 10:172. doi: 10.3390/vaccines10020172
46. Megha, K, and Mohanan, P. Role of immunoglobulin and antibodies in disease management. Int J Biol Macromol. (2021) 169:28–38. doi: 10.1016/j.ijbiomac.2020.12.073
47. Li, Y, Jin, L, and Chen, T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int. (2020) 2020:2032057. doi: 10.1155/2020/2032057
48. Ao, T, Kikuta, J, and Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomol Ther. (2021) 11:1624. doi: 10.3390/biom11111624
49. Wu, C, Ma, H, Lu, S, Shi, X, Liu, J, Yang, C, et al. Effects of bamboo leaf flavonoids on growth performance, antioxidants, immune function, intestinal morphology, and cecal microbiota in broilers. J Sci Food Agr. (2024) 104:7656–67. doi: 10.1002/jsfa.13602
50. Huang, H, Wang, X, Yang, L, He, W, Meng, T, Zheng, K, et al. The effects of fenugreek extract on growth performance, serum biochemical indexes, immunity and NF-κB signaling pathway in broiler. Front Vet Sci. (2022) 9:882754. doi: 10.3389/fvets.2022.882754
51. Manogaran, M, Vuanghao, L, and Mohamed, R. Gynura procumbens ethanol extract and its fractions inhibit macrophage derived foam cell formation. J Ethnopharmacol. (2020) 249:112410. doi: 10.1016/j.jep.2019.112410
52. Miao, ZQ, Dong, YY, Qin, X, Yuan, JM, Han, MM, Zhang, KK, et al. Dietary supplementation of methionine mitigates oxidative stress in broilers under high stocking density. Poult Sci. (2021) 100:101231. doi: 10.1016/j.psj.2021.101231
53. De Almeida, W, Matei, JC, Akiyama Kitamura, RS, Gomes, MP, Leme, DM, Silva De Assis, HC, et al. Alkylphenols cause cytotoxicity and genotoxicity induced by oxidative stress in RTG-2 cell line. Chemosphere. (2023) 313:137387. doi: 10.1016/j.chemosphere.2022.137387
54. Chang, Q, Cai, H, Wei, L, and Lan, R. Chitosan oligosaccharides alleviate acute heat stress-induced oxidative damage by activating ERK1/2-mediated HO-1 and GSH-Px gene expression in breast muscle of broilers. Poult Sci. (2022) 101:101515. doi: 10.1016/j.psj.2021.101515
55. Yang, H, Yu, C, Yin, Z, Guan, P, Jin, S, Wang, Y, et al. Curcumin: a potential exogenous additive for the prevention of LPS-induced duck ileitis by the alleviation of inflammation and oxidative stress. J Sci Food Agric. (2023) 103:1550–60. doi: 10.1002/jsfa.12252
56. Li, C, Li, Y, Li, S, Chen, S, Liu, G, Deng, X, et al. Bacillus subtilis protects the ducks from oxidative stress induced by Escherichia coli: efficacy and molecular mechanism. Antioxidants (Basel). (2022) 11:1951. doi: 10.3390/antiox11101951
57. Ao, X, and Kim, IH. Effects of grape seed extract on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult Sci. (2020) 99:2078–86. doi: 10.1016/j.psj.2019.12.014
58. Tang, X, Liu, X, and Liu, H. Effects of dietary probiotic (Bacillus subtilis) supplementation on carcass traits, meat quality, amino acid, and fatty acid profile of broiler chickens. Front Vet Sci. (2021) 8:767802. doi: 10.3389/fvets.2021.767802
59. Ma, J, Chang, W, Liu, G, Zhang, S, Zheng, A, Li, Y, et al. Effects of flavones of sea buckthorn fruits on growth performance, carcass quality, fat deposition and lipometabolism for broilers. Poult Sci. (2015) 94:2641–9. doi: 10.3382/ps/pev250
60. Yan, Y, Chen, X, Huang, J, Huan, C, and Li, C. H2O2-induced oxidative stress impairs meat quality by inducing apoptosis and autophagy via ROS/NF-kappaB signaling pathway in broiler thigh muscle. Poult Sci. (2022) 101:101759. doi: 10.1016/j.psj.2022.101759
61. Li, F, Lu, Y, He, Z, Yu, D, Zhou, J, Cao, H, et al. Analysis of carcass traits, meat quality, amino acid and fatty acid profiles between different duck lines. Poult Sci. (2024) 103:103791. doi: 10.1016/j.psj.2024.103791
62. Qiao, Y, Guo, Y, Zhang, W, Guo, W, Oleksandr, K, Bozhko, N, et al. Effects of compound polysaccharides derived from Astragalus and Glycyrrhiza on growth performance, meat quality and antioxidant function of broilers based on serum metabolomics and Cecal microbiota. Antioxidants. (2022) 11:1872. doi: 10.3390/antiox11101872
63. Ahmad, S, Yousaf, MS, Tahir, SK, Rashid, MA, Majeed, KA, Naseem, M, et al. Effects of co-supplementation of β-Galacto-oligosaccharides and methionine on breast meat quality, meat oxidative stability and selected meat quality genes in broilers. Pak Vet J. (2023) 43:428–34. doi: 10.29261/pakvetj/2023.043
64. Huff-Lonergan, E, and Lonergan, SM. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. (2005) 71:194–204. doi: 10.1016/j.meatsci.2005.04.022
65. Jin, S, Yang, H, Liu, F, Pang, Q, Shan, A, and Feng, X. Effect of dietary curcumin supplementation on duck growth performance, antioxidant capacity and breast meat quality. Food Secur. (2021) 10:2981. doi: 10.3390/foods10122981
Keywords: Gynura procumbens extract, immunity, antioxidant capacity, meat quality, meat ducks
Citation: Ai G, Xiong P, Chen J, Song W, Song Q, Xu C, Su W, Zou Z, Wei Q and Chen X (2024) Effects of Gynura procumbens extract supplementation on growth performance, carcass traits, antioxidant capacity, immunity and meat quality of meat ducks. Front. Vet. Sci. 11:1508048. doi: 10.3389/fvets.2024.1508048
Edited by:
Moyosore Joseph Adegbeye, University of Africa, Bayelsa State, NigeriaReviewed by:
Aisha Khatoon, University of Agriculture, Faisalabad, PakistanMahmoud M. Azzam, King Saud University, Saudi Arabia
Copyright © 2024 Ai, Xiong, Chen, Song, Song, Xu, Su, Zou, Wei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qipeng Wei, d2VpcXA2NkBzaW5hLmNvbQ==; Xiaolian Chen, eGlhb2xpYW5jaGVuQDEyNi5jb20=
†These authors have contributed equally to this work
 Gaoxiang Ai1,2†
Gaoxiang Ai1,2† Chuanhui Xu
Chuanhui Xu Xiaolian Chen
Xiaolian Chen